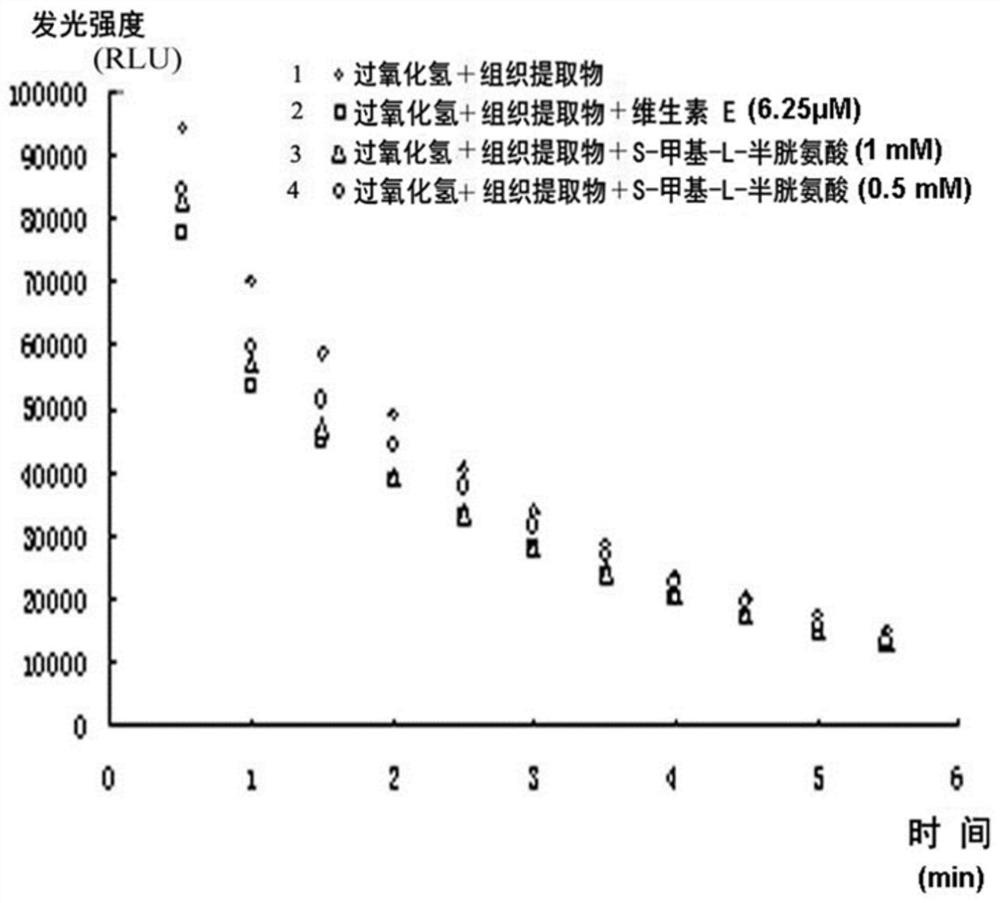
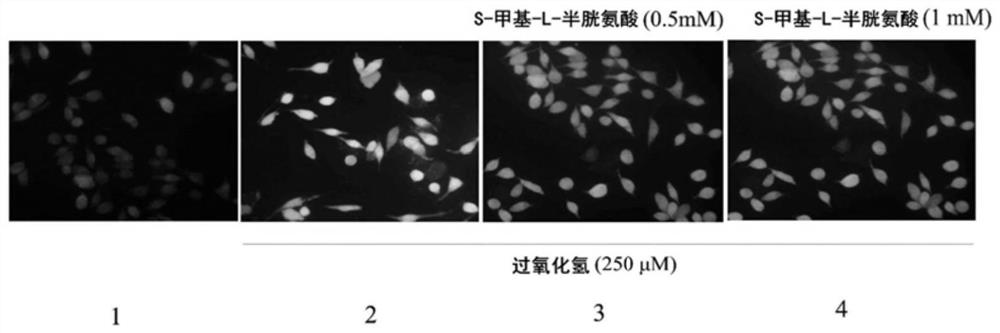
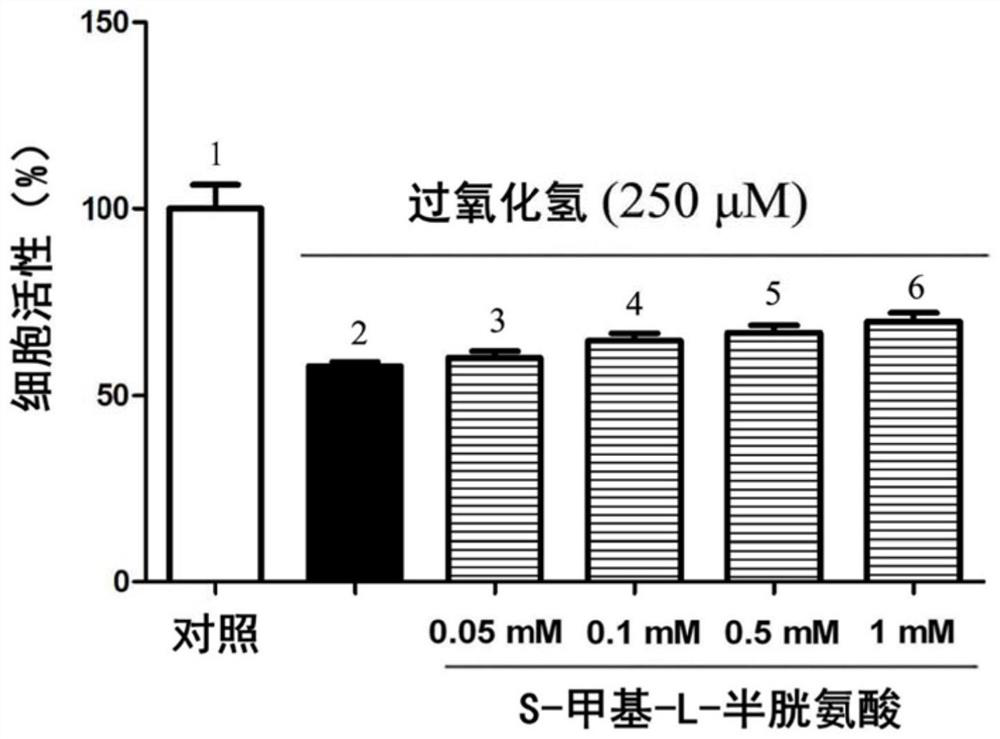
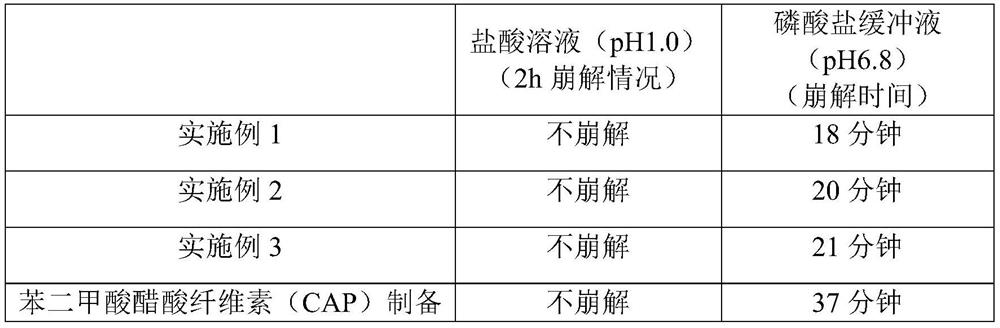
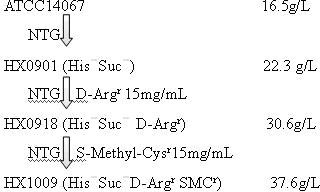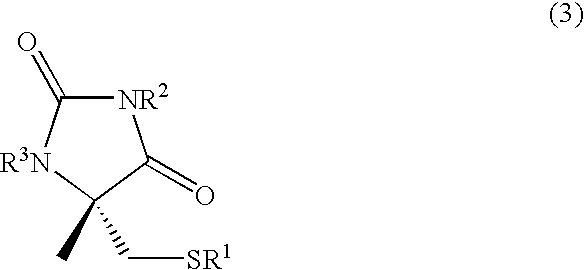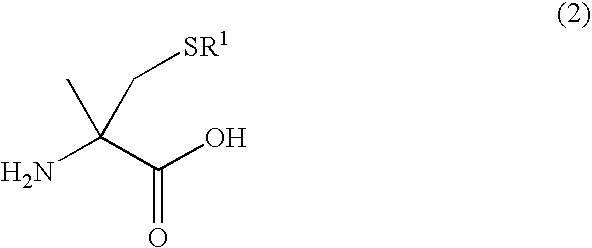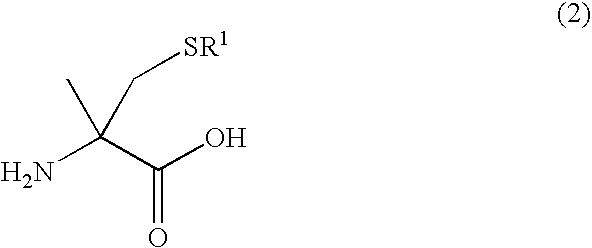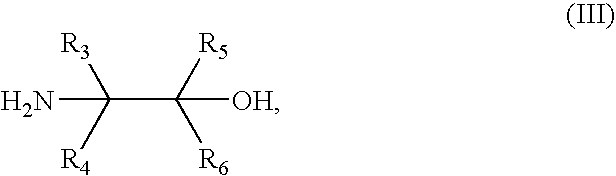Patents
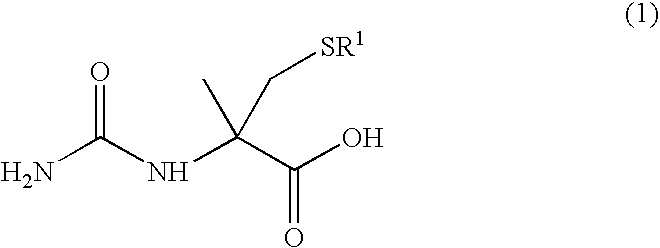
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "S-methylcysteine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
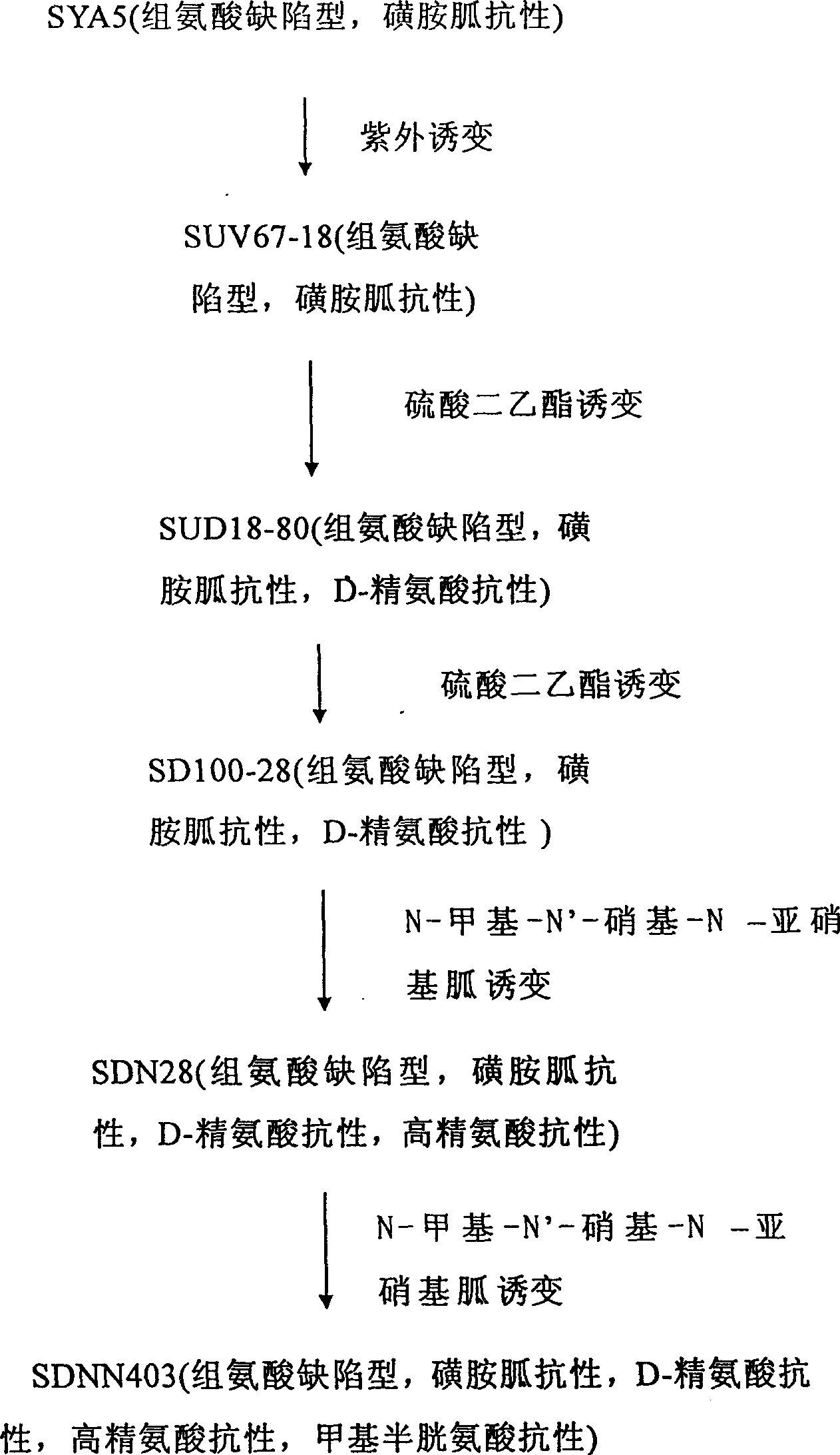
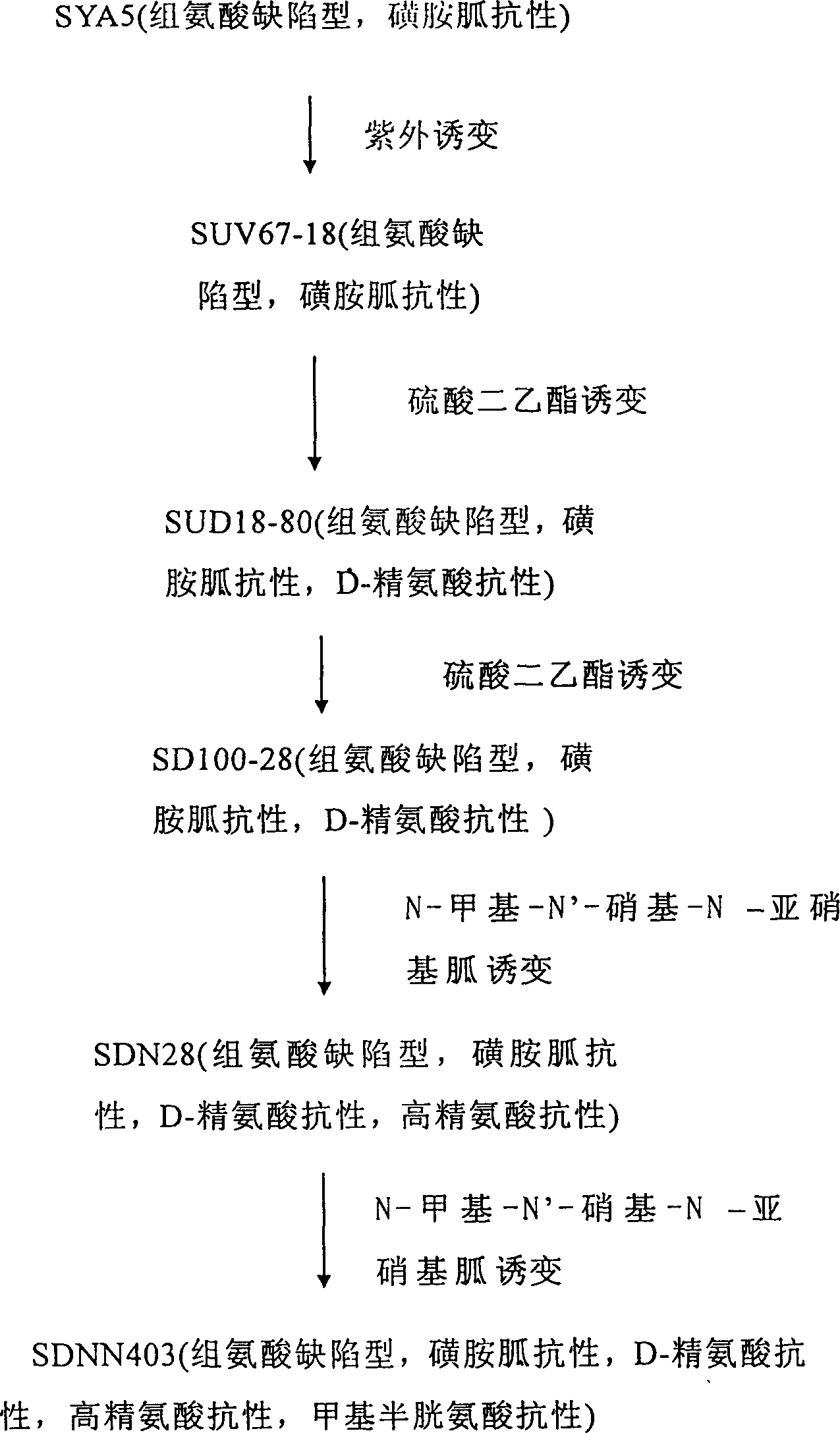
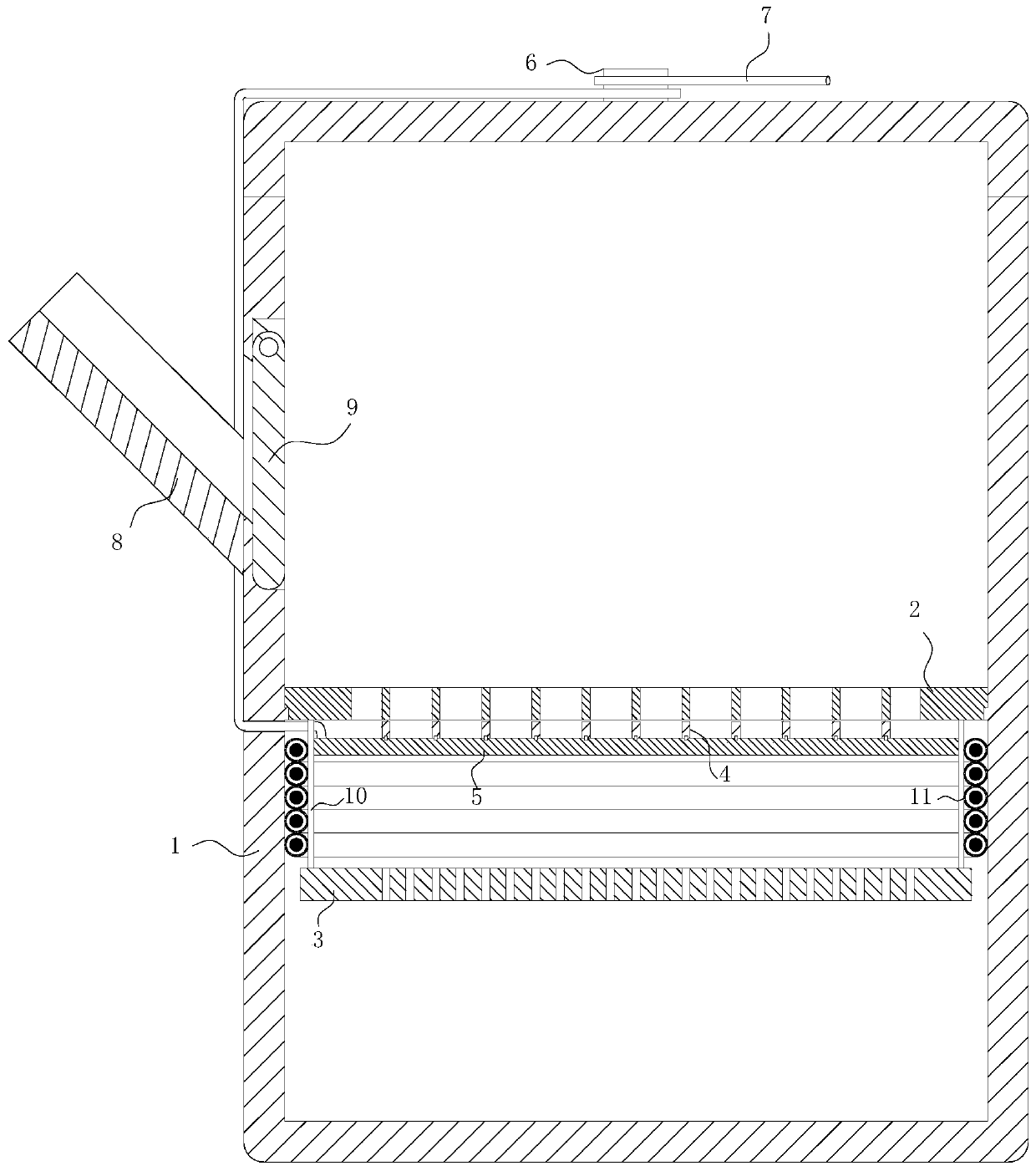
L-arginine producing strain and its mutation method and usage in producing L-arginine
InactiveCN1441055AHigh genetic stabilityGood genetic stabilityBacteriaFermentationBiotechnologyArginine
The present invention relates to the fermentation process of producing L-arginine. By using Corynebacterium crenatum SYA5 screened and preserved by the present lab as parent and through conventional physical and chemical mutation process and multiple structural analog resistance screening, mutant strain SDNN403 is obtained. Through culturing of the mutant strain under optimized condition, L-arginine is produced in the yield level of 30-35 g / L. The strain has high genetic stability, stable yield characteristic, high L-arginine yield level, less produced hetero acids and easy technological amplification, and is suitable four industrial production.
Owner:JIANGNAN UNIV
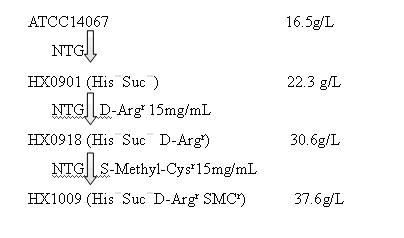
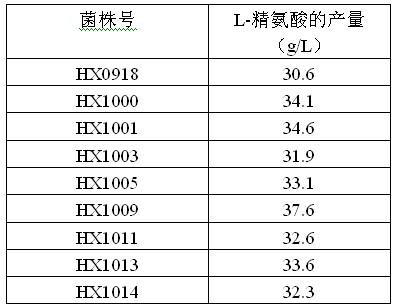
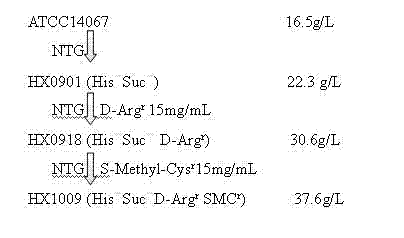
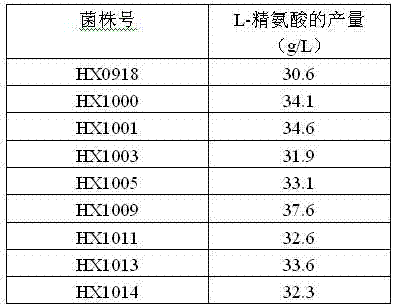
Strain capable of producing L-arginine and method for producing L-arginine by same
The invention relates to a strain capable of producing L-arginine and a method for producing the L-arginine by the strain and belongs to the technical field of biological engineering. For the strain, Brevibacterium flavum ATCC 14067 is used as a starting strain; nitrosoguanidine is adopted to carry out mutagenesis step by step; mutant strains with histidine and succinic acid auxotrophic strains are screened out so as to cut off a competitive metabolic pathway; and mutant strains (His-, Suc-, D-Argr, SMCr) with resistances of arginine structural analogs and cysteine structural analogs are screened out. The strain is named as Brevibacterium flavum HX1009, is preserved in the China general microbiological culture collection center, has the preservation number of CGMCC No.4464 and has the genetic characters of histidine auxotroph His-, succinic acid auxotroph Suc-, D-arginine resistance D-Argr and S-methyl cysteine resistance SMCr for improving the yield of the L-arginine. Under the optimized condition, the L-arginine is produced by fermentation on a fermentation tank with the volume of 5L to 5M3 and the arginine production level achieves 50 to 70g / L.
Owner:FUJIAN GUTIAN PHARMA
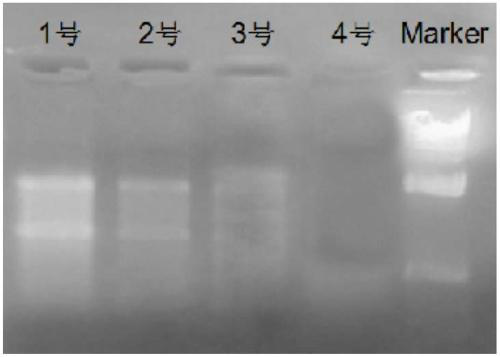
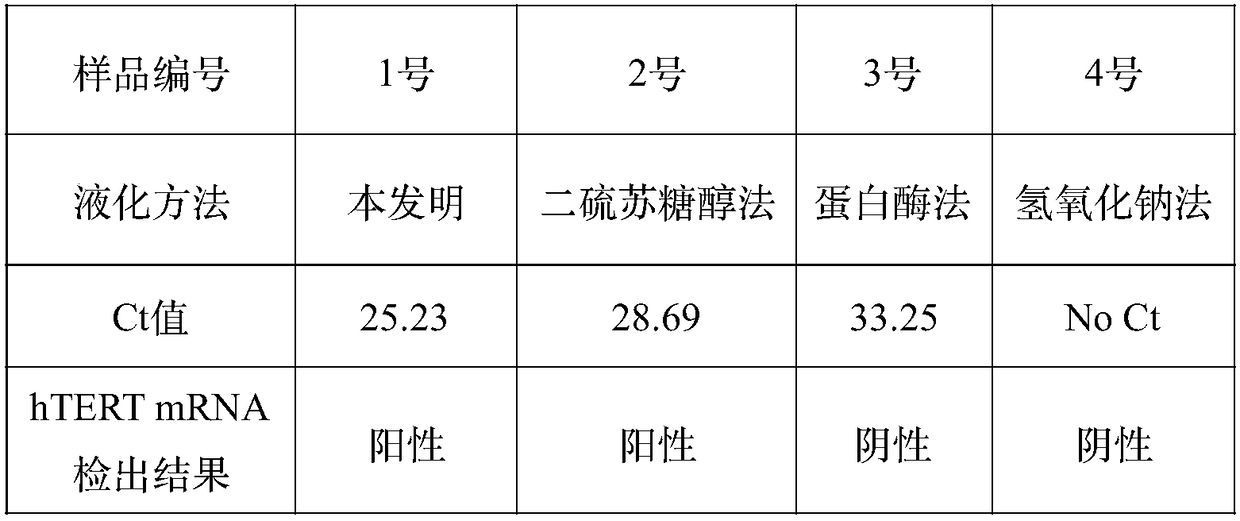
Reagent for liquefying phlegm and protecting nucleic acid
ActiveCN108949748AFully liquefiedProtection from degradationDNA preparationSimple componentPolyvinyl chloride
The invention provides a reagent for simultaneously liquefying phlegm and effectively protecting nucleic acid. The reagent contains guanidine, a cysteine derivative and a macromolecule inert particle,wherein guanidine is one or more of guanidine hydrochloride, guanidinium isothiocyanate, guanidine sulfate and guanidine carbonate; the cysteine derivative is one or more of cysteine, acetylcysteine,homocysteine, methylcysteine and cysteine hydrochloride; and the macromolecule inert particle is one or more of a polypropylene particle, a polyethylene particle, a polyvinyl chloride particle and apolystyrene particle. The reagent is a mixed solid of powder and particles and is capable of well permeating into a phlegm sample, and the phlegm sample is adequately mixed, so that phlegm can be adequately liquefied; and meanwhile, the reagent is capable of protecting nucleic acid in the phlegm sample from being degraded at a normal temperature for a long time. The reagent contains simple components and is low in cost, and the production process is simple, and the reagent is easy to use and operate and very suitable for wide clinical use.
Owner:ZHEJIANG JFK BIOLOGICAL TECH
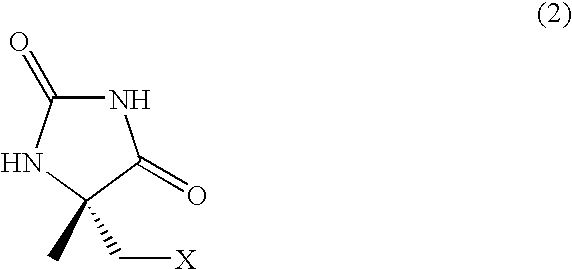
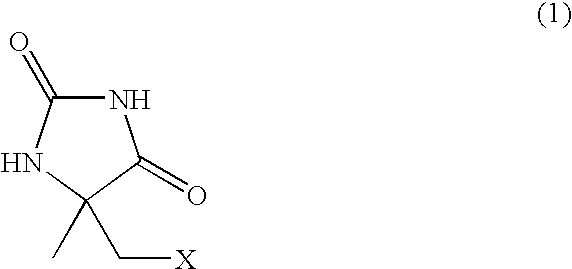
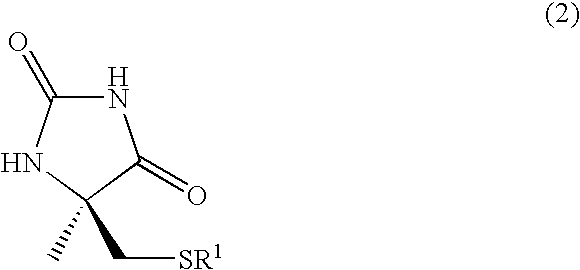
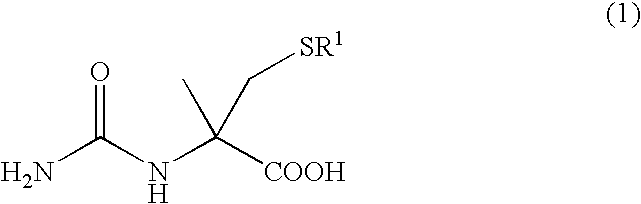
Process for producing l-alpha-methylcysteine derivative
A process for easily producing an L-α-methylcysteine derivative or its salt, which is useful as a drug intermediate, from a cheap easily procurable raw material through an enzymatic D-stereoselective hydrolysis of racemic 5-halomethyl-5-methyl-hydantoin. L-α-methylcysteine derivative or its salt is produced by converting racemic 5-halomethyl-5-methylhydantoin to L-5-halomethyl-5-methylhydantoin through an enzymatic D-stereoselective hydrolysis, reacting the L-5-halomethyl-5-methylhydantoin with a sulfurizing agent into L-5-methyl-5-thiomethylhydantoin and hydrolyzing the L-5-methyl-5-thiomethylhydantoin.
Owner:KANEKA CORP
Injectable parenteral medicinal preparation of temozolomide and preparation method thereof
ActiveCN102342931AImprove stabilityEasy to acceptOrganic active ingredientsPowder deliveryVitamin CPharmaceutical formulation
The invention relates to an injectable parenteral medicinal preparation of temozolomide and a preparation method thereof. The medicinal preparation comprises (1) temozolomide or pharmaceutically acceptable salt thereof, (2) at least one stabilizer, and (3) at least one aqueous diluent, wherein the stabilizer is selected from L-alanine, L-glycine, L-cysteine, L-cysteine hydrochloride anhydride, L-cysteine hydrochloride monohydrate, acetyl cysteine, S-carboxymethyl-L-cysteine, L-ethyl cysteine hydrochloride, L-methyl cysteine hydrochloride, vitamin C or a mixture thereof. The invention further relates to lyophilized power containing the medicinal preparation and products thereof.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Crystalline pharmaceutical compound and preparation method and usage thereof
ActiveCN102863365AUniform particle size distributionStable traitsOrganic active ingredientsRespiratory disorderDiseaseAntioxidant
The invention relates to an S-(carboxymethyl)-L-cysteine ammonium salt water crystalline pharmaceutical compound (having the following structural formula) and a preparation method and the application of the compound in the preparation of medicines for eliminating phlegm as well as medicines for preventing and treating respiratory system diseases such as chronic obstructive pulmonary diseases (COPD) and the like. After the compound is adopted, the COPD model rat airway resistance is remarkably reduced, the generation of oxide is reduced, the antioxidant level is increased, and the damage of oxide, inflammatory mediator and the like to the lung can be relieved.
Owner:GUANGZHOU BAIYUNSHAN PHARMA HLDG CO LTD BAIYUNSHAN PHARMA GENERAL FACTORY +1
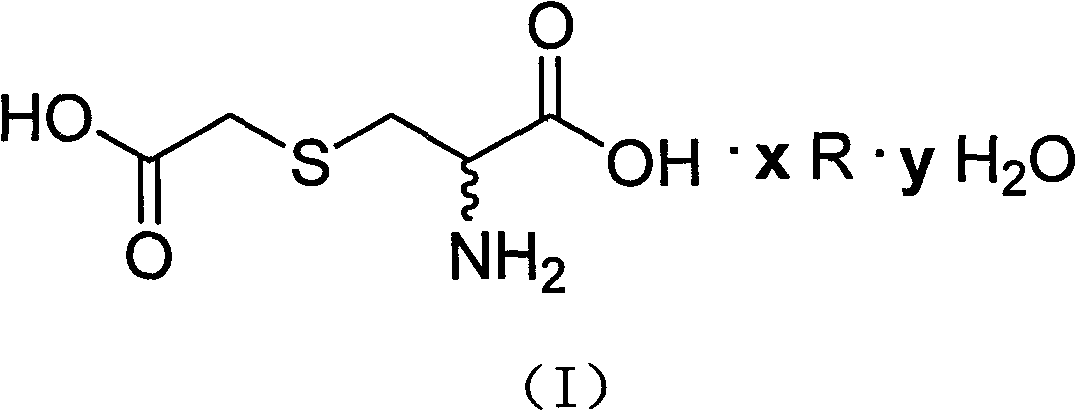
S-(carboxymethyl)-cysteine pharmaceutical compound and preparation method and usage thereof
ActiveCN102863364AThe preparation method is simple and easy to controlHigh yieldOrganic active ingredientsOrganic chemistryDiseaseMedicine
The invention relates to an S-(carboxymethyl)-cysteine pharmaceutical compound and a preparation method and the usage of the compound; the structural formula of the compound is shown by (I); and the compound is especially applied to the preparation of medicines for eliminating phlegm as well as medicines for preventing and treating respiratory system diseases such as chronic obstructive pulmonary diseases (COPD) and the like. After the compound is adopted, the COPD model rat airway resistance is remarkably reduced, the generation of oxide is reduced, the antioxidant level is increased, and the damage of oxide, inflammatory mediator and the like to the lung can be relieved.
Owner:GUANGZHOU BAIYUNSHAN PHARMA HLDG CO LTD BAIYUNSHAN PHARMA GENERAL FACTORY +1
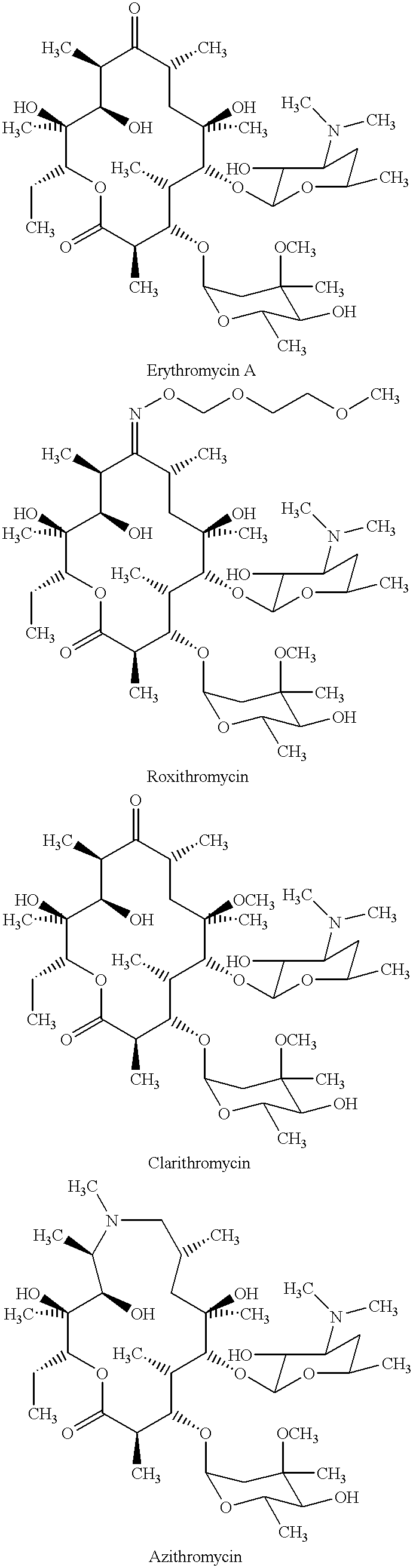
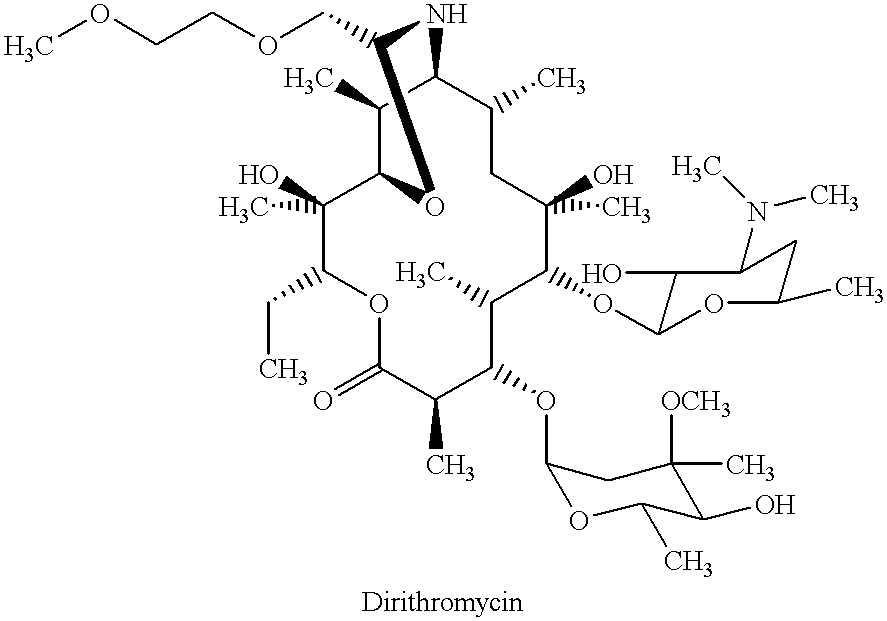
Derivatives of erythromycin, clarithromycin, roxithromycin or azithromycin with antibiotic and mucolytic activity
InactiveUS20010031736A1Improved pharmacological profileGood effectBiocideSugar derivativesRoxithromycinAzithromycin
A pharmaceutical with an enhanced pharmaceutical profile comprises a mucolytic and an antibiotic in which the mucolytic is present in an amount of greater than one molar equivalent of the antibiotic. The antibiotic may be selected from Erythromycin, Roxithromycin, Clarithromycin, Azithromycin, Dirithromycin; and pharmaceutically acceptable salts or esters thereof. The mucolytic is a mucolytically active thiol, especially N-acetylcysteine, mercaptoethanesulfonic acid, tiopronin or methylcysteine. The adducts can be isolated via a simple and efficient process.
Owner:RUSSINSKY
Derivatives of erythromycin, clarithromycin, roxithromycin or azithromycin with antibiotic and mucolytic activity
A pharmaceutical with an enhanced pharmaceutical profile comprises a mucolytic and an antibiotic in which the mucolytic is present in an amount of greater than one molar equivalent of the antibiotic. The antibiotic may be selected from Erythromycin, Roxithromycin, Clarithromycin, Azithromycin, Dirithromycin; and pharmaceutically acceptable salts or esters thereof. The mucolytic is a mucolytically active thiol, especially N-acetylcysteine, mercaptoethanesulfonic acid, tiopronin or methylcysteine. The adducts can be isolated via a simple and efficient process.
Owner:RUSSINSKY
L-arginine producing strain and its mutation method and usage in producing L-arginine
InactiveCN1169946CHigh genetic stabilityGood genetic stabilityBacteriaFermentationBiotechnologyD-Arginine
The present invention relates to the fermentation process of producing L-arginine. By using Corynebacterium crenatum SYA5 screened and preserved by the present lab as parent and through conventional physical and chemical mutation process and multiple structural analog resistance screening, mutant strain SDNN403 is obtained. Through culturing of the mutant strain under optimized condition, L-arginine is produced in the yield level of 30-35 g / L. The strain has high genetic stability, stable yield characteristic, high L-arginine yield level, less produced hetero acids and easy technological amplification, and is suitable four industrial production.
Owner:JIANGNAN UNIV
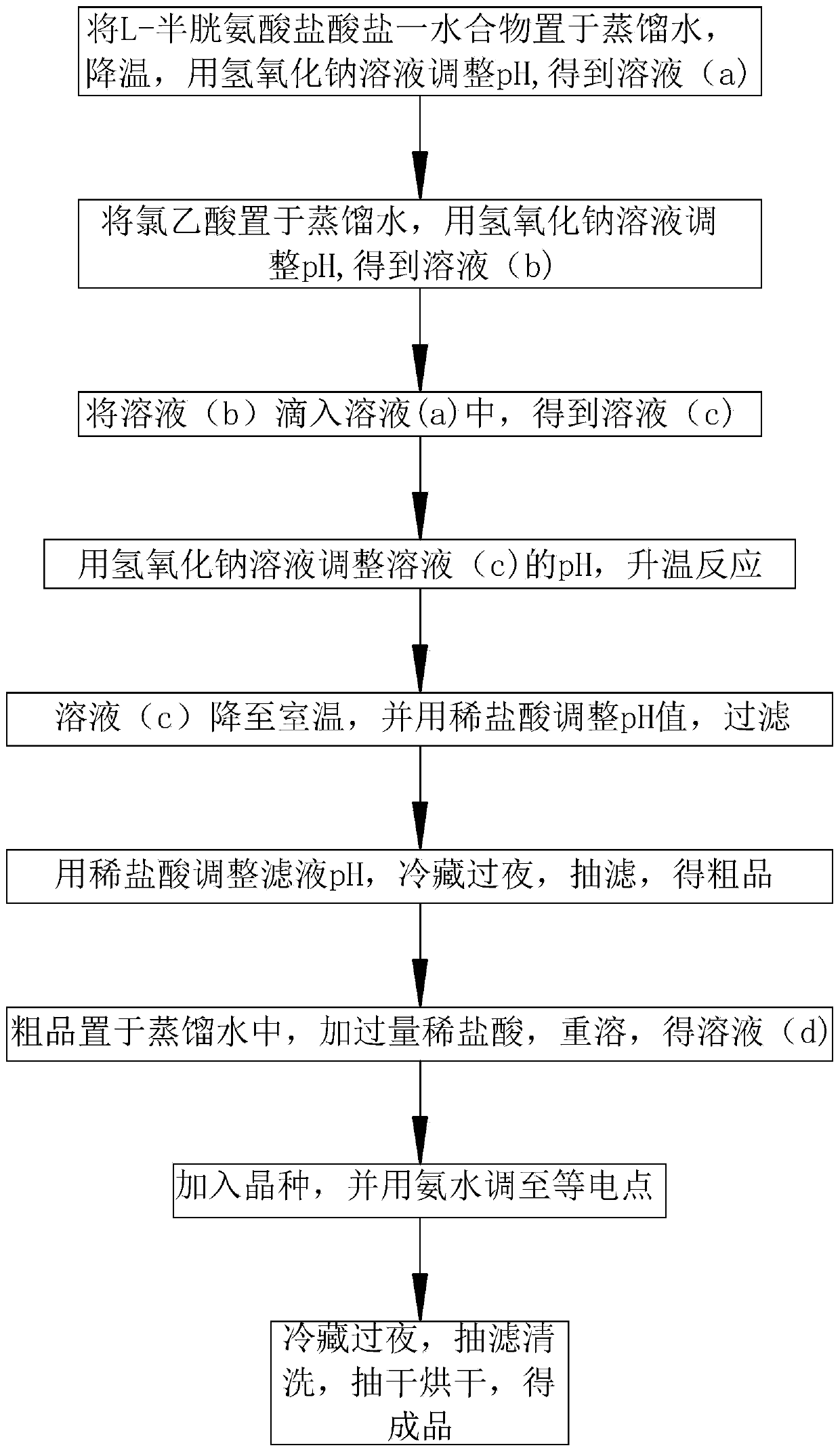
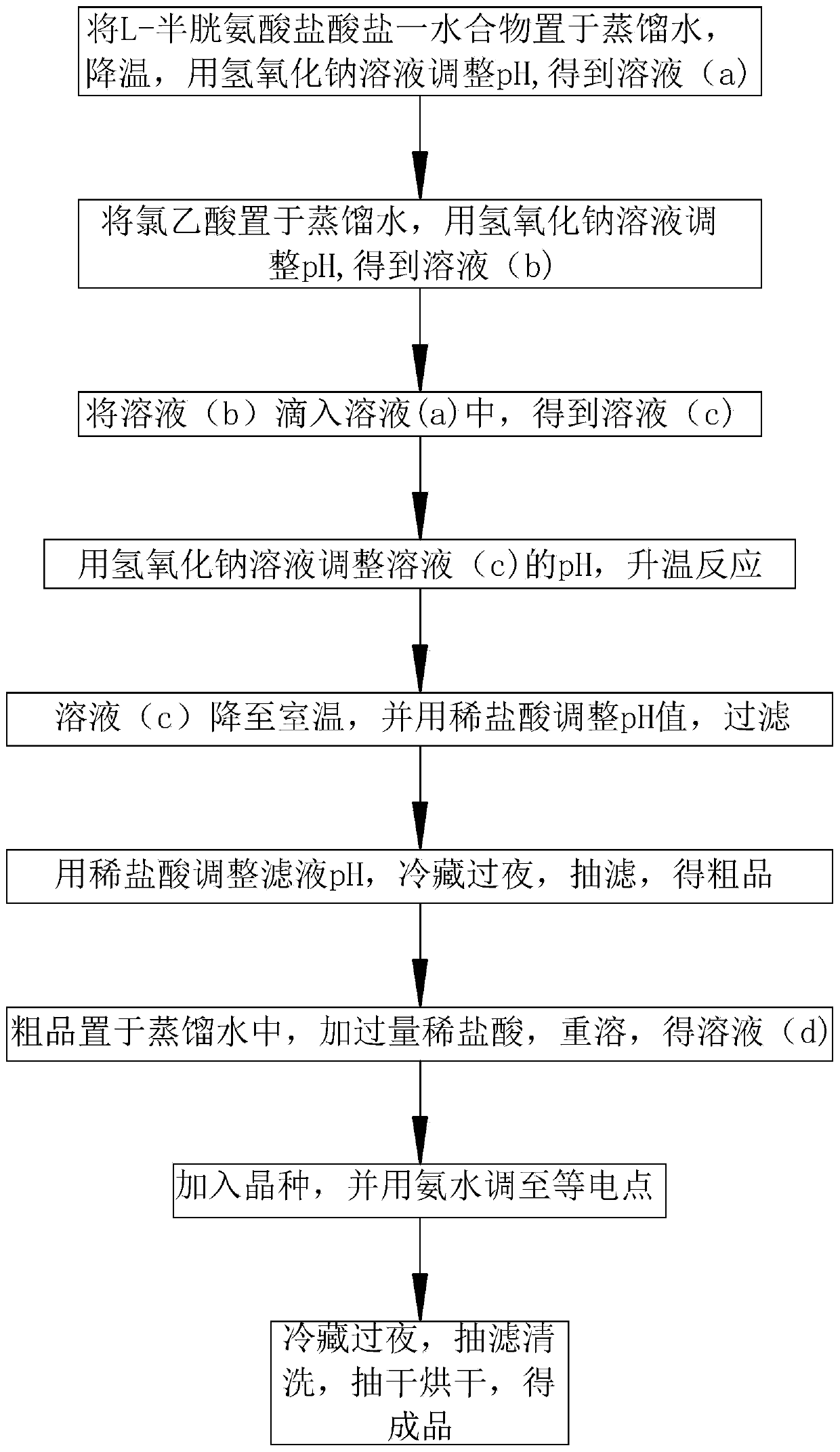
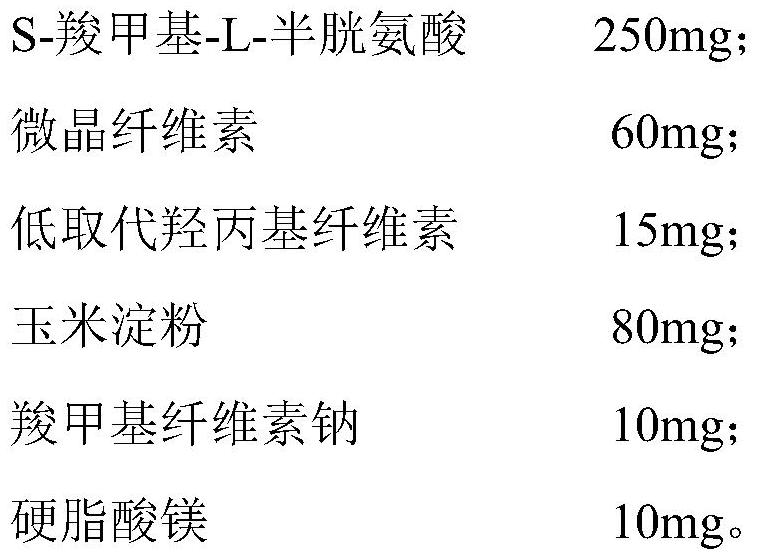
Preparation method of S-carboxymethyl-L-cysteine
InactiveCN111138326AHigh densityReduce packageOrganic compound preparationSulfide preparationPhysical chemistryChloroacetic acids
The invention discloses a preparation method of S-carboxymethyl-L-cysteine, which relates to the field of amino acid production, and mainly comprises the following steps: 1, dissolving L-cysteine hydrochloride monohydrate, cooling the solution to 0-5 DEG C, and regulating the pH value to 9-10 to obtain a solution (a); 2, dissolving chloroacetic acid, and adjusting the pH value to 6-7 to obtain a solution (b); 3, dropwise adding the solution (b) into the solution (a), and carrying out heat preservation to obtain a solution (c); 4, adjusting the pH value of the solution (c) to 8, and heating thesolution to react for 1 hour; 5, cooling the solution (c) to room temperature, adjusting the pH value to 5.5, and filtering the solution; 6, adjusting the pH value of the filtrate to 2.5-3.0, refrigerating the filtrate overnight, carrying out suction filtration, and drying the filtrate to obtain a crude product; 7, dissolving the crude product to obtain a solution (d), and acidifying the solution(d) to an isoelectric point; 8, adding a seed crystal into the solution (d), and adjusting the solution (d) to an isoelectric point; 9, purifying a finish product. By means of the preparation method,S-carboxymethyl-L-cysteine with large particle size, high density and high purity can be obtained, and the preparation method is suitable for being used in multiple fields.
Owner:NINGBO YUANFA BIOENG
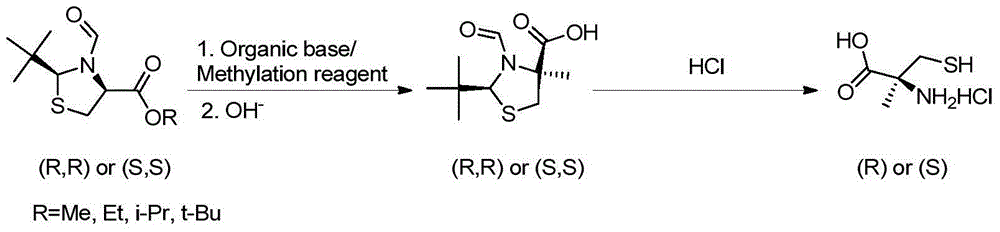
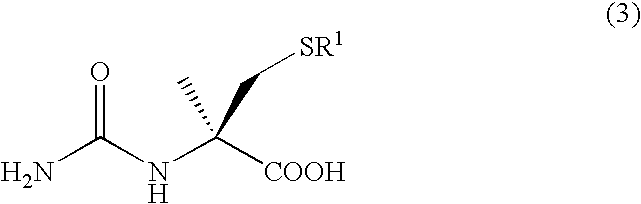
Preparation method of chirality 2-methyl cysteine and hydrochloride thereof
InactiveCN104892477AHigh purityHigh optical purityOrganic chemistryOrganic compound preparationOrganic baseS-methylcysteine
The invention discloses a preparation method of chirality 2-methyl cysteine and hydrochloride thereof. The preparation method comprises the following steps: chirality 2-tert-butyl-3-formoxyl thiaxolidine-4-carboxylic ester, a methylation reagent and organic base are subjected to methylation in organic solvent to obtain reaction solution, inorganic base is added in the reaction solution, chirality-invariable 2-tert-butyl-3-formoxyl thiaxolidine-4-methyl-4-carboxylic acid is obtained through one-pot reaction, then the chirality-invariable 2-tert-butyl-3-formoxyl thiaxolidine-4-methyl-4-carboxylic acid is subjected to reflux reaction in hydrochloric acid solution to obtain chirality 2-methyl cysteine hydrochloride, and finally, chirality 2-methyl cysteine is obtained through aftertreatment. Compared with the prior art, the preparation method disclosed by the invention is simple in operating process, the production cost is lower, the reaction yield and the optical purity of products are higher, and excellent industrial application value is realized.
Owner:SYNCOZYMES SHANGHAI
Process for producing optically active alpha-methylcysteine derivative
The present invention provides a simple industrial process for producing an L- or D-optically active α-methylcysteine derivative or its salt, which is a useful pharmaceutical intermediate, from readily available, inexpensive raw materials. In a process for producing an L- or D-optically active α-methylcysteine derivative or its salt, a racemic N-carbamoyl-α-methylcysteine derivative or its salt is D-selectively cyclized with hydantoinase to produce a D-5-methyl-5-thiomethylhydantoin derivative or its salt and an N-carbamoyl-α-methyl-L-cysteine derivative or its salt, which are then subjected to deprotection of the amino group and the sulfur atom, and hydrolysis.
Owner:KANEKA CORP
Garlic formulation and a process for preparing the same for treatment of diabetes
ActiveUS20140147528A1Avoid tissue damageUseful in treatmentBiocideAnimal repellantsS-AllylmercaptocysteineMedicine
The present invention relates to a garlic formulation enriched with sulphur containing amino acids and a process for enriching sulphur containing amino acids in garlic. The sulphur containing amino acids includes S-allylcysteine, S-methylcysteine, and S-allylmercaptocysteine. The percentage of enriched garlic concentrate after processing is in the range of 7.5-9.5%. The garlic formulation is useful in the treatment of Diabetes Mellitus (DM) and prevention of tissue and organ damage that occurs frequently in diabetic patients.
Owner:DEEPA M A
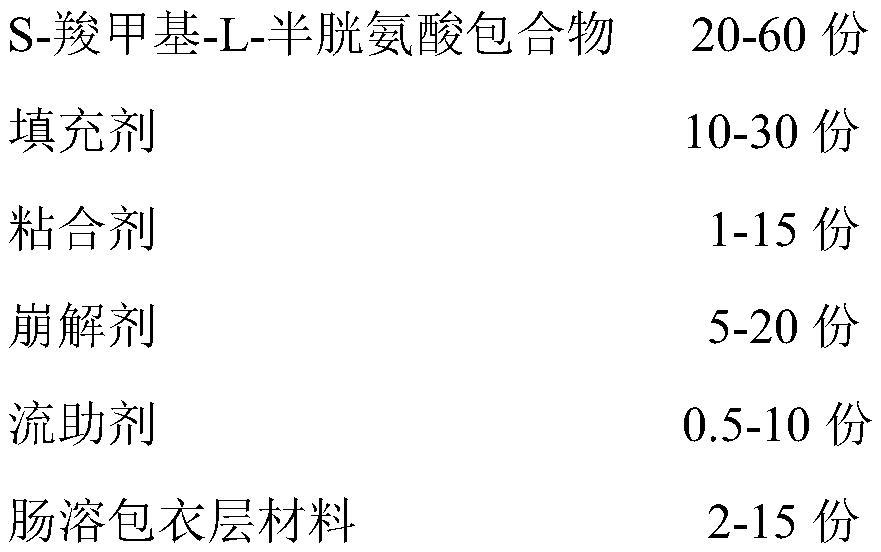
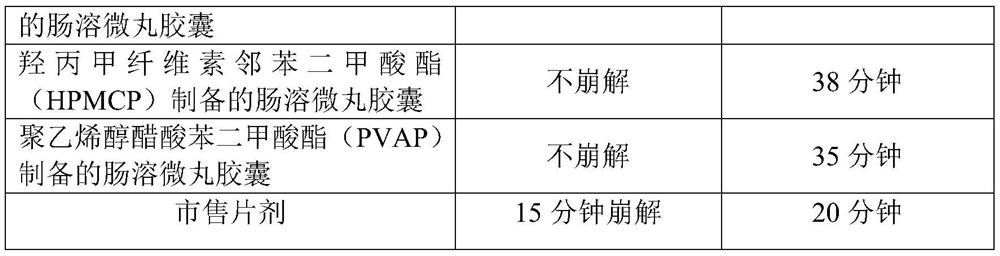
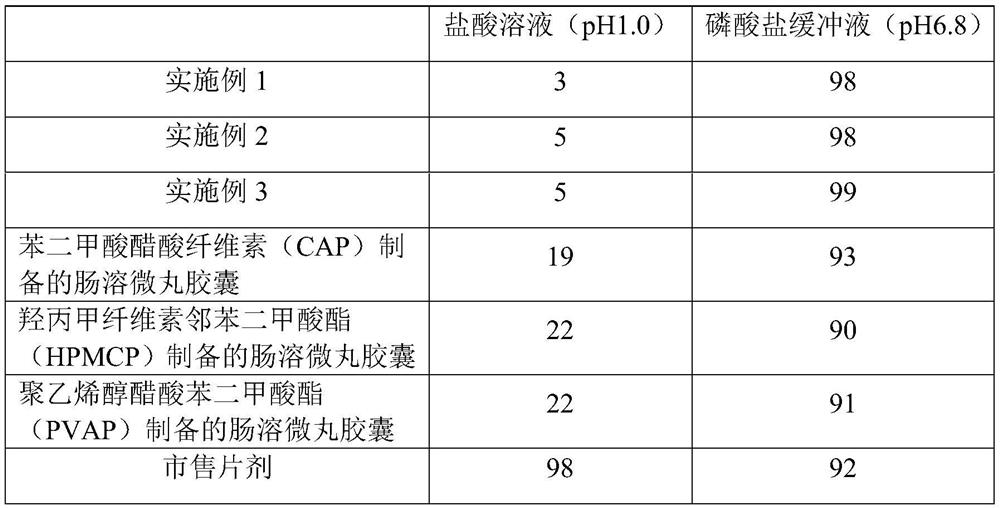
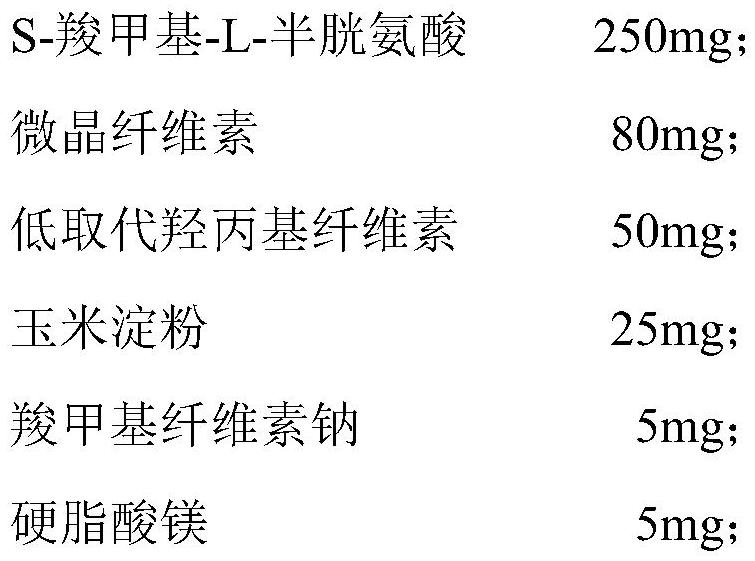
A kind of s-carboxymethyl-l-cysteine inclusion compound and its enteric-coated preparation composition
ActiveCN108324957BImprove bioavailabilityReduce stimulationOrganic active ingredientsPharmaceutical non-active ingredientsS-methylcysteineBULK ACTIVE INGREDIENT
The invention provides an S-carboxymethyl-L-cysteine inclusion compound. The S-(carboxymethyl)-L-cysteine clathrate of the present invention has a good dispersion effect, can effectively reduce the stimulation of S-(carboxymethyl)-L-cysteine to the digestive tract, and increase the and the stability of its formulations. The enteric-coated preparation containing the clathrate can avoid being destroyed in the acidic environment of the stomach and reduce the irritation to the stomach; it directly reaches the small intestine at the absorption site, dissolves rapidly and releases the active ingredient, increasing the S-carboxymethyl-L- Bioavailability of cysteine.
Owner:GUANGZHOU BAIYUNSHAN PHARMA HLDG CO LTD BAIYUNSHAN PHARMA GENERAL FACTORY
Antibacterial peptide lexapeptide and its preparation method and use
ActiveCN106188253BNo inhibitory activityHigh antibacterial activityAntibacterial agentsAntimycoticsHeterologousLanthiopeptin
The invention discloses an antimicrobial peptide Lexapeptide, and a preparation method and application thereof. The lanthiopeptin antimicrobial peptide Lexapeptide is a new compound obtained through separating after heterologously expressing a bacterial artificial chromosome library derived from a Streptomyces rochei Sal35 bacterial strain in Streptomyces lividans SBT5, is formed by 38 amino acids, and is the lanthiopeptin which is reported for the first time and simultaneously contains a C terminal 2-amidogen alkenyl-3-methyl-aminothiopropionic acid structure and an N terminal methylated modification structure. With the highly structural modification, the Lexapeptide has better stability compared with other lanthiopeptin antimicrobial peptides. The Lexapeptide can be used as an anti-gram-positive bacterium preparation so as to provide a solution for a generally clinical dug-resistance problem.
Owner:SHANGHAI JIAO TONG UNIV
Medicine and functional food for preventing and treating post-traumatic stress syndrome
The invention discloses medicine or functional food for preventing and / or treating post-traumatic stress syndrome. The medicine or functional food consists of S-methyl-L-cysteine(thiomethy L-cysteine)or amino acid salts of thiomethy L-cysteine and additives or / and carriers acceptable in pharmacy or food industry. The S-methyl-L-cysteine can be provided by a plant extract which is used as a raw material and is rich in the S-methyl-L-cysteine. The medicine or functional food can generate a dissipation promoting effect on fear memory related to traumatic stress by reducing oxidative stress levelin a body, reducing free radicals and enhancing synaptic plasticity and other characteristics dependent on NMDA receptors in relevant brain regions, thereby treating behavioral abnormalities caused by post-traumatic stress.
Owner:HUAZHONG UNIV OF SCI & TECH
S-(carboxymethyl)-L-cysteine ammonium anhydrous crystal form, and preparation method and applications thereof
ActiveCN111333555ALess irritatingIncrease contentOrganic active ingredientsOrganic chemistry methodsDiseasePharmaceutical drug
The invention discloses an S-(carboxymethyl)-L-cysteine ammonium anhydrous crystal form, and a preparation method and applications thereof. The X-powder diffraction pattern of the crystal form is represented by a pattern shown in the description. The preparation method of the crystal form is mild in reaction condition, simple in process and suitable for industrial production. The invention also discloses an application of the crystal form in the preparation of a phlegm eliminating medicine and an application of the crystal form in the preparation of a medicine for preventing and / or treating respiratory system diseases.
Owner:GUANGZHOU BAIYUNSHAN PHARMA HLDG CO LTD BAIYUNSHAN PHARMA GENERAL FACTORY
Novel purpose of S-(carboxymethyl)-L-cysteine
InactiveCN107865831AImproves oxidative stressImprove inflammatory damageOrganic active ingredientsRespiratory disorderDiseaseAtmosphere
The invention relates to a novel purpose of medicine, in particular to application of S-(carboxymethyl)-L-cysteine and pharmacologically acceptable salts of the S-(carboxymethyl)-L-cysteine to prevention and / or treatment of respiratory system diseases and the like caused by haze. The S-(carboxymethyl)-L-cysteine and the pharmacologically acceptable salts of the S-(carboxymethyl)-L-cysteine are used; and the inflammation damage and oxidation operation of atmosphere PM2.5 on the lungs of mice can be obviously reduced, so that the S-(carboxymethyl)-L-cysteine and the pharmacologically acceptablesalts of the S-(carboxymethyl)-L-cysteine can be applied to preparation of medicine for preventing and treating diseases caused by haze.
Owner:GUANGZHOU BAIYUNSHAN PHARMA HLDG CO LTD BAIYUNSHAN PHARMA GENERAL FACTORY
Method for preparing high-purity chiral 2-smethylcysteine s and hydrochloride of high-purity chiral 2-smethylcysteine s
InactiveCN105061275AHigh purityEasy to operateOrganic chemistryOrganic compound preparationOrganic solventMethylcysteine hydrochloride
The invention discloses a method for preparing high-purity chiral 2-smethylcysteine s and hydrochloride of high-purity chiral 2-smethylcysteine s. The method comprises the steps that crude chiral 2-methyl cysteine hydrochloride and a reducing agent are added to an organic solvent, crude chiral 2-methyl cysteine hydrochloride is dissolved at room temperature or under the heating condition, stirring and cooling crystallization are carried out to obtain high-purity chiral 2-methyl cysteine hydrochloride; aftertreatment is carried out on high-purity chiral 2-methyl cysteine hydrochloride to obtain high-purity chiral 2-smethylcysteine s. Compared with the prior art, the adopted method is simple in operation process, low in production cost and high in product purity, and good industrial application value is achieved.
Owner:SYNCOZYMES SHANGHAI
Medicine and functional food for preventing and treating post-traumatic stress syndrome
The invention discloses a drug or functional food for preventing or / and treating post-traumatic stress syndrome, which is composed of S-methyl-L-cysteine (thiomethyl L-cysteine) or Amino acid salt of thiomethyl L-cysteine and pharmaceutically or food industry-acceptable additives or / and carriers, described S-methyl-L-cysteine can be composed of rich Provided by plant extracts containing S‑Methyl‑L‑Cysteine. The medicine or functional food of the present invention can reduce the level of oxidative stress in the body, reduce free radicals, enhance the synaptic plasticity of relevant brain regions dependent on NMDA receptors, etc., and produce the effect of promoting the dissipation of fear memory related to trauma stress, thereby treating trauma Behavioral abnormalities brought on by post-stress.
Owner:HUAZHONG UNIV OF SCI & TECH
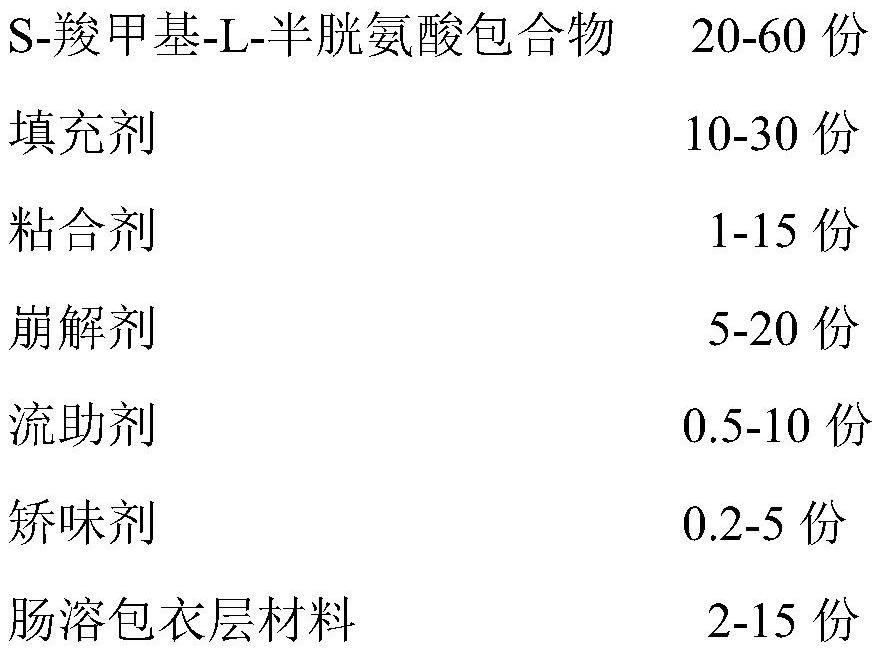
A kind of s-carboxymethyl-l-cysteine enteric-coated pellet capsule
ActiveCN108338978BImprove bioavailabilityReduce stimulationOrganic active ingredientsPharmaceutical non-active ingredientsMedicineS-methylcysteine
The invention discloses an S-carboxymethyl-L-cysteine enteric pellet. The enteric pellet contains a pellet core, an isolation layer and an enteric layer, wherein the enteric layer is obtained by coating and packaging a water-based acrylic acid enteric-coated material. The invention further discloses an S-carboxymethyl-L-cysteine enteric pellet capsule containing the S-carboxymethyl-L-cysteine enteric pellets. The S-carboxymethyl-L-cysteine enteric pellet capsule disclosed by the invention cannot be dissolved in the stomach, has a little stimulation to the stomach, and is stable in quality in astorage process.
Owner:GUANGZHOU BAIYUNSHAN PHARMA HLDG CO LTD BAIYUNSHAN PHARMA GENERAL FACTORY
Strain capable of producing L-arginine and method for producing L-arginine by same
The invention relates to a strain capable of producing L-arginine and a method for producing the L-arginine by the strain and belongs to the technical field of biological engineering. For the strain, Brevibacterium flavum ATCC 14067 is used as a starting strain; nitrosoguanidine is adopted to carry out mutagenesis step by step; mutant strains with histidine and succinic acid auxotrophic strains are screened out so as to cut off a competitive metabolic pathway; and mutant strains (His-, Suc-, D-Argr, SMCr) with resistances of arginine structural analogs and cysteine structural analogs are screened out. The strain is named as Brevibacterium flavum HX1009, is preserved in the China general microbiological culture collection center, has the preservation number of CGMCC No.4464 and has the genetic characters of histidine auxotroph His-, succinic acid auxotroph Suc-, D-arginine resistance D-Argr and S-methyl cysteine resistance SMCr for improving the yield of the L-arginine. Under the optimized condition, the L-arginine is produced by fermentation on a fermentation tank with the volume of 5L to 5M3 and the arginine production level achieves 50 to 70g / L.
Owner:FUJIAN GUTIAN PHARMA
Process for producing optically active alpha -methylcysteine derivative
InactiveUS20060172393A1Readily availableConveniently producedHydrolasesFermentationCombinatorial chemistryPerylene derivatives
A process for conveniently and industrially producing an optically active α-methylcysteine derivative, which is useful as an intermediate of medicines and the like, from an inexpensive and readily available material is provided. The present invention relates to a process for producing a racemic or optically active α-methylcysteine derivative including a step of hydrolyzing a racemic or optically active N-carbamyl-α-methylcysteine derivative by treating with decarbamylase, and a process for producing an optically active α-methylcysteine derivative and an optically active N-carbamyl-α-methylcysteine derivative having a configuration opposite to that of the compound including a step of stereoselectively hydrolyzing a racemic N-carbamyl-α-methylcysteine derivative by treating with decarbamylase.
Owner:KANEKA CORP
Application of S-(carboxymethyl)-L-cysteine to preparation of medicines for preventing and treating respiratory system diseases
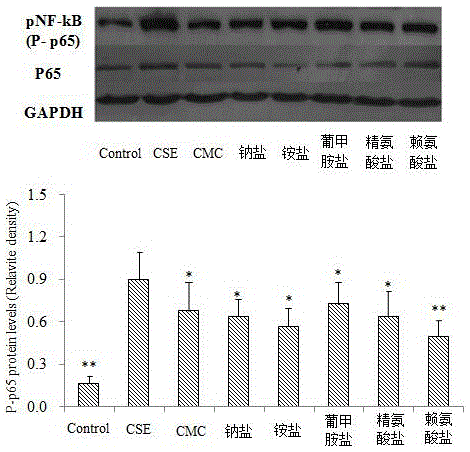
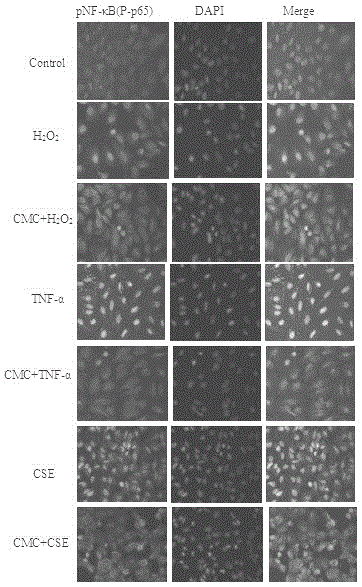
InactiveCN105311009AOrganic active ingredientsRespiratory disorderInflammatory factorsBronchial epithelium
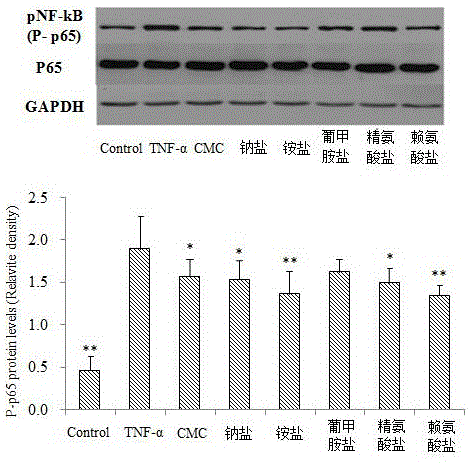
The invention relates to novel application of medicines, in particular to application of S-(carboxymethyl)-L-cysteine and pharmaceutically acceptable salts thereof to preparation of medicines for preventing and treating respiratory system diseases caused by NF-kappa B signal pathway activation. The application has the advantages that the S-(carboxymethyl)-L-cysteine and the pharmaceutically acceptable salts thereof can remarkably inhibit not only NF-kappa B signal pathway activation and release of inflammatory factors interleukin-6 and interleukin-8 due to H2O2, TNF (rumor necrosis factor)-alpha and cigarette smoke extract but also acute lung injury, release of the interleukin-6 and the interleukin-8 in bronchial asthma mice bronchoalveolar lavage fluid and expression of NF-kappa B in lung tissues remarkably, thereby being applied to prevention and treatment of NF-kappa B-mediated respiratory system diseases such as bronchial asthma, acute lung injury and acute respiratory distress syndrome.
Owner:GUANGZHOU BAIYUNSHAN PHARMA HLDG CO LTD BAIYUNSHAN PHARMA GENERAL FACTORY +1
A kind of preparation method of s-(carboxymethyl)-cysteine
ActiveCN110452142BAdjust the degree of deformationShorten the durationOrganic chemistry methodsSulfide preparationOrganic acidChloroacetic acids
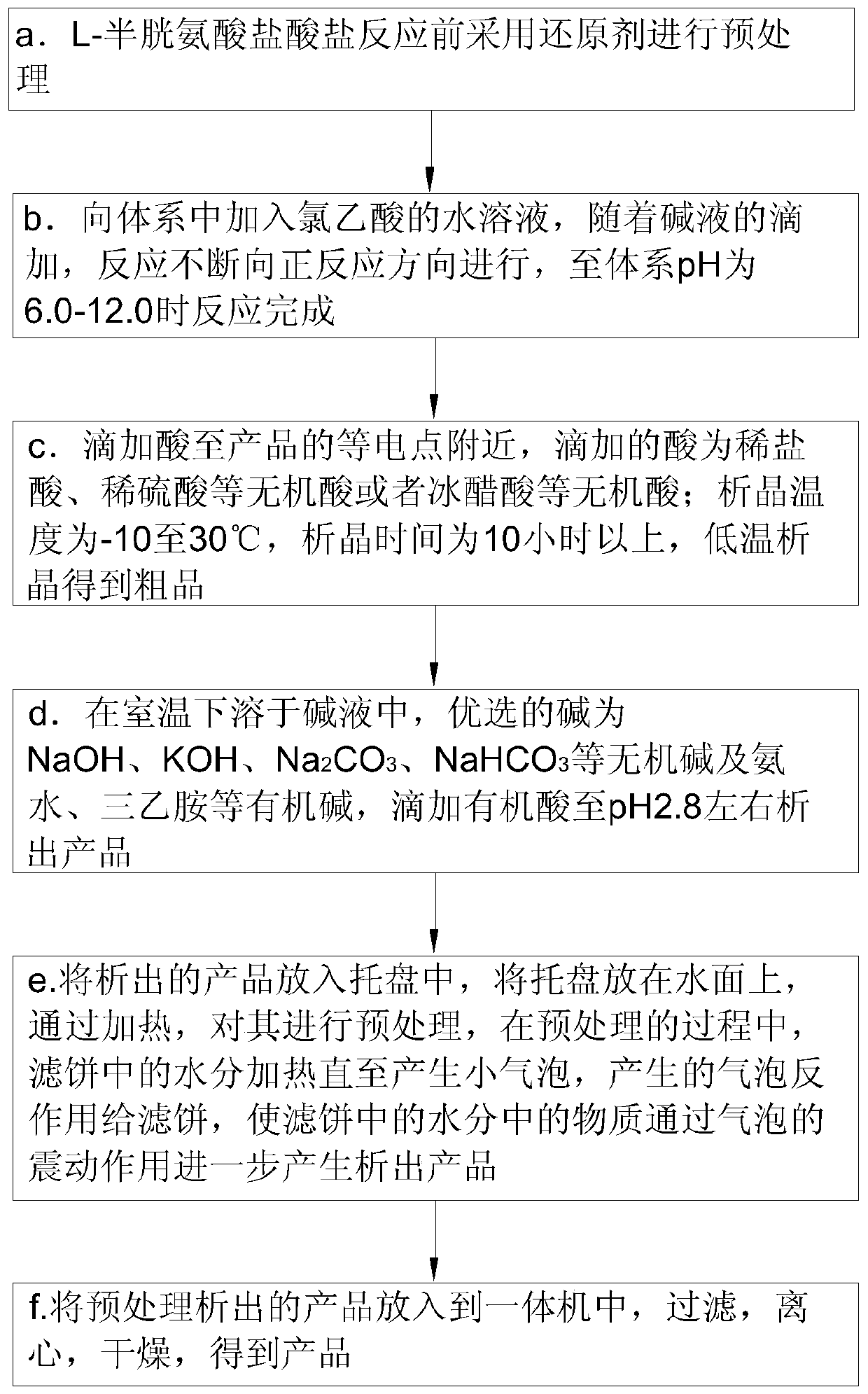
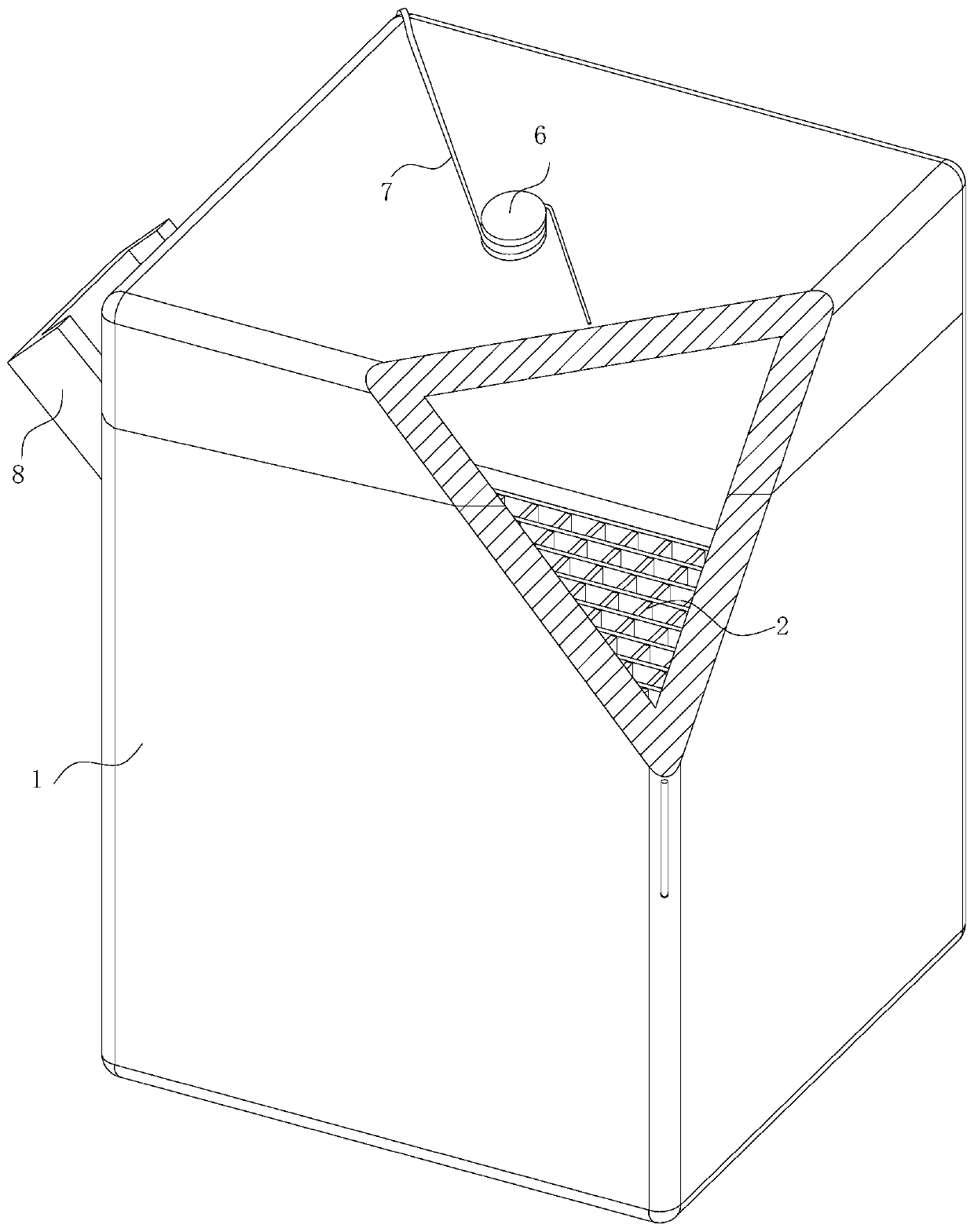
The invention belongs to the technical field of compound preparation and particularly relates to a preparation method of high-purity S-(carboxymethyl)-cysteine. First, L-cysteine hydrochloride is pretreated by a reducing agent before reaction, an aqueous solution of chloroacetic acid is added into the system, and the reaction is continuously carried out in the positive reaction direction with dropping addition of an alkaline solution until the reaction is finished when the pH value of the system is 6.0-12.0, an acid is dropwise added to the vicinity of an isoelectric point of the product, andcrystallization is carried out at low temperature to obtain a crude product; the crude product is dissolved in the alkaline solution at room temperature, an organic acid is dropwise added until a product precipitates when the pH value is about 2.8, the precipitated product is put into a tray, the tray is put on the water surface and is pretreated by heating, moisture in a filter cake is heated until small bubbles are generated in the pretreatment process, and the generated bubbles counter-react with the filter cake, so that substances in the moisture in the filter cake further generate a precipitated product through the vibration effect of the bubbles, and finally the pretreated precipitated product is put into an all-in-one machine to obtain the product.
Owner:北京云鹏鹏程医药科技有限公司
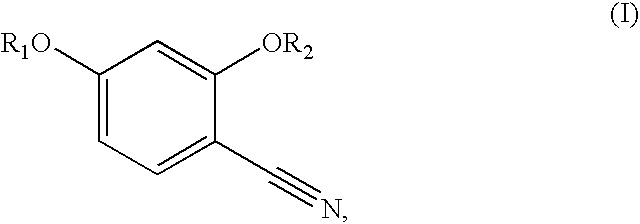
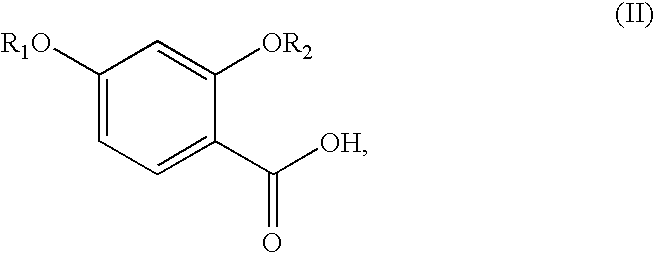
Synthesis of benzonitriles from substituted benzoic acid
InactiveUS6875882B2Inexpensive and readily available starting materialOrganic active ingredientsCarboxylic acid nitrile preparationBenzoic acidSuperoxide
Owner:GENZYME CORP
A kind of enteric-coated tablet containing s-carboxymethyl-l-cysteine
ActiveCN108553439BImprove bioavailabilityReduce stimulationOrganic active ingredientsPharmaceutical non-active ingredientsSmall intestineS-methylcysteine
The invention provides an S-carboxymethyl-L-cysteine containing medicinal composition. The medicinal composition is an enteric tablet comprising a tablet core, an isolating layer and an enteric coating layer. The S-carboxymethyl-L-cysteine containing enteric tablet provided by the invention cannot be dissolved in the stomach and is low irritation to the stomach; the S-carboxymethyl-L-cysteine containing enteric tablet is dissolved in the small intestine as a main medicine absorbing site, improves the bioavailability of S-carboxymethyl-L-cysteine and is stable in quality during storage.
Owner:GUANGZHOU BAIYUNSHAN PHARMA HLDG CO LTD BAIYUNSHAN PHARMA GENERAL FACTORY
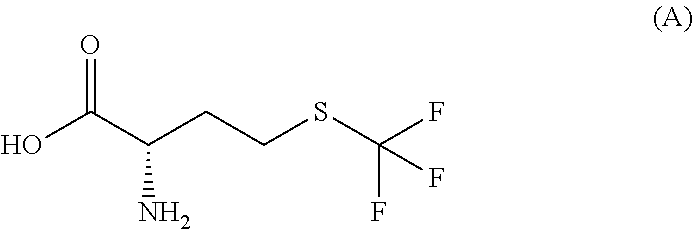
Simple preparation of trifluoromethionine and derivatives thereof
InactiveUS20110015433A1Avoiding the evaporation of ammoniaSimple preparation processOrganic compound preparationAmino-carboxyl compound preparationBirch reductionIodide
Disclosed is a process for the simple preparation of trifluoromethionine, its analogs trifluoromethylcysteine, fluoroalkylhomocysteines, and fluoroalkylcysteines, and derivatives of them. These compounds are drug-candidate compounds or raw materials of drug-candidate compounds. Specifically, trifluoromethionine, trifluoromethylcysteine, a fluoroalkylhomocysteine, or a fluoroalkylcysteine is simply and conveniently prepared directly without passing through homocysteine or cysteine by adding metallic sodium to an optically active or racemic homocystine or cystine in liquid ammonia and further adding a fluoroalkyl iodide thereto under Birch reduction conditions.
Owner:NAGOYA INSTITUTE OF TECHNOLOGY
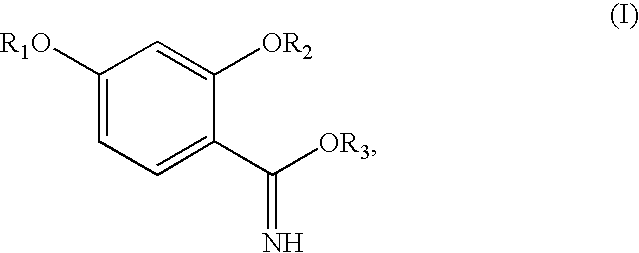
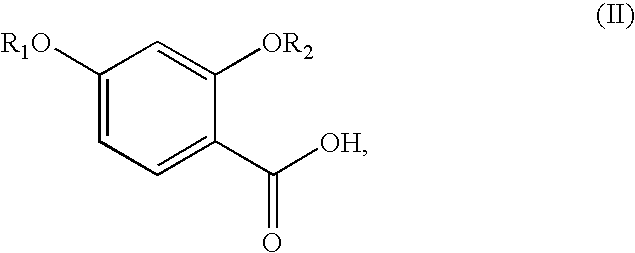
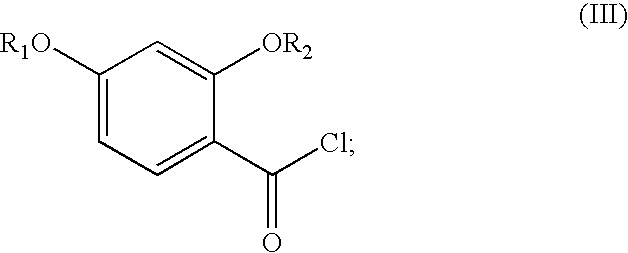
Synthesis of benzimidate from benzoic acid
InactiveUS6846958B2Inexpensive and readily availableInexpensive and readily available starting materialOrganic active ingredientsCarboxylic acid nitrile preparationBenzoic acidEther
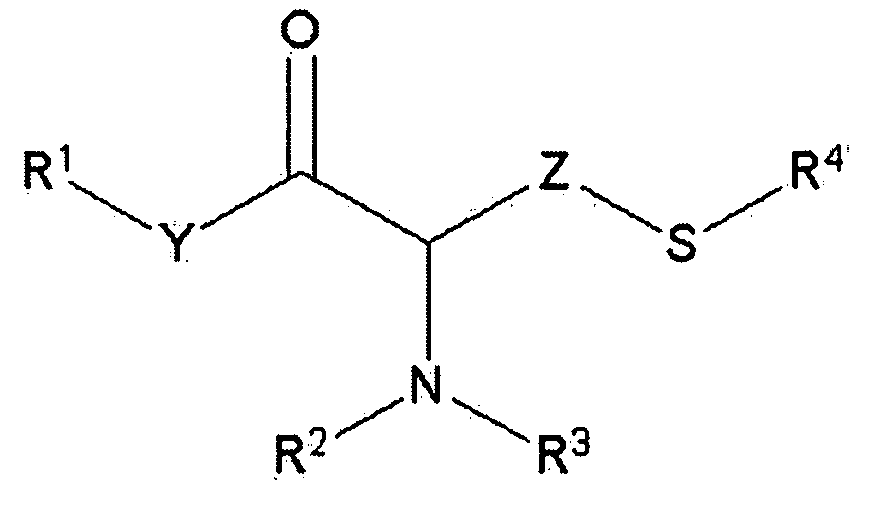
Benzimidates can be reacted with a large number of nucleophiles, leading to a wide variety of products. The present invention discloses a facile synthesis for ethyl 2,4-dihydroxybenzimidate, and ethers and diethers thereof, from 2,4-dihydroxybenzoic acid or 2,4-dibenzyloxybenzoic acid. The present invention also discloses a method of preparing a class of iron chelating agents related to desferrithiocin, all of which contain a thiazoline ring. In this method, 2,4-dihydroxybenzonitrile is condensed with (S)-2-methylcysteine.
Owner:GENZYME CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com