Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
37 results about "Polycystic liver disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
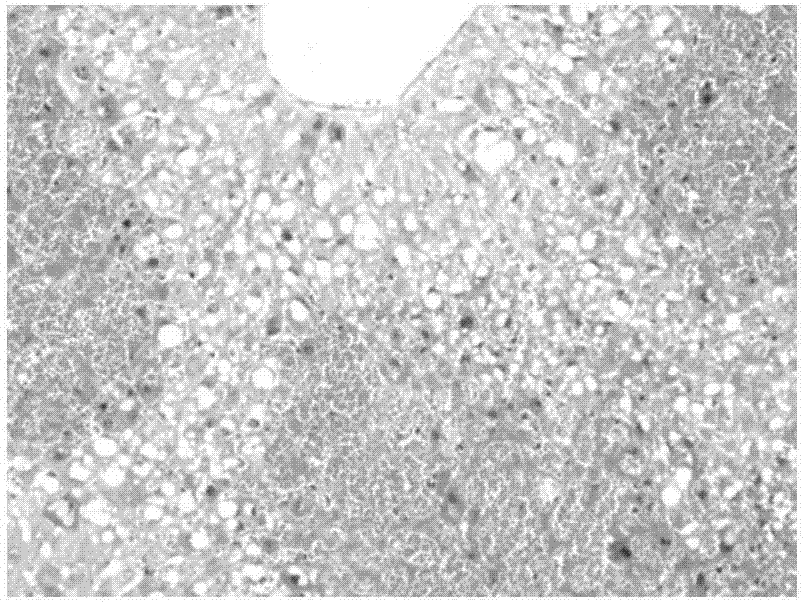
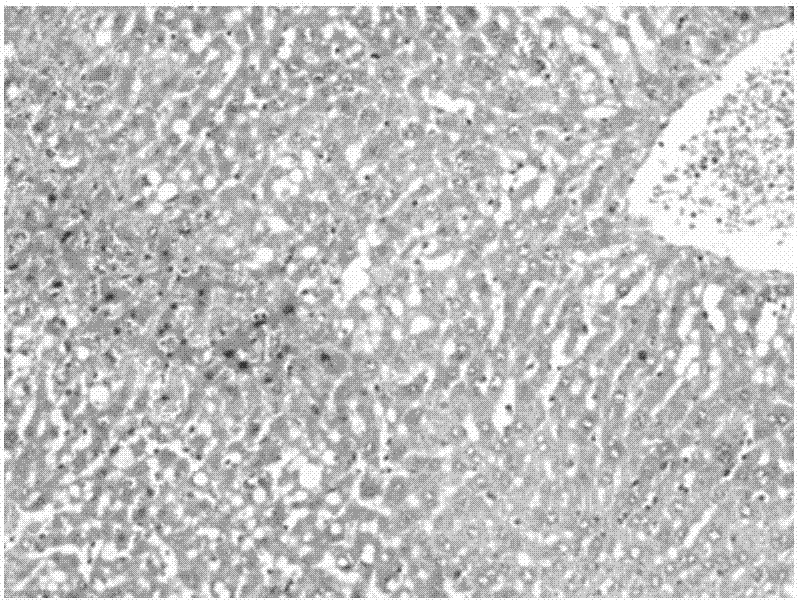
Polycystic liver disease (PLD) usually describes the presence of multiple cysts scattered throughout normal liver tissue. PLD is commonly seen in association with autosomal-dominant polycystic kidney disease, with a prevalence of 1 in 400 to 1000, and accounts for 8–10% of all cases of end stage renal disease. The much rarer autosomal-dominant polycystic liver disease will progress without any kidney involvement.
Method of examining polycystic kidney disease and method of screening for therapeutic agent of the disease
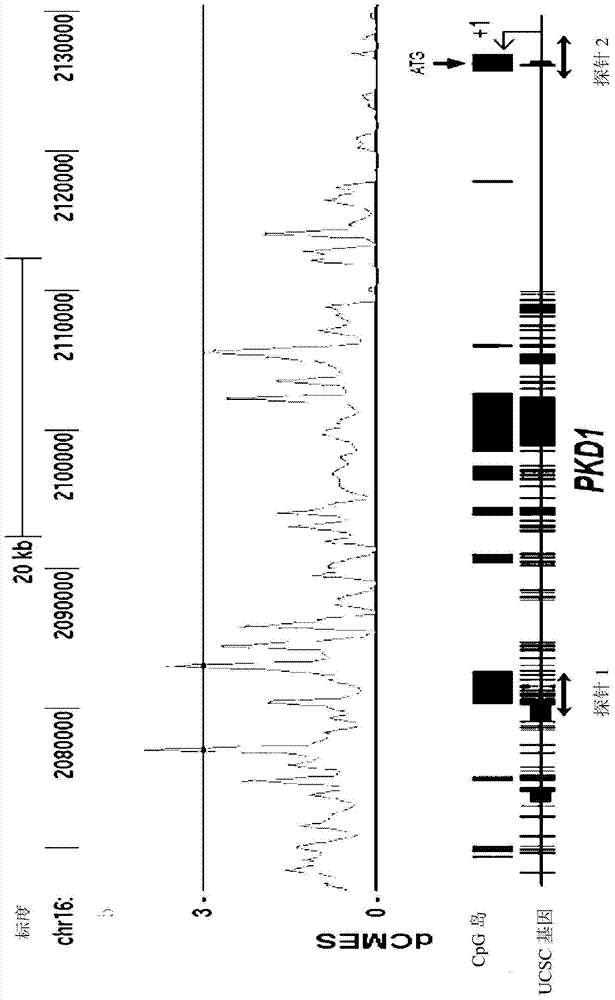
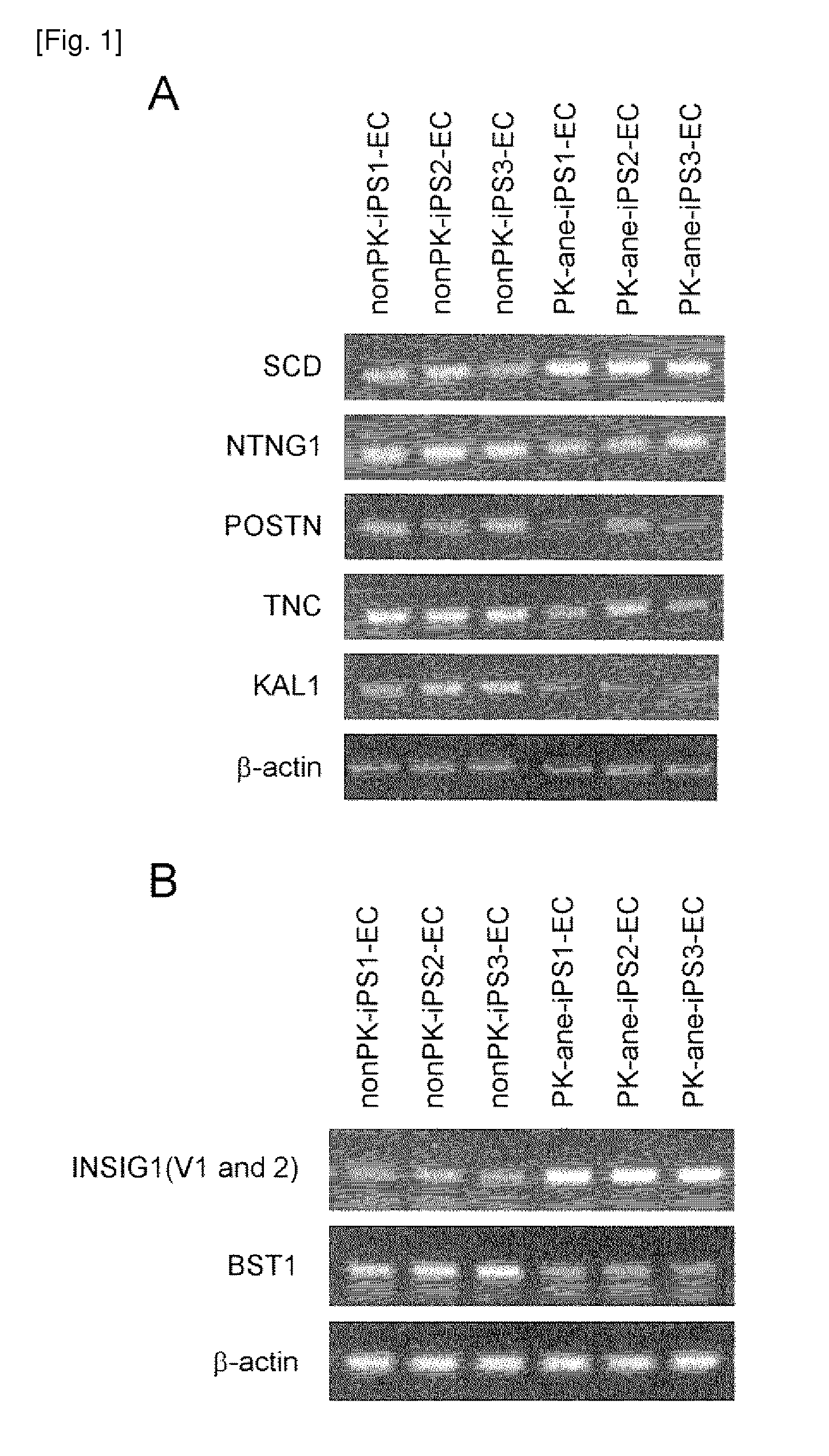
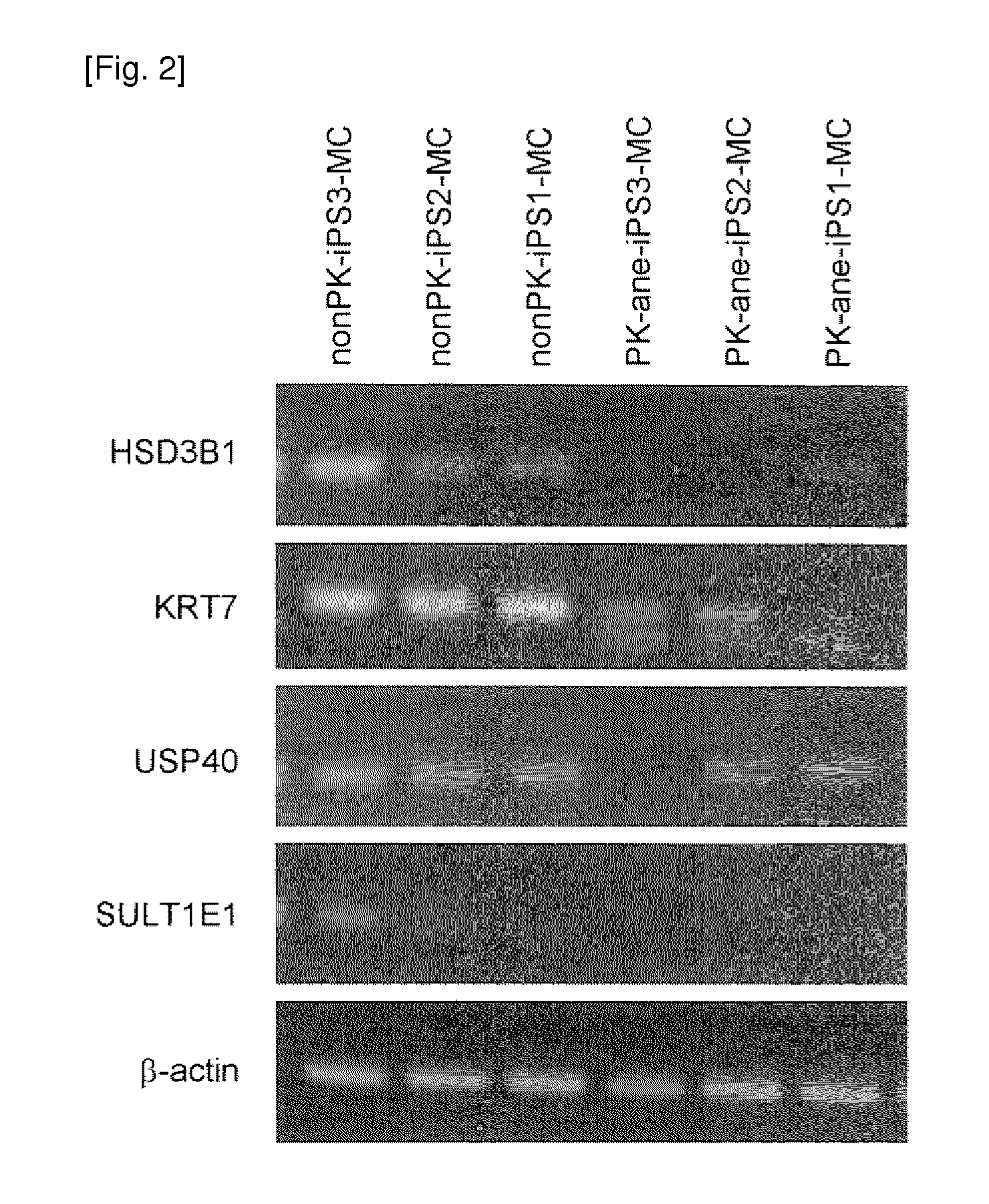
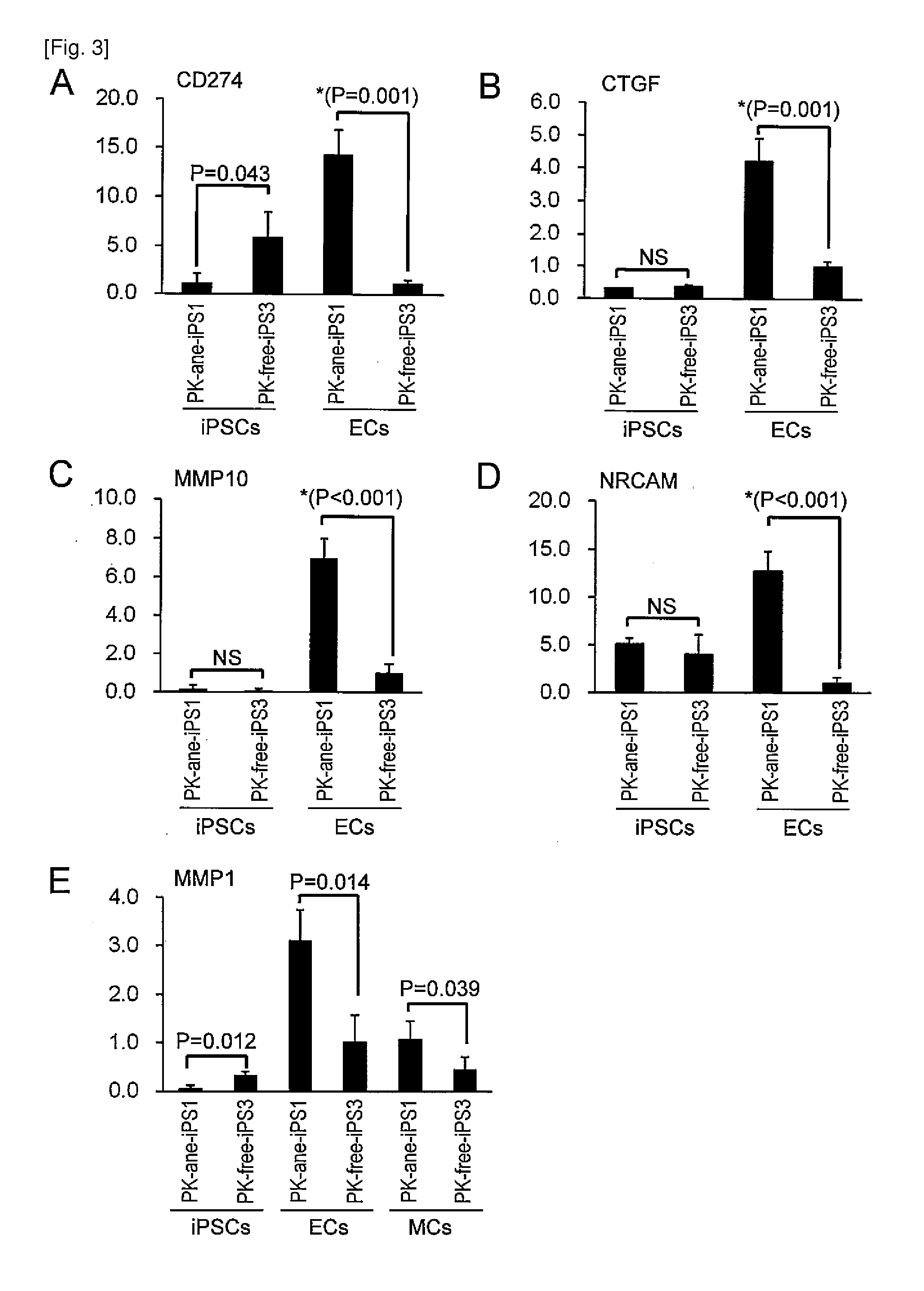
The present invention provides a method of examining polycystic kidney disease or a complication of polycystic kidney disease using a gene(s) selected from the group consisting of NTNG1, POSTN, TNC, KAL1, BST1, ACAT2, INSIG1, SCD, HSD3B1, KRT7, USP40, SULT1E1, BMP6, CD274, CTGF, E2F7, EDN1, FAM43A, FRMD3, MMP10, MYEOV, NR2F1, NRCAM, PDCK1, PLXNA2, SLC30A3, SNAI1, SPOCD1, MMP1, TFPI2, HMGA2, KRTAP4-7, KRTAP4-8, KRTAP4-9, MYPN, RPPH1, and SIAE, and a method of screening for a therapeutic agent or a preventive agent therefore, and further vascular endothelial cells or vascular mural cells obtained via differentiation induction from iPS cells formed from a somatic cell of a subject suffered from polycystic kidney disease and having cerebral aneurysm as a complication.
Owner:KYOTO UNIV
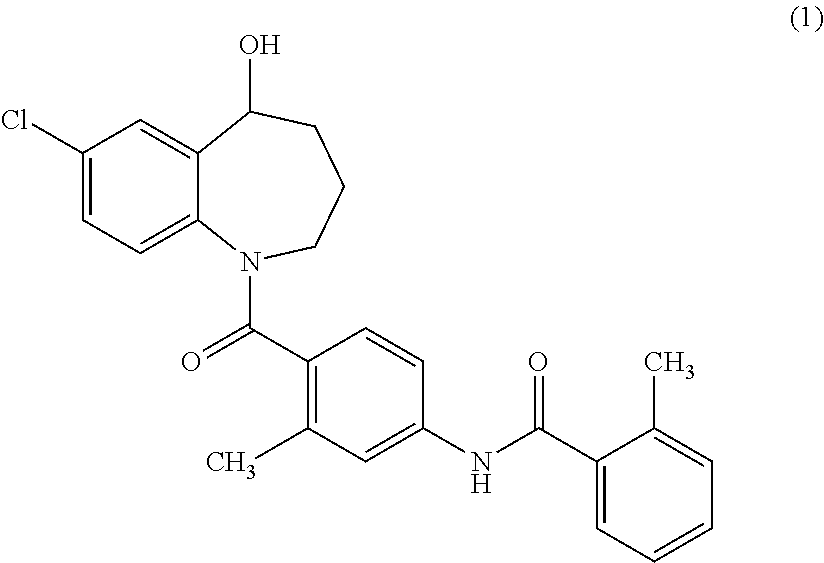
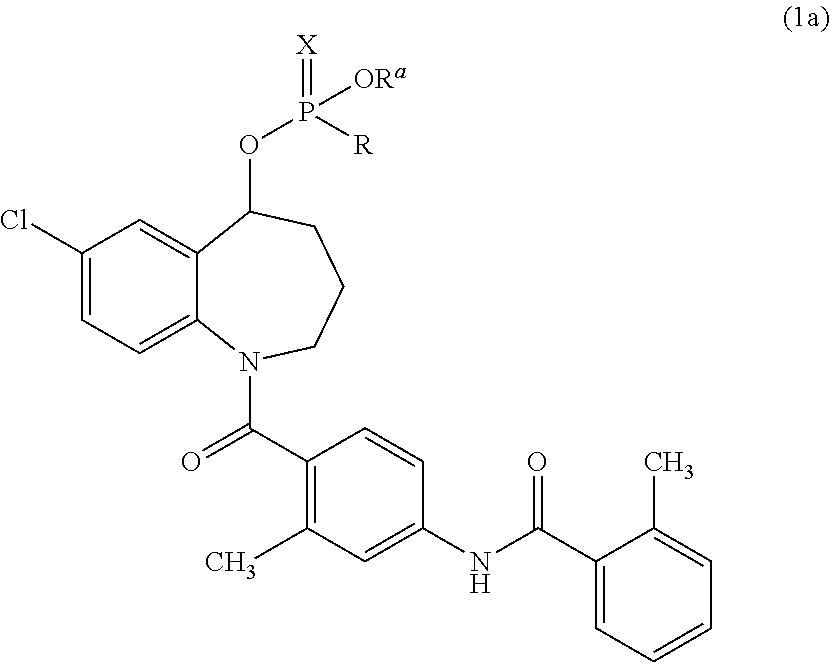
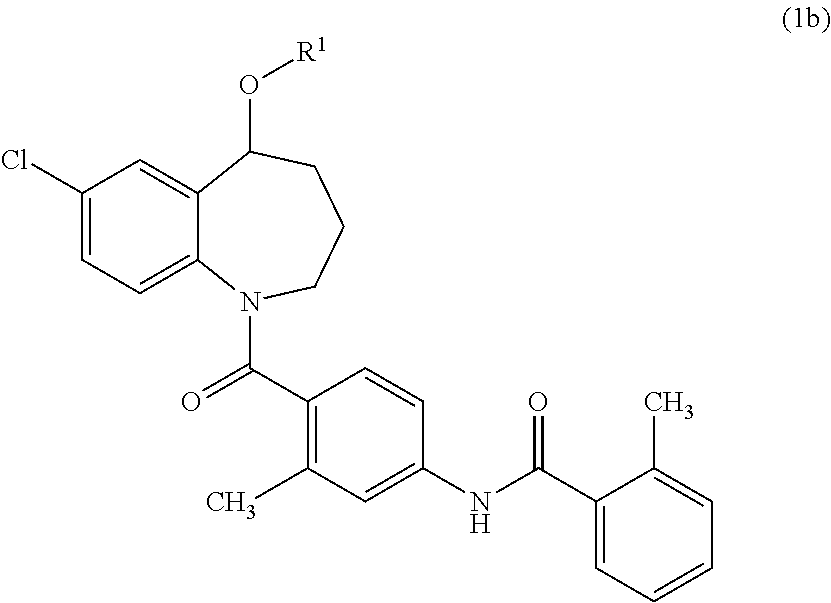
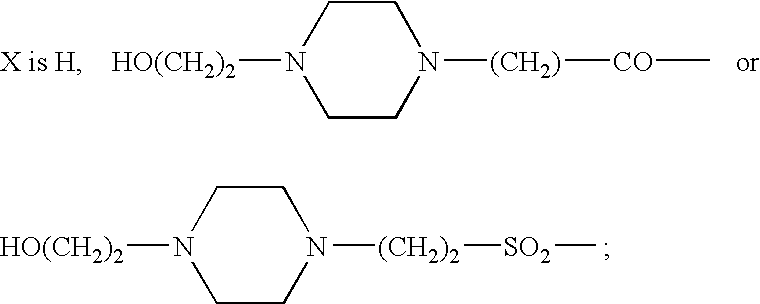
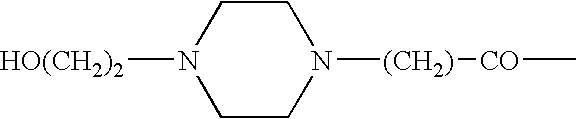
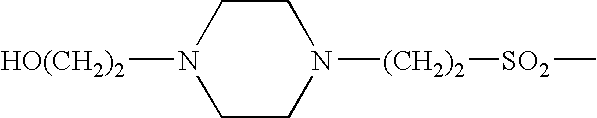
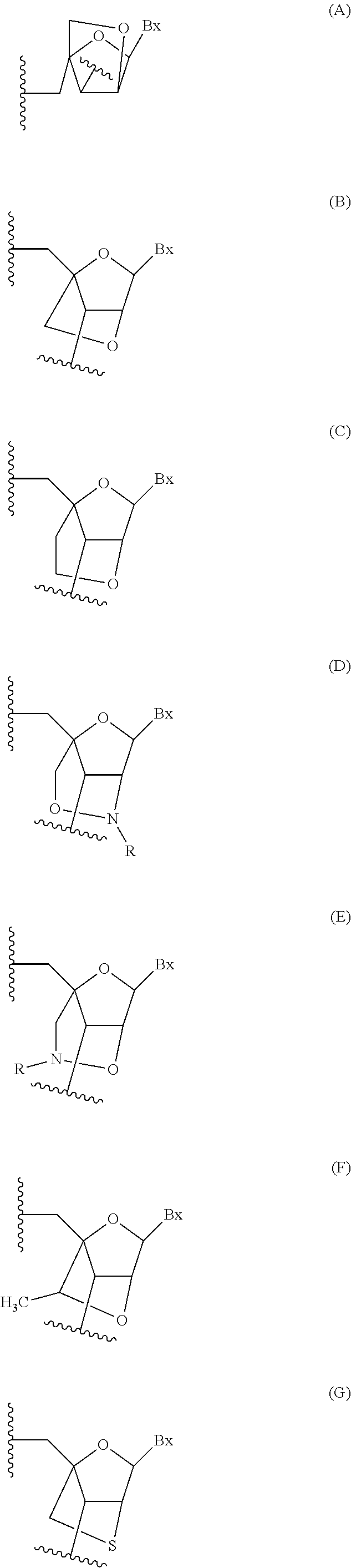
Thieno[3,2-b]pyridine-6-carbonitriles and thieno[2,3-b]pyridine-5-carbonitriles as protein kinase inhibitors
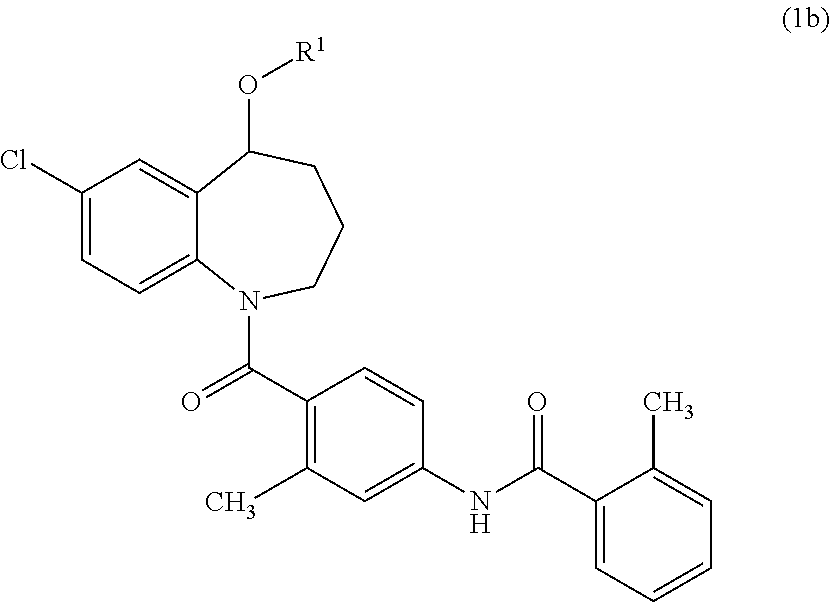
This invention provides compounds of Formula (1a)-(1f) wherein:X, R1, and R2 are defined hereinbefore in the specification, which are useful in the treatment of cancer, stroke, osteoporosis, polycystic kidney disease, autoimmune disease, rheumatoid arthritis, and transplant rejection and process for producing said compounds.
Owner:WYETH LLC
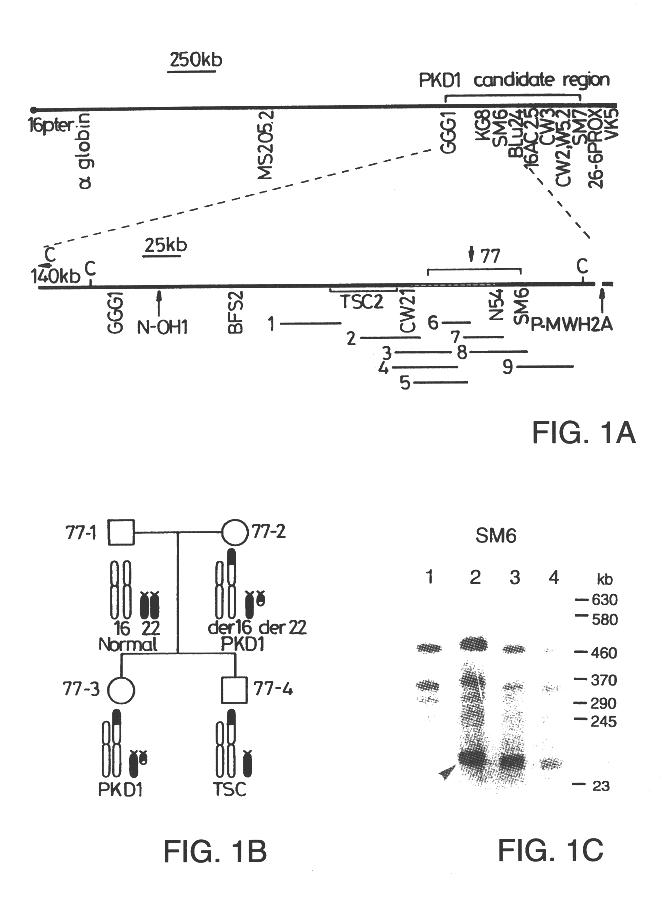
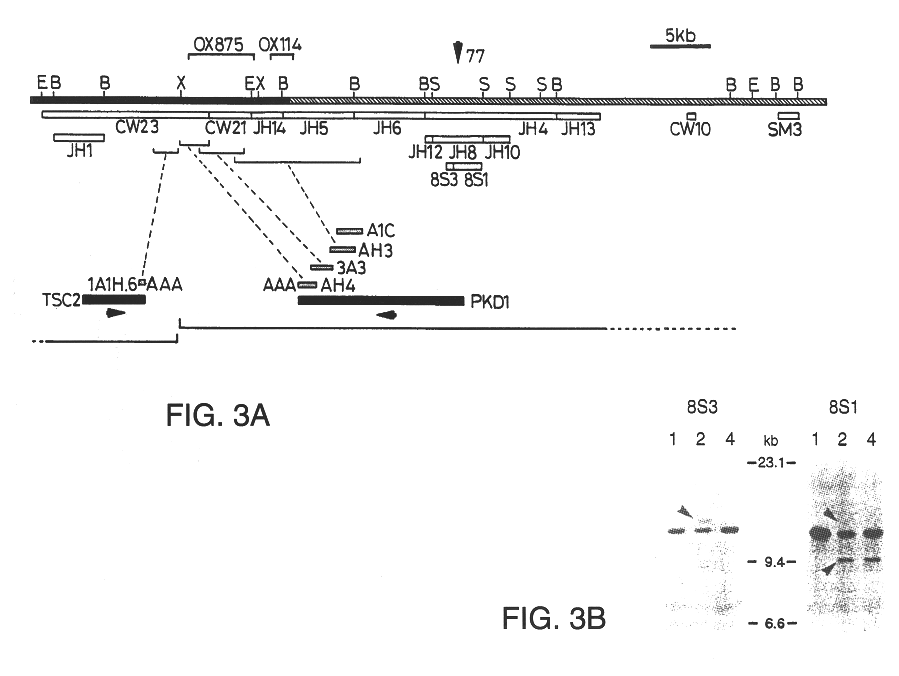
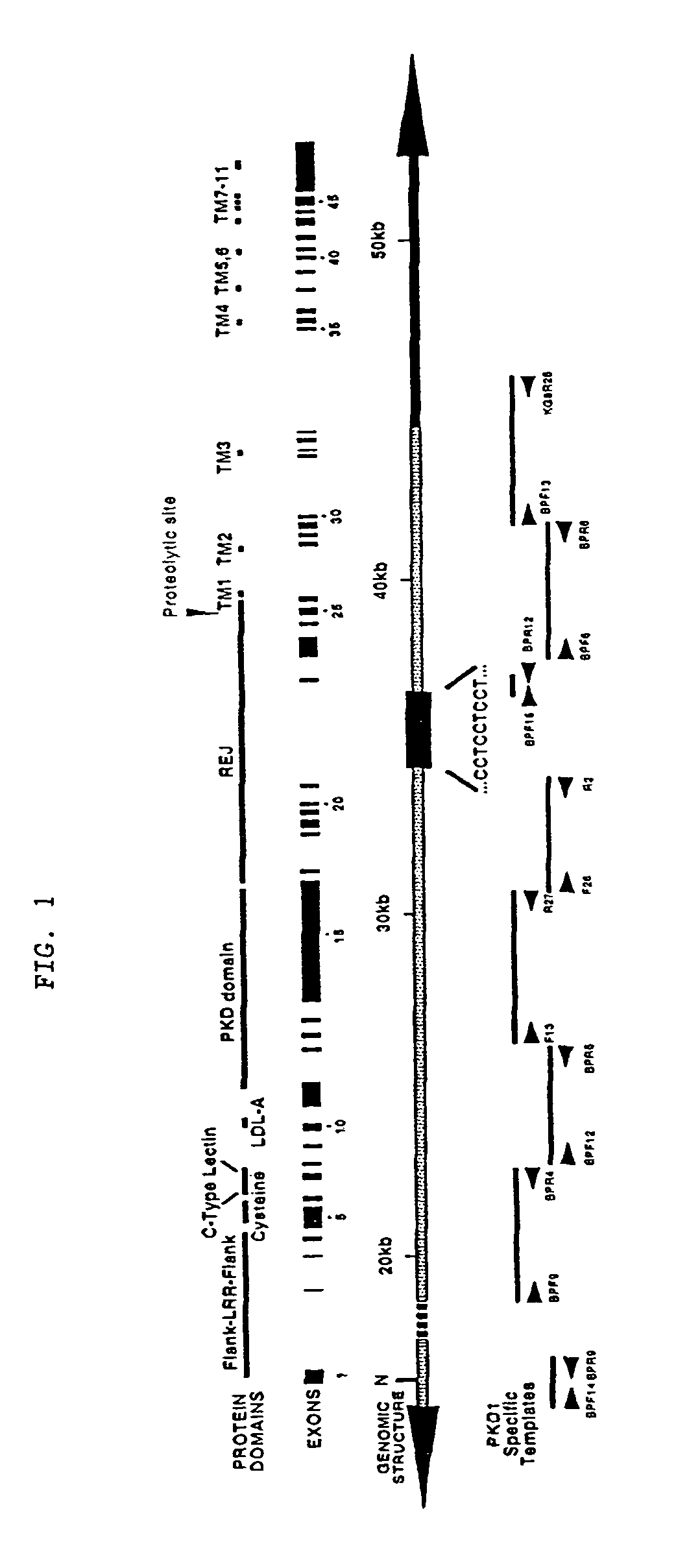
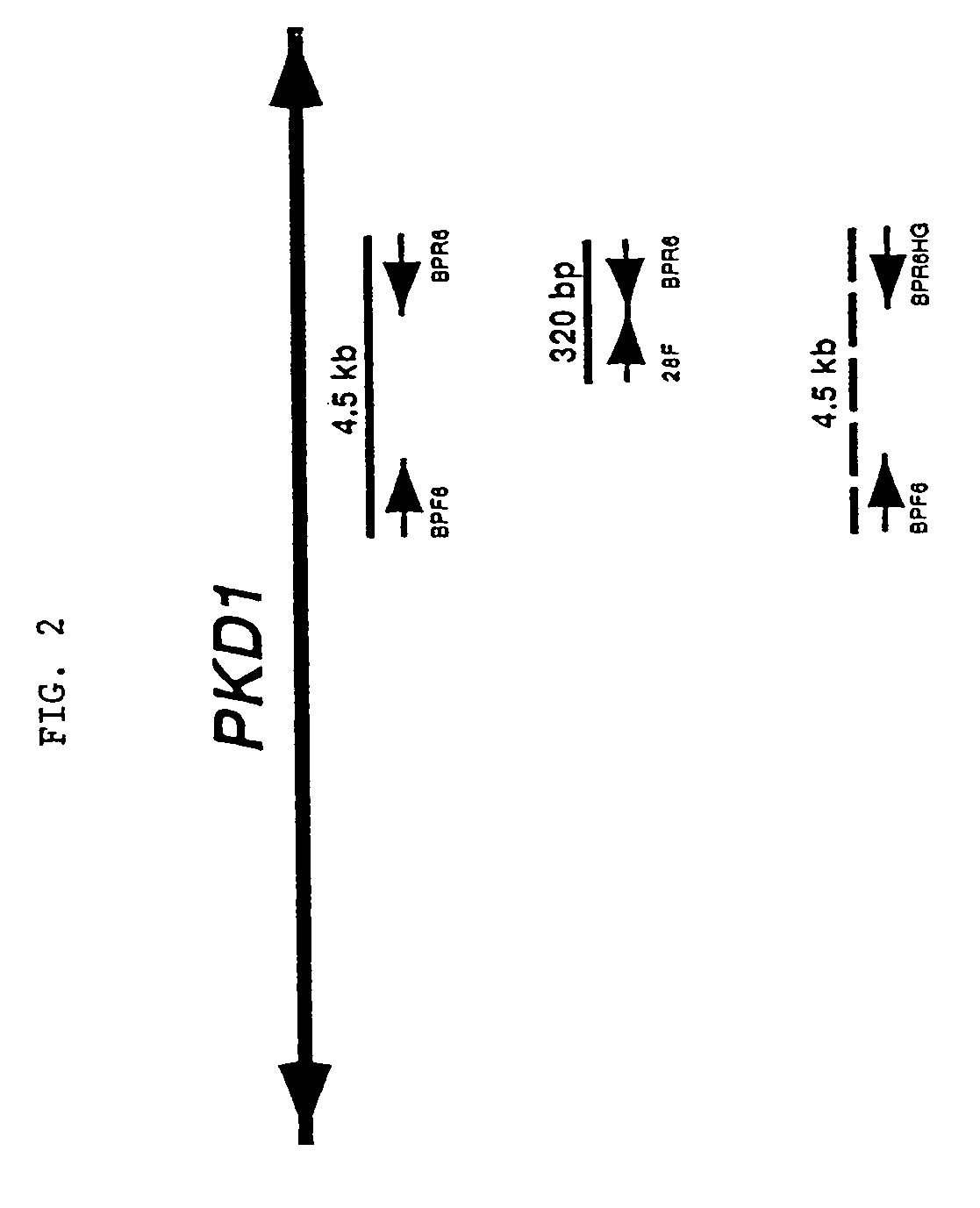
Polycystic kidney disease 1 gene and uses thereof
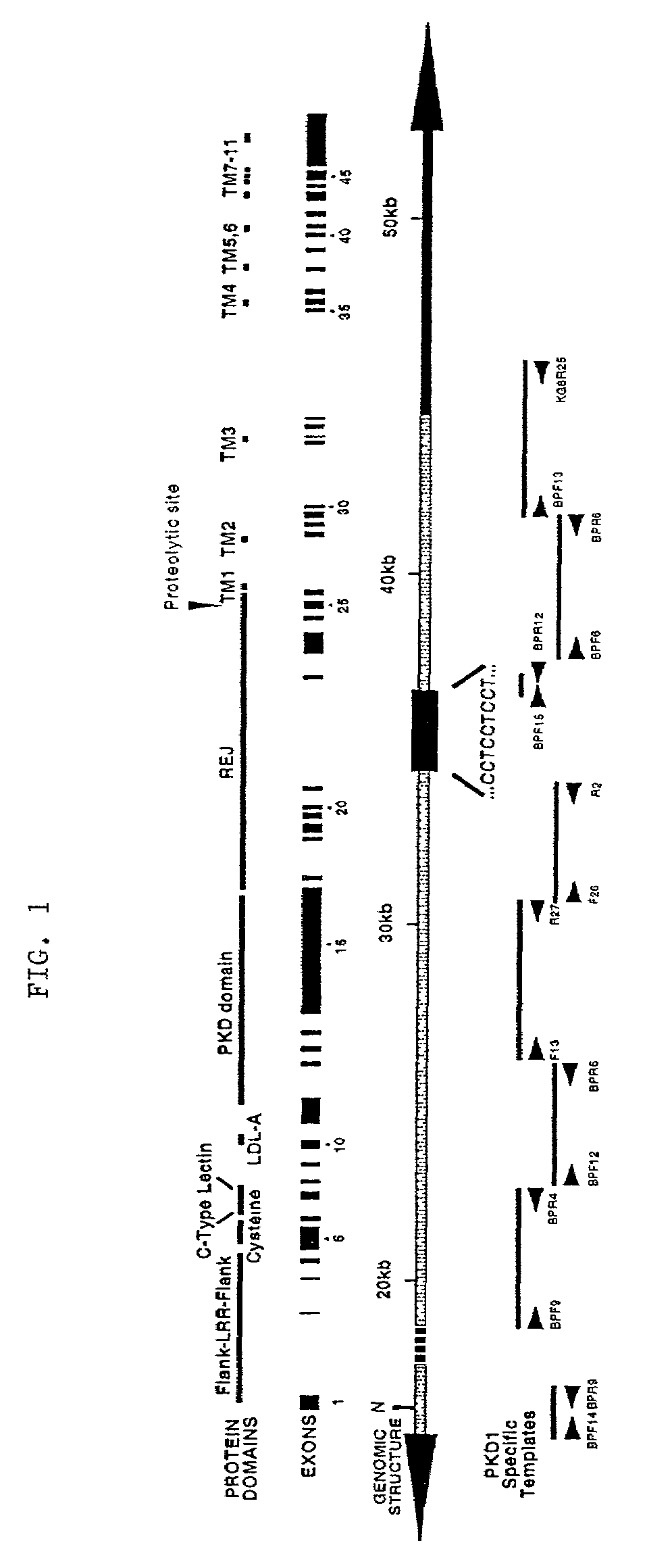
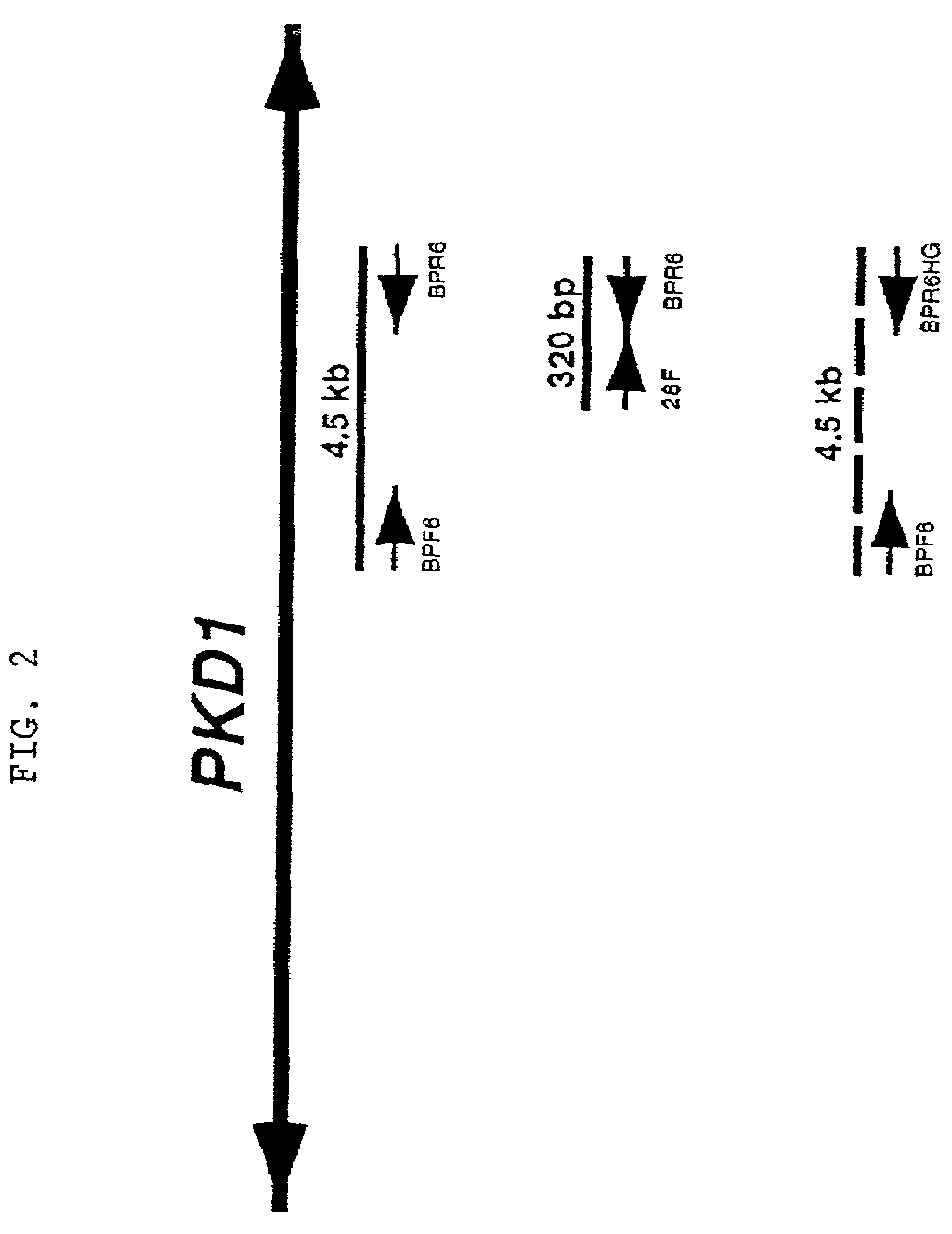
The present invention relates to the polycystic kidney disease 1 (PKD1) gene and its nucleic acid sequence, mutations thereof in patients having PKD1-associated disorders, the protein encoded by the PKD1 gene or its mutants, and their uses in disease diagnosis and therapy.
Owner:ERASMUS UNIVERSITY ROTTERDAM +3
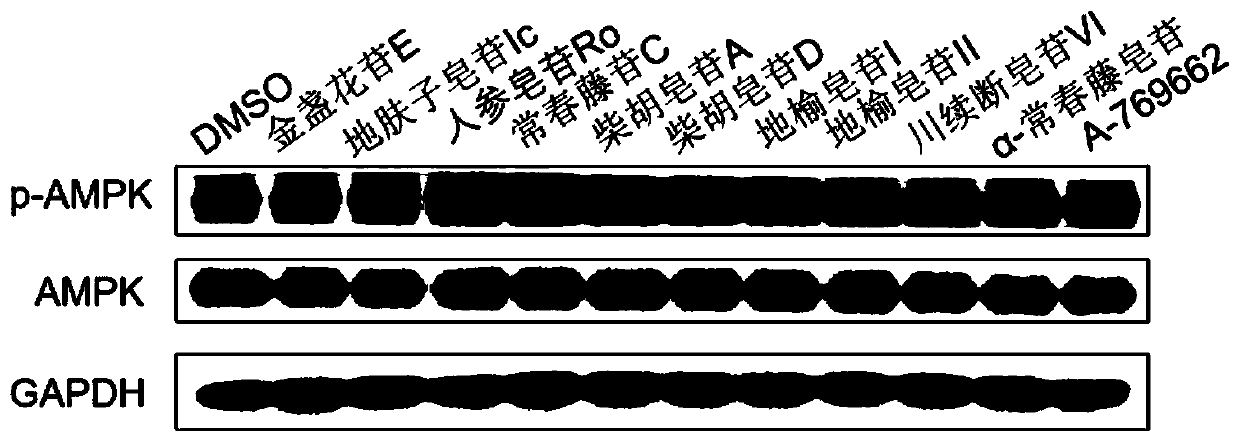
Medical use of pentacyclic triterpenoid saponin and pharmaceutical composition thereof
InactiveCN110200981AImprove securityGood curative effectOrganic active ingredientsSenses disorderBovine respiratory diseaseDiabetic complication
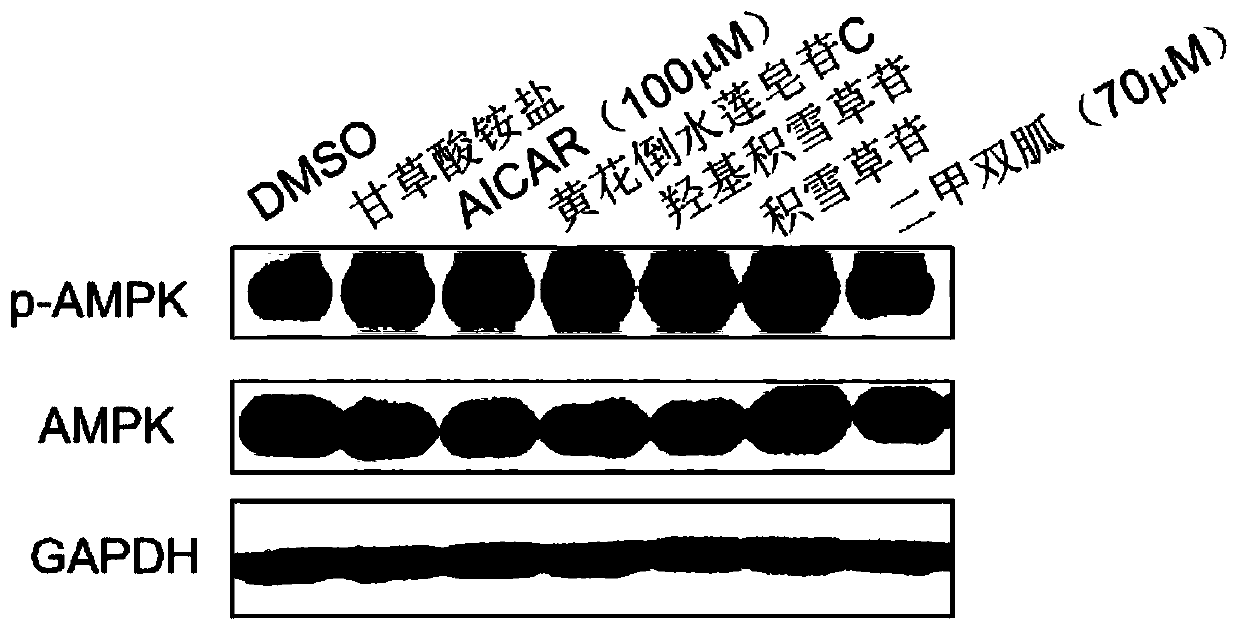
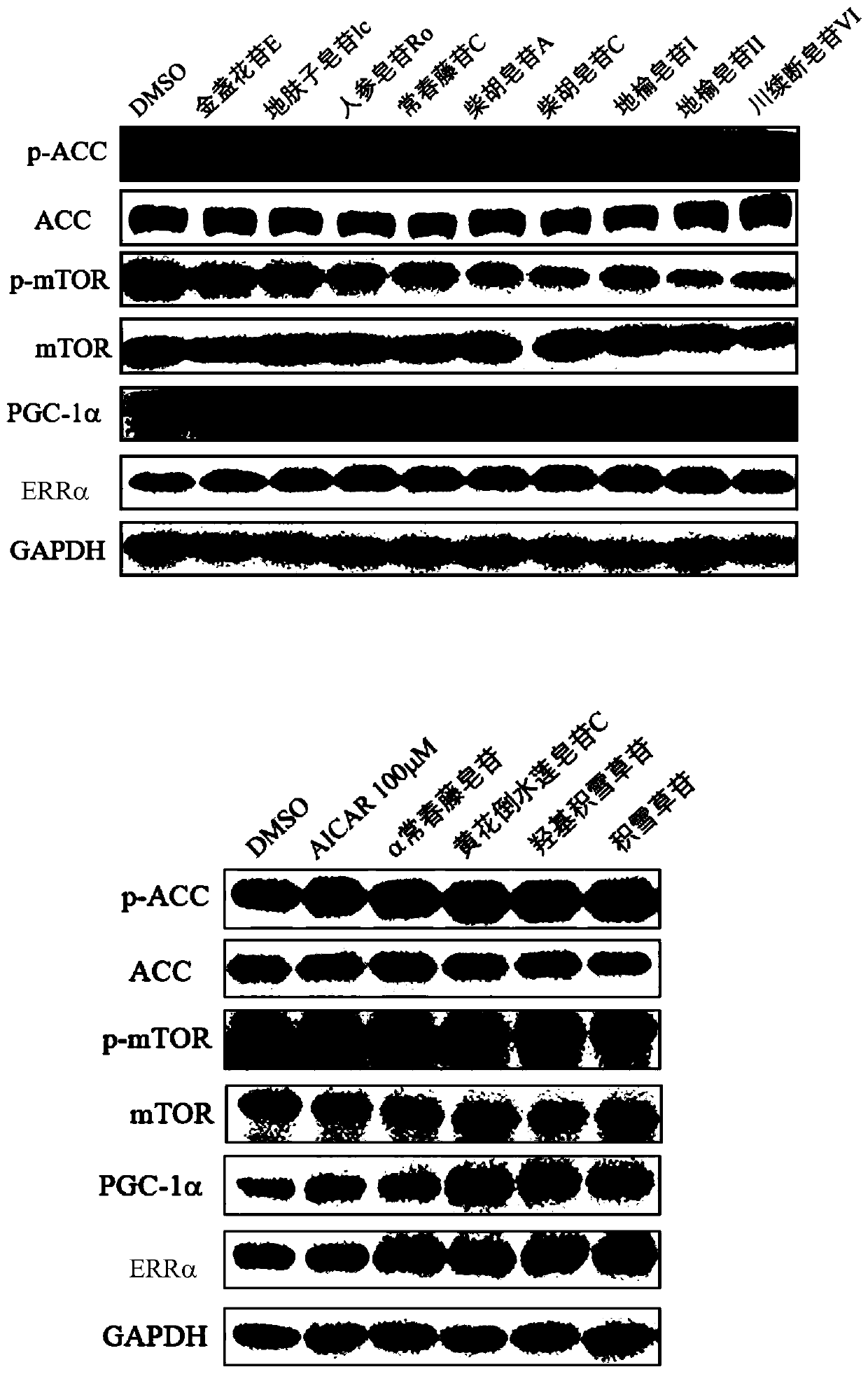
The present invention discloses an application of pentacyclic triterpenoid saponin represented by formulas (I) to (XIII) for the preparation of a medicine for preventing or treating AMPK-mediated diseases including fatty liver disease, inflammatory bowel disease, respiratory disease, diabetic complications and polycystic kidney disease. The compounds of formula (I) to (XIII) are especially usefulfor nonalcoholic simple fatty liver, nonalcoholic steatohepatitis, non-alcoholic steatohepatitis-induced cirrhosis, ulcerative colitis, Crohn's disease, chronic obstructive Pulmonary diseases, asthma,idiopathic pulmonary fibrosis, cystic fibrosis, allergic rhinitis, diabetic nephropathy, diabetic cardiomyopathy, diabetic ulcers, and autosomal dominant polycystic kidney disease. A pharmaceutical composition for preventing and treating the AMPK-mediated diseases of the present invention comprises a therapeutically effective amount of the compounds of the formula (I) to (XIII) or a pharmaceutically acceptable salt or a solvate thereof as an active ingredient and a pharmaceutically acceptable excipient.
Owner:CHINA PHARM UNIV
Detection and treatment of polycystic kidney disease
InactiveUS7553644B2Improve throughputBioreactor/fermenter combinationsFungiPKD1Polycystic liver disease
Compositions useful for examining the PKD1 gene are provided. In addition, methods for detecting mutations of the PKD1 gene, which can be associated with autosomal dominant polycystic kidney disease in humans, are provided. Methods for diagnosing a mutant PKD1 gene sequence in a subject also are provided, as are methods of treating a subject having a PKD1-associated disorder.
Owner:THE JOHNS HOPKINS UNIVERSITY SCHOOL OF MEDICINE
Novobiocin analogues and treatment of polycystic kidney disease
InactiveUS20110082098A1Reduction of cyst formationBiocideCarbohydrate active ingredientsNovobiocinCyst formation
Novobiocin analogues are useful in methods of treating, inhibiting, and / or preventing cyst formation in autosomal dominant polycystic kidney disease (ADPKD) in a subject. The disclosure provides methods of treating ADPKD comprising administering a therapeutically effective amount of a coumarin-3-carboxamide novobiocin analogue. Accordingly, the method can include administering a novobiocin analogue in a therapeutically effective amount for reducing levels of mTOR pathway phosphoproteins P-mTOR, P-Akt and P-S6K, or combinations thereof. Further, the method can include administering a novobiocin analogue in a therapeutically effective amount for reducing levels of Hsp-90 client proteins CFTR, ErbB2, c-Raf and Cdk4, or combinations thereof.
Owner:UNIVERSITY OF KANSAS
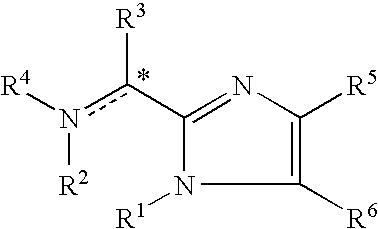
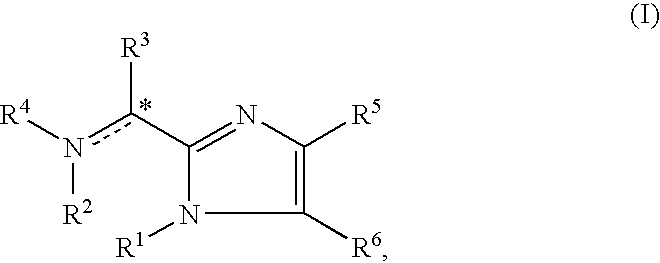
Imidazolyl derivatives
The present invention is directed to imidazolyl derivatives of the formula: where the substituents are defined in the specification, or a pharmaceutically acceptable salt thereof. The derivatives bind selectively to the somatostatin subtype receptors and elicit either an agonist or antagonist effect from the somatostatin subtype receptors. The derivatives are useful for treating a variety of diseases including acromegaly, restenosis, Crohn's disease, systemic sclerosis, external and internal pancreatic pseudocysts and ascites, VIPoma, nesidoblastosis, hyperinsulinism, gastrinoma, Zollinger-Ellison Syndrome, diarrhea, AIDS related diarrhea, chemotherapy related diarrhea, scleroderma, Irritable Bowel Syndrome, pancreatitis, small bowel obstruction, gastroesophageal reflux, duodenogastric reflux, Cushing's Syndrome, gonadotropinoma, hyperparathyroidism, Graves' Disease, diabetic neuropathy, Paget's disease, polycystic ovary disease, cancer, cancer cachexia, hypotension, postprandial hypotension, panic attacks, GH secreting adenomas or TSH secreting adenomas.
Owner:IPSEN PHARMA SAS
Polycystic kidney disease 12 gene and uses thereof
The present invention relates to the polycystic kidney disease 1 (PKD1) gene and its nucleic acid sequence, mutations thereof in patients having PKD1-associated disorders, the protein encoded by the PKD1 gene or its mutants, and their uses in disease diagnosis and therapy.
Owner:UNIV COLLEGE CARDIFF CONSULTANTS LTD +1
Drug for preventing and/or treating polycystic kidney disease
ActiveUS20150141338A1Significant effectHigh therapeutic effectPeptide/protein ingredientsPharmaceutical delivery mechanismTherapeutic effectPolycystic liver disease
An object of the present invention is to provide a combination drug that has remarkably excellent preventive and / or therapeutic effects on polycystic kidney disease. The present invention provides a drug for preventing and / or treating polycystic kidney disease comprising a combination of tolvaptan or a prodrug thereof with a somatostatin derivative, and a method for treating polycystic kidney disease using this drug.
Owner:OTSUKA PHARM CO LTD
Spiroindoline derivatives as gonadotropin- releasing hormone receptor antagonists
Spiroindoline derivatives, process for their preparation and pharmaceutical compositions thereof, their use for the treatment and / or prophylaxis of diseases, and their use for the manufacture of medicaments for the treatment and / or prophylaxis of diseases, especially sex-hormone-related diseases in both men and women, in particularly those selected from the group of endometriosis, uterine fibroids, polycystic ovarian disease, hirsutism, precocious puberty, gonadal steroid-dependent neoplasia such as cancers of the prostate, breast and ovary, gonadotrope pituitary adenomas, sleep apnea, irritable bowel syndrome, premenstrual syndrome, benign prostatic hypertrophy, contraception and infertility (e.g., assisted reproductive therapy such as in vitro fertilization). The present application relates in particular to spiroindoline derivatives as gonadotropin-releasing hormone (GnRH) receptor antagonists.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Detection and treatment of polycystic kidney disease
InactiveUS8530161B2Improve throughputBioreactor/fermenter combinationsFungiPKD1Polycystic liver disease
Compositions useful for examining the PKD1 gene are provided. In addition, methods for detecting mutations of the PKD1 gene, which can be associated with autosomal dominant polycystic kidney disease in humans, are provided. Methods for diagnosing a mutant PKD1 gene sequence in a subject also are provided, as are methods of treating a subject having a PKD1-associated disorder.
Owner:THE JOHNS HOPKINS UNIVERSITY SCHOOL OF MEDICINE
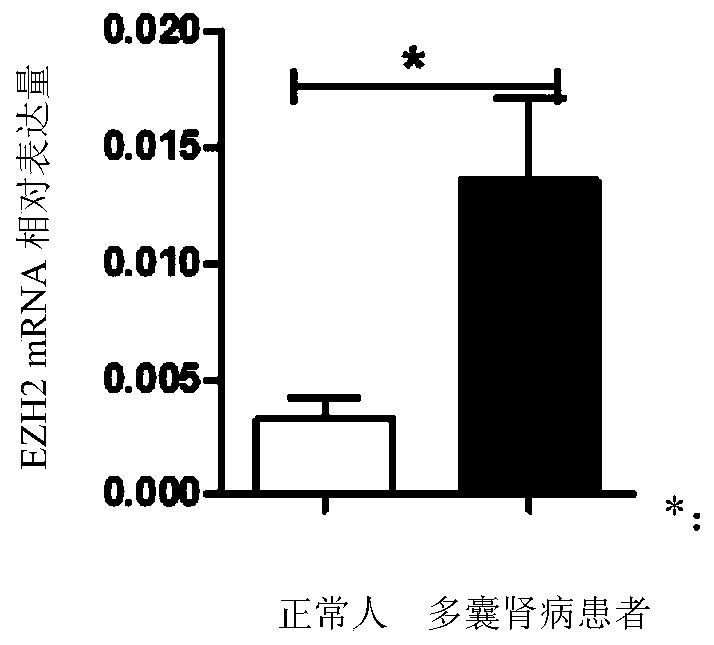
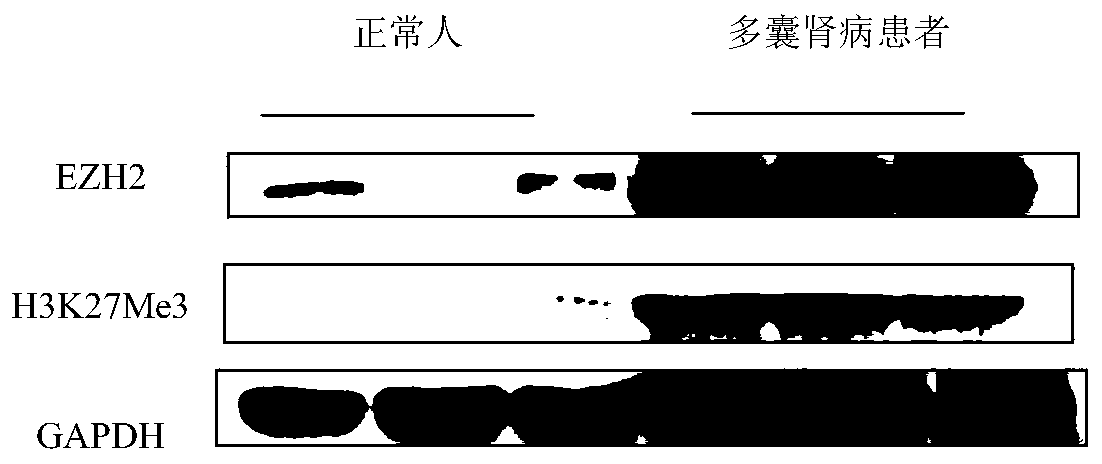
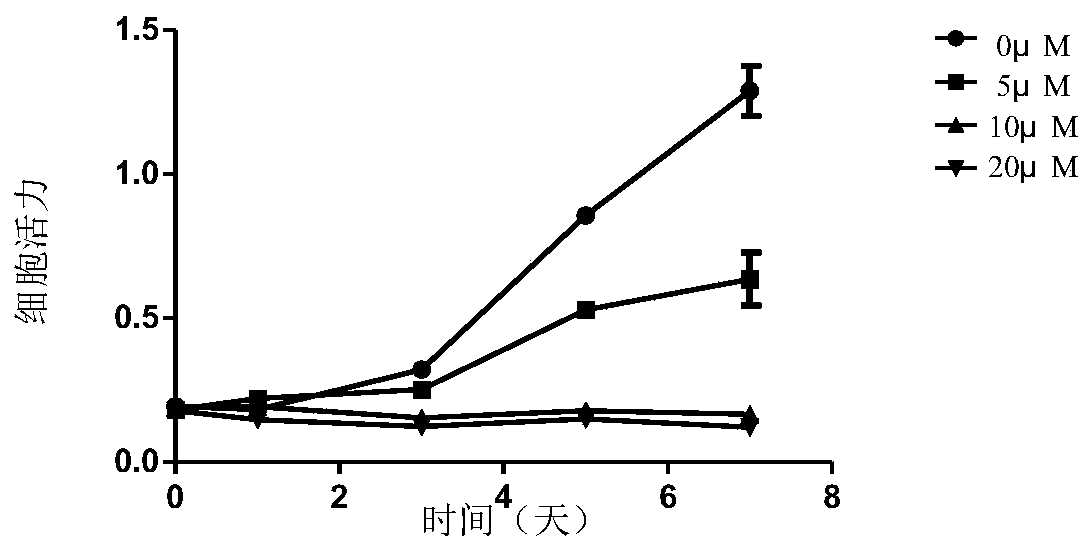
Application of EZH2 (enhancer of zeste homolog 2) in preparation of drugs to prevent or treat polycystic kidney disease
InactiveCN109966479AConfirmed inhibitionEZH2 inhibitory inhibition confirmedPeptide/protein ingredientsTransferasesEZH2Polycystic liver disease
The invention relates to the technical field of medicine, in particular to application of EZH2 (enhancer of zeste homolog 2) in the preparation of drugs to prevent or treat polycystic kidney disease.It is discovered herein that an EZH2 inhibitor can inhibit the proliferation of cyst cells by inducing the apoptosis of polycystic kidney disease cyst lining epithelial cells and regulating cell cycle, and can protect the renal function by inhibiting the growth of renal cysts and enlargement of renal size. It is indicated that EZH2 can act as a novel target for autosomal dominant polycystic kidneydisease and is very significant to the treatment of polycystic kidney disease; the EZH2 inhibitor can be used to develop new pilot compounds to prevent or treat autosomal dominant polycystic kidney disease and can also be used to prepare drugs to prevent or treat autosomal dominant polycystic kidney disease.
Owner:SHANGHAI CHANGZHENG HOSPITAL +1
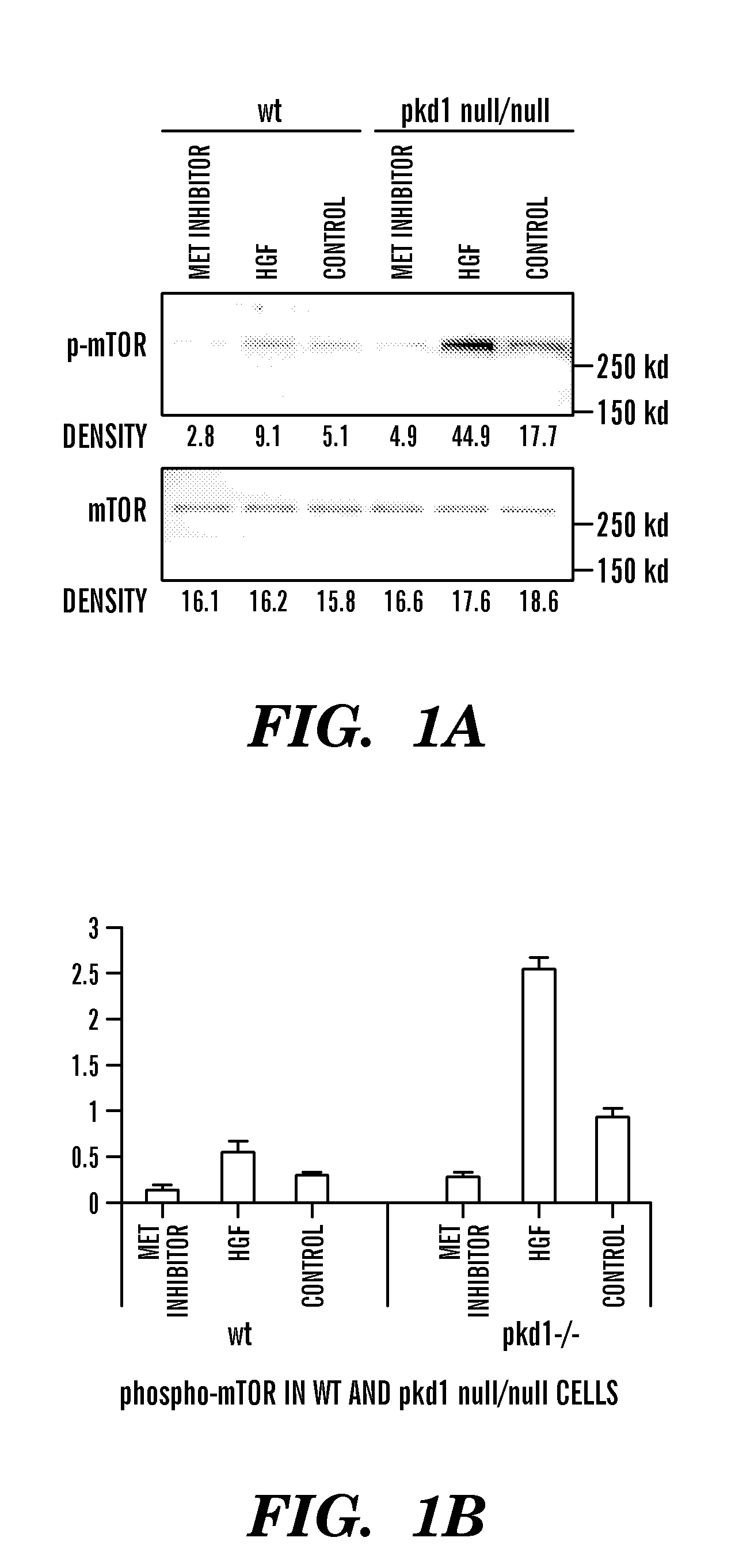
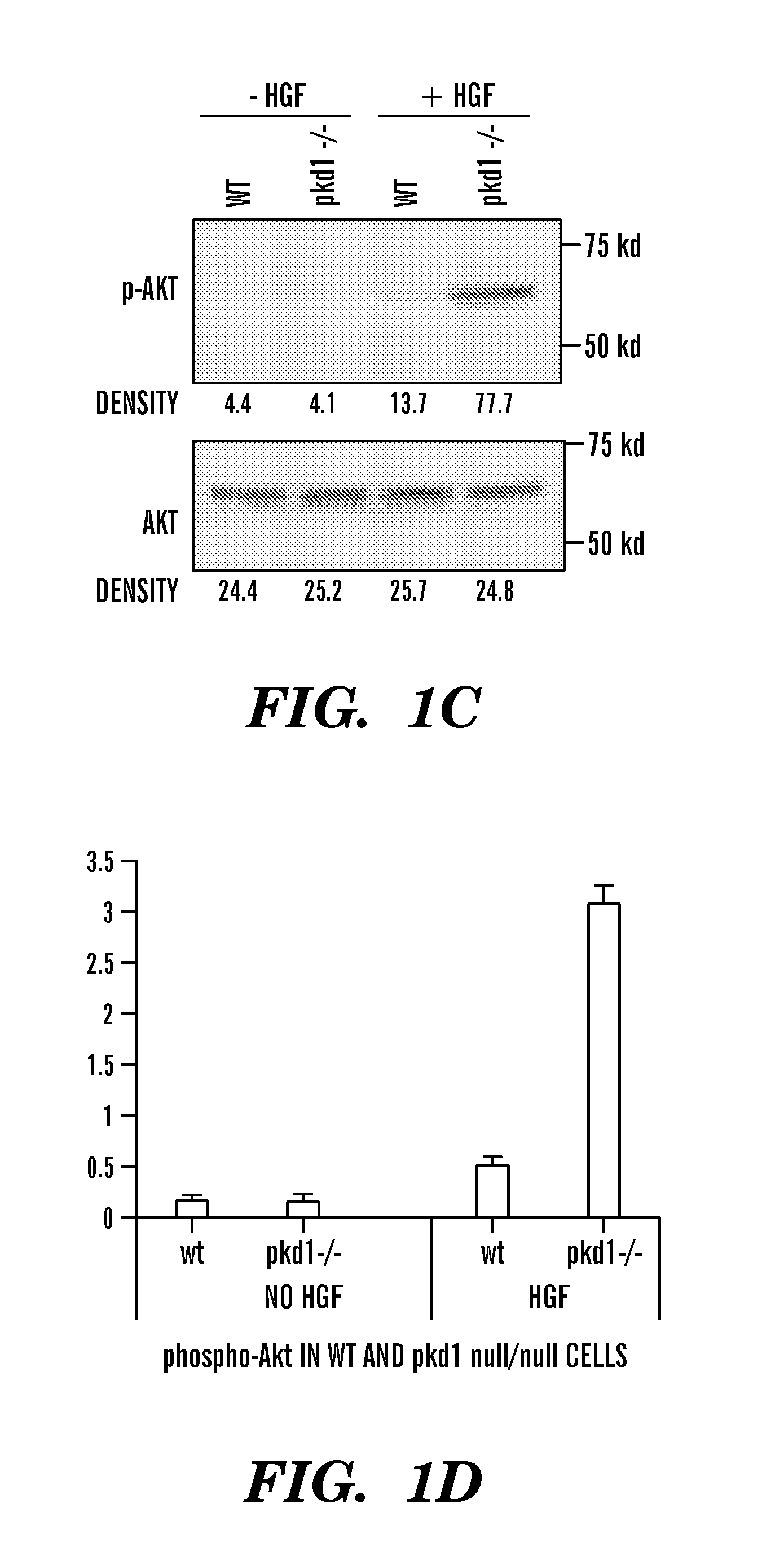
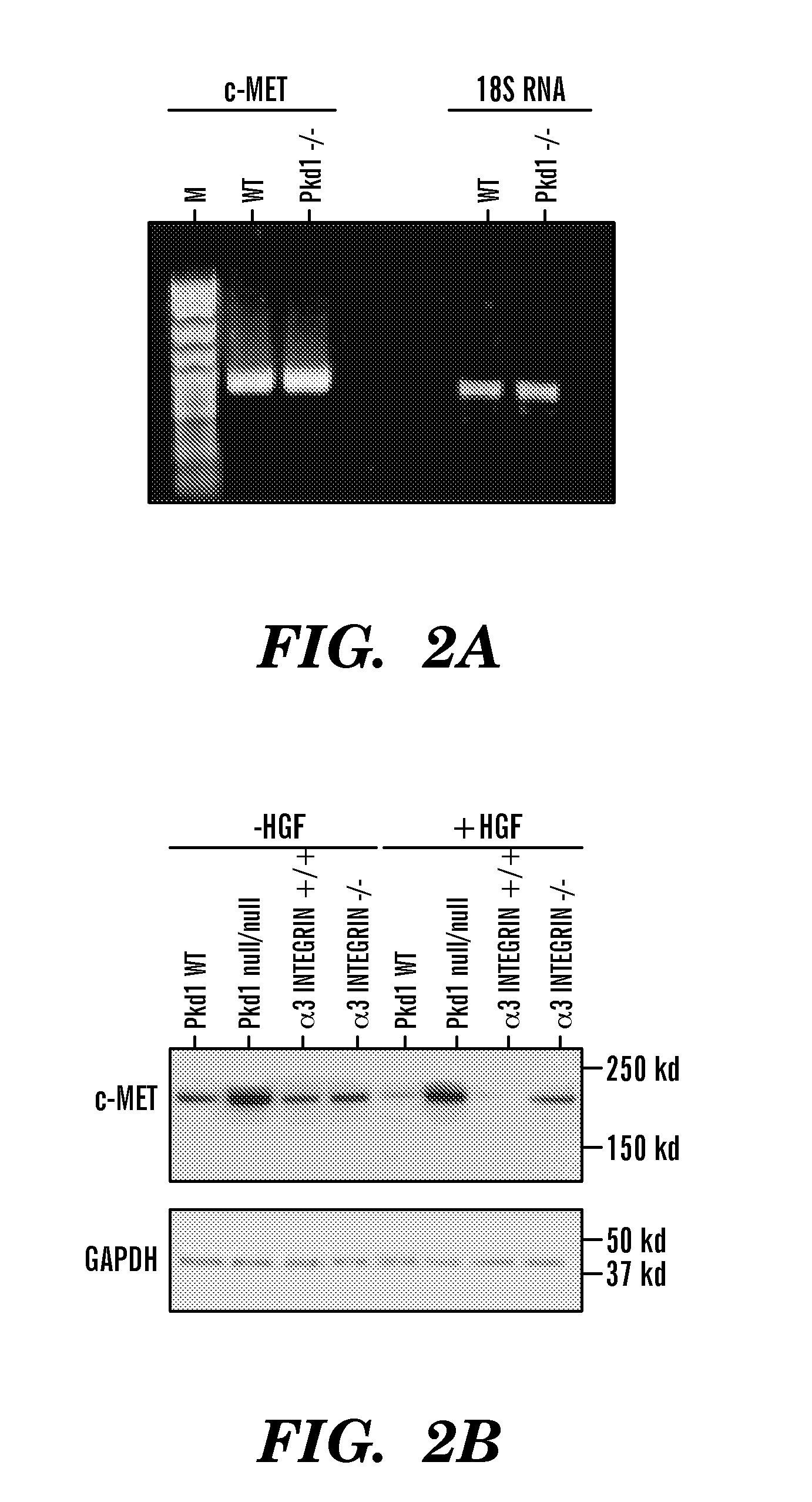
Method of treating polycystic kidney disease
InactiveUS20110020326A1Organic active ingredientsAntibody ingredientsPolycystic liver diseasePolycystic kidney disease
The present invention is directed toward methods for treating, inhibiting the progression of or eradicating polycystic kidney disease in a mammal in need thereof by providing a cMET inhibitor.
Owner:CHILDRENS MEDICAL CENT CORP
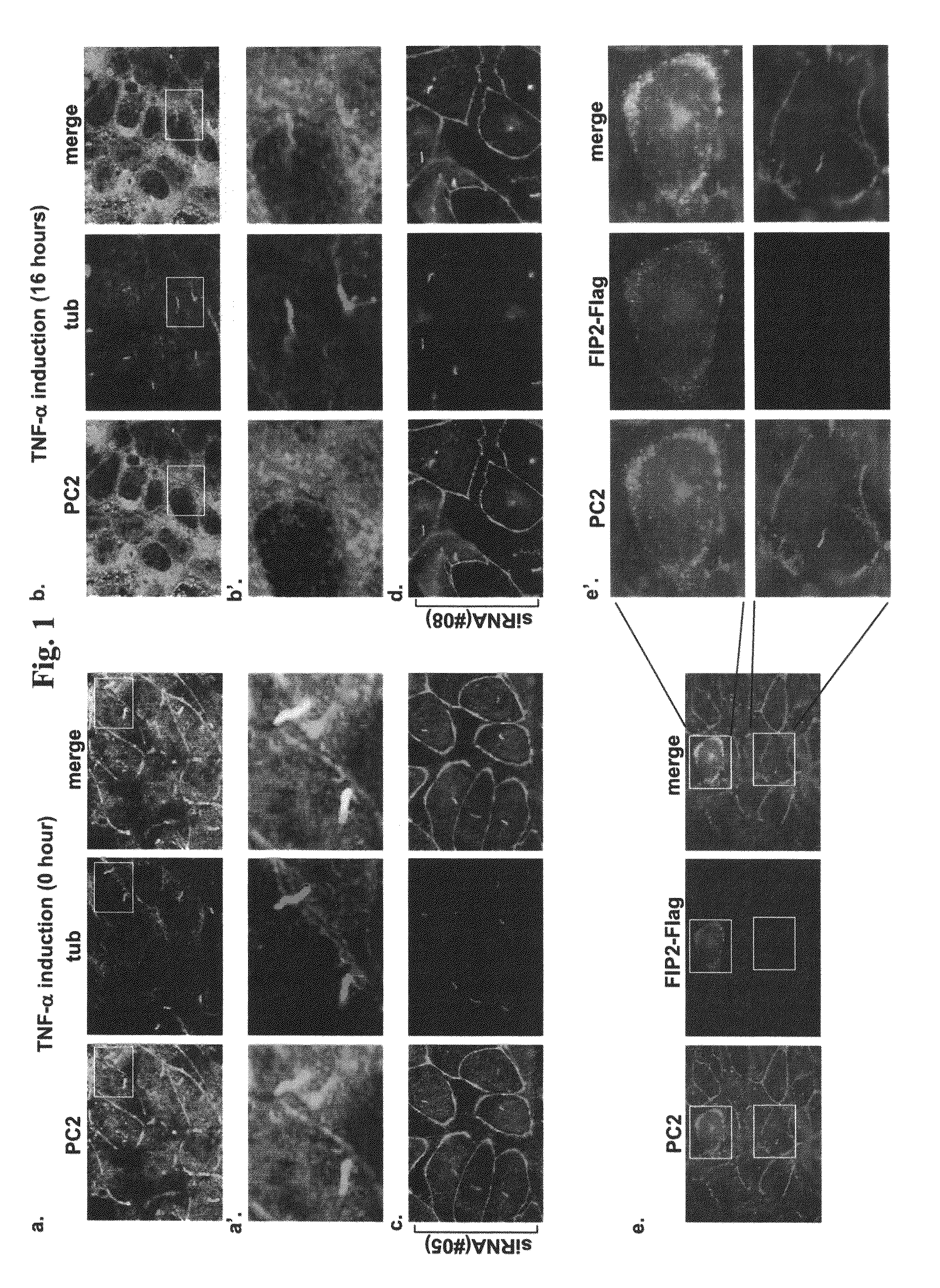
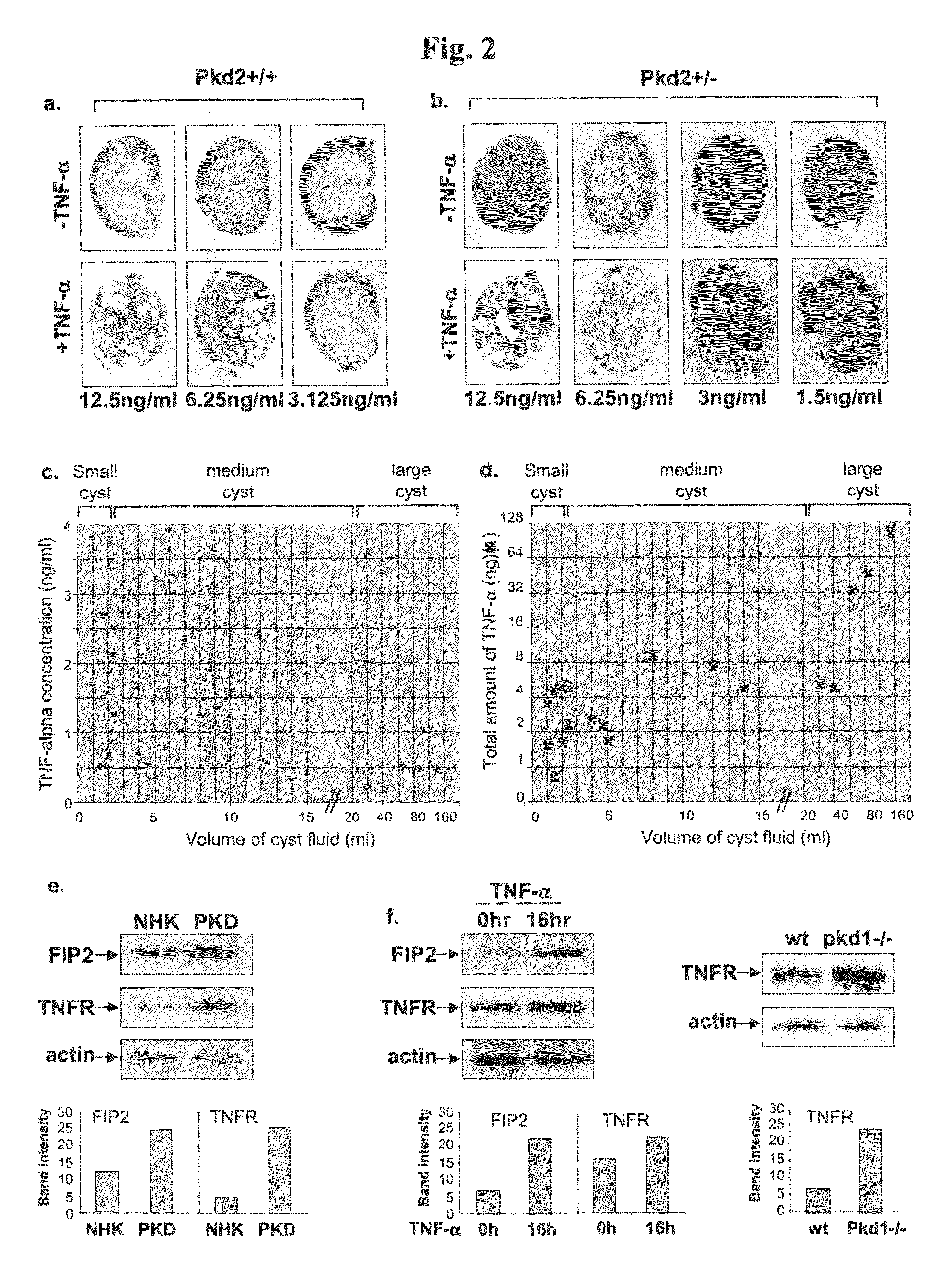
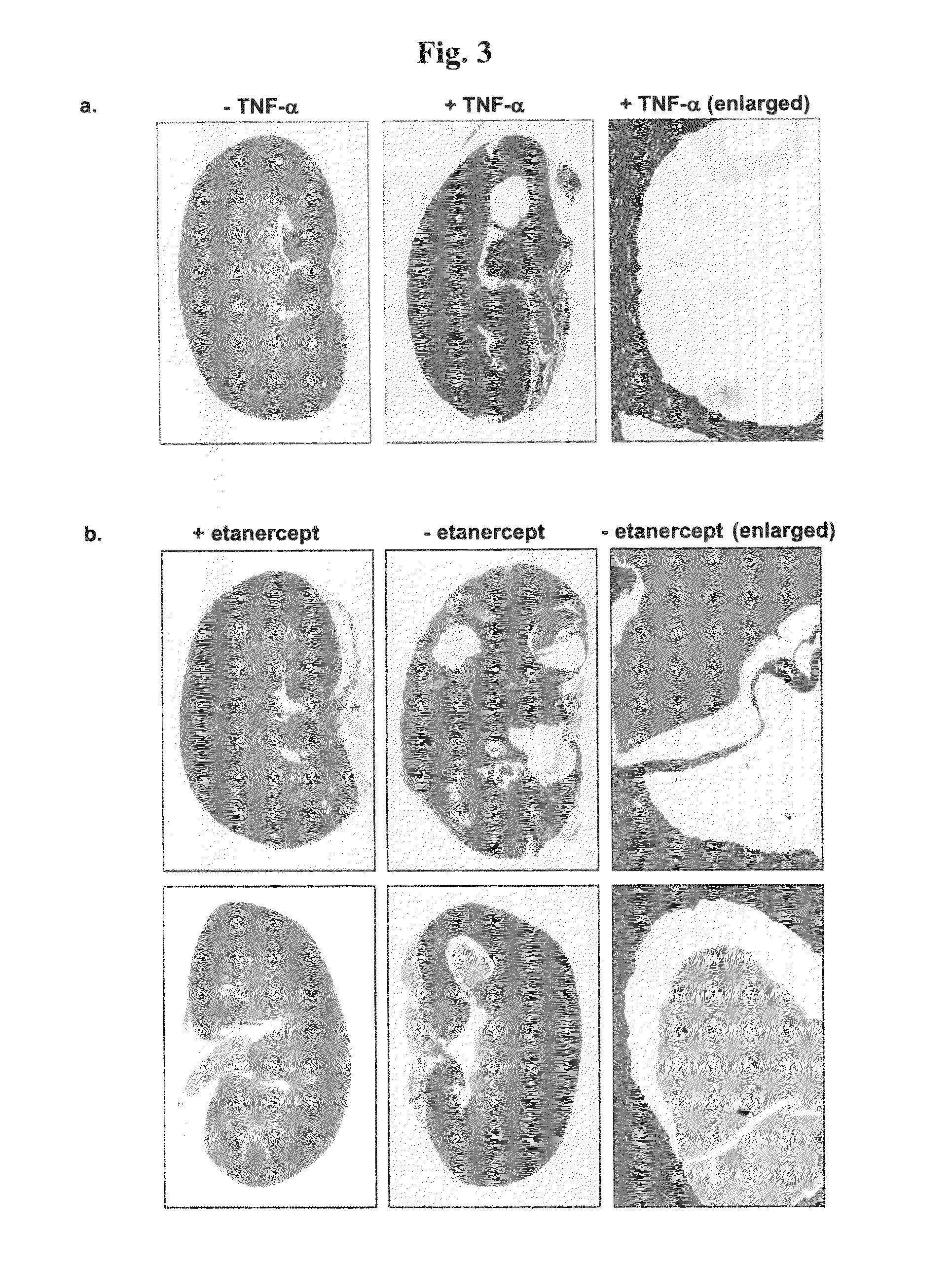
Methods for treating polycystic kidney disease (PKD) or other cyst forming diseases
The present invention is directed to methods of treating or ameliorating an effect of a polycystic disease. More particularly, the methods include administering to a patient in need thereof an amount of a modulator of a tumor necrosis factor (TNF) pathway, which is sufficient to treat or ameliorate an effect of a polycystic disease. Methods of treating or ameliorating an effect of a polycystic kidney disease (PKD) are also provided. Methods are also provided for identifying a candidate compound that may be effective to treat or ameliorate an effect of a polycystic disease or to increase polycystin-2 (PC2) function or decrease Rab11-Family of Interacting Protein2 (FIP2) function. Further provided are methods for identifying a patient having, or who is at risk for developing, a polycystic disease or who would benefit from treatment with a TNF-alpha inhibitor.
Owner:STOWERS INST FOR MEDICAL RES
Drug for preventing and/or treating polycystic kidney disease
ActiveUS20160058841A1Significant effectLife maintenancePeptide/protein ingredientsPharmaceutical delivery mechanismTherapeutic effectPolycystic liver disease
An object of the present invention is to provide a combination drug that has remarkably excellent preventive and / or therapeutic effects on polycystic kidney disease. The present invention provides a drug for preventing and / or treating polycystic kidney disease comprising a combination of tolvaptan or a prodrug thereof with a somatostatin derivative, and a method for treating polycystic kidney disease using this drug.
Owner:OTSUKA PHARM CO LTD
Somatostatin agonists
InactiveUS6864234B1Improve scalabilityLose weightPeptide/protein ingredientsMetabolism disorderVIPomaPercent Diameter Stenosis
The present invention is directed to cyclic peptides of formula (I): X-A1-cyclo(D-Cys-A3-A4-Lys-A6-A7)-A8-Y, or a pharmaceutically acceptable salt thereof. The peptides bind selectively to the somatostatin subtype receptor type-5 and elicit an agonist effect from the somatostatin subtype receptors. The peptides are useful for treating a variety of diseases, including Cushings Syndrome, gonadotropinoma, hyperparathyroidism, Paget's disease, VIPoma, nesidioblastosis, hyperinsulinism, gastrinoma, Zollinger-Ellison Syndrome, hypersecretory diarrhea related to AIDS and other conditions, irritable bowel syndrome, pancreatitis, Crohn's Disease, systemic sclerosis, thyroid cancer, psoriasis, hypotension, panic attacks, sclerodoma, small bowel obstruction, gastroesophageal reflux, duodenogastric reflux, Graves' Disease, polycystic ovary disease, upper gastrointestinal bleeding, pancreatic pseudocysts, pancreatic ascites, leukemia, meningioma, cancer cachexia, acromegaly, restenosis, hepatoma, lung cancer, melanoma, inhibiting the accelerated growth of a solid tumor, decreasing body weight, treating insulin resistance, Syndrome X, prolonging the survival of pancreatic cells, fibrosis, hyperlipidemia, hyperamylinemia, hyperprolactinemia and prolactinemia.
Owner:IPSEN PHARMA SAS
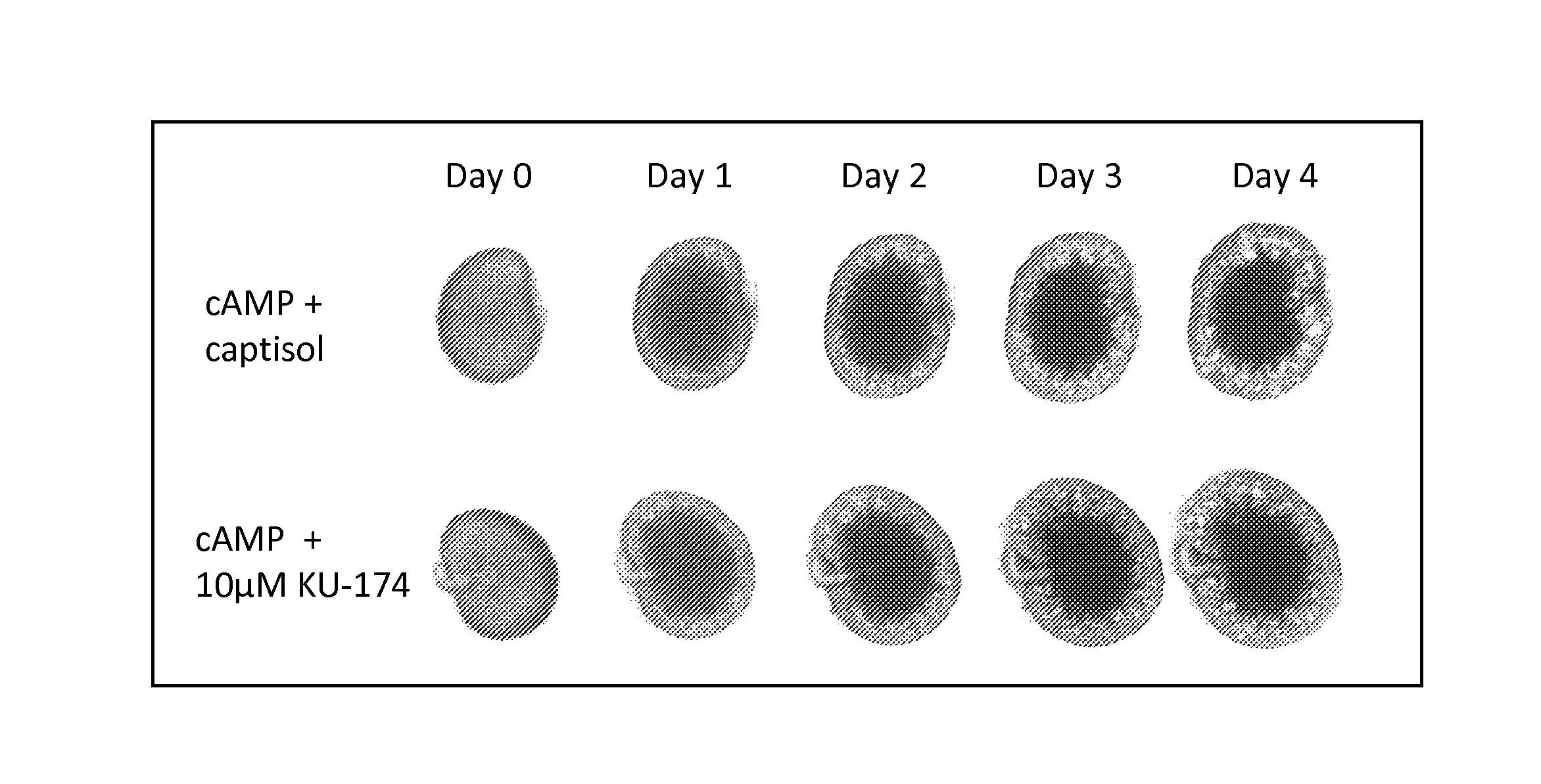
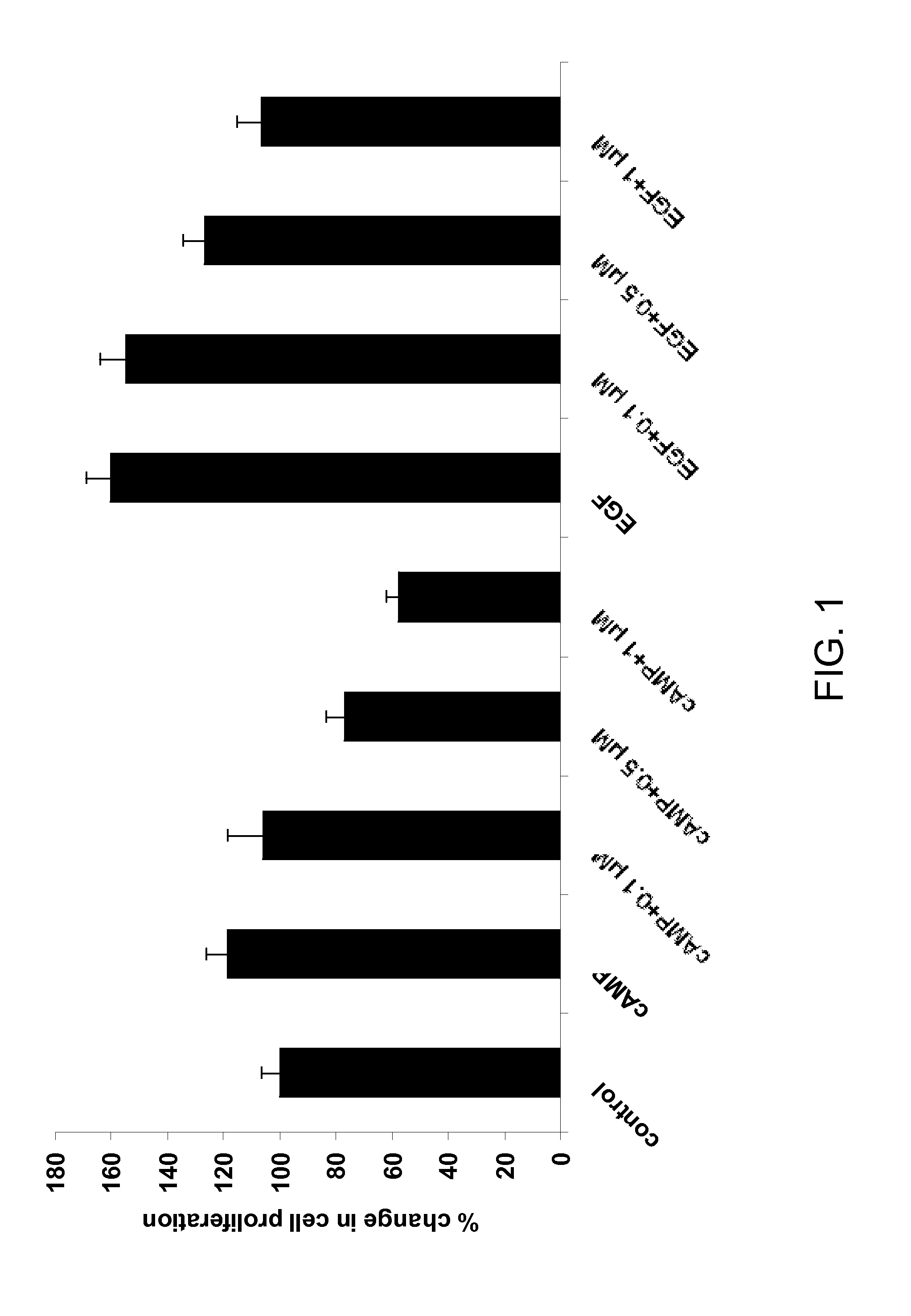
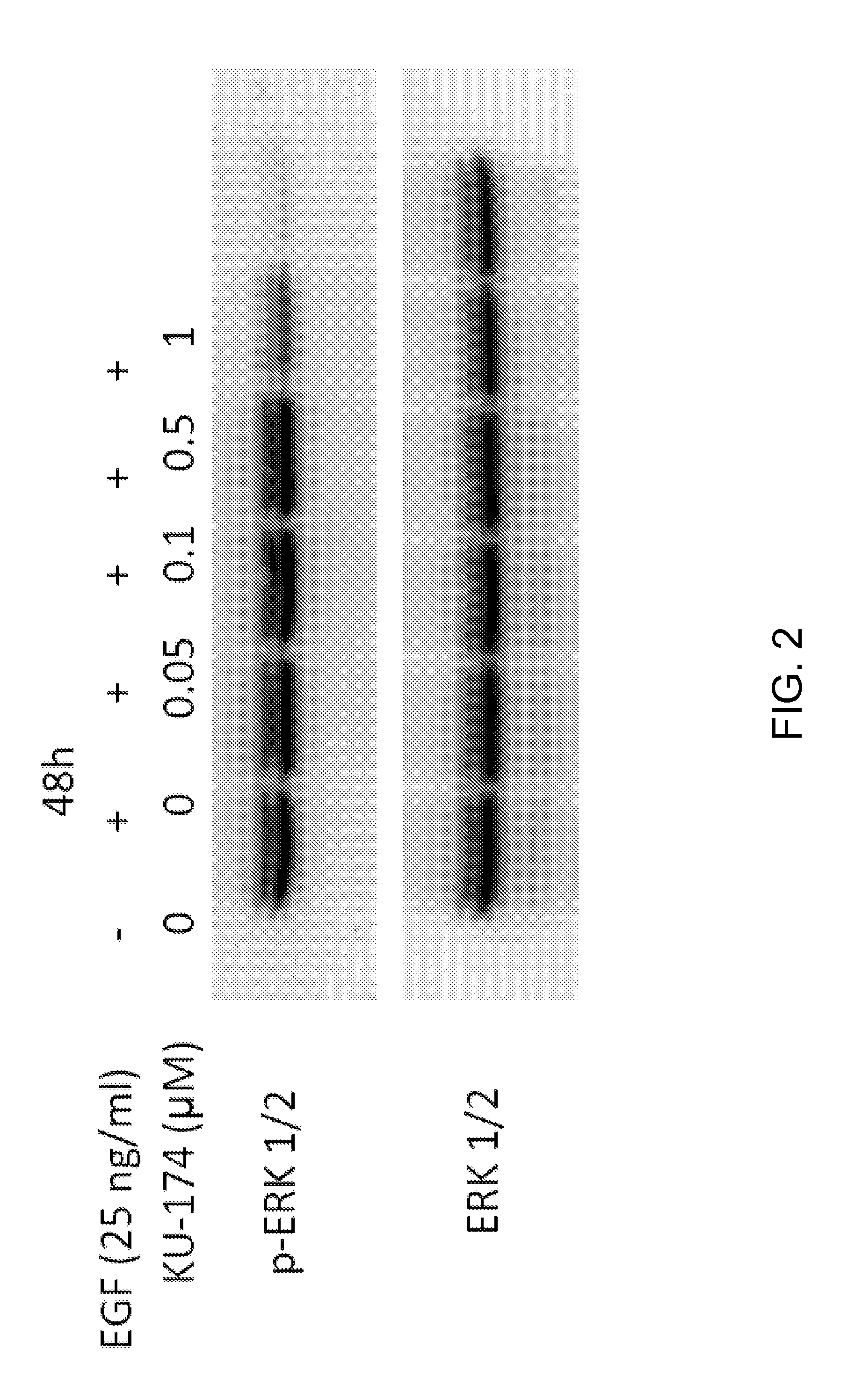
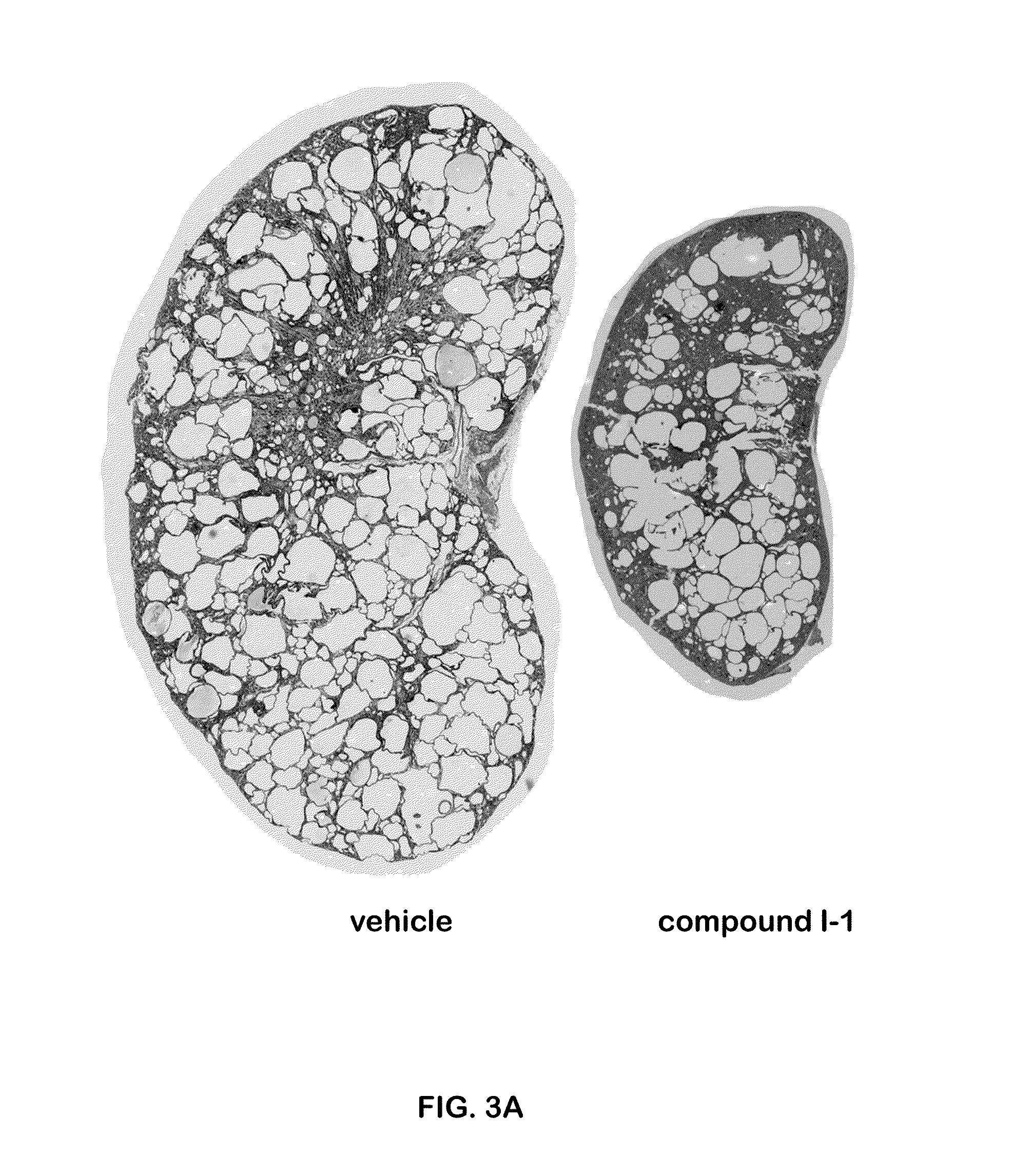
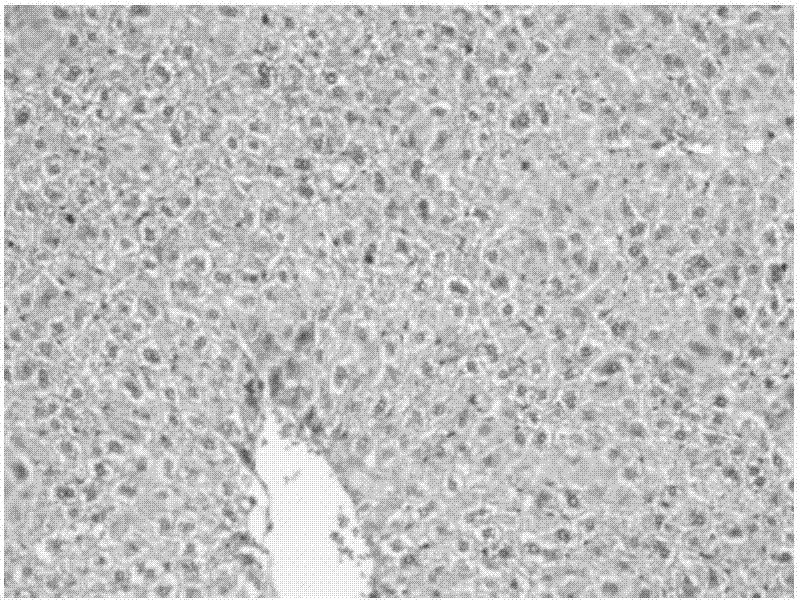
Methods for treating polycystic kidney disease and polycystic liver disease
ActiveUS20150105361A1Reduces and avoids symptom and causeGood curative effectOrganic active ingredientsDigestive systemPhysiologyCyst
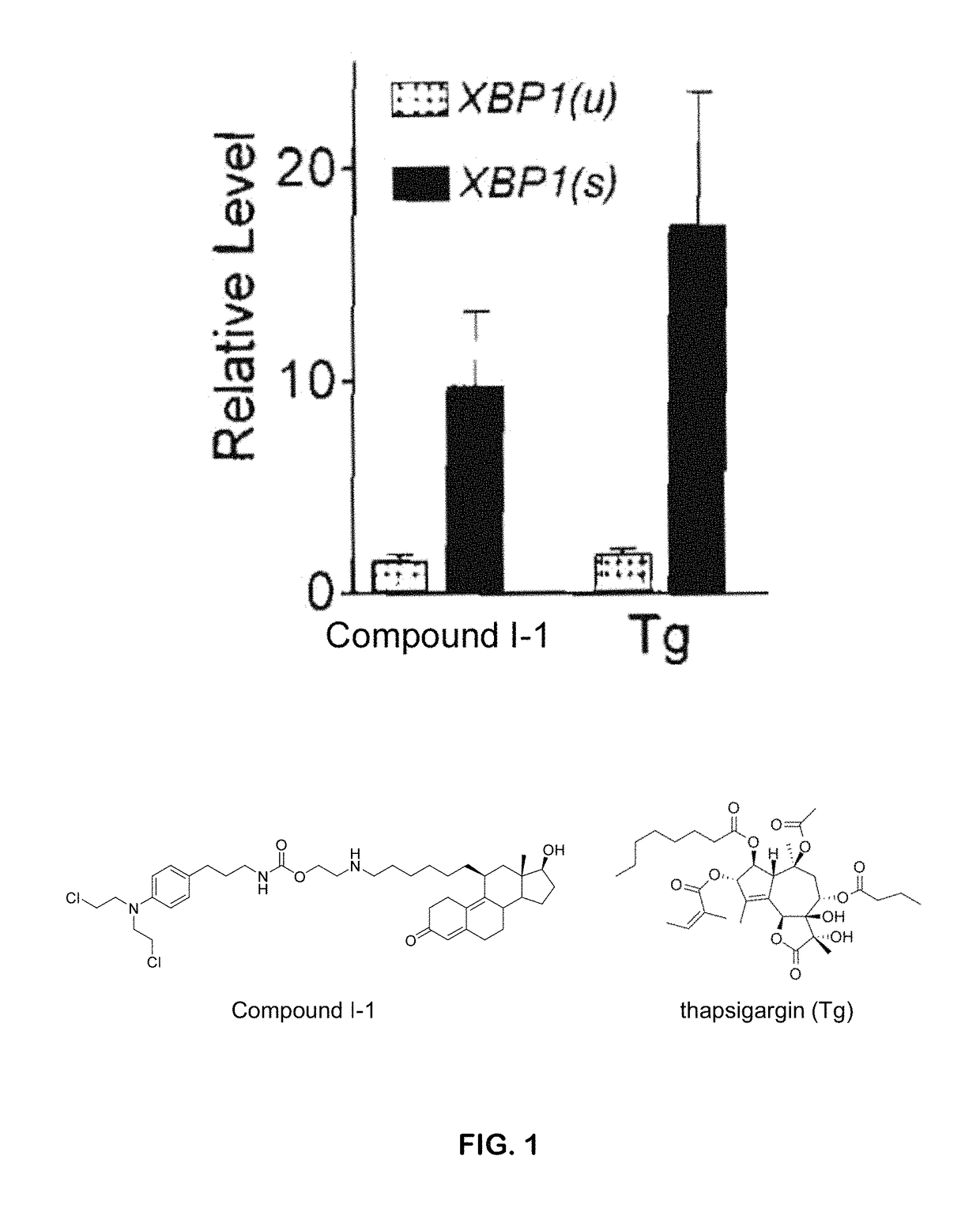
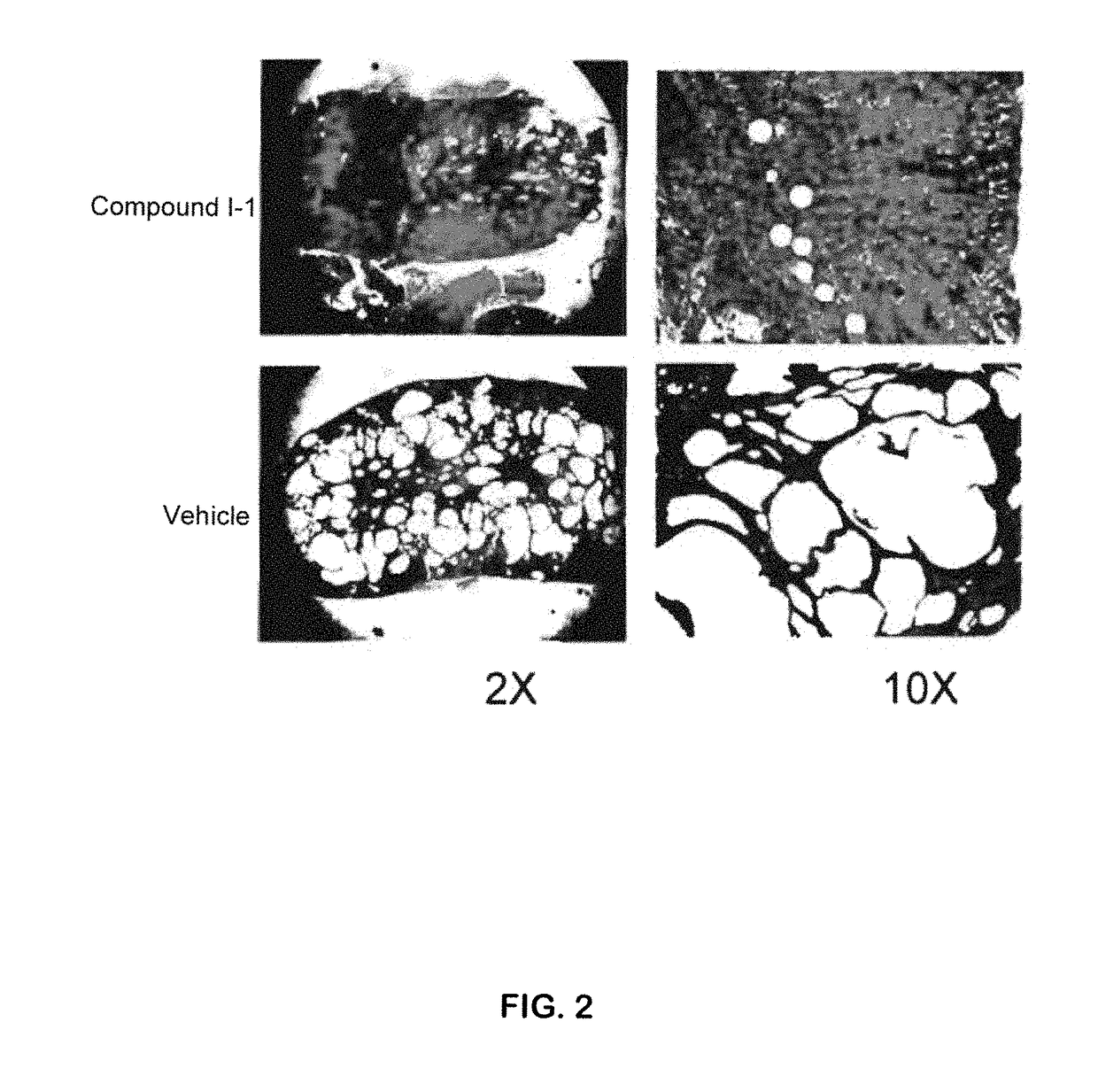
The present invention provides compounds of Formula (I) or (II), which are thought to be able to inhibit mTOR (mammalian target of rapamycin) signaling pathway, induce UPR (unfolded protein response), and / or perturb mitochondrial function of a cyst cell (e.g., a cyst cell causing polycystic kidney disease (PKD, e.g., autosomal dominant PKD (ADPKD) or autosomal recessive PKD (ARPKD)) or polycystic liver disease (PLD, e.g., autosomal dominant PLD (ADPLD) or autosomal recessive PLD (ARPLD)). The invention also provides pharmaceutical compositions, kits, and methods involving the compounds described herein for use in treating PKD or PLD, inhibiting the growth of a cyst cell, and / or killing a cyst cell.
Owner:YALE UNIV +1
Application of tripterine in preparation of medicines for treating cholestatic liver disease
ActiveCN106924265AReduce bile acid contentReduce necrosisOrganic active ingredientsDigestive systemCholic acidBiliary excretion
The invention discloses application of tripterine in preparation of medicines for treating cholestatic liver disease. The tripterine achieves obvious effects of improving cholestasis and alleviating liver damage on intrahepatic cholestasis induced by mouse alpha-naphthylisothiocyanate (ANIT) and cholestatic liver disease induced by thioacetamide (TAA), can obviously lower levels of aspartic transaminase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) in plasma of two models, reduce the cholic acid content in the plasma and promote bile excretion and has the effects of protecting liver and increasing choleresis; and when being applied to research and development of medicines with effects of protecting liver and increasing choleresis, the tripterine can create excellent economic benefits and wide social benefits.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
Screening methods for compounds useful in the treatment of polycystic kidney disease
The present invention provides cell-based screening assays designed to identify agents that regulate the activity of the polycystic kidney disease proteins encoded by the PKD-1 and PKD-2 genes and that may be useful in the treatment of polycystic kidney disease. The assays of the invention comprise the contacting of genetically engineered cells expressing a mutant or truncated PKD gene product with a test agent and assaying for a decrease in the PKD mediated mutant phenotype. Characteristics associated with such a mutant phenotype include increased adherence to type I collagen coated surfaces; apical expression of NaK-ATPase on the cell membrane; increased expression of β-2-NaK-ATPase; and decreased focal adhesion kinase (FAK) incorporation into focal adhesion complexes, and inability to form tubular structures in a gel matrix. To facilitate the screening methods of the invention, cells may be genetically engineered to express epitope tagged PKD gene products and / or epitope tagged PKD interacting proteins (PKD-IP). Such interacting proteins include, for example, focal adhesion complex proteins such as FAK, paxillin, vinculin, talin and the like.
Owner:MT SINAI SCHOOL OF MEDICINE
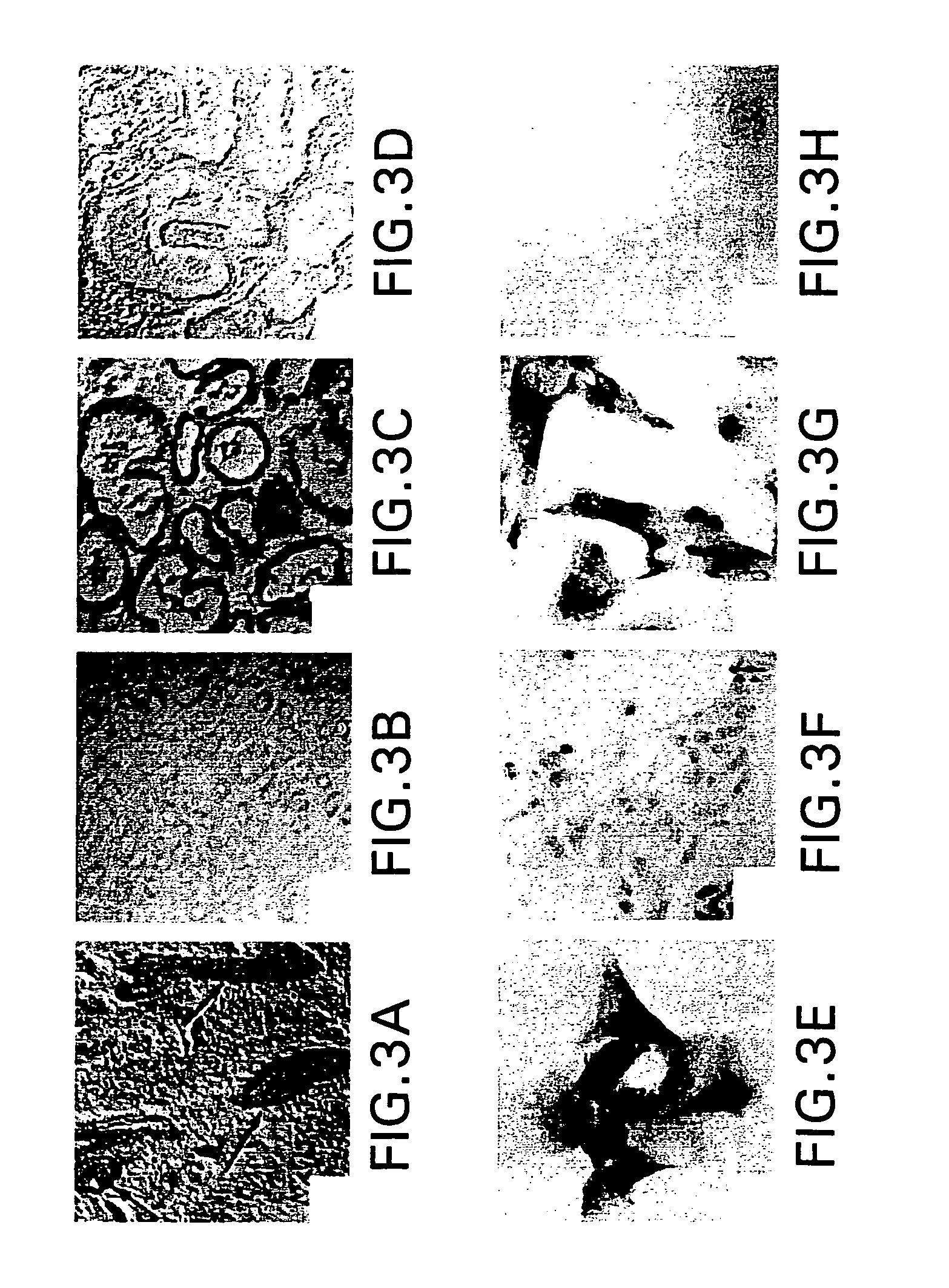
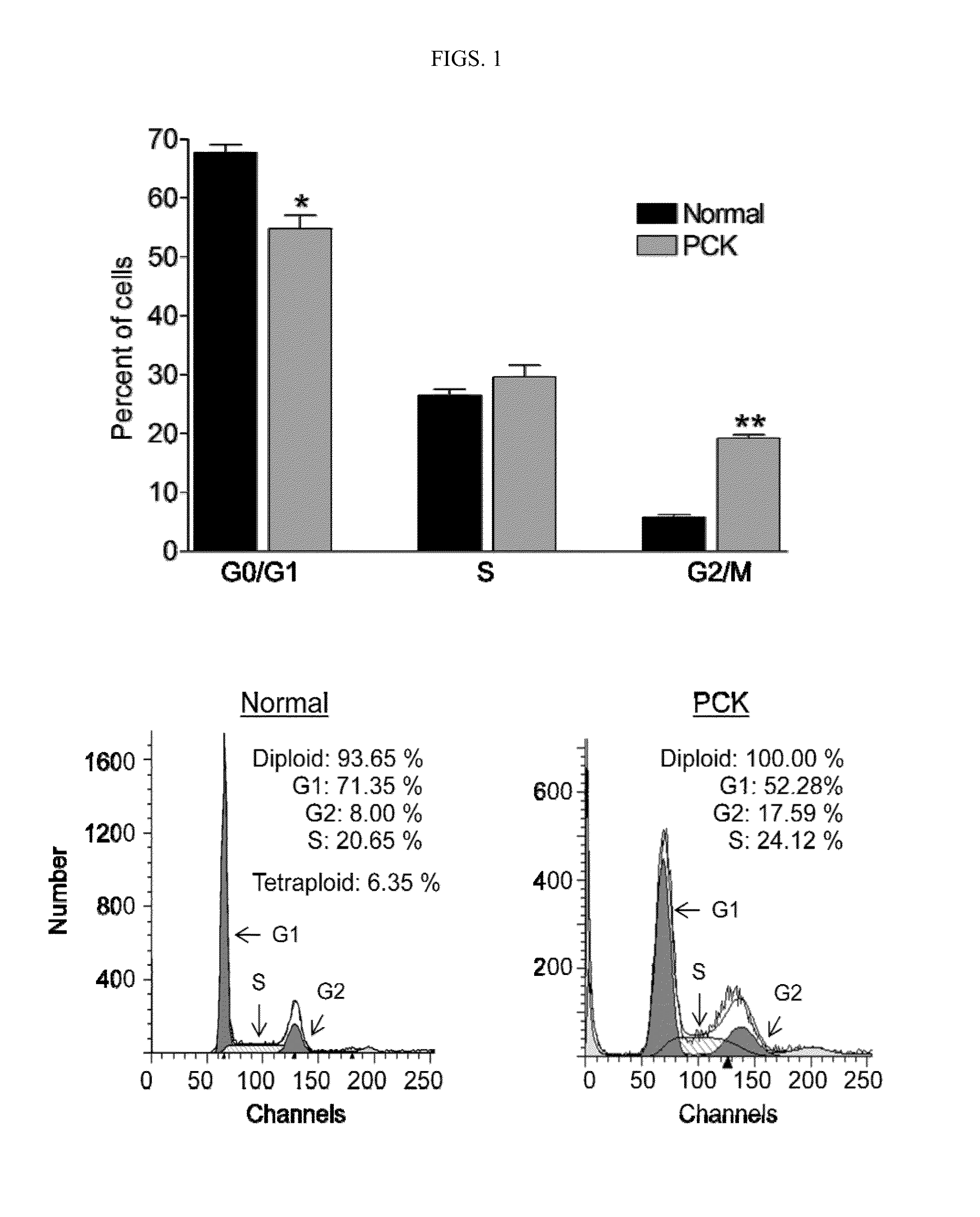
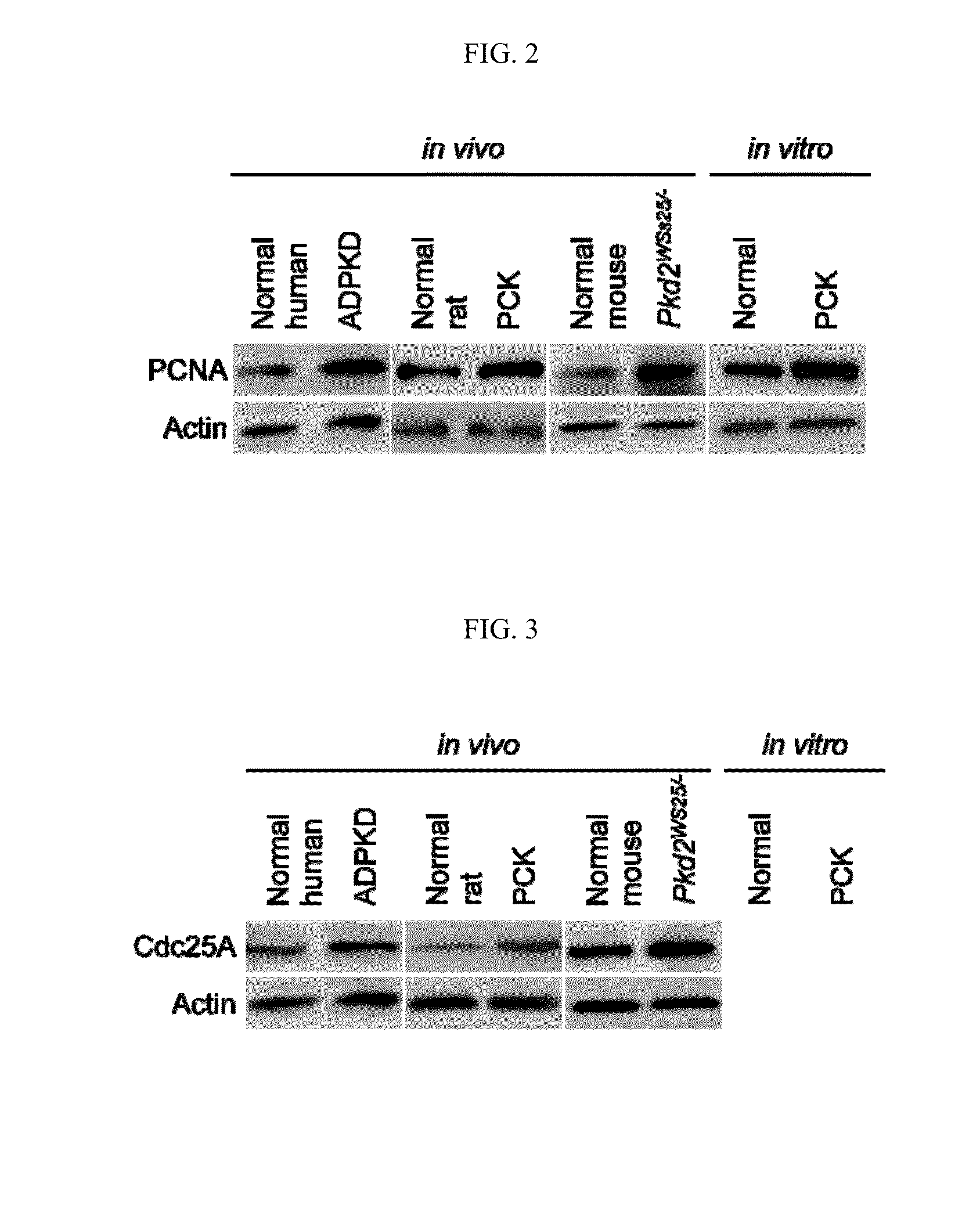
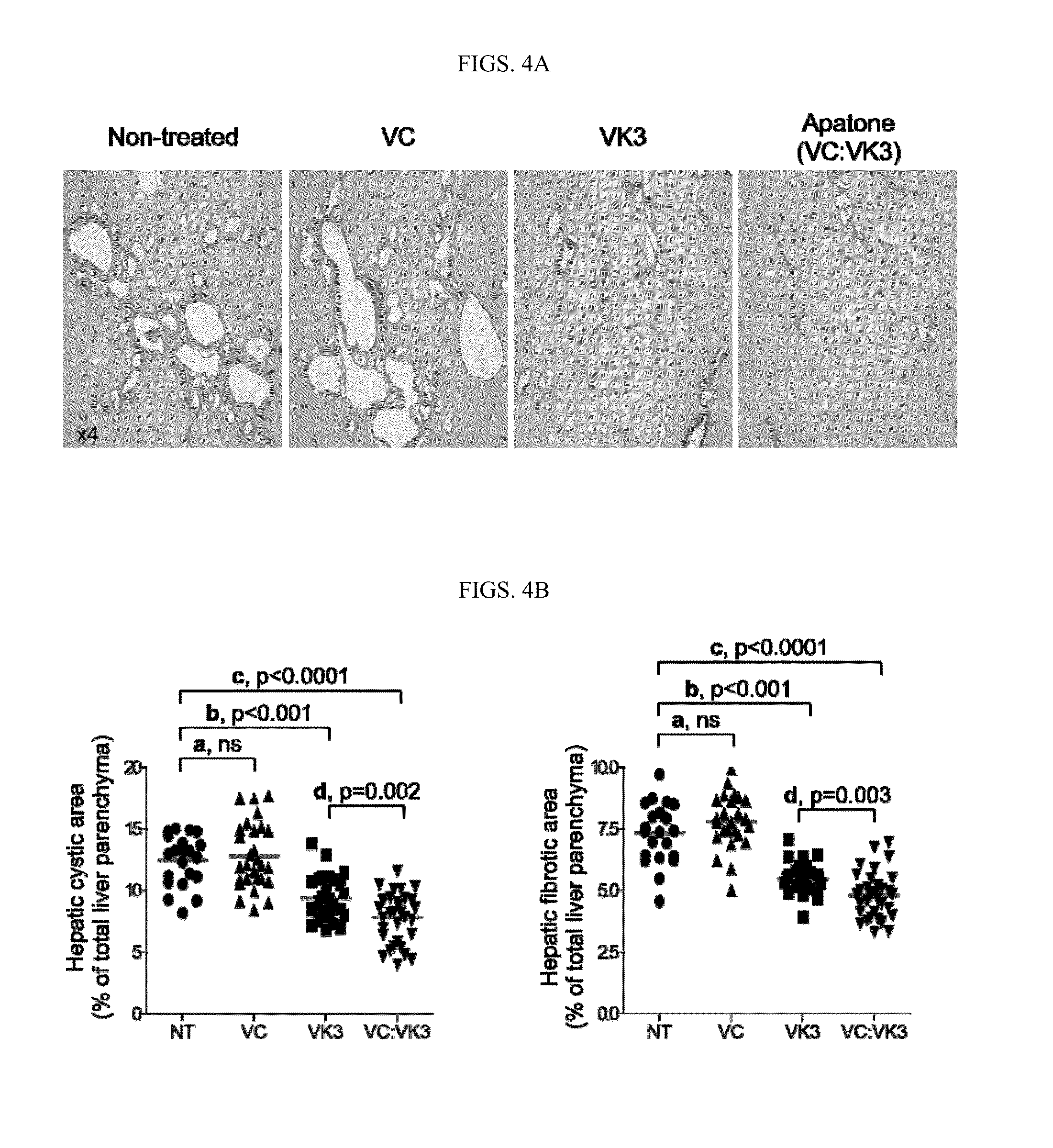
Vitamins c and k for treating polycystic diseases
Provided herein are methods for treating, preventing, or ameliorating one or more symptoms of a polycystic disease in a subject, comprising administering to the subject a therapeutically effective amount of vitamins C and K.
Owner:IC MEDTECH CORP
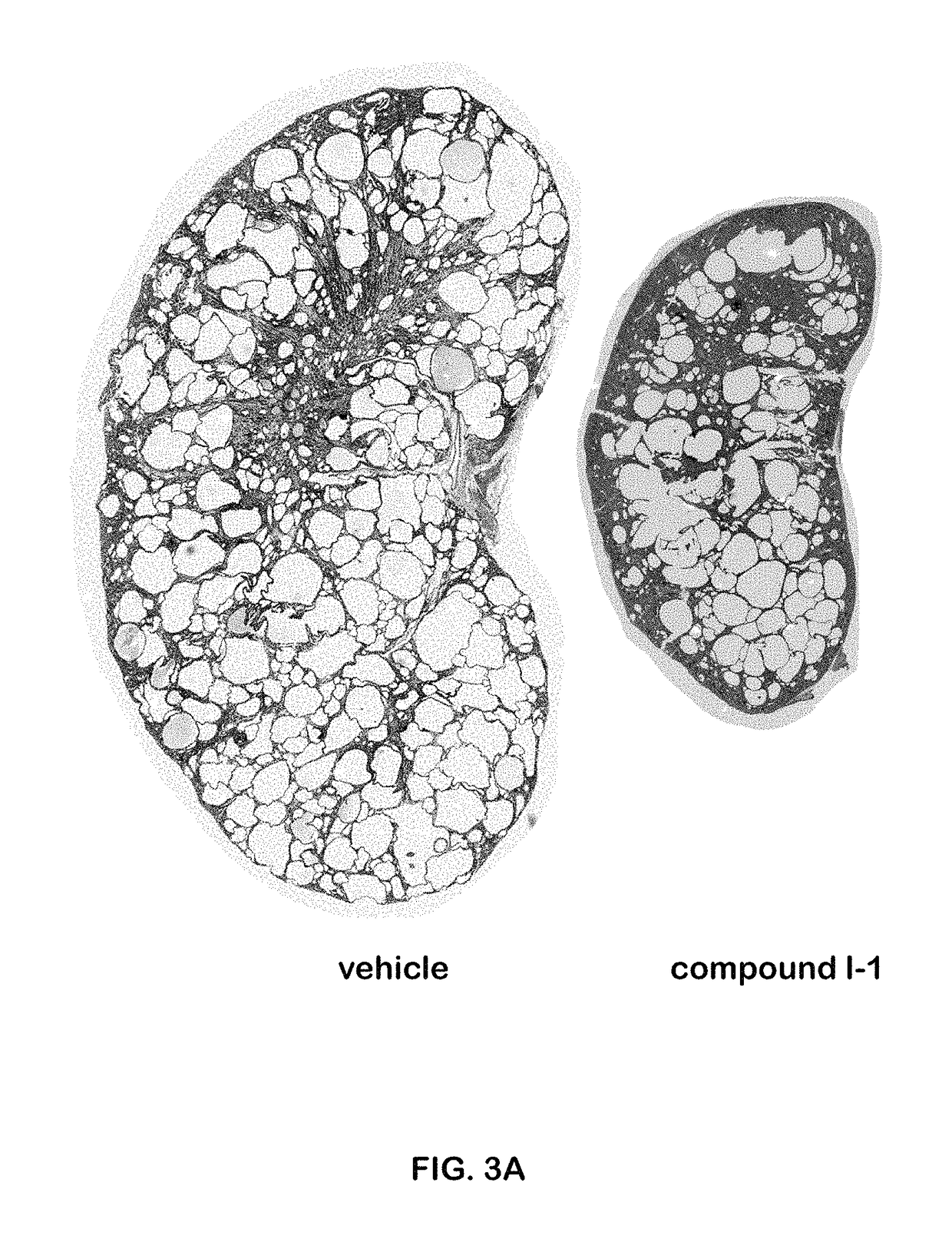
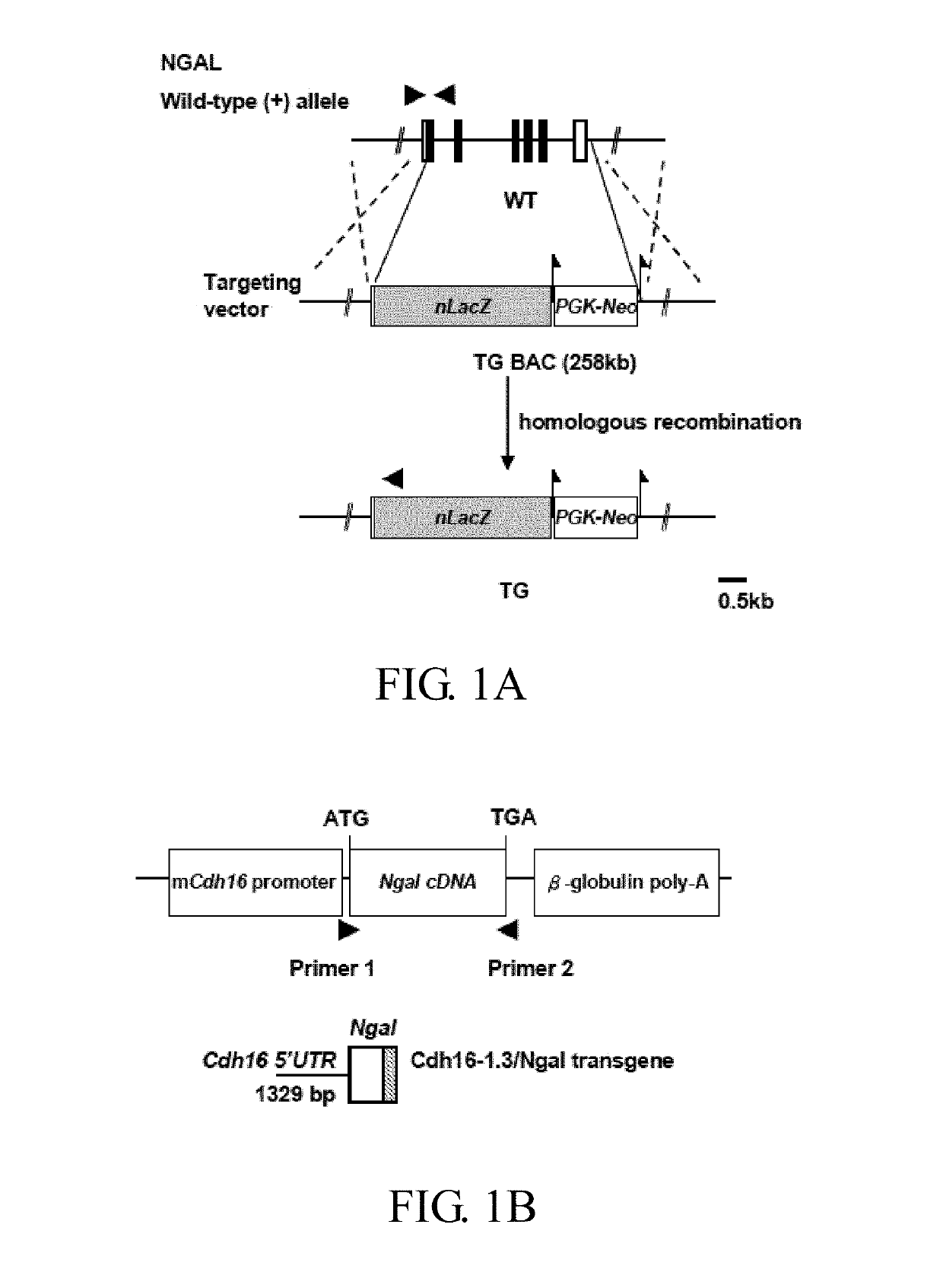
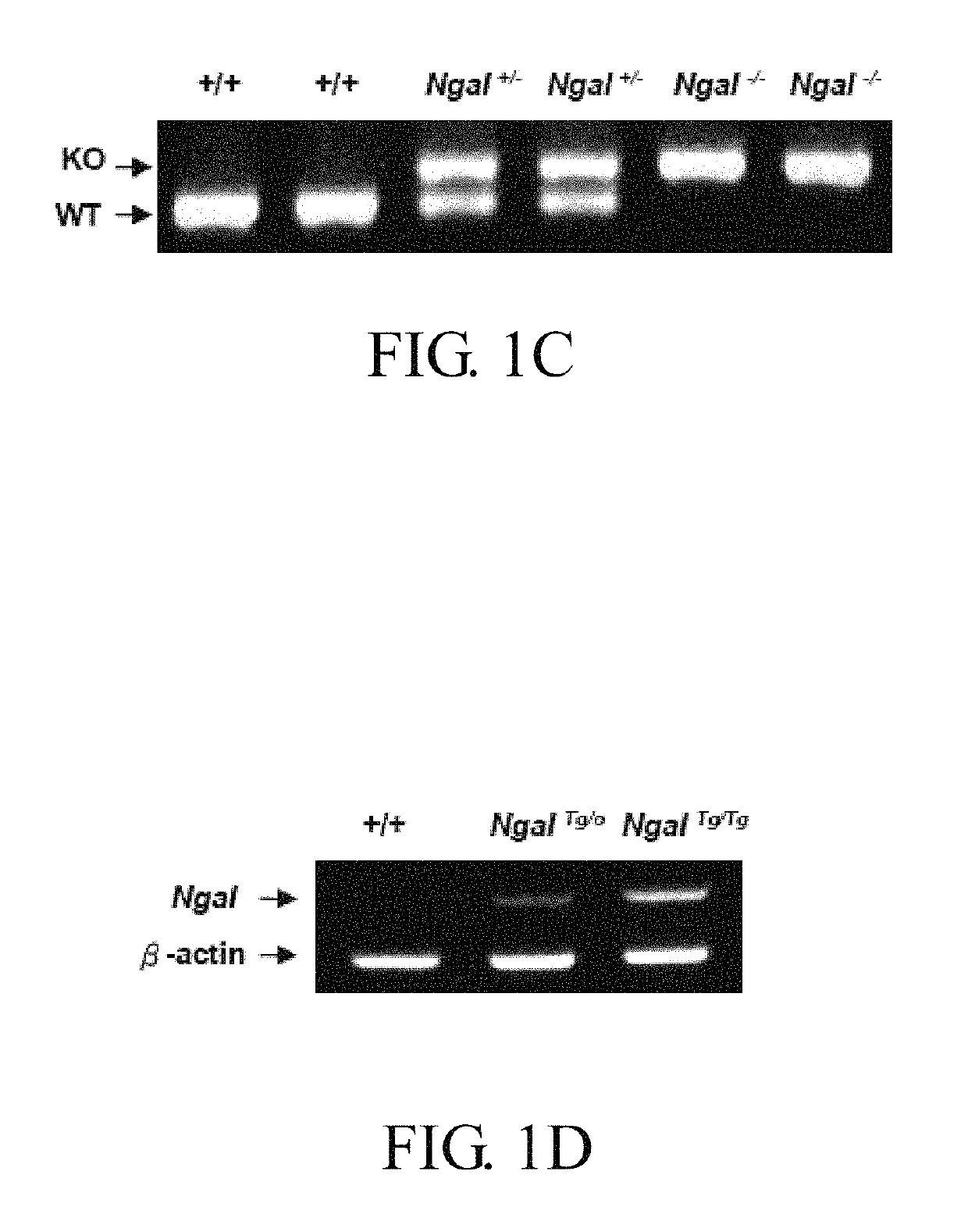
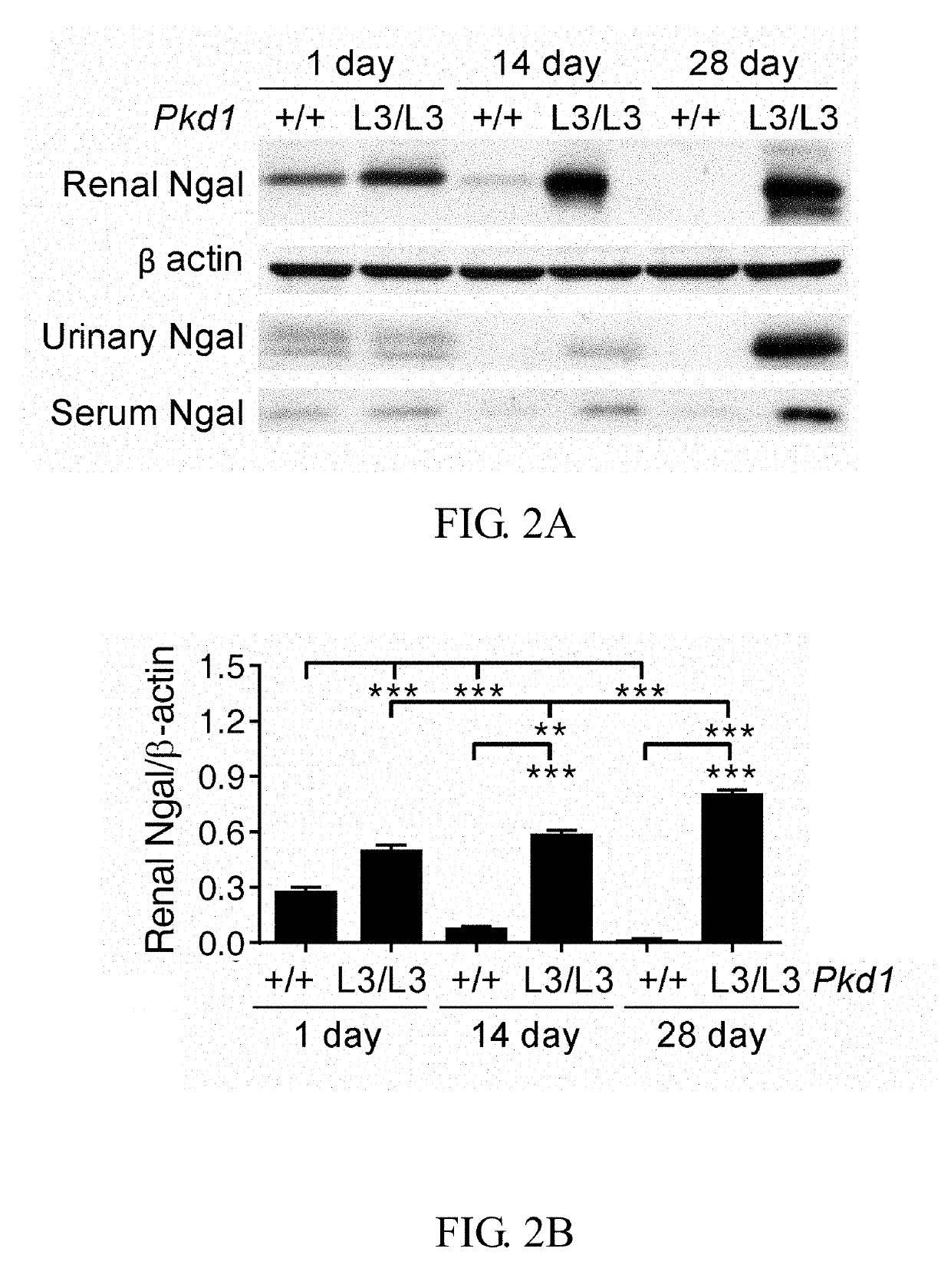
Method of treating or preventing polycystic kidney disease and pkd animal model with exogenous neutrophil gelatinase-associated lipocalin
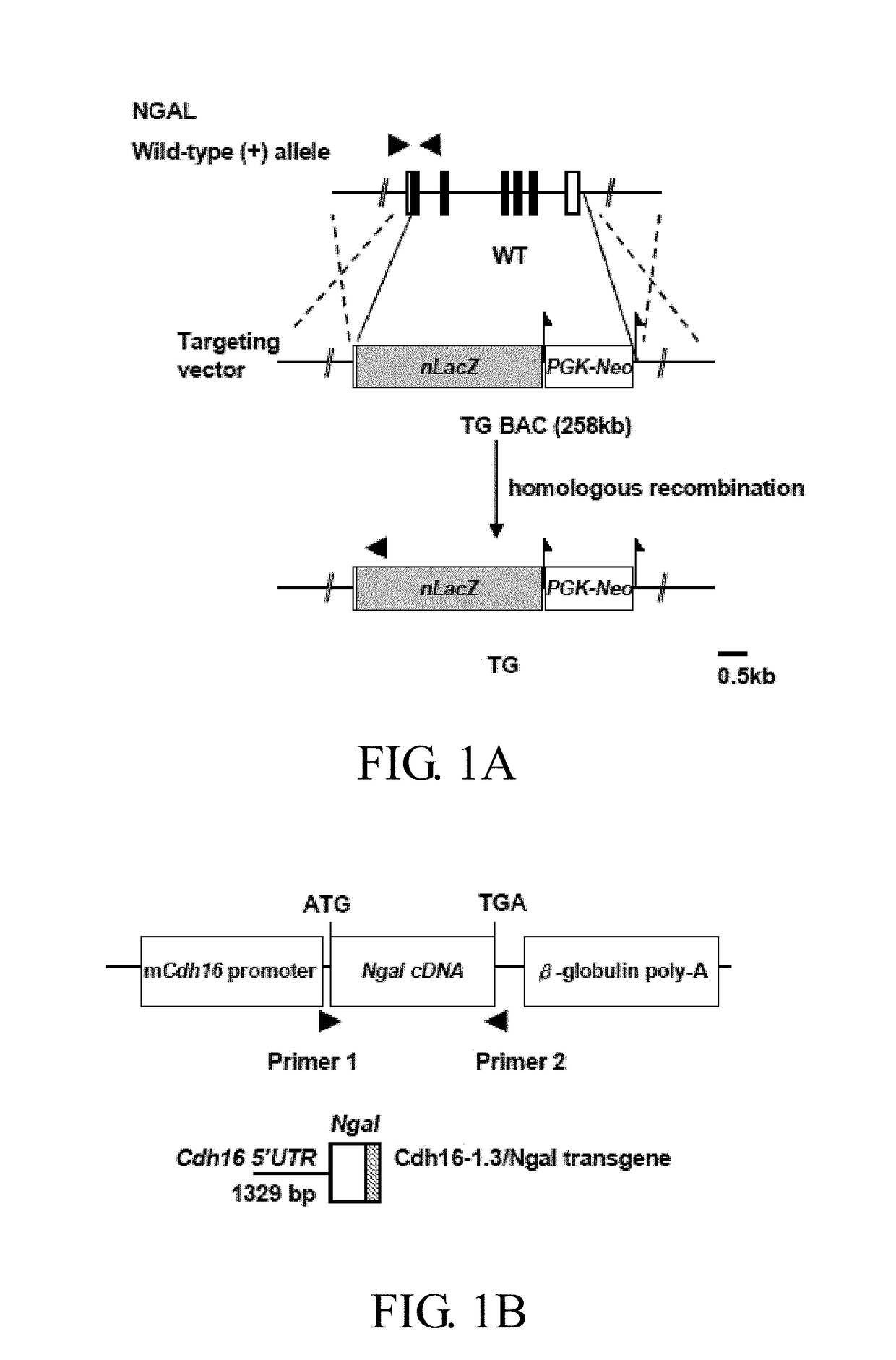
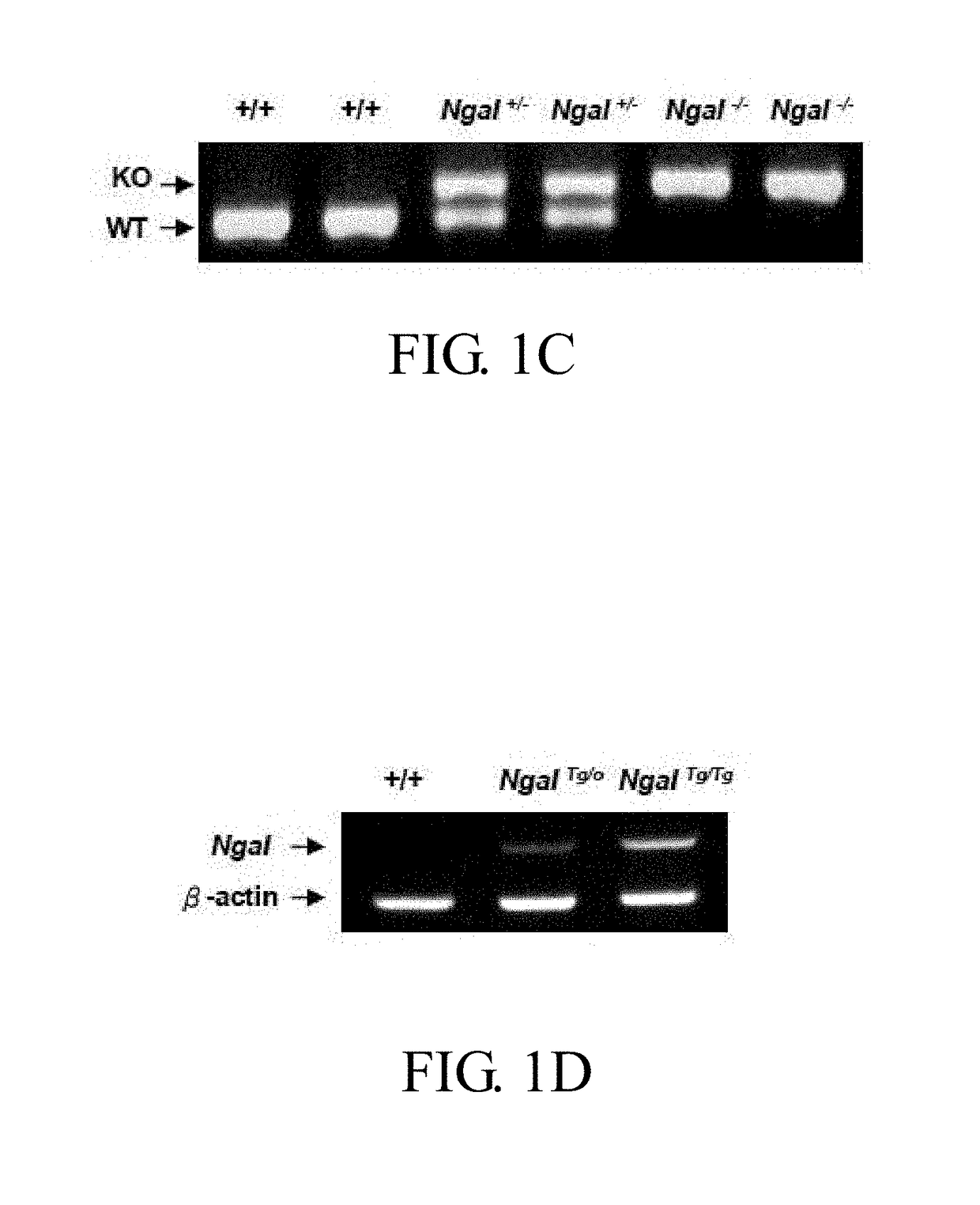
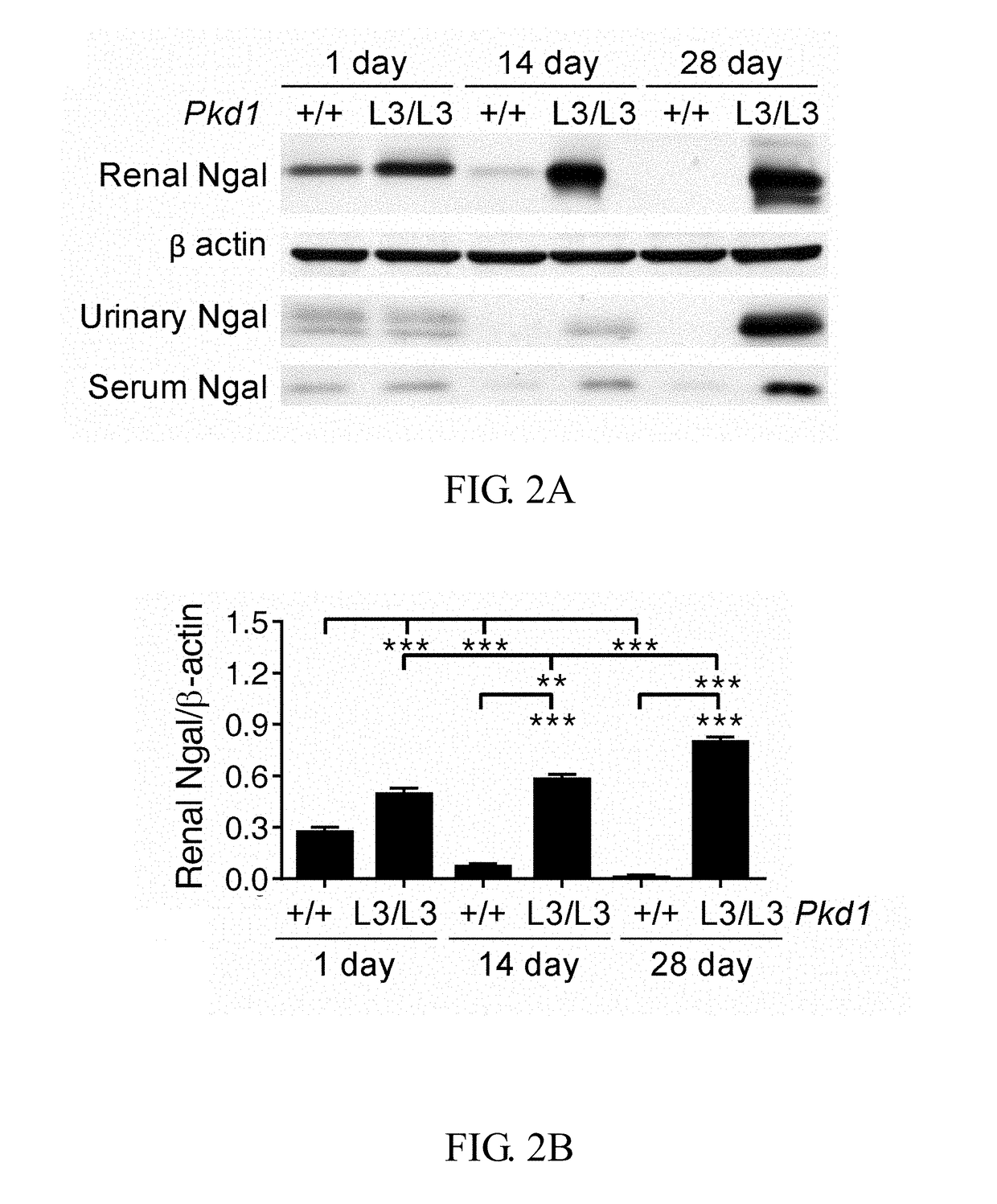
ActiveUS20170182120A1Strong specificityReduce degradationPeptide/protein ingredientsUrinary disorderGelatinaseNGAL Protein
Disclosed is a method of treating or preventing polycystic kidney disease (PKD) by administration of neutrophil geleatinase-associated lipocalin (Ngal) protein. Also, a transgenic non-human animal model is established to investigate the effect of overexpression of exogenous Ngal on PKD progression.
Owner:NATIONAL TAIWAN NORMAL UNIVERSITY
Methods for treatment of polycystic kidney disease
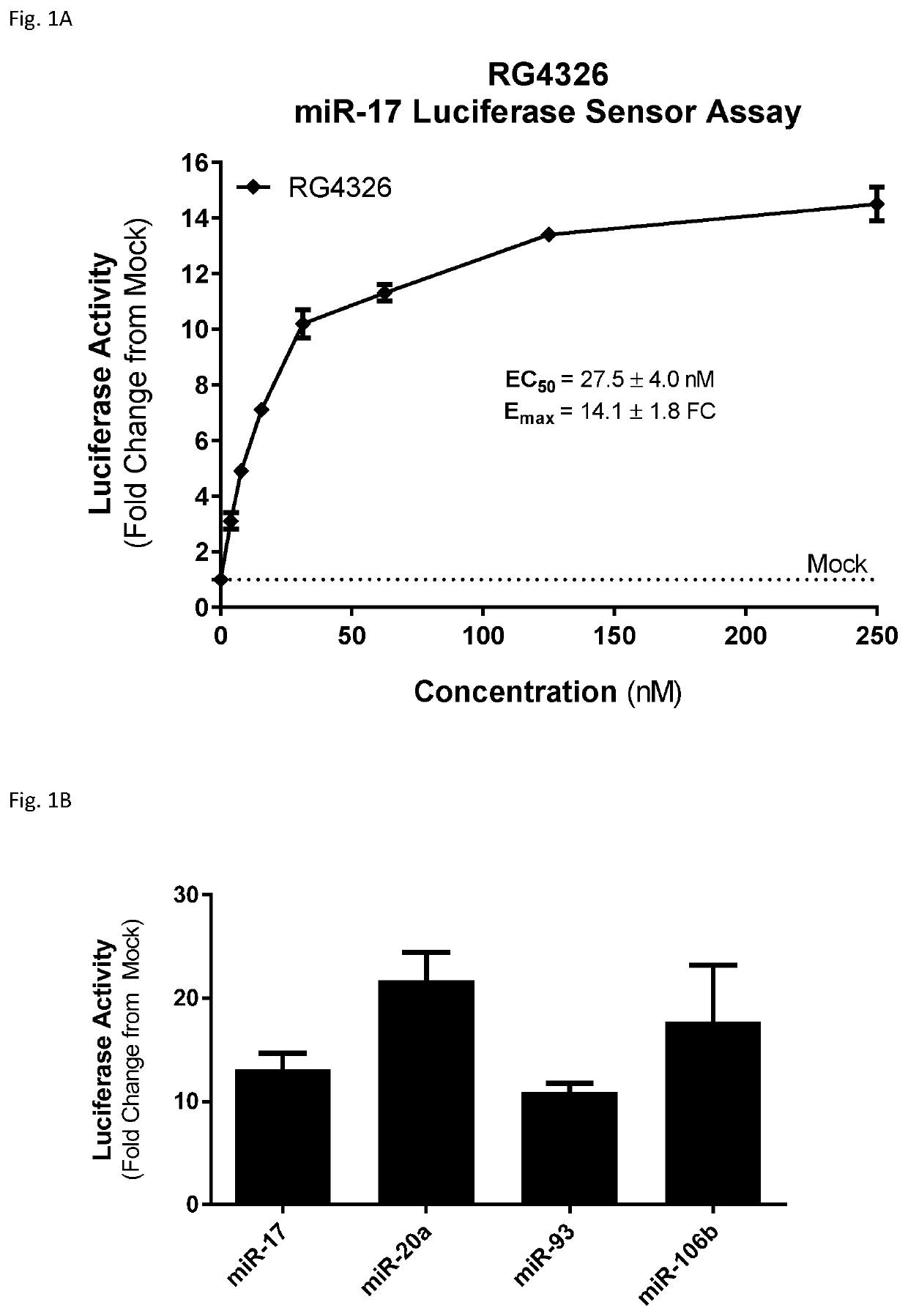
InactiveUS20190345494A1Increase slowlySlows rate of decline of glomerular filtration ratePeptide/protein ingredientsPharmaceutical delivery mechanismPhysiologyPolycystic liver disease
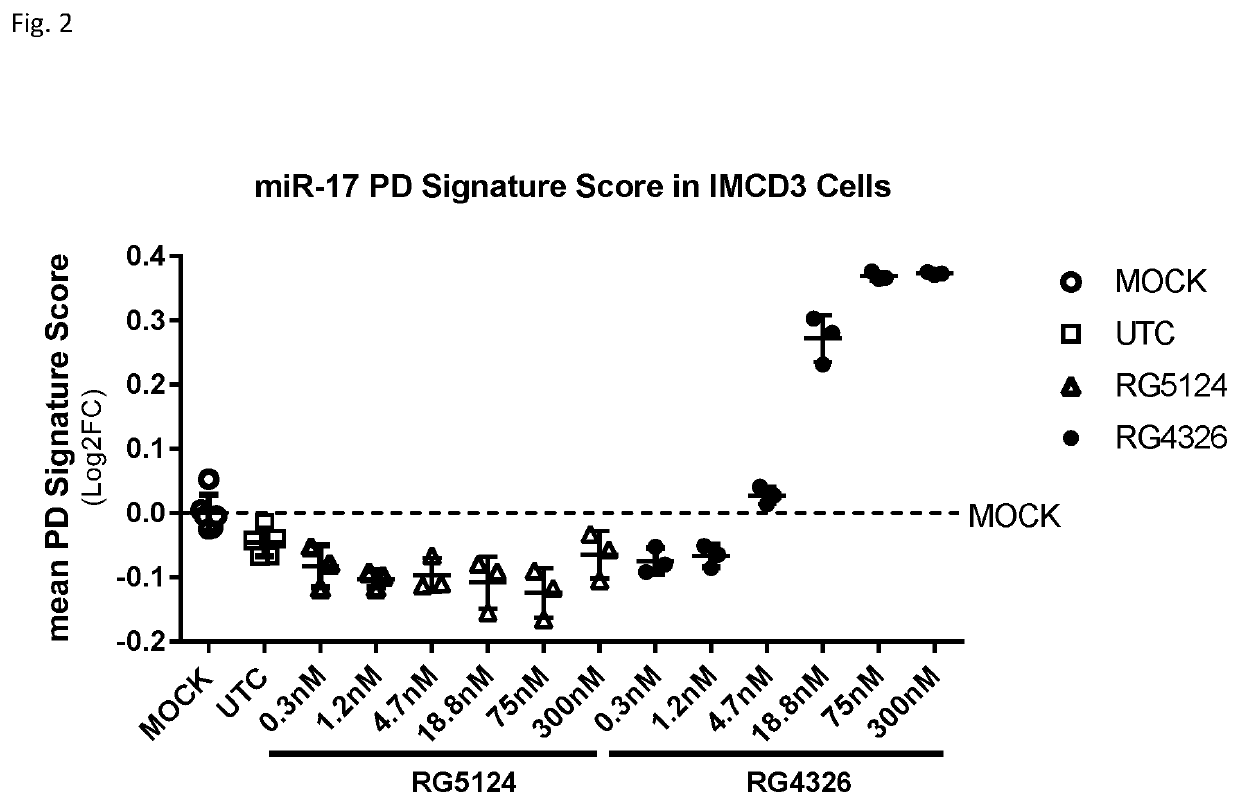
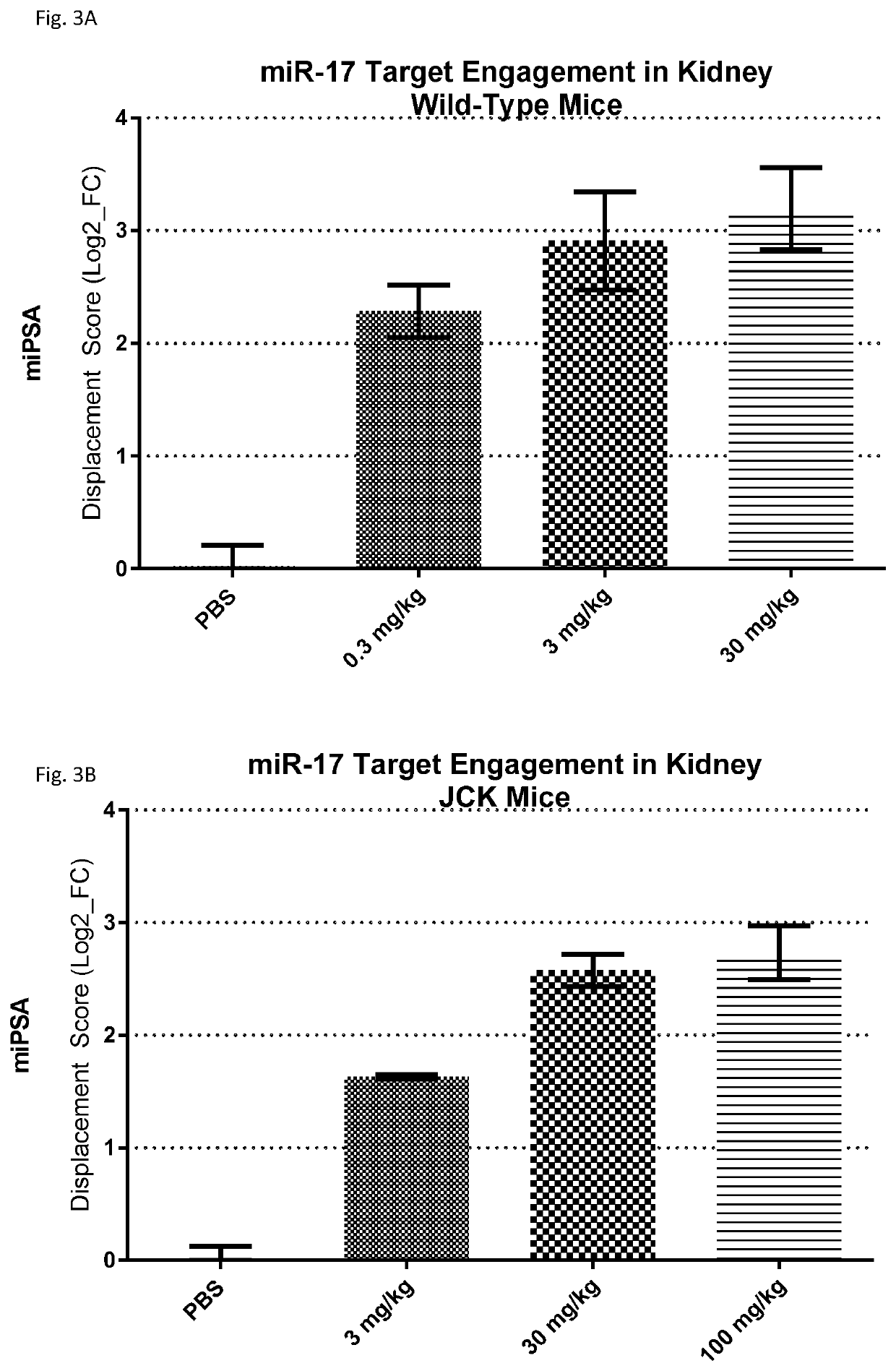
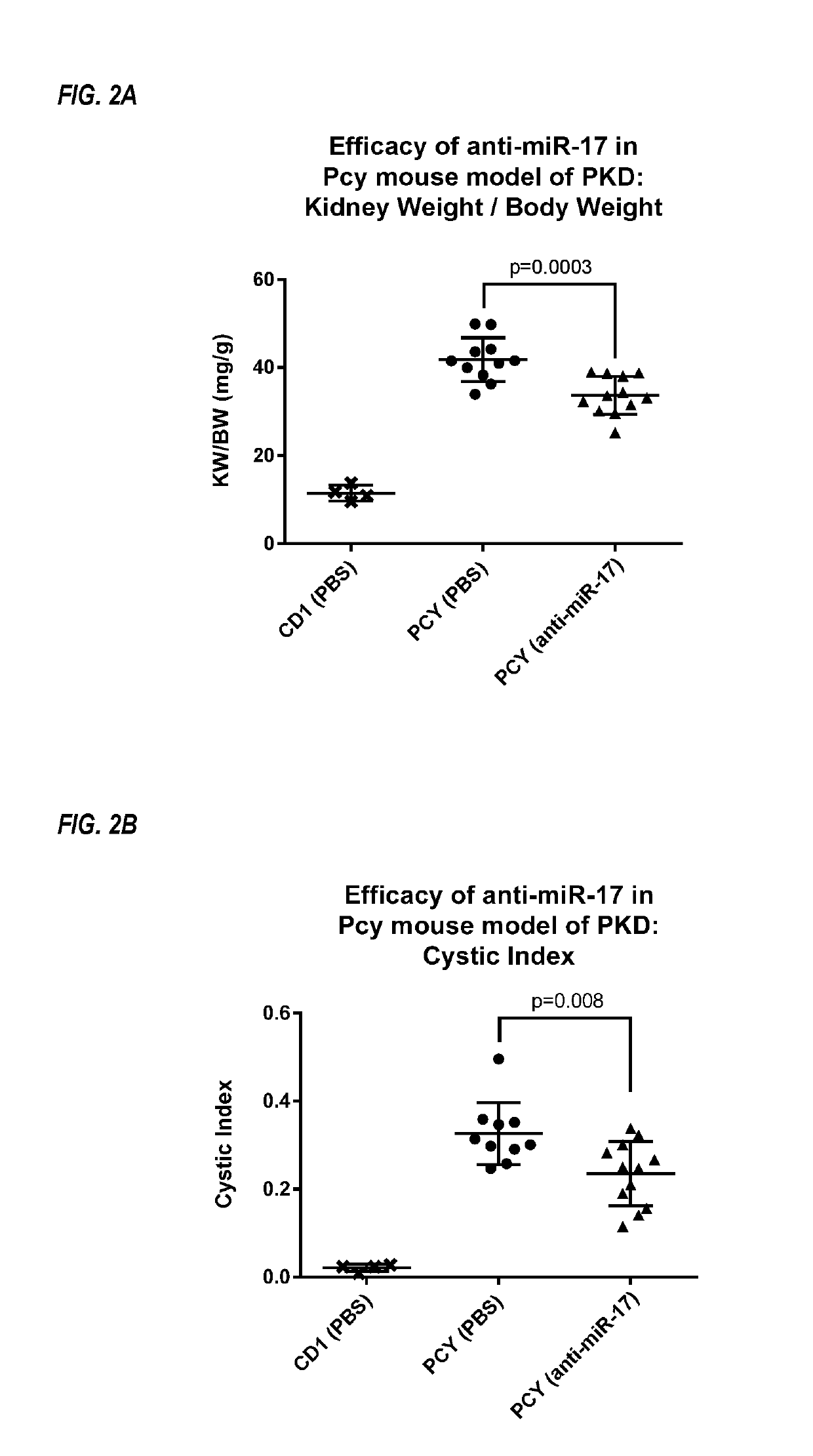
Provided herein are methods for the treatment of polycystic kidney disease, including autosomal dominant polycystic kidney disease, using modified oligonucleotides targeted to miR-17.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Methods for treating polycystic kidney disease and polycystic liver disease
ActiveUS9982009B2Reduces and avoids symptom and causeGood curative effectOrganic active ingredientsDigestive systemPhysiologyCyst
Owner:YALE UNIV +1
Method of preventing polycystic kidney disease and PKD animal model with exogenous neutrophil gelatinase-associated lipocalin
ActiveUS10307461B2Strong specificityReduce degradationPeptide/protein ingredientsUrinary disorderPhysiologyGelatinase
Disclosed is a method of treating or preventing polycystic kidney disease (PKD) by administration of neutrophil geleatinase-associated lipocalin (Ngal) protein. Also, a transgenic non-human animal model is established to investigate the effect of overexpression of exogenous Ngal on PKD progression.
Owner:NATIONAL TAIWAN NORMAL UNIVERSITY
Methods for treatment of polycystic kidney disease
ActiveUS20190153442A1Increased total kidney volumeImprove kidney functionOrganic active ingredientsUrinary disorderPhysiologyPolycystic liver disease
Provided herein are methods for the treatment of poly-cystic kidney disease, including autosomal dominant polycystic kidney disease, using modified oligonucleotides targeted to miR-17.
Owner:REGULUS THERAPEUTICS INC +1
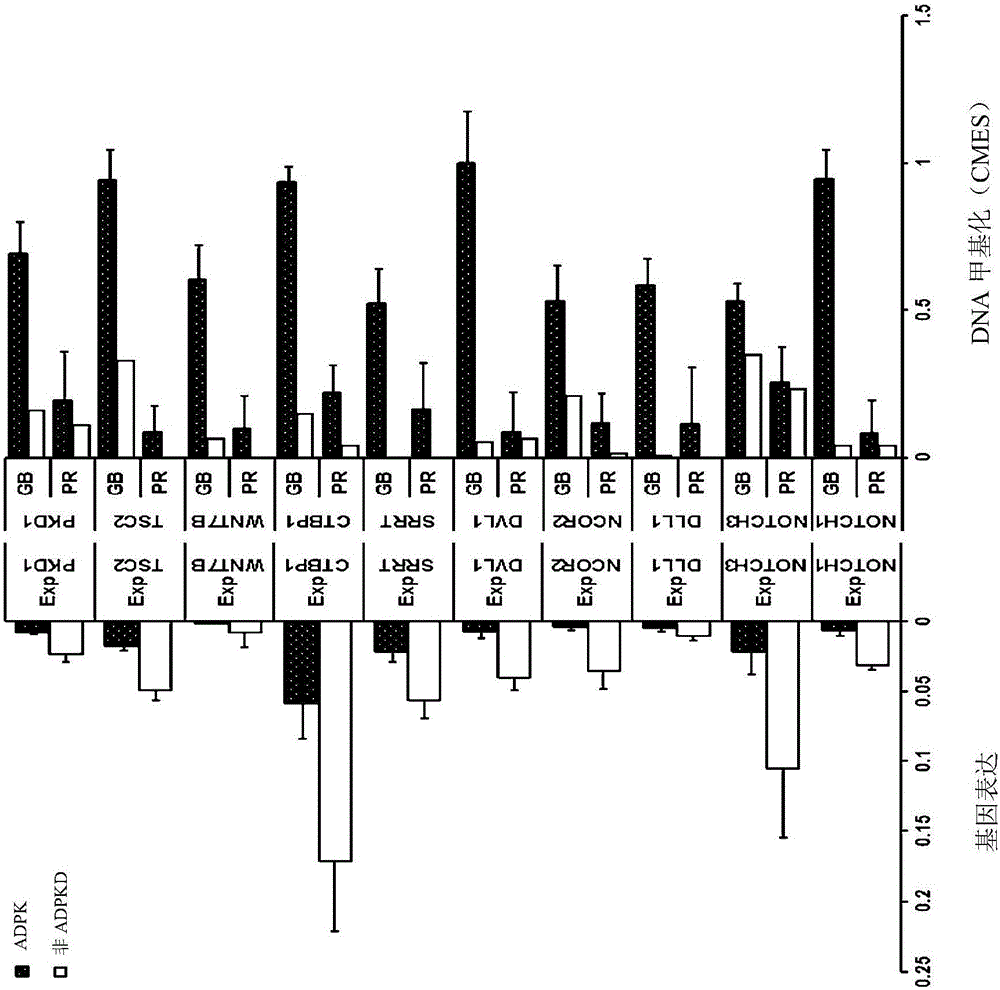
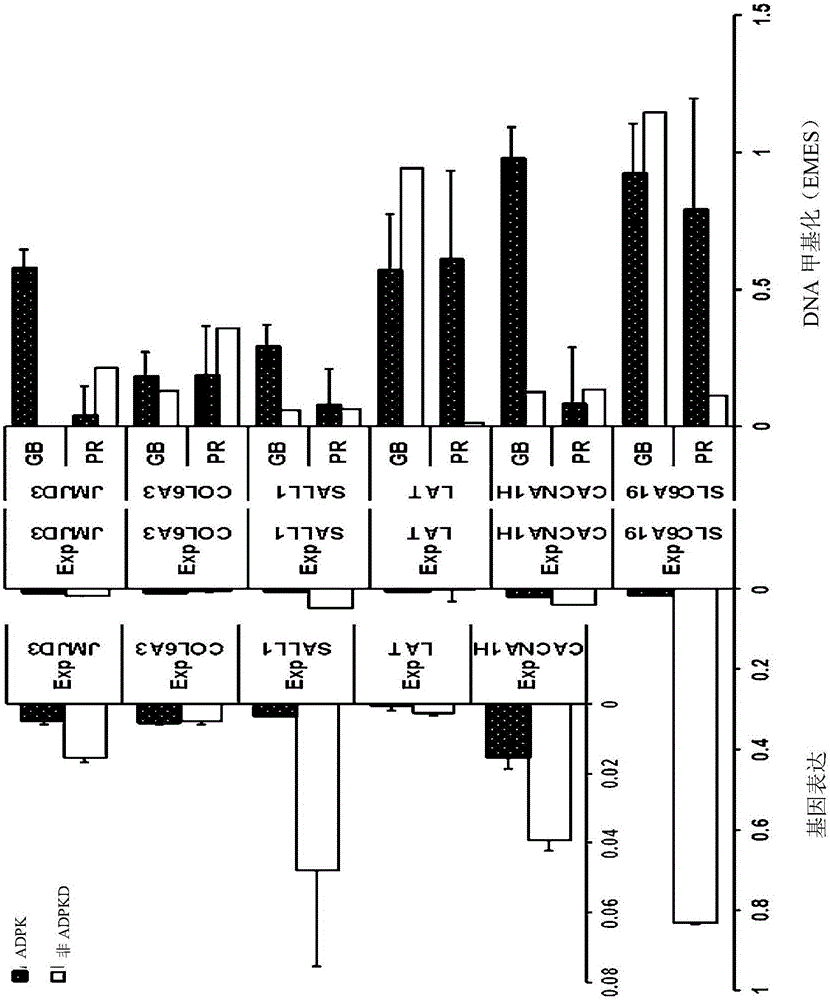
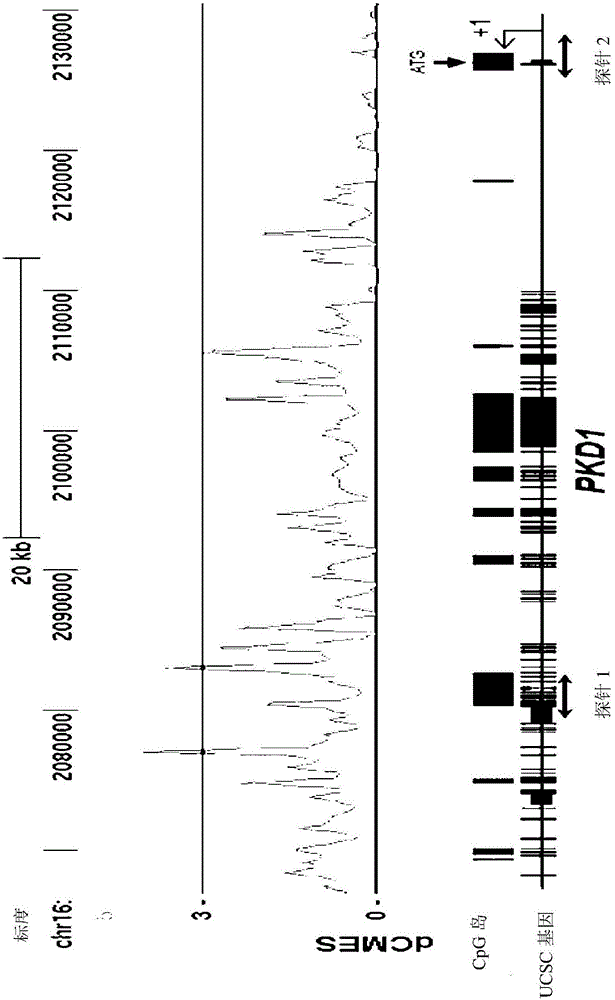
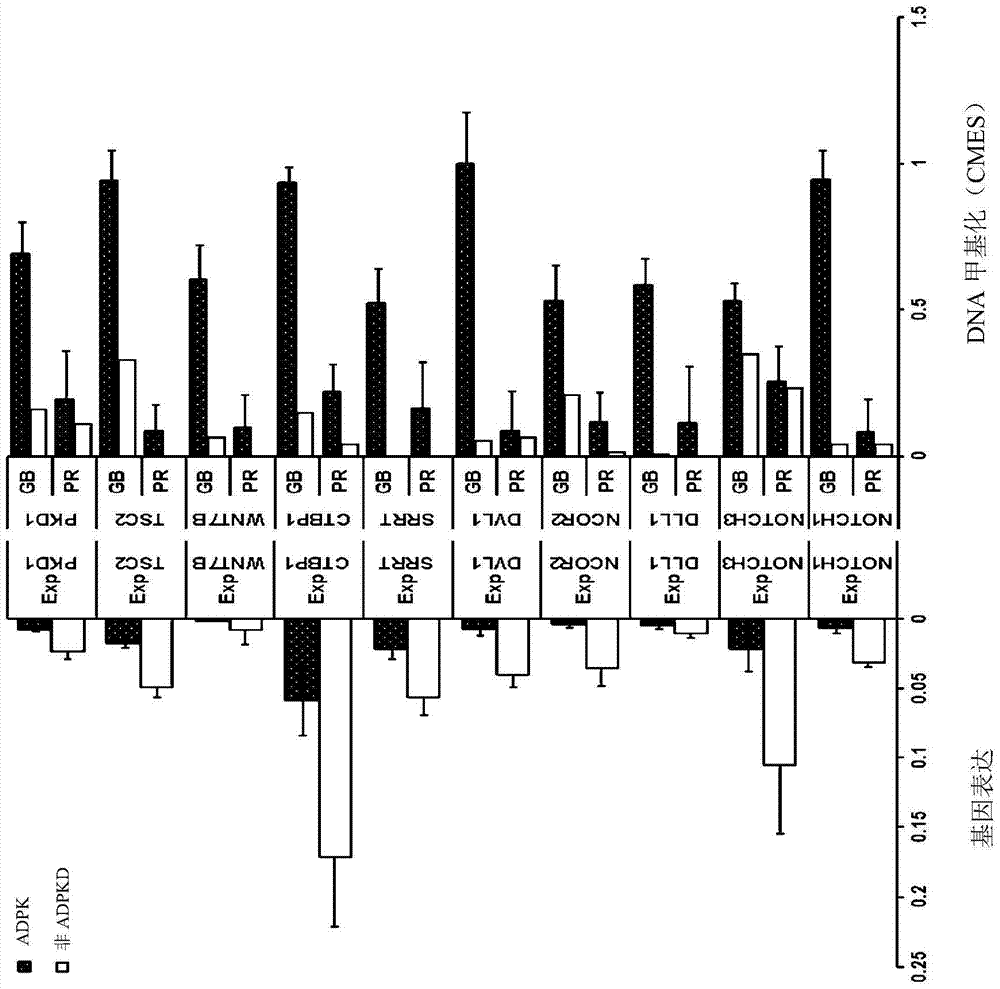
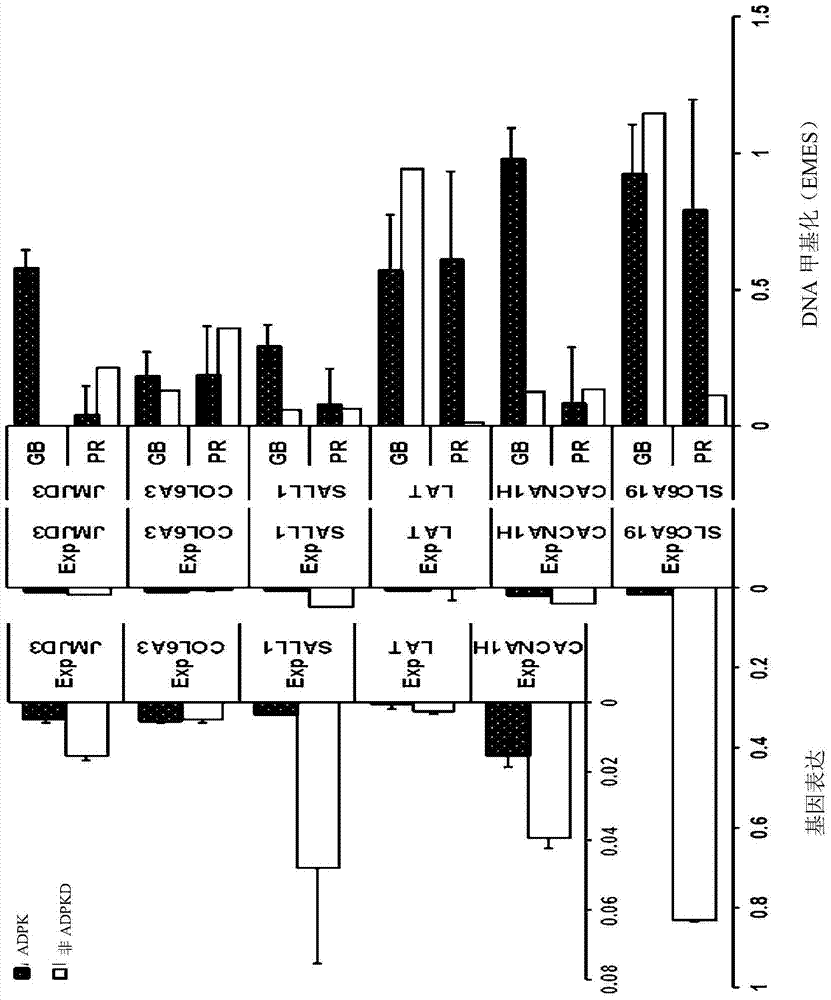
Pharmaceutical composition for alleviating or treating autosomal dominant polycystic kidney disease comprising dna methylation inhibitor
ActiveCN105163741ADelayed cyst formationPeptide/protein ingredientsGenetic material ingredientsCanine kidneyHereditary Mutation
In order to determine epigenetic variations of autosomal dominant polycystic kidney disease and functional association therebetween, the present inventors have subjected individuals with polycystic kidney disease and without polycystic kidney disease to analysis through methylation profiling in random fashion of the genome as a whole. Interestingly, in PKD1 and other genes associated with ion transport and cell adhesion, there was hypermethylation in the gene-body region, and the expression of these genes was down-regulated in polycystic kidney disease. In particular, in PKD1, there was hypermethylation in the polycystic kidney disease gene-body region, and this was associated with MBD2 (methyl-CpG-binding domain 2) protein binding. In addition, DNA methylation inhibitor treatment was accompanied by up-regulation of PKD1 expression and caused a delay in cyst formation in MDCK (Madin-Darby Canine Kidney) cells. This therefore demonstrates that, in the present invention, hypermethylation of PKD1 and regulator genes associated with cyst formation plays a decisive role in cyst formation and shows that the present invention can be used in therapeutic applications for autosomal dominant polycystic kidney disease.
Owner:SOOKMYUNG WOMENS UNIV IND ACADEMIC COOPERATION FOUND
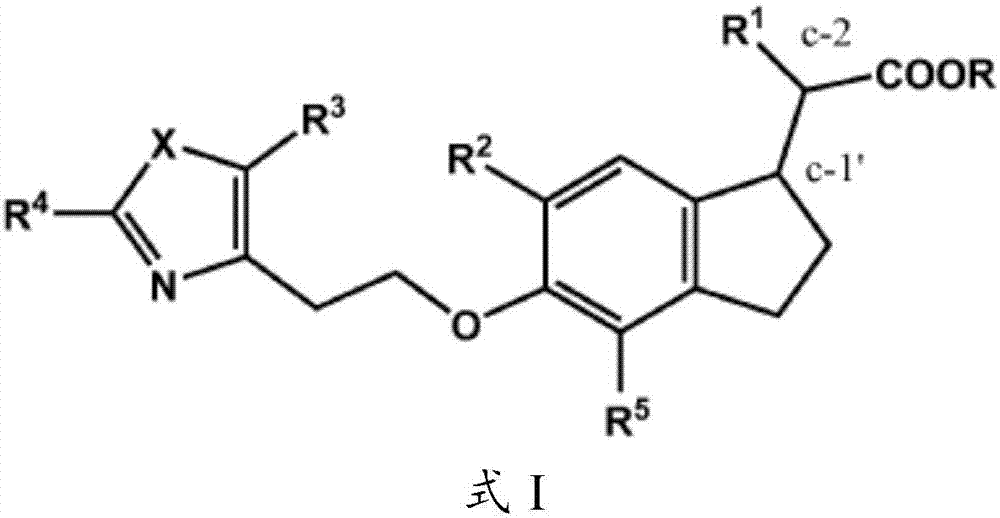
Methods of treating liver disease using indane acetic acid derivatives
InactiveCN107530352AOrganic active ingredientsDigestive systemSevere hypertriglyceridemiaSustained viral response
This invention describes the use of indane acetic acid derivatives which are dual PPAR delta / gamma agonists for the treatment of liver diseases including one or more of the following: NAFLD (Non Alcoholic Fatty Liver Disease), NASH (Non Alcoholic Steatohepatitis), Farber's Disease, ACLF (Acute-on-Chronic Liver Failure), CLF (Chronic Liver Failure), POLT-HCV-SVR (Post-Orthotopic Liver Transplantdue to Hepatitis C Virus infection after Sustained Viral Response following anti-HCV therapy), Alagille syndrome, PFIC (Progressive Familial Intrahepatic Cholestasis), PBC (Primary Biliary Cirrhosis),Primary Sclerosing Cholangitis, ADPCLD (Autosomal Dominant Polycystic Liver Disease), Treatment of liver transplant patients with reestablished fibrosis, CESD (Cholesteryl Ester Storage Disease), SHTG (Severe Hypertriglyceridemia), HoFH (Homozygous Familial Hypercholesterolemia), HE (Hepatic Encephalopathy), or Alcoholic Liver Disease.
Owner:T3D THERAPEUTICS INC
Medicine composition for treating polycystic kidney disease and use thereof
The invention relates to a pharmaceutical composition for treating polycystic kidney disease. The active ingredients of the pharmaceutical composition comprise effective doses of thiazolidinediones and diuretics. The preferential combination is as follows: the thiazolidinediones are selected from rosiglitazone and the diuretics are selected from amiloride hydrochloride. The animal experiments prove that the efficacy of the composition of the rosiglitazone and the diuretics on the treatment of the polycystic kidney disease is better than the single use of one drug. The effect of the pharmaceutical composition for treating the polycystic kidney disease is better than the single use of one drug, thereby being a drug for treating the polycystic kidney disease and being worth expecting.
Owner:CHANGZHOU HI TECH DISTRICT MULTIPLE DIMENSION IND TECH INST
Kit for use in treatment of polycystic ovarian disease
Agents which increase the levels of human insulin-like growth factor-1 binding protein (h-ICFBP-1), such as an estrogen, are used in conjunction with a gonadotropin releasing hormone (GnRH) analogue in the treatment of PCOD and associated infertility.
Owner:LAB SERONO SA
Pharmaceutical composition for improving or treating autosomal dominant polycystic kidney disease comprising dna methylation inhibitor
ActiveCN105163741BDelayed cyst formationPeptide/protein ingredientsGenetic material ingredientsCanine kidneyHereditary Mutation
In order to determine the epigenetic mutation of autosomal dominant polycystic kidney disease and its functional relevance, the present inventors randomly analyzed the whole genome of polycystic kidney disease and non-polycystic kidney disease individuals by methylation profiling method and compared with their performance data. Interestingly, PKD1 and other genes associated with ion transport and cell binding were hypermethylated at the gene body, which was downregulated in polycystic kidney disease. In particular, PKD1 is partially hypermethylated in the polycystic kidney gene body, which is associated with MBD2 (methyl‑CpG‑binding domain 2) protein binding. Furthermore, DNA methylation inhibitor treatment was accompanied by an upregulation of PKD1 expression and delayed cyst formation in MDCK (Madin‑Darby Canine Kidney) cells. Thus, hypermethylation of PKD1 and cyst formation-associated regulatory genes of the present invention is decisive for cyst formation and revealed to be useful as a treatment for autosomal dominant polycystic kidney disease.
Owner:SOOKMYUNG WOMENS UNIV IND ACADEMIC COOPERATION FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Thieno[3,2-b]pyridine-6-carbonitriles and thieno[2,3-b]pyridine-5-carbonitriles as protein kinase inhibitors Thieno[3,2-b]pyridine-6-carbonitriles and thieno[2,3-b]pyridine-5-carbonitriles as protein kinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/3e7f9e76-68bb-4d09-9032-296c9f6ec5ae/US06987116-20060117-C00001.png)
![Thieno[3,2-b]pyridine-6-carbonitriles and thieno[2,3-b]pyridine-5-carbonitriles as protein kinase inhibitors Thieno[3,2-b]pyridine-6-carbonitriles and thieno[2,3-b]pyridine-5-carbonitriles as protein kinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/3e7f9e76-68bb-4d09-9032-296c9f6ec5ae/US06987116-20060117-C00002.png)
![Thieno[3,2-b]pyridine-6-carbonitriles and thieno[2,3-b]pyridine-5-carbonitriles as protein kinase inhibitors Thieno[3,2-b]pyridine-6-carbonitriles and thieno[2,3-b]pyridine-5-carbonitriles as protein kinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/3e7f9e76-68bb-4d09-9032-296c9f6ec5ae/US06987116-20060117-C00003.png)