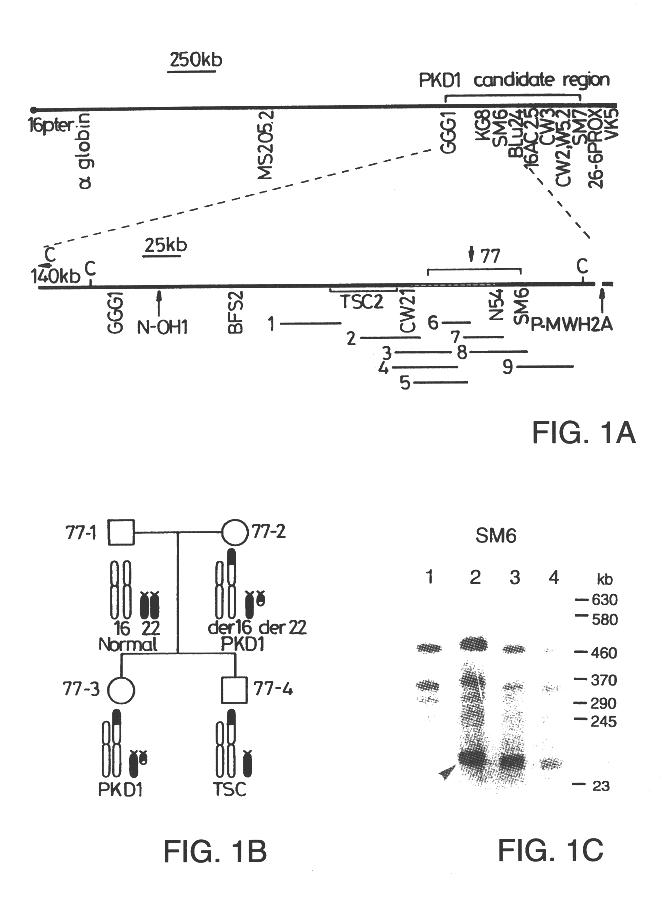
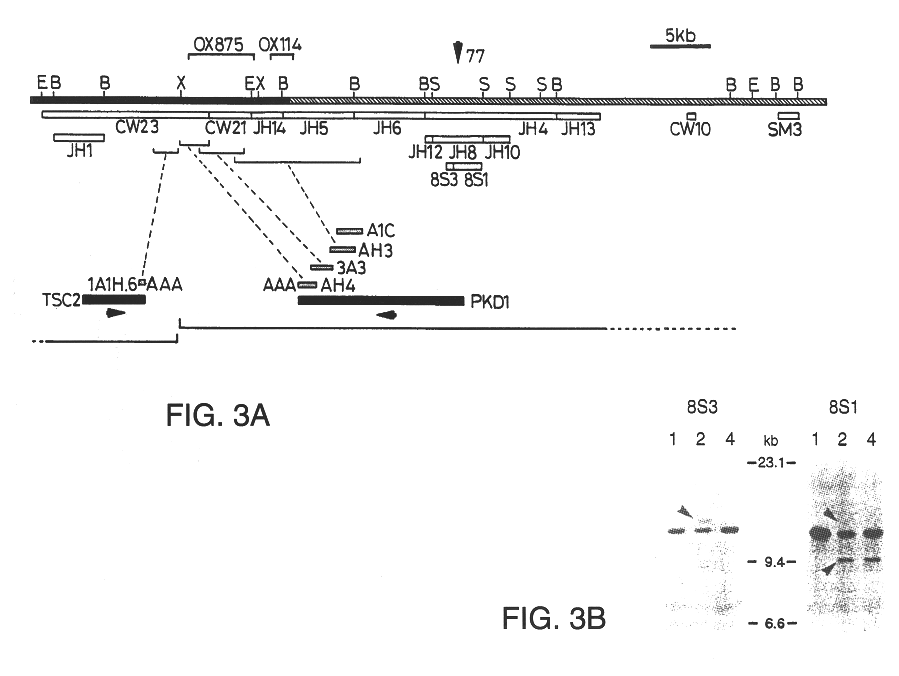
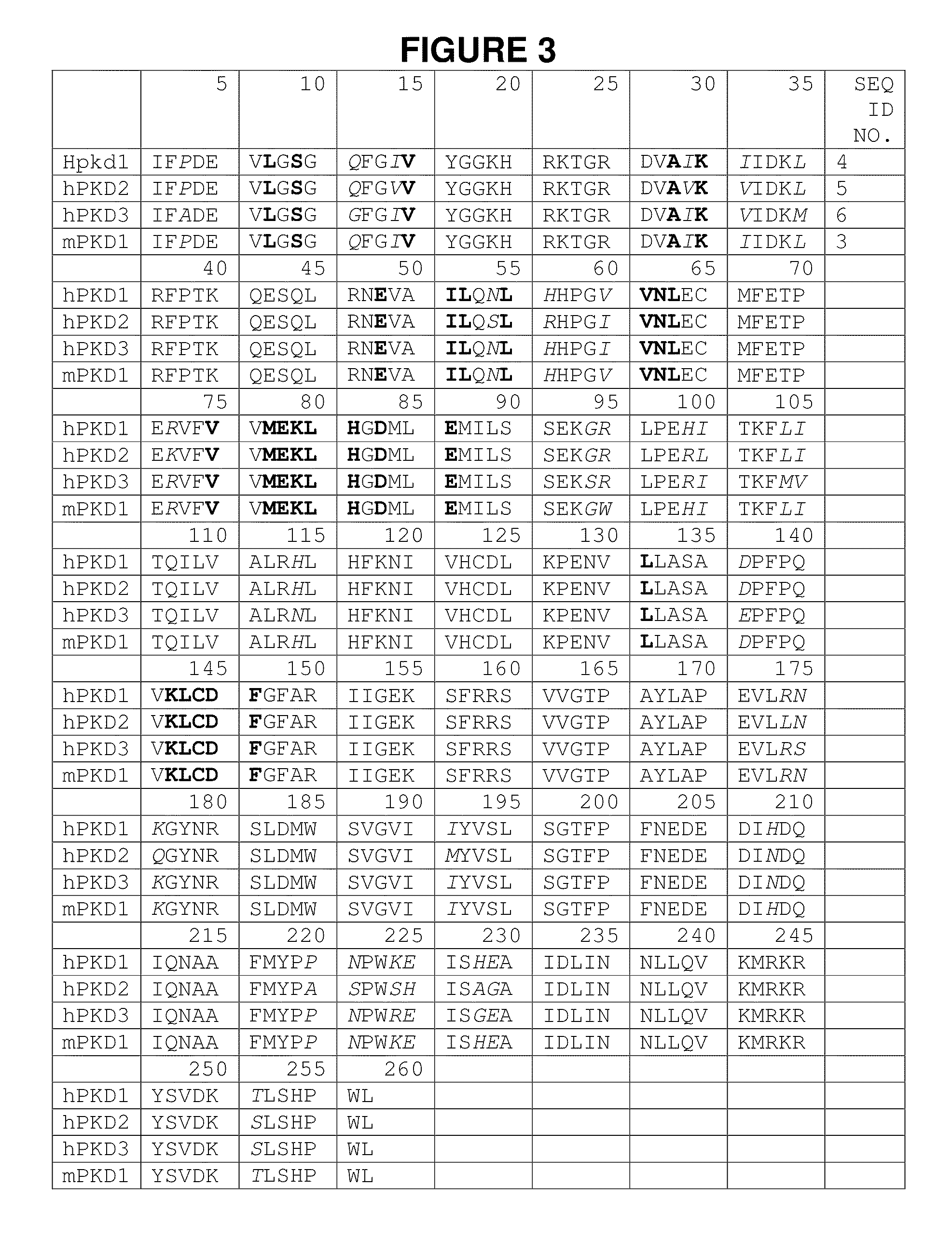
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
39 results about "PKD1" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
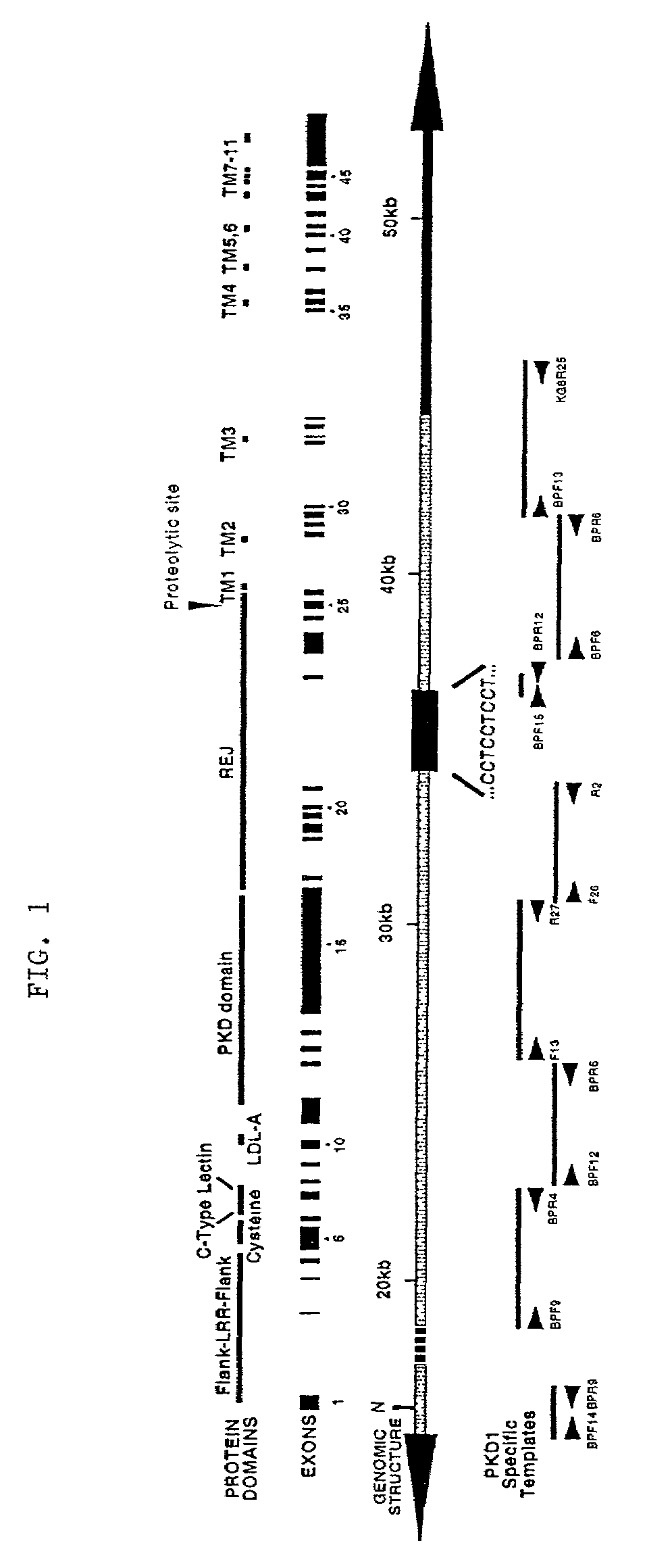
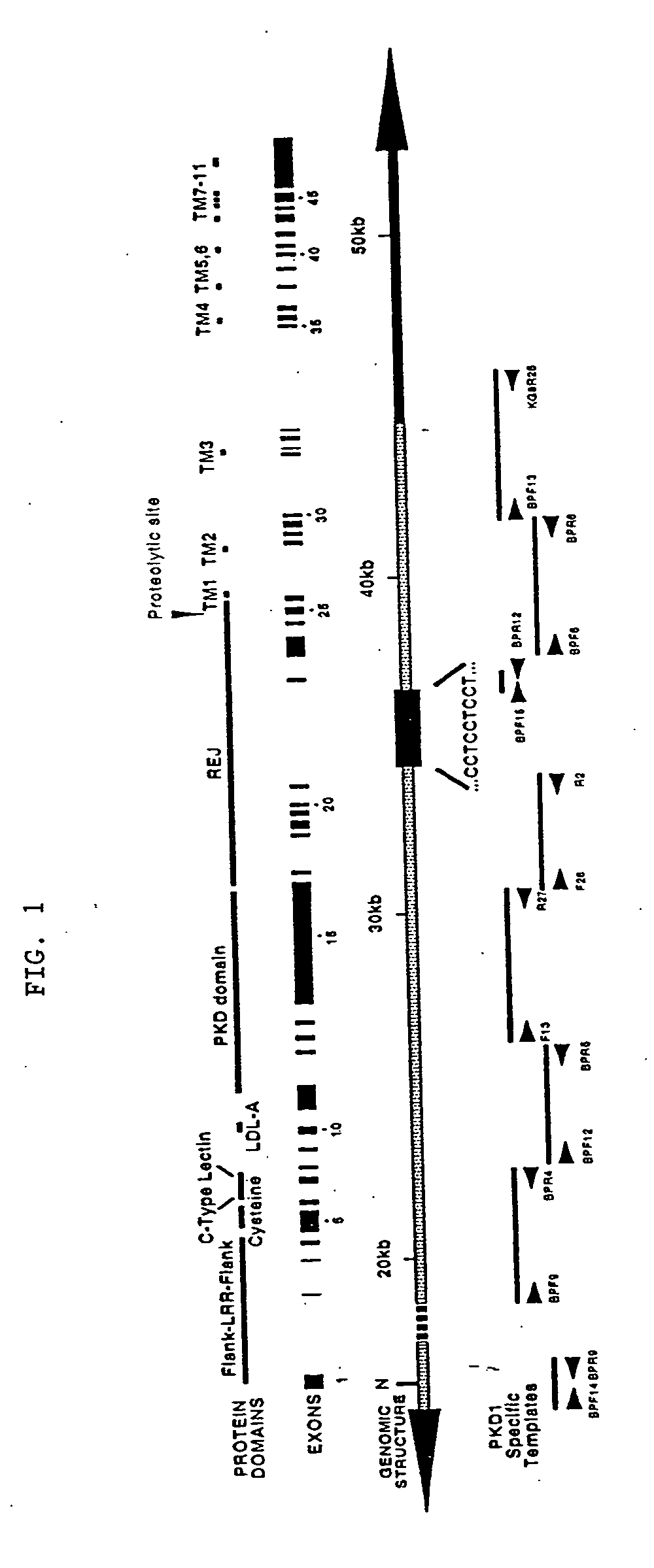
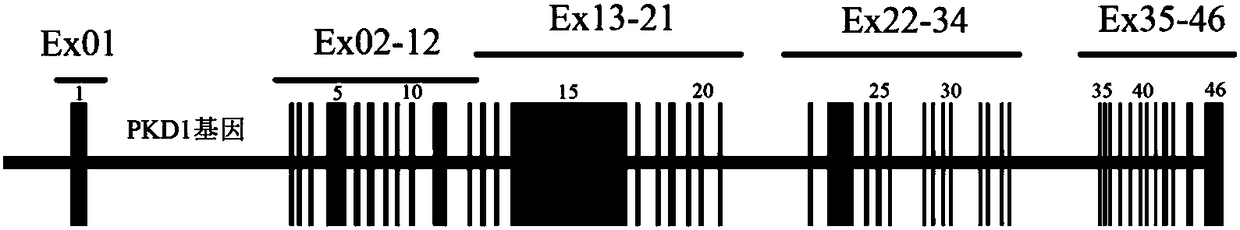
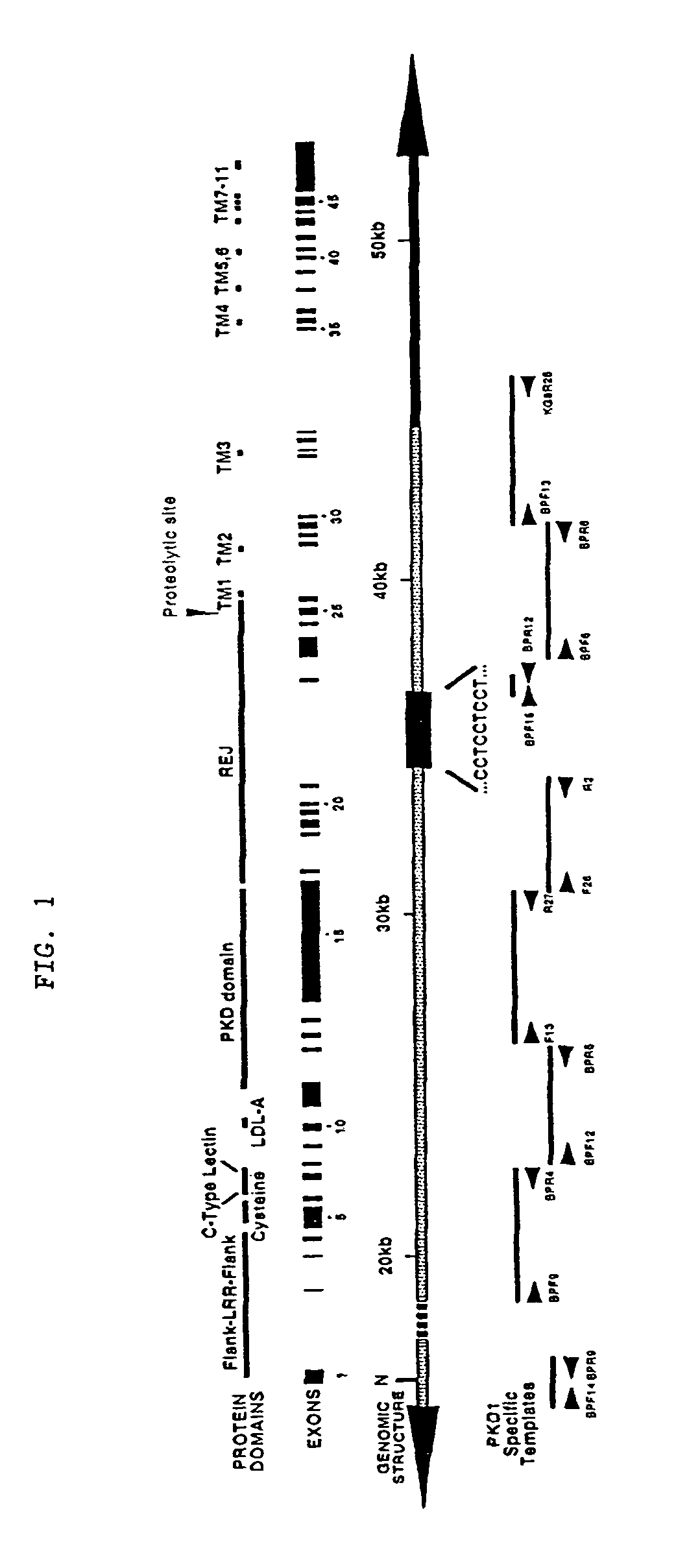
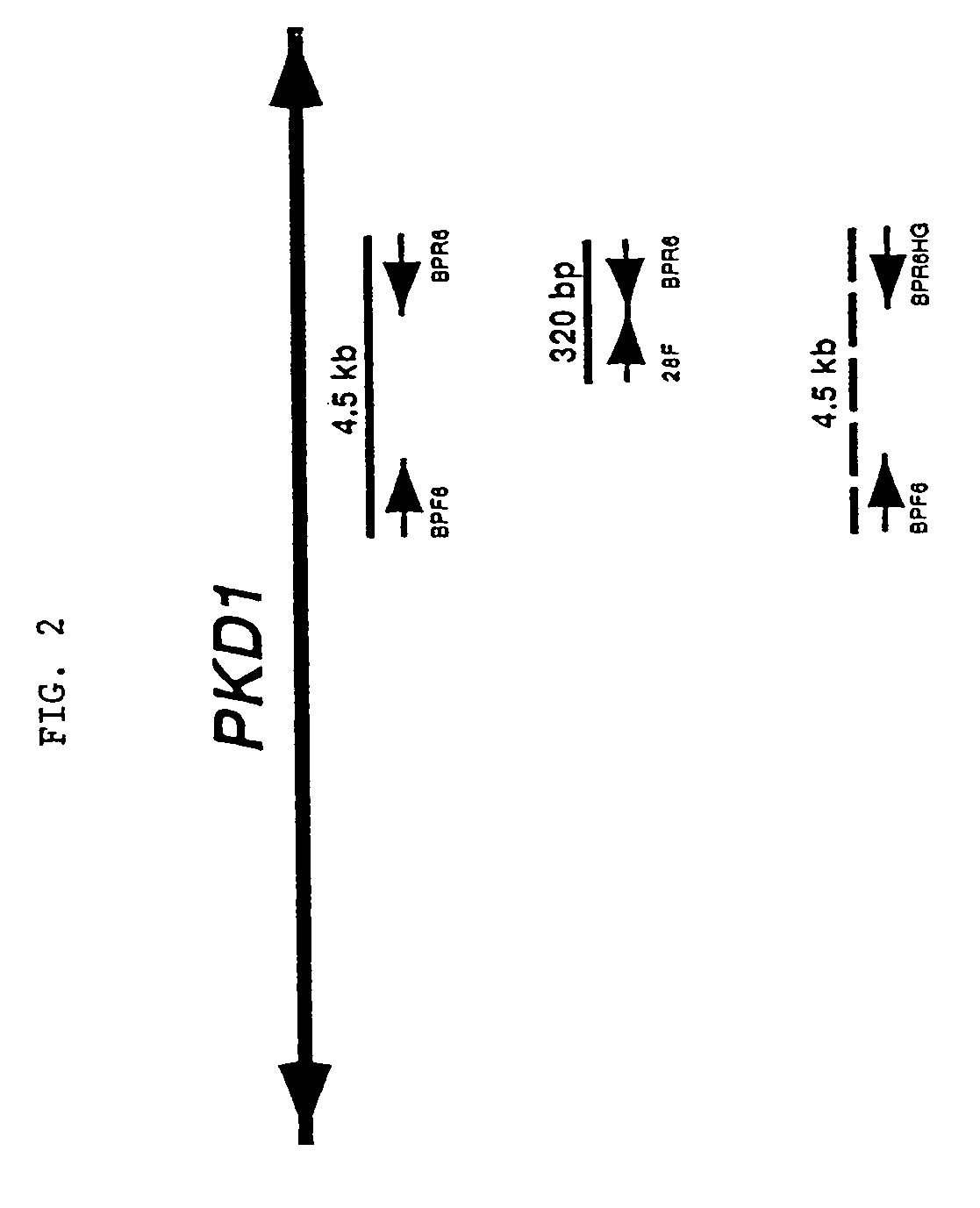
Polycystin 1 (often abbreviated to PC1) is a protein that in humans is encoded by the PKD1 gene . Mutations of PKD1 are associated with most cases of autosomal dominant polycystic kidney disease, a severe hereditary disorder of the kidneys characterised by the development of renal cysts and severe kidney dysfunction .
Polycystic kidney disease 1 gene and uses thereof
The present invention relates to the polycystic kidney disease 1 (PKD1) gene and its nucleic acid sequence, mutations thereof in patients having PKD1-associated disorders, the protein encoded by the PKD1 gene or its mutants, and their uses in disease diagnosis and therapy.
Owner:ERASMUS UNIVERSITY ROTTERDAM +3
Pyridine benzamides and pyrazine benzamides used as pkd inhibitors
The present invention pertains generally to the field of therapeutic compounds, and more specifically to certain pyridine benzamide and pyrazine benzamide compounds (referred to herein as PDBA and PZBA compounds) which, inter alia, inhibit protein kinase D (PKD) (e.g., PKD1, PKD2, PKD3). The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit PKD, and in the treatment of diseases and conditions that are mediated by PKD, that are ameliorated by the inhibition of PKD, etc., including proliferative conditions such as cancer, etc.
Owner:CANCER RES TECH LTD
Detection and treatment of polycystic kidney disease
InactiveUS7553644B2Improve throughputBioreactor/fermenter combinationsFungiPKD1Polycystic liver disease
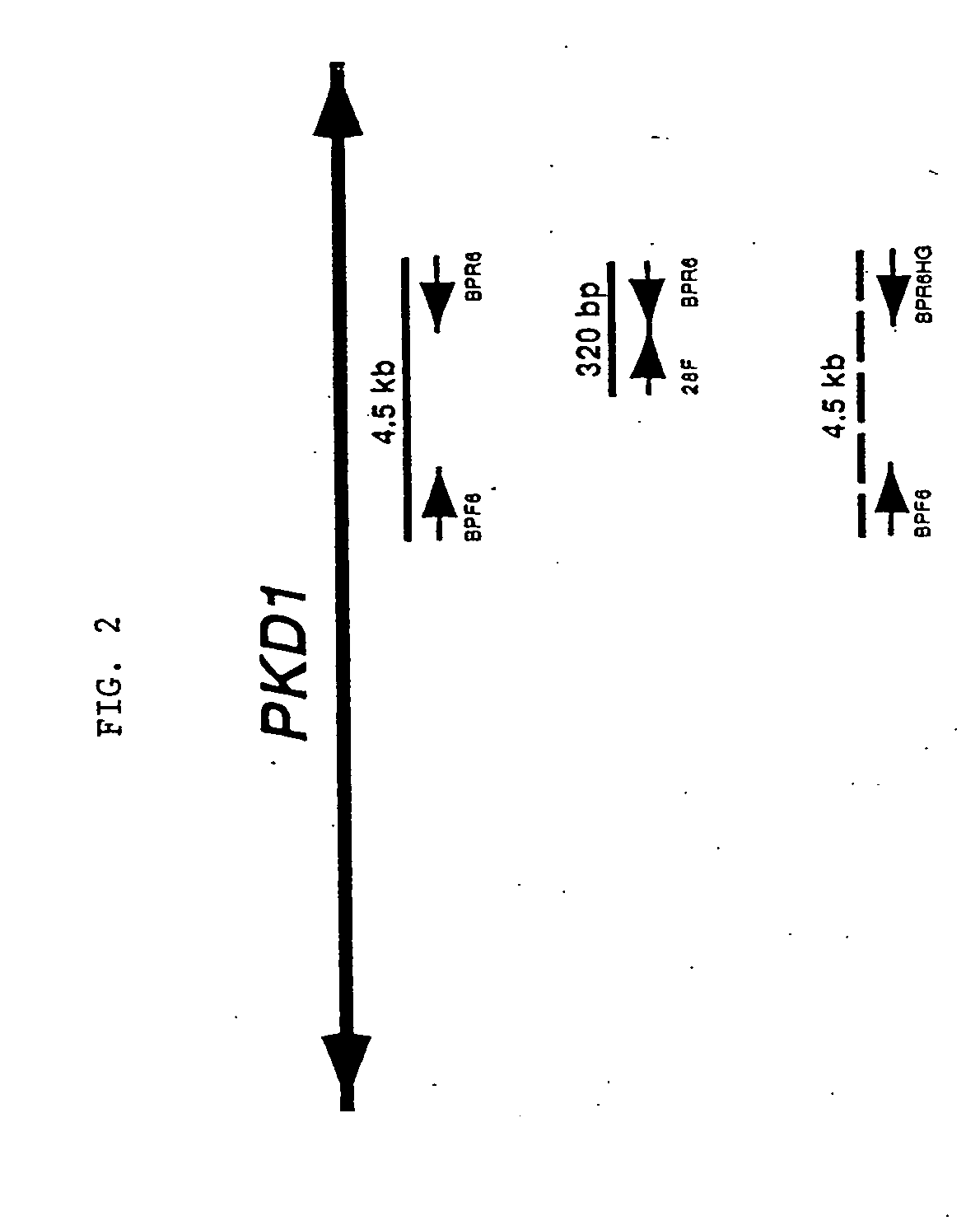
Compositions useful for examining the PKD1 gene are provided. In addition, methods for detecting mutations of the PKD1 gene, which can be associated with autosomal dominant polycystic kidney disease in humans, are provided. Methods for diagnosing a mutant PKD1 gene sequence in a subject also are provided, as are methods of treating a subject having a PKD1-associated disorder.
Owner:THE JOHNS HOPKINS UNIVERSITY SCHOOL OF MEDICINE
Amplimers, kit and method for detecting PKD1 gene mutation
ActiveCN104975081AAchieve separate markingAchieving Parallel SequencingMicrobiological testing/measurementDNA/RNA fragmentationPKD1Pcr ctpp
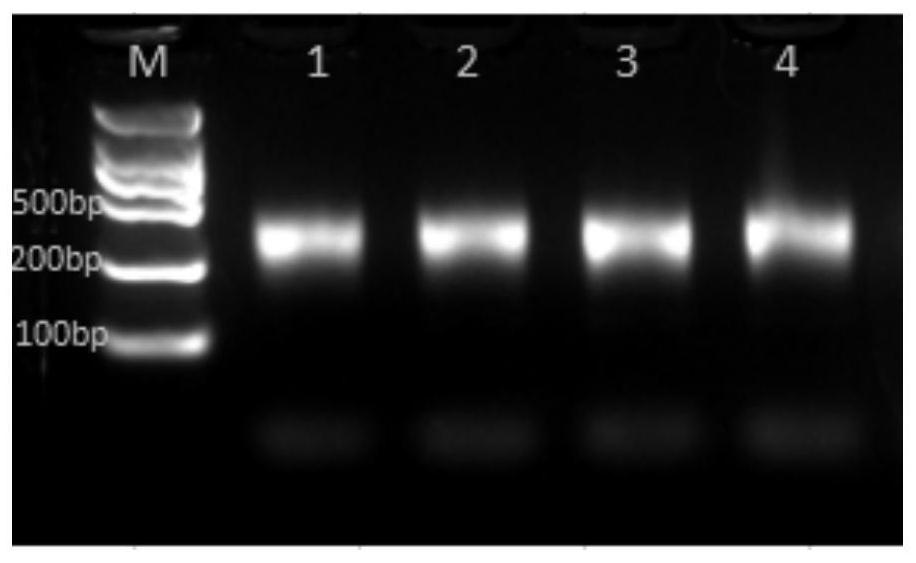
The present invention relates to a method for detecting PKD1 gene mutation. The method utilizes sequence information of the PKD1 gene, designs nine pairs of PCR tag primers, and uses a long-chain PCR technology for targeting amplification of PKD1 gene; while amplification products are labeled, PCR products are purified and mixed for direct construction of a single SMRTBell library; a PacBio RSII sequencer is employed for sequencing; and bioinformatic analysis is carried out to obtain PKD1 gene mutation information. The invention has the beneficial effects that a simplified pair of primers is designed and combined with tag primer technology to realize respective marking of PCR products of multiple DNA samples; the amplified PCR products do not need to perform knockout and can be directly used for the library construction, and the library construction does not need extra PCR, so that a plurality of samples are mixed into one library and processed simultaneously by a PacBio RSII sequencing library construction link, and the experimental operation is greatly simplified.
Owner:NANJING MATERNITY & CHILD HEALTH CARE HOSPITAL
PKD1 gene mutation detection kit and detection method
ActiveCN104531883AComprehensive detection rangeLow mismatch rateMicrobiological testing/measurementSequence analysisNephrosisPKD1
The invention provides a primer set, a kit and a detection reaction system for detecting PKD1 gene mutation through the long fragment PCR and high-throughput sequencing technology, a non-diagnosis-purpose method for external PKD1 gene mutation detection, a non-diagnosis-purpose method for external PKD1 gene analysis and a method for detecting new mutation sites on the PKD1 gene. According to the method, the primer set is used for carrying out long fragment PCR amplification on the PKD1 gene of a sample, and detecting or analyzing is carried out through high-throughput sequencing. The autosomal dominant genetic polycystic nephrosis (adult polycystic nephrosis) can be diagnosed through the assistance of a detection result, previous unknown new mutation on a plurality of PKD1 real genes can be obtained and supplied to doctors or researchers so that the relevance between the mutation and the adult polycystic nephrosis can be studied.
Owner:北京圣谷智汇医学检验所有限公司
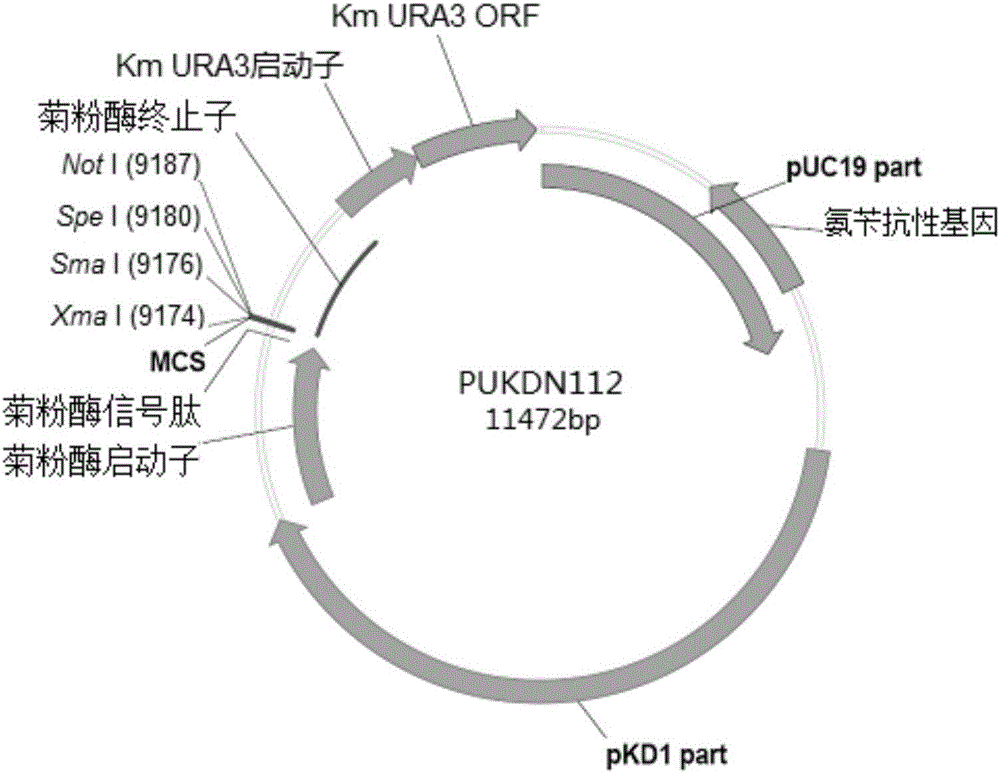
Recombinant vector used for carrying out foreign gene secretory expression in auxotrophic kluyveromyces marxianus strain
The invention provides a recombinant vector used for carrying out foreign gene secretory expression in an auxotrophic kluyveromyces marxianus strain as well as a preparation method and application of the recombinant vector. The recombinant vector comprises ampicillin resistance genes, a PKD1 vector, inulase promoters, signal peptides, multiple cloning sites, inulase terminators, nutritional gene promoters and a nutritional gene open reading flame according to the sequence. The constructed recombinant vector used for carrying out foreign gene secretory expression and the preparation method of the recombinant vector can be used for constructing a transformant to achieve secretory expression of foreign genes.
Owner:FUDAN UNIV
Polycystic kidney disease 12 gene and uses thereof
The present invention relates to the polycystic kidney disease 1 (PKD1) gene and its nucleic acid sequence, mutations thereof in patients having PKD1-associated disorders, the protein encoded by the PKD1 gene or its mutants, and their uses in disease diagnosis and therapy.
Owner:UNIV COLLEGE CARDIFF CONSULTANTS LTD +1
Amino-ethyl-amino-aryl (AEAA) compounds and their use
The present invention pertains generally to the field of therapeutic compounds, and more specifically to certain amino-ethyl-amino-aryl (AEAA) compounds which, inter alia, inhibit protein kinase D (PKD) (e.g., PKD1, PKD2, PKD3). The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit PKD, and in the treatment of diseases and conditions that are mediated by PKD, that are ameliorated by the inhibition of PKD, etc., including proliferative conditions such as cancer, etc.
Owner:CANCER RES TECH LTD
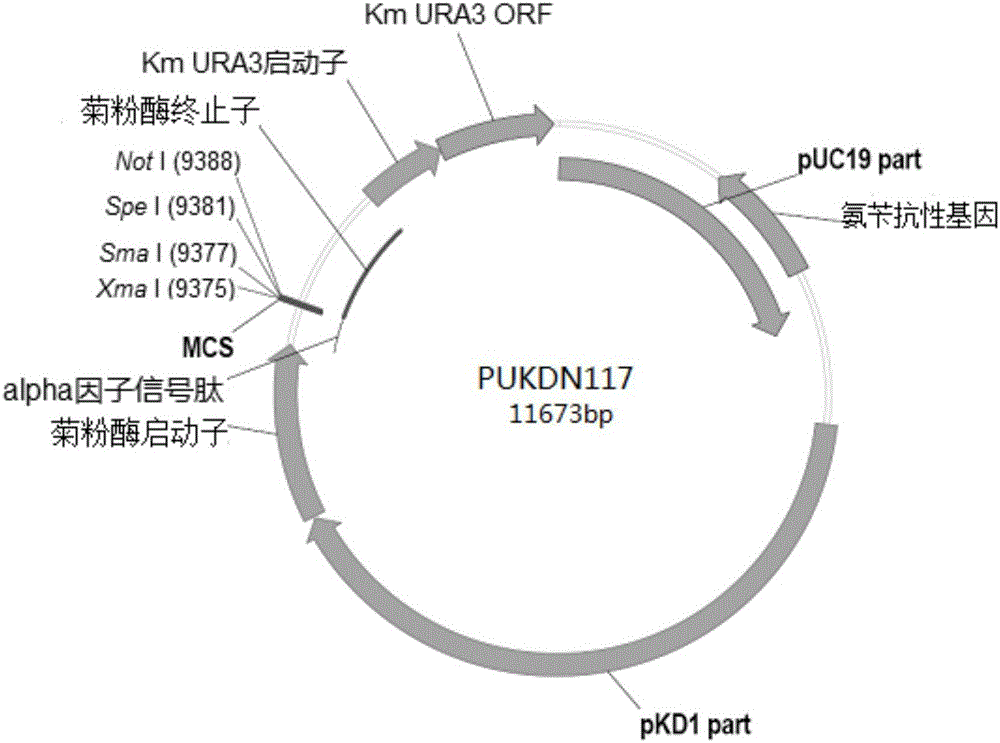
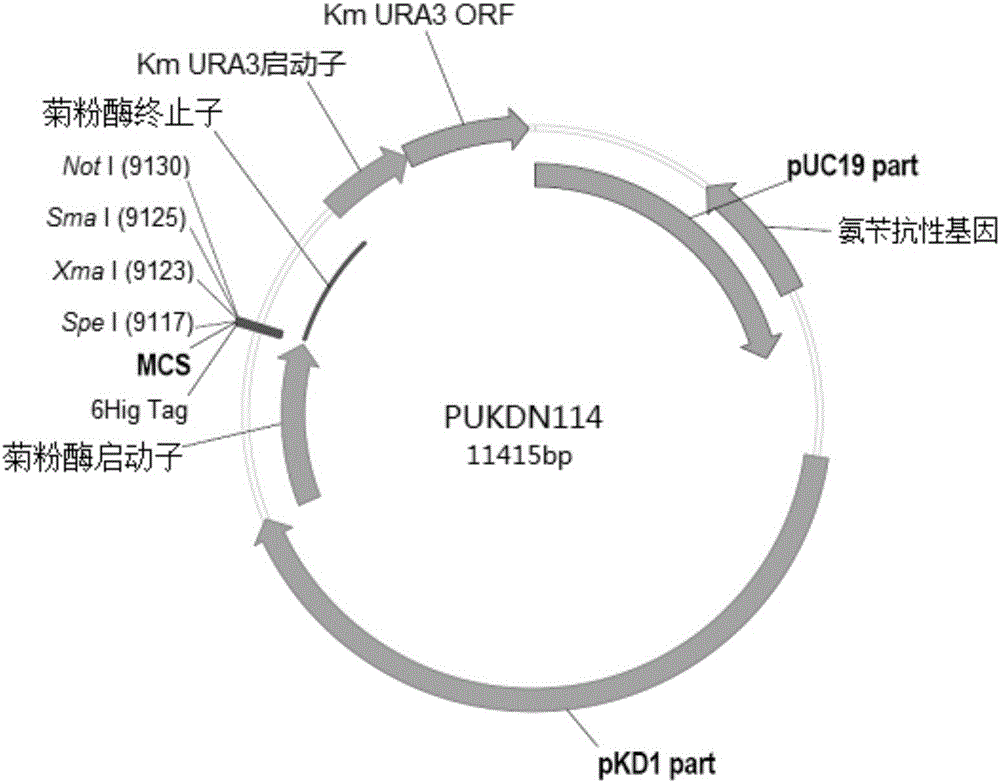
Recombinant vector for expressing histidine-tag-fused foreign gene in Kluyveromyces marxianus nutritional deficient strain
The invention provides a recombinant vector for expressing a histidine-tag-fused foreign gene in a Kluyveromyces marxianus nutritional deficient strain, and a preparation method and application of the recombinant vector. The recombinant vector sequentially comprises an ampicillin resistance gene, a PKD1 vector sequence, an inulase promotor, multiple cloning sites, an inulase terminator, a nutritional gene promotor and a nutritional gene open reading frame, and further comprises histidine tags located at C ends or N ends of the multiple cloning sites. The recombinant vector provided by the invention can be used for building a transformant to realize expression of the foreign gene.
Owner:FUDAN UNIV
Detection and treatment of polycystic kidney disease
InactiveUS20060246504A1High throughput formatImprove throughputBioreactor/fermenter combinationsFungiPKD1Gene mutation
Compositions useful for examining the PKD1 gene are provided. In addition, methods for detecting mutations of the PKD1 gene, which can be associated with autosomal dominant polycystic kidney disease in humans, are provided. Methods for diagnosing a mutant PKD1 gene sequence in a subject also are provided, as are methods of treating a subject having a PKD1-associated disorder.
Owner:THE JOHNS HOPKINS UNIVERSITY SCHOOL OF MEDICINE
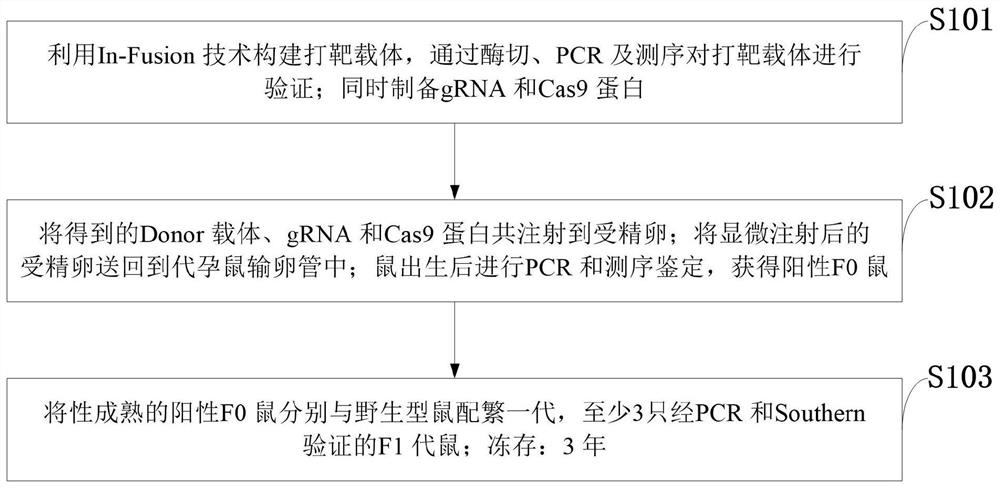
CRISPR Cas9 conditional gene knockout mouse and establishment method
PendingCN111961685AImprove knockout effectMicroinjection basedNucleic acid vectorKnockout animalSexual maturity
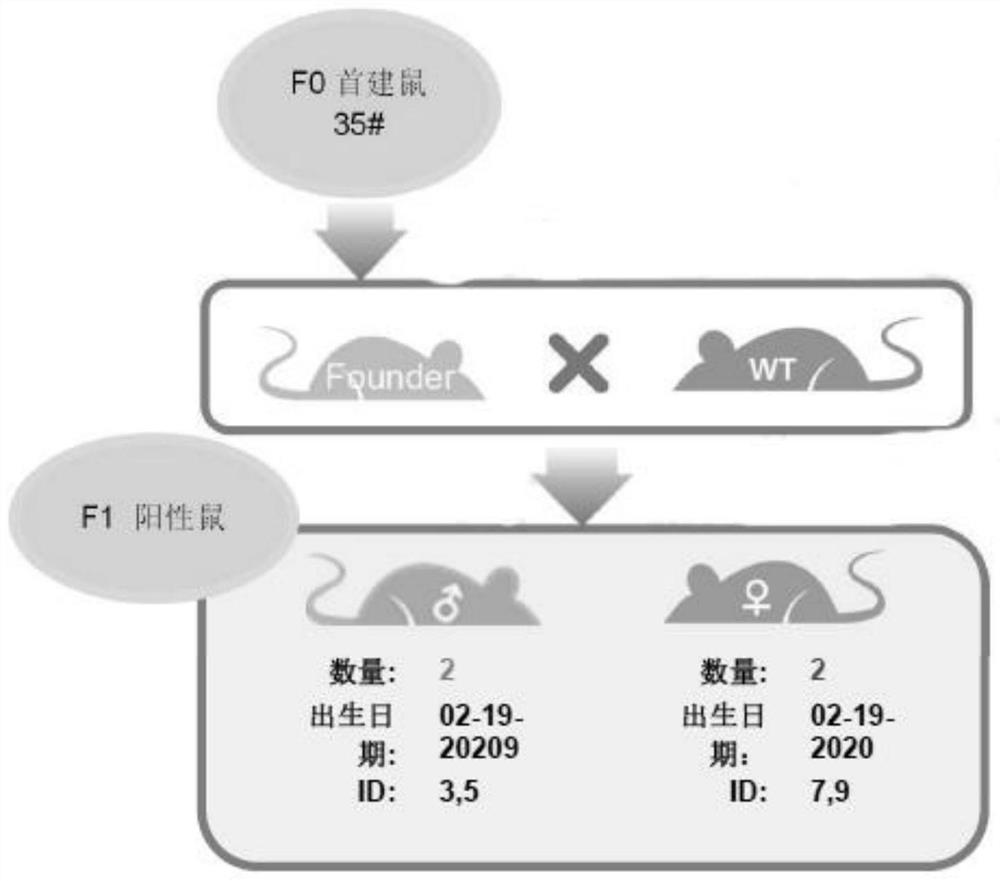
The invention belongs to the technical field of gene knockout, and discloses a CRISPR Cas9 conditional gene knockout mouse and an establishment method. According to the establishment method, a vectoris designed and constructed, and gRNA and Cas9 protein are prepared; a gRNA sequence and a targeting vector are designed; the targeting vector is constructed by the In-Fusion technology, and the targeting vector is verified by restriction digestion, PCR and sequencing; the gRNA and Cas9 protein are prepared at the same time; microinjection and F0 mouse identification are performed; the obtained Donor vector, gRNA and Cas9 protein are co-injected into a fertilized egg; the fertilized egg after microinjection is returned to the oviduct of a surrogate mouse; PCR and sequencing identification areperformed after a mouse is born, and a positive F0 mouse is obtained; the reproduction and identification of a F1 generation mouse are performed; the sexually mature positive F0 mice are matched withwild-type mice respectively for reproducing the first generation, and a polycystic kidney gene PKD1 knockout mouse is obtained successfully.
Owner:JIANGXI UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
PCR (polymerase chain reaction) primer for amplifying PKD (polystic kidney disease)1 exon ultra-long fragment, kit for detecting PKD1 gene mutation and application
ActiveCN106995851AAchieving Simultaneous DetectionImprove throughputMicrobiological testing/measurementDNA/RNA fragmentationNephropathyPKD1
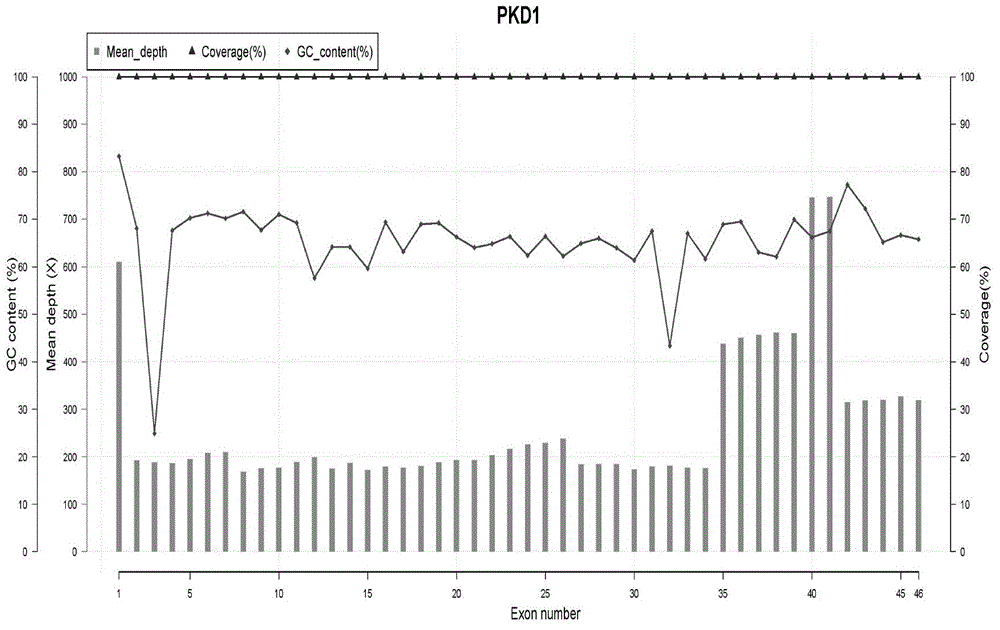
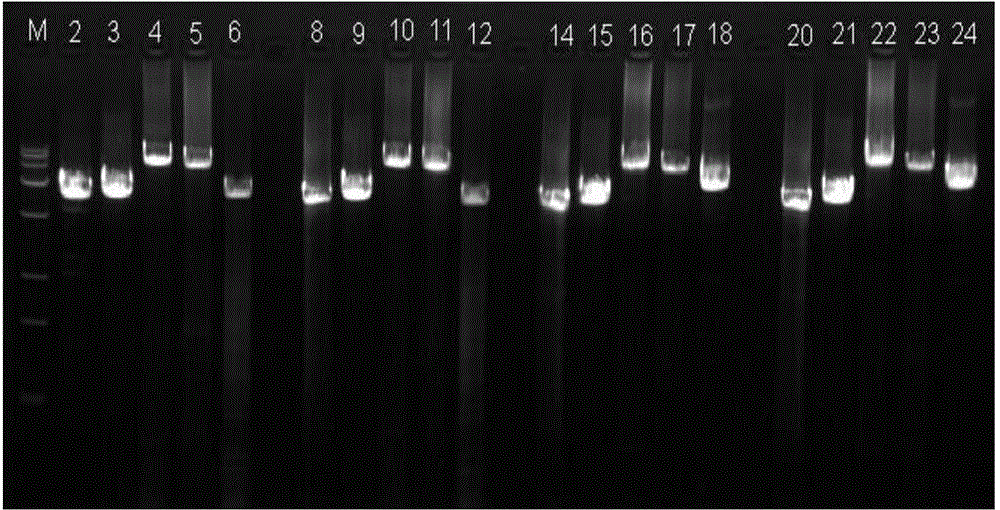
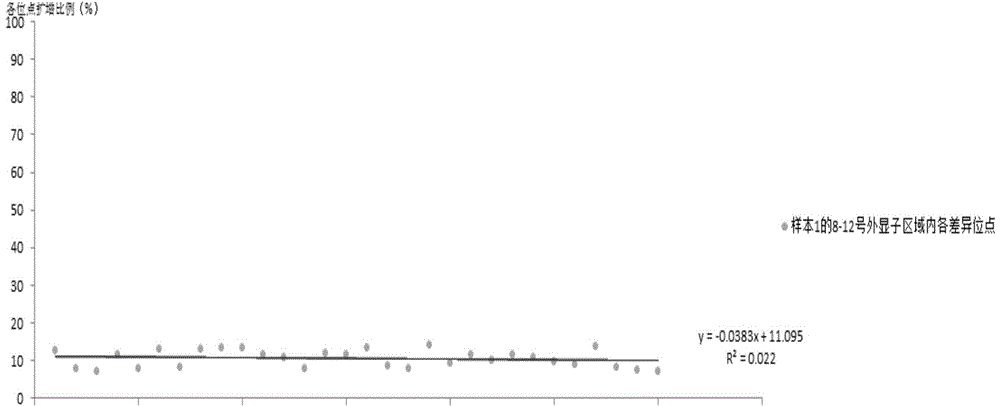
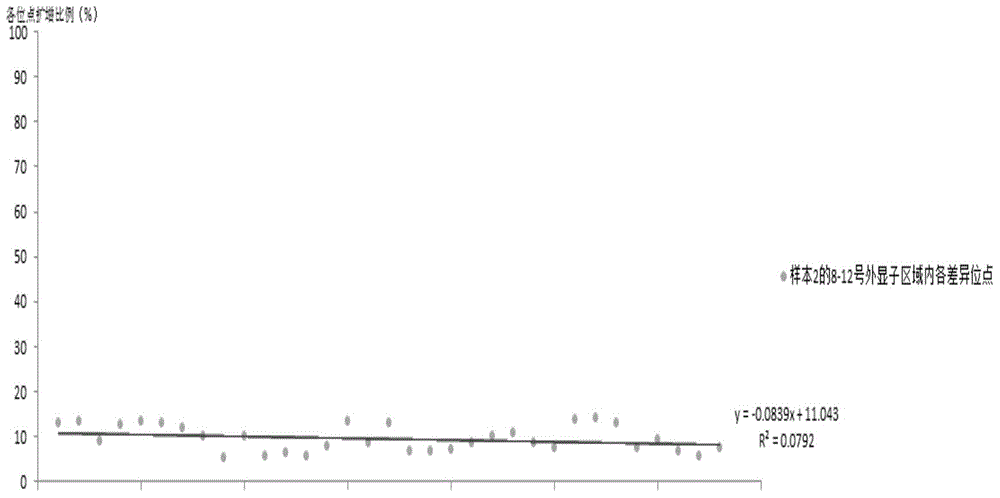
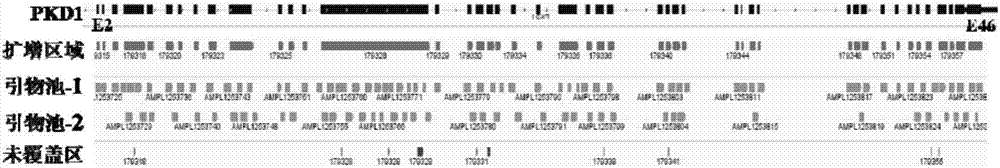
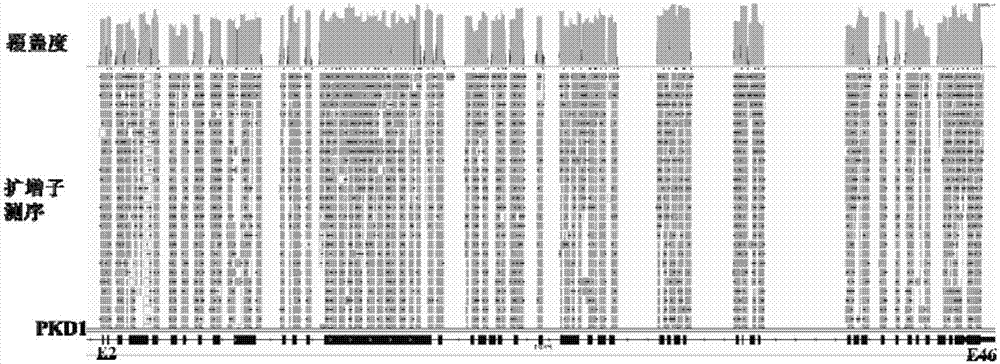
The invention relates to the field of disease transmission gene detection, in particular to a PCR (polymerase chain reaction) primer for amplifying a PKD (polystic kidney disease)1 exon ultra-long fragment, a kit for detecting PKD1 gene mutation and application. PKD1 exons are 2nd to 46th exons; the PCR primer has the primer with the base sequences shown by SEQ ID NO:1 and SEQ ID NO:2. The kit has the advantages that the diagnosis accuracy is high; the operation flow process is simple, convenient and efficient.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Compositions and methods relating to polycystic kidney disease
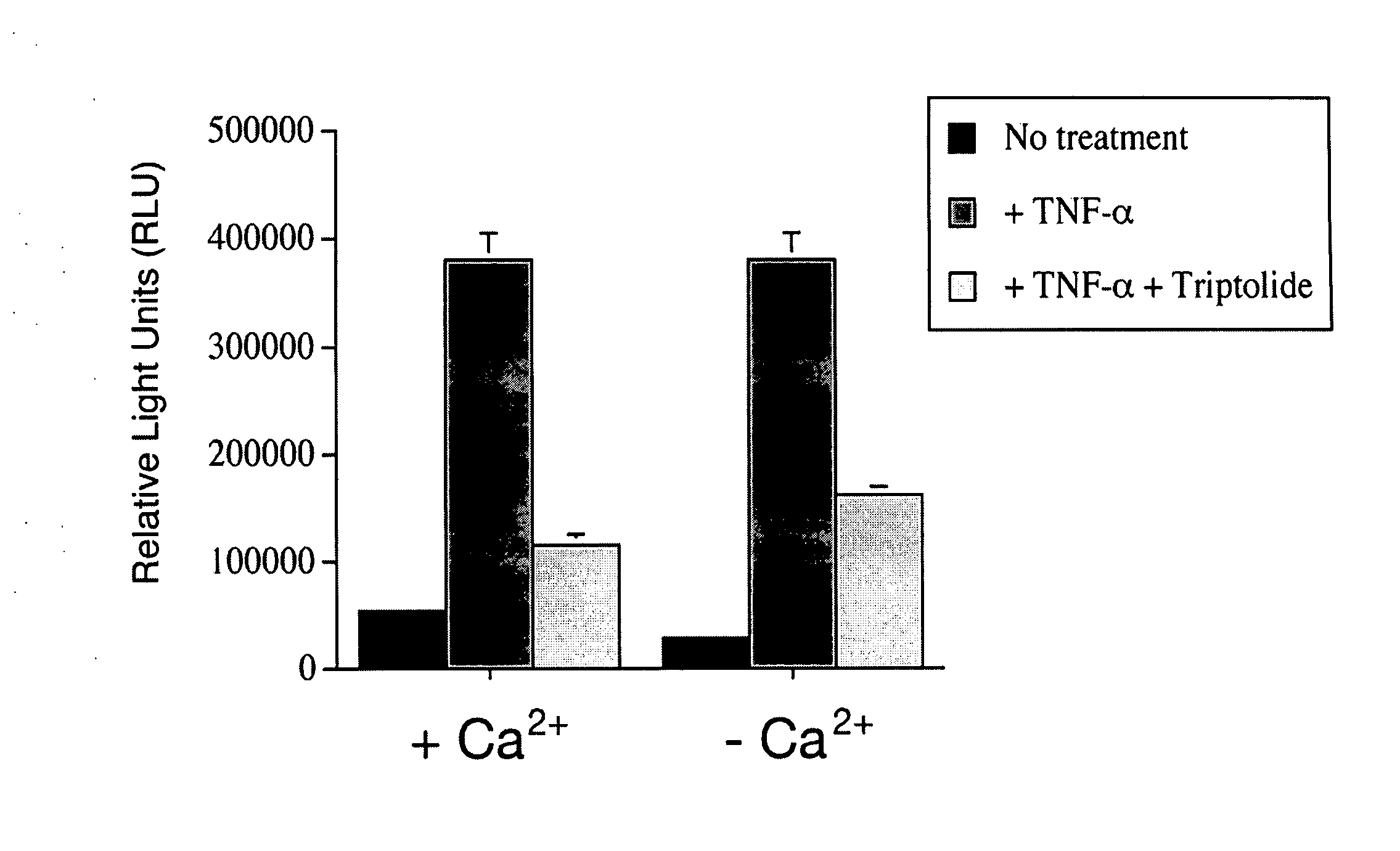
Described herein are therapeutic strategies (methods and compositions) useful for treating conditions in which cilia are affected and which manifest with cysts and / or fibrosis, such as conditions in which the kidney, pancreas, liver and / or spleen are affected and contain cysts. Particular embodiments described herein are therapeutic strategies in which PC-2 agonists, particularly agonists (calcium channel agonists) that target PC-2 directly and / or selectively, are administered to individuals with mutations in PKD1, in order to alter the course of polycystic kidney disease, particularly ADPKD. In specific embodiments, the invention relates to use of PC-2 agonists triptolide and triptolide derivatives to regulate calcium release. In other aspects, the invention relates to use of PC-2 agonists to treat or aid in the treatment of any condition in which a calcium channel, such as the gene product of PKD1 and / or PKD2, is mutated; calcium signaling is abnormal; or both.
Owner:YALE UNIV
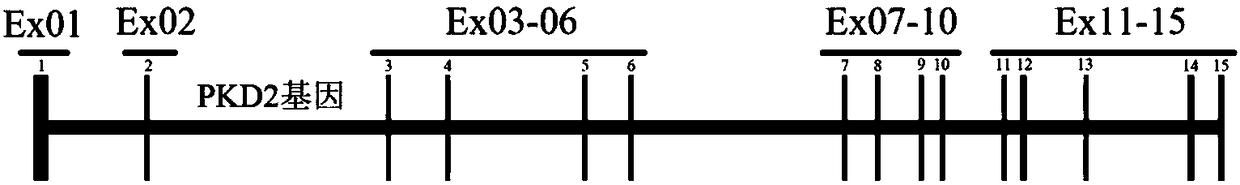
A detection method and a detection kit for PKD1 gene and PKD2 gene mutation
InactiveCN108707648AImprove detection accuracyAvoid amplificationMicrobiological testing/measurementHuman DNA sequencingHigh homology
The invention discloses a detection method and a detection kit for PKD1 gene and PKD2 gene mutation. Amplification is performed by creatively designing multiple pairs of specific long fragment PCR primers, and when amplification is performed by adopting the human genome DNA as a template, amplification of pseudogenes with high homology can be avoided, and therefore the appearance of the false positive and false negative result brought by the pseudogenes can be avoided, and the detection accuracy of mutation of the PKD1 gene and PKD2 gene can be greatly improved. A high-throughput sequencing technique is combined, and the method and the kit when compared with a traditional medical exon trapping technique, can simplify a technical process, reduce the detection cost and shorten the detectionperiod.
Owner:GUANGZHOU JIAJIAN MEDICAL TESTING CO LTD
Detection method of adult type polycystic kidney
InactiveCN107201402AFacilitate process detectionAvoid interferenceMicrobiological testing/measurementAdult typePKD1
The invention relates to a detection method of adult type polycystic kidney. The treatment mutation of adult type polycystic kidney disease-causing genes PKD1 and PKD2 is detected and identified by adopting a long fragment PCR (Polymerase Chain Reaction) capturing, second generation sequencing and first generation identification. Compared with an existing probe capturing technology, true and false genes can be distinguished, real genes are specifically captured and interference caused by the false genes is reduced; the ratio of all components is optimized and the consistency of capturing is ensured; the conditions that the uniformity of data is poor and the data quantity of partial segments is not enough are avoided; an optimized database building scheme has good stability and automatic database building is convenient to carry out; after an existing analysis method is tested, a detection result can be processed very well and a correct result is found.
Owner:杭州博圣医学检验实验室有限公司
Detection and treatment of polycystic kidney disease
InactiveUS8530161B2Improve throughputBioreactor/fermenter combinationsFungiPKD1Polycystic liver disease
Compositions useful for examining the PKD1 gene are provided. In addition, methods for detecting mutations of the PKD1 gene, which can be associated with autosomal dominant polycystic kidney disease in humans, are provided. Methods for diagnosing a mutant PKD1 gene sequence in a subject also are provided, as are methods of treating a subject having a PKD1-associated disorder.
Owner:THE JOHNS HOPKINS UNIVERSITY SCHOOL OF MEDICINE
Primer group and kit for amplifying PKD1 gene
ActiveCN112195238AAccurate detectionExtend amplicon lengthMicrobiological testing/measurementDNA/RNA fragmentationMedicinePseudogene
The invention relates to the field of genetic disease gene detection, in particular to a PCR primer group for amplifying a PKD1 gene. The PCR primer group comprises one or more pairs in a first primerpair, a second primer pair, a third primer pair and a fourth primer pair. According to the primer group disclosed by the invention, the length of an amplicon covering an exon No.1 is increased, stable amplification of an exon No.1 region is successfully realized, and an amplification product covers all exon regions and most of intron regions of PKD1; a PKD1 gene sequence is specifically obtained,and a pseudogene sequence of PKD1 cannot be completely amplified; and under the condition that pseudogene interference is completely eliminated, the variation of the PKD1 gene, including known variation and unknown variation, can be detected more accurately.
Owner:上海韦翰斯生物医药科技有限公司
Primer set and method for amplifying exons of pkd1 gene and pkd2 gene
ActiveUS20180037955A1Efficient amplificationGrowth inhibitionMicrobiological testing/measurementLibrary creationPKD1Exon
Owner:OTSUKA PHARM CO LTD
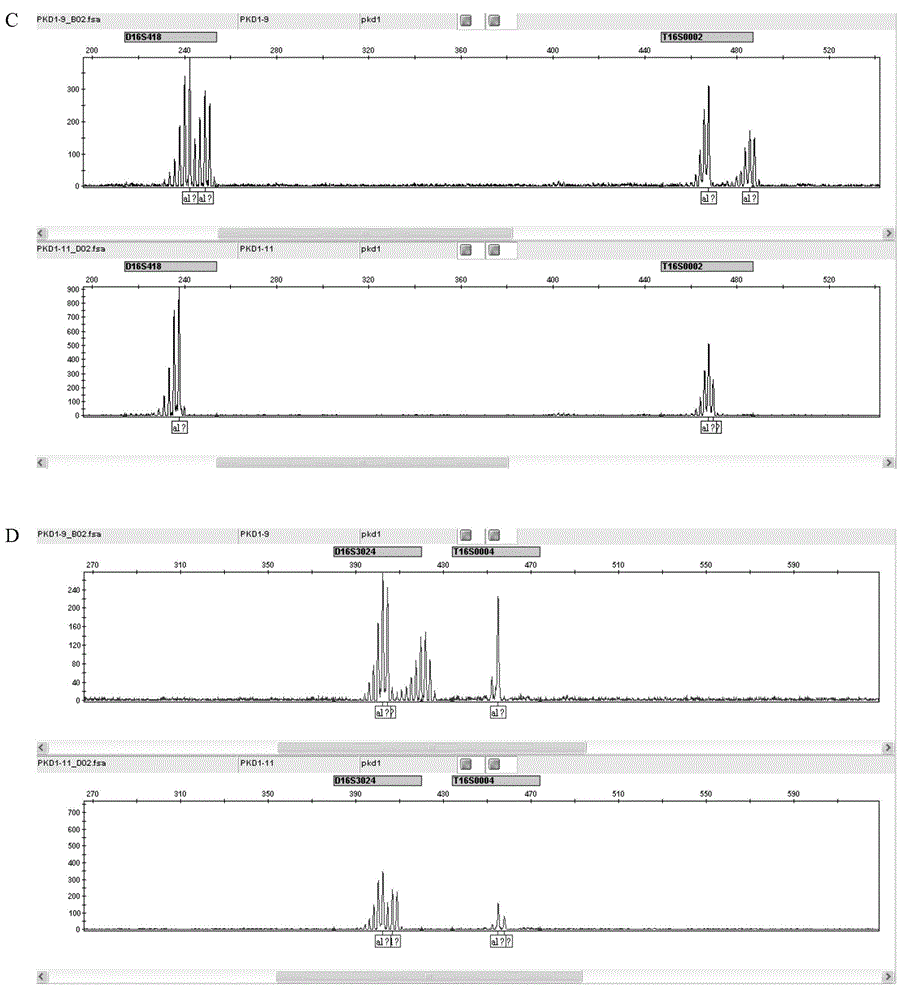
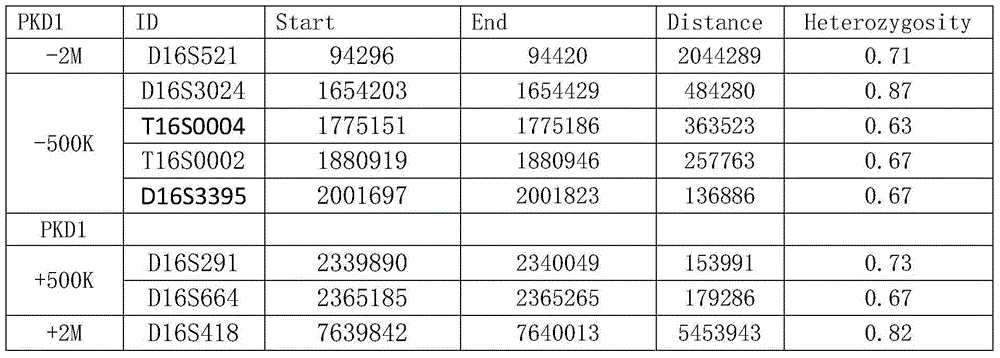
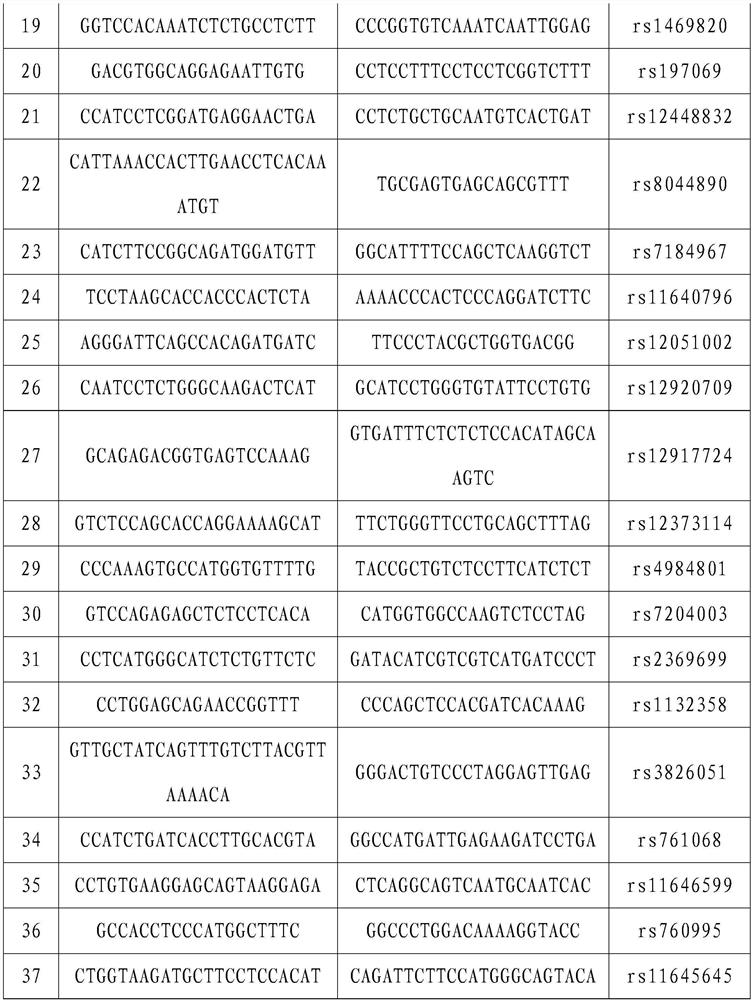
STR locus of PKD1 gene and application thereof
ActiveCN106282172AImprove compatibilityWide range of choicesMicrobiological testing/measurementDNA/RNA fragmentationPrenatal diagnosisNucleic acid sequencing
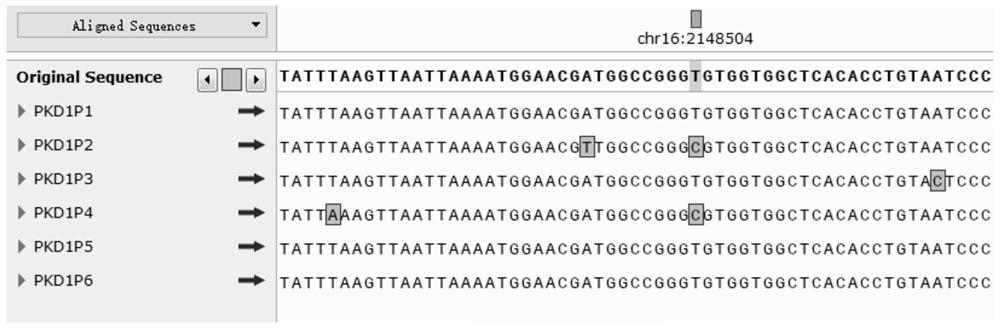
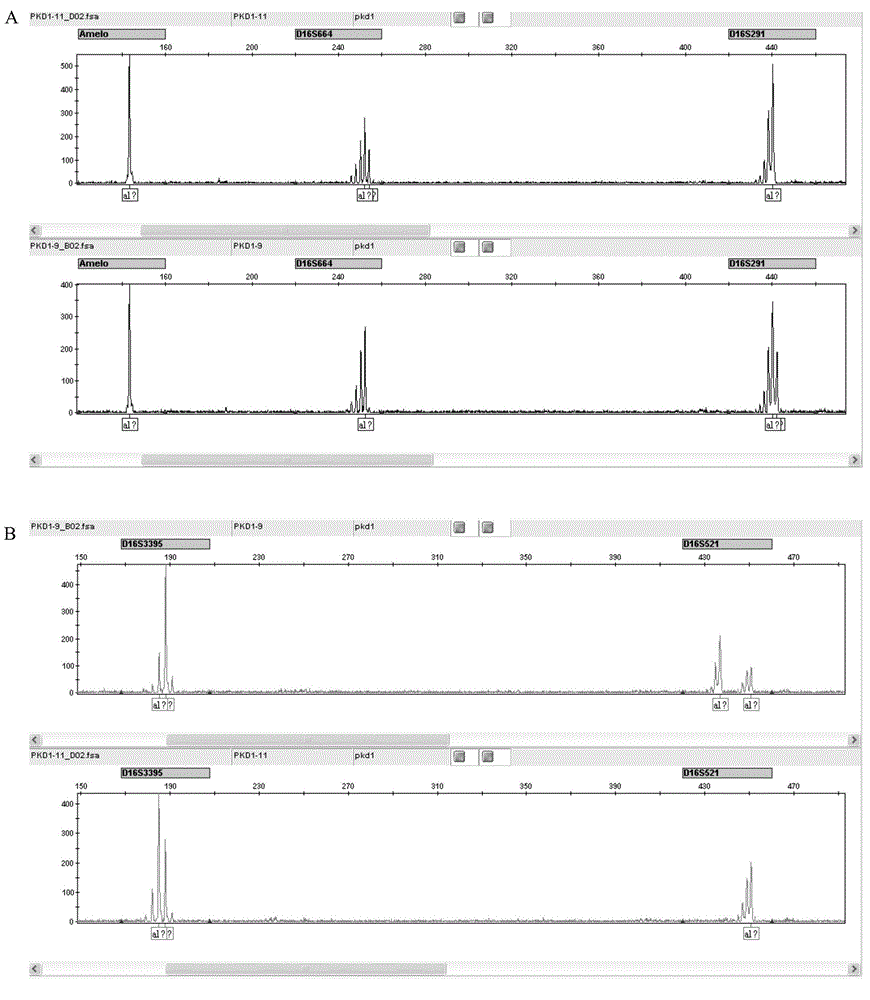
The invention discloses an STR locus of a PKD1 gene. The STR locus has a nucleic acid sequence shown by SEQ ID NO.1 or SEQ ID NO.2. The invention also discloses an application of the STR locus of the PKD1 gene, wherein the STR locus is applied to the preplantation genetile diagnosis or prenatal diagnosis of autosomal dominant polycystic kidney. With higher resolution, the STR locus of the PKD1 gene disclosed by the invention is applied to the linkage heredity analysis of the PKD1 gene, can be used for remarkably improving the typing recognition efficiency and accuracy, and provides a basis for clinical preplantation genetile diagnosis of autosomal dominant polycystic kidney.
Owner:THE INT PEACE MATERNITY & CHILD HEALTH HOSPITAL OF CHINA WELFARE INST
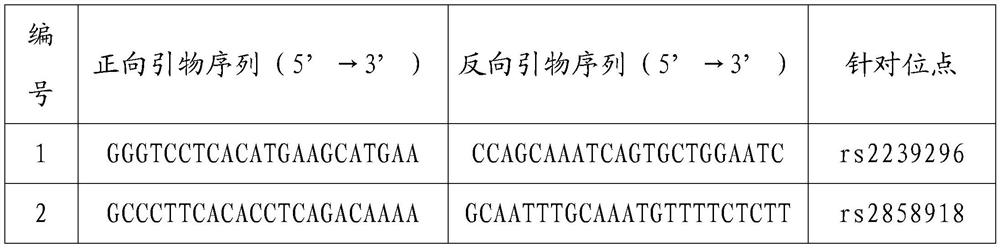
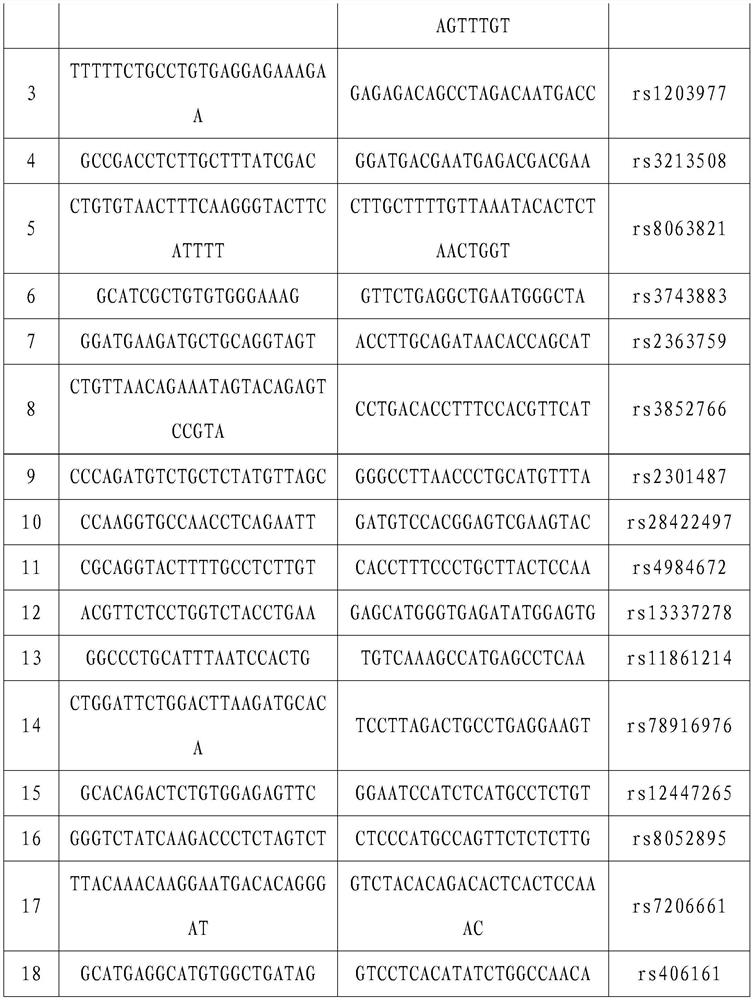
Primer composition, product and method for detecting PKD1 variant single sperm
PendingCN113388672AEasy to getAvoid deficienciesMicrobiological testing/measurementDNA/RNA fragmentationMedicinePathogenicity
The invention provides a primer composition, a product and a method for detecting a PKD1 variant single sperm. The primer composition comprises primers capable of specifically amplifying a PKD1 gene and SNP in a 2Mb upstream and downstream range of the PKD1 gene. The primer composition can be used for genetic marking of variant sperms and therefore distinguish high-pathogenicity-risk haplotypes of men.
Owner:PEKING JABREHOO MED TECH CO LTD
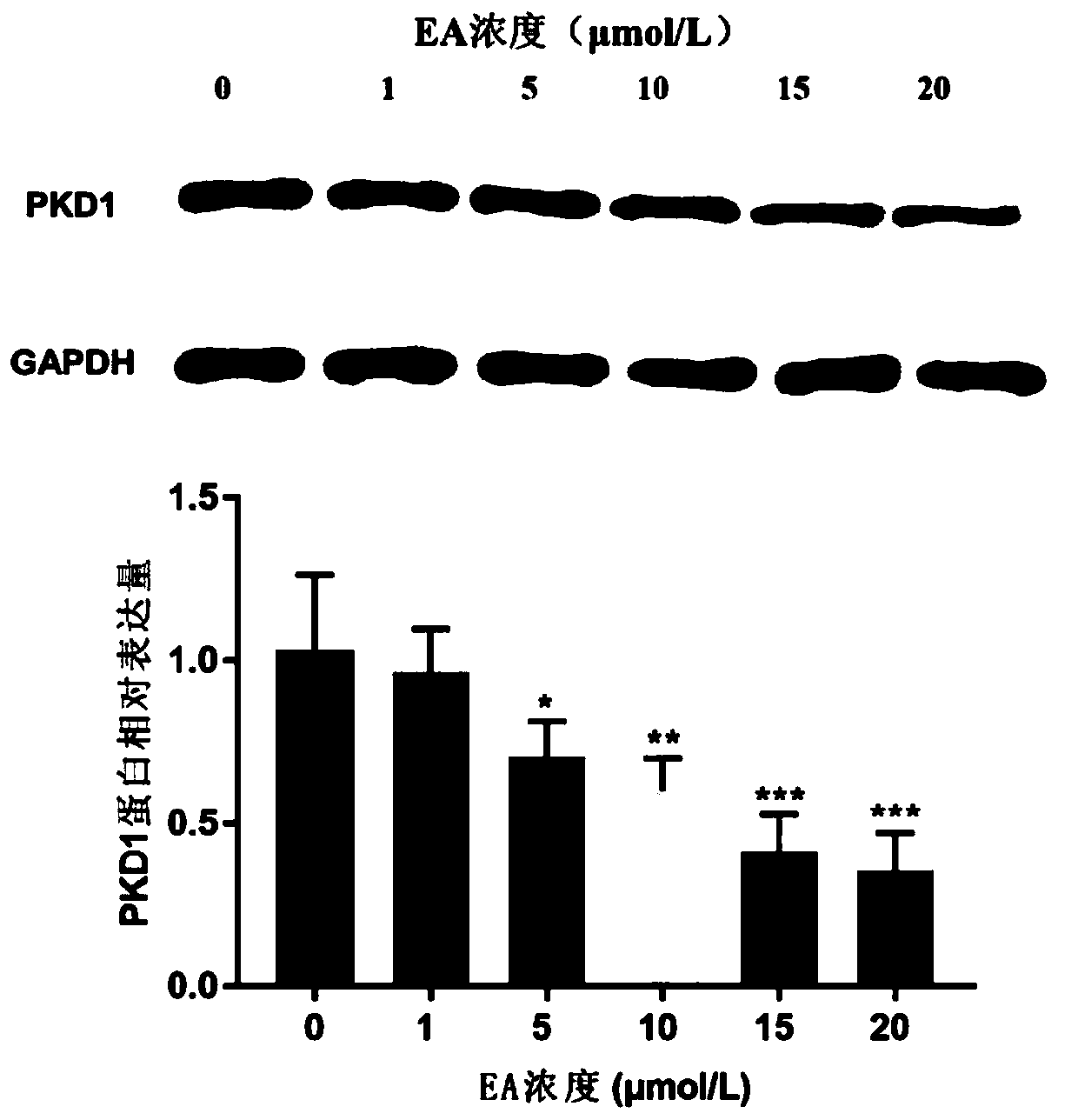
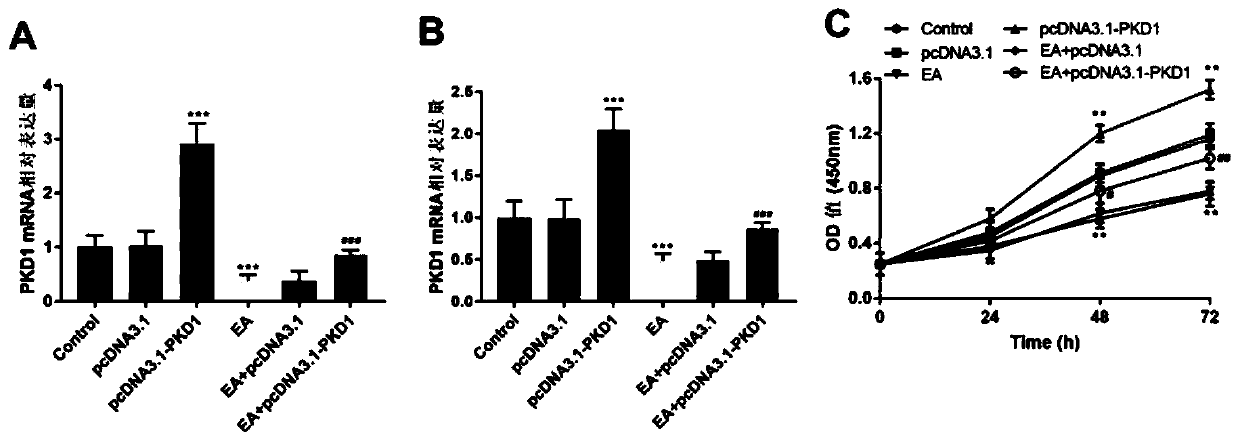
Application of ebracteolatain A in preparing drugs for treating breast cancer
InactiveCN111084766ARecovery inhibitionProliferative effectKetone active ingredientsAntineoplastic agentsCancer cellAkt signalling
The present invention belongs to the technical field of medicines, specifically relates to an application of ebracteolatain A (EA) in preparing drugs for treating breast cancer and reveals for the first time that the ebracteolatain A can inhibit expression of PKD1 protein in breast cancer cells in a dose-dependent manner. At the same time, the overexpression of PKD1 can significantly restore an inhibitory effect of the ebracteolatain A on proliferation of breast cancer cells. Finally, through mechanism research and analysis, the ebracteolatain A is confirmed to inhibit the proliferation of thebreast cancer cells by inhibiting MEK / ERK and PI3K / AKT signaling pathways mediated by the specific target PKD1. The research finds that the PKD1 plays a very important role in process of inhibiting the proliferation of breast cancer by the ebracteolatain A and is a key target in treatment process of the breast cancer.
Owner:赵亮 +1
Reagent composition and detection method for detecting autosomal dominant polycystic kidney disease
PendingCN114480603ASolve puzzlesThe experimental method is simpleMicrobiological testing/measurementGeneticsGenomic DNA
The reagent composition for detecting the autosomal dominant polycystic kidney disease comprises a primer group, a first reagent for extracting genomic DNA from a sample; the second reagent is used for carrying out long fragment PCR reaction by using the primer group; a third agent for processing the amplification product such that the amplification product can be used in a high-throughput sequencing technique; and the fourth reagent is used for performing high-throughput sequencing on the treated amplification product. Comprising the following steps: S1, amplifying a PKD1 gene by using a reagent composition according to any one of claims 1-7 through long-fragment PCR (Polymerase Chain Reaction); s2, performing high-throughput sequencing on the amplified sequence obtained in the step (1); s3, comparing the sequencing result obtained in the step (2) with a PKD1 gene reference sequence, and determining a mutation site; s4, eliminating false gene locus interference through bioinformatics analysis, and determining a PKD1 true gene mutation locus; and S5, comparing the true gene mutation site determined in the step S4 with a known PKD1 gene mutation site, and determining a new mutation site on the PKD1 gene.
Owner:广州源古纪科技有限公司
PKD mutations and evaluation of same
ActiveUS8771946B2Improve certaintyAid in diagnosisSugar derivativesMicrobiological testing/measurementPKD1Novel mutation
The present invention relates to methods of detecting novel mutations in a PKD1 and / or PKD2 gene that have been determined to be associated with autosomal dominant polycystic kidney disease (ADPKD) in order to detect or predict the occurrence of ADPKD in an individual.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Pkd mutations and evaluation of same
ActiveUS20100047785A1Inexpensively evaluatedImprove certaintyMicrobiological testing/measurementPKD1Novel mutation
The present invention relates to methods of detecting novel mutations in a PKD1 and / or PKD2 gene that have been determined to be associated with autosomal dominant polycystic kidney disease (ADPKD) in order to detect or predict the occurrence of ADPKD in an individual.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
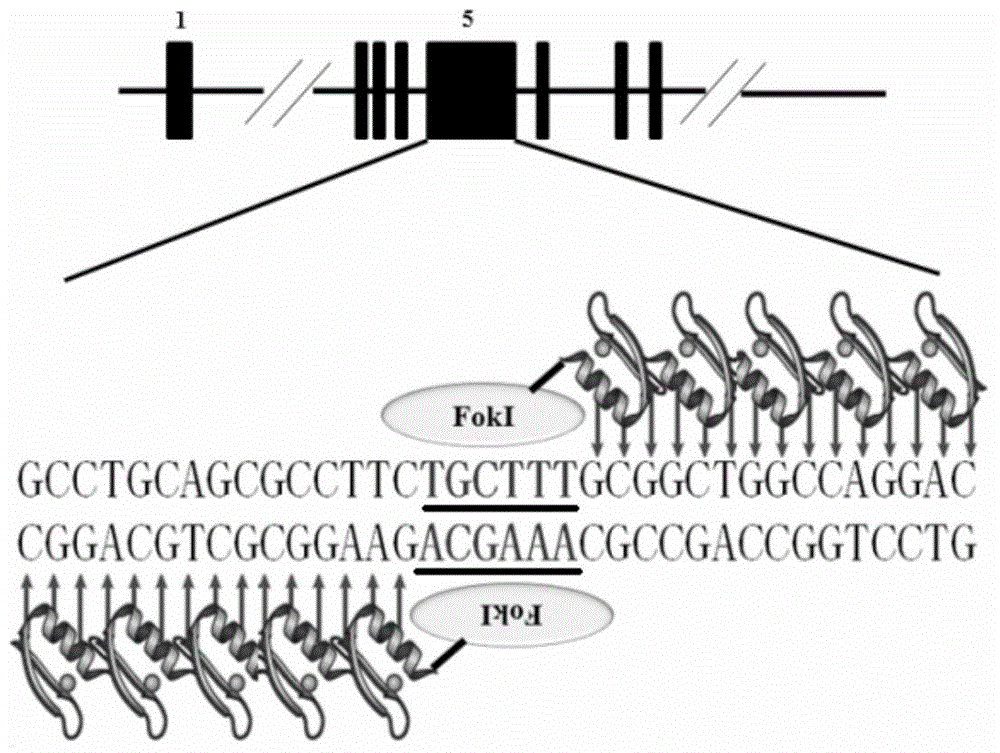
Production method and applications of autosomal dominant polycystic kidney disease gene mutation pig
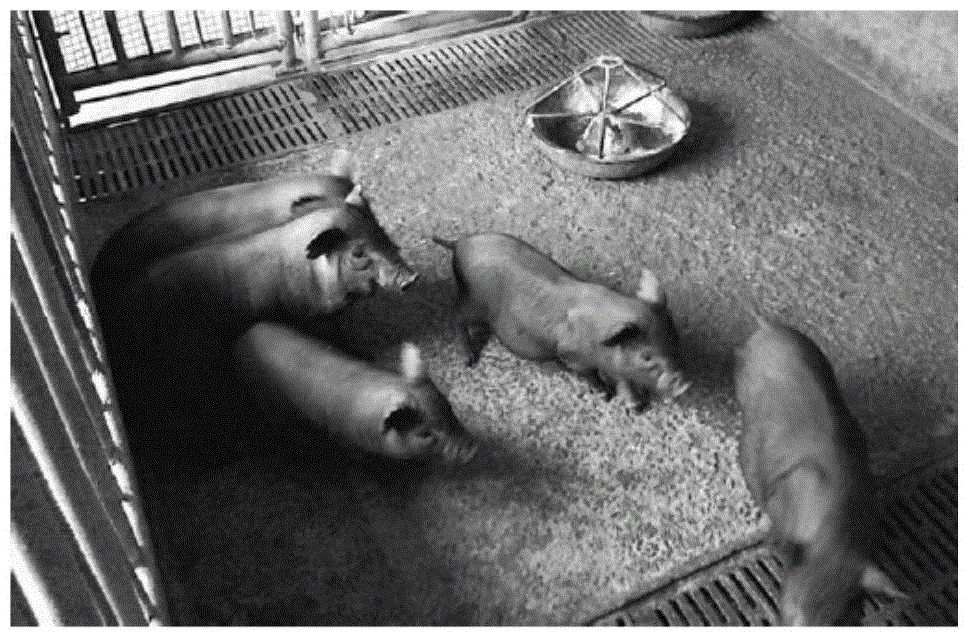
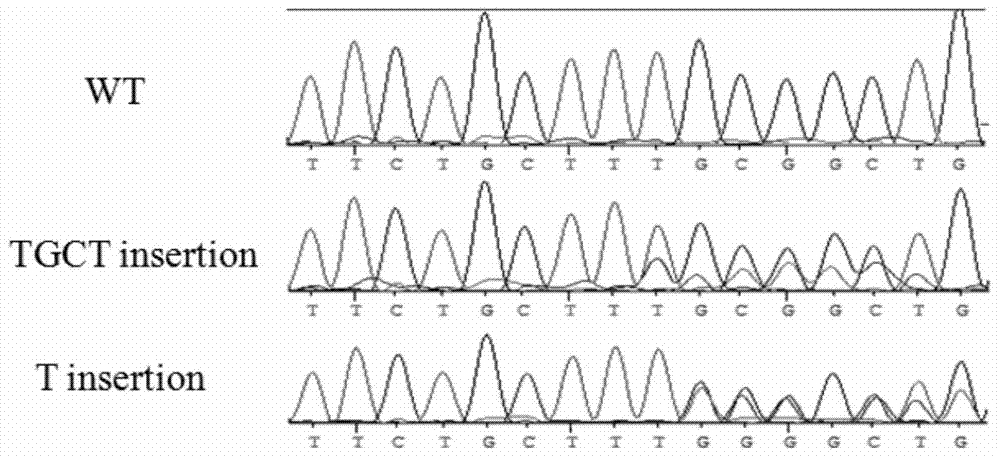
The present invention provides a production method and applications of an autosomal dominant polycystic kidney disease gene mutation pig, wherein a gene editing technology is used to introduce insertion mutation into the 5# exon of the pig PKD1 gene and knockout the pig PKD1 gene to construct an autosomal dominant polycystic kidney disease gene mutation pig model. The constructed gene mutation pig model of the present invention can be used for research and drug screening of the autosomal dominant polycystic kidney disease.
Owner:CHINA AGRI UNIV
A kind of production method and application of autosomal dominant polycystic kidney disease gene mutation pig
The invention provides a production method and application of a pig with autosomal dominant polycystic kidney disease gene mutation. Gene editing technology is used to introduce an insertion mutation at exon 5 of the pig PKD1 gene, and the pig PKD1 gene is knocked out to construct a pig PKD1 gene A pig model of chromosomal dominant polycystic kidney disease gene mutation. The gene mutation pig model established by the invention can be used for the research and drug screening of autosomal dominant polycystic kidney disease.
Owner:CHINA AGRI UNIV
Detection kit and detection method for pkd1 gene mutation
ActiveCN104531883BComprehensive detection rangeLow mismatch rateMicrobiological testing/measurementSequence analysisNephrosisPKD1
The invention provides a primer set, a kit and a detection reaction system for detecting PKD1 gene mutation through the long fragment PCR and high-throughput sequencing technology, a non-diagnosis-purpose method for external PKD1 gene mutation detection, a non-diagnosis-purpose method for external PKD1 gene analysis and a method for detecting new mutation sites on the PKD1 gene. According to the method, the primer set is used for carrying out long fragment PCR amplification on the PKD1 gene of a sample, and detecting or analyzing is carried out through high-throughput sequencing. The autosomal dominant genetic polycystic nephrosis (adult polycystic nephrosis) can be diagnosed through the assistance of a detection result, previous unknown new mutation on a plurality of PKD1 real genes can be obtained and supplied to doctors or researchers so that the relevance between the mutation and the adult polycystic nephrosis can be studied.
Owner:北京圣谷智汇医学检验所有限公司
Application of apigenin in preparation of medicine for treating and/or preventing autosomal dominant hereditary polycystic kidney disease
PendingCN112546039AInhibition formationGrowth inhibitionOrganic active ingredientsUrinary disorderPharmacometricsApigetrin
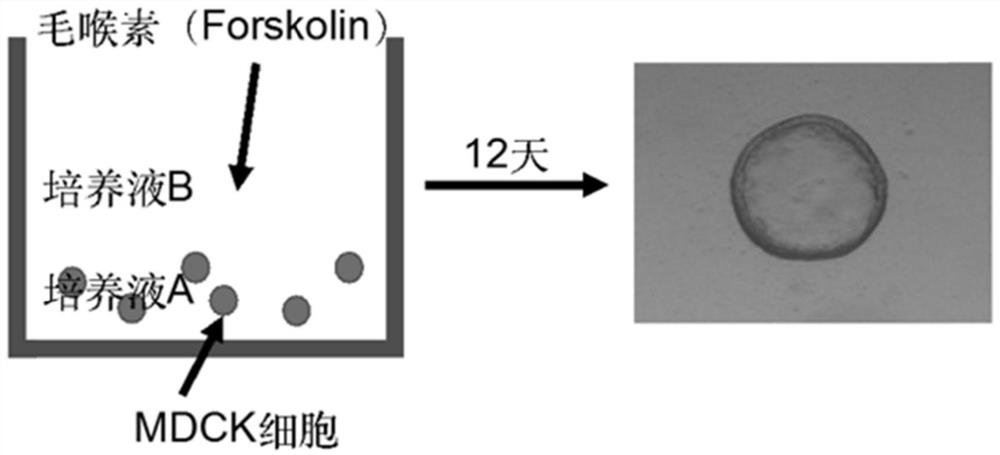
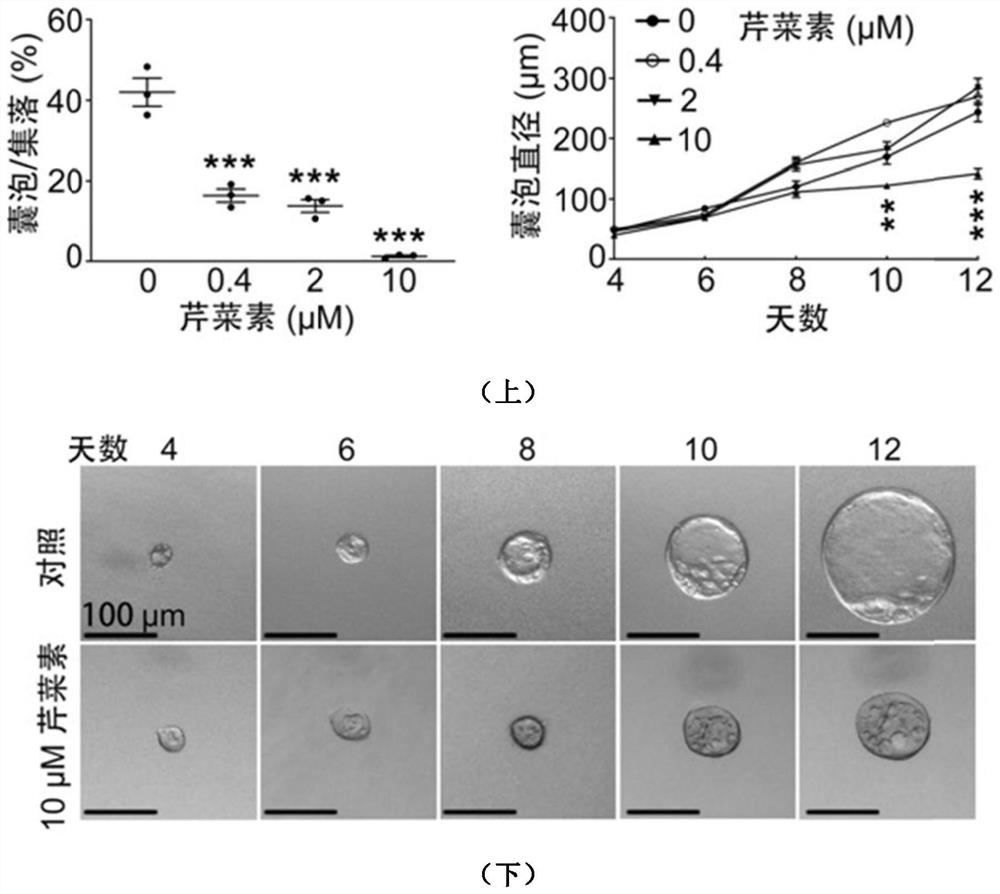
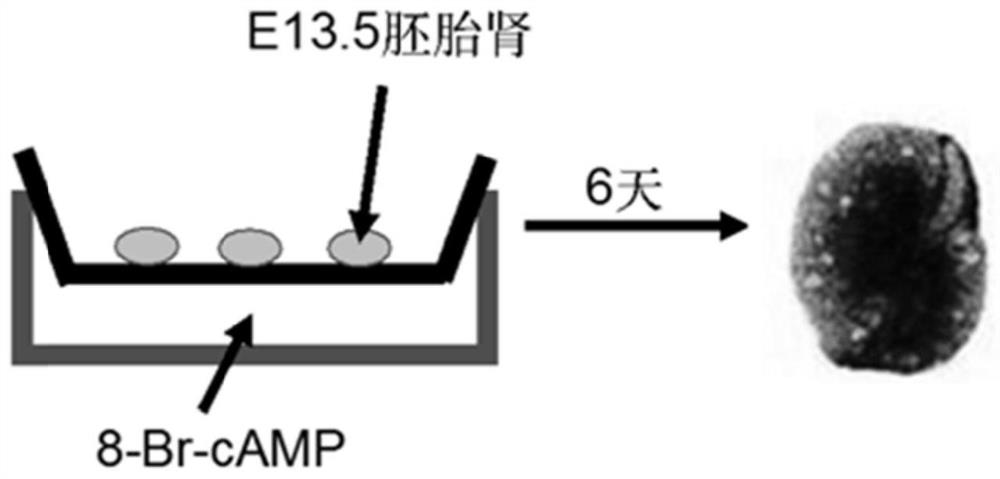
The invention discloses application of apigenin in preparation of a medicine for treating and / or preventing autosomal dominant hereditary polycystic kidney disease. The application of an MDCK vesiclemodel proves that the apigenin can inhibit the formation and growth of vesicles, the intrarenal pharmacological activity of the apigenin is determined through an embryo kidney vesicle model, and the development of kidney vesicles is remarkably inhibited under the condition that the normal growth of the kidney is not influenced. Finally, in a Pkd1 gene knockout polycystic kidney mouse model, it isfurther proved that the apigenin can effectively inhibit generation and development of the kidney vesicles in the mouse body. The invention also shows that the apigenin has no cytotoxicity and has noobvious influence on kidney cell viability, that is, the vesicle inhibiting effect of the apigenin is irrelevant to cytotoxicity; and meanwhile, the apigenin can inhibit a signal path related to proliferation in kidney cells, and is one of important mechanisms for inhibiting generation and development of the kidney vesicles. The result indicates that the apigenin can be used for treating the autosomal dominant hereditary polycystic kidney disease.
Owner:PEKING UNIV
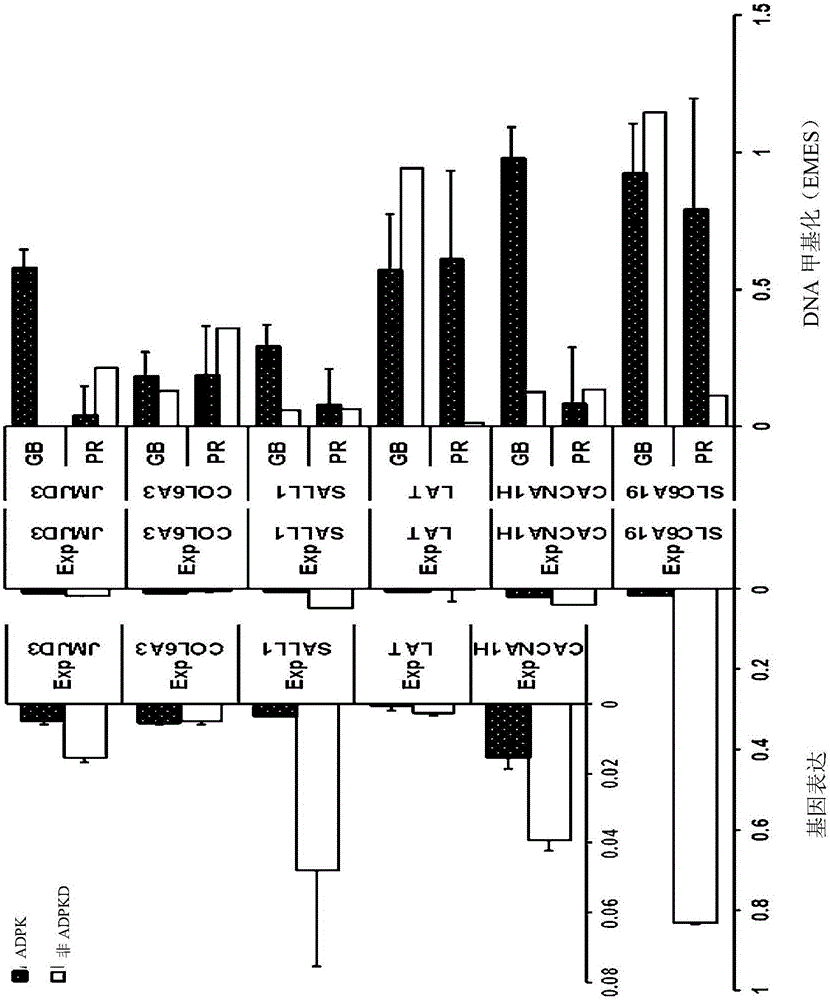
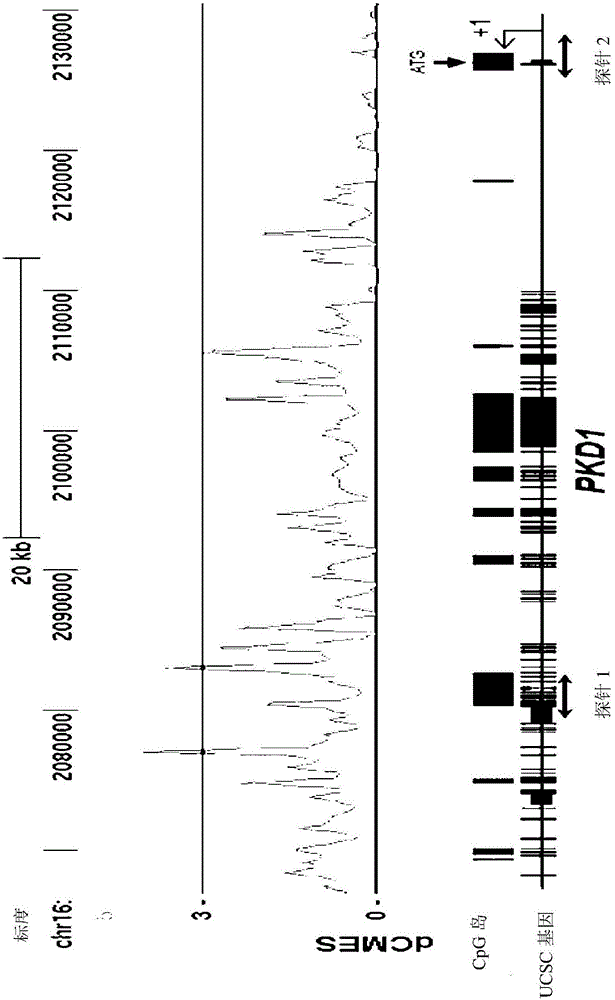
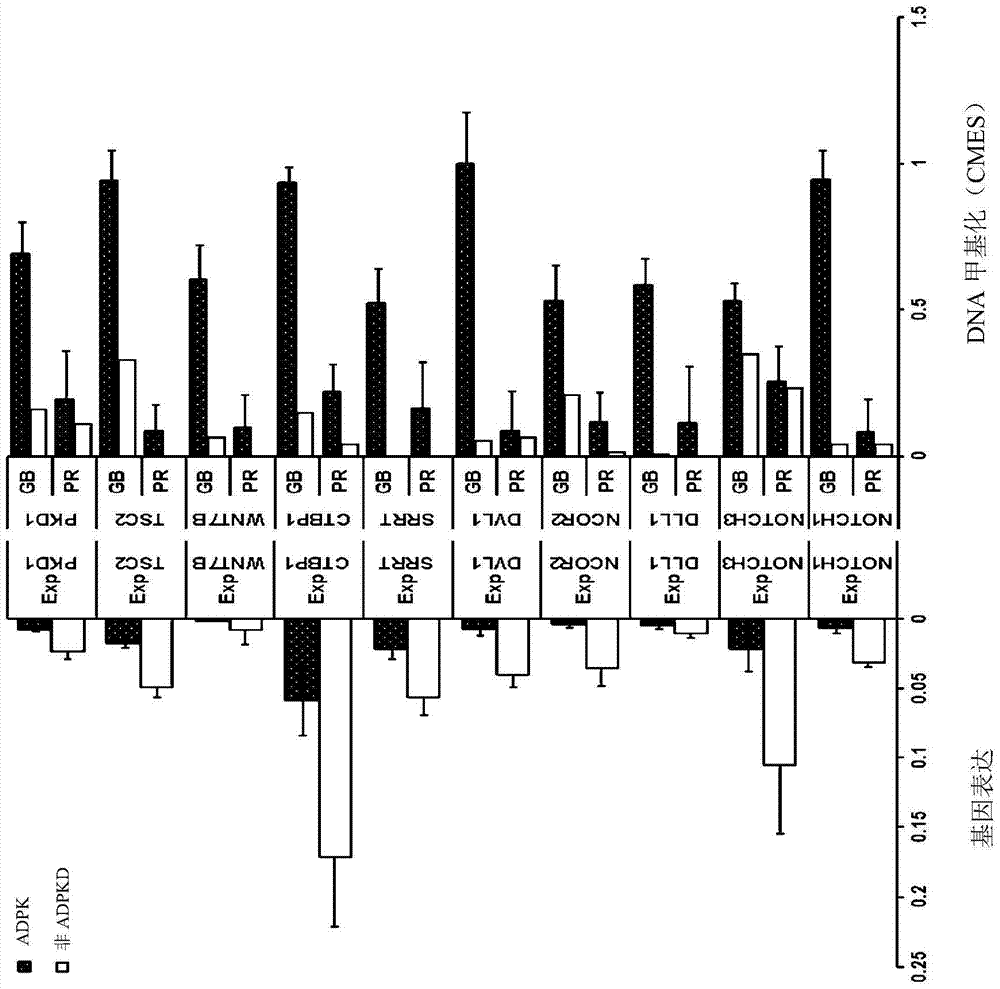
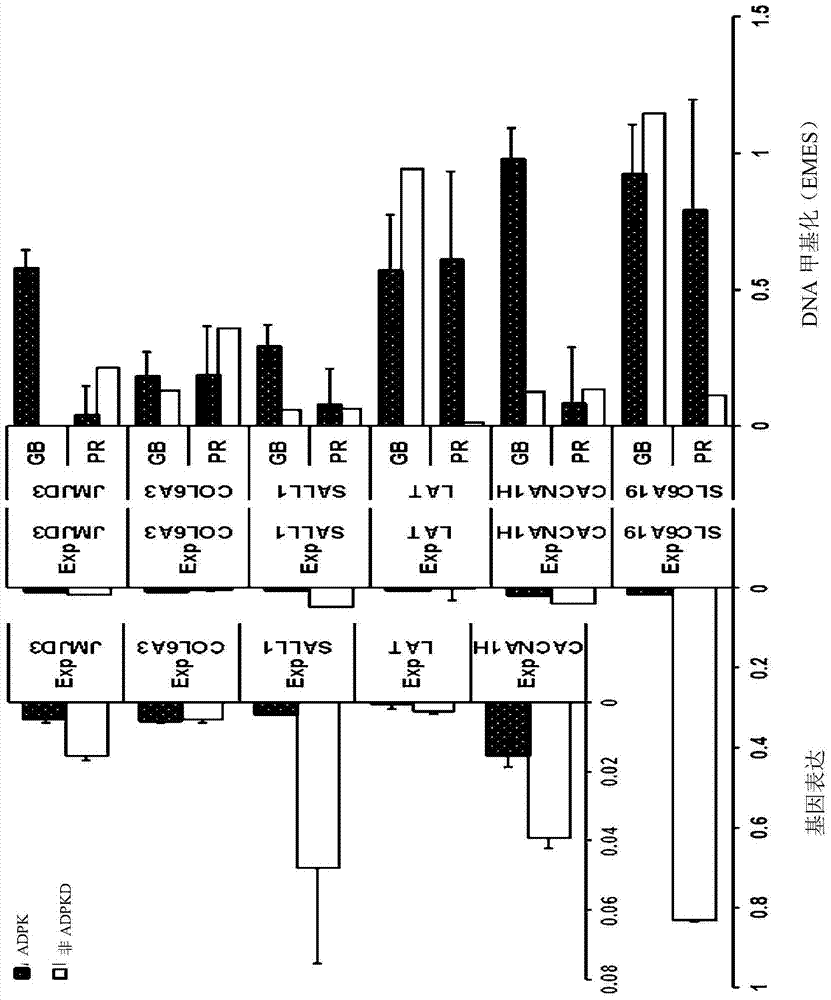
Pharmaceutical composition for alleviating or treating autosomal dominant polycystic kidney disease comprising dna methylation inhibitor
ActiveCN105163741ADelayed cyst formationPeptide/protein ingredientsGenetic material ingredientsCanine kidneyHereditary Mutation
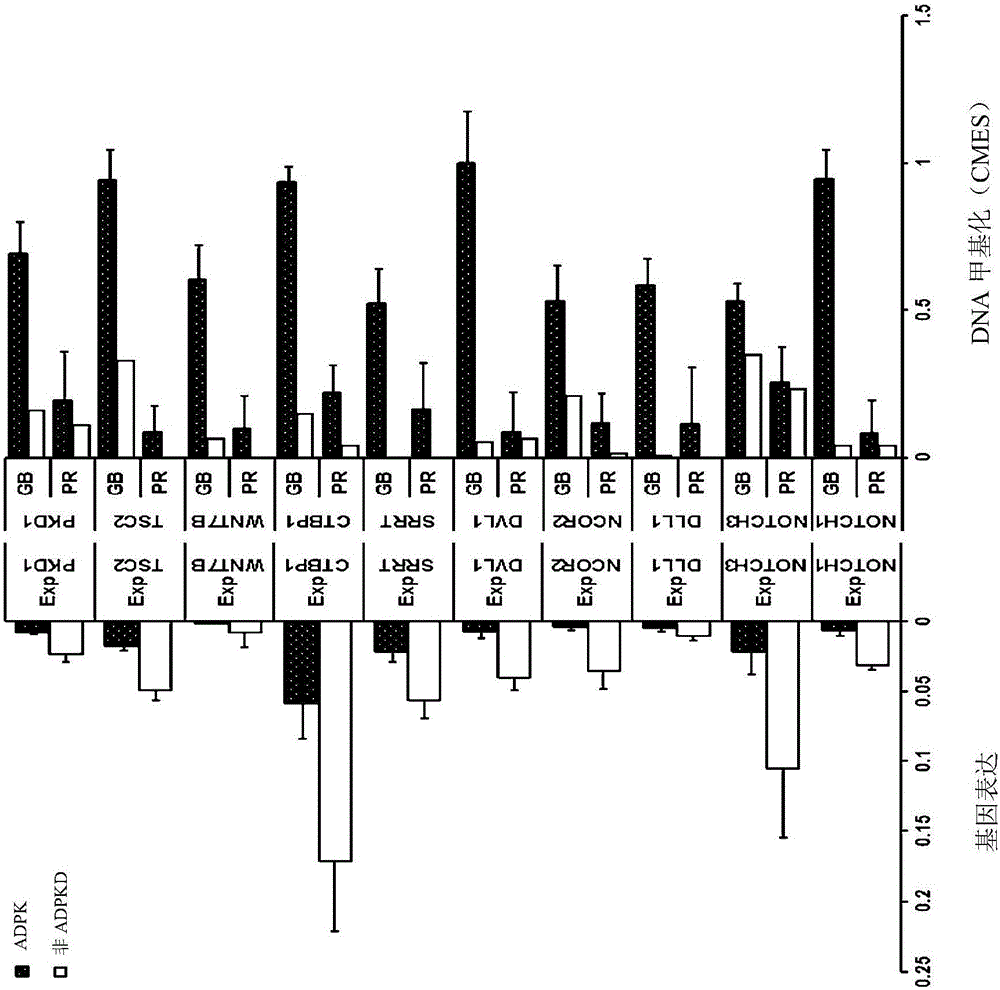
In order to determine epigenetic variations of autosomal dominant polycystic kidney disease and functional association therebetween, the present inventors have subjected individuals with polycystic kidney disease and without polycystic kidney disease to analysis through methylation profiling in random fashion of the genome as a whole. Interestingly, in PKD1 and other genes associated with ion transport and cell adhesion, there was hypermethylation in the gene-body region, and the expression of these genes was down-regulated in polycystic kidney disease. In particular, in PKD1, there was hypermethylation in the polycystic kidney disease gene-body region, and this was associated with MBD2 (methyl-CpG-binding domain 2) protein binding. In addition, DNA methylation inhibitor treatment was accompanied by up-regulation of PKD1 expression and caused a delay in cyst formation in MDCK (Madin-Darby Canine Kidney) cells. This therefore demonstrates that, in the present invention, hypermethylation of PKD1 and regulator genes associated with cyst formation plays a decisive role in cyst formation and shows that the present invention can be used in therapeutic applications for autosomal dominant polycystic kidney disease.
Owner:SOOKMYUNG WOMENS UNIV IND ACADEMIC COOPERATION FOUND
Pharmaceutical composition for improving or treating autosomal dominant polycystic kidney disease comprising dna methylation inhibitor
ActiveCN105163741BDelayed cyst formationPeptide/protein ingredientsGenetic material ingredientsCanine kidneyHereditary Mutation
In order to determine the epigenetic mutation of autosomal dominant polycystic kidney disease and its functional relevance, the present inventors randomly analyzed the whole genome of polycystic kidney disease and non-polycystic kidney disease individuals by methylation profiling method and compared with their performance data. Interestingly, PKD1 and other genes associated with ion transport and cell binding were hypermethylated at the gene body, which was downregulated in polycystic kidney disease. In particular, PKD1 is partially hypermethylated in the polycystic kidney gene body, which is associated with MBD2 (methyl‑CpG‑binding domain 2) protein binding. Furthermore, DNA methylation inhibitor treatment was accompanied by an upregulation of PKD1 expression and delayed cyst formation in MDCK (Madin‑Darby Canine Kidney) cells. Thus, hypermethylation of PKD1 and cyst formation-associated regulatory genes of the present invention is decisive for cyst formation and revealed to be useful as a treatment for autosomal dominant polycystic kidney disease.
Owner:SOOKMYUNG WOMENS UNIV IND ACADEMIC COOPERATION FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com