Production method and applications of autosomal dominant polycystic kidney disease gene mutation pig
A production method and autosomal technology, applied to the production method and application field of autosomal dominant polycystic kidney disease gene mutation pigs, can solve the problems of low targeting efficiency, not very wide application of gene targeting technology, lack of embryonic stem cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Cloning of porcine polycystic kidney disease gene and screening of mutation sites
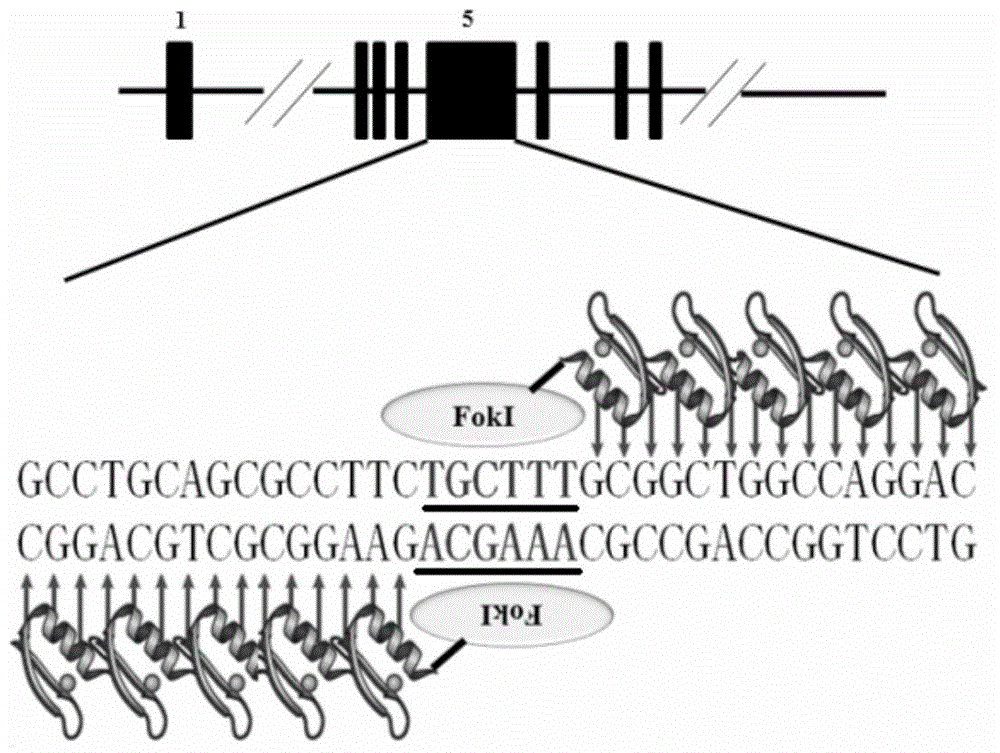
[0031] Based on the mRNA sequence of the porcine polycystic kidney disease gene PKD1 (GenBank Accession No.: NM_001246202), the inventor used ZFN, Talen and CRISPR-Cas9 gene editing techniques to locate the 642-643 site of exon 5 of PKD1 Introducing insertion mutations ( figure 1 ). As a target site for gene editing, the resulting mutant protein would fail to retain most of its functional domains, thus rendering PKD1 non-functional.
Embodiment 2
[0032] Example 2 Screening of PKD1 knockout fibroblasts
[0033] According to the target sites determined in Example 1, ZFNs for gene editing can be designed. Talen and CRISPR-Cas9 technologies can also be used for gene editing operations. ZFN technology is preferred in this embodiment. After obtaining ZFN, it is first necessary to optimize the amount of fibroblast transfected. We used 0.3 μg, 0.5 μg, 1 μg, 2 μg, 4 μg of ZFN plasmids (ZFN transfection is a pair of plasmids, and the amount at this time represents the amount of each plasmid) to transfect 1×10 6a fibroblast. The culture condition of fibroblasts was 37°C, the medium was DMEM (Sigma) plus 10% fetal bovine serum (Gibco), and the medium was changed 24 hours before transfection, so that the cells were in a state of exponential growth. Good transfection conditions are based on the following two criteria: 1) The fibroblasts are in good condition after transfection, with normal morphology and no large number of deaths;...
Embodiment 3
[0037] Example 3 Preparation of PKD1 Gene Knockout Minipigs by Somatic Cell Nuclear Transfer Technology
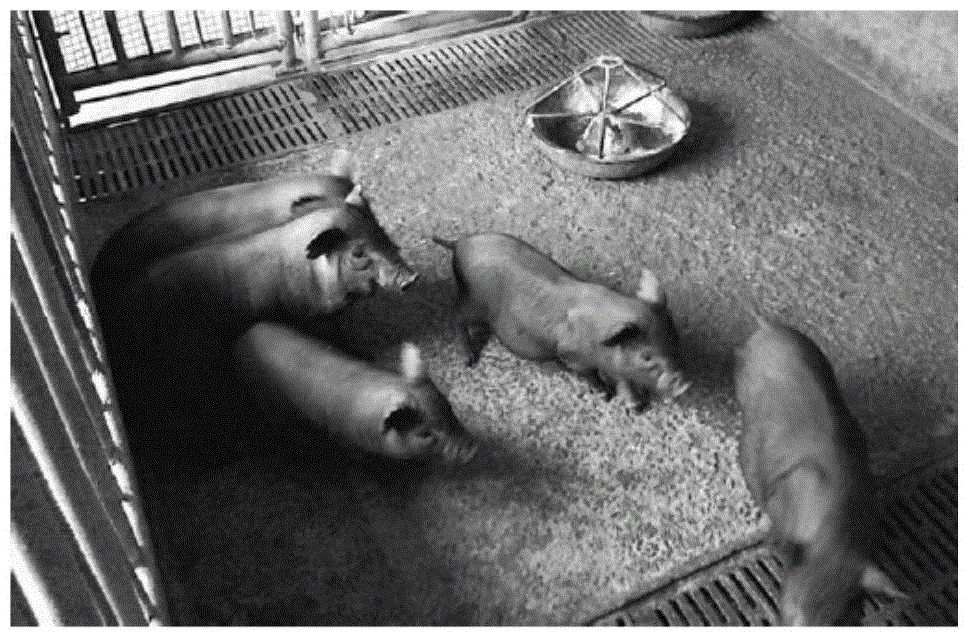
[0038] The nuclei of the screened positive clones were transferred into the enucleated oocytes by somatic cell nuclear transfer technology, cultured in vitro to form reconstituted embryos, and then transplanted into each recipient pig with an average of 384 reconstituted embryos In the oviducts of 13 recipient pigs. After about 114 days, 5 surrogate sows gave birth to 20 cloned miniature pigs ( image 3 ).
[0039] The operation steps are as follows: Retrieve the pig ovary from the slaughterhouse → extract the follicles with a needle tube → put the follicles into the maturation solution for in vitro maturation → after the fibroblasts at the mutant clone point fill the culture wells, digest, wash and resuspend → recipient oocytes Denucleate under the microscope, then inject the donor cells into the perivitelline space so that the cell membranes of the two are in close con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com