Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
52 results about "Methylone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methylone (also known as "3,4-methylenedioxy-N-methylcathinone", "MDMC", "βk-MDMA" and by the slang term "M1") is an empathogen and stimulant psychoactive drug. It is a member of the substituted amphetamine, substituted cathinone and substituted methylenedioxyphenethylamine classes.
2-pyridinyl[7-(substituted-pyridin-4-yl) pyrazolo[1,5-a]pyrimidin-3-yl]methanones
The present invention provides novel 2-pyridinyl[7(pyridin-4-yl)pyrazolo[1,5-a]pyrimidin-3-yl]methanones with at least one substituent on both the 2- and 4-pyridinyl ring having the chemical structure of formula I: The invention further provides compositions and methods employing the novel 2-pyridinyl[7-(pyridin-4-yl)pyrazolo[1,5-a]pyrimidin-3-yl]methanones of formula I to modulate GABA and GABAA receptor physiology to elicit therapeutic responses in mammalian subjects to alleviate neurological or psychiatric disorders, including stroke, head trauma, epilepsy, pain, migraine, mood disorders, anxiety, post traumatic stress disorder, obsessive compulsive disorders, mania, bipolar disorders, schizophrenia, seizures, convulsions, tinnitus, neurodegenerative disorders including Alzheimer's disease, amyotrophic lateral sclerosis and Parkinson's disease, Huntington's chorea, depression, bipolar disorders, mania, trigeminal and other neuralgia, neuropathic pain, hypertension, cerebral ischemia, cardiac arrhythmia, myotonia, substance abuse, myoclonus, essential tremor, dyskinesia and other movement disorders, neonatal cerebral hemorrhage, and spasticity, as well as other psychiatric and neurological disorders mediated by GABA and / or GABAA receptors.
Owner:SKOLNICK PHIL +1
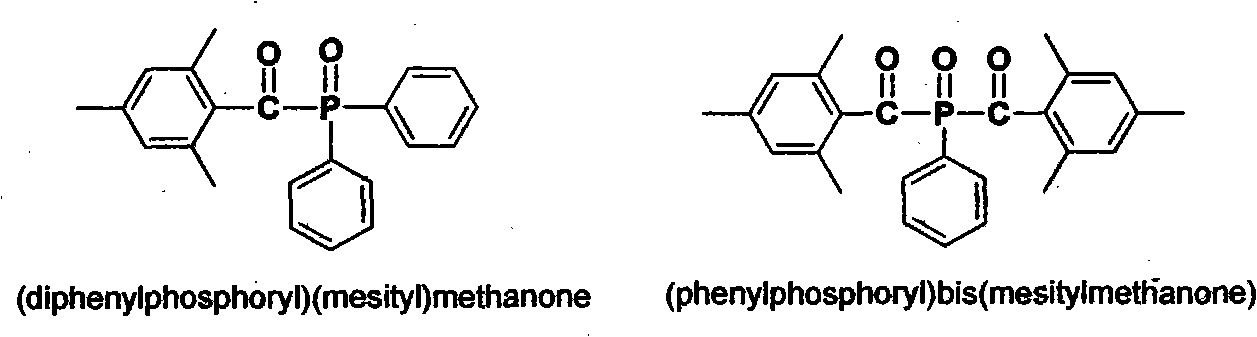
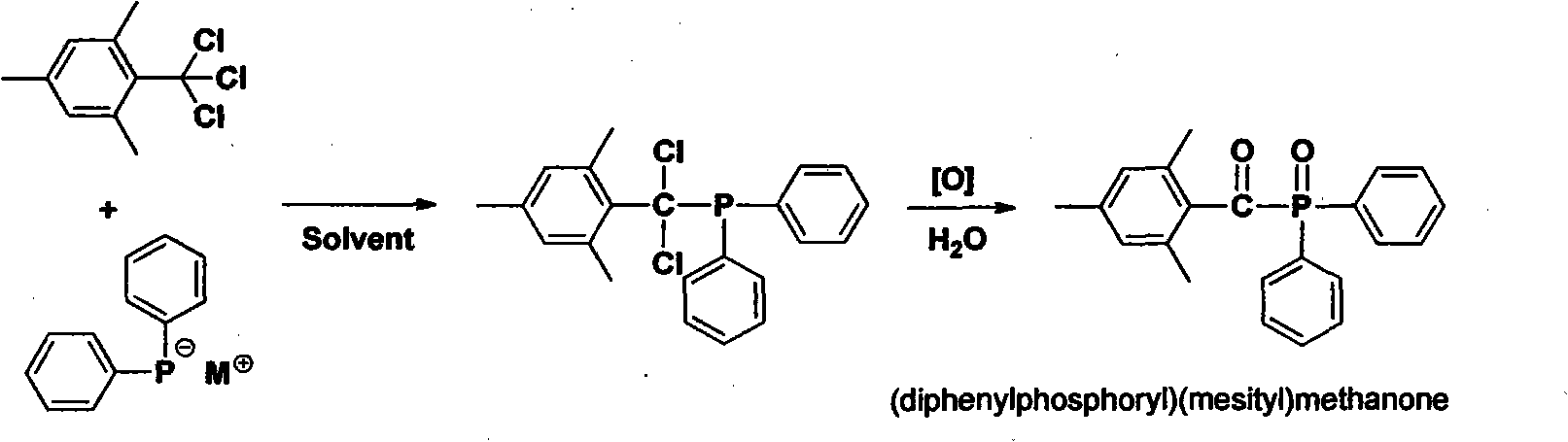
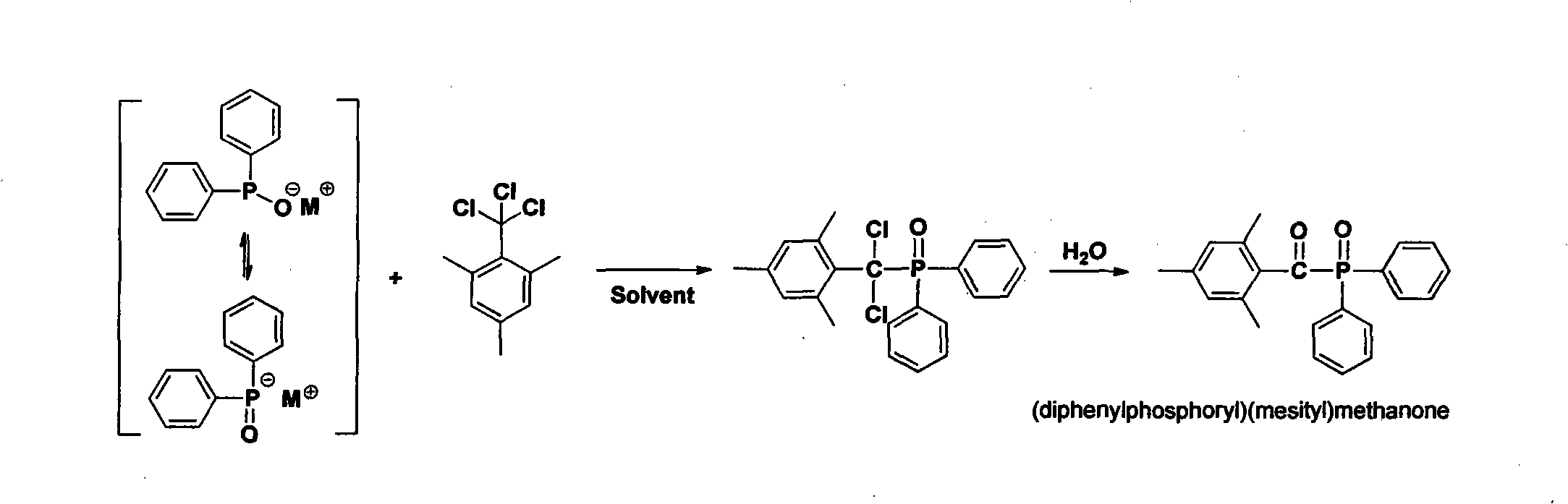
Preparation method of (diphenylphosphine oxide)(mesitylene)ketone and (phenylphosphine oxide)bis(mesitylene ketone)
The invention relates to the technical field of optical radiation free-radical polymerized new materials, especially to a novel synthesis technological process of commercial photoinitiator compounds of (diphenylphosphine oxide)(mesitylene)ketone and (phenylphosphine oxide)bis(mesitylene ketone). According to the invention, 1,3,5-trimethyl-2-(trichloromethyl)-benzene is used as a key raw material and is respectively subjected to a condensation reaction with corresponding organic phosphine precursors so as to prepare the target compounds. In comparison with a known technological route, the technology disclosed in the application has significant advantages such as novelty of the chemical reaction process, economical cost, competitiveness and environmental friendliness.
Owner:SHENZHEN UV-CHEMTECH CO LTD
[4-(5-aminomethyl-2-fluoro-phenyl)-piperidin-1-yl]-[7-fluoro-1-(2-methoxy-ethyl)-4-trifluoromethoxy-1h-indol-3-yl]-methanone as an inhibitor of mast cell tryptase
The present invention is directed to an indole benzylamine compound of formula I:useful as an inhibitor of tryptase. In addition, the present invention is directed to the use of the compound for treating a patient suffering from, or subject to, a physiological condition in need of amelioration by inhibition of tryptase, comprising administering to the patient of a therapeutically effective amount of the compound, and to a pharmaceutical composition comprising a pharmaceutically effective amount of the compound of formula I, and a pharmaceutically acceptable carrier.
Owner:SANOFI SA
Preparation method of 2-p-chlorobenzyl pyridine
The invention relates to a preparation method of 2-p-chlorobenzyl pyridine, comprising the following steps of: firstly adding chlorobenzene and alchlor to 2-pyridine formyl chloride hydrochloride for reaction so as to obtain 1-(4-chlorphenyl)-1-(2-pyridyl) ketone; and then adding diglycol, hydrazine hydrate and potassium hydroxide to the prepared 1-(4-chlorphenyl)-1-(2-pyridyl) ketone for reaction so as to obtain the 2-p-chlorobenzyl pyridine. The 2-p-chlorobenzyl pyridine produced by the method is used as an intermediate for preparing chlorpheniramine and has high product purity and simple and convenient process, and the chlorpheniramine prepared by using the 2-p-chlorobenzyl pyridine accords with Chinese pharmacopoeia; in addition, the invention has low cost, easy industrialization and outstanding economic benefit and social benefit.
Owner:GUANGXI UNIV
Hydroxyalkyl benzophenone photocuring agent and its preparation and use
A novel hydroxyalkyl phenyl ketone: (1-hydroxycylohexyl)[4-(2- hydroxy ethoxy)phenyl] methanone, its preparing process and its application as the trigger for optical solidification are disclosed. Said trigger can effectively and optical trigger the polymerization between unsaturated compound and resin. Its special molecular structure can prevent migration, odoring and yellowing.
Owner:XIANGTAN UNIV
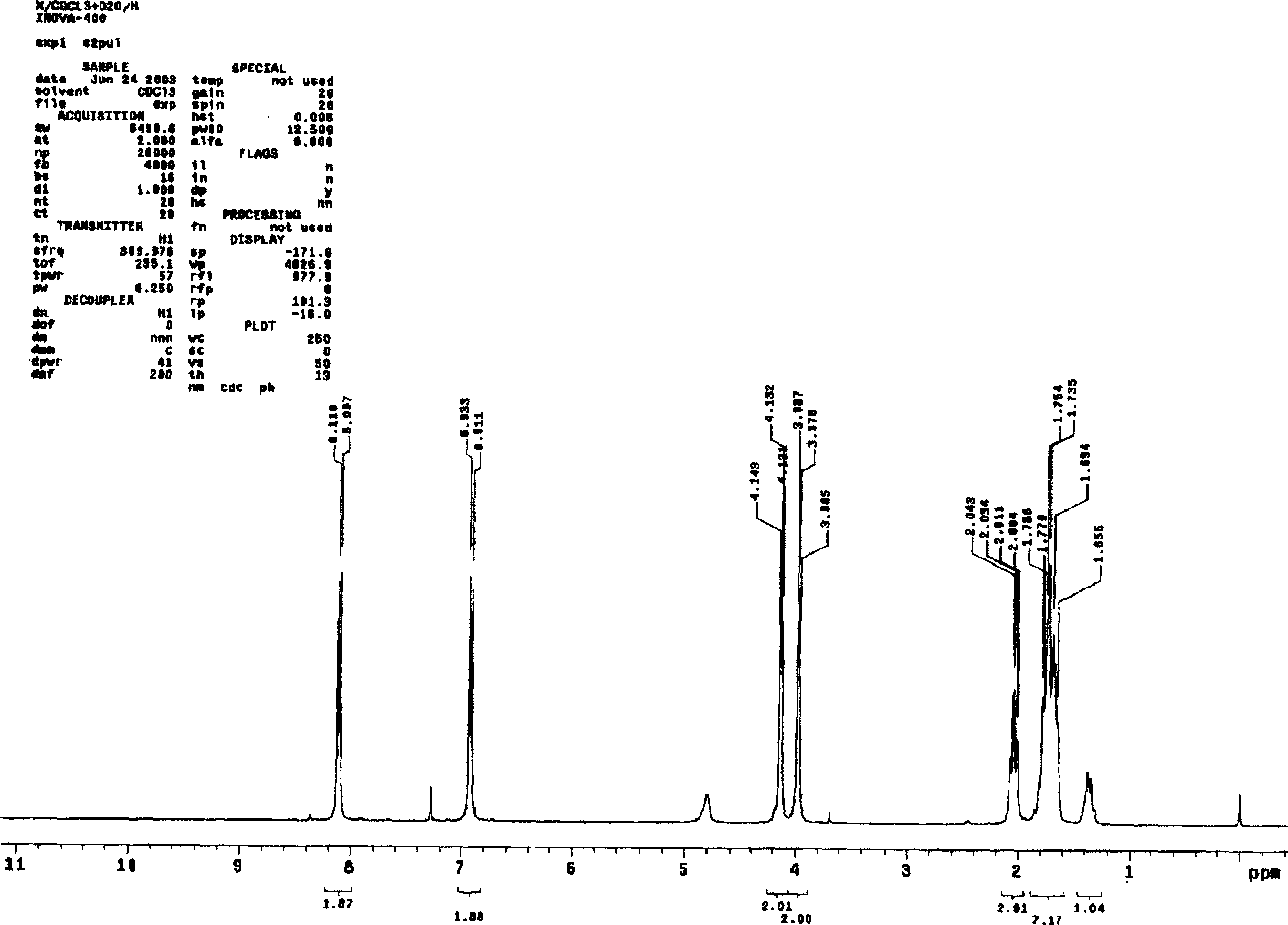
Asymmetric synthesis method for anti-allergy drug carbinoxamine
The invention relates to an asymmetric synthesis method for an anti-allergy drug carbinoxamine. The method specifically comprises the following steps: oxidizing (4-chlorophenyl)(2-pyridyl)ketone to obtain (4-chlorophenyl)(2-pyridyl)ketone-N-oxide; by taking monosulfonylated chiral diamine and metal ruthenium / rhodium / iridium complex as catalysts and sodium formate or a formic acid and triethylamine mixture or isopropanol as a hydrogen source, reducing the (4-chlorophenyl)(2-pyridyl)ketone-N-oxide through asymmetry transfer hydrogenation to prepare (S)-(4-chlorophenyl)(2-pyridyl)methanol-N-oxide; reducing the (S)-(4-chlorophenyl)(2-pyridyl)methanol-N-oxide to obtain (S)-(4-chlorophenyl)(2-pyridyl)methanol; and performing etherification reaction on the (S)-(4-chlorophenyl)(2-pyridyl)methanol and 2-chloro-N,N-dimethylethylamine to obtain the product, wherein the overall yield is 74.6%.
Owner:CHINA THREE GORGES UNIV
Substituted (e)-n'-(1-phenylethylidene) benzohydrazide analogs as histone demethylase inhibitors
Substituted (E)-N-(1-phenylethylidene)benzohydrazide analogs or (3-(5-chloro-2-hydroxyphenyl)-4-METHYL-1 H-pyrazol-1-YL)(3- (morpholinosulfonyl)phenyl)methanone, derivatives thereof, and related compounds are useful as inhibitors of lysine-specific histone demethylase, including LSD1. Synthetic methods for making the compounds; pharmaceutical compositions comprising the compounds; and methods of using the compounds and compositions to treat disorders associated with dysfunction of the LSD1 (lysine-specific demethylase). A pharmaceutically acceptable salt, hydrate, solvate, or polymorph thereof, and one or more of: (a) at least one agent known to increase histone demethylase activity; (b) at least one agent known to decrease histone demethylase activity; (c) at least one agent known to treat a disorder of uncontrolled cellular proliferation; (d) at least one agent known to treat a neurodegenerative disorder; (e) instructions for treating a neurodegenerative disorder; or (f) instructions for treating a disorder associated with uncontrolled cellular proliferation.
Owner:UNIV OF UTAH RES FOUND
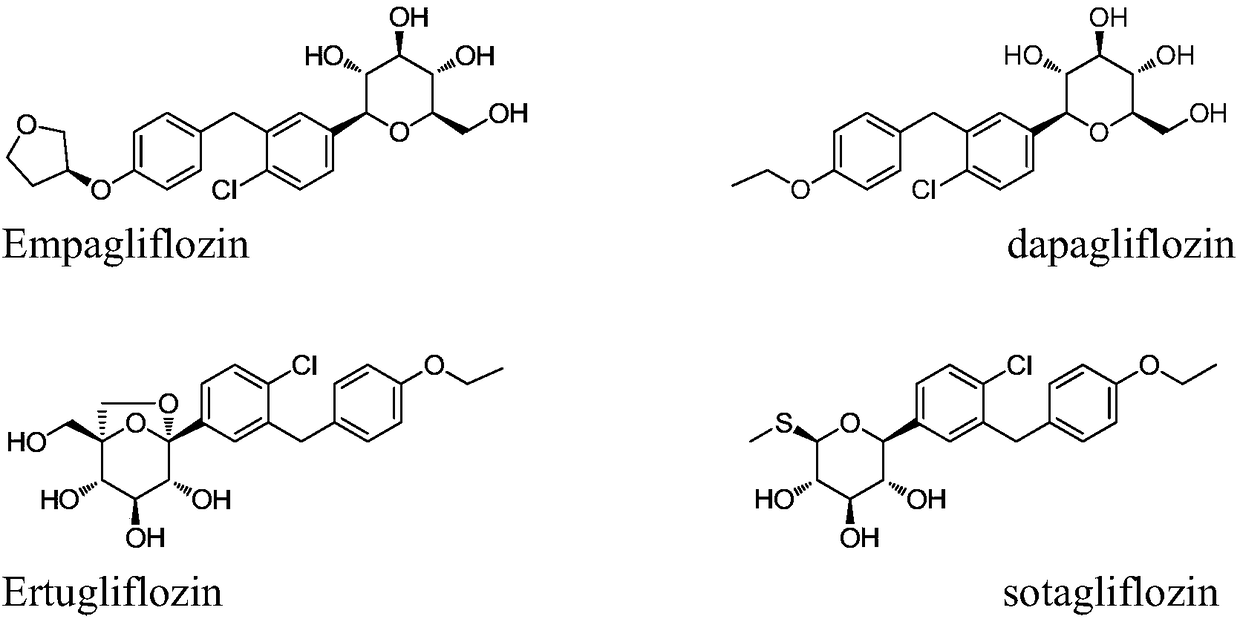
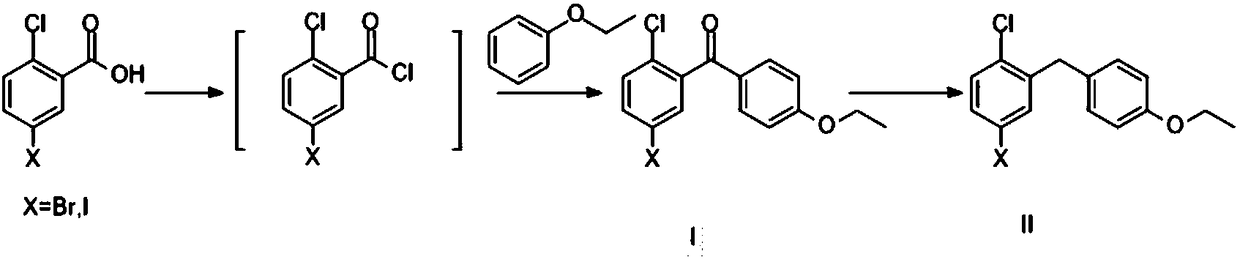
Method for preparing SGLT2 (Sodium-Dependent Glucose Transporters 2) inhibitor intermittent
ActiveCN108752184AReduce usageKOH reductionOrganic compound preparationCarbonyl compound preparation by condensation2-Chlorobenzoic acidKetone
The invention discloses a method for preparing an SGLT2 (Sodium-Dependent Glucose Transporters 2) inhibitor intermittent. The method comprises the following steps: (1) carrying out a Friedel-Crafts reaction on 5-halogen-2-chlorobenzoic acid and fluorobenzene so as to obtain (5-halogen-2-chlorphenyl) (4-fluorophenyl) ketone; (2) under the action of an organic alkali, carrying out a substitution reaction on (5-halogen-2-chlorphenyl) (4-fluorophenyl) ketone and ethanol, and after the reaction is completed, treating so as to obtain (5-halogen-2-chlorphenyl) (4-ethyoxyl phenyl) ketone; (3) carryingout a carbonyl reduction reaction on the (5-halogen-2-chlorphenyl) (4-ethyoxyl phenyl) ketone under the action of a reducing agent, thereby obtaining the SGLT2 inhibitor intermittent. By adopting themethod, an ethoxy metal reagent and DMSO (Dimethyl Sulfoxide) (or DMF (Dimethyl Formamide)) are replaced by the inorganic alkali and ethanol, so that not only is the cost effectively reduced, but also the environment can be protected.
Owner:杭州科耀医药科技有限公司
(4-phenyl-piperidin-1-yl)-[5-(1H-pyrazol-4-yl)-thiophen-3-yl]-methanone compounds and their use
The present invention pertains generally to the field of therapeutic compounds. More specifically the present invention pertains to certain (4-phenyl-piperidin-1-yl)-[5-(1H-pyrazol-4-yl)-thiophen-3-yl]-methanone compounds that, inter alia, inhibit 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1). The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit 11β-hydroxysteroid dehydrogenase type 1; to treat disorders that are ameliorated by the inhibition of 11β-hydroxysteroid dehydrogenase type 1; to treat the metabolic syndrome, which includes disorders such as type 2 diabetes and obesity, and associated disorders including insulin resistance, hypertension, lipid disorders and cardiovascular disorders such as ischaemic (coronary) heart disease; to treat CNS disorders such as mild cognitive impairment and early dementia, including Alzheimer's disease; etc.
Owner:THE UNIV OF EDINBURGH
Photoprotective system
InactiveCN108464951AFlawless application formNon stickyOrganic active ingredientsCosmetic preparationsBenzoic acidEthyl group
The present invention concerns a topical, cosmetic or pharmaceutical preparation containing a combination of 3 or 4 solar filters comprising: -one or two UVA filters to obtain a critical wavelength >370 nm, chosen from among: ( i) - 5, 6, 5, 6-tetraphenyl-3, 3'-(1,4-phenylene)-bis[1, 2, 4]triazine; (ii) 1,1'-(1,4-piperazinediyl)bis[1-[2-[4-(diethylamino)-2-hydroxybenzoyl]phenyl]-methanone; (iii)- Butyl Methoxydibenzoylmethane (BMDBM), in a quantity less than 2% by weight with regard to the total weight of said composition; (iv) - Hexyl -[4-(diethylamino)-2- hydroxybenzoyl]benzoate, -2,4-Bis[4-(2-ethylhexyloxy)-2-hydroxyphenyl]-6- (4-methoxyphenyl)-1, 3, 5-triazine = (BEMT), - one or two selected from the group consisting of diethylhexyl butamido triazone, ethylhexyl triazone, and filtersof tris-biphenyl triazine, ethylhexyl salicylate, phenylbenzimidazole sulfonic acid and TiO2 in an amount of from 3% by weight to 7% by weight relative to the total weight of the composition.
Owner:PIERRE FABRE DERMO COSMETIQUE CORP
Disubstituted [4-(5-aminomethyl-phenyl)-piperidin-1-yl]-1h-indol-3-yl]-methanones
InactiveCN102470132AEasy to understandOrganic active ingredientsSenses disorderPharmaceutical medicineMethylone
Owner:SANOFI SA
Production method of 4-(4-chlorophenoxy)-2-chloro phenyl-methyl ketone
InactiveCN1493557AThe temperature of the reaction condition is loweredReduce manufacturing costOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsP-ChlorophenolMethylone
A process for preparing 4-(4-chlorophenoxy)-2-chlorophenyl-methylone as the intermediate of difenoconazole includes reacting between p-chlorophenol and 2,4-dichloro phenyl ethanone at 100 deg.C under catalysis of copper match, and separating. Its advantages are low reaction temp, and high output rate up to more than 95%.
Owner:JIANGSU CHANGQING AGROCHEMICAL CO LTD
Crystalline form of (s)-(2-(6-chloro-7-methyl-1h-benzo[d]imidazol-2-yl)-2-methylpyrrolidin-1 -yl)(5-methoxy-2-(2h-1,2,3-triazol-2-yl)phenyl)methanone and its use as orexin receptor antagonists
The invention relates to crystalline forms of (S)-(2-(6-chloro-7-methyl-1H-benzo[d]imidazol-2- yl)-2-methylpyrrolidin-1-yl)(5-methoxy-2-(2H-1,2,3-triazol-2-yl)phenyl)methanone, processes for the preparation thereof, pharmaceutical compositions containing such crystalline forms, pharmaceutical compositions prepared from such crystalline forms, and their use as a medicament, especially as orexin receptor antagonists.
Owner:IDORSIA PHARM LTD
Use of (4-hydroxy-2-methyl-1,1-dioxido-2h-benzo[e][1,2]thiazine-3-yl)(naphthalene-2-yl) methanone in the prevention and/or treatment of non-alcoholic steatohepatitis
InactiveCN107530355AOrganic active ingredientsMetabolism disorderAlcoholic steatohepatitisInternal medicine
The present invention relates to (4-hydroxy-2-methyl-1,1-dioxido-2H-benzo [e][1,2]thiazine-3-yl)(naphthalen-2-yl)methanone or one of the salts thereof, pharmaceutically acceptable for use in the prevention and / or treatment of hepatic steatosis, including non-alcoholic steatohepatitis or one of the complications of same.
Owner:PIERRE FABRE MEDICAMENT SAS
New method for preparing 1-(6-methylpyridine-3-yl)-2-[4-(methylsulfanyl)phenyl]acetone
ActiveCN103664754AIncrease hydroxylamine lengthAvoid it happening againOrganic chemistryGrignard reagentKetone
The invention relates to a new method for preparing 1-(6-methylpyridine-3-yl)-2-[4-(methylsulfanyl)phenyl]acetone. The method comprises the step of reacting Grignard reagent of formula (II) with isoxazole-2-yl-(6-methylpyridine-3-yl)-ketone of formula (III) at minus 20 to 0 DEG C to obtain the 1-(6-methylpyridine-3-yl)-2-[4-(methylsulfanyl)phenyl]acetone of formula (I). Compared with the prior art, the method has the advantage of lowering the explosive risk of the reaction system.
Owner:SHANGHAI JIAO TONG UNIV +1
Phenyl-(pyrazolo[1,5-alpha]pyridin-3-yl)methanone derivatives
The invention discloses phenyl-(pyrazolo[1,5-alpha]pyridin-3-yl)methanone derivatives, which are compounds shown as a general formula (I) or pharmaceutically acceptable salts thereof. The compounds orthe pharmaceutically acceptable salts thereof can be applied to uric acid excretion promotion for treating or preventing hyperuricemia and gout, or can be used for treating diabetes.
Owner:JIANGSU ATOM BIOSCI & PHARMA CO LTD
Phenyl-3-aza-bicyclo[3.1.0]hex-3-yl-methanones and the use thereof as medicament
Substituted phenyl-3-aza-bicyclo[3.1.0]hex-3-yl-methanones which are glycine transporter-1 (GlyT1) inhibitors. These are useful for the treatment of schizophrenia, Alzheimer's Disease and other neurological and psychiatric disorders.
Owner:BOEHRINGER INGELHEIM INT GMBH
Substituted (e)-n'-(1-phenylethylidene)benzohydrazide analogs as histone demethylase inhibitors
Substituted (E)‑N'‑(1‑phenylethylidene)phenylhydrazide analogs or (3‑(5‑chloro‑2‑hydroxyphenyl)‑4‑methyl‑1H‑pyrazole -1-yl)(3-(morpholinosulfonyl)phenyl)methanone, derivatives thereof, and related compounds are useful as inhibitors of lysine-specific histone demethylases including LSD1 of. Synthetic methods of making the compounds; pharmaceutical compositions comprising the compounds; methods of using the compounds and compositions to treat disorders associated with LSD1 (lysine specific demethylase) dysfunction. A pharmaceutically acceptable salt, hydrate, solvate or polymorph thereof, and one or more of the following: (a) at least one agent known to increase the activity of histone demethylase; ( b) at least one agent known to reduce the activity of histone demethylases; (c) at least one agent known to treat disorders of uncontrolled cell proliferation; (d) at least one agent known to treat neurodegeneration (e) instructions for treating neurodegenerative disorders; or (f) instructions for treating disorders associated with uncontrolled cell proliferation.
Owner:UNIV OF UTAH RES FOUND
Preparation method of (4,6-diaryl-tetrahydropyridine-3-yl)(aryl) ketone
The invention relates to a preparation method of (4,6-diaryl-tetrahydropyridine-3-yl)(aryl)ketone. The method includes the following steps that (1) aryl propargyl alcohol, chalcone derivatives, acid and a solvent are mixed and heated for reaction under reflux conditions; (2) amine compounds are added into a reaction system where the reaction is completed for reaction for 9-17 hours under reflux conditions, and the (4,6-diaryl-tetrahydropyridine-3-yl)(aryl)ketone is obtained. Compared with the prior art, the synthesis method has the advantages that the method is simple, the condition is mild, the yield is relatively high, and the atom utilization rate can reach 100%. The synthesis method greatly optimizes synthesis of the compound and provides a new synthesis idea for the synthesis of compounds with similar structures.
Owner:SHANGHAI INST OF TECH
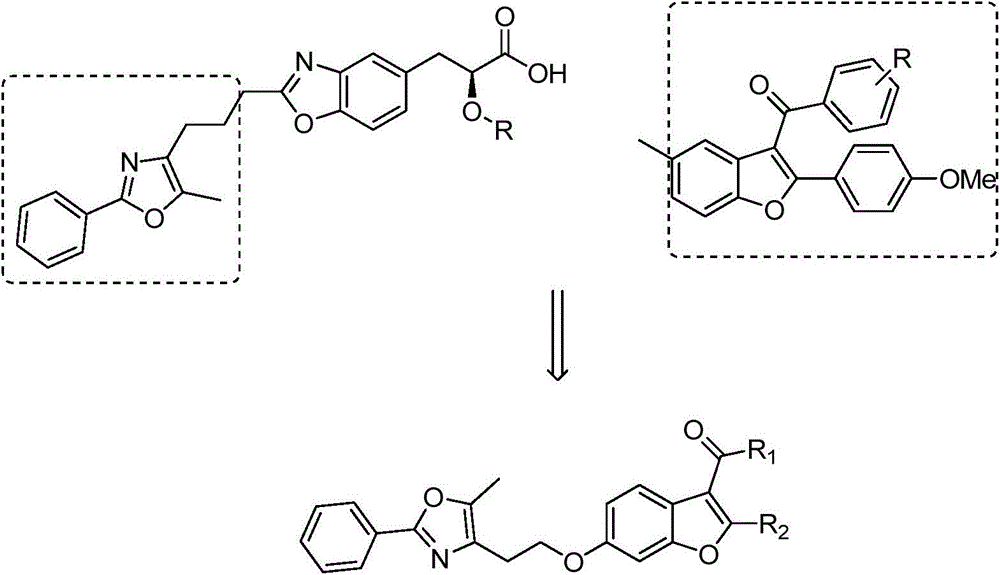
2-substituted-3-arylketone-6-(5-methyl-2-phenyl-4-ehtyoxyloxazole)benzofuran compound
InactiveCN103588763AEstablish and optimize preparation methodsEasy to prepareAntibacterial agentsOrganic active ingredientsKetoneAntibacterial activity
The invention relates to a 2-substituted-3-arylketone-6-(5-methyl-2-phenyl-4-ehtyoxyloxazole)benzofuran compound with the structural formula as shown in the specification. Compared with the prior art, the novel compound is prepared by introducing (5-methyl-2-phenyloxaole-4-yl)ethyoxyl to the 6 site of benzofuran by taking 3-ketone substituent benzofuran as the center of an aromatic ring; a preparation method of the compound is established and optimized; the prepared novel compound is subjected to an antibacterial screening experiment; the primary in-vitro antibacterial experiment confirms that the prepared novel compound has excellent broad-spectrum antibacterial activity and can be used for preparing novel antibacterial drugs.
Owner:SHANGHAI JIAO TONG UNIV
Crystals of [2-(1-methyl-1H-pyrazol-4-yl)-6(morpholin-4-yl)-9H-purin-8-yl][4-(morpholin-4-yl)piperidin-1-yl]methanone and pharmaceutically acceptable salt thereof
ActiveUS10822336B2Good storage stabilitySatisfactory physical propertyOrganic active ingredientsAntipyreticMorpholinePurine
Provided are novel crystals of [2-(1-methyl-1H-pyrazol-4-yl)-6-(morpholin-4-yl)-9H-purin-8-yl][4-(morpholin-4-yl)piperidin-1-yl]methanone and a pharmaceutically acceptable salt thereof having advantageous characteristics. [2-(1-Methyl-1H-pyrazol-4-yl)-6-(morpholin-4-yl)-9H-purin-8-yl][4-(morpholin-4-yl)piperidin-1-yl]methanone and a pharmaceutically acceptable salt thereof provided by the present invention are excellent in stability and have satisfactory physical properties as a drug substance of a pharmaceutical product.
Owner:DAIICHI SANKYO CO LTD
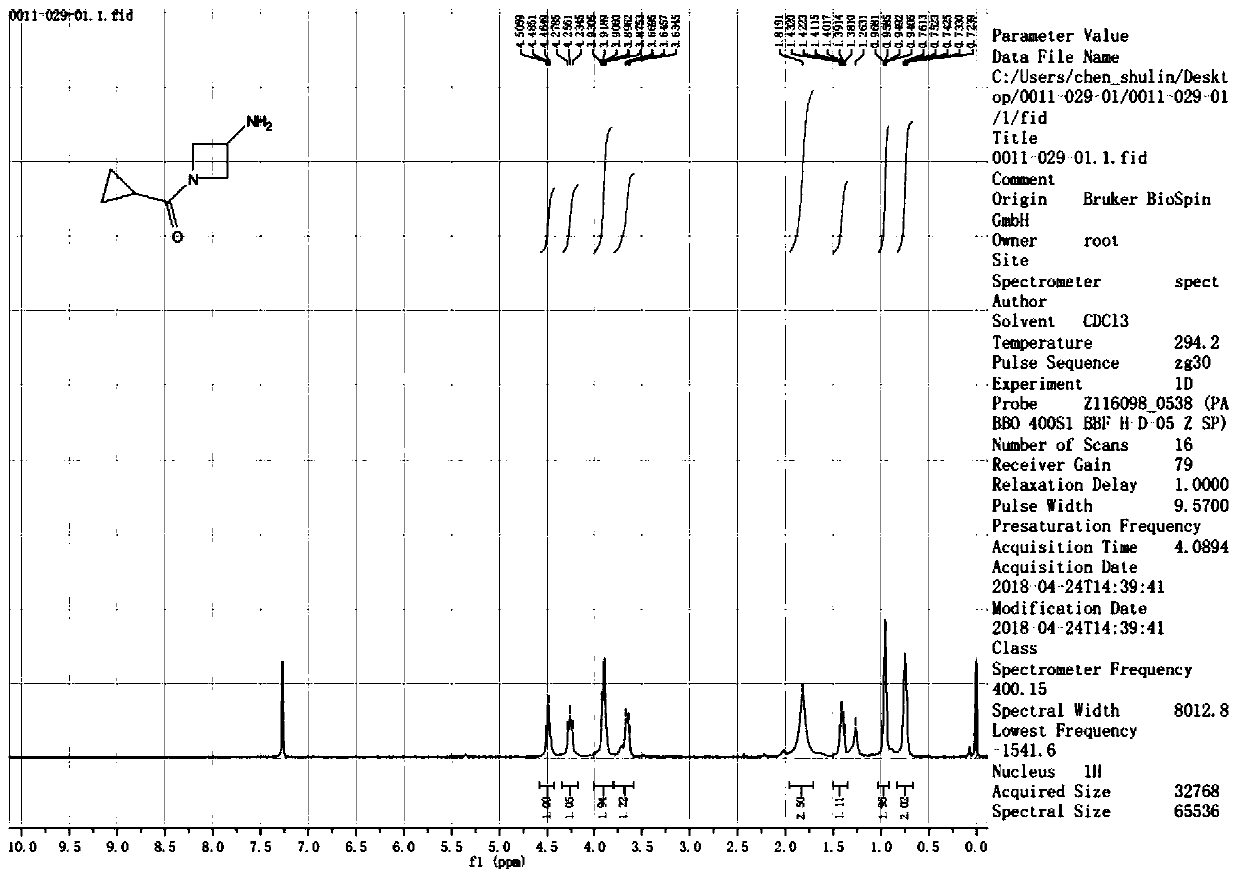
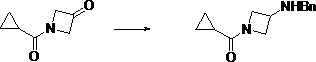
Preparation method of (3-amino-azetidine-1-yl)-cyclopropyl-ketone
The invention discloses a preparation method of (3-amino-azetidine-1-yl)-cyclopropyl-ketone. The preparation method comprises the following steps: dissolving azetidine-3-ketone hydrochloride in a solvent under an alkaline condition, adding an acylation reagent for reaction to obtain 1-cyclopropanecarbonyl-azetidine-3-ketone; dissolving the 1-cyclopropanecarbonyl-azetidine-3-ketone in a solvent, adding benzylamine, stirring and dissolving the benzylamine, and adding a reducing agent for reaction at-5-5 DEG C to obtain (3-benzylamino-azetidine-1-yl)-cyclopropyl-ketone; dissolving 3-boroamino-azetidine-1-yl)-cyclopropyl-ketone in a solvent to react with a reducing agent in the presence of a catalyst to obtain (3-amino-azetidine-1-yl)-cyclopropyl-ketone. The raw materials used in the preparation method provided by the invention are low in price and wide in source, six steps of reaction of an original synthesis method are reduced to three steps of reaction, dangerous reagents are not used,reaction steps are shortened, and reaction efficiency is improved.
Owner:南京合巨药业有限公司
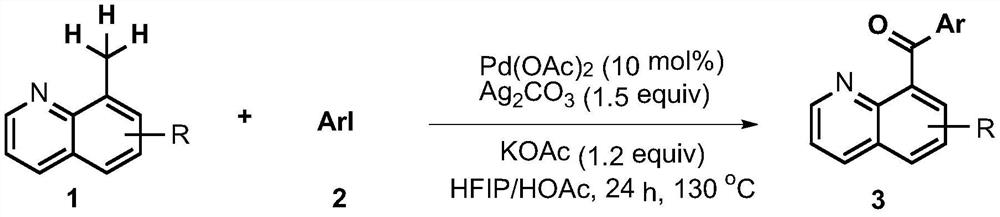
Synthesis method of phenyl(quinolin-8-yl)-one derivative
A synthesis method of a phenyl(quinolin-8-yl)-one derivative comprises the following steps: by taking 8-methylquinoline or substituted 8-methylquinoline and aryl iodide as raw materials, Ag2CO3 as an additive, KOAc as alkali and Pd(OAc)2 as a catalyst, reacting the raw materials in a solution formed by mixing hexafluoroisopropanol and glacial acetic acid according to a volume ratio of 3: 7, sealing a tube at 130 DEG C, and reacting for 24 hours to generate the target product. The synthesis process route is simple, and the highest yield reaches 87%.
Owner:XUZHOU NORMAL UNIVERSITY
3,4-methylenedioxy methcathinone detection methods
InactiveCN110346470AFast waySimple methodComponent separationGas chromatography flame ionization detectorDischarge ionization detector
The invention discloses 3,4-methylenedioxy methcathinone detection methods. One method in the invention performs organic solvent extraction on Methylone in a suspected drug sample, performs qualitative analysis by using gas chromatography-mass spectrography (GC-MS), and performs quantitative analysis on Methylone by using a gas chromatography-flame ionization detector (GC-FID). The other method inthe method performs organic solvent extraction on Methylone in the suspected drug sample, performs qualitative analysis in a multi-reaction monitoring (MRM) mode by using a liquid chromatography-tandem mass spectrometry (LC-MS / MS), and performs quantitative analysis on Methylone by using a liquid chromatography-diode array or ultraviolet detector (LC-DVD) or LC-UV).
Owner:INST OF FORENSIC SCI OF MIN OF PUBLIC SECURITY
Production method of 4-(4-chlorophenoxy)-2-chloro phenyl-methyl ketone
InactiveCN100358855CThe temperature of the reaction condition is loweredReduce manufacturing costOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsChlorobenzeneP-Chlorophenol
A process for preparing 4-(4-chlorophenoxy)-2-chlorophenyl-methylone as the intermediate of difenoconazole includes reacting between p-chlorophenol and 2,4-dichloro phenyl ethanone at 100 deg.C under catalysis of copper match, and separating. Its advantages are low reaction temp, and high output rate up to more than 95%.
Owner:JIANGSU CHANGQING AGROCHEMICAL CO LTD
Preparation of 2-([1,2,3]triazol-2-yl)-benzoic acid derivatives
PendingCN110612291AOrganic chemistry methodsMetal/metal-oxides/metal-hydroxide catalystsBenzoic acidPerylene derivatives
The present invention relates to a process for the preparation of particular 2-(2H-[1,2,3]triazol-2-yl)-benzoic acid derivatives of formula (I), to certain crystalline forms of potassium salts of said2-(2H-[1,2,3]triazol-2-yl)-benzoic acid derivatives of formula (IK), to certain crystalline forms of said 2-(2H-[1,2,3]triazol-2-yl)-benzoic acid derivatives of formula (I), and to their use in the preparation of pharmaceuticals such as (S)-(2-(5-chloro-4-methyl-1H-benzo[d]imidazol-2-yl)-2-methylpyrrolidin-1-yl)-(5-methoxy-2-(2H-1,2,3-triazol-2-yl)phenyl)methanone.
Owner:IDORSIA PHARM LTD
Application of substituted piperazine compound in preparation of medicament for resisting fungal infection
InactiveCN103006659BGrowth inhibitionConvenient sourceOrganic active ingredientsAntimycoticsDiseaseMethylone
The invention relates to the application of a substituted piperazine compound in the preparation of a medicament for resisting fungal infection and particularly discloses a compound which is (4-chlorophenyl)-(piperazin-1-yl)-methanone or pharmaceutically acceptable salt thereof as well as a preparation method and medicinal application of the (4-chlorophenyl)-(piperazin-1-yl)-methanone or medicinally acceptable salt thereof. The steps for synthesizing the compound are simple, the method for preparing the compound is simple and practical, the sources of raw materials are easy to obtain, the cost is low, and less pollution is caused. The compound has an obvious activity of inhibiting the growth of fungi and has the MIC value of 62.5mcg / ml. The pharmacodynamic results show that the substituted piperazine compound or medicinally acceptable salt is expected to be used to prepare the medicament for preventing and treating the diseases caused by fungal infection.
Owner:DALI UNIV
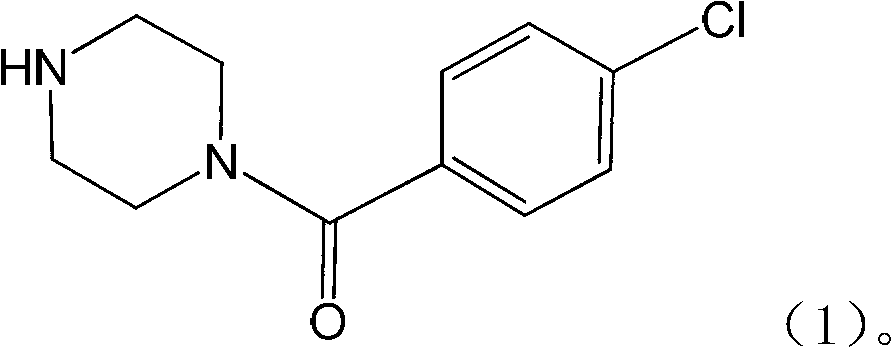
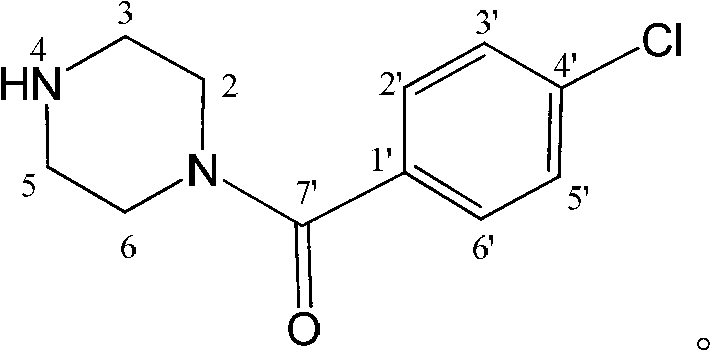
Application of substituted piperazine compound in preparation of medicament for resisting fungal infection
InactiveCN103006659AGrowth inhibitionConvenient sourceOrganic active ingredientsAntimycoticsDiseasePhenyl group
The invention relates to the application of a substituted piperazine compound in the preparation of a medicament for resisting fungal infection and particularly discloses a compound which is (4-chlorophenyl)-(piperazin-1-yl)-methanone or pharmaceutically acceptable salt thereof as well as a preparation method and medicinal application of the (4-chlorophenyl)-(piperazin-1-yl)-methanone or medicinally acceptable salt thereof. The steps for synthesizing the compound are simple, the method for preparing the compound is simple and practical, the sources of raw materials are easy to obtain, the cost is low, and less pollution is caused. The compound has an obvious activity of inhibiting the growth of fungi and has the MIC value of 62.5mcg / ml. The pharmacodynamic results show that the substituted piperazine compound or medicinally acceptable salt is expected to be used to prepare the medicament for preventing and treating the diseases caused by fungal infection.
Owner:DALI UNIV
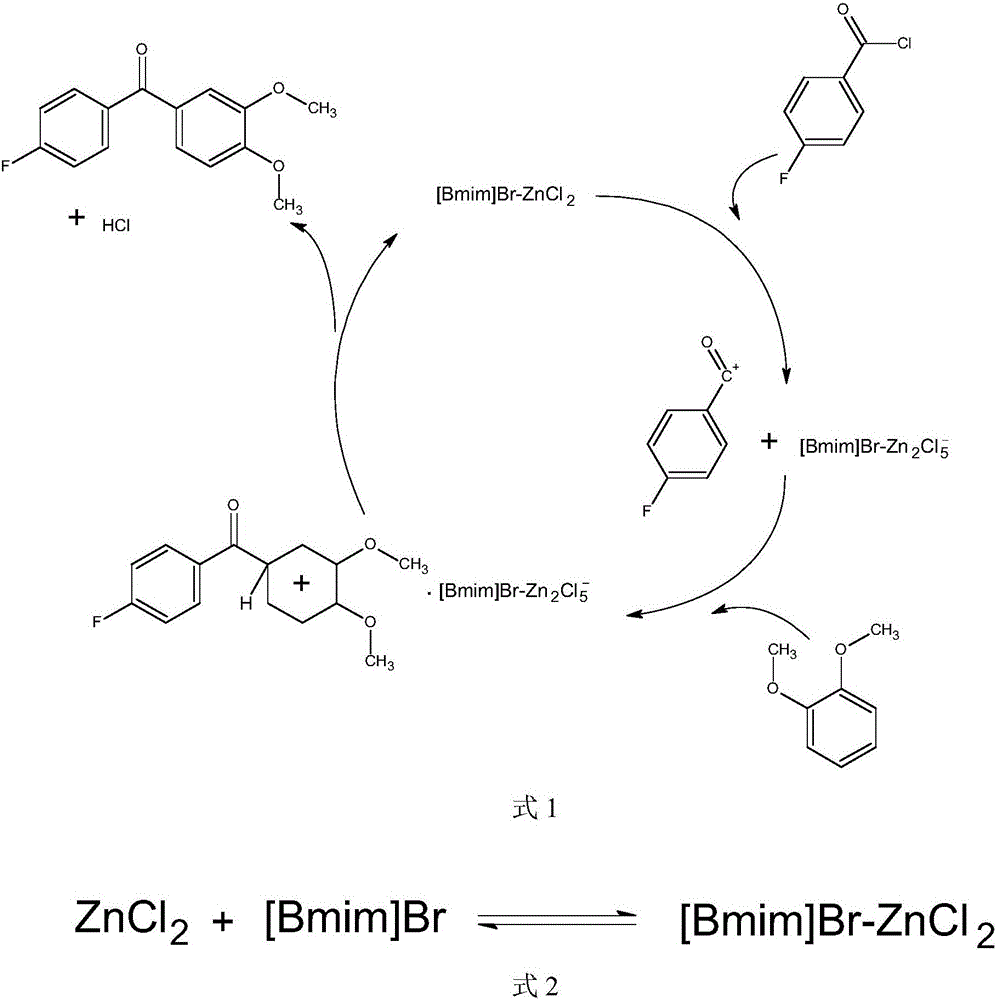
Synthesis method of (3,4-dimethoxyphenyl)(4-p henyl fluoride) ketone
InactiveCN106699529AEmission reductionReduce pollutionCarbonyl compound preparation by condensationSynthesis methodsSlag
The invention relates to a synthesis method of (3,4-dimethoxyphenyl)(4-p henyl fluoride) ketone, and solves the problem that in the prior art, the synthesis method brings large amout of pollution and the cost is high. According to the method, acidic ionic liquid is applied as the catalyst which is polymerized by an ionic liquid precursor containing positive electricity and metal halides with negative electricity, wherein, the ionic liquid precursor is [Bmim] C1 or [Bmim] Br, the metal halide is AlCl3, FeC13, ZnCl2 or CuCl2. The synthesis method of (3,4-dimethoxyphenyl)(4-p henyl fluoride) ketone has the advantages of largely reducing the use amout of catalysts, lowering the emission of the waste water, saving the neutralization process of sulfuric acid, eliminating the waste water generated in the step, avoiding the corrosion caused by strong acid. Compared with the original reaction, the emission of slag is omitted. The reaction product is easy to be separated. The post-treatment is simpler. The reaction temperature is low. The energy cost is low. The product yield rate can reach as high as 97% or higher.
Owner:BEIJING UNIV OF CHEM TECH
2-Substituted-3-arylmethanone-6-(5-methyl-2-phenyl-4-ethoxyoxazole)benzofuran compound
InactiveCN103588763BEstablish and optimize preparation methodsEasy to prepareAntibacterial agentsOrganic active ingredientsAntibacterial activityKetone
The invention relates to a 2-substituted-3-arylketone-6-(5-methyl-2-phenyl-4-ehtyoxyloxazole)benzofuran compound with the structural formula as shown in the specification. Compared with the prior art, the novel compound is prepared by introducing (5-methyl-2-phenyloxaole-4-yl)ethyoxyl to the 6 site of benzofuran by taking 3-ketone substituent benzofuran as the center of an aromatic ring; a preparation method of the compound is established and optimized; the prepared novel compound is subjected to an antibacterial screening experiment; the primary in-vitro antibacterial experiment confirms that the prepared novel compound has excellent broad-spectrum antibacterial activity and can be used for preparing novel antibacterial drugs.
Owner:SHANGHAI JIAO TONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![2-pyridinyl[7-(substituted-pyridin-4-yl) pyrazolo[1,5-a]pyrimidin-3-yl]methanones 2-pyridinyl[7-(substituted-pyridin-4-yl) pyrazolo[1,5-a]pyrimidin-3-yl]methanones](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d5295254-0381-4a85-b180-3ea833a7a1eb/US20050245517A1-20051103-C00001.png)
![2-pyridinyl[7-(substituted-pyridin-4-yl) pyrazolo[1,5-a]pyrimidin-3-yl]methanones 2-pyridinyl[7-(substituted-pyridin-4-yl) pyrazolo[1,5-a]pyrimidin-3-yl]methanones](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d5295254-0381-4a85-b180-3ea833a7a1eb/US20050245517A1-20051103-C00002.png)
![2-pyridinyl[7-(substituted-pyridin-4-yl) pyrazolo[1,5-a]pyrimidin-3-yl]methanones 2-pyridinyl[7-(substituted-pyridin-4-yl) pyrazolo[1,5-a]pyrimidin-3-yl]methanones](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d5295254-0381-4a85-b180-3ea833a7a1eb/US20050245517A1-20051103-C00003.png)
![[4-(5-aminomethyl-2-fluoro-phenyl)-piperidin-1-yl]-[7-fluoro-1-(2-methoxy-ethyl)-4-trifluoromethoxy-1h-indol-3-yl]-methanone as an inhibitor of mast cell tryptase [4-(5-aminomethyl-2-fluoro-phenyl)-piperidin-1-yl]-[7-fluoro-1-(2-methoxy-ethyl)-4-trifluoromethoxy-1h-indol-3-yl]-methanone as an inhibitor of mast cell tryptase](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c1d2ed5a-8f5b-4813-997c-fef82533a849/US20110201647A1-20110818-D00001.png)
![[4-(5-aminomethyl-2-fluoro-phenyl)-piperidin-1-yl]-[7-fluoro-1-(2-methoxy-ethyl)-4-trifluoromethoxy-1h-indol-3-yl]-methanone as an inhibitor of mast cell tryptase [4-(5-aminomethyl-2-fluoro-phenyl)-piperidin-1-yl]-[7-fluoro-1-(2-methoxy-ethyl)-4-trifluoromethoxy-1h-indol-3-yl]-methanone as an inhibitor of mast cell tryptase](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c1d2ed5a-8f5b-4813-997c-fef82533a849/US20110201647A1-20110818-D00002.png)
![[4-(5-aminomethyl-2-fluoro-phenyl)-piperidin-1-yl]-[7-fluoro-1-(2-methoxy-ethyl)-4-trifluoromethoxy-1h-indol-3-yl]-methanone as an inhibitor of mast cell tryptase [4-(5-aminomethyl-2-fluoro-phenyl)-piperidin-1-yl]-[7-fluoro-1-(2-methoxy-ethyl)-4-trifluoromethoxy-1h-indol-3-yl]-methanone as an inhibitor of mast cell tryptase](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c1d2ed5a-8f5b-4813-997c-fef82533a849/US20110201647A1-20110818-C00001.png)








![(4-phenyl-piperidin-1-yl)-[5-(1H-pyrazol-4-yl)-thiophen-3-yl]-methanone compounds and their use (4-phenyl-piperidin-1-yl)-[5-(1H-pyrazol-4-yl)-thiophen-3-yl]-methanone compounds and their use](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6bf0a989-bb3d-45b0-9cbb-64f93c11bc1e/US08642621-20140204-C00001.png)
![(4-phenyl-piperidin-1-yl)-[5-(1H-pyrazol-4-yl)-thiophen-3-yl]-methanone compounds and their use (4-phenyl-piperidin-1-yl)-[5-(1H-pyrazol-4-yl)-thiophen-3-yl]-methanone compounds and their use](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6bf0a989-bb3d-45b0-9cbb-64f93c11bc1e/US08642621-20140204-C00002.png)
![(4-phenyl-piperidin-1-yl)-[5-(1H-pyrazol-4-yl)-thiophen-3-yl]-methanone compounds and their use (4-phenyl-piperidin-1-yl)-[5-(1H-pyrazol-4-yl)-thiophen-3-yl]-methanone compounds and their use](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6bf0a989-bb3d-45b0-9cbb-64f93c11bc1e/US08642621-20140204-C00003.png)


![Disubstituted [4-(5-aminomethyl-phenyl)-piperidin-1-yl]-1h-indol-3-yl]-methanones Disubstituted [4-(5-aminomethyl-phenyl)-piperidin-1-yl]-1h-indol-3-yl]-methanones](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0c63ccae-6e5a-4d24-819b-d0fbf898f99a/BDA0000136712300000021.PNG)
![Disubstituted [4-(5-aminomethyl-phenyl)-piperidin-1-yl]-1h-indol-3-yl]-methanones Disubstituted [4-(5-aminomethyl-phenyl)-piperidin-1-yl]-1h-indol-3-yl]-methanones](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0c63ccae-6e5a-4d24-819b-d0fbf898f99a/BDA0000136712300000031.PNG)
![Disubstituted [4-(5-aminomethyl-phenyl)-piperidin-1-yl]-1h-indol-3-yl]-methanones Disubstituted [4-(5-aminomethyl-phenyl)-piperidin-1-yl]-1h-indol-3-yl]-methanones](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0c63ccae-6e5a-4d24-819b-d0fbf898f99a/BDA0000136712300000032.PNG)

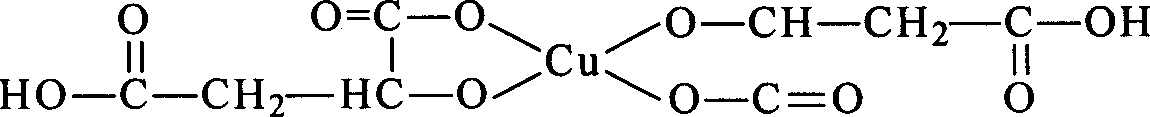

![Crystalline form of (s)-(2-(6-chloro-7-methyl-1h-benzo[d]imidazol-2-yl)-2-methylpyrrolidin-1 -yl)(5-methoxy-2-(2h-1,2,3-triazol-2-yl)phenyl)methanone and its use as orexin receptor antagonists Crystalline form of (s)-(2-(6-chloro-7-methyl-1h-benzo[d]imidazol-2-yl)-2-methylpyrrolidin-1 -yl)(5-methoxy-2-(2h-1,2,3-triazol-2-yl)phenyl)methanone and its use as orexin receptor antagonists](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c68bd4d8-0f9f-4a80-b47a-ece6cb049e02/HDA0001003418360000011.PNG)
![Crystalline form of (s)-(2-(6-chloro-7-methyl-1h-benzo[d]imidazol-2-yl)-2-methylpyrrolidin-1 -yl)(5-methoxy-2-(2h-1,2,3-triazol-2-yl)phenyl)methanone and its use as orexin receptor antagonists Crystalline form of (s)-(2-(6-chloro-7-methyl-1h-benzo[d]imidazol-2-yl)-2-methylpyrrolidin-1 -yl)(5-methoxy-2-(2h-1,2,3-triazol-2-yl)phenyl)methanone and its use as orexin receptor antagonists](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c68bd4d8-0f9f-4a80-b47a-ece6cb049e02/HDA0001003418360000012.PNG)
![Crystalline form of (s)-(2-(6-chloro-7-methyl-1h-benzo[d]imidazol-2-yl)-2-methylpyrrolidin-1 -yl)(5-methoxy-2-(2h-1,2,3-triazol-2-yl)phenyl)methanone and its use as orexin receptor antagonists Crystalline form of (s)-(2-(6-chloro-7-methyl-1h-benzo[d]imidazol-2-yl)-2-methylpyrrolidin-1 -yl)(5-methoxy-2-(2h-1,2,3-triazol-2-yl)phenyl)methanone and its use as orexin receptor antagonists](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c68bd4d8-0f9f-4a80-b47a-ece6cb049e02/HDA0001003418360000021.PNG)
![Use of (4-hydroxy-2-methyl-1,1-dioxido-2h-benzo[e][1,2]thiazine-3-yl)(naphthalene-2-yl) methanone in the prevention and/or treatment of non-alcoholic steatohepatitis Use of (4-hydroxy-2-methyl-1,1-dioxido-2h-benzo[e][1,2]thiazine-3-yl)(naphthalene-2-yl) methanone in the prevention and/or treatment of non-alcoholic steatohepatitis](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d1b87a92-4a0f-41bc-a597-aa3d62101de9/HDA0001440190770000011.png)
![Use of (4-hydroxy-2-methyl-1,1-dioxido-2h-benzo[e][1,2]thiazine-3-yl)(naphthalene-2-yl) methanone in the prevention and/or treatment of non-alcoholic steatohepatitis Use of (4-hydroxy-2-methyl-1,1-dioxido-2h-benzo[e][1,2]thiazine-3-yl)(naphthalene-2-yl) methanone in the prevention and/or treatment of non-alcoholic steatohepatitis](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d1b87a92-4a0f-41bc-a597-aa3d62101de9/HDA0001440190770000021.png)
![Use of (4-hydroxy-2-methyl-1,1-dioxido-2h-benzo[e][1,2]thiazine-3-yl)(naphthalene-2-yl) methanone in the prevention and/or treatment of non-alcoholic steatohepatitis Use of (4-hydroxy-2-methyl-1,1-dioxido-2h-benzo[e][1,2]thiazine-3-yl)(naphthalene-2-yl) methanone in the prevention and/or treatment of non-alcoholic steatohepatitis](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d1b87a92-4a0f-41bc-a597-aa3d62101de9/HDA0001440190770000031.png)
![New method for preparing 1-(6-methylpyridine-3-yl)-2-[4-(methylsulfanyl)phenyl]acetone New method for preparing 1-(6-methylpyridine-3-yl)-2-[4-(methylsulfanyl)phenyl]acetone](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d8feac81-880e-4ee2-a233-a2d4d3a5861b/BDA0000410787320000011.PNG)
![New method for preparing 1-(6-methylpyridine-3-yl)-2-[4-(methylsulfanyl)phenyl]acetone New method for preparing 1-(6-methylpyridine-3-yl)-2-[4-(methylsulfanyl)phenyl]acetone](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d8feac81-880e-4ee2-a233-a2d4d3a5861b/BDA0000410787320000012.PNG)
![New method for preparing 1-(6-methylpyridine-3-yl)-2-[4-(methylsulfanyl)phenyl]acetone New method for preparing 1-(6-methylpyridine-3-yl)-2-[4-(methylsulfanyl)phenyl]acetone](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d8feac81-880e-4ee2-a233-a2d4d3a5861b/BDA0000410787320000013.PNG)
![Phenyl-(pyrazolo[1,5-alpha]pyridin-3-yl)methanone derivatives Phenyl-(pyrazolo[1,5-alpha]pyridin-3-yl)methanone derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5f4b8a0f-550d-4275-af9c-99a6a57218e8/FDA0001383354120000011.png)
![Phenyl-(pyrazolo[1,5-alpha]pyridin-3-yl)methanone derivatives Phenyl-(pyrazolo[1,5-alpha]pyridin-3-yl)methanone derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5f4b8a0f-550d-4275-af9c-99a6a57218e8/FDA0001383354120000012.png)
![Phenyl-(pyrazolo[1,5-alpha]pyridin-3-yl)methanone derivatives Phenyl-(pyrazolo[1,5-alpha]pyridin-3-yl)methanone derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5f4b8a0f-550d-4275-af9c-99a6a57218e8/BDA0001383354130000041.png)
![Phenyl-3-aza-bicyclo[3.1.0]hex-3-yl-methanones and the use thereof as medicament Phenyl-3-aza-bicyclo[3.1.0]hex-3-yl-methanones and the use thereof as medicament](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/786369a2-125a-46ac-90a9-6b57c9761eb1/US20130197011A1-20130801-C00001.png)
![Phenyl-3-aza-bicyclo[3.1.0]hex-3-yl-methanones and the use thereof as medicament Phenyl-3-aza-bicyclo[3.1.0]hex-3-yl-methanones and the use thereof as medicament](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/786369a2-125a-46ac-90a9-6b57c9761eb1/US20130197011A1-20130801-C00002.png)
![Phenyl-3-aza-bicyclo[3.1.0]hex-3-yl-methanones and the use thereof as medicament Phenyl-3-aza-bicyclo[3.1.0]hex-3-yl-methanones and the use thereof as medicament](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/786369a2-125a-46ac-90a9-6b57c9761eb1/US20130197011A1-20130801-C00003.png)
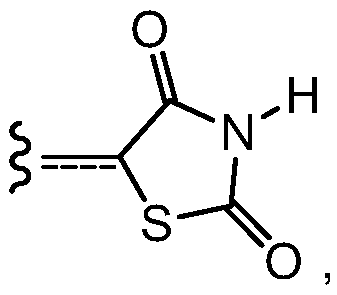
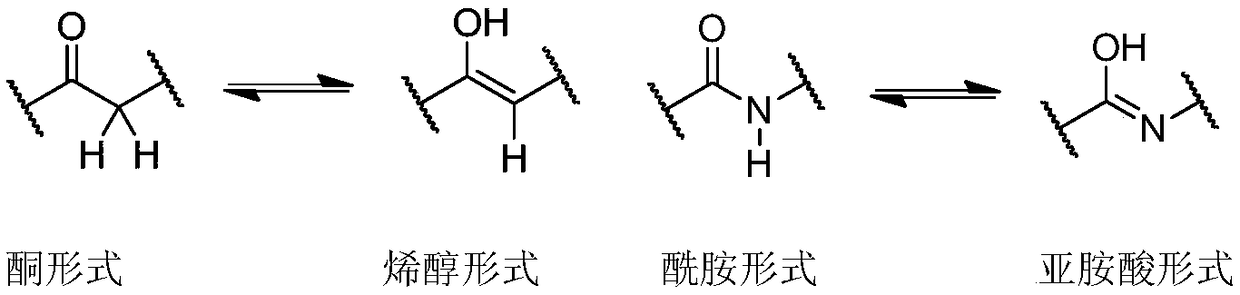






![Crystals of [2-(1-methyl-1H-pyrazol-4-yl)-6(morpholin-4-yl)-9H-purin-8-yl][4-(morpholin-4-yl)piperidin-1-yl]methanone and pharmaceutically acceptable salt thereof Crystals of [2-(1-methyl-1H-pyrazol-4-yl)-6(morpholin-4-yl)-9H-purin-8-yl][4-(morpholin-4-yl)piperidin-1-yl]methanone and pharmaceutically acceptable salt thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/07ba5625-4d97-4ac9-8323-cb8fe3970087/US10822336-D00001.png)
![Crystals of [2-(1-methyl-1H-pyrazol-4-yl)-6(morpholin-4-yl)-9H-purin-8-yl][4-(morpholin-4-yl)piperidin-1-yl]methanone and pharmaceutically acceptable salt thereof Crystals of [2-(1-methyl-1H-pyrazol-4-yl)-6(morpholin-4-yl)-9H-purin-8-yl][4-(morpholin-4-yl)piperidin-1-yl]methanone and pharmaceutically acceptable salt thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/07ba5625-4d97-4ac9-8323-cb8fe3970087/US10822336-D00002.png)
![Crystals of [2-(1-methyl-1H-pyrazol-4-yl)-6(morpholin-4-yl)-9H-purin-8-yl][4-(morpholin-4-yl)piperidin-1-yl]methanone and pharmaceutically acceptable salt thereof Crystals of [2-(1-methyl-1H-pyrazol-4-yl)-6(morpholin-4-yl)-9H-purin-8-yl][4-(morpholin-4-yl)piperidin-1-yl]methanone and pharmaceutically acceptable salt thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/07ba5625-4d97-4ac9-8323-cb8fe3970087/US10822336-D00003.png)







![Preparation of 2-([1,2,3]triazol-2-yl)-benzoic acid derivatives Preparation of 2-([1,2,3]triazol-2-yl)-benzoic acid derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2e02e7cc-0bc9-4346-9571-8a6dec68fa89/HDA0002255090390000011.png)
![Preparation of 2-([1,2,3]triazol-2-yl)-benzoic acid derivatives Preparation of 2-([1,2,3]triazol-2-yl)-benzoic acid derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2e02e7cc-0bc9-4346-9571-8a6dec68fa89/HDA0002255090390000012.png)
![Preparation of 2-([1,2,3]triazol-2-yl)-benzoic acid derivatives Preparation of 2-([1,2,3]triazol-2-yl)-benzoic acid derivatives](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2e02e7cc-0bc9-4346-9571-8a6dec68fa89/HDA0002255090390000021.png)