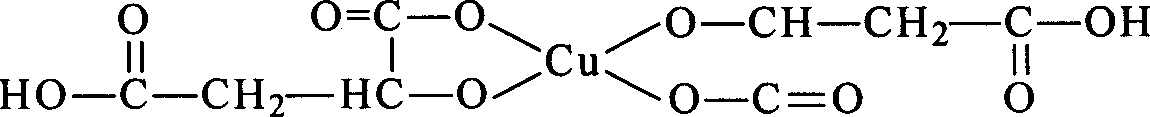Production method of 4-(4-chlorophenoxy)-2-chloro phenyl-methyl ketone
A technology for the production of chlorophenoxy, which is applied in the field of intermediate synthesis of the agricultural fungicide oxafeconazole, can solve the problems of long reaction cycle and high efficiency, and achieve the effects of reducing production costs, reducing energy, and improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] In a 250 ml reaction flask equipped with stirring, a thermometer, a reflux condenser and a dropping funnel, add 13.0 g (0.10 mole) of p-chlorophenol, 100 ml of xylene, and add 13.4 g (0.12 mole) of 50% potassium hydroxide aqueous solution dropwise at room temperature. ), after the dropwise addition, heat up and reflux to divide the water, and after the moisture is exhausted, decompress and go out xylene, drop into 80ml dimethyl sulfoxide in the dry p-chlorophenol salt that makes, 0.1g two (malate) copper ( II),
[0013]
[0014] Raise the temperature to 50°C, slowly add 19.2g (0.10mole) 2,4-dichloroacetophenone dropwise within 1.5 hours, then slowly raise the temperature to 100°C after the addition, and keep the reaction at this temperature for 3 hours, the reaction After the end, part of the DMSO was evaporated under negative pressure, and then quantitative toluene, water and dilute alkali solution were added, stirred, allowed to stand, and layered; the organic phas...
Embodiment 2
[0016] In a 250 ml reaction flask equipped with stirring, a thermometer, a reflux condenser and a dropping funnel, add 13.0 g (0.10 mole) of p-chlorophenol, 100 ml of xylene, and add 13.4 g (0.12 mole) of 50% potassium hydroxide aqueous solution dropwise at room temperature. ), after the dropwise addition, heat up and reflux to divide the water, after the moisture is exhausted, decompress and go out xylene, drop into 80ml dimethyl sulfoxide in the dry p-chlorophenate that makes, 0.5g two (malate) copper ( II),
[0017]
[0018] Raise the temperature to 50°C, slowly add 19.2g (0.10mole) 2,4-dichloroacetophenone dropwise within 1.5 hours, then slowly raise the temperature to 100°C after the addition, and keep the reaction at this temperature for 3 hours, the reaction After the end, part of the DMSO was evaporated under negative pressure, and then quantitative toluene, water and dilute alkali solution were added, stirred, allowed to stand, and layered; the organic phase was se...
Embodiment 3
[0020] In a 250 ml reaction flask equipped with stirring, a thermometer, a reflux condenser and a dropping funnel, add 13.0 g (0.10 mole) of p-chlorophenol, 100 ml of xylene, and add 13.4 g (0.12 mole) of 50% potassium hydroxide aqueous solution dropwise at room temperature. ), after the dropwise addition is completed, heat up and reflux to divide the water. After the moisture is exhausted, the dimethylbenzene is decompressed, and 80ml dimethyl sulfoxide, 0.1g bis(2-benzylaminopyridine) are dropped into the obtained dry p-chlorophenol salt ) copper(II),
[0021]
[0022] Raise the temperature to 50°C, slowly add 19.2g (0.10mole) 2,4-dichloroacetophenone dropwise within 1.5 hours, then slowly raise the temperature to 100°C after the addition, and keep the reaction at this temperature for 3 hours, the reaction After the end, part of the DMSO was evaporated under negative pressure, and then quantitative toluene, water and dilute alkali solution were added, stirred, allowed to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com