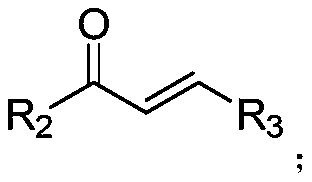
Preparation method of (4,6-diaryl-tetrahydropyridine-3-yl)(aryl) ketone
A tetrahydropyridine and diaryl technology, which is applied in the field of preparation of ketones, can solve problems such as difficulty in obtaining and are complicated, and achieve the effects of simple operation, mild conditions and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The preparation method of general (4,6-diaryl-tetrahydropyridin-3-yl) (aryl) ketone 4 specifically comprises the following steps: add aryl propynyl alcohol (0.10 mmol), solvent (0.50~1.00mL), acid (0.01~0.04mmol) and chalcone derivatives (0.10~0.20mmol), heating, reacting under reflux conditions, monitoring the reaction by TLC, waiting for the reaction of aryl propynyl alcohol After completion, add amine compound (0.10~0.20mmol), react under reflux conditions, the reaction duration is about 9~11h, then add water to the reaction solution to quench the reaction, add ethyl acetate to extract the organic phase, the obtained The organic phase was washed with saturated sodium chloride, dried over anhydrous sodium sulfate, and then concentrated on a rotary evaporator, and the obtained concentrate was purified by column chromatography.
[0043] Method 2: Preparation of (4,6-diaryl-tetrahydropyridin-3-yl)(aryl)methanone 5
[0044]The preparation method of general (4,6-diaryl-te...
Embodiment 1
[0061] (4,6-diphenyl-1-toluenesulfonyl-1,2,3,4-tetrahydropyridin-3-yl) (phenyl) ketone preparation method, the molecular formula of the target product is:
[0062]
[0063] The process of the preparation method refers to Method 1. The types and amounts of the specific aryl propynyl alcohol, solvent, catalyst, chalcone compound and amine are respectively: phenylpropynyl alcohol (0.10mmol), 1,2-dichloro Ethane (0.50 mL), bismuth triflate (0.02 mmol) and chalcone (0.12 mmol) and p-toluenesulfonamide (0.12 mmol), reaction time after addition of amine was 9 hours.
[0064] The synthesized product was characterized, and the obtained NMR data was HRMS calculated for C 31 h 29 NO 3 S([M+H] + ):494.1790; found: 494.1791.
[0065] The calculated yield of the target product was 73%.
Embodiment 2
[0067] (4,6-diphenyl-1-methylsulfonyl-1,2,3,4-tetrahydropyridin-3-yl) (phenyl) ketone preparation method, the molecular formula of the target product is:
[0068]
[0069] The process of the preparation method refers to Method 1. The consumption and types of specific aryl propargyl alcohol, solvent, acid, chalcone compound and amine are respectively: phenylpropargyl alcohol (0.10mmol), 1,2-dichloroethane Alkane (1.0 mL), copper triflate (0.03 mmol), chalcone (0.12 mmol) and benzenesulfonamide (0.12 mmol), reaction time after addition of amine was 9 hours.
[0070] The synthesized product was characterized, and the obtained NMR data was HRMS calculated for C 30 h 26 NO 3 S([M+H] + ):480.1633; found: 480.1632.
[0071] The calculated yield of the target product was 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com