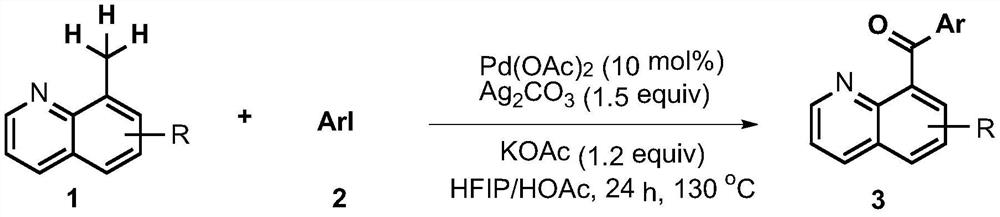
Synthesis method of phenyl(quinolin-8-yl)-one derivative
A synthesis method and technology of derivatives, applied in the direction of organic chemistry, etc., can solve the problems of difficult availability, high price of metal catalysts and raw materials, and achieve the effects of simple operation and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] 4-(quinoline-8-formyl)methyl benzoate preparation: add 8-methylquinoline (0.1mmol), 4-iodobenzoic acid methyl ester (0.15 mmol), 0.01 mmol of palladium acetate, 0.15 mmol of silver carbonate, 0.12 mmol of potassium acetate, then add 1 mL of a mixed solution of hexafluoroisopropanol and glacial acetic acid (V / V=3:7), seal the tube, Heat the reaction at 130°C for 24 hours, add the reaction mixture to 30 mL of ethyl acetate to dilute, then wash with saturated sodium carbonate solution, dry the separated organic layer with anhydrous magnesium sulfate, filter to remove magnesium sulfate, and obtain the crude product The organic solution was evaporated under reduced pressure to remove the solvent, and the residue was purified by column chromatography to obtain the target product (eluent V / V: petroleum ether: ethyl acetate = 8:1).
Embodiment 2
[0012] Preparation of 4-(5-bromoquinoline-8-formyl)methyl benzoate: Add 5-bromo-8-methylquinoline (0.1mmol) and 4-iodo Methyl benzoate (0.15mmol), the palladium acetate of 0.01 mmol, the silver carbonate of 0.15 mmol, the potassium acetate of 0.12 mmol, then add the mixed solution of 1mL hexafluoroisopropanol and glacial acetic acid (V / V=3: 7), seal the tube, heat and react at 130°C for 24 hours, add the reaction mixture to 30mL ethyl acetate to dilute, then wash with saturated sodium carbonate solution, dry the separated organic layer with anhydrous magnesium sulfate, filter to remove sulfuric acid Magnesium, to obtain the organic solution of the crude product, the solvent was removed by rotary evaporation under reduced pressure, and the residue was purified by column chromatography to obtain the target product (eluent V / V: petroleum ether: ethyl acetate=8:1).
Embodiment 3-10
[0014] Embodiment 3-0 is similar to Embodiment 1 and 2. The reaction raw materials, reaction conditions and yields of each embodiment are shown in Table 1 below.
[0015] The reaction raw material of table 1 embodiment 1-10, reaction condition and productive rate
[0016]
[0017] As can be seen from Table 1, the method of the present invention has easy-to-obtain raw materials, simple and safe operation, high yield and convenient post-treatment, thus having great implementation value and potential social and economic benefits.
[0018] The characterization data of the obtained compound are as follows.
[0019] Methyl 4-(quinoline-8-formyl)benzoate (3a)
[0020] 1 H NMR (400MHz, CDCl 3 )δ8.83(dd, J=4.4Hz, 1.6Hz, 1H), 8.26(dd, J=8.4Hz, 1.6Hz, 1H), 8.08-8.06(m, 2H), 8.01(dd, J=8.2Hz ,1.4Hz,1H),7.88-7.86(m,2H),7.80(dd,J=7.0Hz,1.4Hz,1H),7.67(dd,J=8.0Hz,7.2Hz,1H),7.45(dd, J=8.0,4.0Hz,1H),3.93(s,3H); 13 C{ 1 H}NMR (100MHz, CDCl 3 )δ197.3, 166.3, 150.8, 145.7, 141.1, 138.4, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com