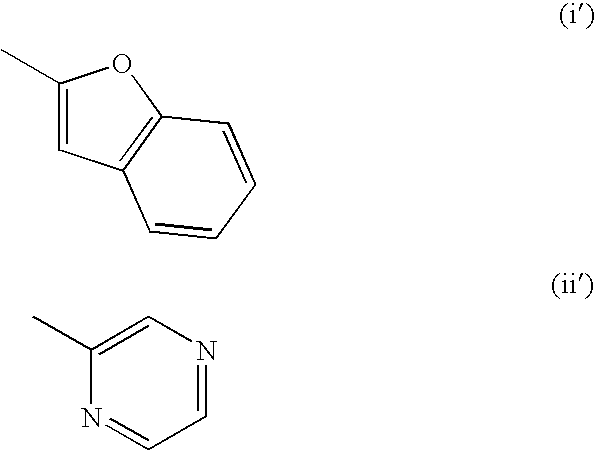
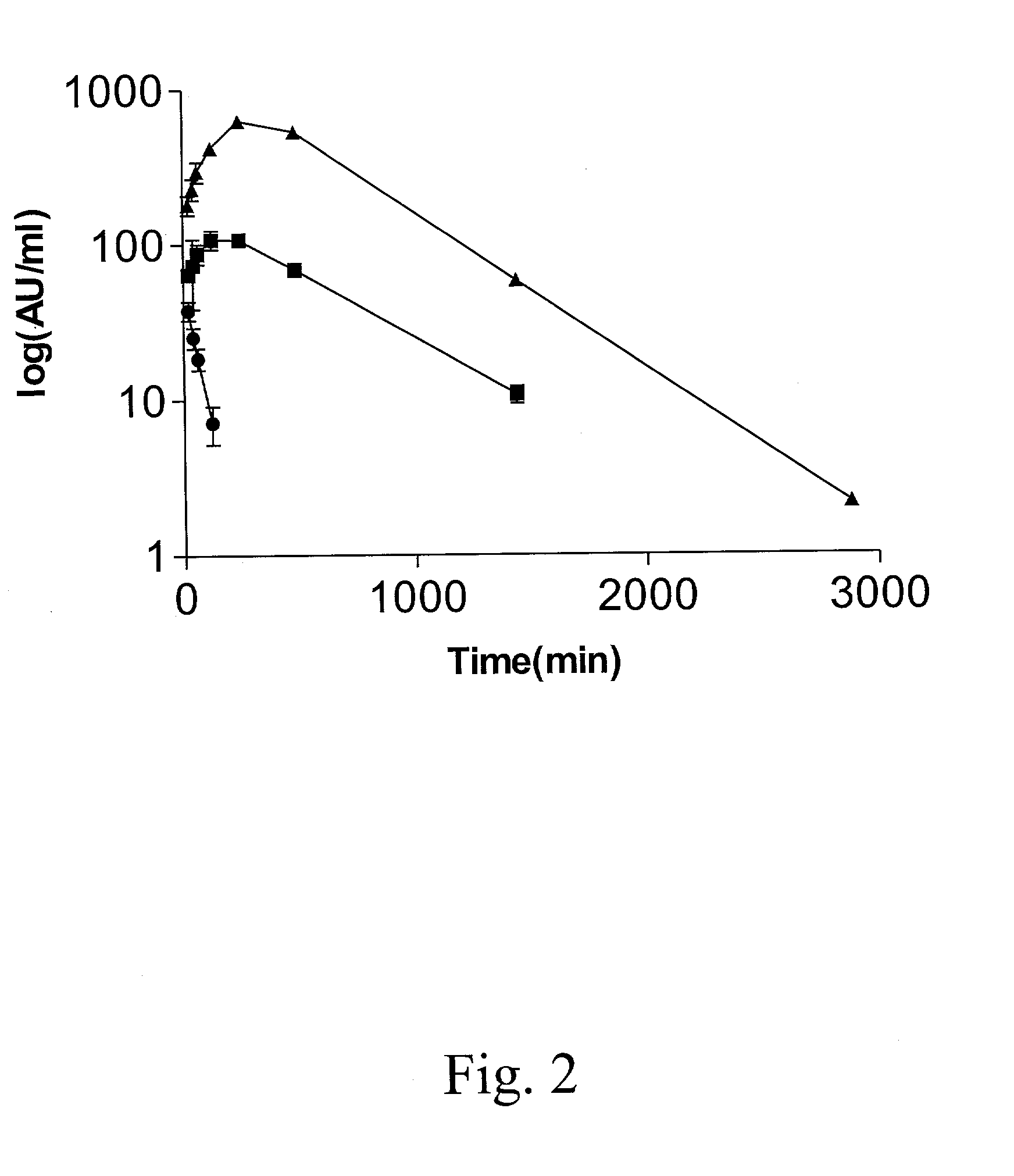
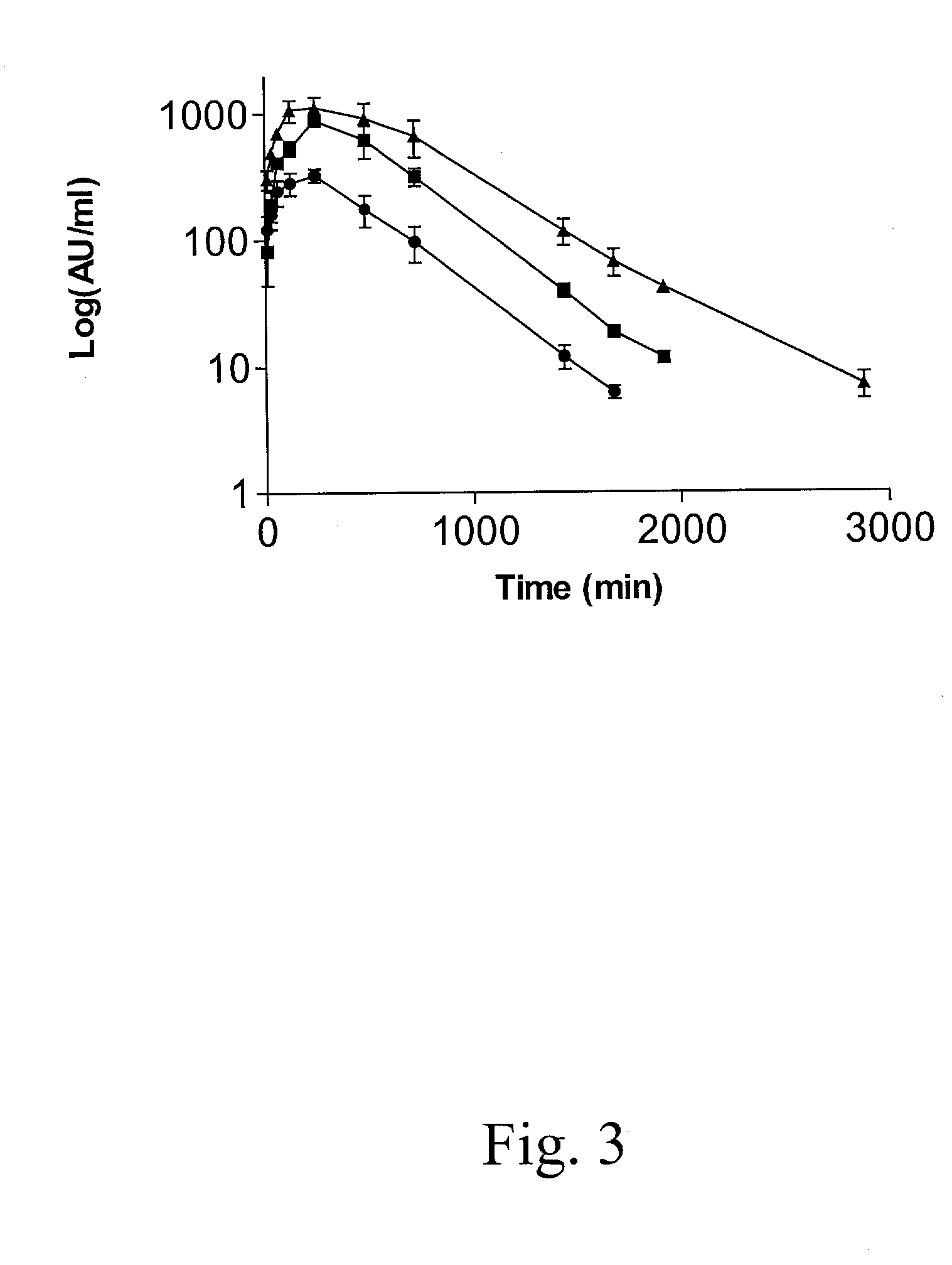
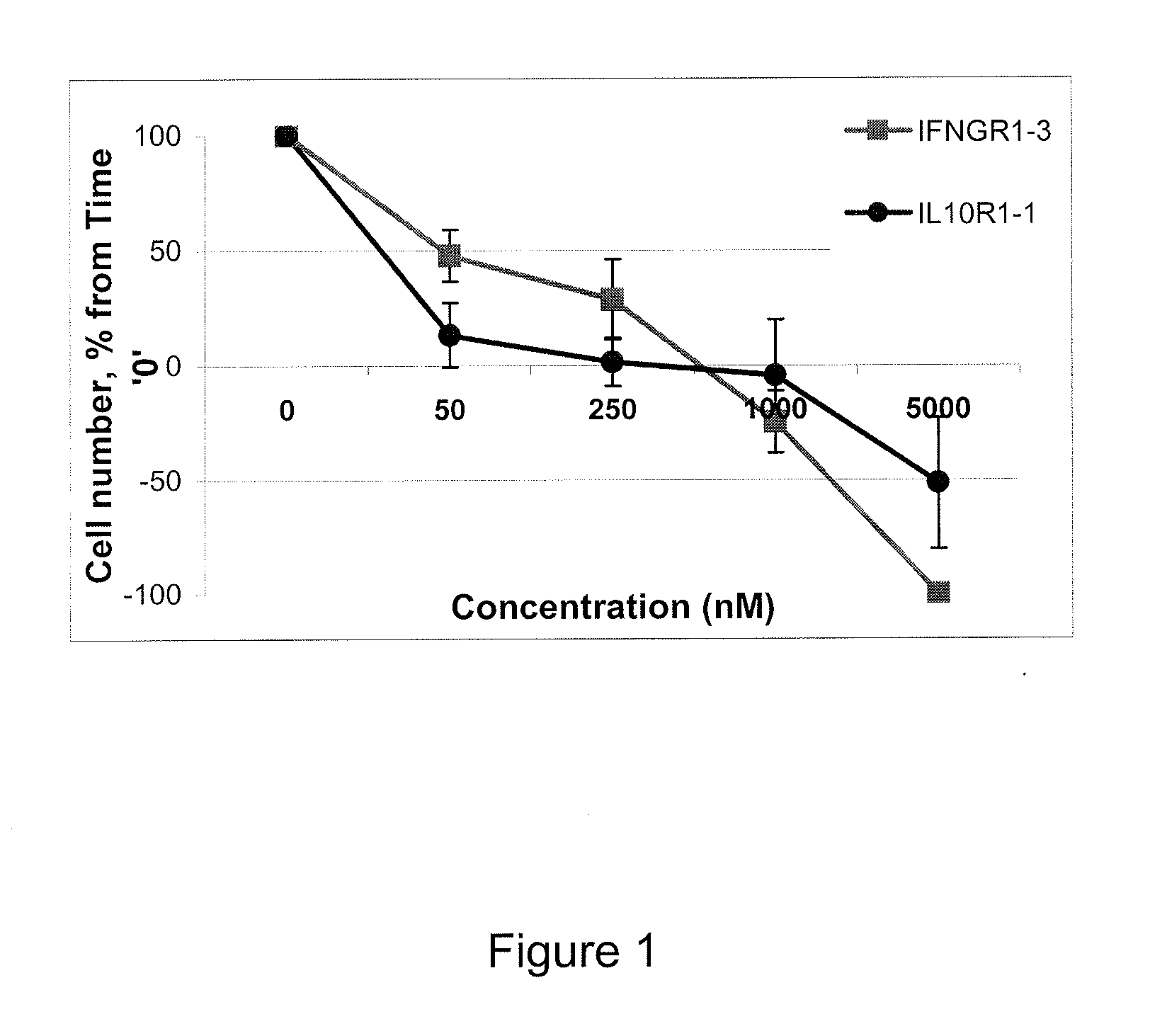
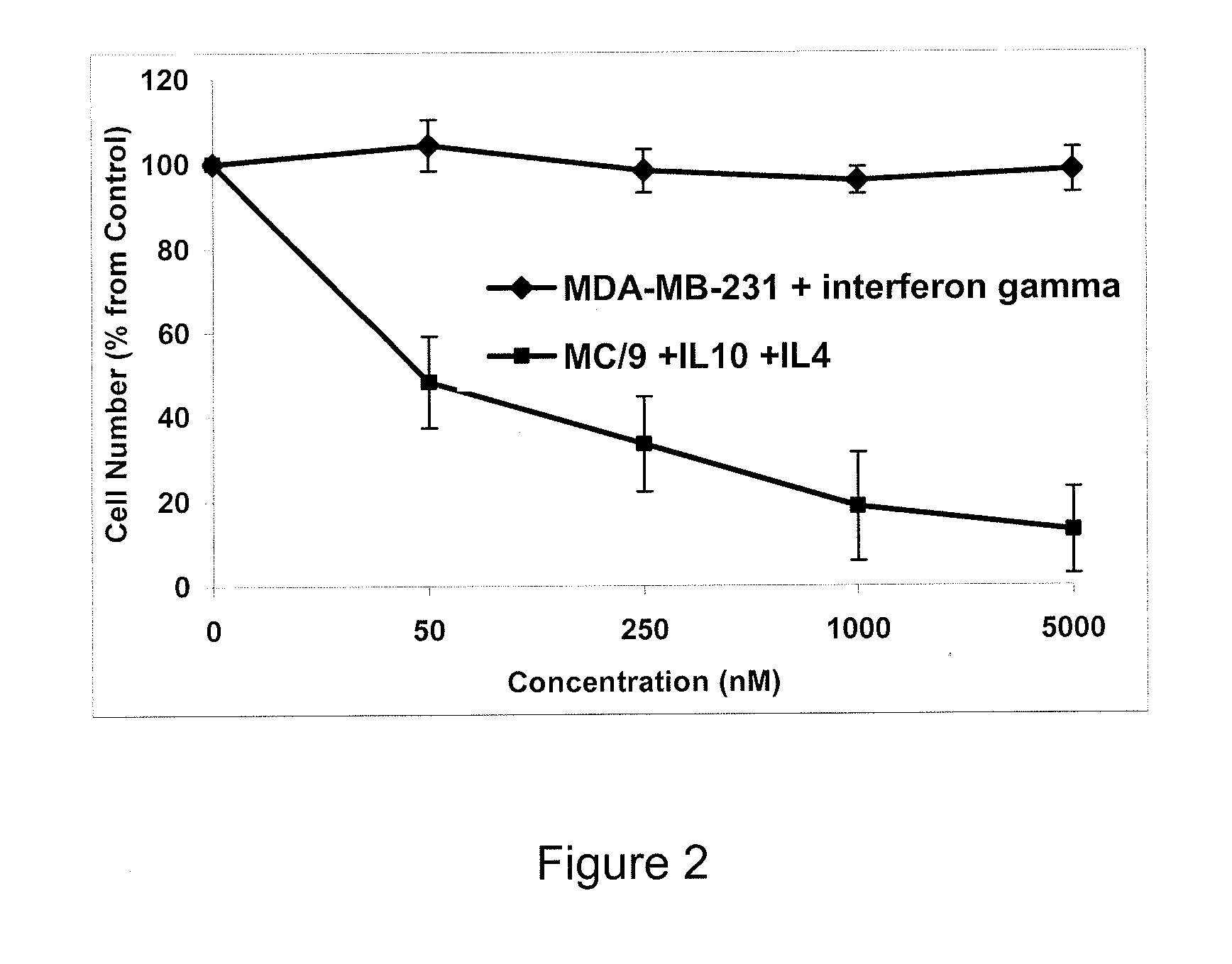
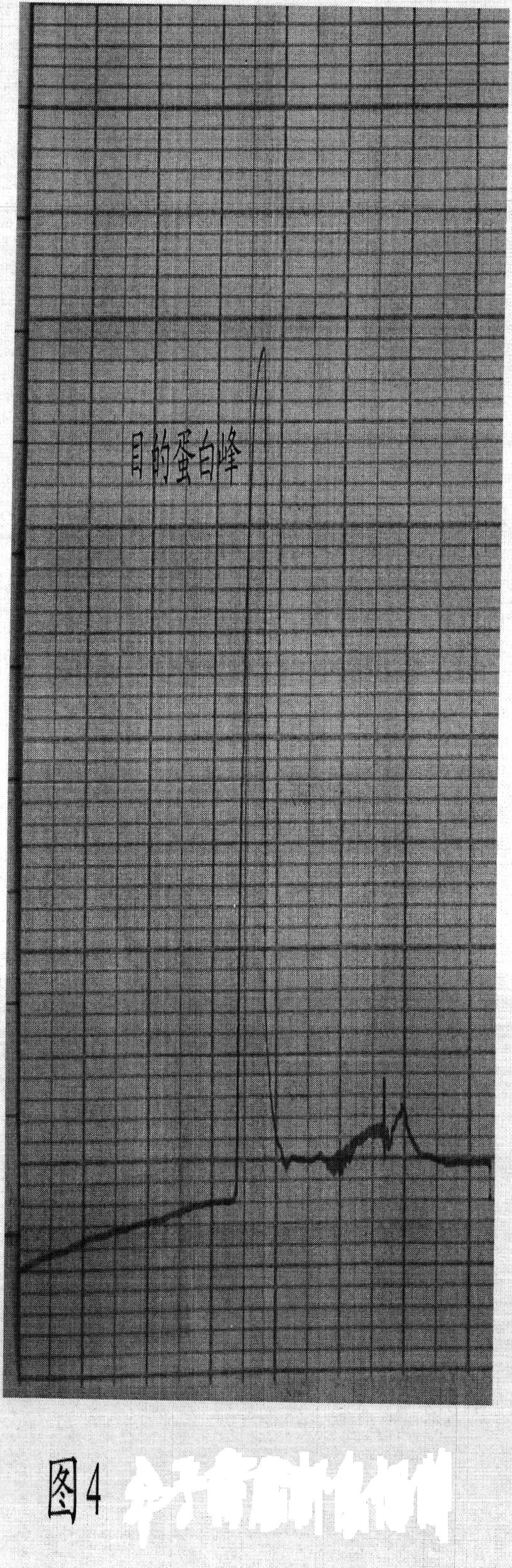
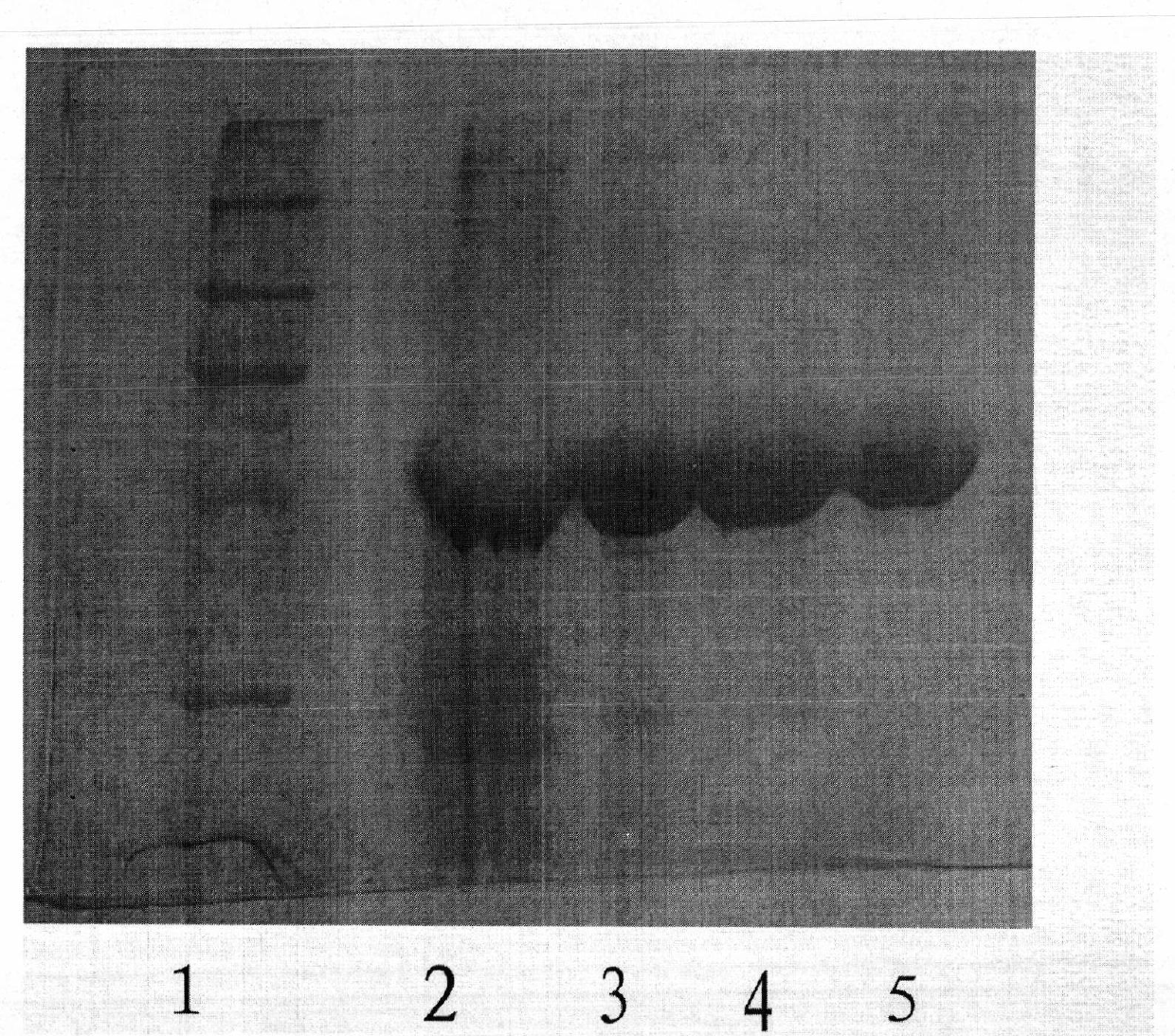
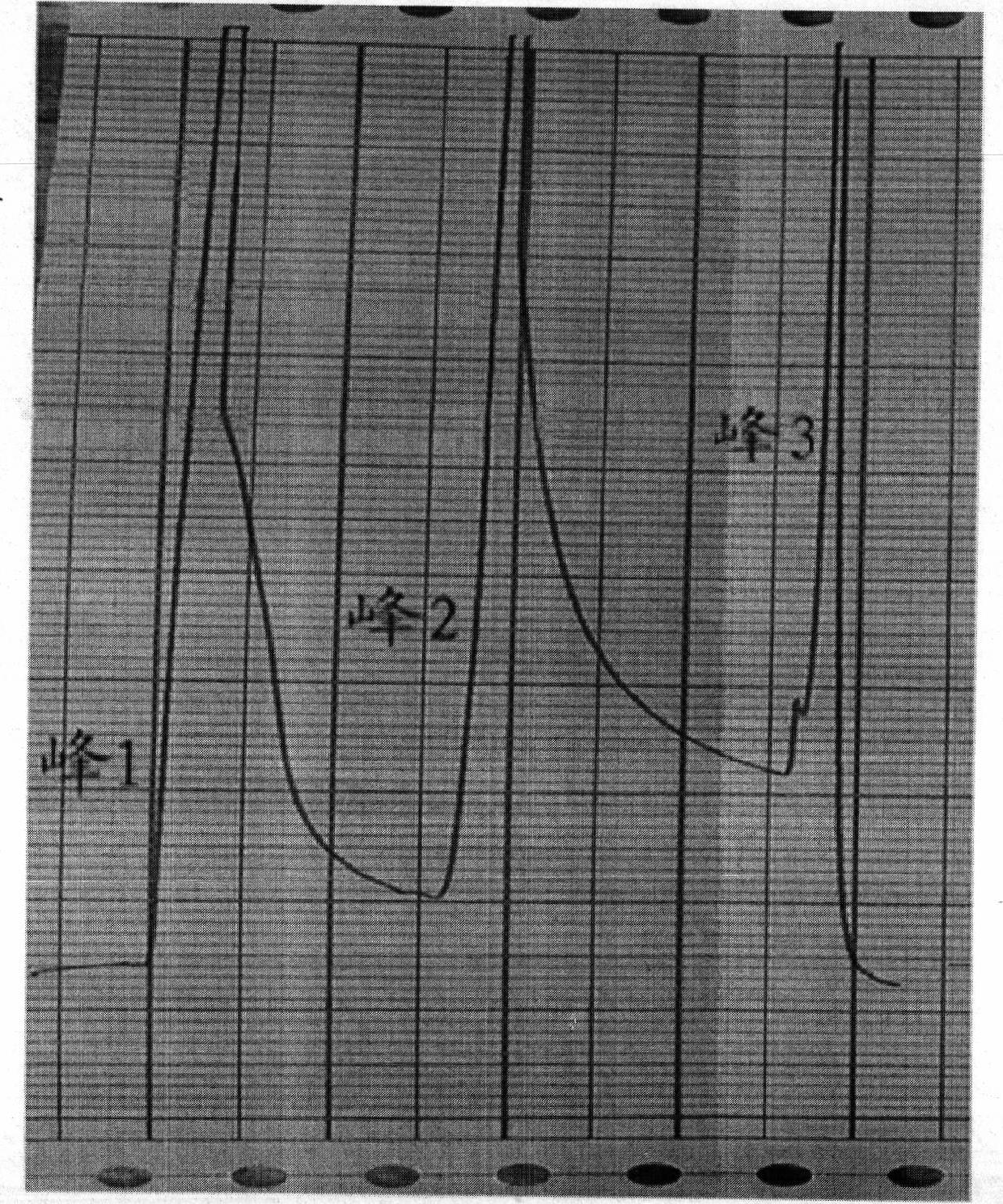
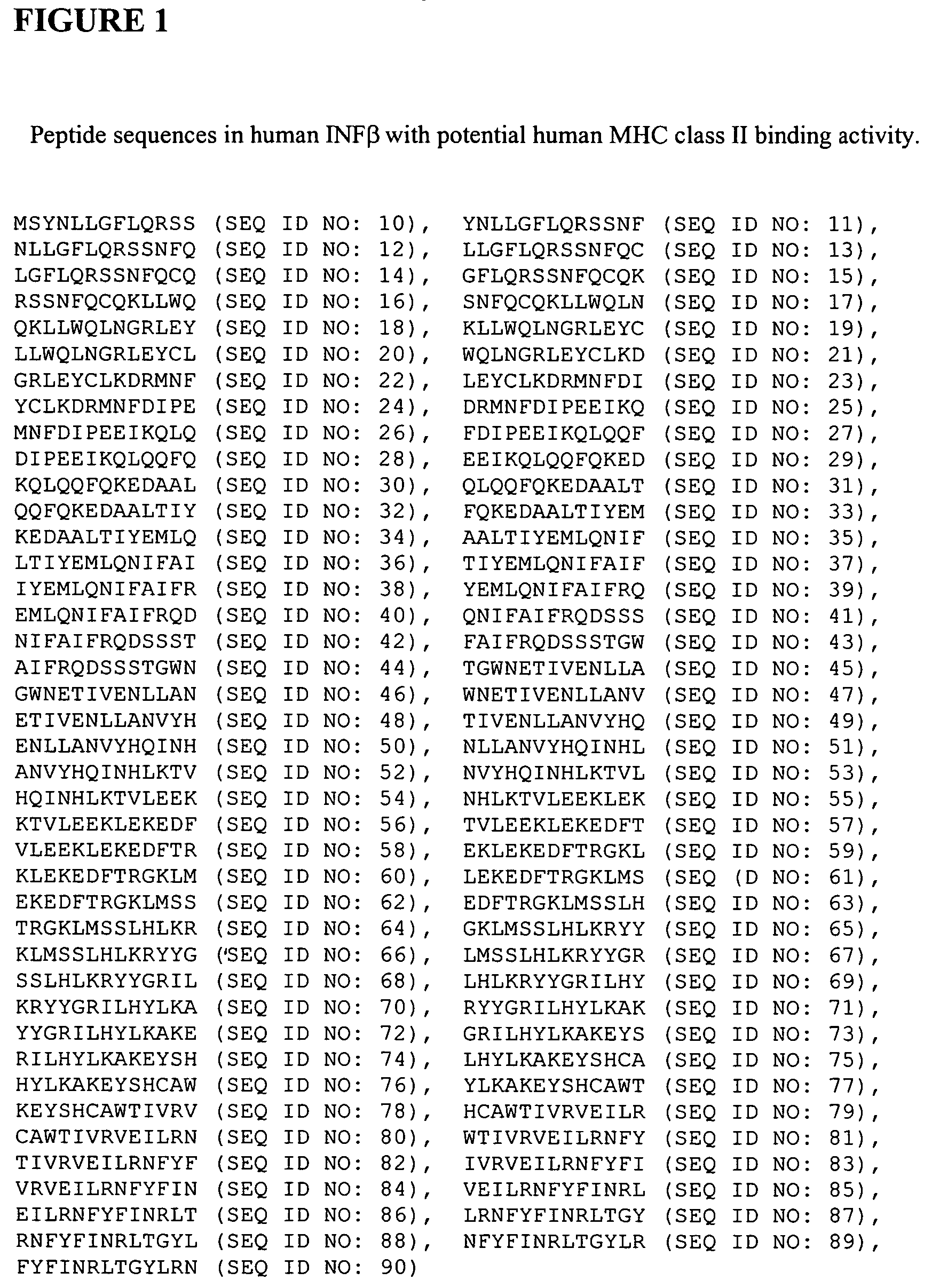
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
99 results about "Interferon epsilon" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Interferon epsilon, IFN-epsilon, interferon epsilon 1, interferon tau-1 GeneRIFs: Gene References Into Functions T Allele of nonsense polymorphism (rs2039381, Gln71Stop) of interferon-epsilon is a risk factor for the development of intracerebral hemorrhage.
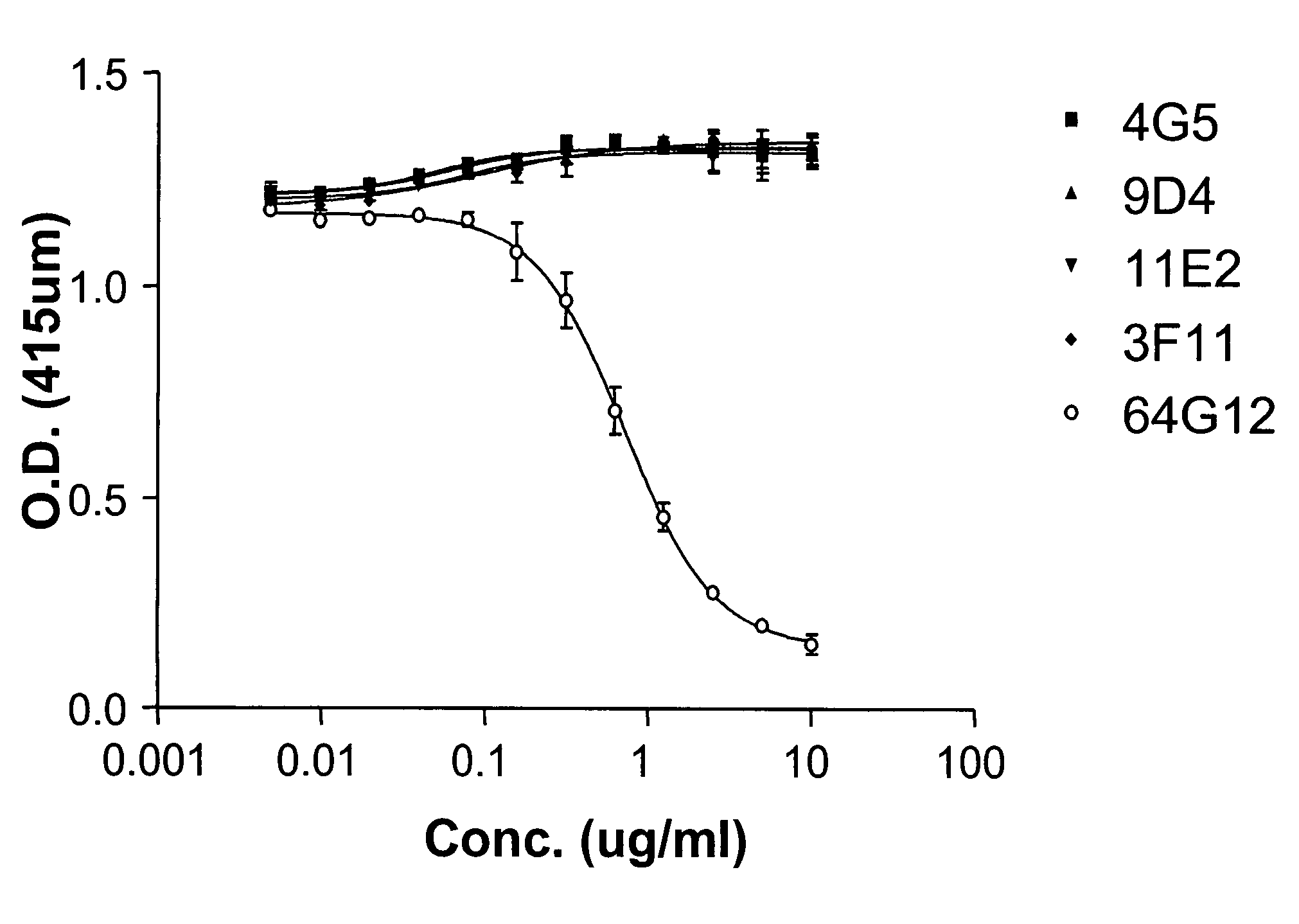
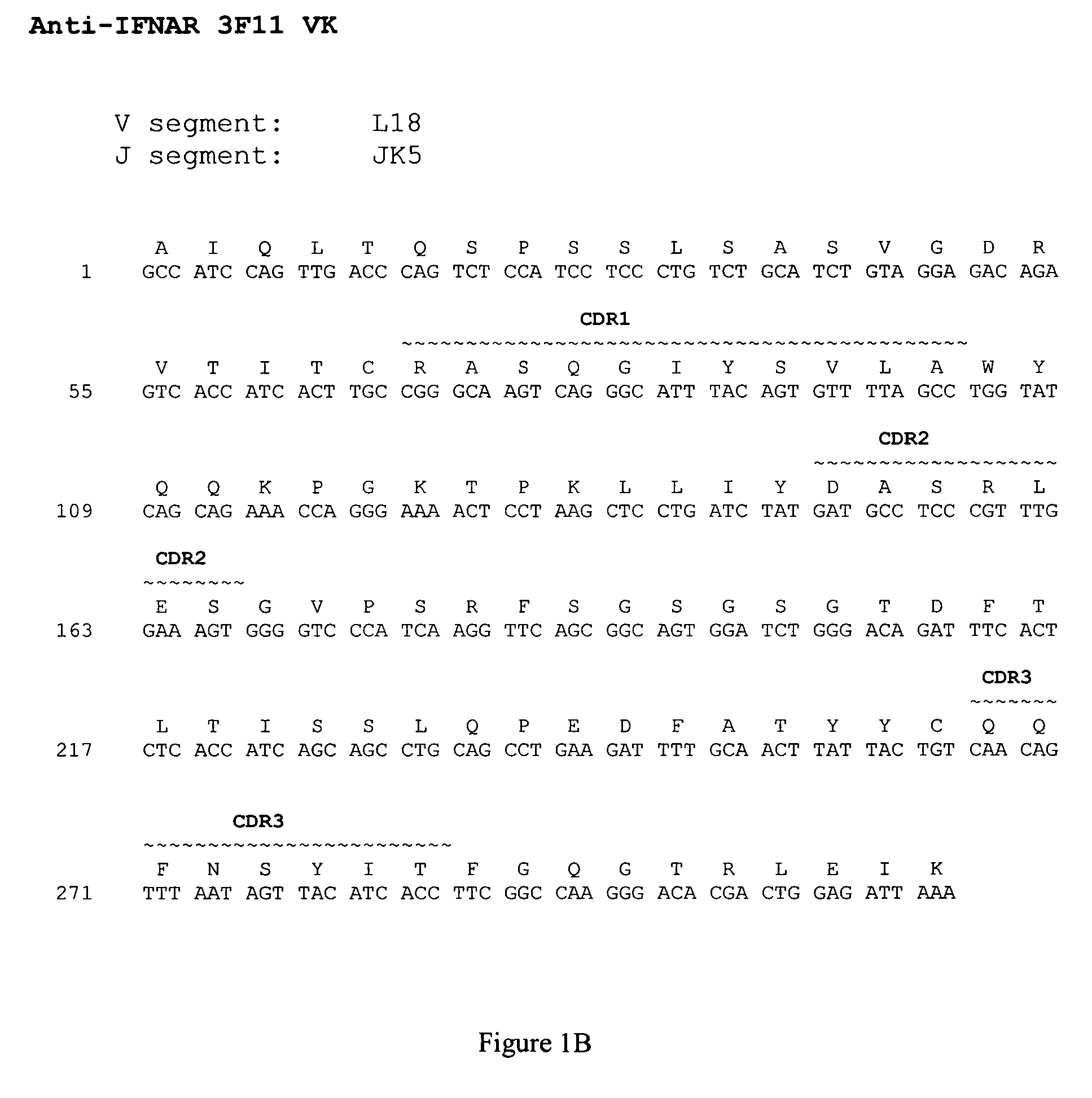
Interferon alpha receptor 1 antibodies and their uses
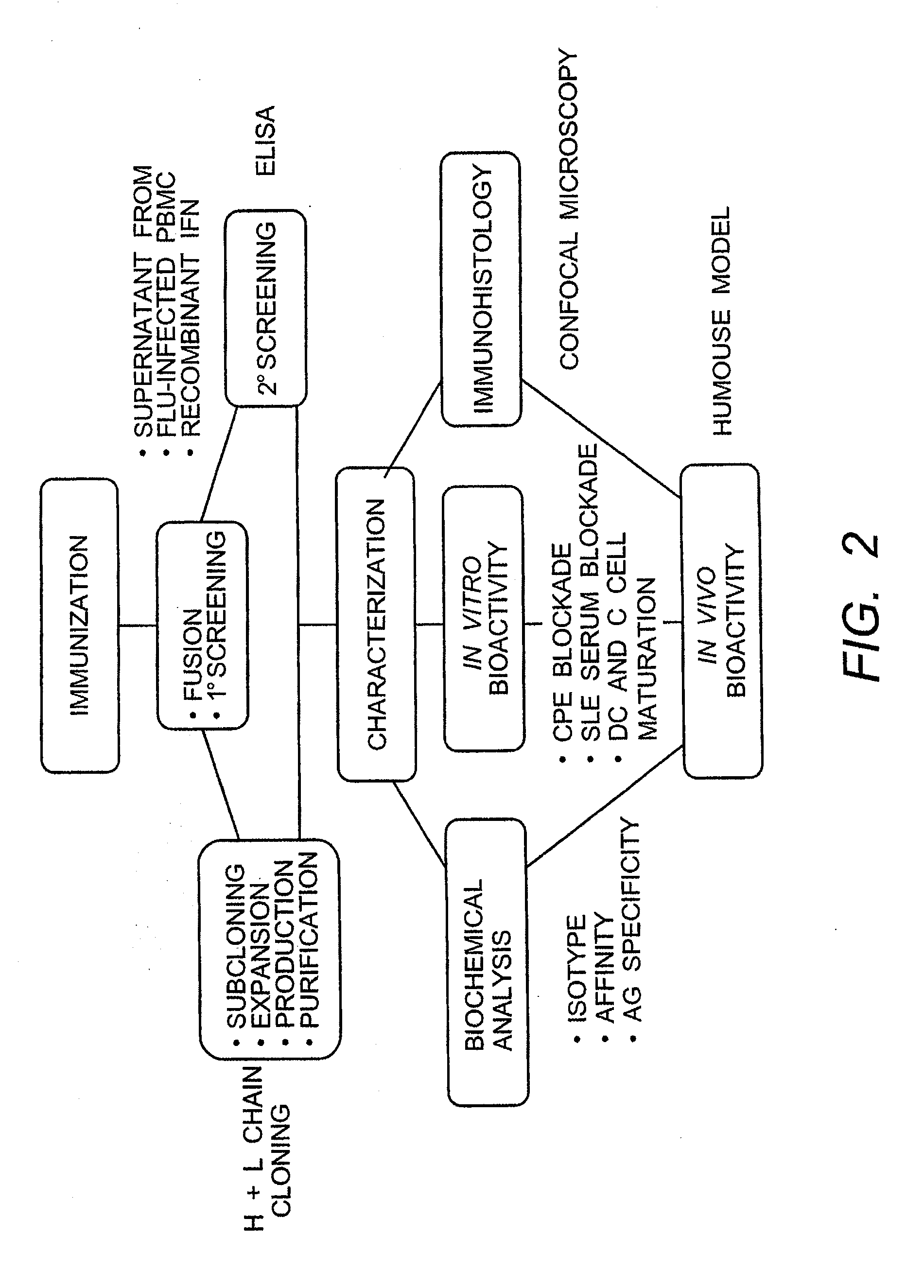
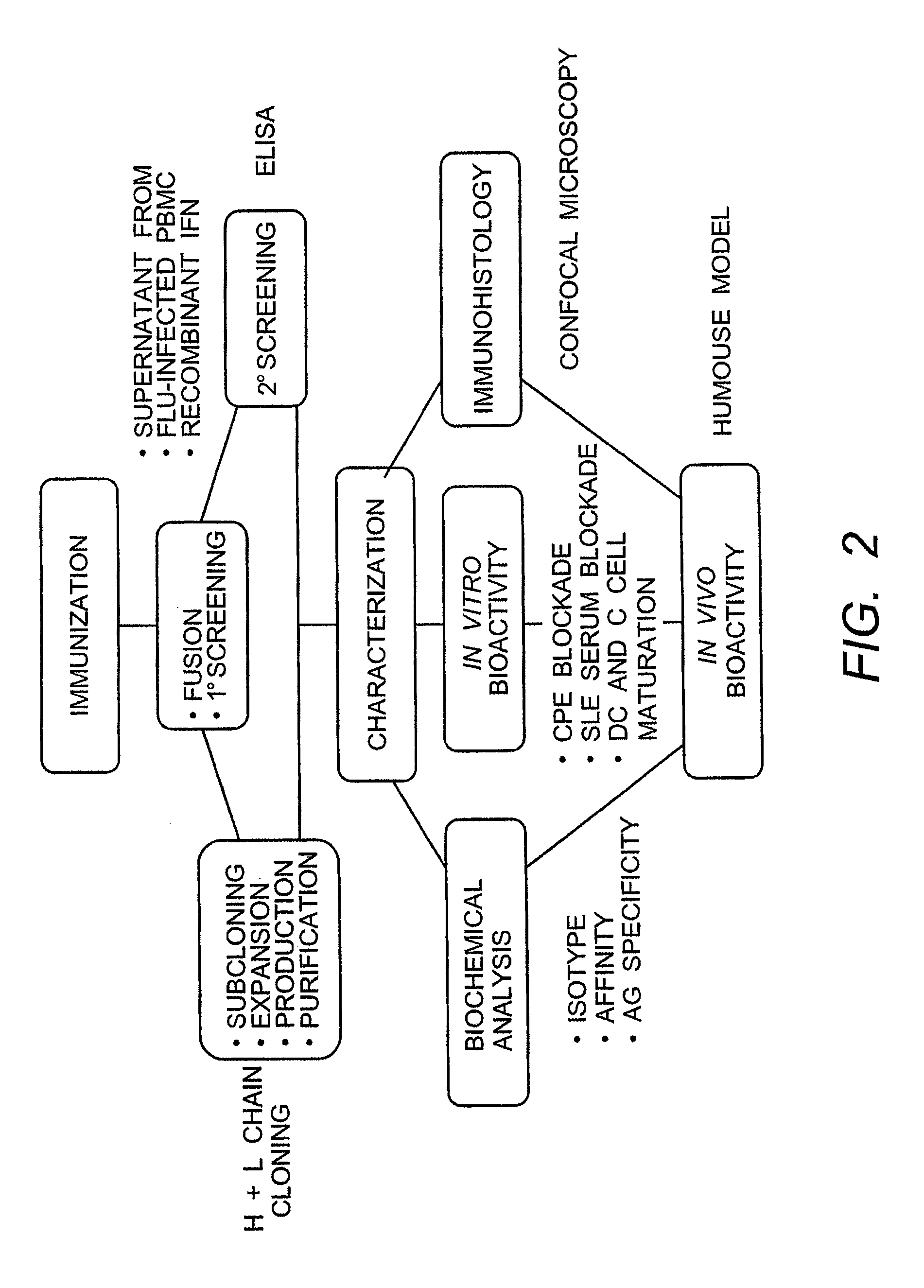
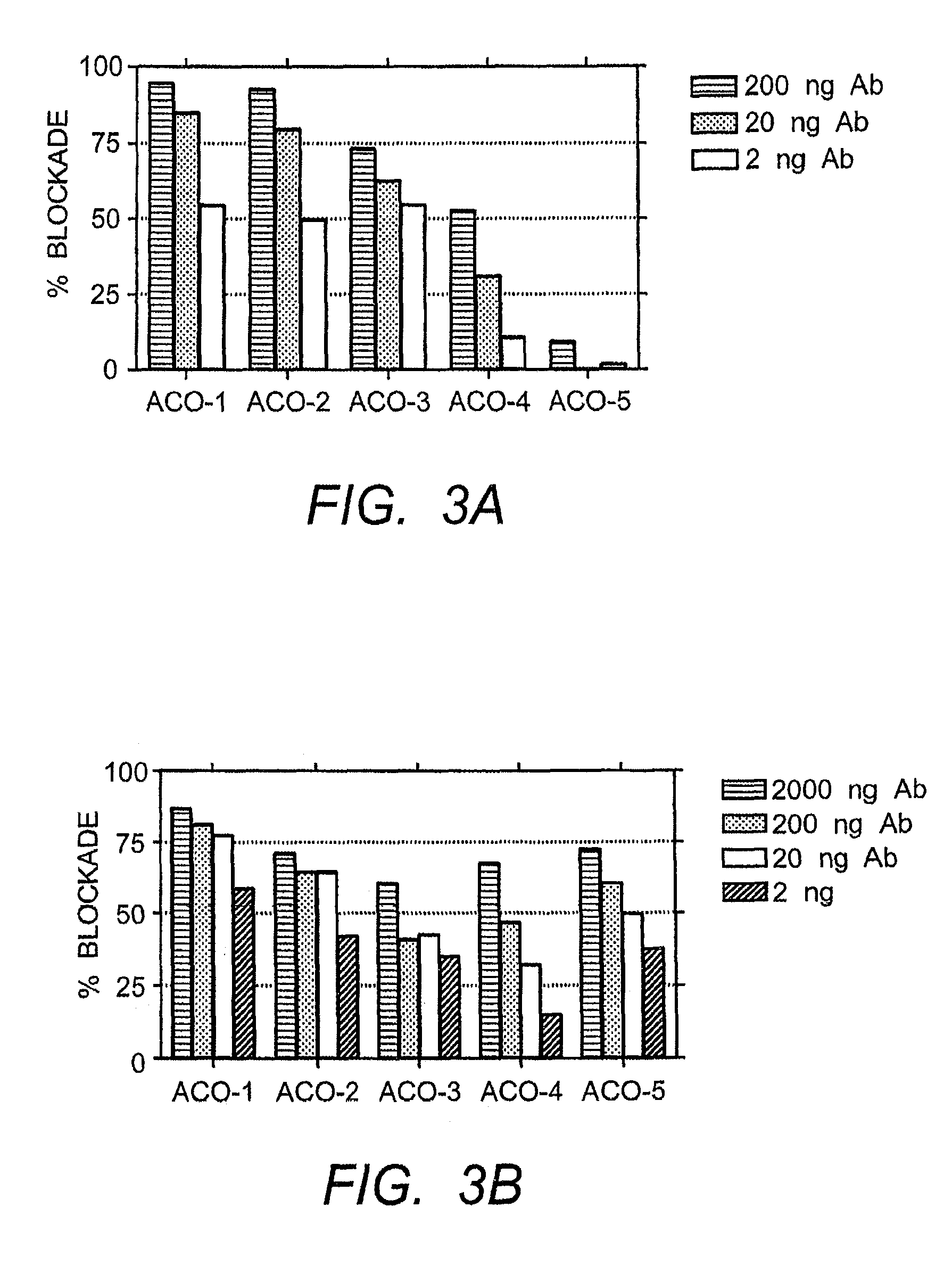
The present invention provides isolated human monoclonal antibodies that bind to IFNAR-1 and that are capable of inhibiting the biological activity of Type I interferons. Immunoconjugates, bispecific molecules and pharmaceutical compositions comprising the antibodies of the invention are also provided. The invention also provides methods for inhibiting Type I interferon-mediated disorders using the antibodies of the invention, including methods for treating autoimmune disorders, transplant rejection or Graft Versus Host Disease using the antibodies of the invention.
Owner:ER SQUIBB & SONS INC
Dihydroorotate dehydrogenase inhibitors for the treatment of viral-mediated diseases
InactiveUS6841561B1Potent activityBiocideOrganic chemistryDiseaseDihydroorotate Dehydrogenase Inhibitor
Flavivirus, rhabdovirus and paramyxovirus infections may be treated by administering an inhibitor of the enzyme dihydroorotate dehydrogenase such as 6-fluoro-2-(2′-fluoro-1,1′-biphenyl-4-yl)-3-methyl-4-quinolinearcarboxylic acid sodium salt (Brequinar). A synergistic effect can be obtained if an interferon such as interferon α2, interferon α8 or interferon β, or an inhibitor of a second enzyme selected from inosine monophosphate dehydrogenase, guanosine monophosphate synthetase, cytidine triphosphate synthetase and S-adenosylhomocysteine hydrolase, is also administered.
Owner:INST OF MOLECULAR & CELL BIOLOGY
Colorectal cancer biomarker
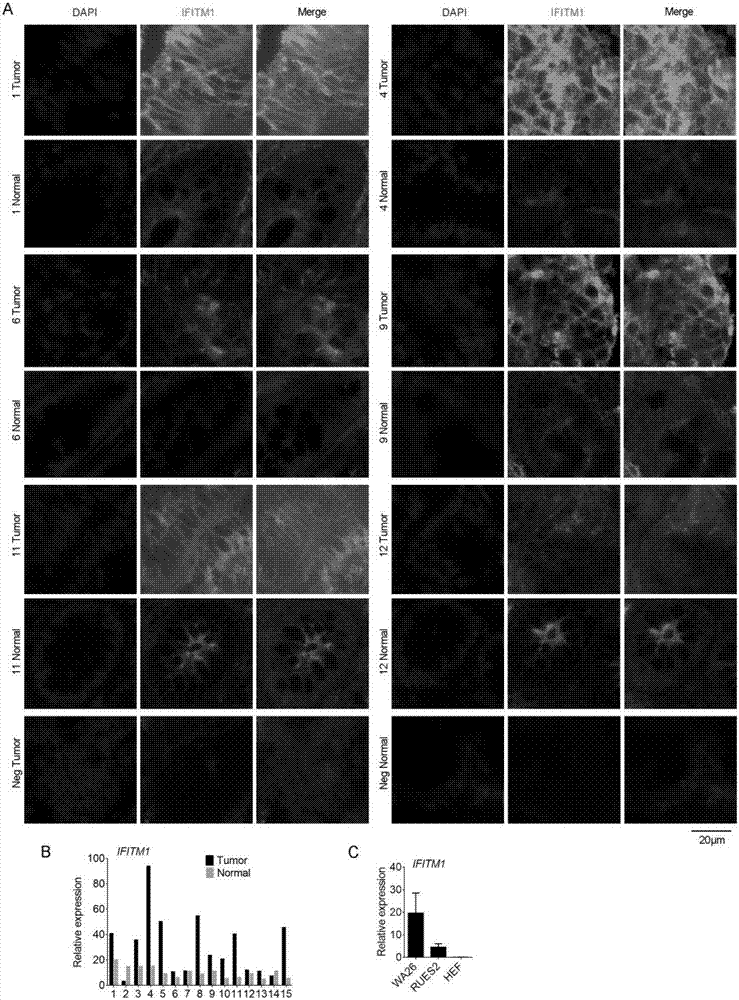
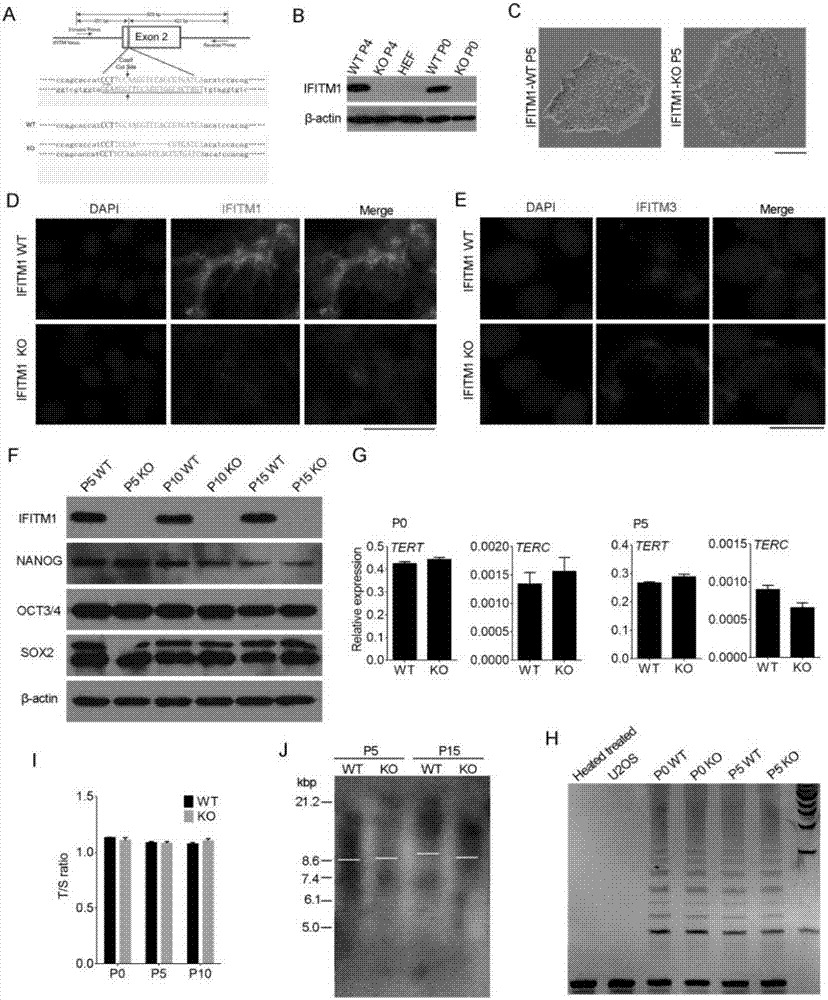
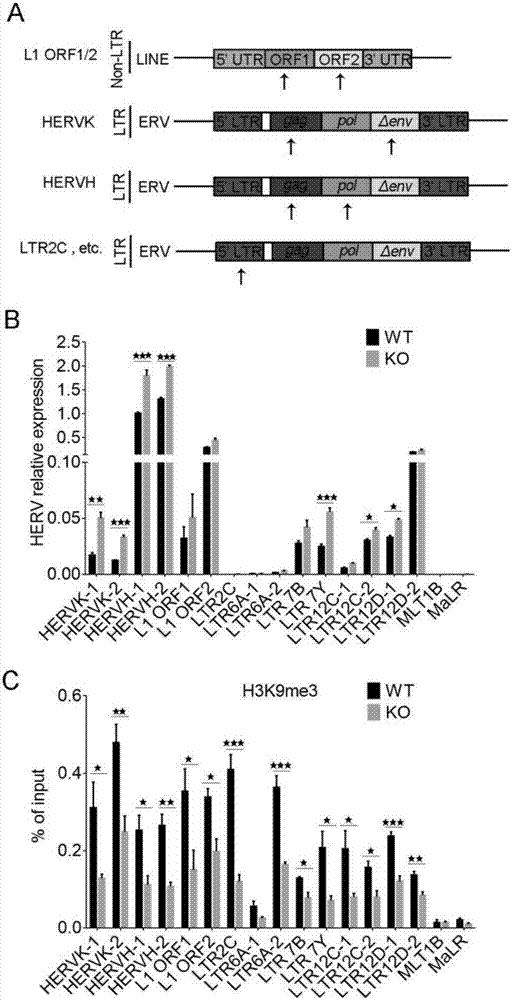
The invention discloses a colorectal cancer biomarker, relating to application of interferon-induced transmembrane protein 1 in inhibition of endogenous retrovirus in colorectal cancer. The hESCs of IFITM1-KO is constructed by using CRISPR / Cas9 technology so as to study the functions of IFITM1. Studies find that the IFITM1-deleted human embryonic stem cell (hESCs) is identical with IFITM1-WT in the aspects of cell proliferation, cell pluripotency, telomere length, telomerase activity and the like. The expression of human endogenous retrovirus in hESCs of IFITM1-KO increases, and expression in para-carcinoma tissue is higher than that in colorectal carcinoma tissue. ChIP-qPCR detection finds that the enrichment of H3K9me3 in hESCs of IFITM1-KO is reduced at the HERVs site. Data shows that the expression level of IFITM1 in hESCs and colorectal carcinoma is in negative correlation to HERVs expression, and the IFITM1 can inhibit the expression of HERVs by regulating epigenetic inheritance.
Owner:NANKAI UNIV
Interferon antagonists useful for the treatment of interferon related diseases
InactiveUS20030138404A1Extended half-lifeLow immunogenicityBiocideNervous disorderDiseaseAutoimmune responses
The present invention relates to a process for ameliorating or preventing diseases that are caused, in part, by an increased level of, and / or an abnormal responsivity to, interferon. Alzheimer's disease, HIV infection, Down syndrome, transplant rejection, autoimmune disease, and infant encephalitis are examples of such diseases. Specifically, the invention provides a method for treating subjects suffering from, or at risk for, such diseases by the administration of a pharmacological preparation of interferon binding proteins of mammalian and / or viral origin that antagonize interferon's action. This invention comprises compositions of interferon binding proteins that can inhibit the activity of interferon gamma plus interferon alpha such compositions along with their method of production and modification being described herein.
Owner:MEIOGEN BIOTECH CORP
Carboxymethylated retroviral regulatory proteins and interferon-α
InactiveUS7022326B1Simple and efficientPeptide/protein ingredientsAntibody mimetics/scaffoldsRetroviral infectionMammal
This invention relates to retroviral regulatory proteins or fragments thereof, or interferon alpha protein or fragments thereof, which are carboxymethylated. This chemical modification leads to new proteins or fragments which are biologically inactive but preserve their immunogenicity (toxoids). These proteins or fragments thereof, or interferon alpha or fragments thereof, can be utilized in the treatment and prevention of retroviral infections. The invention also relates to a pharmaceutical composition comprising at least one carboxymethylated protein or fragment of the invention, together with a pharmaceutically acceptable carrier. The invention also relates to a vaccine comprising at least one of the carboxymethylated proteins or fragments of the invention, together with an immunologically acceptable carrier. The invention also relates to a process for obtaining an immunogenic yet not toxic retroviral regulatory protein or fragment, or interferon alpha or fragment. The invention also relates to a method of inducing an immune response in a mammal, comprising administering the vaccine of the invention to a mammal in an immunologically effective amount.
Owner:BIOVACS +1
Humanized antibodies to interferon alpha receptor-1 (IFNAR-1)
Owner:ER SQUIBB & SONS INC
Fusion protein comprised of human serum and interferon and its coding gene and application
InactiveCN101062952AImprove stabilityExtended half-lifePeptide/protein ingredientsAntiviralsInterferon alphaFhit gene
The invention discloses a fusing protein, encoding gene and appliance, which is characterized by the following: comprising with albuminar and interferon; comprising (a) and (b) protein; choosing amino acid residue sequence from sequences 3 in sequence table as the protein (a); replacing or deleting or adding amino acid residue sequence from sequences 1 trough one or several amino acid residue sequence; possessing interferon alpha 2b active; deriving from the protein (a) as the protein (b). The interferon part of the fusing protein is placed N on end of the fusing protein, but a part of albuminar is placed on the end of C. This fusing protein possesses better homogeneity, higher stability and higher receiving rate, which can be used to cure multiple tumors and virus disease.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Interferon-alfa sensitivity biomarkers
InactiveUS20120035347A1Lower levelPeptide/protein ingredientsMicrobiological testing/measurementDiseaseInterferon alpha
The present invention provides biomarkers of sensitivity to interferon alfa (IFN-α). These IFN-α sensitivity biomarkers are useful, inter alia, to identify patients who are most likely to benefit from treatment with pharmaceutical compositions of IFN-α, in methods of treating patients having a disease susceptible to treatment with interferon alfa, and in methods for selecting the most appropriate therapy for such patients.
Owner:SCHERING CORP
Production process of fusion expression recombinant chicken interferon alpha
InactiveCN106399321AStrong antiviral activityEfficient expressionFermentationInterferonsEscherichia coliInclusion bodies
The invention discloses a production process of fusion expression recombinant chicken interferon alpha. The process includes the steps of: S1, according to the preference of Escherichia coli codon, conducting codon optimization on a chicken interferon alpha gene sequence published in Genebank, and artificially synthesizing the chicken interferon alpha gene; S2, according to the codon optimized chicken interferon alpha gene, designing three specific primers; S3, constructing recombinant chicken interferon alpha plasmid containing ProS2 dissolution promoting label; S4, transforming and identifying the recombinant expression plasmid; S5, conducting inducible expression of recombinant chicken interferon alpha; S6, extracting an expression product and conducting protein renaturation purification: S61, inclusion body extraction and treatment; S62, inclusion body denaturation; S63, denaturation solution renaturation; and S64, nickel column affinity purification. By means of cell cytopathic inhibition, the invention detects that the interferon has the activity of inhibiting vesicular stomatitis virus proliferation, and the activity unit reaches 7.32*10<7>UI / mg.
Owner:SOUTH CHINA AGRI UNIV +1
Modified interferon beta polypeptides and their uses
InactiveUS20120197006A1Improve stabilityImprove solubilityNervous disorderPeptide/protein ingredientsInterferon alphaInterferon beta
Owner:AMBRX
Anti-Interferon Alpha Monoclonal Antibodies and Methods For Use
ActiveUS20090214565A1Metabolism disorderImmunoglobulins against cytokines/lymphokines/interferonsSystemic lupus erythematosusInterferon alpha
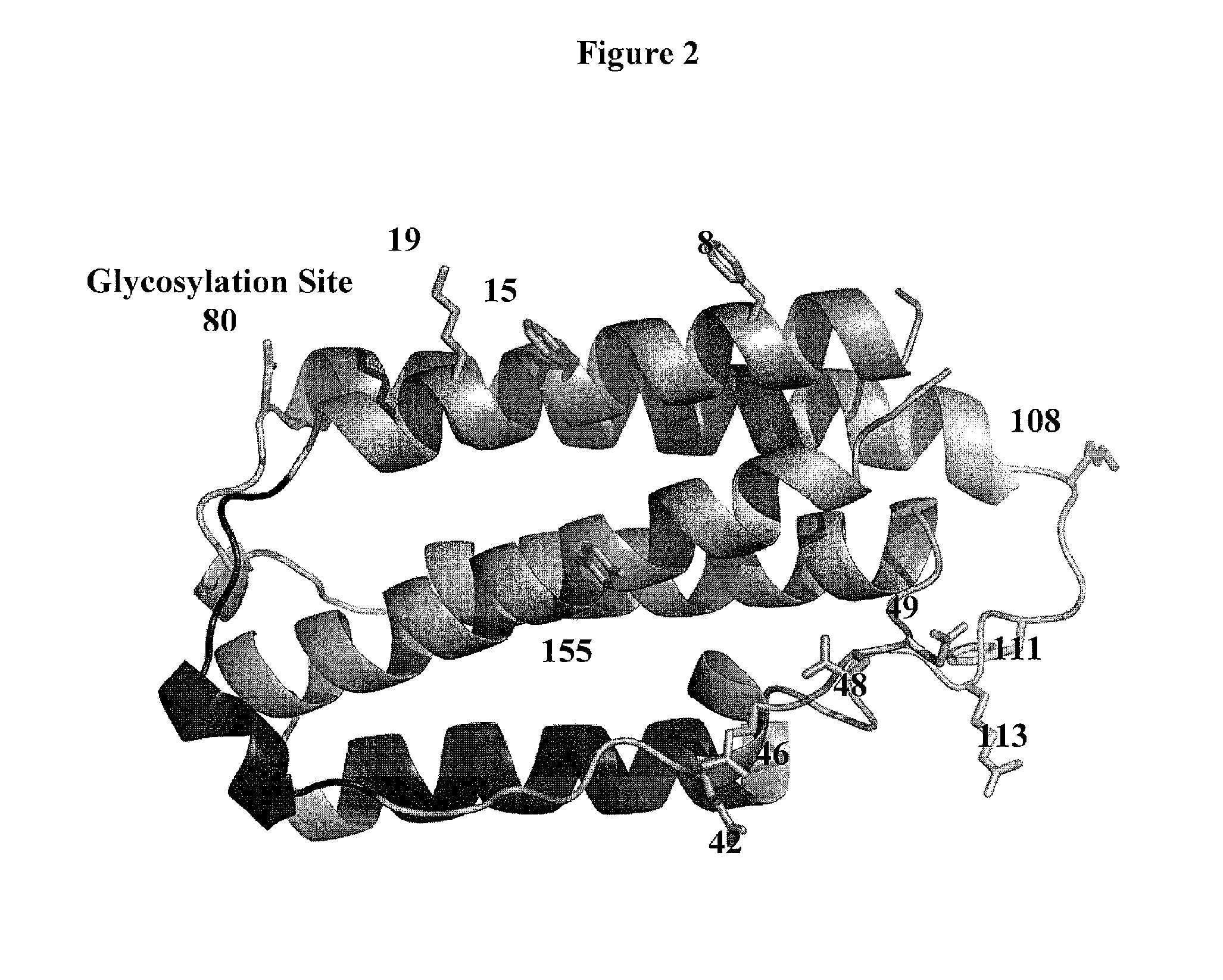
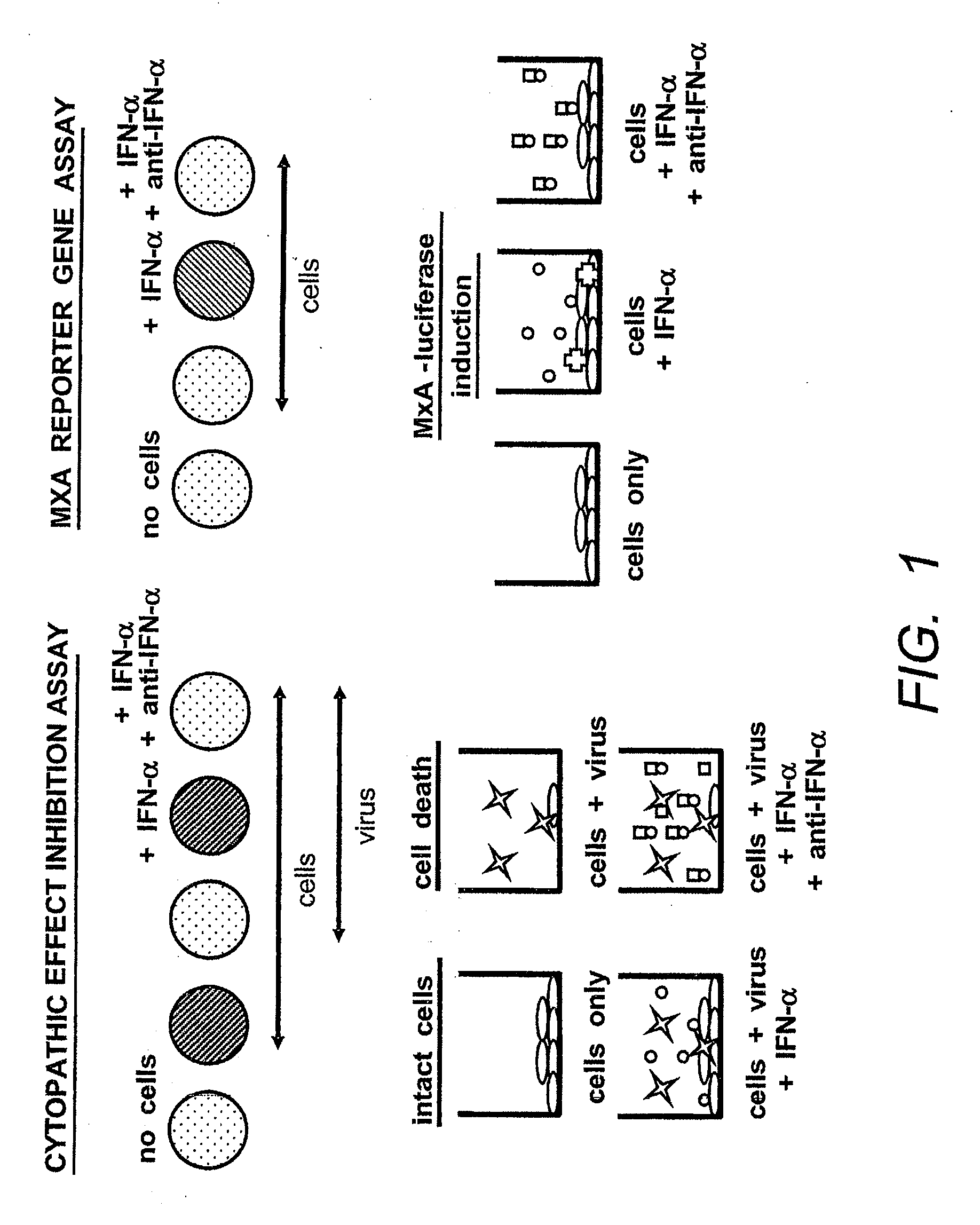
The present invention includes compositions and methods that include antibodies that selectively neutralize a bioactivity of at least two interferon alpha (“IFNα”) protein subtypes for the protein subtypes A, 2, B2, C, F, G, H2, I, J1, K, 4a, 4b and WA, but does not neutralize at least one bioactivity of IFNα protein subtype D. Examples of bioactivity for measurement include activation of the MxA promoter or antiviral activity and variants, derivatives and fragments thereof. The invention also includes host cells, hybridomas and plasmacytomas that produce antibodies. Because of their unique selectivity and affinity, the antibodies of the present invention are useful to detect IFNα subtypes in sample or tissue and / or for therapeutic applications that include, but are not limited to the treatment and / or amelioration of an IFNα related disorder such as SLE, lupus, type I diabetes, psoriasis, AIDS and Graft versus Host Disease.
Owner:BAYLOR RES INST
Novel Vectors for Production of Interferon
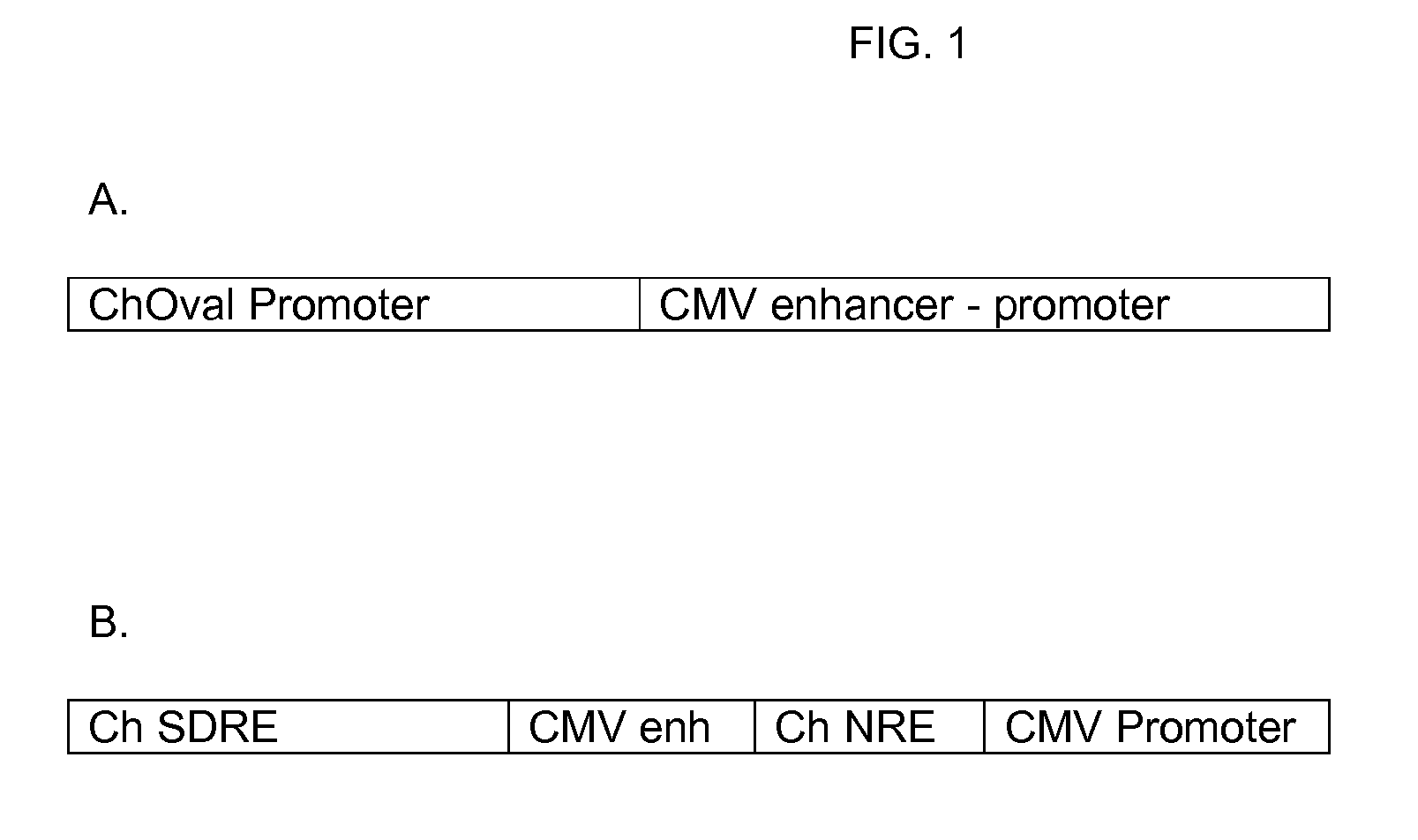
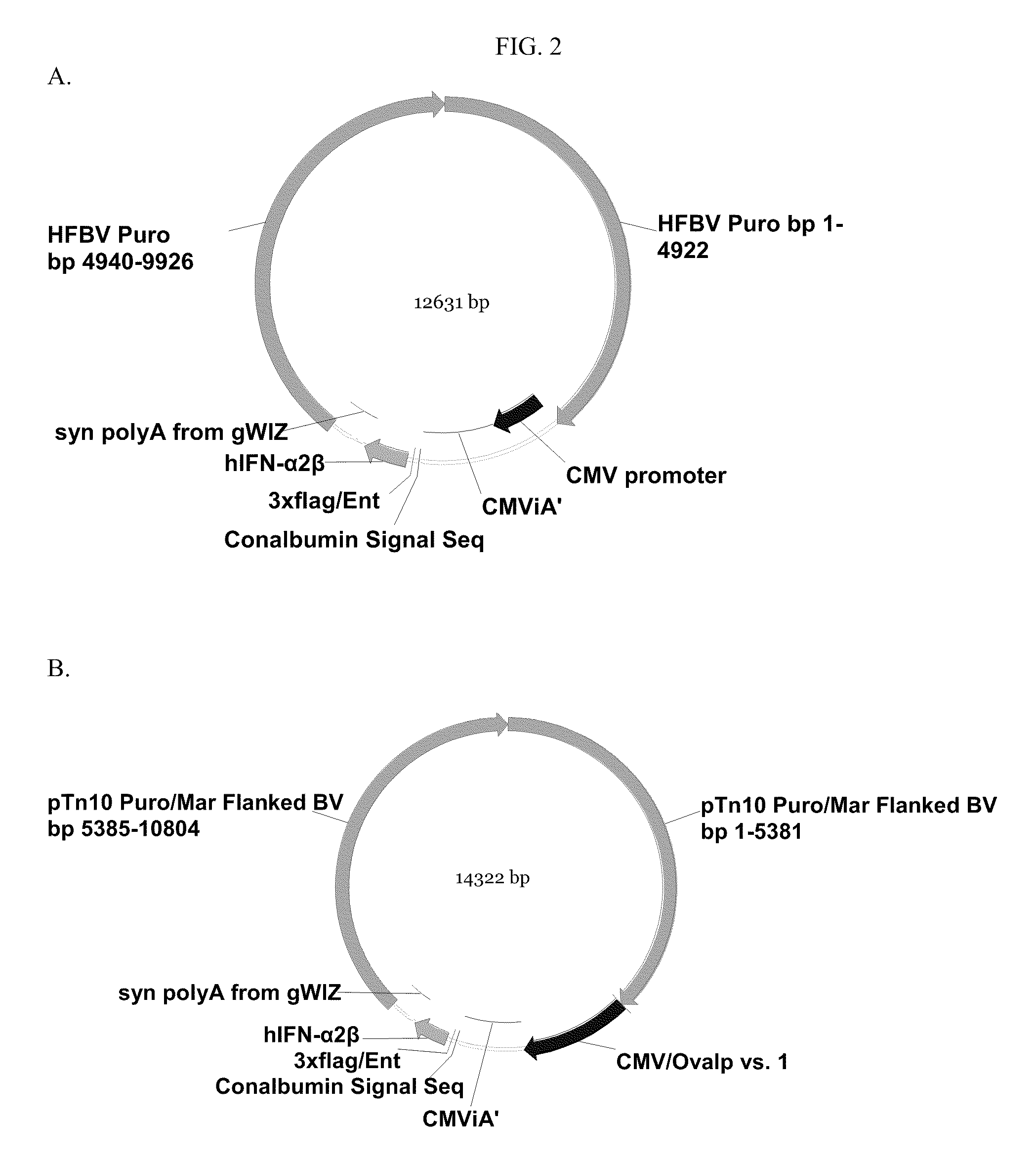
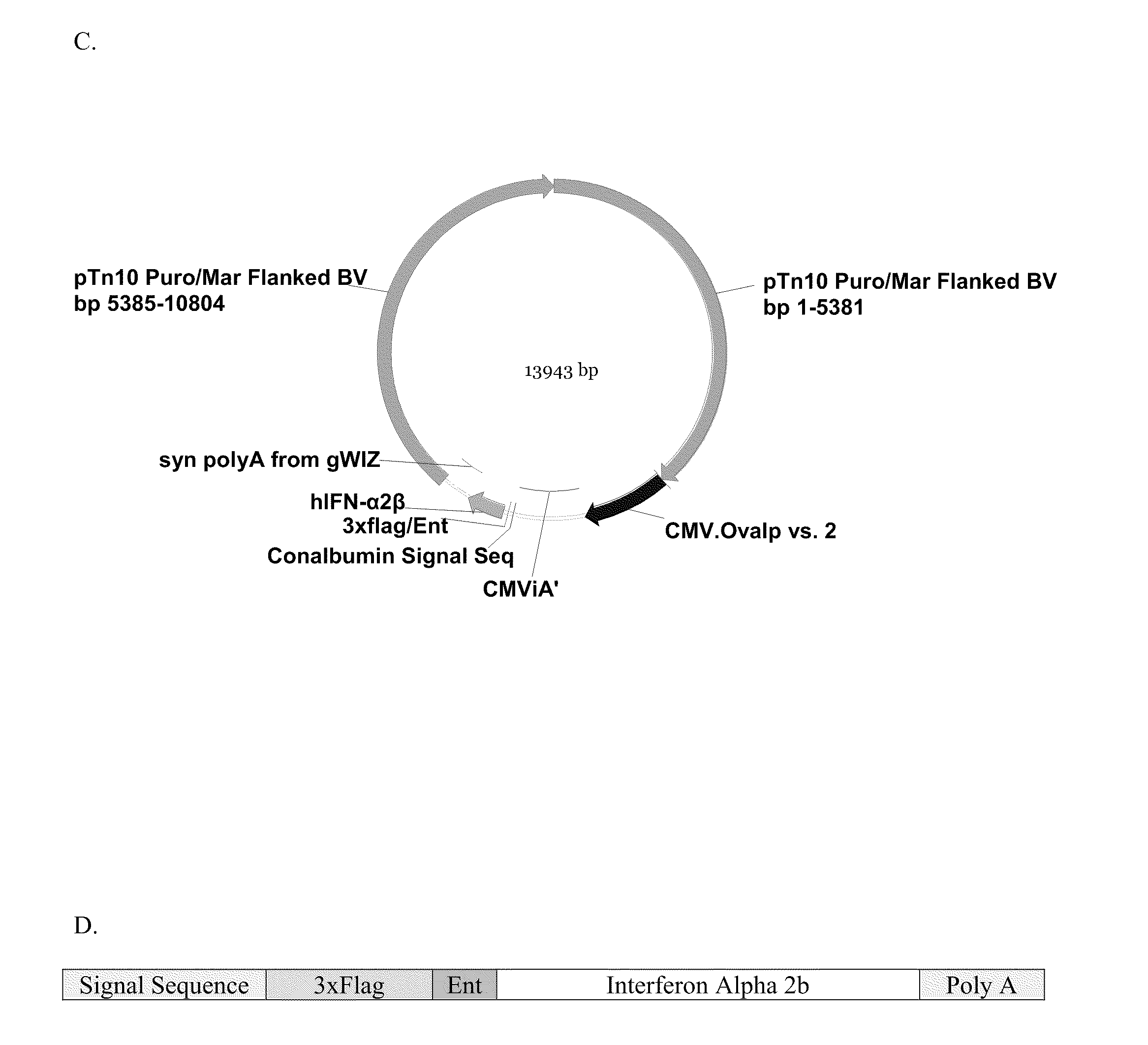
InactiveUS20100081789A1Efficient productionSugar derivativesAntibody mimetics/scaffoldsInterferon β 1aInterferon α-2b
Novel compositions for the production of interferons such as interferon-α 2a, interferon-α 2b, or interferon-β 1a (IFN-α 2a, IFN-α 2b, or IFN-β 1a) are provided. The compositions comprise components of vectors, such as a vector backbone, a promoter, and a gene of interest that encodes an interferon such as IFN-α 2a, IFN-α 2b, or IFN-β 1a, and the vectors comprising these components. In certain embodiments, these vectors are transposon-based vectors. Also provided are methods of making these compositions and methods of using these compositions for the production of an interferon such as IFN-α 2a, IFN-α 2b, or IFN-β1a.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE +1
Polynucleotides encoding interferon gamma peptide variants
InactiveUS20050201982A1Reducing or avoiding C-terminal heterogeneity of an IFNG polypeptideSugar derivativesPeptide/protein ingredientsNucleotideNucleotide level
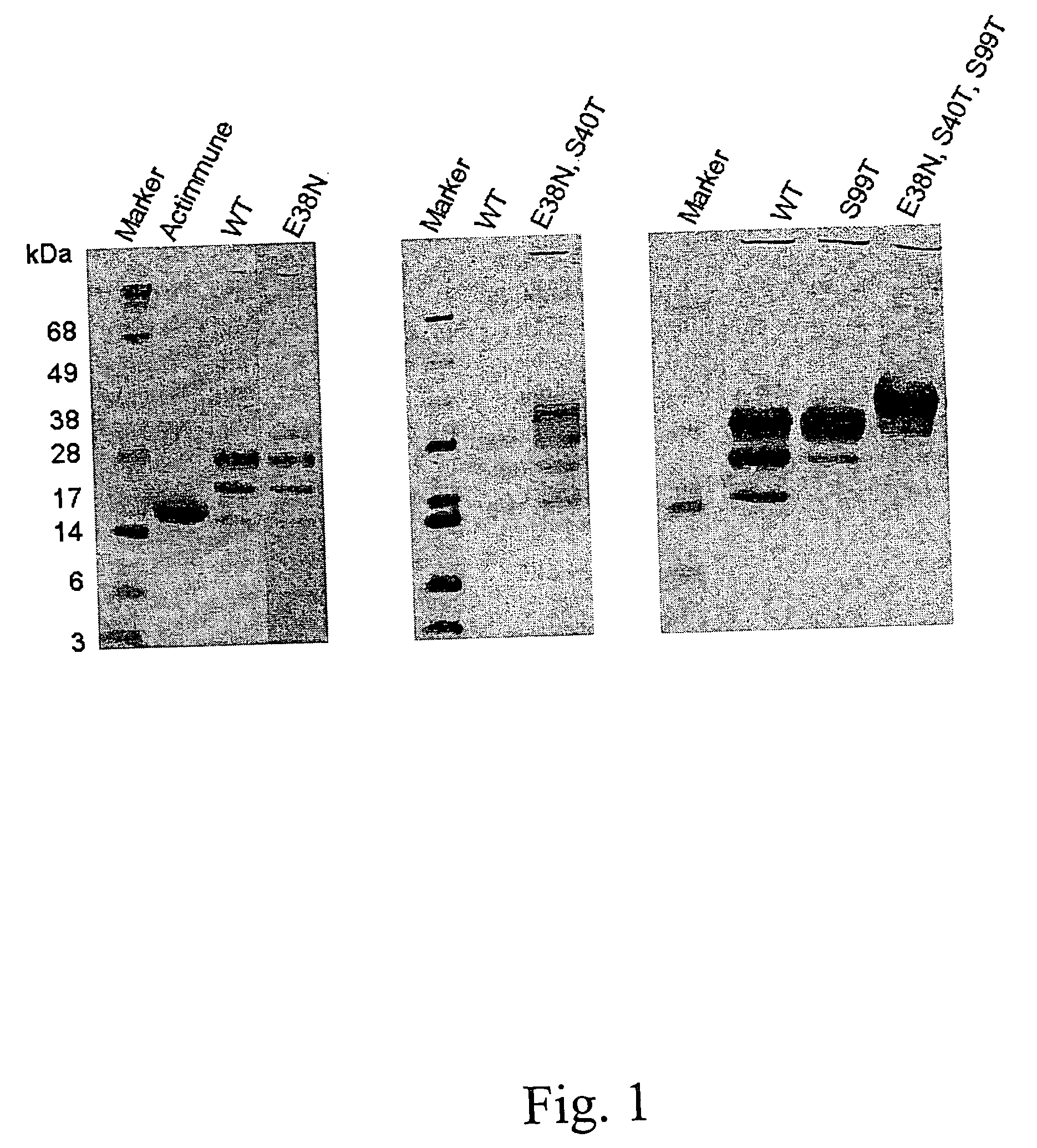
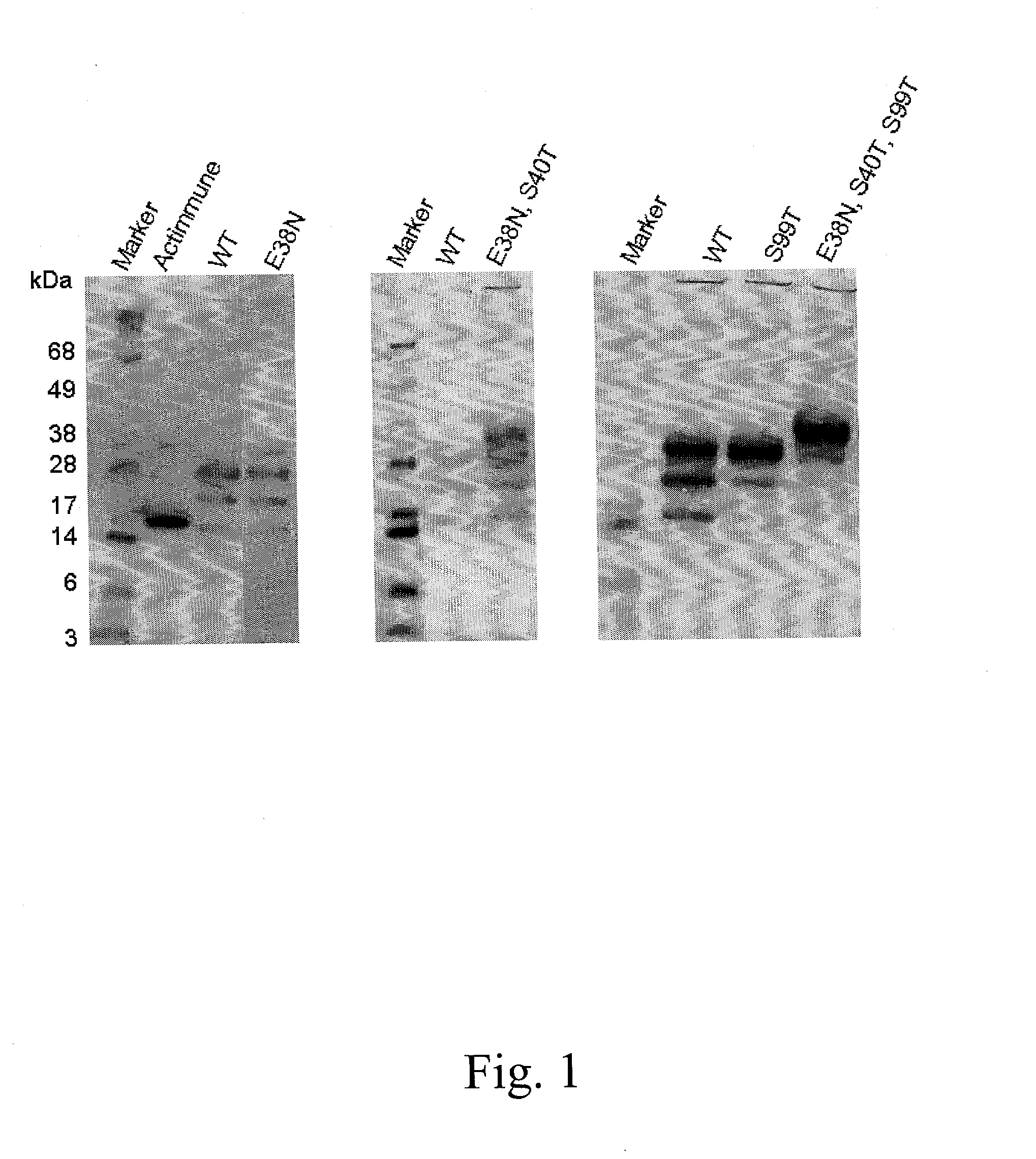
When interferon gamma (IFNG) is produced in mammalian cell lines a heterogenous population of IFNG polypeptides is obtained due to C-terminal processing of the IFNG polypeptide. Clearly, this constitutes a severe problem in that valuable polypeptide material is lost and, further, it is necessary to carry out time-consuming and cumbersome purification in order to obtain a homogenous population of active IFNG polypeptides having the desired length. It has now been found that an IFNG fragment containing 132 amino acid residues (truncated at the nucleotide level by introducing a stop-codon after the codon encoding amino acid residue no. 132) does not undergo C-terminal truncation or, at least, is not significantly C-terminally truncated. Furthermore, as the IFNG fragment containing 132 amino acid residues is active, this opens up the possibility of producing a homogenous active IFNG polypeptide in eukaryotic host cells, such as CHO cells. More particularly, the present invention relates to an IFNG polypeptide variant exhibiting IFNG activity and having the amino acid sequence shown in SEQ ID NO:12. In a highly preferred embodiment of the invention, the variant comprises at least one further modification, such as 1-10 further modifications, relative to the amino acid sequence shown in SEQ ID NO:12. A particular preferred further modification is E38N+S40T.
Owner:PERSEID THERAPEUTICS
Blood-based biomarkers of tumor sensitivity to pd-1 antagonists
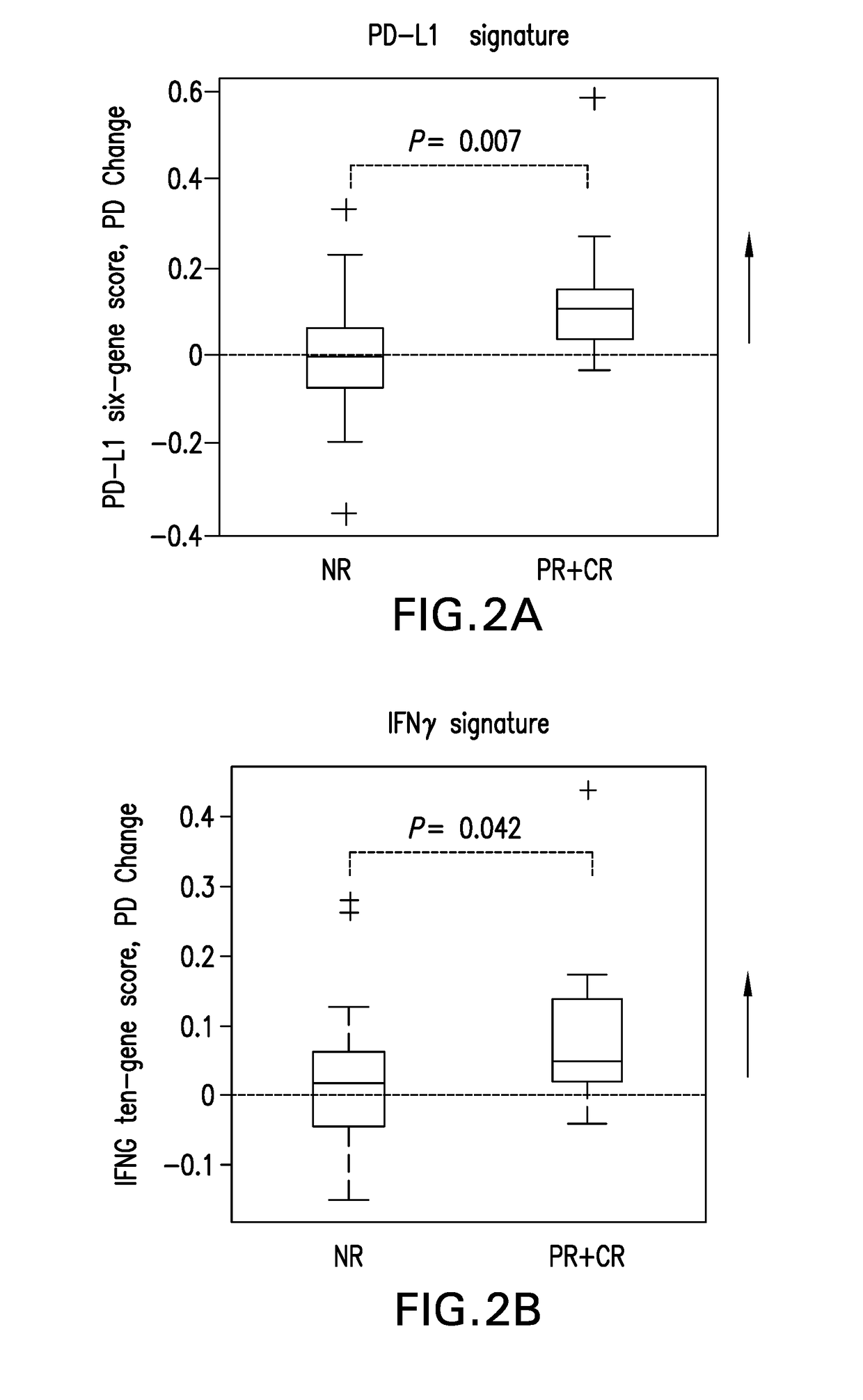
ActiveUS20180148790A1Inhibit bindingMicrobiological testing/measurementDisease diagnosisPhosphorylationPD-L1
The present disclosure describes baseline and on treatment blood-based gene signature biomarkers that are predictive of tumor sensitivity to therapy with a PD-1 antagonist. The on-treatment biomarkers comprise a PD-L1 gene signature or an interferon gamma gene signature and the baseline gene signature biomarker comprises genes associated with the oxidative phosphorylation pathway. The disclosure also provides methods and kits for testing tumor samples for these biomarkers, as well as methods for treating subjects with a PD-1 antagonist based on the test results.
Owner:MERCK SHARP & DOHME LLC
Anti-interferon alpha monoclonal antibodies and methods for use
Owner:BAYLOR RES INST
Enhancement of Immune Response to Vaccine by Interferon Alpha
InactiveUS20090022761A1Enhance immune responseSsRNA viruses positive-senseSugar derivativesDiseaseAdjuvant
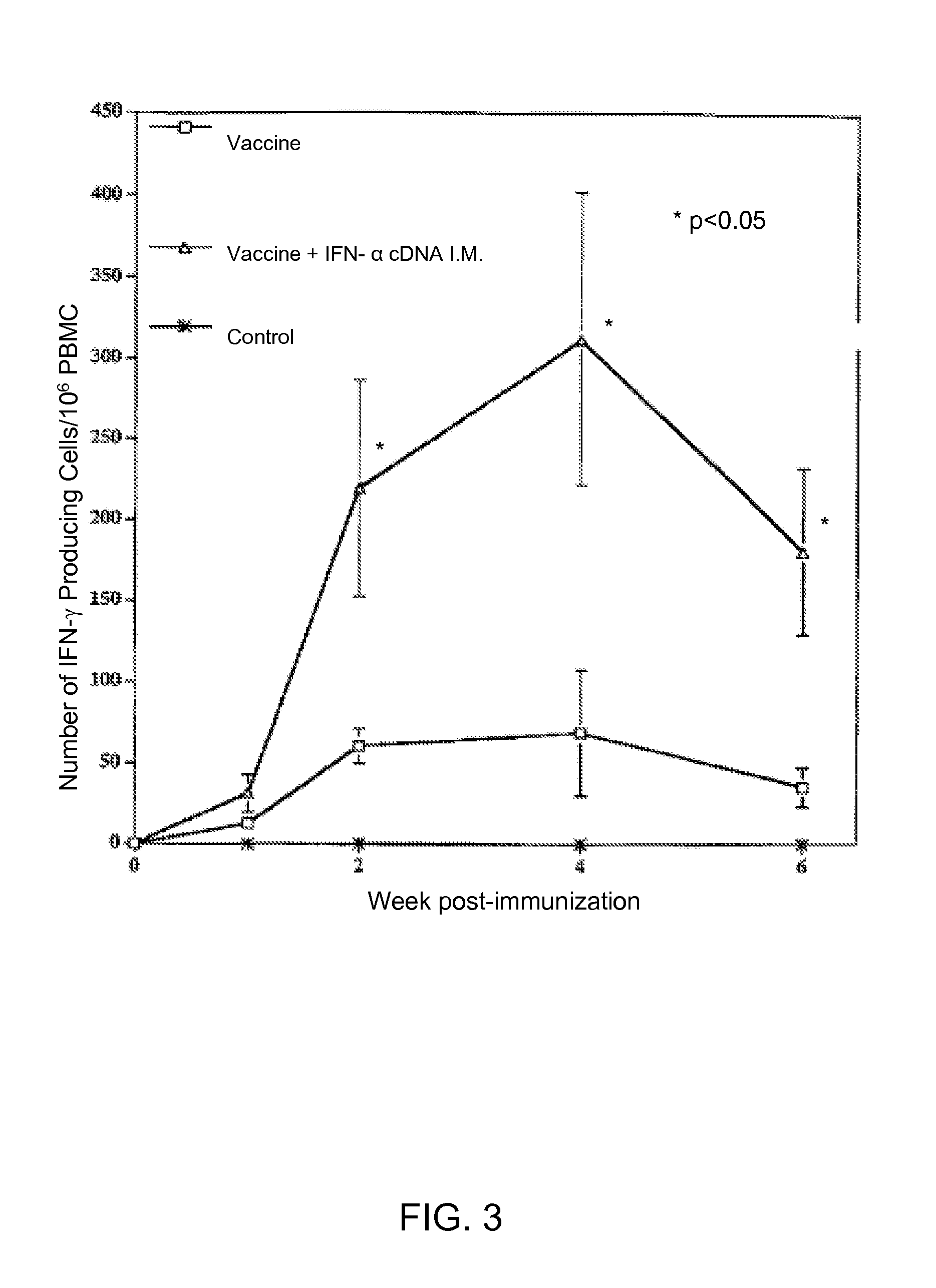
Exogenous cDNA capable of expressing interferon″ activity, exogenous interferon″ protein, inducers of endogenous interferon″ protein activity, inducers of endogenous interferon $ protein activity, inducers of endogenous interferon′ activity, or inducers of other immune-enhancing activity can be combined with a vaccine to enhance an immune response. Specifically disclosed are adjuvant and vaccine combinations where the adjuvant comprises a cDNA capable of expressing interferon″ activity, a complex comprising polyriboinosinic-polyribocytidilic acid, or a complex comprising polyriboinosinic-polyribocytidilic acid, poly-L-lysine, and carboxymethylcellulose and where the vaccine is a live vaccine virus derived from a virus causing porcine reproductive and respiratory syndrome disease.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Interferon gamma polypeptide variants
InactiveUS20020192183A1Renal clearance is reducedHigh molecular weightSugar derivativesPeptide/protein ingredientsInterstitial lung diseaseDisease
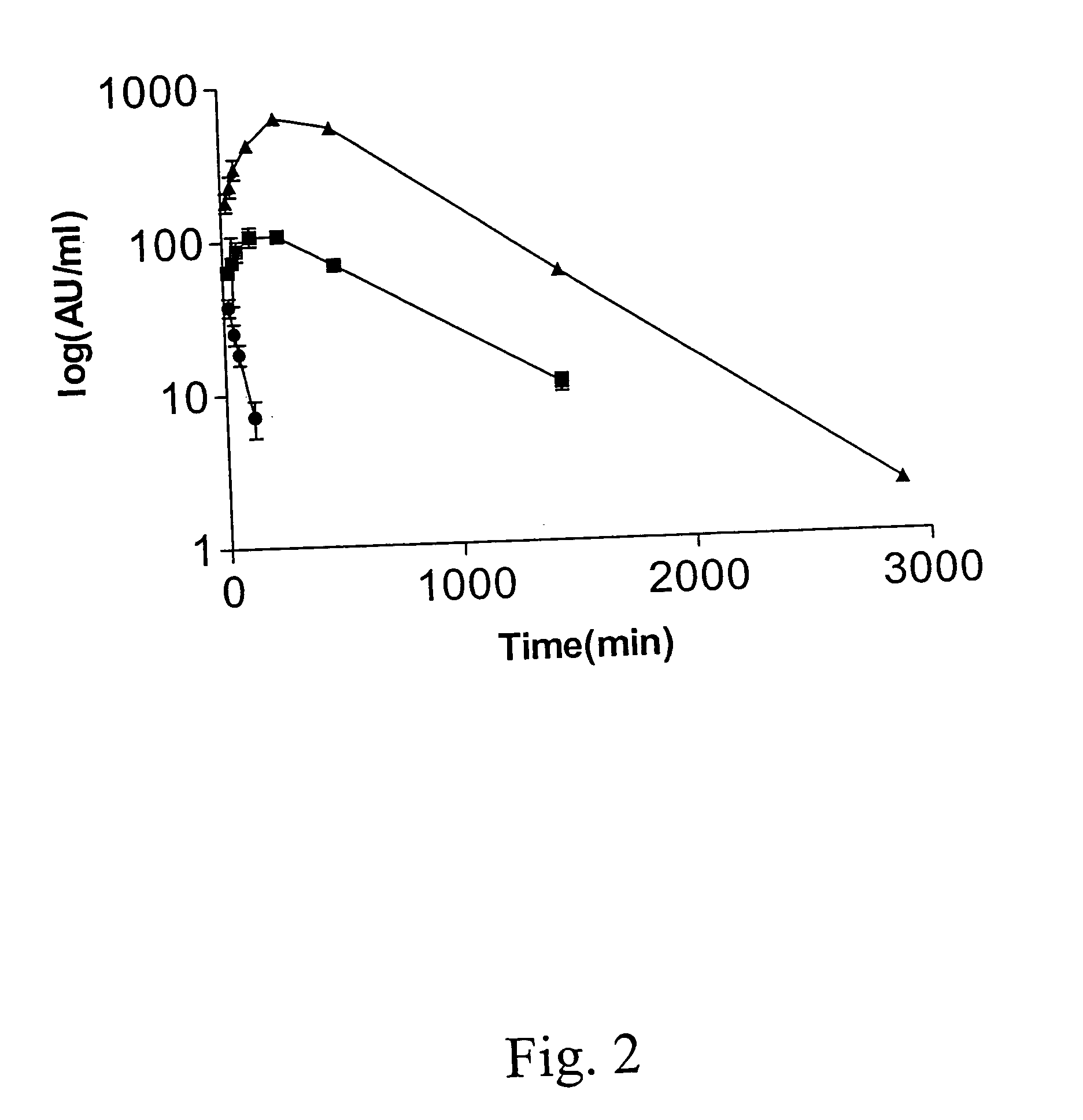
The present invention relates to novel interferon gamma polypeptide variants having interferon gamma (IFNG) activity, methods for their preparation, pharmaceutical compositions comprising the polypeptide variants and their use in the treatment of diseases, in particular for the treatment of interstitial pulmonary diseases, such as idiopathic pulmonary fibrosis. These novel polypeptide variants all comprise the substitution S99T as compared to the amino acid sequence of huIFNG or fragments thereof. By performing this mutation the naturally occurring N-glycosylation site present at position 97 is significantly better utilized. Preferably, the variants comprise further modifications, e.g. in order to increase the AUC of such variants when administered subcutaneously.
Owner:PERSEID THERAPEUTICS
Peptide-based inhibitor of interleukin-10 or interferon-gamma signaling
ActiveUS20130109619A1Suppression problemInhibition of activationBiocideAntipyreticInterleukin 10White blood cell
A peptide or peptidomimetic comprising an amino acid sequence based on conserved regions of IL10 or IFN-gamma receptor sequences, and related compounds and compositions, as well as methods for the use thereof to inhibit cytokine signaling.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Method for producing recombinant human serum albumin-interferon alpha 2b
ActiveCN101768601AImprove the purification effectEasy to operateMicroorganism based processesAntiviralsPichia pastorisYeast
The present invention relates to a method for producing recombinant human serum albumin-interferon alpha 2b of secretory expression in Pichia pastoris system, particularly to efficient expression of recombinant human serum albumin-interferon alpha 2b in Pichia pastoris and expression product purification. In the present invention, Pichia pastoris engineering bacteria containing recombinant human serum albumin-interferon alpha 2b are fermented in high density, fermented liquid is subsequently centrifuged by a high-speed centrifugal machine, and fermented supernatant liquid is collected and purified by cation exchange chromatography, hydrophobic layer chromatography and molecular sieve chromatography in a combined mode. Recombinant human serum albumin-interferon alpha 2b fusion protein produced meets the regulations of Chinese Pharmacopoeia. The present invention has the advantages of high fermentation expression rate, reasonable purification technical combination, simplified operation procedure, high yield and low production cost. The present invention meets industrial requirements.
Owner:山东泉港药业有限公司
Modified interferon beta with reduced immunogenicity
InactiveUS7381795B2Modify characteristicExtended circulation timeSugar derivativesPeptide/protein ingredientsInterferon alphaIn vivo
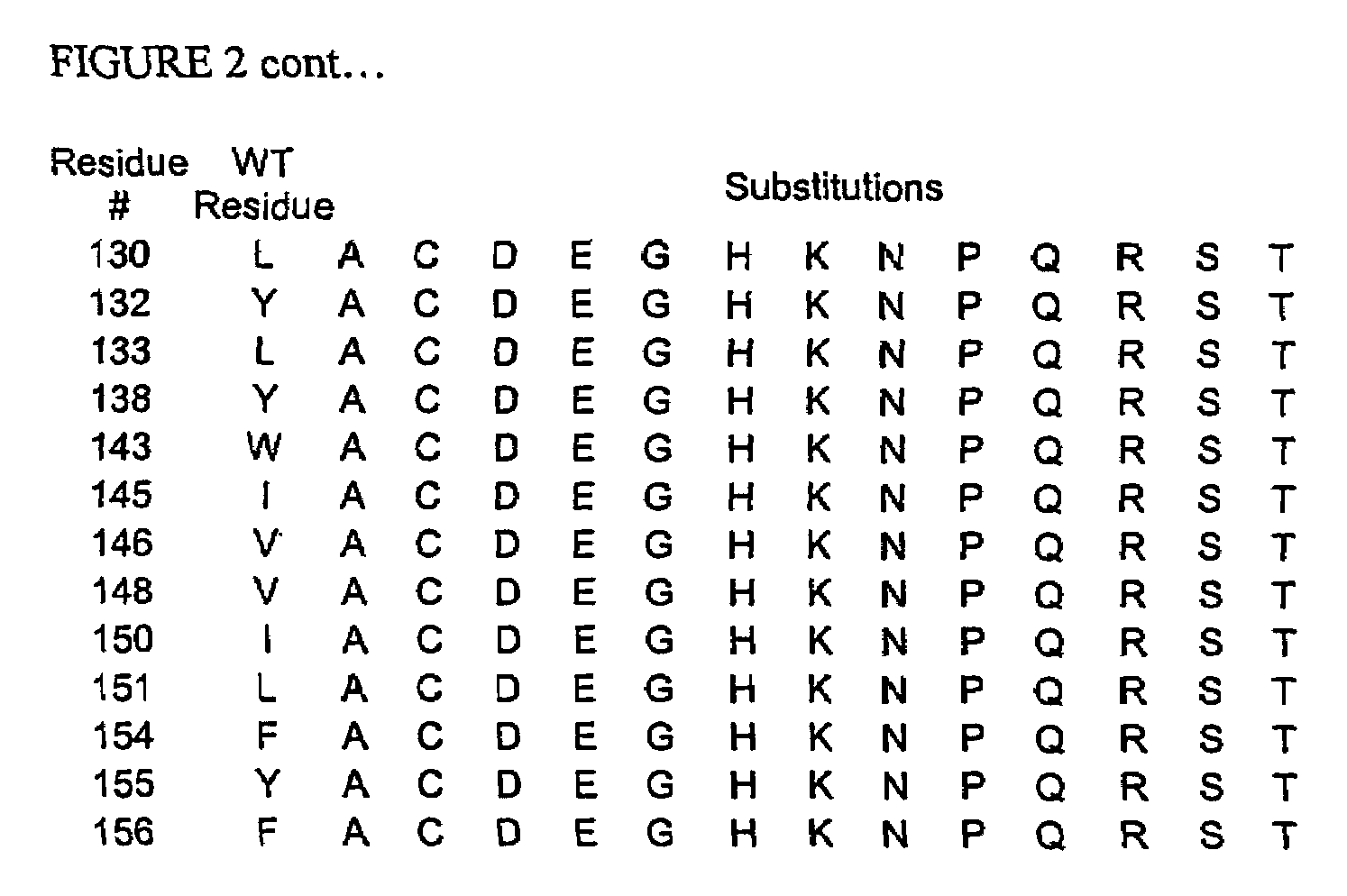
A modified interferon beta (INFβ) is provided, which is less immunogenic than human INFβ (SEQ ID NO: 1) when administered to a human in vivo. The modified INFβ comprises an amino acid residue sequence that differs from SEQ ID NO: 1 by a substitution at one or more residues of SEQ ID NO: 1. Preferred substitutions are at residues selected from the group consisting of residue 50, 59, 60, 62, 63, 66, 67, 69, 70, 125, 126, 129, 130, 132, 133, and 138. Examples of suitable substitutions include F50A, L57A, I59A, Y60N, M62A, L63A, I66T, F67H, I69A, F70A, Y125A, Y126A, I129A, L130A, Y132S, L133A, Y138H, and Y138A.
Owner:MERCK PATENT GMBH
Recombinant fusion interferon for animals
ActiveUS20140030222A1Treating and inhibiting virus infectionHybrid immunoglobulinsPeptide/protein ingredientsInterferon alphaAntibody
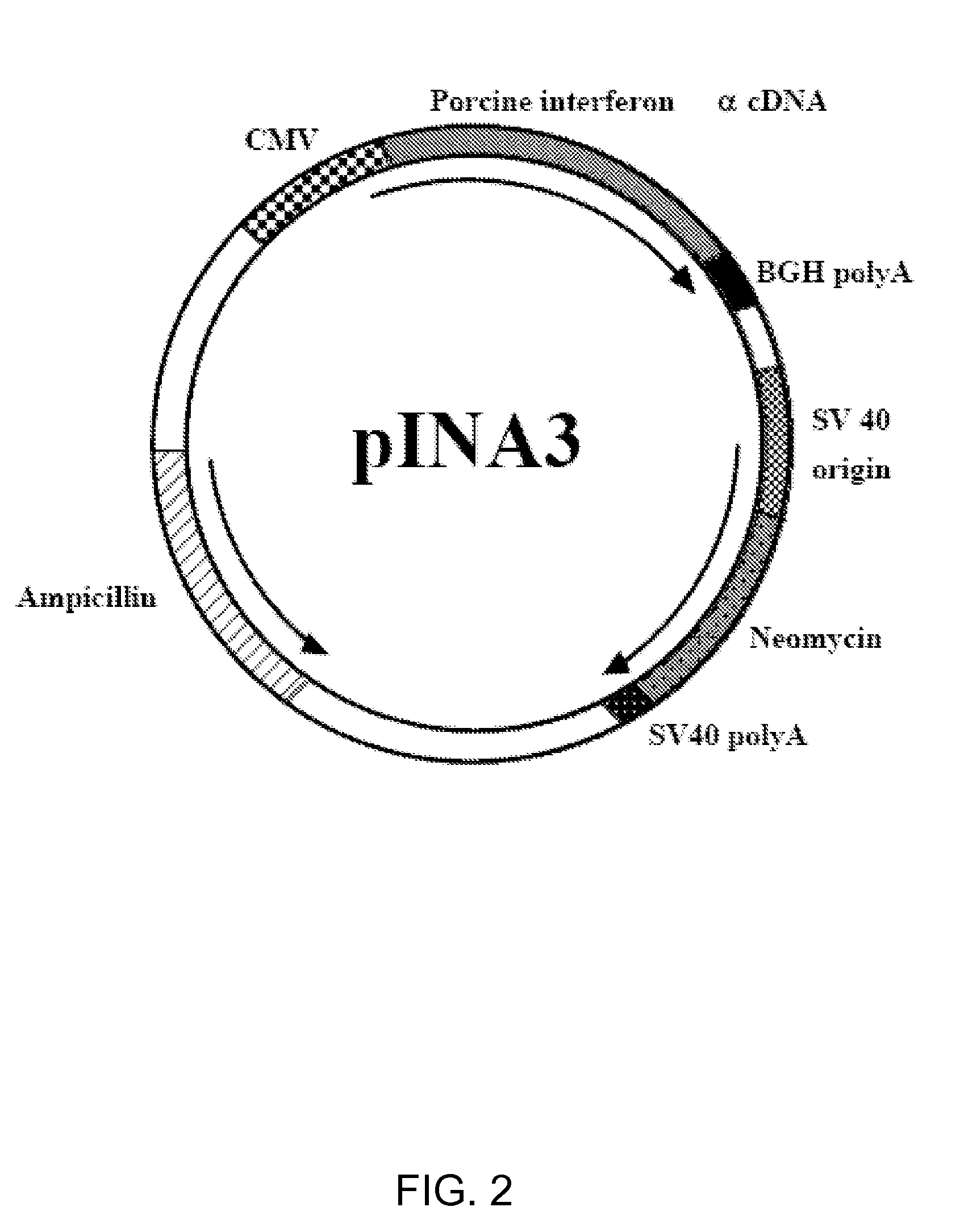
The present invention relates to a recombinant fusion interferon for animals, a pharmaceutical composition thereof, and the use of the recombinant fusion interferon. The recombinant fusion interferon is represented by formula (I) or formula (II),(Porcine interferon)-(Linker)n-(Porcine immunoglobulin Fc fragment) (I)(Porcine immunoglobulin Fc fragment)-(Linker)n-(Porcine Interferon) (II)wherein n is 0 or a positive integer between 1 to 10, the recombinant fusion IFN specifically binds an antibody that specifically binds porcine interferon and an antibody that specifically binds porcine immunoglobulin Fc fragment.
Owner:VIRBAC H K TRADING LTD
Interferon alpha responsive protein
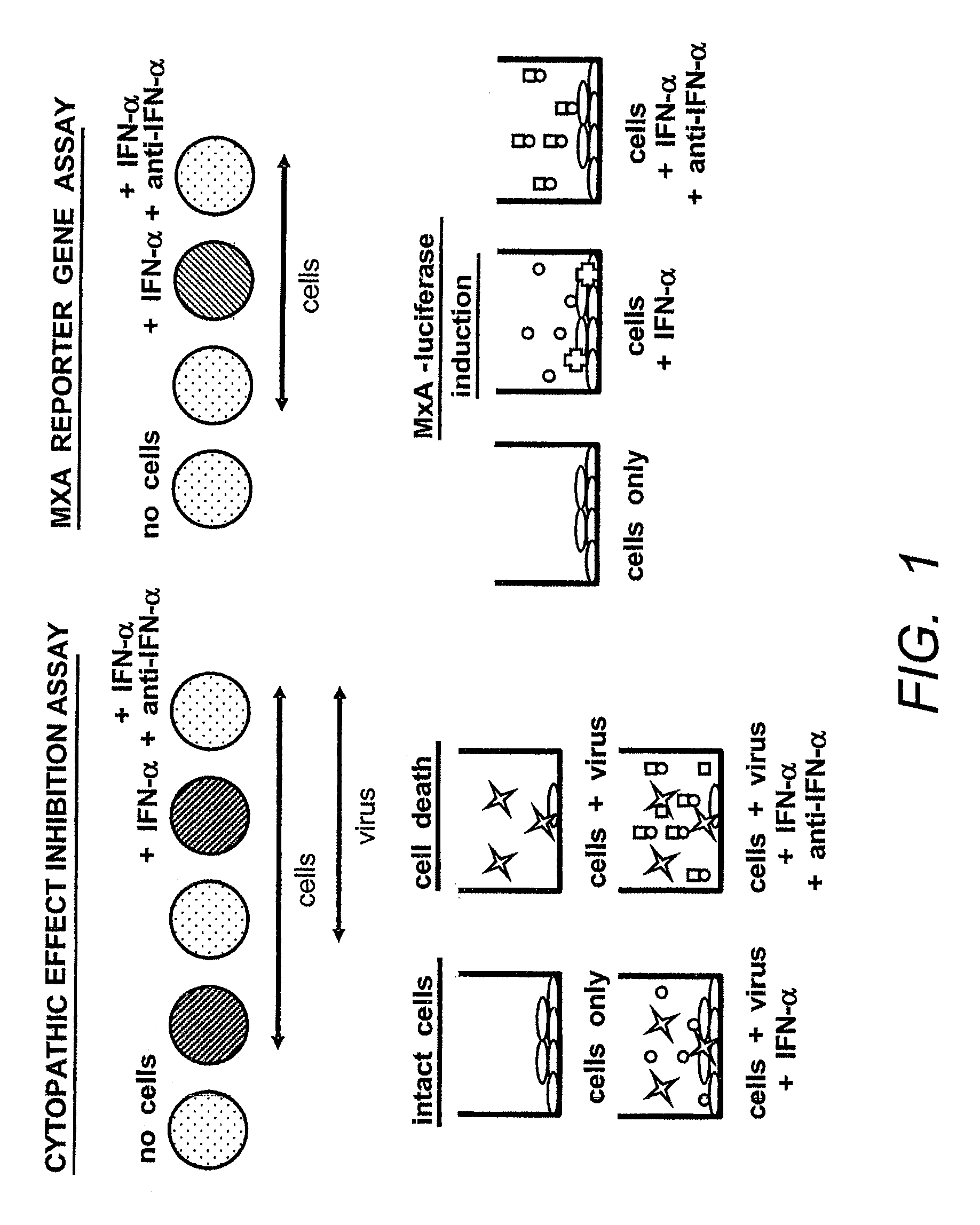
The present invention relates to identification of a gene upregulated by interferon-α administration corresponding to the cDNA sequence set forth in SEQ ID NO: 1. Determination of expression products of this gene is proposed as having utility in predicting responsiveness to treatment with interferon-a and other interferons which act at the Type 1 interferon receptor. Therapeutic use of the protein encoded by the same gene is also envisaged.
Owner:PHARM PACIFIC PTY LTD
Antiviral Activity of Bovine Type III Interferon Against Foot-and-Mouth Disease Virus
ActiveUS20120164171A1Reduce degreeReduce rateOrganic active ingredientsSugar derivativesVaccinationInterferon alpha
Interferons are the first line of defense against viral infections and administration of interferons as biotherapeutics has been demonstrated to be effective in controlling several viral infections. Here we report for the first time the identification and characterization of a member of the bovine type III IFN family, boIFN-λ3. We have expressed boIFN-λ3 using a recombinant replication defective human adenovirus type 5 (Ad5) and demonstrated antiviral activity against foot-and-mouth disease virus (FMDV) and vesicular stomatitis virus (VSV) in bovine cells in vitro. Furthermore, we have tested the efficacy of boIFN-λ3 against FMDV in vivo by inoculation of cattle with Ad5-boIFN-λ3 followed by intradermolingual or aerosol virus challenge. Our results demonstrate that the type III IFN family is conserved in bovines and that treatment of cattle with boIFN-λ3 alone or in combination with IFN-α is able to confer delayed and reduced severity of FMD. Furthermore inoculation with Ad5-boIFN-λ3 alone conferred full protection against aerosol challenge for at least 7 days after administration suggesting that type III IFN used in combination with FMD vaccines could fill one of the current gaps in emergency vaccination against FMDV.
Owner:US SEC AGRI
Chicken alpha interferon/interleukin 2 chimeric gene
ActiveCN102041263ARapid purificationPurify from goodPeptide/protein ingredientsAntiviralsProtein activityIn vivo
The invention belongs to the technical field of biologic genetic engineering, and discloses a chicken alpha interferon (ChIFN-alpha) / interleukin 2(ChIL-2) chimeric gene, the technology comprises using an overlap extension PRC technique to constitute a mature peptide gene of the ChIFN-alpha and the ChIL-2 as a ChIFN-alpha-linker-ChIL-2 chimeric gene through a gene flexible linker (linker)(G4S), and cloning the constituted gene into a pGEM-TEasy carrier, subcloning the chimeric gene in a pQE-30 expression carrier to perform prokaryotic expression and perform rapid renaturation and purification to the expressed recombinant fusion protein. The activities of the purified rChIFN-alpha-liner-ChIL-2 fusion protein on the cell and in vitro and in vivo of the chicken are all provided with the superposition of the protein activity of the ChIFN-alpha and the ChIL-2, and the antiviral activity thereof is superior to the rChIFN-alpha protein. The chicken alpha interferon (ChIFN-alpha)interleukin 2(ChIL-2) chimeric gene is used as the main component of the antiviral preparation for preventing and treating the viral disease of the chicken, which is efficient, broad-spectrum, safe and low in cost.
Owner:HENAN CENT FOR ANIMAL DISEASE CONTROL & PREVENTION
Interferon-beta-1a-immunoglobulin fusion proteins and uses
InactiveUS7527946B2High activityNo effective loss in activitySenses disorderNervous disorderBeta interferonsMutant
A fusion polypeptide is described having the amino acid sequence X-Y-Z, or portion thereof, comprising the amino acid sequence of a glycosylated interferon-beta (X); Y is an optional linker moiety; and Z is a polypeptide comprising at least a portion of a polypeptide other than glycosylated interferon-beta. It is preferred that X is human interferon-beta-1a. Mutants of interferon-beta-1a are also described.
Owner:BIOGEN MA INC
Enhancement of immune response to vaccine by interferon alpha
Exogenous cDNA capable of expressing interferon α activity, exogenous interferon α protein, inducers of endogenous interferon α protein activity, inducers of endogenous interferon β protein activity, inducers of endogenous interfereon Γ activity, or inducers of other immune-enhancing activity can be combined with a vaccine to enhance an immune response. Specifically disclosed are adjuvant and vaccine combinations where the adjuvant comprises a cDNA capable of expressing interferon α activity, a complex comprising polyriboinosinic-polyribocytidilic acid, or a complex comprising polyriboinosinic-polyribocytidilic acid, poly-L-lysine, and carboxymethylcellulose and where the vaccine is a live vaccine virus derived from a virus causing porcine reproductive and respiratory syndrome disease.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Efficiently expressed series porcine alpha and gamma interferon genes and application of expressed protein thereof
ActiveCN102212539AHigh expression activityPrevent proliferationPeptide/protein ingredientsAntiviralsPig farmsSynthesis methods
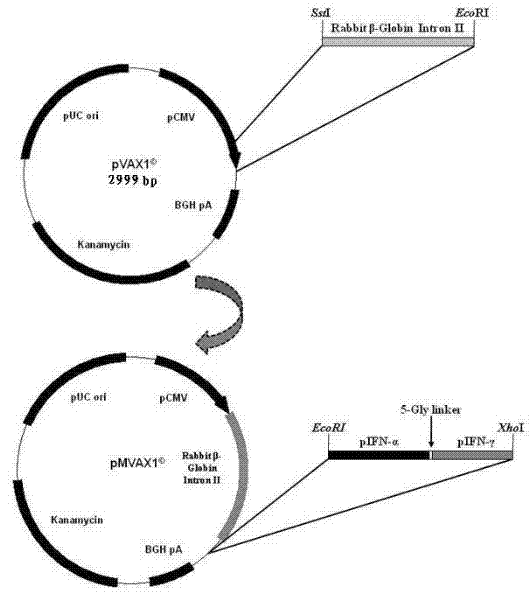
The invention relates to the field of gene expression, in particular to efficiently expressed series porcine alpha and gamma interferon genes. The nucleotide sequence of the interferon genes is shown as a sequence 2. The genes of the sequence 2 are cloned to eukaryotic expression vectors to obtain recombinant plasmids. A synthesis method of the series porcine alpha and gamma interferon genes comprises the following steps of: inserting Rabbit beta-GlobinIntronII, a pCMV immediate early promoter and a T7 promoter into pVAX1 to obtain pMVAX1, and inserting fusion protein genes pIFN-alpha / gamma into pMVAX1 to obtain pMVAX1-pIFN-alpha / gamma. The invention also relates to application of expression protein of the interferon genes in preparation of a medicament for treating porcine reproductive and respiratory syndrome. Through the interferon genes, the limitations of low expression quantity and poor activity when pIFN-alpha and pIFN-gamma are expressed through the pVAX1 are broken, the activity reaches 1*10<8.0>U / 0.1mL, the multiplication of high pathogenic porcine reproductive and respiratory syndrome viruses (PRRSV) can be obviously inhibited, and the economic loss of a pig farm is reduced.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Pre-filled recombinant human interferon injection
The invention relates to a pre-filled recombinant human interferon injection which is prepared from recombinant human interferon, arginine hydrochloride or lysine hydrochloride, acetic acid-acetate buffer solution with the pH value of 4.5 to 5.5 and tween-80; the injection prepared by the formula of the invention can be immediately filled into a small glass bottle for injection, or can be directly filled into a prefilled syringe; the injection of the invention is filled into the small glass bottle, the problems of a large amount of protein aggregation and greatly reduced interferon titer caused by the surface absorption of the small glass bottle are solved, and after being stored for a long time, interferon still has quite high titer.
Owner:BEIJING KAWIN TECH SHARE HLDG +1
Mature chicken interferon-alpha gene capable of high-efficiency expression and preparation method of polypeptide thereof
InactiveCN102121013AGood refoldingImprove the purification effectMicroorganism based processesFermentationEscherichia coliCompetent cell
The invention provides a preparing method of a mature chicken interferon-alpha polypeptide, relates to a preparing method of a chicken interferon-alpha gene polypeptide, and the problems that the conventional recombinant chicken interferon-alpha gene cannot be expressed in escherichia coli, or has low expression level, and the recombinant chicken interferon-alpha has poor renaturation effect in preparation are solved. The method comprises the following steps of: 1, synonymously substituting all rare codons in the mature chicken interferon-alpha gene into escherichia coli preference codons, synthesizing to obtain optimized chicken interferon-alpha gene; 2, cloning to an expression vector, and then converting into an escherichia coli competent cell, inductively expressing after screening, centrifuging to obtain humidin, and extracting and dissolving an inclusion body; and 3, renaturing and purifying to collect protein. The mature chicken interferon-alpha gene can be expressed in high efficiency in the escherichia coli, the expression level can reach 30 percent of total mycoprotein, and the renaturation effect is good.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Fusion protein of chicken interferon IFN-lambda and IFN-alpha
ActiveCN106674354AHigh concentration proteinGood antiviral effectPeptide/protein ingredientsAntibody mimetics/scaffoldsEscherichia coliAnti virus
The invention belongs to the technical field of biological engineering, and discloses fusion expression of chicken interferon genes lambda and alpha of an interferon fusion preparation, a production method and clinical application thereof. According to the interferon fusion preparation of biological engineering, total RNA (Ribonucleic Acid) of CEF cells is extracted, a specific primer is designed, the chicken interferon genes lambda and alpha are cloned and are fused by using a complementary hydrophobic flexible amino acid connector, and thus the complete chicken interferon fusion genes lambda and alpha can be obtained; the chicken interferon fusion genes lambda and alpha are cloned to a 19-T carrier, is massively cloned and expressed successfully, and is further connected with a pET-32a carrier for massive expression; a product is identified to ensure that the product is expressed in an inclusion body, and then modification, purification and renaturation are implemented; the anti-virus activity of the product is detected, and the clinical application effect of the product is evaluated. Prokaryotic expression plasmid pET32a-chIFN-lambda+alpha of the recombinant chicken interferon genes lambda and alpha is successfully established, and 1:1 fusion expression of the chicken interferon genes lambda and alpha on an escherichia coli prokaryotic expression system can be achieved.
Owner:SOUTH CHINA AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com