Method for producing recombinant human serum albumin-interferon alpha 2b
A technology of human serum albumin and human serum albumin, which is applied in the field of recombinant DNA technology to produce genetically engineered protein drugs, can solve the problems of virus suppression, restriction, and short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
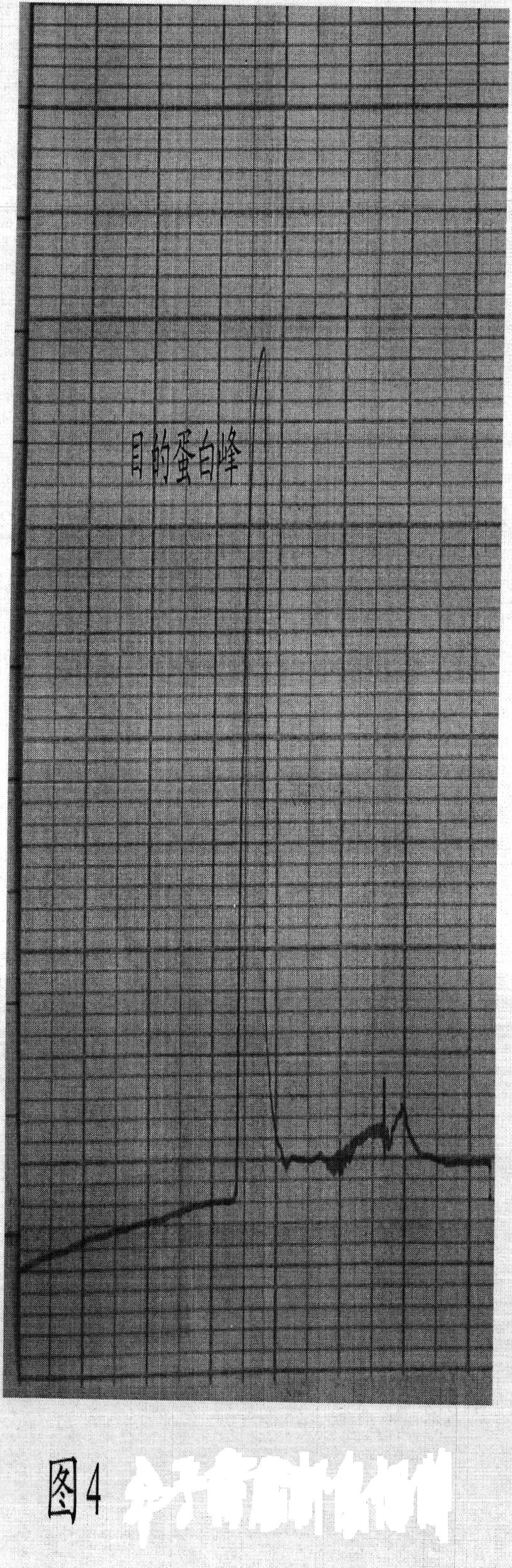
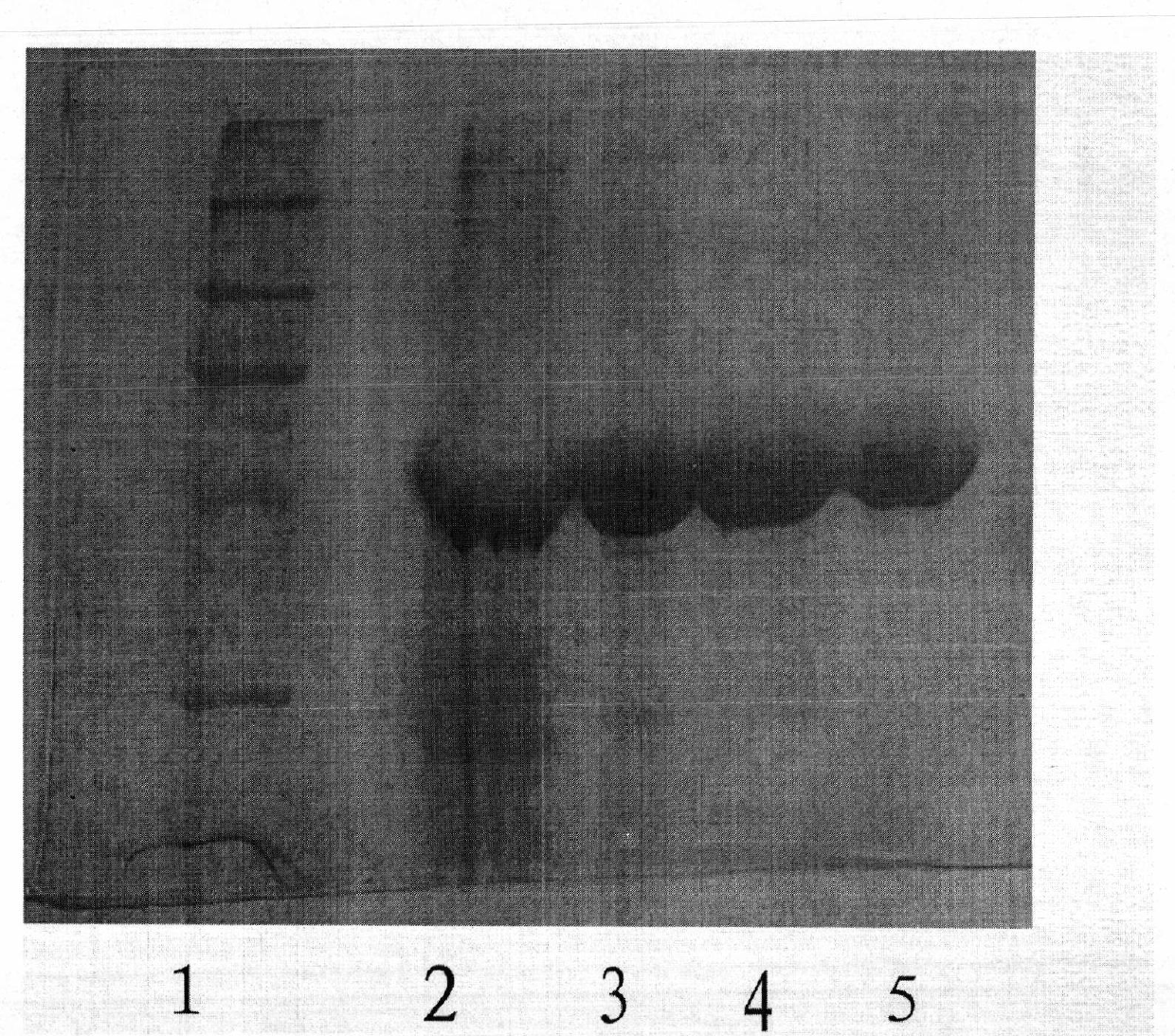
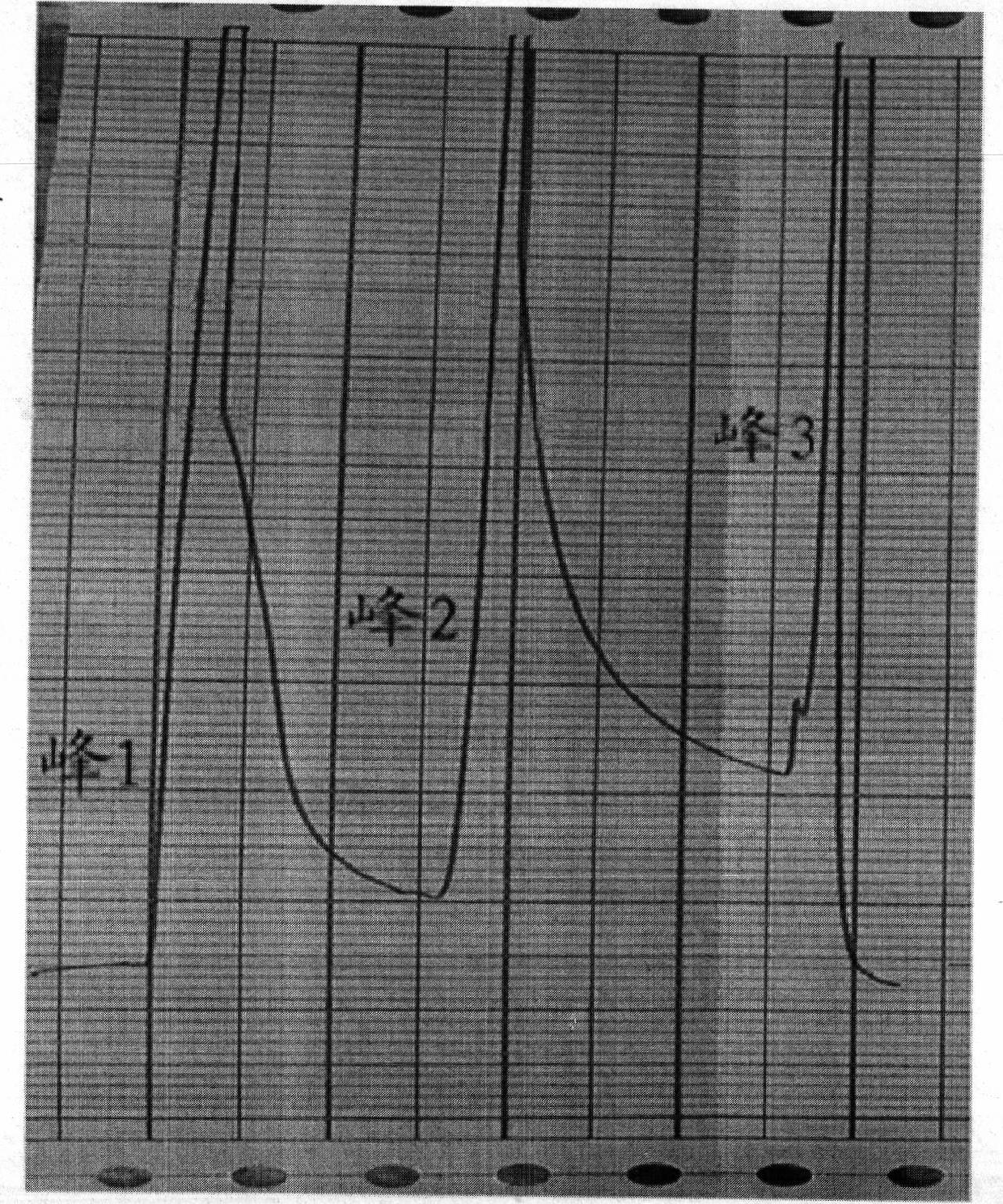
Image
Examples
Embodiment Construction
[0025] 1. Construction of recombinant human serum albumin-interferon α2b Pichia pastoris engineering strain
[0026] 1. Acquisition of the target gene
[0027] According to the sequence information of the HSA gene published in GeneBank, design PCR primers
[0028] XhoI-HSA-P5: 5'-cat ctc gag (endonuclease XhoI site) aaa aga (Kex2 protease cleavage site) gat gca cac aag agt gag gtt-3'
[0029] BamHI-HSA-P3: 5'-cat gga tcc (BamHI endonuclease site) taa gcc taa ggc agc ttgact tgc agc-3'
[0030] According to the sequence information of plasmid pHC16IFN-α2b from the existing interferon α2b engineering bacteria, design PCR primer BamHI-2b-P5: 5'-cat gga tcc (endonuclease BamHI site) tgt gat ctg cct caa accca-3'
[0031] NotI-2b-P3: 5'-cat gcggccgc (NotI site of endonuclease) tca ttc ctt act tct taaact ttc-3'
[0032] Use primers XhoI-HSA-P5 and BamHI-HSA-P3 to amplify the human HSA gene from the cDNA first-strand library, and XhoI-HSA-P5 primers add restriction endonuclease XhoI...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com