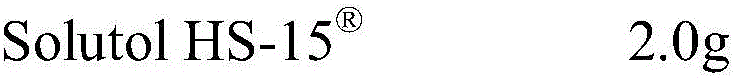Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
54 results about "Injection drug use" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Injecting drug use is associated with many local and systemic complications for the individual and is also associated with the transmission of infectious diseases via needle sharing and sexual activity. The most commonly injected drug is heroin, but amphetamines, buprenorphine, benzodiazepines, barbiturates, cocaine,...
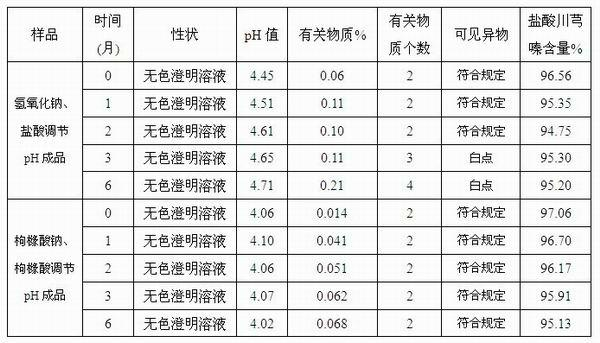
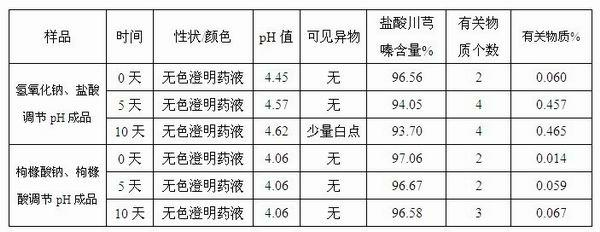
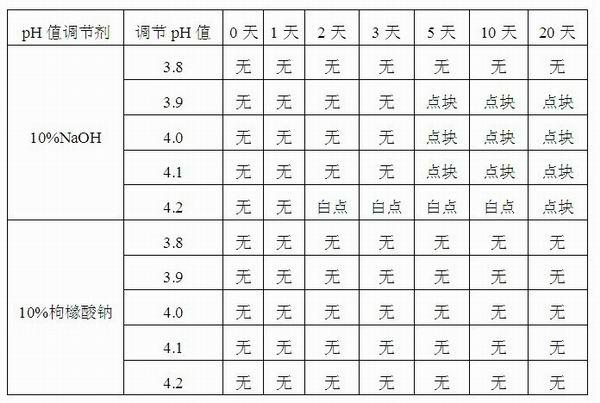
Injection-purpose medicine composition for improving stability of ligustrazine medicine injection formulation and preparation method of injection-purpose medicine composition
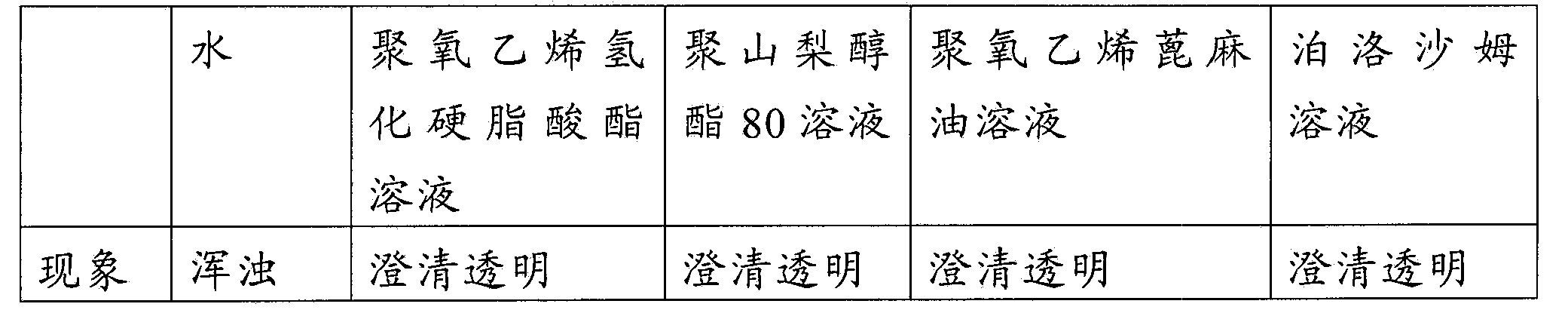
ActiveCN102008727AStable pHDecreased substancesOrganic active ingredientsPharmaceutical non-active ingredientsMedication injectionUse medication
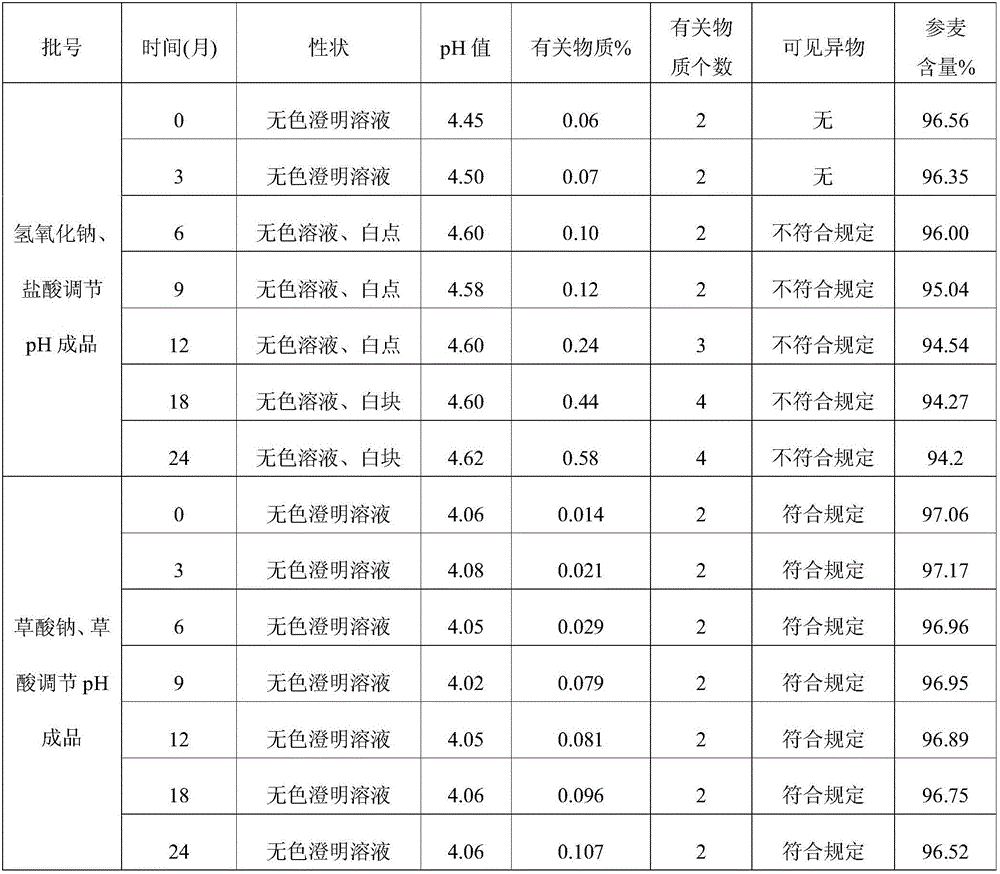
The invention discloses an injection-purpose medicine composition for improving stability of ligustrazine medicine injection formulation and a preparation method of the injection-purpose medicine composition. The injection-purpose medicine composition is prepared by the method comprising following steps: dissolving ligustrazine salt in water for injection, adjusting the pH value of the liquid medicine by adding citric acid and / or sodium citrate used as a pH regulator, wherein the dosage of the citric acid and / or sodium citrate ranges from (0.1mg to 200.0mg) / 100ml. In the invention, pH value of the injection liquid can be more stable, the content of ligustrazine degradation substance is greatly reduced compared with that of the prior art, the clarity of the ligustrazine injection liquid is improved under the condition of not using other cosolvents to increase the clinical application risk, particularly, the problems of small white spots, white blocks and solution turbidity are solved under the condition that the ligustrazine injection liquid is stored for a long time by adopting the prior art, and the medicine composition ensures that the inspection of visible foreign substances of the product is in accordance to the formulation of medicine quality standard, and is convenient for clinical medication and popularization.
Owner:SICHUAN SUNNYHOPE PHARM CO LTD
Pharmaceutical composition for improving safety of Shenmai injection and method for preparing same
ActiveCN101518617ADelays the problem of significant drops in pHImprove stabilityPowder deliveryPharmaceutical non-active ingredientsMedicineHydroxystearic Acid
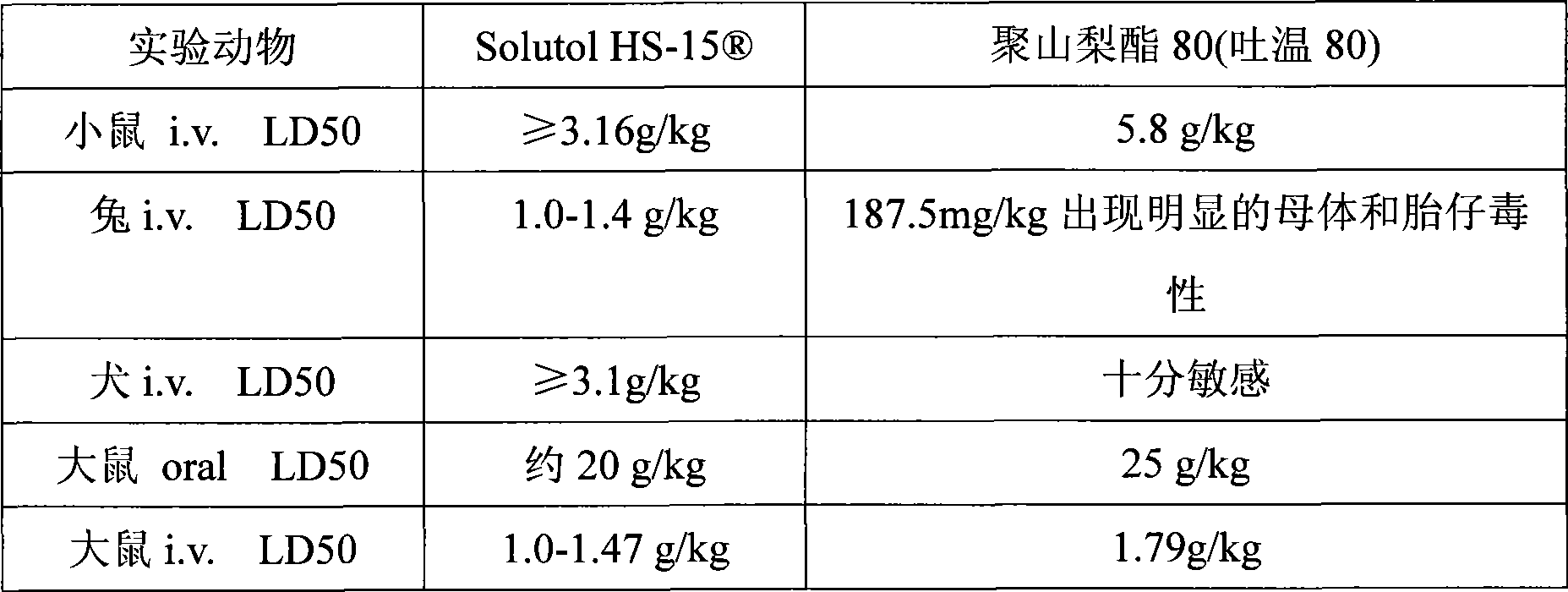
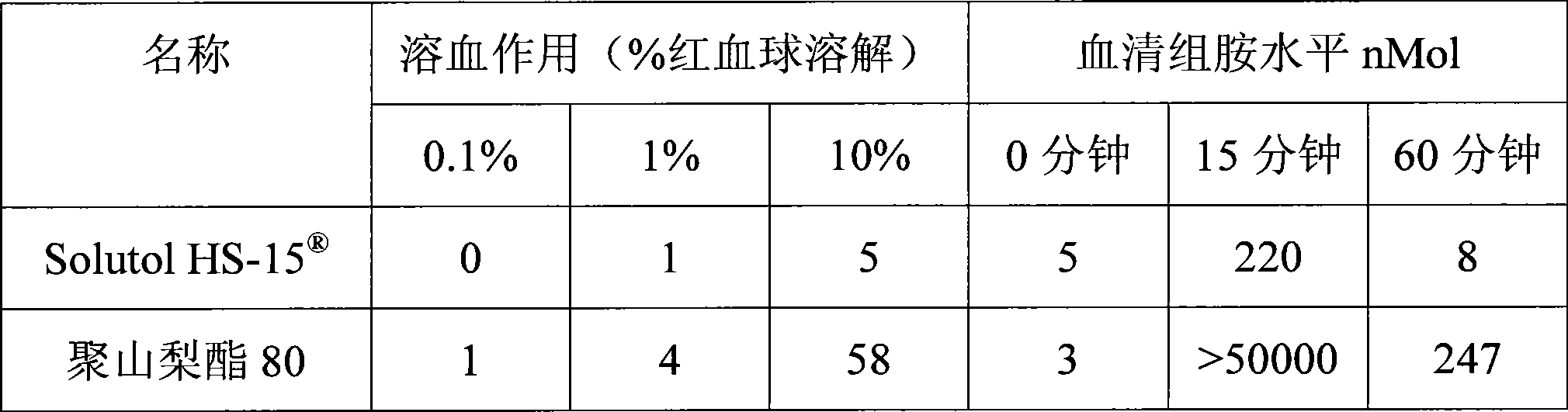
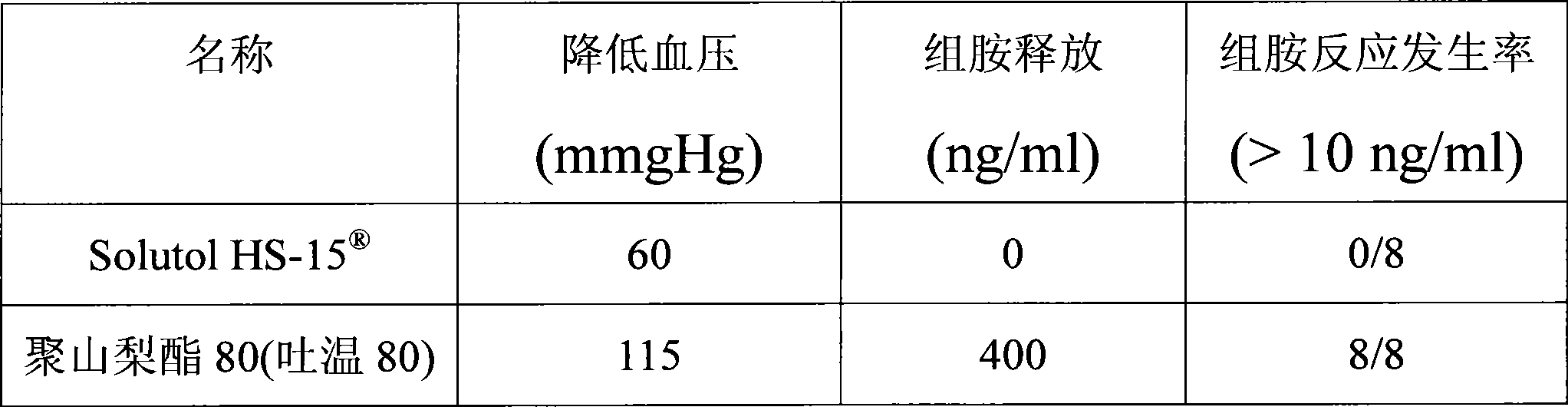
The invention discloses a pharmaceutical composition for improving the safety of Shenmai injection and a method for preparing the same. The pharmaceutical composition is a pharmaceutical composition for injection and is mainly prepared from red ginseng extract, dwarf lilyturf root extract and polyethylene glycol 12-hydroxy stearic acid ester. The pharmaceutical composition adopts a latent solvent with better safety and more obvious hydrotropy to replace the latent solvent polysorbate-80 which has potential safety hazard and influences the product quality, thus the reduction of the pH value of the Shenmai injection in the processes of storing and high temperature sterilization is obviously delayed, and the stability of the pharmaceutical composition is increased; besides, the safety of the polyethylene glycol 12-hydroxy stearic acid ester is higher than that of the polysorbate-80 and the dosage thereof is lower, thus the probability and the risk of untoward reactions of the pharmaceutical composition is reduced, and the safety for clinical application is improved.
Owner:SICHUAN SUNNYHOPE PHARM CO LTD
Injectable medicine composition capable of improving stability of puerarin medicine injection preparation and preparation method of injectable medicine composition
InactiveCN105106110ADecreased substancesStable pHOrganic active ingredientsNervous disorderPuerarinPharmaceutical drug
The invention discloses an injectable medicine composition capable of improving the stability of a puerarin medicine injection preparation and a preparation method of the injectable medicine composition. The injectable medicine composition is mainly prepared by dissolving a puerarin salt in water for injection to obtain a liquid medicine, and adding citric acid and / or sodium citrate as a pH regulator to regulate the pH value of the liquid medicine, wherein the dose of citric acid and / or sodium citrate is 0.1-200.0 mg / 100 ml. Through adoption of the injectable medicine composition, the pH value of the puerarin medicine injection preparation can be more stable; compared with those in the prior art, degraded substances in puerarin are greatly reduced; under the circumstance that other solubilizing agents increasing the clinical application risk are avoided, the clarity of the puerarin medicine injection preparation is improved; particularly, the problem that when the conventional puerarin injection product is stored for a relatively long time, small white dots and white blocks are generated to result in solution turbidity can be solved. Therefore, examination of visible foreign matters in the puerarin medicine injection preparation can be guaranteed to accord with the medicine quality standard regulation, and clinical medication and promotion are facilitated.
Owner:CHENGDU AIBIKE BIOTECH
Esomeprazole sodium polymorph and application of esomeprazole sodium polymorph in drugs for injection
InactiveCN102746273AImprove solubilityImprove stabilityOrganic active ingredientsOrganic chemistryEsomeprazole SodiumDisease
The present invention relates to an esomeprazole sodium polymorph and an application of the esomeprazole sodium polymorph in drugs for injection. According to the present invention, esomeprazole sodium compositions for injection have characteristics of good stability and high purity, a preparation process of the esomeprazole sodium compositions is easily industrialized, and the esomeprazole sodium compositions can be used for treatments of diseases related to gastric acid parasecretion, wherein the esomeprazole sodium compositions are prepared from the esomeprazole sodium polymorph having good stability and excellent solubility in the present invention.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Rabeprazole sodium for injection as well as preparation method and detection method thereof
InactiveCN102440967ANo apparent in vitro hemolysisNo obvious agglutinationAntibacterial agentsPowder deliveryDiseaseDuodenal ulcer
The invention relates to rabeprazole sodium for injection as well as a preparation method and a detection method thereof. The rabeprazole sodium for injection is a medical preparation for injection, which is prepared from rabeprazole sodium as an active ingredient, a stabilizing agent, a pH value regulator and pharmaceutically acceptable auxiliary materials. The rabeprazole sodium for injection, disclosed by the invention, is rapid in effect taking and high in bioavailability, is used for treating gastrohelcosis, duodenal ulcer, erosive gastroesophageal reflux disease, helicobacter pylori injection, Zollinger-Ellision syndrome and the like and is more suitable for being used as a substitutive medicament when oral preparations for treating the diseases have no effect.
Owner:SHANDONG DANHONG PHARMA
Safe medicine composition for compound gastrodin injection and preparation method of safe medicine composition
InactiveCN105106111AImprove securityReduced responseAntipyreticAnalgesicsPolyethylene glycolHydroxystearic Acid
The invention discloses a safe medicine composition for a compound gastrodin injection and a preparation method of the safe medicine composition. The safe medicine composition used for injection is mainly prepared from a gastrodin extract, a Szechuan lovage rhizome extract, a carthamus tinctorius extract and 12-hydroxystearic acid-polyethylene glycol. The safe medicine composition has the advantages that a solubilizing agent with higher safety and a more obvious solubilizing effect is utilized for replacing polysorbate 80 in the conventional compound gastrodin injection; polysorbate 80 has a potential safety hazard and affects the quality of the conventional compound gastrodin injection, 12-hydroxystearic acid-polyethylene glycol has higher safety than polysorbate 80, and the dose of 12-hydroxystearic acid-polyethylene glycol is lower than that of polysorbate 80, so that the possibility and risk of an adverse medicine reaction are reduced, and the safety of clinical medication is improved.
Owner:CHENGDU AIBIKE BIOTECH
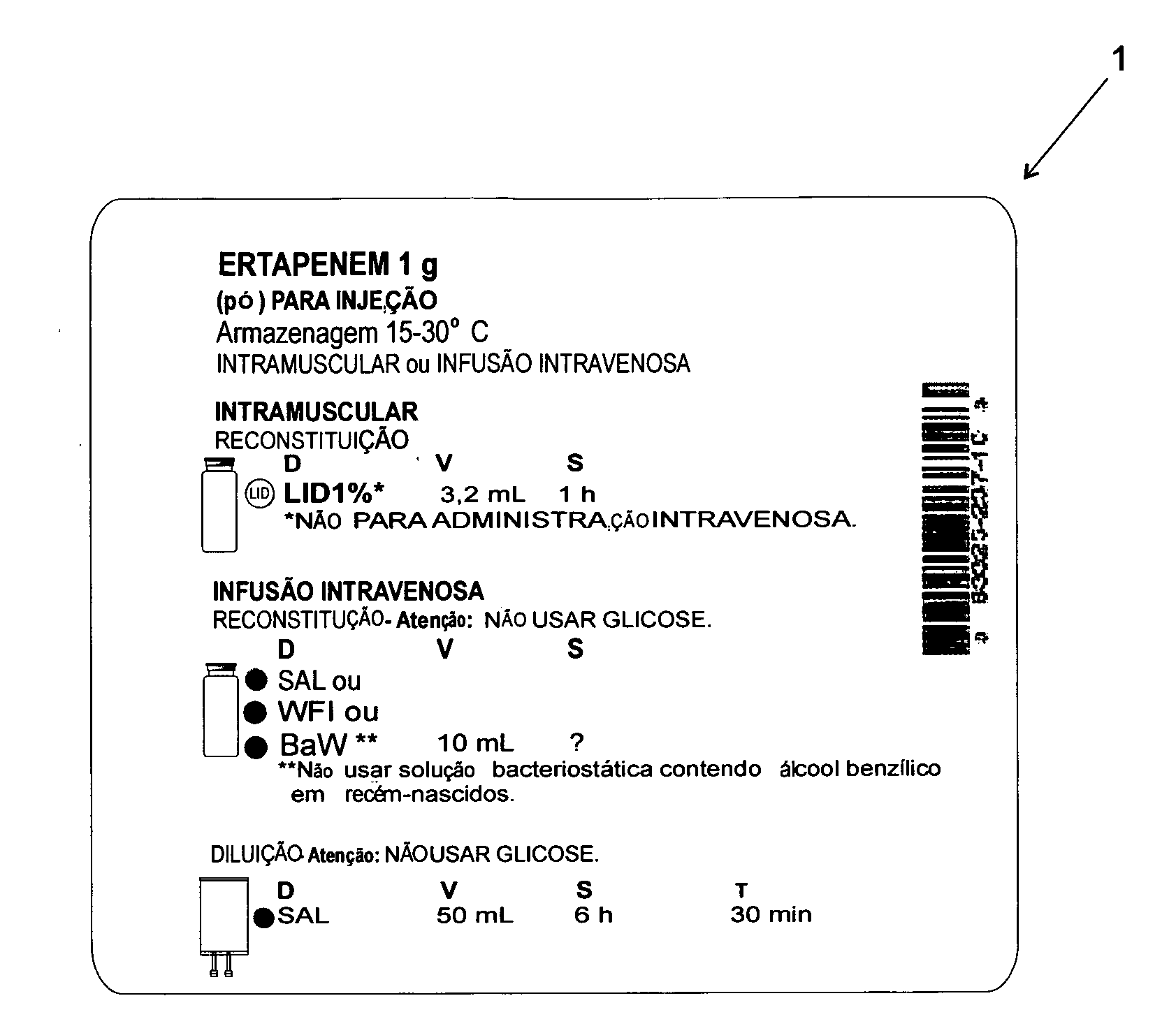
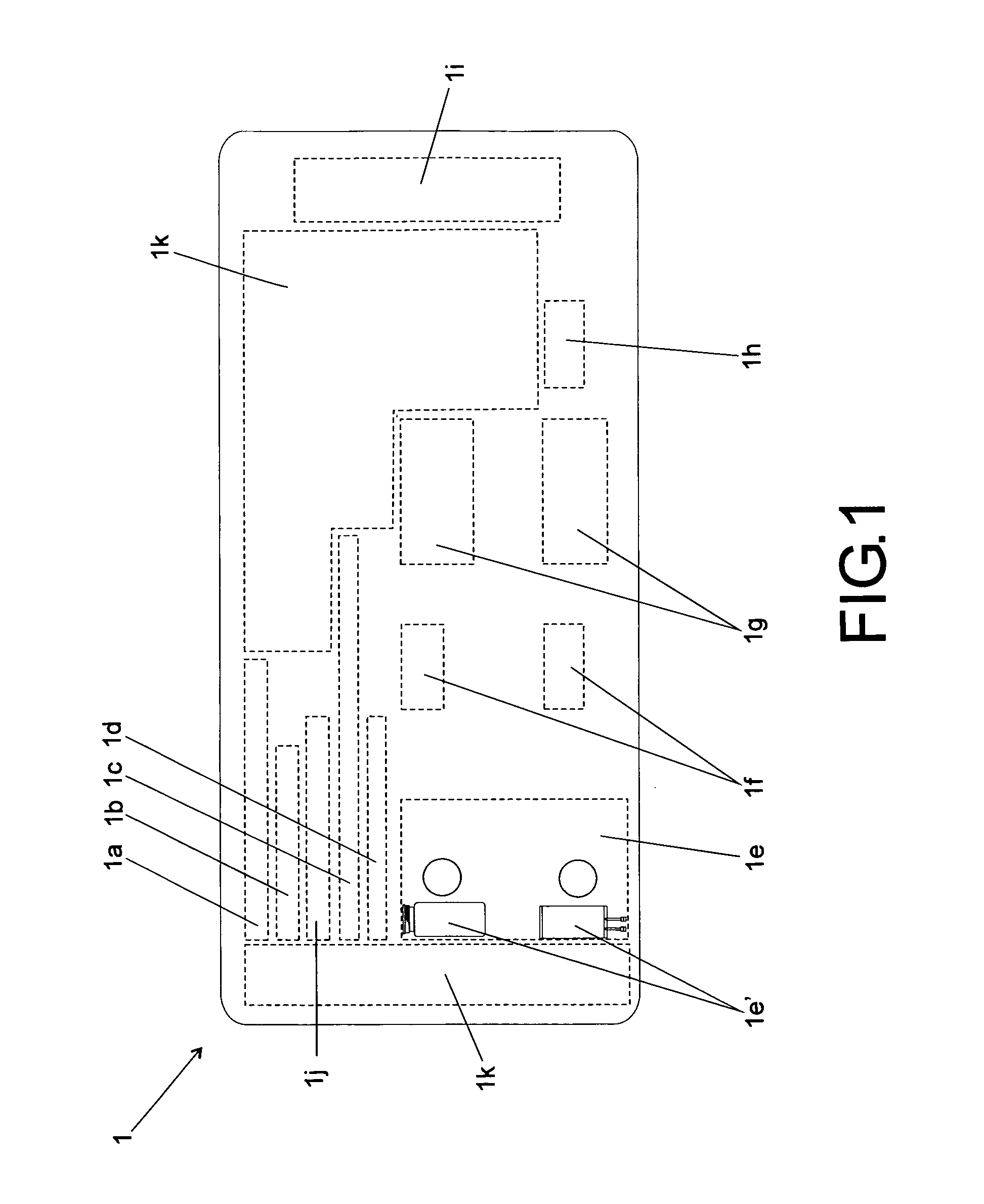
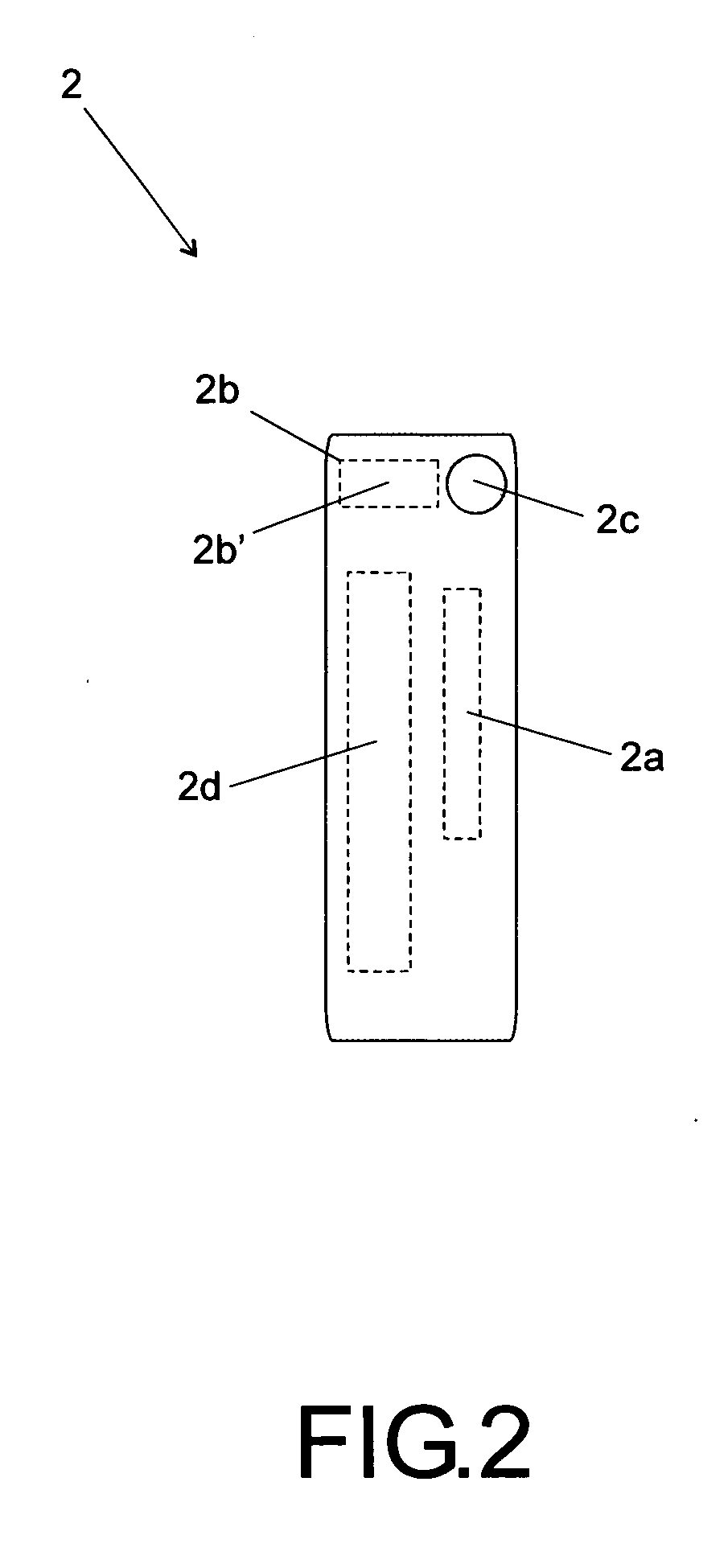
Method of standardization of injectalbe medicines and their diluents
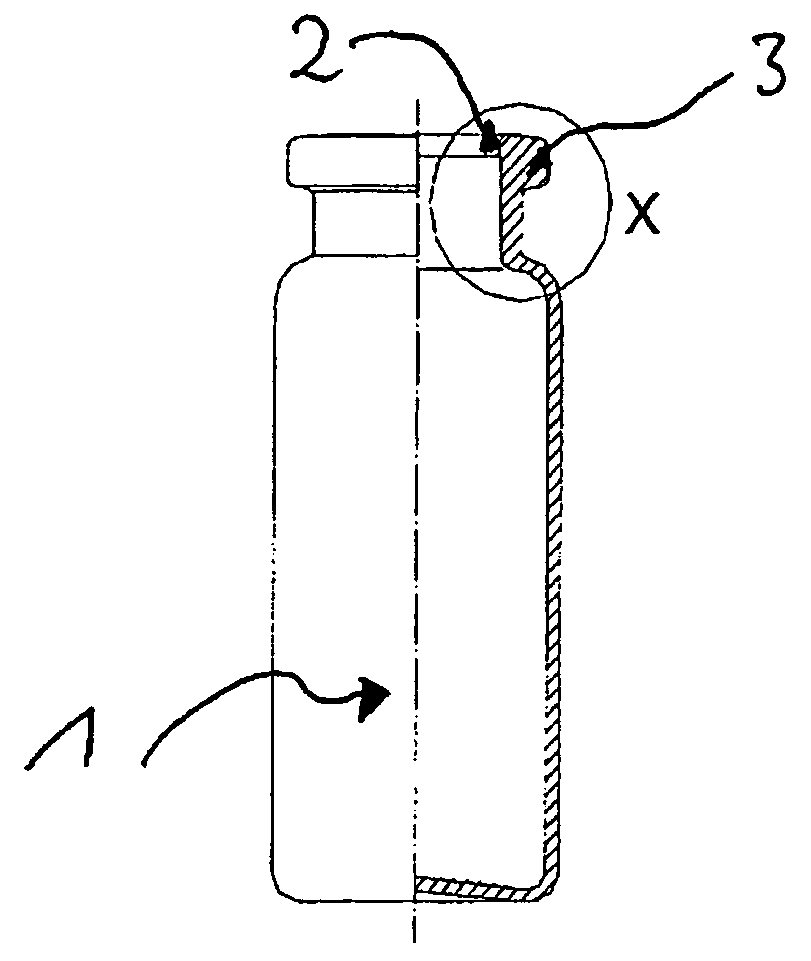
“METHOD OF STANDARDIZATION OF INJECTABLE MEDICINES AND THEIR DILUENTS” is particularly intended to eliminate the possibility of confusion arising from the determination of said injectable medicines and their diluents in the general context of health care, such as hospitals, clinics and similar health-related facilities. The method is essentially intended to allow an injectable medicine to be administered following the correct definition of the appropriate diluent required by each product, said method comprising the standardization of a label (1) designed to be attached to vials or containers of injectable medicines, as well as a label (2) specifically designed to be attached to vials or containers of diluents; said label (1) includes information fields for various relevant information that should be corrected in the event of codification.
Owner:NORIVAL CAETANO
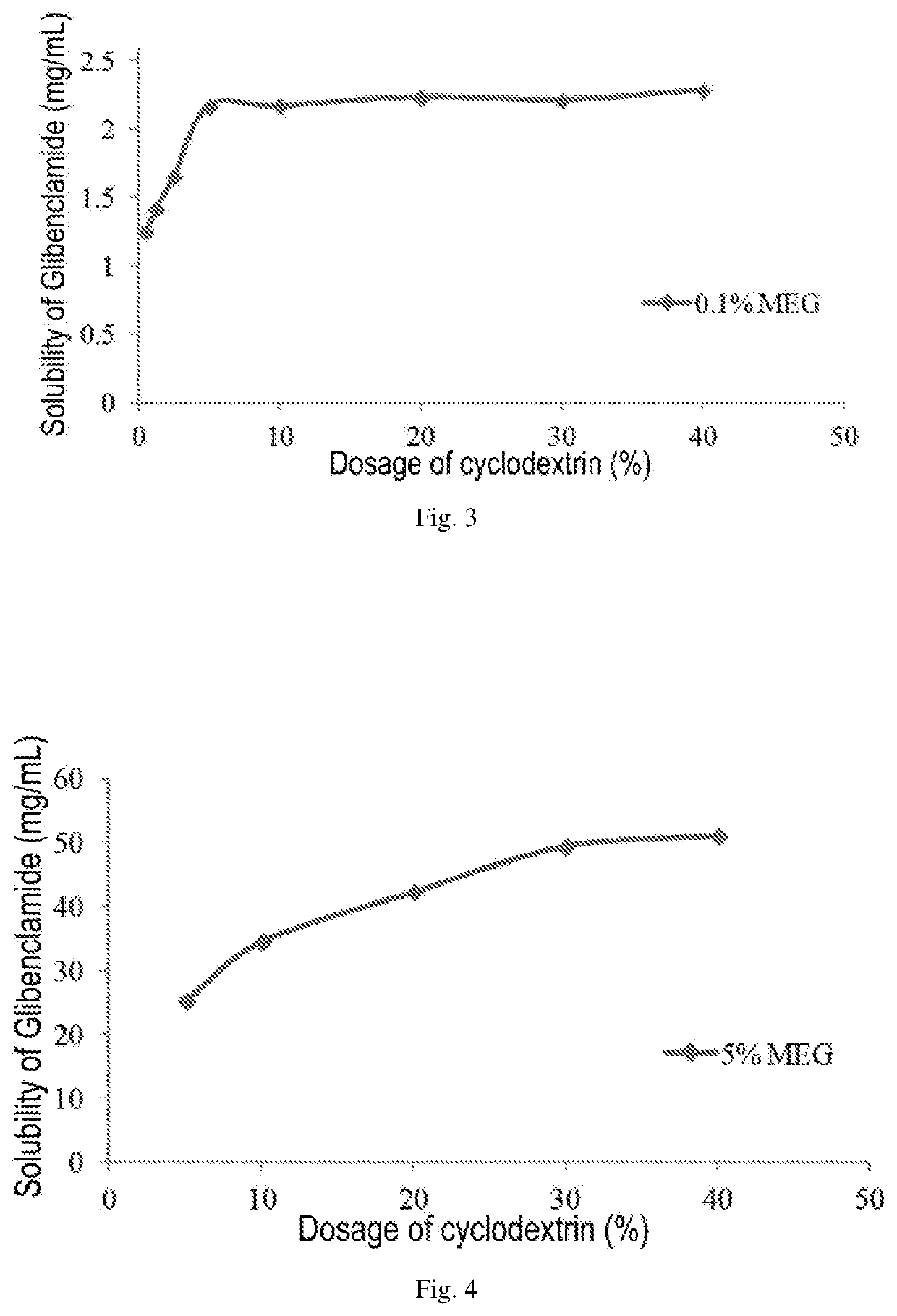
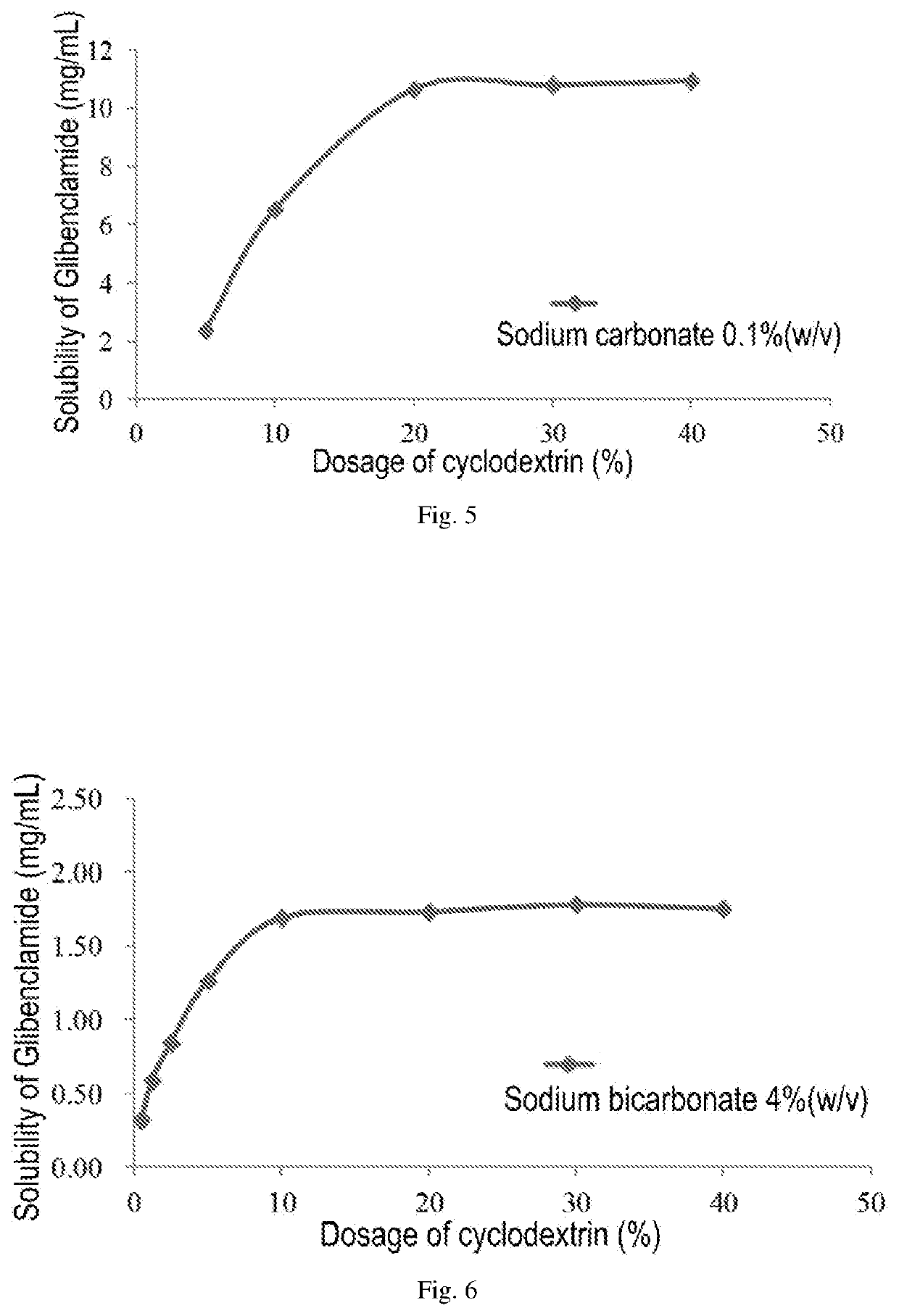
A pharmaceutical composition comprising a sulfonylurea drug and preparation method thereof
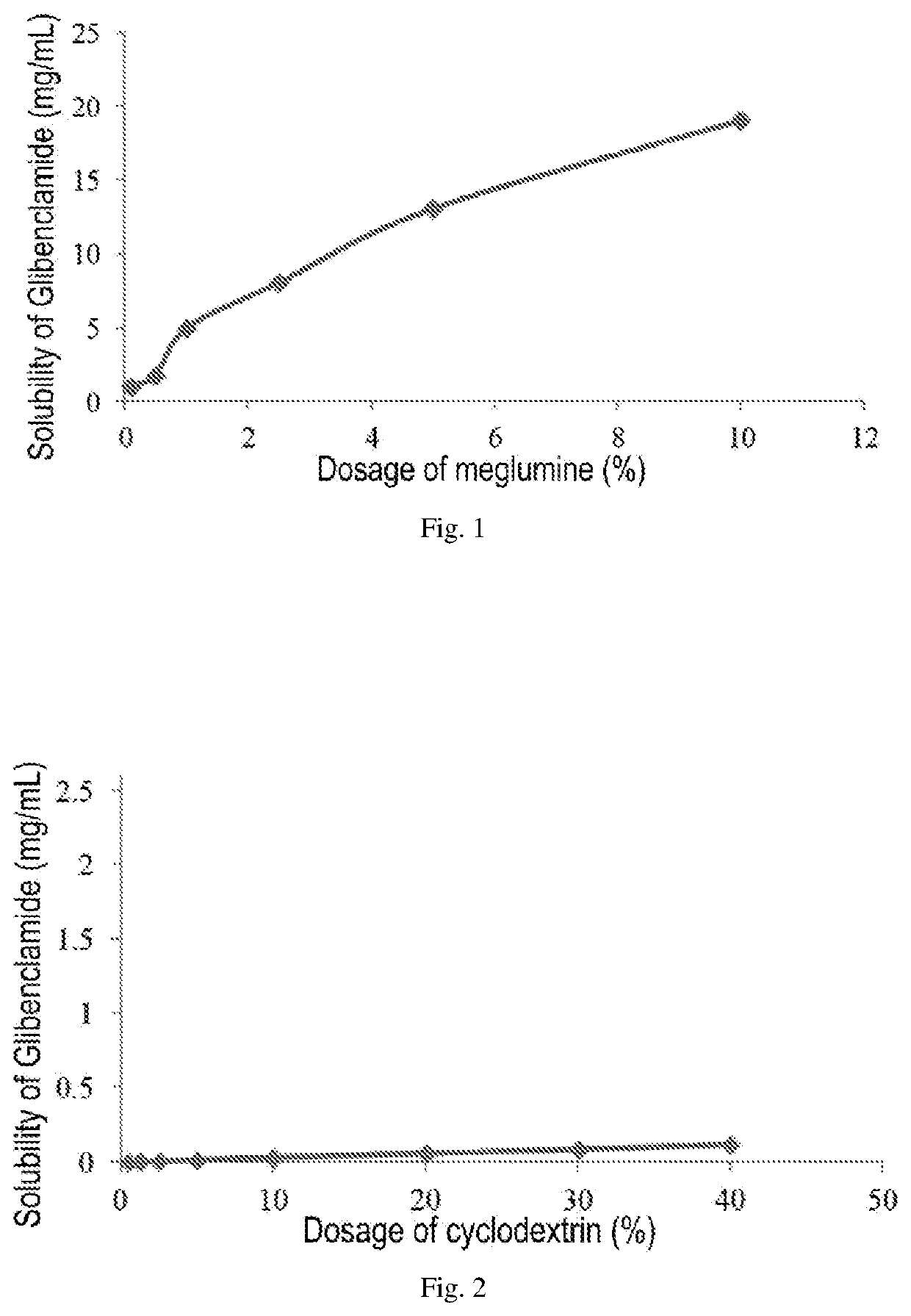
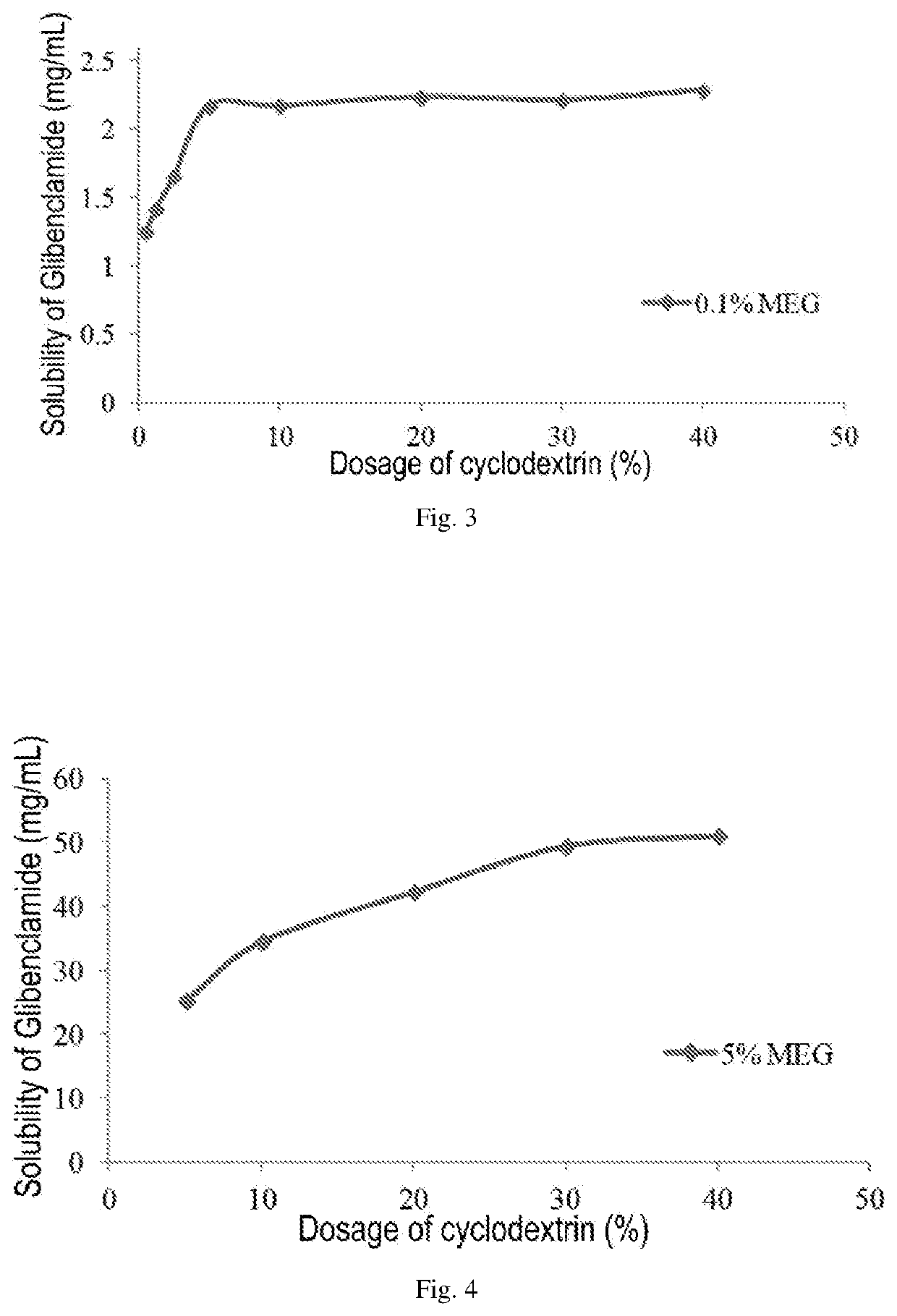
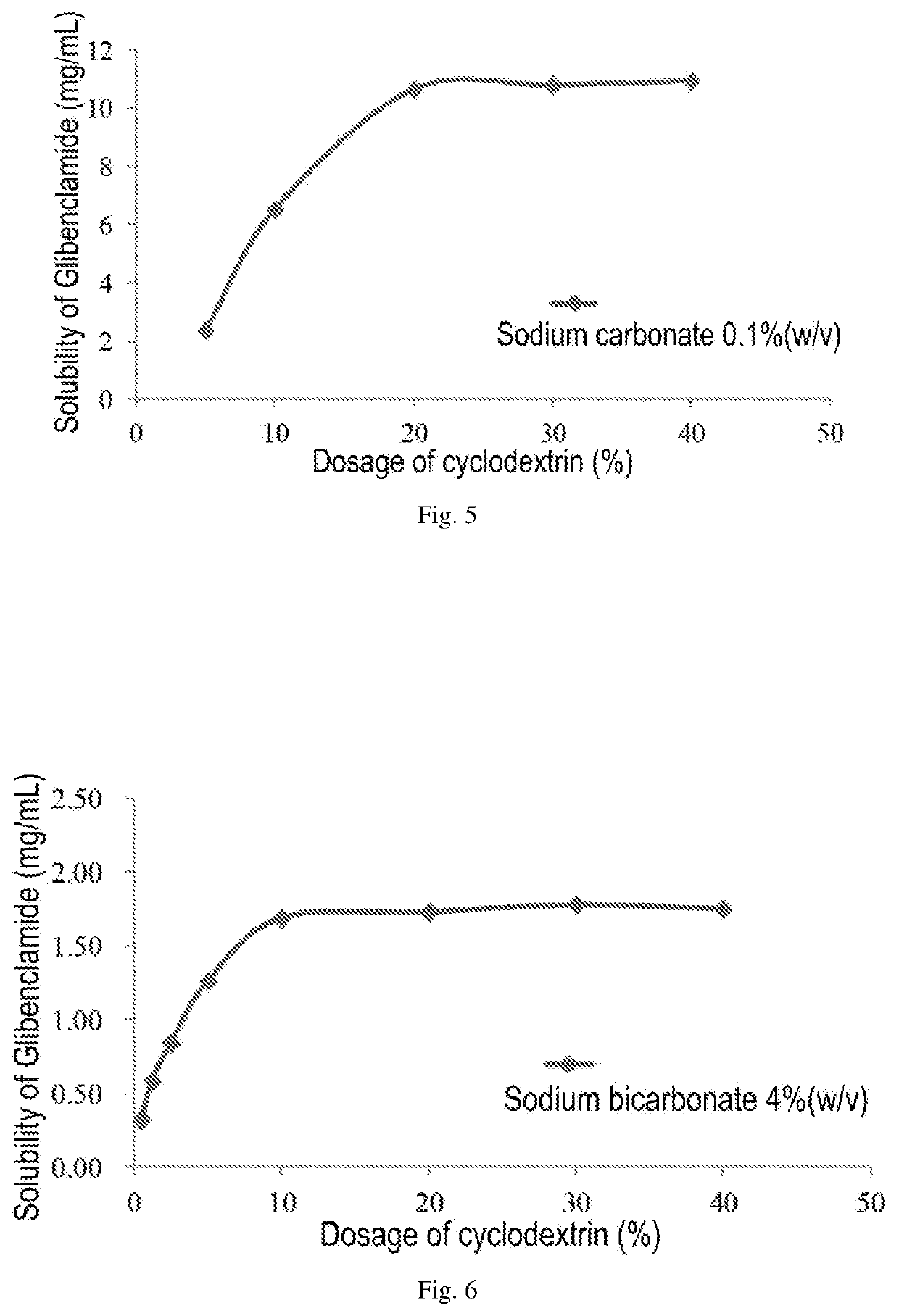
ActiveUS20200022993A1Improve solubilityImprove stabilityMetabolism disorderSulfonylurea active ingredientsSulfonylureaCyclodextrin
An injectable pharmaceutical composition of a sulfonylurea drug and a preparation method thereof were described. The pharmaceutical composition contains a sulfonylurea drug, a cyclodextrin and an additive.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Polyoxyethylene-660-12-hydroxy stearate containing mulberry ginger cold injection preparation and preparation method thereof
InactiveCN104117026AHigh physiological toleranceImprove securityNervous disorderAntipyreticMedicinePolyethylene glycol
The invention provides a mulberry ginger cold injection preparation with higher security and a preparation method thereof, the mulberry ginger cold injection preparation is an injection drug mainly prepared by together dissolving a mulberry extract, a chrysanthemum extract, a perilla extract, a forsythia extract, a semen armeniacae amarae extract, a dried ginger extract, phenylcarbinol and polyoxyethylene-660-12-hydroxy stearate (solutol-HS15) as a solubilizer into injection water, and the use amount of the used polyoxyethylene-660-12-hydroxy stearate (solutol-HS15) is 0.3g / 100ml. The literature research in the prior art confirms that the security of the polyoxyethylene-660-12-hydroxy stearate (solutol-HS15) used in the preparation is far better than that of polysorbate-80; moreover, safety experiment shows that the security of a mulberry ginger cold injection liquid using the polyoxyethylene-660-12-hydroxy stearate (solutol-HS15) as the solubilizer is obviously better than that of a mulberry ginger cold injection liquid using the polysorbate-80 as a solubilizer.
Owner:CHENGDU LIST PHARMA
Application of resveratrol to preparation of drugs for treating or relieving chronic airway inflammation
InactiveCN107998110ASignificant effectReduce dosagePowder deliverySpray deliverySide effectCurative effect
The invention discloses application of resveratrol to the preparation of drugs for treating or relieving chronic airway inflammation (asthma and chronic obstructive pulmonary). According to the method, chronic airway inflammation, such as asthma, is treated in a mode of aerosol inhalation of resveratrol, the curative effect is obvious, and the dosage of resveratrol can be greatly reduced; in addition, compared with oral or injection drugs, the mode of inhalation of resveratrol can increase the local drug concentration, namely the drug concentration at the lungs of asthma mice is increased, a drug directly acts on the lungs of the mice, the target is clear, the drugs directly act on the lungs, damage to other visceral organs is reduced, the drug loss is small, and the drugs have a good application prospect.
Owner:SHANGHAI PUTUO DISTRICT CENT HOSPITAL
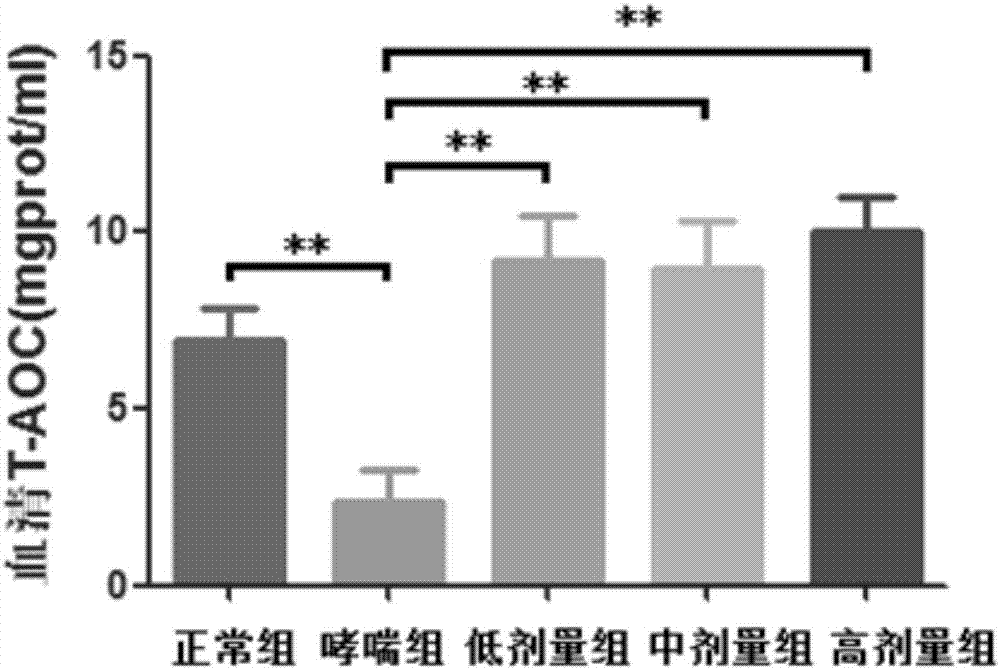
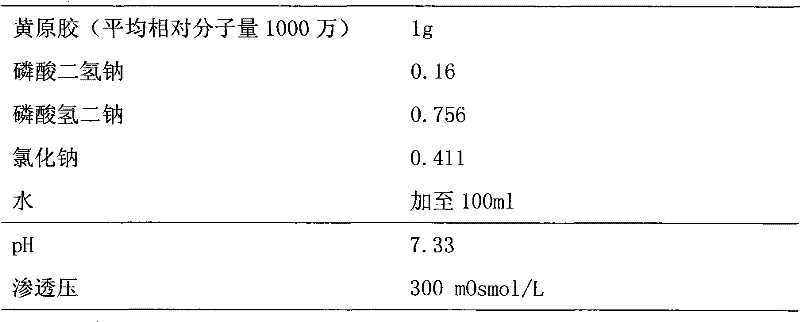
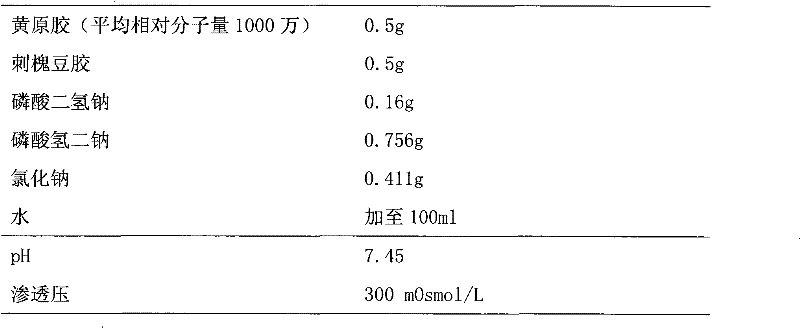
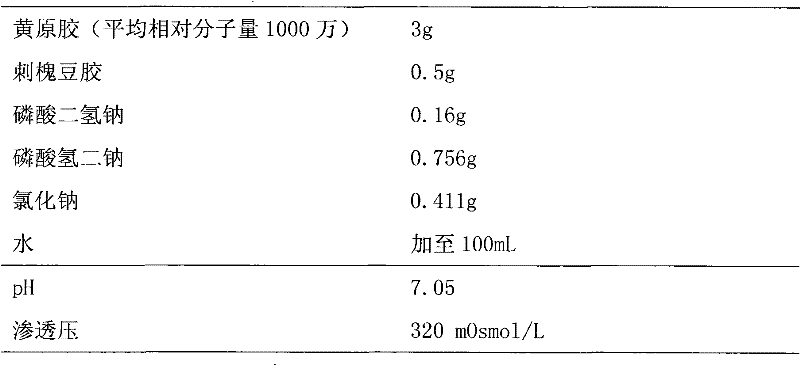
Xanthan-gum-containing pharmaceutical preparation for joint intracavity injection
ActiveCN101940587BIncrease viscosityViscosity recoveryOrganic active ingredientsSkeletal disorderJoint cavityRheumatism
Owner:INST OF BIOPHARM OF SHANDONG PROVINCE
Injectable formulation of antibiotic and solution for intravenous administration thereof
InactiveUS7968588B2Remarkable antibacterial activityReduce the burden onAntibacterial agentsBiocideHigh concentrationAntibiotic Y
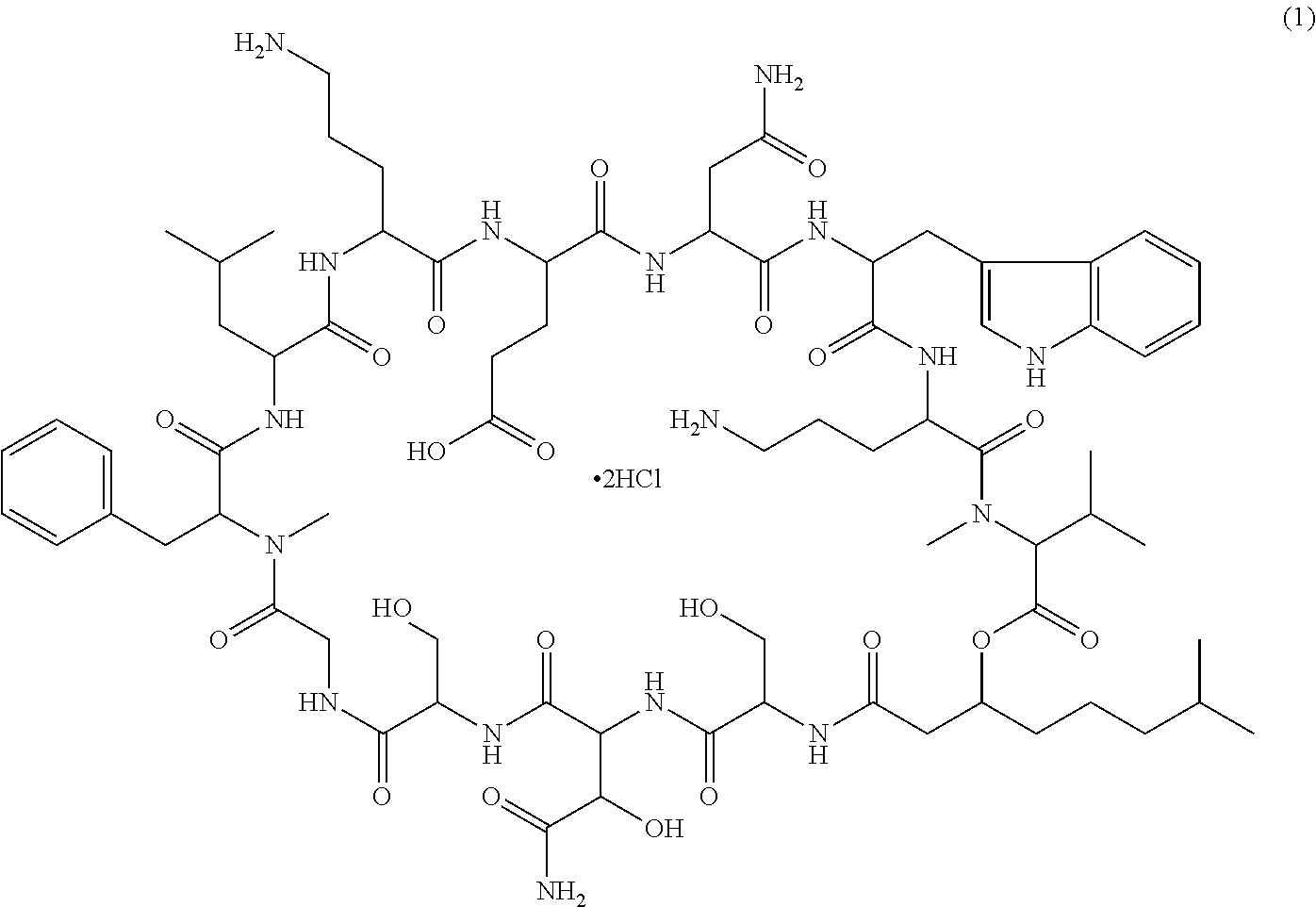
A pharmaceutical composition for injection comprising a depsipeptide antibiotic, WAP-8294A2, as an active ingredient, which is stable and contains WAP-8294A2 in high concentrations is provided. This composition comprises WAP-8294A2 of the following structural formula (1) as an active ingredient and is characterized in that 2-hydroxypropyl-β-cyclodextrin or β-cyclodextrin is contained as a stabilizer or solubilizer and the pH of the composition is not adjusted. This composition is mixed with a pH-adjusting agent such as dextrose and with an infusion or diluent comprising a solution of disodium hydrogen phosphate, sodium dihydrogen phosphate, and sodium hydroxide at the time of use to prepare a solution for intravenous administration of WAP-8294A2.
Owner:KYOTO BIOPHARMA INC
Application of 5'-AMP for treating urarthritis
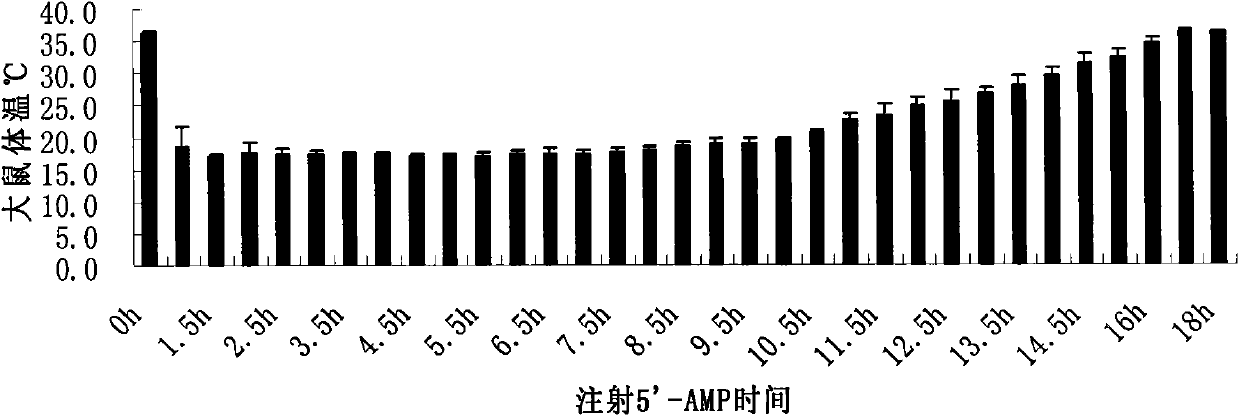
InactiveCN101947230AReduce swellingReduce infiltrationOrganic active ingredientsAntipyreticSide effectConnective tissue fiber
The invention discloses application of 5'-AMP in preparing a drug for treating urarthritis. Proved by relevant tests, on one hand, the 5'-AMP can obviously reduce the swelling degree of a urarthritis joint cavity; and on the other hand, the 5'-AMP can obviously reduce the infiltration of a connective tissue inflammatory cell in the urarthritis joint cavity when being used for treating the urarthritis. The 5'-AMP is used as an injection drug to treat the urarthritis and does not have obvious damage to animals, and the adverse side effect is not discovered. The 5'-AMP is a drug which is safe and reliable and can be used for treating the urarthritis.
Owner:苗志敏 +2
Medicine composite of honeysuckle hydrochloric acid extract injection and preparation method thereof
InactiveCN105079107AImprove securityReduced responsePowder deliveryNervous disorderPolyethylene glycolHydroxystearic Acid
The invention discloses a medicine composite of honeysuckle hydrochloric acid extract injection and a preparation method thereof. The medicine composite for injection is mainly prepared from honeysuckle extract, scutellaria extract, licorice extract and polyethylene glycol dodecahydroxyl stearic acid ester. A hydrotropic agent higher in safety and more obvious in hydrotropic effect is adopted for replacing a hydrotropic agent-polysorbate 80, causing potential safety hazards and influencing product quality, in compound honeysuckle injection, and the polyethylene glycol dodecahydroxyl stearic acid ester is higher in safety and smaller in dosage compared with polysorbate 80, so that the probability and risk of adverse drug reactions are reduced, and clinical drug use safety is improved.
Owner:CHENGDU AIBIKE BIOTECH
Polyoxyethylene-660-12-hydroxy stearate containing breviscapine injection preparation and preparation method thereof
InactiveCN104116749AHigh physiological toleranceImprove securityOrganic active ingredientsPharmaceutical delivery mechanismPolythylene glycolHydroxystearic Acid
The invention provides a breviscapine injection preparation with higher security and a preparation method thereof, the breviscapine injection preparation is an injection drug mainly prepared by together dissolving a breviscapine extract and polyoxyethylene-660-12-hydroxy stearate (solutol-HS15) as a solubilizer into injection water, and the use amount of the used polyoxyethylene-660-12-hydroxy stearate (solutol-HS15) is 0.01g / 100ml. The literature research in the prior art confirms that the security of the polyoxyethylene-660-12-hydroxy stearate (solutol-HS15) used in the preparation is far better than that of polysorbate-80; moreover, safety experiment shows that the security of a breviscapine injection liquid using the polyoxyethylene-660-12-hydroxy stearate (solutol-HS15) as the solubilizer is obviously better than that of a breviscapine injection liquid using the polysorbate-80 as a solubilizer.
Owner:CHENGDU LIST PHARMA
Use of 5'-AMP in septicemia treatment
InactiveCN102895254AReduce infiltrationImprove expression levelAntibacterial agentsOrganic active ingredientsInflammatory factorsSide effect
The present invention discloses a use of 5'-AMP in preparation of drugs for treatment or prevention of septicemia. Related test results show that: with application of the 5'-AMP in septicemia treatment, an inflammatory factor expression level in septicemia can be significantly reduced, an anti-inflammatory factor expression level can be improved, and lung tissue inflammation cell infiltration of the septicemia can be significantly reduced. In addition, the 5'-AMP is adopted as an injection drug for septicemia treatment, such that no obvious damage is generated to animals, no adverse side effect is generated, and the 5'-AMP is a safe and reliable drug for septicemia treatment.
Owner:王云龙 +2
Polyoxyethylene-660-12-hydroxy stearate containing compound musk injection preparation and preparation method thereof
InactiveCN104117007AAvoid adverse reactionsImprove securityHydroxy compound active ingredientsPharmaceutical delivery mechanismPolythylene glycolHydroxystearic Acid
The invention provides a compound musk injection preparation with higher security and a preparation method thereof, the compound musk injection preparation is an injection drug mainly prepared by together dissolving a compound musk extract, a pogostemon cablin extract, a radix curcumaeextract, a rhizoma acori gramineiextract, borneol, menthol and polyoxyethylene-660-12-hydroxy stearate (solutol-HS15) as a solubilizer into injection water, and the use amount of the used polyoxyethylene-660-12-hydroxy stearate (solutol-HS15) is 2g / 100ml. The literature research in the prior art confirms that the security of the polyoxyethylene-660-12-hydroxy stearate (solutol-HS15) used in the preparation is far better than that of polysorbate-80; moreover, safety experiment shows that the security of a compound musk injection liquid using the polyoxyethylene-660-12-hydroxy stearate (solutol-HS15) as the solubilizer is obviously better than that of a compound musk injection liquid using the polysorbate-80 as a solubilizer.
Owner:CHENGDU LIST PHARMA
Preparation method of drug composition for improving stability of Shengmai drug injection preparation
InactiveCN106361969AStable pHDecreased ginseng degraded substancesPharmaceutical delivery mechanismPharmaceutical non-active ingredientsDrug injectionCLARITY
The invention discloses a preparation method of a drug composition for improving the stability of a Shengmai drug injection preparation. The drug composition for injection is mainly prepared by dissolving Shengmai salt in water for injection and adding oxalic acid and / or sodium oxalate to serve as a pH regulator for regulating a pH value of medicinal liquid. The usage amount of the oxalic acid and / or sodium oxalate is 1-100.0 mg / 100ml. The pH value of the injection can be stable, substance degradability of the Shengmai is greatly reduced compared with the prior art, the clarity of the Shengmai injection is improved under the situation that other cosolvents increasing clinical application risks are not used, especially the problem that the Shengmai injection adopting a product in the prior art produces small white points, white blocks and is turbid under the condition of long storage time is solved, and it can be ensured that visible foreign matter inspection of the product meets the stipulations of the drug quality standard and the drug composition is convenient to clinically apply and popularize.
Owner:CHENGDU XIANXIANXIAN BIOTECH CO LTD
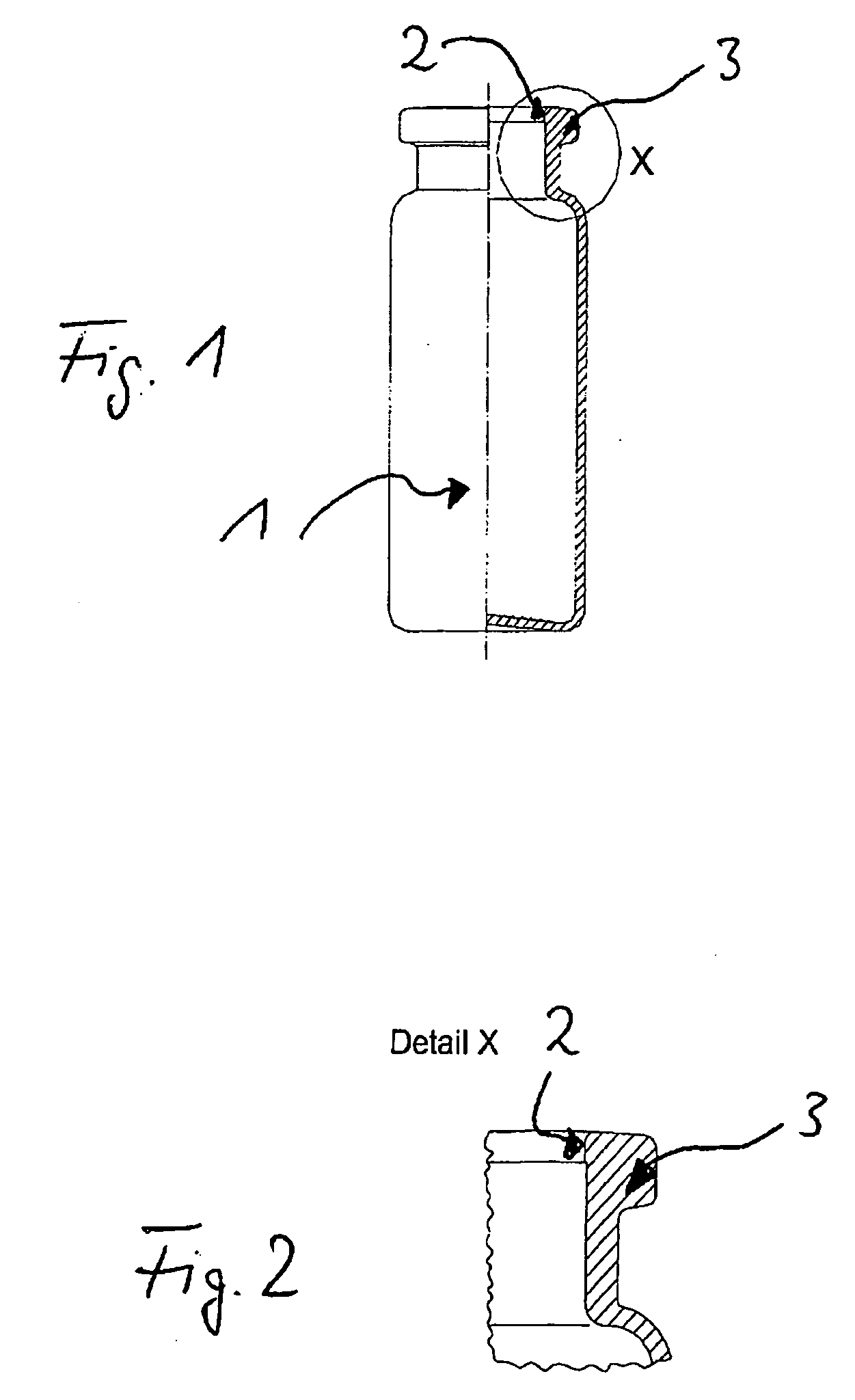
Pharmaceutical Product For Injection
The present invention is related to a pharmaceutical product for injection comprising a container including a closure suitable for preparations for injection, the container containing an acid labile proton pump inhibitor, a salt thereof, a solvate of the acid labile proton pump inhibitor or a salt thereof, wherein the container and closure are made of material which essentially does not release zinc ions.
Owner:NYCOMED GMBH
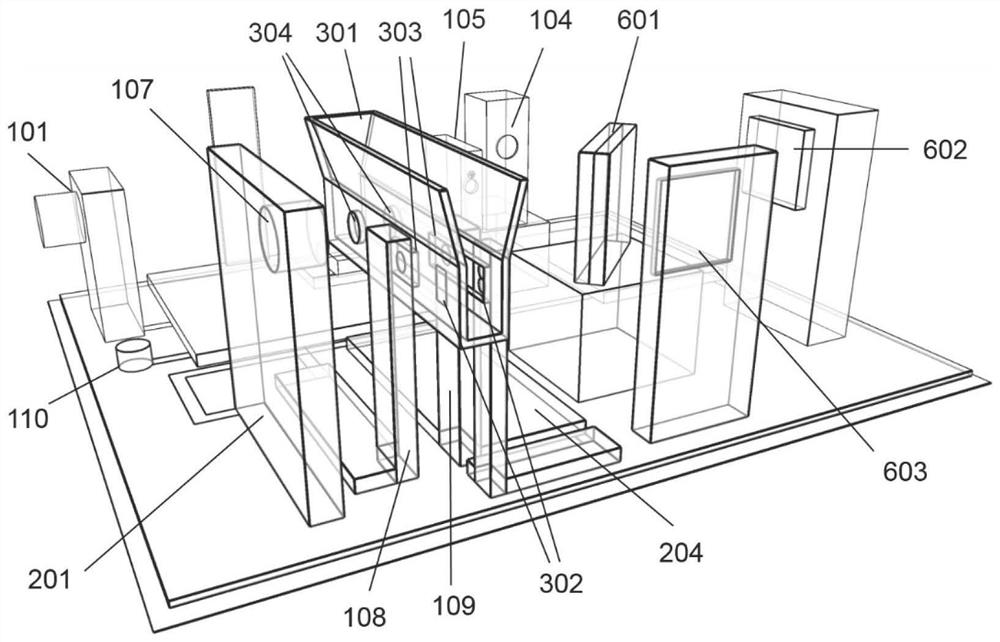
Intelligent photoelectric identification system for intravenous fluid detection
InactiveCN112881331AImprove work efficiencyReduce security risksColor/spectral properties measurementsSignal processing circuitsUltraviolet lights
The invention discloses an intelligent photoelectric identification system for intravenous injection liquid detection, which utilizes information of a functional group region and a fingerprint region of an intermediate infrared spectrum to identify drug components, and utilizes ultraviolet absorbance to calculate drug concentration, so as to realize component and concentration identification of drugs for intravenous injection. The system comprises an image recognition module, a mid-infrared light source module, a laser module, a mid-infrared interferometer, a mid-outer red light detector, an infusion bag fixing assembly, an ultraviolet light source module, an ultraviolet light detector, a mid-infrared spectrum collecting and processing circuit, a comprehensive signal processing circuit, a buzzer and a display module. The system disclosed by the invention can simultaneously identify medicine components and concentration, can quickly complete detection of intravenous fluid, is adaptive to infusion bags of various specifications, is suitable for manual fluid preparation and automatic fluid preparation robot equipment and checking of infusion finished products, has an abnormal early warning and prompting function, improves the working efficiency of intravenous fluid preparation, and reduces medical accidents at the same time.
Owner:SUZHOU WEIJIE MEDICAL SCI & TECH
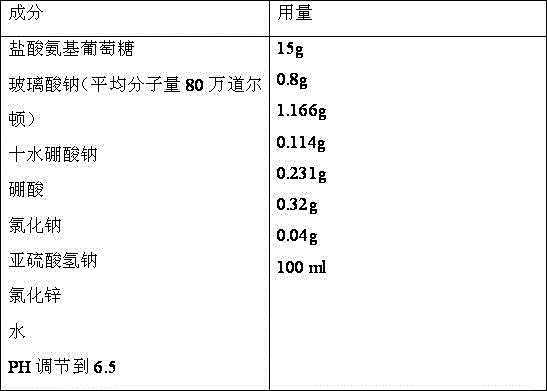
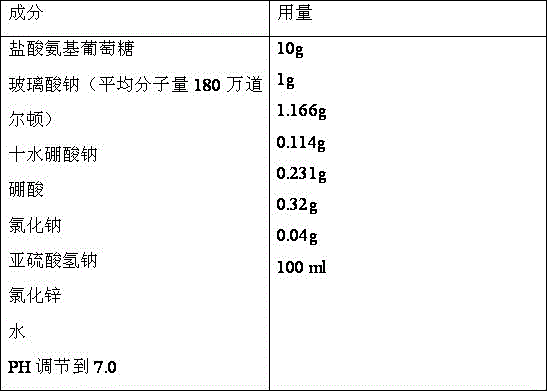
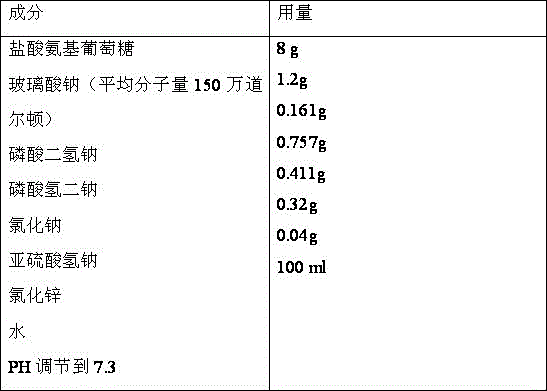
Medicine composition containing glucosamine and used for articular cavity injection
ActiveCN105796588ASpeed up the repair processPromote repairOrganic active ingredientsInorganic non-active ingredientsPharmaceutical drugArticular cavity
The invention discloses medicine composition containing glucosamine and used for articular cavity injection. The medicine composition contains glucosamine hydrochloride and sodium hyaluronate, wherein the concentration of glucosamine hydrochloride ranges from 5% to 20%, the molecular weight of sodium hyaluronate is 800,000-2000,000 Dalton and the concentration of sodium hyaluronate is 0.5%-1.5%. The medicine composition has the advantages that micromolecular glucosamine is rapidly absorbed and has a rapid repairing function, also has the advantages of good viscoelasticity and buffering property relative to high-molecular-weight sodium hyaluronate and capability of promoting slow release of glucosamine and can be used for treating arthritis through articular cavity injection.
Owner:THIRD INST OF OCEANOGRAPHY STATE OCEANIC ADMINISTATION
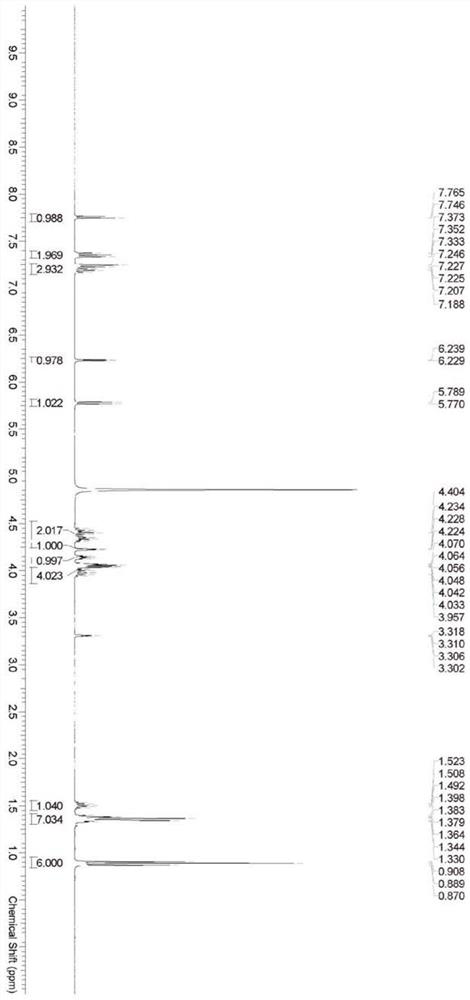
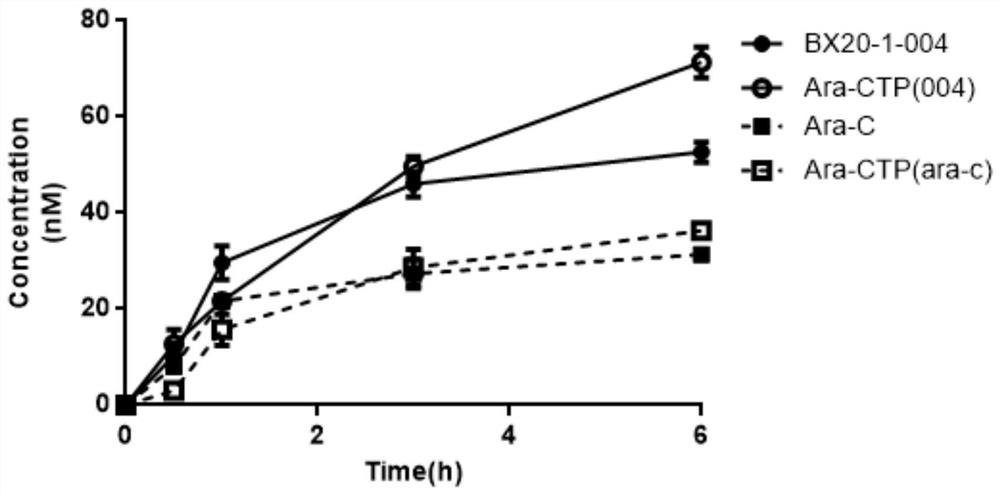
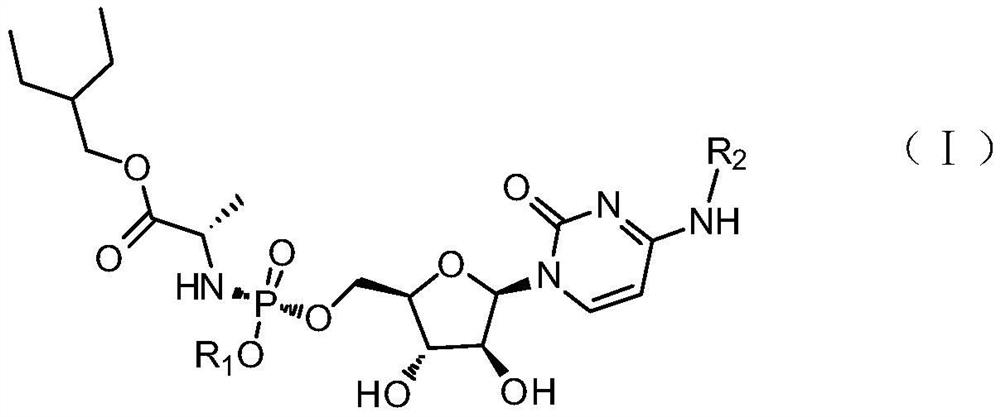
Cytarabine analogue and preparation method and application thereof
ActiveCN112409431AEnhanced inhibitory effectExtended half-lifeSugar derivativesSugar derivatives preparationCytarabineBlood drug concentration
The invention discloses a cytarabine analogue and a preparation method and application thereof. The cytarabine structural analogues P1-P4 are synthesized for the first time and can inhibit proliferation of leukemia cells HL60, and particularly, compared with Ara-C, the cytarabine structural analogues P4 are higher in leukemia cell proliferation inhibition capacity and cell penetration capacity, the concentration of the drug entering the cells and the concentration of the active metabolite cytarabine triphosphate generated in the cells are higher, and the bioavailability is higher; the cytosinearabinoside can be converted into a parent drug cytosine arabinoside in cells, so that the blood concentration of target cells in vivo is remarkably improved, and toxic and side effects and drug resistance caused by large-dose medication can be avoided; and the half-life period of cytarabine is remarkably prolonged, the effective blood concentration is maintained for a long time, oral administration can be achieved, and inconvenience caused by injection medication can be avoided. The preparation method is simple, easy to operate and suitable for industrial production.
Owner:武汉伯瑞恒医药科技有限公司
Pre-filled parenteral drug inspection station and method of using the same
ActiveUS20210293725A1Improve detectabilityAffordable costProgramme controlProgramme-controlled manipulatorFormularyDrug utilisation
The invention is a flexible and configurable inspection system for the inspection of container units that combines and integrates a holding assembly for multiple containers integrating servo-controlled rotation of the units, transport and positioning of the containers that simulate human handling, and camera stations employing automated vision inspection. The system performs horizontal inspection for particulate and any other container defect that promotes particulate to better locate within the inspection area of the cameras. Inspection sequences and product recipes combine the typical manual inspection agitation with automated inspection rotational techniques to optimize detection. The system allows for semi-automatic operation with the operator at the front of the station feeding and out-feeding material manually or fully automated with conveyance system feeding and out-feeding material from the back of the station.
Owner:SOTO MANUEL
Polyoxyethylene-660-12-hydroxy stearate (solutol-HS15) containing radix bupleuri injection drug preparation and preparation method thereof
InactiveCN104116768AHigh physiological toleranceImprove securityAntipyreticAnalgesicsPolyethylene glycolHydroxystearic Acid
The invention provides a radix bupleuri injection drug preparation with higher security and a preparation method thereof, the radix bupleuri injection drug preparation is an injection drug mainly prepared by together dissolving a fresh radix bupleuri extract and polyoxyethylene-660-12-hydroxy stearate (solutol-HS15) as a solubilizer into injection water, and the use amount of the used polyoxyethylene-660-12-hydroxy stearate (solutol-HS15) is 0.3g / 100ml. The literature research in the prior art confirms that the security of the polyoxyethylene-660-12-hydroxy stearate (solutol-HS15) used in the preparation is far better than that of polysorbate-80; moreover, safety experiment shows that the security of a radix bupleuri injection liquid using the polyoxyethylene-660-12-hydroxy stearate (solutol-HS15) as the solubilizer is obviously better than that of a radix bupleuri injection liquid using the polysorbate-80 as a solubilizer.
Owner:CHENGDU LIST PHARMA
Polyoxyethylene-660-12-hydroxy stearate containing collateral-generating injection preparation and preparation method thereof
InactiveCN104116907AHigh physiological toleranceImprove securityPharmaceutical delivery mechanismAntiinfectivesPolythylene glycolHydroxystearic Acid
The invention provides a collateral-generating injection preparation with higher security and a preparation method thereof, the collateral-generating injection preparation is an injection drug mainly prepared by together dissolving a red ginseng extract, a dwarf lilyturf root extract, a fructus schisandrae extract and polyoxyethylene-660-12-hydroxy stearate (solutol-HS15) as a solubilizer into injection water, and the use amount of the used polyoxyethylene-660-12-hydroxy stearate (solutol-HS15) is 0.5g / 100ml. The literature research in the prior art confirms that the security of the polyoxyethylene-660-12-hydroxy stearate (solutol-HS15) used in the preparation is far better than that of polysorbate-80; moreover, safety experiment shows that the security of a collateral-generating injection liquid using the polyoxyethylene-660-12-hydroxy stearate (solutol-HS15) as the solubilizer is obviously better than that of a collateral-generating injection liquid using the polysorbate-80 as a solubilizer.
Owner:CHENGDU LIST PHARMA
Injectable medicine composition used for resisting cancer
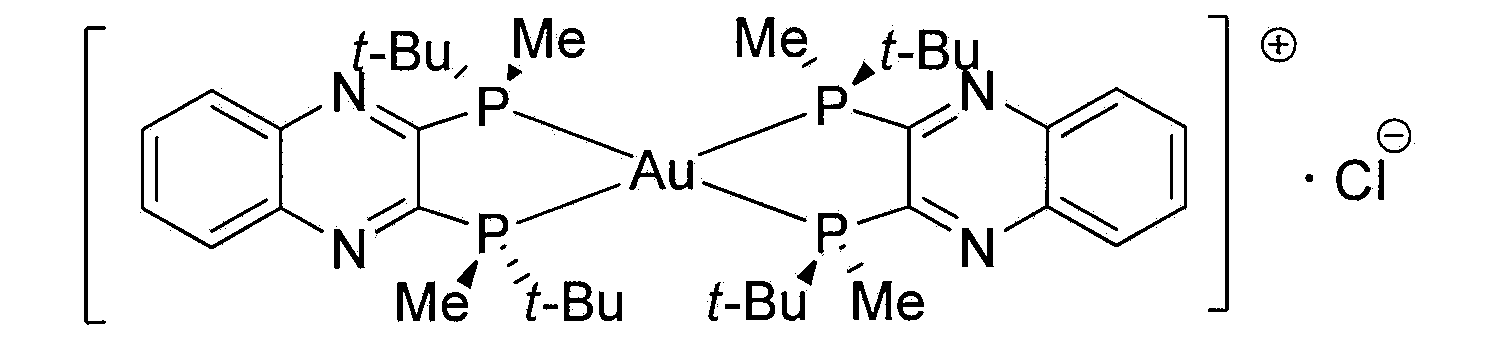
InactiveCN103385851AImprove solubilityLess irritatingOrganic active ingredientsPowder deliveryQuinoxalineActive agent
The invention relates to an injectable medicine composition used for resisting cancer. The composition comprises: (i) bis(2,3-bis(tertbutylmethylphosphino)quinoxaline)gold chloride; (ii) a pharmaceutically acceptable injection surfactant; and (iii) a pharmaceutically acceptable lyophilization bracket agent or solvent. A weight ratio of bis(2,3-bis(tertbutylmethylphosphino)quinoxaline)gold chloride to the surfactant is 1:1-1:50. With the composition, the solubility of bis(2,3-bis(tertbutylmethylphosphino)quinoxaline)gold chloride can be increased, and the irritation of bis(2,3-bis(tertbutylmethylphosphino)quinoxaline)gold chloride can be reduced.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Preparation method of vernakalant hydrochloride
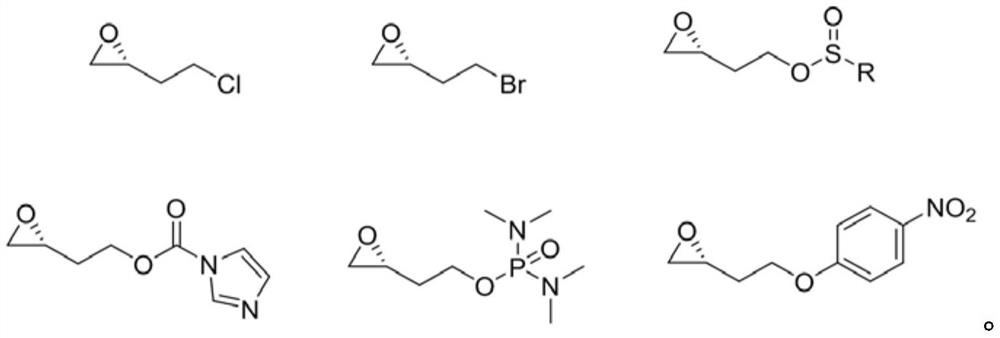
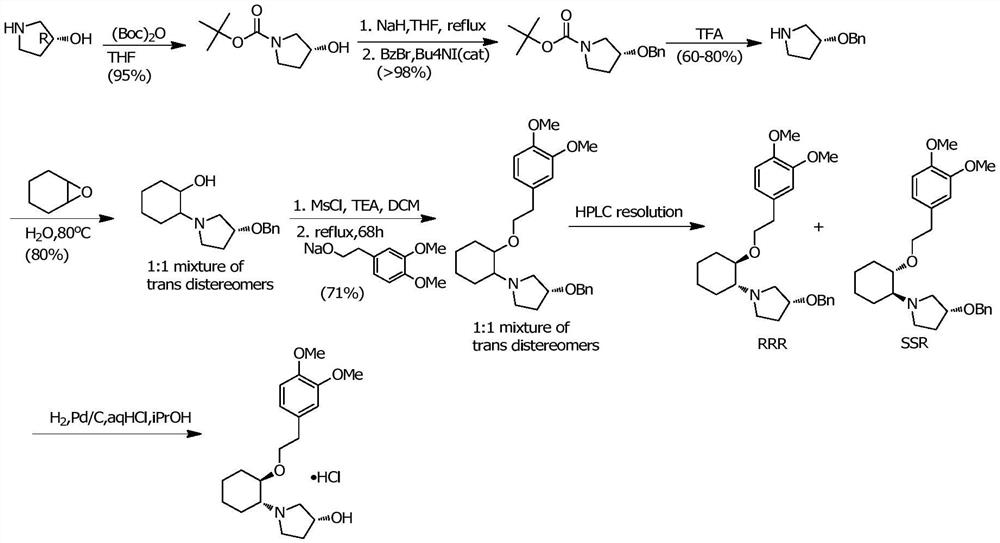
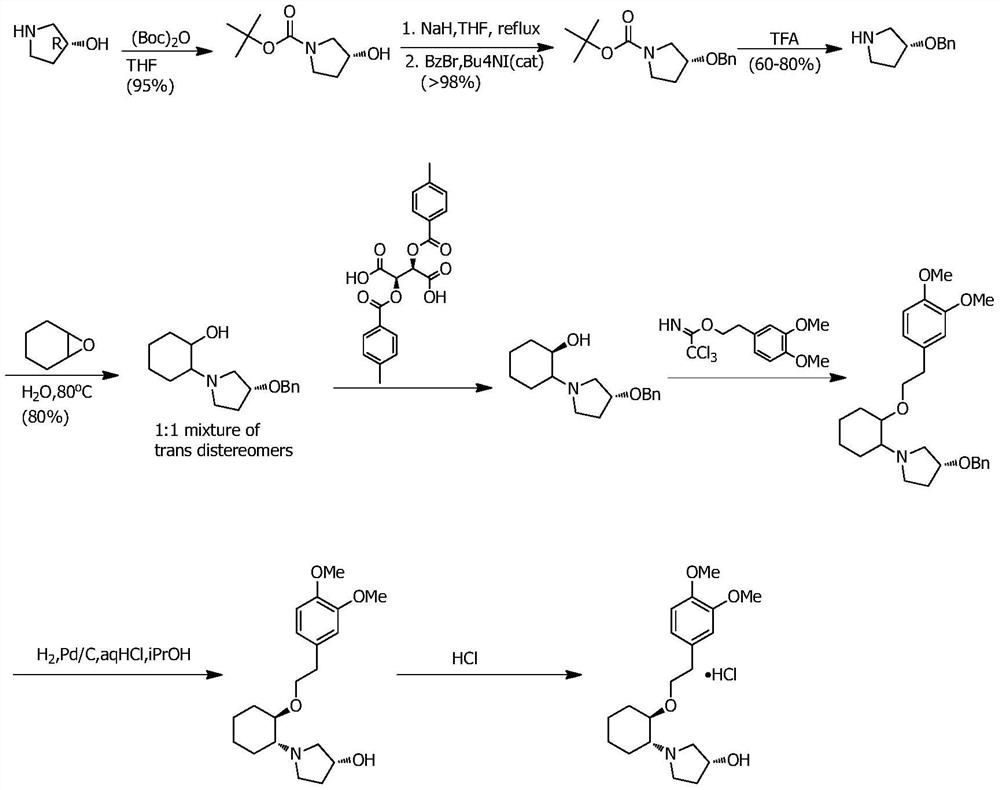
InactiveCN112457232AReduce manufacturing costSuitable for industrial productionAsymmetric synthesesEpoxyCyclohexylamines
The invention discloses a preparation method of vernakalant hydrochloride. The preparation method comprises the following steps: (1) adding a substrate (1R, 2R)- 2- (3, 4-dimethoxyphenethoxy) cyclohexylamine into a solvent; (2) adding an alkaline reagent, and controlling the temperature of the system; (3) adding an epoxy compound, controlling the temperature of the system, and raising the temperature to carry out cyclization reaction for a period of time; and (4) filtering to obtain a vernakalant solution, adding a hydrochloric acid solution to salify, concentrating under reduced pressure, andadding a crystallization solvent to crystallize, thereby obtaining the vernakalant hydrochloride. According to the invention, a chiral epoxy compound is adopted for cyclization reaction, the characteristics of high yield, short reaction time, simplicity and convenience in operation, mild conditions, convenience in post-treatment, easiness in industrial production and the like are realized, the use of heavy metals is avoided, and the development of injection medicines is facilitated.
Owner:BEIJING MEDISAN TECH +1
Cefdinir acid double salt and method for producing the same
ActiveUS20110257388A1Improve stabilityImprove bioavailabilityAntibacterial agentsOrganic active ingredientsSolubilityDouble salt
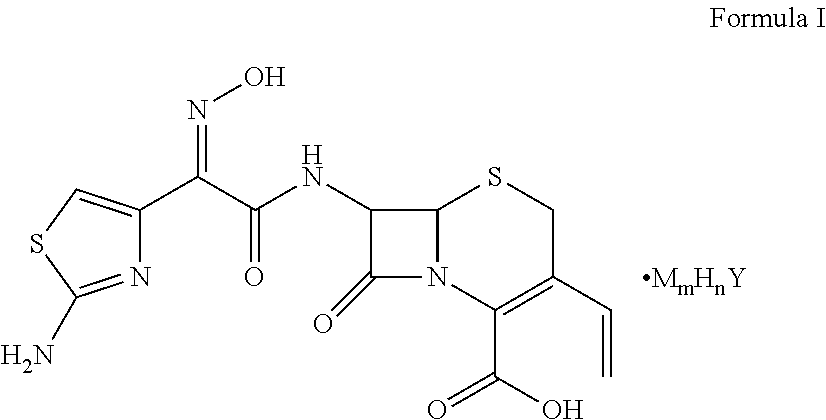
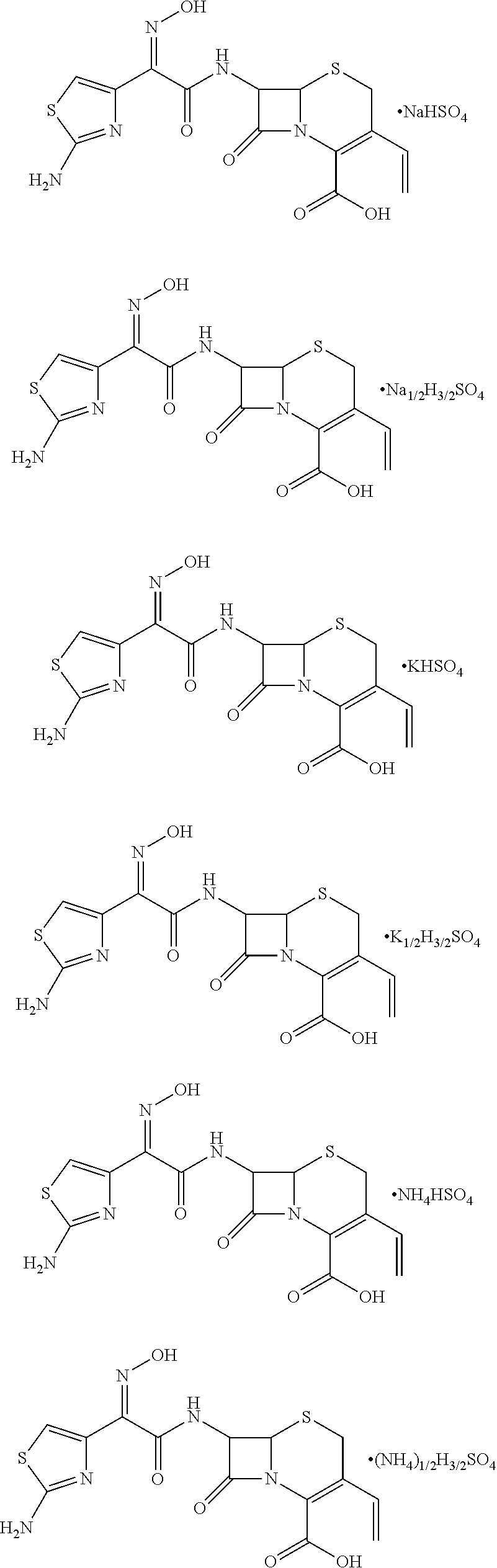
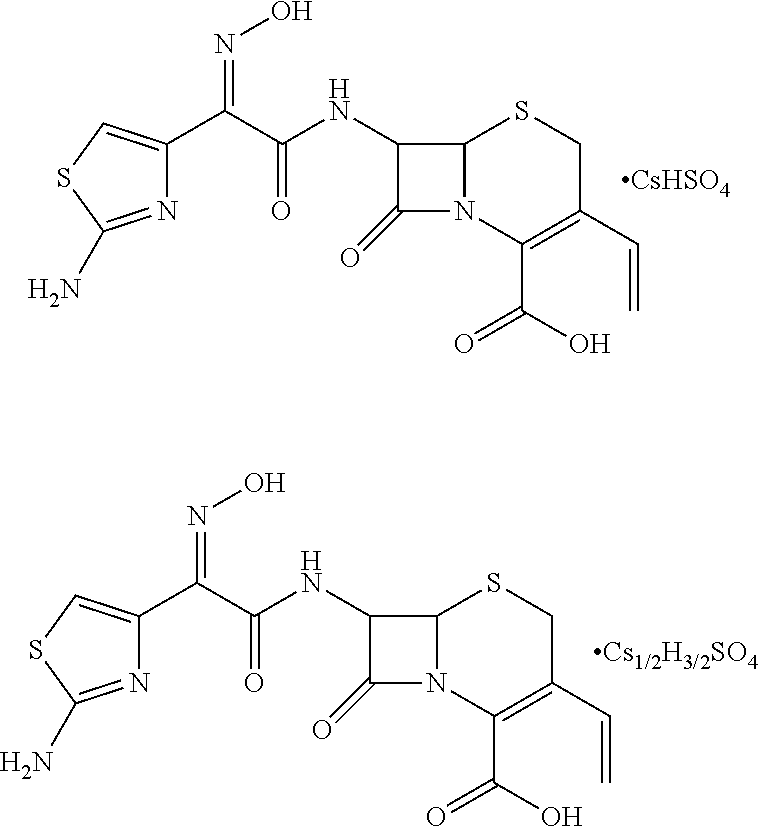
A compound represented by Formula I, wherein M represents Na+, K+, NH4+, or Cs+; and 1) when Y represents SO42−: when m=1, then n=1; and when m=0.5, then n=1.5; and 2) when Y represents PO42−, when m=1, then n=2. The compound has good solubility and high bioavailability and can be formulated into oral pharmaceutical preparations and pharmaceutical preparations for injections.
Owner:ZHEJIANG YONGNING PHARMA +1
Pharmaceutical composition comprising a sulfonylurea drug and preparation method thereof
ActiveUS10966993B2Improve solubilityImprove stabilityPowder deliveryMetabolism disorderMedicineSulfonylurea
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Drug composition for improving safety of compound ginseng injection
InactiveCN106361813AImprove securityReduced responsePowder deliveryAntipyreticSalvia miltiorrhizaPolyethylene glycol
This invention discloses a drug composition for improving the safety of a compound ginseng injection. The drug composition for injection is mainly prepared from a ginseng extract, salvia miltiorrhiza extract, a euphorbia humifusa extract and polyoxyl 15 hydroxystearate. A cosolvent good in safety and obvious in solubilizing-aiding effect is adopted to replace a cosolvent polysorbate 80 having potential safety hazard and affecting the product quality existing in the compound ginseng injection, the safety of the polyoxyl 15 hydroxystearate is higher than that of the polysorbate 80, the usage amount of the polyoxyl 15 hydroxystearate is lower, the probability and risk of adverse reaction of drugs are reduced, and the safety of clinical medication is improved.
Owner:CHENGDU XIANXIANXIAN BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com