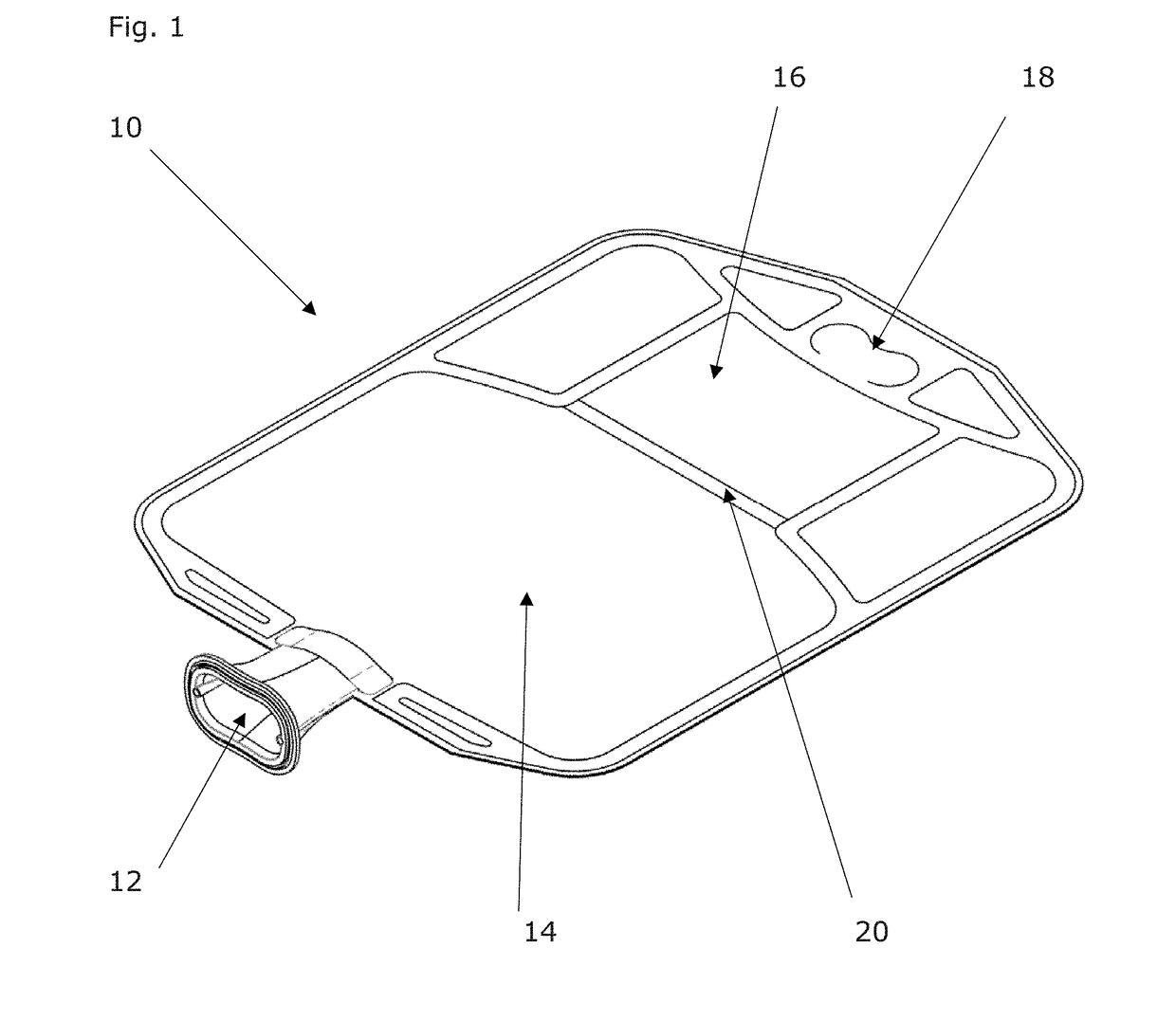
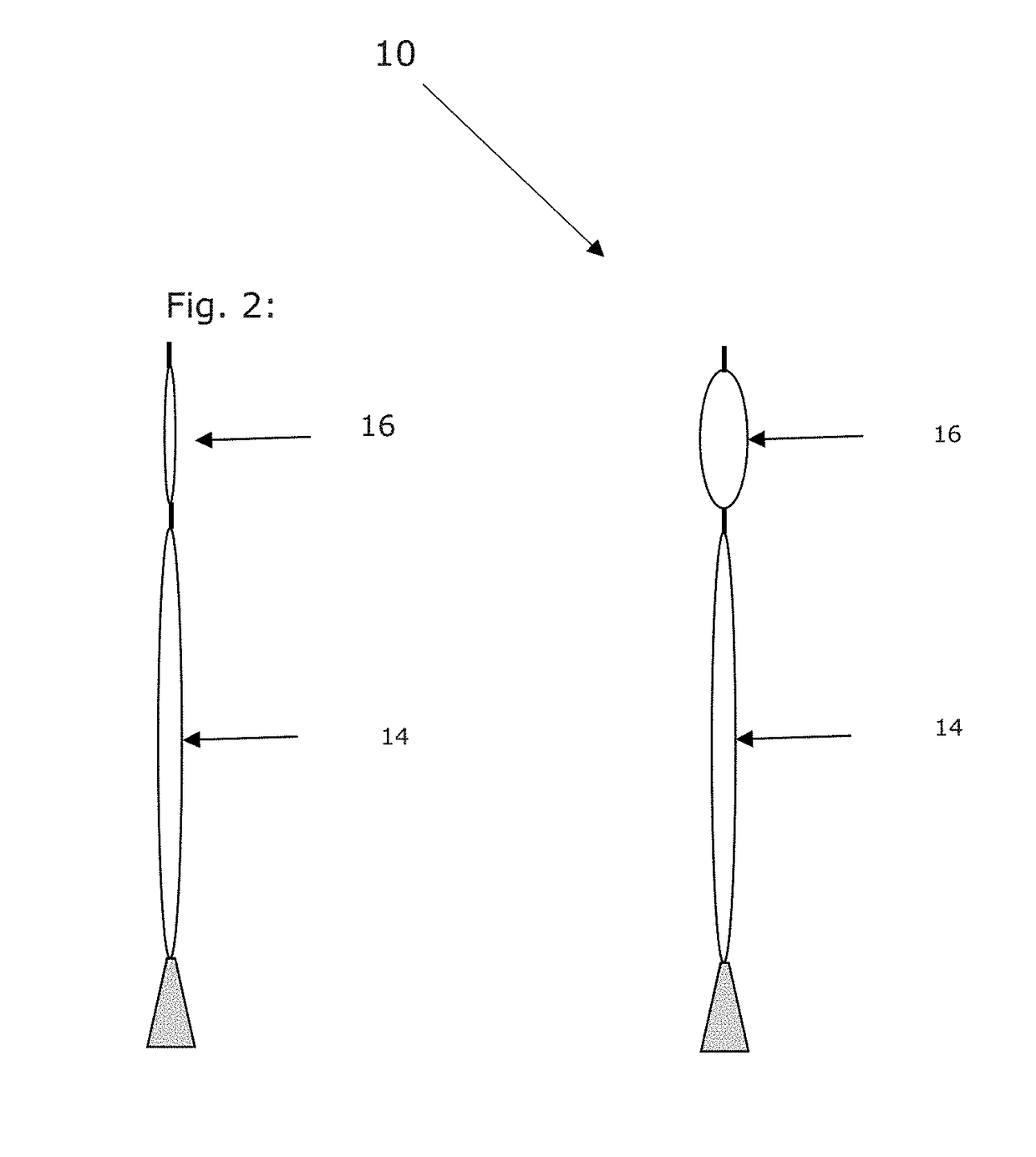
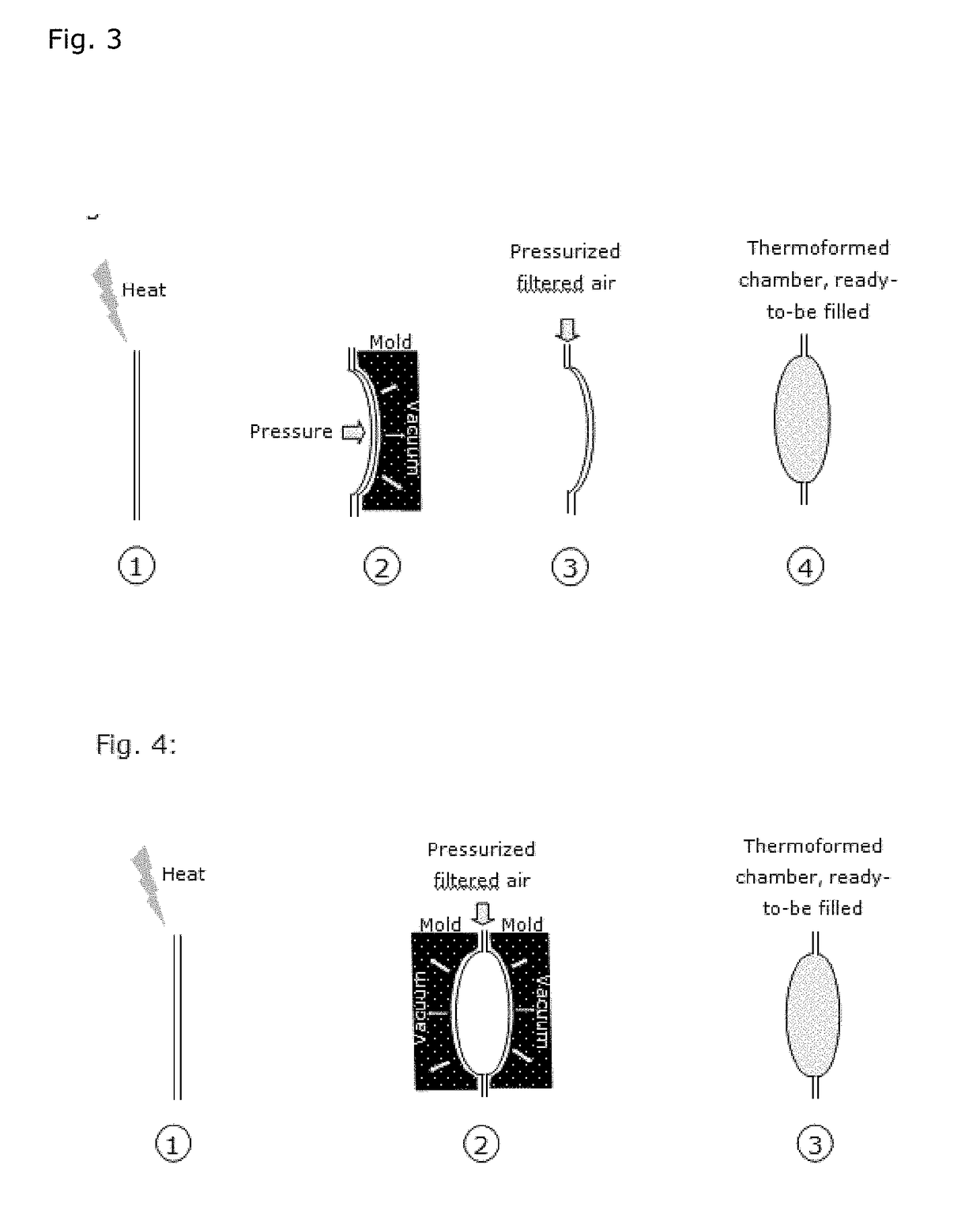
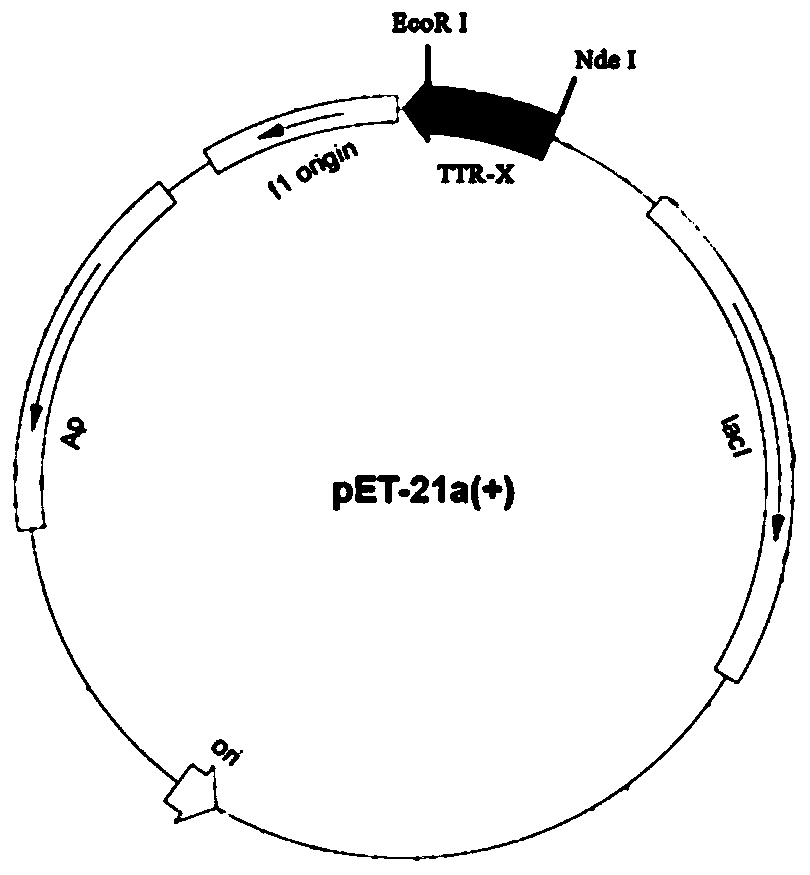
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
183 results about "Injected drug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A wide variety of drugs are injected. Among the most popular in many countries are morphine, heroin, cocaine, amphetamine, and methamphetamine. Prescription drugs—including tablets, capsules, and even liquids and suppositories—are also occasionally injected.
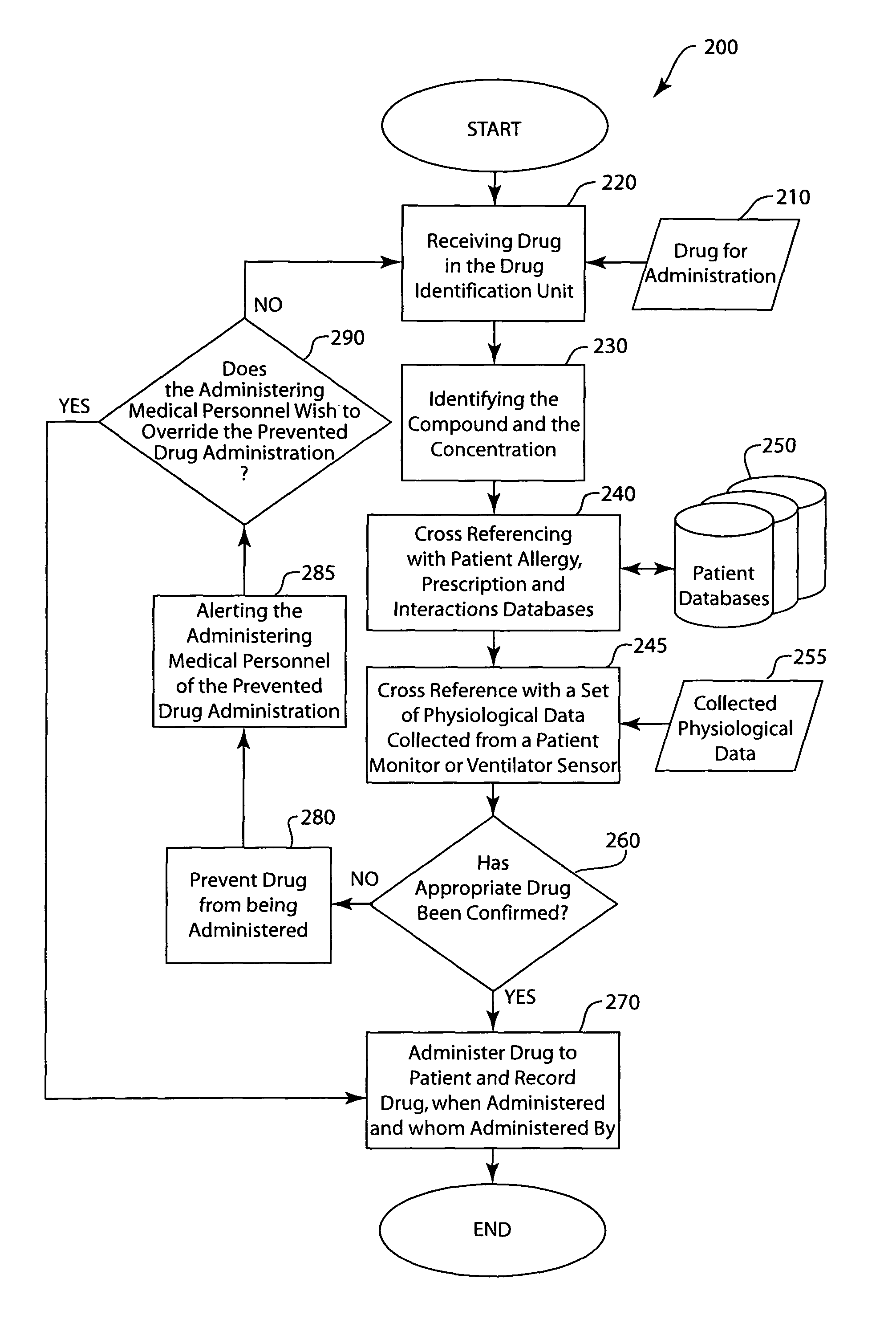
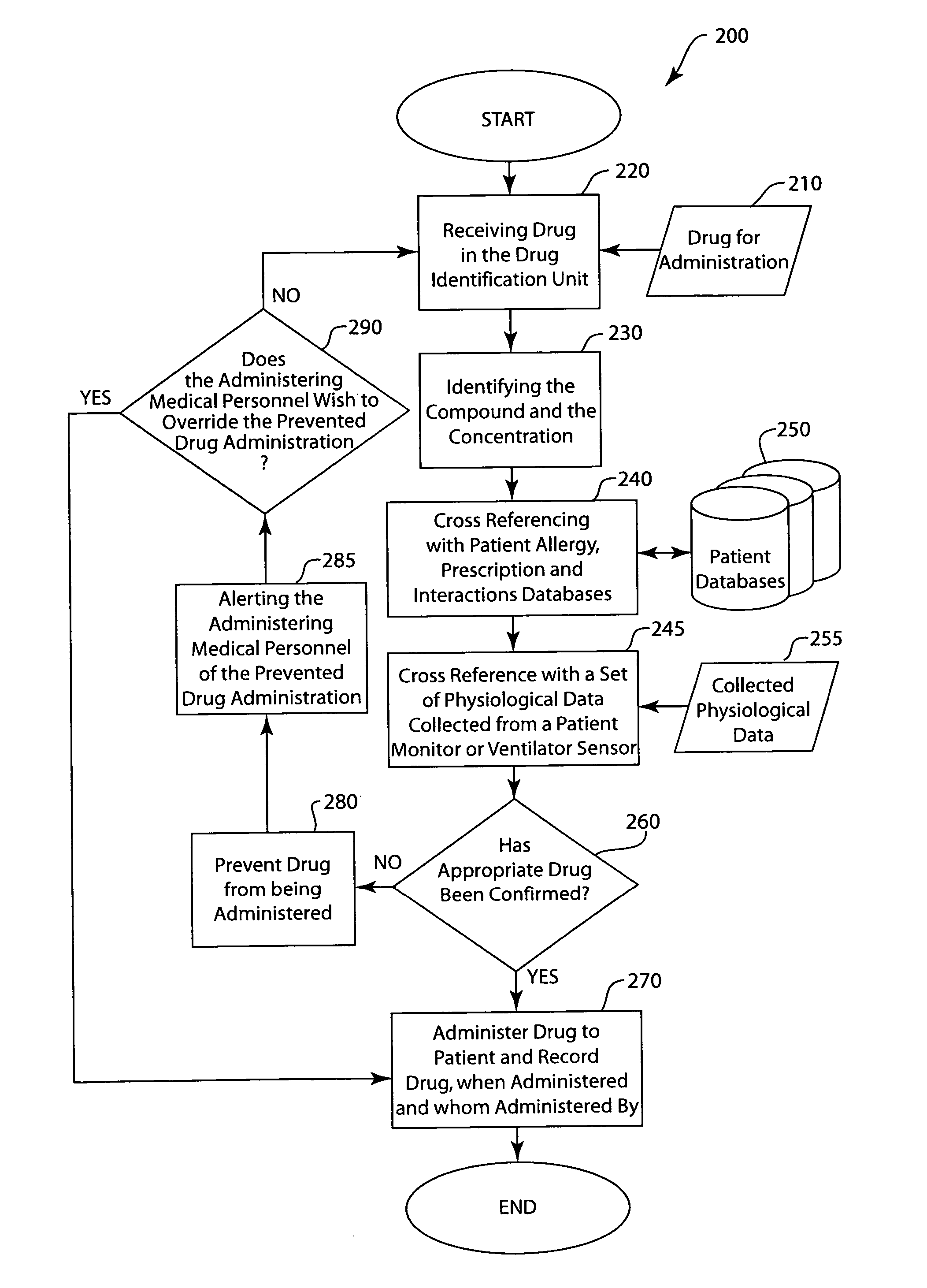
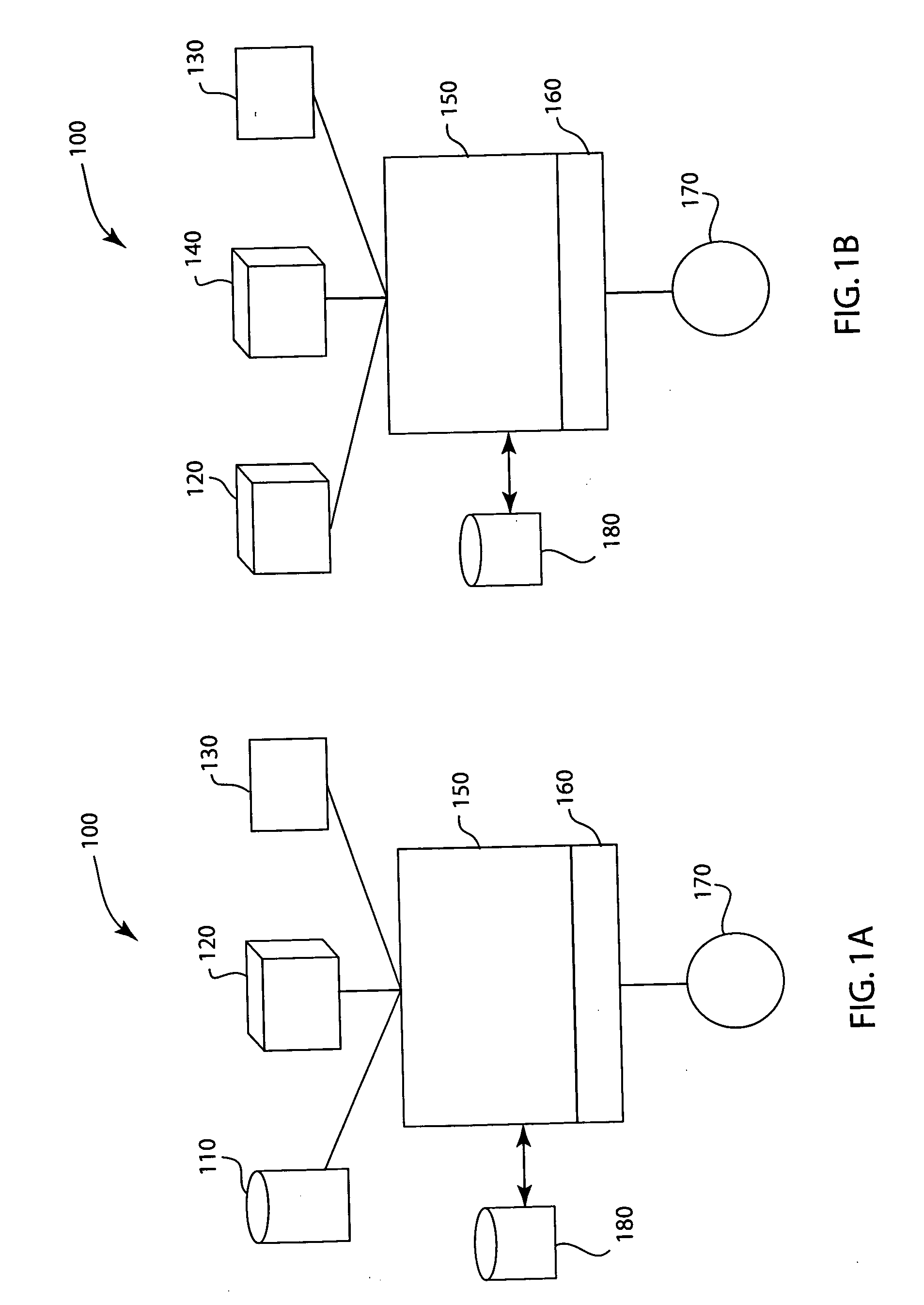
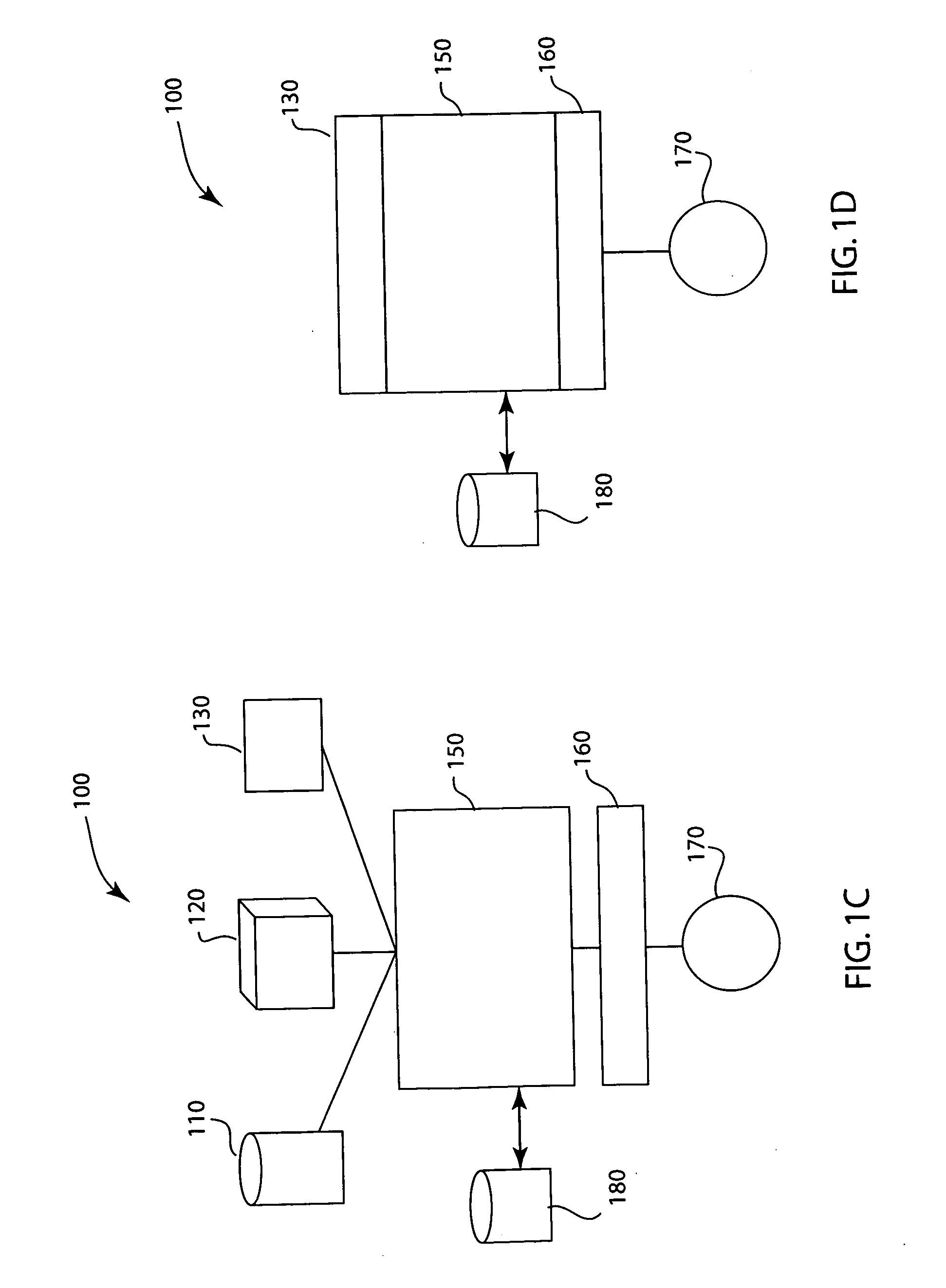
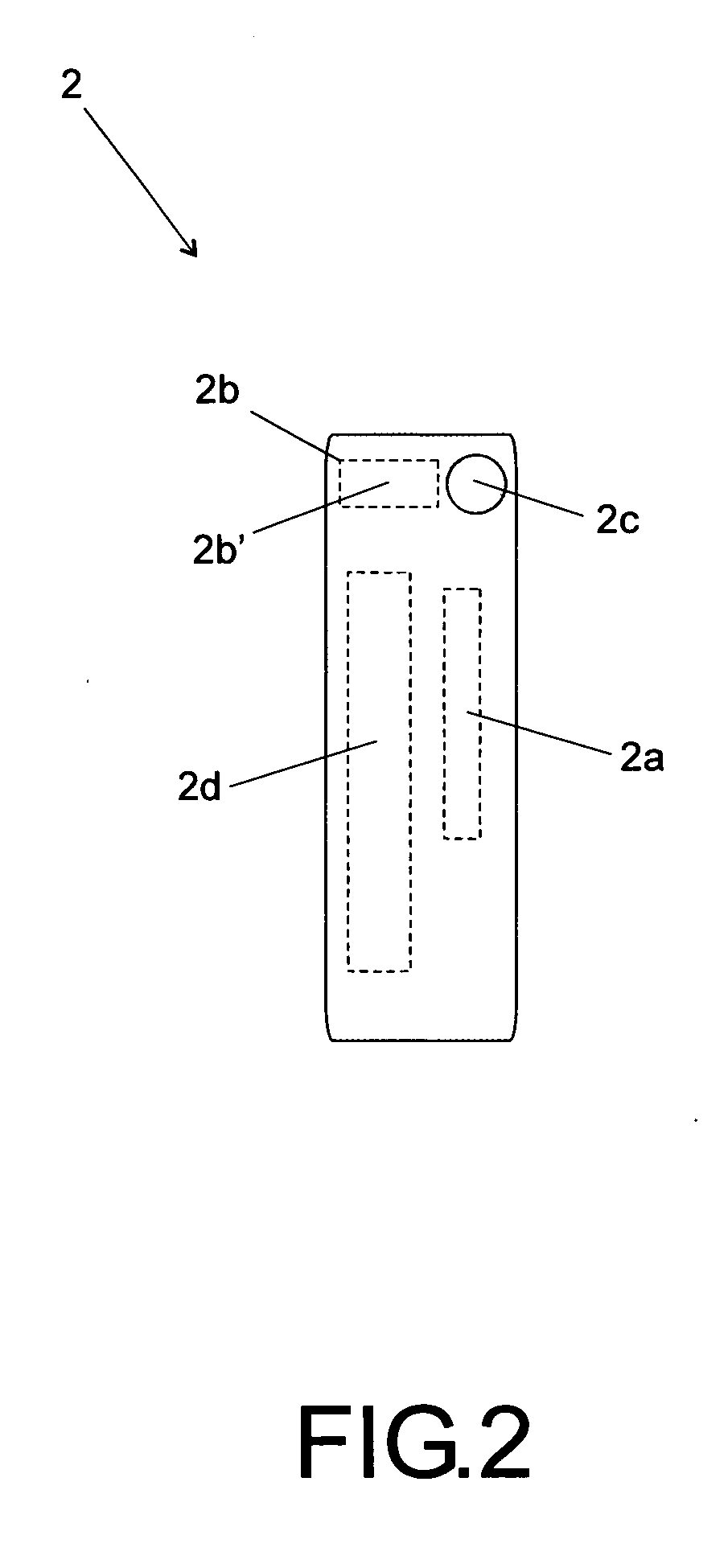
Injected drug identification and fail-safe system
The present invention is a system and method of pre-delivery drug identification. The system and method is implemented where drugs are being administered, such as in a hospital or clinic, and identifies the drug being administered to the patient before the drug reaches the patient. The system and method may utilize a sensor used for other physiologic monitoring to identify the drug. After identification, the system and method is configured to cross-reference the identified drug with the patient's prescription and allergy information, and to prevent delivery to the patient, if necessary. The system and method also utilizes data collected from the patient with a physiological monitor or a ventilator sensor to further determine whether the drug is appropriate for the patient. The method and system may also include an override system for medical personnel. The method described may be carried out by a software application.
Owner:GENERAL ELECTRIC CO
Injected drug identification and fail-safe system
ActiveUS20060287887A1Prevented being administeredData processing applicationsDrug and medicationsAllergyInjected drug
The present invention is a system and method of pre-delivery drug identification. The system and method is implemented where drugs are being administered, such as in a hospital or clinic, and identifies the drug being administered to the patient before the drug reaches the patient. The system and method may utilize a sensor used for other physiologic monitoring to identify the drug. After identification, the system and method is configured to cross-reference the identified drug with the patient's prescription and allergy information, and to prevent delivery to the patient, if necessary. The system and method also utilizes data collected from the patient with a physiological monitor or a ventilator sensor to further determine whether the drug is appropriate for the patient. The method and system may also include an override system for medical personnel. The method described may be carried out by a software application.
Owner:GENERAL ELECTRIC CO
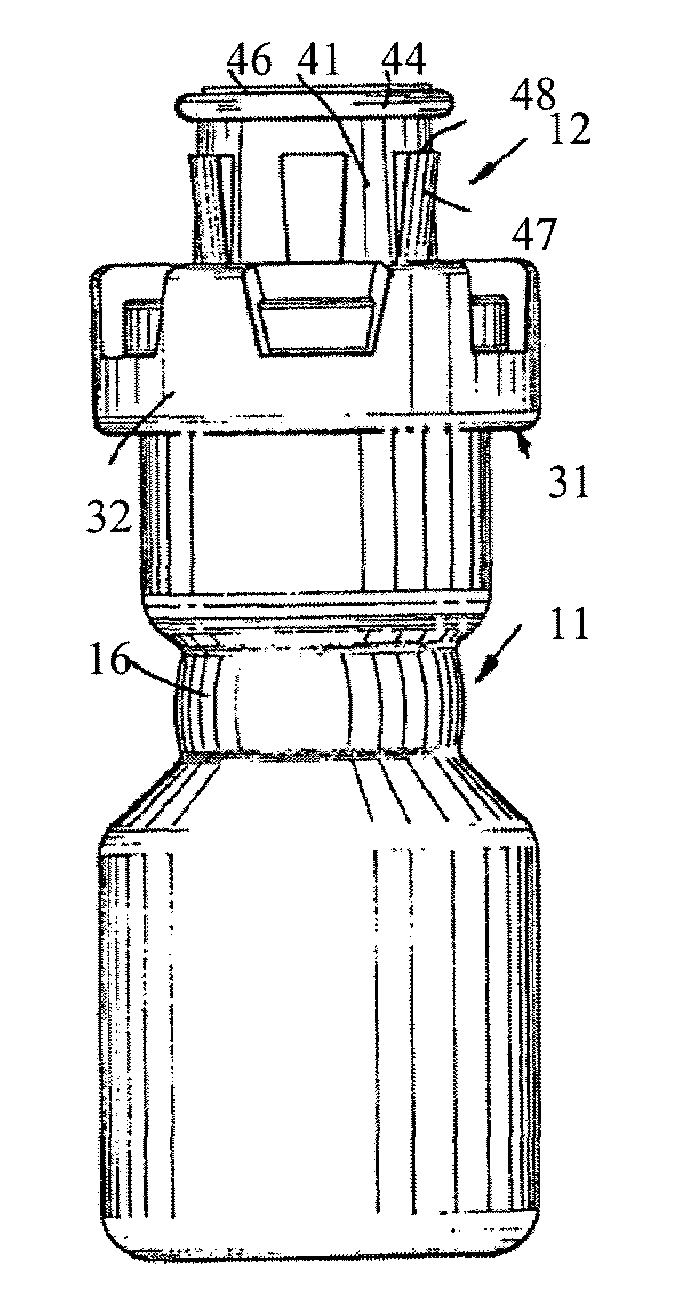
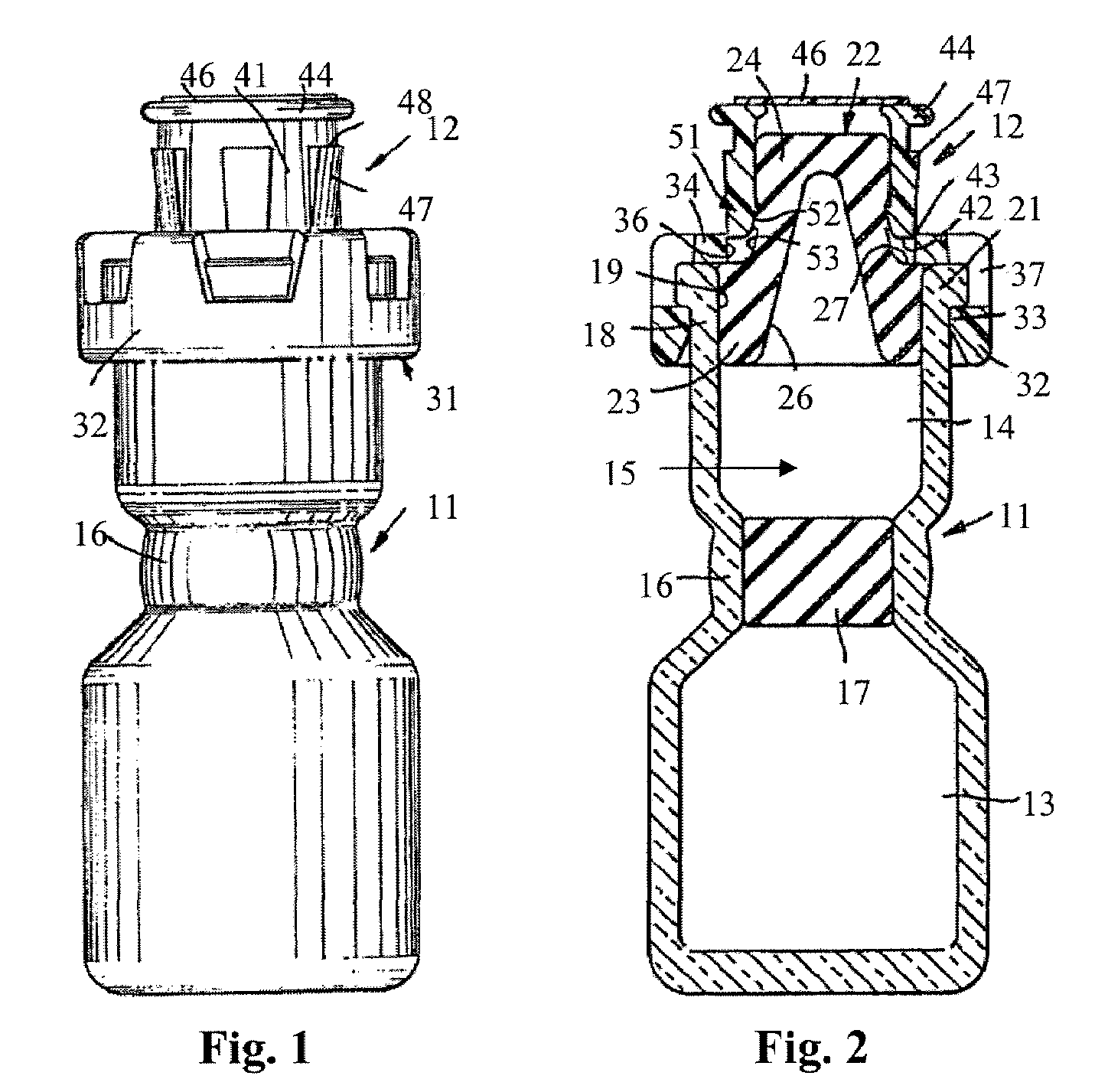
Injectable pharmaceutical suspension in a two-chamber vial
An article of manufacture is provided comprising a vial having (a) a first chamber that is substantially filled with an injectable pharmaceutical formulation; (b) a second chamber that is substantially empty but for a gaseous medium; (c) a septum, impermeable to the gaseous medium, separating the first and second chambers; and (d) actuating means effective to bring the formulation and the gaseous medium into contact by breach of the septum such that the gaseous medium acts as an effective headspace for agitation of the formulation. The formulation comprises an aqueous medium, a drug in solid particulate form in a therapeutically effective amount suspended in the medium, and one or more wetting and / or suspending agents in an amount effective to provide controlled flocculation of the drug, at least one ingredient of the formulation being susceptible to oxidative degradation.
Owner:PHARMACIA CORP
Biopsy method and gun set devices
InactiveUS20140228661A1Reduce transmissionCut evenlySurgical needlesDiagnostics using spectroscopyBiopsy methodsDensitometry
A novel set of biopsy-gun related tools are developed in order to make a better safer sampling and diagnosis, comprising a set of tools that takes the sample, releases a drug impregnated plug and additionally may inject drugs, measure pH and the molecular content of the penetrated tissues.A novel technology aiming to minimize the penetrated tissue damage, using a small diameter needle with capabilities for immediate spectroscopic analysis of the tissue, and followed by sampling and plugging of the tissue when needed.The invention describes a set of variable diameter thin tubes used to guide and insulate the penetrated organs, and a final operation of plugging the wounds with absorbable substances, impregnated with drugs.The supplementary low intensity radiation sources could increase the processes' accuracy and safety, offering the opportunity of gathering supplementary x-densitometry information.
Owner:VARIABLE GAUGE CATHETER INC
Injectable compositions and process of preparation thereof
Novel and highly stable injectable pharmaceutical compositions comprising at least one cyclooxygenase-II enzyme (COX-II) inhibitor or non-steroidal anti-inflammatory drug (NSAID) or COX / LOX inhibitor, or its tautomeric forms, analogues, isomers, polymorphs, solvates, prodrugs or salts thereof as active ingredient suitable for parenteral administration preferably by intramuscular (IM) or intravenous (IV) route; process of preparing such compositions and therapeutic methods of using such compositions are provided. The analgesic and anti-inflammatory injectable compositions of the present invention are very useful in mammals particularly in humans for the treatment of acute painful conditions like one or more of post-operative trauma, pain associated with cancer, sports injuries, migraine headache, neurological pain and pain associated with sciatica and spondylitis, and the like, and / or chronic painful conditions, and / or a variety of painful and inflammatory conditions like postoperative pain, primary dysmenorrhea and painful osteoarthritis, and / or other associated disorders such as inflammation, fever, allergy, or the like.
Owner:PANACEA BIOTEC
Injectable pharmaceutical formulation of melphalan
InactiveUS20130131174A1High viscosityBiocideOrganic active ingredientsPharmaceutical formulationOrganic solvent free
An injectable pharmaceutical formulation of Melphalan comprising a solid composition of melphalan hydrochloride lyophilized with a content of impurities up to 1.3% (p / p)and a pH buffer solution; a process to prepare said solid composition. Also a reconstituted solution of melphalan comprising a solid composition of melphalan lyophilized reconstituted wherein said solution is aqueous, a perfusion free of organic solvent and a kit.
Owner:ERIOCHEM SA
Young garlic shoot harvester
ActiveCN106941868ASolving the problem of mechanized harvestingPrecious harvest timeHarvestersShootTurning frames
The invention relates to the field of agricultural machinery, in particular to a young garlic shoot harvester which comprises a car frame. A swing cutter device for cutting down young garlic shoots from bottoms is arranged at the front end of the car frame, a conveying device for conveying the young garlic shoots cut down by the swing cutter device to a harvesting box on the car frame is arranged above the swing cutter device on the car frame, advancing wheels are arranged on the two sides of the car frame, a land supporting drag wheel is arranged at the tail end of the car frame, a young garlic shoot drawing device for gathering and lifting young garlic shoots up in an advancing process of the car frame is arranged on the car frame and between the conveying device and the swing cutter device, and a peeling device for peeling the young garlic shoots in a lifting process through the young garlic shoot drawing device is arranged on the car frame and between the young garlic shoot drawing device and the swing cutter device. The young garlic shoot harvester disclosed by the invention solves the problem of mechanically harvesting the young garlic shoots, further has a reasonable structure, small volume, flexibility and convenience to operate and high work efficiency, wins precious harvesting time for young garlic shoot farmers and effectively reduces harvesting cost.
Owner:HENAN UNIV OF SCI & TECH
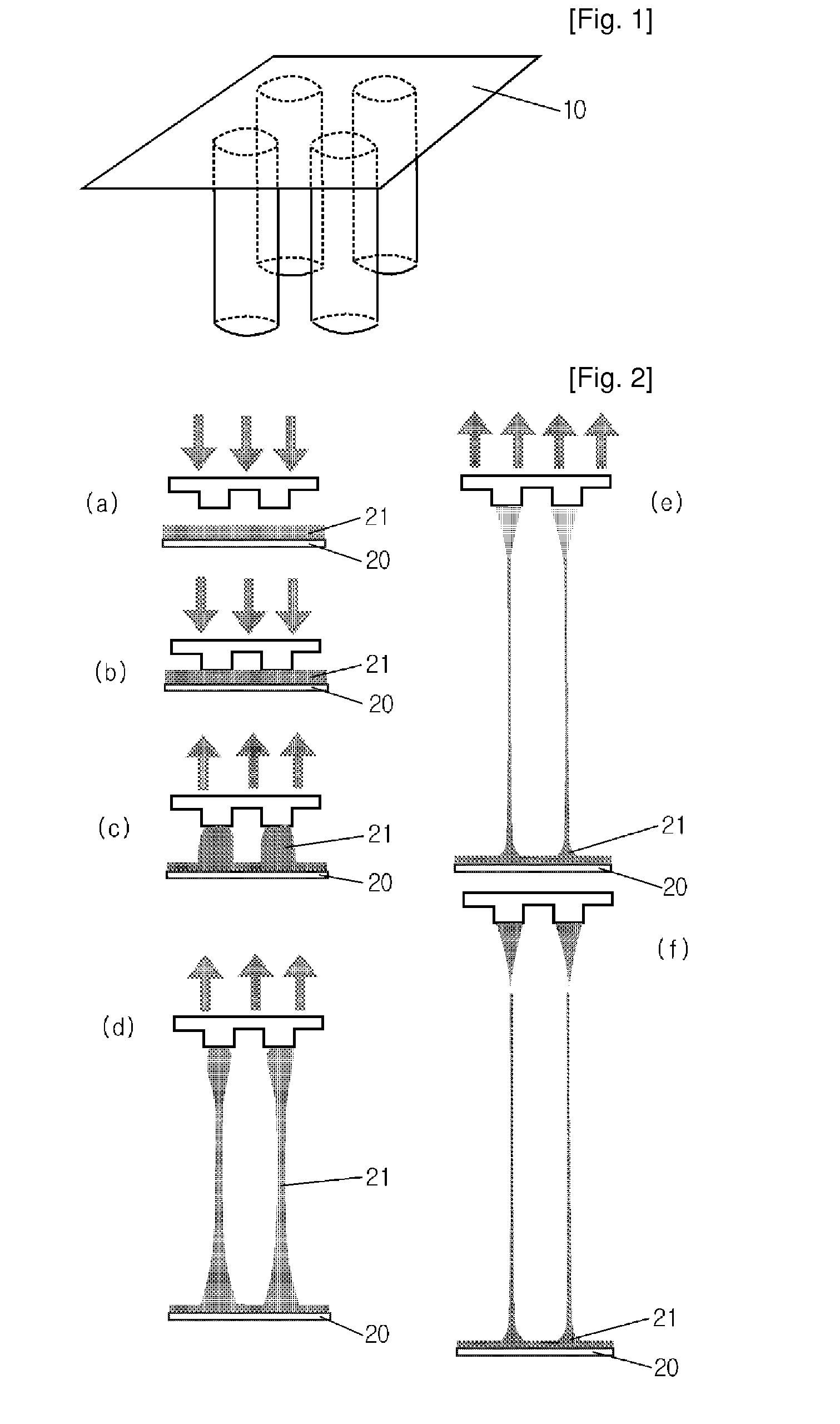
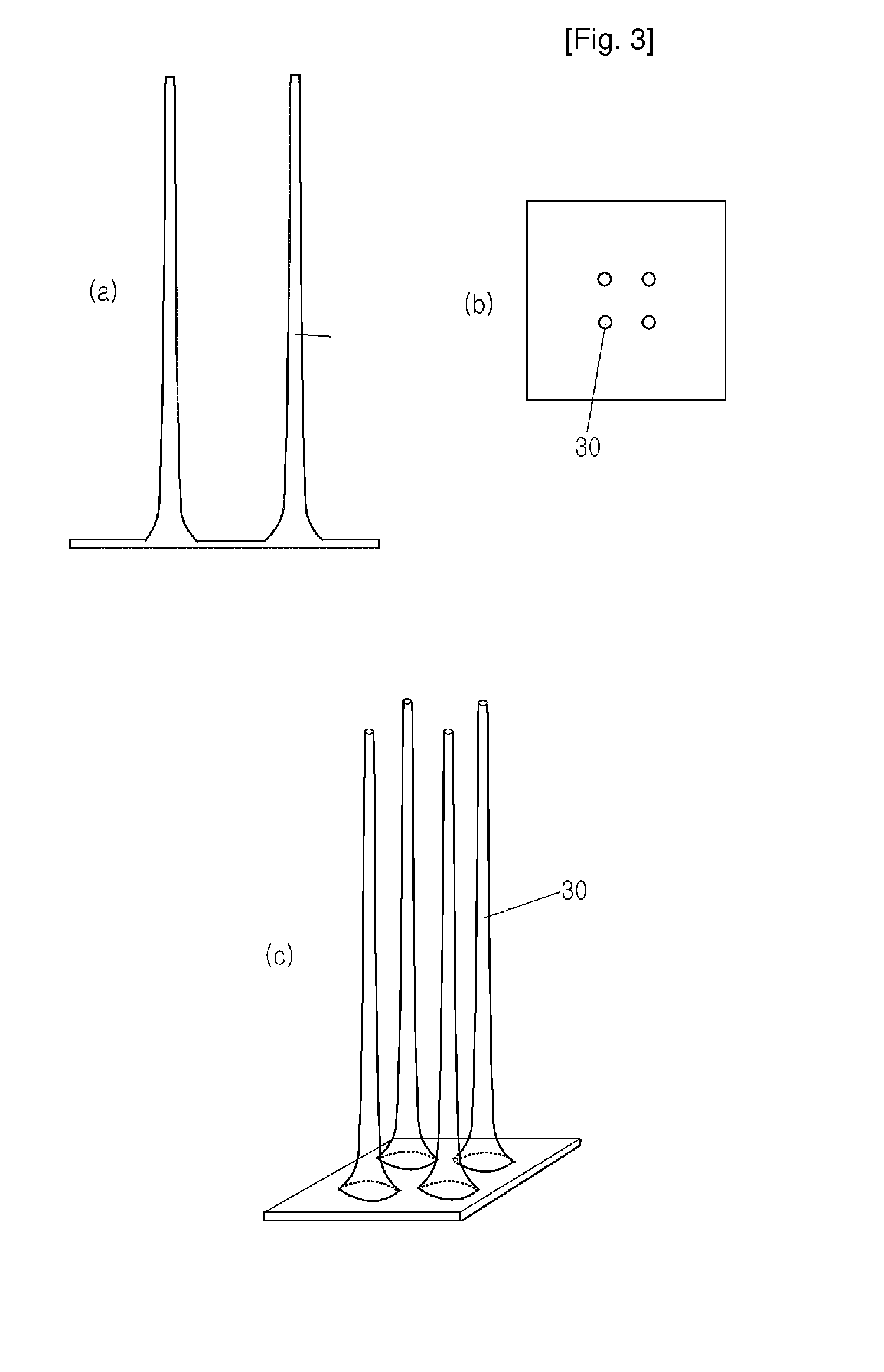
Hollow Type Microneedle and Methods for Preparing It
ActiveUS20100114043A1Slender structureImprove drawing speedGlovesMicroneedlesDrug injectionStratum corneum
Disclosed herein are hollow microneedles and a fabrication method thereof. The microneedles are small in diameter and are long and hard enough to pass through the stratum corneum. Therefore, the hollow microneedle of the present invention can be used in blood sampling or drug injection through the skin.
Owner:IND ACADEMIC CORP FOUND YONSEI UNIV
Injectable controlled release composition comprising high viscosity liquid carrier
Compositions may include a pharmaceutical active agent, a high viscosity liquid carrier material (HVLCM), a lactic acid-based polymer, and an organic solvent. Related compositions and methods are also disclosed. For instance, a carrier formulation for controlled release of injectable drugs is disclosed. The formulation may include a non-water soluble high viscosity liquid which may be sucrose acetate isobutyrate, a lactic-acid based polymer which may be a poly(lactic acid)(glycolic acid), and an organic solvent which maintains the composition in a monophasic form at 25° C. in one atmosphere. Drug in the formulation may be released upon administration such that less than 10% (e.g. 2-8%) of drug is released in the first 5 hours; 10% to 80% of the drug is released during a period of 5 hours to 7 days after administration; and 10% to 40% of the drug is released gradually over a period of 7 days to 28 days from initial administration. The drug may be an anti-schizophrenia agent delivered by injection.
Owner:DURECT CORP
Biological cell ultrasonic atomic force microscopic detection system and method
ActiveCN105910560ASuitable for non-destructive inspectionAvoid destructionUsing subsonic/sonic/ultrasonic vibration meansCancer cellSonification
The invention relates to a biological cell ultrasonic atomic force microscopic detection system and method under the physiological environment. The system is composed of a detector, a piezoelectric transducer, a probe control module, a phase-locked amplifier and an ultrasonic ranging module. The detector is arranged in the physiological environment to scan biological cells, and the piezoelectric transducer vibrates at ultrasonic frequency. Ultrasonic signals penetrate through the biological cells to generate different signal responses. The system acquires the response signals so as to obtain the biological cell morphology and internal maps. The ultrasonic ranging module records and processes echo delay signals and quantitatively expresses an acoustic path of a vertical direction so as to reconstruct the depth map of the biological cells. Nanoscale ultra-micro internal structural analysis of the biological cells can be realized, a probe can be accurately located and guided to inject drug loading nanoparticles into cancer cells and the stress changes of the morphology and the internal structure can be observed so that the system and the method can be used for nanoscale lossless measurement of the living cells under the physiological environment and have a directive significance for nano-biotechnology and nano-medicine.
Owner:CHANGCHUN UNIV OF SCI & TECH
Lyophilization process
The present invention provides a process for producing a lyophilized pharmaceutical composition containing a protein. The present invention further provides a product produced by the process. The present invention further provides a process for producing an injectable pharmaceutical composition. The present invention further provides a method of treating a patient with a therapeutic protein composition.
Owner:TEVA PHARMA IND LTD
Insulin liquid formulations for nose administration
InactiveCN1919338AImprove adhesionPeptide/protein ingredientsMetabolism disorderEnteral administrationNasal cavity
The invention discloses insulin providing liquid agent through nose, which is characterized by the following: avoiding side-effect due to injecting drug, increasing drug adsorbing area because the nose possesses bigger nasal area (150-180cm<2>) with large scale of microvillus and thinner nasal mucosa (2-4mm), adsorbing drug rapidly due to abundant capillary and capillary lymphangiolog.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Injectable pharmaceutical compositions comprising adrenaline and citric acid
ActiveUS9433589B2Good storage stabilityGood choiceOrganic active ingredientsInorganic non-active ingredientsCitric acidEpinephrine
The present invention relates to injectable pharmaceutical compositions showing improved storage stability said compositions comprising adrenaline and citric acid showing improved storage stability.
Owner:FREMONT GRP LTD
A kind of photosensitizer and its preparation method and application
InactiveCN102284059AEvenly dispersedImprove targetingOrganic active ingredientsEnergy modified materialsCancer cellPhotosens
The invention relates to a photosensitizer and its preparation method and application. The photosensitizer is a liposome preparation composed of phthalocyanine zinc complex. The liposome preparation can be uniformly dispersed in an aqueous solution and exists stably for a long time, so it can be conveniently used as an injection drug, has high targeting, and can better exert drug effects. At the same time, the preparation has a wide range of killing and inhibiting effects on various types of bacteria, and has a strong killing effect on various cancer cells. item requirements.
Owner:FUZHOU UNIV
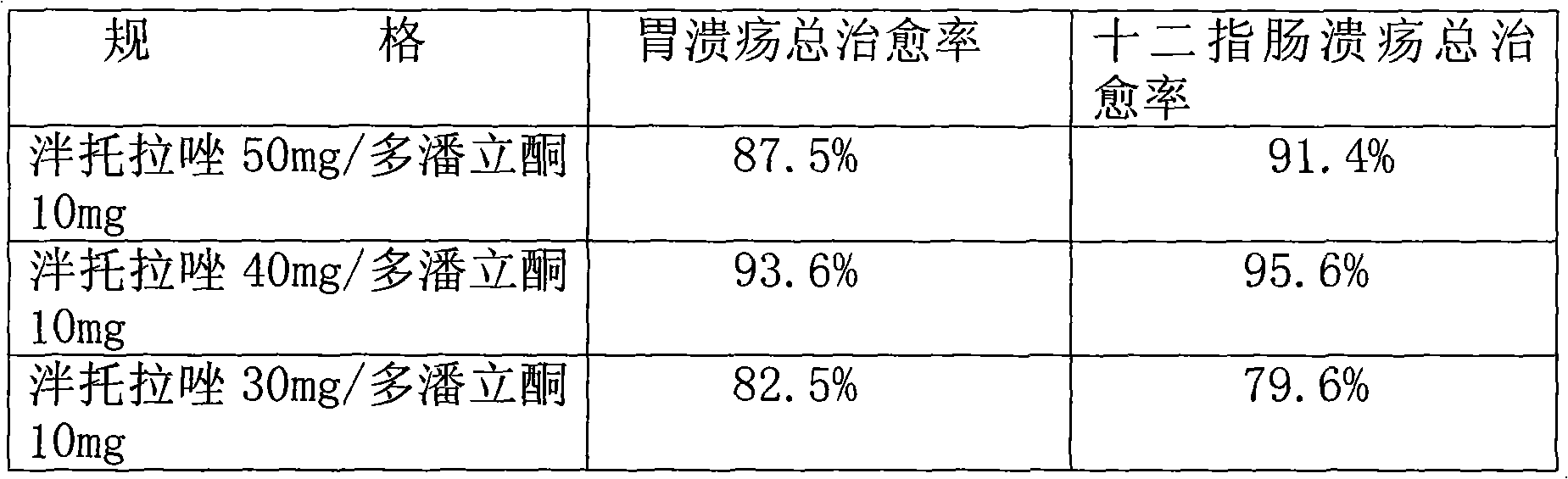
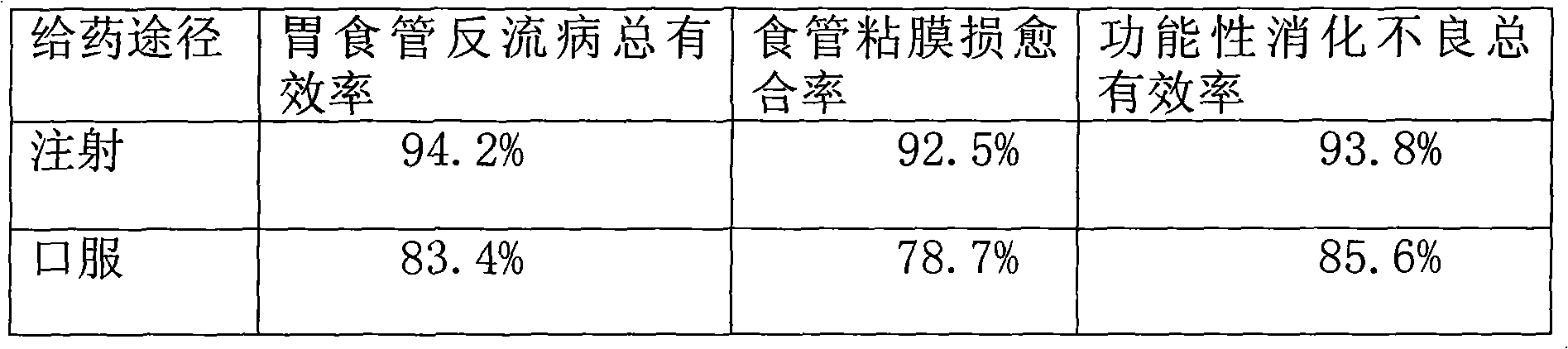
Combined drug of pantoprazole and domperidone
InactiveCN101961334AHas an acid-suppressing effectInhibition of secretionPowder deliveryOrganic active ingredientsTreatment effectDuodenal ulcer
The invention provides a combined drug of pantoprazole and domperidone and in particular provides application of pantoprazole and domperidone to preparing combined injections for treating gastric ulcer, duodenal ulcer, gastro-esophageal reflux diseases or functional dyspepsia. The invention also provides the combined injections for treating gastric ulcer, duodenal ulcer, gastro-esophageal reflux diseases or functional dyspepsia, including different specifications of unit preparations, pantoprazole and domperidone for simultaneous, respective or successive administration and preparations prepared by pharmaceutically acceptable vectors. The weight ratio of pantoprazole and domperidone is 1:1-5:1, and the pantoprazole and domperidone contain excipients, stabilizing agents, etc. Through combined injection administration, the invention improves the bioavailability of pantoprazole and domperidone and ensures the treatment effects to be obviously better.
Owner:CHENGDU ZIHAO PHARMA
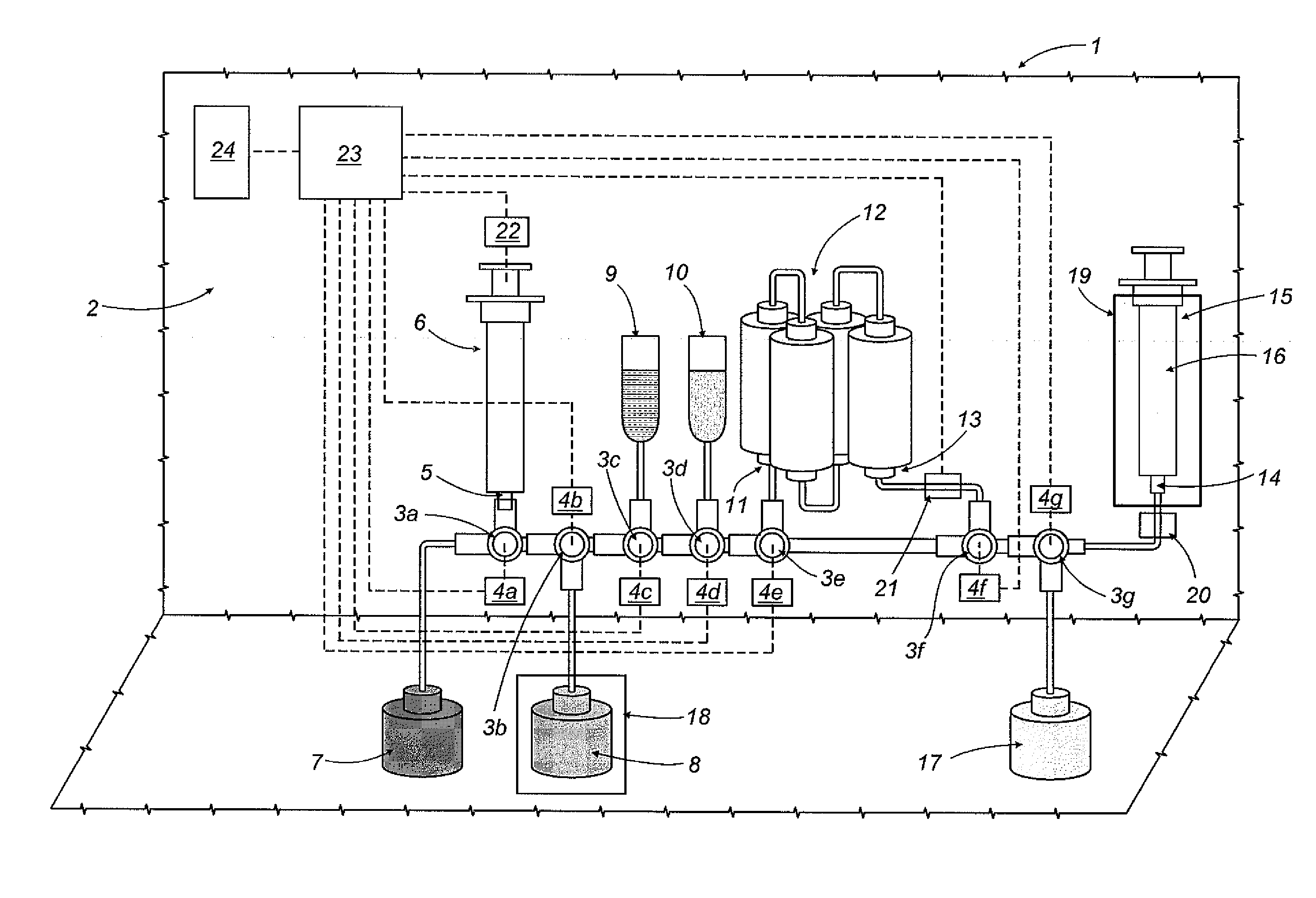
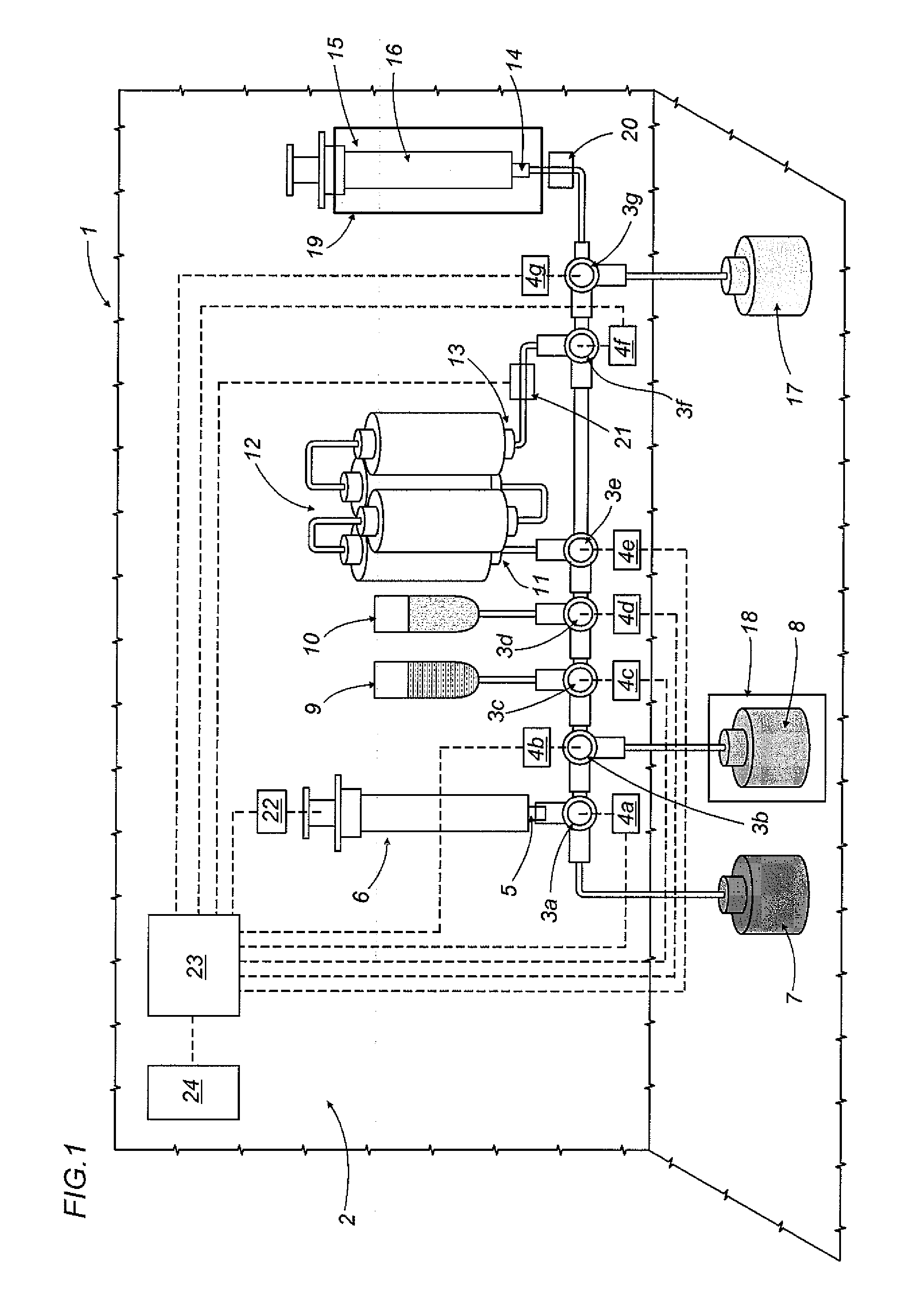
Apparatus and method for preparing medicines containing radioactive substances
InactiveUS20110020224A1Rapid and easy to useRapid and safeRadioactive preparation carriersPeptide preparation methodsChromatographic separationMedicine
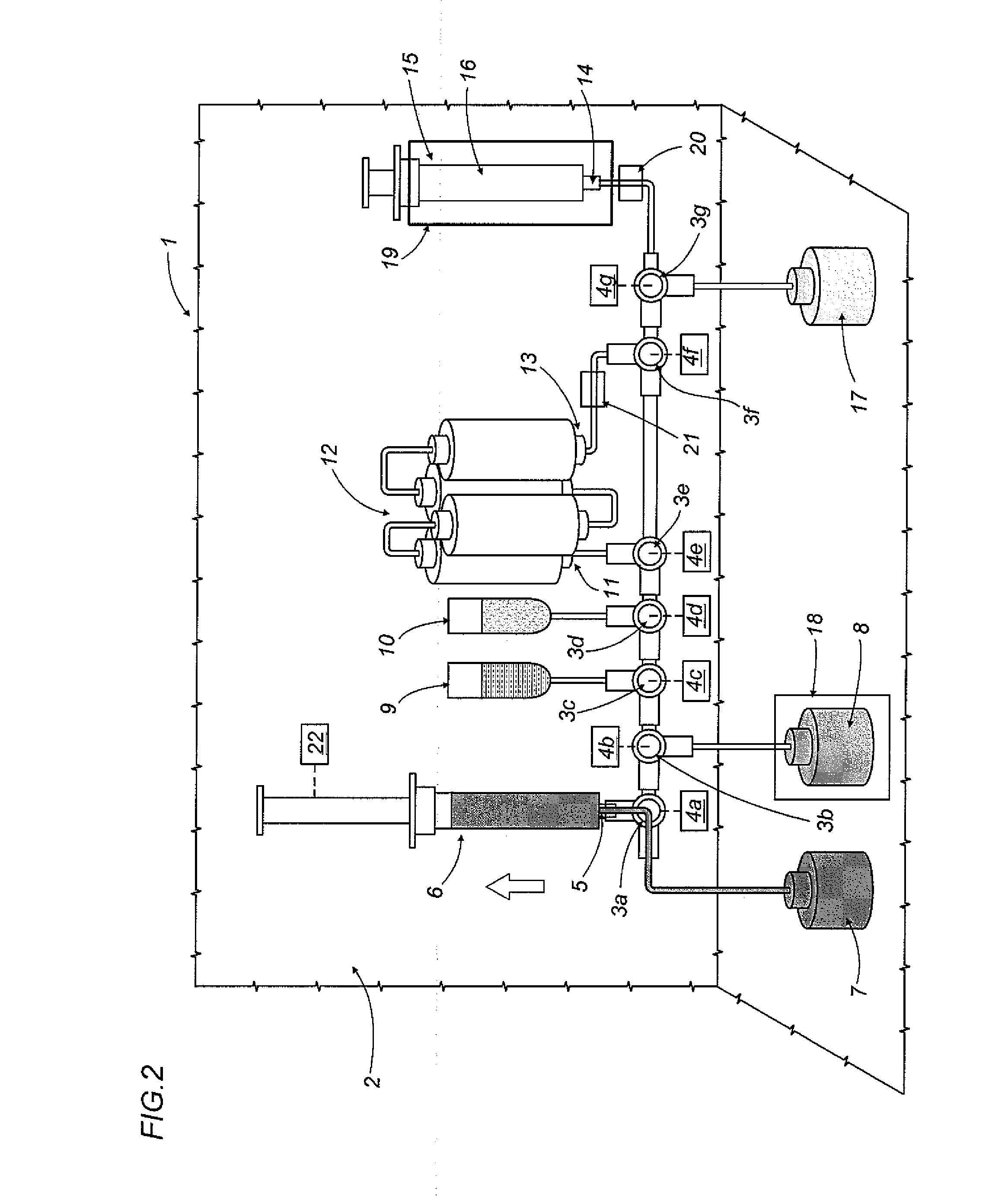
An apparatus (1) for preparing medicines containing radioactive substances, more specifically injectable medicines containing beta-emitting substances, in which a radioactive element is combined, in a mixture, with a protein to be labelled using the radioactive element; the mixture is then subjected to a chromatographic separation step which separates the mixture and isolates the medicine (16).
Owner:PHILOGEN SRL LLC
Rapid bloating-relieving and digestion-promoting device for treating bloating in rumen
ActiveCN104739538ARestore blood circulationPromote blood circulationVibration massageEvaluation of blood vesselsDrug injectionPostoperative recovery
The invention discloses a rapid bloating-relieving and digestion-promoting device for treating bloating in rumen and belongs to the technical field of veterinary apparatuses and instruments. The rapid bloating-relieving and digestion-promoting device is capable of taking both drug injection and bloating-relieving into account, and also capable of effectively and mechanically breaking bubbles in the rumen while injecting drugs and preventing chyme attached on the bubbles from blocking a bloating-relieving channel; while injecting drugs, the rotating speed of a drug injection part can be controlled manually, and meanwhile, drugs can be injected into the rumen at a large area and in all directions; the device is further provided with a sucking pump and a cardiac rhythm tester; the pressure in the rumen and the heart conditions of a silk animal can be monitored in real time during an operation, the pressure in the rumen can be reduced in real time quickly and the heart conditions of the silk animal can be monitored in real time during the operation, and complications during the operation can be prevented; furthermore, the device is provided with a massager which is capable of helping bloating-relieving during the operation and postoperative recovery.
Owner:南通香境生物医药科技有限公司
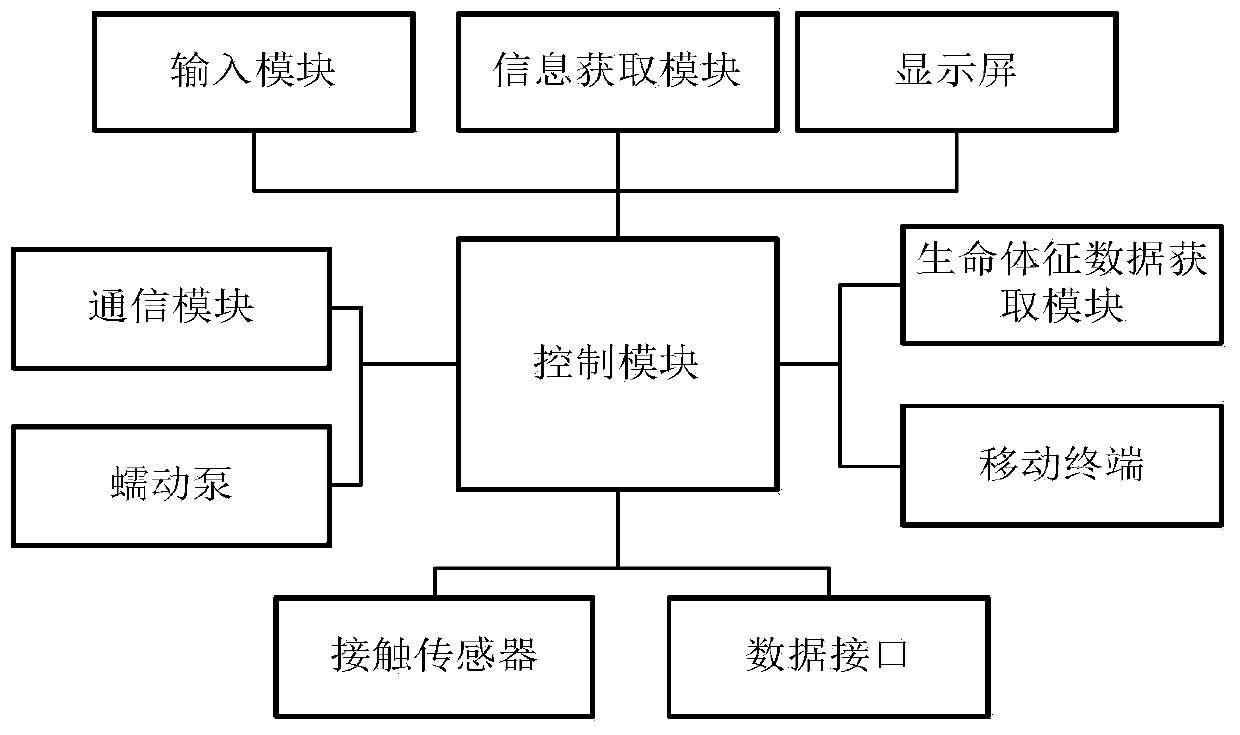
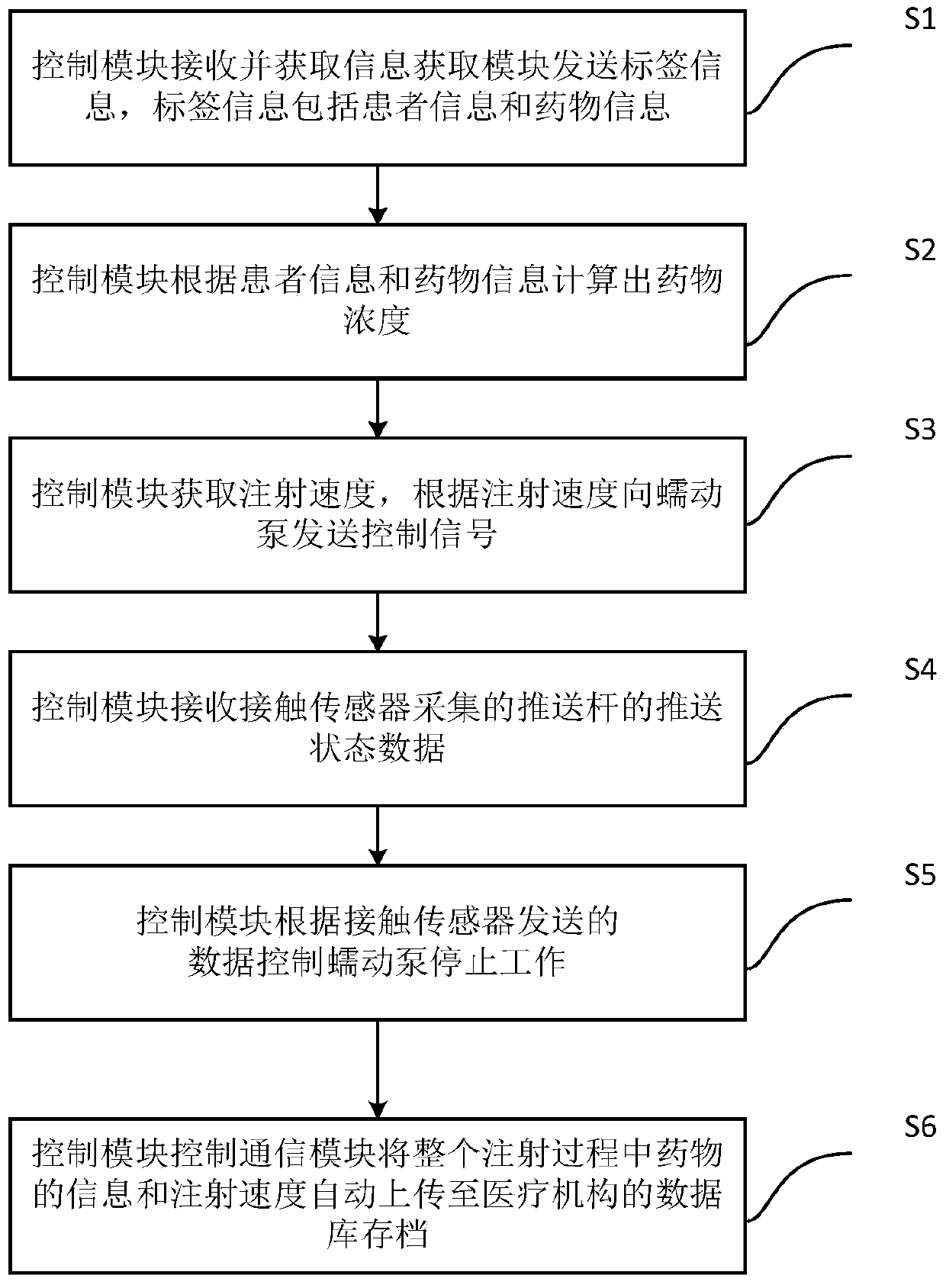
Intelligent injection pump system, control method thereof, and medium
PendingCN111430000AReduce workloadMedical communicationDrug and medicationsPeristaltic pumpMedication information
The invention discloses an intelligent injection pump system, a control method thereof, and a medium. The injection pump system includes an information acquiring module, a control module, a display screen, a peristaltic pump, a contact sensor, and a communication module, wherein the information acquiring module reads the label information on the injector and acquires information of patients and drugs through the label information; the control module calculates concentration of the drug and sets injection speed according to the information of patients and drugs and sends a control signal to theperistaltic pump; the peristaltic pump drives the injector to inject the drug according to the control signal; the contact sensor sends collected data to the control module; the control module controls the peristaltic pump to stop working; the communication module automatically uploads the information of drugs and injection speed to a database of a medical organization for storage. The system canrapidly recognize the label information of the injection drug and automatically control the injector to precisely inject the drug. In addition, the information of drugs and injection speed are automatically uploaded to a database for storage during the whole injection process, thus reducing working load of medical workers.
Owner:领先医疗科技(广州)有限责任公司
Intelligent bed system
InactiveCN107007412AImprove automationImprove completenessNursing bedsAmbulance serviceNursing techniquesAutomatic control
The invention provides an intelligent bed system. Movement and operation of different bed body units can be independently controlled through a splicing structure of the bed body units, a diversified functional mechanism design and an automatic control design of a servo system, the nursing actions such as prostration, reclining, foot lifting, standing and turnover of a nursed person on a bed surface, and complicated nursing functions such as dinning, cleaning, defecating, drug injection, drug attachment, drug replacement, physiological signal collection, heating, cooling and ventilating are achieved in an auxiliary manner; various bed body units can be separately shifted out to carry out treatment such as energy debugging or maintenance, charging, overall replacement and local maintenance or replacement; removal and replacement operations of a bedding layer material can be completed through automatic control; automatic and intelligent degrees of nursing work of nursed persons such as old people and patients are greatly improved; the nursing work efficiency is improved; and the intelligent bed system has profound significance in promotion of an automatic and intelligent nursing technology.
Owner:金倚天
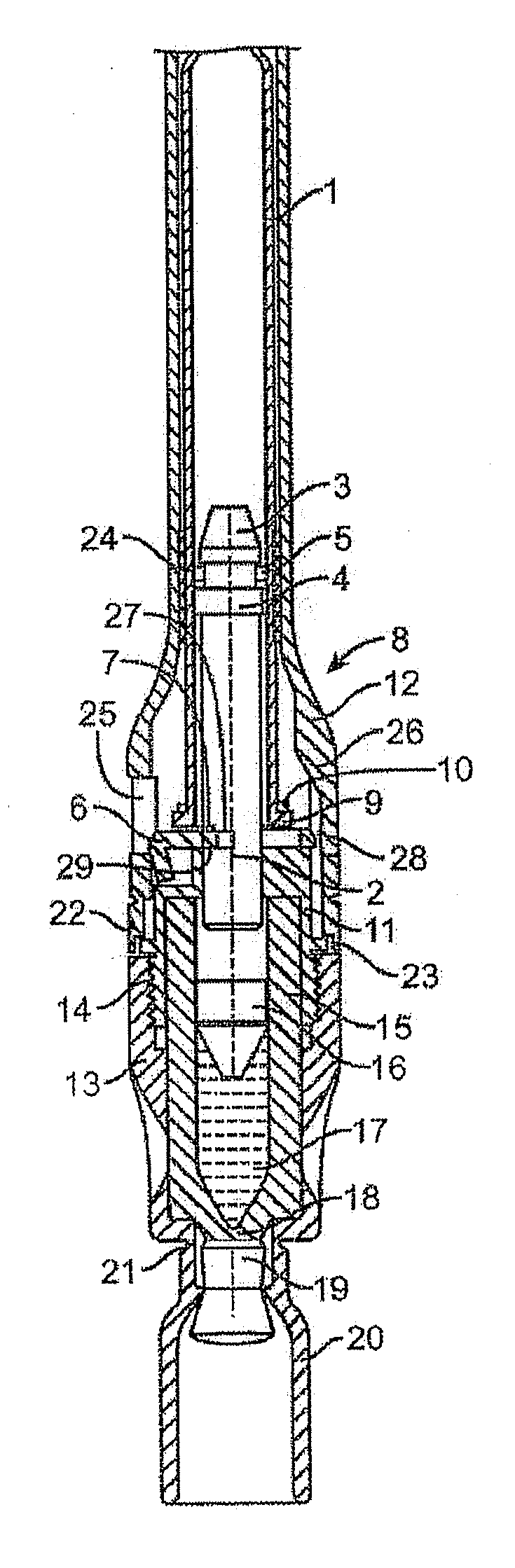
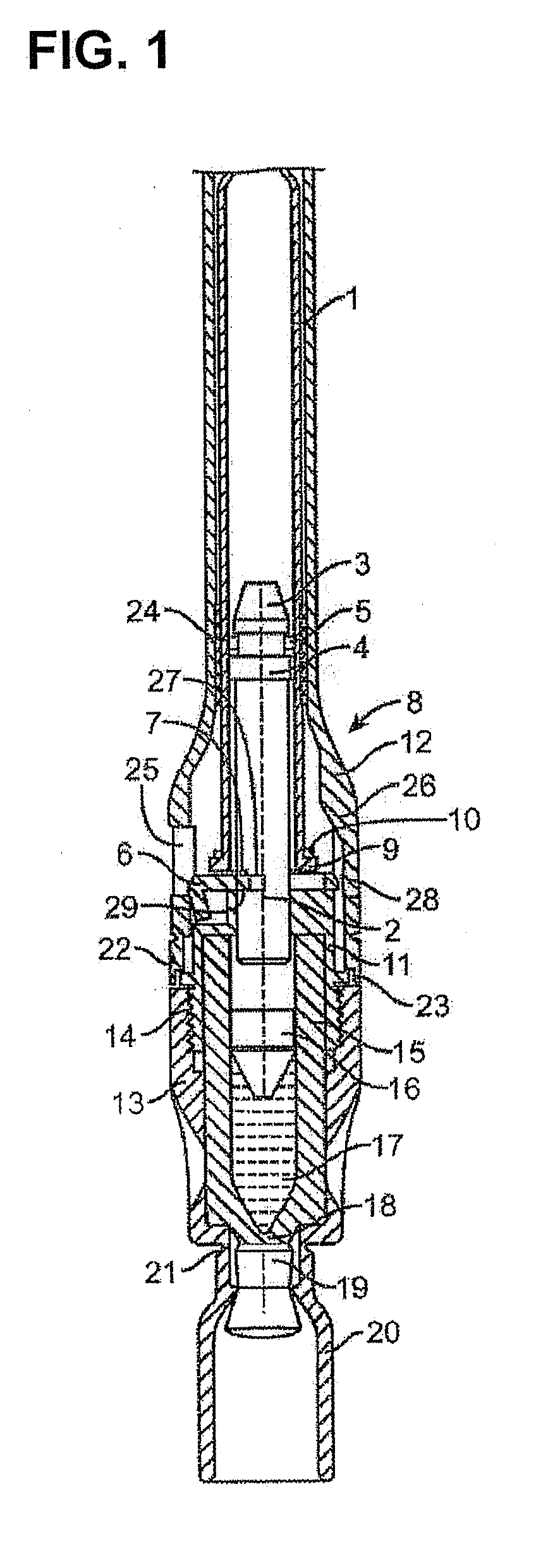
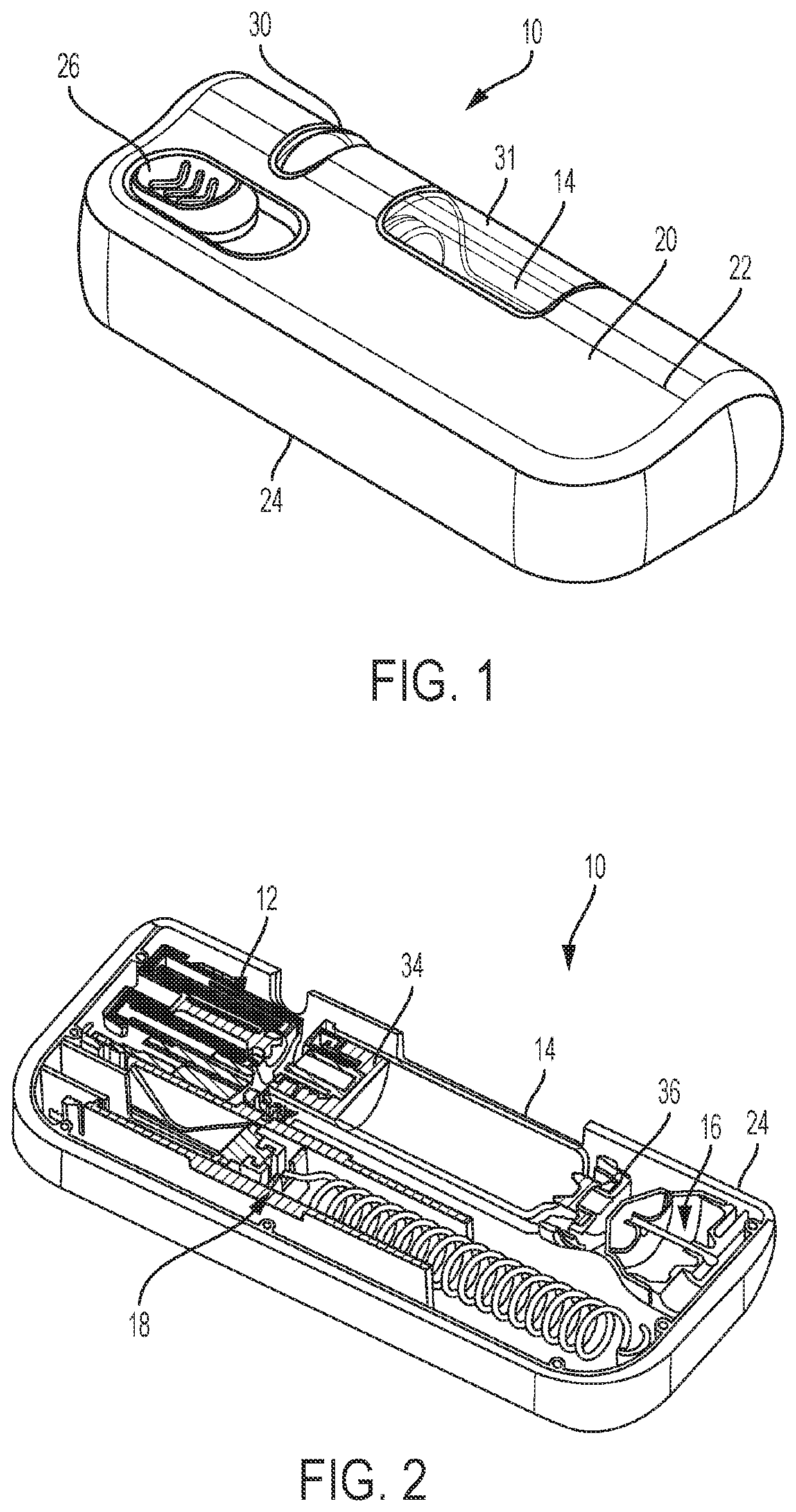
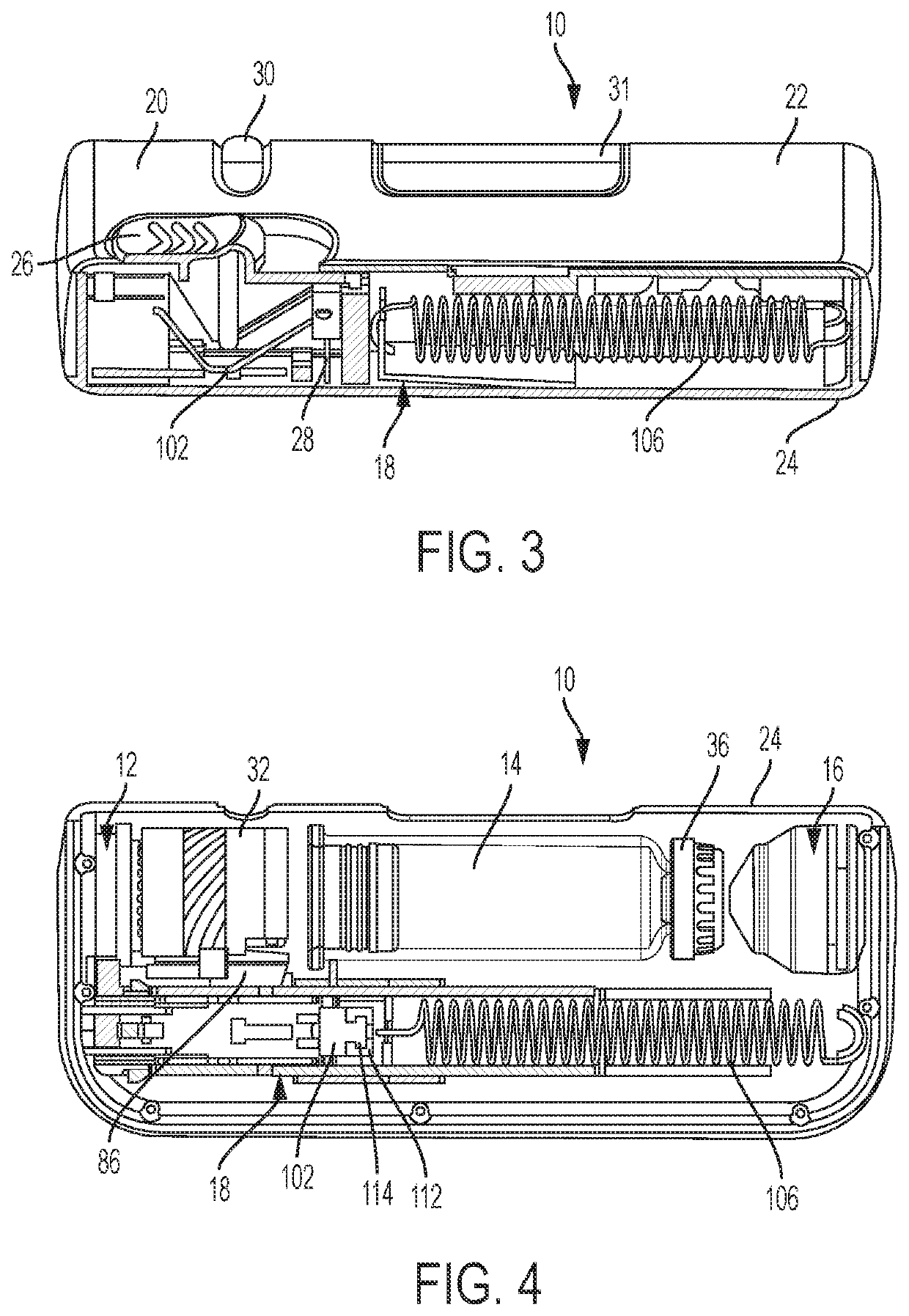
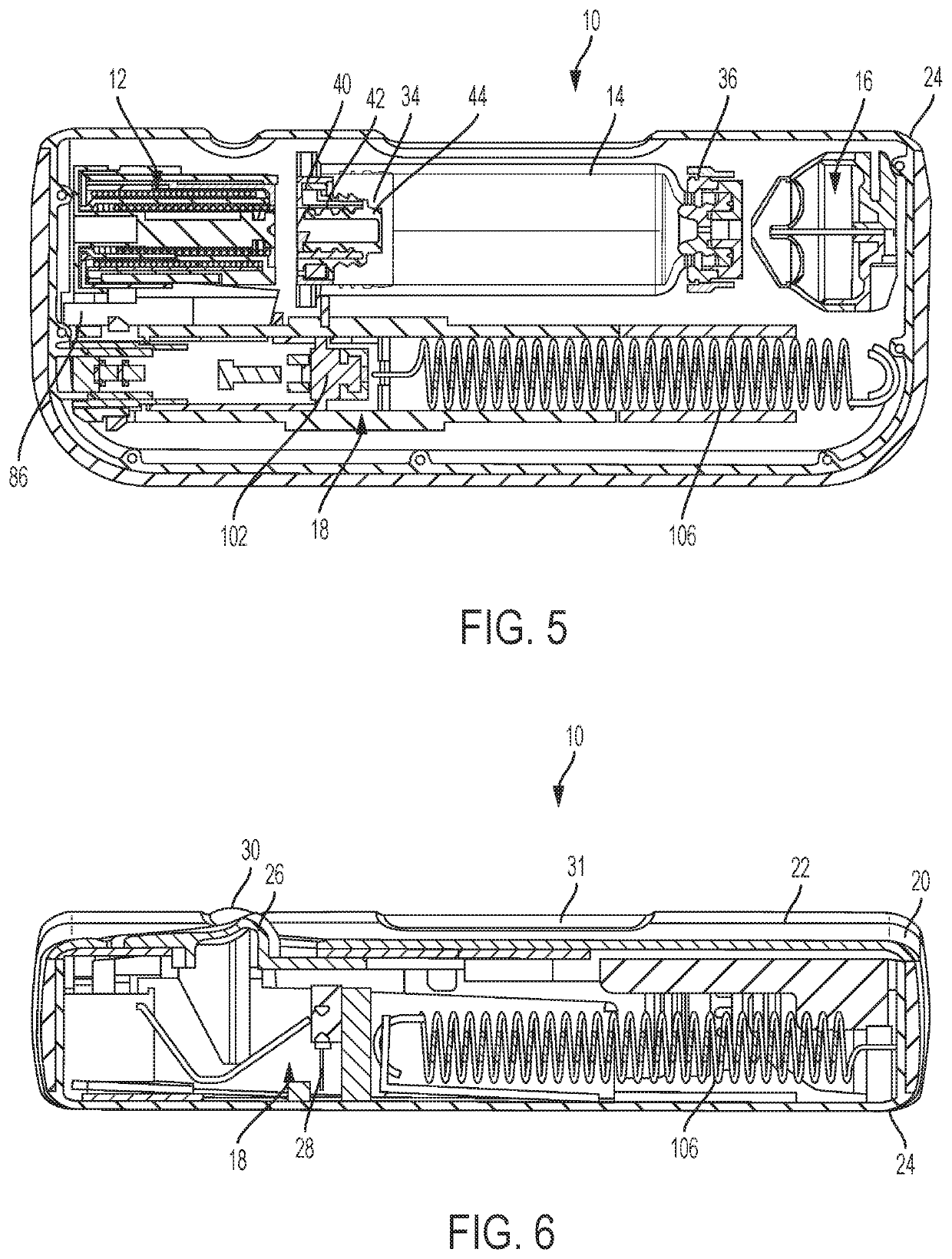
Tube crimping arrangement for drug delivery device
A drug delivery system for injecting a medicament is provided. The system includes a container configured to receive a medicament; a drive assembly which, upon actuation, is configured to expel the medicament from the container; a needle for injecting the medicament to a patient; a fluid path assembly comprising a tube in fluid communication with the container and the needle for conducting fluid from the container to the needle; and a tube crimping arrangement configured to engage the tube to block fluid flow through the tube. The drive assembly causes the tube crimping arrangement to engage the tube after a dose of the medicament has been delivered to the patient through the needle.
Owner:BECTON DICKINSON & CO
Controllable release system of glucocorticoid and its preparation and use
InactiveCN1470240AEffectively control the release rateEffective control of stabilityOrganic active ingredientsSenses disorderBlood concentrationSide effect
The invention discloses a controllable release system of glucocorticoid, the preparing method and the application. The controllable release sytem is the glucocorticoid microcapsule, uses natural biodegradable high-molecular material as the base material of glucocorticoid drug, and adopts ultrasonic micro-capsulizating technique of combination of emulsification and mechanical mixing to wrap the drug in the microsphere composed of the base material to make microcapsule; further prepare the drug's powder, capsule, plaster, tablet and injection or pill, able to effectively control the release rate and stability of the drug, maintain the needed blood concentration, and on the premise of assuring the cure effect, largely reduce the frequency of dosing or injecting and the side effect of the drug.
Owner:JINAN UNIVERSITY
Device and method for drug delivery
ActiveUS8961458B2Improve efficiencyThe drug effect is goodAutomatic syringesDiagnosticsDrug injectionTherapeutic treatment
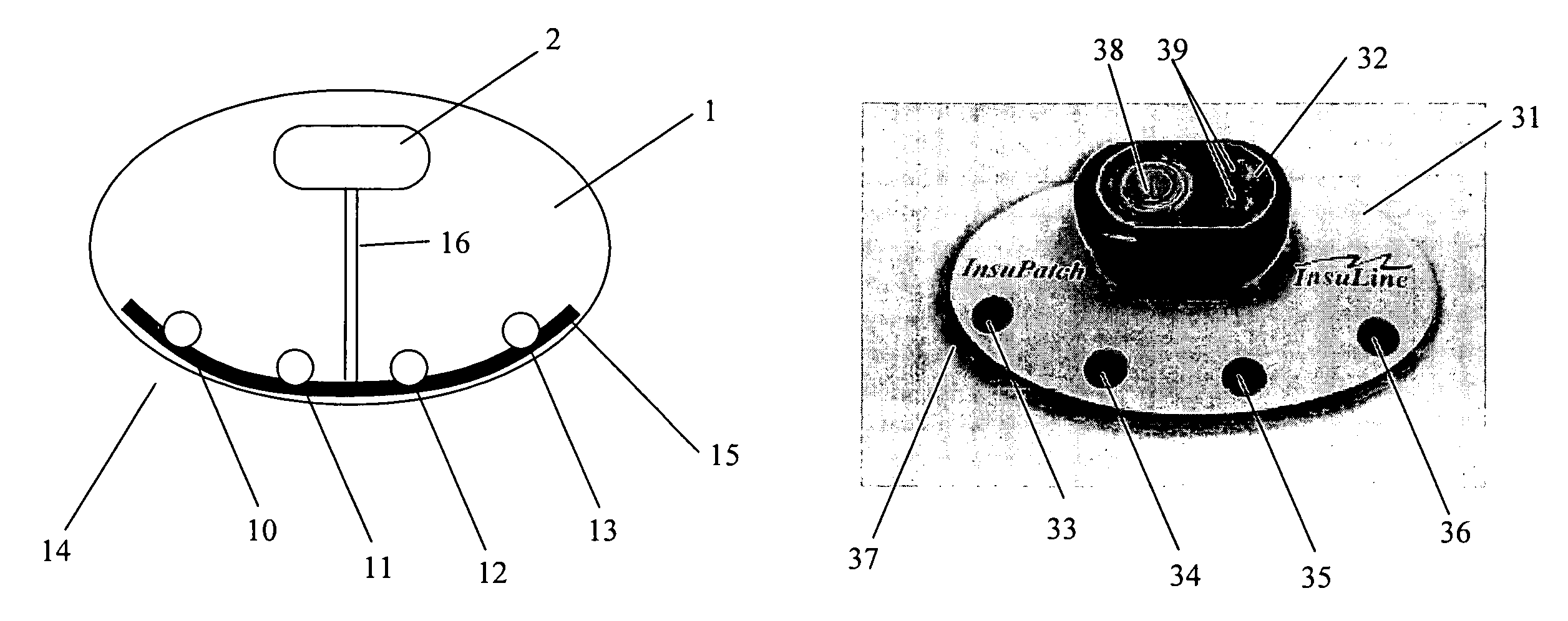
A therapeutic treatment device, system and method for improving administration of a temperature sensitive drug into a tissue on the body of a patient at a drug injection site are disclosed. The device includes a treatment element with a controllable heating element in temperature communicative contact with the tissue adjacent to the drug injection site. The controllable heating element is configured to heat the tissue adjacent to the drug injection site to a controllable temperature but does not heat the injected drug above a predetermined limiting temperature, above which degradation of the injected drug may occur.
Owner:INSULINE MEDICAL
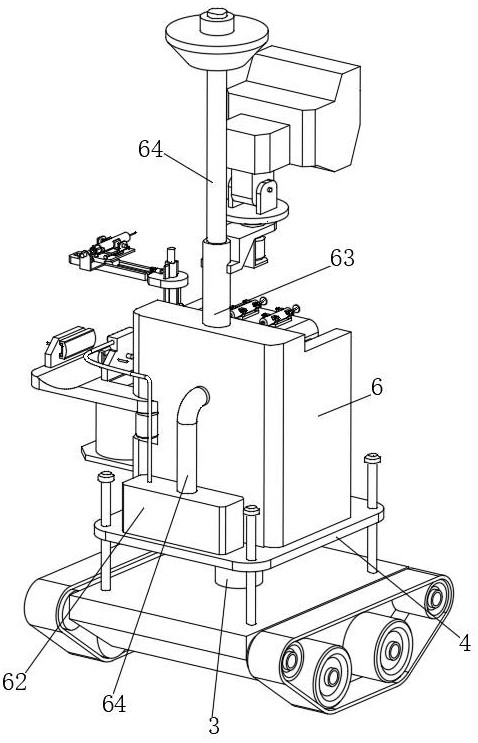
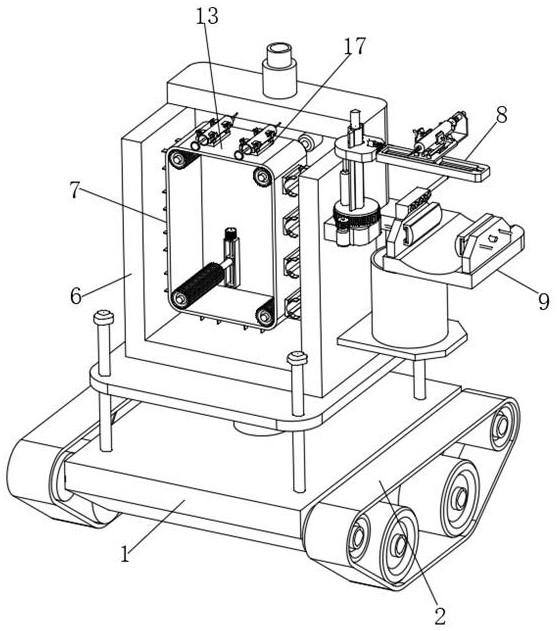
Injection robot used for epidemic prevention
InactiveCN111975792AReduce the chance of infectionReduce laborInfusion syringesMedical applicatorsPhysical medicine and rehabilitationPharmaceutical drug
The invention discloses an injection robot used for epidemic prevention. The injection robot comprises a moving base; the bottom part of the moving base is provided with a crawler chassis, and the toppart of the moving base is provided with a first electric push rod; moreover, a support flat plate is fixed to the top part of the first electric push rod; and the top part of the support flat plateis provided with an injection device for injecting drugs into a patient instead of a medical worker to reduce the infection probability of the medical worker. The injection robot can drive on ground surfaces with different conditions through the crawler chassis; the injection device on the support flat plate can replace the medical worker to perform drug injection on the patient suffering from infectious viruses including the novel coronavirus and the like, and accordingly, the contact between the medical worker and the patient can be effectively reduced, the probability that the medical worker is infected is lowered, and the workload of the medical worker is also reduced.
Owner:MEI HOSPITAL UNIV OF CHINESE ACAD OF SCI
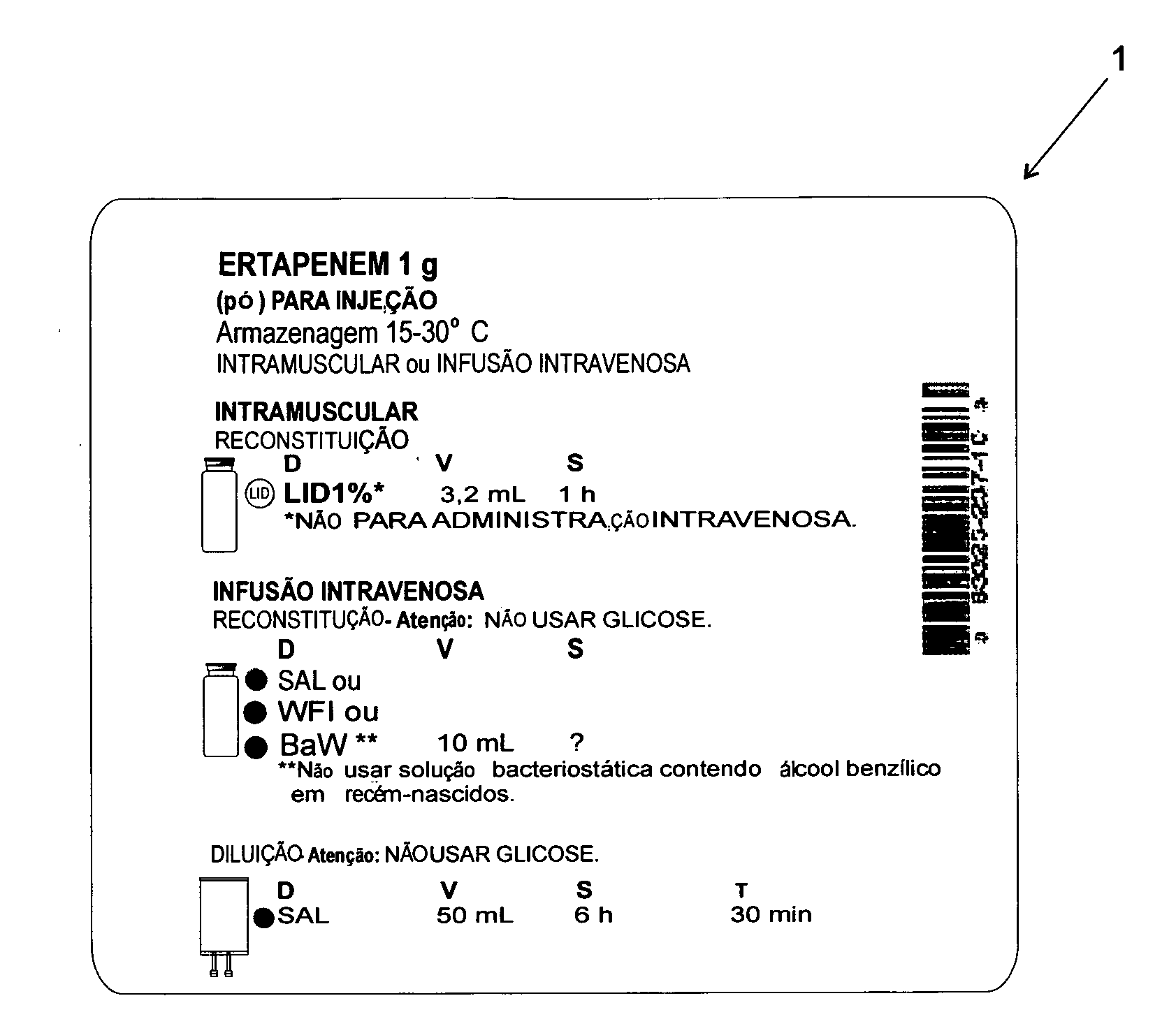
Method of standardization of injectalbe medicines and their diluents
“METHOD OF STANDARDIZATION OF INJECTABLE MEDICINES AND THEIR DILUENTS” is particularly intended to eliminate the possibility of confusion arising from the determination of said injectable medicines and their diluents in the general context of health care, such as hospitals, clinics and similar health-related facilities. The method is essentially intended to allow an injectable medicine to be administered following the correct definition of the appropriate diluent required by each product, said method comprising the standardization of a label (1) designed to be attached to vials or containers of injectable medicines, as well as a label (2) specifically designed to be attached to vials or containers of diluents; said label (1) includes information fields for various relevant information that should be corrected in the event of codification.
Owner:NORIVAL CAETANO
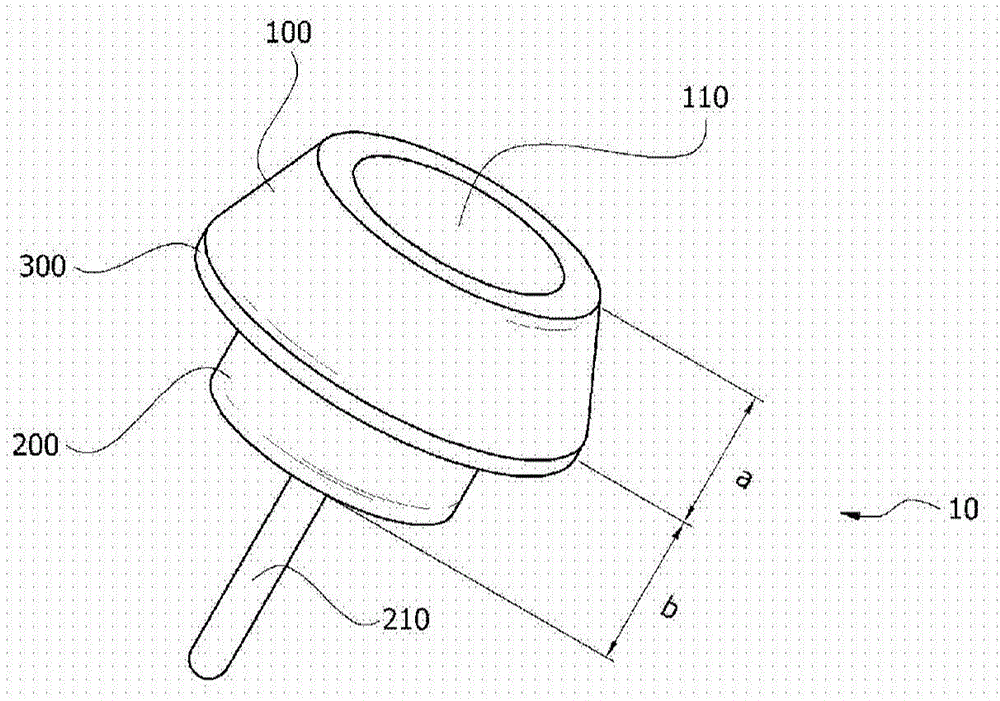
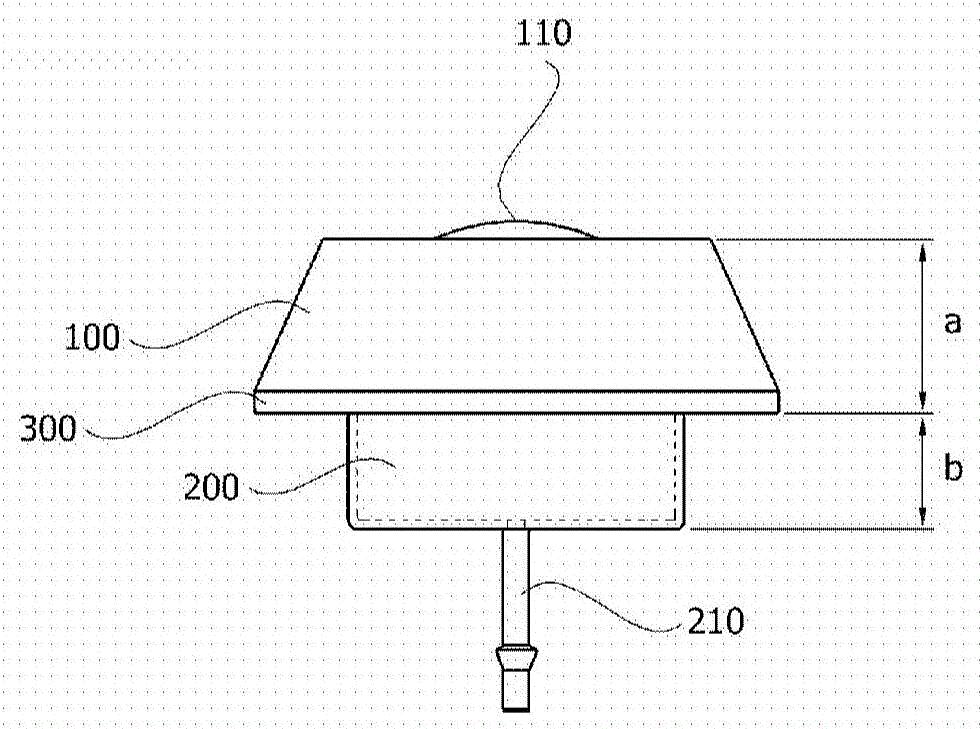
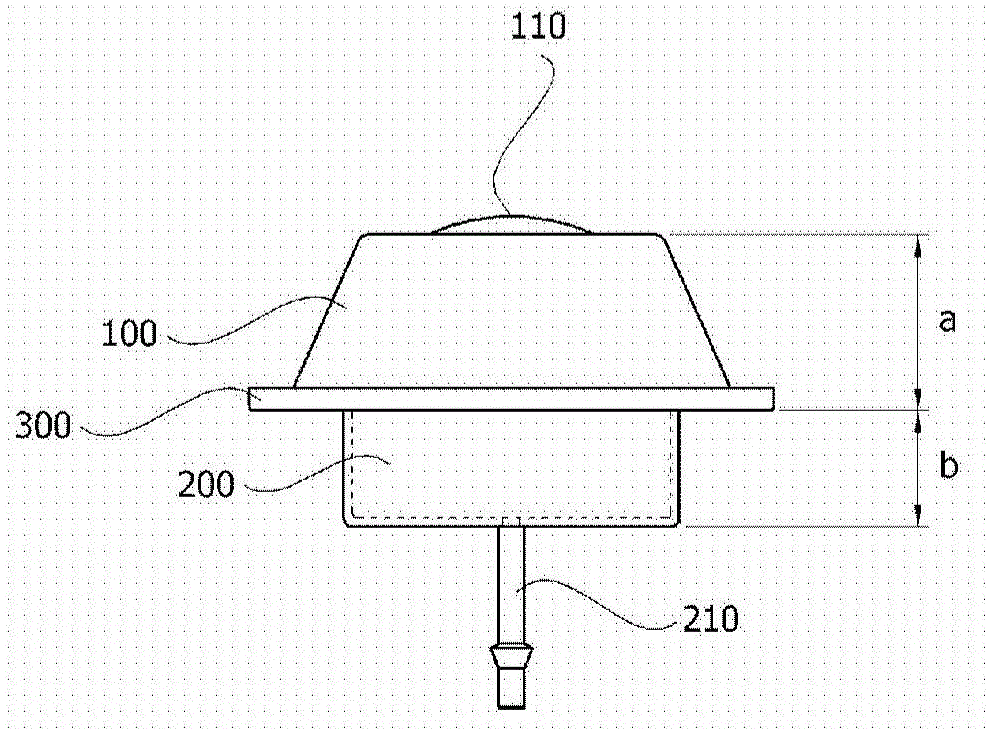
Skull implant type drug injection port
ActiveCN105611956ASure easyImplanted accuratelyInfusion devicesMedical devicesDrug injectionSkull bone
The present invention relates to a skull implant type drug injection port comprising: a holder which is placed on the upper section of a port insertion hole formed in the skull of a patient and has a drug injection hole formed on the upper surface thereof; a drug injection diaphragm which is provided so as to seal the drug injection hole of the holder and into which a syringe needle for injecting a drug is inserted; a drug storage part combined with the lower section of the holder, for storing the drug inserted through the drug injection diaphragm; a drug discharge pipe which is connected to the drug storage part so as to discharge the drug stored in the drug storage part; and a rib which is formed on the outskirt between the holder and the drug storage part and has a diameter larger than the inner diameter of the port insertion hole, wherein the height ratio of the holder to the drug storage part is 1:0.5 to 0.6 on the basis of the rib. The present invention relates to a skull implant type drug injection system comprising: a skull implant type drug injection port; and a guide needle which is attachable to and detachable from the drug discharge pipe by passing through the drug injection diaphragm of the skull implant type drug injection part, wherein the guide needle has a protruding section formed on an area which is separated at a predetermined distance from a cutting-edge section.
Owner:NAT CANCER CENT
Pharmacy bag with integrated flush option
InactiveUS20190021953A1Reduce amountPrecise deliveryPharmaceutical containersMedical packagingPharmacyDiluent
The present invention relates to a bag containing preprepared injectable drug(s) and / or diluents for injectable drugs to be administered by intravenous (IV) application to a patient, said bag having an integrated flushing option.
Owner:B BRAUN MELSUNGEN AG
Application of transthyretin in transferring fusion protein into eyes
InactiveCN110960687AEfficient deliveryGood biocompatibilitySenses disorderAntibody mimetics/scaffoldsProtein engineeringBiocompatibility
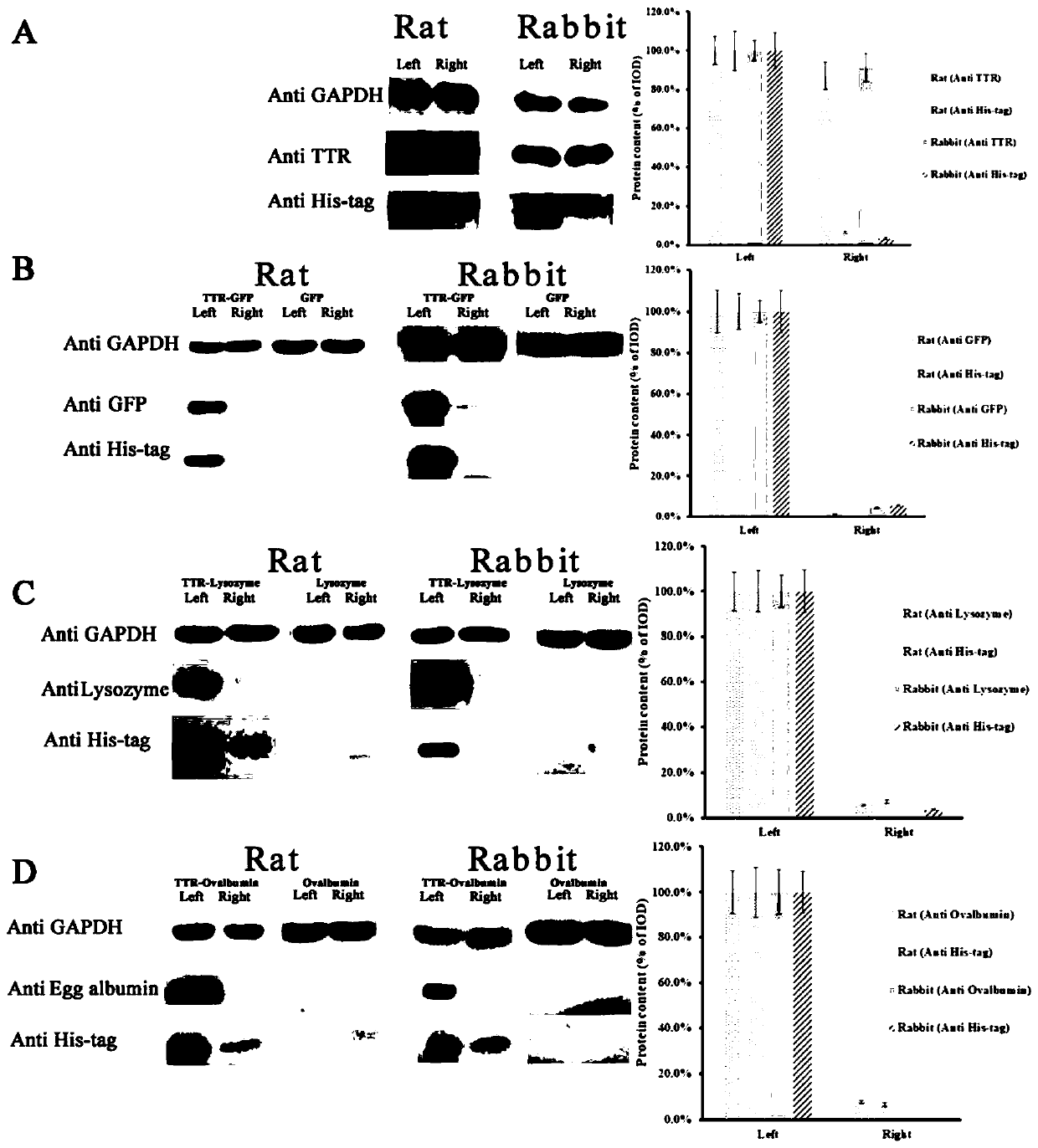
The invention discloses application of transthyretin in transferring fusion protein into eyes, belonging to the technical field of protein engineering. The invention provides a method for allowing foreign protein to pass through eye barriers and to be conveyed into eyes through fusion expression of transthyretin as shown in SEQ ID NO.1 and the foreign protein. The results of animal experiments prove that as the fusion protein prepared in the invention is used for eye drop treatment on rats and rabbits, foreign protein can be rapidly and effectively conveyed to fundus. The transthyretin used inthe method is derived from a human body, has good biocompatibility and safety in the human body, and has important application prospects when used as a substitute of injection drugs in the field of medicine.
Owner:TONGYAN (SHANGHAI) MEDICAL EQUIPMENT CO LTD
Device for injecting a pharmaceutical active principle
InactiveUS8348881B2Risk of outWithout any fear of the implant falling outMedical devicesAdditive ingredientInjection device
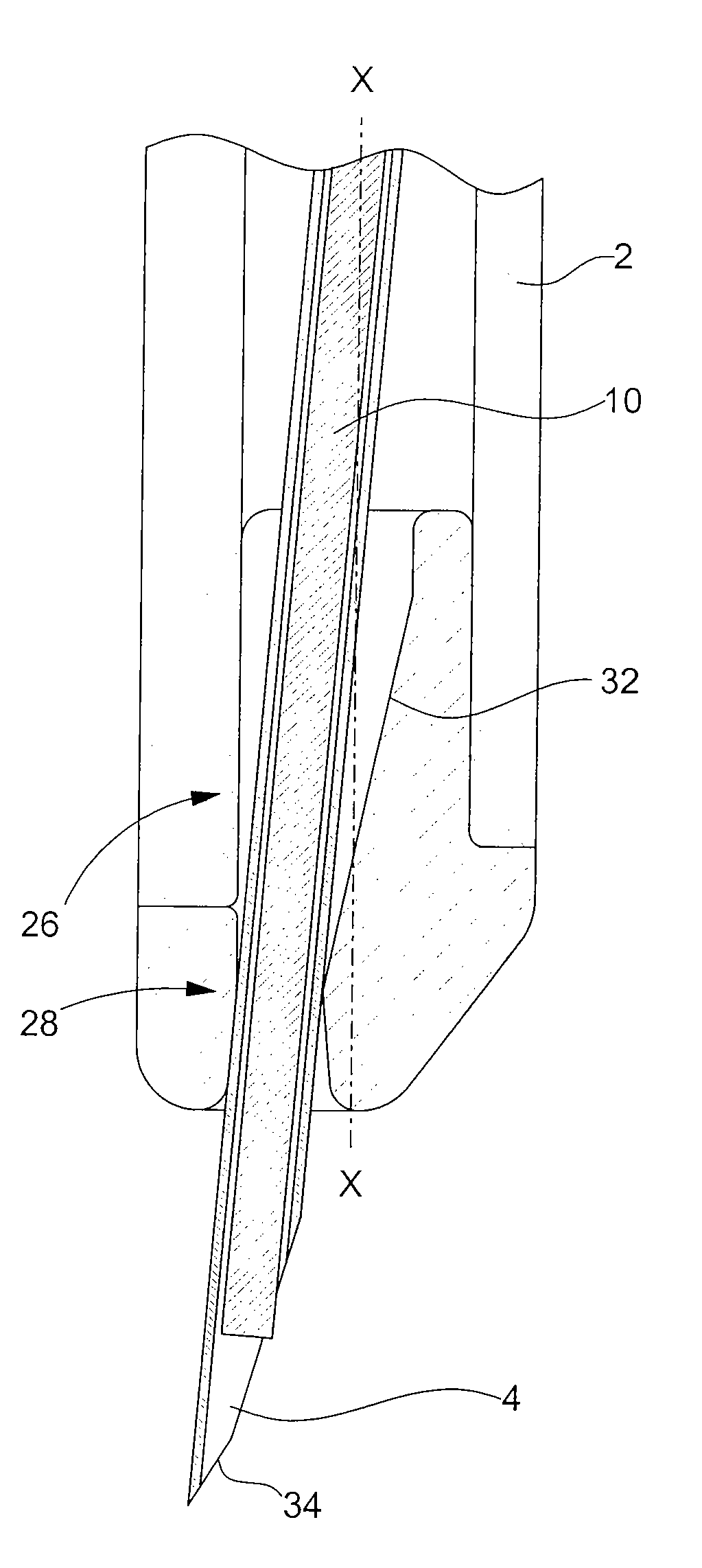
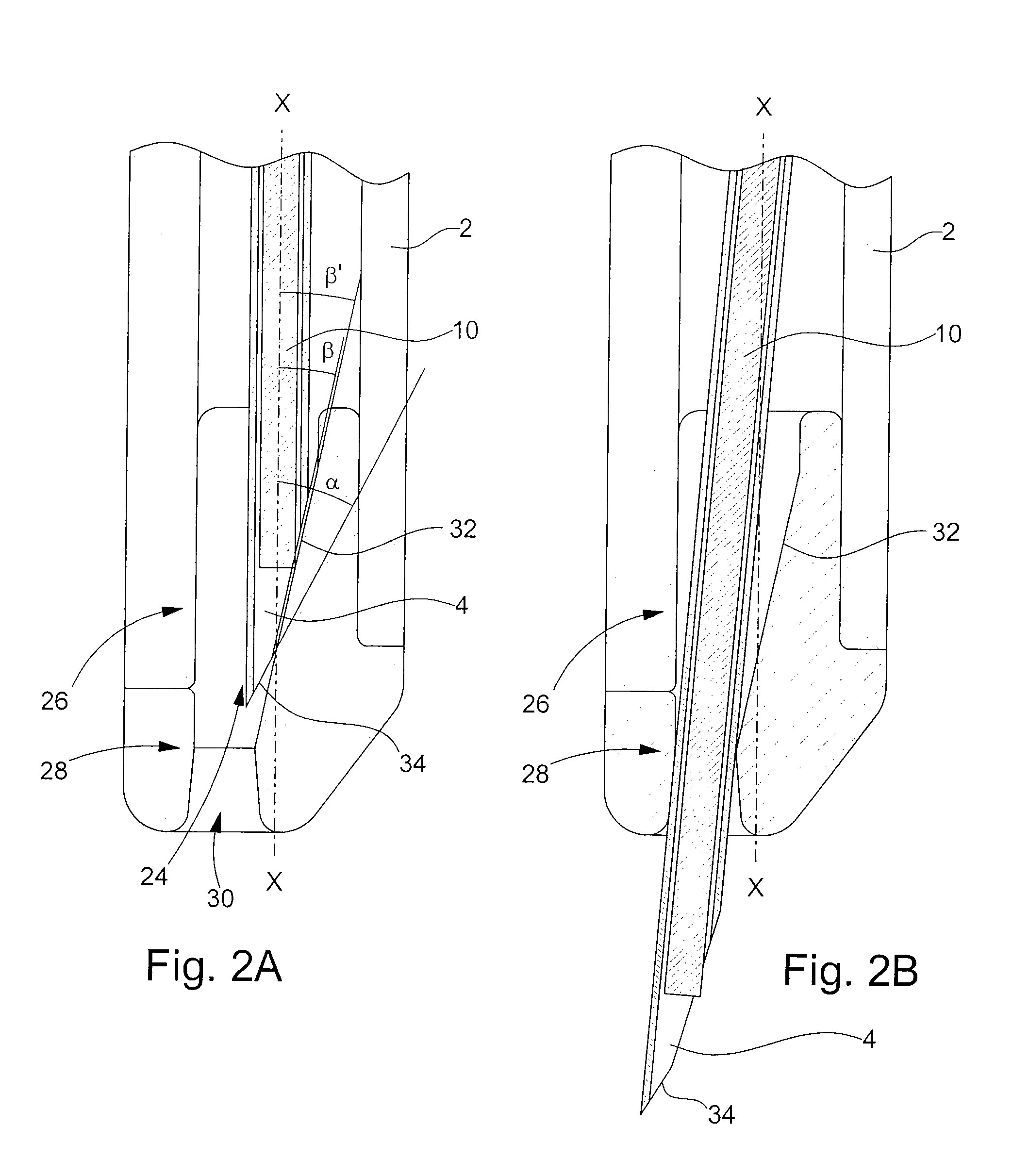
The invention concerns a device for injecting a solid medicine (10) comprising a body (2) inside which moves along a general forward moving axis (X-X) a bevelled (34) needle (4) wherein is introduced the medicine (10), said injection device (1) further comprising retaining means for preventing the medicine from falling (10) prior to being injected. The invention is characterized in that the medicine (10) is retained through an elastic deformation imparted to the needle (4) by the retaining means or by an elastic deformation of the retaining means themselves, or still by the combined flexibility of those two means.
Owner:IPSEN PHARMA SAS
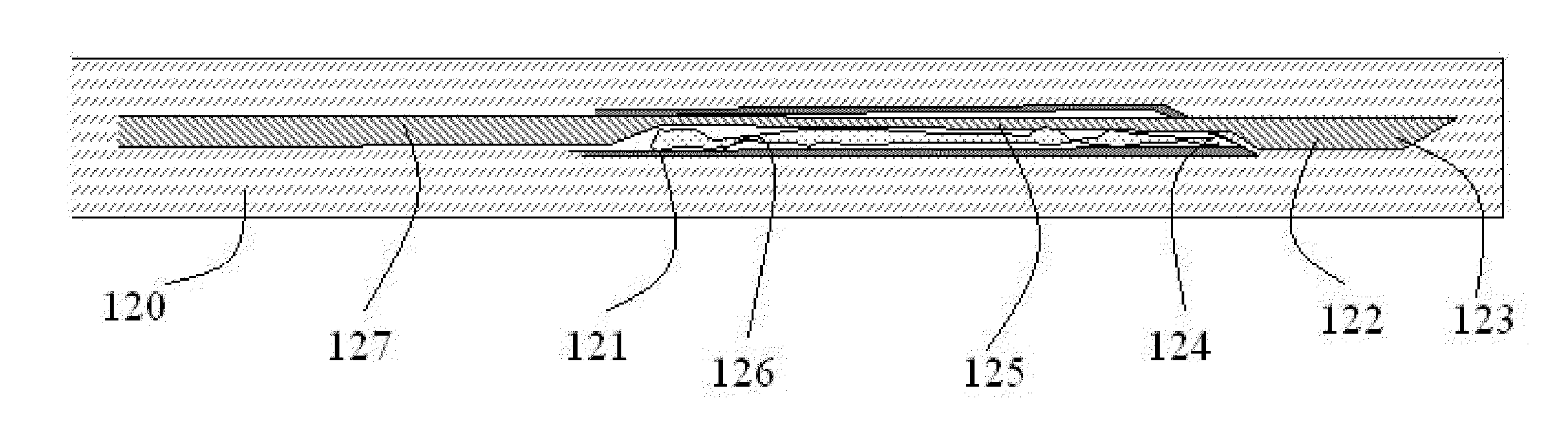
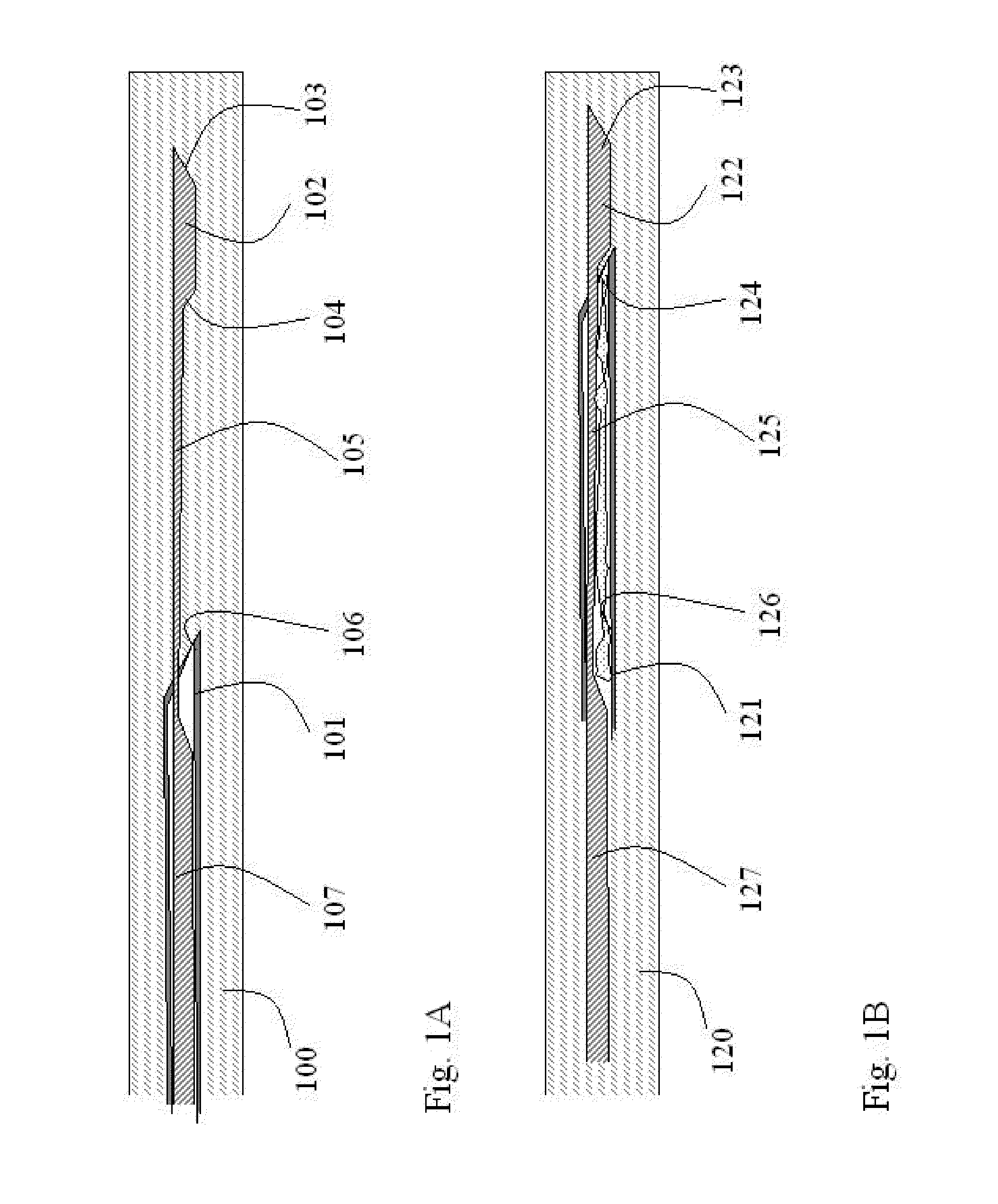
Sterilization Arrangement for Drug Delivery Device
Owner:BECTON DICKINSON & CO
Spacer assembly for drug delivery system
A drug delivery system for injecting a medicament includes a container including a closure and a stopper configured to move within the container from a pre-use position to a post-use position. The system includes a drive assembly configured to move the stopper between first and second positions, and a spacer assembly engaged with the stopper configured to be engaged by the drive assembly. The spacer assembly includes a first spacer portion received within the stopper, a second spacer portion spaced from the first spacer portion, an inner plunger, and a spacer shuttle received by the inner plunger. The inner plunger, the spacer shuttle, and the second portion are configured to move relative to the stopper, where movement of the second spacer portion is restricted by the spacer shuttle, movement of the spacer shuttle is restricted by the inner plunger, and movement of the inner plunger is restricted by the stopper.
Owner:BECTON DICKINSON & CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com