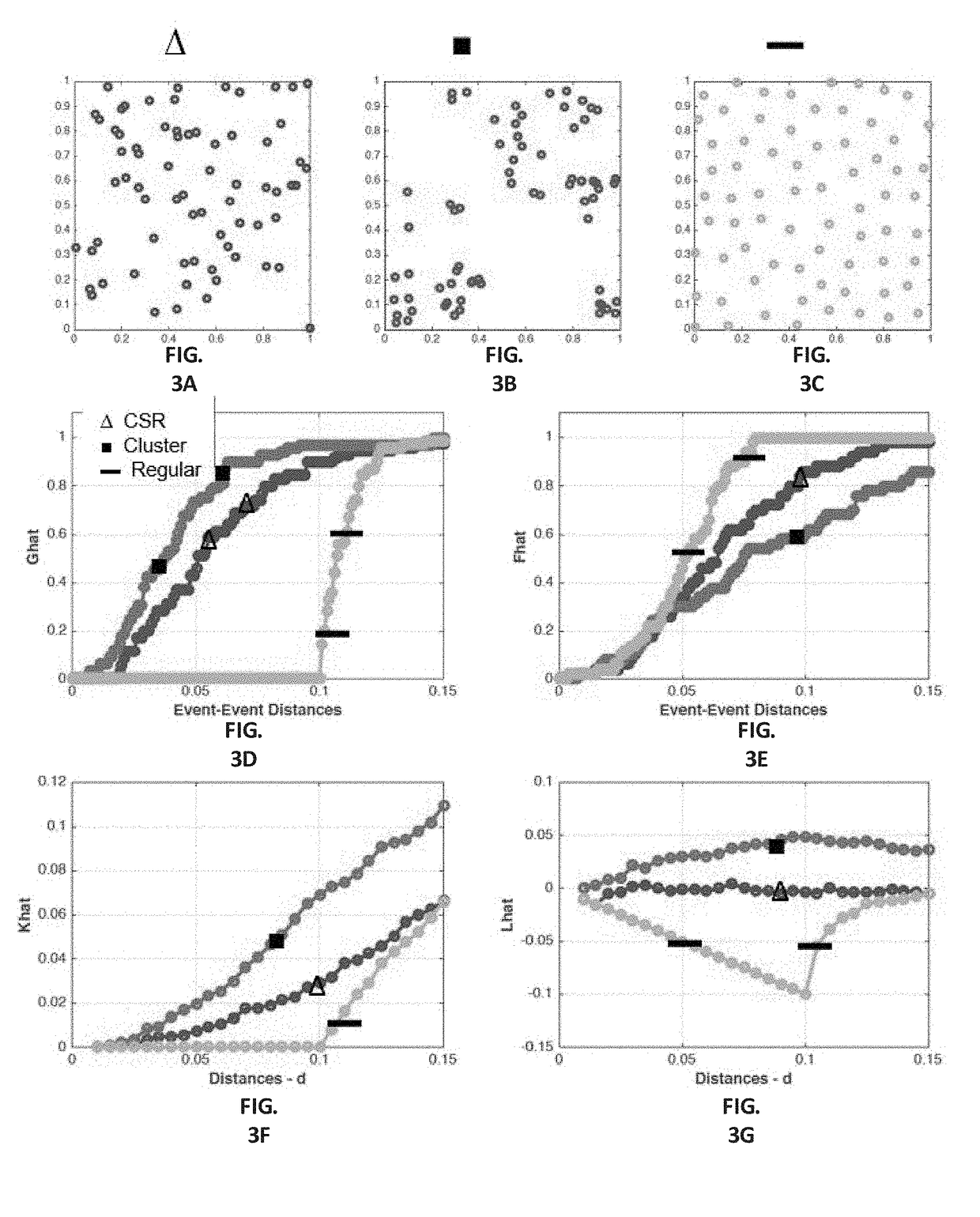
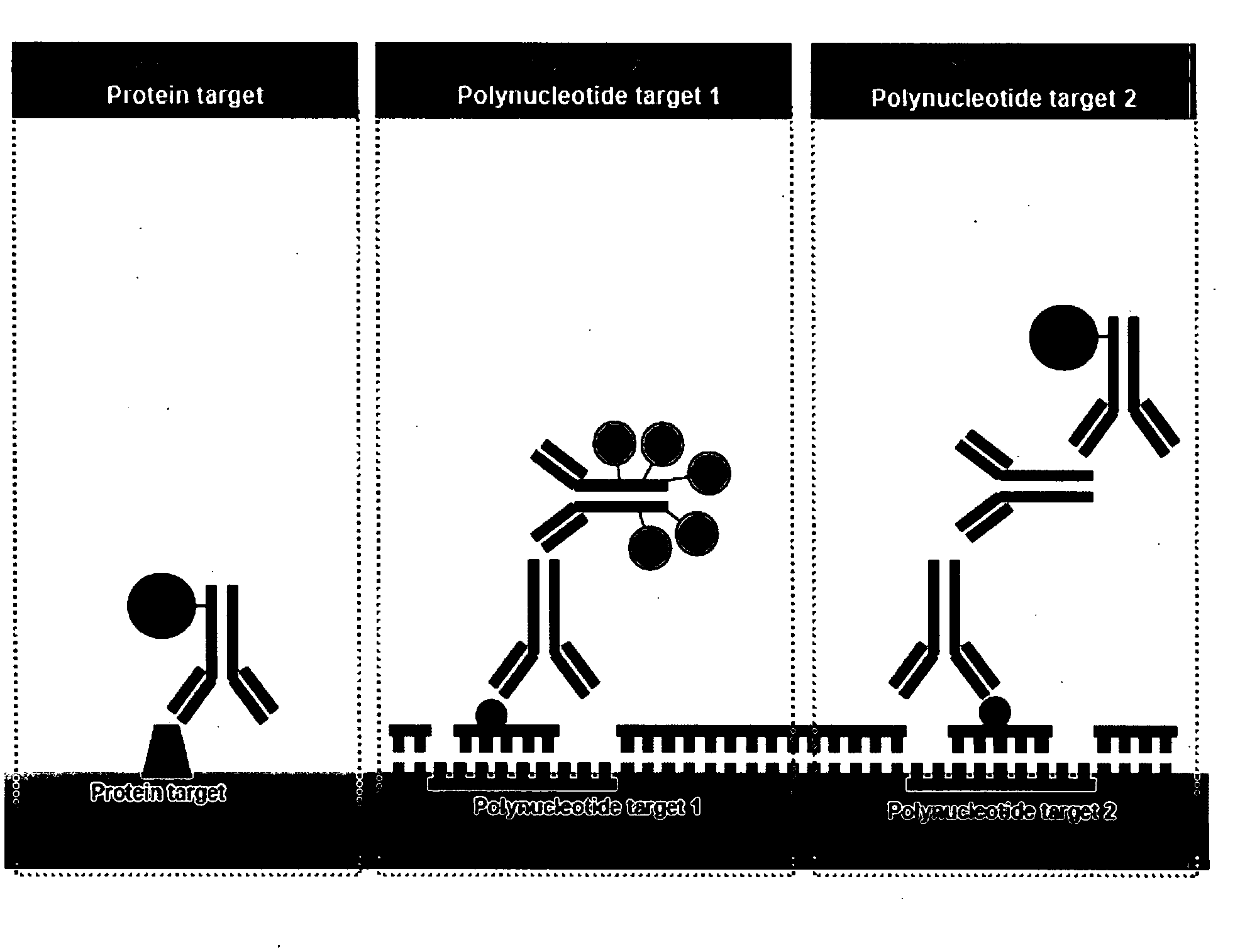
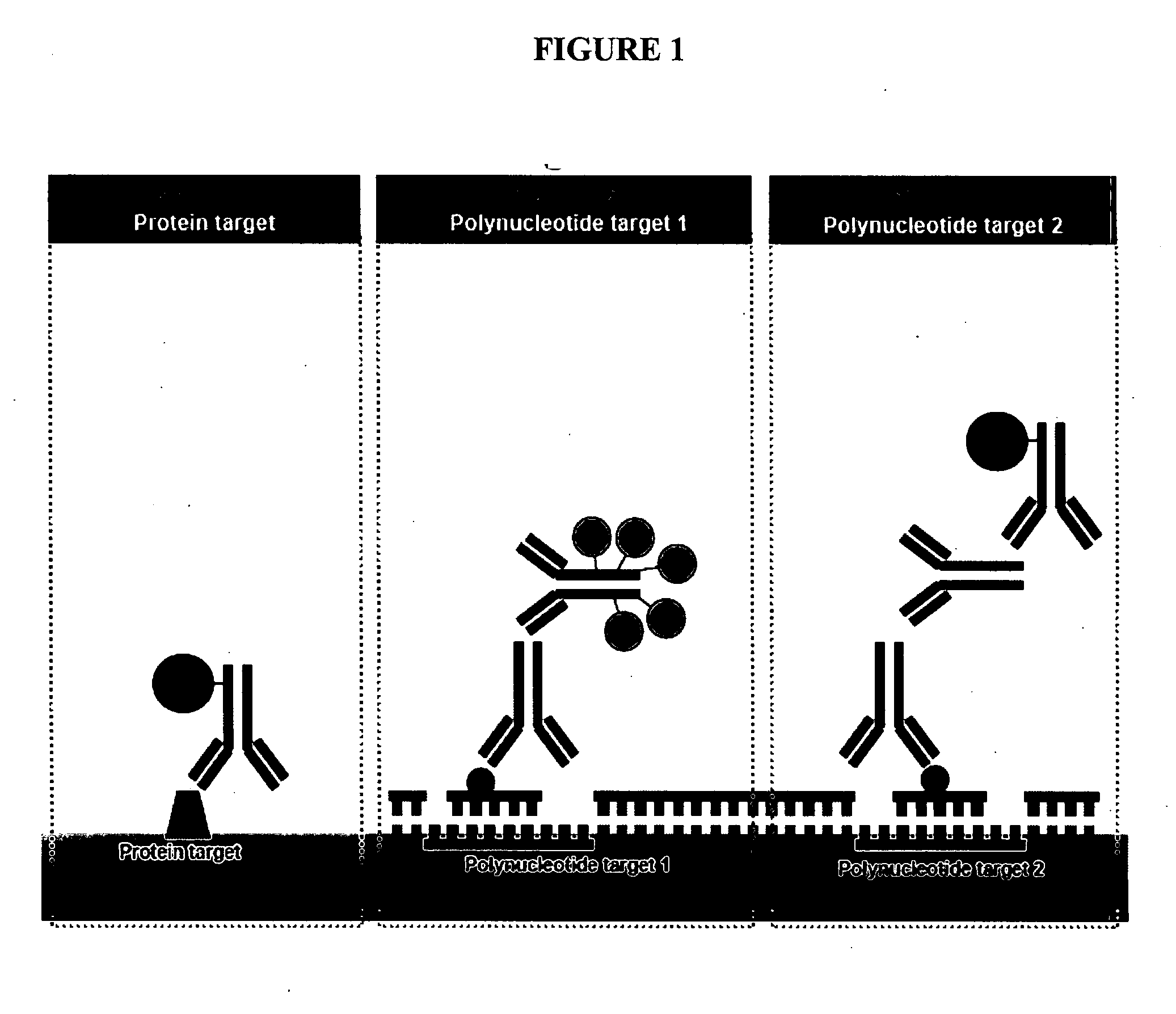
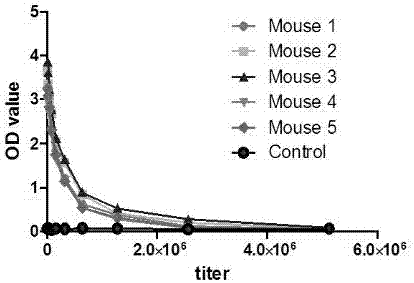
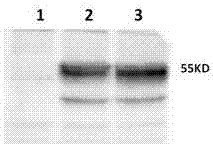
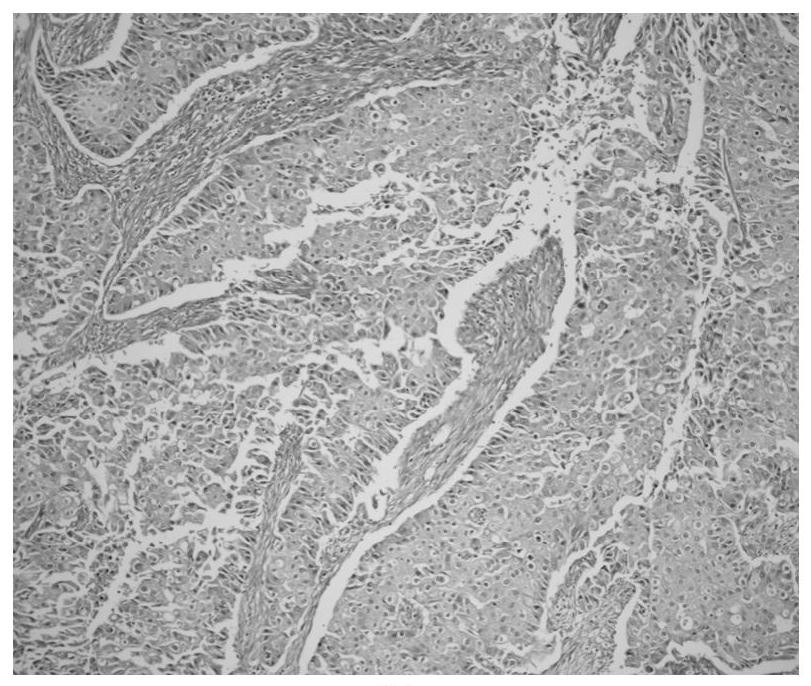
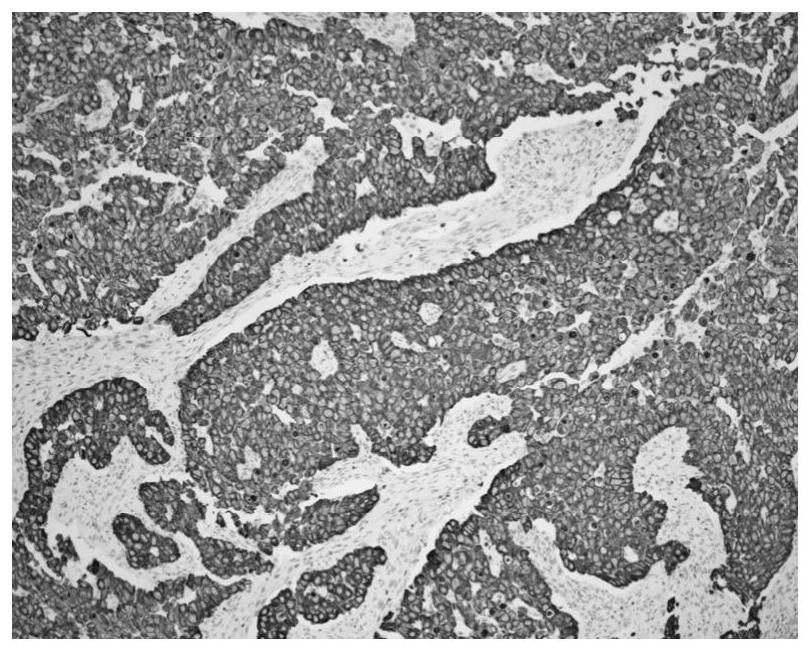
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
114 results about "Immuno histochemistry" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Antibody conjugates
InactiveUS20060246523A1Easy to detectIntense stainingHybrid immunoglobulinsHydrolasesIn situ hybridisationAntibody conjugate
Antibody / signal-generating moiety conjugates are disclosed that include an antibody covalently linked to a signal-generating moiety through a heterobifunctional polyalkyleneglycol linker. The disclosed conjugates show exceptional signal-generation in immunohistochemical and in situ hybridization assays on tissue sections and cytology samples. In one embodiment, enzyme-metallographic detection of nucleic acid sequences with hapten-labeled probes can be accomplished using the disclosed conjugates as a primary antibody without amplification.
Owner:VENTANA MEDICAL SYST INC
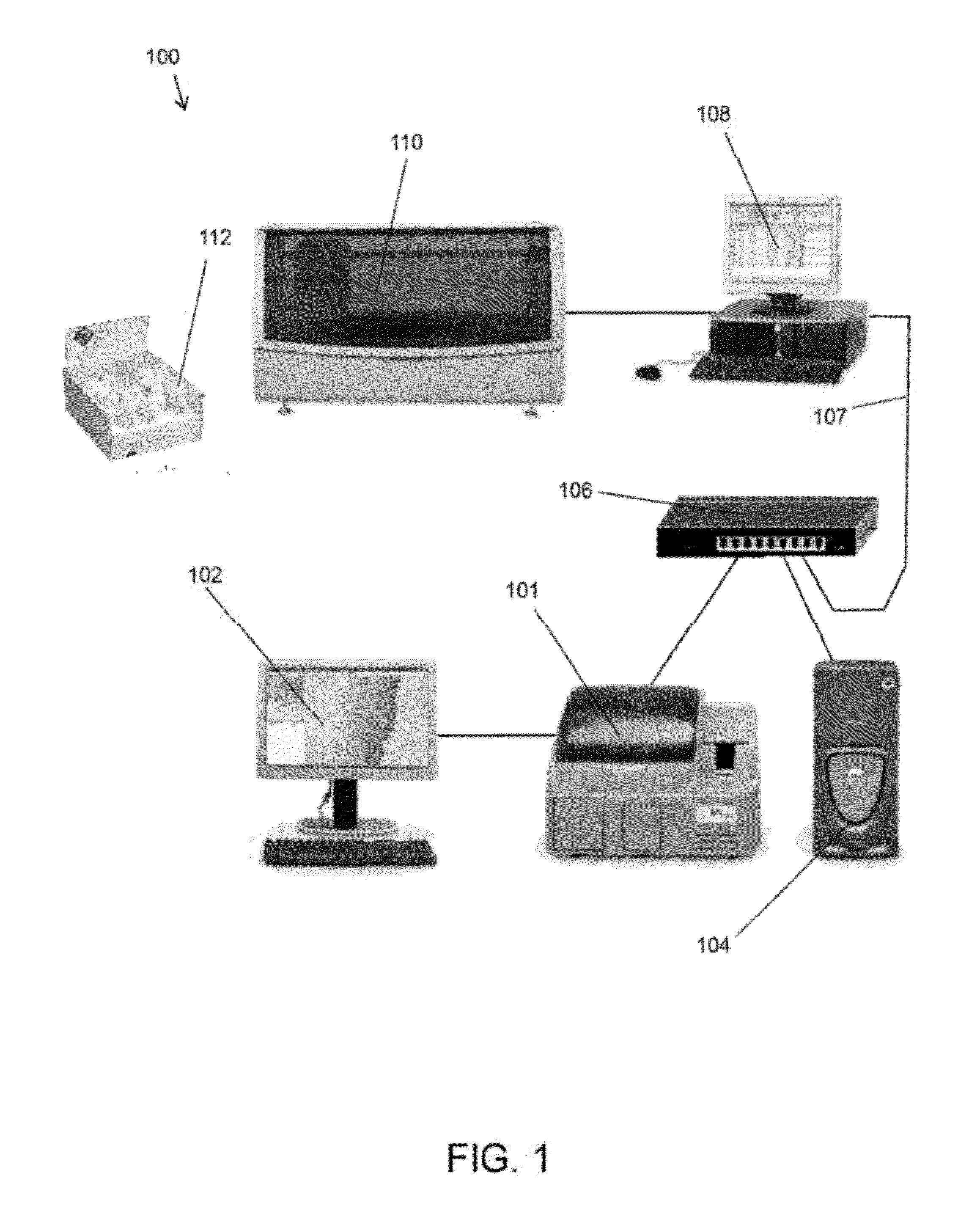
Methods and systems for analyzing images of specimens processed by a programmable quantitative assay
ActiveUS20120163681A1Material analysis by optical meansCharacter and pattern recognitionTissue sampleBioinformatics
Disclosed are methods and systems for analyzing images of specimens processed by a programmable quantitative assay or more specifically a robust programmable quantitative dot assay, PDQA, that enable specimens to be imaged and assessed across a wide variety of conditions and applications. Specific embodiments directed to immunohistochemical applications provide more quantitative methods of imaging and assessing biological samples including tissue samples.
Owner:AGILENT TECH INC
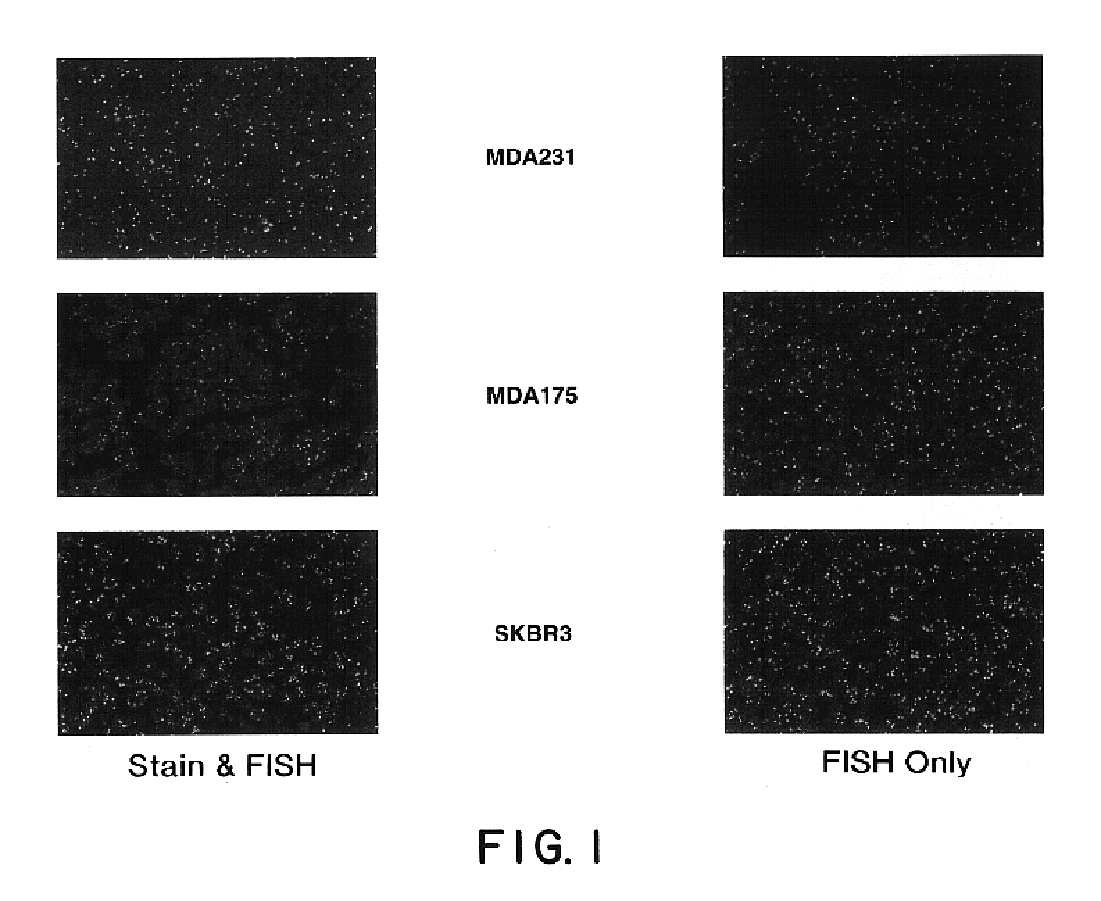
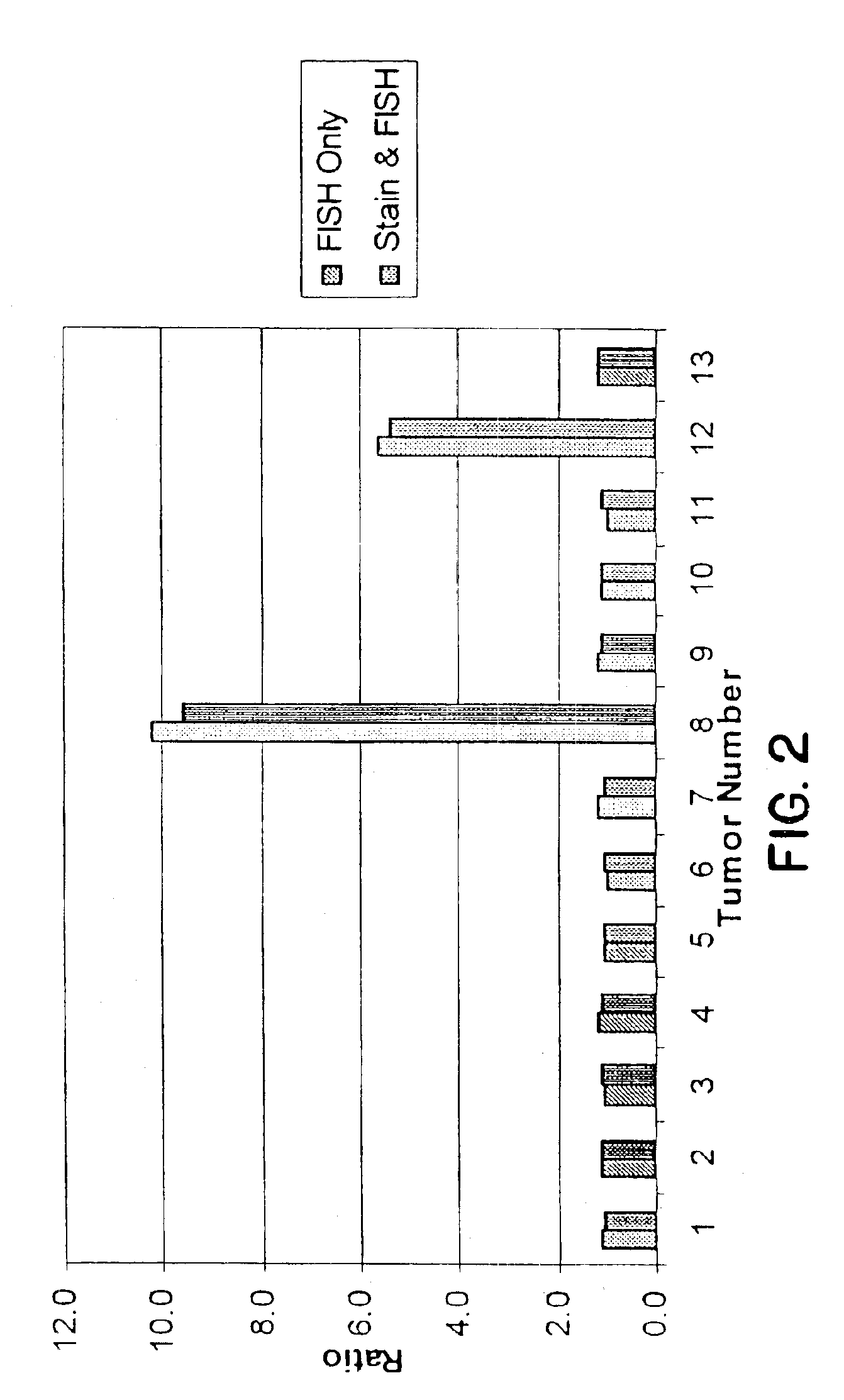
Tissue analysis and kits therefor
InactiveUS6905830B2Microbiological testing/measurementPreparing sample for investigationIn situ hybridisationStaining
This invention relates to methods of analyzing a tissue sample from a subject. In particular, the invention combines morphological staining and / or immunohistochemistry (IHC) with fluorescence in situ hybridization (FISH) within the same section of a tissue sample. The analysis can be automated or manual. The invention also relates to kits for use in the above methods.
Owner:GENENTECH INC +1
Antibodies that bind to human programmed death ligand 1 (pd-l1)
The present disclosure provides isolated antibodies that specifically bind to human PD-L1, as well as antigen binding fragments of such antibodies, and kits comprising the anti-PD-L1 antibodies or binding fragments and a set of reagents for detecting a complex of the antibody, or antigen binding fragment thereof, bound to human PD-L1. The antibodies and antigen binding fragments of this disclosure are useful for immunohistochemical detection of human PD-L1 expression in tissue samples. Nucleic acid molecules encoding the antibodies and antigen binding fragments of this disclosure, as well as expression vectors and host cells for expression thereof, are also provided.
Owner:MERCK SHARP & DOHME LLC
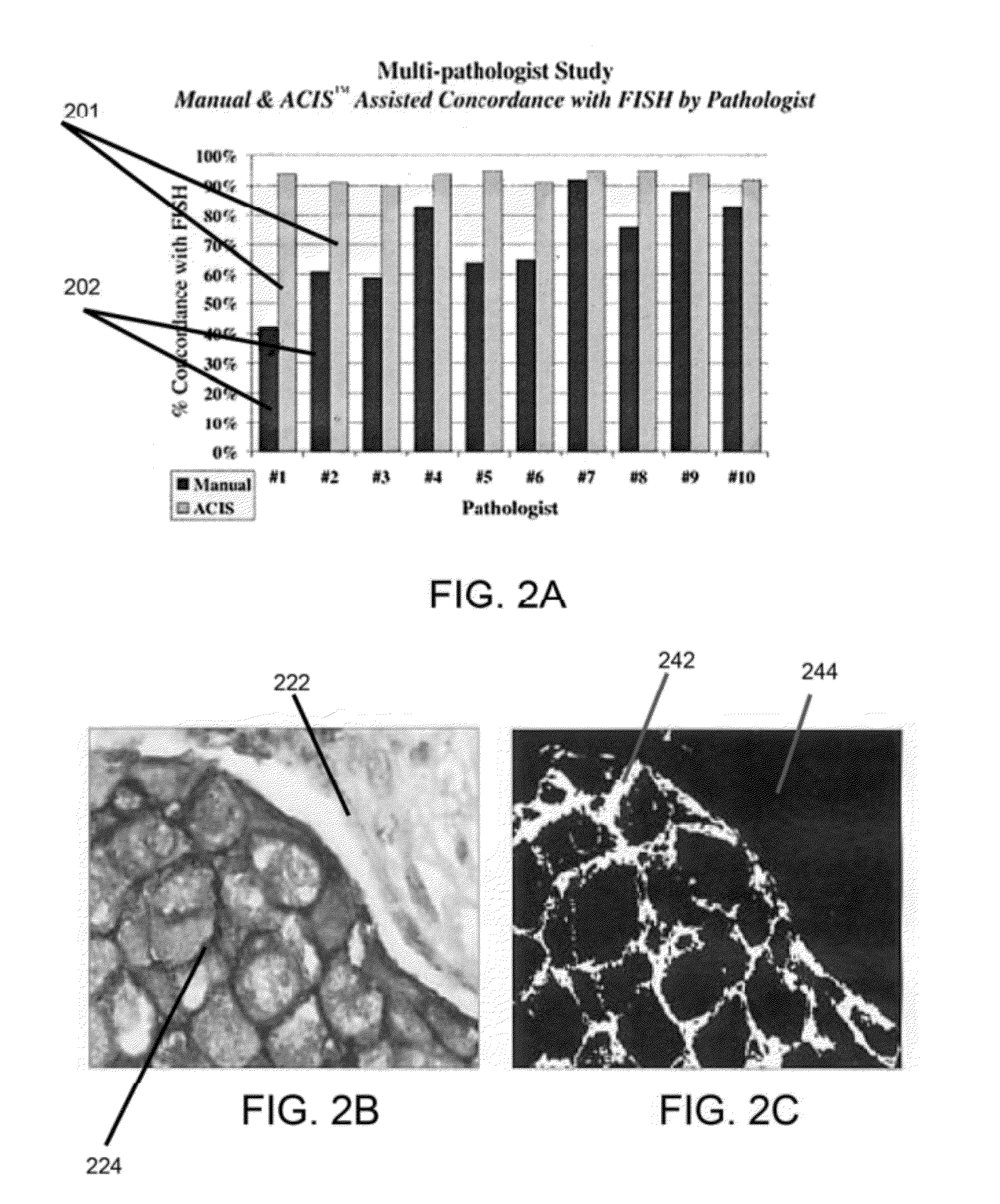
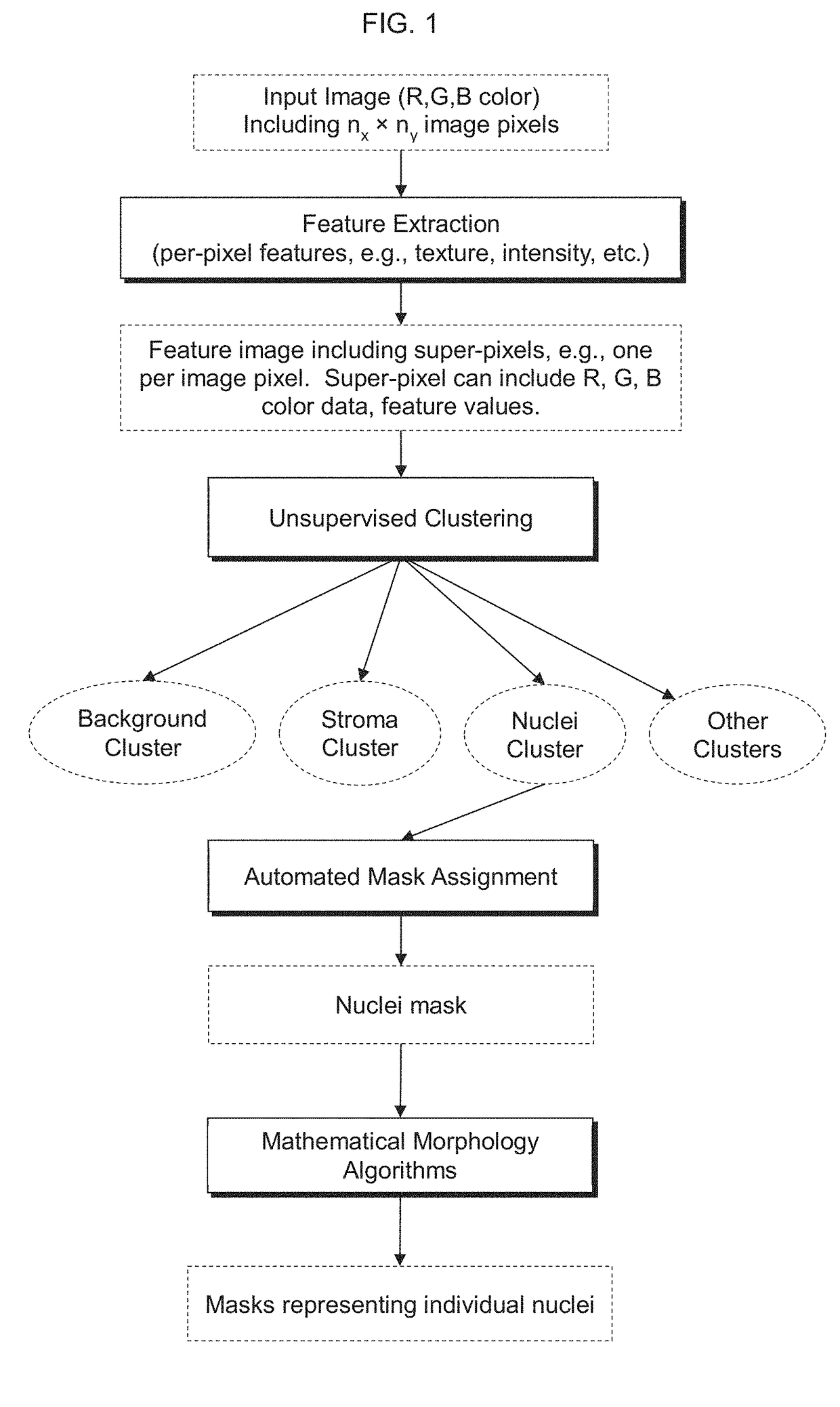
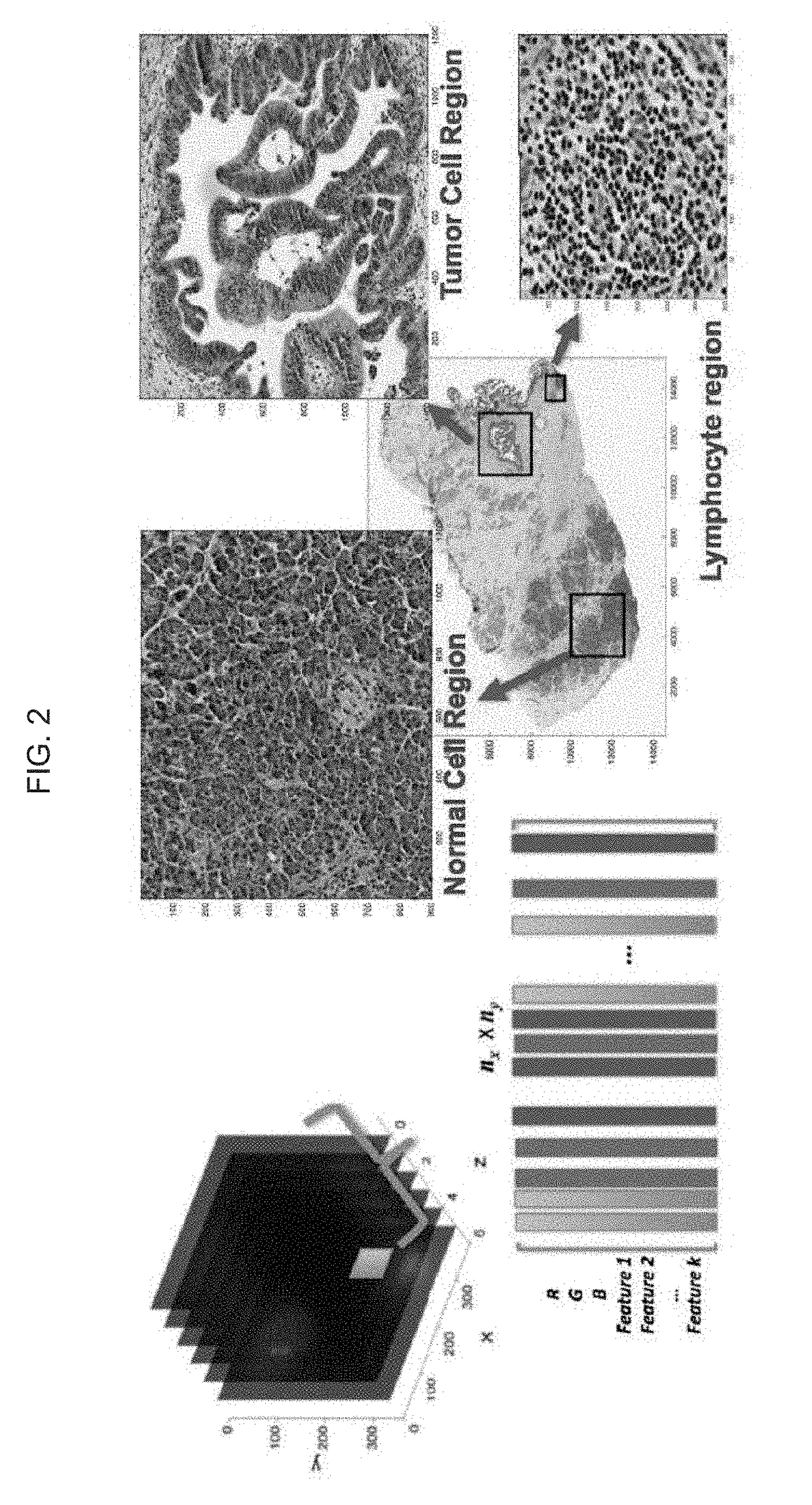
Automatic nuclei segmentation in histopathology images
Provided herein are systems and computer-implemented methods for quantitative analyses of tissue sections (including, histopathology samples, such as immunohistochemically labeled or H&E stained tissue sections), involving automatic unsupervised segmentation of image(s) of the tissue section(s), measurement of multiple features for individual nuclei within the image(s), clustering of nuclei based on extracted features, and / or analysis of the spatial arrangement and organization of features in the image based on spatial statistics. Also provided are computer-readable media containing instructions to perform operations to carry out such methods. A quantitative image analysis pipeline for tumor purity estimation is also described
Owner:OREGON HEALTH & SCI UNIV
Multicolor chromogenic detection of biomarkers
InactiveUS20080299555A1Facilitates chromogenic detection of signalThe result is accurateMicrobiological testing/measurementBiological testingHistocytochemistryChemiluminescence
The present invention provides compositions, kits, assembles of articles and methodology for detecting multiple target molecules in a sample, such as in a tissue sample. In particular, site-specific deposition of elemental metal is used in conjunction with other means of detection, such as other chromogenic, radioactive, chemiluminescent and fluorescent labeling, to simultaneously detect multiple targets, such a gene, a protein, and a chromosome, in a biological sample. More particularly the multiple targets may be labeled with the specifically deposited metal and other chromogenic labels to allow chromogenic immunohistochemical (IHC) detection in situ by using bright field light microscope.
Owner:VENTANA MEDICAL SYST INC
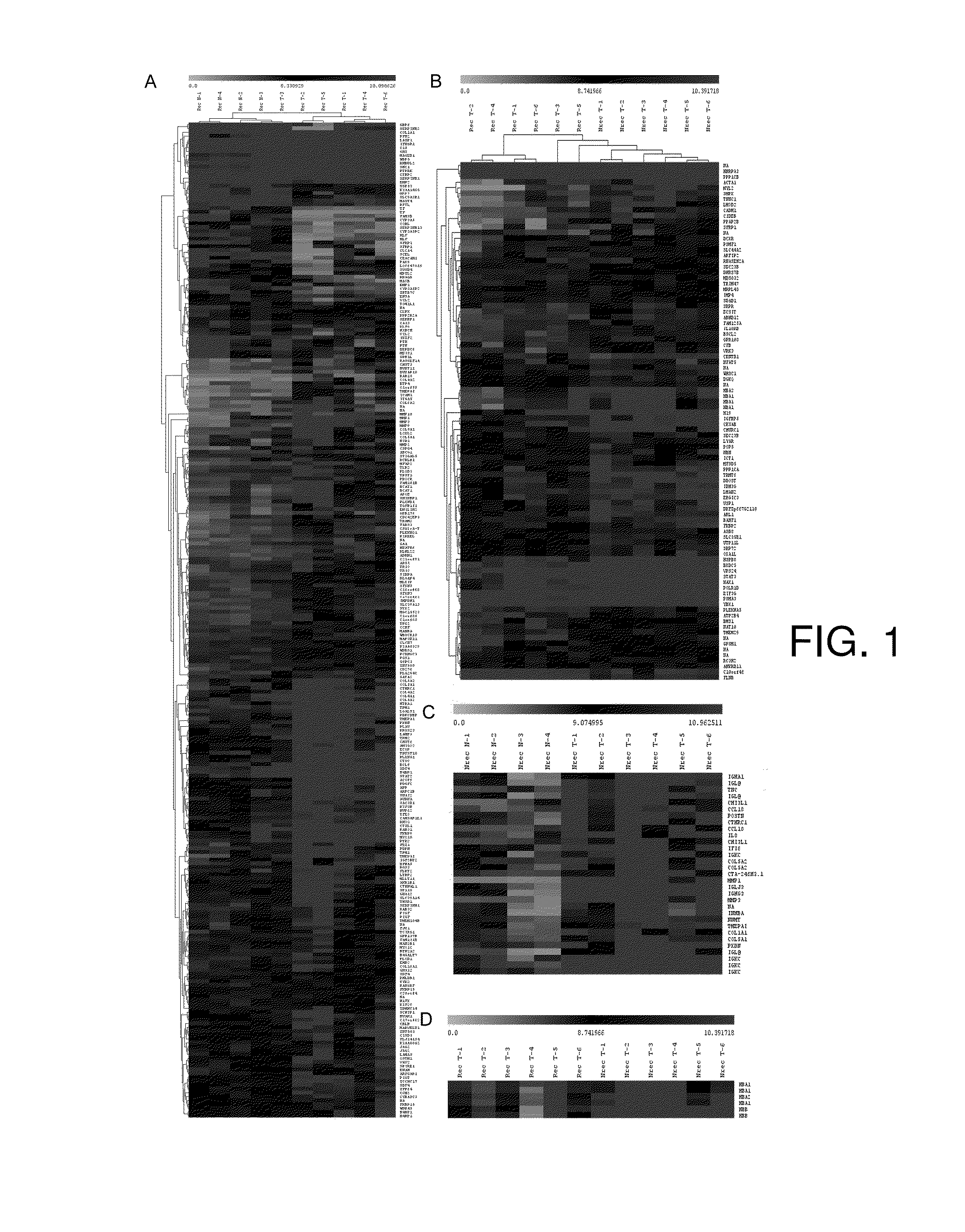
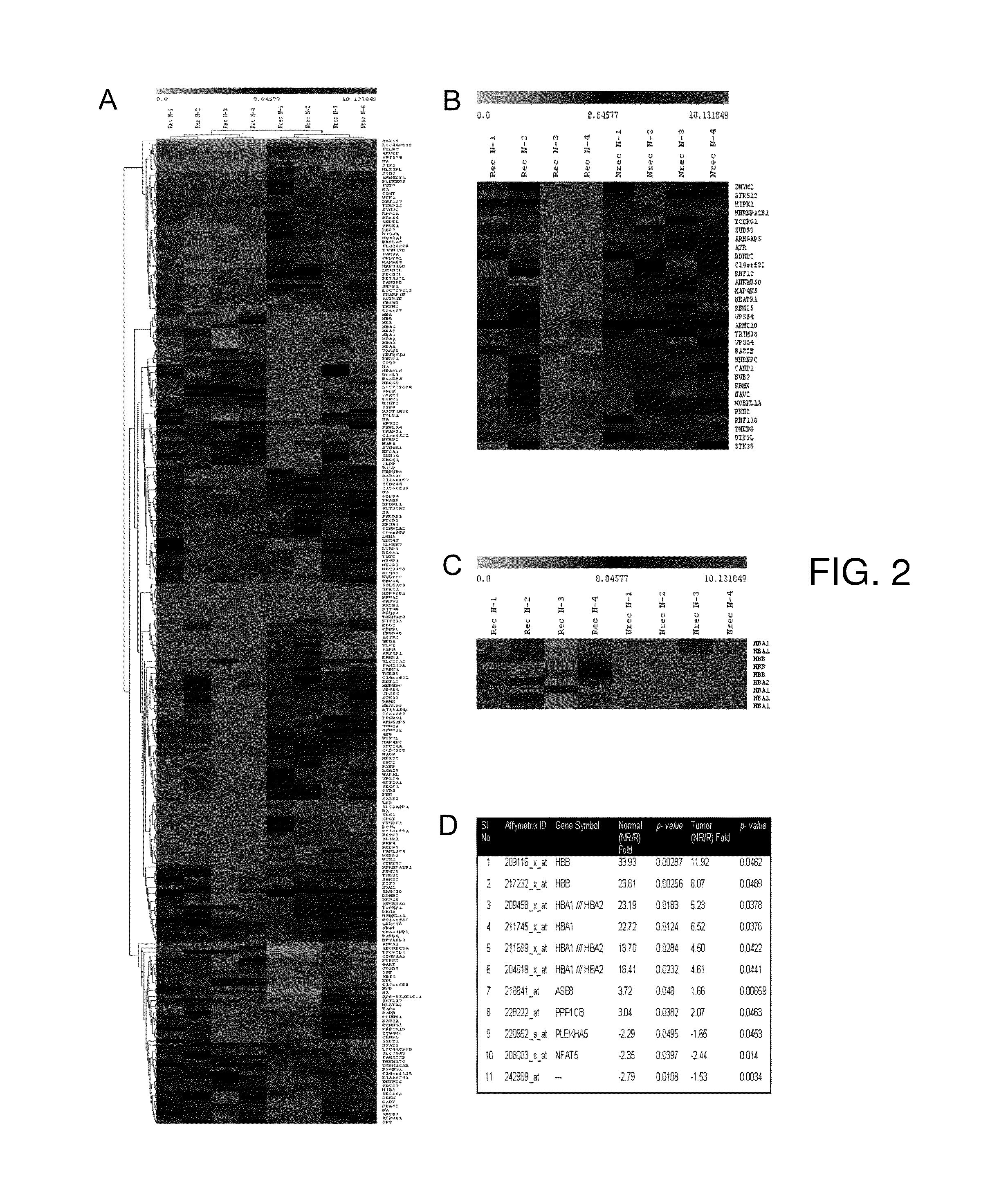
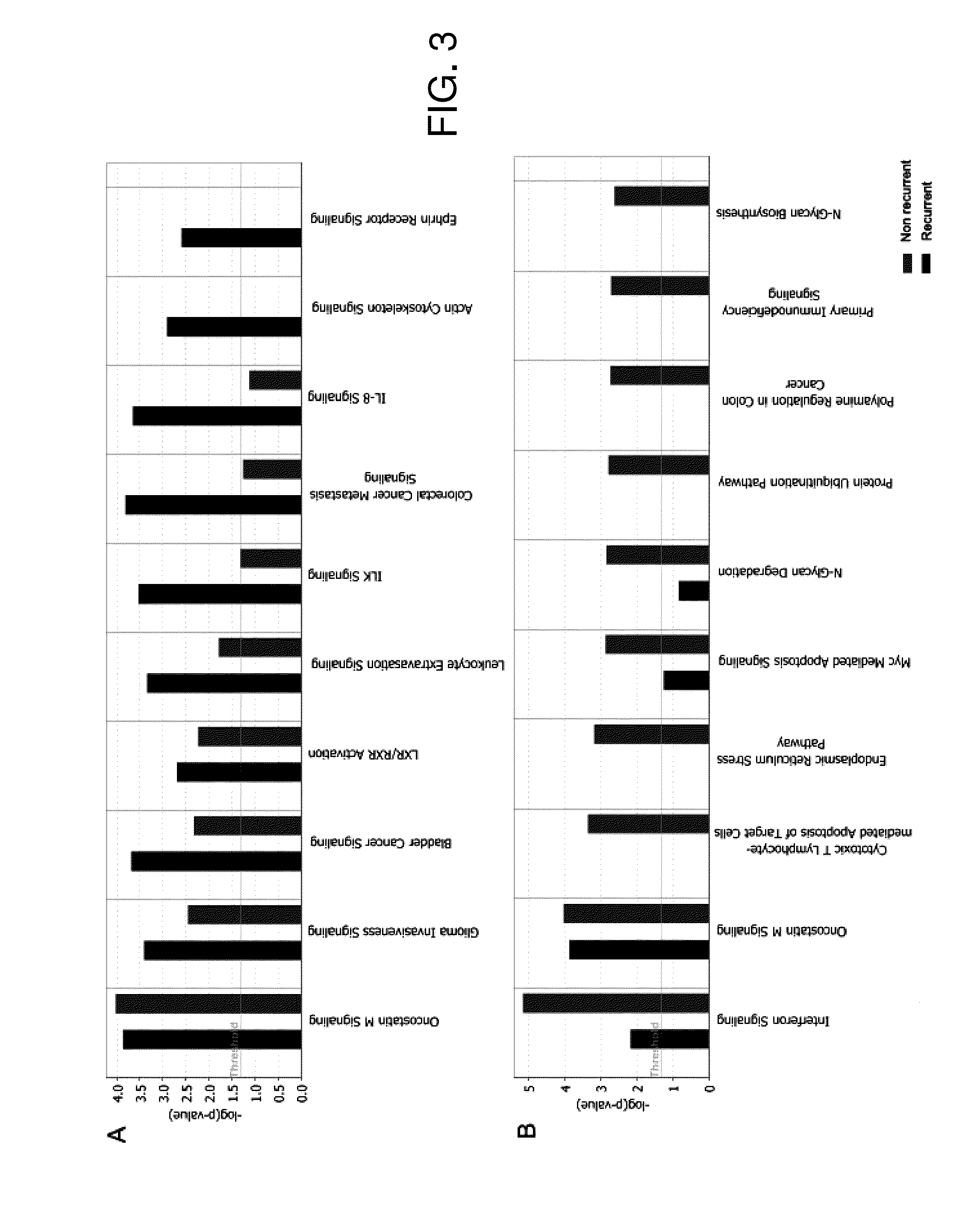
Diagnostic tests for predicting prognosis, recurrence, resistance or sensitivity to therapy and metastatic status in cancer
InactiveUS20140342946A1Low costReduce morbidityMicrobiological testing/measurementProtein nucleotide librariesParanasal Sinus CarcinomaDiagnostic test
The present invention describes a method utilizing a set of genes or gene products whose altered expression in cancer tissue, particularly head and neck cancer and other carcinomas, or its adjacent normal tissues predicts (a) probability of recurrence in time after treatment (b) sensitivity or resistance to therapies or (c) probability of metastasis at the time of initial discovery of the tumor. Furthermore, the invention describes methods of determining the molecular signature in tumor tissues, tissues adjacent to the tumor, or in saliva by using DNA microarray techniques, quantitative real-time PCR, immunohistochemistry or other methods that are used for determining gene or gene product expression levels.
Owner:KURIAKOSE MONI ABRAHAM +1
Low temperature deparaffinization
InactiveUS20060252025A1Easy to explainImproving stainability and readabilityPreparing sample for investigationDead animal preservationCytochemistryBatch processing
Methods and apparatuses for gently removing embedding media from biological samples at temperatures below the embedding medium melting point with liquid composition using batch methods or automated instruments prior to immunohistochemical (IHC), in situ hybridization (ISH) or other special staining or histochemical or cytochemical manipulations.
Owner:VENTANA MEDICAL SYST INC
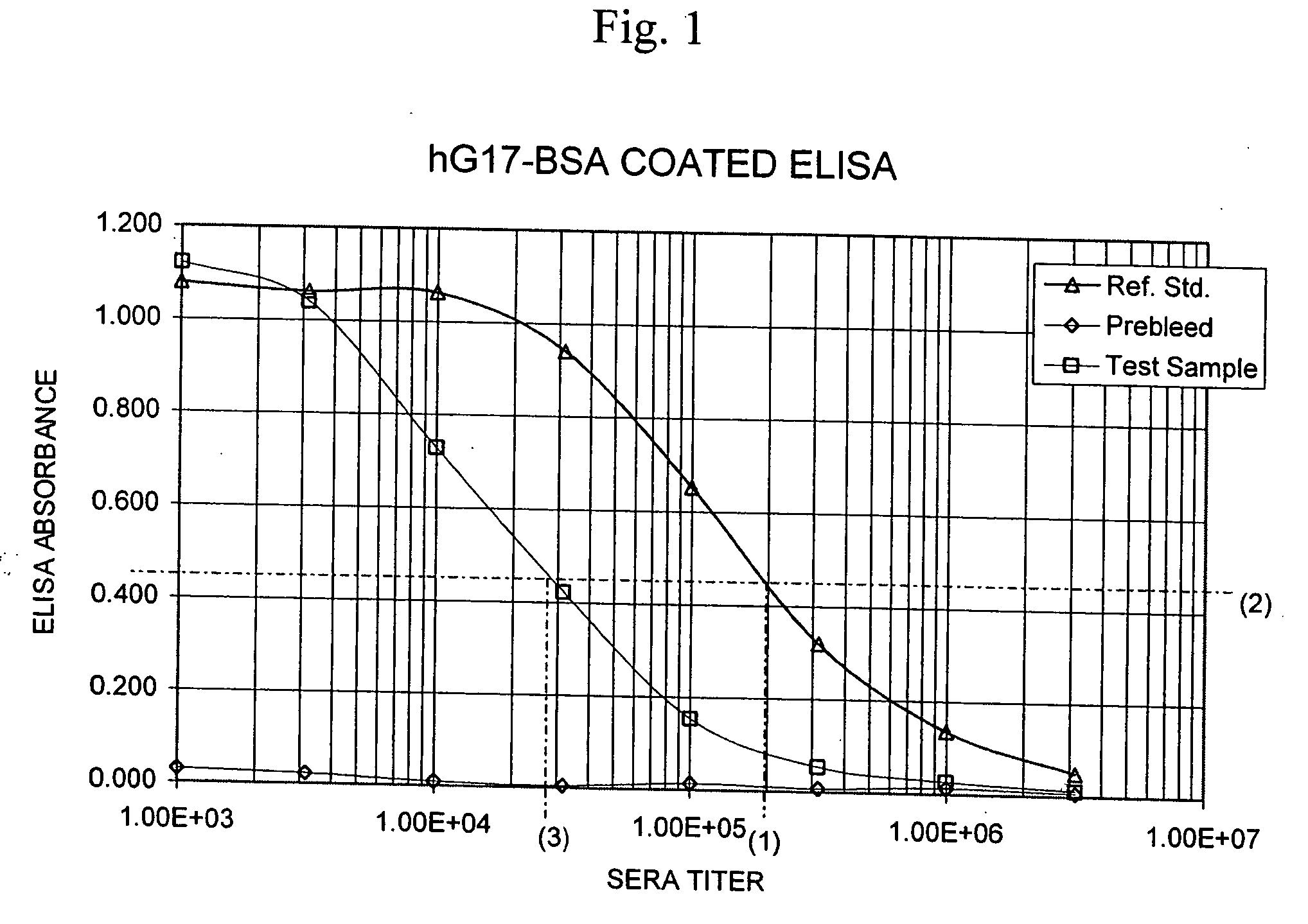
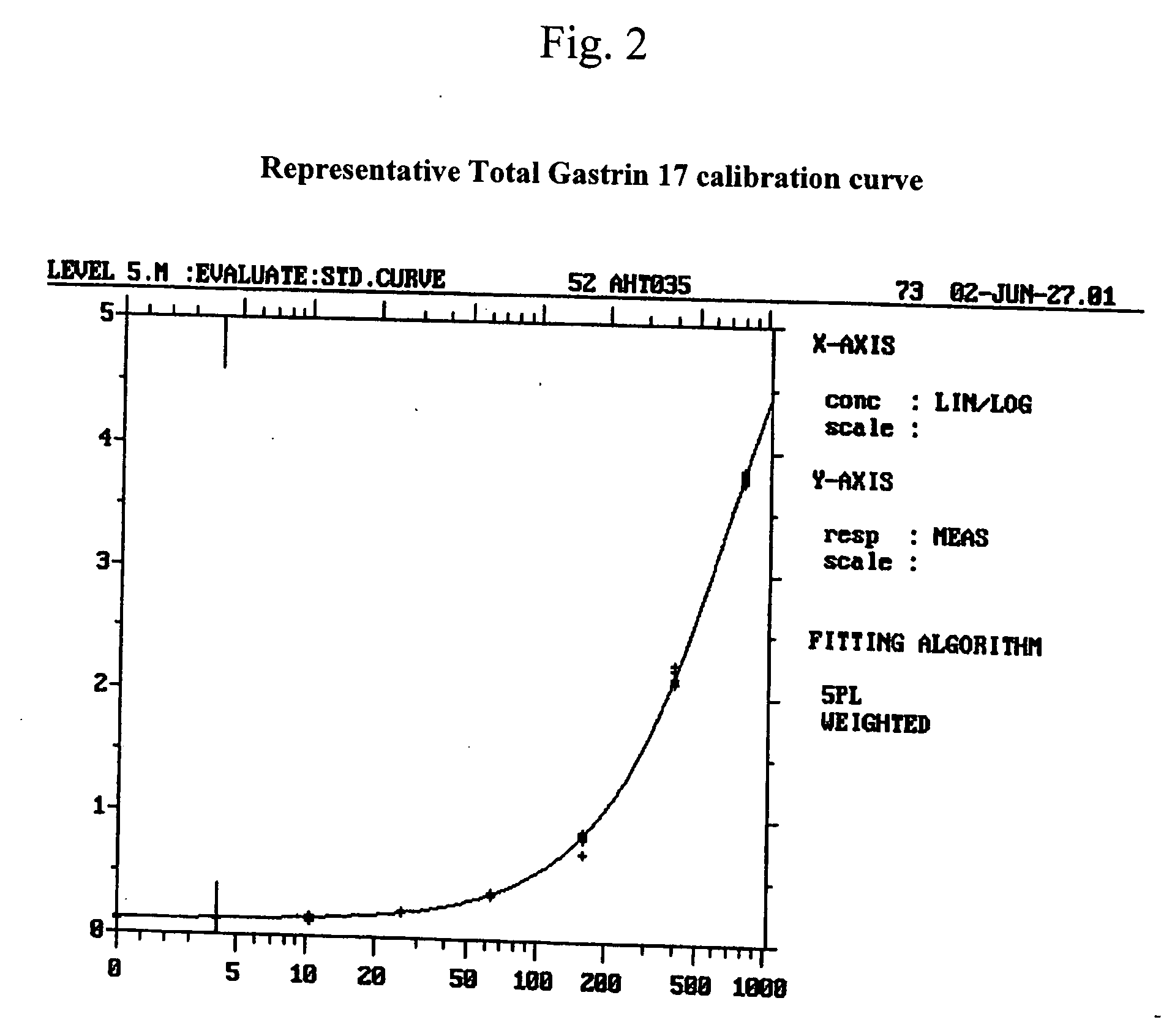
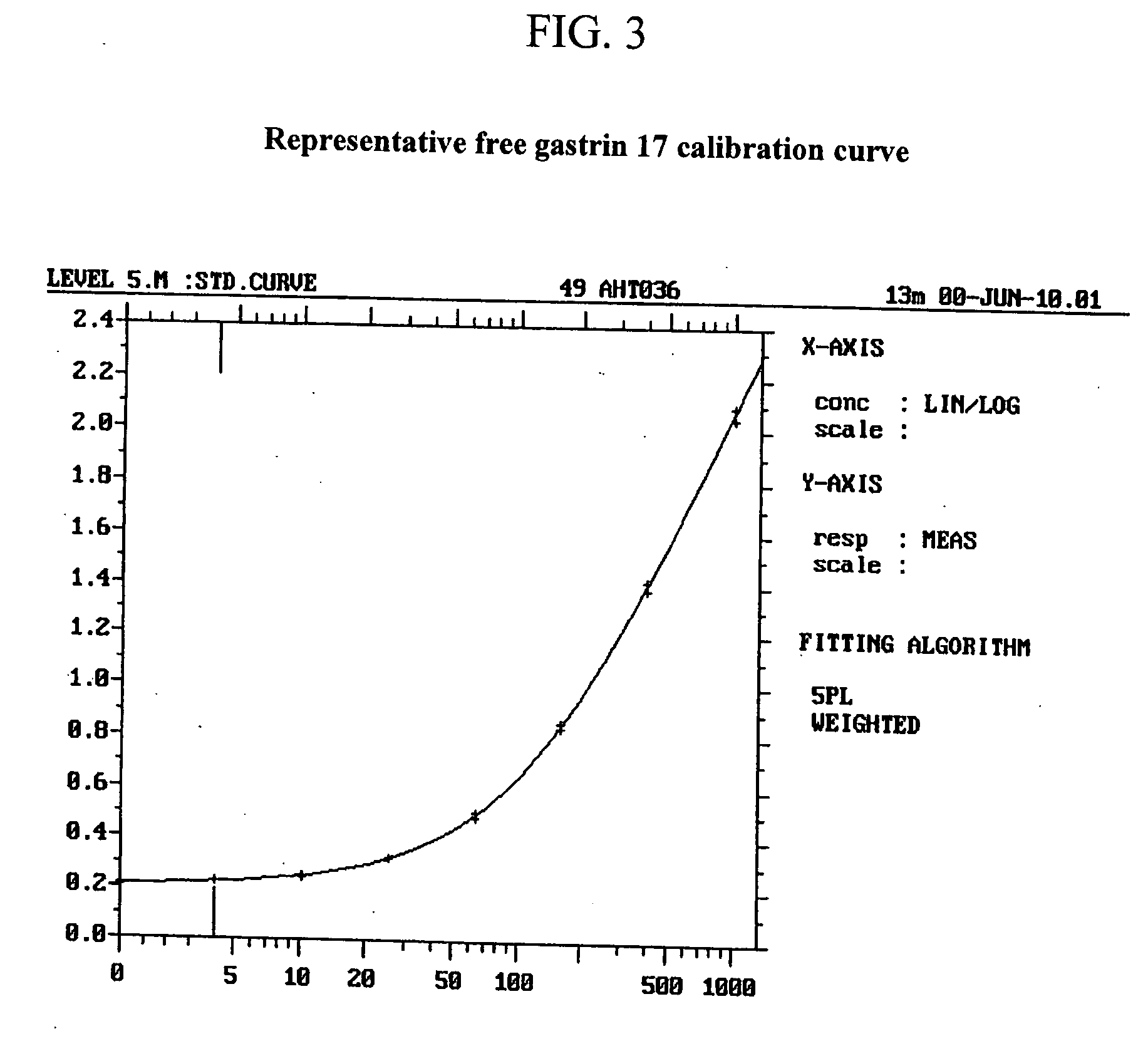
Monoclonal Antibodies to Progastrin
The present invention provides progastrin-binding molecules specific for progastrin that do not bind gastrin-17(G17), gastrin-34(G34), glycine-extended gastrin-17(G17-Gly), or glycine-extended gastrin-34(G34-Gly). Further, the invention provides monoclonal antibodies (MAbs) selective for sequences at the N-terminus and the C-terminus of the gastrin precursor molecule, progastrin and the hybridomas that produce these MAbs. Also provided are panels of MAbs useful for the detection and quantitation of progastrin and gastrin hormone species in immuno-detection and quantitation assays. These assays are useful for diagnosing and monitoring a gastrin-promoted disease or condition, or for monitoring the progress of a course of therapy. The invention further provides solid phase assays including immunohistochemical (IHC) and immunofluorescence (IF) assays suitable for detection and visualization of gastrin species in solid samples, such as biopsy samples or tissue slices. The progastrin-binding molecules are useful therapeutically for passive immunization against progastrin in progastrin-promoted diseases or conditions. Also provided are surrogate reference standard (SRS) molecules that are peptide chains of from about 10 to about 35 amino acids, wherein the SRS molecule comprises at least two epitopes found in a protein of interest of greater than about 50 amino acids. Such SRS molecules are useful as standards in place of authentic proteins of interest.
Owner:CANCER ADVANCES INC
Immunohistochemical assay for detecting expression of programmed death ligand 1 (pd-l1) in tumor tissue
InactiveUS20160084839A1Improve standardizationAntibody ingredientsDisease diagnosisProgrammed deathPD-L1
The present disclosure provides processes for describing and quantifying the expression of human programmed death ligand-1 (PD-L1) in tumor tissue sections as detected by immunohistochemical assay using an antibody that specifically binds to PD-L1. The results generated using these processes have a variety of experimental, diagnostic and prognostic applications.
Owner:MERCK SHARP & DOHME CORP
Method for chromogenic detection of two or more target molecules in a single sample
ActiveUS20110136130A1Low backgroundReduce and preventMicrobiological testing/measurementBiological testingSingle sampleTissue sample
The present invention provides a method and kit for detection of two or more target molecules in a single tissue sample, such as for gene and protein dual detection in a single tissue sample. Methods comprise treating a tissue sample with a first binding moiety that specifically binds a first target molecule. Methods further comprise treating the tissue sample with a solution containing a soluble electron-rich aromatic compound prior to or concomitantly with contacting the tissue sample with a hapten-labeled binding moiety and detecting a second target molecule. In one example, the first target molecule is a protein and the second is a nucleic acid sequence, the first target molecule being detected by immunohistochemistry and the second by in situ hybridization. The disclosed method reduces background due to non-specific binding of the hapten-labeled specific binding moiety to an insoluble electron rich compound deposited near the first target molecule.
Owner:VENTANA MEDICAL SYST INC
Tissue dewaxing transparent agent free of benzene
InactiveCN104155160ASoft effectNot easy to shrink and deformPreparing sample for investigationLiquid base cytologyStaining
The invention relates to a tissue dewaxing transparent agent free of benzene, which is used in biological histology, histopathology or forensic science, can make a tissue transparent, and can make the tissue dewaxed. The tissue dewaxing transparent agent belongs to the technical field of in vitro diagnostic reagents. The tissue dewaxing transparent agent mainly contains 95%-100% of alicyclic hydrocarbon, and the balance of accessories; the alicyclic hydrocarbon is a monocyclic alicyclic hydrocarbon or a bicyclic alicyclic hydrocarbon. The tissue dewaxing transparent agent free of benzene has the advantages of being non-toxic, soft in effect, and not easy to cause shrinkage, deformation, hardening and embrittlement of a tissue material, capable of improving the section intact rate, insensitive to the humid environment, not easy to muddy due to absorption of moisture in the air, and the like; not only can be used for liquid based cytology, immunohistochemistry, biological histology, routine pathological diagnosis, and immunohistochemical diagnostic techniques, is also suitable for special staining method and histochemical techniques. The refractive index of used alicyclic hydrocarbon transparent agent (cyclohexane) is 1.42, the (methyl cyclohexane) refractive rate is 1.42, and is basically consistent with the tissue refractive index (1.418), and the transparent effect is better.
Owner:陆可望 +1
Methods and compounds for detection of molecular targets
ActiveUS8435735B2Faster and more sensitive and precise detectionEasy to detectSugar derivativesMicrobiological testing/measurementIn situ hybridisationChemical compound
The present invention relates to methods and compounds for detection of molecular targets, such as biological or chemical molecules, or molecular structures, in samples using a host of experimental schemes for detecting and visualizing such targets, e.g. immunohistochemistry (IHC), in situ hybridization (ISH), ELISA, Southern, Northern, and Western blotting, etc.
Owner:AGILENT TECH INC
Double-stained kit for auxiliary diagnosis of benign or malignant hepatocellular tumor and application thereof
InactiveCN104730242AIncrease the amount of informationImprove differential stainingMaterial analysisInformation quantityDifferential diagnosis
The invention discloses a double-stained kit for auxiliary diagnosis of benign or malignant hepatocellular tumor and application thereof. The kit comprises the following components: mixed primary antibody of Arginase-1 and Glypian-3, mixed second antibody of alkaline phosphatase conjugated goat anti-rabbit second antibody and horse radish peroxidase labeled goat anti -mouse second antibody, chromogenic reagent AP-Red and DAB (Diaminobenzidine), and TBS (Tris buffer saline) buffer solution. Two immunohistochemical labels provided by the invention are used for auxiliary diagnosis of benign or malignant hepatocellular tumor and differentiation degrees of tumor, the two labels are displayed through staining label by different detection systems and utilizing the differences of target cells stained by each label, information quantity and differential staining on the same radiograph are improved, readability and accuracy of radiograph reading are increased, so that the double-stained kit has important significance to identification of benign or malignant hepatocellular tumor and differentiation degrees of tumor.
Owner:GUANGZHOU LBP MEDICINE SCI & TECH
Site-specific enzymatic deposition of metal in situ
InactiveUS20080213783A1Easily rate of reactionMore sensitive detectionMicrobiological testing/measurementEnzymesBiologic markerHistocytochemistry
The present invention provides compositions, kits, assembles of articles and methodology for carrying out processes that permit biological enzymes to act directly on metals and metal particles. In particular, the invention relates to use of enzymes to selectively deposit metal to the vicinity of a target molecule. The invention also relates to linking of metals to enzyme substrates, control of enzymatic metal deposition and applications of enzymatic metal deposition to sensitively and selectively detect target molecules such as biomarkers in various biological samples, such as chromogenic immunohistochemical (IHC) detection in situ by using bright field light microscope.
Owner:NANOPROBES
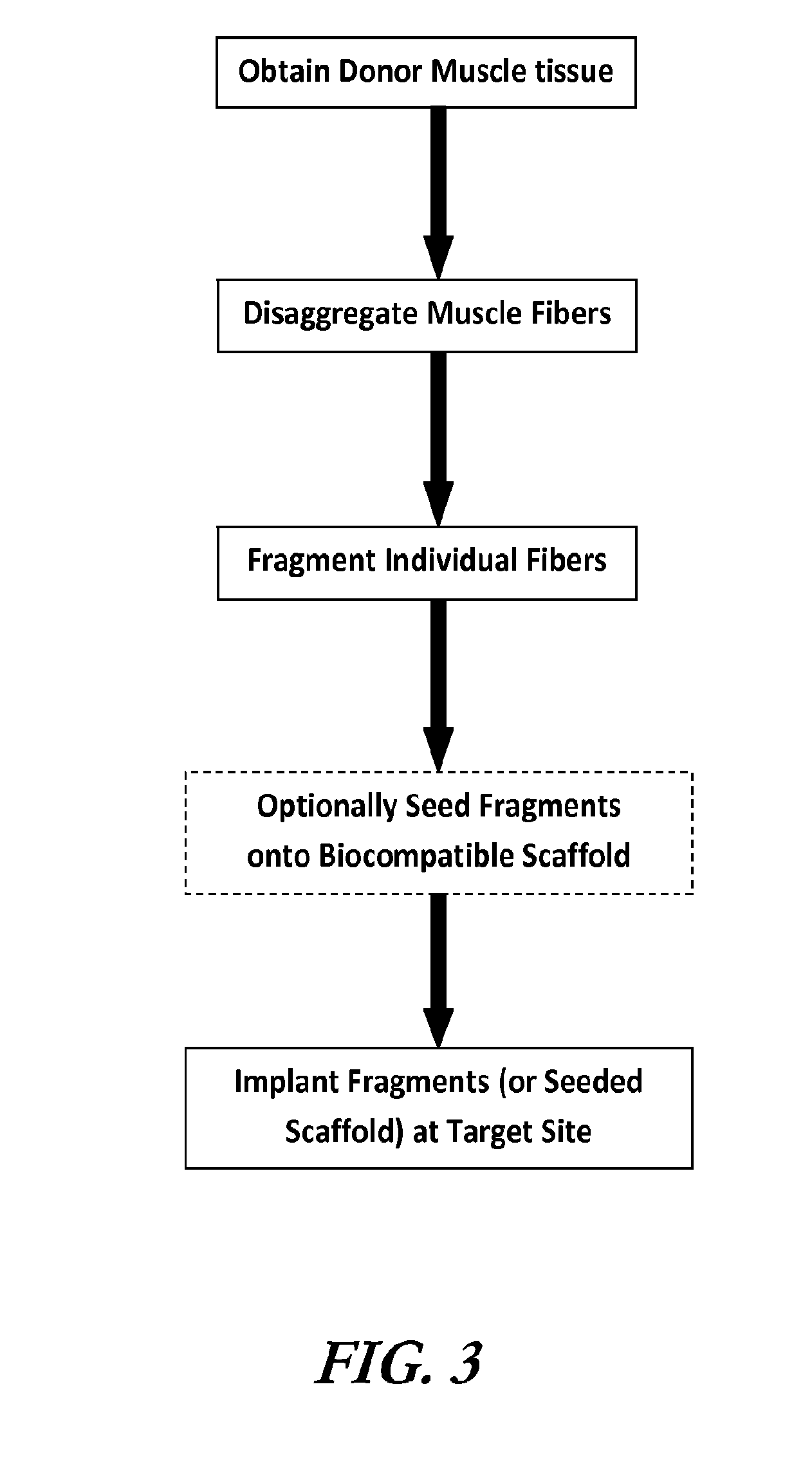
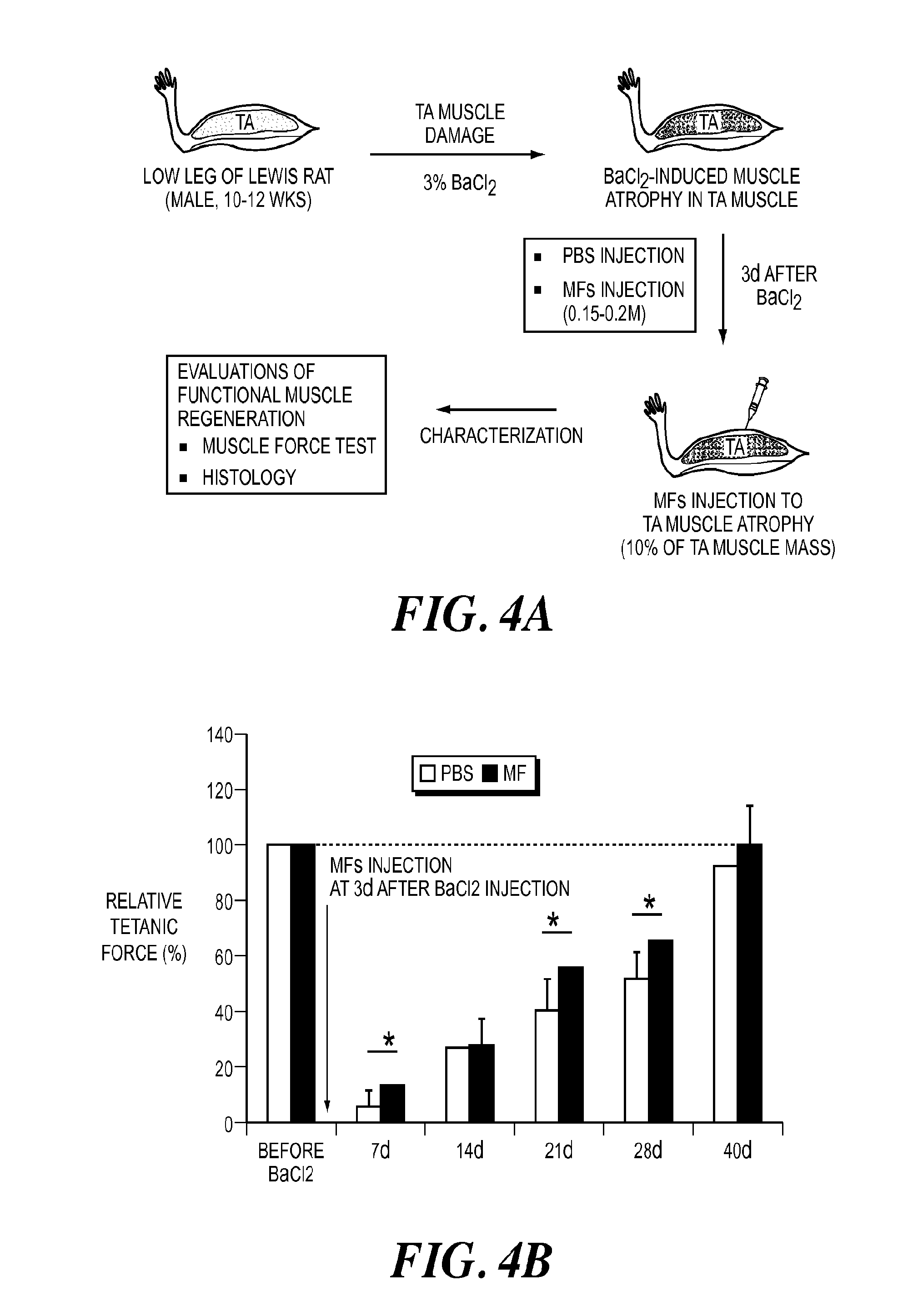
Muscle tissue regeneration using muscle fiber fragments
ActiveUS20140242125A1Efficient functional muscle regenerationEfficient regenerationBiocidePeptide/protein ingredientsFiberMuscle tissue
The invention is directed to methods and compositions for obtaining uniform sized muscle fiber fragments for transplantation. These muscle fiber fragments are able to reconstitute into long fibers that are oriented along native muscle. The implanted muscle cells integrate with native vascular and neural network, as confirmed by histology and immunohistochemistry. This invention is particularly advantageous because autologous muscle can be harvested from a donor site, processed and injected into target sites in the operating room. The fragmented muscle fibers can be readily integrated within the host.
Owner:WAKE FOREST UNIV HEALTH SCI INC
Anti-CK20 protein monoclonal antibody and cell line and preparing method and application thereof
ActiveCN109734805AStrong specificityIncreased sensitivityImmunoglobulins against animals/humansMicroorganism based processesHeavy chainProtein.monoclonal
The invention relates to the field of biological detection, provides an anti-CK20 protein monoclonal antibody, further provides a preparing method of the anti-CK20 protein monoclonal antibody, and further provides a hybridoma cell line for secreting anti-CK20 proteins. A heavy-chain variable region and a light-chain variable region of the antibody have the amino acid sequence shown in SEQ ID NO.4and the amino acid sequence shown in SEQ ID NO.5. The 411th-site to 424th-site proteins of the human CK20 amino acid are selected to be coupled with KLH to form an immunogen. The cell line is a rat hybridoma cell line 10F6B12A9 and has the preservation number of CGMCC NO.16204. The anti-CK20 protein monoclonal antibody has high specificity and sensitivity, can specifically recognize cells of expressing CK20 proteins, and is suitable for immunological detection, especially immuno-histochemistry.
Owner:FUZHOU MAIXIN BIOTECH CO LTD
Tumor necrosis factor-alpha induced protein 8 L3 (TIPE3) immunohistochemistry detection kit for diagnosing lung cancer
InactiveCN102707058AEffective diagnosisDiagnosis, opens up new effective lung cancer diagnosisPreparing sample for investigationAntigenStaining
The invention discloses a tumor necrosis factor-alpha induced protein 8 L3 (TIPE3) immunohistochemistry detection kit for diagnosing lung cancer. Contents of the kit comprise a reagent which is used for inactivating endogenous peroxidase and biotin in a human tissue section, a non-specific protein blocking agent which is used for blocking non-specific staining of cross protein reaction in the human tissue section, a rabbit anti-human initial antibody which is combined with human tissue antigen, an antibody diluent, a goat anti-rabbit monoclonal connection antibody which can be connected with the initial antibody and is marked by horse radish peroxidase, a developing reagent which can be reacted with the horse radish peroxidase and used for developing, and a reagent which can specifically stain nuclei in tissues. TIPE3 is positively expressed in various kinds of pathological type lung cancer, tumors can be early detected and found, the kit is high in specificity and high in sensitivity and can be applied to early diagnosis of the lung cancer, a patient can be effectively and timely treated, unnecessary medical cost is reduced, the survival quality of the patient is improved, survival time is prolonged, and the survival rate of the patient who suffers from the lung cancer is improved.
Owner:SHANDONG UNIV
MSI prediction model construction method based on gastric cancer histopathology image texture features
InactiveCN112183557ARealize visualizationHighlight tumor featuresBiostatisticsRecognition of medical/anatomical patternsImage extractionNonzero coefficients
The invention discloses an MSI prediction model construction method based on gastric cancer histopathological image texture features. The method comprises the following steps: obtaining histopathological images of a gastric cancer patient, marks of focus parts and clinical pathological information; extracting texture features of the original image and texture features after wavelet transform for the histopathological image of the patient with gastric cancer; performing feature selection on the obtained texture features by using LASSO, and selecting non-zero coefficient features corresponding to the lambda value when 10 times of cross validation error is minimum to obtain screened texture features; carrying out linear fitting according to the characteristic value of the selected texture characteristic and the coefficient weight of the characteristic value so as to obtain an MSI label of the gastric cancer patient; and combining the MSI label and the clinical pathological information ofthe patient to construct an MSI prediction model. According to the method, the MSI state of the gastric cancer patient is directly predicted on the basis of the histopathological image which is easy to obtain, an additional laboratory is not required to carry out gene detection and immunohistochemical analysis, and the MSI state can be detected at a lower cost.
Owner:SHANXI MEDICAL UNIV
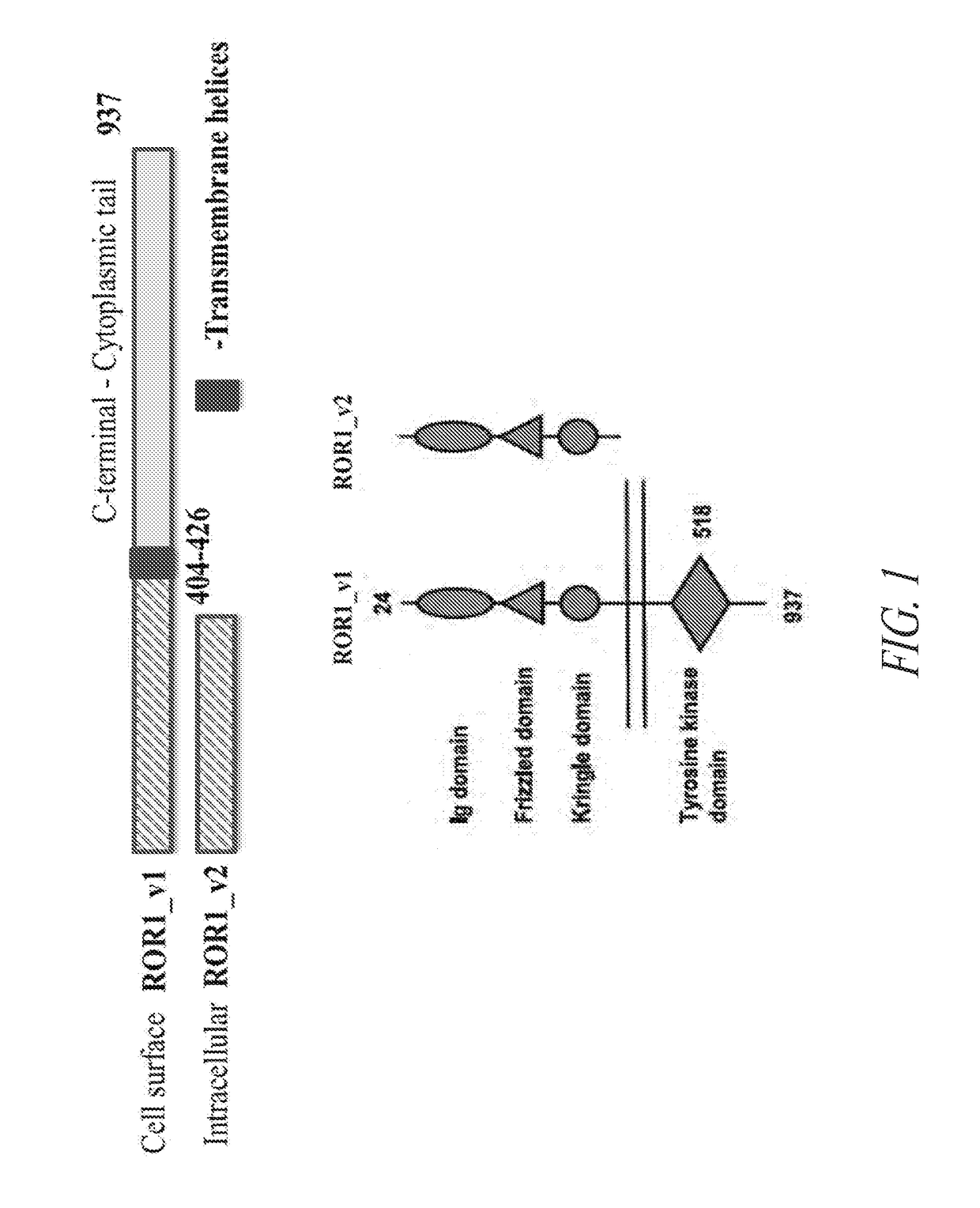
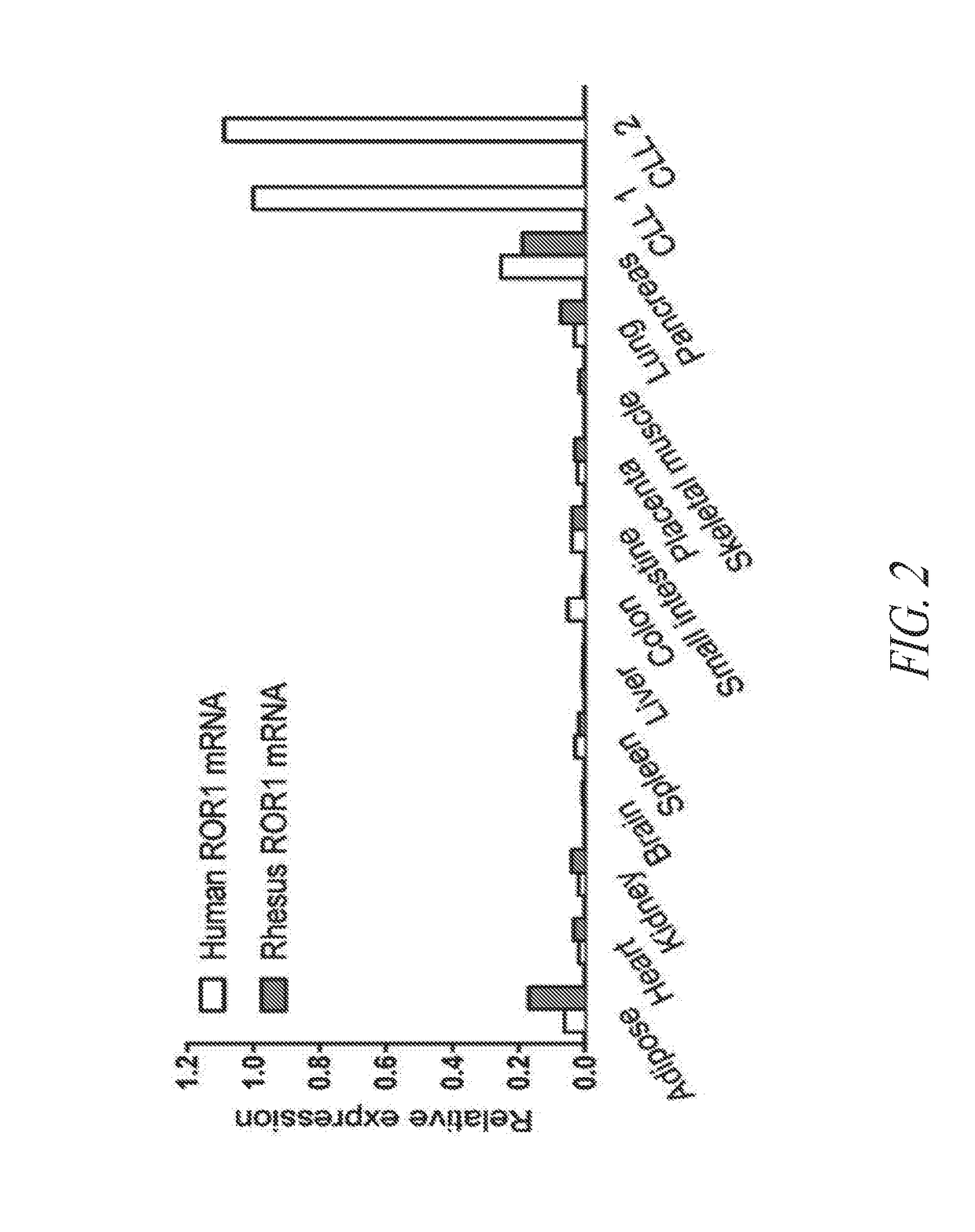
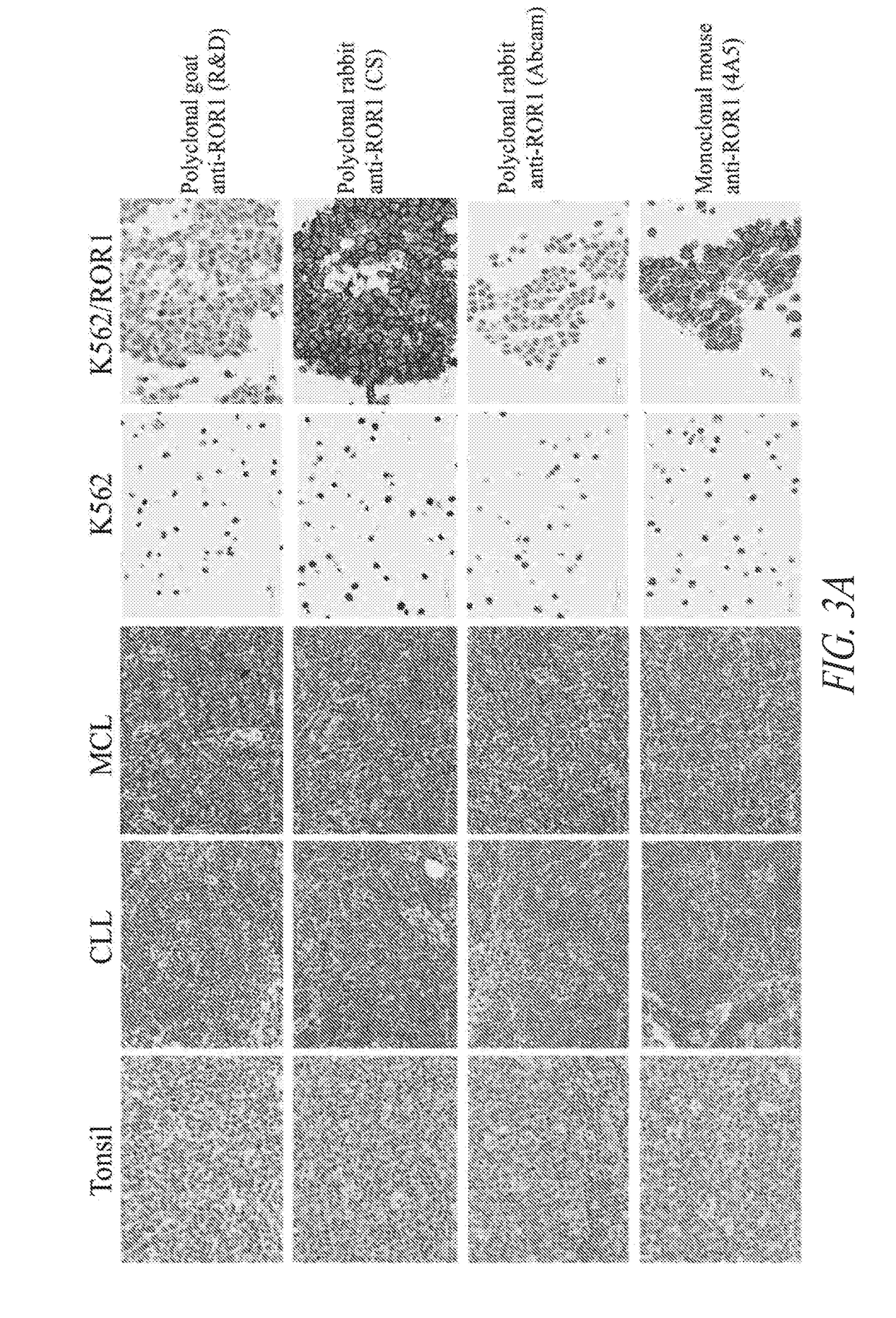
Anti-ror1 antibodies and uses thereof
ActiveUS20190040132A1Polypeptide with localisation/targeting motifImmunoglobulin superfamilyDiseaseAntiendomysial antibodies
The present disclosure relates to anti-ROR1 binding proteins, including those that bind to a ROR1 or portion thereof such as an intracellular C terminal portion of a ROR1 protein, and the use of such binding proteins in immunohistochemical and diagnostic methods. Related kits and methods of using the binding proteins are also provided, as are methods of treatment of subjects having diseases or conditions determined to be candidates for such treatments by the binding proteins or methods of this disclosure.
Owner:FRED HUTCHINSON CANCER CENT
Monoclonal antibodies to gastrin hormone
The present invention provides monoclonal antibodies (MAbs) selective for the N-termini and C-termini of the gastrin hormone forms, gastrin-17 (G17), glycine-extended gastrin-17 (G17-Gly), gastrin-34 (G34) and glycine-extended gastrin-34 (G34-Gly); and the hybridomas that produce these MAbs. Also provided are panels of MAbs useful for the detection and quantitation of gastrin-17 (G17), glycine-extended gastrin-17 (G17-Gly), gastrin-34 (G34) and glycine-extended gastrin-34 (G34-Gly). These assays are useful for monitoring a gastrin-mediated disease or condition, or for monitoring the progress of a course of therapy. The invention further provides solid phase assays including immunohistochemical (IHC) and immunofluorescence (IF) assays suitable for detection and visualization of gastrin species in solid samples, such as biopsy samples or tissue slices. Pharmaceutical compositions of the MAbs of the invention are also provided, along with methods of diagnosis, prevention and treatment of gastrin-mediated diseases or conditions. Methods of evaluating a gastrin hormone-blocking treatment are described. The course of a gastrin-mediated disease or condition may be monitored in a patient by means of assay methods provided.
Owner:CANCER ADVANCES INC
Rapid and sensitive method for detection of biological targets
ActiveUS20100285467A1Attached tightlyPrecise positioningOrganic chemistryMicrobiological testing/measurementIn situ hybridizationBiological target
The present invention relates to a method of biological labeling that occurs via a free radical chain reaction. The labeling occurs due to deposition of a detectable reporter molecule from a media comprising a substance comprising at least two moieties of a peroxidase enzyme substrate (termed herein ‘cross-linker’) in a target site comprising peroxidase activity and a biological marker. The labeling reaction described herein may generally be used to detect targets in a host of experimental schemes for detecting and visualizing a biological or chemical target, including immunohistochemistry (IHC), in situ hybridization (ISH), antibody-based staining methods such as ELISA, Southern, Northern, and Western blotting, and others.
Owner:AGILENT TECH INC
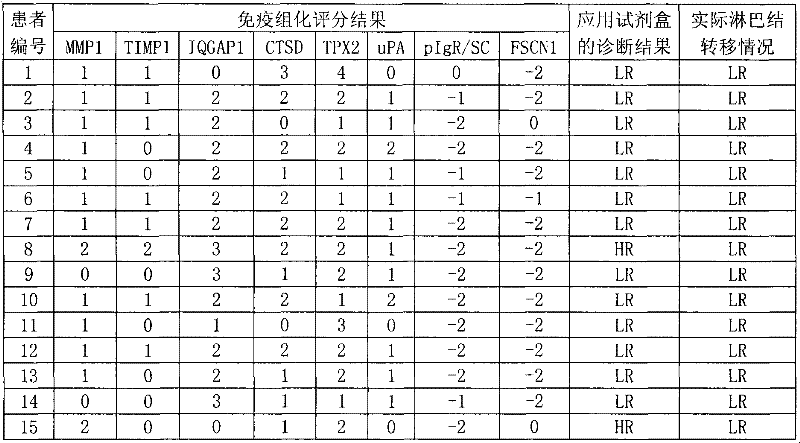
Reagent for auxiliarily diagnosing lung cancer lymph node metastasis
InactiveCN102445543AIncrease credibilityPracticalBiological testingStainingPolymeric immunoglobulin receptor
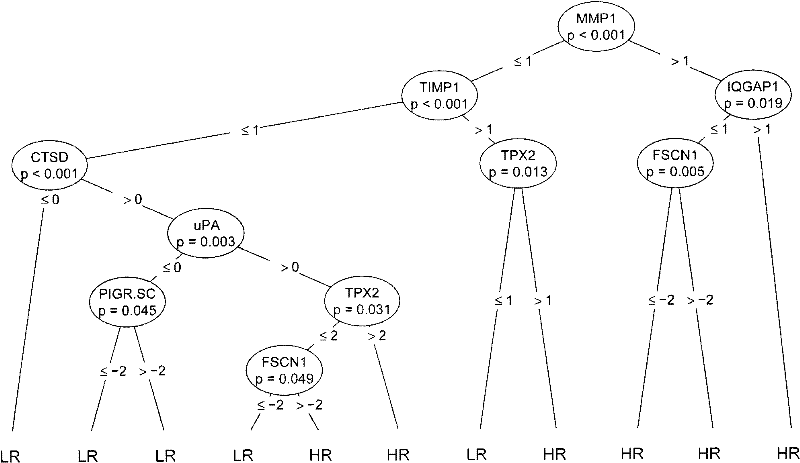
The invention discloses a reagent for auxiliarily diagnosing lung cancer lymph node metastasis, comprising 8 antibodies which are used for detecting 8 protein markers which are MMP1 (matrix metalloproteinase-1), TIMP1 (tissue inhibitor of metalloproteinases metallopeptidase inhibitor 1), IQGAP1 (IQ motif containing GTPase activating protein 1), TPX2 (targeting protein for Xklp2), uPA (Urokinase-type plasminogen activator), Cathepsin-D, Fascin and pIgR / SC (polymeric immunoglobulin receptor / secretory component). By adopting the 8 antibodies and an immunohistochemical staining result, lung cancer lymph node metastasis can be auxiliarily diagnosed, and the reagent is expected to be used for the risk estimation of lung squamous cell cancer lymph node metastasis and the prognostic prediction. The reagent has high creditability, strong practicability and clinical use value based on the clinical routine immunohistochemical staining technology when the reagent is used for auxiliary diagnosis.
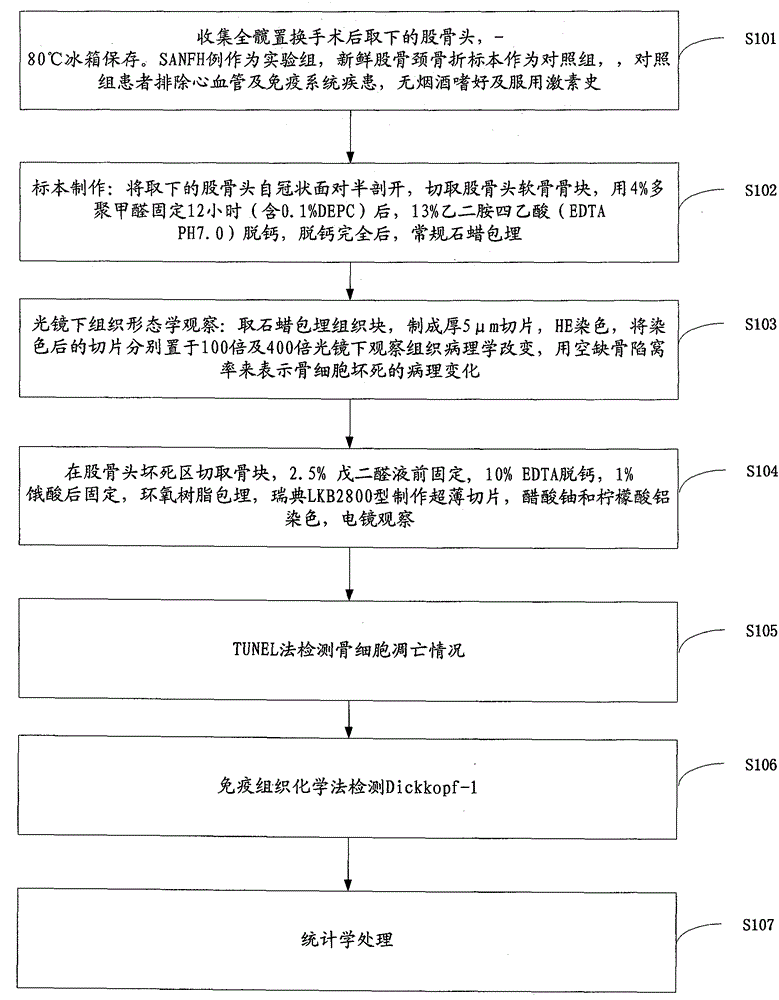
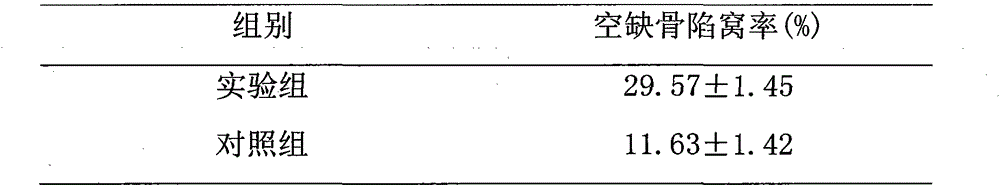
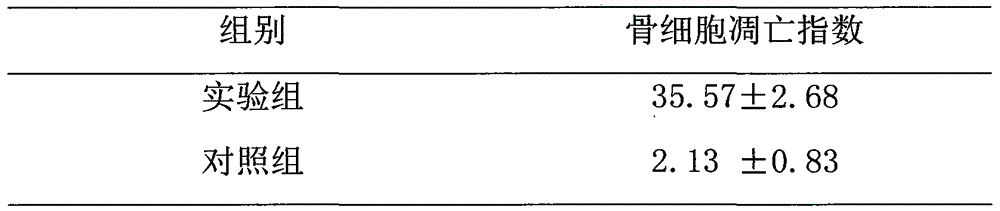
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
Double-labeled immunohistochemical staining kit for micro invasive lung adenocarcinoma
The invention belongs to the technical field of immunohistochemistry and relates to a double-labeled immunohistochemical staining kit for micro invasive lung adenocarcinoma. In order to solve the problem that invasive components and non-invasive components of micro invasive lung adenocarcinoma in HE staining films are difficultly distinguished clearly, the CD34 and beta-Tubulin-III double-labeled immunohistochemical staining kit is prepared and is applied to identification of the invasive lesions and the non-invasive components in the early stage of the lung adenocarcinoma, and overdiagnosis or under-diagnosis of the lung adenocarcinoma, caused by artificial interpretation difference, can be avoided.
Owner:FUZHOU MAIXIN BIOTECH CO LTD
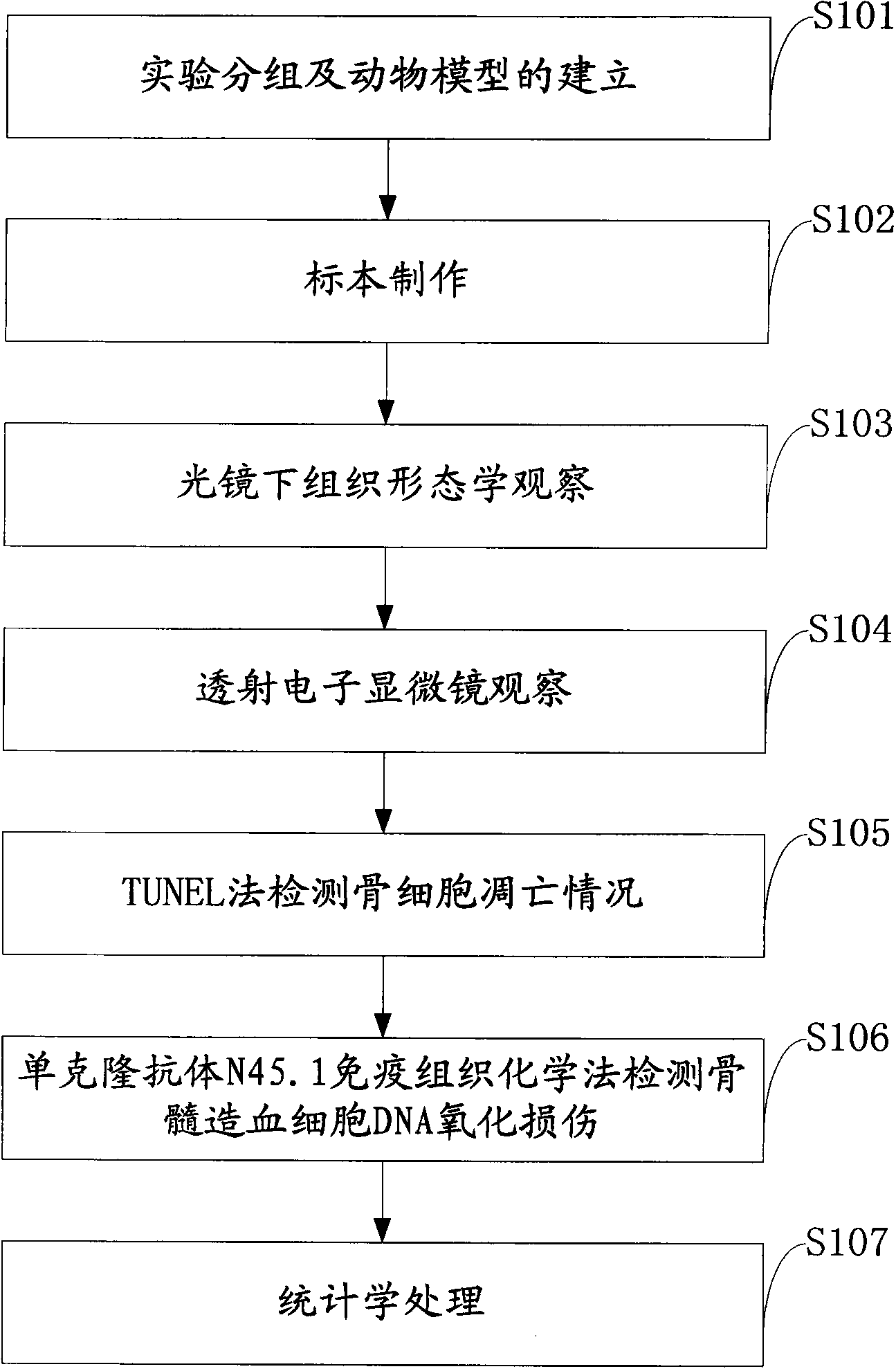
Experimental method of effects of DNA oxidative damage and bone cell apoptosis on avascular necrosis
The invention discloses an experimental method of effects of DNA oxidative damage and bone cell apoptosis on avascular necrosis, the method comprises the following steps: experimental grouping and establishment of an animal model, preparation of specimens, histomorphology observation under a light microscope, observation by a transmission electron microscope, bone cell apoptosis situation detection by TUNEL (terminal dexynucleotidyl transferase(TdT)-mediated dUTP nick end labeling) method, bone marrow hematopoietic cell DNA oxidative damage detection by monoclonal antibody N45.1 immunohistochemistry method, and statistical analysis. According to the method, experiment animals are respectively killed in the second week and the fourth week after the last time of administration, and five animals are killed for each time in each group; bilateral femoral heads are taken for conventional fixed decalcification for stand-by testing, the number of vacant bone lacunas is counted by HE (hematoxylin-eosin) staining, the bone cell morphological change is observed by the electron microscope, the bone cell apoptosis situation is detected by the TUNEL method, the bone marrow hematopoietic cell DNA oxidative damage is detected by the immunohistochemistry method, and the relationship between SANFH ((steroid-induced avascular necrosis of femoral head)) and the bone marrow hematopoietic cell DNA oxidative damage and the bone cell apoptosis is explored, and provides new ideas for the further study of pathogenesis of the steroid induced avascular necrosis of femoral head.
Owner:刘万林
Anti-human B7-H4 extracellular monoclonal antibody secreting hybridoma cell strain, anti-human B7-H4 monoclonal antibody and application thereof
ActiveCN107299085AGood passaging abilityStrong specificityBiological material analysisMicroorganism based processesAntiendomysial antibodiesWestern blot
The invention relates to the technical field of immune application, in particular to an anti-human B7-H4 extracellular monoclonal antibody secreting hybridoma cell strain, an anti-human B7-H4 monoclonal antibody and application thereof. The hybridoma cell strain comprises five hybridoma cells capable of generating high-potency monoclonal antibody and has good passaging capability. The antibody is the monoclonal antibody generated by the anti-human B7-H4 extracellular monoclonal antibody secreting hybridoma cell strain. The antibody can recognize a prokaryotic expression product, a eukaryotic expression product of human B7-H4 and expressed extracellular functional domain protein. The antibody is applied to immunological detection of the B7-H4, including detection of the B7-H4 expressed on the surfaces of the cells by a flow cytometry, detection of the B7-H4 expressed in tissues or the cells by an immunohistochemistry and a Western blot technology and detection of the soluble B7-H4 by an immunofluorescence technology, ELISA and chemiluminescence immunoassay; the antibody is also applied to antibody drug design.
Owner:GUANGDONG MEDICAL UNIV
Immunohistochemical kit for rapidly identifying lung cancer and sclerotic lung cytoma during operation
PendingCN111766385ARapid identificationComprehensive and reliable diagnosis basisMaterial analysisAbzymeStaining
The invention relates to an immunohistochemical kit for rapidly identifying a lung cancer and sclerotic lung cytoma in an operation. The kit comprises a frozen stationary liquid, a peroxidase sealingagent, a CK wide (AE1 / AE3) antibody, a P40 antibody, a TTF1 antibody, a VIM antibody, an enzyme-labeled goat anti-mouse / rabbit polymer, a DAB chromogenic substrate, a DAB chromogenic buffer solution,a hematoxylin redyeing solution and a cleaning buffer solution, and the invention also provides a use method of the immunohistochemical detection kit. According to the kit, quick immunohistochemical staining is carried out on frozen sections in the operation; CK wide, VIM, TTF1 and p40 detection indexes are used for assisting in distinguishing the sclerotic lung cytoma and the lung cancer; and anintraoperative pathological judgment basis is increased from single morphological HE staining to combination of immunohistochemistry and morphology, and a comprehensive and reliable diagnosis basis isprovided for pathologists so that diagnosis accuracy is improved, and a misdiagnosis rate and a missed diagnosis rate are reduced.
Owner:河南赛诺特生物技术有限公司
Constructing method of bracket-free engineering cartilaginous tissue and product thereof
InactiveCN101543644AWith normal chondrocytesUniform planting densityBone implantBiocompatibility ProblemCell type
The invention discloses a constructing method of a bracket-free engineering cartilaginous tissue and a product therefrom. The constructing method comprises the following steps: inducing and differentiating human umbilical mesenchymal stem cells into cartilaginous cells and then carrying out aggregation inducing culture so that the cartilaginous cells excrete stroma towards the outside of cells and form membranoid substance; continuously inducing and culturing the membranoid substance in a cell culture box and then transferring the membranoid substance into a centrifugal tube for inducing culture to form loose cartilaginous tissue agglomerate; and continuously inducing and culturing the loose cartilaginous tissue agglomerate in a rotating wall type bioreactor to obtain dense cartilaginous-like tissue. The cytology, the histology or the immunohistochemistry dyeing shows that the constructed engineering tissue has a normal cartilaginous cell type. The constructed bracket-free engineering cartilaginous tissue does not relate to the composite problem of the cells and an exogenous bracket, is absent from the biocompatibility problem between the cells and the bracket, is safe to human body, has even cell planting density and simple clinical application operation and can be applied to the treatment or the repair of cartilage loss.
Owner:GENERAL HOSPITAL OF PLA
Immunohistochemical assay for detecting expression of programmed death ligand 1 (pd-l1) in tumor tissue
The present disclosure provides processes for describing and quantifying the expression of human programmed death ligand-1 (PD-L1) in tumor tissue sections as detected by immunohistochemical assay using an antibody that specifically binds to PD-L1. The results generated using these processes have a variety of experimental, diagnostic and prognostic applications.
Owner:MERCK SHARP & DOHME LLC
Method for researching effects of Dickkopf-1 and cell apoptosis in steroid-induced avascular necrosis of femoral head (SANFH)
InactiveCN105043981AMaterial analysis by optical meansMaterial analysis by transmitting radiationStaining techniqueRight femoral head
The invention discloses a method for researching the effects of Dickkopf-1 and cell apoptosis in steroid-induced avascular necrosis of femoral head (SANFH). The method comprises the following steps: by taking the femoral head sample of SANFH taken through total hip replacement as an experiment group and taking the sample after the hip replacement for fracture of neck of femur as a contrast group, cutting the femoral head sample, fixing and decalcifying the cut femoral head sample to prepare slices, observing the morphologic change of osteocytes by an electronic microscope, observing the pathological change of the osteocytes by a light microscope, counting the vacant bone lacuna, detecting the expression of Dickkopf-1 by an immunohistochemical staining technique, thus exploring the effects of Dickkopf-1 and cell apoptosis in SANFH. The method can be used for providing assistance for early diagnosis and curing of SANFH and also providing a theoretical basis for clinical prevention and curing of SANFH in future.
Owner:刘万林
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com