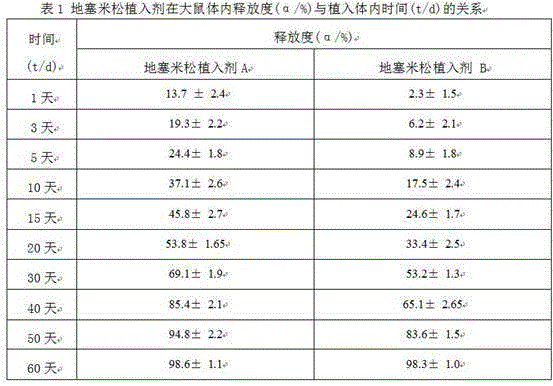
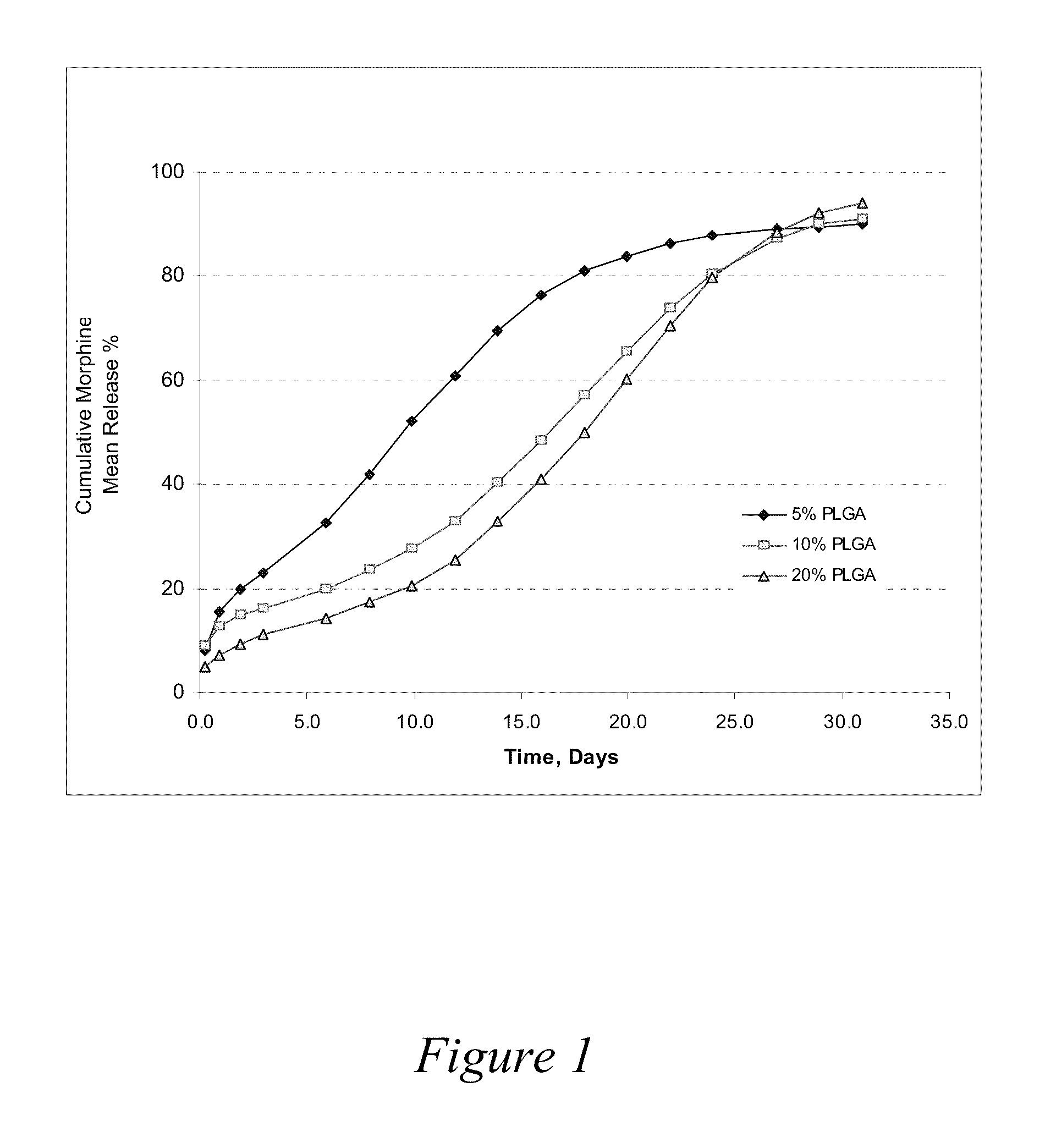
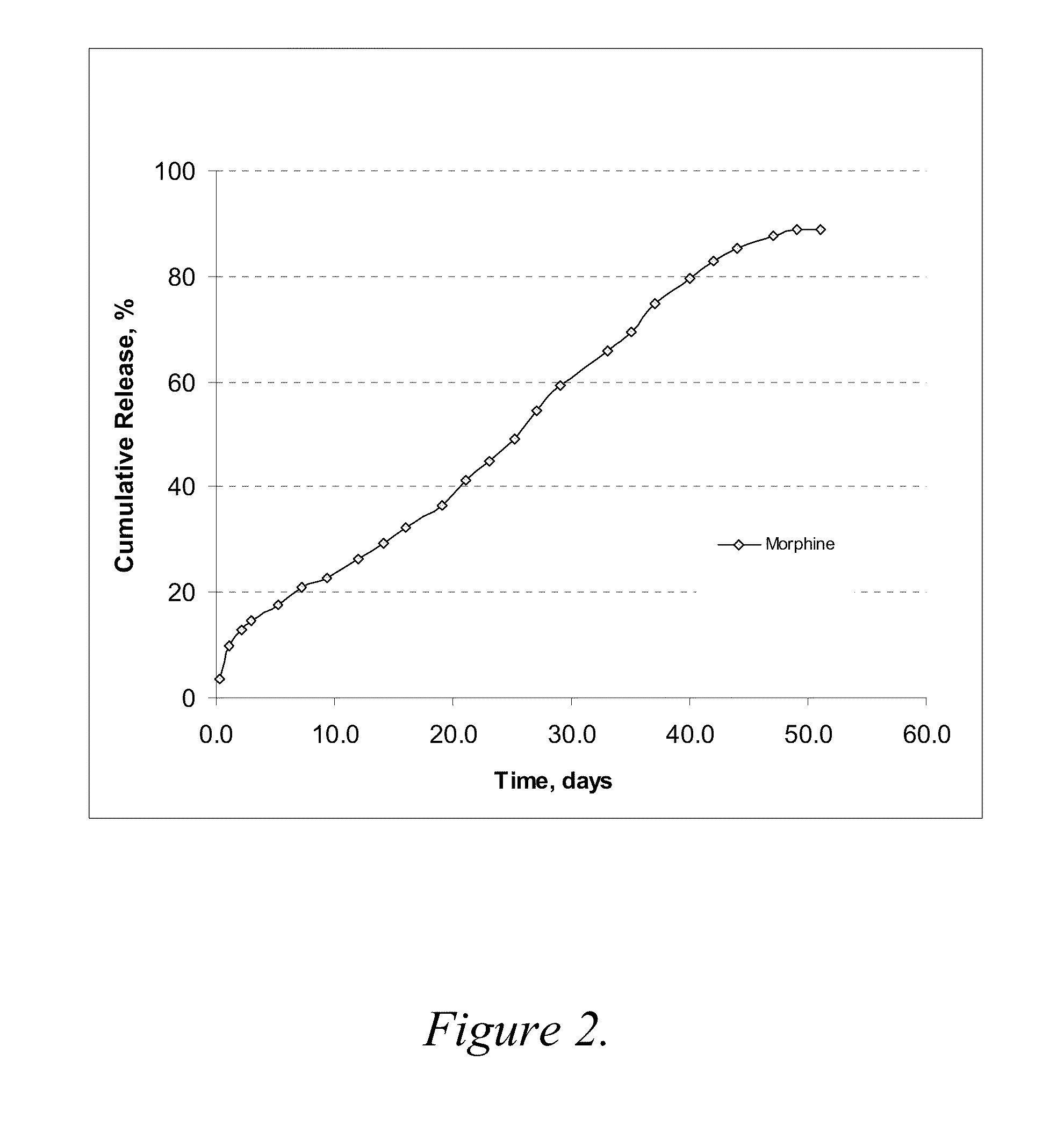
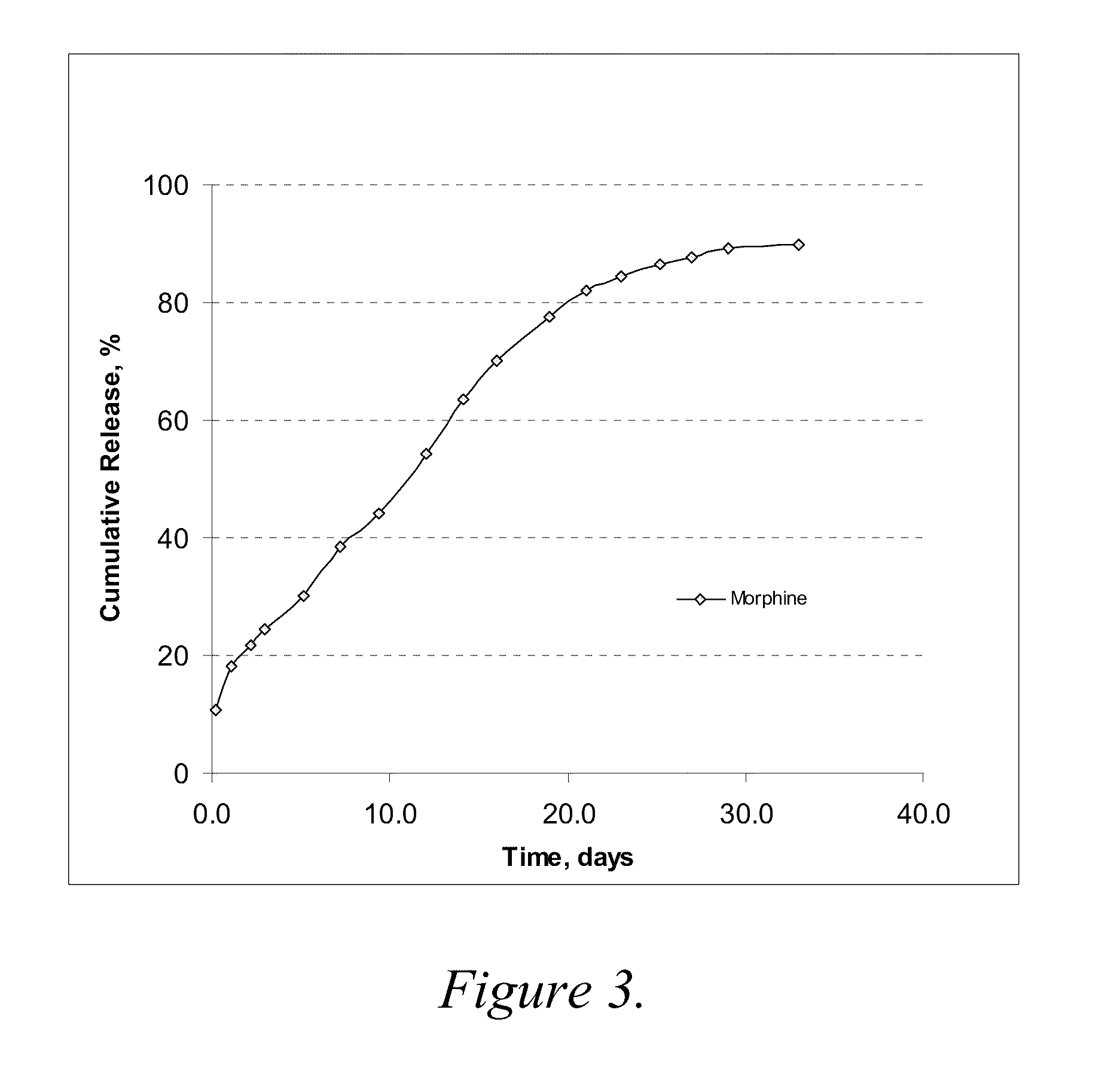
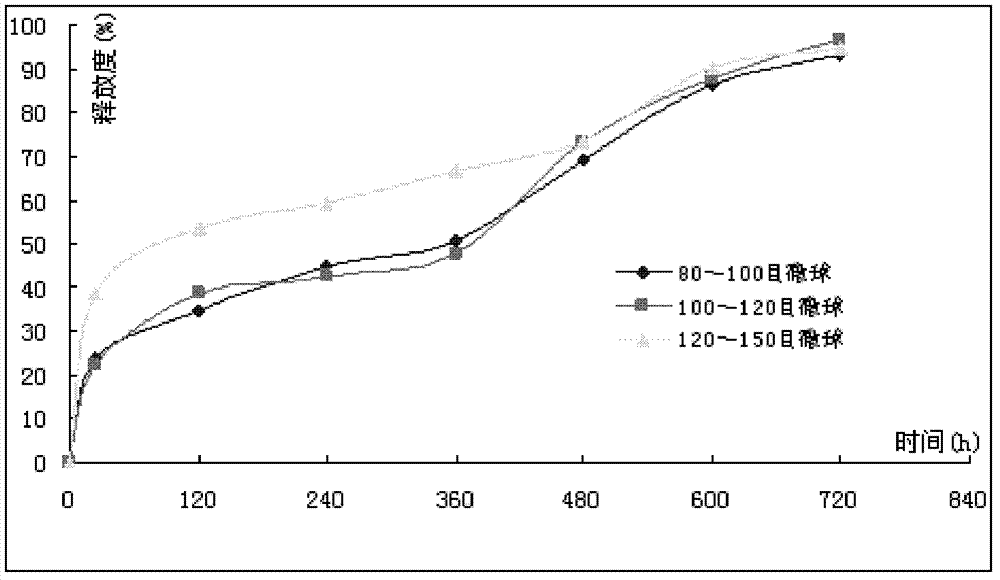
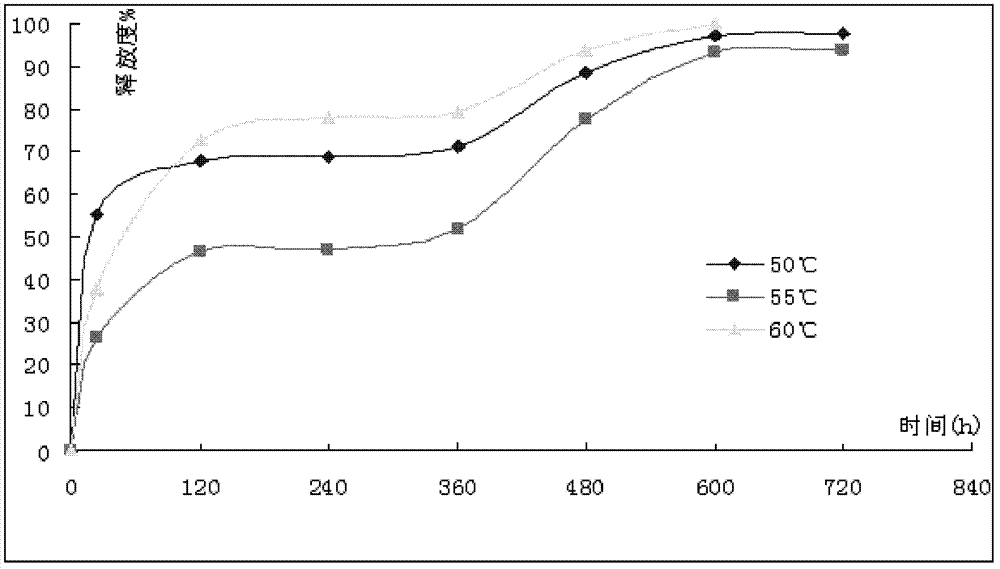
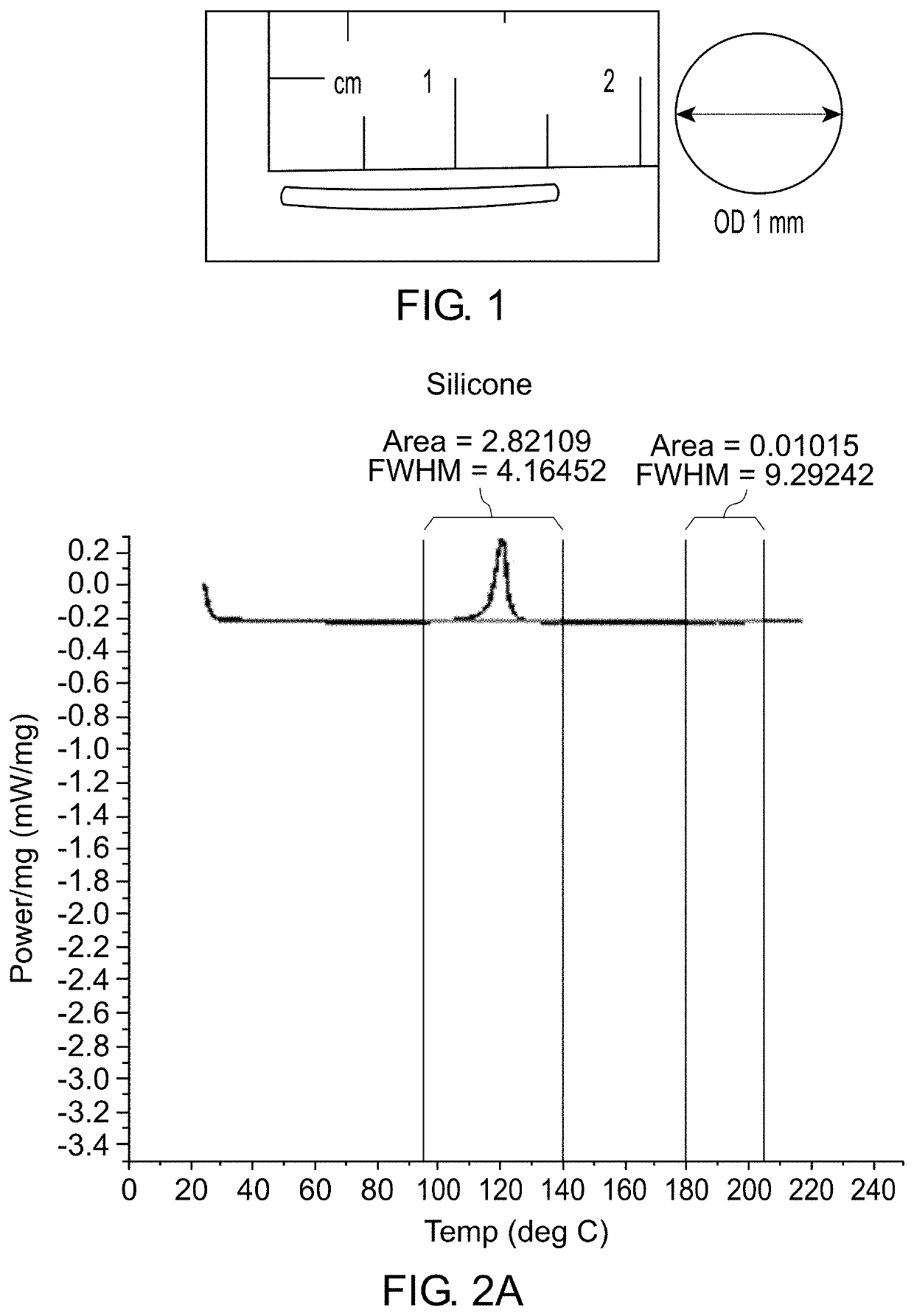
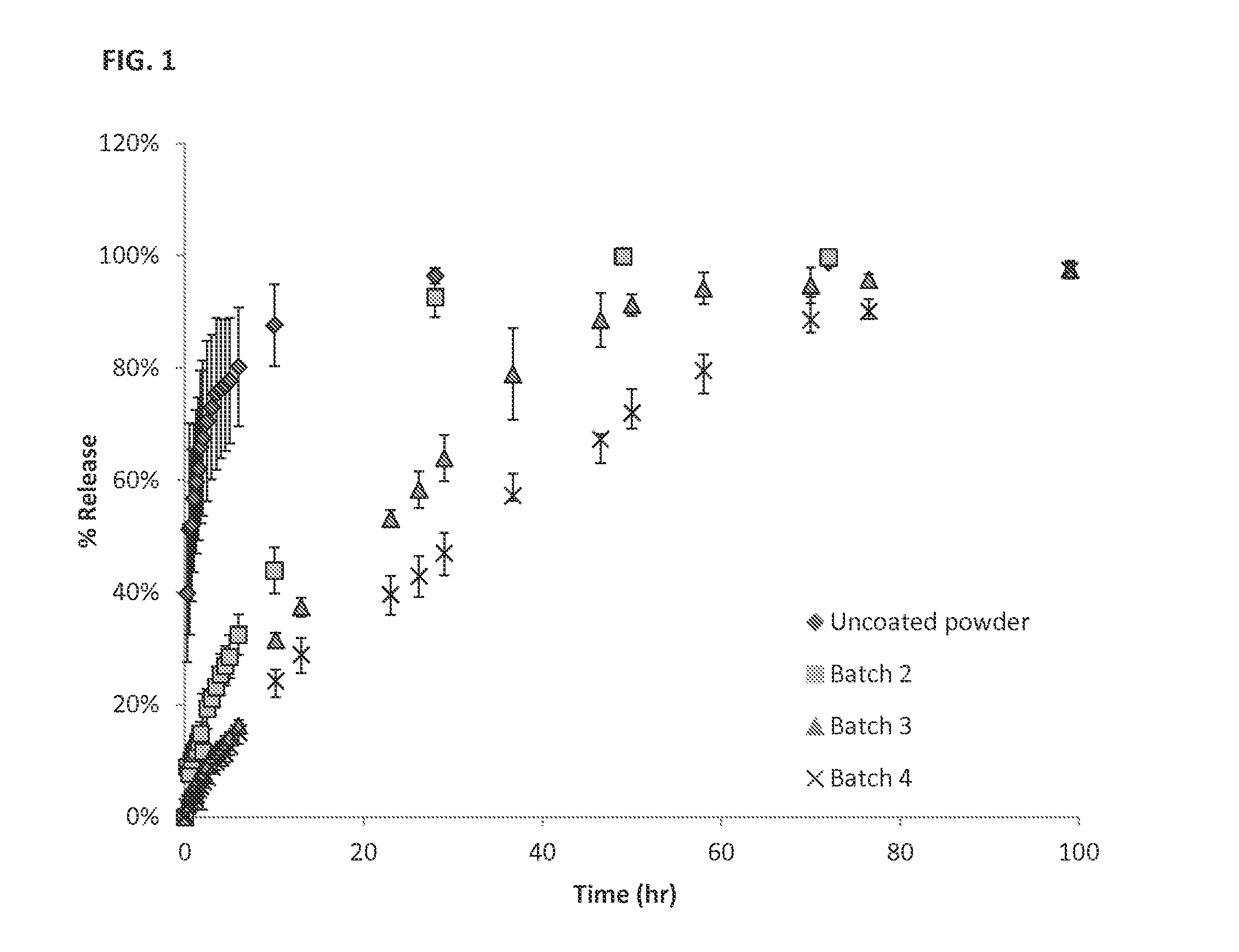
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
39 results about "Drug Implants" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
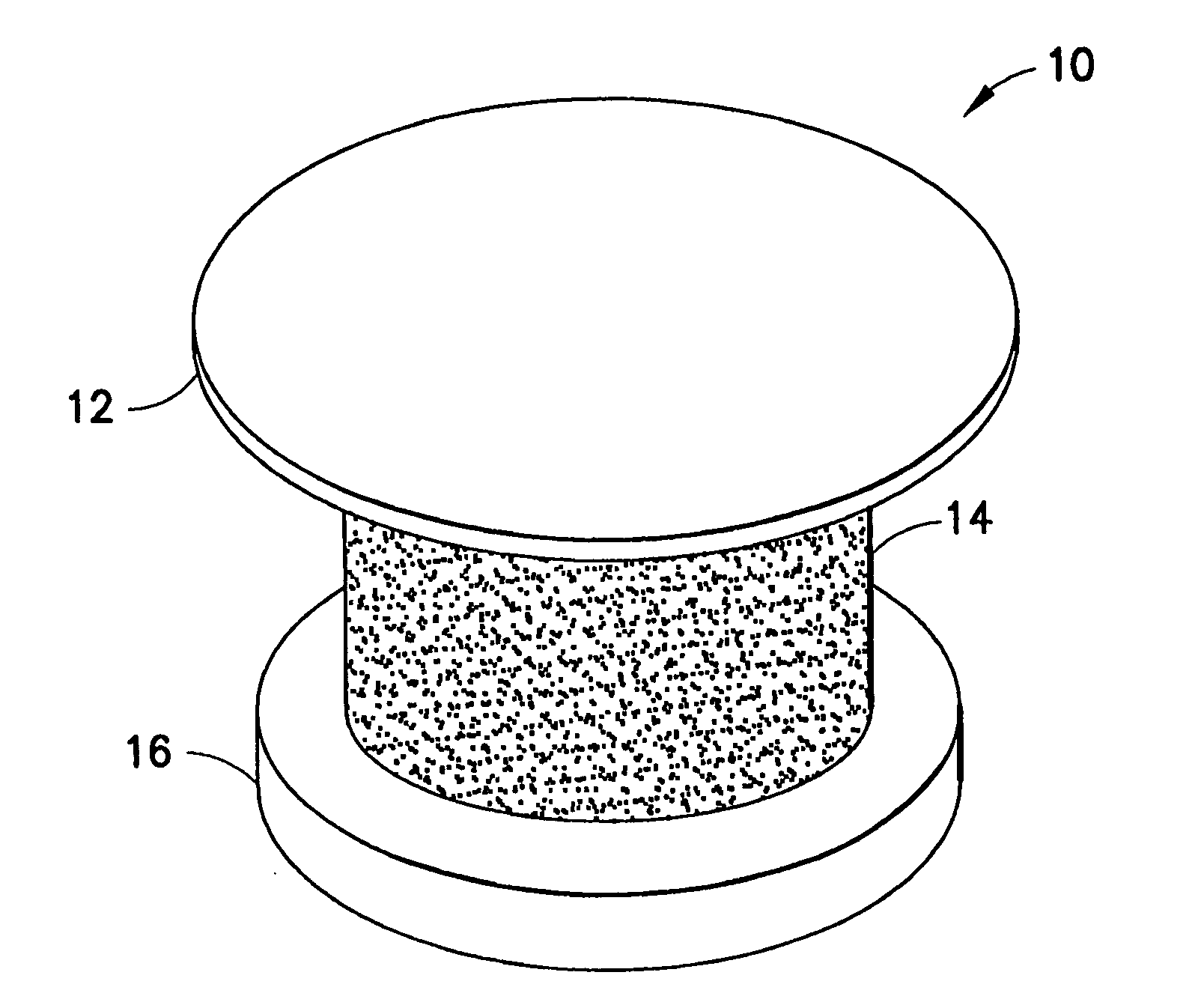
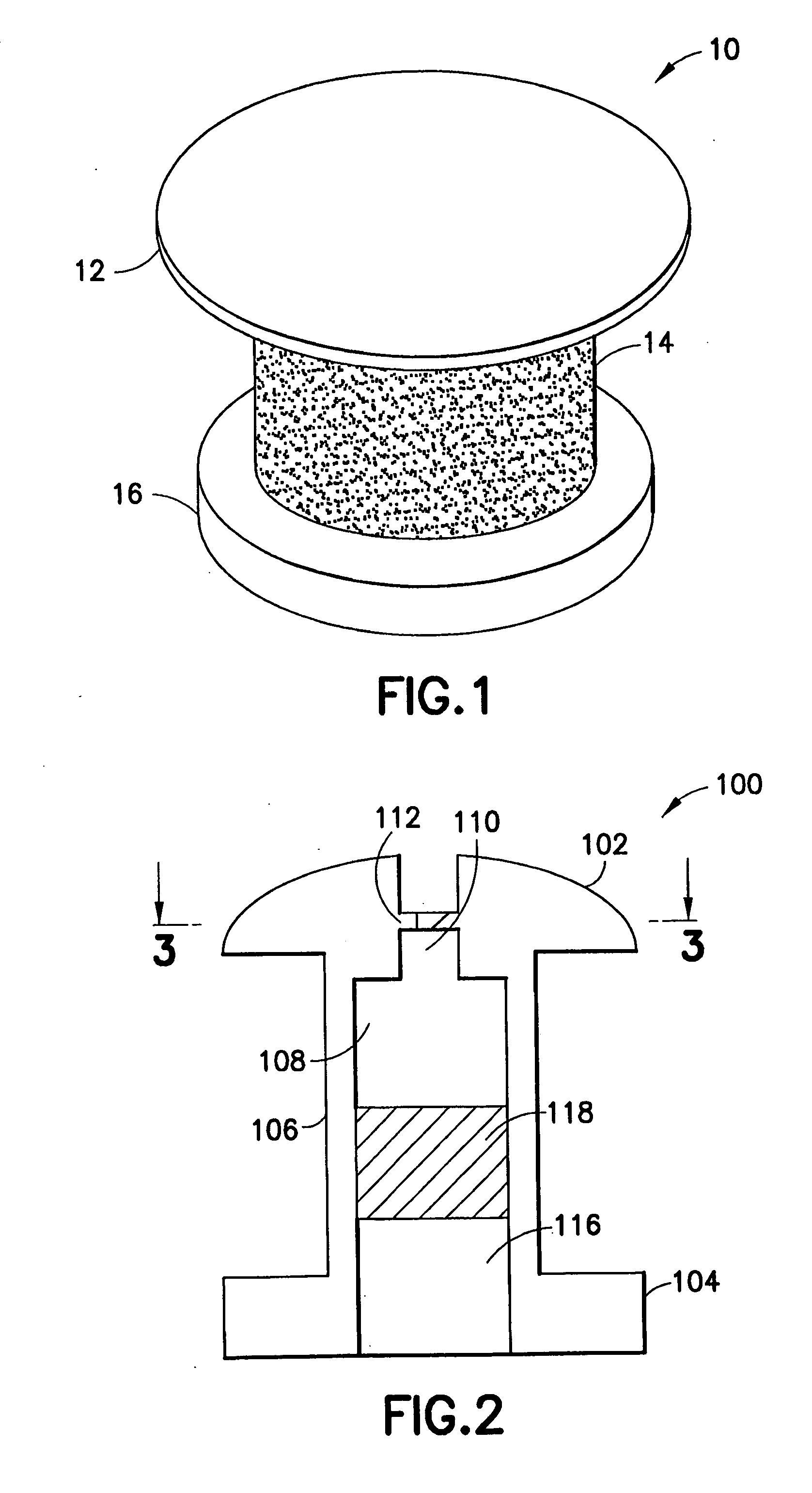
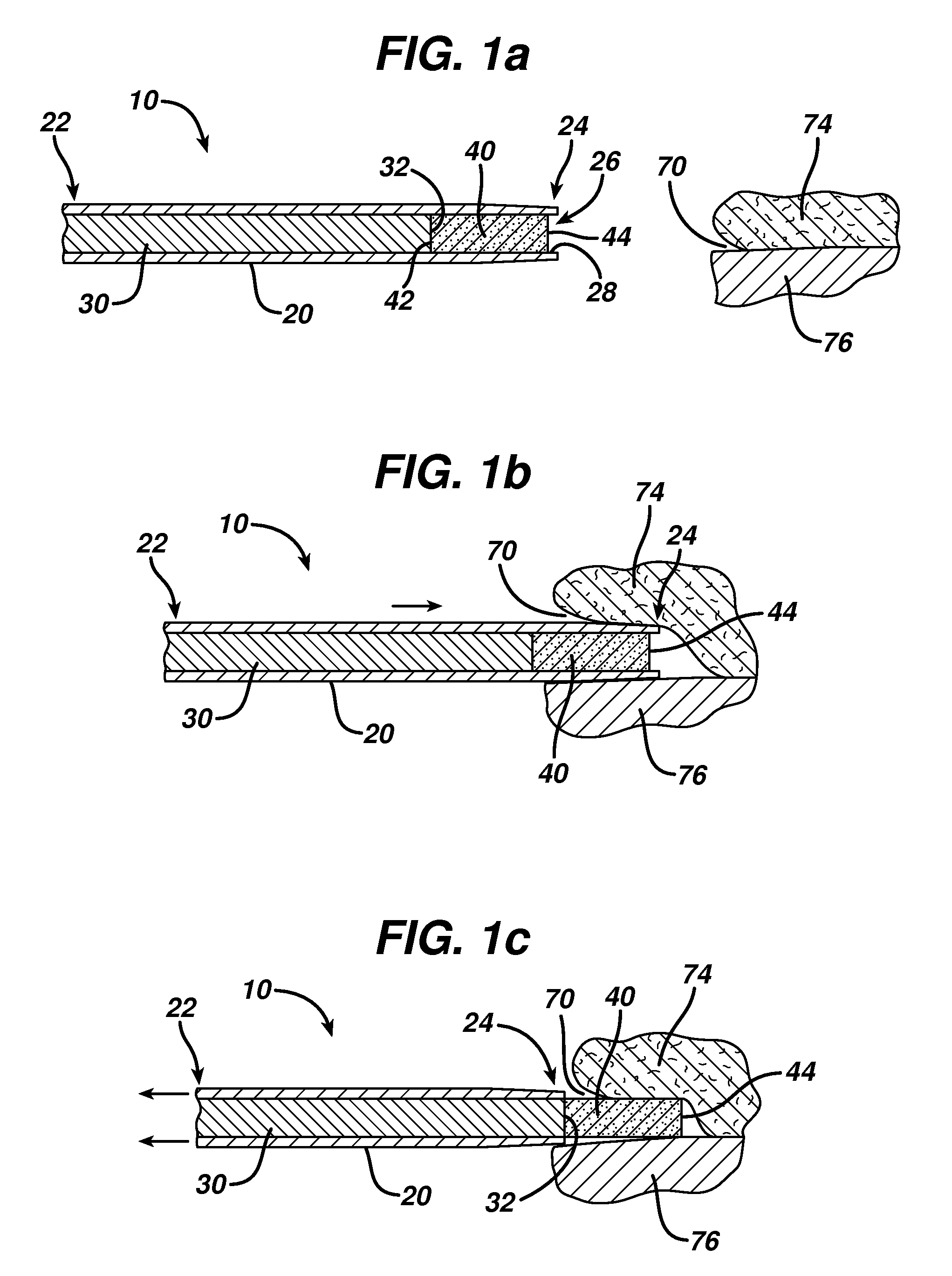
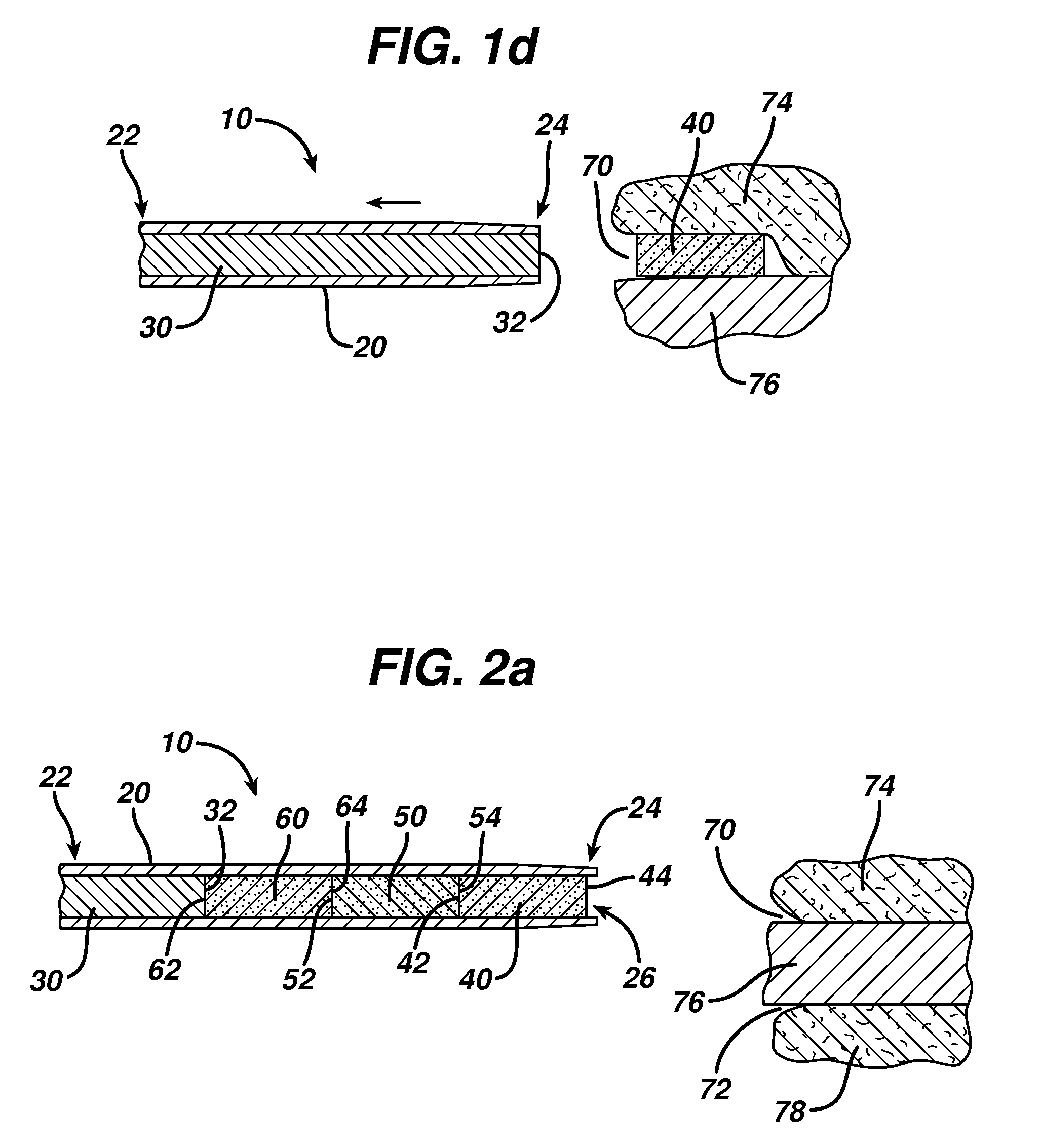
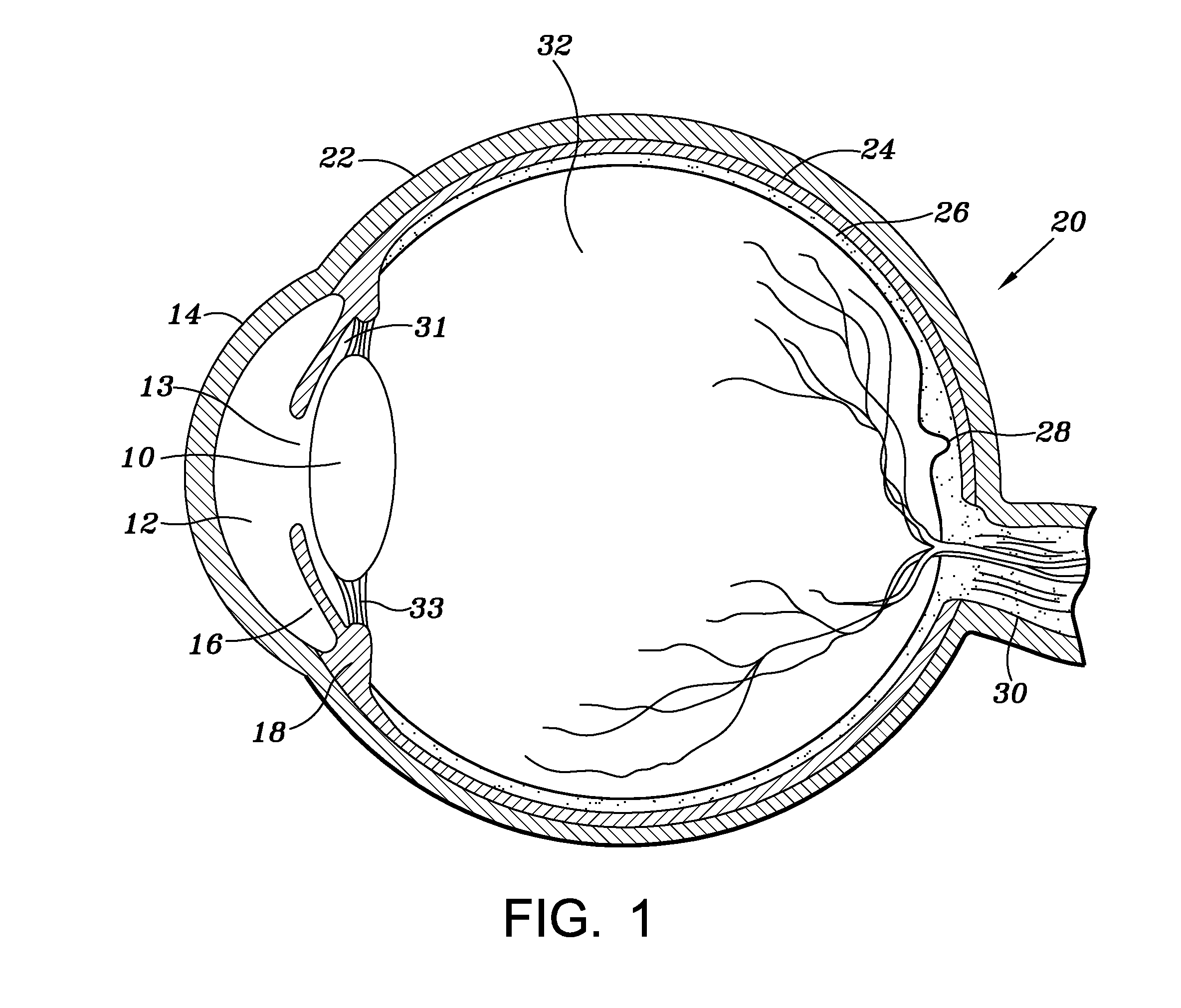
Ocular implant and methods for making and using same
InactiveUS20050119737A1MinimizeLower eye pressureEye implantsEye surgeryAqueous humorImplanted device
An ocular implant device that is insertable into either the anterior or posterior chamber of the eye to drain aqueous humor and / or to introduce medications. The implant can include a substantially cylindrical body with a channel member that regulates the flow rate of aqueous humor from the anterior chamber or introduces medications into the posterior chamber, and simultaneously minimizes the ingress of microorganisms into the eye.
Owner:BECTON DICKINSON & CO
Pharmaceutical Composition for Delivery of Receptor Tyrosine Kinase Inhibiting (RTKi) Compounds to the Eye
InactiveUS20100227904A1Bioavailability of drugLow toxicityOrganic active ingredientsBiocideActive agentPolyethylene glycol
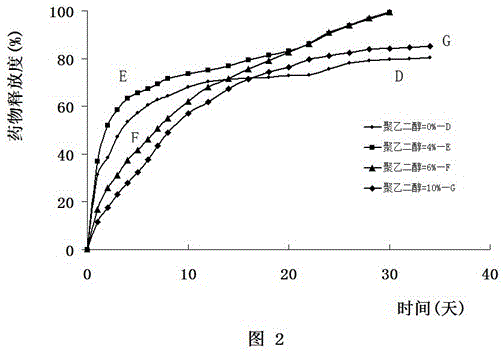
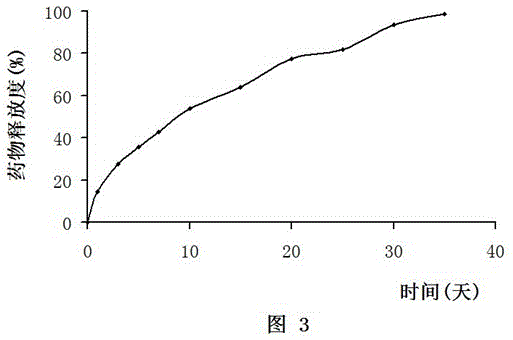
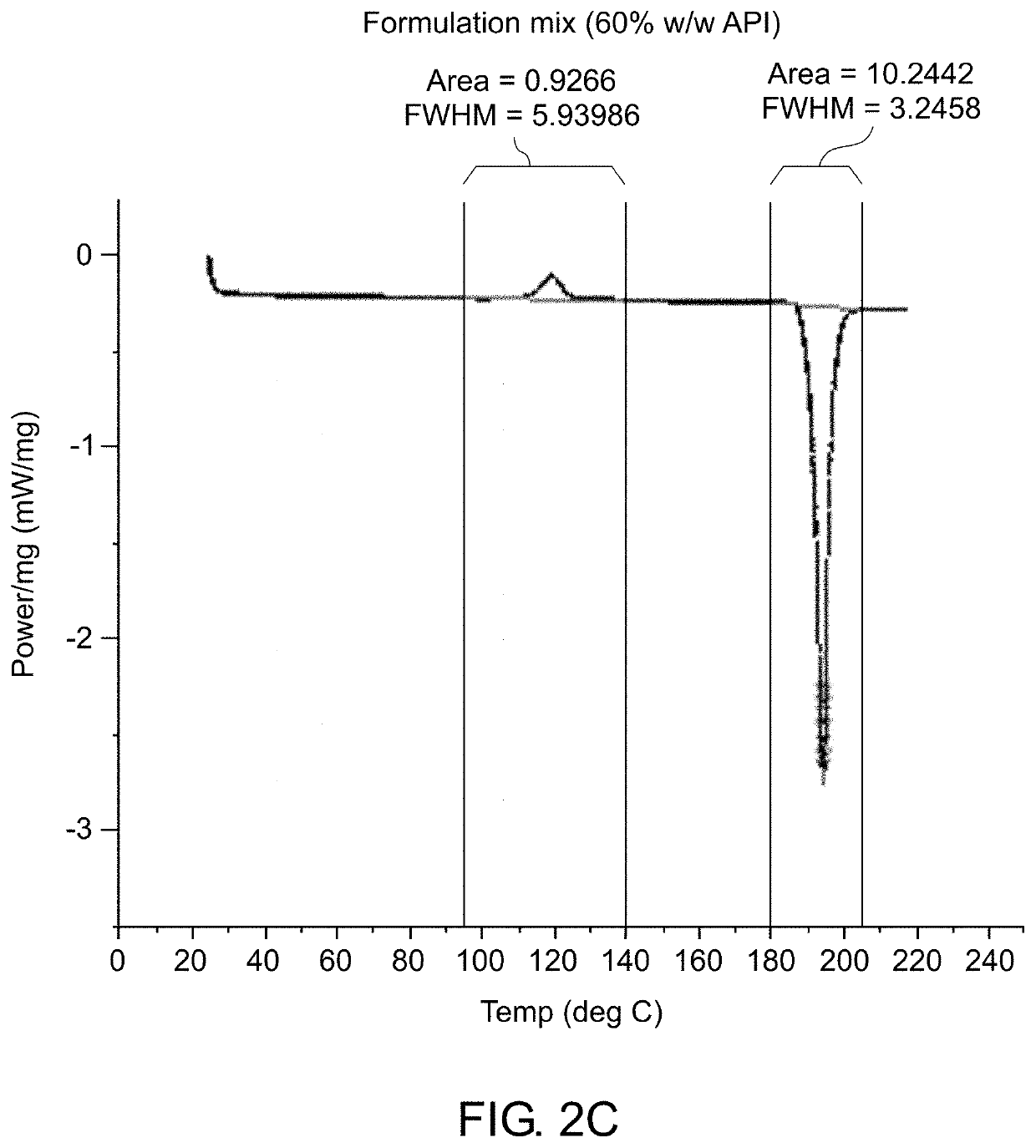
The present invention relates to development of efficacious pharmaceutical implant compositions comprising an active agent in a therapeutically effective amount and a polyethylene glycol having a molecular weight of at least 2000.
Owner:ALCON RES LTD
Resorbable Polyetheresters and Medicinal Implants Made Therefrom
InactiveUS20070014848A1Reduce contentSurgeryPharmaceutical delivery mechanismPolyesterAbsorption kinetics
The invention relates to the use of absorbable block copolymers with polyether and polyester units for preparing surgical implants which are suitable for the human or animal body, and the block copolymers in question. The block copolymers used according to the invention and those which are new according to the invention are characterised by high mechanical strength and rapid absorption kinetics.
Owner:EVONIK ROEHM GMBH
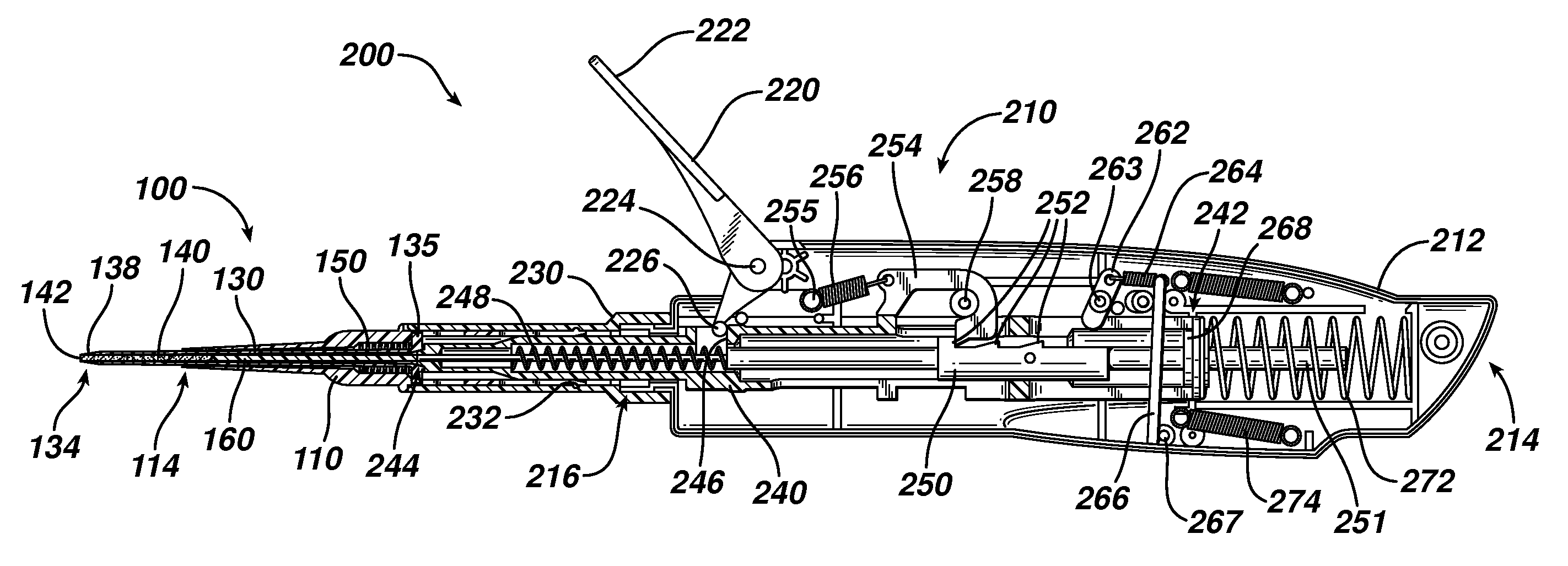
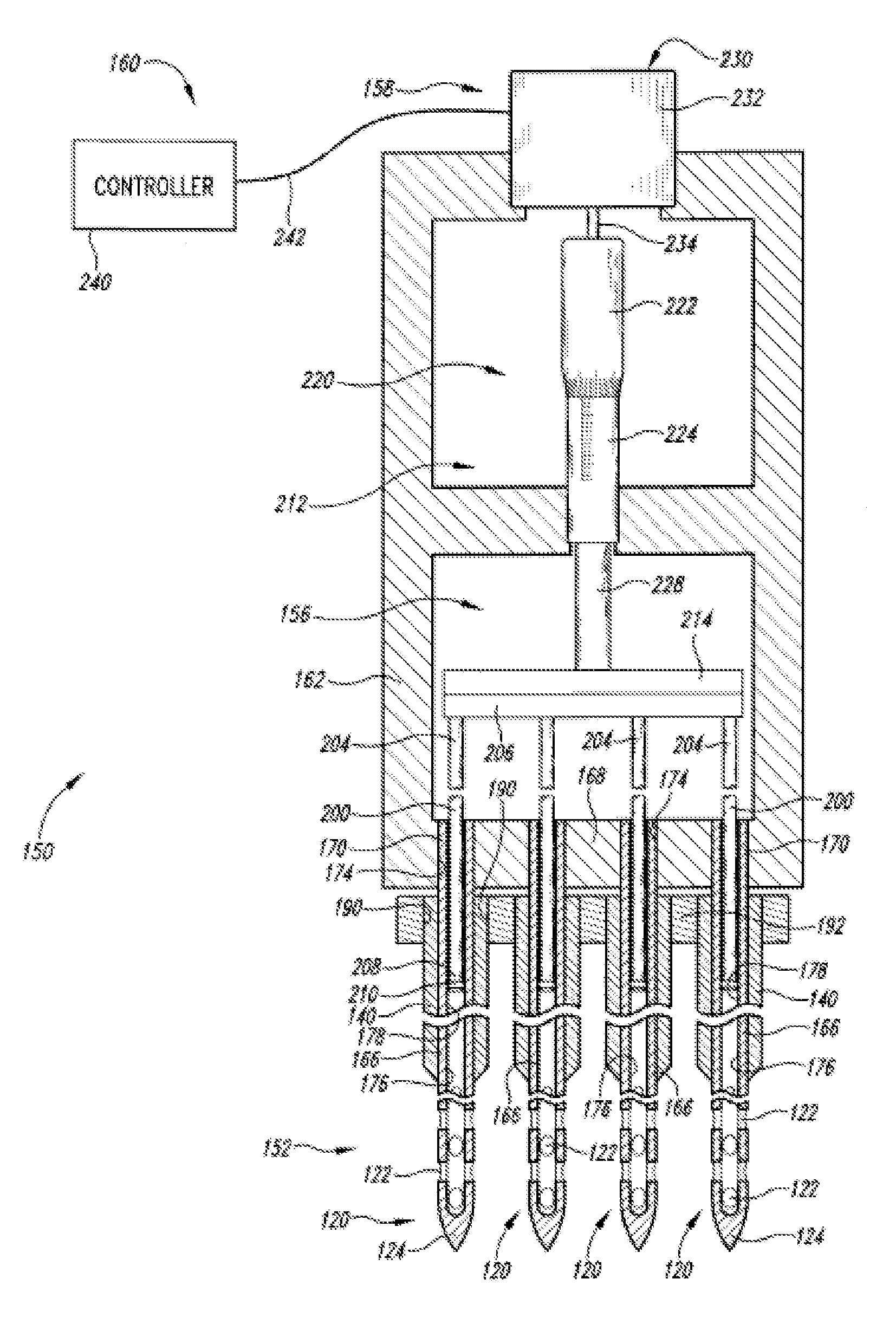
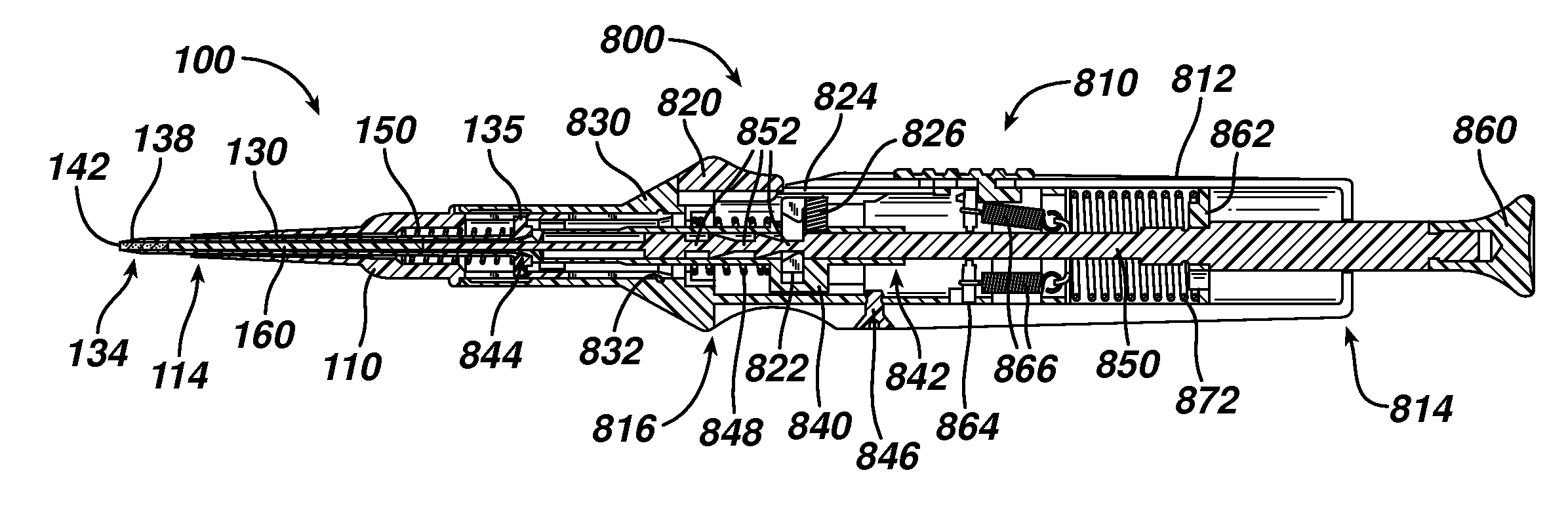
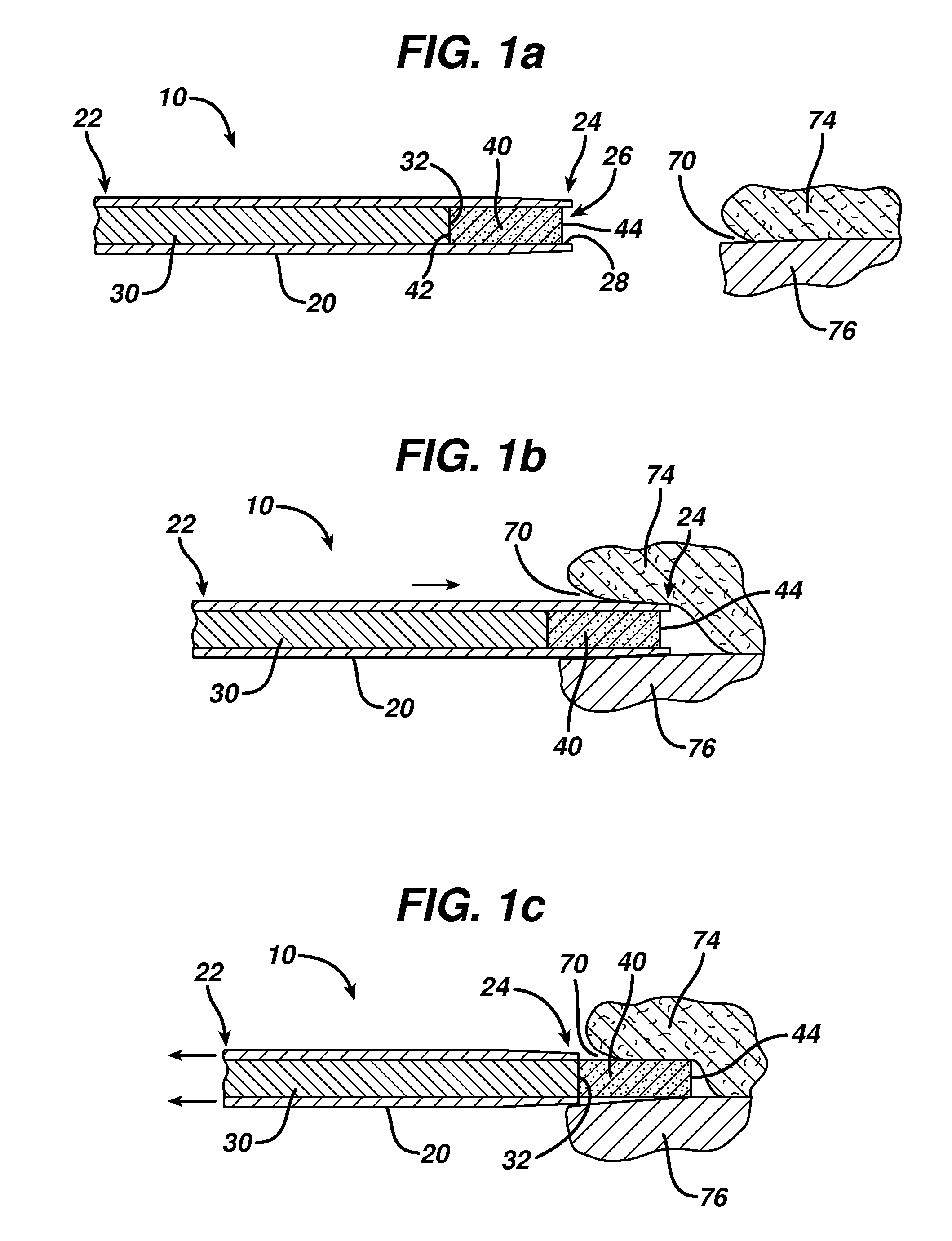
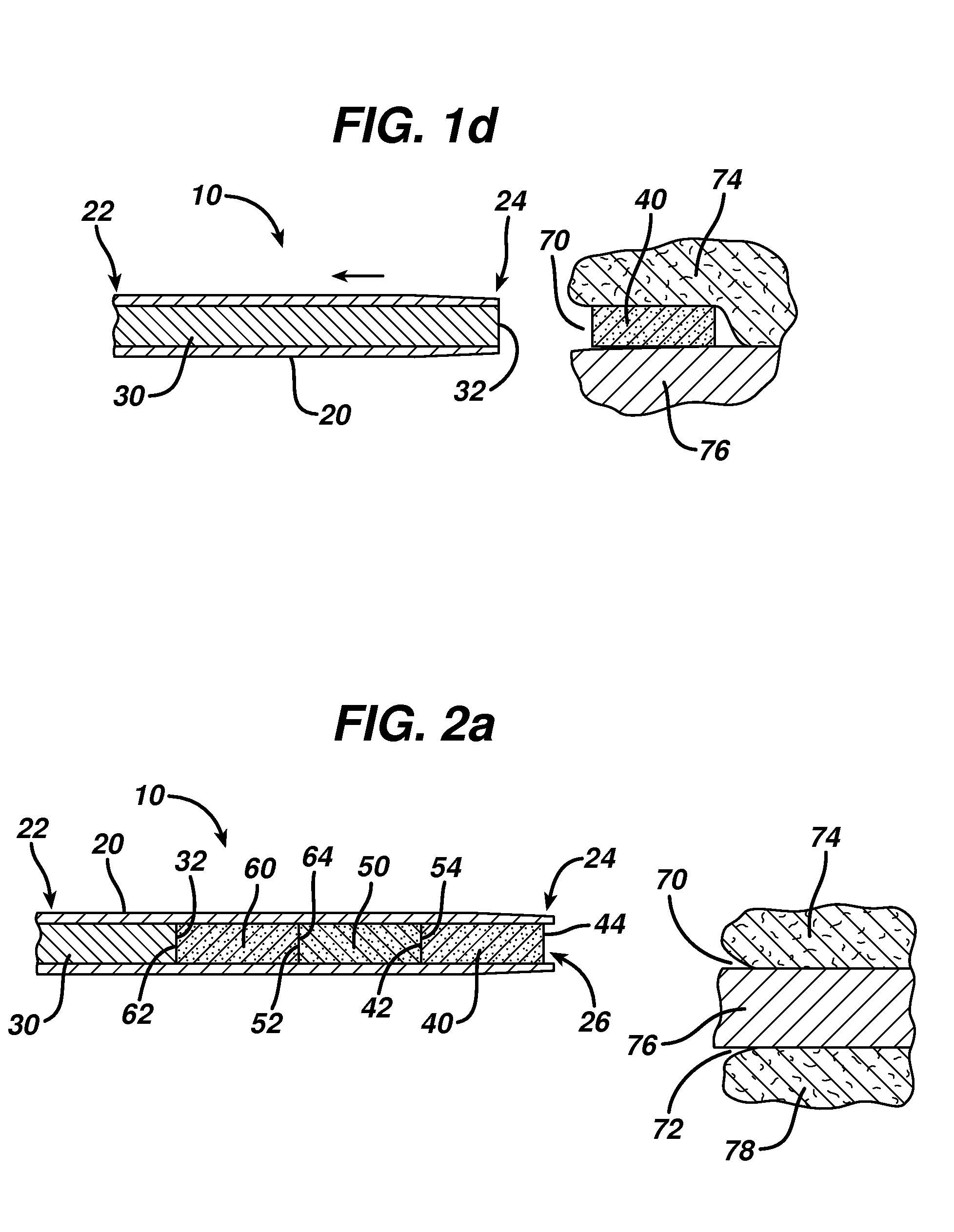
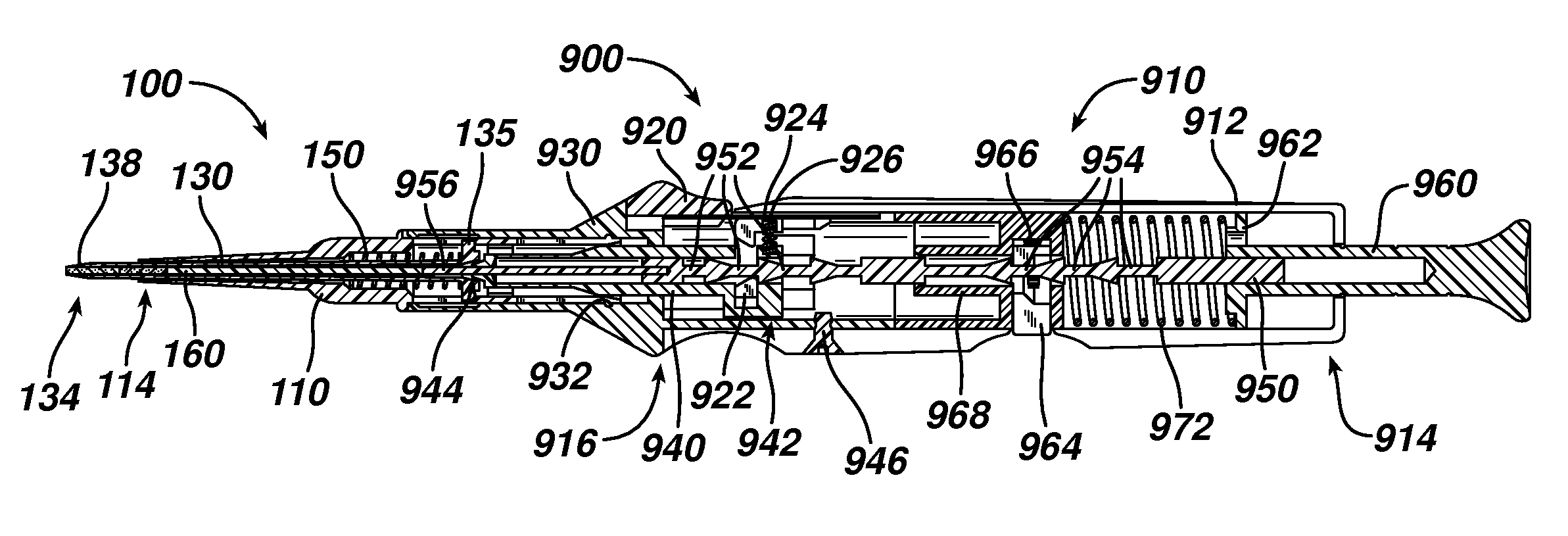
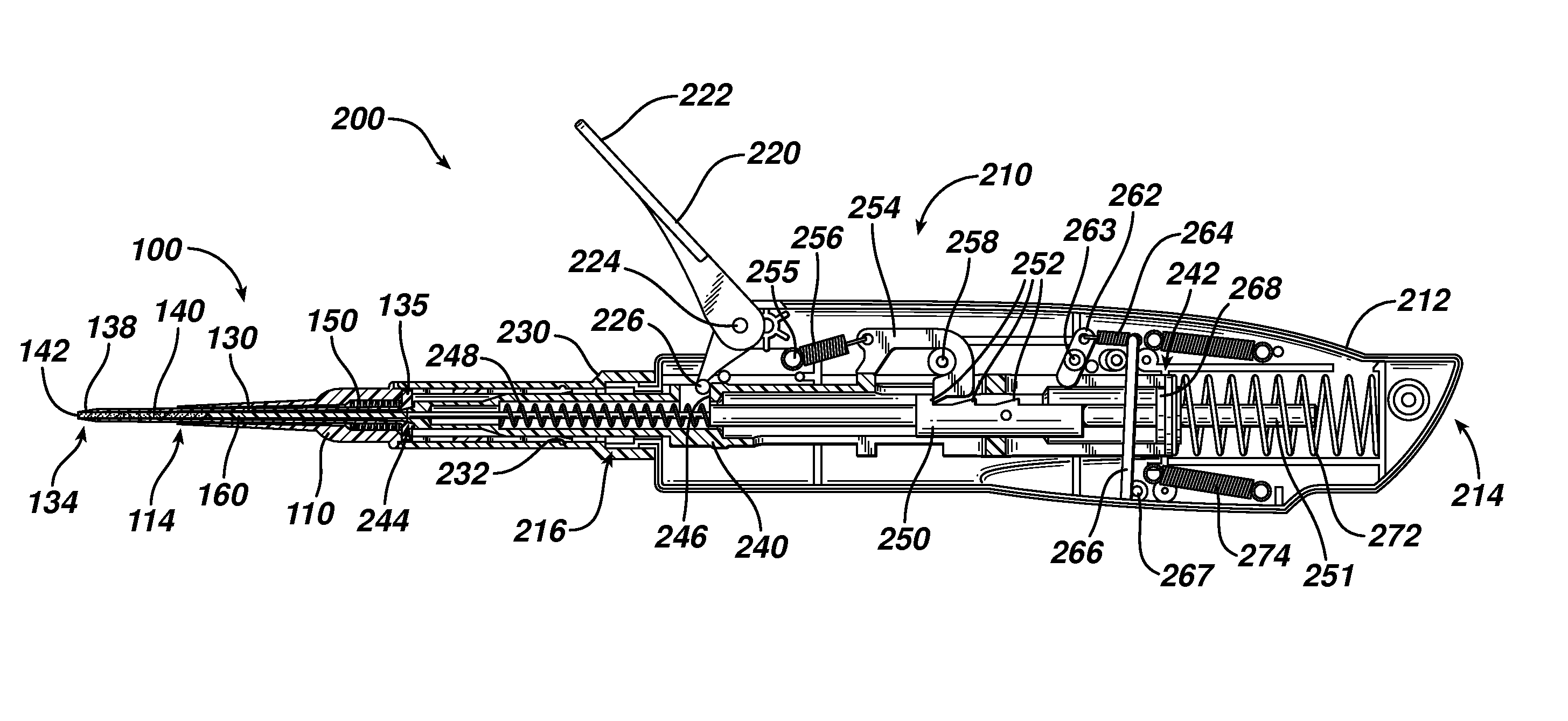
Device for delivering medicinal implants
The invention includes a device for inserting a medicament within a body cavity of a mammal, the device including a cartridge for containing the medicament therein, the cartridge including a housing, a retractable chamber disposed within the housing and having a lumen, a substantially stationary member disposed within the lumen of the retractable chamber and having a uniform cross-section sized to provide a sliding fit within the lumen to provide for retraction of the retractable chamber about the substantially stationary member upon actuation of the device, and means for retracting the retractable chamber about the substantially stationary member while maintaining the substantially stationary member in a substantially stationary position upon actuation of the device; the device further including means for activating the means for retracting the retractable chamber.
Owner:ORAPHARMA
Modulation neural pathways
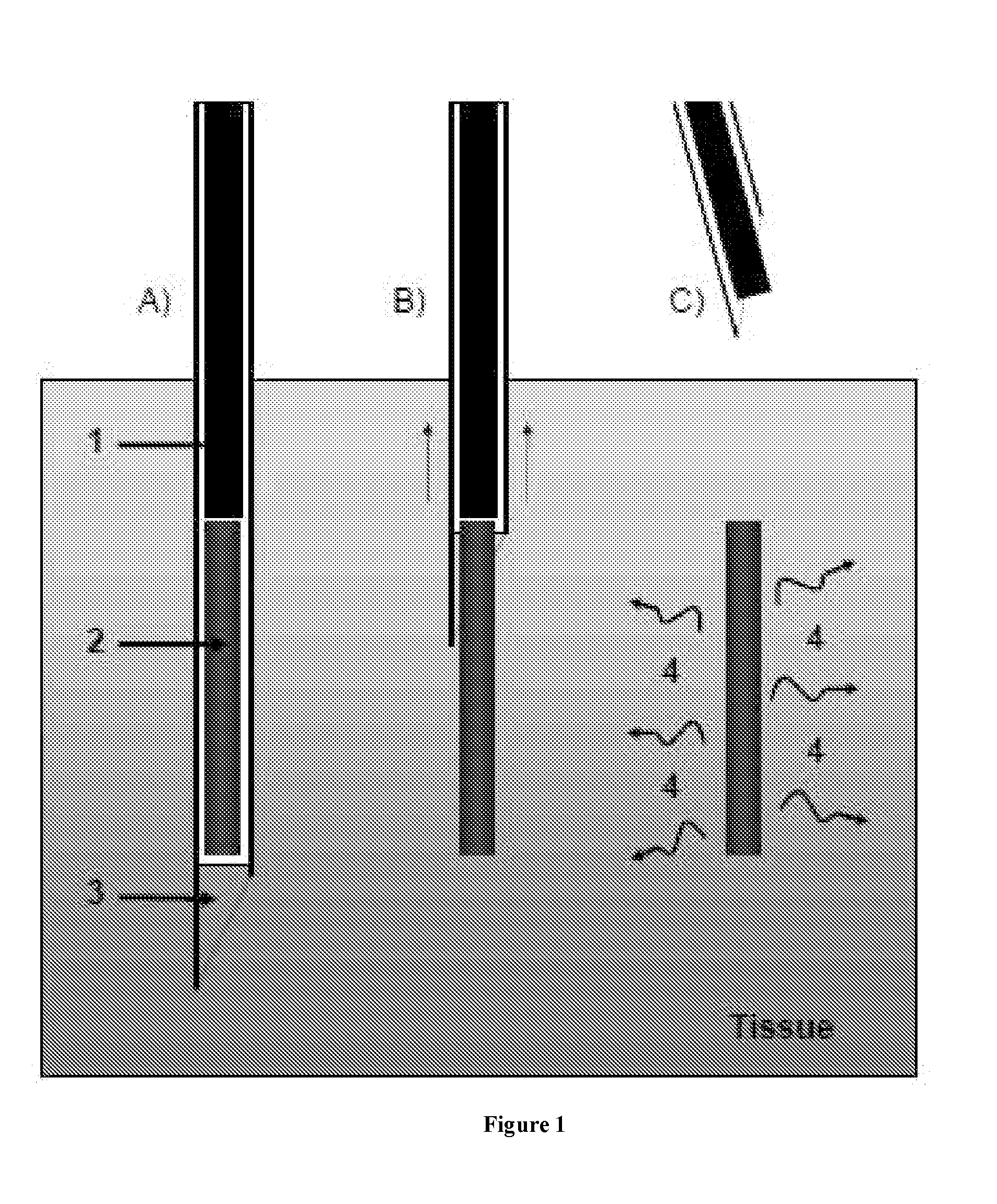
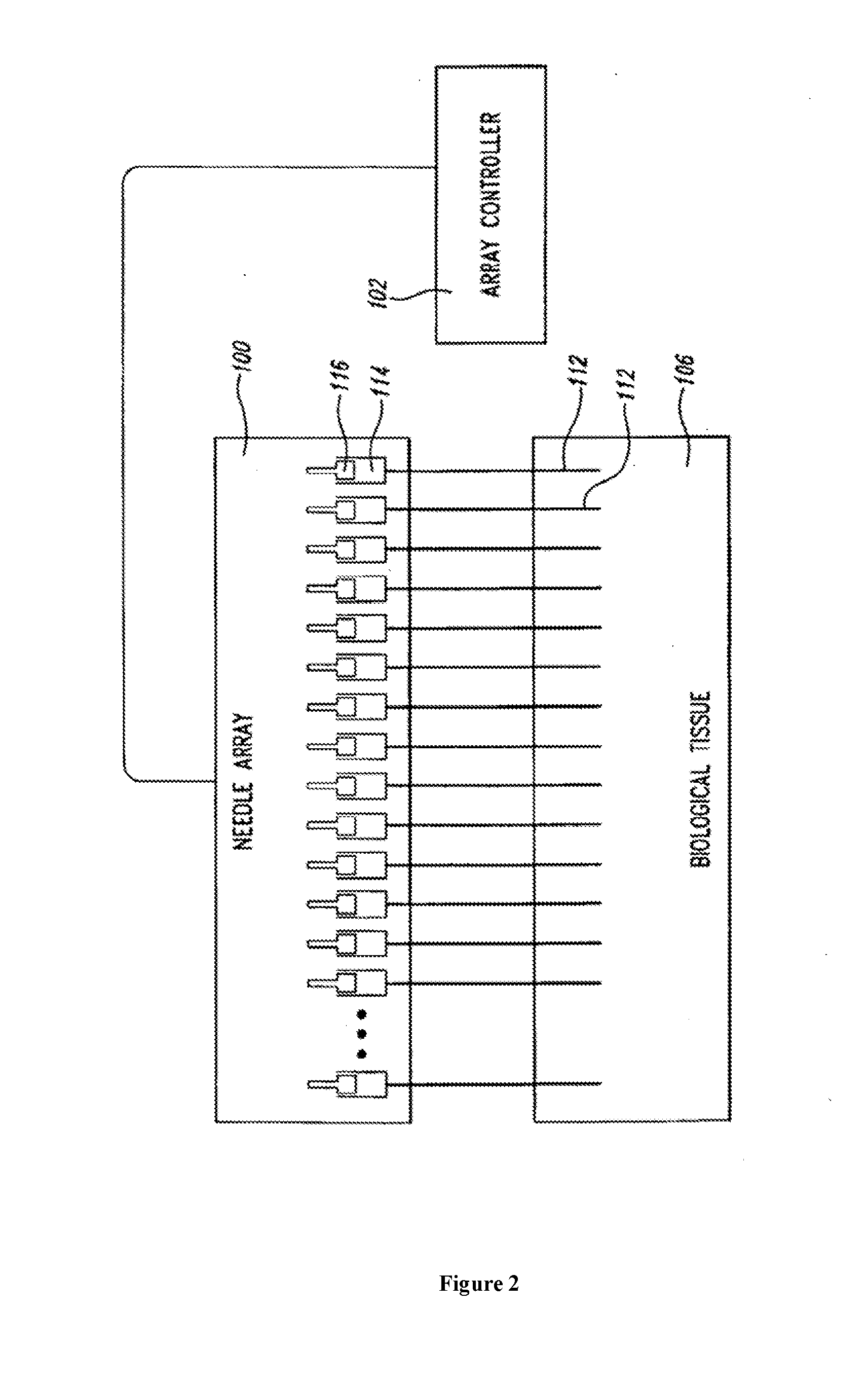
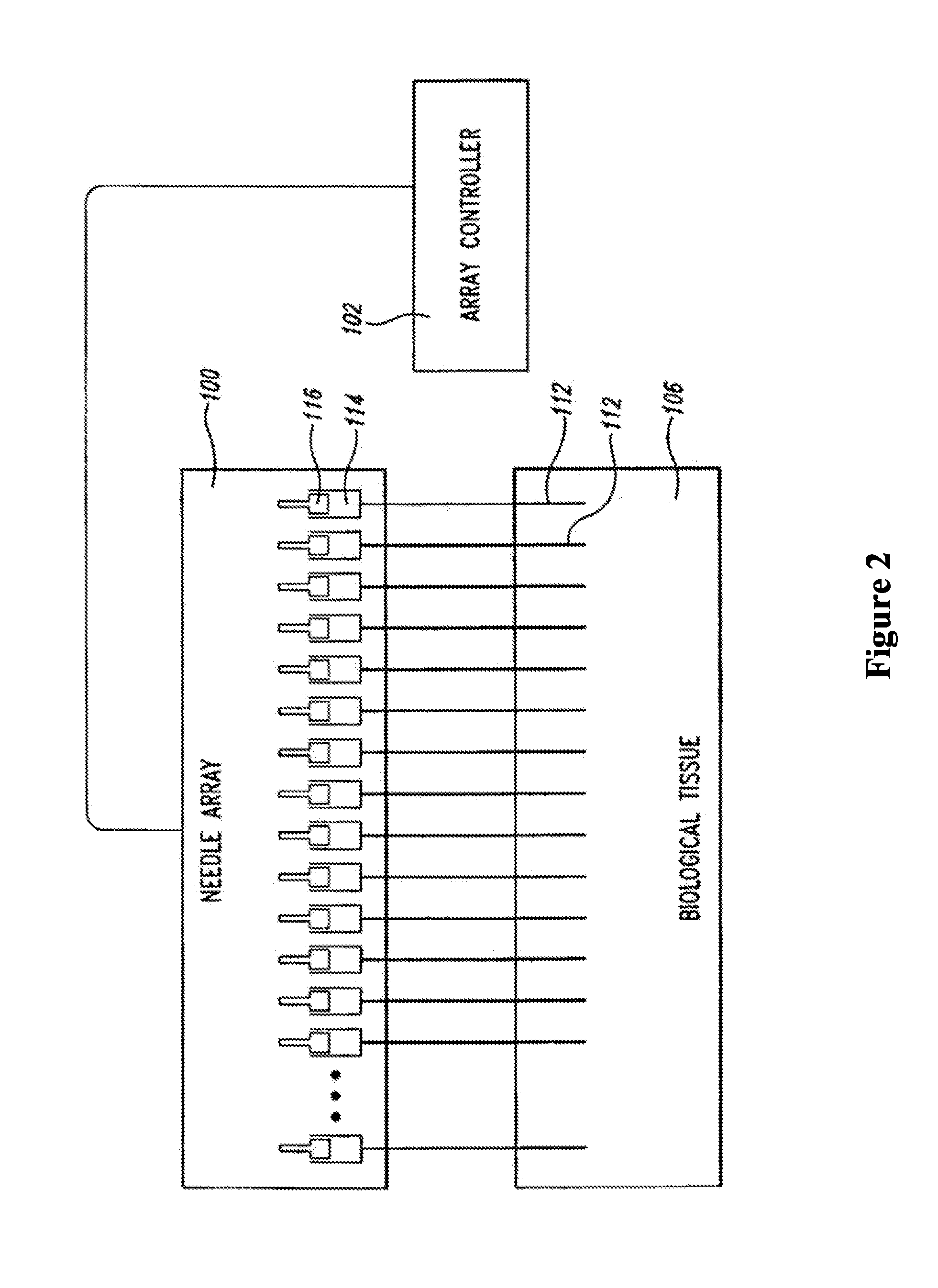
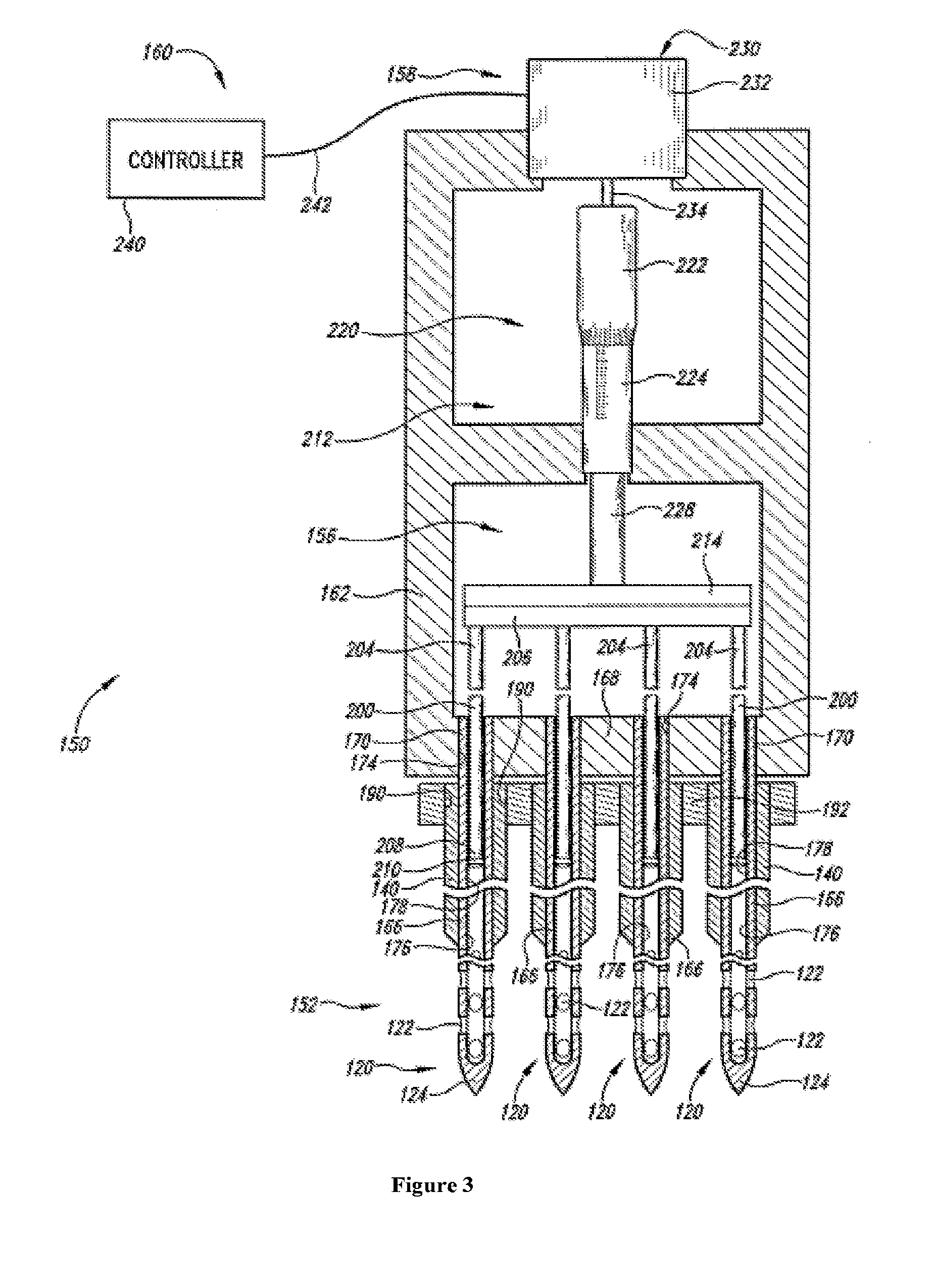
An administration device comprising an array of needles, one or more fluid agents, and at least one hydrogel is described. The device can simultaneously deliver a plurality of fluid agents along respective axes into a tissue. The use of hydrogel leads to constrained delivery of the fluid agents. The constrained delivery of an agent is also achieved by depositing a drug implant into a tissue. The effect of an agent on the tissue can be evaluated thereafter. In addition, the invention is directed to treating muscle diseases by delivering a therapeutic agent in vivo, and the use of reporter tissues for candidate drug evaluation, detecting and characterizing resistance.
Owner:PRESAGE BIOSCI
Implants for postoperative pain
InactiveUS20130071463A1Relieve and prevent painReduce riskOrganic active ingredientsPeptide/protein ingredientsPostoperative PainsPharmaceutical drug
Medical implants and methods useful in treating postoperative pain are described. The implants comprise one or more electrospun drug-loaded fibers, which fibers comprise a drug useful in the treatment of pain. The implants are implanted at sites of interest including joint capsules, bones, and subcutaneous spaces, and are secured with tissue flaps or fasteners.
Owner:ARSENAL MEDICAL
Device for delivering medicinal implants
The invention includes a device for inserting a medicament within a body cavity of a mammal, the device including a cartridge for containing the medicament therein, the cartridge including a housing, a retractable chamber disposed within the housing and having a lumen, a substantially stationary member disposed within the lumen of the retractable chamber and having a uniform cross-section sized to provide a sliding fit within the lumen to provide for retraction of the retractable chamber about the substantially stationary member upon actuation of the device, and means for retracting the retractable chamber about the substantially stationary member while maintaining the substantially stationary member in a substantially stationary position upon actuation of the device; the device further including means for activating the means for retracting the retractable chamber.
Owner:ORAPHARMA
Actuators for device for delivering medicinal implants
The invention includes an actuator for actuating a device for inserting a medicament within a body cavity of a mammal, the actuator including a handle case having a proximal portion for gripping and a distal portion including means for attaching the actuator to a cartridge for containing medicament, and means for creating a proximal to distal linear motion with respect to the handle case, wherein the cartridge includes a retractable chamber for containing the medicament disposed therein, a substantially stationary member and means for retracting the retractable chamber about the substantially stationary member while maintaining the substantially stationary member in a substantially stationary position.
Owner:ORAPHARMA
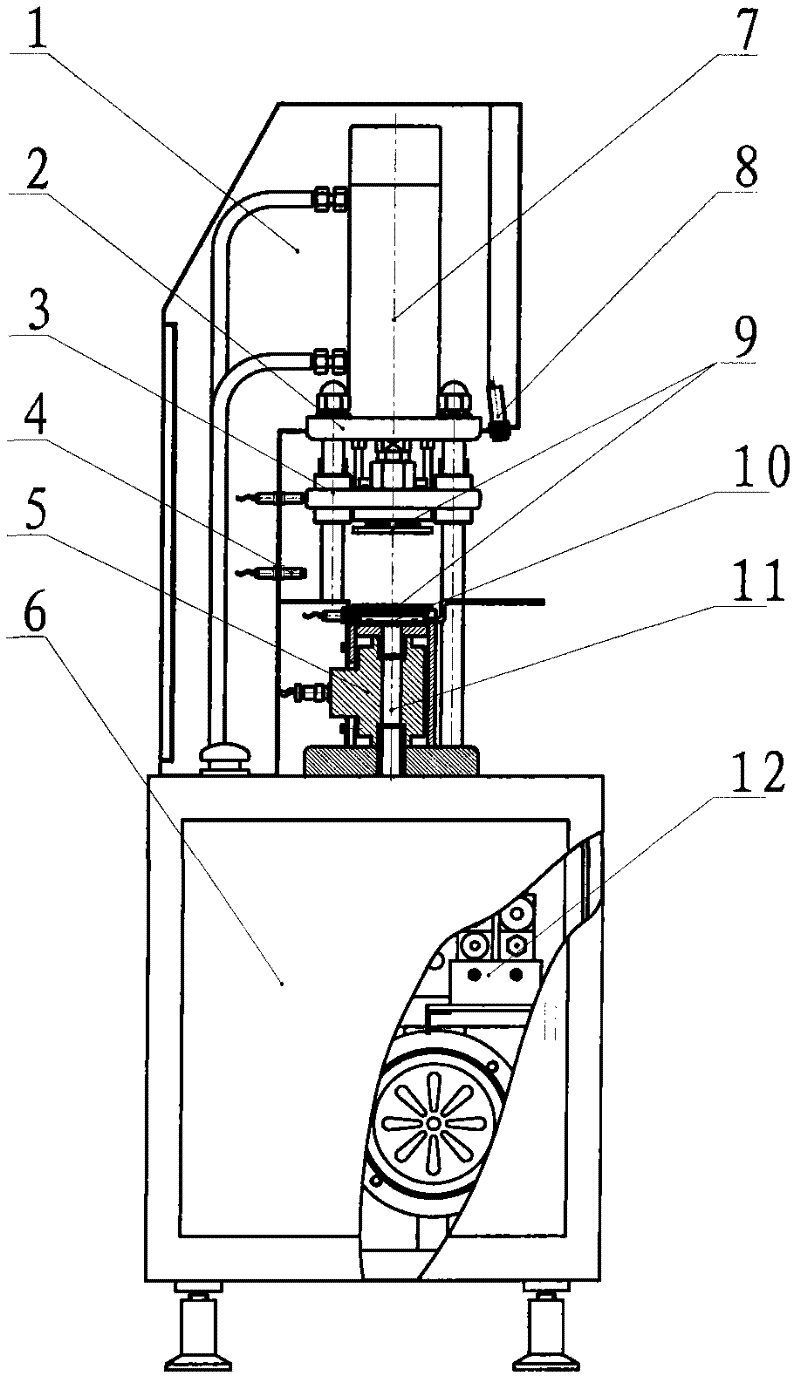
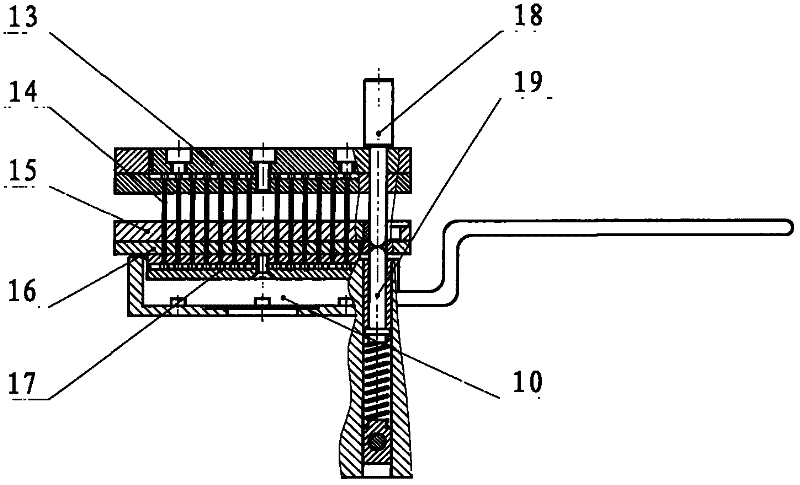
A drug pressing machine for preparing small-diameter granular implants
The invention discloses a drug pressing machine for preparing small-diameter granular implants from powdery raw materials, including a machine frame, a mold frame, a hydraulic system, a forming mold, a vacuum drug collecting device, a control system and the like. The molding die is composed of a die, a punching pin, a punching pin fixing plate, and a guiding device. The vacuum drug collection device includes components such as a vacuum pump, a vacuum channel, and a drug collection box, which can realize the automatic collection of preparation particles. The control system is composed of programmable controller, photoelectric sensor, force sensor and instrument, etc., which can precisely control the pressure, mold movement direction and speed, time and position. The medicine pressing machine uses the powdery mixture as the raw material, presses it into granular implants with a diameter of 0.5-2mm, and puts them into the medicine collection box through vacuum suction, and the whole process is automatically completed. This medicine pressing machine can adjust various process parameters, mold hole number and hole diameter according to needs, and press dozens to hundreds of granular implants at a time. The extruded particles have high hardness, stable shape and smooth appearance.
Owner:ANHUI ZHONGREN TECH
Actuators for device for delivering medicinal implants
The invention includes an actuator for actuating a device for inserting a medicament within a body cavity of a mammal, the actuator including a handle case having a proximal portion for gripping and a distal portion including means for attaching the actuator to a cartridge for containing medicament, and means for creating a proximal to distal linear motion with respect to the handle case, wherein the cartridge includes a retractable chamber for containing the medicament disposed therein, a substantially stationary member and means for retracting the retractable chamber about the substantially stationary member while maintaining the substantially stationary member in a substantially stationary position.
Owner:ORAPHARMA
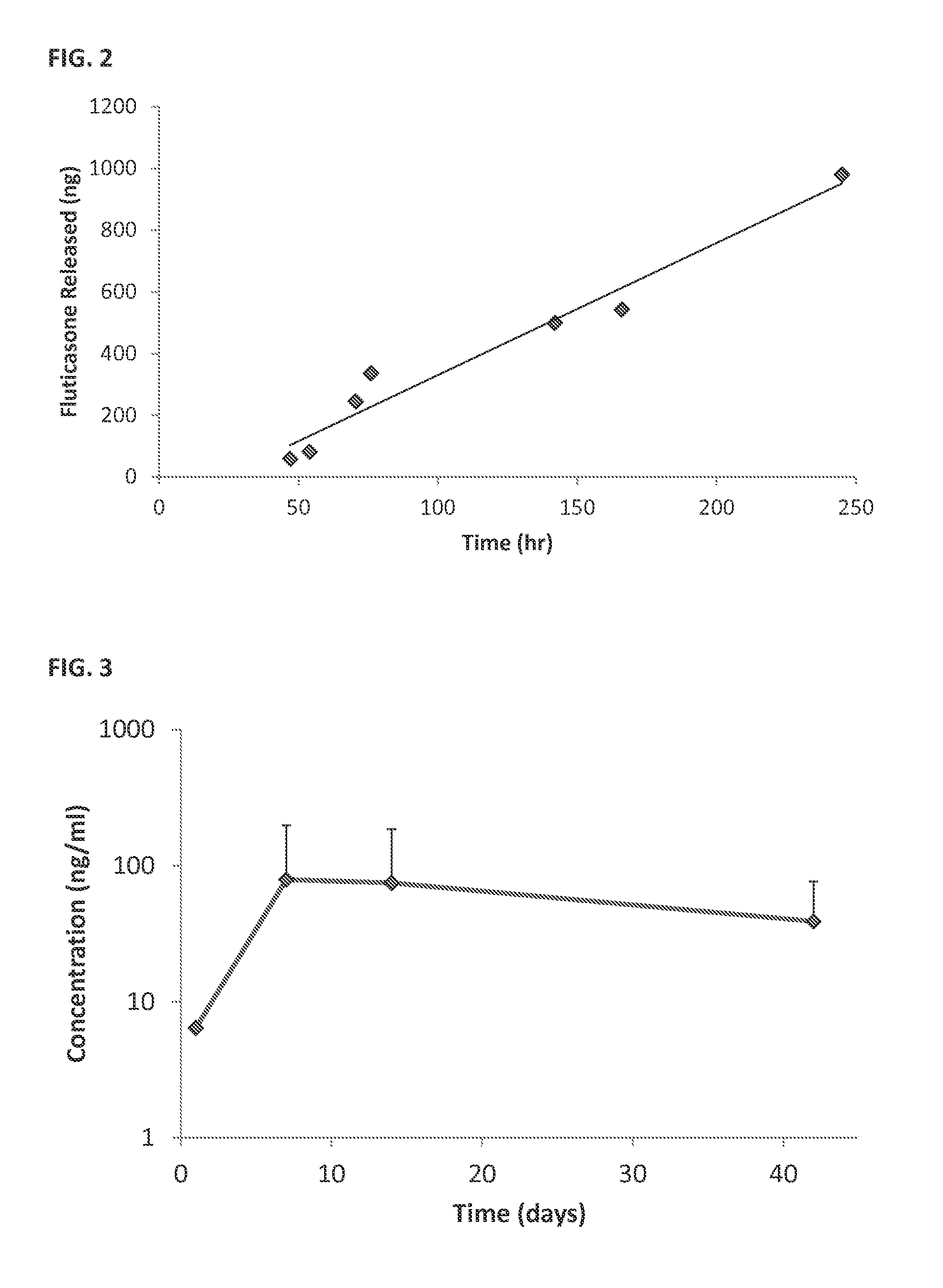
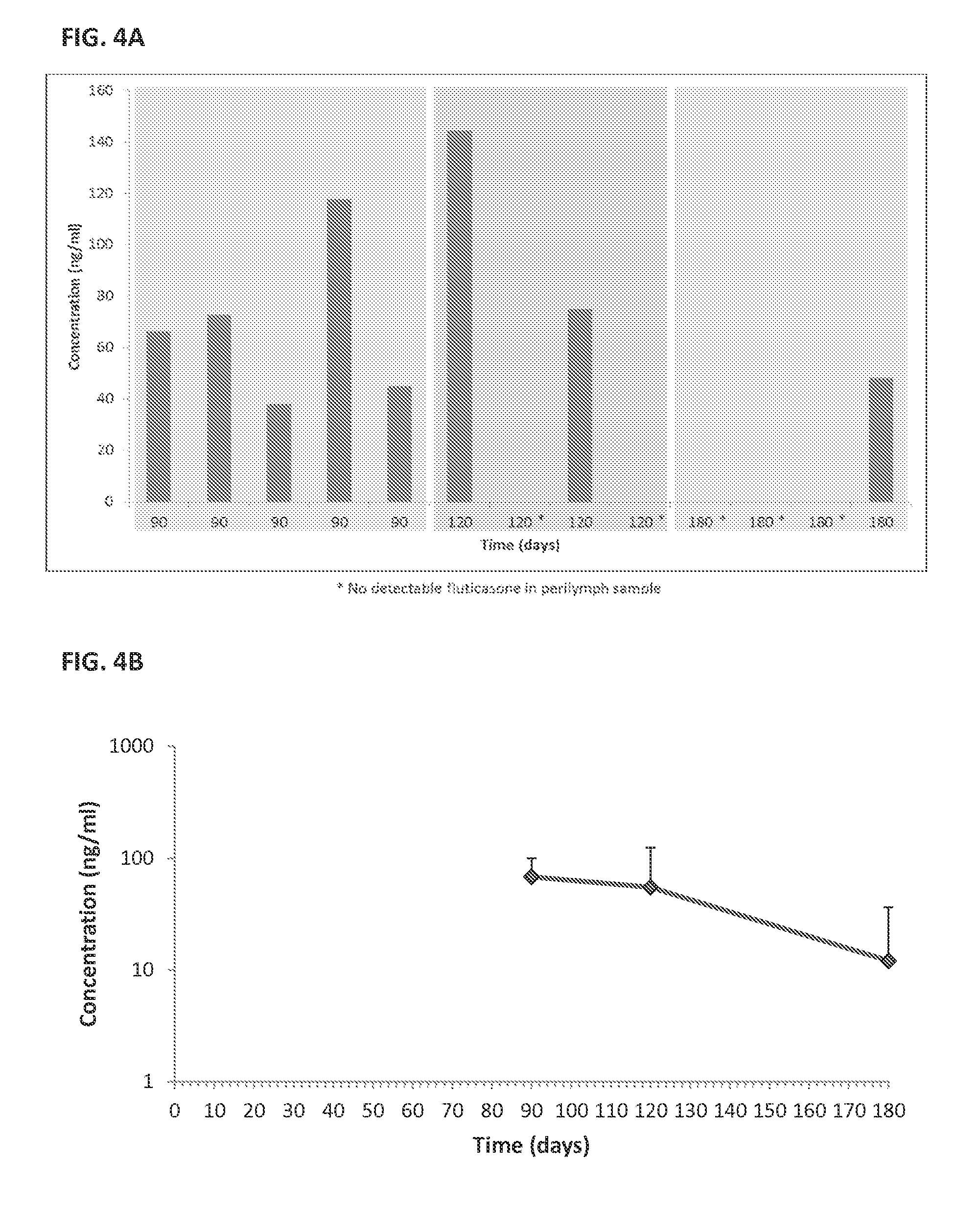
Solid drug implants for intracochlear delivery of therapeutics for the treatment of otic disorders
InactiveUS20150044271A1Preventing and reducing ototoxicityReduce the impactOrganic active ingredientsGogglesDiseaseDrug release
The present invention provides for pharmaceutical preparations, devices, systems and methods for the treatment of otic diseases and conditions. In various embodiments, the preparations, devices, systems and methods enable sustained drug release for the treatment or prevention of hearing loss, infections, and other pathological conditions of cochlea and inner ear.
Owner:O RAY PHARMA
Degradable removable implant for the sustained release of an active compound
A degradable, removable, pharmaceutical implant for the sustained release of one or more drugs in a subject, wherein the pharmaceutical implant is composed of a tube comprising an outer wall made of a degradable polymer completely surrounding a cavity, wherein the outer wall has a plurality of openings and wherein the cavity contains one or more sets of micro-particles, which micro-particles contain an active agent or a combination of two or more active agents, and wherein the size of the microparticles is selected such that the majority of the microparticles cannot pass through the openings.
Owner:JANSSEN SCI IRELAND UC
Painless drug implanter
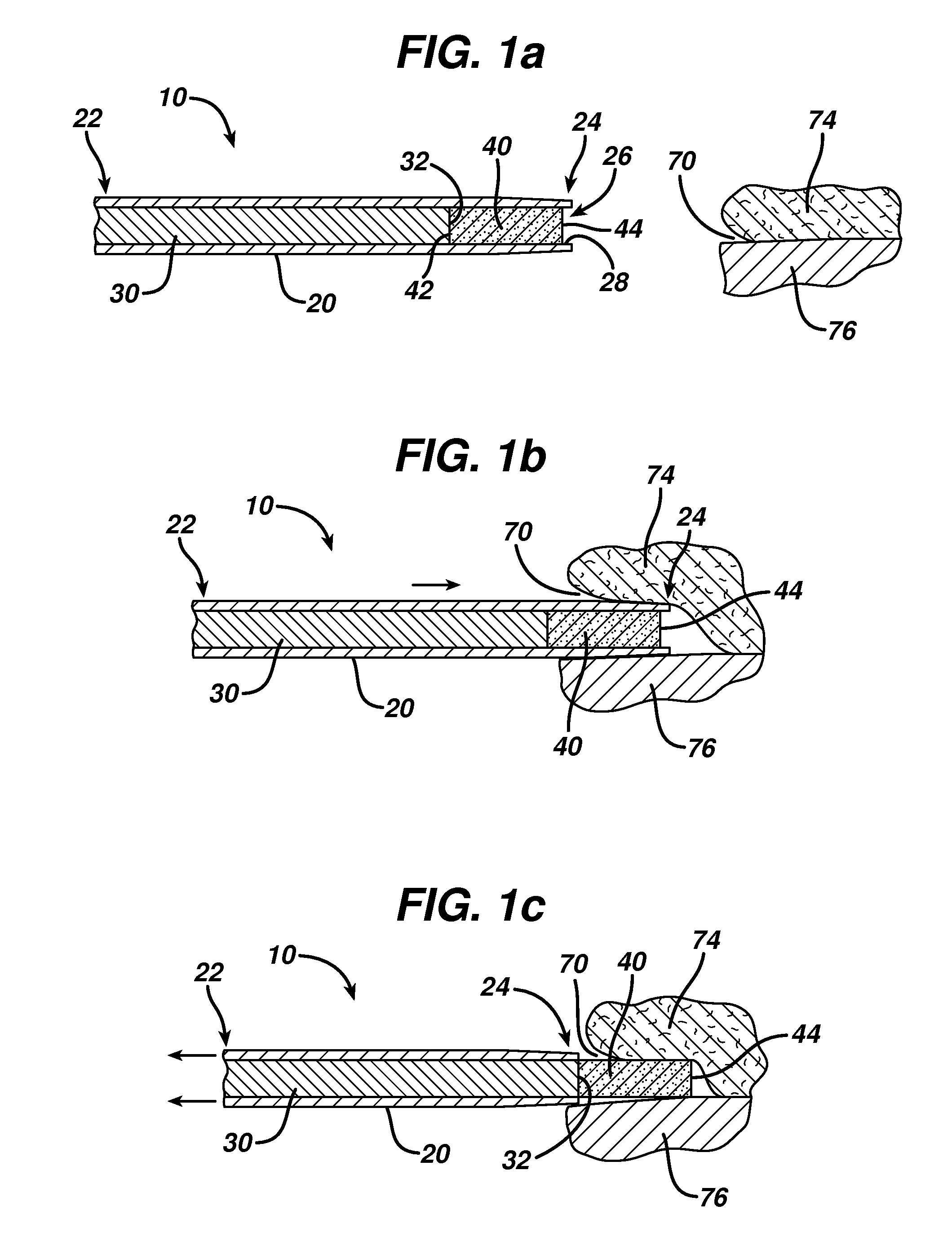
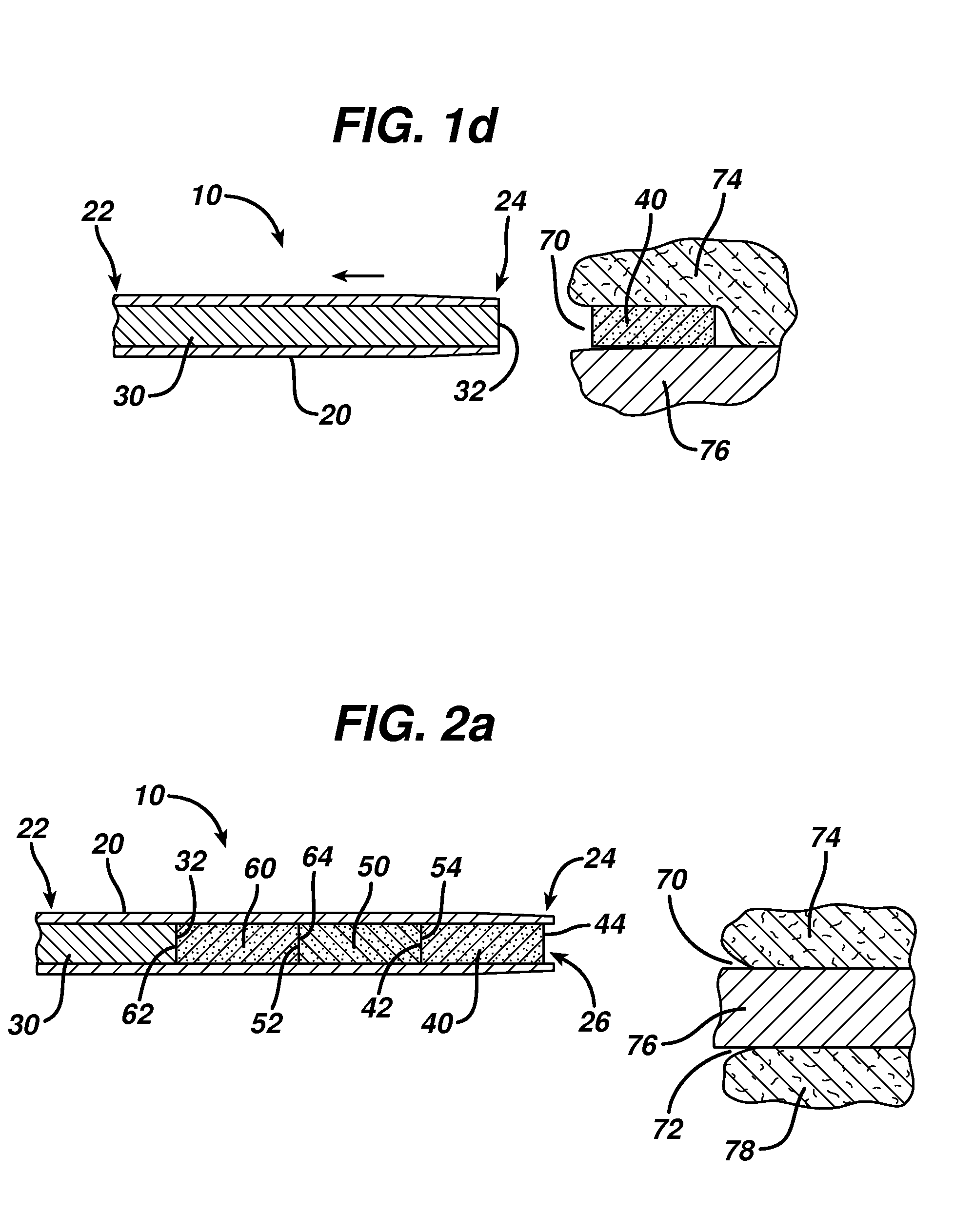
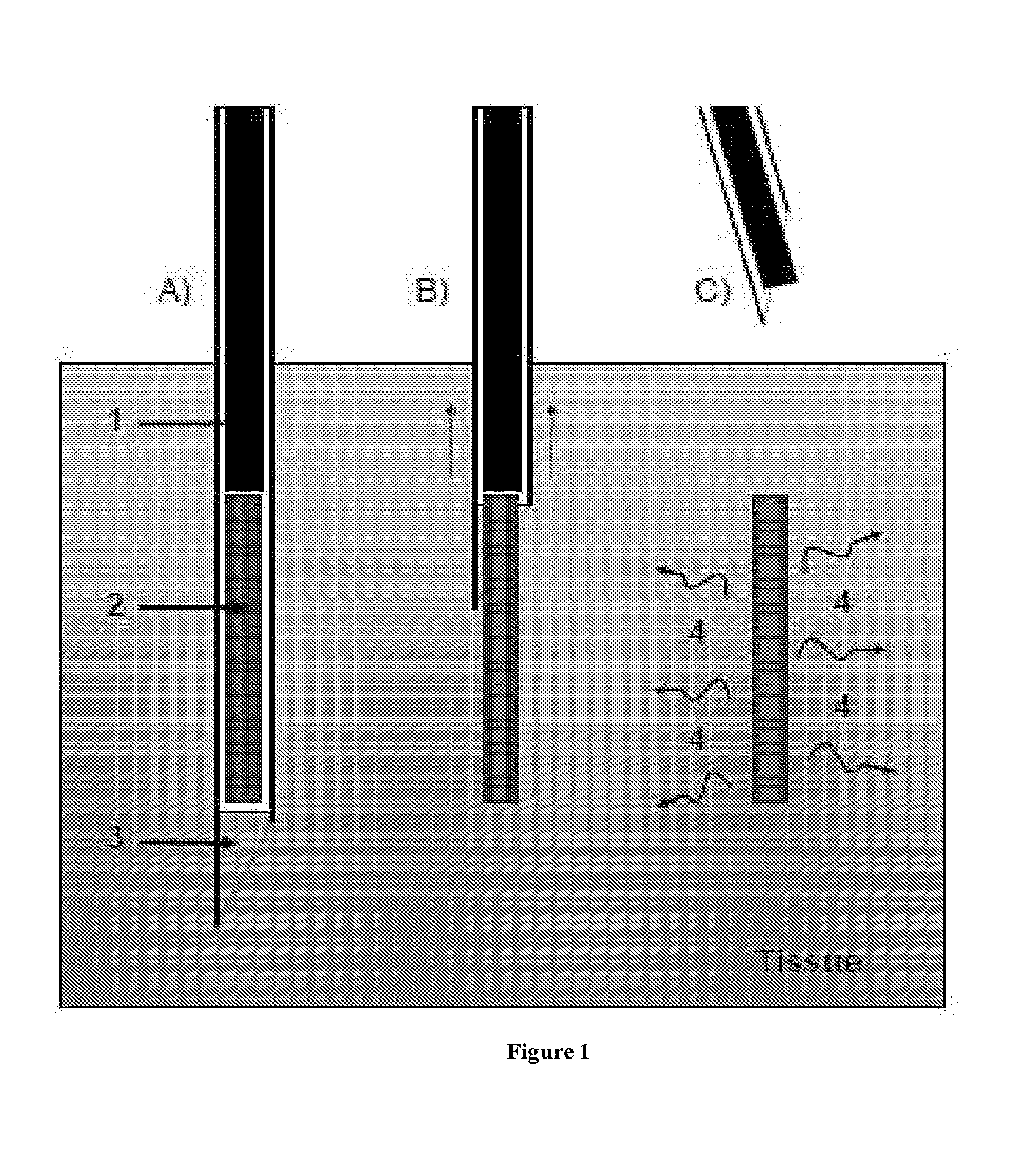
InactiveUS20180021556A1Rapid perpendicular insertionReduce occupied volumeInfusion syringesMicroneedlesBiomedical engineeringDrug Implants
The present invention relates to a drug implant device which delivers a drug load to the body painlessly. The present invention achieves the painless drug implantation by adopting two principles: (1) rapid perpendicular insertion of fine cannula is painless and that (2) pain is incurred only when the occupied volume caused by the implant process is increased. Therefore, instead of inserting a cannula and injection a volume of drug, which increases the occupied volume of the injection process due to additional volume of the drug, the present invention retracts the cannula in order to dispose the drug into the body. The retraction of cannula does not increase the occupied volume therefore incurs no pain. In the preferred embodiment, the drug implant device (100) comprises a cannula (300) with a bevelled tip, a drug load (320) and an inner rod (340), wherein the drug load (320) and the inner rod (340) are slidably disposed within the cannula (300), and that the drug load (320) is disposed at the bevelled end of the cannula (300) and that the inner rod (340) is disposed adjacent to the drug load (320).
Owner:LIM CHEE YEN
Preparation method of dexamethasone implant for kidney
ActiveCN105560161APromote meltingEasy extrusionOrganic active ingredientsPharmaceutical delivery mechanismDexamethasone acetateEngineering
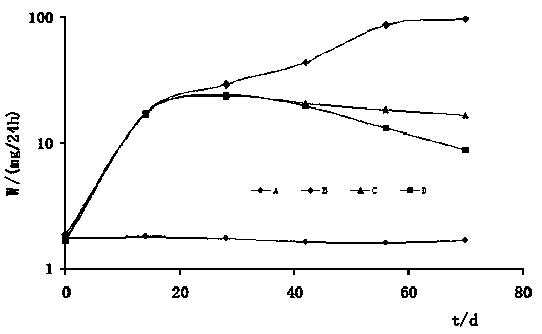
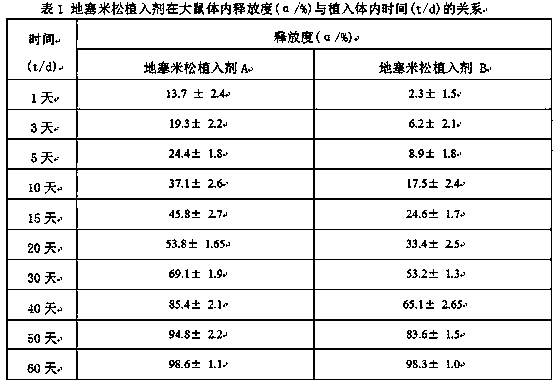
The present invention relates to a preparation method, uses and a use method of a dexamethasone implant for kidney, wherein the dexamethasone implant comprises dexamethasone or dexamethasone acetate, a degradable polymer material, and a water soluble auxiliary material. The preparation method comprises: crushing various materials, mixing, carrying out micro-spheroidization, carrying out mold pressing molding, and heating for a certain time at a proper temperature to prepare the cylindrical implant with a diameter of 0.2-0.9 mm and a length of 0.8-4 mm. According to the present invention, the implant has characteristics of smooth surface and uniform drug release in vivo, wherein the time for releasing 90% of the drug is 1 month to 1 year; and the implant can be implanted into the renal sac through a drug implanting needle so as to treat nephrotic syndrome, nephritis and other chronic kidney diseases.
Owner:ANHUI ZHONGREN TECH +2
In situ gelling drug delivery system
The invention provides liquid controlled-release drug delivery compositions which gel upon injection into the body to form, in situ, controlled-release drug implants. The compositions of the invention feature a gel-forming polymer that is insoluble in water, a polyethylene glycol solvent in which the polymer is dissolved, and the drug substance to be delivered.
Owner:PSIVIDA US INC
Eye implanted gel containing cyclosporin
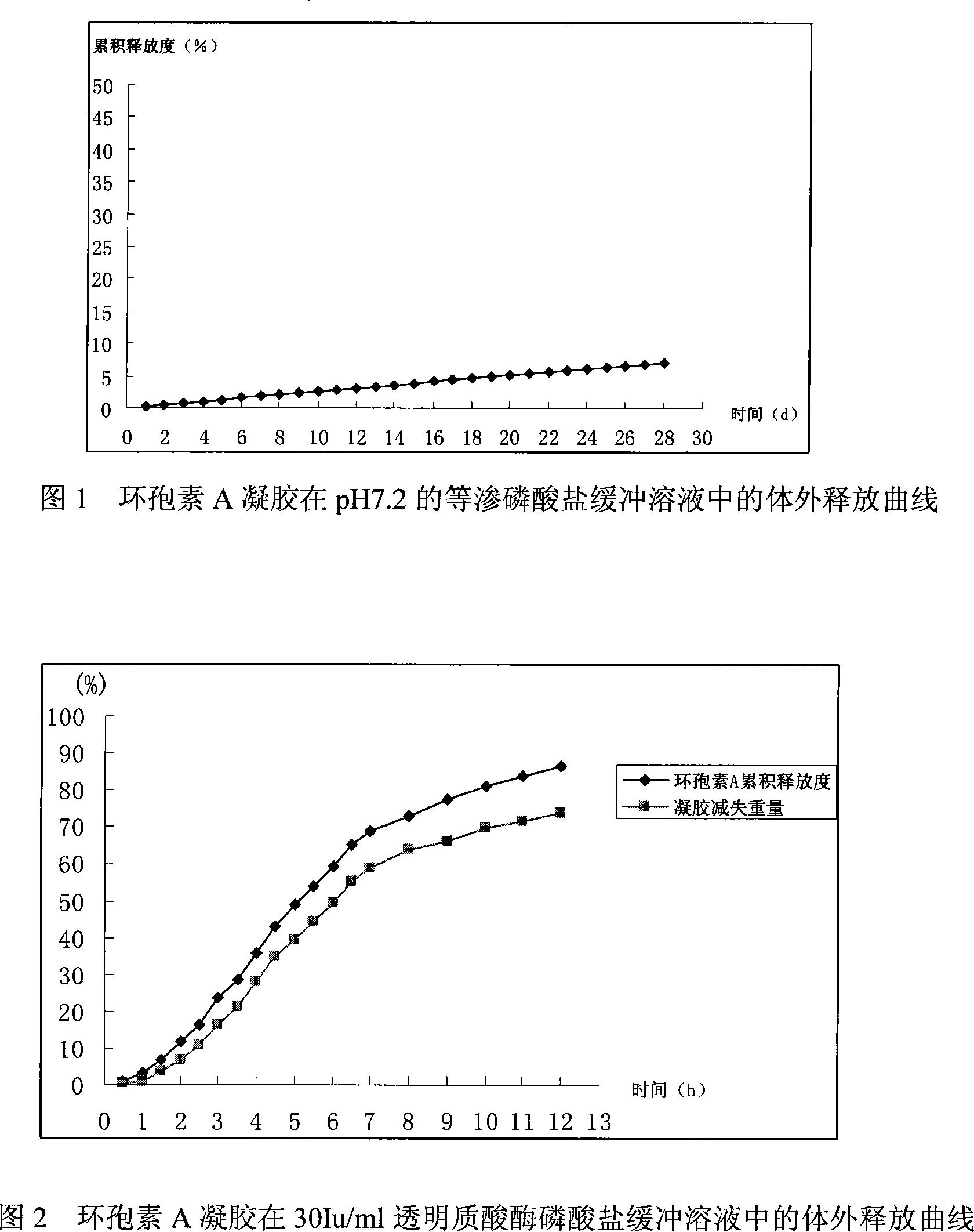
InactiveCN101085341APromote formationAvoid scaringPharmaceutical delivery mechanismCyclic peptide ingredientsCross-linkDisease
The invention relates to ocular implantation gel containing ciclosporin and its preparation method and application. The gel is characterized in that ciclosporin disperses in cross linked hyaluronic acid gel, and the weight ratio of cross linked hyaluronic acid and ciclosporin is 1:5-5:1.The preparation method of the gel comprises suspending ciclosporin in sodium hyaluronateaqueous solution, mixing with cross-linking agent for reaction to form gel, purifying, cutting, packaging, and sterilizing to obtain final product. The gel can be be used for preparation of ocular drug implants for preventing adhesion of humor aquosus through filtering channel in non penetrable joist operation of glaucoma, improving filterability and keeping filtering channel, controlling intraocular pressure after operation, preventing rejection reaction after corneal transplantation and some ocular autoimmune disease.
Owner:凌沛学
Ocular biodegradable drug implant and method of its use
A biodegradable drug implant includes a PLG matrix and a non-steroid anti inflammation drug, for example diclofenac sodium. The implant is inserted into the eye in the anterior chamber, for example in the ciliary sulcus, following eye surgery. A method for treating and or preventing inflammation of the eye following eye surgery includes placing the implant in the anterior portion of the eye.
Owner:LENSTEC BARBADOS
Therapeutic methods and compositions for solid delivery
An administration device comprising an array of needles, one or more fluid agents, and at least one hydrogel is described. The device can simultaneously deliver a plurality of fluid agents along respective axes into a tissue. The use of hydrogel leads to constrained delivery of the fluid agents. The constrained delivery of an agent is also achieved by depositing a drug implant into a tissue. The effect of an agent on the tissue can be evaluated thereafter. In addition, the invention is directed to treating muscle diseases by delivering a therapeutic agent in vivo, and the use of reporter tissues for candidate drug evaluation, detecting and characterizing resistance.
Owner:PRESAGE BIOSCI
A kind of preparation method of dexamethasone implant for kidney
ActiveCN105560161BPromote meltingEasy extrusionOrganic active ingredientsPharmaceutical delivery mechanismDexamethasone acetateDrug release
The present invention relates to a preparation method, uses and a use method of a dexamethasone implant for kidney, wherein the dexamethasone implant comprises dexamethasone or dexamethasone acetate, a degradable polymer material, and a water soluble auxiliary material. The preparation method comprises: crushing various materials, mixing, carrying out micro-spheroidization, carrying out mold pressing molding, and heating for a certain time at a proper temperature to prepare the cylindrical implant with a diameter of 0.2-0.9 mm and a length of 0.8-4 mm. According to the present invention, the implant has characteristics of smooth surface and uniform drug release in vivo, wherein the time for releasing 90% of the drug is 1 month to 1 year; and the implant can be implanted into the renal sac through a drug implanting needle so as to treat nephrotic syndrome, nephritis and other chronic kidney diseases.
Owner:ANHUI ZHONGREN TECH +2
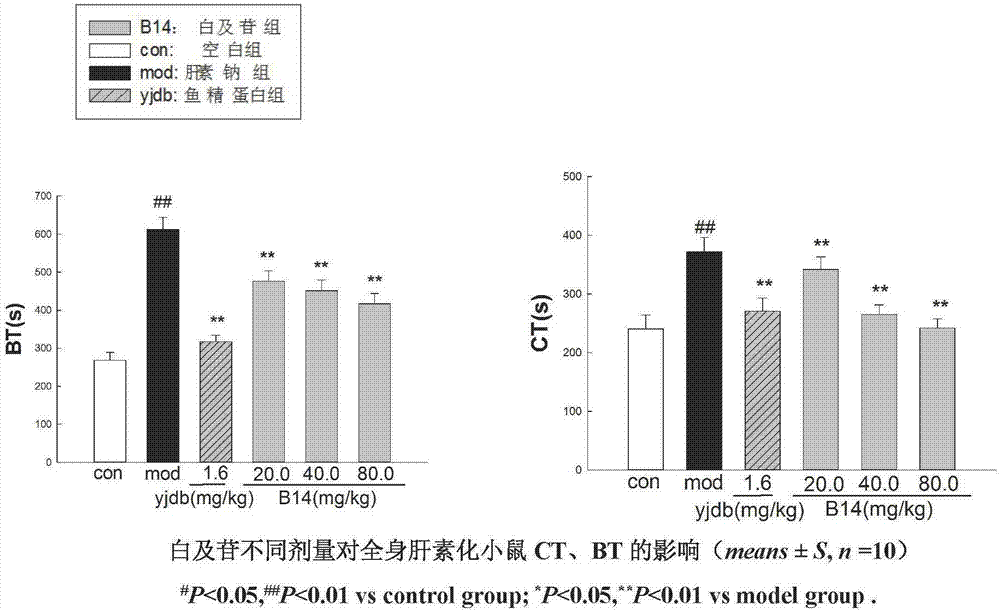
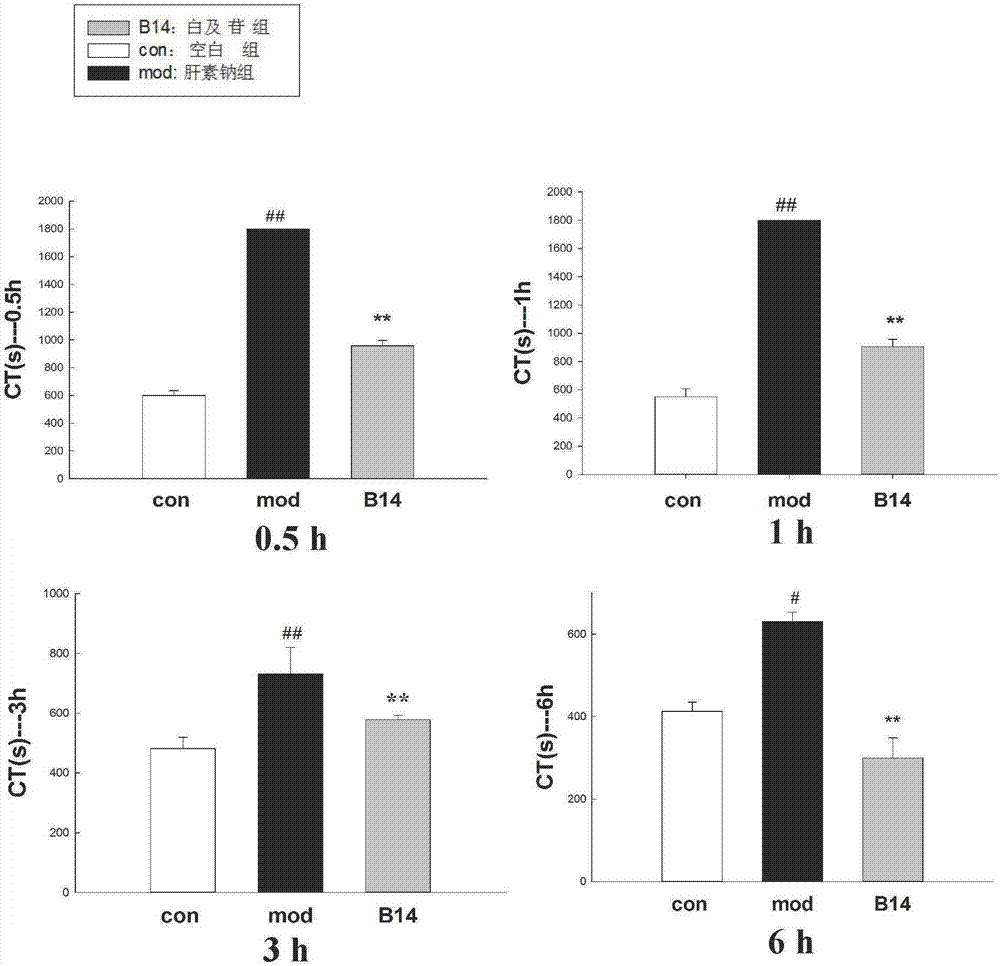
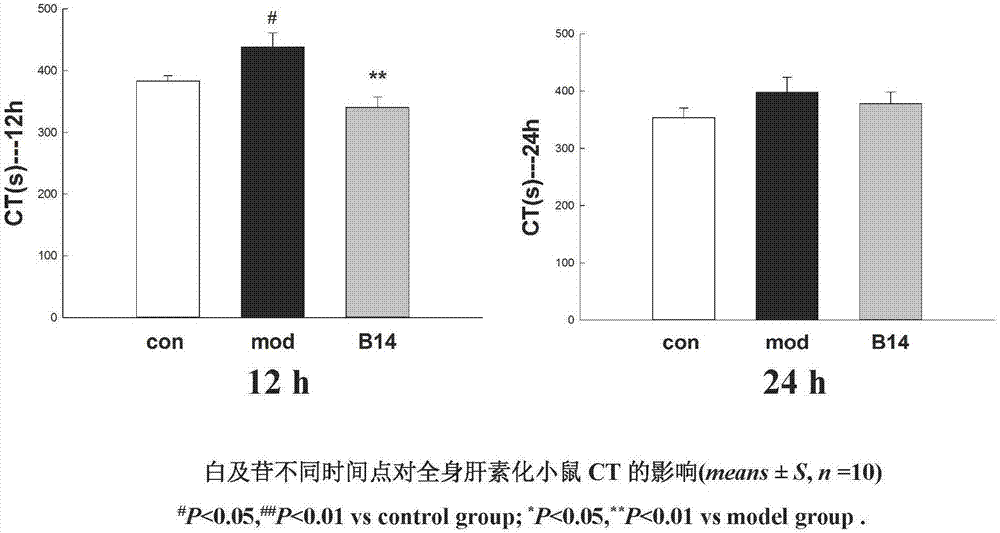
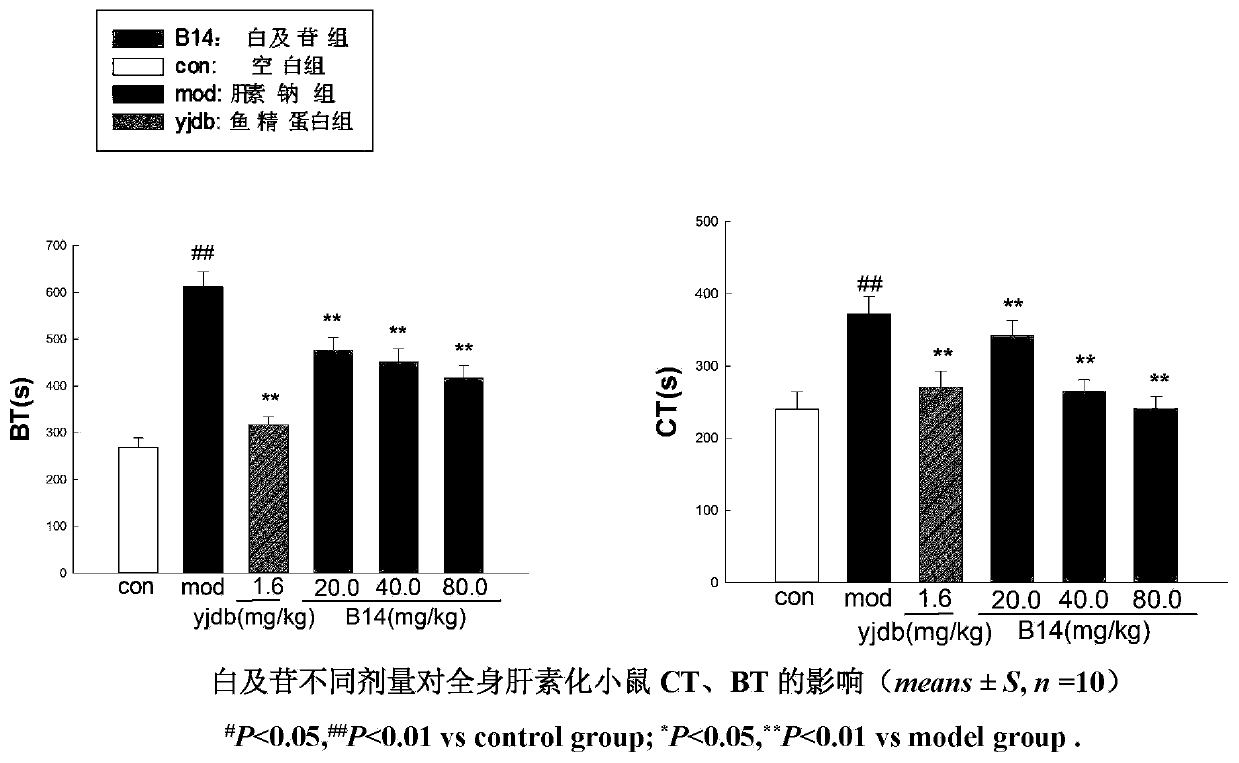
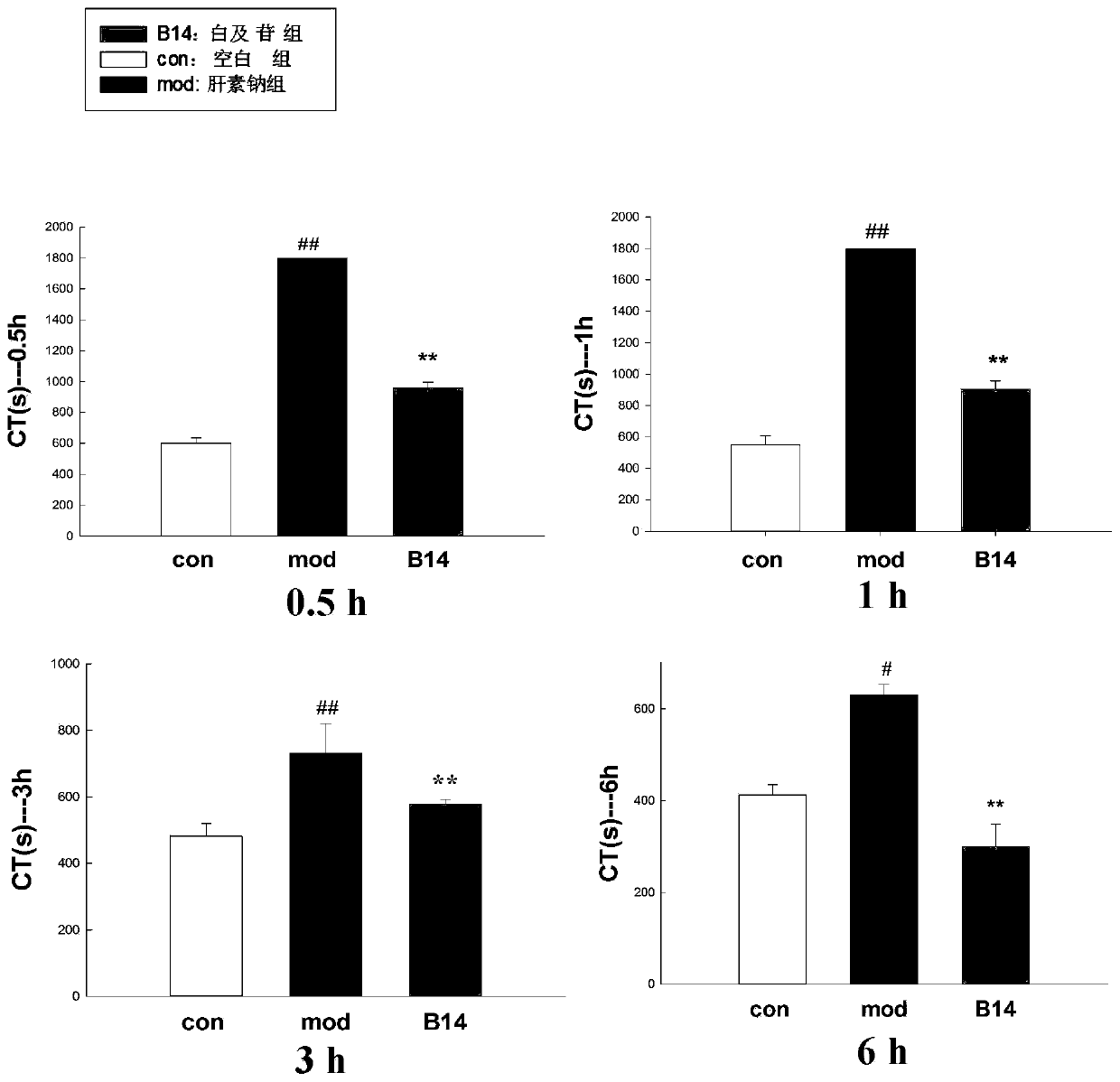
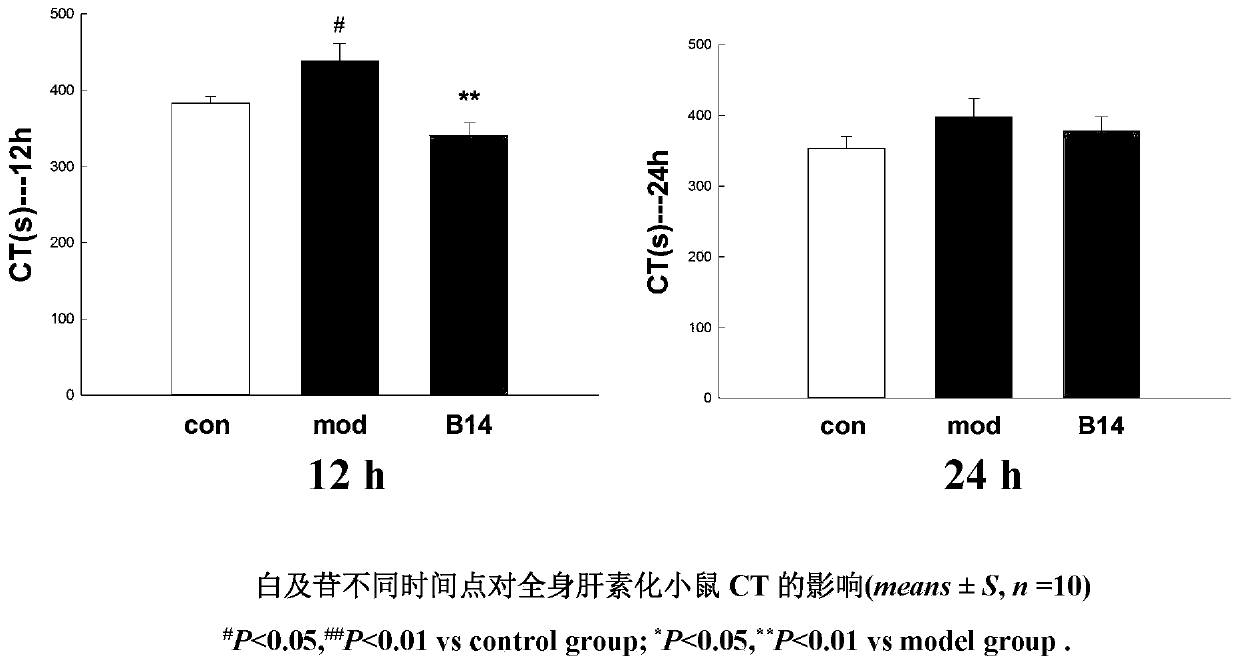
Application of militarine in preparation of heparin antagonist
ActiveCN106924273ADoes not cause allergiesSignificantly antagonizes heparinOrganic active ingredientsAntinoxious agentsAllergyMilitarine
The invention discloses new application of militarine in preparation of heparin antagonist. The molecular formula of the militarine in the invention is C34H46O17 and the chemical name is bis [4-(beta-D-glucopyranosoxy) benzyl]-2-isobutyl malate. The militarine provided by the invention can be prepared into dosage form suitable for clinical application, mainly including injection, granule, capsule, tablet, powder, pill, drug implants and etc. The effect of antagonize heparin is obvious. The pharmacological effect is similar to or identical with protamine. The invention has the advantage of no adverse reactions to human such as allergy and safety, and can be developed into the substitute of the protamine to be applied in clinic with good application prospect.
Owner:GUIZHOU MEDICAL UNIV
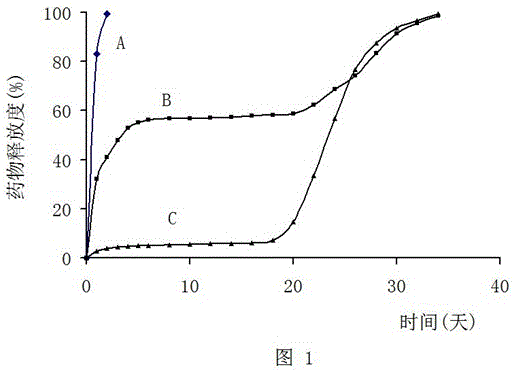
Implant capable of releasing doxorubicin continuously for long term, and preparation method thereof
ActiveCN106692031AFlat surfaceImprove toughnessOrganic active ingredientsPharmaceutical delivery mechanismLactideCylindroma
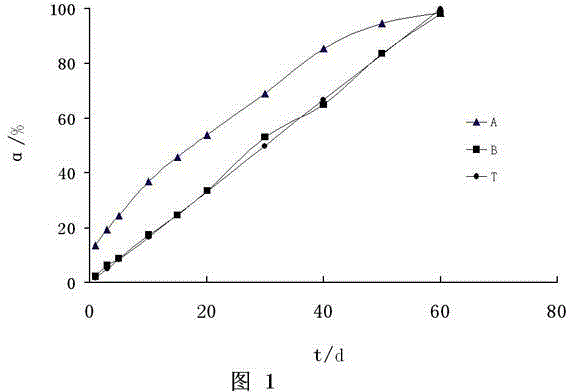
The invention relates to an implant capable of releasing doxorubicin continuously for a long term, and a preparation method thereof. The implant is composed of doxorubicin hydrochloride, a glycolide-lactide copolymer, and polyethylene glycol. According to the preparation method, melting method is adopted to prepare the cylindrical implant with a diameter ranging from 0.3 to 1.6mm and a length ranging from 0.8 to 5mm; in in-vivo test, 5 to 21% of the coated drug is released in one day, 25 to 51% of the coated drug is released in 5 days, 35 to 63% of the coated drug is released in 10 days, and 80 to 100% of the coated drug is released in 30 days. The surface of the implant is smooth; the implant can be implanted in tumor, or tumor surrounding tissue, or administration places using a drug implanting needle via percutaneous puncture; the implant is suitable for treatment of solid tumor; the preparation process is simple; quality control is convenient to realize; cost is low; no pollution is caused; and the preparation method is convenient for industrialization.
Owner:ANHUI ZHONGREN TECH
Implantable drug delivery devices for localized drug delivery
ActiveUS11338119B2Reduce and eliminate toxicityIncrease concentrationPharmaceutical delivery mechanismMedical devicesDiseaseActive agent
Provided herein are drug implants comprising a therapeutically active agent for the treatment of disease in a subject. In some cases, the drug implant may comprise a polymer matrix and a therapeutically active agent disposed therein. Additionally provided are methods for manufacturing the drug implants and methods of treating diseases with the implants. In some cases, the drug implant may comprise bicalutamide, e.g., for use in the treatment of prostate cancer.
Owner:ALESSA THERAPEUTICS INC +1
Application of militarine in the preparation of heparin antagonistic drugs
ActiveCN106924273BObvious antagonismDoes not cause allergiesOrganic active ingredientsAntinoxious agentsCaplet Dosage FormAllergy
The invention discloses new application of militarine in preparation of heparin antagonist. The molecular formula of the militarine in the invention is C34H46O17 and the chemical name is bis [4-(beta-D-glucopyranosoxy) benzyl]-2-isobutyl malate. The militarine provided by the invention can be prepared into dosage form suitable for clinical application, mainly including injection, granule, capsule, tablet, powder, pill, drug implants and etc. The effect of antagonize heparin is obvious. The pharmacological effect is similar to or identical with protamine. The invention has the advantage of no adverse reactions to human such as allergy and safety, and can be developed into the substitute of the protamine to be applied in clinic with good application prospect.
Owner:GUIZHOU MEDICAL UNIV
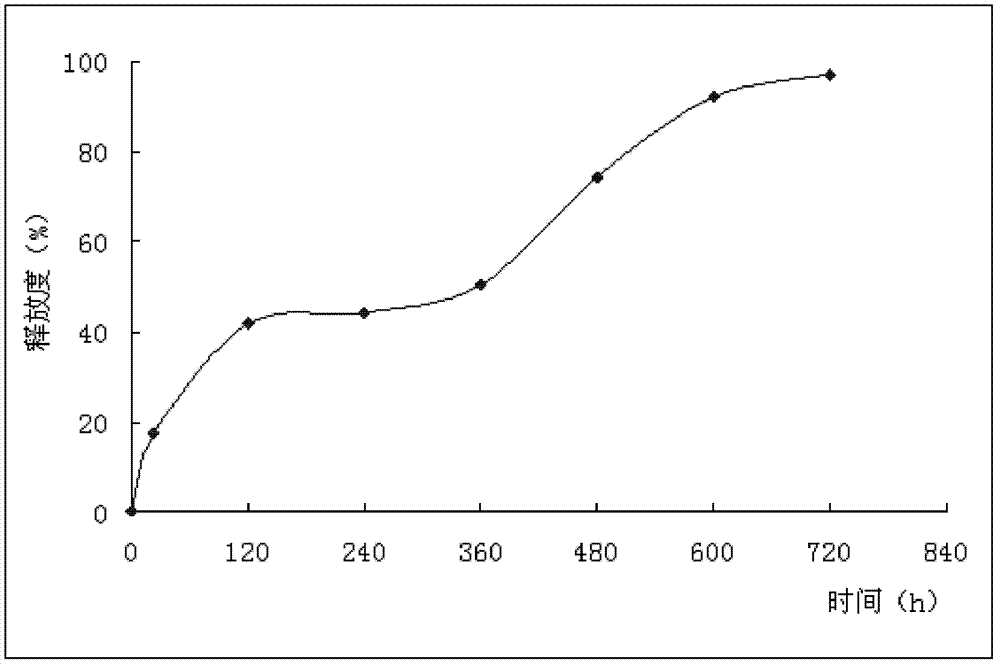
Chemotherapy drug pulse sustained-release implant agent and preparation method thereof
ActiveCN102670520BProlonged local effective drug concentrationAvoid secondary damagePharmaceutical non-active ingredientsGranular deliveryCarboplatinSolvent
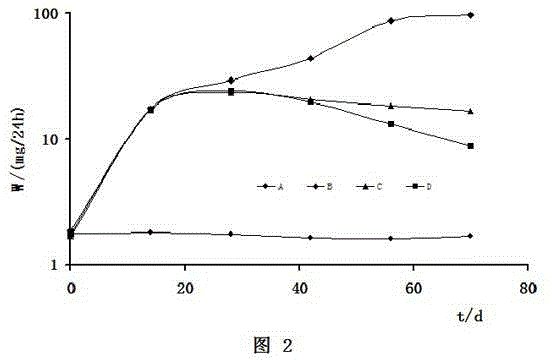
The invention provides a chemotherapy drug pulse sustained-release implant agent and a preparation method thereof. The sustained-release implant agent comprises a chemotherapy drug active component such as any one of gemcitabine, gemcitabine hydrochloride, doxorubicin, 5-fluorouracil, cisplatin, carboplatin and paclitaxel, and an excipient such as a polylactic-glycolic acid copolymer. The preparation method provided by the invention comprises the following steps of: evenly mixing the chemotherapy drug active component with the excipient in a solvent, and then drying the mixture of the chemotherapy drug active component and the excipient in a vacuum after removing the solvent; and then sequentially crushing, sieving, melting and blending the mixture to obtain the sustained-release implant agent. The sustained-release implant agent provided by the invention has the advantages that a second drug release is carried out after tumor growth is controlled by a first drug release for a certain period, and the local effective concentration of a drug is effectively increased, so that the effect of administering once and releasing twice is achieved, and the side injury caused by secondary drug implanting is avoided.
Owner:普华赛尔生物医疗科技有限公司
Implantable Drug Delivery Devices for Localized Drug Delivery
ActiveUS20210290920A1Reduce and eliminate toxicityIncrease concentrationPowder deliverySurgical needlesDiseaseActive agent
Provided herein are drug implants comprising a therapeutically active agent for the treatment of disease in a subject. In some cases, the drug implant may comprise a polymer matrix and a therapeutically active agent disposed therein. Additionally provided are methods for manufacturing the drug implants and methods of treating diseases with the implants. In some cases, the drug implant may comprise bicalutamide, e.g., for use in the treatment of prostate cancer.
Owner:ALESSA THERAPEUTICS INC +1
Solid drug implants for intracochlear delivery of therapeutics for the treatment of otic disorders
ActiveUS20160250231A1Preventing and reducing ototoxicityIncrease doseOrganic active ingredientsMicrocapsulesDiseaseDrug release
The present invention provides for pharmaceutical preparations, devices, systems and methods for the treatment of otic diseases and conditions. In various embodiments, the preparations, devices, systems and methods enable sustained drug release for the treatment or prevention of hearing loss, infections, and other pathological conditions of cochlea and inner ear.
Owner:O RAY PHARMA
External auditory canal directional delivery anti-epidemic device and preparation process
PendingCN113244048AAmplify active information signalTrace lowEar treatmentMedical devicesExternal Auditory CanalsDisease
The invention relates to an external auditory canal directional delivery anti-epidemic device with a height of 20-30mm, a lower part diameter of 12-22mm and a upper part diameter of 6-12mm, the anti-epidemic device has an upper part with a round solitary, is in the shape of a bullet and implanted with drugs. The anti-epidemic device provided by the invention is pinched into a slender strip, the slender strip is plugged into external auditory canals of two ears, and the anti-epidemic device automatically rebounds in the external auditory canals and makes contact with the walls of the external auditory canals and ear hairs. According to the invention, the drug implanted of the anti-epidemic device delivered in external auditory canal is not only absorbed by external auditory canal skin cells, but also the drug information of implanted drug smell (wave) , the drug smell (wave) is sensed by the external auditory canal cells and ear hairs, and the drug smell (wave) is delivered to a human body and is further delivered to nasal olfactory nerve cells through eustachian tubes of ears, so that the human body biological information effect is generated to effectively treat human body diseases.
Owner:杨孟君
Implantable Drug Delivery Devices for Localized Drug Delivery
ActiveUS20210290584A1Reduce and eliminate toxicityIncrease concentrationPharmaceutical delivery mechanismPharmaceutical non-active ingredientsDiseaseActive agent
Provided herein are drug implants comprising a therapeutically active agent for the treatment of disease in a subject. In some cases, the drug implant may comprise a polymer matrix and a therapeutically active agent disposed therein. Additionally provided are methods for manufacturing the drug implants and methods of treating diseases with the implants. In some cases, the drug implant may comprise bicalutamide, e.g., for use in the treatment of prostate cancer.
Owner:ALESSA THERAPEUTICS INC +1
Radionuclide particle implantation medicine for cancer, use and preparation method thereof
InactiveCN101337080BSolve the problem of not being able to exert an anti-cancer effectGood curative effectRadioactive preparation carriersAntineoplastic agentsEthanolDrug Implants
The invention relates to the drug implant of radionuclide particles for cancer therapy. The drug implant comprises composition which is composed of radionuclide which can emit Beta rays, and biodegradable high-molecular material. The compounding ratio is as follows: each 0.9-1.1 mg of the biodegradable high molecular material is proportional to radionuclide which can emit Beta rays with the radioactivity of 18.5 to 37.0 MBq. The composition of <32>P-CP and poly-L-lactic acid is taken as the drug implant of the radionuclide particles for the therapy of malignant solid tumors. The preparation method comprises the following steps: selecting colloidal chromic phosphate or calcium phosphate with the radioactive concentration of 3700 to 7400 MBq / ml and the chemical concentration of 0.5 to 4.14 mg / mal as the <32>P-CP, according to the proportion that <32>P-CP: PLLA is 18.5 to 37.0 MBq: 0.9 to 1.1 mg and according to the proportion that each gram of poly-L-lactic acid is proportional to 3 to 6 ml of anhydrous alcohol, adding anhydrous alcohol into the poly-L-lactic acid and mixing, then adding the <32>P-CP and mixing uniformly, obtaining the paste composition, grinding the paste composition to uniformly distribute the radioactivity into the medium, drying the composition at 60 DEG C to 70 DEG C, then further grinding the composition into powder, positioning the powder into a pressing machine, and pressing particles with the diameter of 0.8 to 1 mm and the length of 2 to 4 mm.
Owner:江苏三和生物工程股份有限公司 +1
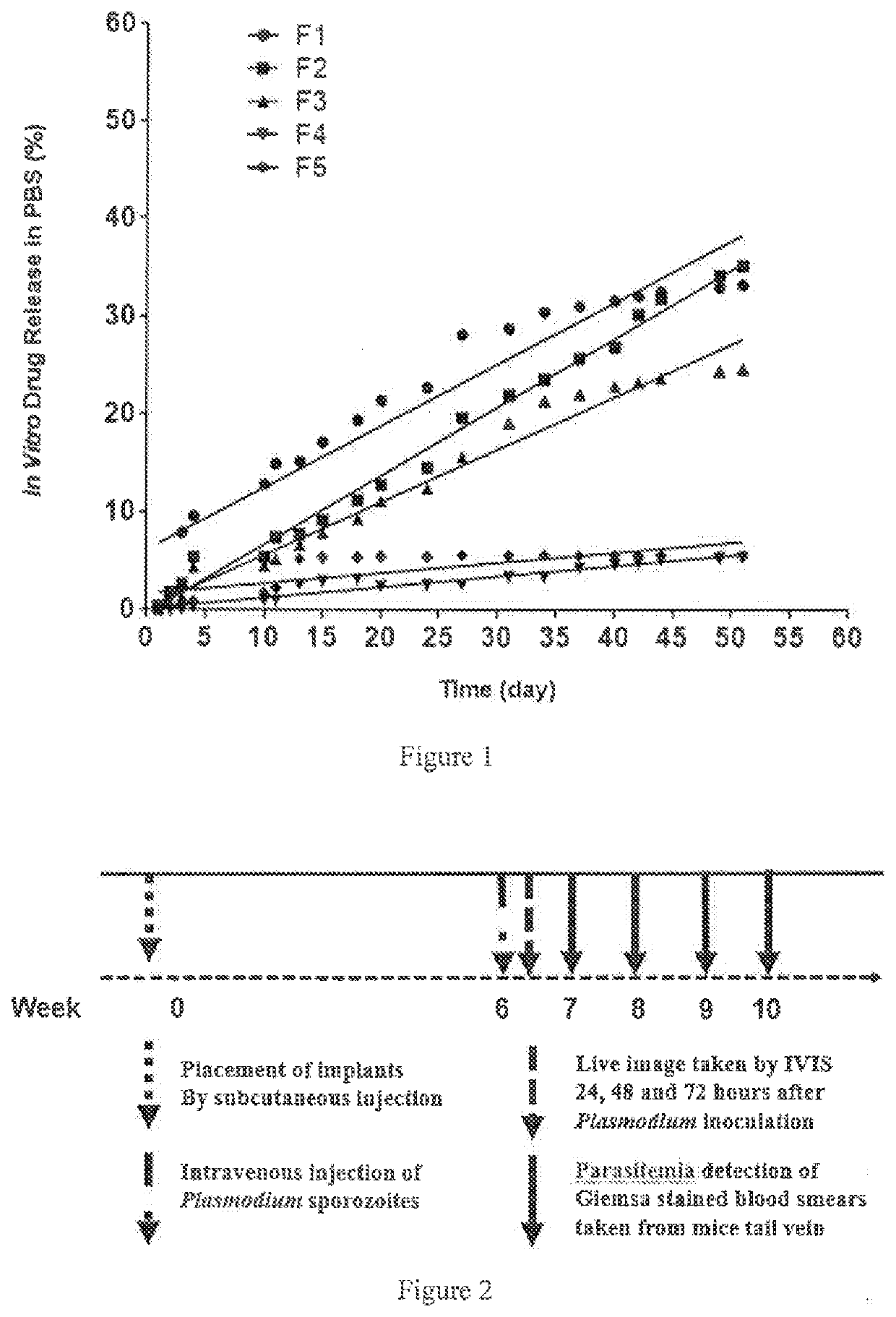
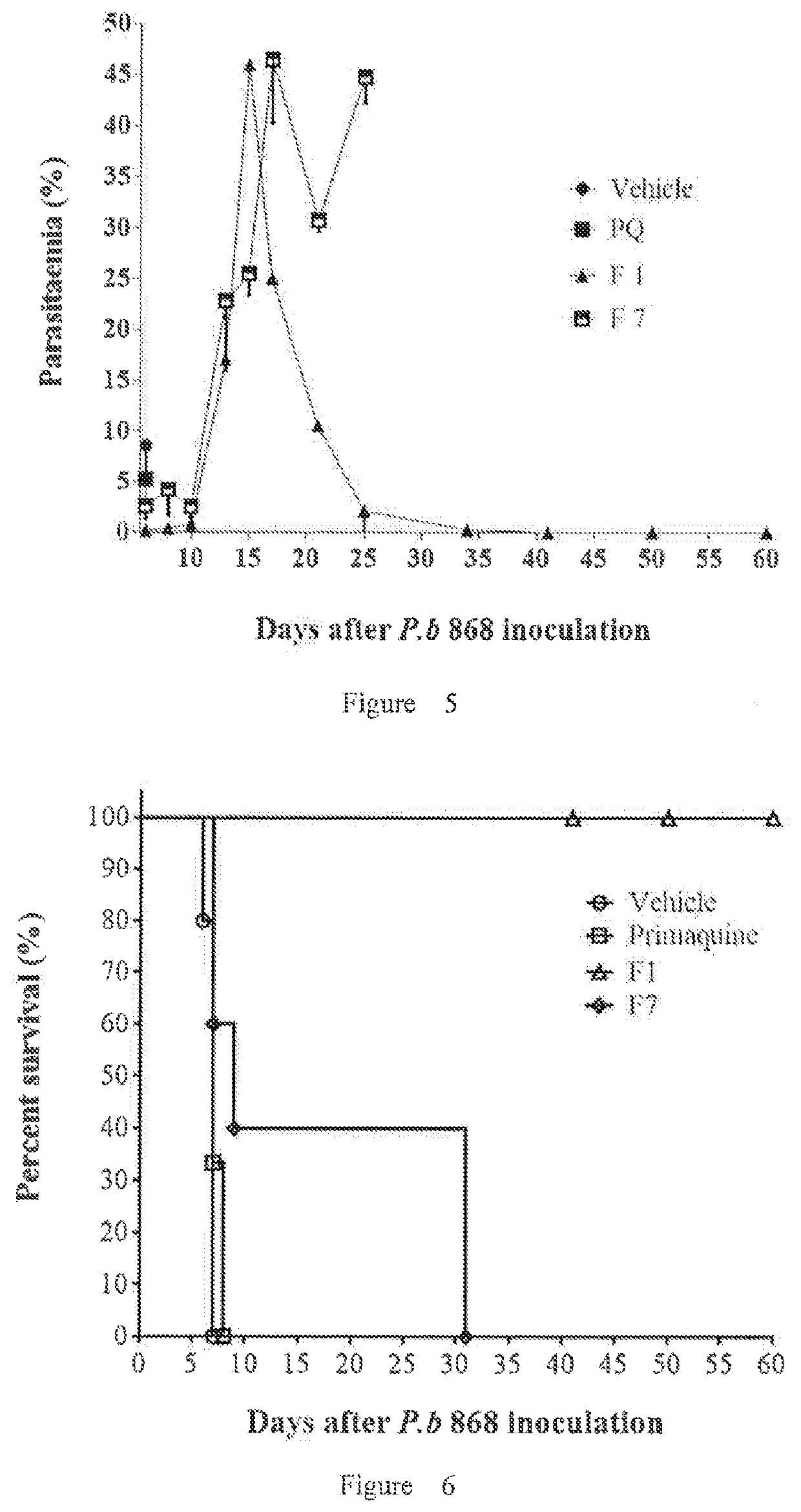
Intramuscular depot of decoquinate compositions and method of prophylaxis and treatment thereof
ActiveUS20210059946A1No adverse effectsAdequate levelPill deliveryPharmaceutical non-active ingredientsDiseaseMonoglyceride
Implants comprising an antimalarial agent such as decoquinate or another therapeutic drug are disclosed. The implants can be in the form of a complex comprising a drug, a lipid or lipids and optionally a polymer carrier. The present invention provides methods for preparing implants wherein the implants comprise decoquinate or another therapeutic drug, and a lipid or lipids such as cholesterol, monoglycerides, or diglycerides, and optionally a small percentage of a biocompatible polymer based on the total implant weight. The implants are useful for releasing a therapeutic drug at a constant level and maintaining a prophylactic or therapeutic level of the drug in a subject, for preventing a malarial infection or treating other diseases or disorders.
Owner:CAS LAMVAC (GUANGZHOU) BIOMEDICAL TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com