Preparation method of dexamethasone implant for kidney
A technology of dexamethasone and dexamethasone acetate, which is applied to the composition of renal dexamethasone implants, the preparation of renal dexamethasone implants, the size, the drug release characteristics and the preparation process, and the shape field, which can solve the problem of thermal stability. It can solve the problems of poor performance, more degradation, and damage to the end face of the implant, so as to achieve the effect of high intensity of action, low blood drug concentration, and high kidney concentration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Example 1 Implant preparation
[0092] Component parts by weight
[0093] Dexamethasone 10
[0094] Polylactic-glycolic acid (75:25; molecular weight 9,000)87
[0095] Polyethylene glycol (molecular weight 6,000)3
[0096] The above-mentioned materials were crushed separately, passed through a 900-mesh sieve, weighed according to the prescription amount, 5 g in total, mixed evenly, and made into filaments with a micro-extruder. Crush filaments, sieve, make microspheres with a particle size of 30 microns or more, put them into a stainless steel mold with a pore size of 0.9 mm, put them in a ZR-Ⅱ medicine press (developed by Anhui Zhongren Technology Co., Ltd.), and place them under a pressure of 480 MPa Molding: demoulding to obtain dexamethasone implant A, and heat preservation at 56° C. for 15 minutes to obtain dexamethasone implant B, which is the dexamethasone implant of the present invention.
[0097] During the preparation process, the micro-extrusion machine...
Embodiment 2
[0098] Example 2 In vivo release test
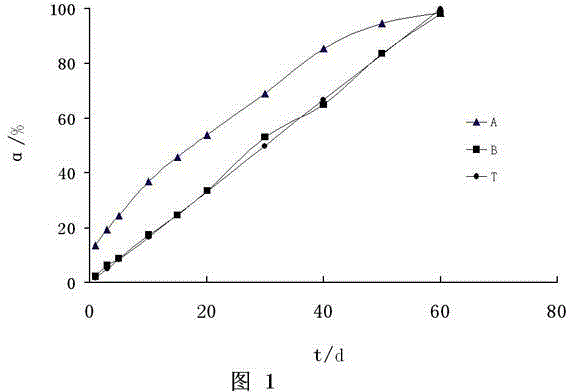
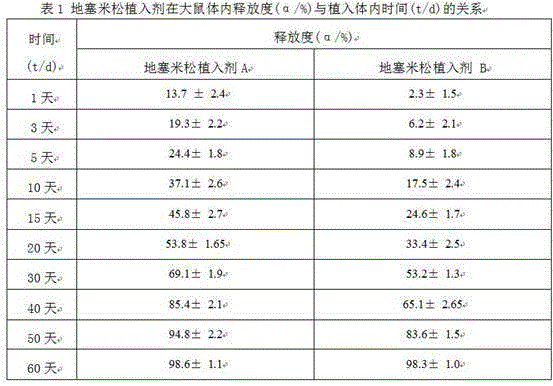
[0099] Take 120 Wista rats and divide them into two groups: Ⅰ and Ⅱ, 60 rats in each group, and set 10 sampling time points, 6 rats in each point. Group Ⅰ was implanted with dexamethasone implant A, and group Ⅱ was implanted with dexamethasone implant A. For dexamethasone implant B, 1 piece of the corresponding implant after weighing was implanted into the inner muscle of the right hind leg of each rat, and the remaining implants were taken from 6 rats in each group at different time points after implantation , the remaining drug amount of dexamethasone in the residual implant was measured by high performance liquid chromatography, and the release rate was calculated according to the implanted drug amount and the remaining drug amount. Table 1 and figure 1 .
[0100]
[0101] Table 1 with figure 1 Show: the dexamethasone implant A (A) that embodiment 1 prepares, the difference of the release degree of dexamethasone implant B (B...
Embodiment 3
[0105] Embodiment 3 pharmacodynamics test
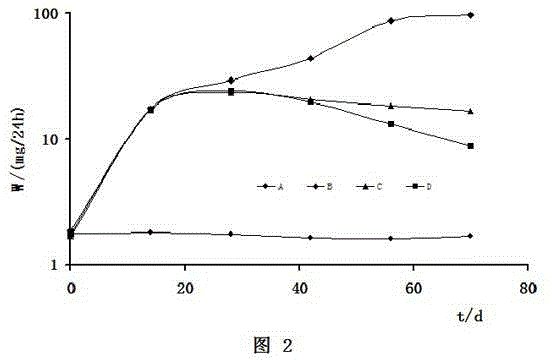
[0106] Replication of the nephrotic syndrome model: male SD rats weighing 180-220 g were injected with 4 mg / kg of doxorubicin hydrochloride through the tail vein on the 1st and 8th day after the start of the experiment (doxorubicin was dissolved in normal saline at 2 g / L) Medium, namely 2mL / kg), they were put into metabolic cages and fed and drank freely.
[0107]Grouping of animals: 10 male SD rats from the same batch without modeling were taken as the normal control group A. On day 14, 30 male SD rats successfully modeled by the above-mentioned tail vein injection of Adriamycin hydrochloride were taken and randomly divided into B, C, and D Three groups, 10 rats in each group, group B is the renal disease model group, group C is the renal disease model dexamethasone implant A treatment group, and group D is the renal disease model dexamethasone implant B treatment group.
[0108] Treatment: No treatment in groups A and B, weighin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com