Implant capable of releasing doxorubicin continuously for long term, and preparation method thereof
A technology of doxorubicin and implants, which can be applied to non-active ingredients medical preparations, medical preparations containing active ingredients, and pharmaceutical formulas, etc., can solve the problems of low release degree, reduced systemic toxicity, and high tumor concentration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
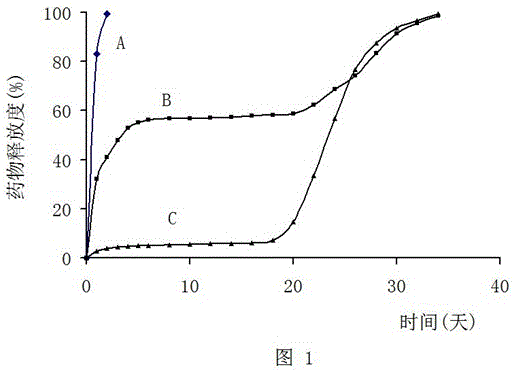
[0133] Example 1 Doxorubicin implant A, B, C
[0134] (1) For prescription, see Table 1.
[0135] Table 1 Doxorubicin implant A, B, C components and weight ratio
[0136]
[0137] (2) Preparation
[0138] ① Pulverize the materials in Table 1 respectively and pass through a 120-mesh drug sieve to obtain adriamycin hydrochloride fine powder and PLGA fine powder;
[0139] ② Mix the fine powder of ① according to the ratio in the above table 1, and make implant A, B, C mixed powder respectively;
[0140] ③ Dry the mixed powder of ② in a vacuum drying oven at a temperature of 22°C and a pressure of 9KPa, so that the content of water and volatile components in the mixed powder is less than 0.1%, and the mixed powder of dry implants A, B, and C is obtained;
[0141] ④ Place each dry mixed powder of ③ in a stainless steel container and melt at 110°C for 9 minutes, pour it into a mold (diameter of the mold hole is 0.9mm, length 4mm) for molding, cooling, demoulding, and sterili...
Embodiment 2
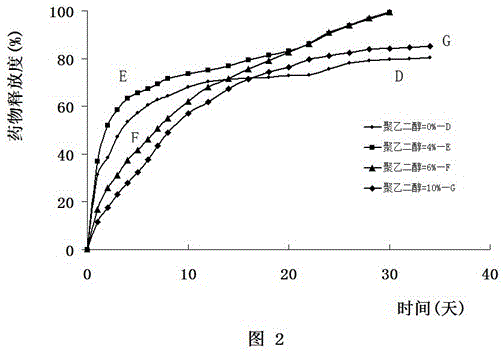
[0142] Example 2 Doxorubicin implants D, E, F, G
[0143] (1) For prescription, see Table 2.
[0144] Table 2 Doxorubicin implant D, E, F, G components and weight ratio
[0145]
[0146] (2) preparation
[0147] ① Pulverize the materials in Table 2 respectively and pass through a 120-mesh drug sieve to obtain adriamycin hydrochloride fine powder and PLGA fine powder;
[0148] ② Mix the fine powder of ① according to the ratio in the above table 2, and make implant D, E, F, G mixed powder respectively;
[0149] ③Dry the mixed powder of ② in a vacuum drying oven at a temperature of 26°C and a pressure of 11KPa, so that the content of water and volatile components in the mixed powder is less than 0.1%, and the mixed powders of dry implants D, E, F, and G are prepared ;
[0150] ④ Place the mixed powder of each dry implant in ③ into a stainless steel container and melt at 130°C for 9 minutes, inject it into a mold (diameter of the mold hole is 0.9mm, length 4mm) for moldi...
Embodiment 3
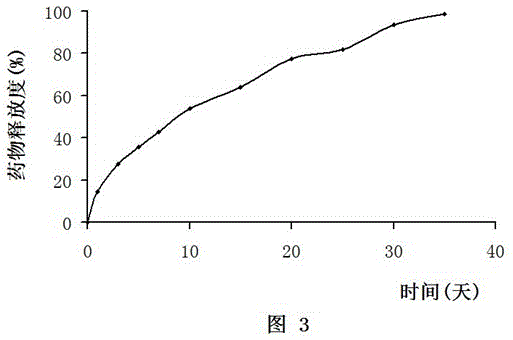
[0151] Example 3 In vitro release test
[0152] (1) Determine the content of doxorubicin implants A, B, C, D, E, F, and G by spectrophotometry;
[0153] (2) Take 3 doxorubicin implants A, weigh them accurately, put each into a 10mL clean test tube with stopper, add 5mL purified water (pH=7.0) to each test tube, and make the implant The particles are immersed in water and kept in a water bath at (37±0.5)°C to keep warm, which is the in vitro release device for doxorubicin implant A.
[0154] (3) Make the in vitro release devices of doxorubicin implants B, C, D, E, F, and G in the same way.
[0155] (4) Take out all the liquid in each test tube every day, add the same volume of fresh purified water at the same time, and put it in a water bath at (37±0.5 )°C to keep warm;
[0156] (5) Use spectrophotometry to detect the amount of doxorubicin hydrochloride in the liquid taken out every day, and calculate the release rate according to the input amount and release amount. The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| compressive strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com