Solid drug implants for intracochlear delivery of therapeutics for the treatment of otic disorders
a technology of intracochlear and solid drug implants, which is applied in the direction of medical preparations, goggles, microcapsules, etc., can solve the problems of snhl, loud noise, hearing deficits, etc., and achieve the effects of preventing or reducing ototoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Intracochlear Fluticasone Proprionate Levels Following Implantation, Study A
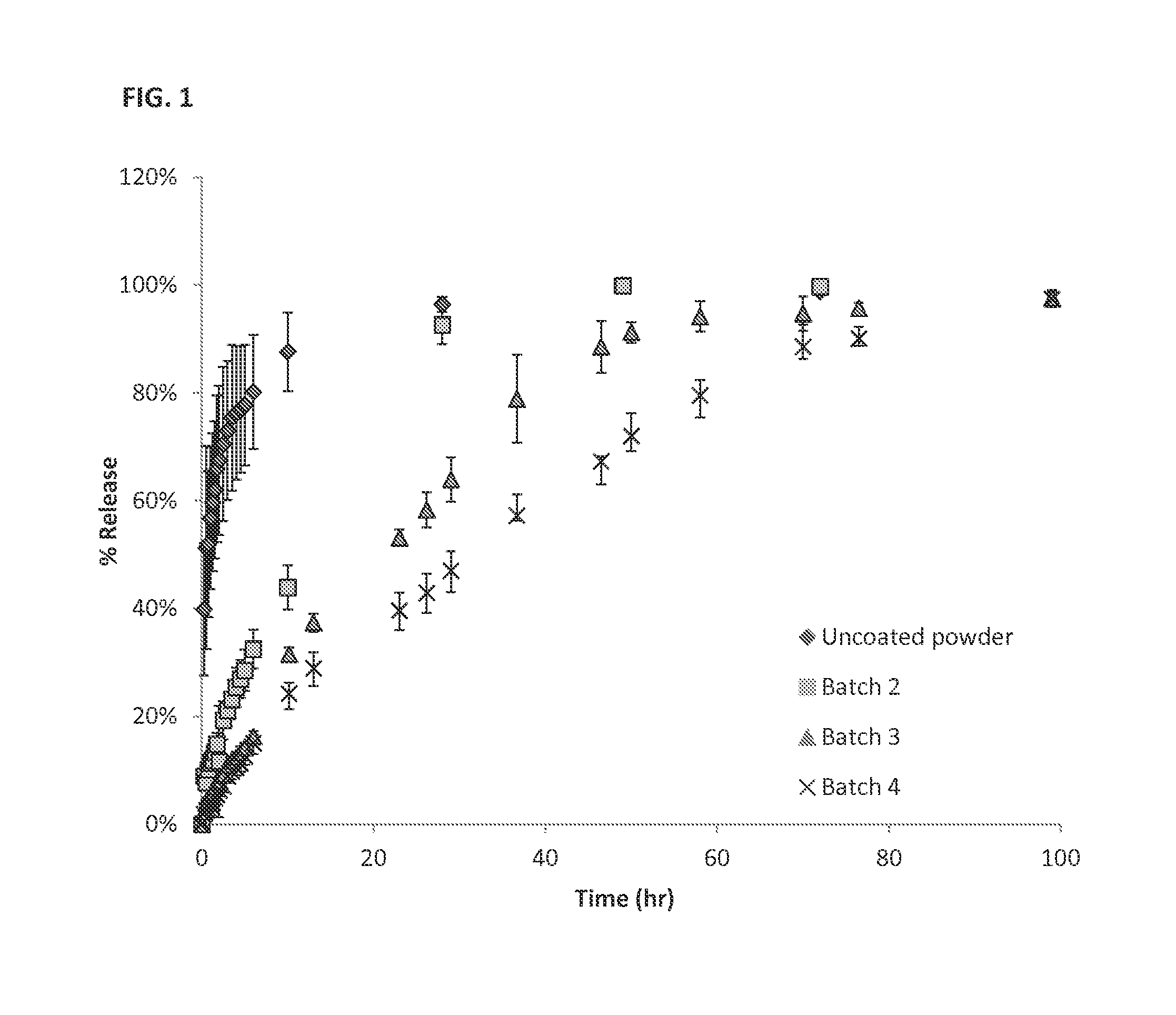
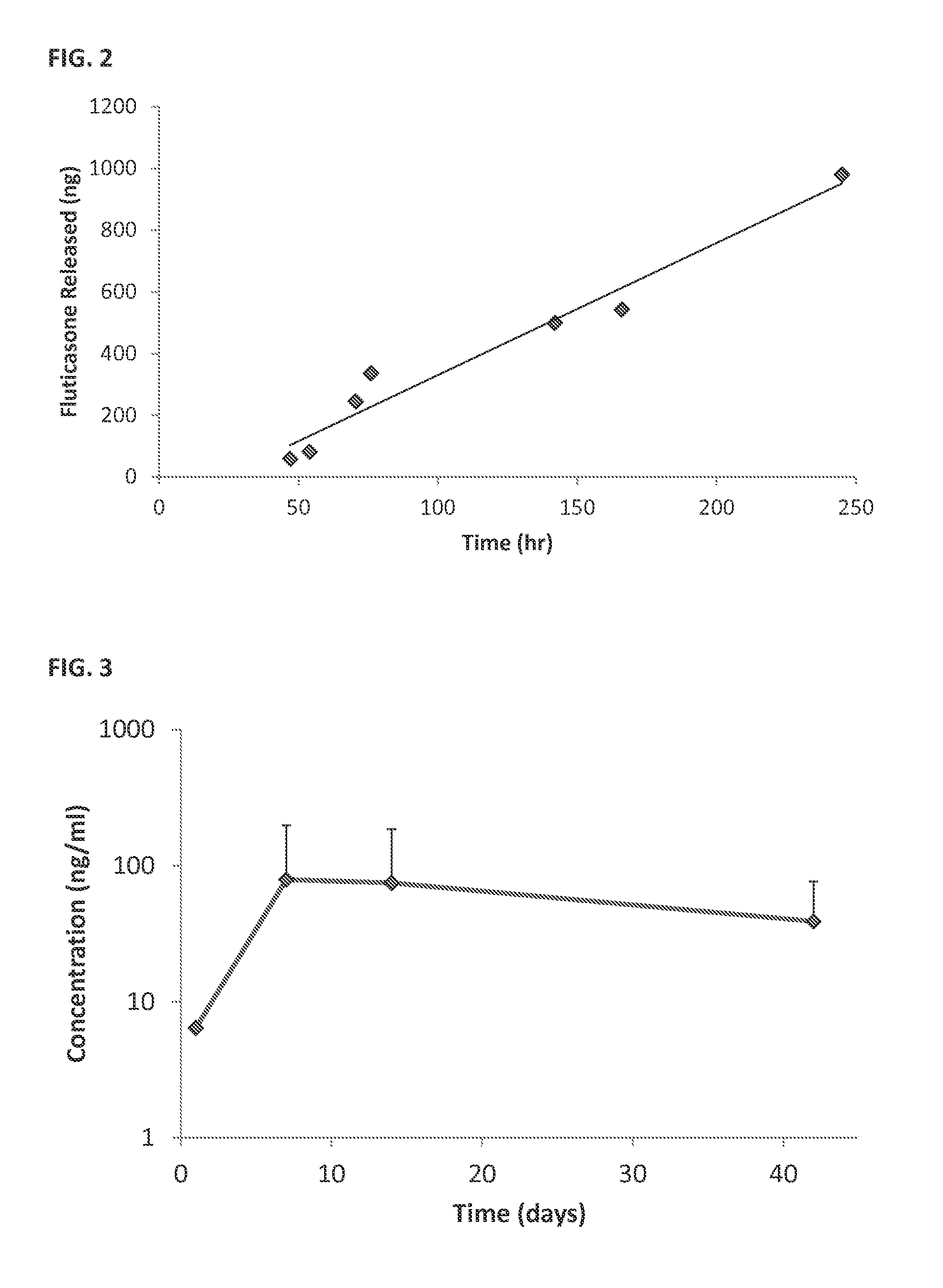
[0201]Sustained Release Coating: Fluticasone propionate crystals were coated with polyvinyl alcohol (PVA).
[0202]In vivo studies; see FIG. 3:
[0203]Surgery: Albino guinea pigs were used for the single dose intracochlear implantation experiment. The guinea pigs received a single implant in one of their ears, and the other ear was used as internal control. Under general anesthesia, a postauricular incision was made over the bulla in the experimental ear. A small hole was drilled through the bulla using a posterior approach. The ear was positioned with the round window facing horizontally and superiorly. A 32-gauge needle was used to puncture the round window and the particle was put in contact with the perilymph in the scala tympani. A blood drop was placed over the round window injection site to avoid leakage of perilymphatic fluids and a suture was used to close the wound.
[0204]Pharmacokinetic: Perilymph sampl...
example 2
Intracochlear Fluticasone Proprionate Levels Following Implantation, Study B
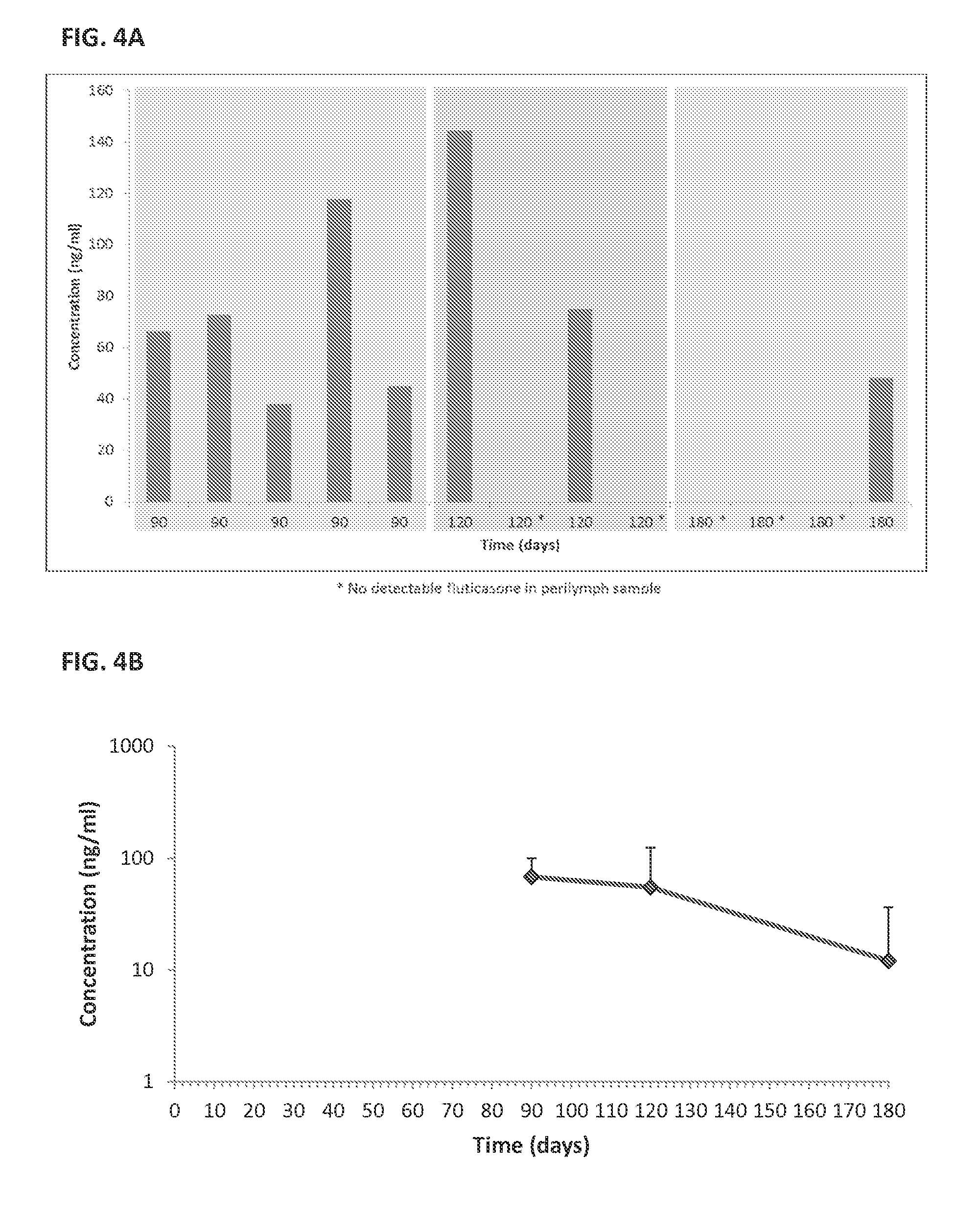
[0205]Sustained Release Coating: Fluticasone propionate crystals were coated with polyvinyl alcohol (PVA).
[0206]In vivo studies; see FIGS. 4A and 4B:
[0207]Surgery: Albino guinea pigs were used for the single dose intracochlear implantation experiment. The guinea pigs received a single implant in one of their ears, and the other ear was used as internal control. Under general anesthesia, a postauricular incision was made over the bulla in the experimental ear. A small hole was drilled through the bulla using a posterior approach. The ear was positioned with the round window facing horizontally and superiorly. A 32-gauge needle was used to puncture the round window and the particle was put in contact with the perilymph in the scala tympani. A blood drop was placed over the round window injection site to avoid leakage of perilymphatic fluids and a suture was used to close the wound.
[0208]Pharmacokinetic: Perily...
example 3
Hearing Tests Following Intracochlear Fluticasone Proprionate Particle Implantation
[0209]Study Design The animals from Example 2 underwent hearing tests to ascertain the safety of the extended release fluticasone proprionate implant. Hearing was tested pre-implant (N=15), 90 (N=5), 120 (N=5), and 180 (N=5) days post-implant. Implanted ears were compared to ears that did not undergo surgery.
[0210]Auditory Measurements; see FIGS. 5A and 5B: Animals were anesthetized for auditory testing. Briefly, ABRs were measured under computer control in response to clicks 50 μs duration from levels below threshold to 80 dB SPL in 5 dB steps. Responses were detected with subcutaneous needle electrodes placed at the vertex and ventrolateral to the left and right pinna. Response was amplified (10,000 times), filtered (0.1-3 kHz bandpass) and averaged (across 512 sweeps at each frequency-level combination). On visual inspection of stacked waveforms, “threshold” was defined as the lowest stimulus level...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com