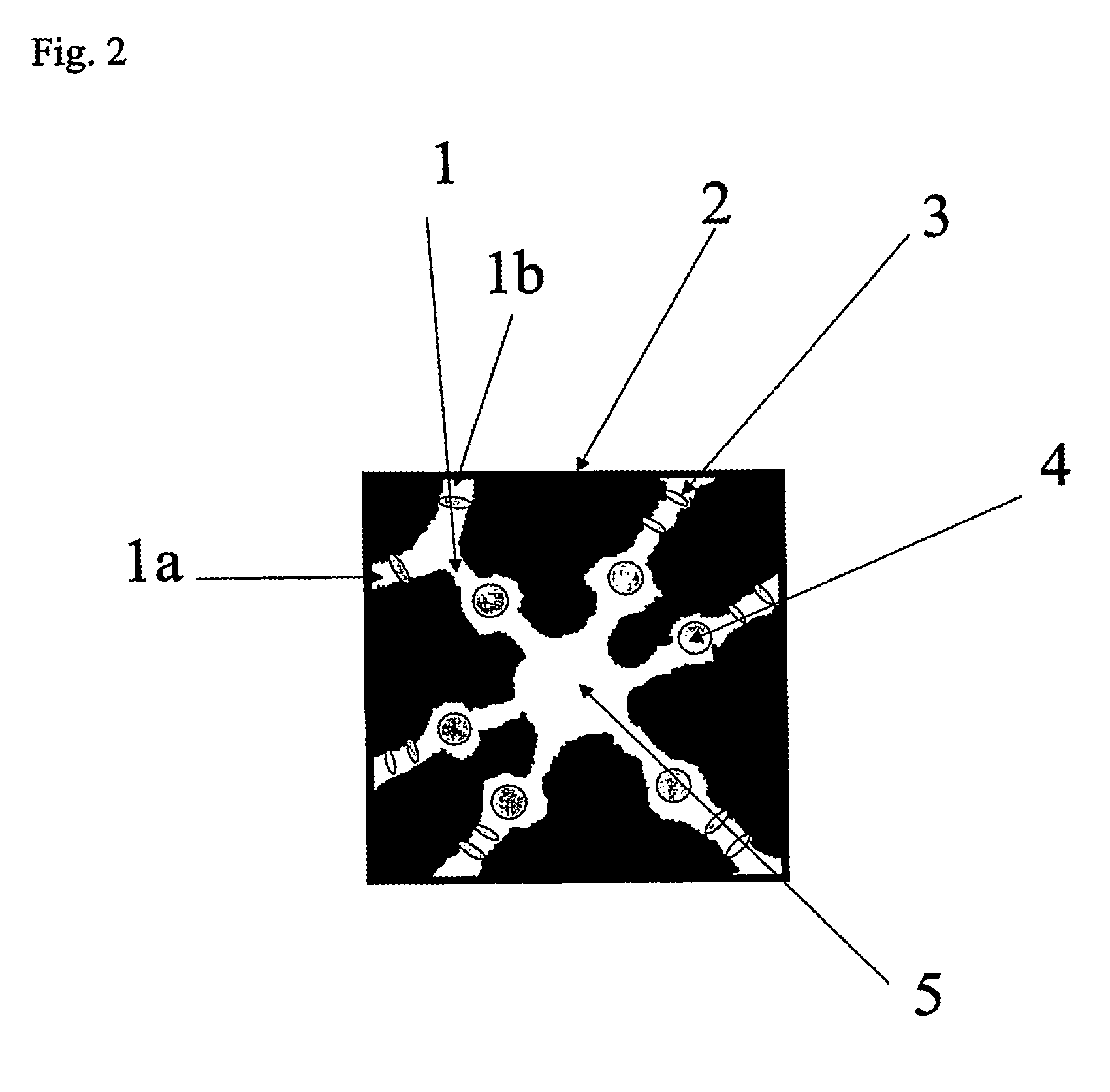
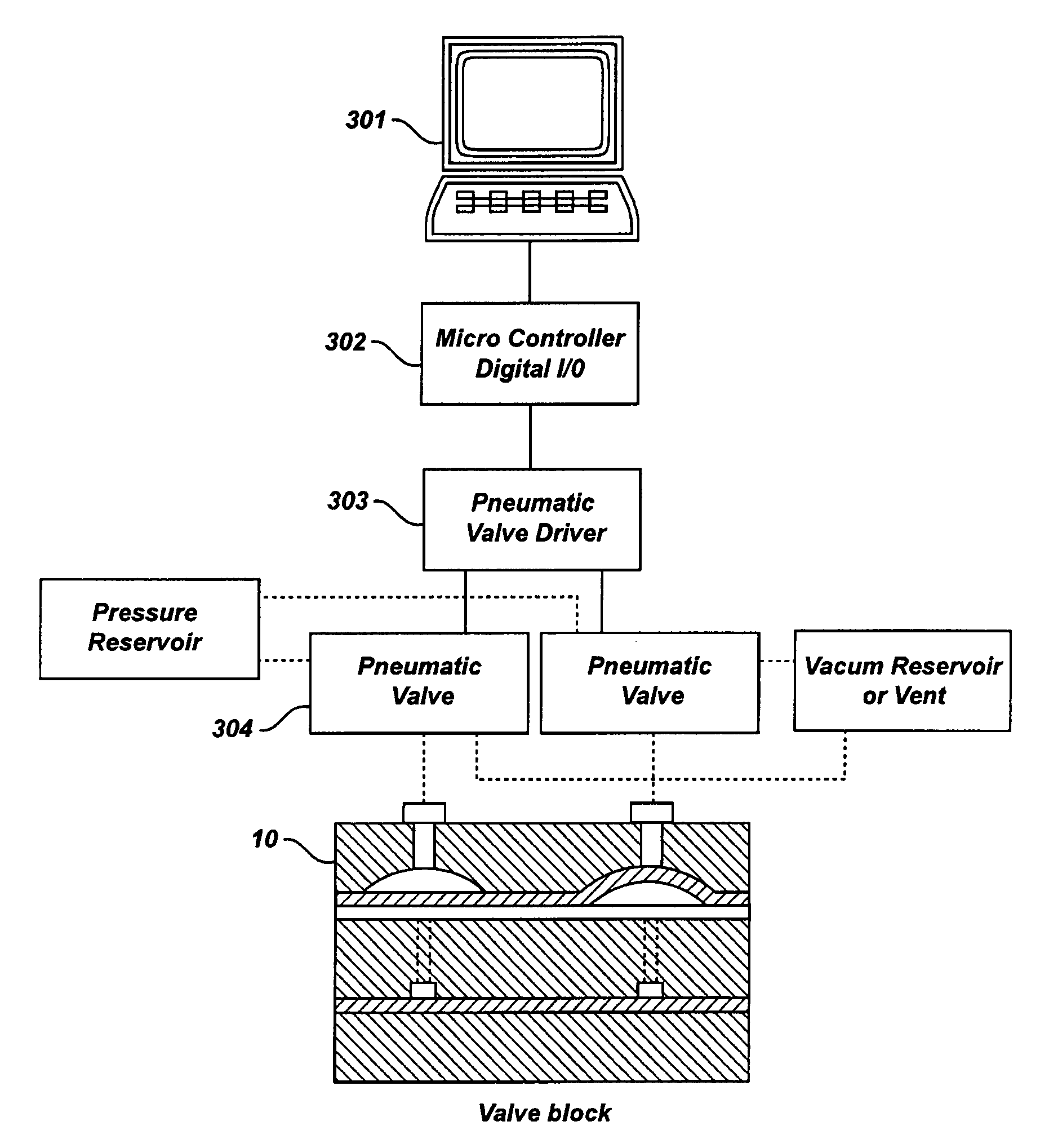
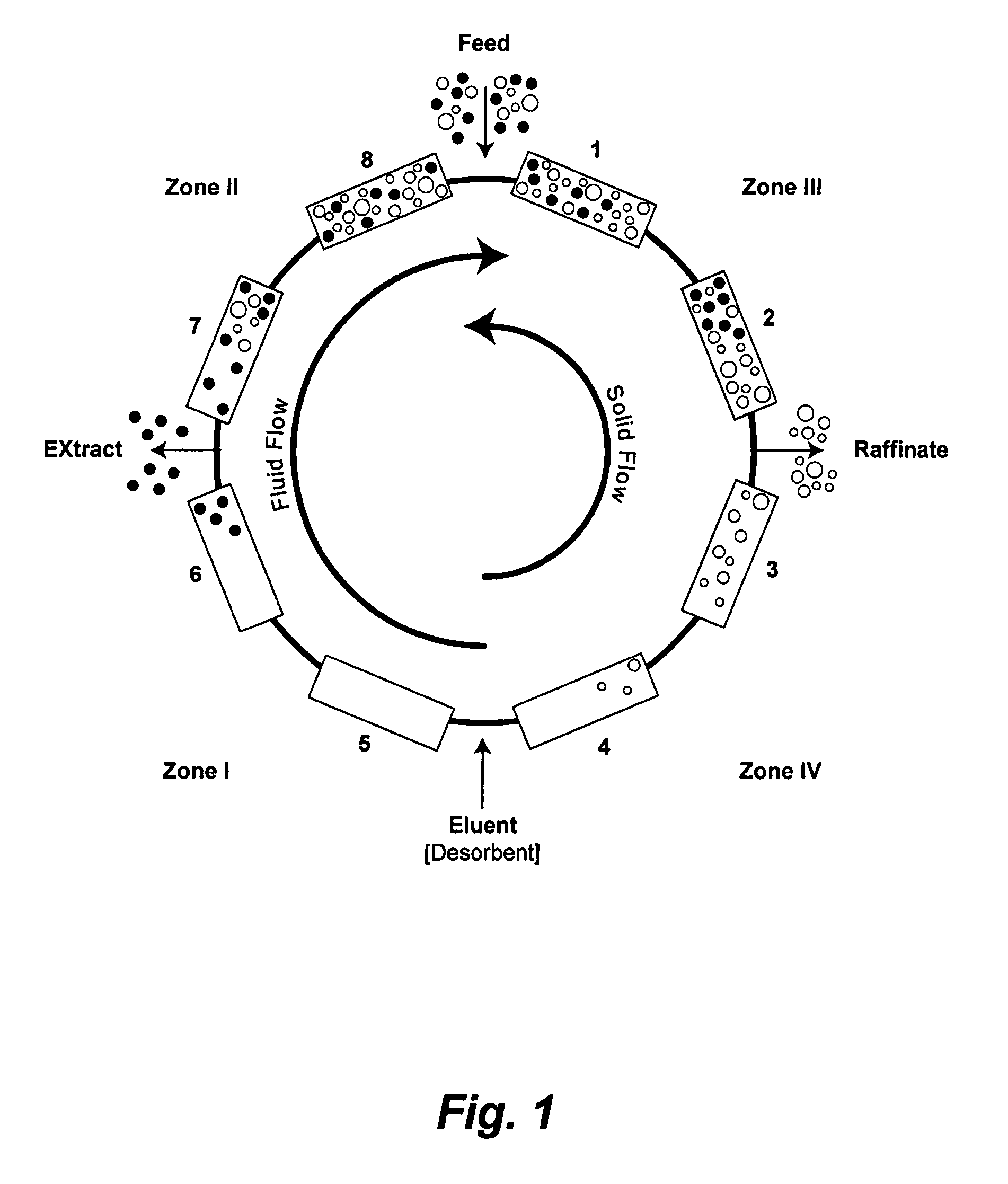
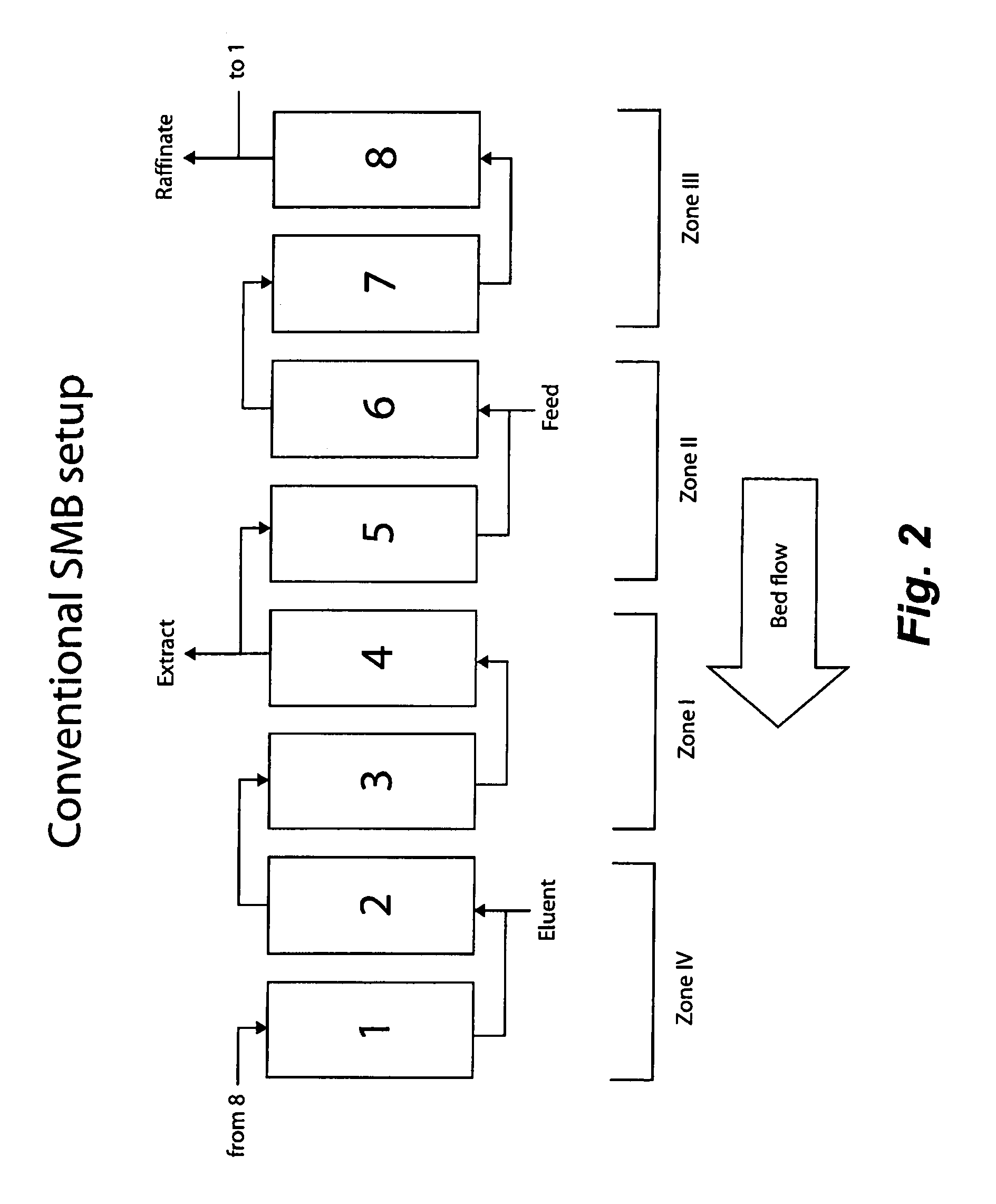
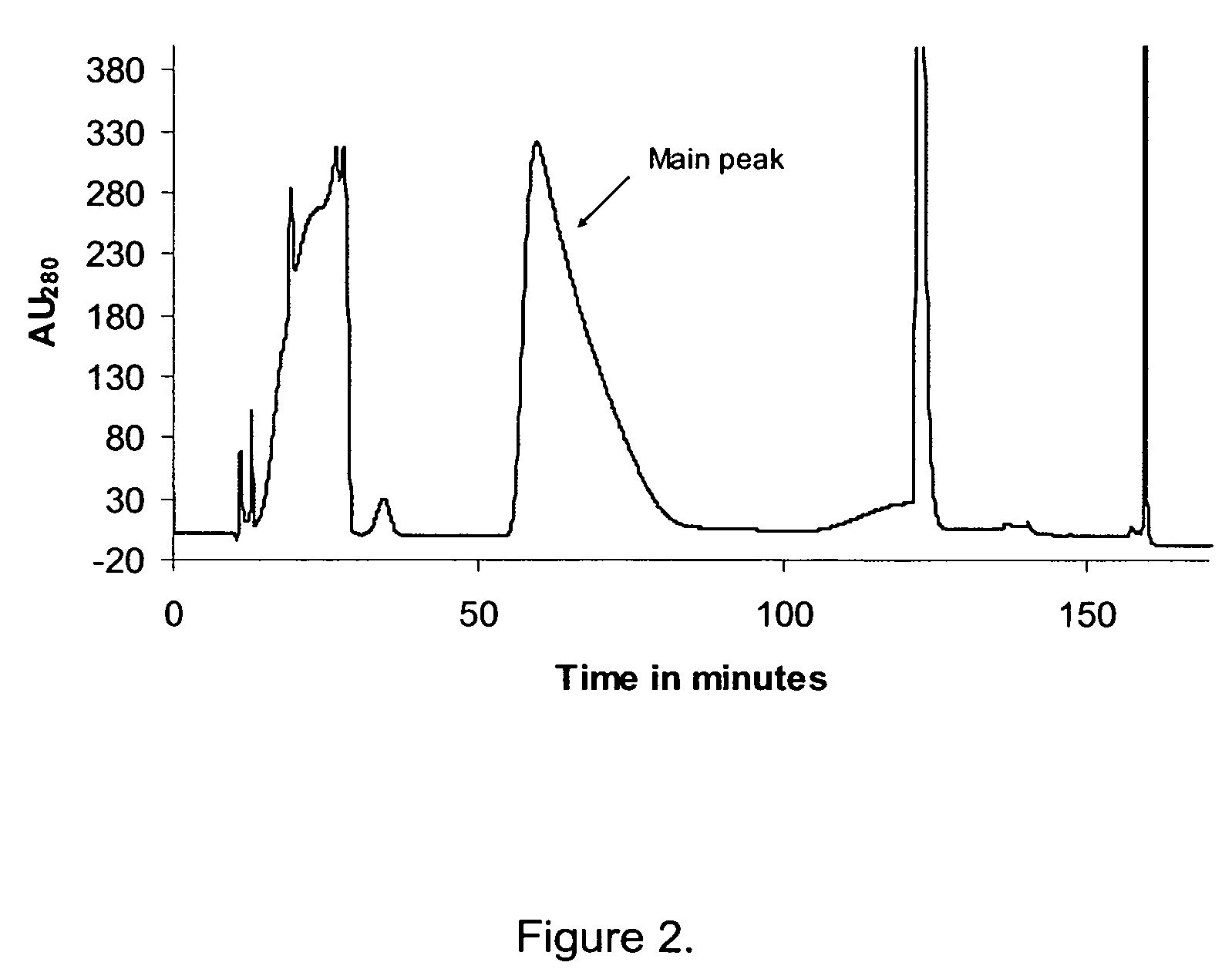
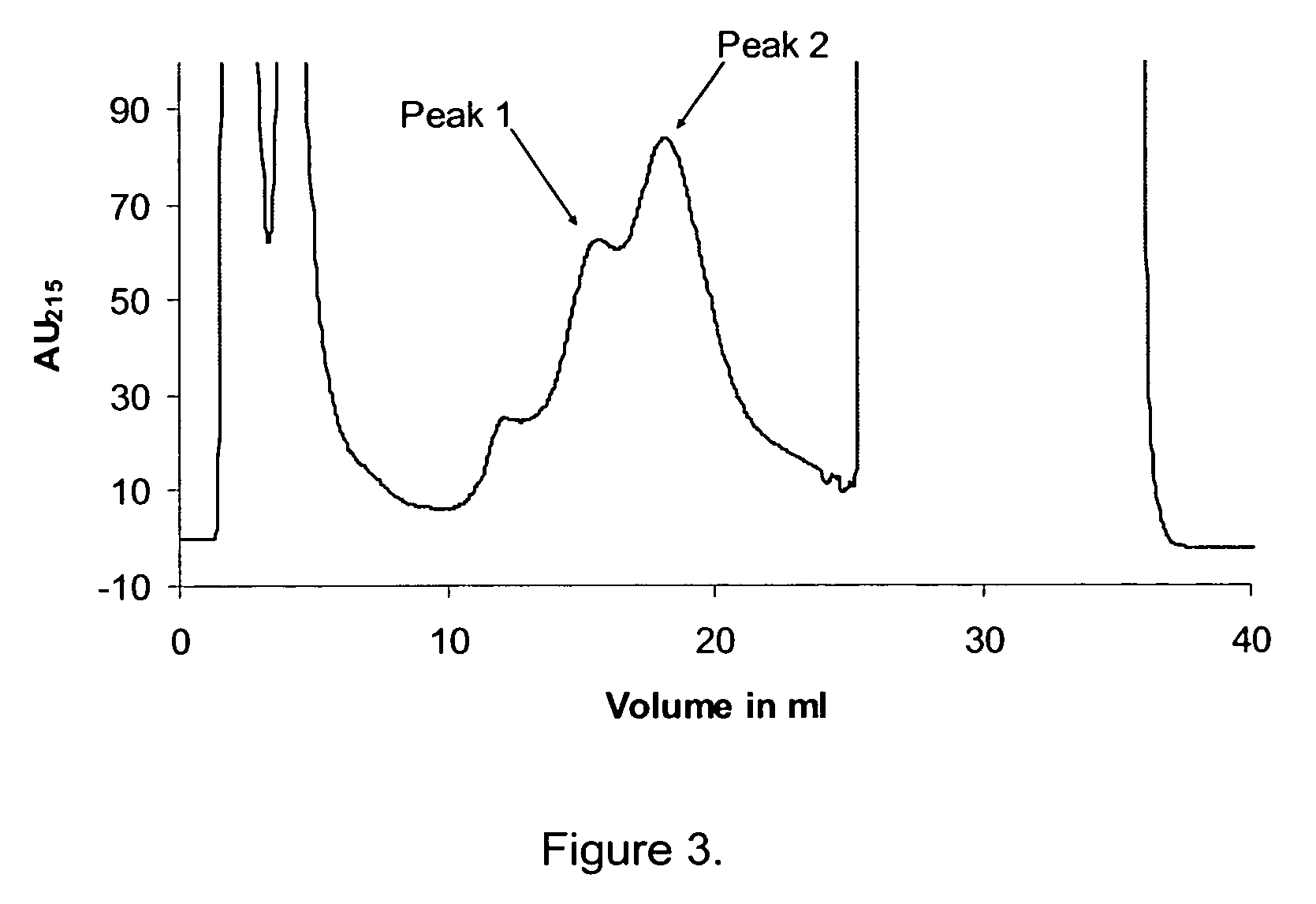
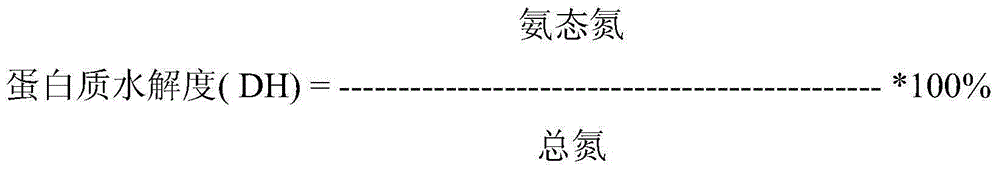
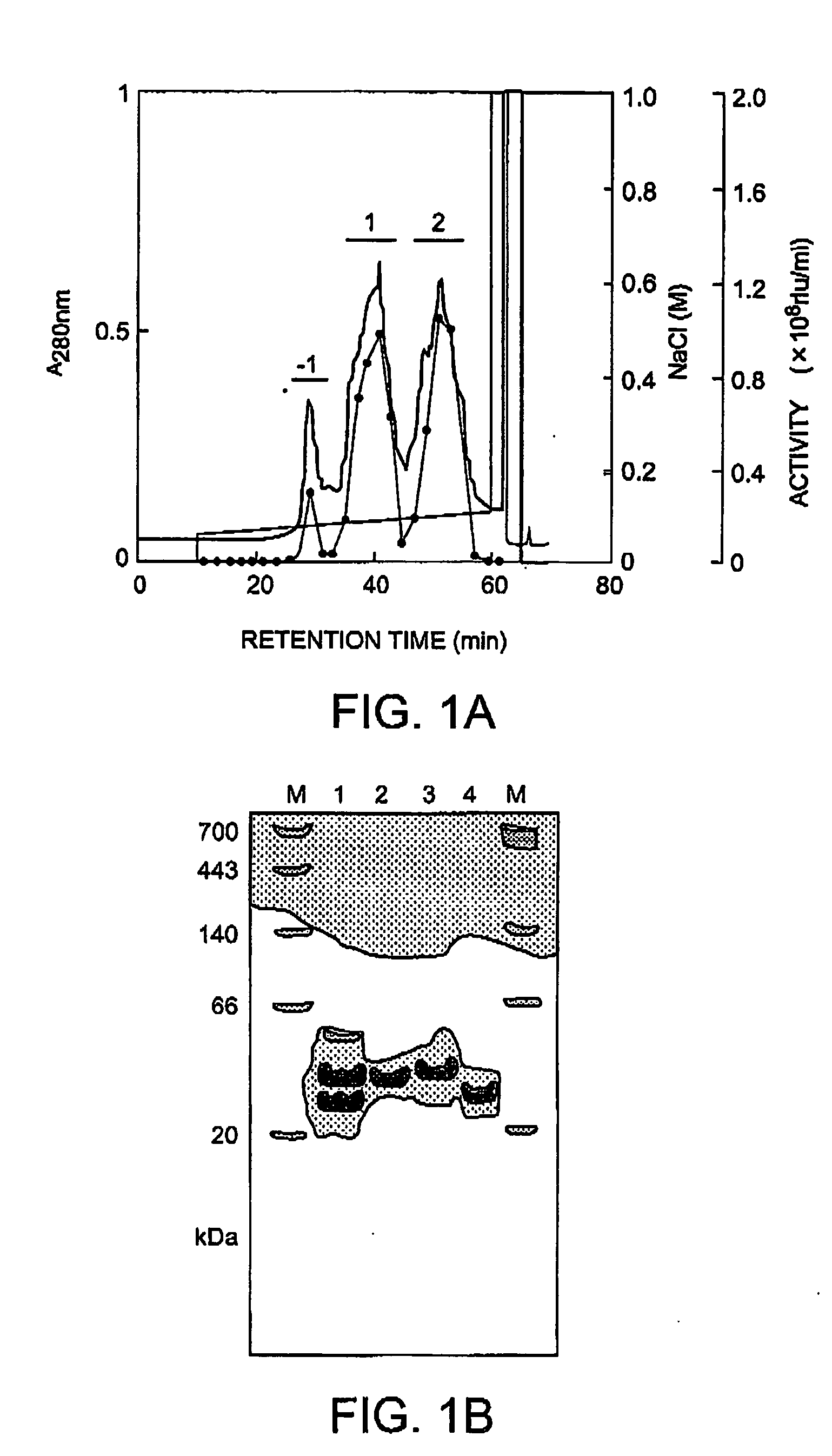
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
806 results about "CMC chromatography" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
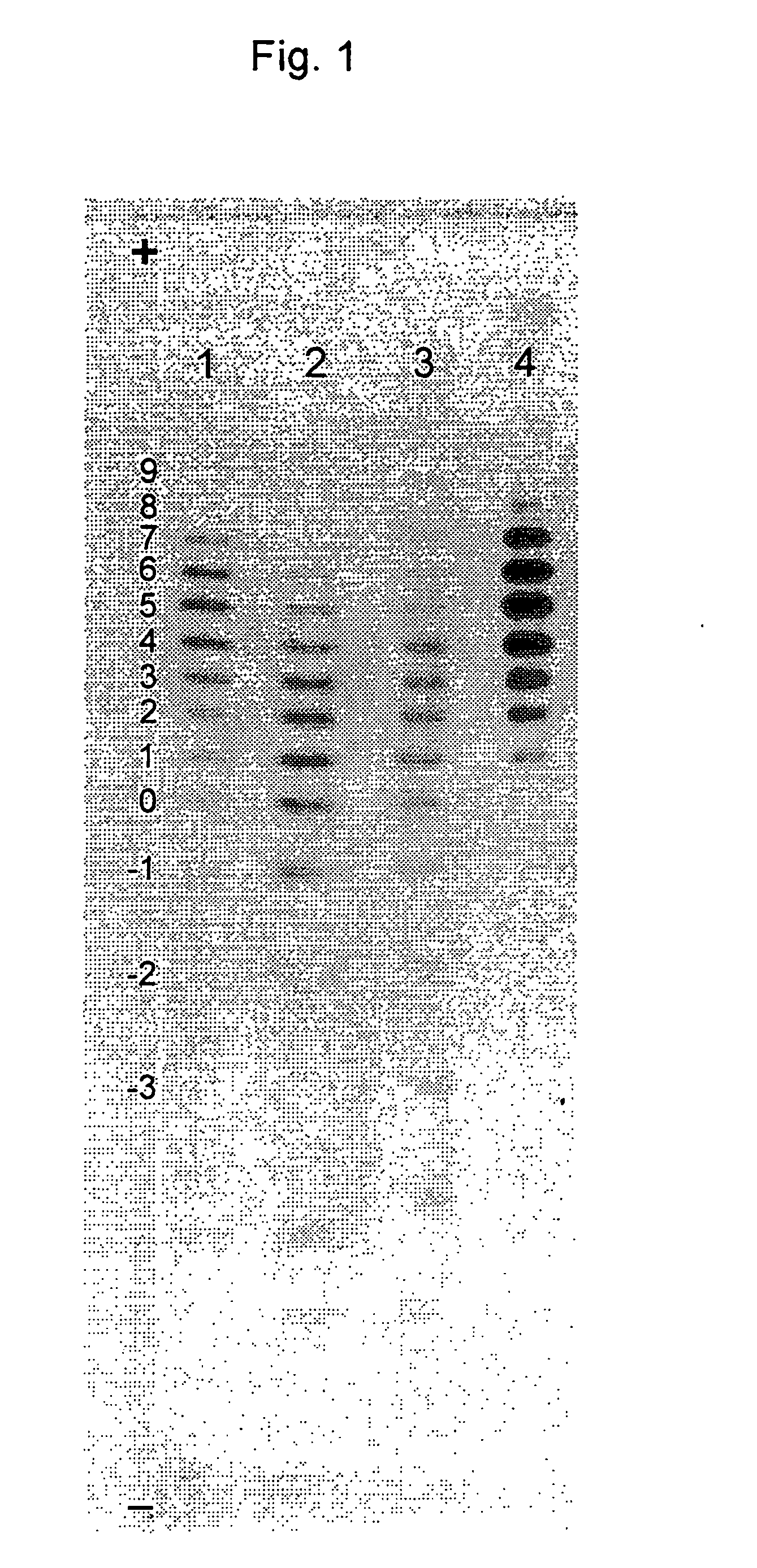
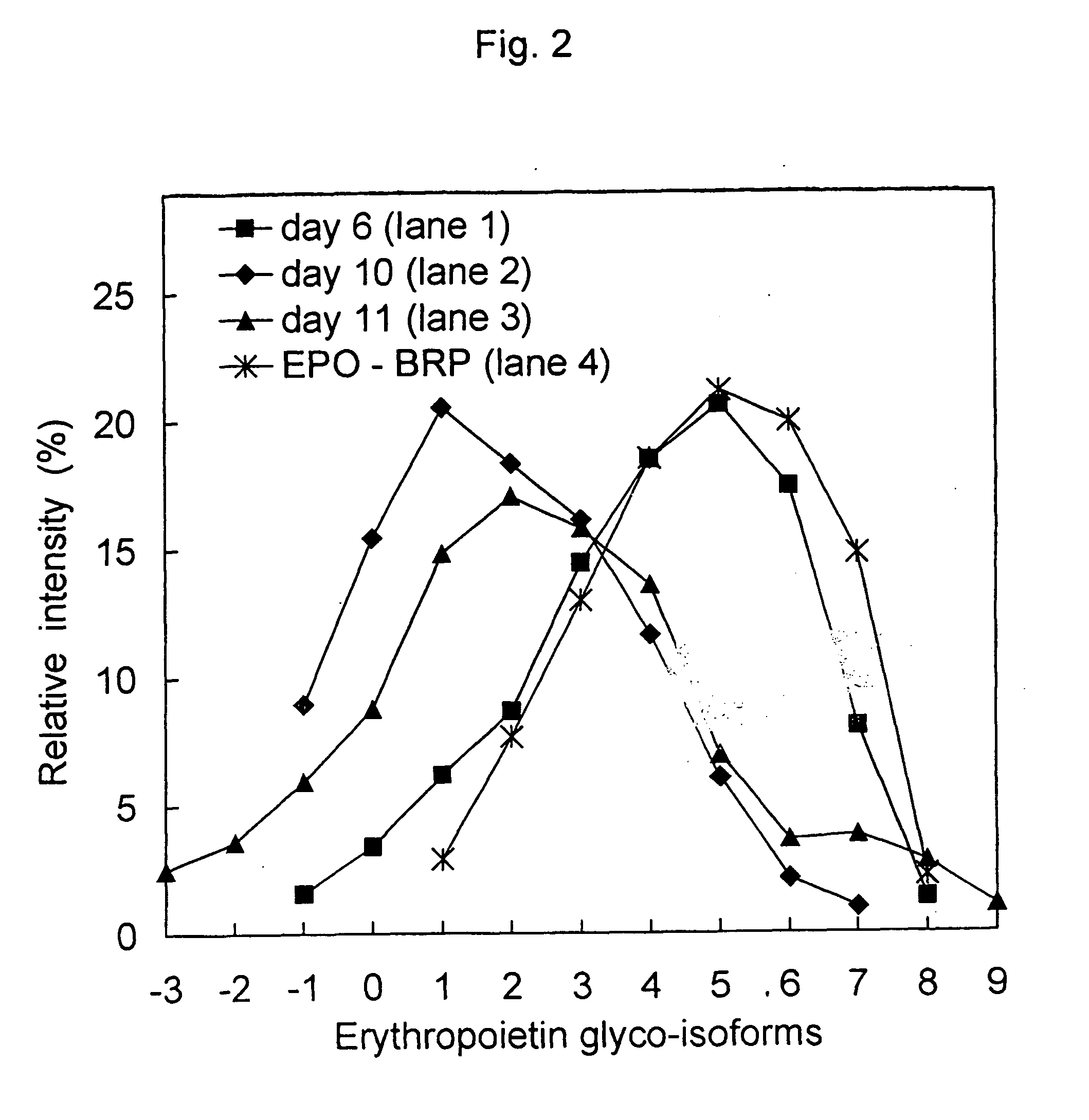
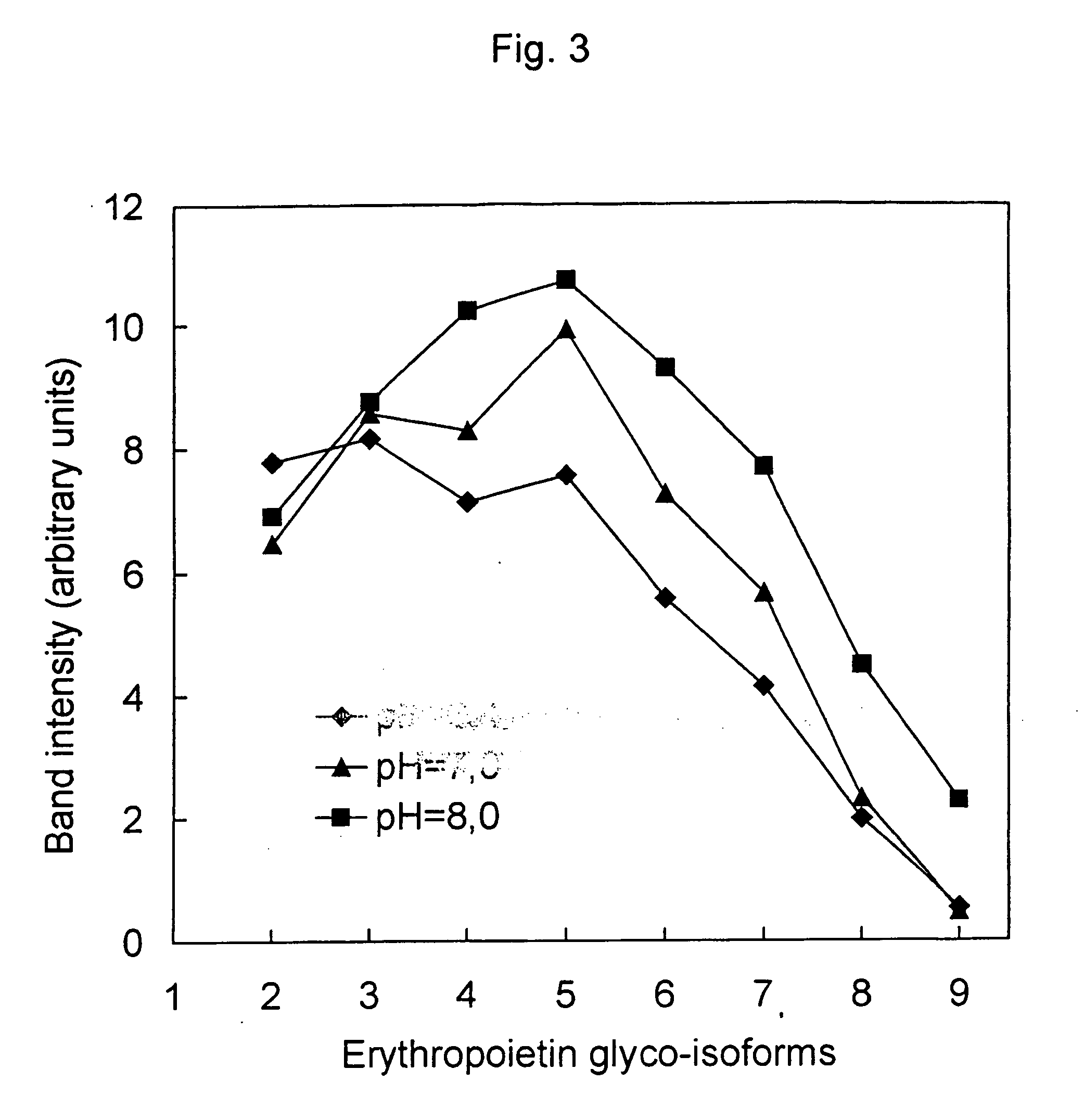
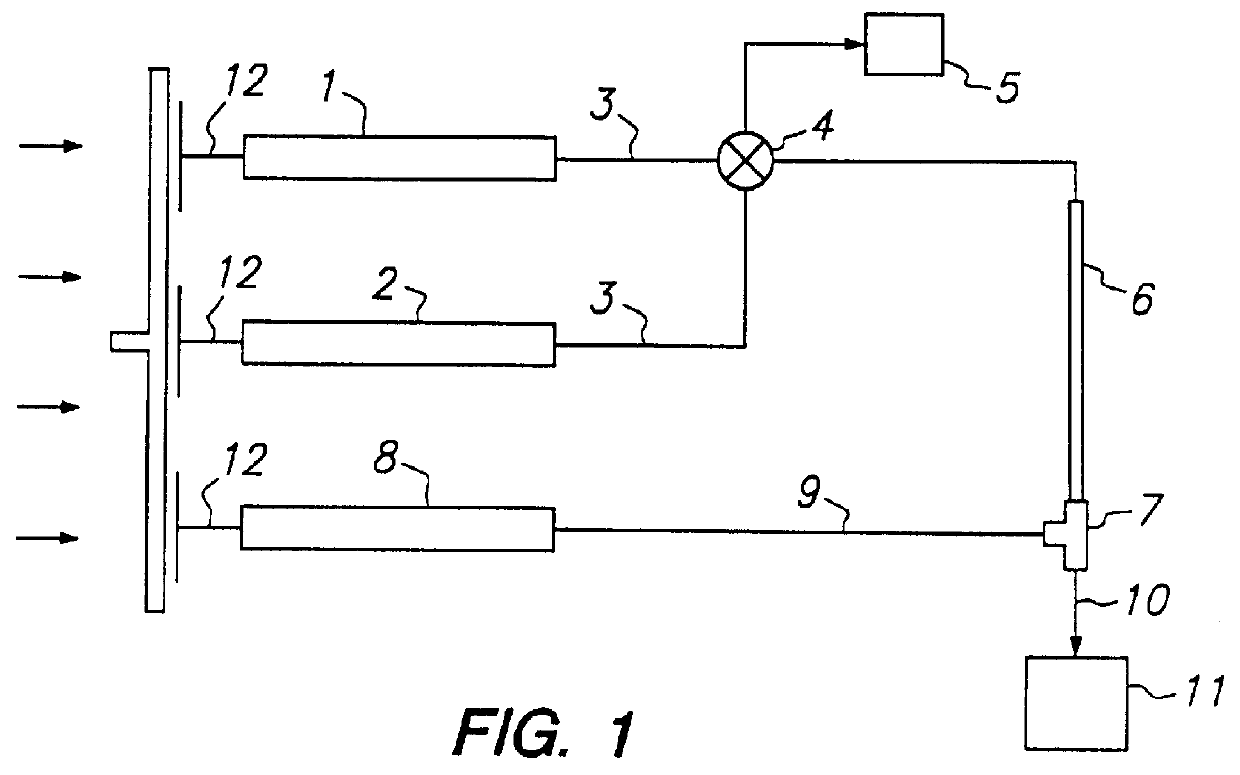
Process for the preparation of a desired erythropoietin glyco-isoform profile
InactiveUS20050153879A1High and uniform product specificityImprove product qualityPeptide/protein ingredientsComponent separationFiltrationRed blood cell
The present invention provides a process for the production of erythropoietin (EPO) with high purity and with a desired profile of EPO glycol-isoforms by using a combination of specific chromatographic steps in such a manner that the starting EPO glycol-isoform profile is changed or modified. The applied chromatographic steps includes at least (a) dye affinity chromatography, and (b) hydrophobic chromatography and / or (c) anion-exchange chromatography. In a preferred embodiment, the process further includes (d) gel filtration chromatography. The present invention also provides a process for the determination of erythropoietin (EPO) glycol-isoform profile in an EPO containing composition.
Owner:SVETINA MONICA +4
Enhanced purification of antibodies and antibody fragments by apatite chromatography
ActiveUS20090187005A1Peptide/protein ingredientsPeptide preparation methodsApatiteAntibody fragments
Methods are disclosed for use of apatite chromatography, particularly without reliance upon phosphate gradients, for purification or separation of at least one intact non-aggregated antibody, or at least one immunoreactive antibody fragment, from an impure preparation. Integration of such methods into multi-step procedures with other fractionation methods are additionally disclosed.
Owner:BIO RAD LAB INC
Apparatus for screening compound libraries
InactiveUS6054047AComponent separationIon-exchanger regenerationChemical compoundCombinatorial chemistry
Disclosed are apparatus for screening compound libraries using frontal chromatography in combination with mass spectrometry to identify and rank those members of the library that bind to a target receptor. The apparatus of this invention also permit a compound library to be rapidly screened to determine if any member of the library has a higher affinity for the target receptor relative to a pre-selected indicator compound.
Owner:SYNSORB BIOTECH INC
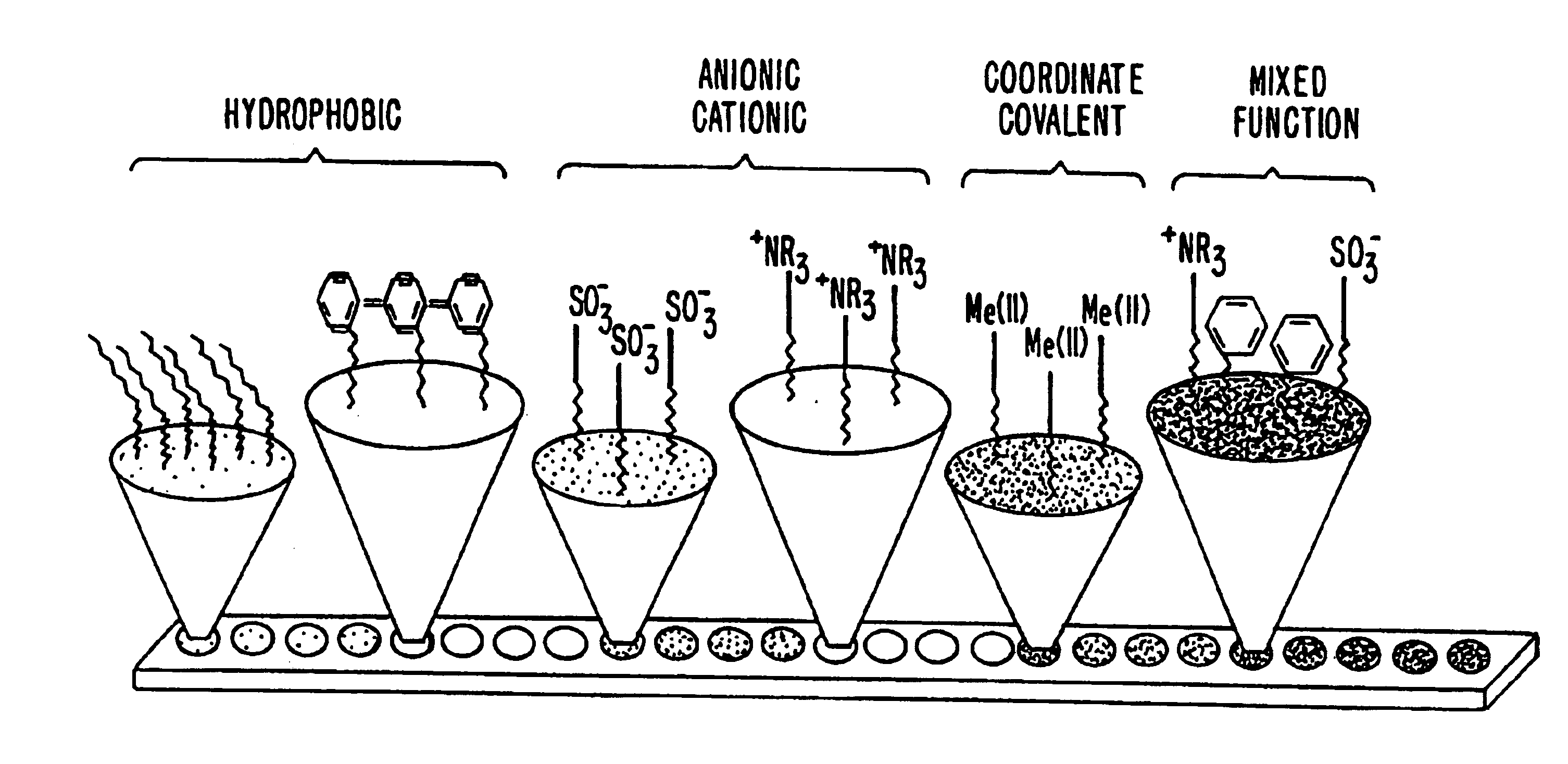
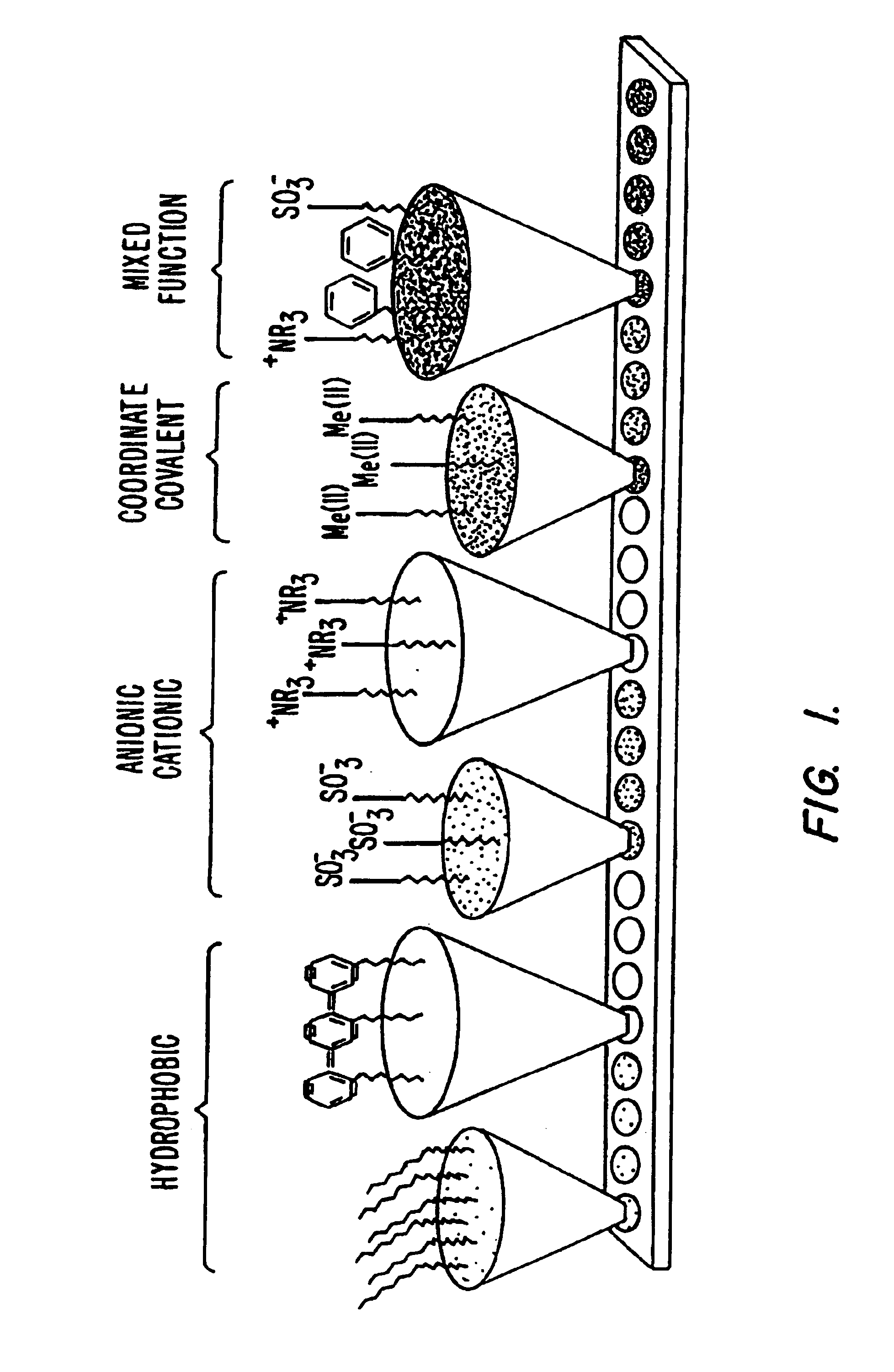
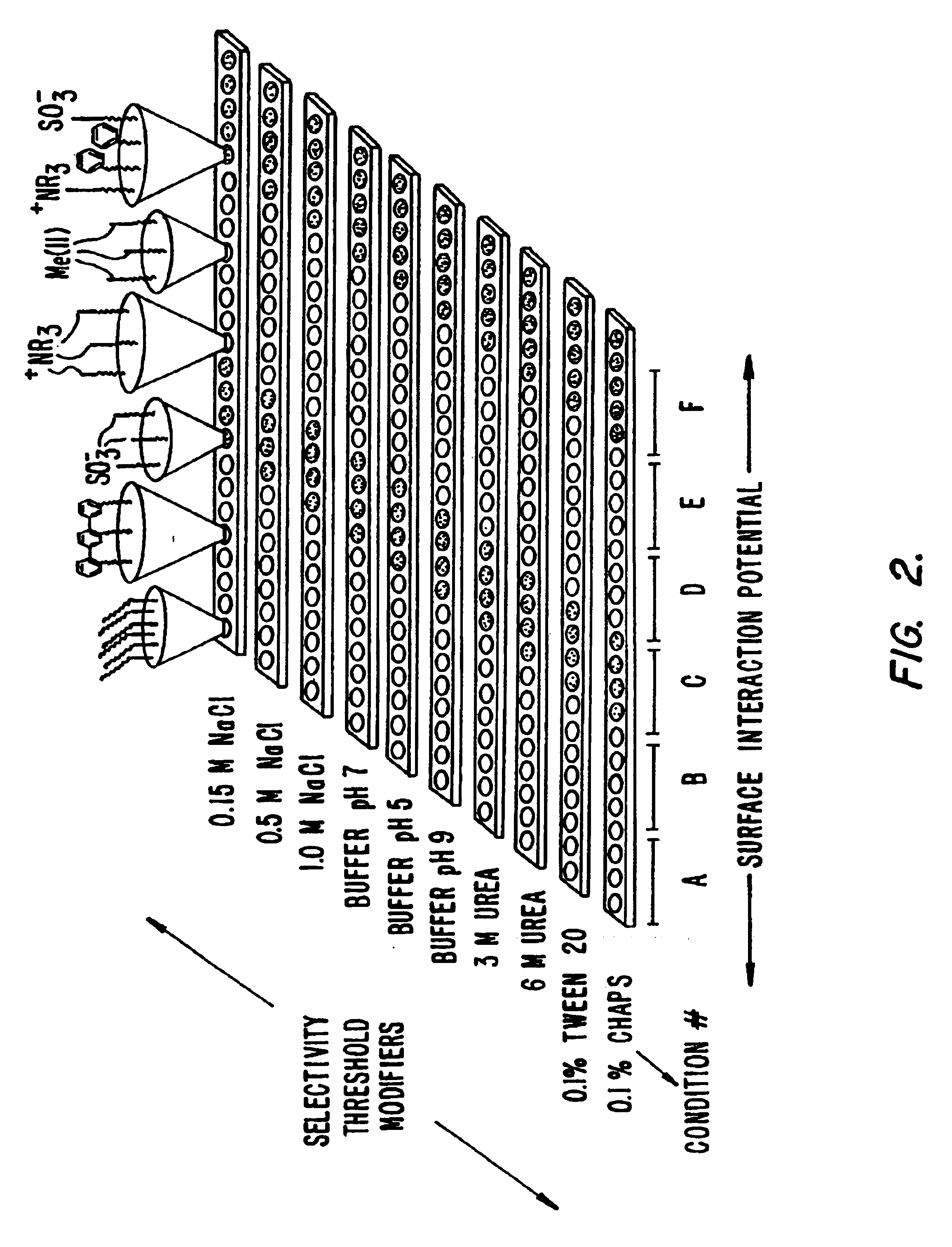
Retentate chromatography and protein chip arrays with applications in biology and medicine
InactiveUS6881586B2Rapid and multi-dimensional characterizationHigh resolutionBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteDesorption
This invention provides methods of retentate chromatography for resolving analytes in a sample. The methods involve adsorbing the analytes to a substrate under a plurality of different selectivity conditions, and detecting the analytes retained on the substrate by desorption spectrometry. The methods are useful in biology and medicine, including clinical diagnostics and drug discovery.
Owner:BIO RAD LAB INC
Purification of proteins using hydrophobic interaction chromatography
ActiveUS8946395B1Great purification and recovery of proteinGreat purification and recoveryVirus peptidesImmunoglobulins against cytokines/lymphokines/interferonsProtein proteinChemistry
The present invention is directed to methods for purifying a protein of interest, e.g., an antibody, from a sample comprising the protein of interest and at least one impurity, e.g., an aggregate, by employing a hydrophobic interaction chromatography (HIC) method that allows for binding of both the protein of interest and the at least one impurity under strong binding conditions. The present invention is based, at least in part, on the finding that both flow through and bind-elute techniques can be combined to achieve greater purification and recovery of a protein of interest, e.g., an antibody, under isocratic wash conditions and strong binding conditions.
Owner:ABBVIE INC
Process for producing immunoglobulins for intravenous administration and other immunoglobulin products
InactiveUS7138120B2Improve administeringAdminister intravenouslySerum immunoglobulinsAntiviralsChemistryIntrathecal
The present invention relates to a process for purifying immunoglobulin G from a crude immunoglobulin-containing plasma protein fraction. Said process includes a number of steps of which the anion exchange chromatography and the cation exchange chromatography are preferably connected in series. An acetate buffer having a pH of about 5.0-6.0 and having a molarity of about 5-25 mM is preferably used throughout the purification process. The invention further comprises an immunoglobulin product which is obtainable by this process. The invention also relates to an immunoglobulin product which has a purity of more than 98%, has a content of IgG monomers and dimers of more than 98.5%, has a content of IgA less than 4 mg of IgA / l, and contains less than 0.5% polymers and aggregates. Said product does not comprise detergent, PEG or albumin as a stabilizer. The product is stable, virus-safe, liquid and ready for instant intravenous administration.
Owner:CSL BEHRING AG
Method for the preparation of a high-temperature stable oxygen-carrier-containing pharmaceutical composition and the use thereof
ActiveUS7989593B1Increase oxygenationHigh sensitivityPeptide/protein ingredientsMammal material medical ingredientsArteriolar VasoconstrictionWhite blood cell
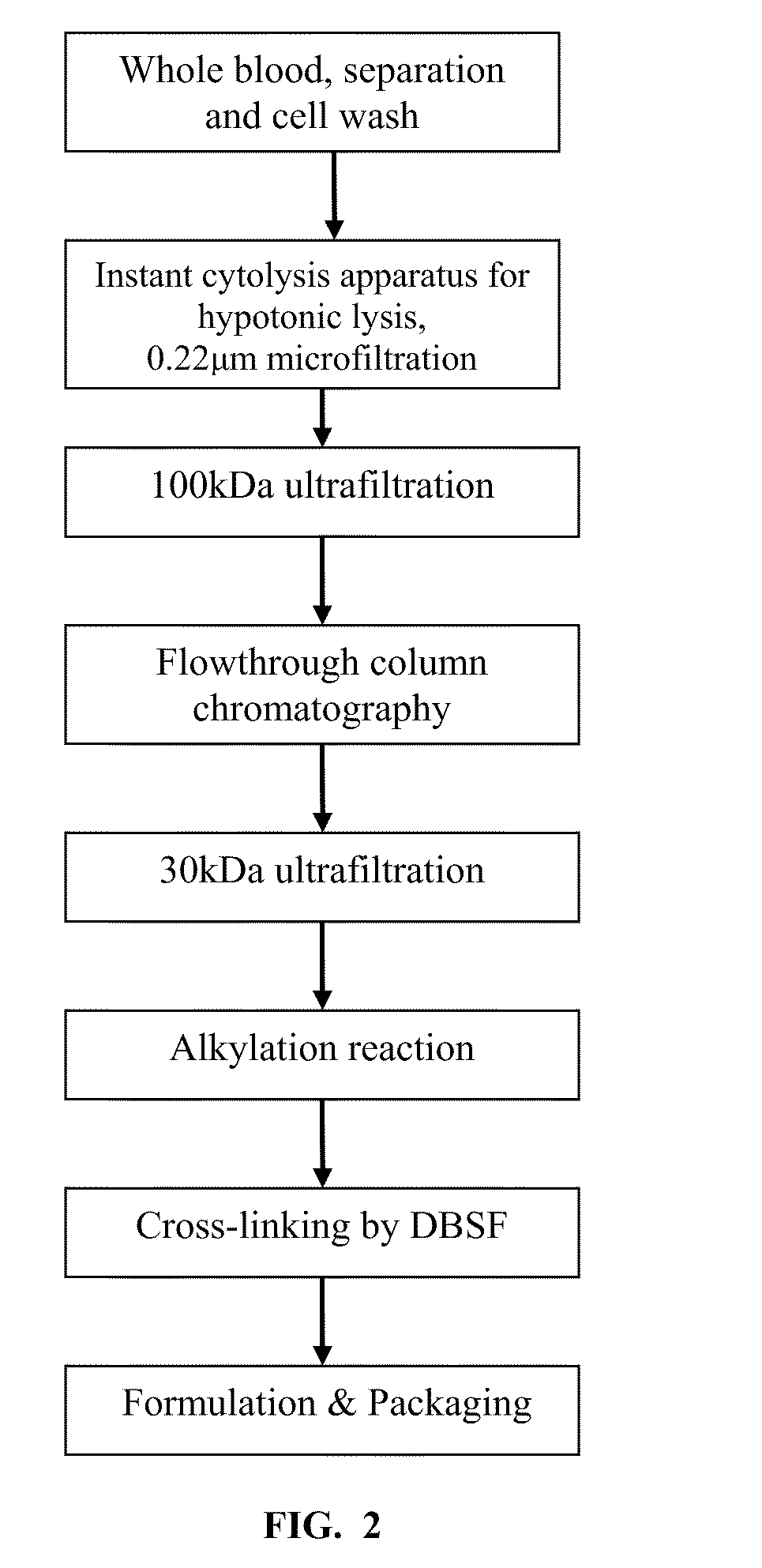
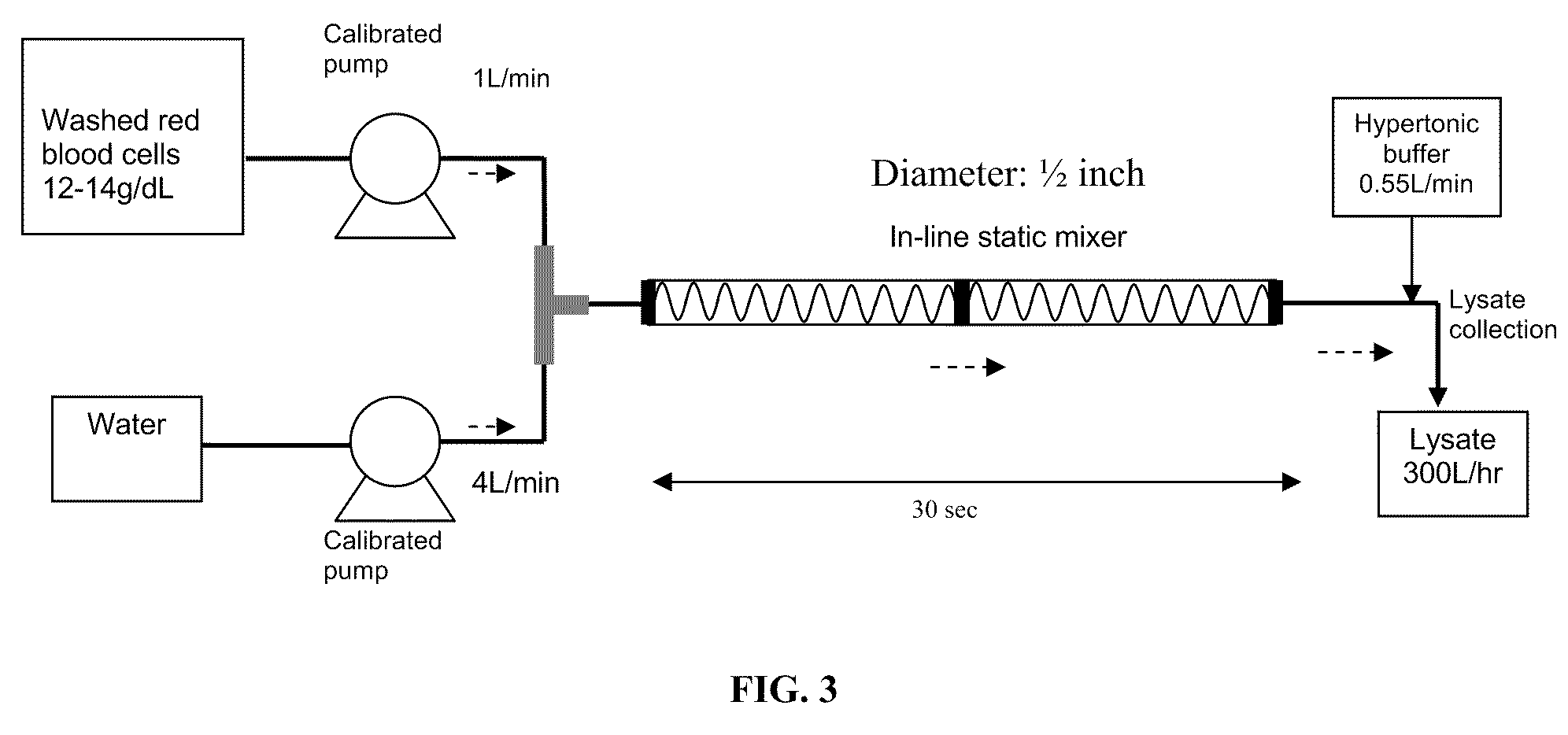
A high temperature-stable and highly purified cross-linked (optionally ≧70% β-β linked) tetrameric hemoglobin with high efficiency of oxygen delivery suitable for use in mammals without causing renal injury and vasoconstriction is provided. The dimeric form of hemoglobin is degenerated and purification processes are performed on red blood cells from whole blood. Controlled hypotonic lysis in an instant cytolysis apparatus prevents lysis of white blood cells. Nucleic acids from white blood cells and phospholipids impurities are not detected. Blocking of reactive sulfhydryl groups by a sulfhydryl reagent is performed in an oxygenated environment. Flowthrough column chromatography removes different plasma protein impurities. N-acetyl cysteine is added to the cross-linked tetrameric hemoglobin to maintain a low level of met-hemoglobin. The stabilized hemoglobin is preserved in an infusion bag with aluminum overwrap to prevent formation of inactive met-hemoglobin from oxygen intrusion. The product finds use in tissue oxygenation and cancer treatment.
Owner:BILLION KING INT
Hydroxyl carthamus tinctorius yellow colour A, preparation method and application thereof
ActiveCN101195647AGood product qualityFine particleOrganic active ingredientsSugar derivativesCarthamusChemistry
The invention relates to a hydroxysaffloryellow A extracted from Chinese medicinal material safflower, a relative preparation method and application thereof. The inventive extraction method of hydroxysaffloryellow A is stuffed into chromatography column according to different diameter-height ratios, the invention uses the upper sample to process column chromatography according to different ratios between upper sample volumes and bed volumes, to separate and purify safflower extract. The invention has simple process, few steps, low cost, high yield, no environment pollution and suitability for industrialized and large-scale production. The yield of extracted hydroxysaffloryellow A is higher than 50%, while the hydroxysaffloryellow A content tested by high-effect liquid hydroxysaffloryellow A reaches 99.5%. The drug prepared from the hydroxysaffloryellow A can effectively prevent and treat cerebrovascular diseases as cerebral infraction and hypertensive cerebral hemorrhage or the like.
Owner:山西德元堂药业有限公司
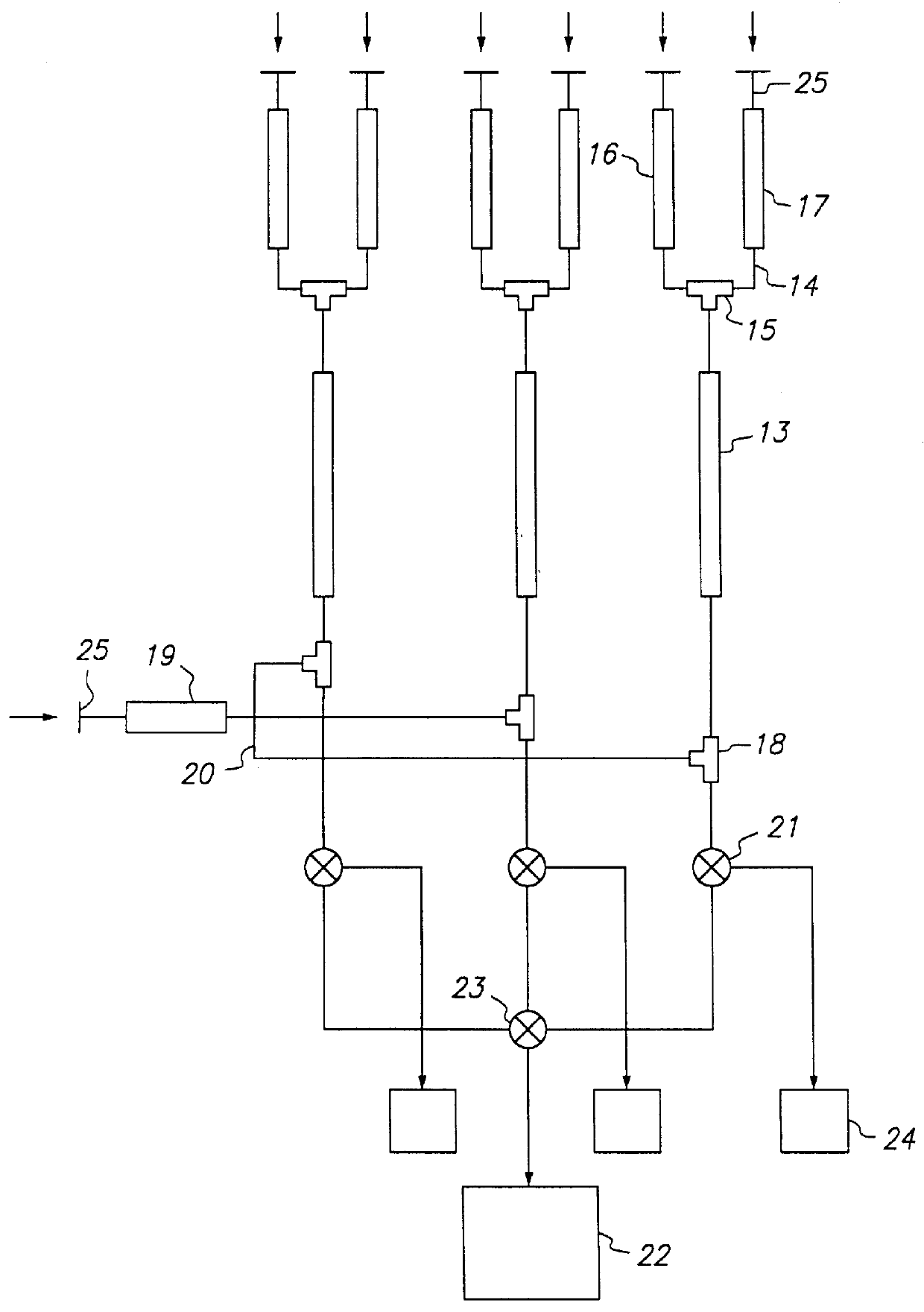
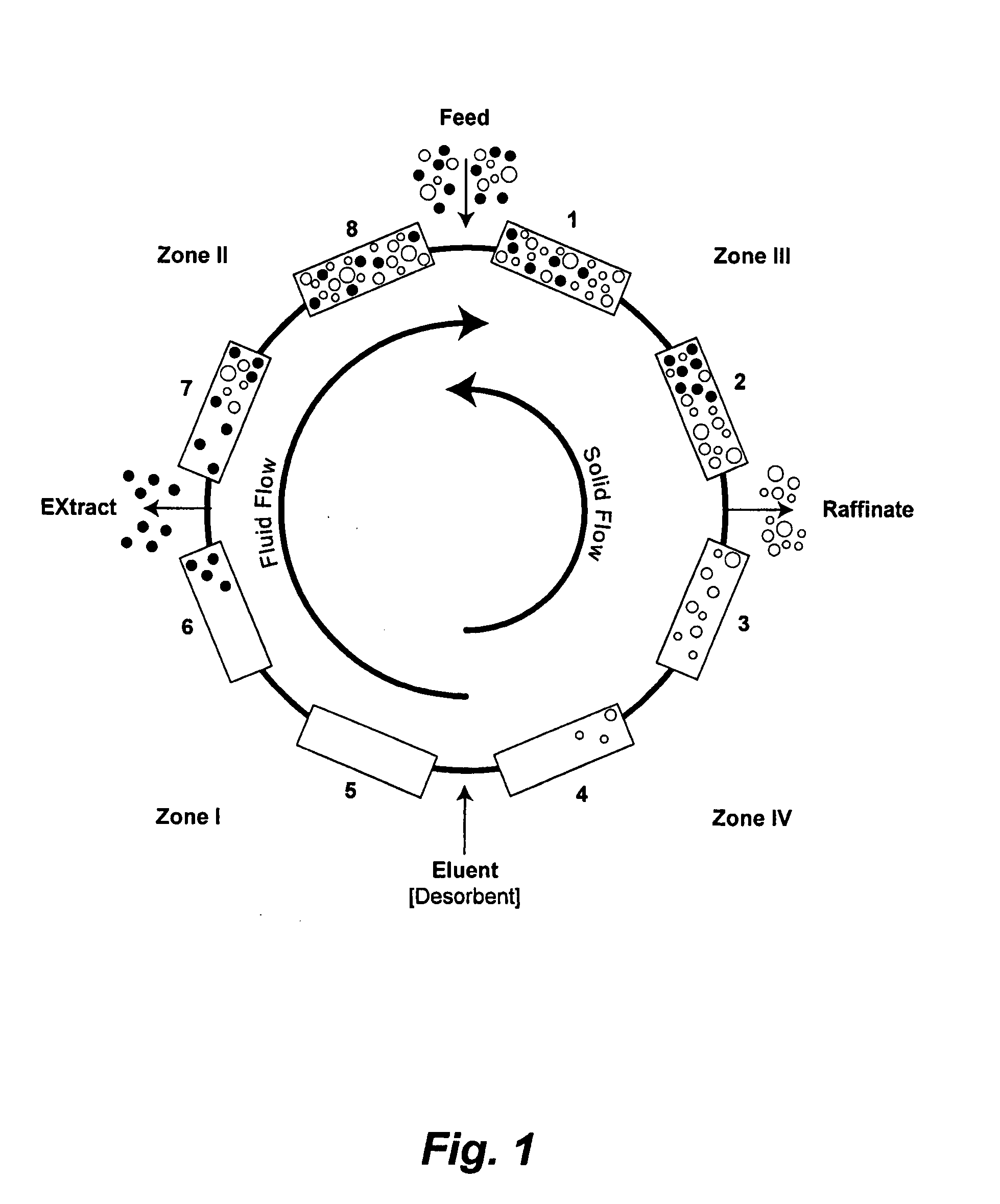
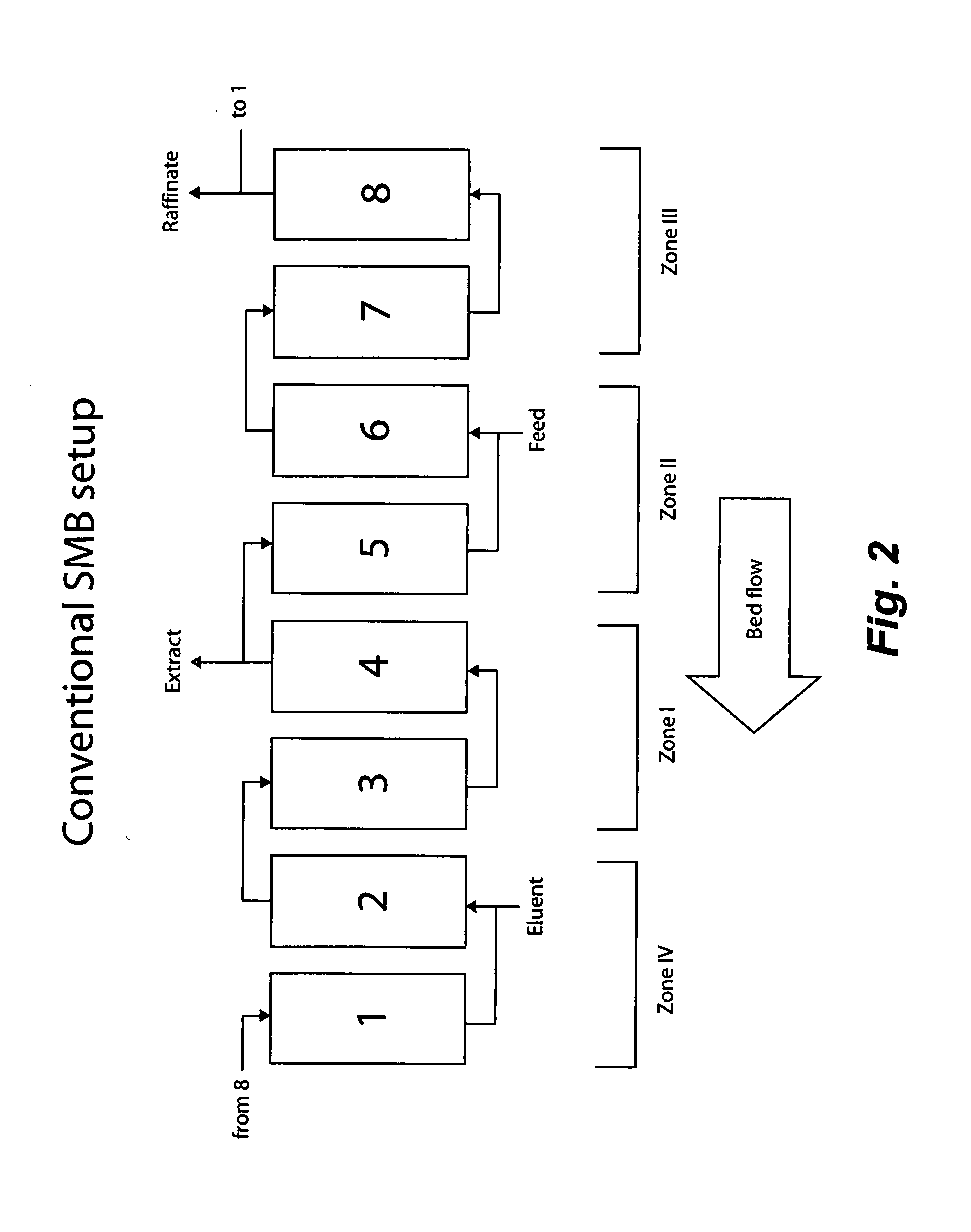
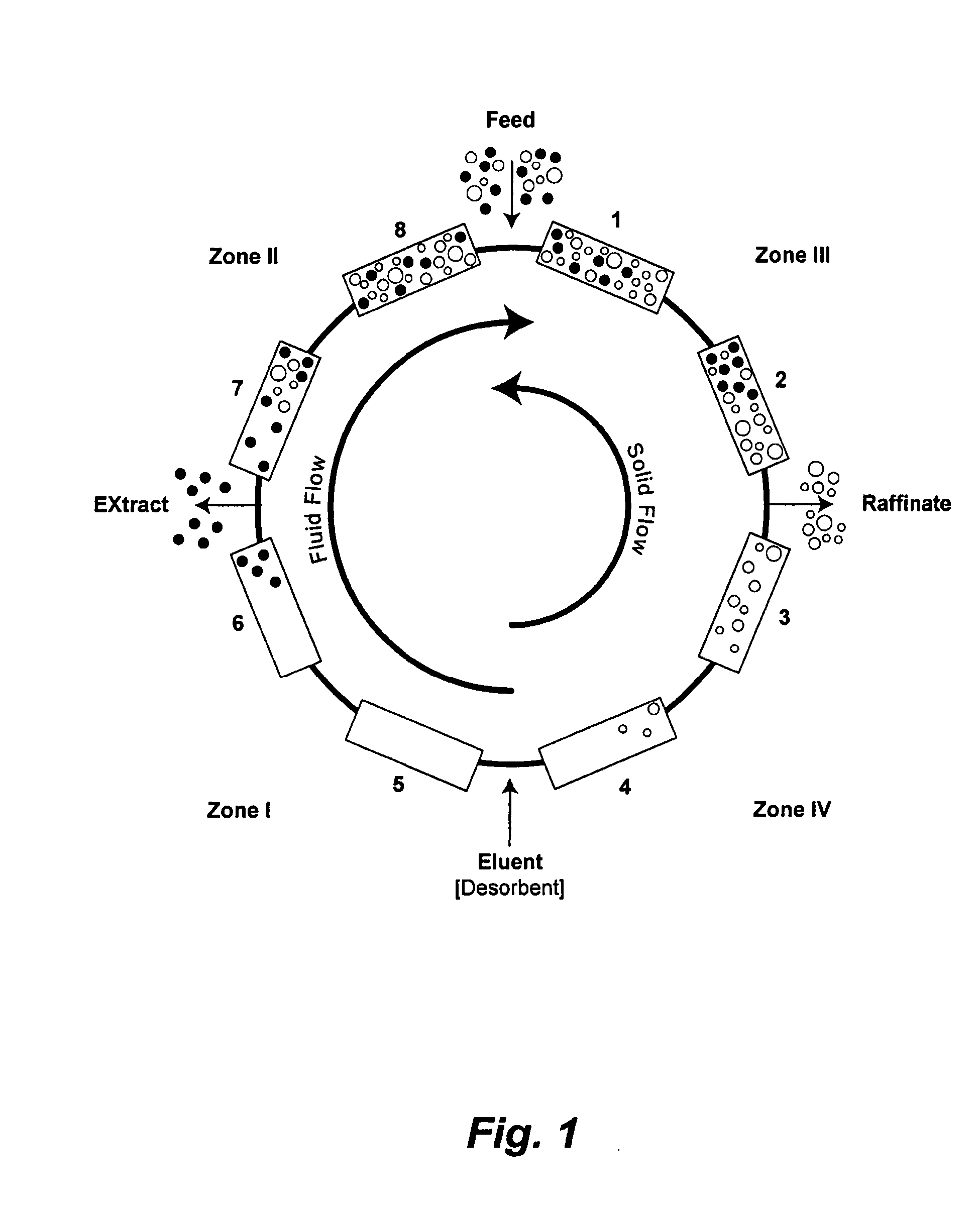
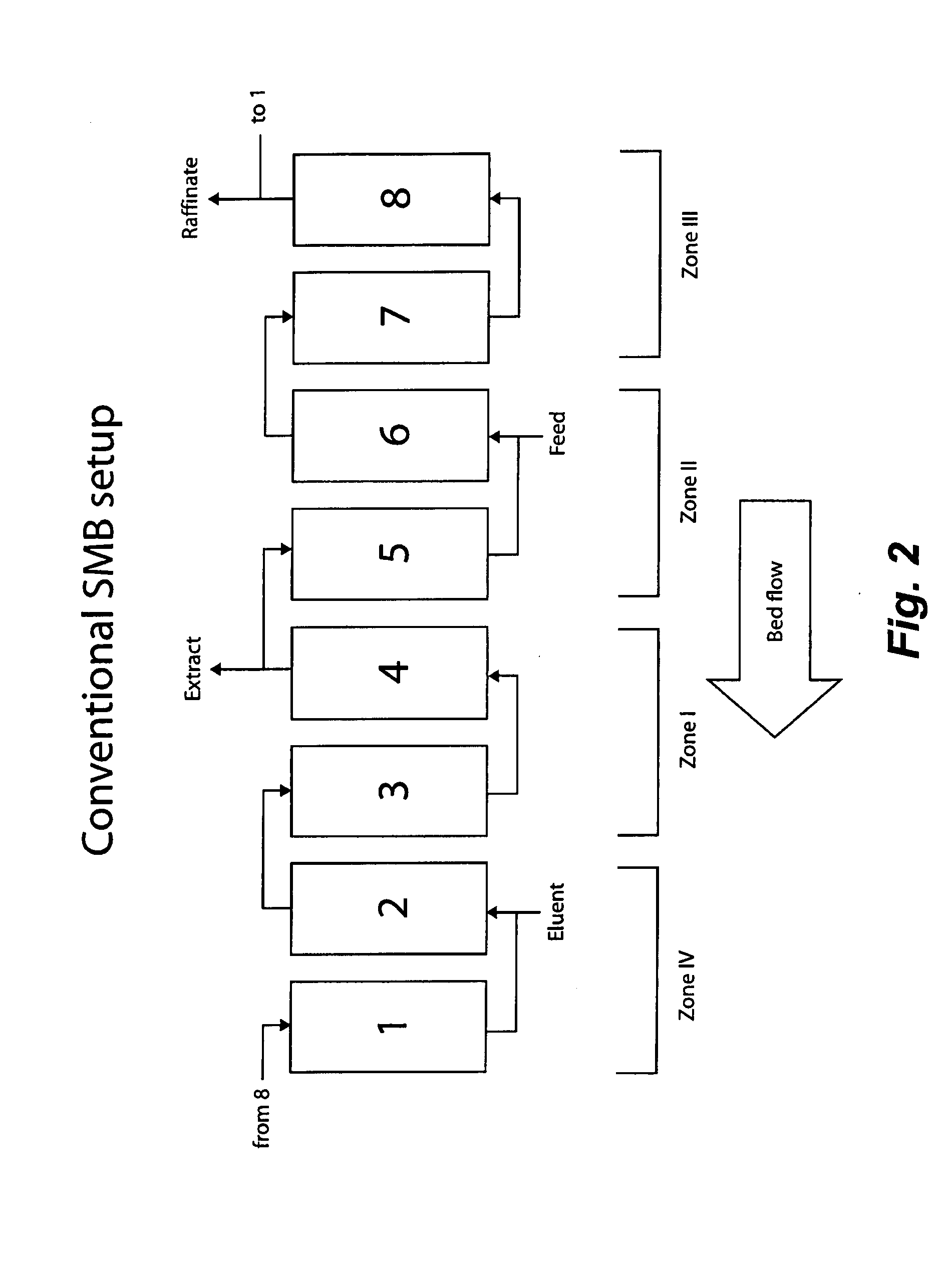
Control System For Simulated Moving Bed Chromatography
ActiveUS20080053917A1Simple and easily programmable controlPromote repairSolid sorbent liquid separationWater/sewage treatmentControl systemSimulated moving bed
The present invention provides devices and methods for micro-scale simulated moving bed chromatography (SMB) for continuous preparation of analytic quantities of highly pure fractions of target molecules. The present apparatus and method of the invention is adapted in a preferred embodiment to separations by affinity chromatography involving three discontinuous liquid flow loops. An alternative embodiment of affinity chromatography utilizes standard SMB operating under isocratic conditions.
Owner:TOSOH BIOSCIENCE LLC
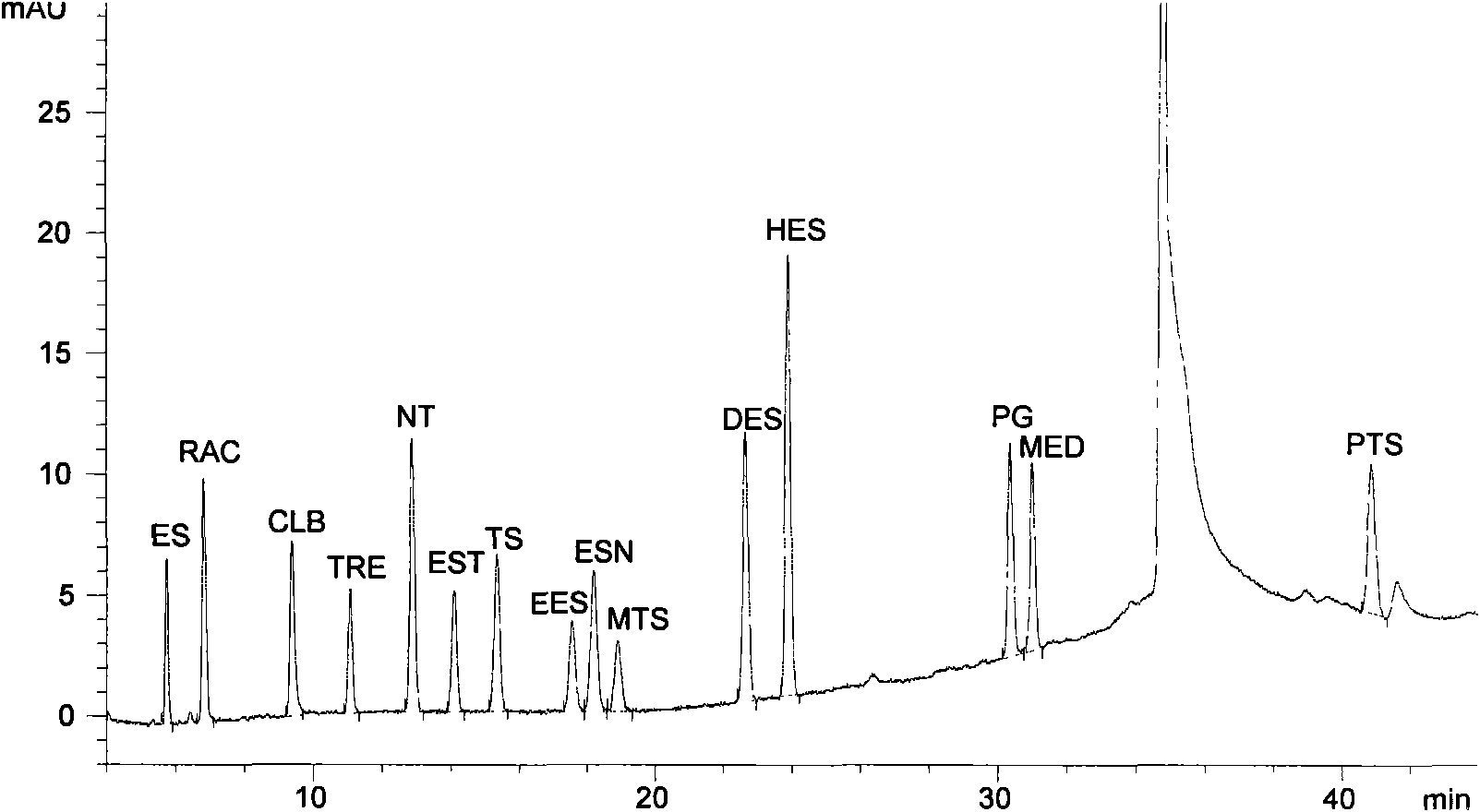
Liquid chromatography for synchronously detecting 15 anabolic hormone residues in food
ActiveCN101551362ALow toxicityLow costComponent separationPreparing sample for investigationWater bathsHplc method
The invention relates to a liquid chromatography for detecting anabolic hormone drug residue in animal-derived food, which is characterized by first sampling: animal musculature is taken off the fat and connective tissue, then minced and evenly ground, and the sample is weighed; extracting: anabolic hormone extract is extracted from the weighed sample by methanol ultrasonic extraction, the extract is evaporated to dryness in water bath through a rotary evaporator, and the residue is dissolved by methanol aqueous solution; purifying: C18 Solidoid extraction column on the extract is carried out solid phase extraction; and testing by devices: the filtrate is tested by opposite phase high efficiency liquid chromatography, quantified by external reference method-peak area, and then carried out with binary gradient elution. In the invention, the detected sample is complex biological sample; the established method can complete one detection in about one hour and is simple and fast, with reliable sensitivity and low cost; the method can analyze more veterinary hormone drug species than the synchronous usage of the existing GC or HPLC methods with easier operation, and has lower cost than the existing GC / MS and LC / MS or LC / MS / MS methods with low solvent toxicity.
Owner:上海国矗生物科技有限公司
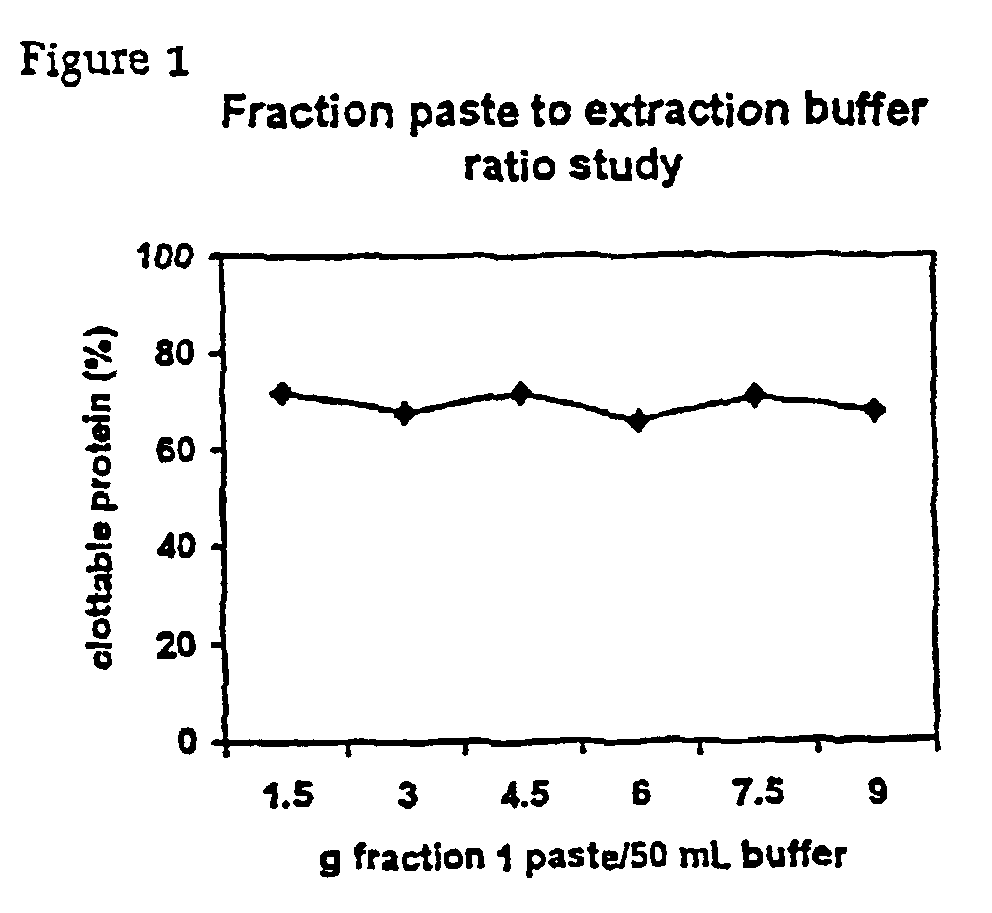
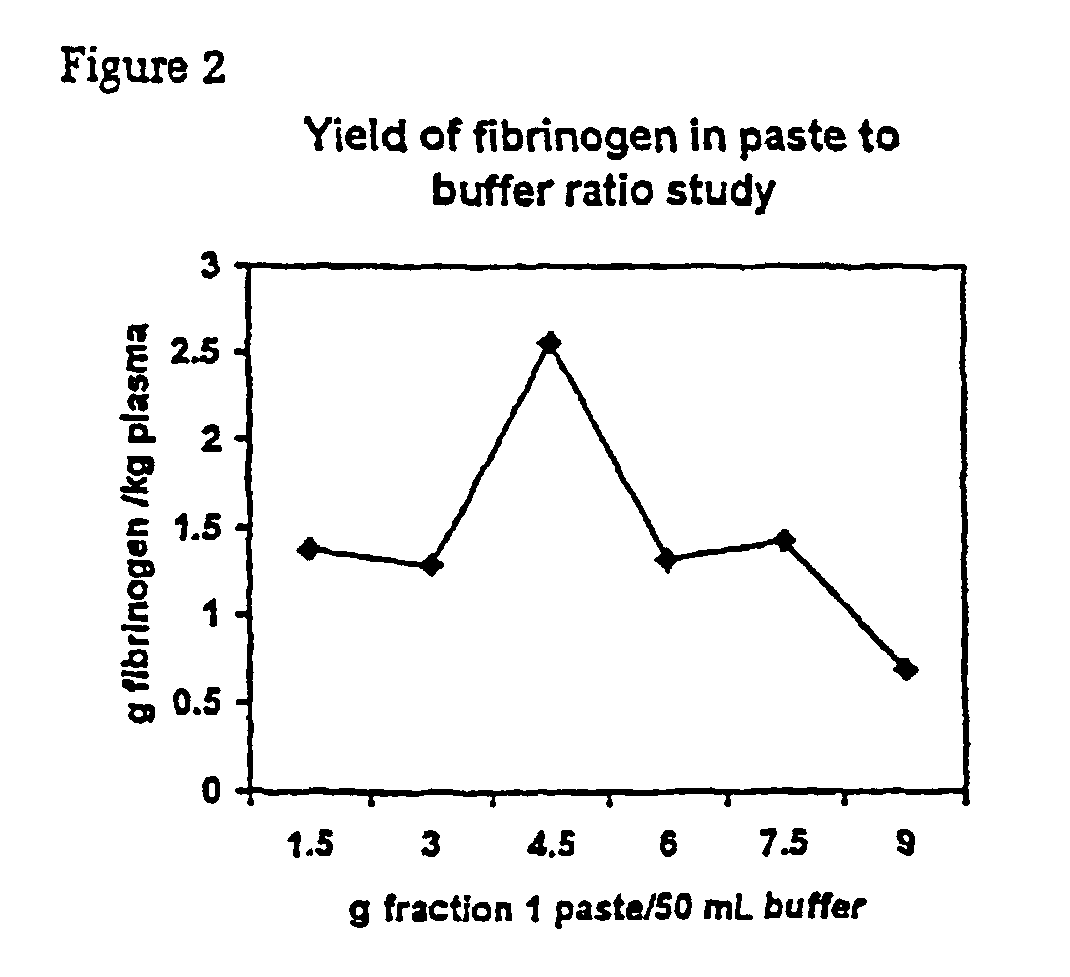
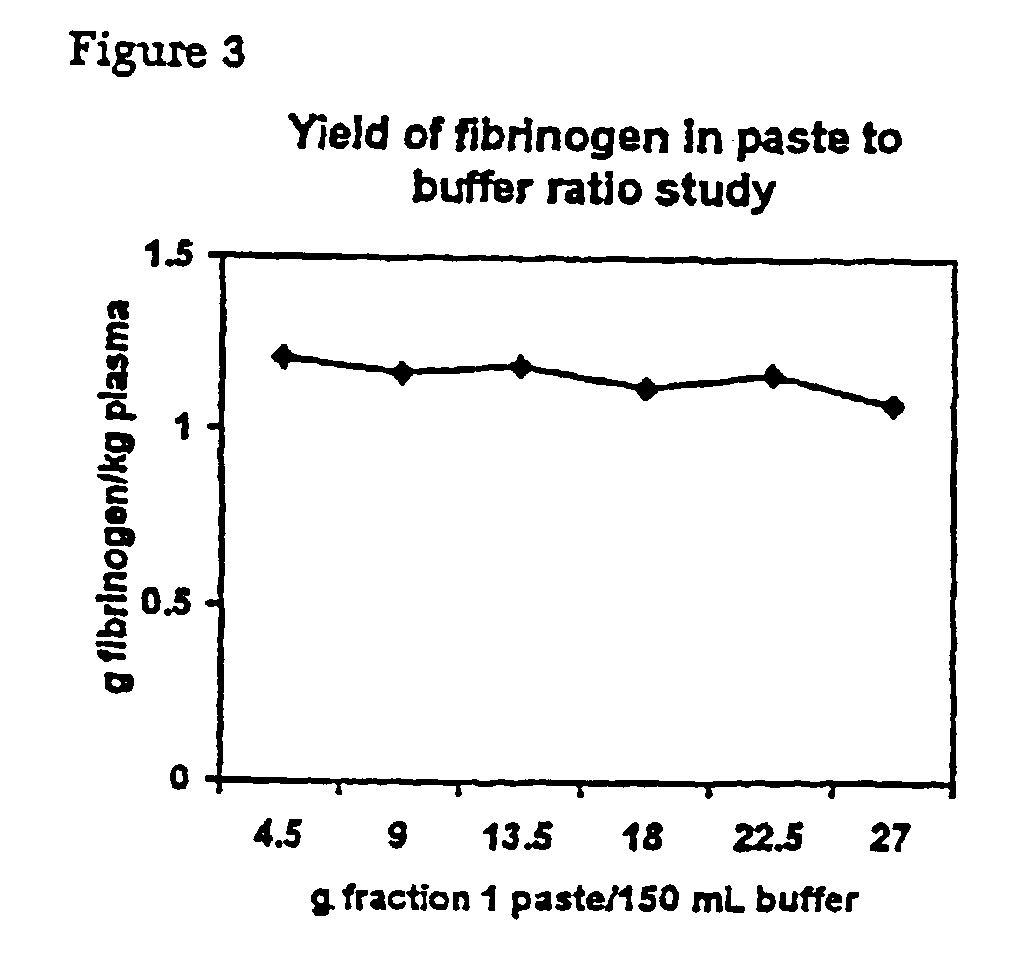
Separation of fibrinogen from plasma proteases
InactiveUS6960463B2Simple manufacturing methodHigh purityFibrinogenHydrolasesProteinase activityIon exchange
The present invention relates to methods for purifying fibrinogen. In one aspect, the present invention relates to a method of separating fibrinogen from plasma fraction I precipitate. In another aspect, the invention relates to the purification of fibrinogen using ion exchange chromatography.
Owner:CSL LTD
Continuous Isocratic Affinity Chromatography
ActiveUS20080053901A1Simple and easily programmable controlPromote repairIon-exchange process apparatusIon-exchanger regenerationSimulated moving bedMoving bed
The present invention provides devices and methods for micro-scale simulated moving bed chromatography (SMB) for continuous preparation of analytic quantities of highly pure fractions of target molecules. The present apparatus and method of the invention is adapted in a preferred embodiment to separations by affinity chromatography involving three discontinuous liquid flow loops. An alternative embodiment of affinity chromatography utilizes standard SMB operating under isocratic conditions.
Owner:TOSOH BIOSCIENCE LLC
Systems and methods for characterization of molecules
InactiveUS20060269964A1Molecular entity identificationBiological testingMolecular compositionLiquid liquid partition
The present invention generally provides systems and methods for the detection, identification, or characterization of differences between properties or behavior of corresponding species in two or more mixtures comprised of molecules, including biomolecules and / or molecules able to interact with biomolecules, using techniques such as partitioning. The experimental conditions established as distinguishing between the mixtures of the molecules using the systems and methods of the invention can also be used, in some cases, for further fractionation and / or characterization of the biomolecules and / or other molecules, using techniques such as single-step or multiple-step extraction, and / or by liquid-liquid partition chromatography. The methods could also be used for discovering and identifying markers associated with specific diagnostics, and can be used for screening for such markers once discovered and identified during diagnostics screening.
Owner:ANALIZA
Protein Purification Using HCIC and Ion Exchange Chromatography
ActiveUS20090105465A1Low costHigh level of proteinImmunoglobulins against cell receptors/antigens/surface-determinantsPeptide preparation methodsFiltrationIon exchange
The present invention provides methods for purifying proteins. In particular, the methods employ a two-step non-affinity chromatography process without the use of an in-process tangential flow filtration step.
Owner:MEDAREX LLC
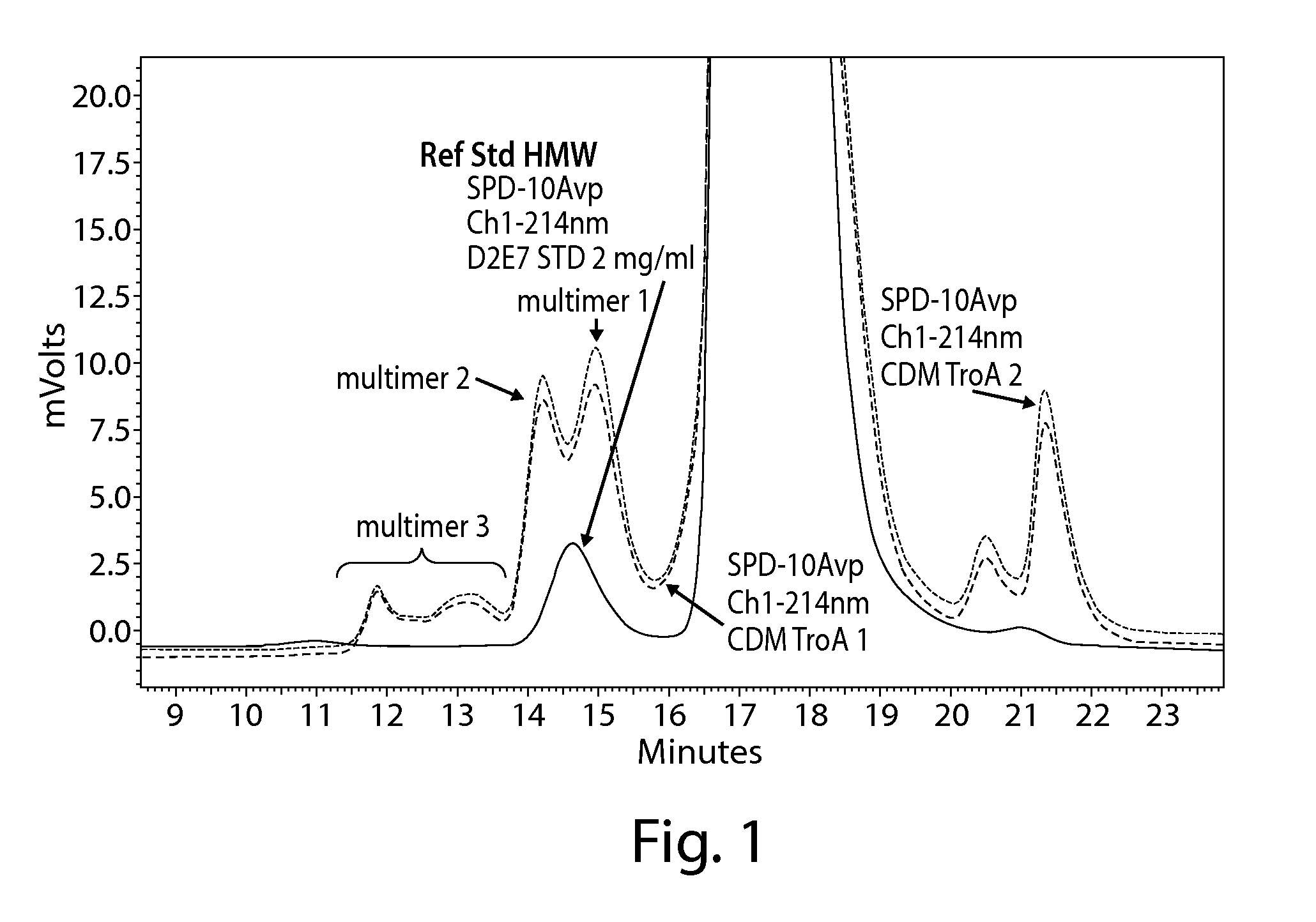
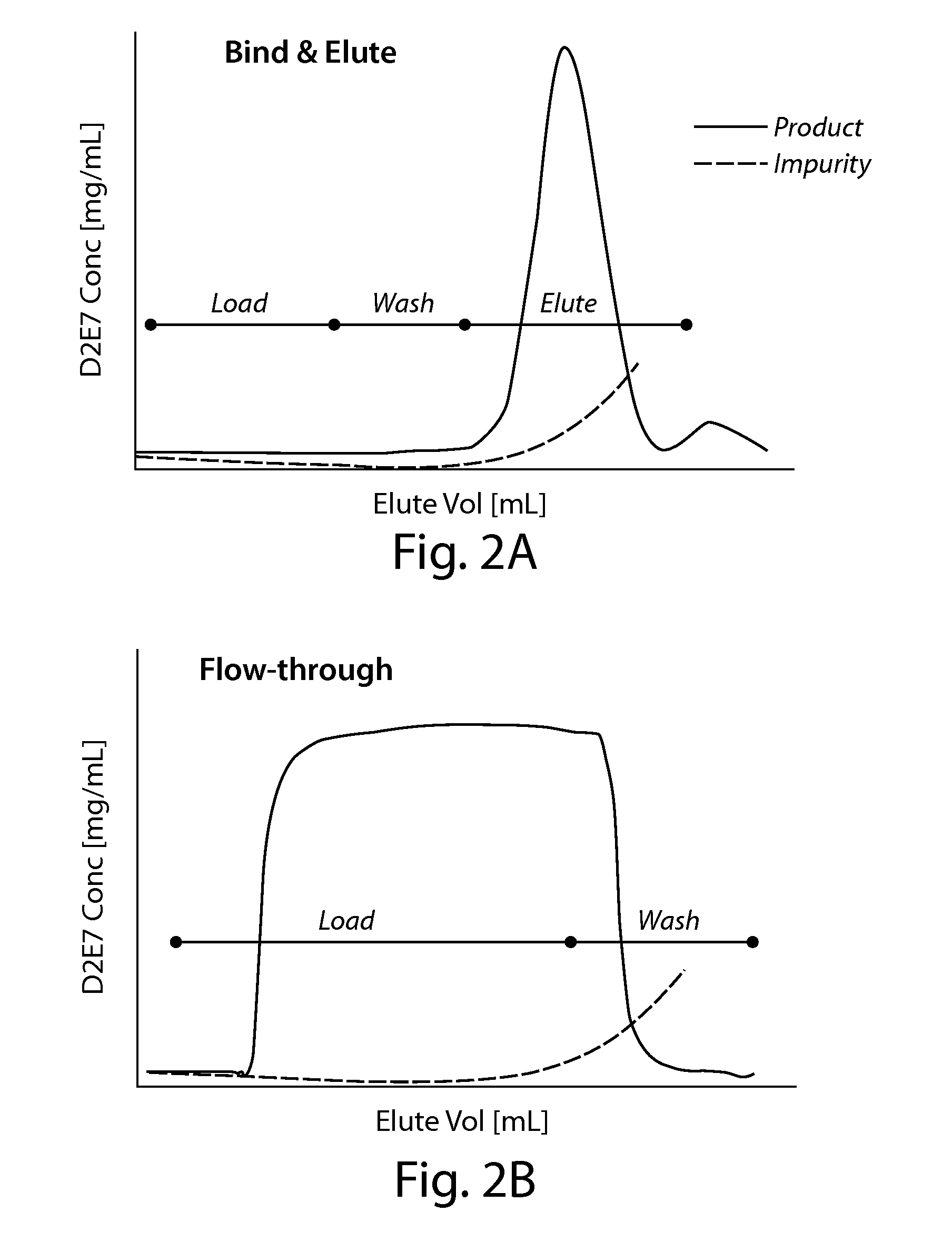
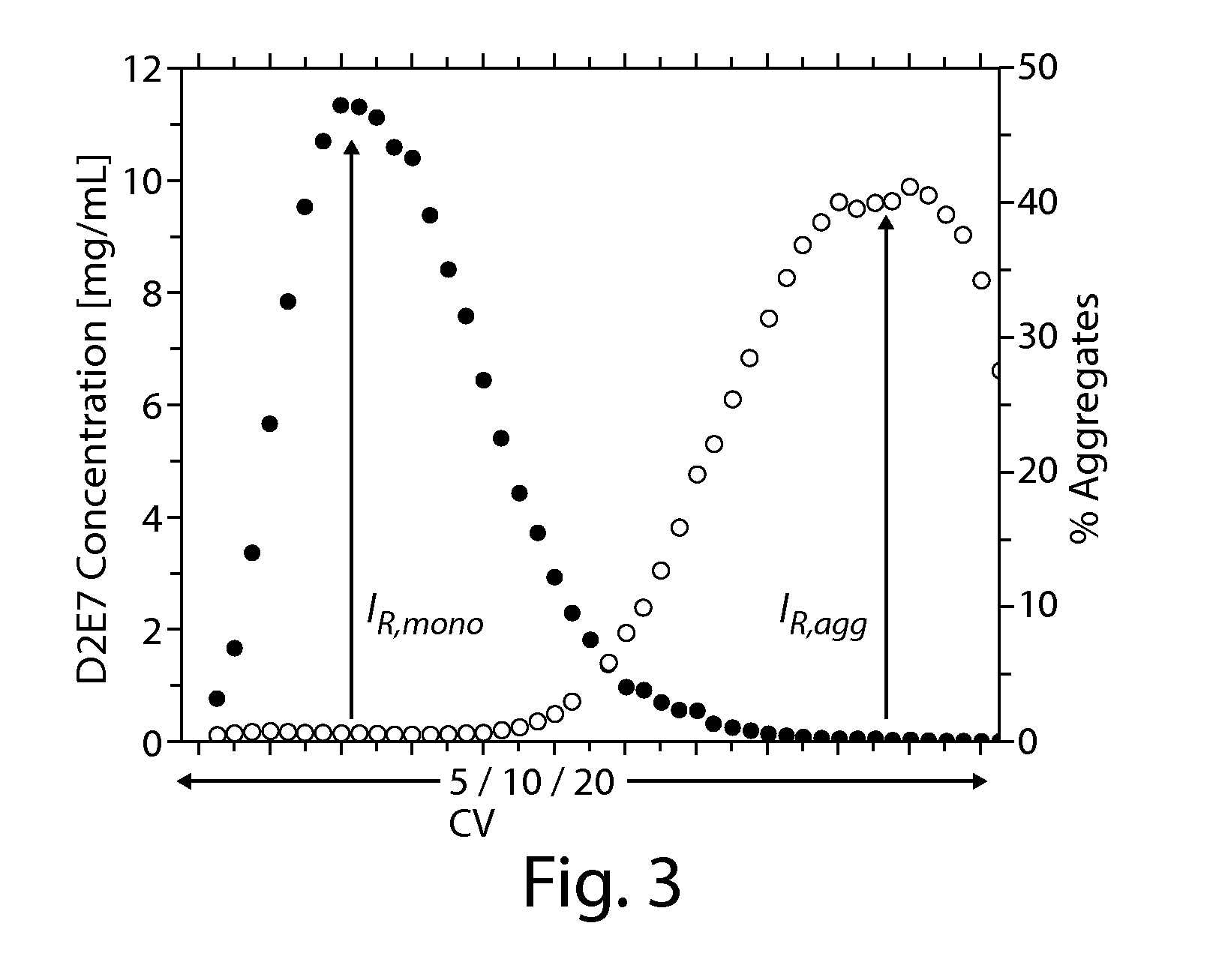
Method For The Removal Of Aggregate Proteins From Recombinant Samples Using Ion Exchange Chromatography
InactiveUS20080058507A1Efficient removalEfficient separationSerum immunoglobulinsDepsipeptidesRetention timeProtein aggregation
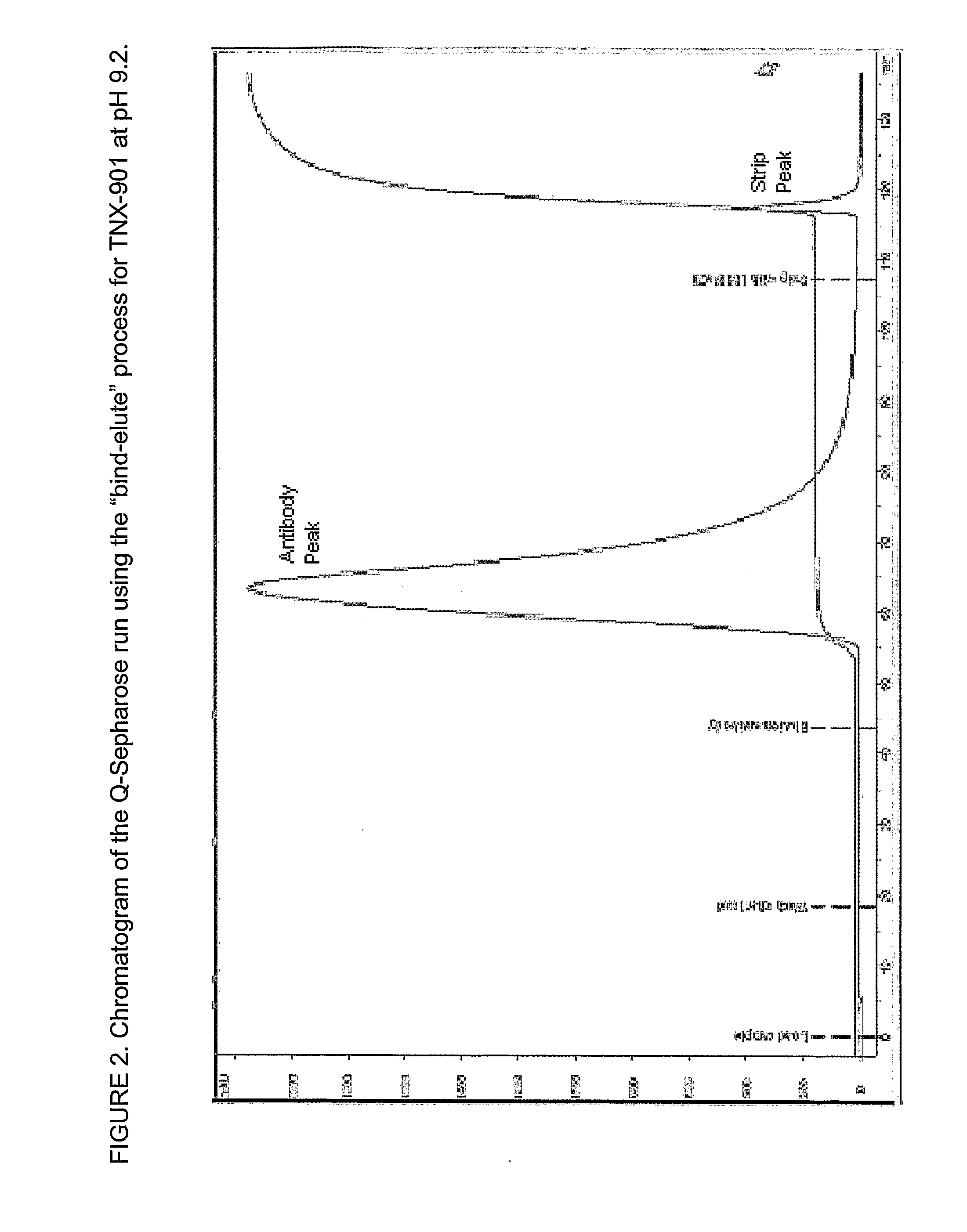
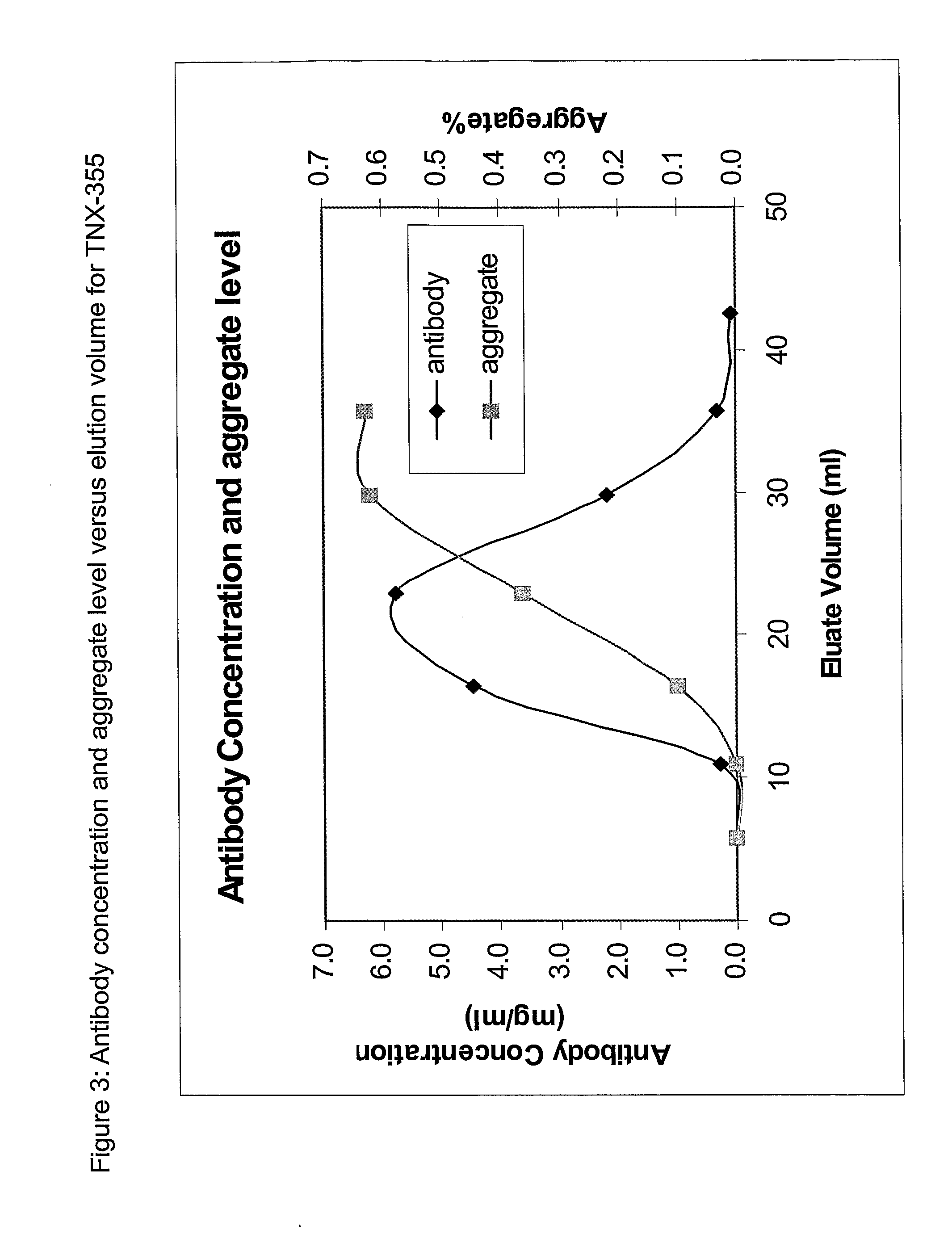
The present invention relates to processes for the removal of unwanted protein aggregates from antibody preparations. One process involves removal when the aggregate and antibody are very close in pI value (“Bind-Elute” process). Another process involves the removal when the aggregate and antibody are very close in net electric charge and retention time on ion exchange resin (“Bind-Washout” process). Using either process, at least 90% of the antibody is recovered and 70-90% of the aggregate is removed.
Owner:GENENTECH INC
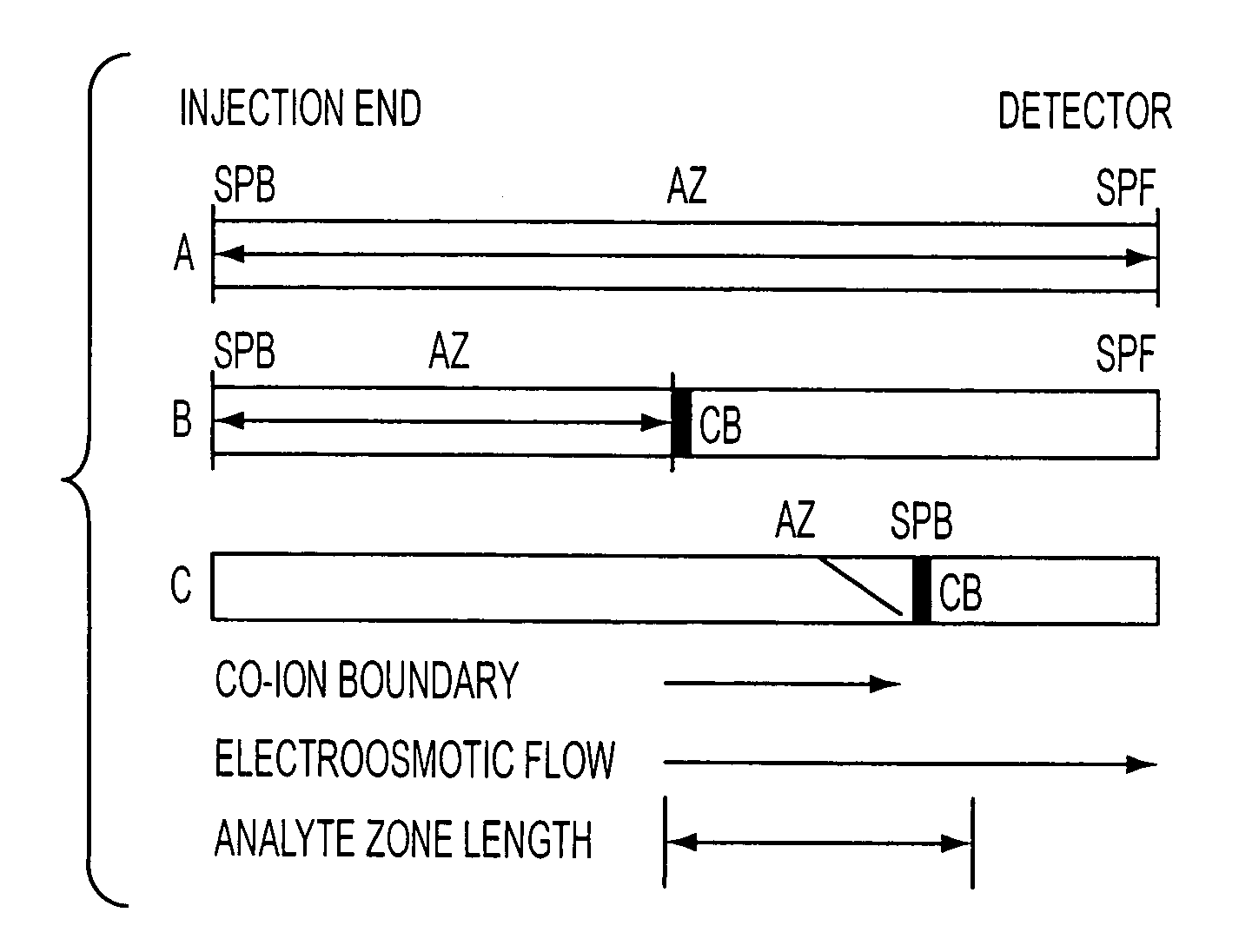
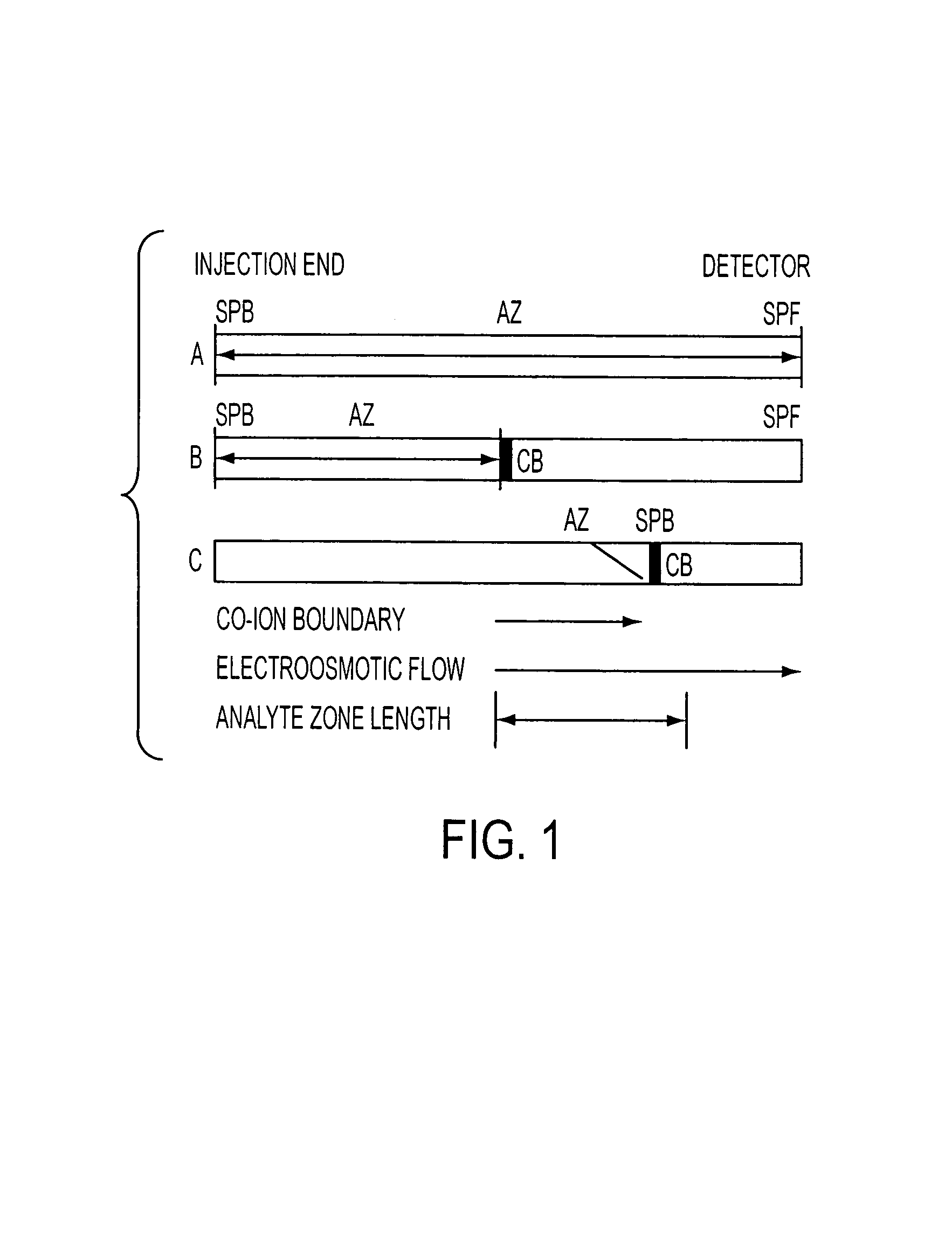
Method for orthogonal analyte stacking/injection systems in electrophoresis
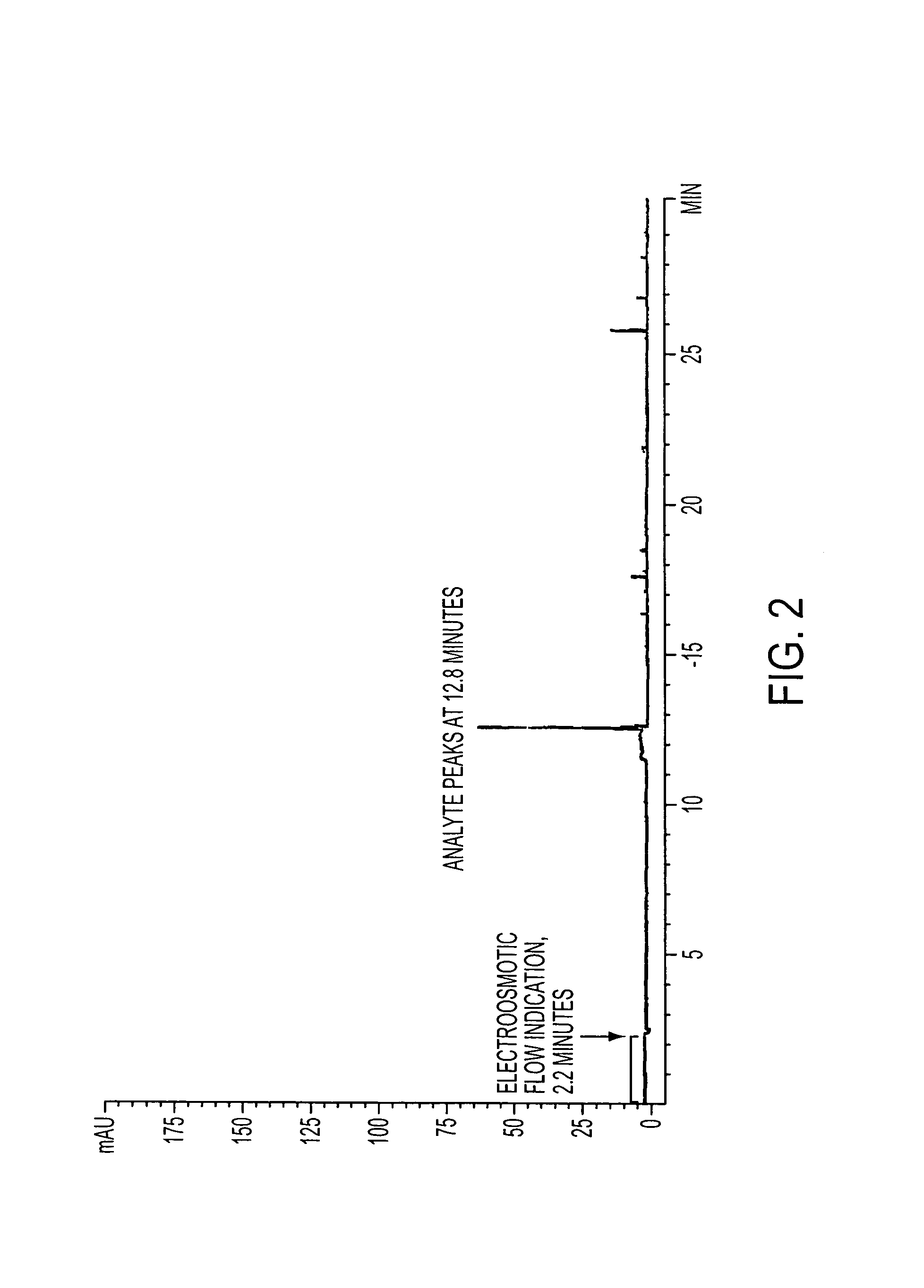
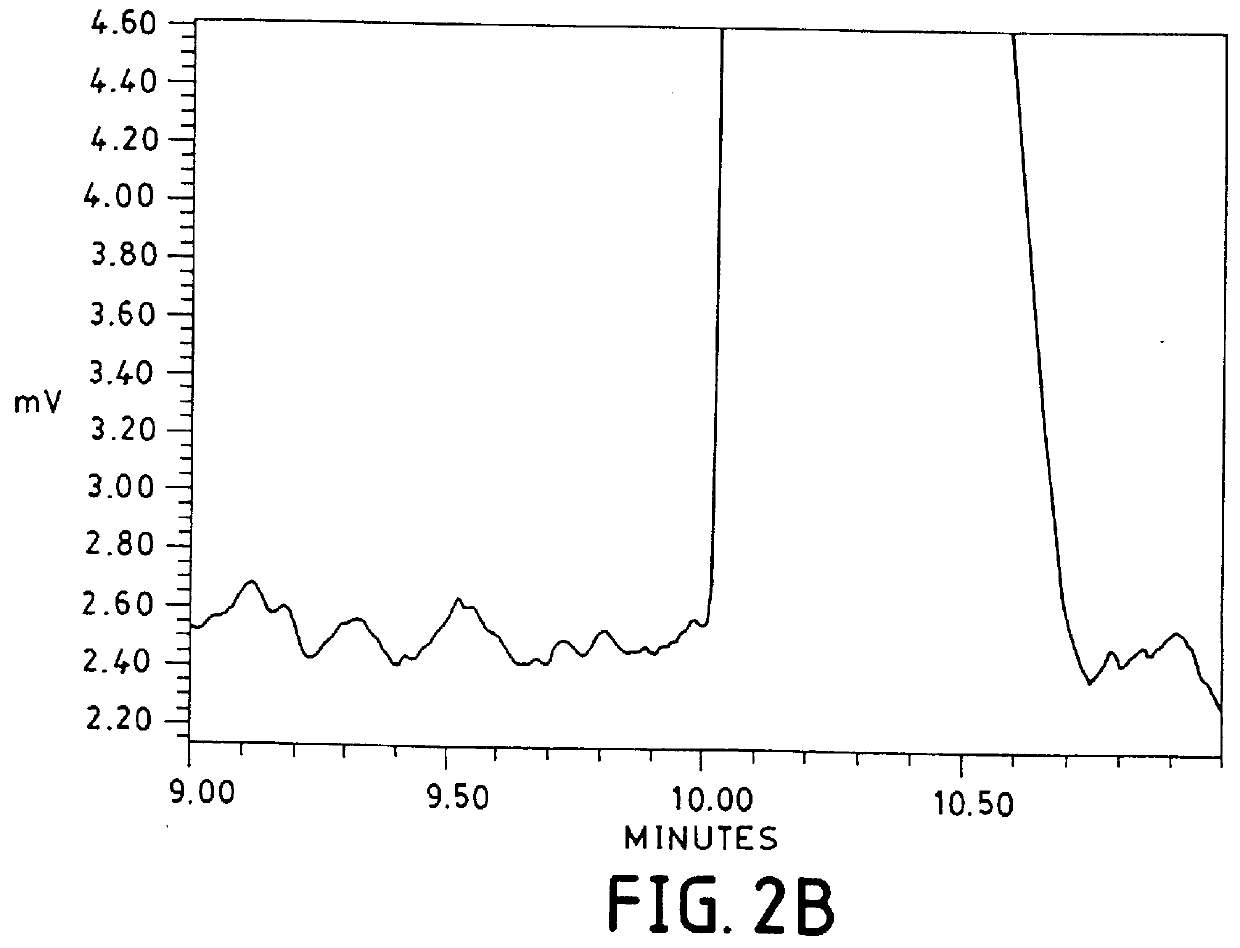
InactiveUS7223325B2High resolutionFaster and high injectionSludge treatmentVolume/mass flow measurementCapillary volumePresent method
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Reagents and methods for performing electrokinetic chromatography
The invention is in the field of electrokinetic chromatography. In particular, the invention is directed to reagents and methods of performing electrokinetic chromatography. The reagents and methods afford fast, high resolution separations with increased detectability.
Owner:WATERS TECH CORP
Yellow pigment of safflower preparation method and application
This invention provides safflower uranidin prepd. from the Chinese herb medicine-safflower which isu sed for promoting blood circulation and removing blood disturbance, and the prepn. method and usage therefor. Said safflower uranidin is tested by high efficiency liquid phase chromatography with its content of 70-99.6%. It can be used for treating and preventing various kinds of diseases, such as cardiovascular disease, cerebrovascular disease and other blood circulation disturbance disease.
Owner:ZHEJIANG YONGNING PHARMA
Dispersion of carbon nanotubes by nucleic acids
Carbon nanotubes dispersed by a stabilized solution of nucleic acid molecules formed separated nanotube-nucleic acid complexes and were separated according to standard chromatographic methods, including gel electrophoresis, two phase solvent systems and ion exchange chromatography.
Owner:EI DU PONT DE NEMOURS & CO
Taurolidine quality checking method
InactiveCN101285813AAvoid product qualityGuarantee product qualityWeighing by removing componentComponent separationGas phaseTaurolidine
The invention provides a quality detection method for Taurolidine. The method uses thin-layer chromatography, gas phase chromatography and titering process to detect the quality of the Taurolidiene. The quality detection method for Taurolidine has the advantages of rapidness, accuracy, convenient operation and excellent reproduction quality, and ensures the product quality of the Taurolidine.
Owner:CHANGCHUN MAILING BIOLOGICAL ENG CO LTD
Purification of recommbined human urokinase zymogen
ActiveCN1680550AEasy to fillLarge amount of processingPeptide preparation methodsPeptidasesZymogenUrokinase Plasminogen Activator
The invention is about a method to purify the recombined human urokinase by the chromatography. The invention relates to recovery the gene engineering production from the mammal cell culture using the Streamline-SP, Sephacryl S-200, Sepharose Fast Flow and DEAE-Sepharose Fast Flow method. The recombined human urokinase can meet the SFDA standard and the percent recovery is above 70%, the purity is above 99% by using the method.
Owner:TASLY BIOPHARMACEUTICALS CO LTD
Coumarin compounds, and preparation method and application thereof
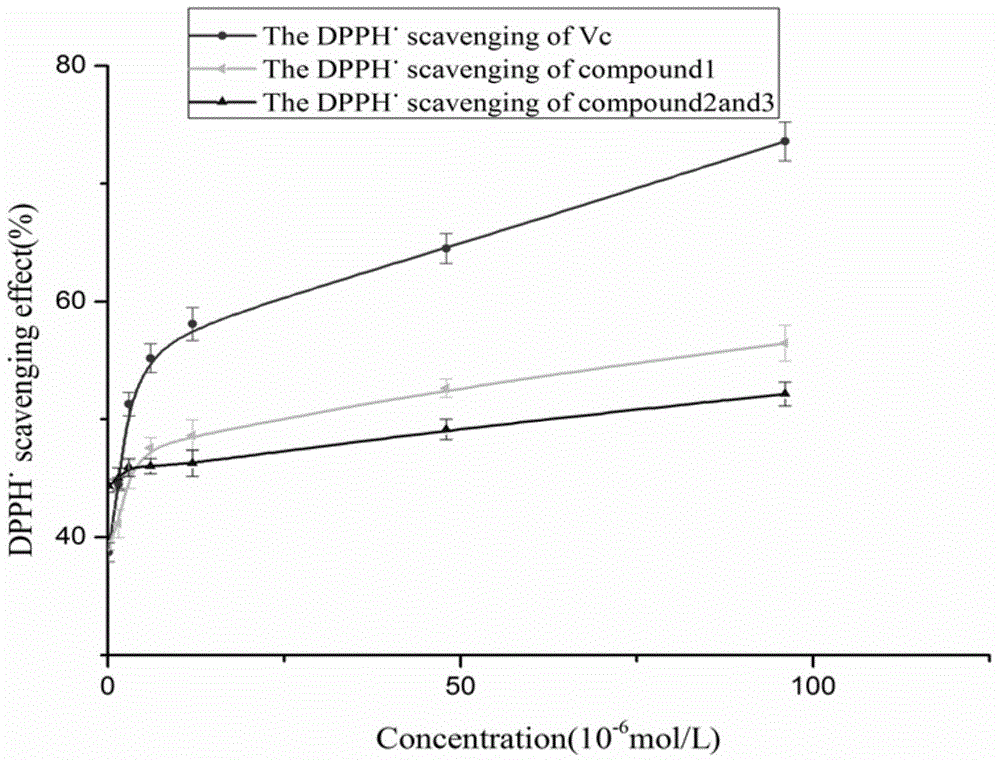
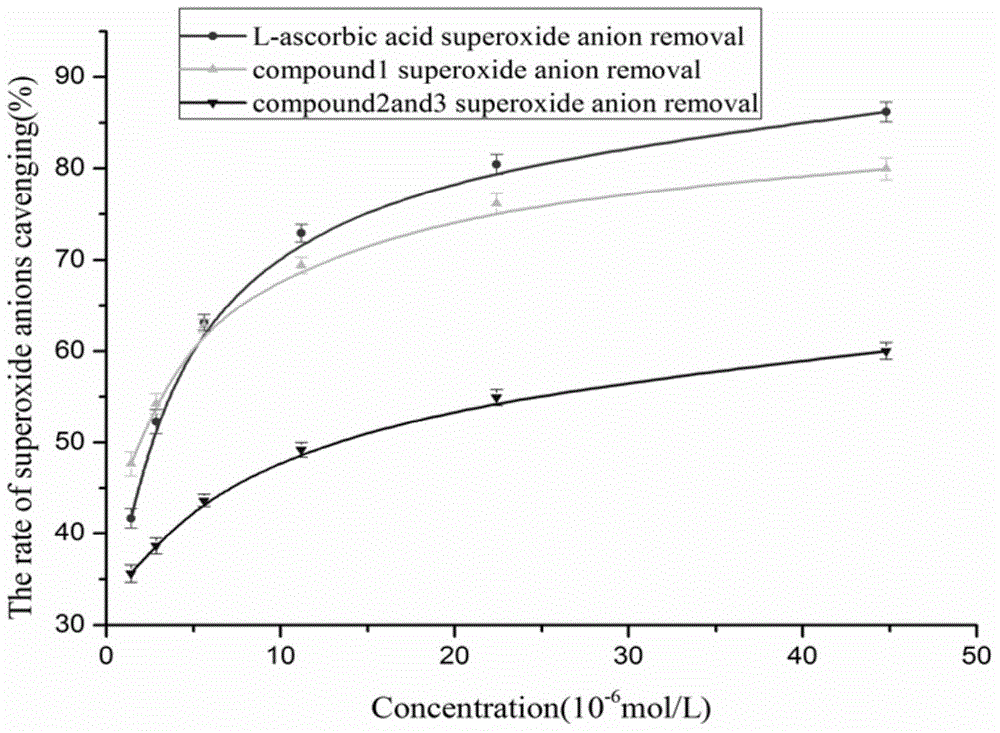
The invention discloses coumarin compounds. The preparation method comprises the following steps: extracting from rattan of Derris eriocarpa How by the inventor; according to the detection introduction of thin-layer chromatography, carrying out reversed-phase silica gel column chromatography, carrying out gradient elution by a petroleum ether-ethyl acetate system for further systematic separation, and recrystallizing. The antioxidation activity experiment proves that the compounds 1 have favorable antioxidation actions, the IC50 value for superoxide anion removal activity is less than that of the positive drug control ascorbic acid, the IC50 value DPPH.free radical removal activity has no great difference from the positive drug, and the compounds 1 have excellent antioxidation activity as compared with the coumarin compounds 6,7-dihydroxycoumarin, daphnetin and daphnoretin which are reported in documents. Therefore, the coumarin compounds disclosed by the invention can be used as a pharmaceutical or non-pharmaceutical antioxidant and the like.
Owner:GUANGXI UNIV FOR NATITIES
Multiple-channel test device, method for producing the same and use thereof
InactiveUS20070042444A1Improve storabilityLaboratory glasswaresMaterial analysisIgm antibodyCardiac infarction
The object of the invention is a multiple-channel test devise based on immunodiffusion and immunochromatography, which enables the simultaneous or parallel determination of several analytes. In the test devise, it is possible to group together different combinations of markers recognizing allergens, myocardial infarction markers, venereal disease analytes, blood screening analytes, respiratory infection producing agents, IgG, IgA and IgM antibodies, other infectious disease producing agents as well as various cancer markers. The multiple-channel test devise comprises a porous carrier material on which a channel network has been formed by etching the carrier material by laser to form a shaped figure that contains several channels. In the channels, various specific binding reagents have been immobilized, which enable the diagnoses of a target illness and / or syndrome. The sample application point is optionally provided with a filter and optionally contains a label mobilizable by the analyzable sample and a specific binding reagent. Also the method for the production of the test device and its use are disclosed in the invention.
Owner:ANI BIOTECH
Valve module and methods for simulated moving bed chromatography
ActiveUS8807164B2Simple and easily programmable controlPromote repairOperating means/releasing devices for valvesComponent separationSimulated moving bedMoving bed
The present invention provides devices and methods for micro-scale simulated moving bed chromatography (SMB) for continuous preparation of analytic quantities of highly pure fractions of target molecules. The present apparatus and method of the invention is adapted in a preferred embodiment to separations by affinity chromatography involving three discontinuous liquid flow loops. An alternative embodiment of affinity chromatography utilizes standard SMB operating under isocratic conditions.
Owner:TOSOH BIOSCIENCE LLC
Quality testing method for fingerprint of herbal medicine musk
The invention discloses a quality testing method for fingerprint of herbal medicine musk. The method includes establishing a gas chromatographic fingerprint of the musk by gas chromatography, and analyzing chemical components of different types of musk by gas chromatography and mass spectrometry. A test sample is processed by specific process, chromatographic detection is optimized, and tests show that the method is stable, precise and repeatable. Three established reference musk fingerprints are well specific. Similarity of the three types of musk is researched under integration of non-integration conditions of muscone, and more accurate basis is provided for testing quality of the three different types of musk. In addition, common compounds of the three types of musk are obtained by identification by GC-MS (gas chromatography and mass spectrometry) and can be used as basis for further identification of synthetic musk, domestic musk and natural musk.
Owner:ZHANGZHOU PIEN TZE HUANG PHARM
Medicine for treating infantile anorexia, its preparing method and quality control method
A Chinese medicine for treating anorexia of baby is prepared from 12 Chinese-medicinal materials including tuckahoe, tangerine peel, haw, rhubarb, etc through decocting, filtering, concentrating, mixing, etc. Its quality control method including thin-layer chromatography and high-effect liquid-phase chromatography is also disclosed.
Owner:GUANGDONG ZHONGSHENG PHARMA
Separation of polypeptides comprising a racemized amino acid
Owner:NOVO NORDISK AS
Walnut peptide having ACE inhibitory activity and preparation method thereof
ActiveCN105111282AAbundant raw materialsLow pricePeptide preparation methodsFermentationFreeze-dryingAmino acid
The invention discloses walnut peptide having the ACE inhibitory activity and a preparation method thereof. The amino acid sequence of the walnut peptide is EPNGLLLPGY, and the preparation method of the walnut peptide comprises the steps of taking walnut pomace as raw materials, selecting appropriate protease, preparing walnut antihypertensive peptide coarse products according to the optimal enzymolysis technology, adopting the coupling chromatographic technique of the membrane technology for conducting fine preparation on the walnut antihypertensive peptide, adopting the high performance liquid chromatography for conducting further purification, and adopting the vacuum freeze drying or low-temperature spray drying technology for preparing final products. According to the walnut peptide, the walnut pomace is adopted as the raw materials for developing the protein peptide, the raw materials are abundant, the price is low, and the large-scale industrialized production can be satisfied; the walnut peptide has important significance in walnut protein product diversification and development and utlization of walnut biological active peptide in functional foodstuff, health food or medicine.
Owner:HARBIN INST OF TECH
Preparing method of human serum albumin
ActiveCN105037487AReduce yield lossReduce processing timePeptide preparation methodsWhole blood productUltrafiltration
The invention relates to the technical field of biological product and blood product production, and mainly relates to a separation and purification method of human serum albumin in the blood product production, in particular to a preparing method of human serum albumin. According to the method, healthy plasma supernatant is used as raw materials; a Kistler-Nitchmann low-temperature ethanol method is adopted for precipitating and separating ingredients FI+II+III and ingredients FIV; supernatant after the ingredient FIV separation is subjected to dealcoholization treatment; then, one-step ion exchange chromatography and ultrafiltration are further performed; a proper amount of sodium caprylate is added as a stabilizer; after the Pasteur virus inactivation treatment is performed, the human serum albumin finished product is obtained. Through efficient liquid chromatography detection, the purity of the human serum albumin prepared by the process is higher than 99 percent; the polymer content is lower than or equal to 1 percent; the yield of the plasma reaches 28 to 30g / L. The purity of the product is higher; the impurity protein content is lower, so that the clinical medication is safer.
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD
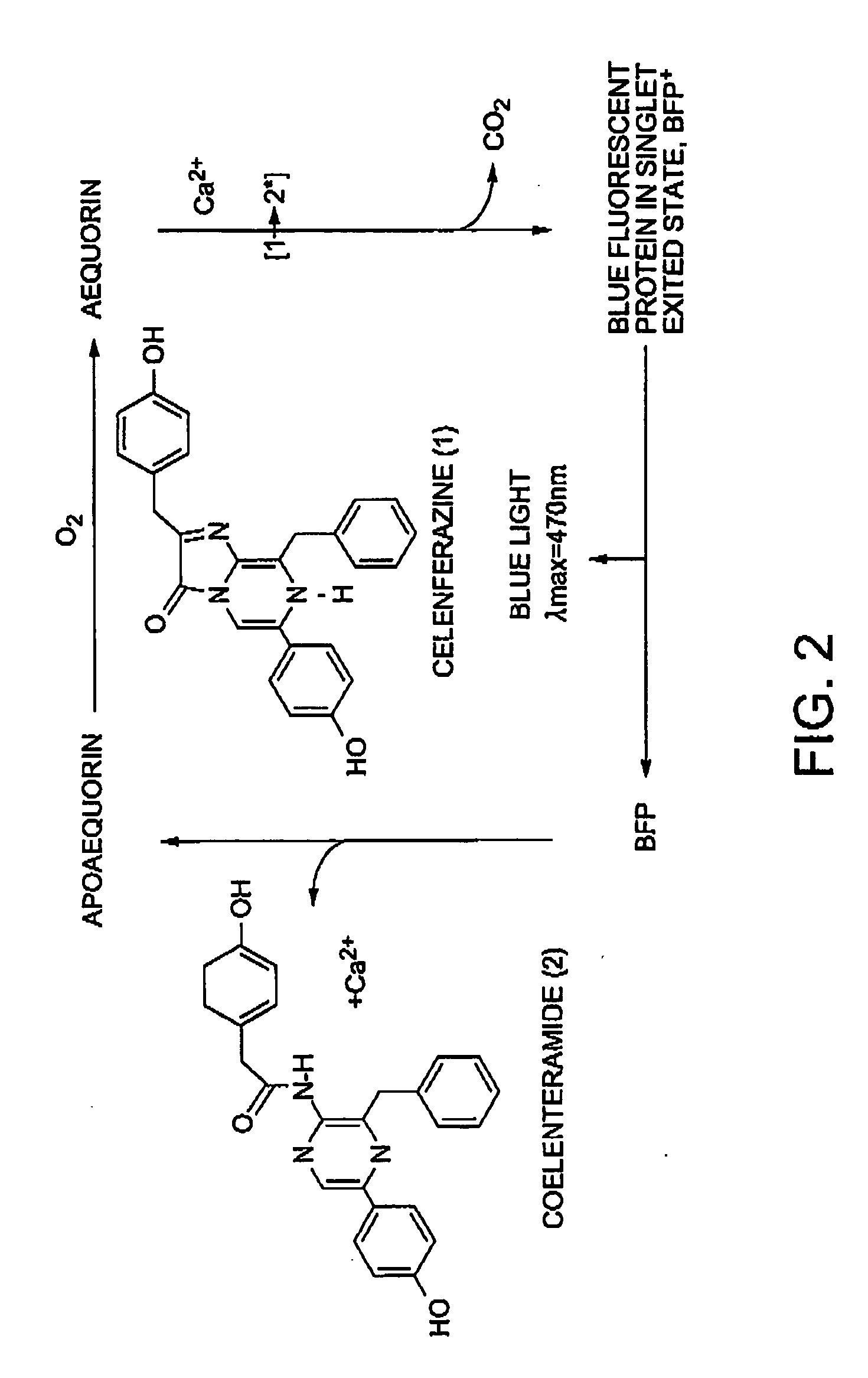
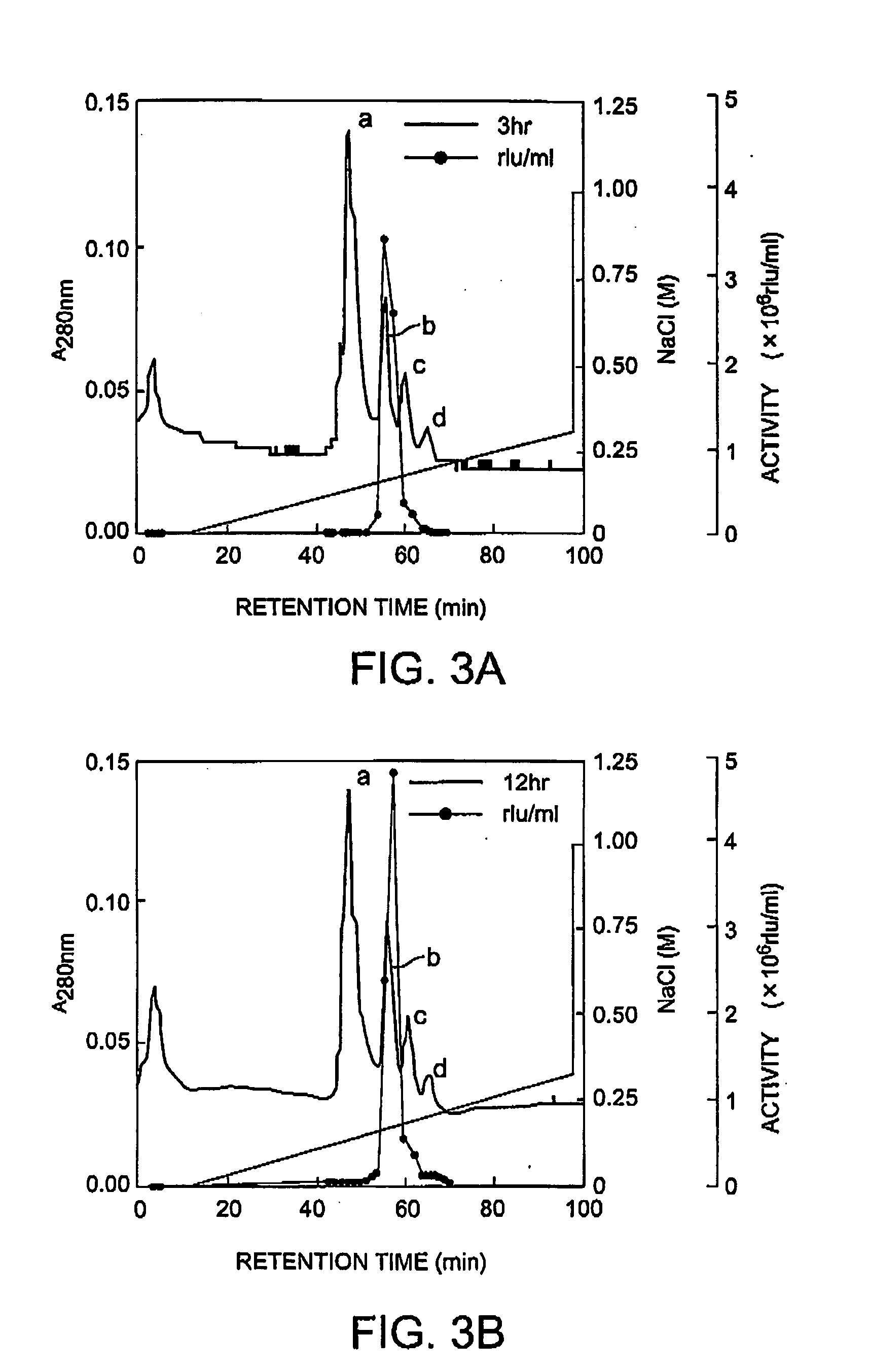
Method of isolating and purifying aequorin, aequorin produced by the method, and process for detecting calcium ions with aequorin
InactiveUS20050054838A1Improve accuracyHigh precision measurementComponent separationMaterial analysis by observing effect on chemical indicatorGradient elutionChromatography
Isoforms of apoaequorin and isoforms of aequorin are isolated and purified from recombinant apoaequorin and a solution containing regenerated aequorin, respectively, by gradient elution chromatography. As a result, aequorin can be isolated and purified.
Owner:NEC SOLUTION INNOVATORS LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com