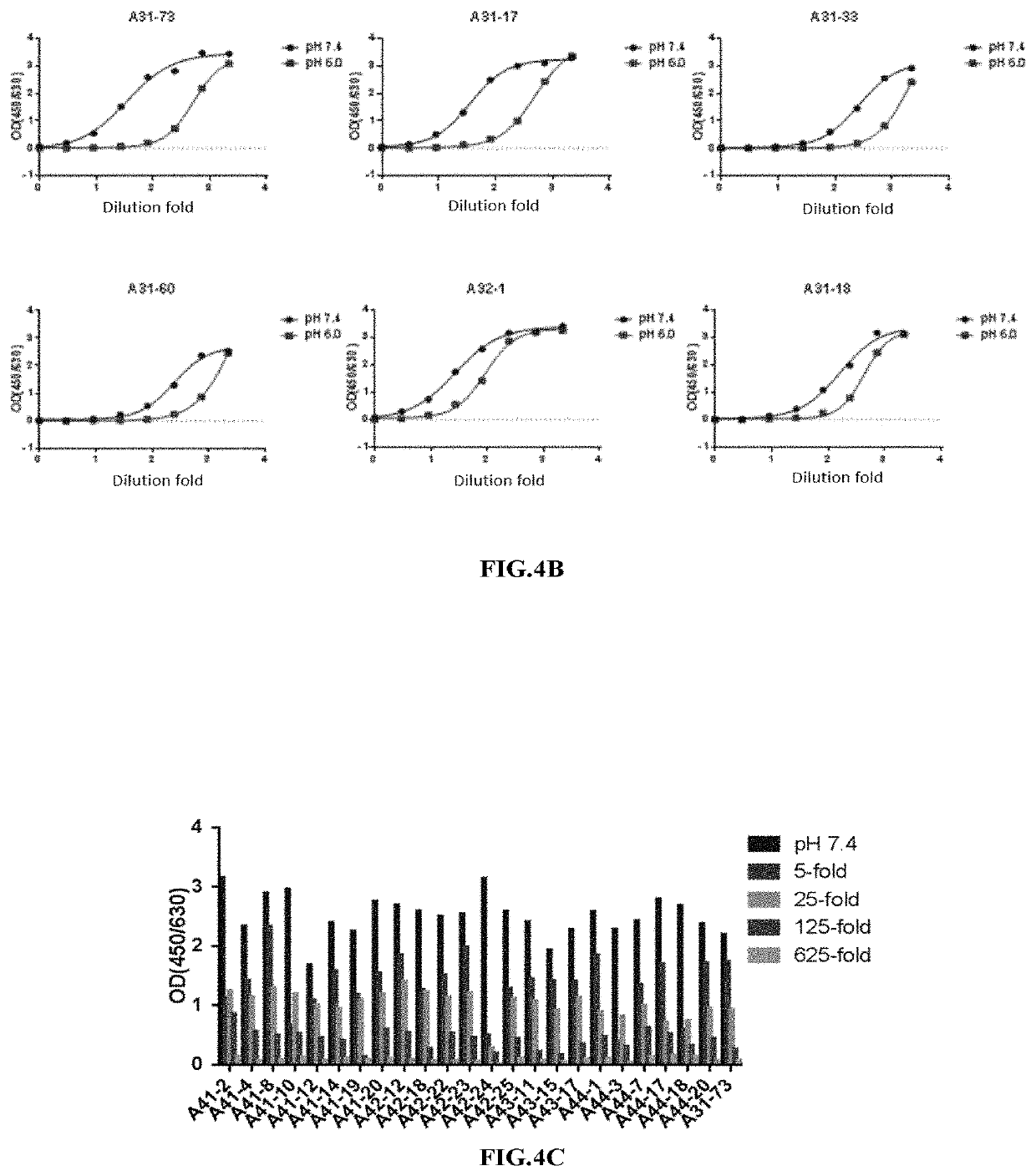
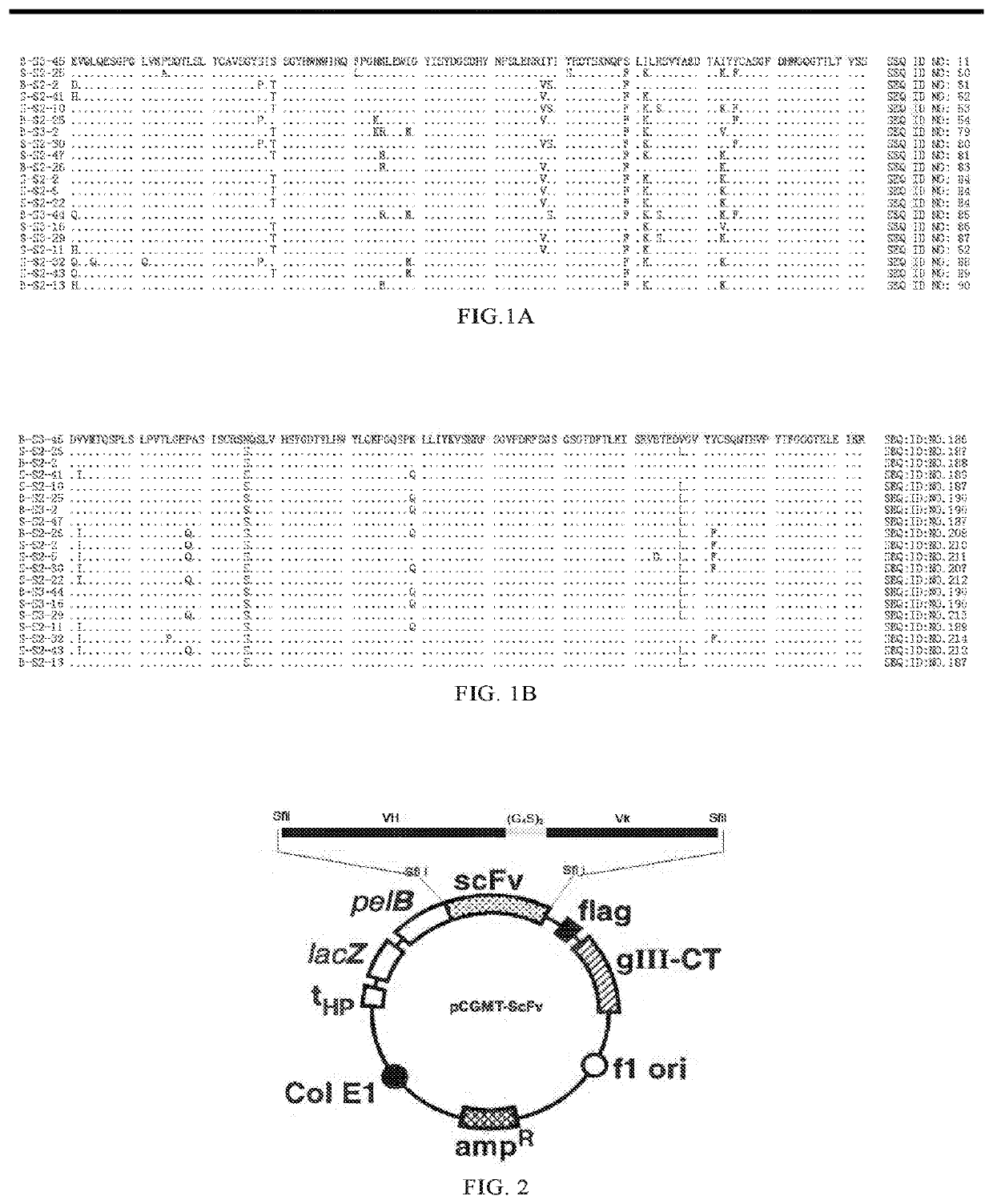
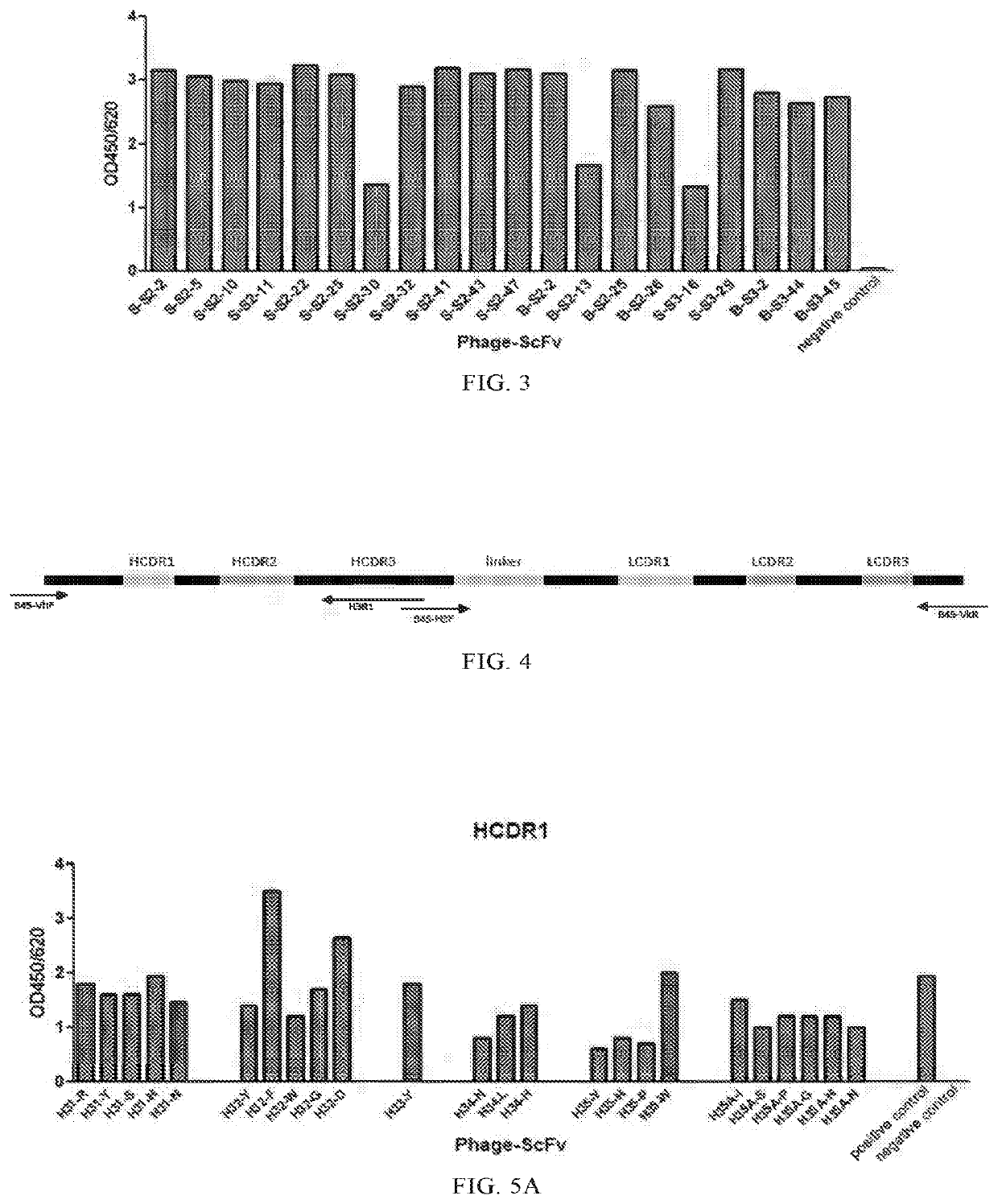
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38 results about "B hepatitis surface" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
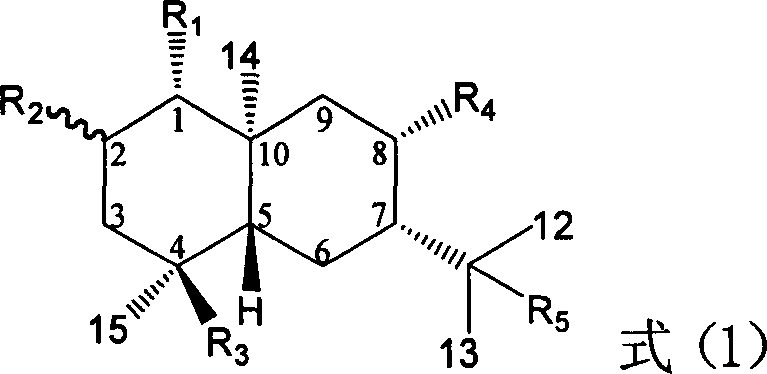
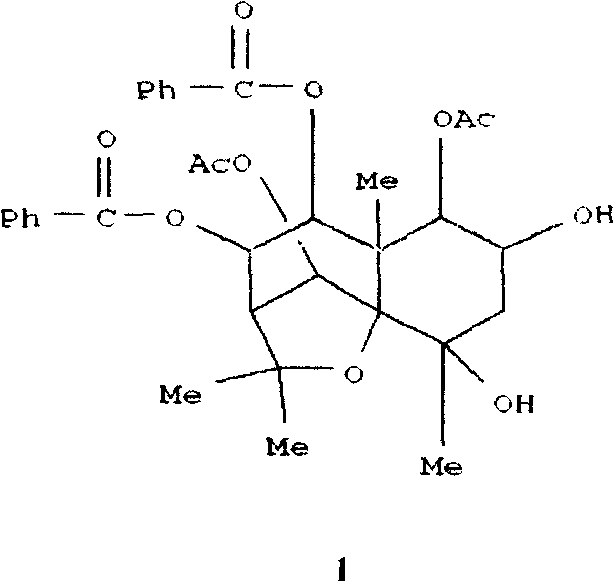
Pharmaceutical use of ent-eudesmane alcohol type sesquiterpene for inhibiting hepatitis virus
InactiveCN1935762APrevention and treatment of viral hepatitis BHBsAg reductionSugar derivativesHydroxy compound active ingredientsDiseaseSolvent
The invention relates to an enantiomorphic amine alkyl sesquiterpene alcohol and glucoside and the medicated salt or solvent thereof, as well as the effect and activity of the composed medicine combination, mainly relating to the medical use in reducing HBV-DNA replication activity. And it has considerably strong inhibiting effect on HBsAG screted by HepG2.2.15 and HBV-DNA replication as compared with positive contrast Lamivudine; and it has obvious inhibition activity to HBV-DNA replication at large dosage (100 mug / mL) and medium dosage(20 mug / mL) as contrasted with Lamivudine, and can be expected to apply to preparing medicines for curing HB virus infection disease.
Owner:赵昱
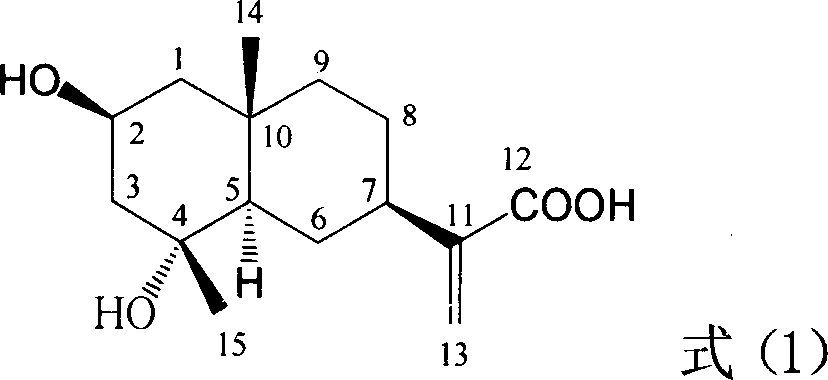
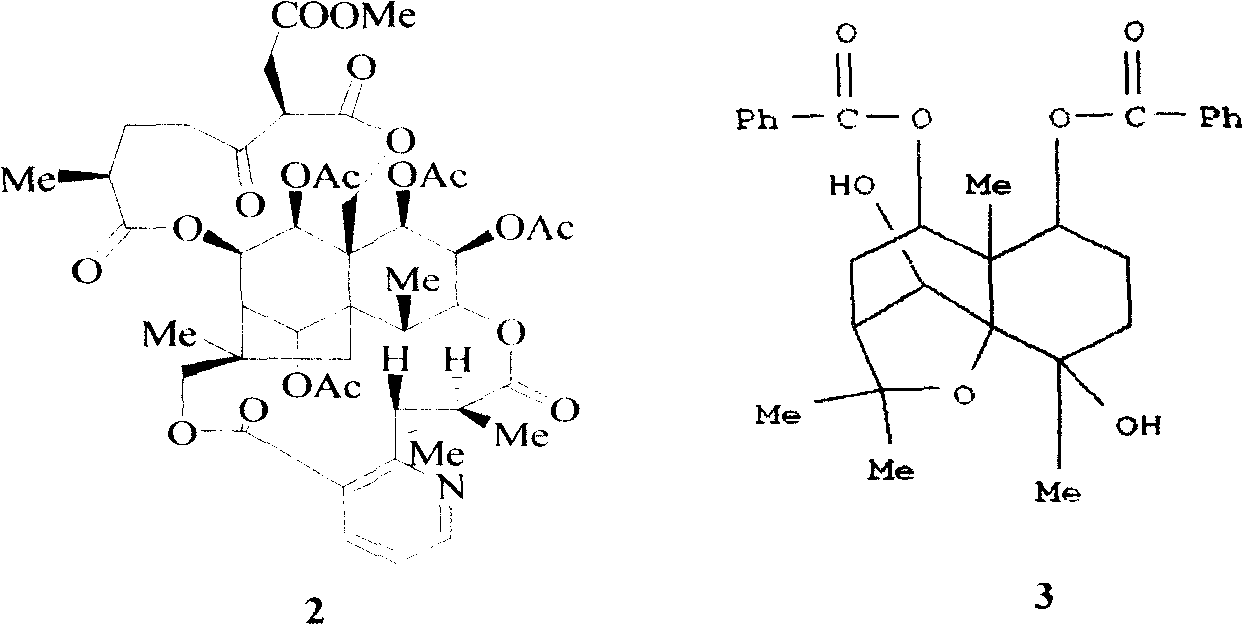
Medical usage of 2beta-hydroxyilicicacid in inhibiting hepatitis B
InactiveCN1951378APrevention and treatment of viral hepatitis BHBsAg reductionOrganic active ingredientsAerosol deliveryDiseaseHepatitis b surface antigen
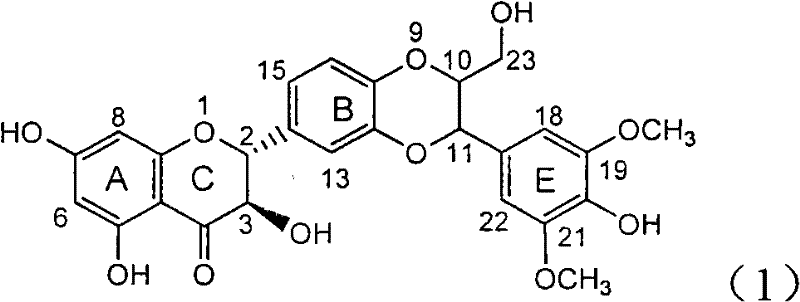
The invention relates to a hemiterpene derivant 2-Hydroxyilicic acid, as formula (1) 2beta-hydroxy-5alphaH-eudesmane-11(13)-allyl-12-acid and relative compounds which can be used to prepare the drug treating hepatitis B disease. The inventive compound can restrain the copy of hepatitis B surface antigen (HBsAg) and the hepatitis B deoxyribonucleic acid (HBV-DNA), while its HBsAg restrain ability is higher than positive contrast difuradin; in the density as 100mug / ml, 20mug / ml, and 4mug / ml, it can restrain the copy of hepatitis B virus HBV-DNA.
Owner:WENZHOU MEDICAL UNIV +1
Pharmaceutical use of 1 beta-hydroxy ilexolic acid for inhibiting hepatitis virus
InactiveCN1935131APrevention and treatment of viral hepatitis BHBsAg reductionOrganic active ingredientsOrganic chemistryChemical structureDisease
The present invention relates to an eudesmane type sesquiterpene derivative 1 beta-hydroxyilicic acid, namely 1 beta-hydroxy-5 alpha H-eudesmane-11 (13)-ethylene-12-acid, its medicineal salt or solvent compound and its medicine composition and medicinal application for preparing medicine capable of curing hepatitis B virus infective disease and resisting hepatitis B virus. Said invention also provides its chemical structure formula.
Owner:WENZHOU MEDICAL UNIV
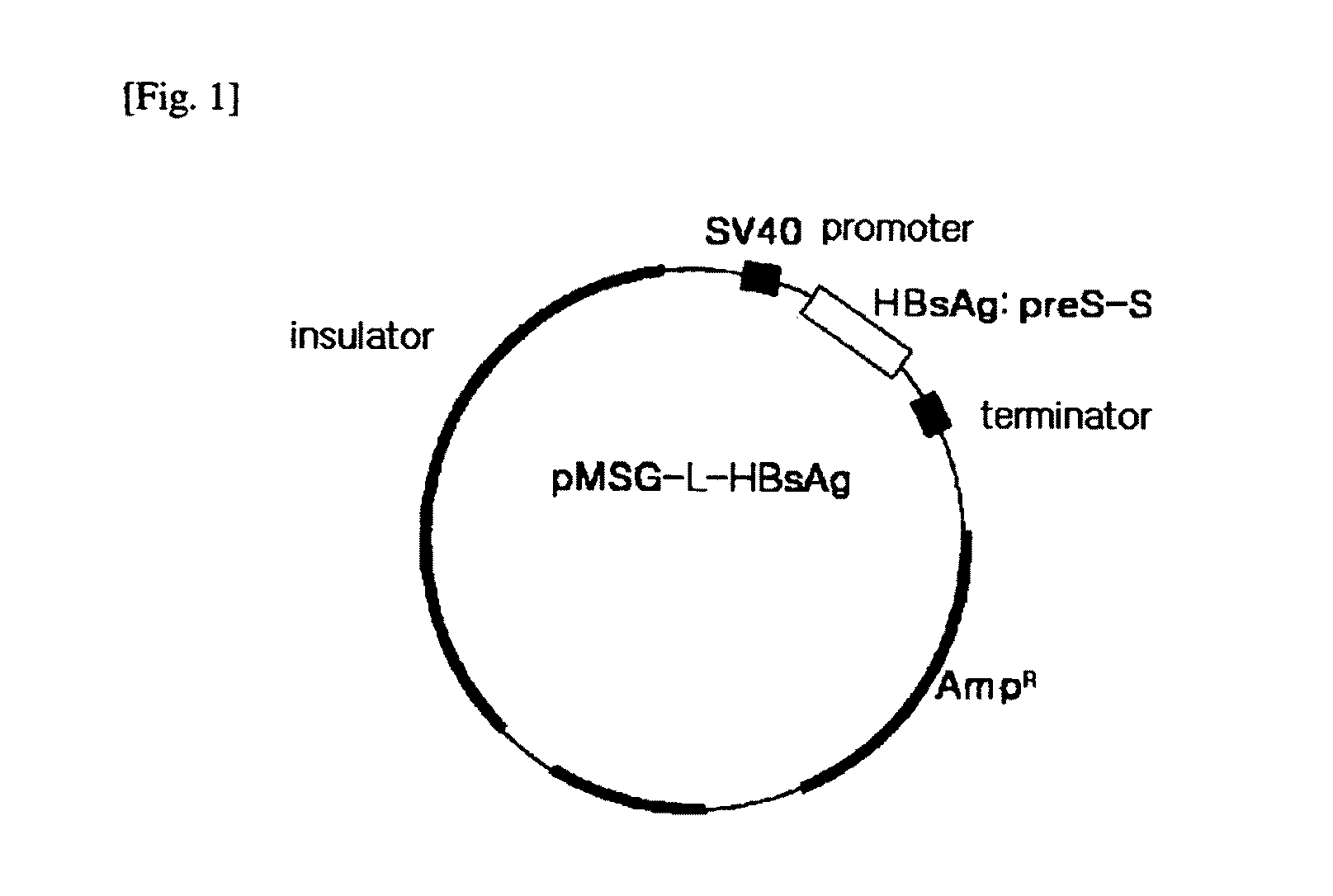
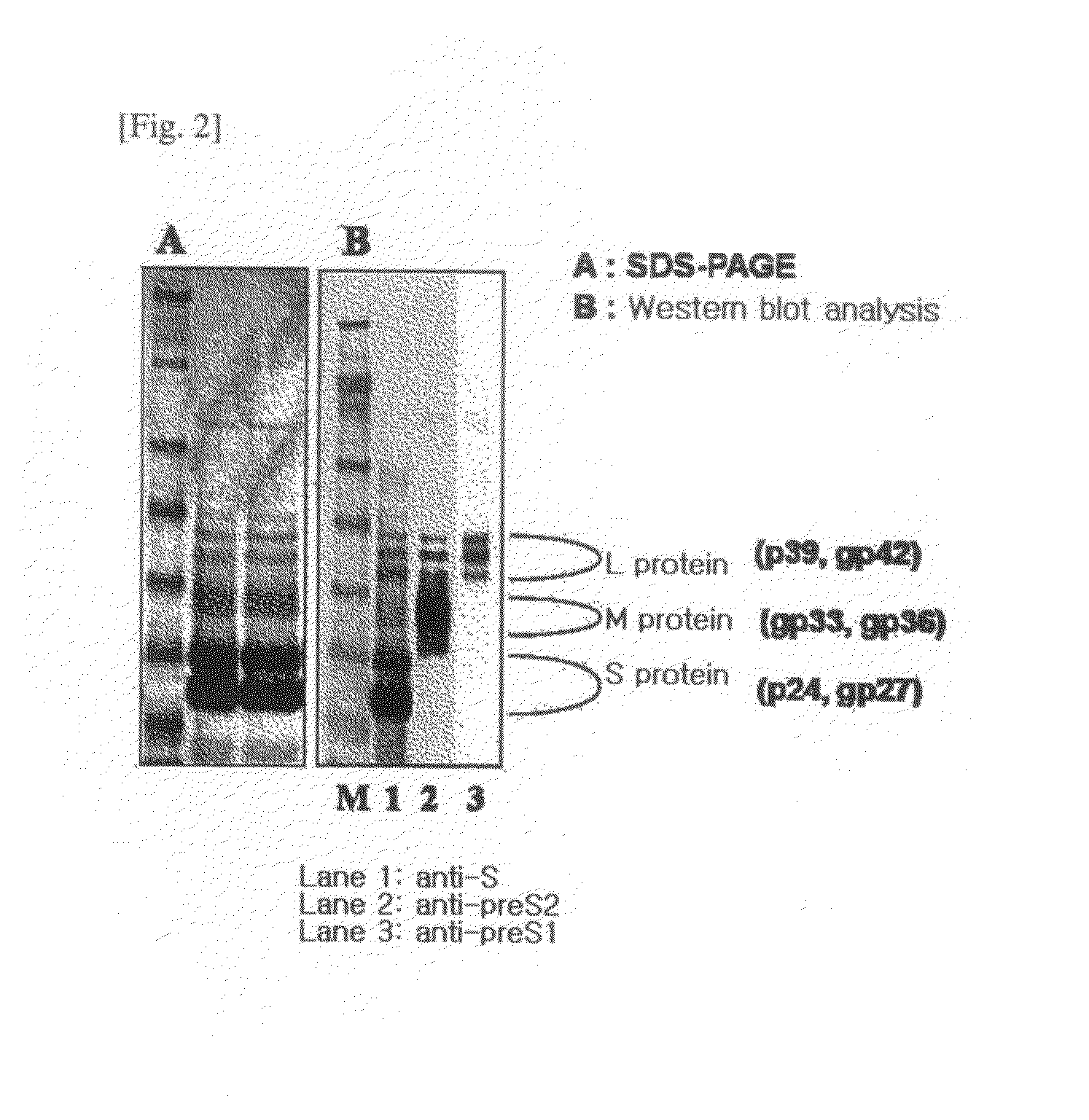
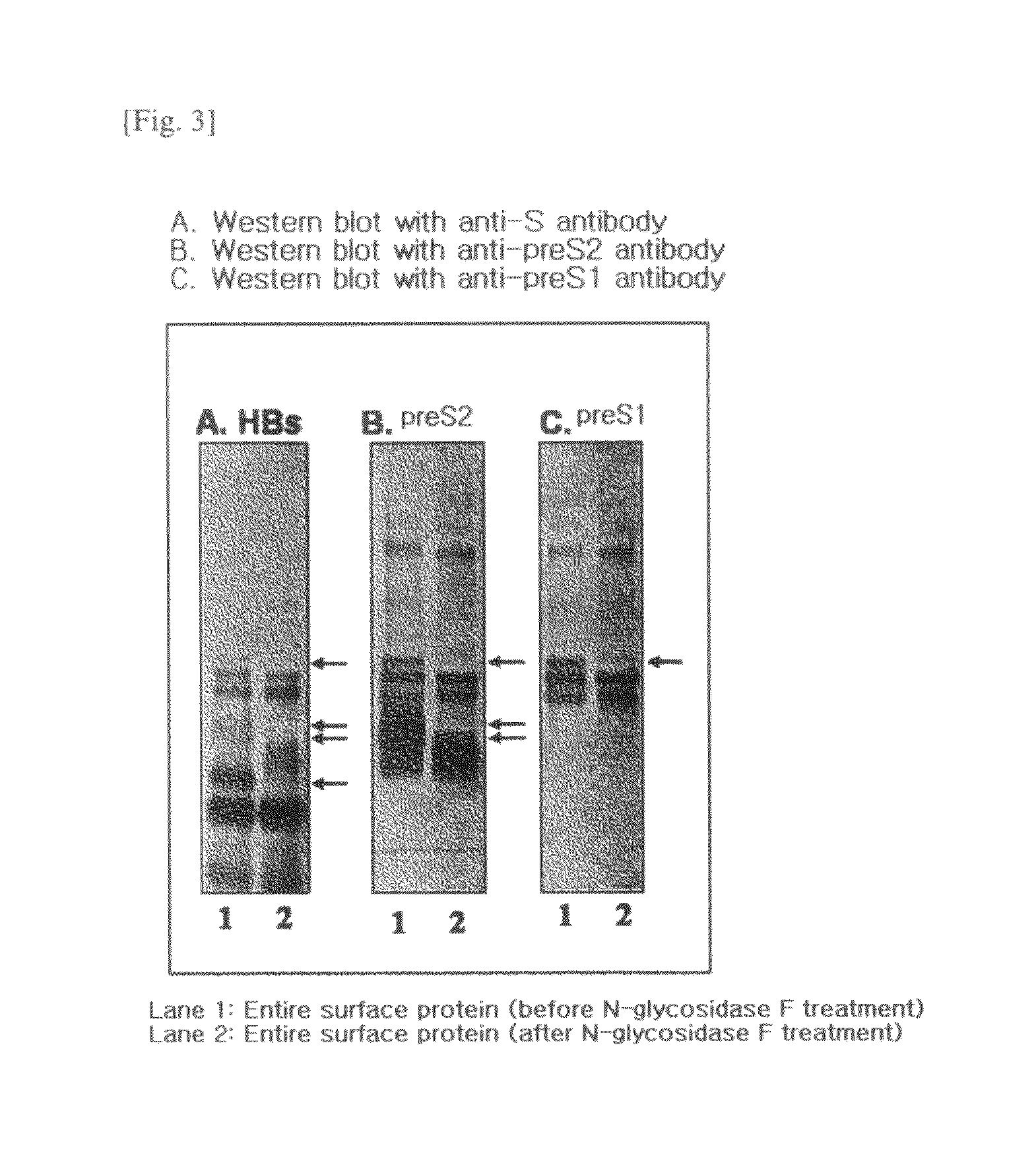
HBV vaccine and a process of preparing the same
The present invention relates to an HBV vaccine comprising an entire hepatitis B surface antigen of L protein, M protein and S protein, in which the produced antigens form virus-like particles, and a multi-antigen vaccine further comprising an HBV core antigen in addition to the entire surface antigen, and a method for preparing the same. The vaccines provide various epitopes and have excellent immunogenicity to induce a strong humoral immune response as well as a cell-mediated immune response.
Owner:CHA VACCINE RES INST CO LTD
Hepatitis B virus surface antigen detection particles, preparation thereof and use thereof
InactiveCN101666801AReduce sensitivityHigh sensitivityChemiluminescene/bioluminescenceBiological testingSerum igeAntigen testing
The invention relates to a reagent for the diagnosis of hepatitis B and discloses hepatitis B surface antigen detection particles, which are luminous particles coated by a hepatitis B surface antigen.The invention also discloses the preparation and use of the hepatitis B surface antigen detection particles. In addition, the invention also discloses an in-vitro diagnostic kit for determining the hepatitis B surface antigen in a sample and also discloses the using method of the kit. The kit of the invention can be used in combination with other serums and clinic information to diagnose the acute or chronic hepatitis B infection condition of an individual and can also be used for screening hepatitis B in perinatal females to judge the hepatitis B infection risk of the newborns.
Owner:BEYOND DIAGNOSTICS (SHANGHAI) CO LTD
Rabbit monoclonal antibodies to hepatitis B surface antigens and methods of using the same
ActiveUS20060008798A1Simple and accurate and efficient diagnosisAvoid deathMicrobiological testing/measurementImmunoglobulins against virusesB hepatitis surfaceHepatitis b surface antigen
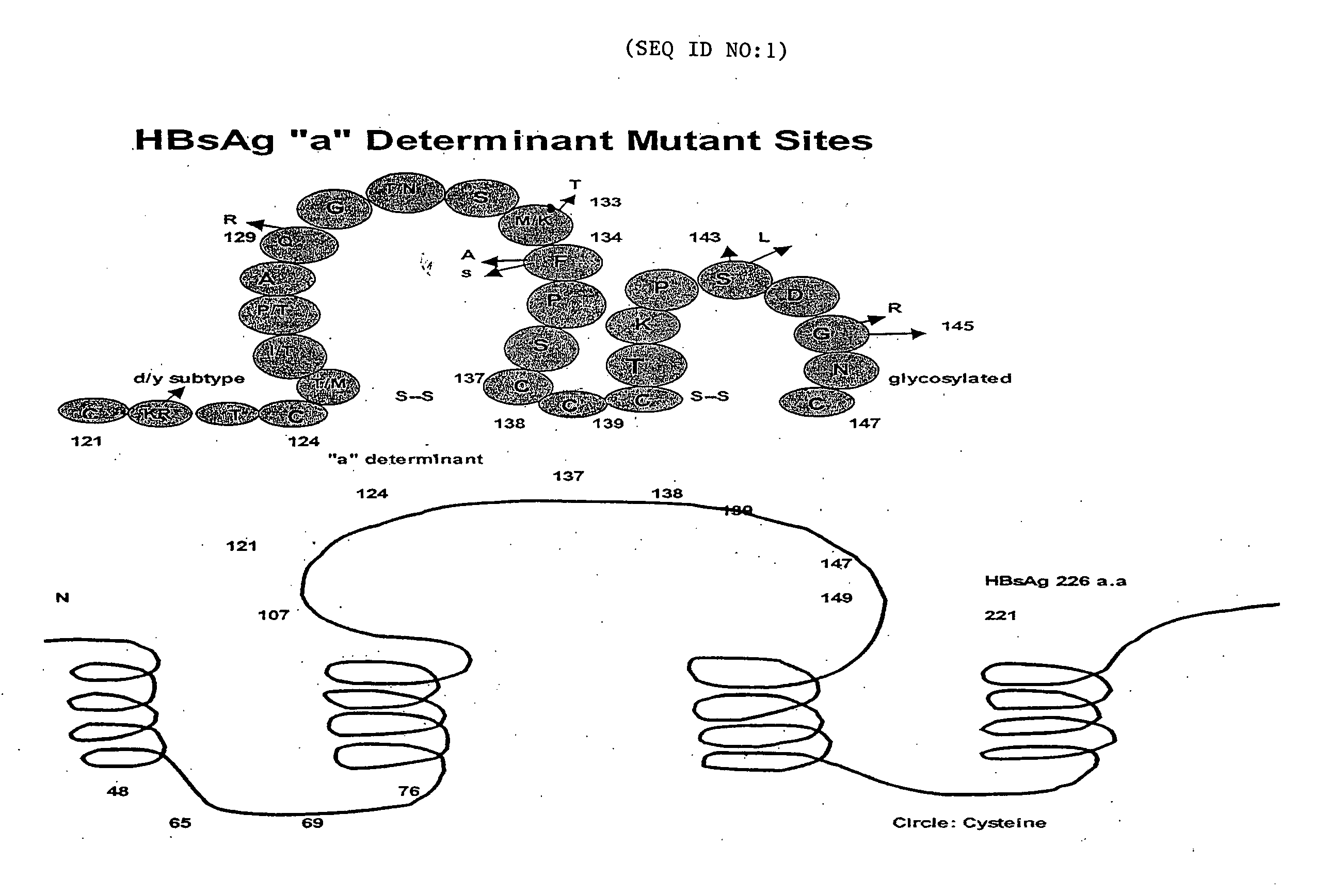
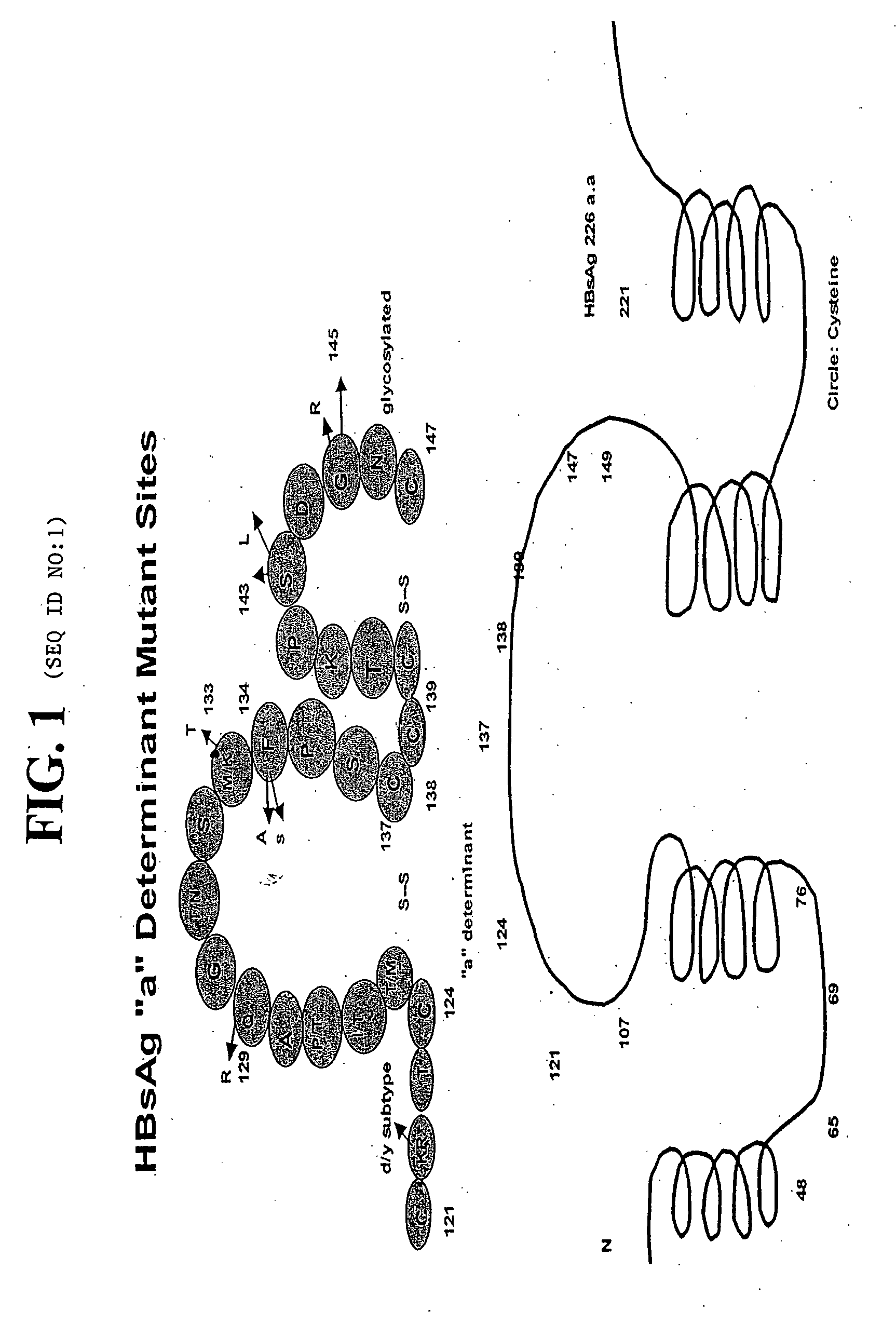
Reagents, methods and immunodiagnostic test kits for the accurate detection of hepatitis B virus (HBV) infection are disclosed. The methods and kits employ novel rabbit monoclonal antibodies directed against HBV surface antigens (HBsAg) with mutations in the “a” determinant region of HBsAg.
Owner:GRIFOLS WORLDWIDE OPERATIONS
Methods for the treatment of hepatitis b and hepatitis d infections
It is disclosed a method for the treatment of hepatitis B (HBV) infection or HBV / hepatitis D (HDV) co-infection, the method comprising administering to a subject in need of treatment a first pharmaceutically acceptable agent that removes the hepatitis B surface antigen from the blood and a second pharmaceutically acceptable agent which stimulates immune function.
Owner:REPLICOR INC
Lucid ganoderma-Cordyceps sinensis granule and preparation method thereof
InactiveCN103505478AHas immunomodulatory functionSolve difficult problemsOrganic active ingredientsGranular deliveryB hepatitis surfaceMedicine
The invention discloses a lucid ganoderma-Cordyceps sinensis granule. A preparation method for the granule comprises the following steps: subjecting purely natural lucid ganoderma to reflux extraction with 70 to 90% ethanol, carrying out pressure reduced condensation, settlement with a ZTC clarifying agent and combination of extracts obtained after extraction three times, filtering the combined extract, then carrying out standing for 10 to 25 h and centrifugation, collecting a precipitate, adding distilled water to dissolve the precipitate, boiling the dissolved precipitate, filtering out insoluble substances while the dissolved precipitate is hot, adding ethanol into an obtained filtrate with stirring until alcohol content reaches 80%, carrying out standing to allow a grayish purple precipitate to be precipitated, drying the grayish purple precipitate at a low temperature to obtain a crude lucid ganoderma polysaccharide product, subjecting the crude lucid ganoderma polysaccharide product to repeated washing and precipitation with 50 to 80% ethanol, carrying out continuous elution and dissolution by using distilled water, concentrating an eluate, adding ethanol until alcohol content reaches 70%, carrying out standing to allow a precipitate to be filtered and drying the precipitate at a low temperature so as to obtain lucid ganoderma polysaccharide; and processing a lucid ganoderma extract particle from 50 to 80% of the lucid ganoderma polysaccharide and mixing the particle and Cordyceps sinensis mycelia according to a ratio of 1: 9 so as to prepare the lucid ganoderma-Cordyceps sinensis granule. The lucid ganoderma-Cordyceps sinensis granule provided by the invention has an immunoloregulation function, enables negative conversion of a hepatitis B surface antigen to be realized and has energy invigorating, foundation strengthening, liver and kidney nourishing, blood circulation promoting and blood stasis removing effects.
Owner:朱文峰
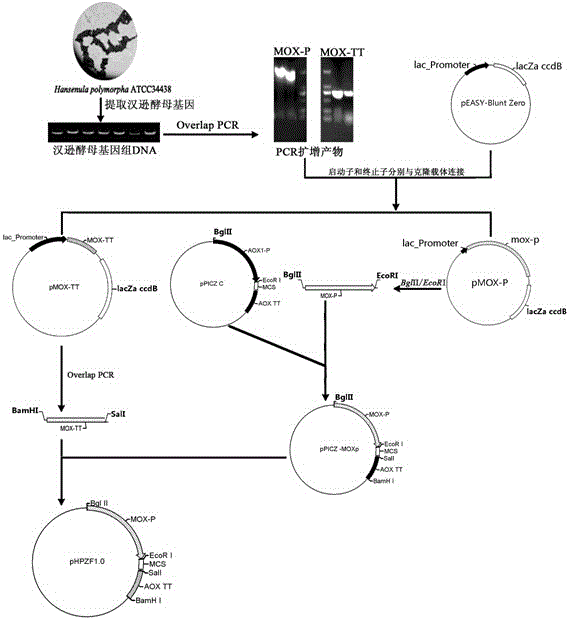
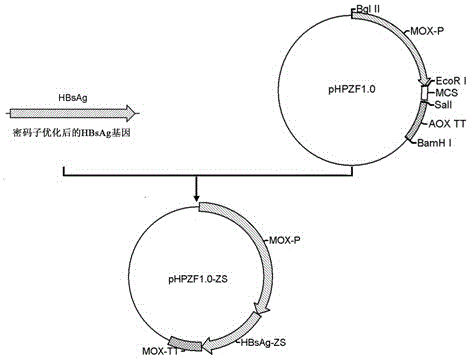
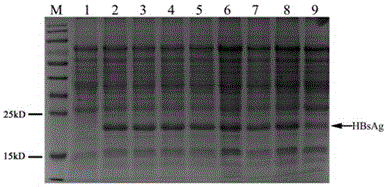
Method for constructing eukaryon Hansenula polymorpha engineering bacteria with recombinant hepatitis B virus genes and method for producing hepatitis B surface antigens
The invention discloses a method for constructing eukaryon Hansenula polymorpha with recombinant hepatitis B virus genes and a method for producing hepatitis B surface antigens, and belongs to the technical field of bioengineering. The method for constructing the eukaryon Hansenula polymorpha includes steps of 1, constructing plasmids pHPZF1.0-ZS with the recombinant hepatitis B virus HBsAg genes; 2, screening the plasmids to obtain the eukaryon Hansenula polymorpha engineering bacteria with the recombinant hepatitis virus HBsAg genes and the highest expression level. The methods have the advantages that HBsAg recombinant proteins can be stably and efficiently expressed in a methanol induction mode by Hansenula polymorpha engineering bacterial strains of the hepatitis B surface antigens which can be obtained by the aid of the methods, and the methods are suitable for producing the HBsAg recombinant proteins on a large scale.
Owner:ANHUI ZHIFEI LONGCOM BIOPHARM CO LTD +2
Four-in-one combined detection test paper box and preparation process
InactiveCN106290829ASave materialSample requirement is smallMaterial analysisHepatitis B immunizationEngineering
The invention relates to a four-in-one combined detection test paper box. A coating film of a test paper strip is provided with a quality control region and a detection region in sequence from top to bottom; the quality control region and the detection region are provided with a quality control line and a detection line respectively; and the detection line is composed of an AIDS (Acquired Immune Deficiency Syndrome) virus 1 / 2 type antibody detection line, a hepatitis B surface antigen detection line, a hepatitis C antibody detection line and a treponema pallidum antibody detection line, which are arrayed in sequence from top to bottom. The invention further relates to a preparation process of the test paper box. The preparation process comprises the steps of preparing a label pad, preparing the detection line, preparing the quality control line, preparing a sample pad and preparing a sample diluted solution. According to the product provided by the invention, four infectious diseases can be simultaneously detected on one test paper strip in one step; and the required amount on samples is less and the detection is rapid and accurate.
Owner:HANGZHOU BIOTEST BIOTECH CO LTD
Hepatitis B vaccine preparation
A novel hepatitis B vaccine contains the genetically engineered surface antigen of hepatitis B virus, human surface antigen antibody of heptitis B, Al adjuvant and A(MPL). Its advantage is high immunogenicity.
Owner:BEIJING LUZHU BIOTECH
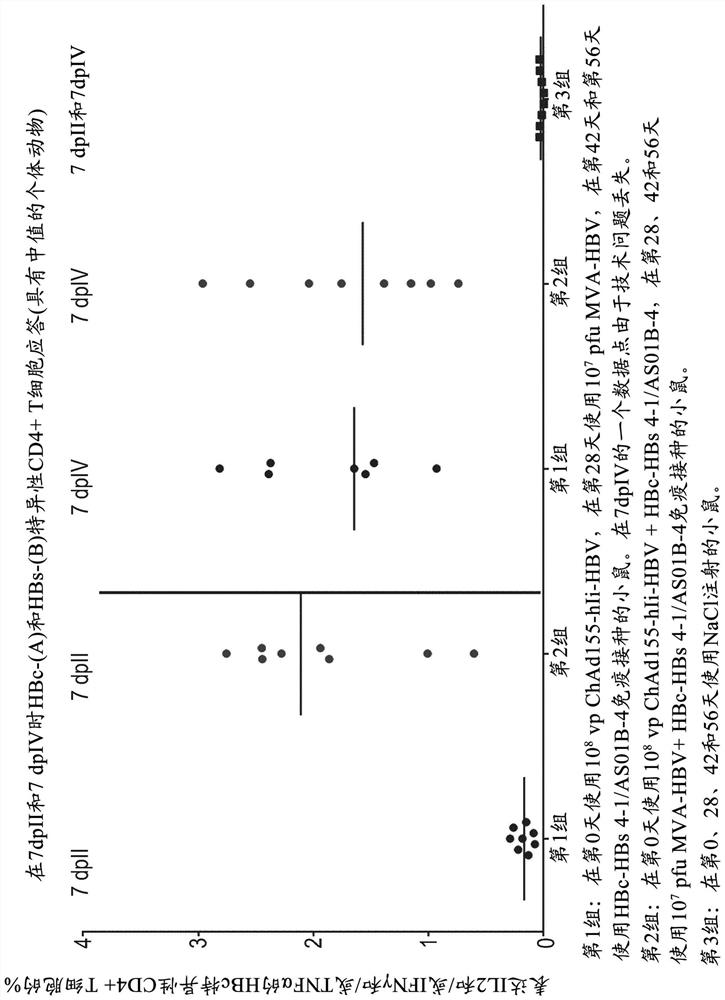
Hepatitis b immunisation regimen and compositions
PendingCN113573730AViral antigen ingredientsWhole-cell/virus/DNA/RNA ingredientsHepatitis B virus core AntigenAdjuvant
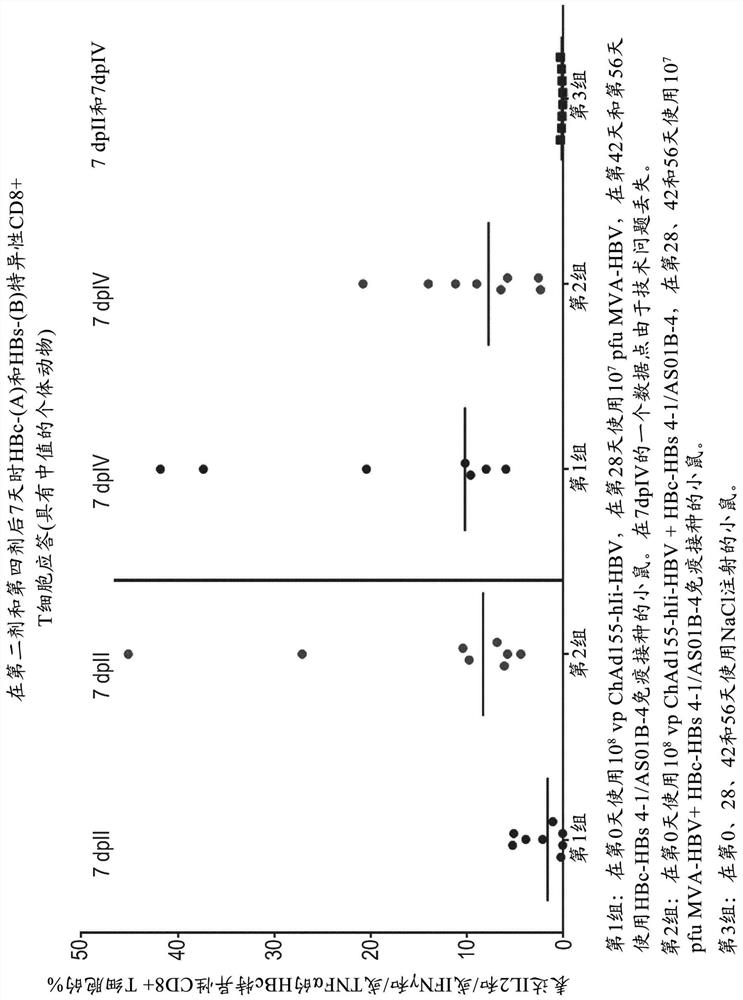
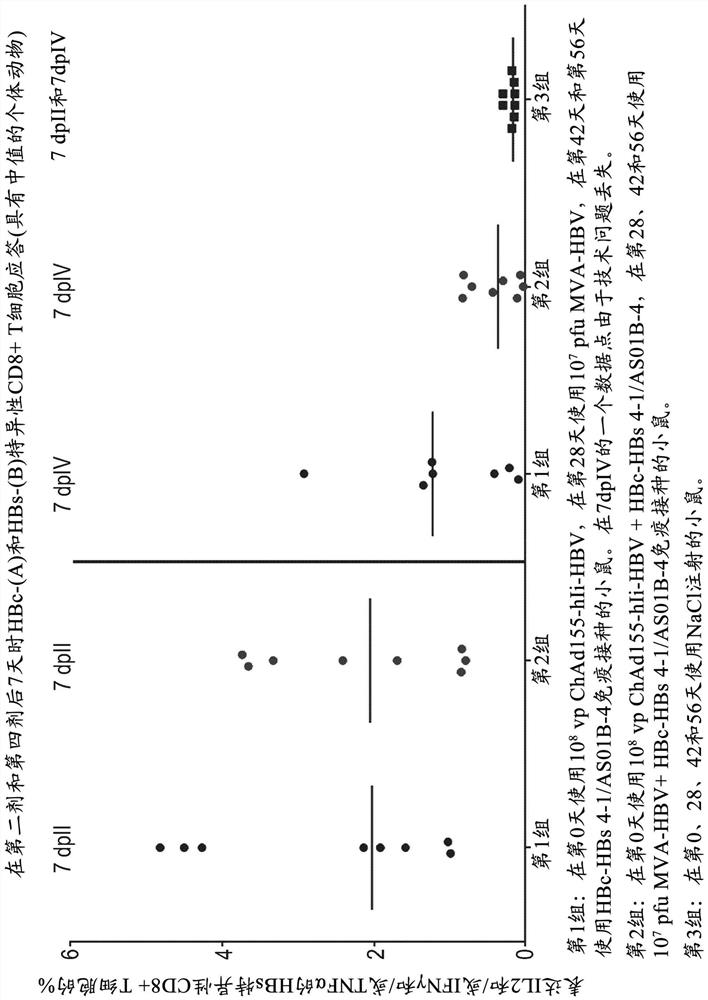
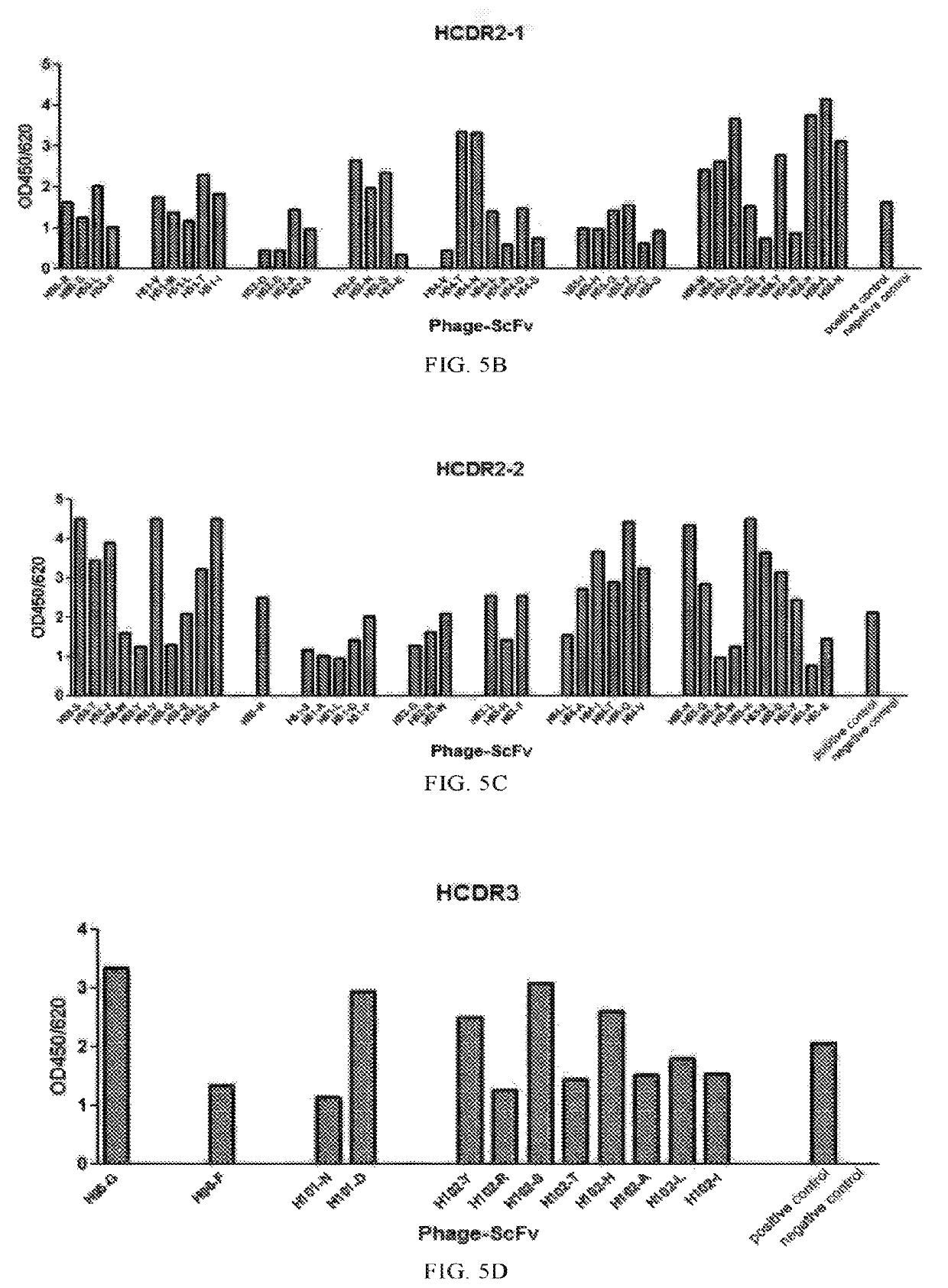
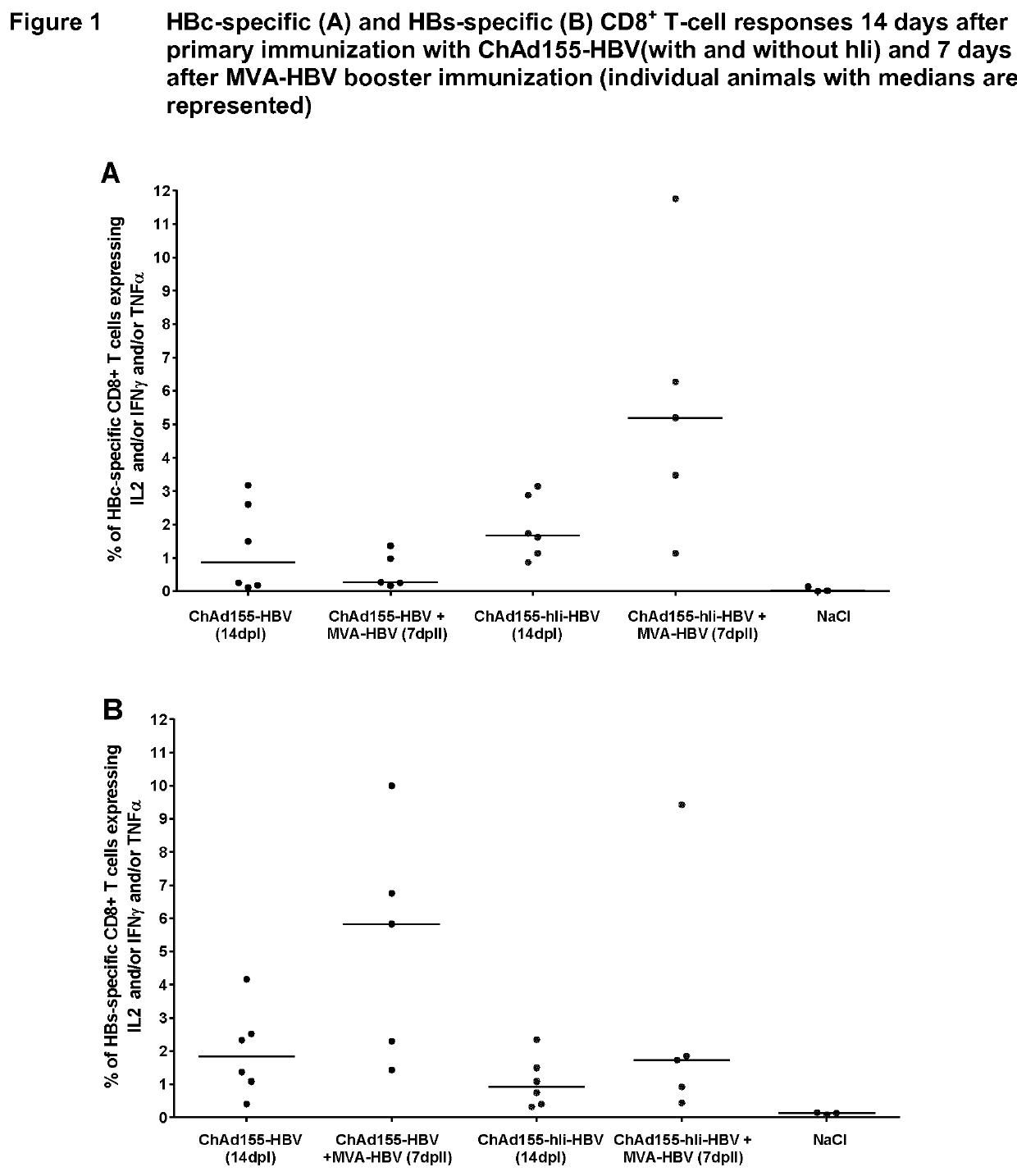
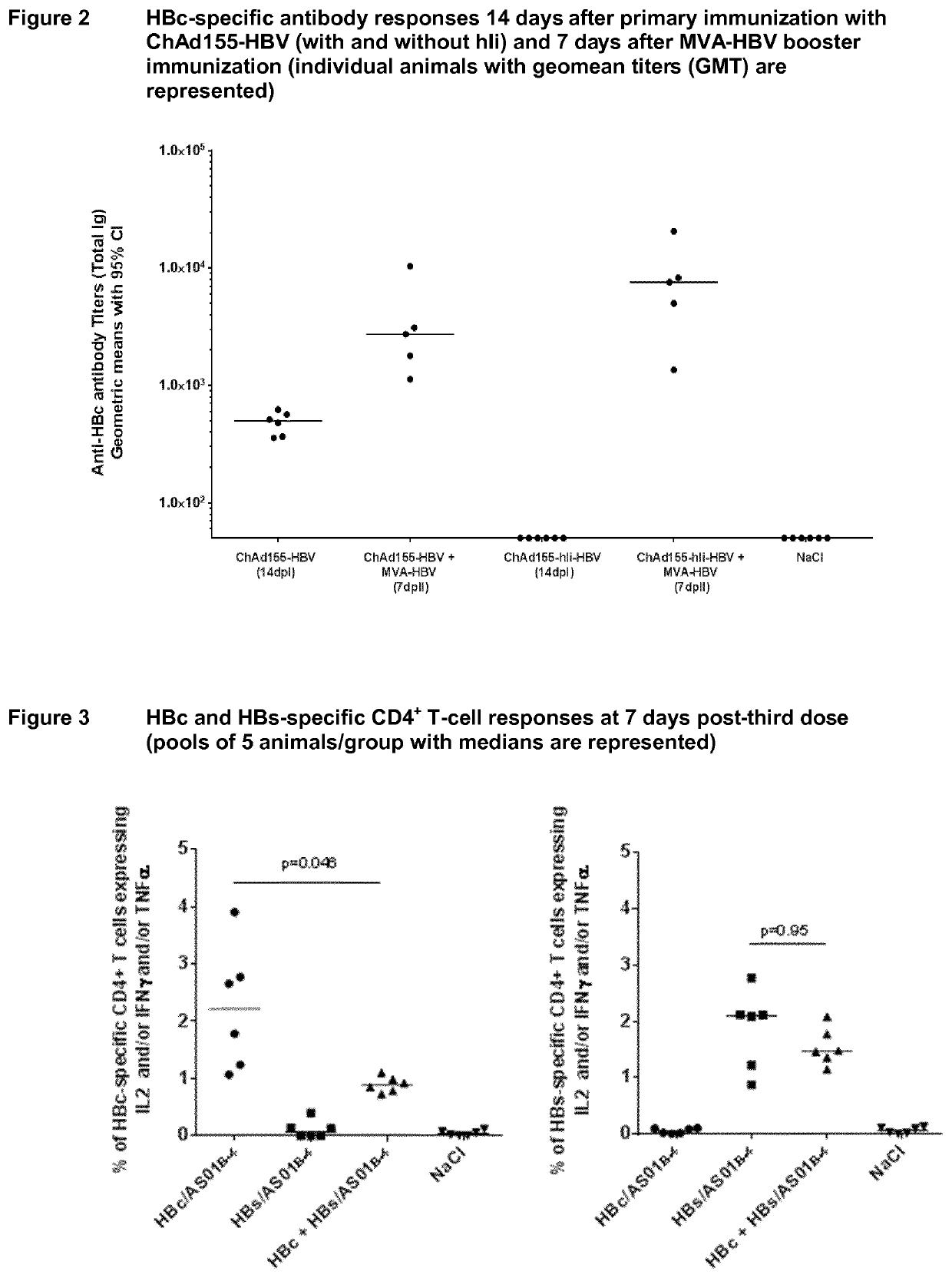
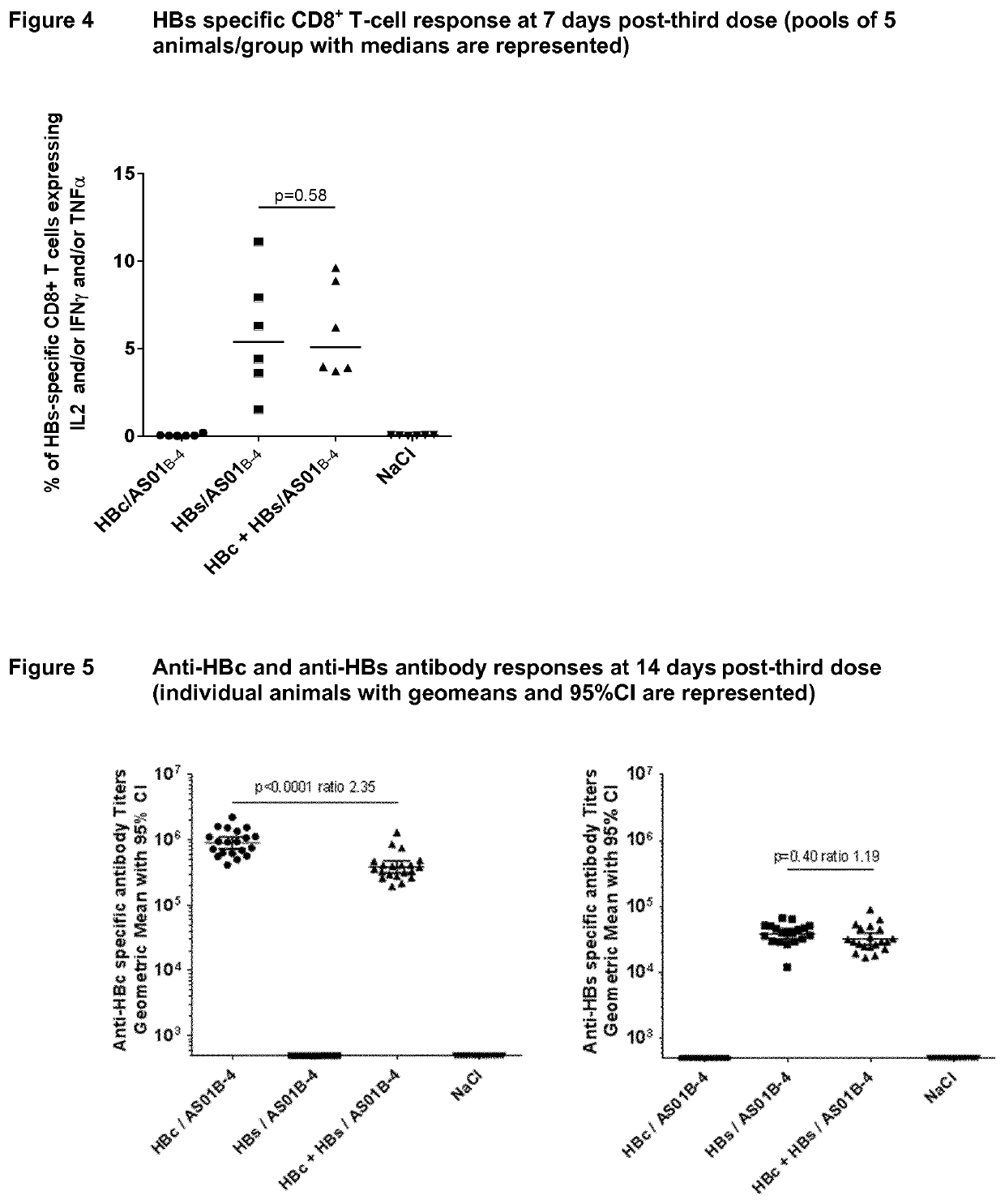
There is provided a method of treating chronic hepatitis B infection (CHB) and / or chronic hepatitis D infection (CHD) in a human, comprising the steps of: a) administering to the human a composition comprising an antisense oligonucleotide (ASO) 10 to 30 nucleosides in length, targeted to a HBV nucleic acid (an HBV ASO); b) administering to the human a composition comprising a replication-defective chimpanzee adenoviral (ChAd) vector comprising a polynucleotide encoding a hepatitis B surface antigen (HBs) and a nucleic acid encoding a hepatitis B virus core antigen (HBc); c) administering to the human a composition comprising a Modified Vaccinia Virus Ankara (MVA) vector comprising a polynucleotide encoding a hepatitis B surface antigen (HBs) and a nucleic acid encoding a hepatitis B virus core antigen (HBc); and d) administering to the human a composition comprising a recombinant hepatitis B surface antigen (HBs), recombinant hepatitis B virus core antigen (HBc) and an adjuvant.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA +1
An immunogenic composition having improved stability, enhanced immunogenicity and reduced reactogenicity and process for preparation thereof
ActiveUS20200206331A1Suitable preventionSuitable treatmentBacterial antigen ingredientsSsRNA viruses positive-senseHepatitis B immunizationAdjuvant
An immunogenic composition comprising of Diphtheria toxoid antigen (D), tetanus toxoid (T) antigen, Hepatitis B surface antigen (HBsAg), inactivated whole-cell B. pertussis (wP) antigen, Haemophilus influenzae type B (Hib) capsular saccharide conjugated to a carrier protein, Inactivated Polio Virus (IPV) antigen and additionally one or more antigens and the method of preparing the same. A fully liquid combination vaccine, showing improved immunogenicity, reduced reactogenicity and improved stability. Improved methods of formaldehyde inactivation, improved adsorption profile of Diphtheria toxoid antigen (D), tetanus toxoid (T) antigen and Hepatitis B (HepB) surface antigen adsorbed individually onto aluminium phosphate adjuvant, minimum total aluminum content (Al3+) and optimized concentration of 2-phenoxyethanol (2-PE) as preservative.
Owner:SERUM INST OF INDIA PTE LTD
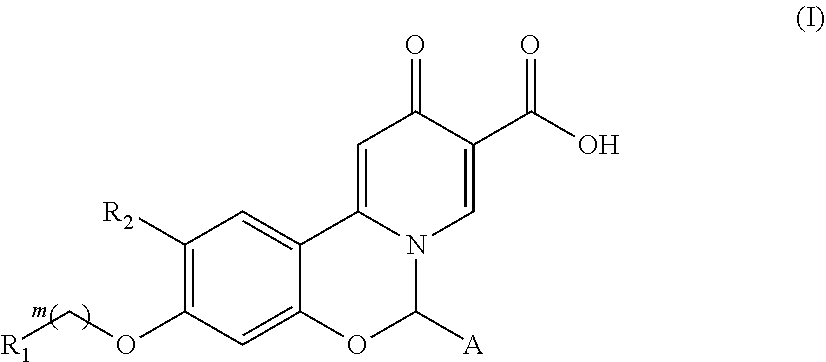
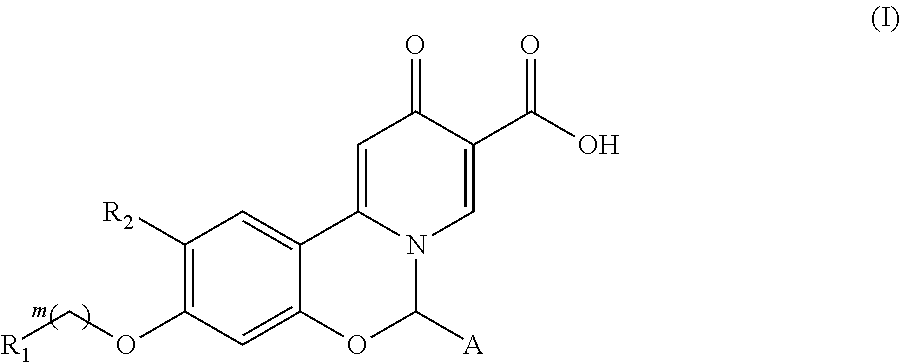
Hepatitis B virus surface antigen inhibitor
ActiveUS11008331B2Improve stabilityEasily hydrolyzedOrganic active ingredientsOrganic chemistryHepatitis B immunizationCarboxylic acid
10-oxo-6,1-dihydrobenzo[e]pyrido[1,2-c][1,3]oxazine-9-carboxylic acid derivatives of formula (I) as hepatitis B surface antigen inhibitors or pharmaceutically acceptable salts thereof, and uses of a compound of formula (I) or pharmaceutically acceptable salts thereof and pharmaceutical compositions thereof in preparation of medicaments for treatment of viral hepatitis B.
Owner:FUJIAN AKEYLINK BIOTECHNOLOGY CO LTD
4-pyridone compound or salt thereof, and pharmaceutical composition and formulation including same
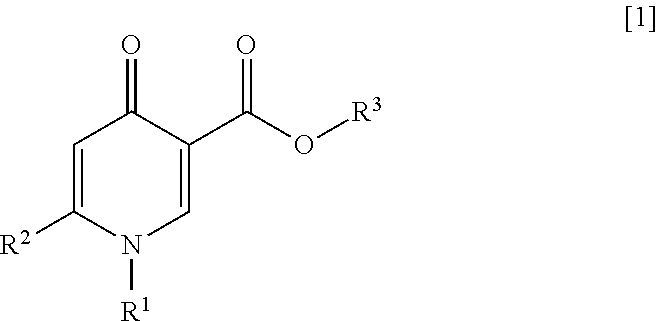
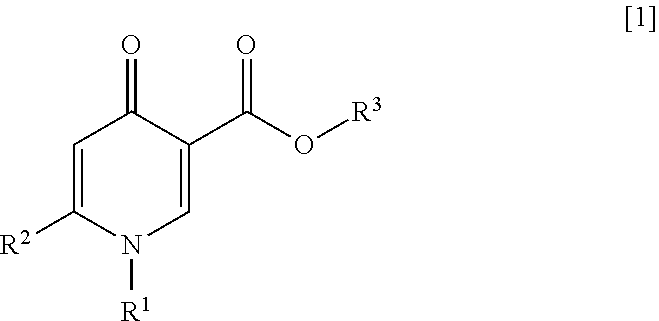
ActiveUS20200017459A1Superior anti-HBV activityHigh activityOrganic active ingredientsOrganic chemistryHepatitis B immunizationB hepatitis surface
An object of the present invention is to provide a compound or a salt thereof having anti-HBV activity, a pharmaceutical composition, an anti-hepatitis B virus agent, a production inhibitor of DNA of a hepatitis B virus, and a production or secretion inhibitor of a hepatitis B surface antigen. According to the present invention, provided are a compound represented by General Formula [1] or a salt thereof:(in the formula, R1 represents a benzothiazolyl group which may be substituted (in which a carbon atom constituting the 6-membered ring of the benzothiazolyl group of R1 is bonded to the nitrogen atom of the pyridone ring); R2 represents a C2-6 alkenyl group which may be substituted, or the like; and R3 represents a hydrogen atom or the like).
Owner:FUJIFILM CORP
Antibody against hepatitis b surface antigen and use thereof
ActiveUS20190389939A1Neutralize HBV virulenceLow serum levelsImmunoglobulins against virusesAntiviralsDiseaseHepatitis B immunization
The invention provides an antibody (in particular, a humanized antibody) against hepatitis B surface antigen (HBsAg), a nucleic acid molecule encoding the same, a method for preparing the same, and a pharmaceutical composition comprising the same. The invention also provides use of the antibody and pharmaceutical composition. The antibody and pharmaceutical composition according to the invention can be used for preventing and / or treating HBV infection or a disease associated with HBV infection (such as Hepatitis B), for neutralizing HBV virulence in a subject (such as human), or for reducing the serum level of HBV DNA and / or HBsAg in a subject.
Owner:XIAMEN UNIV +1
Hepatitis B virus adsorbent based on nanostructure as well as preparation method and application of hepatitis B virus adsorbent
ActiveCN112844331AImprove adsorption efficiencyImprove adsorption capacityOther chemical processesOther blood circulation devicesAptamerSpecific adsorption
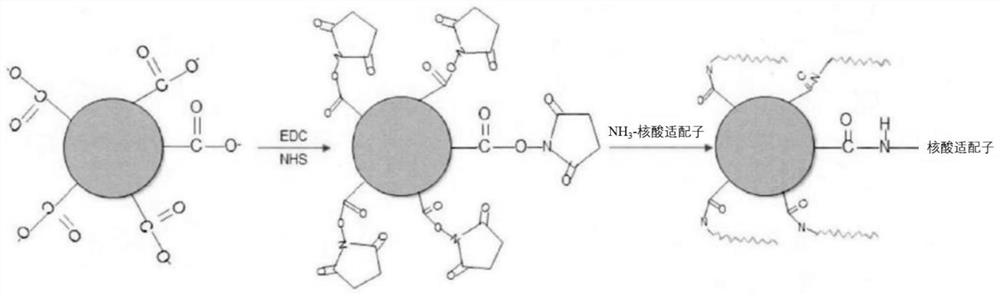
The invention provides a hepatitis B virus adsorbent based on a nanostructure as well as a preparation method and an application of the hepatitis B virus adsorbent. The method comprises the following steps: synthesizing a DNA nano-material with a specific structure, and connecting the DNA nano-material with an aptamer to obtain a DNA nano-material connected with the aptamer; and loading the DNA nano material connected with the aptamer on a specific adsorption carrier to obtain the hepatitis B virus adsorbent based on the nano structure. Through the mode, the DNA nano material can be used as an intermediate structure for connecting the aptamer and the adsorption carrier, so that complex operation required when the aptamer and the adsorption carrier are directly coupled can be avoided, and the preparation process is simplified; meanwhile, the adsorption efficiency of the adsorbent can be effectively improved while the aptamer and the adsorption carrier are stably connected, so that efficient adsorption of the hepatitis B surface antigen is realized, the content of the hepatitis B surface antigen in blood is greatly reduced, and the adsorbent has a relatively high practical application value.
Owner:WUHAN RUIFA MEDICAL DEVICES CO LTD
Hepatitis B virus adsorbent as well as preparation method and application thereof
ActiveCN112717902AThe preparation process is convenient and efficientImprove adsorption capacityOther chemical processesOther blood circulation devicesAptamerHepatitis B immunization
The invention provides a hepatitis B virus adsorbent as well as a preparation method and application thereof. The preparation method comprises the following steps: mixing Nhydroxysuccinimide and 1 (3-dimethylaminopropyl) 3ethylcarbodiimide hydrochloride, and dissolving the mixture in a buffer solution to prepare a cross-linking catalyst; adding the obtained cross-linking catalyst into a carboxylated agarose gel microsphere suspension, fully and uniformly mixing, then adding a dissolved amino-modified nucleic acid aptamer, and directly coupling the carboxylated agarose gel microsphere with the amino-modified nucleic acid aptamer by virtue of amidation reaction, and therefore, the hepatitis B virus adsorbent is simply, conveniently and efficiently prepared. By means of the mode, the nucleic acid aptamer can replace a traditional antibody to serve as a ligand, the prepared adsorbent has higher adsorption efficiency, effective adsorption of the hepatitis B surface antigen is achieved, and therefore the content of the hepatitis B surface antigen in blood is effectively reduced, and high practical application value is achieved.
Owner:WUHAN RUIFA MEDICAL DEVICES CO LTD
Hepatitis B surface antigen detection method
PendingCN111024950AThe detection sample size is smallDetection background is clearMaterial analysisAntiendomysial antibodiesVenous blood
The invention provides a hepatitis B surface antigen detection method, which comprises the following steps: (1) preparing a serum sample, namely, collecting venous blood, separating the venous blood to obtain serum, and carrying out high-speed centrifugation on the serum 1-2 times to obtain a serum sample to be detected, wherein the high-speed centrifugal conditions are as follows: the temperatureis 4-10 DEG C, the speed is 10000-15000rpm / min, and the time is 3-8min; (2) adding a sample, namely, adding 40-50 [mu] L of the serum sample to be detected into a reaction hole coated with a monoclonal antibody aiming at the hepatitis B surface antigen, wherein the clone number of the monoclonal antibody is 86C; (3) adding an enzyme-labeled antibody, namely, adding 50+ / -1 [mu] L of the enzyme-labeled antibody into the reaction hole, then covering a reaction plate with a plate sealing film, and incubating the reaction plate at 37+ / -1 DEG C for 35-45min; (4) washing; (5) developing; (6) terminating; (7) measuring. The detection method can realize accurate detection of the hepatitis B surface antigen, and particularly has the advantages of smaller sample size required, clear and clean reaction hole body and low background when being used for detecting people with hyperlipidemia.
Owner:JILIN JINYU MEDICAL SCI INSPECTION CO LTD
Method for suppression of hepapitis b virus replication and hepapitis b virus surface antigen secretion
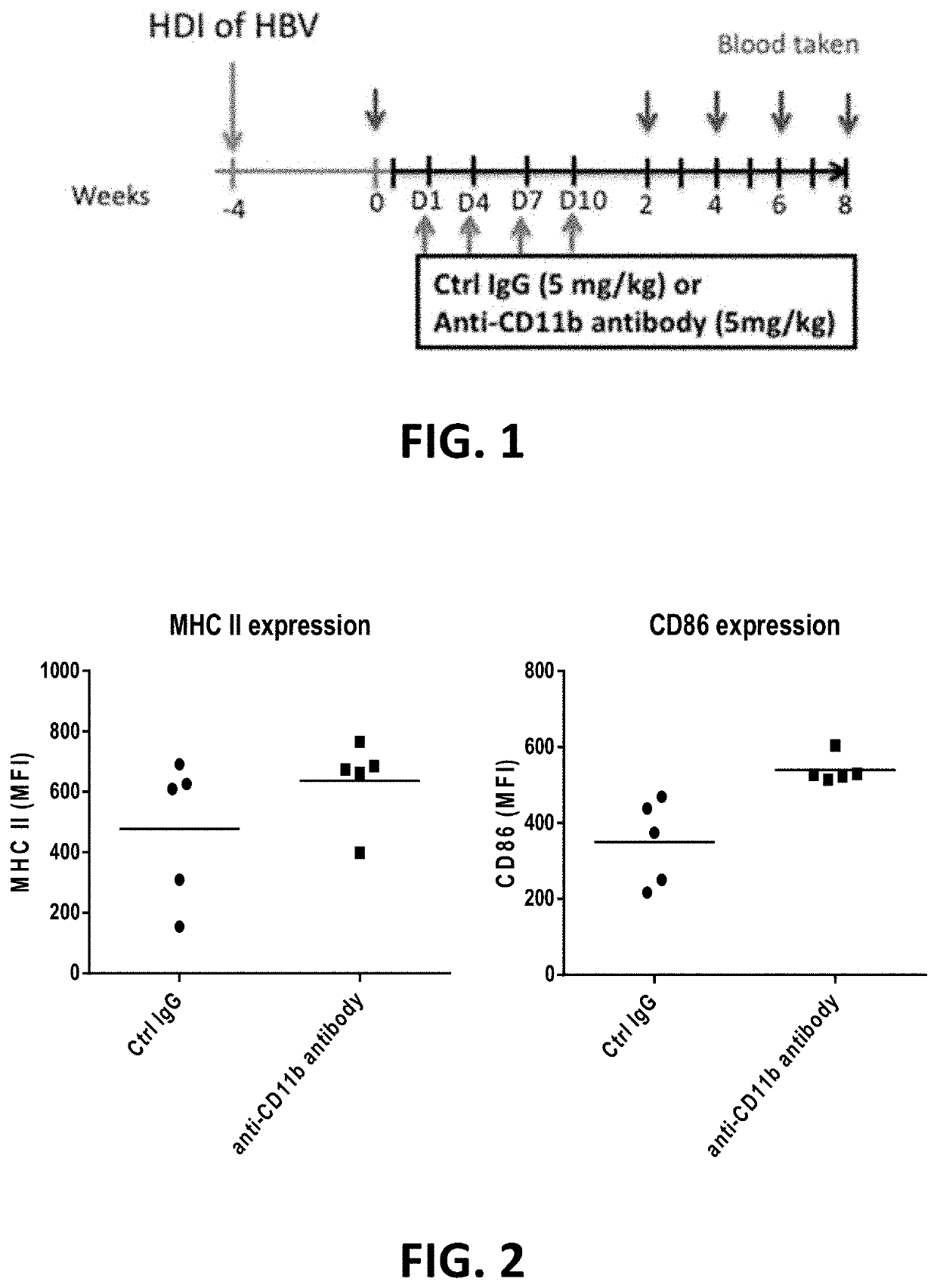
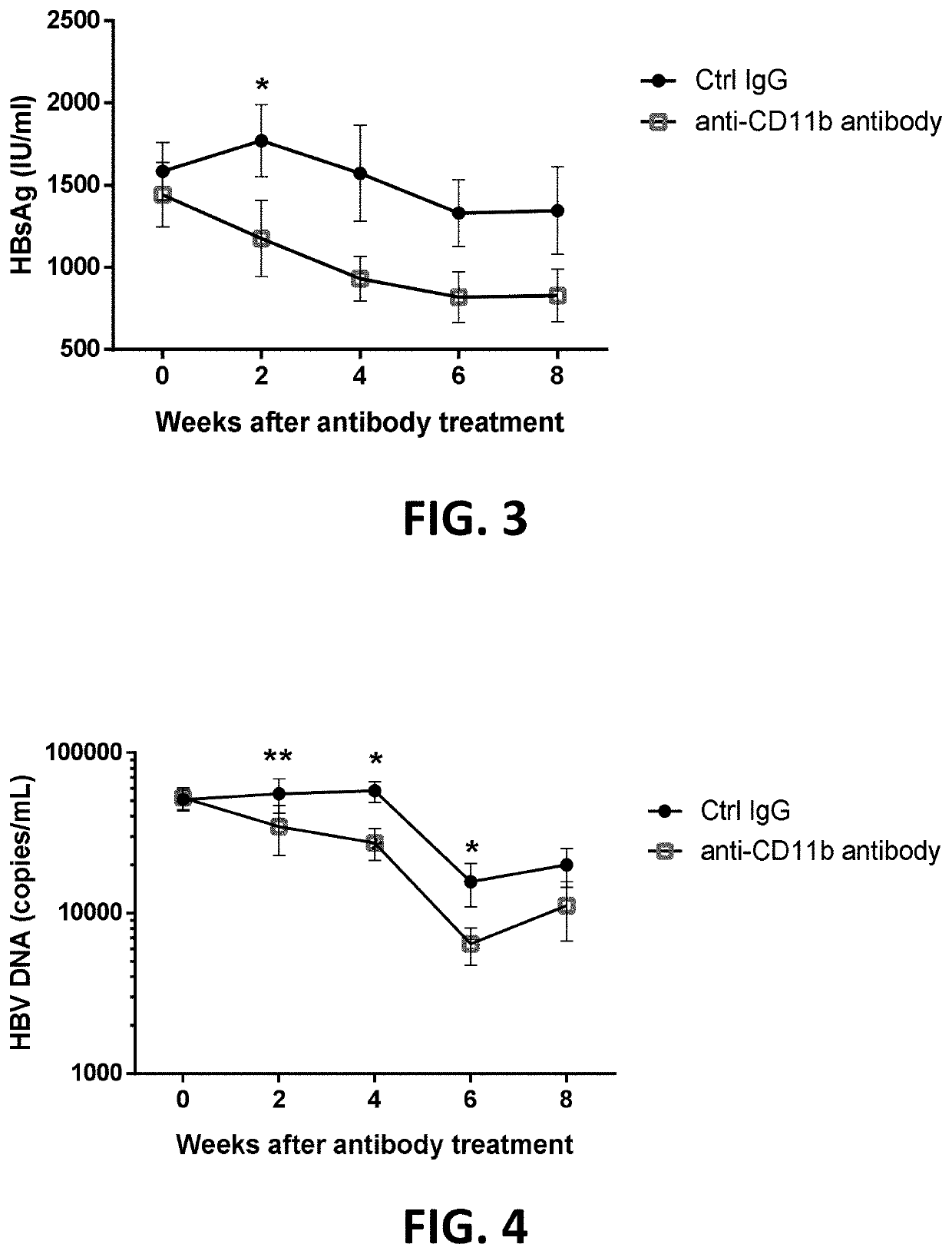
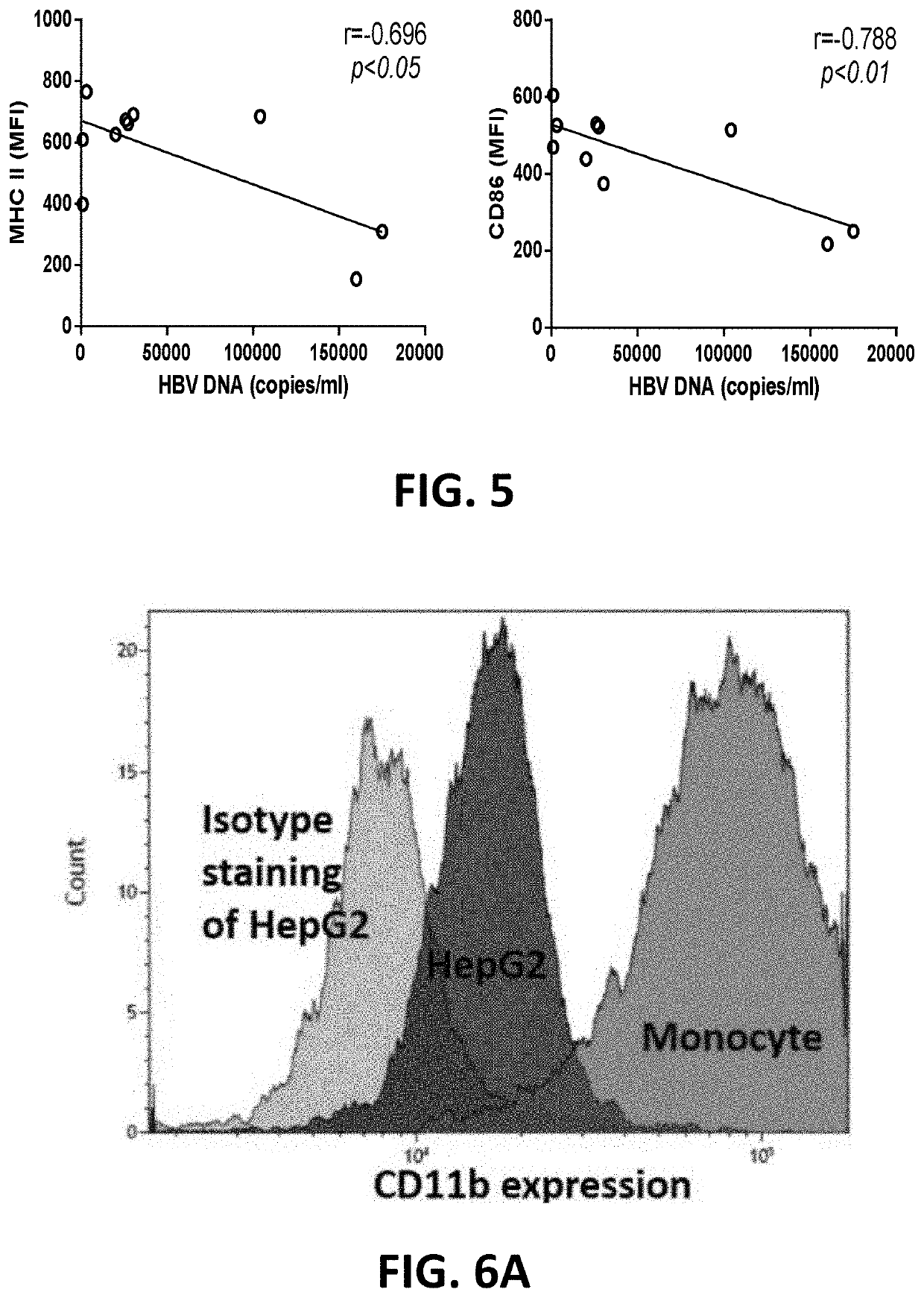
PendingUS20210324084A1Increased surface expressionSuppressed levelAntisepticsAntiviralsPeripheral blood mononuclear cellHepatitis B immunization
A pharmaceutical composition for use in treating hepatitis B virus (HBV) infection includes an effective amount of an antibody against CD11b or a binding fragment thereof. A method for treating hepatitis B virus infection includes administering to a subject in need thereof an antibody against CD11b. Anti-CD11b antibody binding to CD11b may trigger immunostimulatory responses, as evidenced by the following observations: increased surface expression of MHC II and CD86 in CD11b+ peripheral blood mononuclear cells (PBMCs); suppressed level of hepatitis B surface antigen (HBsAg) and HBV DNA in the blood; and accelerated clearance of HBV from liver.
Owner:ASCENDO BIOTECHNOLOGY INC
Hepatitis b immunisation regimen and compositions
There is provided a method of treating chronic hepatitis B infection (CHB) in a human, comprising the steps of: a) administering to the human a composition comprising a replication-defective chimpanzee adenoviral (ChAd) vector comprising a polynucleotide encoding a hepatitis B surface antigen (HBs) and a nucleic acid encoding a hepatitis B virus core antigen (HBc); b) administering to the human acomposition comprising a Modified Vaccinia Virus Ankara (MVA) vector comprising a polynucleotide encoding a hepatitis B surface antigen (HBs) and a nucleic acid encoding a hepatitis B virus core antigen (HBc); and c) administering to the human a composition comprising a recombinant hepatitis B surface antigen (HBs), recombinant hepatitis B virus core antigen (HBc) and an adjuvant.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Novel Anti-hepatitis b virus antibody and uses thereof
PendingUS20220235117A1Reduce frequencyReduce dosageDigestive systemImmunoglobulins against virusesDiseaseChronic hepatitis
Disclosed are antibodies to anti-hepatitis B surface antigen (HBsAg) (especially humanized antibodies), nucleic acid molecules encoding same, methods for preparing same, and pharmaceutical compositions containing same. The antibodies have higher affinity for HBsAg at neutral pH than at acidic pH, thereby significantly enhancing the virus clearance efficiency and prolonging the virus inhibition time. The antibodies and the pharmaceutical compositions can be used for preventing and / or treating HBV infections or diseases related to HBV infections (e.g., hepatitis B), for neutralizing the virulence of HBV in a subject (e.g., a human), for reducing the serum level of HBV DNA and / or HBsAg in the body of the subject, or for activating the humoral immune response of the subject (e.g., a chronic HBV infected or chronic hepatitis B patient) against HBV.
Owner:XIAMEN UNIV +1
Antibody against hepatitis B surface antigen and use thereof
ActiveUS10689434B2Neutralize HBV virulenceLow serum levelsImmunoglobulins against virusesAntiviralsDiseaseHepatitis B immunization
The invention provides an antibody (in particular, a humanized antibody) against hepatitis B surface antigen (HBsAg), a nucleic acid molecule encoding the same, a method for preparing the same, and a pharmaceutical composition comprising the same. The invention also provides use of the antibody and pharmaceutical composition. The antibody and pharmaceutical composition according to the invention can be used for preventing and / or treating HBV infection or a disease associated with HBV infection (such as Hepatitis B), for neutralizing HBV virulence in a subject (such as human), or for reducing the serum level of HBV DNA and / or HBsAg in a subject.
Owner:XIAMEN UNIV +1
Immunogenic composition having improved stability, enhanced immunogenicity and reduced reactogenicity and process for preparation thereof
ActiveUS11179453B2Reduced reactogenicityImprove effectivenessBacterial antigen ingredientsSsRNA viruses positive-senseHepatitis B immunizationAdjuvant
An immunogenic composition comprising of Diphtheria toxoid antigen (D), tetanus toxoid (T) antigen, Hepatitis B surface antigen (HBsAg), inactivated whole-cell B. pertussis (wP) antigen, Haemophilus influenzae type B (Hib) capsular saccharide conjugated to a carrier protein, Inactivated Polio Virus (IPV) antigen and additionally one or more antigens and the method of preparing the same. A fully liquid combination vaccine, showing improved immunogenicity, reduced reactogenicity and improved stability. Improved methods of formaldehyde inactivation, improved adsorption profile of Diphtheria toxoid antigen (D), tetanus toxoid (T) antigen and Hepatitis B (HepB) surface antigen adsorbed individually onto aluminium phosphate adjuvant, minimum total aluminum content (Al3+) and optimized concentration of 2-phenoxyethanol (2-PE) as preservative.
Owner:SERUM INST OF INDIA PTE LTD
Hepatitis b immunisation regimen and compositions
PendingUS20210069322A1Viral antigen ingredientsVirus peptidesHepatitis B virus core AntigenModified vaccinia Ankara
There is provided a method of treating chronic hepatitis B infection (CHB) in a human, comprising the steps of:a) administering to the human a composition comprising a replication-defective chimpanzee adenoviral (ChAd) vector comprising a polynucleotide encoding a hepatitis B surface antigen (HBs) and a nucleic acid encoding a hepatitis B virus core antigen (HBc);b) administering to the human a composition comprising a Modified Vaccinia Virus Ankara (MVA) vector comprising a polynucleotide encoding a hepatitis B surface antigen (HBs) and a nucleic acid encoding a hepatitis B virus core antigen (HBc); andc) administering to the human a composition comprising a recombinant hepatitis B surface antigen (HBs), recombinant hepatitis B virus core antigen (HBc) and an adjuvant.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Medical use of 2 alpha, 3 beta-dihydroxy-5, 11(13)- diallyl eudesmane-12 acid for inhibiting hepatitis B virus
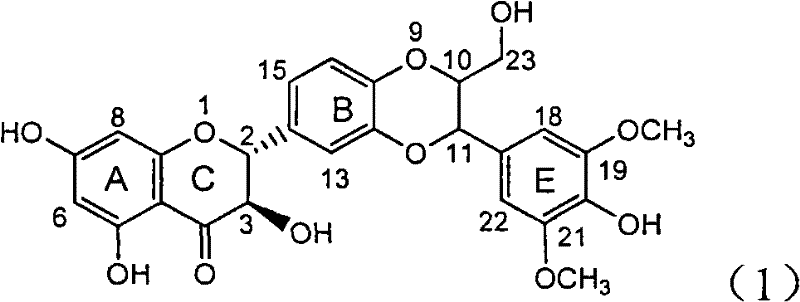
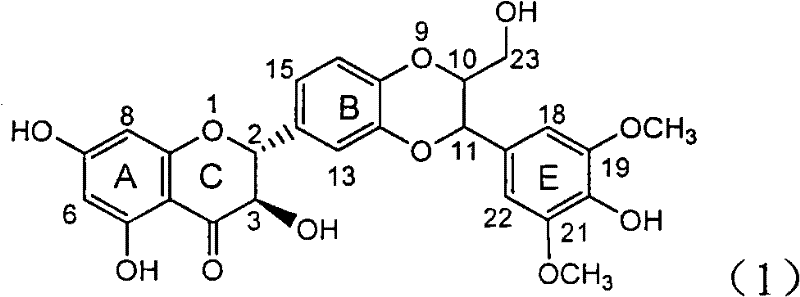
InactiveCN100413495CPrevention and treatment of viral hepatitis BHBsAg reductionOrganic active ingredientsDigestive systemDiseaseHepatitis B immunization
The invention relates to a sesterterpane as formula (1) and relative medical drug or solvent, and relative drug compound, which can reduce hepatitis B surface antigen and restrain hepatitis B HBVDNA copy activity. Wherein, said invention has strong restrain on hepatitis B surface antigen (HBsAg) generated by HepG2.2.15 cell and the copy of hepatitis B deoxyribonucleic acid (HBV-DNA), while it restrain ability is higher than positive contrast difuradin; and the copy restrain activity at large amount (100 mug / mL) and middle amount (20 mug / mL) on the hepatitis B HBV-DNA are both higher then difuradin.
Owner:赵昱
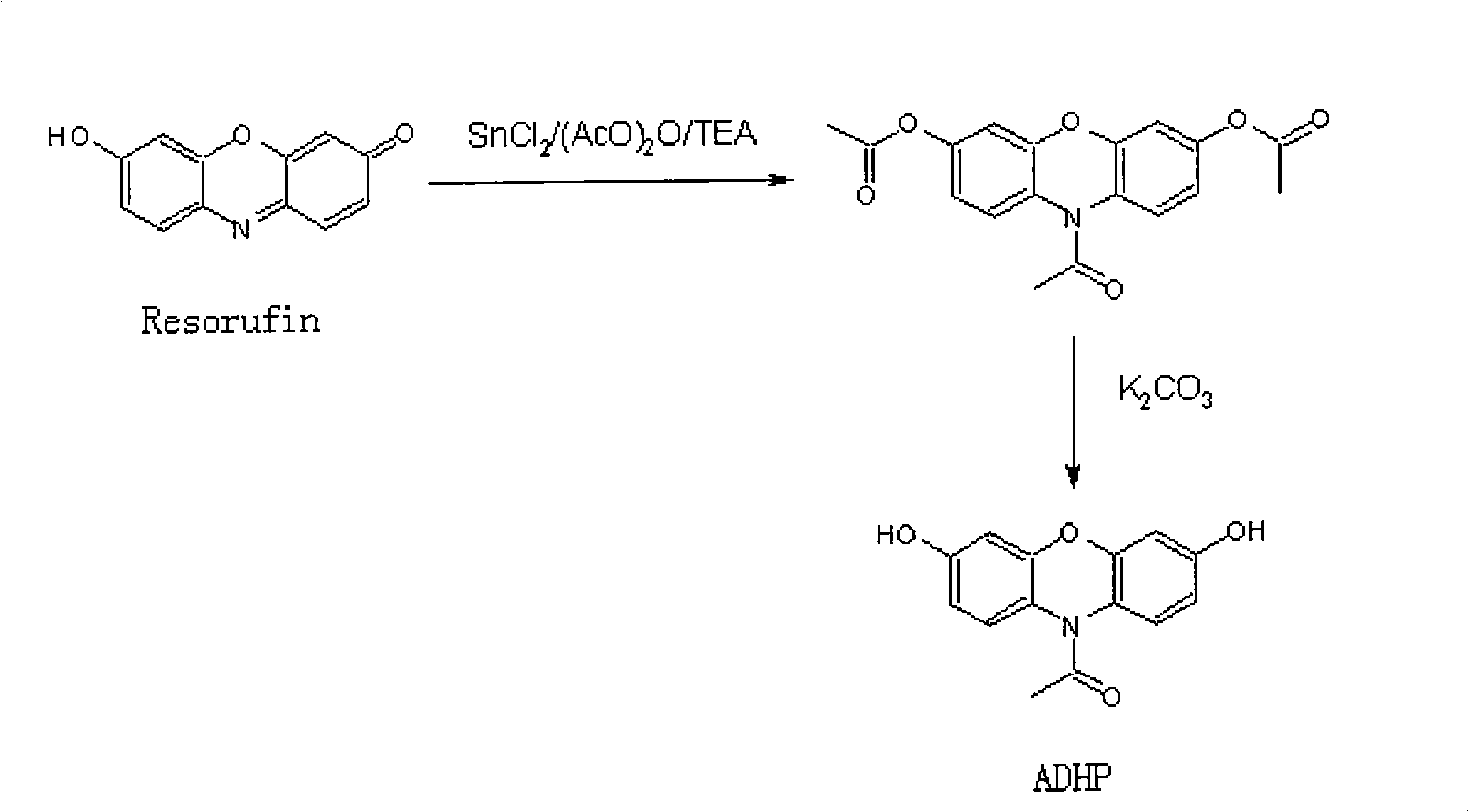
Hepatitis b surface antigen and antibody detection method
InactiveCN101334407AEliminate health hazardsLow costBiological testingHepatitis surface antigenChemistry
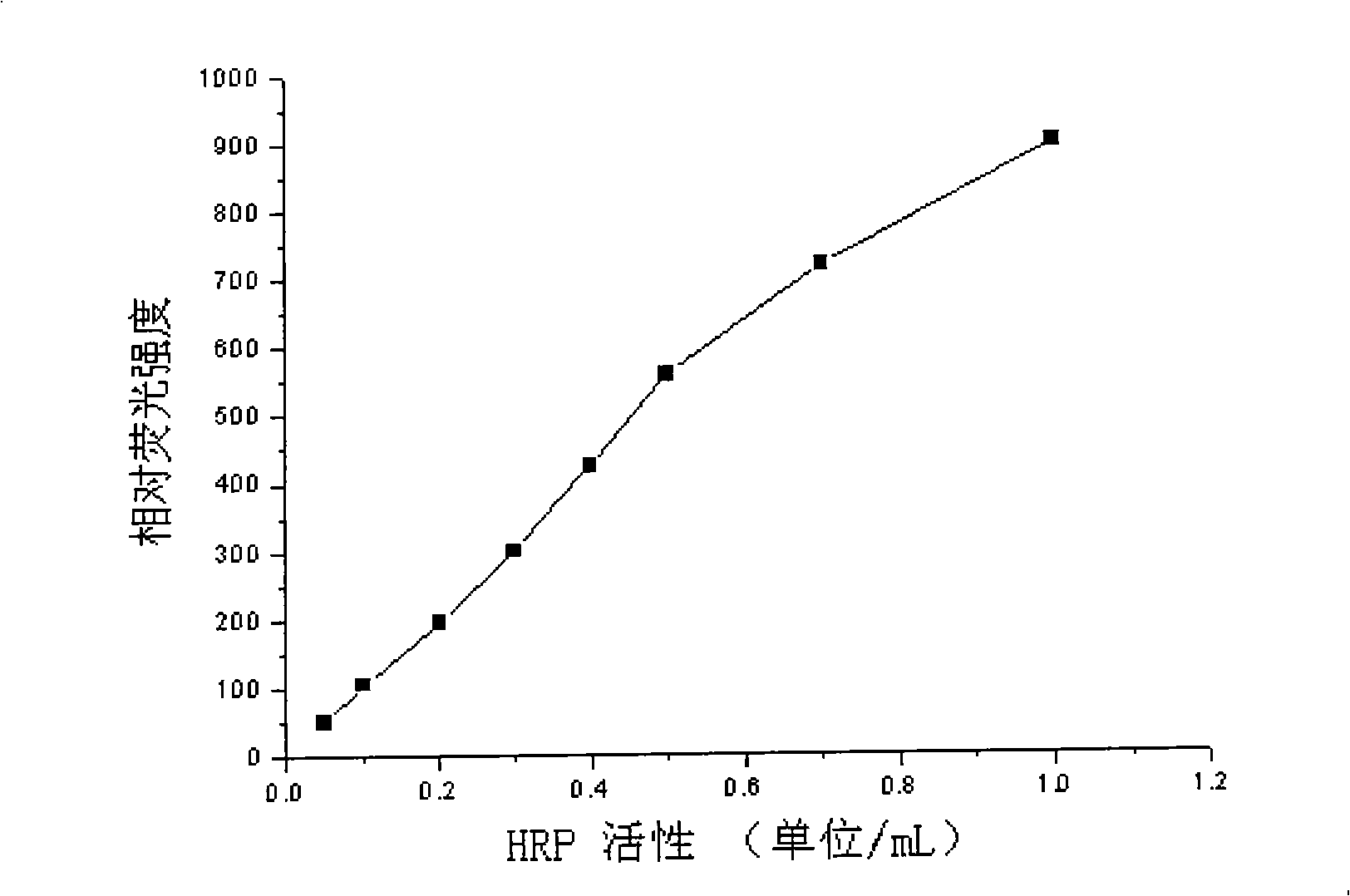
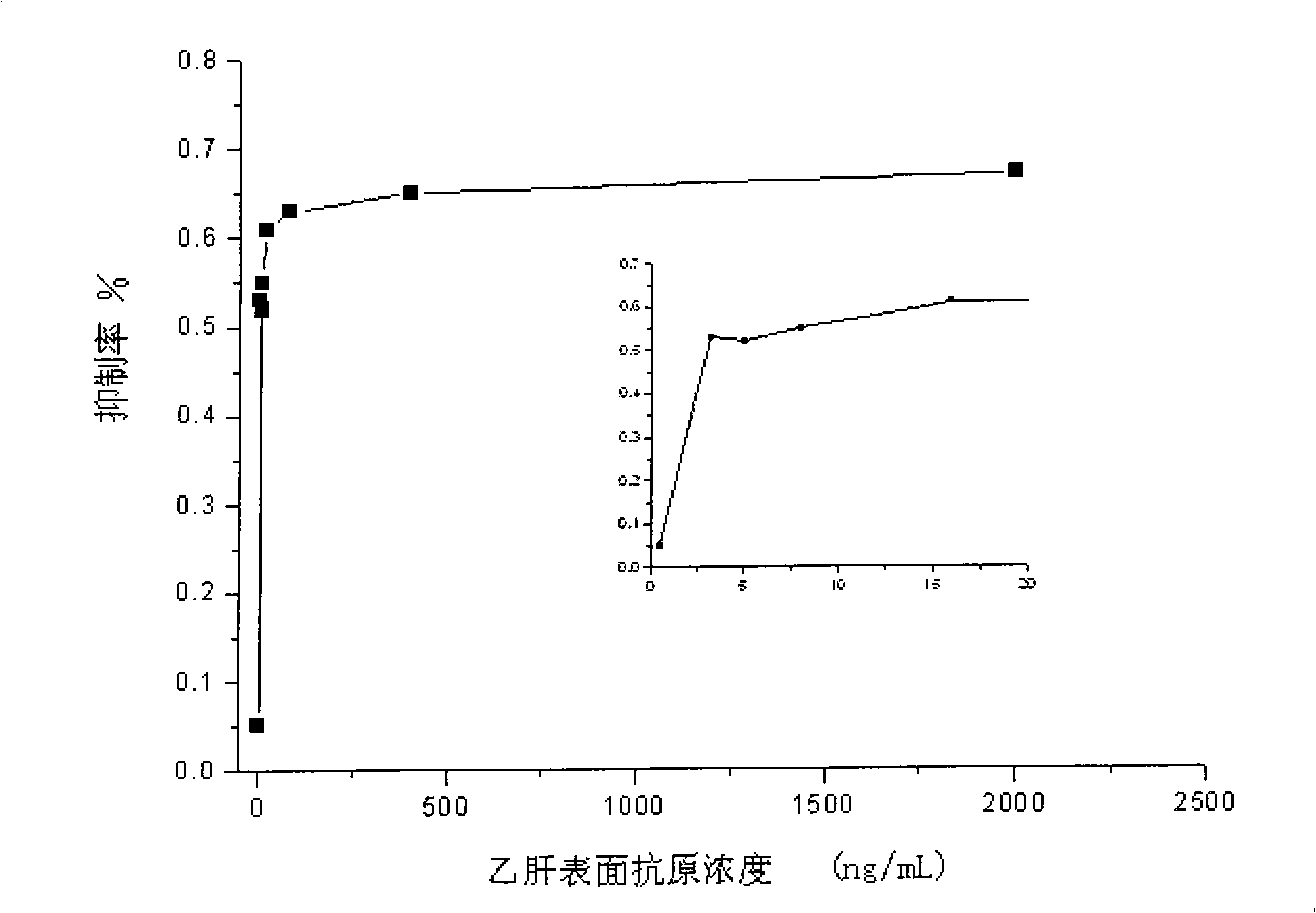
The invention discloses a covalent conjugate of polypeptide and horseradish peroxidase (HRP), the covalent conjugate is reacted with a novel fluorescent substrate of the HRP to generate very strong fluorescent signals, the HRP-labeled polypeptide can be used for the diagnosis of hepatitis B surface antigen or antibody, and the conjugate is a product formed by the covalent conjugation of the polypeptide with less than 50 amino acids and the horseradish peroxidase (HRP). The HRP-labeled polypeptide with the covalent bond connection is easily identified and combined by the antibody, the existence of the hepatitis B surface antibody or antigen in a sample can be measured through various modes. The HRP-labeled polypeptide conjugate of the invention is reacted with the fluorescent substrate ADHP of the HRP, thereby being used for the fluorescence detection of the hepatitis B surface antigen and antibody.
Owner:BEIJING FANBO BIOCHEM
Application of flavone lignan to preparing medicaments for treating virus B hepatitis
InactiveCN101912384BInhibitory activityThe source is easy to getOrganic active ingredientsDigestive systemBenzeneB hepatitis surface
Owner:DALI UNIV
Test strip for detecting hepatitis B surface antigen and preparation method thereof
PendingCN114414807ABodily injuryImprove protectionMaterial analysisHepatitis B immunizationEngineering
The invention discloses a test strip for detecting hepatitis B surface antigen and a preparation method thereof, and the preparation method comprises the following steps: S1, preparing a sample pad treating fluid, and treating a glass fiber membrane through the sample pad treating fluid to prepare a sample pad; s2, preparing a gold-labeled pad treating fluid, and treating the glass fiber membrane through the gold-labeled pad treating fluid to obtain a gold-labeled pad; and S3, preparing monoclonal antibody immune gold for resisting the hepatitis B virus surface antigen, and spraying the prepared immune gold on the treated gold label pad according to 3 [mu] l / cm by using a gold spraying and membrane scratching all-in-one machine. Based on the immune lateral flow chromatography technology, instruments are not needed in the detection process, and whether the hepatitis B surface antigen exists in blood or not can be judged through naked eyes; the detection result is accurate, the detection time is short, and the body of a detected person cannot be injured; because the detection time is short, acute detection can be carried out on a patient before an operation, so that the protection of medical personnel is facilitated, and the transmission of infectious diseases is effectively blocked.
Owner:南京珀尔泰生物技术有限公司
A kind of bispecific recombinant anti-hbsag antibody, its preparation method and use
ActiveCN104592390BInhibition releaseHigh activityHybrid immunoglobulinsImmunoglobulins against virusesHepatitis B immunizationAntiendomysial antibodies
The invention belongs to the field of biotechnology and discloses a bispecific antibody specifically binding to different epitopes of hepatitis B surface antigen (HBsAg). The present invention also provides the amino acid sequence of the antibody, the DNA sequence encoding the antibody, the expression vector containing the DNA sequence and the host cell containing the expression vector. The invention also discloses the preparation method of the above-mentioned antibody and its application in preventing hepatitis B virus infection.
Owner:SHENZHEN SCIPROGEN BIO PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com