A kind of bispecific recombinant anti-hbsag antibody, its preparation method and use
A bispecific antibody and carrier technology, which is applied in the field of prevention, bispecific recombinant anti-HBsAg antibody, and treatment of hepatitis B virus infection, can solve the problems of not meeting clinical needs, high development costs, and limiting the development process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Preparation of HBsAg-specific single B cells
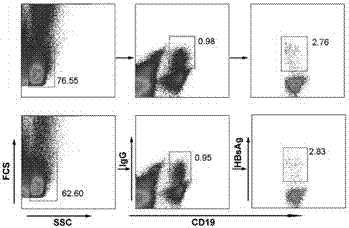
[0041] By separating lymphocytes from the peripheral blood of volunteers who had been injected with hepatitis B vaccine, HBsAg was separated with CD19-PE / Cy5, IgG-PE, HBsAg-biotin, Streptavidin-FITC fluorescent antibodies + CD19 + IgG + B cells. Such as figure 1 As shown, a small population of HBsAg-positive cells (approximately 0.01-0.02% of the entire B cell population) can be seen. Each HBsAg sorted by MoFlo XDP ultrafast flow cytometry sorting system + CD19 + IgG + B cells were injected into 96-well Single cell PCR plates, ensuring one cell per PCR well. Then, the lysate was added, frozen in liquid nitrogen, and stored in ice at -80°C for later use.
Embodiment 2
[0042] Embodiment 2: RT-PCR and nested PCR clone antibody variable region gene and gene expression
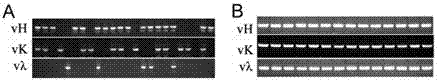
[0043] Take the 96-well Single cell PCR plates frozen in a -80°C refrigerator, thaw slowly on ice, and then add reverse transcriptase to synthesize cDNA. Since the cDNA synthesized by a single cell is extremely rare, the antibody gene contained in it must be amplified by nested PCR. After two rounds of PCR, obvious PCR band amplification (about 400bp in size) can be seen. The paired PCR bands of the light and heavy chains were recovered and sequenced. Such as figure 2 As shown, after analyzing its sequence, specific PCR amplifies the variable region band of the antibody, and introduces restriction enzyme cutting sites. The PCR product of the amplified variable region of the antibody was connected with the constant region of the antibody and then inserted into the expression vector pcDNA3.1(+).
Embodiment 3
[0044] Example 3: Monoclonal Antibody Gene Expression
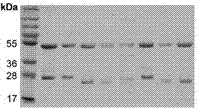
[0045] Paired antibody light chain and heavy chain plasmids were transiently transfected into Freestyle 293F cells cultured in serum-free medium, and the cell supernatant was collected 7 days after transfection, and the IgG antibody in the cell supernatant was purified by Protein A. A total of 42 anti-HBsAg monoclonal antibody strains were obtained. SDS-PAGE electrophoresis identification such as image 3 As shown, the purified IgG antibody shows two bands on 10% SDS-PAGE, one of which is about 50KD (heavy chain), and the other is about 25KD (light chain).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com