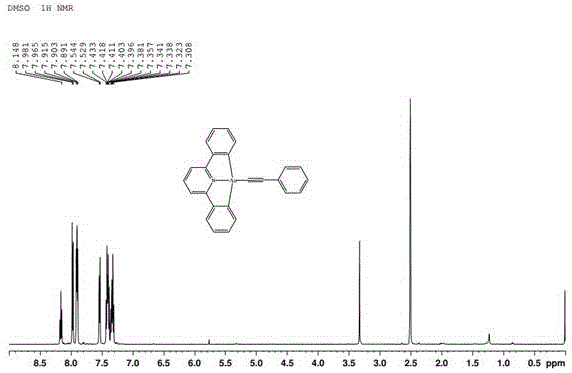
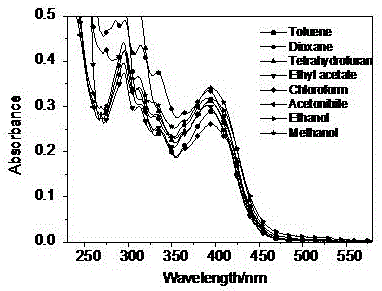
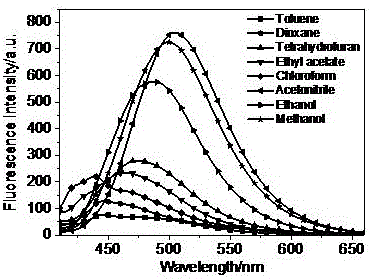
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
96 results about "9H-carbazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The section 8(d) model rule requires manufacturers, importers, and processors of listed chemical substances and mixtures to submit to EPA copies and lists of unpublished health and safety studies. 9H-Carbazole is included on this list.
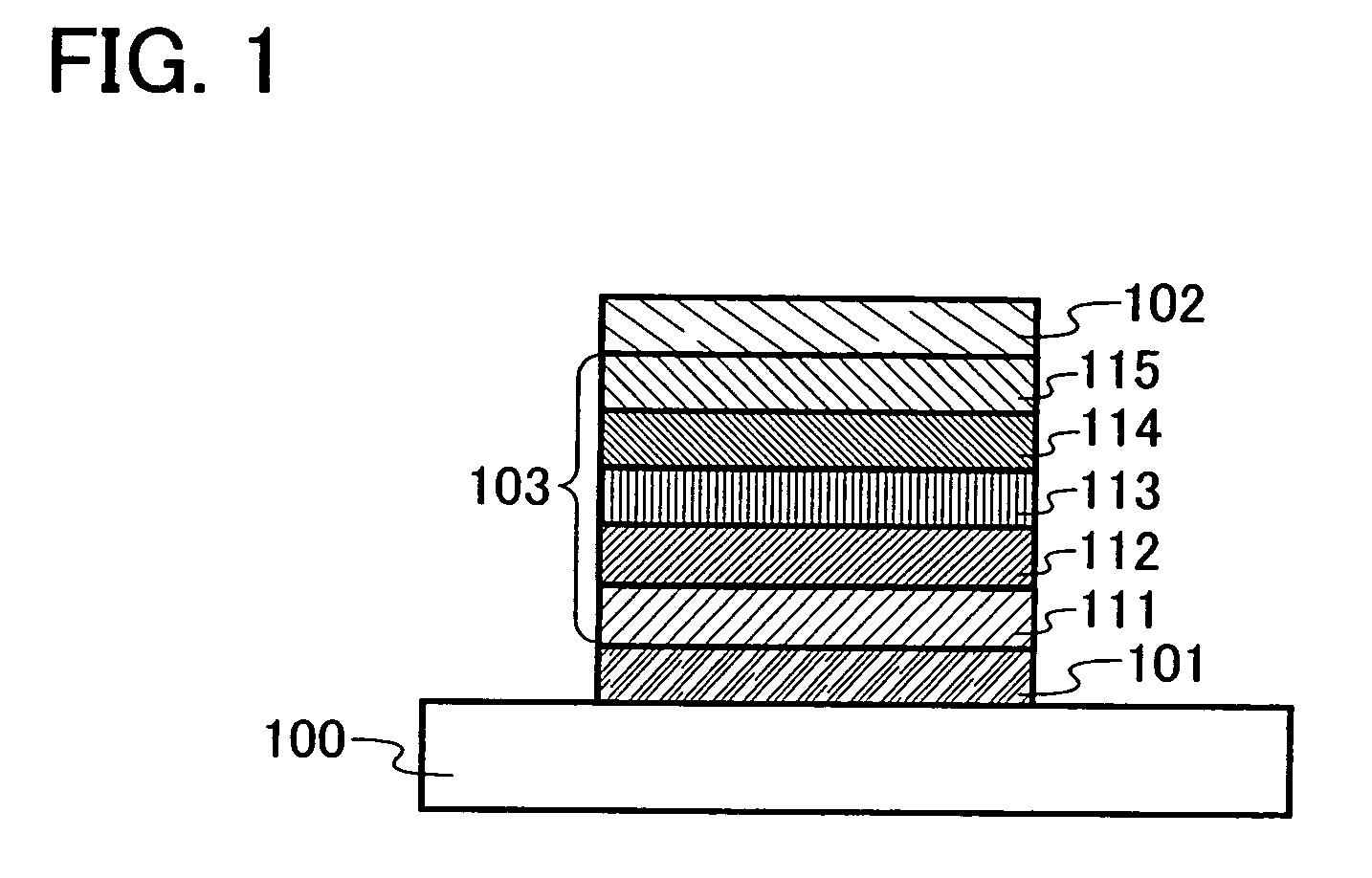
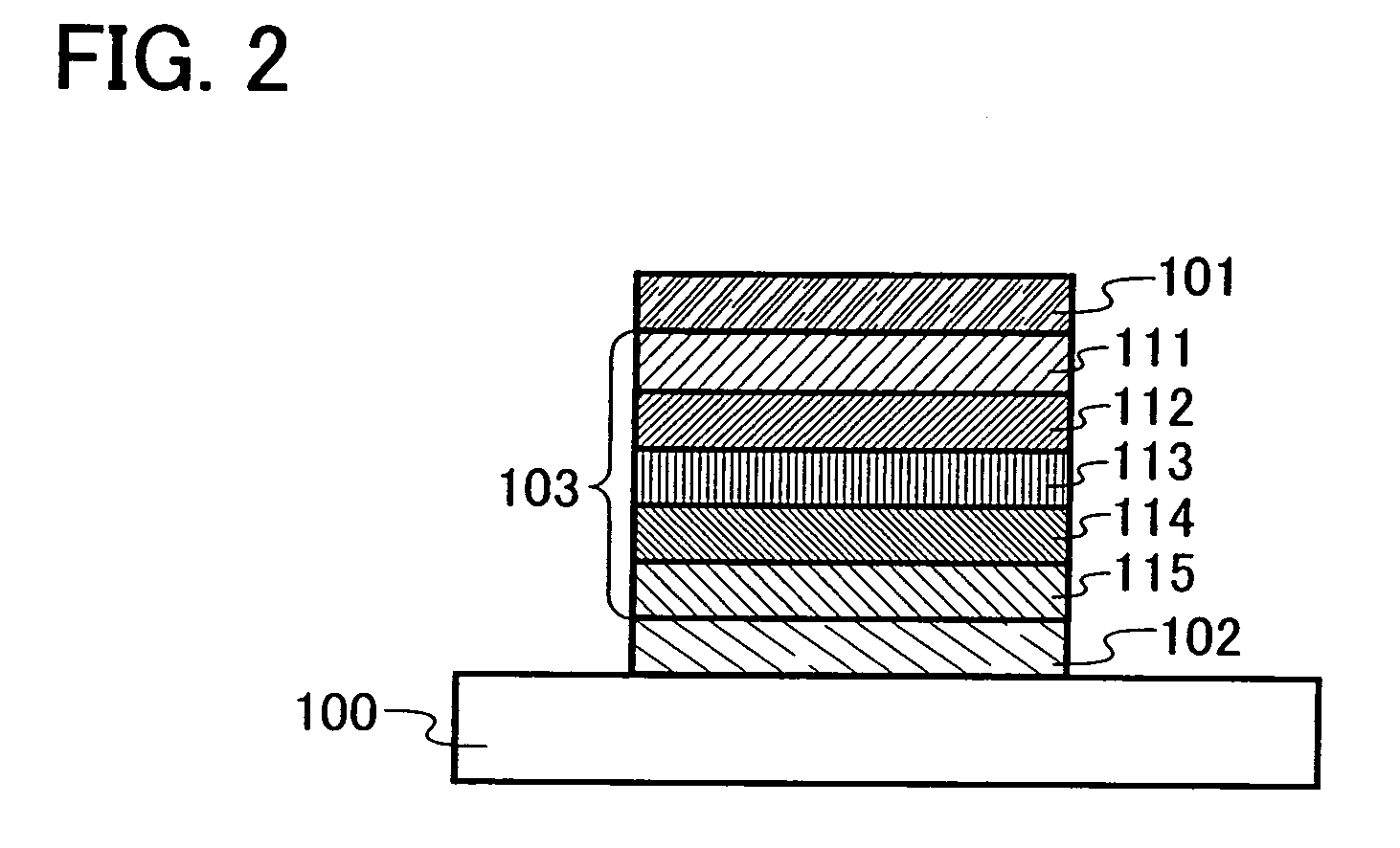
Carbazole Derivative and Method for Producing the Same
InactiveUS20100076201A1Rich varietySimple methodSolid-state devicesGroup 3/13 element organic compoundsArylReactive site
To provide a method for producing a wide variety of carbazole derivatives which have a simple and uncomplicated process and in which variations in the yield, purity, etc. of a desired substance which are caused by an aryl group introduced is reduced as much as possible. A method for producing a carbazole derivative represented by General Formula (1) is provided, in which 9-[4-(10-phenyl-9-anthryl)phenyl]-9H-carbazole having an active site at the 3-position of the carbazole skeleton and an aromatic compound having an active site are coupled.In the formula, Ar1 represents an aryl group with 6 to 13 carbon atoms in a ring, and Ar1 may have a substituent.
Owner:SEMICON ENERGY LAB CO LTD
Triazole derivative, and light-emitting device, and electronic device with the use of triazole derivative
ActiveUS20080286607A1High triplet excitation energySolve low luminous efficiencyOrganic chemistryDischarge tube solid anodesArylTriazole derivatives
It is an object of the present invention to provide a novel triazole derivative. Further, it is another object of the present invention to provide a light-emitting element having high luminous efficiency with the use of the novel triazole derivative. Moreover, it is still another object of the present invention to provide a light-emitting device and electronic devices which have low power consumption. A light-emitting element having high luminous efficiency can be manufactured with the use of a triazole derivative which is a 1,2,4-triazole derivative, in which an aryl group or a heteroaryl group is bonded to each of 3-position, 4-position, and 5-position, and in which any one of the aryl group or heteroaryl group has a 9H-carbazol-9-yl group.
Owner:SEMICON ENERGY LAB CO LTD
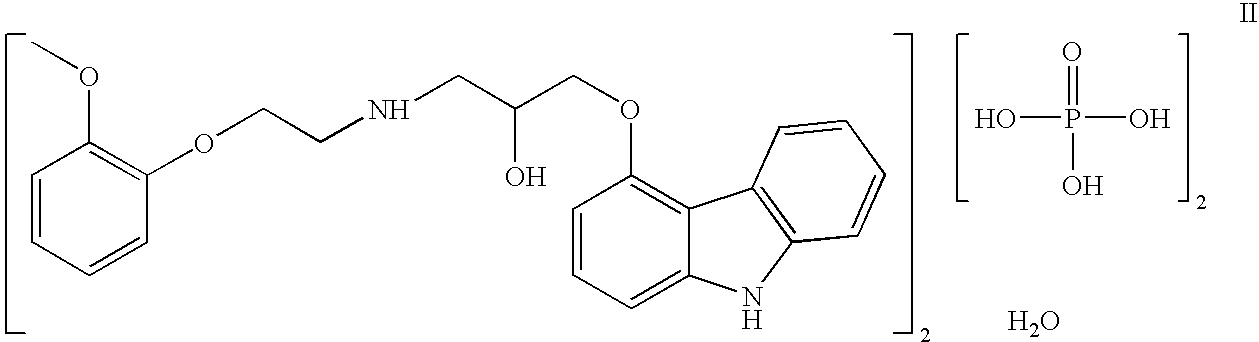
Efficient process for production of carvedilol phosphate
InactiveUS7777053B2High purityHigh yieldOrganic chemistryDipotassium hydrogen phosphateCarvedilol Phosphate
A novel cost effective process for the synthesis of phosphate salts of 1-(9H-carbazol-4yloxy)-3-[[2-(2-methoxyphenoxy)ethyl]amino]-propan-2-ol, (carvedilol phosphate) of formula (II) with high yields and purity is disclosed. More particularly, the invention discloses a process for preparation of crystalline phosphate salts of carvedilol using various phosphate forming reagents such as phosphorous pentoxide, polyphosphoric acid, dipotassium hydrogen phosphate, ammonium dihydrogen ortho phosphate, and sodium dihydrogen ortho phosphate in solvents selected from Acetonitrile, acetone and tetrahydrofuran. The solvents used to prepare solvates of carvedilol dihydrogen phosphate are methanol, ethanol and isopropyl alcohol.
Owner:WANBURY
Condensed heterocyclic compound
InactiveUS20140228409A1Superior RORγt inhibitory actionImprove efficacyBiocideNervous disorder9H-carbazoleStereochemistry
The present invention provides a fused heterocyclic compound having an RORγt inhibitory action. The present invention relates to a compound represented by the formula (I′):wherein each symbol is as defined in the specification, provided that 2-(2-((4-cyanophenyl)amino)-2-oxoethoxy)-N-(9-ethyl-9H-carbazol-3-yl)acetamide and N-(4-cyanophenyl)-N′-(9-ethyl-9H-carbazol-3-yl)-3-methylpentanediamide are excluded, or a salt thereof.
Owner:TAKEDA PHARMA CO LTD
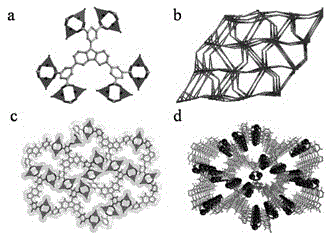
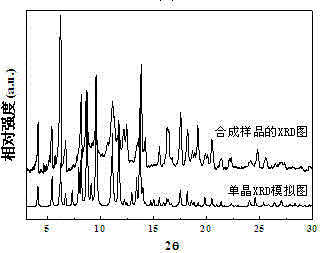
Micro-porous metal organic framework material for methane and acetylene adsorption and storage, and preparation method of micro-porous metal organic framework material
ActiveCN104549164AImprove adsorption capacityIncrease storage capacityOther chemical processesCopper organic compoundsMetal-organic frameworkCarboxylic acid
The invention discloses a micro-porous metal organic framework material for methane and acetylene adsorption and storage, and a preparation method of the micro-porous metal organic framework material. The metal organic framework material is a three-dimensional crystal material prepared from self-assembling transition-metal ions and a polycarboxylic acid organic ligand 5,5',5''-(9H-carbazole-3,6,9-tri-yl)-m-phthalic acid by virtue of coordinate bonds. The metal organic framework material has the relatively large specific surface area and pore volume, the specific surface area can be 1200-1400m<2> / g, the pore volume is 0.6-0.8cm<3> / g, and the metal organic framework material is high in thermal stability and simple in preparation process. The material has high-density open metal sites, can increase the methane and acetylene adsorption and storage amount under 273K and 298K and can be expected to serve as a novel high-efficiency methane and acetylene adsorption and storage material.
Owner:ZHEJIANG UNIV
Process for preparation of carvedilol
InactiveUS20060167077A1High technical contentReduce contentBiocideOrganic chemistryCarvedilolSolvent
The invention solves a new method of preparation of Carvedilol for pharmaceutical use. In the synthesis of Carvedilol a reaction of 4-(oxirane-2-ylmethoxy)-9H-arbazole (II) with 2-(2-methoxyphenoxy)ethylamine salts (IV) in the presence of a base, in an alcohol having the number of carbons C2 to C5 as a solvent, at an elevated temperature, is used. After processing of the crude reaction mixture crude Carvedilol is obtained, which is purified by crystallization from ethylacetate with an addition of activated carbon and the final substance is formulated by crystallization from ethylacetate.
Owner:SANECA PHARMA
9H-carbazole compounds and electroluminescent devices involving them
InactiveCN104039778AImprove luminous efficiencyExtend working lifeGroup 4/14 element organic compoundsLuminescent compositionsOrganic electroluminescence9H-carbazole
The present invention relates to a novel organic luminescent compound and an organic electroluminescent device containing the same. The compounds according to the present invention have high luminous efficiency and long operation lifetime. Therefore, they can produce an organic electroluminescent device having an improved power consumption.
Owner:ROHM & HAAS ELECTRONICS MATERIALS LLC
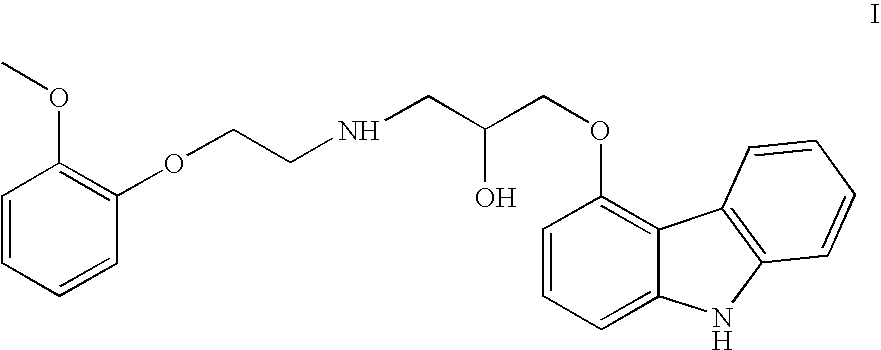
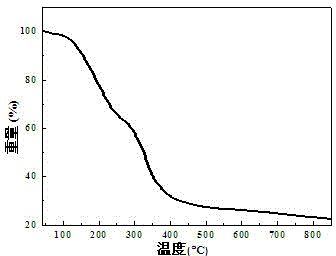
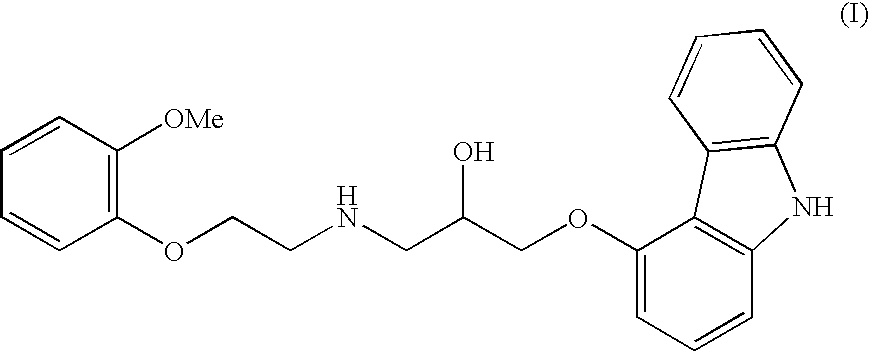
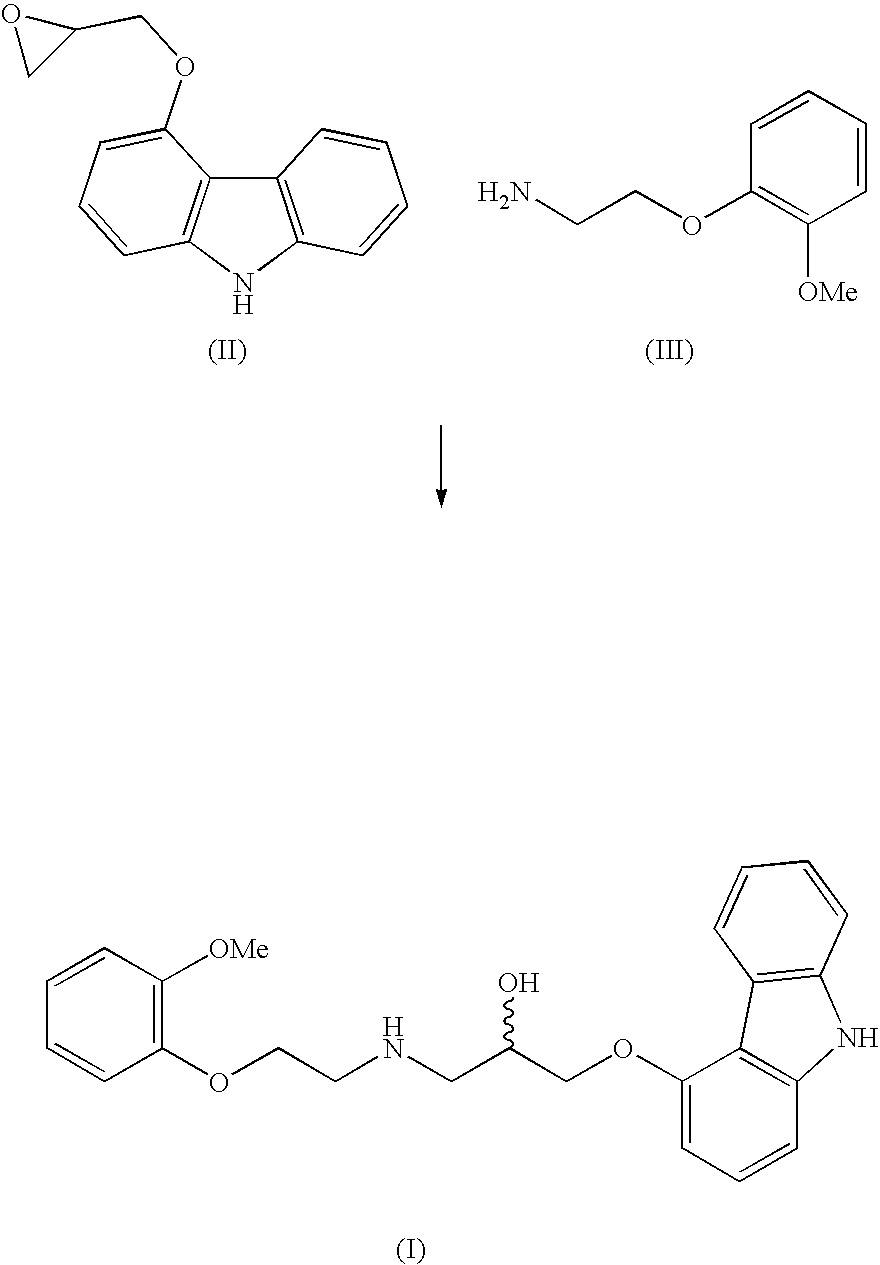
A process for preparation of 1-[9h-carbazol-4-yloxy]-3-[{2-(2-(methoxy)phenoxy)-ethyl}-amino]-propan-2-ol
InactiveUS20060270858A1High yieldShort timeOrganic chemistryAnthracene dyes9H-carbazoleCatalytic hydrogenation
The present invention provides a process for preparation of 1-[9H-carbazol-4-yloxy]-3-[{2-(2-(methoxy)phenoxy)-ethyl}-amino]-propan-2-ol, a compound of formula 1 in racemic form or in the form of optically active R or S enantiomer or its pharmaceutically acceptable salt, comprising, reacting 4-(oxiranylmethoxy)-9H-carbazole, a compound of formula (2) or the R or S enantiomer thereof with a compound of formula (5), wherein R1 is benzyl or substituted benzyl group, in an aprotic organic solvent in presence of a catalyst to obtain a compound of formula (6), or the R or S enantiomer thereof, wherein R1 is as defined above. The resultant compound of formula (6) is subjected to debenzylation reaction by catalytic hydrogenation to obtain the compound of formula (1), if desired converting the resultant compound of formula (1) to a pharmaceutically acceptable salt thereof.
Owner:SUN PHARMA INDS
Fluorescent probe of reversible sulfur dioxide/sulphurous acid (hydrogen) salt
InactiveCN108117544AThe synthesis steps are simpleRaw materials are easy to getOrganic chemistryFluorescence/phosphorescenceHydrogenOxonium ion
The invention provides a fluorescent probe of reversible sulfur dioxide / sulphurous acid (hydrogen) salt, with the chemical name of 7-diethylin-2-(9-ethyl-9H-carbazole-3) benzo iso-pyran oxonium ion. The fluorescent probe can detect sulfur dioxide / sulphurous acid (hydrogen) salt in a solution, cells, tissues or a living body, wherein the living body includes fish, mice, rat, guinea pig and rabbit;and reversibility is realized by formaldehyde. The fluorescent probe is simple in synthesis steps, easily available in material, high in yield and suitable for industrial application.
Owner:UNIV OF JINAN
Compound comprising 9,9-dimethyl-9.10-dihydracridine and preparation and application thereof
ActiveCN107721981AImprove performanceImprove thermal stabilityOrganic chemistrySolid-state devicesAcridineBromine
The invention provides a compound comprising 9,9-dimethyl-9,10-dihydracridine and preparation and an application of the compound. The name of the compound is (3,5-dicarbazyl-9-phenyl)-(4-(9,9-dimethyl-9H-acridine-10)-phenyl)-ketone. A preparation method comprises the following steps: firstly preparing 9,9'-(5-bromine-1,3-phenylene)di(9H-carbazole); enabling the 9,9'-(5-bromine-1,3-phenylene)di(9H-carbazole), t-BuLi, 4-bromobenzaldehyde and PCC to react to obtain (3,5-dicarbazyl-9-phenyl)-(4-bromophenylacetone)-ketone; and finally enabling the (3,5-dicarbazyl-9-phenyl)-(4-bromophenylacetone)-ketone and the 9,10-dihydro-9,9-dimethyl acridine to react to obtain the (3,5-dicarbazyl-9-phenyl)-(4-(9,9-dimethyl-9H-acridine-10)-phenyl)-ketone. The compound is excellent in thermal stability, electrochemical property, charge transfer performance and luminescence property, and can be used as a green organic electroluminescent material.
Owner:SHANXI UNIV
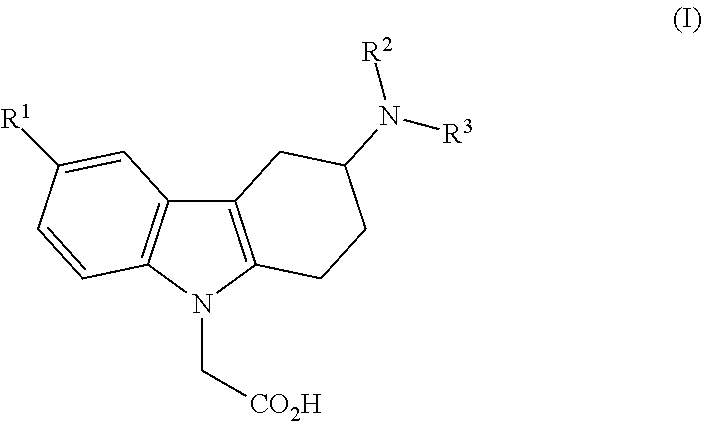
3-(heteroaryl-amino)-1,2,3,4-tetrahydro-9h-carbazole derivatives and their use as prostaglandin d2 receptor modulators
The present invention relates to 3-(heteroaryl-amino)-1,2,3,4-tetrahydro-9H-carbazole derivatives of the formula (I),wherein R1, R2 and R3 are as described in the description and their use as prostaglandin receptor modulators, most particularly as prostaglandin D2 receptor modulators, in the treatment of various prostaglandin-mediated diseases and disorders, to pharmaceutical compositions containing these compounds and to processes for their preparation.
Owner:IDORSIA PHARM LTD
Phosphine-oxygen red/orange thermally activated delayed fluoresce material as well as synthesis method and application thereof
ActiveCN107011379AImprove efficiencyAvoid interactionGroup 5/15 element organic compoundsSolid-state devicesBenzaldehydeSynthesis methods
The invention discloses a phosphine-oxygen red / orange thermally activated delayed fluoresce material as well as a synthesis method and application thereof, relates to the thermally activated delayed fluoresce material as well as the synthesis method and the application thereof, and aims to solve the problems that a conventional red / orange TADF (Thermally Activated Delayed Fluoresce) material is slightly low in concentration quenching and electroluminescent device efficiency and rapid in attenuation because of high molecular polarity. The structural formula of the material is as shown in the specification. The synthesis method comprises the following steps: preparing 9-(2-bromine-4-(1,3-dioxolane-2-yl) phenyl-9H-carbazole or 9-(2-bromine-4-(1,3-dioxolane-2-yl) phenyl)-3,6-ditert-butyl-9H-carbazole, preparing 4-(9H-carbazole-9-yl)-3-(diphenylphosphine oxide) benzaldehyde or 4-(3,6-ditert-butyl-9H-carbazole-9-yl)-3-(diphenylphosphine oxide) benzaldehyde, and preparing a final product. By adopting the material disclosed by the invention, the efficiency of an electroluminescent device is remarkably improved, the quenching effect is reduced, and the efficiency stability of the electroluminescent device is improved. The material belongs to the field of fluorescence material preparation.
Owner:JIANGSU FUXIN ELECTRONICS LIGHTING TECH
Carbazole derivative and method for producing the same
InactiveUS8669373B2Simple and uncomplicated processRich varietyOrganic chemistrySolid-state devicesArylReactive site
To provide a method for producing a wide variety of carbazole derivatives which have a simple and uncomplicated process and in which variations in the yield, purity, etc. of a desired substance which are caused by an aryl group introduced is reduced as much as possible. A method for producing a carbazole derivative represented by General Formula (1) is provided, in which 9-[4-(10-phenyl-9-anthryl)phenyl]-9H-carbazole having an active site at the 3-position of the carbazole skeleton and an aromatic compound having an active site are coupled.In the formula, Ar1 represents an aryl group with 6 to 13 carbon atoms in a ring, and Ar1 may have a substituent.
Owner:SEMICON ENERGY LAB CO LTD
Fluorescent probe for identifying hydrogen sulfide in lysosome and application thereof
InactiveCN105038763ANoveltyWith simplicityOrganic chemistryFluorescence/phosphorescenceFluorescenceLysosome
The invention discloses a fluorescent probe for identifying hydrogen sulfide in lysosome. The fluorescent probe is 2-(9-(2-ethoxy ethyl ester)-2-(pyridyl-4-yl)vinyl)-9H-carbazolyl-3-yl)vinyl)-1-ethyl-3,3-dimethyl-3H-indolyl-1-iodine which is called PCAI for short, and the chemical structural formula is disclosed as Formula (I). The invention also discloses application of the fluorescent probe in detecting H2S in lysosome. The experiment proves that the fluorescent probe can identify lysosome and H2S in the lysosome through fluorescence enhancement; and the probe has potential application value in the field of targeted molecular markers of acidic regions of living cells.
Owner:UNIV OF JINAN
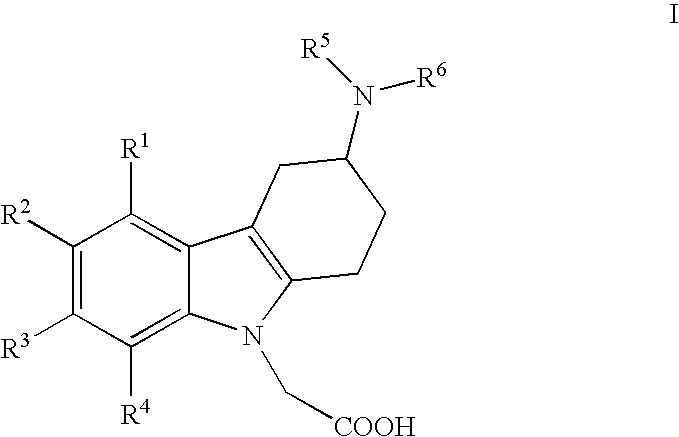
(3-amino-1,2,3,4-tetrahydro-9 H-carbazol-9-yl)-acetic acid derivatives
The present invention relates to (3-amino-1,2,3,4-tetrahydro-9H-carbazol-9-yl)-acetic acid derivatives of Formula (I) wherein R1, R2, R3, R4, R5 and R6 are as described in the description and their use as prostaglandin receptor modulators, most particularly as prostaglandin D2 receptor modulators, in the treatment of various prostaglandin-mediated diseases and disorders, to pharmaceutical compositions containing these compounds and to processes for their preparation.
Owner:IDORSIA PHARM LTD
Flocculating agent and preparation method thereof
ActiveCN108408863ASimple manufacturing methodRaw materials are easy to getBiocideSpecific water treatment objectivesIon exchangeWater quality
The invention discloses a preparation method of a flocculating agent. The preparation method includes the steps of firstly, preparing polymeric-form aminopropyl ascorbyl phosphate; secondly, preparingthe copolymer of the polymeric-form aminopropyl ascorbyl phosphate, 2-(9H-carbazole-9-yl)ethyl acrylate, ethoxylated trimethylolpropane triacrylate and 1-( acryloyloxy)-3-(methacryloyloxy)-2-propanol; thirdly, allowing the copolymer to have ion exchange with sodium sulfobutylether-beta-cyclodextrin. The invention further discloses the flocculating agent prepared by the method. The prepared flocculating agent is evident in flocculating effect, fast in settling, good in discharged-water quality, low in use amount, moderate in intrinsic viscosity, stable, wide in application range, economical, green, environmentally friendly and capable of achieving functions such as sterilizing and alga removing.
Owner:刘鹏
3-(heteroaryl-amino)-1,2,3,4-tetrahydro-9H-carbazole derivatives and their use as prostaglandin D2 receptor modulators
The present invention relates to 3-(heteroaryl-amino)-1,2,3,4-tetrahydro-9H-carbazole derivatives of the formula (I),wherein R1, R2 and R3 are as described in the description and their use as prostaglandin receptor modulators, most particularly as prostaglandin D2 receptor modulators, in the treatment of various prostaglandin-mediated diseases and disorders, to pharmaceutical compositions containing these compounds and to processes for their preparation.
Owner:IDORSIA PHARM LTD
Preparation method and application of amphiphilic photoelectric active branched macromolecules
InactiveCN104650288AOptimal Control StructureEasy to operateCoatingsMaterial electrochemical variablesDispersion stabilityFiltration
A preparation method of amphiphilic photoelectric active branched macromolecules belongs to the field of macromolecular polymerization. The preparation method comprises the following steps: (1) under normal pressure condition, 7-(4-vinyl benzyloxy)-4-methylcoumarin, N-(4-vinyl benzyl)-9H-carbazole, 4-vinyl benzyl mercaptan, maleic anhydride, a radical initiator and a solvent are placed in a 250ml four-neck flask; (2) nitrogen is introduced into the container in the step (1) for 30 min, sealed stirring is carried out to fully dissolve the nitrogen, solution temperature rises to 65-70 DEG C and a thermostatic reaction is carried out for 24h; and (3) after the reaction, the solution obtained from the step (2) is precipitated in a precipitating agent and undergoes suction filtration, and a solid obtained after filtration undergoes dissolution, precipitation and suction filtration repeatedly for 3 times, and the solid obtained finally undergoes vacuum drying to constant weight. According to the invention, polymerization conditions are mild, and the preparation method is simple to operate. The polymer obtained can be used to remarkably raise dispersion stability of carbon nano-tube in an aqueous solvent.
Owner:JIANGNAN UNIV
An organic photomultiplier detector based on surface plasmon polariton
ActiveCN109037454AQuick responseSolid-state devicesSemiconductor/solid-state device manufacturingPolystyreneMetal thin film
The invention relates to the field of organic photomultiplier detectors, an organic photomultiplier detector based on surface plasmon polariton uses an indium tin oxide ITO as an anode layer and poly(3, 4-ethylenedioxythiophene)-polystyrene sulfonic acid (PEDOT: PSS) as an anode layer, and uses any one of 3-hexylthiophene P3HT, poly[[9-(1-octylnonyl)-9H-carbazole-2, 7-dibasic]-2, 5-thiophene diyl-2, 1, 3-benzothiadiazole-4, 7-dibasic-2, 5-thienyl diyl (PCDTBT, PSBTBT: PC71BM] as a donor material of an organic active layer, and uses fullerene derivative PCBM as an acceptor material of the organic active layer, the donor material and the acceptor material constitute the organic active layer, and an integrated metal nano-grating matrix and a metal thin film are used as a cathode layer, and the nano-grating matrix is inlaid in the organic active layer.
Owner:TAIYUAN UNIV OF TECH
Negative light-sensitive resin combination
InactiveCN101571672AHigh resolutionImprove insulation performanceOrganic chemistryPhotomechanical apparatusEpoxyEthyl group
The present invention provides a negative light-sensitive resin combination, having excellent resolving capability, insulativity, flatness, chemical resistance and cementation force, especially having excellent light sensitivity, plastic residue rate and UV permeation rate comparing with the existing light-sensitive resin, thus the combination is suitable to be used in a mode of an organic insulation film. Especially, the invention relates to a negative light-sensitive resin combination containing the following components: (a) acrylic copolymer obtained by the copolymerization of (i) unsaturated carboxyl acid, unsaturated carboxylic acid anhydrides or their mixture, (ii) unsaturated compound containing epoxy group and (iii) ethylene series unsaturated compound; (b) photo-initiator containing [1-[9-ethyl group-6-(2-methylbenzene formyl group )-9H-carbazole -3-group]-1-(O-acetyl oxime); (C) polyfunctional monomer containing olefinic bond type unsaturated linkage; (d) silicon series compound containing epoxy group or amido; and (e) dissolvent.
Owner:DONGJIN SEMICHEM CO LTD
Neuroprotective Compounds and Use Thereof
InactiveUS20160272619A1Enhance net magnitudeEffective treatmentNervous disorderOrganic chemistryNeuron cell deathDisease
Provided herein are compositions and methods for reducing one or both of axonal degeneration and neuronal cell death associated with a disease or traumatic brain injury. An aminopropyl carbazole agent with potent neuroprotective properties is described. Specifically, (−)-(S)—N-(3-(3,6-dibromo-9H-carbazol-9-yl)-2-fluoropropyl)-6-methoxypyridin-2-amine, is proposed as a new pharmacological agent for protecting patients against axonal degeneration and chronic consequences of TBI.
Owner:UNIV OF IOWA RES FOUND +1
Trivalent gold complex and application thereof to hydrogen manufacturing through photocatalytic reduction water
ActiveCN105646551AOrganic-compounds/hydrides/coordination-complexes catalystsHydrogen productionTert butylLight response
The invention relates to a trivalent gold complex. The general chemical formula of the trivalent gold complex is MAuL, wherein M refers to 2,6-diphenyl pyridine or 2,6-bis(4-tertiary butyl phenyl) pyridine, L refers to phenylacetylene or 3,6-2-tertiary butyl-9-(4-acetenyl phenyl)-9H-carbazole. A preparation method comprises the steps that a chlorine gold (III) precursor and L are dissolved in a mixed solution formed by cuprous iodide, dichloromethane and triethylamine, stirring is carried out for 6 h to 12 h at room temperature, and after the reaction is finished, the reaction product is purified to obtain the trivalent gold complex. The trivalent gold complex achieves visible-light responses, and is a catalyst capable of fast reducing water to prepare hydrogen.
Owner:NANJING UNIV
Polycarboxylic acid carbazole ligand-based indium-organic skeleton material and preparation method thereof
InactiveCN106588751ANovel structureImprove performanceOther chemical processesGroup 3/13 organic compounds without C-metal linkagesBenzoic acidMetal-organic framework
The invention relates to a polycarboxylic acid carbazole ligand-based indium-organic skeleton material and a preparation method thereof, and belongs to the technical field of crystalline materials. The chemical molecular formula is [(CH3)2NH2][InL].5H2O, wherein L is organic ligand 4,4',4'',4'''-(9H-carbazole-1,3,6,8-tetrasubstituted) para-benzoic acid radical. Organic ligand 4,4',4'',4'''-(9H-carbazole-1,3,6,8-tetrasubstituted) para-benzoic acid and indium nitrate are dissolved into a solution of N,N'-dimethylacetamide and tetrafluoroboric acid, and solvent thermal reaction is conducted to obtain the metal organic skeleton material. The metal organic skeleton material has a dye adsorption property and can be applied to industrial dye treatment.
Owner:BEIJING UNIV OF TECH
Carbazole-contained benzimidazole-substituted quinoline derivative, preparation method and application thereof
ActiveCN105968098AImprove complexation effectQuick responseOrganic chemistryFluorescence/phosphorescenceQuinolineSolvent
The invention belongs to the technical field of analytical chemistry, and in particular relates to a carbazole-contained benzimidazole-substituted quinoline derivative, a preparation method and an application thereof. The carbazole-contained benzimidazole-substituted quinoline derivative is 3-(2-(8-(1H-benzo[d] imidazole-2-yl) quinoline-2-yl) ethenyl)-9-benzyl-9H-carbazole. The preparation method includes the steps of placing 2-methyl-8-(1H-benzimidazole) quinoline and 9-benzyl-9H-carbazole-3-formaldehyde into polar solvents, adding catalysts, stirring and performing backflow reaction; performing purification after the backflow reaction to obtain a target compound carbazole and benzimidazole-substituted quinoline derivative L. A benzimidazole quinoline derivative zinc ion fluorescent probe is excellent in complexing action with zinc ions and rapid in response, the response time of the Zn<2+>fluorescent probe for Zn<2+> is 50s, the lowest detection limit is 6.69*10-8mol / L, and the fluorescent probe can be used for quantitative trace detection of the zinc ions by fluorescence spectrophotometry.
Owner:CHINA THREE GORGES UNIV
Novel carbazole fluorescent thiol marking reagent as well as synthesis method and application thereof
InactiveCN108484478AQuick tagMark accuratelyOrganic chemistryFluorescence/phosphorescenceBenzaldehydeSynthesis methods
The invention provides a novel carbazole fluorescent thiol marking reagent as well as a synthesis method and application thereof. The florescent marking reagent adopts carbazole as a fluorescent basering and activated C=C double bonds as reaction activity groups, is simple and convenient in synthesis step, easy to operate and can be synthesized in a large scale through two steps of reactions as follows: (1) enabling carbazole to react with 4-bromobenzaldehyde so as to obtain an intermediate (4-(9H-carbazole-9-yl) benzaldehyde; (2) enabling the intermediate (4-(9H-carbazole-9-yl) benzaldehydeto react with malononitrile so as to obtain a target product (4-(9H-carbazole-9-yl) benzaldehyde malononitrile, and performing recrystallization for three times with acetonitrile, thereby obtaining ayellow needle-shaped crystal. The chemical purity of the fluorescent marking reagent provided by the invention is up to 99%, and the fluorescent marking reagent is stable in chemical property. The novel carbazole fluorescent thiol marking reagent provided by the invention is capable of rapidly and accurately marking degradation thiol products of organic thiol ester insecticides under a gentle condition, and quantitative analysis on contents of organic thiol ester insecticides in reagent samples can be achieved.
Owner:QUFU NORMAL UNIV
Preparation method of phenylalanine compound
ActiveCN107868033AHigh puritySuitable for industrial productionOrganic chemistry9H-carbazoleChemistry
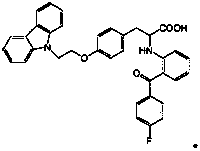
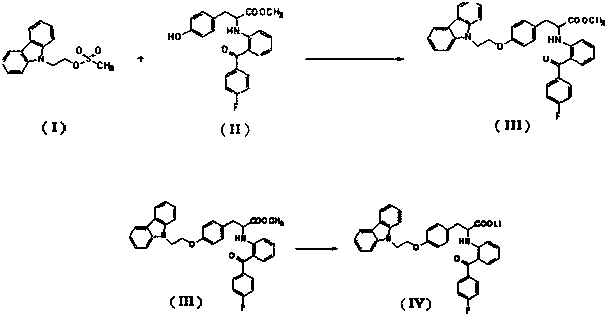
The invention discloses a preparation method of 2-(2-(4-fluorobenzoyl)phenylamino)-3-(4-(2-(9H-carbazole-9-yl)ethoxy)phenyl) propionic acid. The method comprises the step: carrying out condensation, hydrolysis and acidification on 9-carbazole ethyl alcohol methanesulfonate and 2-[(2-(4-fluorobenzoyl)phenyl)amino]-3-(4-hydroxyphenyl) methyl propionate serving as initial raw materials to obtain a target compound. The preparation method disclosed by the invention is suitable for industrial production, and the obtained target compound is high in purity.
Owner:SHENZHEN CHIPSCREEN BIOSCIENCES CO LTD
Fluorescent probe DCCO and preparation method and application thereof
ActiveCN110272731AThe synthesis steps are simpleLow costOrganic chemistryFluorescence/phosphorescenceFluorescenceCROCUS SATIVUS FLOWER
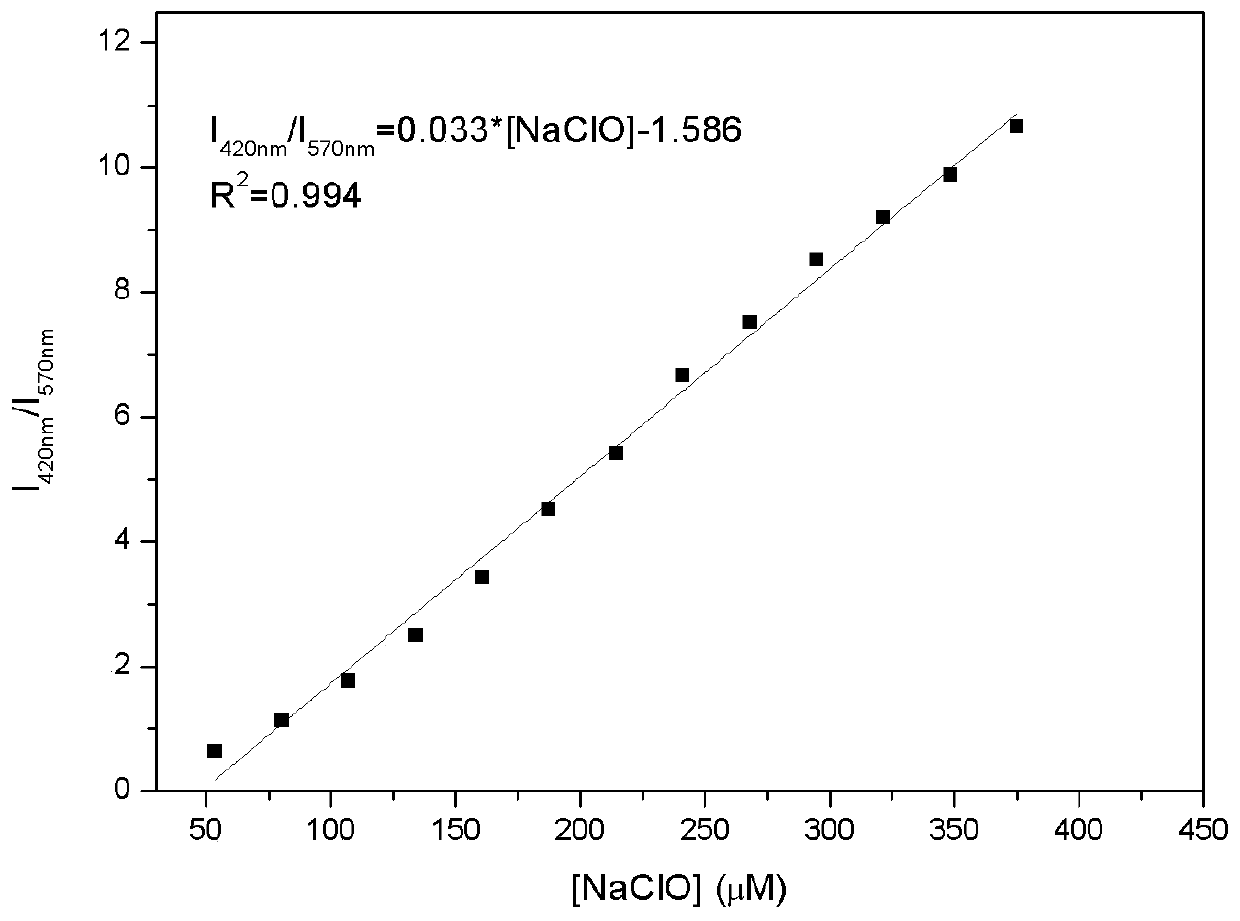
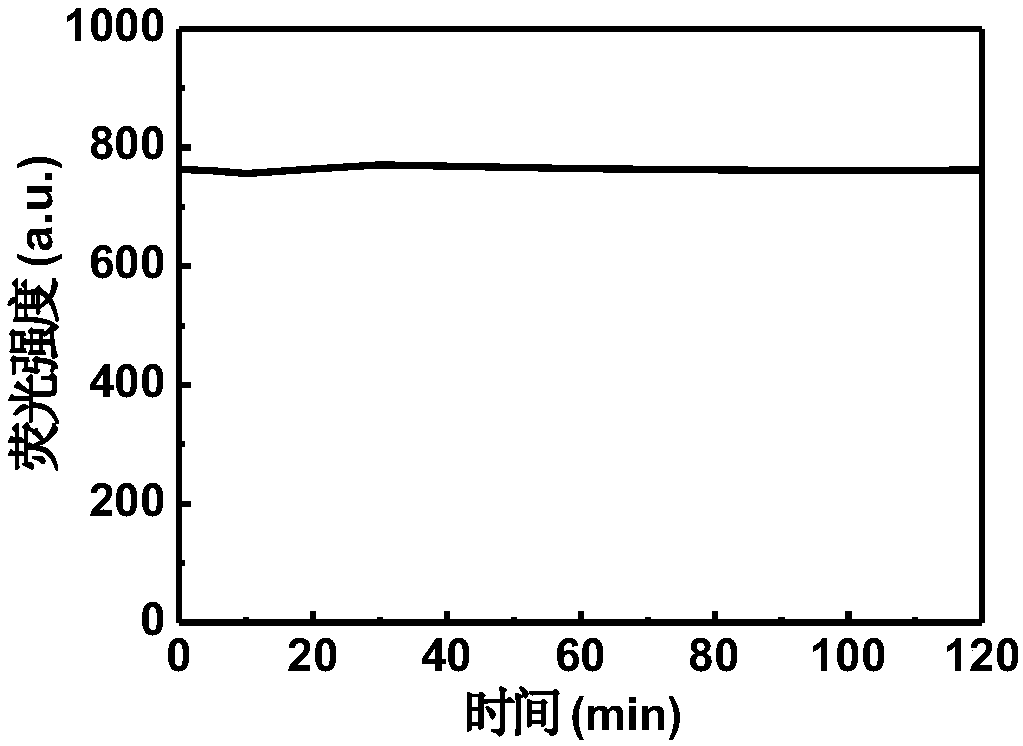
The invention provides a fluorescent probe DCCO and a preparation method and application thereof. The fluorescent probe is 7-(diethylamino)-3-(E)-3-(9-ethyl-9H-carbazole-3-base)acryloyl-2H-pyran-2-ketone. The preparation method of the fluorescent probe DCCO comprises the steps that 3-acetyl-7-(diethylamino)-2H-pyran-2-ketone and 9-ethyl-9H-carbazole-3-formaldehyde amine are dissolved in an ethyl alcohol / acetonitrile (1 / 1, V / V) mixed solution in an equimolar ratio, piperidine is dripped, heating reflux is carried out, and the solution is changed from saffron yellow to red; decompression is carried out to remove a solvent, column chromatography isolation is carried out through a gradient elution method, and a crude product of the fluorescent probe is obtained; the crude product of the fluorescent probe is dissolved, filtered and dried to obtain a pure product. The detection of ClO<-> by the fluorescent probe is of a ratio type, the high sensitivity and good selectivity and stability are shown, and the fluorescent probe has the advantages that the detection process is simple, convenient and rapid, and detection results are accurate. The fluorescent probe DCCO can be applied in the detection of ClO<-> of biological samples.
Owner:SHANXI UNIV
Carbazole-containing discotic liquid crystal compound and preparation method thereof
InactiveCN111825598AStrengthen the p-p effectStrong p-p interactionLiquid crystal compositionsOrganic chemistryCrystallographyAlkoxy group
The invention relates to fusion of 10-octyl-2,3,6,7,13,14,17,18-octa(alkoxy)-10H-bis-phenanthro[9,10-b:9',10'-h]carbazole, substitution of a carbazole-containing discotic liquid crystal triad compound, and substitution of 9-octyl-2,7-di(3',4,4',5-tetra(alkoxy)-[1,1'-biphenyl]-9-yl)-9H carbazole, wherein the compound has structures represented by general formulas (I), (II) and (III), R1 is an alkylchain of C6-C16, R2 is an alkyl chain of C1-C16, the compound represented by the general formula (I) can be self-assembled into a hexagonal columnar liquid crystal intermediate phase in a wide temperature range, and the compound represented by the general formula (II) can be self-assembled into a columnar liquid crystal intermediate phase and a nematic phase within a certain temperature range. The invention further provides a preparation method of the structure represented by the general formulas (I), (II) and (III). The compound represented by the general formula (I) can be obtained by oxidizing and cyclizing the compound represented by the general formula (III) in FeCl3 molecules.
Owner:SICHUAN NORMAL UNIVERSITY
Low-cost composite fluorescent nano-fiber thin film with high selectivity for detecting TNT as well as preparation method and application thereof
ActiveCN109023722AThin diameterLarge specific surface areaMaterial nanotechnologyFluorescence/phosphorescenceFiberFluorescence
The invention discloses a low-cost composite fluorescent nano-fiber thin film with high selectivity for detecting TNT as well as the preparation method and the application of the low-cost composite fluorescent nano-fiber thin film, and particularly provides a composite fluorescent nano-fiber thin film, wherein poly (n-isopropylacrylamide) (PNIPAM) is adopted as the matrix of the composite fluorescent nano-fiber thin film and the 9-(pyrenyl-1-yl)-9H-carbazole (PyCz) is adopted as the fluorescent group of the composite fluorescent nano-fiber thin film. The nano-fiber thin film is applied to thefield of explosive detection for the first time. Due to the fact that a large number of amide groups are arranged on a PNIPAM molecular chain, the interaction between hydrogen bond reinforcement and TNT can be formed by virtue of the PNIPAM molecular chain together with TNT. The fiber can be used for identifying 2, 4 and 6-trinitrotoluene (TNT) steam in a low-cost, high-sensitivity and high-selectivity manner. However, the fiber has no obvious response to naphthalene, urea, toluene, 1,2-dichlorobenzene, sodium nitrite, cigarettes and the like.
Owner:NANJING UNIV OF POSTS & TELECOMM
Carbazolyl triarylamine hole transmission material and synthesis method thereof
InactiveCN104628624AGood hole transport performanceSimple purification methodOrganic chemistrySynthesis methodsAniline
The invention relates to a carbazolyl triarylamine hole transmission material and a synthesis method thereof. The method comprises the following steps: 1) preparing ethylcarbazole; 2) preparing 3-bromo-N-ethyl-carbazole; 3) preparing 4-(N-phenyl-carbazolyl)aniline; and 4) preparing di-[3-(9-ethyl)-9H-carbazolyl]-[4-(3-(9-phenyl)-9H-carbazolyl)-phenyl]amine. The chemical general formula is disclosed in the specification, wherein Q1 is C2-C6 alkyl-substituted 9-site carbazolyl group, and Q2 is C2-C6 alkyl-substituted carbazolyl group or substituent group with the same structure as Q1. The synthesized compound has excellent hole transmission performance, and has potential application value.
Owner:SHAANXI LIGHTE OPTOELECTRONICS MATERIAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![A process for preparation of 1-[9h-carbazol-4-yloxy]-3-[{2-(2-(methoxy)phenoxy)-ethyl}-amino]-propan-2-ol A process for preparation of 1-[9h-carbazol-4-yloxy]-3-[{2-(2-(methoxy)phenoxy)-ethyl}-amino]-propan-2-ol](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c0ff40d6-b239-448b-84f8-d26728b439dc/US20060270858A1-20061130-C00001.png)
![A process for preparation of 1-[9h-carbazol-4-yloxy]-3-[{2-(2-(methoxy)phenoxy)-ethyl}-amino]-propan-2-ol A process for preparation of 1-[9h-carbazol-4-yloxy]-3-[{2-(2-(methoxy)phenoxy)-ethyl}-amino]-propan-2-ol](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c0ff40d6-b239-448b-84f8-d26728b439dc/US20060270858A1-20061130-C00002.png)
![A process for preparation of 1-[9h-carbazol-4-yloxy]-3-[{2-(2-(methoxy)phenoxy)-ethyl}-amino]-propan-2-ol A process for preparation of 1-[9h-carbazol-4-yloxy]-3-[{2-(2-(methoxy)phenoxy)-ethyl}-amino]-propan-2-ol](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c0ff40d6-b239-448b-84f8-d26728b439dc/US20060270858A1-20061130-C00003.png)