Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
121 results about "2-Furoic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
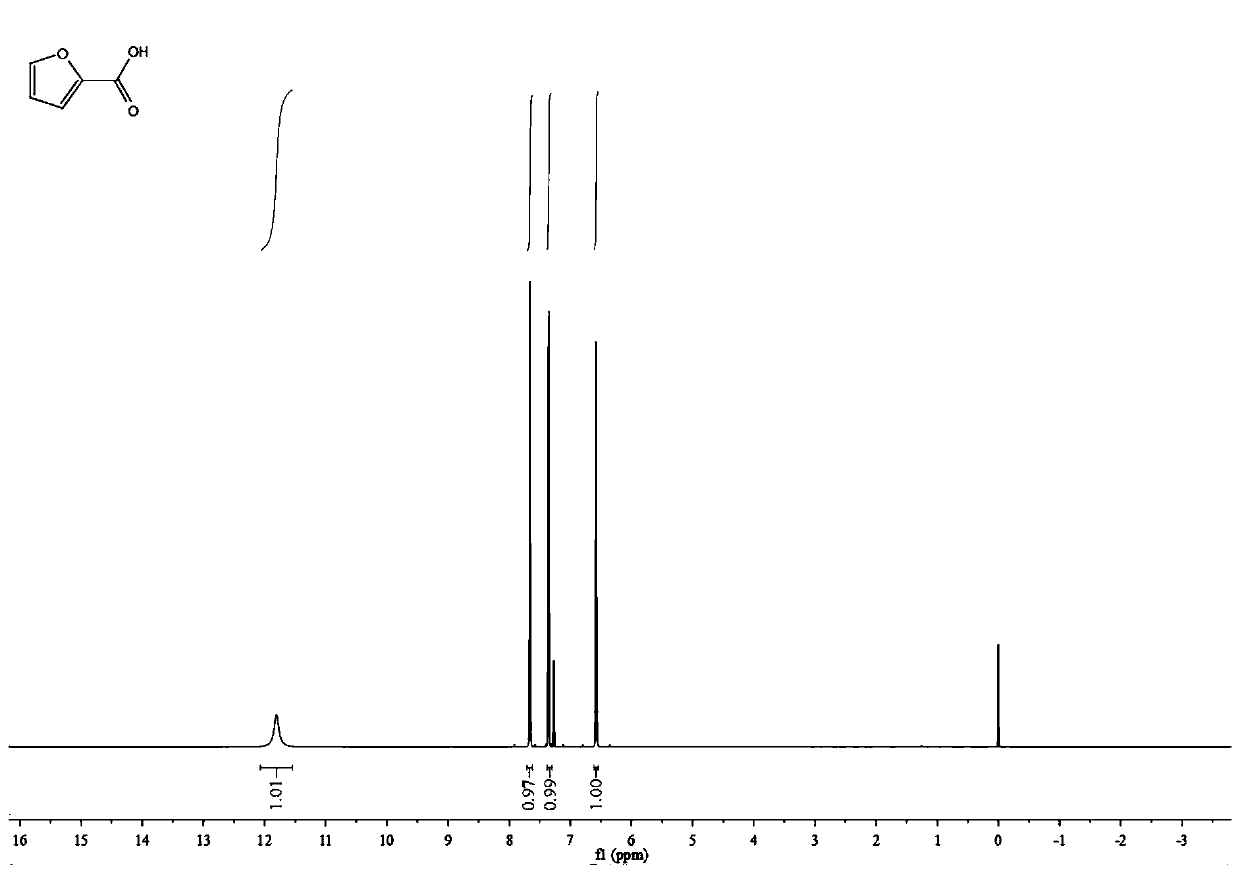
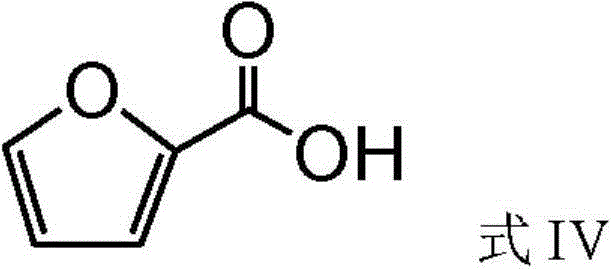
2-Furoic acid is a heterocyclic carboxylic acid, consisting of a five-membered aromatic ring and a carboxylic acid group. Its name is derived from the Latin word furfur, meaning bran. The salts and esters of furoic acids are known as furoates.
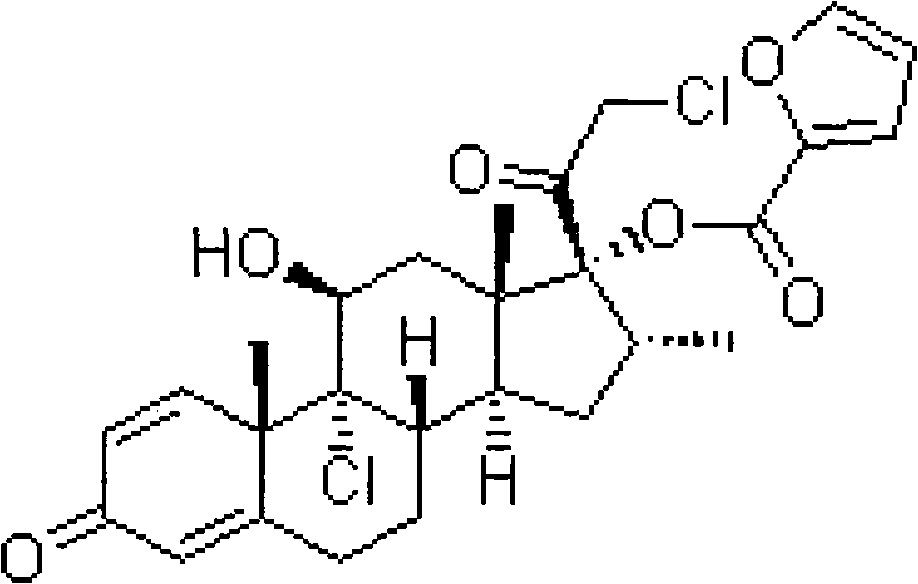
Novel mometasone compositions and methods of making and using the same
The present invention is directed to mometasone furoate compositions comprising mometasone furoate and at least one surface stabilizer. The mometasone furoate particles of the composition preferably have an effective average particle size of less than about 2000 nm.
Owner:ALKERMES PHARMA IRELAND LTD
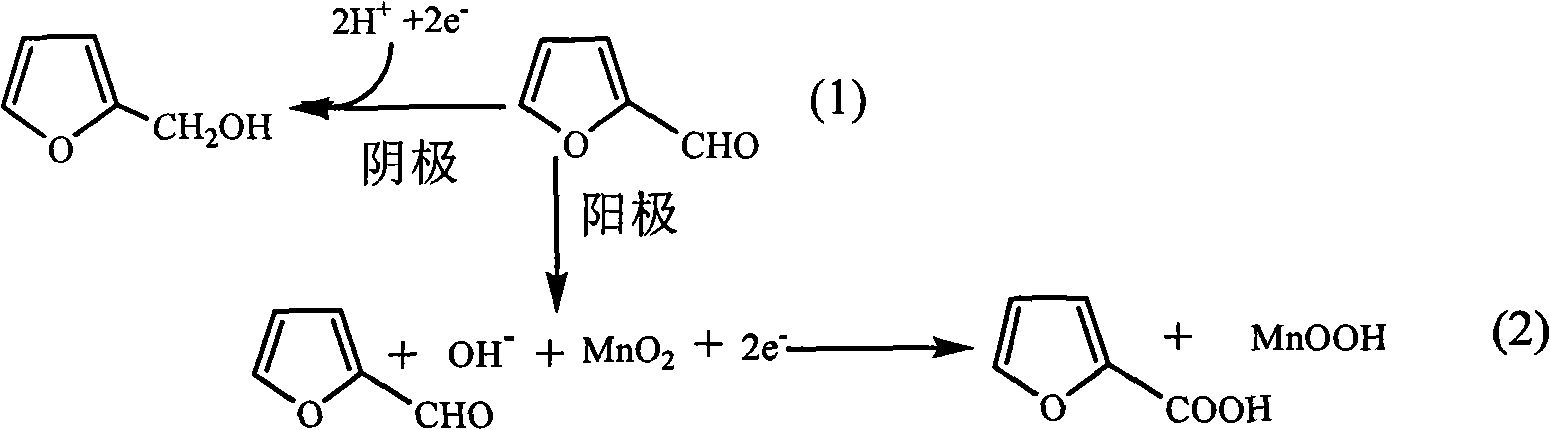
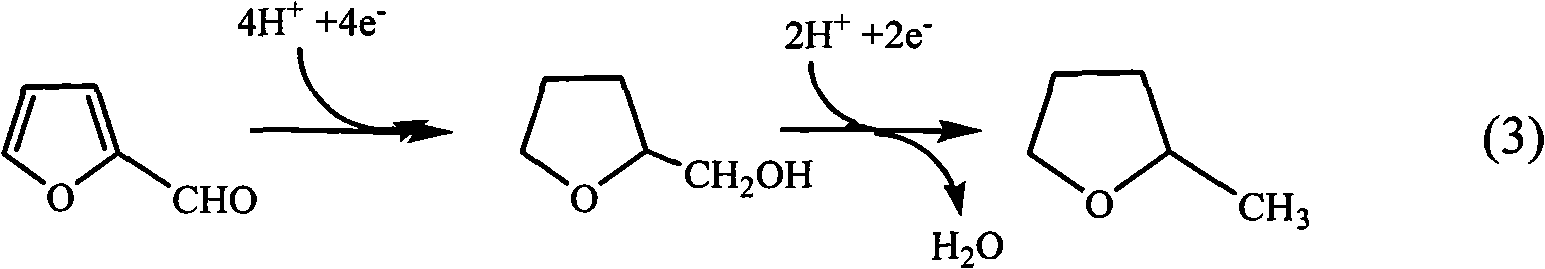
Method for simultaneously preparing furfuryl alcohol and furoic acid on the basis of bipolar membrane technology
InactiveCN101649465AAchieve energy saving effectOrganic chemistryElectrolysis componentsFurfuralPollution
The invention relates to the field of electrochemical synthesis, in particular to a method for simultaneously preparing furfuryl alcohol in a cathode chamber and preparing furoic acid in an anode chamber on the basis of bipolar membrane technology, which has the effects of environmental protection, energy saving and emission reduction. By utilizing the principle that bipolar membranes are dissociated under the action of an electric field, hydrogen ions and hydroxide ions produced after water dissociation are respectively introduced into a cathode chamber and an anode chamber so as to regulatethe pH value of the reaction medium. The method electrooxidizes furfurol to prepare furoic acid in the anode chamber by utilizing an electro-catalyst MnO2 / MnOOH, and the electro-catalyst can be repetitively used so as to have the effects of environmental protection, energy saving and emission reduction; the method reduces the furfurol to prepare furfuryl alcohol in the cathode chamber by utilizingthe furfurol as the raw material. Compared with the traditional process, the method eliminates the pollution of the catalyst chromium in the production of the furfuryl alcohol and the furoic acid, has the advantages of mild production condition and simple equipment, and is a novel process having the advantages of environmental protection and energy saving.
Owner:FUJIAN NORMAL UNIV
Process and intermediates for the production of furan-2,5-dicarboxylic acid or derivatives thereof
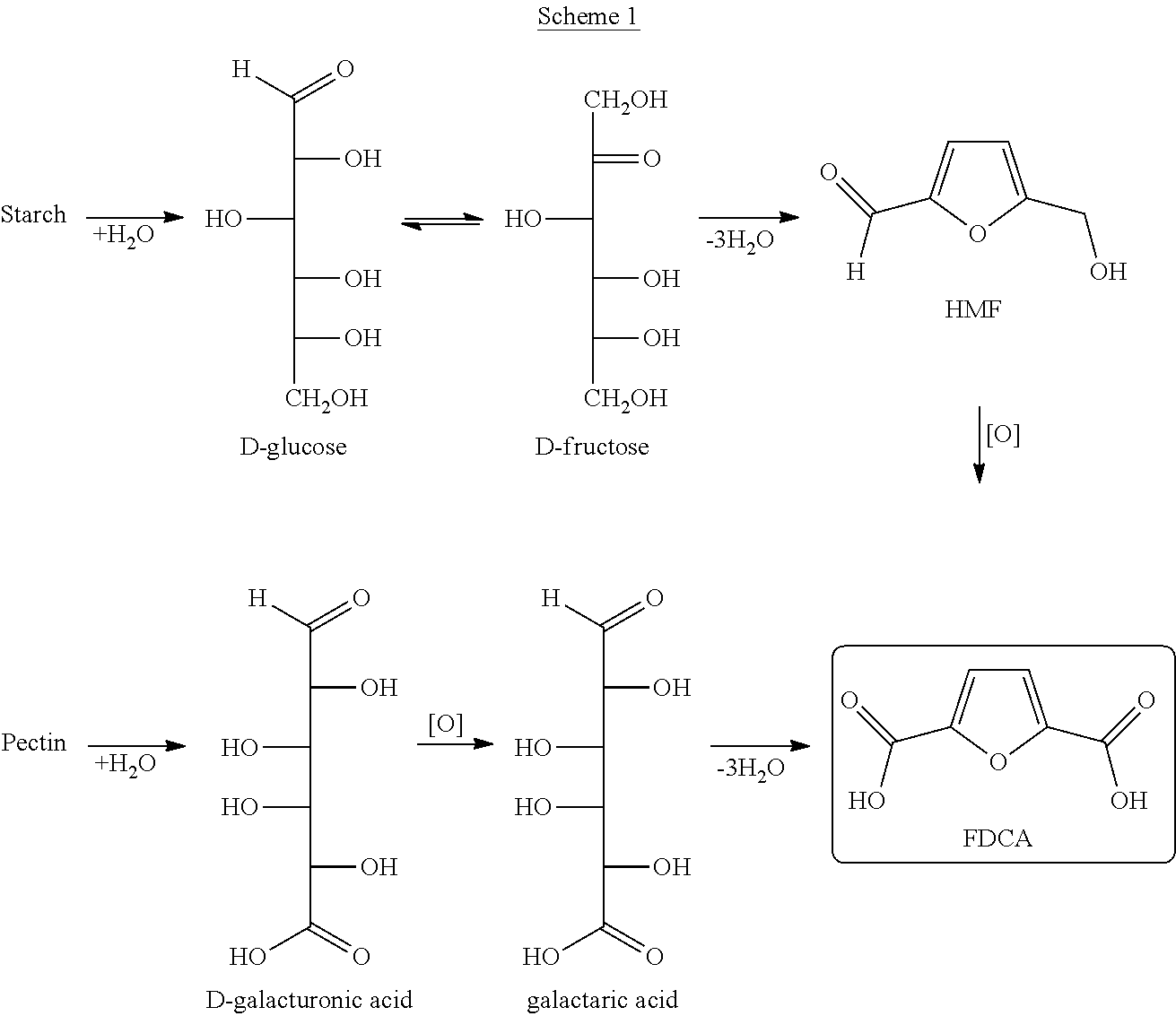
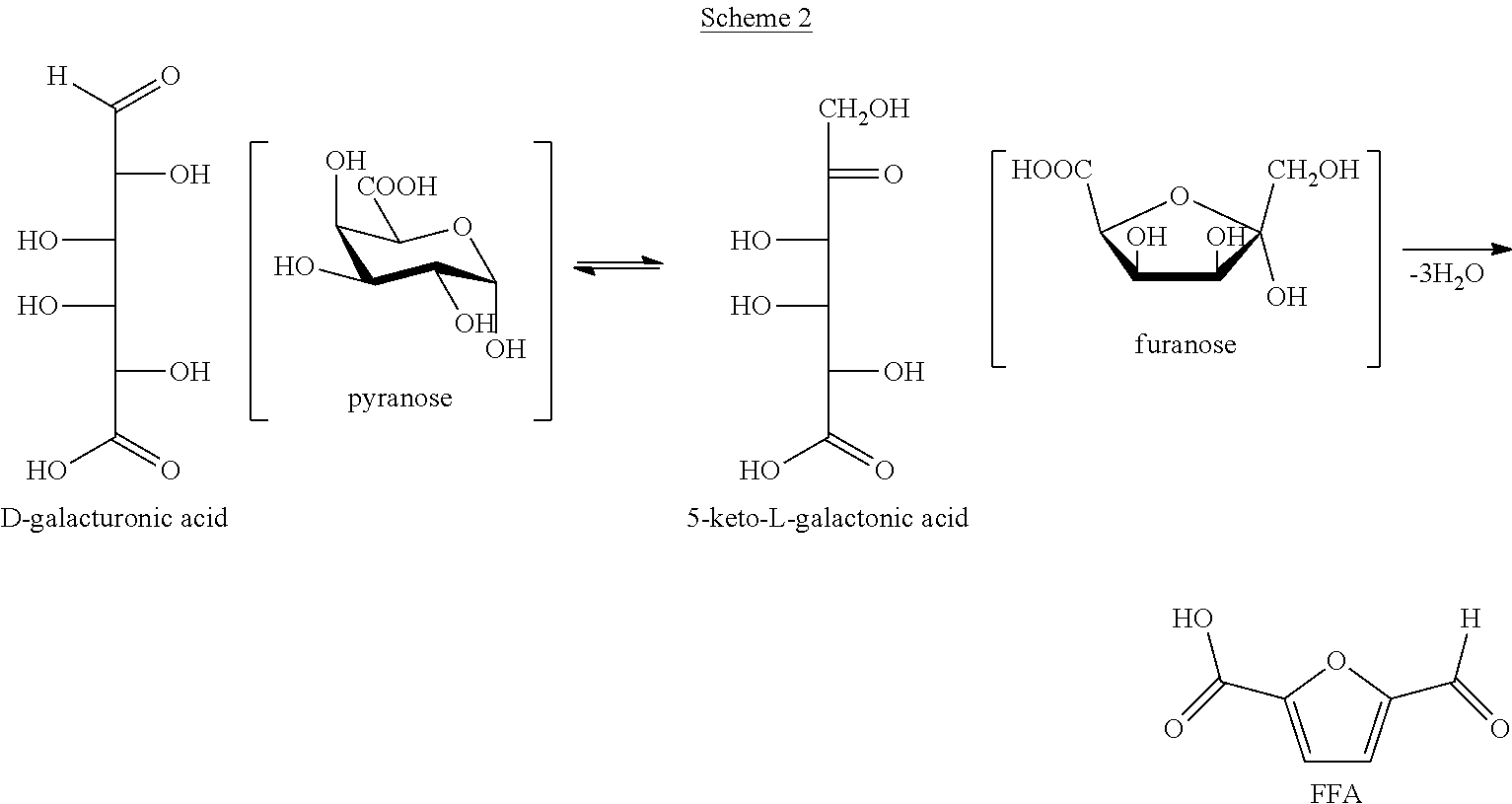
Disclosed is a method of making 5-formyl-2-furoic acid and furan-2,5-dicarboxylic acid. The method involves the use of 5-keto-aldonic acids as intermediates, as these can be subjected to ring formation by a cyclodehydration reaction under mild conditions. The 5-formyl-2-furoic acid or carboxylic derivative thereof is subjected to oxidation so as to form furan-2,5-dicarboxylic acid. The 5-keto-aldonic acid intermediates can be obtained by isomerization of uronic acids which can be obtained from sugar beet pulp, chicory pulp, fruit peals including orange peels, or non-terrestrial sources like seaweeds. A preferred source is sugar beet pulp.
Owner:STICHTING WAGENINGEN RES
Topically applied composition containing povidone iodine and mometasone furoate
InactiveCN102078326AOvercoming Prejudice That Doesn't Stabilize ExistenceOvercoming contraindications not applicable to skin inflammation caused by fungal infectionAntipyreticAnalgesicsIodineMometasone furoate
The invention relates to a topically applied composition containing povidone iodine and mometasone furoate, in particular to a topically applied pharmaceutical composition which contains the micronized mometasone furoate, the povidone iodine and one or more physiologically acceptable excipients applicable to topical application; and in the pharmaceutical composition, the content of the povidone iodine is 1%-10%, and the content of the mometasone furoate is 0.01%-0.1%.
Owner:TIANJIN JINYAO GRP
External use antifungal compound composition and its use
The present invention relates to antifungal composition with two or more active components and its application in medicine, cosmetics and sanitary articles. The externally applied antifungal composition butenafine, butenafine hydrochloride or ketoconazole in 0.1-10 wt%, and mometasone furoate in 0.001-10 wt%, except substrate. Animal test shows that the antifungal composition has obvious synergistic effect in resisting Trichophyton mentagrophytes infection.
Owner:DIHON PHARMA GROUP
Method for appraising royal jelly quality
InactiveCN101363828AEfficient separationSensitive separationComponent separationColor/spectral properties measurementsAnalysis methodHydroxymethyl
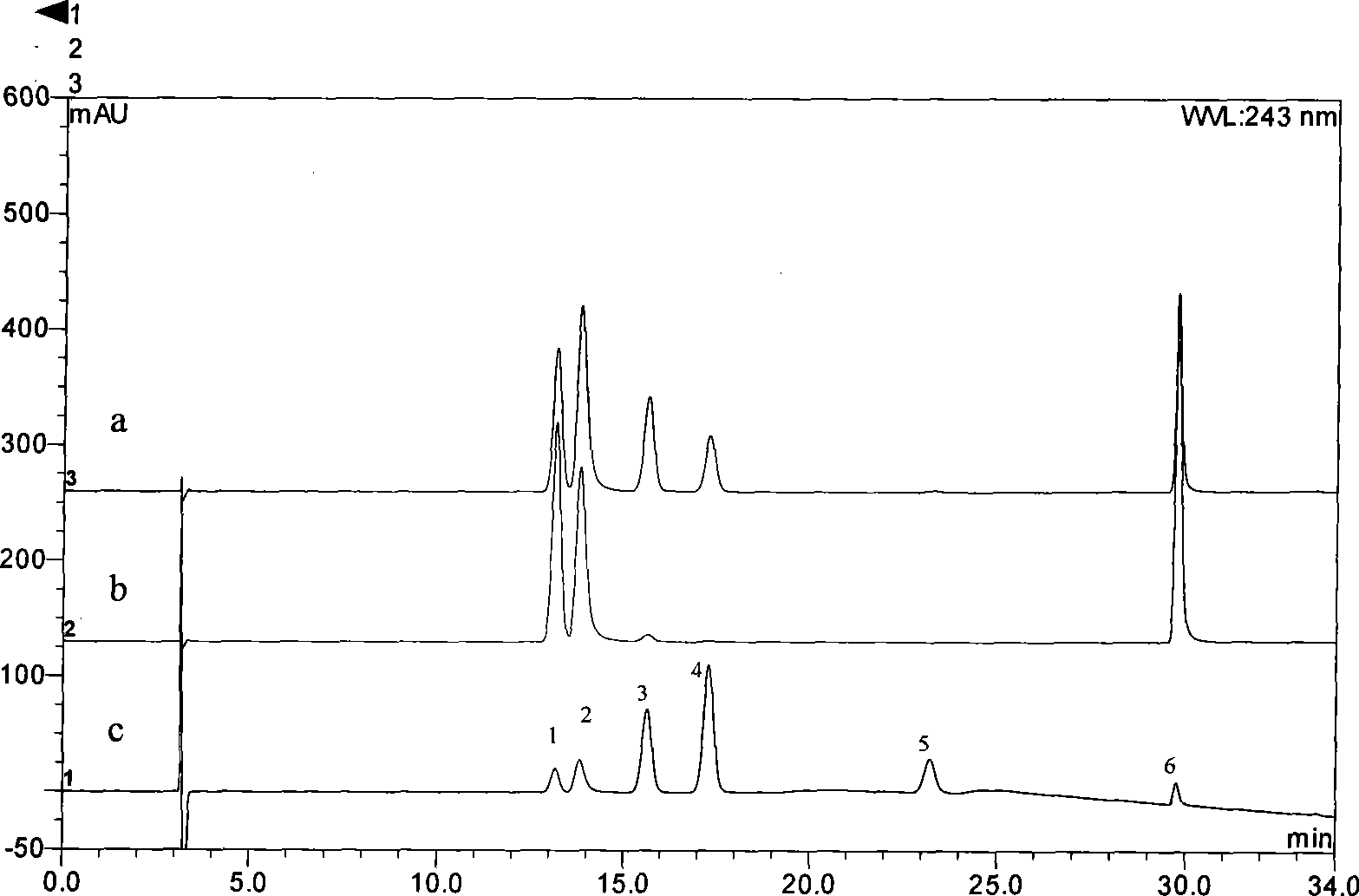
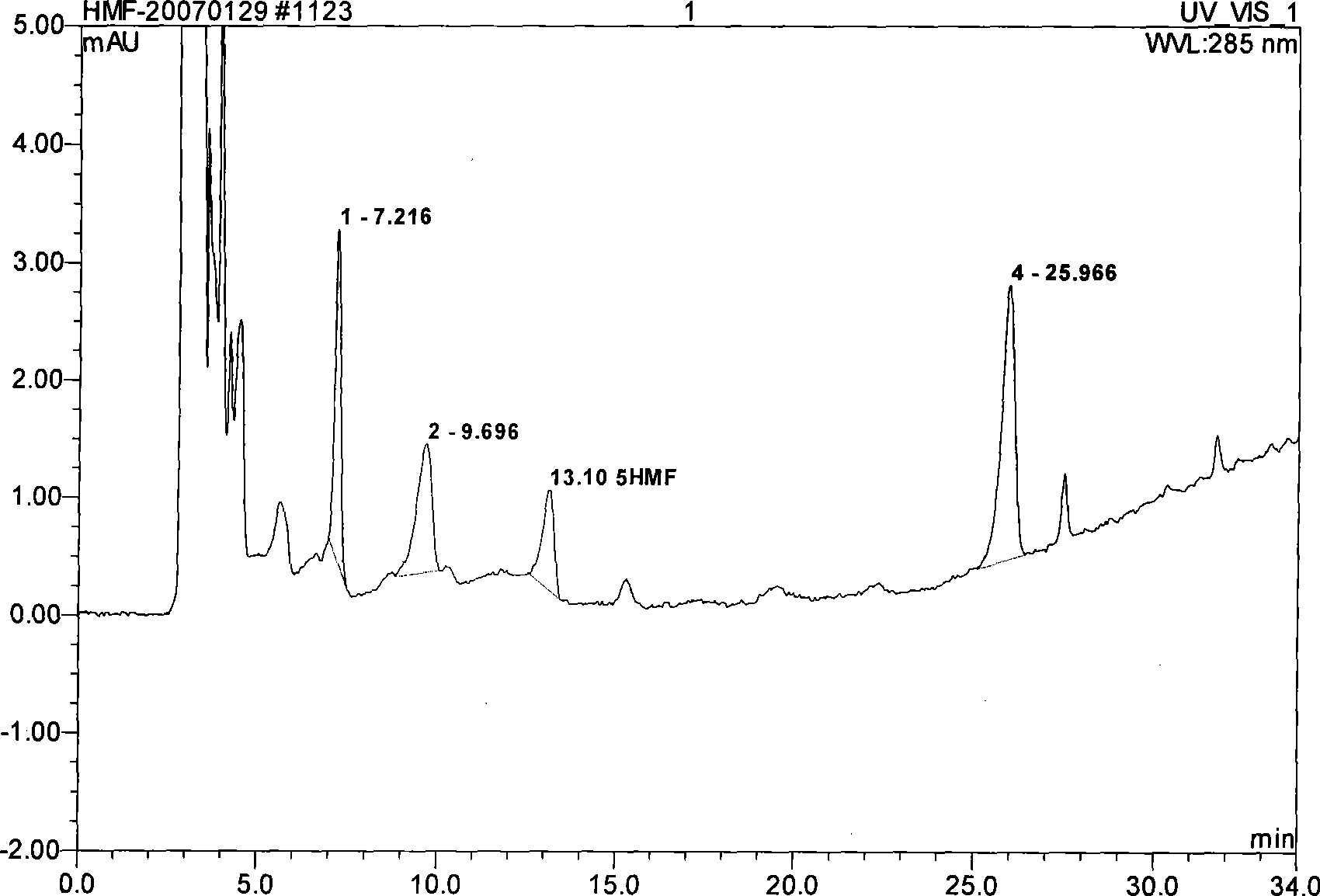
The invention provides an evaluation method for the quality of royal jelly. The quality of royal jelly is judged by detecting the content of a furfurol substance in the royal jelly, namely, 5- hydroxymethyl-2-furfurol. The invention has the advantages that the sensitivity is high, the analysis method is simple, six furfurol substances namely, 5-hydroxymethyl-2-furfurol, 5-methyl hydroxymethyl, 2-hydroxymethyl, 2-furoic acid, 3-furoic acid, 5-hydroxymethyl-2-furoic acid can be effectively, sensitively and accurately separated through the method for detecting the furfurol substances, and accurate quantification for 5-hydroxymethyl-2-furfurol in the royal jelly can be realized.
Owner:BEE RES INST CHINESE ACAD OF AGRI SCI
Process and intermediates for the production of furan-2,5-dicarboxylic acid or derivatives thereof
Disclosed is a method of making 5-formyl-2-furoic acid and furan-2,5-dicarboxylic acid. The method involves the use of 5-keto-aldonic acids as intermediates, as these can be subjected to ring formation by a cyclodehydration reaction under mild conditions. The 5-formyl-2-furoic acid or carboxylic derivative thereof is subjected to oxidation so as to form furan-2,5-dicarboxylic acid. The 5-keto-aldonic acid intermediates can be obtained by isomerization of uronic acids which can be obtained from sugar beet pulp, chicory pulp, fruit peals including orange peels, or non-terrestrial sources like seaweeds. A preferred source is sugar beet pulp.
Owner:STICHTING WAGENINGEN RES
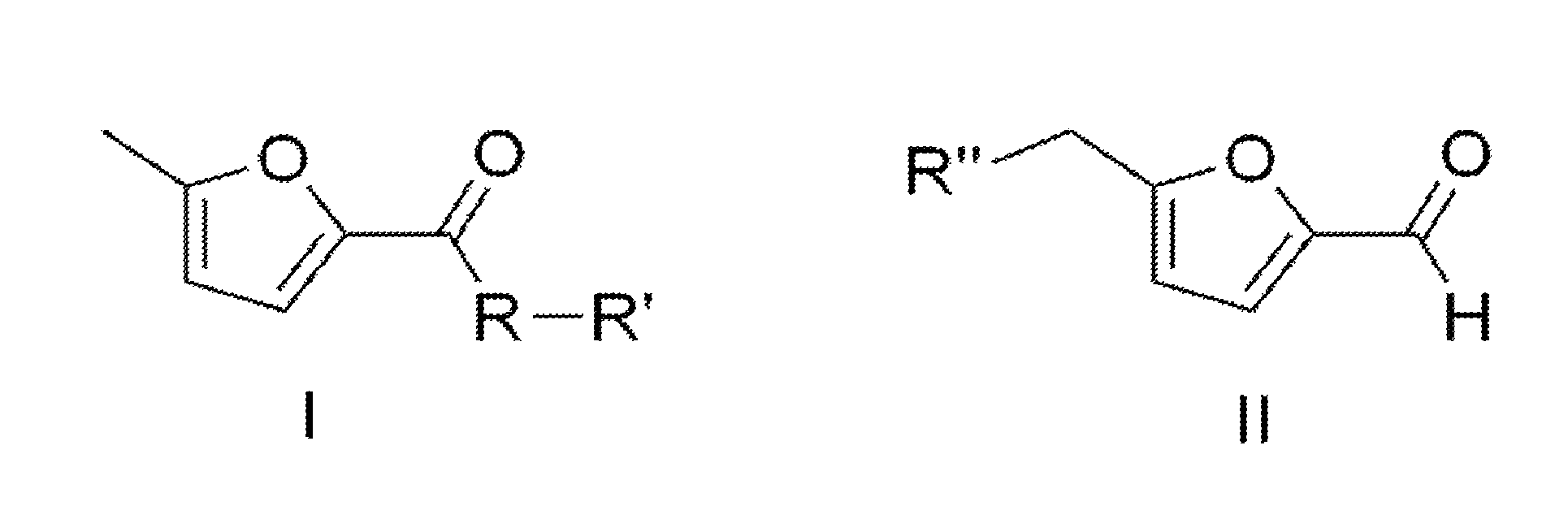
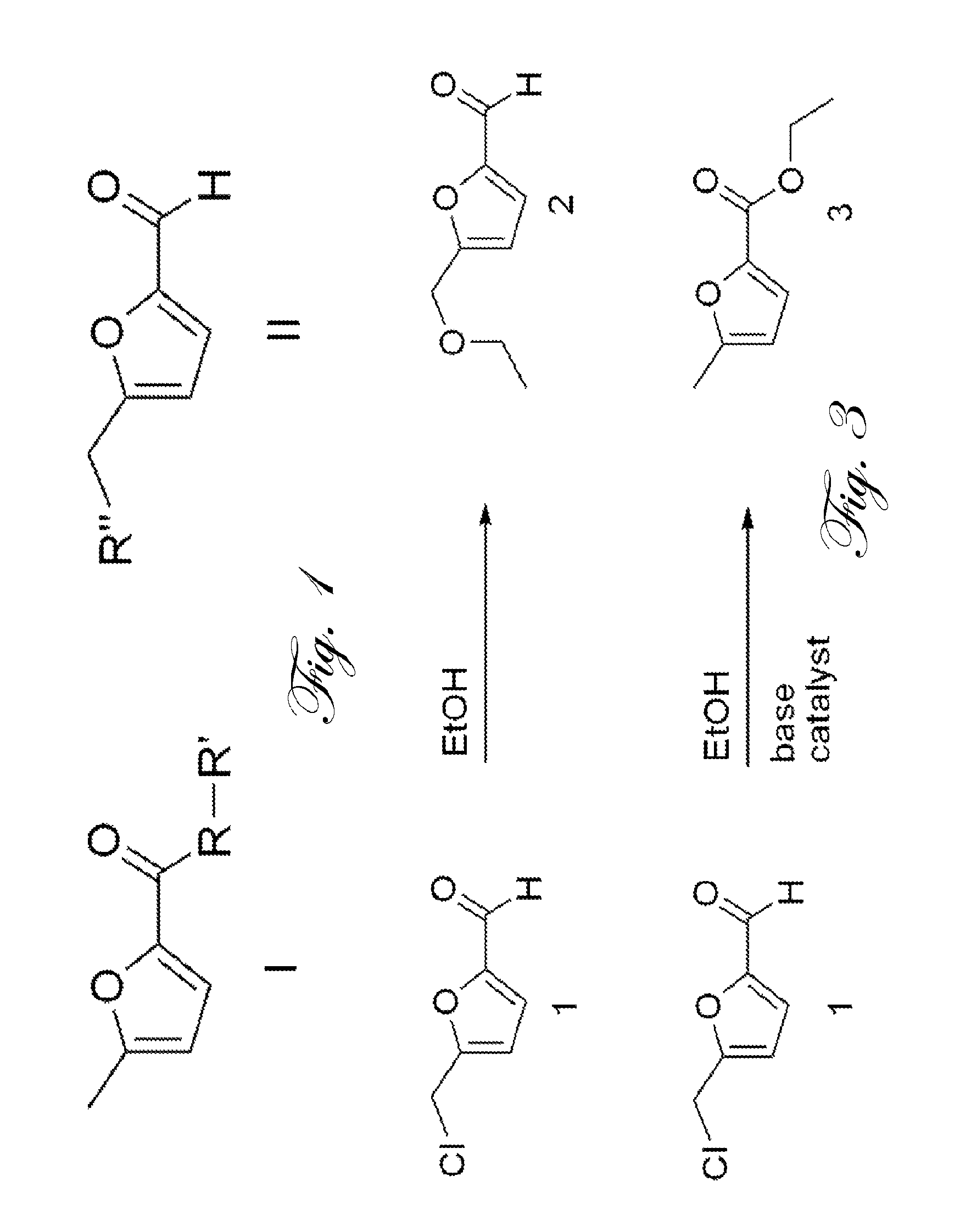
Conversion of 5-(chloromethyl)-2-furaldehyde into 5-methyl-2-furoic acid and derivatives thereof
The present invention concerns the synthesis of 5-methyl-2-furoic acid, including ester, amide, and thioester derivatives of such from 5-(chloromethyl)-2-furaldehyde (CMF). The molecules so prepared are useful as intermediates for pharmaceutical, food, and fragrance molecules; as well as fuel or fuel additives.
Owner:XF TECH INC
Composite fluorine removing agent for removing high-concentration fluorine-containing wastewater and application method thereof
InactiveCN102464394AReduce dosageLow running costWater contaminantsWater/sewage treatmentTrifluoromethanesulfonic anhydrideMagnesium salt
The invention discloses a composite fluorine removing agent for removing high-concentration fluorine-containing wastewater and an application method thereof, which belong to the field of sewage treatment in environment protection. The composite fluorine removing agent is prepared by compounding polyhydroxyl or polycarboxyl compounds and inorganic matters according to a certain proportion, wherein the polyhydroxyl or polycarboxyl compounds include one or more than two of trifluoromethanesulfonic anhydride, caprylyl chloride, 1R-(-)-camphorsulfonic acid, 3-furoic acid, pentanoyl chloride, and butanetetracarboxylic acid; and the inorganic matters include two or more than two of calcium salt, magnesium salt, phosphate, and ferric salt. The composite fluorine removing agent is added into the high-concentration fluorine-containing wastewater in a weight ratio of 1:100 to 1:1000 according to the concentration of fluorine in the wastewater; the pH value of the wastewater is adjusted to 9.0-11.0 with sodium hydroxide; and the wastewater is stirred for 15-30min, so that the yielding water can meet the national emission standard I. The composite fluorine removing agent and the application method thereof disclosed by the invention have the characteristics of simple technological process, smaller dosage of chemical agent, lower running expense, no sludge, high removal rate, and the like; and the removal rate can reach above 99.9%.
Owner:CHANGZHOU YAHUAN ENVIRONMENTAL PROTECTION TECH
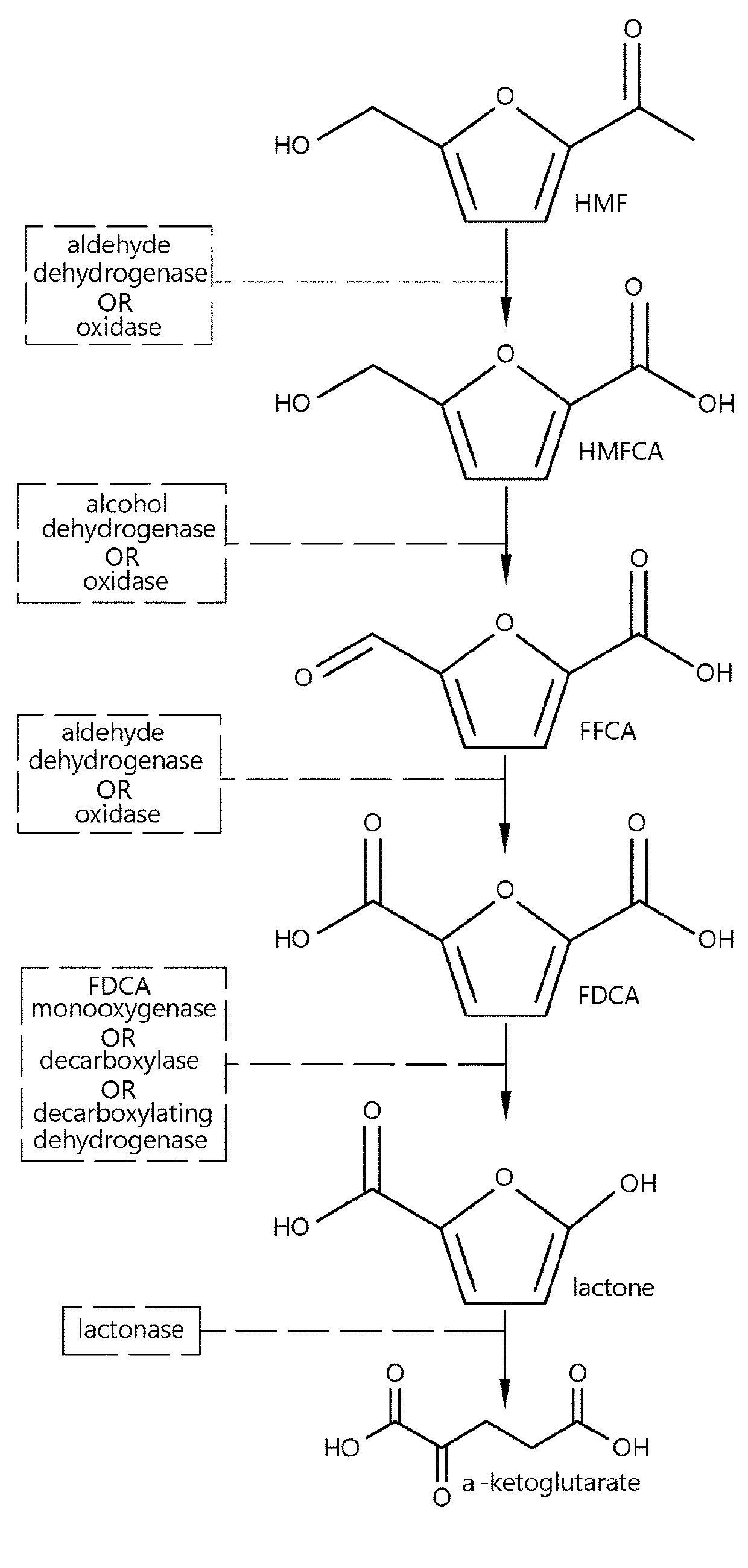
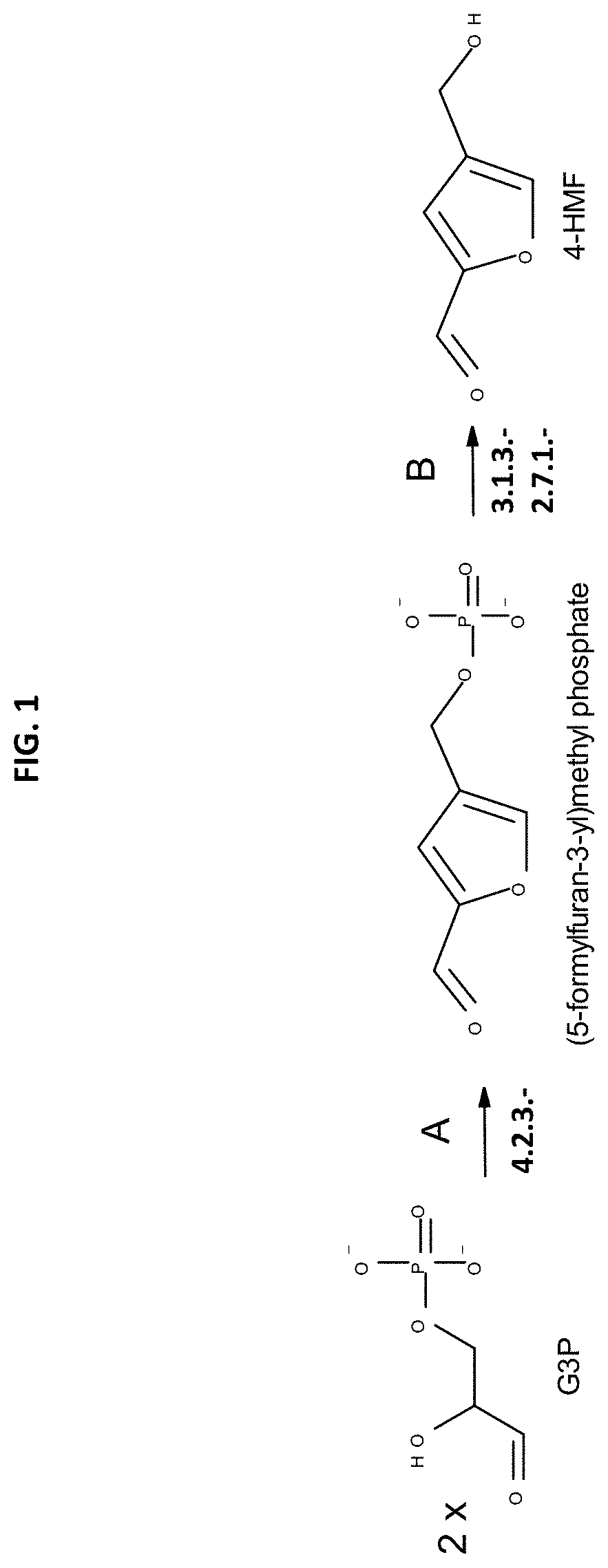
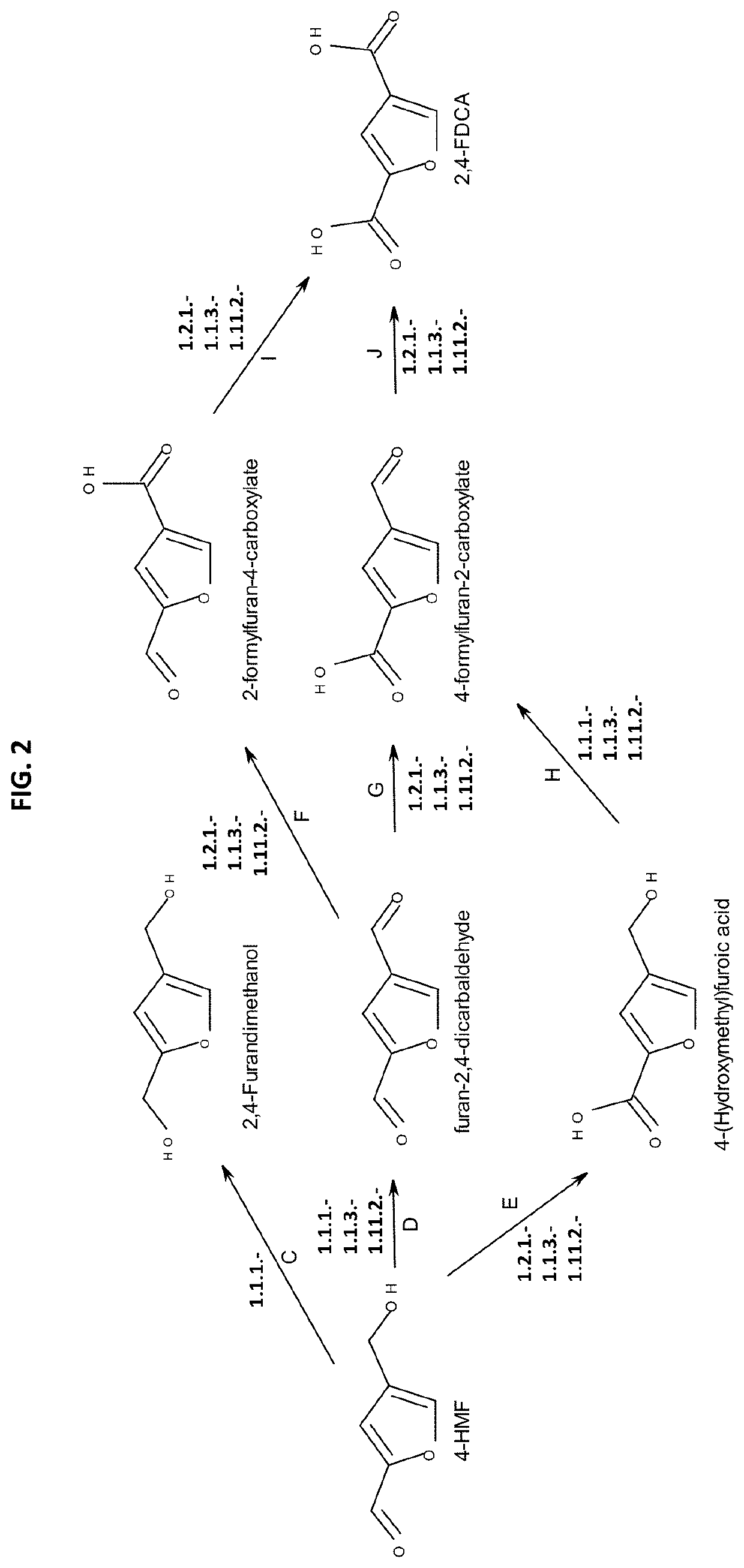
Method for the in vivo synthesis of 4-hydroxymethylfurfural and derivatives thereof
The present disclosure provides recombinant microorganisms and methods for the production of 4-HMF, 2,4-furandimethanol, furan-2,4-dicarbaldehyde, 4-(hydroxymethyl)furoic acid, 2-formylfuran-4-carboxylate, 4-formylfuran-2-carboxylate, and / or 2,4-FDCA from a carbon source. The method provides for engineered microorganisms that express endogenous and / or exogenous nucleic acid molecules that catalyze the conversion of a carbon source into 4-HMF, 2,4-furandimethanol, furan-2,4-dicarbaldehyde, 4-(hydroxymethyl)furoic acid, 2-formylfuran-4-carboxylate, 4-formylfuran-2-carboxylate, and / or 2,4-FDCA. The disclosure further provides methods of producing polymers derived from 4-HMF, 2,4-furandimethanol, furan-2,4-dicarbaldehyde, 4-(hydroxymethyl)furoic acid, 2-formylfuran-4-carboxylate, 4-formylfuran-2-carboxylate, and / or 2,4-FDCA.
Owner:BRASKEM SA
Nasal in-situ gel containing mometasone furoate and H1 receptor antagonist
InactiveCN102078612AImprove bioavailabilityGood treatment effectOrganic active ingredientsPharmaceutical delivery mechanismNK1 receptor antagonistBULK ACTIVE INGREDIENT
The invention discloses nasal in-situ gel containing mometasone furoate and an H1 receptor antagonist. The nasal in-situ gel is prepared from mometasone furoate serving as an active ingredient and / or a hydrate of mometasone furoate, one or more H1 receptor antagonists, an environmentally-sensitive hydrophilic gel material, other pharmaceutically acceptable auxiliary materials and the balance of water.
Owner:TIANJIN JINYAO GRP
Intranasal medicinal composition containing bilastine and mometasone furoate
InactiveCN103736098ARelieve bitternessRelieve irritationOrganic active ingredientsAerosol deliveryThaumatinMedicine
The invention provides an intranasal medicinal composition. The intranasal medicinal composition contains bilastine, mometasone furoate, and thaumatin serving as a bitterness and irritation alleviator.
Owner:于运红
Conversion of 5-(chloromethyl)-2-furaldehyde into 5-methyl-2-furoic acid and derivatives thereof
The present invention concerns the synthesis of 5-methyl-2-furoic acid, including ester, amide, and thioester derivatives of such from 5-(chloromethyl)-2-furaldehyde (CMF). The molecules so prepared are useful as intermediates for pharmaceutical, food, and fragrance molecules; as well as fuel or fuel additives.
Owner:XF TECH INC
Method for preparing methyl furoate by directly oxidizing and esterifying furfural
PendingCN109824634AImprove responseMild reaction conditionsOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsTwo stepFurfural
The invention relates to a method for preparing methyl furoate by directly oxidizing and esterifying furfural. The methyl furoate is prepared by using the furfural as the substrate and a cobalt-basedparticle supporter as the catalyst. The method specifically includes: dissolving the furfural into methanol, adding the cobalt-based particle supporter catalyst and auxiliaries, and performing stirring reaction at the temperature of 40-180 DEG C and in the oxygen atmosphere of 0.01-4MPa for 1-10 hours to prepare the methyl furoate through the one-step oxidization and esterification of the furfural. The method has the advantages that a two-step reaction including furfural oxidization and methanol esterification is shortened to the one-step oxidization and esterification, and the method is promising in application prospect; the cobalt-based particle supporter catalyst used by the method is mild in catalyzing conditions, good in effect, long in service life, easy to separate and recycle, capable of lowering production cost and evident in industrial application value.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
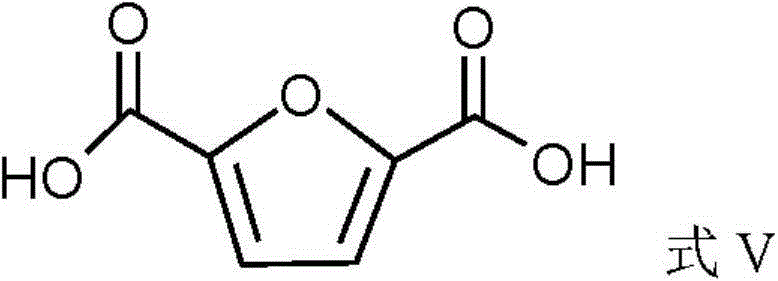
Method for preparing 2,5-furandicarboxylicacid (FDCA) by catalytic carbonylation
ActiveCN108148026AHigh selectivityMild reaction conditionsOrganic chemistryPalladium catalystReaction temperature
The invention discloses a method for preparing 2,5-furandicarboxylicacid (FDCA) by catalytic carbonylation. The method comprises the following steps: taking 5-bromo-2-furoic acid as a raw material, carrying out stirring reaction on a reaction system which simultaneously contains 5-bromo-2-furoic acid, solvent, alkali and palladium catalyst under the atmosphere of carbonic oxide at the temperatureof 50-120 DEG C for 4-20h; then, removing the solvent, adding acidified reagent to regulate a pH (Potential of Hydrogen) value to acidity, and separating a target product FDCA, wherein the palladium catalyst contains a palladium element, the solvent is water or a mixture of water and organic solvent. According to the method, the 5-bromo-2-furoic acid is taken as the raw material, and the whole technical process design of the preparation method and the reaction condition and parameter (including the category and the ratio of reaction raw materials, reaction temperature, time and the like) of each step can be improved. Compared with the prior art, the method disclosed by the invention solves the problem that human grain resources which need to be consumed for preparing FDCA is solved.
Owner:HUAZHONG UNIV OF SCI & TECH
A method of preparing furan-2,5-dicarboxylic acid from furoic acid
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
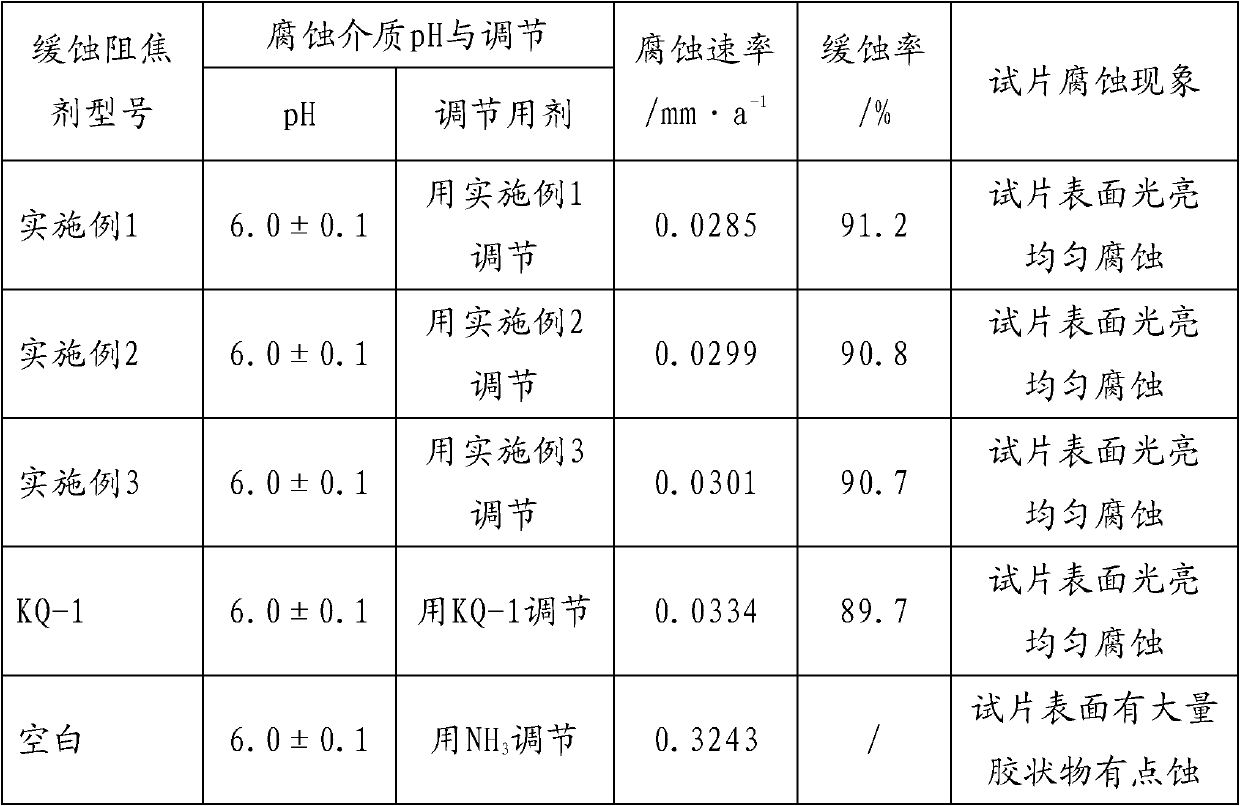
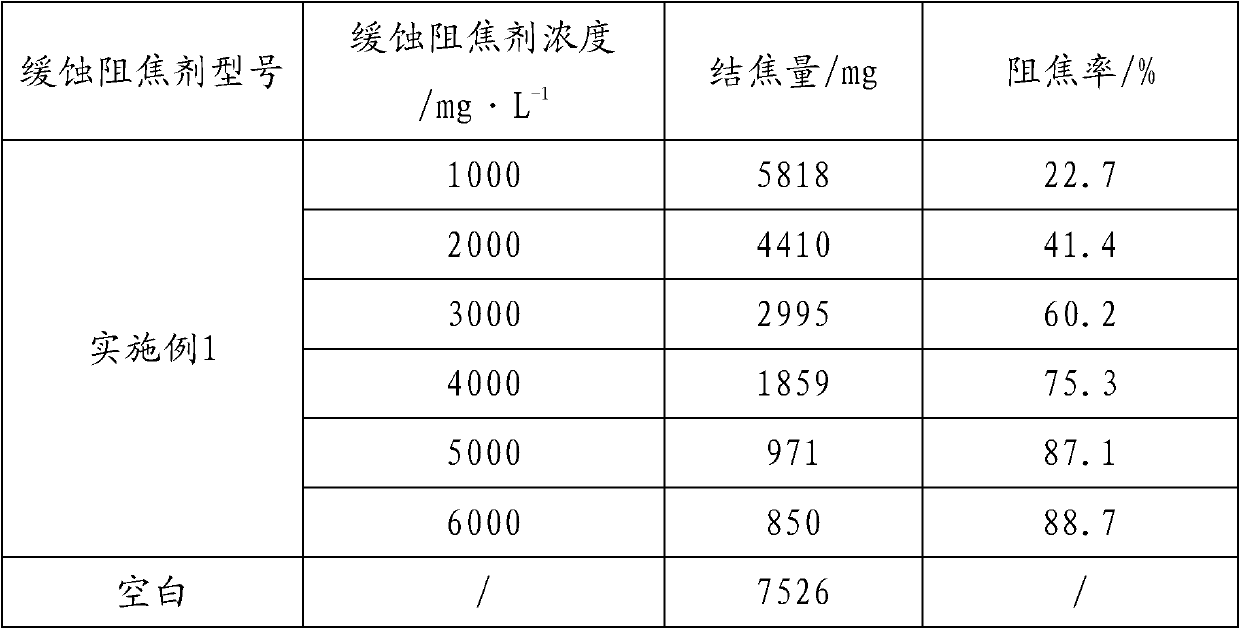
Corrosion-inhibition anti-coking agent for furfural refining device
ActiveCN102888246AStop steamingGood corrosion inhibitionTreatment apparatus corrosion/fouling inhibitionAntioxidantSolvent
The invention relates to the field of chemical additives and discloses a corrosion-inhibition anti-coking agent for a furfural refining device. The corrosion-inhibition anti-coking agent for a furfural refining device comprises: by weight, 1 to 5% of a dispersed component, 5 to 10% of one or more neutral organic amines, 8 to 15% of a water-soluble imidazoline compounds, 1 to 5% of alkynol compounds, 8 to 15% of a nitrogen-containing adsorption-type corrosion inhibitor compound, 8 to 15% of cyclammonium corrosion inhibitors, 3 to 8% of an amine antioxidant and 20 to 55% of a compound solvent. The corrosion-inhibition anti-coking agent has effects of oxidation resistance and coking inhibition, can slow down formation of furfural acid and coke (or a resinoid oxide), can neutralize produced furfural acid, can reduce an acid value, can coordinate with furoic acid, can destroy furfural-furfural acid-water ternary azeotrope formation, and can form an anti-corrosion film on a metal surface without influence on quality of a refined oil product.
Owner:WUHAN RUNERHUA TECH
Fungal production of fdca
The invention relates to fungal cells for the production of FDCA. The fungal cell is genetically modified to have at least one of a) a genetic modification that confers to or increases in the cell theability to oxidize 5-hydroxymethyl-2-furancarboxylic acid to 5-formyl-2-furoic acid; and, b) a genetic modification that reduces catabolism of 2,5-furandicarboxylic acid in the cell. The fungal cellcan further be genetically modified to increase the cell's ability to oxidize furanic aldehydes to the corresponding furanic carboxylic acids. The invention also relates to a process for the production of 2,5-furandicarboxylic acid (FDCA) wherein the cells of the invention are used for oxidation of a furanic precursors of FDCA.
Owner:PURAC BIOCHEM
Mometasone furoate emulsifiable paste and preparation method thereof
ActiveCN112754990AAdvanced technologySimple methodOrganic active ingredientsAntipyreticWhitening AgentsMedicine
Owner:GUANGZHOU BAIYUNSHAN PHARM CO LTD
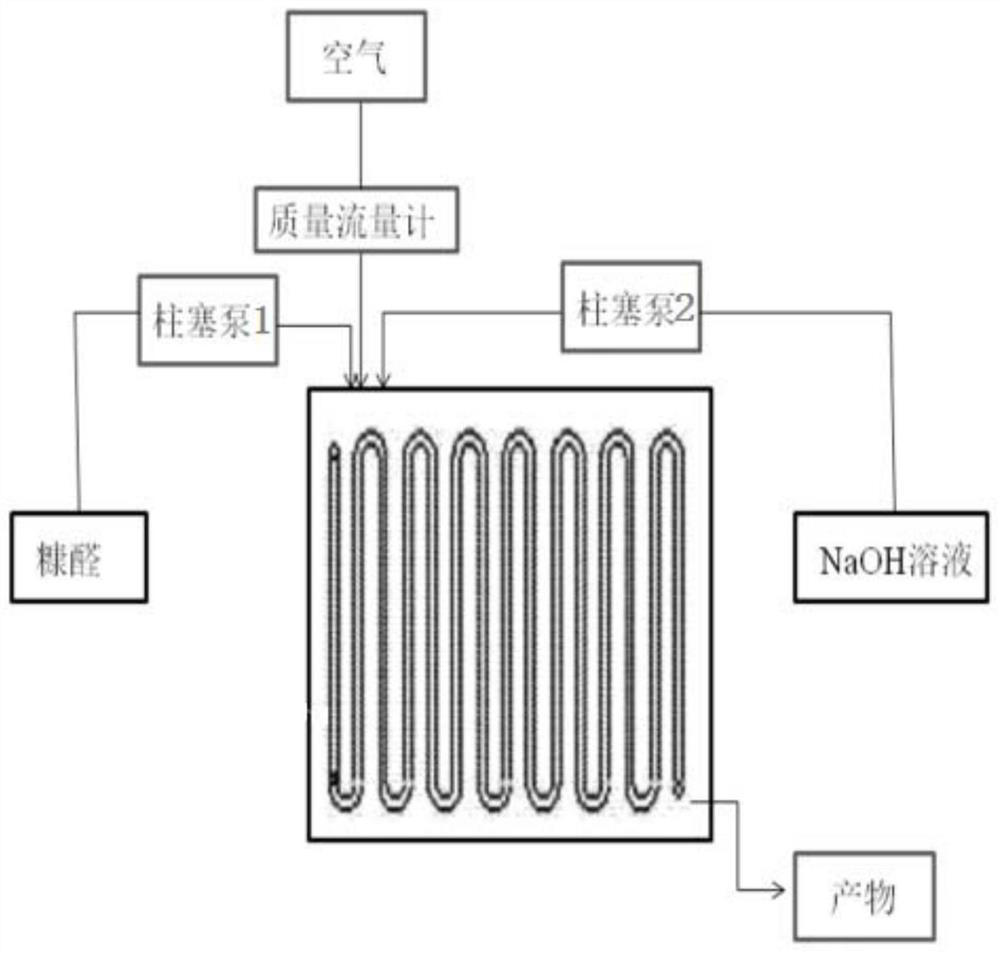
Method for preparing furoic acid by oxidizing furfural on micro-channel reactor
ActiveCN110845456AImprove mixing efficiencyEfficient mixingOrganic chemistryChemical/physical/physico-chemical microreactorsIce waterPhysical chemistry
The invention relates to a method for preparing furoic acid by oxidizing furfural on a micro-channel reactor. The method comprises the following steps: (1) conveying furfural and a NaOH solution intoa micro-channel reactor by a plunger pump, introducing air into the micro-channel reactor, and obtaining a reaction solution from the outlet of the reactor; (2) performing acidification, crystal growing, filtering and ice water leaching to obtain a furoic acid wet product; and (3) performing vacuum drying to obtain solid furoic acid. According to the invention, by utilizing the micro-channel reactor, the reaction heat can be well controlled, the continuous production of furoic acid is realized, the reaction speed is high, the residence time is short, the purity of the prepared furoic acid is more than 99.5%, and the yield is more than 97.6%.
Owner:浙江清和新材料科技有限公司
Preparation method of furoic acid
ActiveCN112778251AHigh selectivitySimplified post-processing stepsOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsPtru catalystCatalytic oxidation
The invention provides a preparation method of furoic acid, and the method comprises the following steps of: under the condition of air and / or oxygen, contacting an aqueous solution of furfural with a catalyst for catalytic oxidation reaction to prepare furoic acid, wherein the catalyst is obtained by the following steps of: mixing a carrier, an alkaline nitrogen-containing compound and an active component precursor and placing the mixture in a solvent, performing heating under a water bath condition and carrying out reflux stirring treatment; sequentially carrying out drying treatment and roasting reduction treatment on the mixture subjected to the reflux stirring treatment to obtain the catalyst; wherein the active component precursor is selected from one or more of rhodium chloride, palladium chloride, chloroplatinic acid and ruthenium chloride, and the carrier is selected from one or more of activated carbon, graphite, fullerene and graphene oxide. The catalyst adopted by the method is high in activity, the product yield is high, the reaction does not need to be carried out in an alkaline environment, the reaction process is environment-friendly, the product is easy to separate, and the problem of complicated subsequent acid treatment in the traditional furoic acid preparation process is solved.
Owner:CHINA PETROLEUM & CHEM CORP +1
Mometasone furoate cream and preparation method thereof
ActiveCN106138066AReduce permeationImprove stabilityOrganic active ingredientsAerosol deliverySkin penetrationMometasone furoate
The invention discloses mometasone furoate cream and a preparation method thereof. The mometasone furoate cream is prepared through the technology method comprising the steps that mometasone furoate is mixed with a mixture of Tween-80 and anhydrous citric acid, and then the obtained mixture is added into an aqueous propylene glycol solution. The mometasone furoate cream has the better stability, the appropriate retention amount in the skin and the lower skin penetration amount.
Owner:REGENEX PHARMA LTD
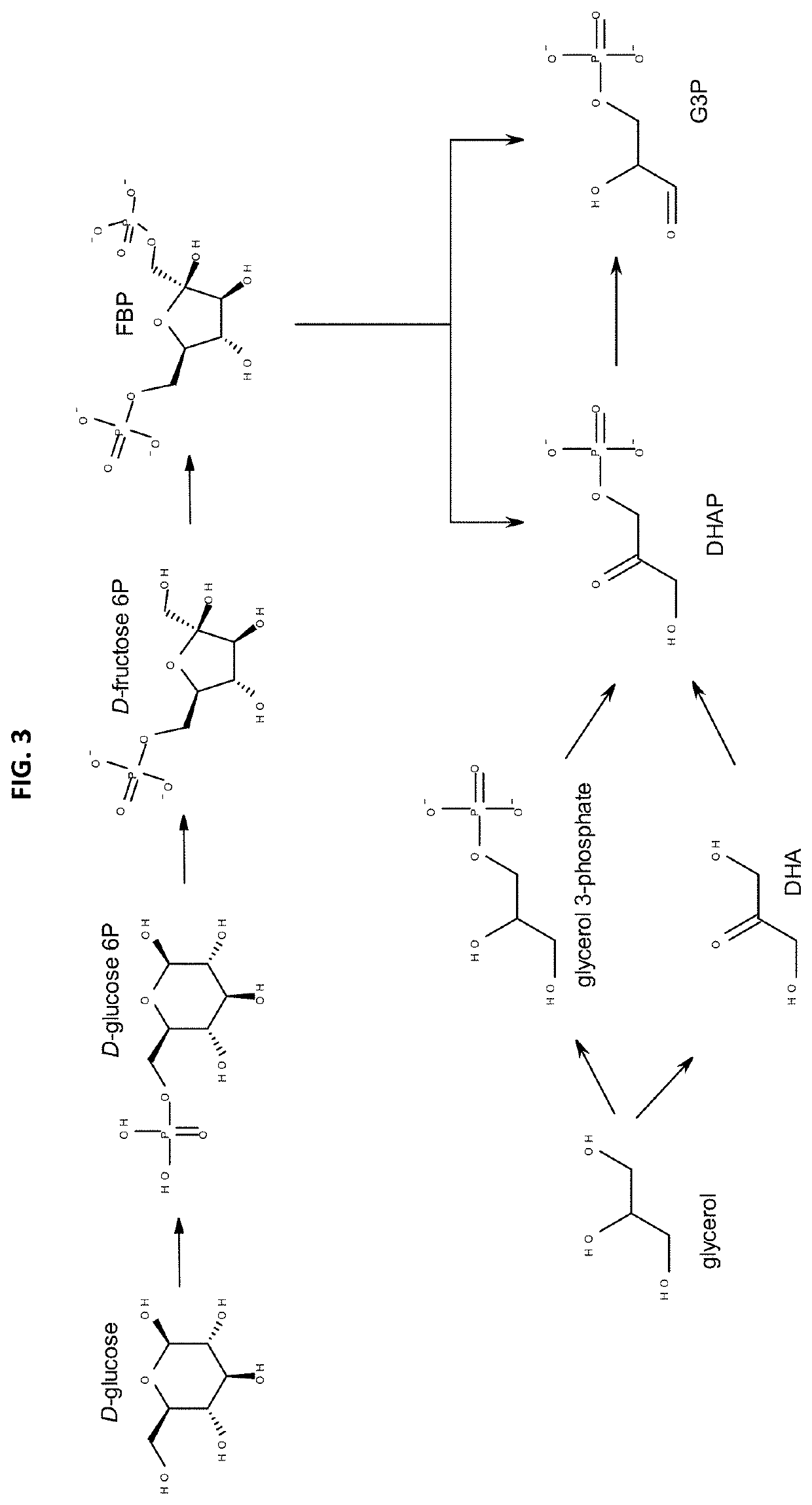
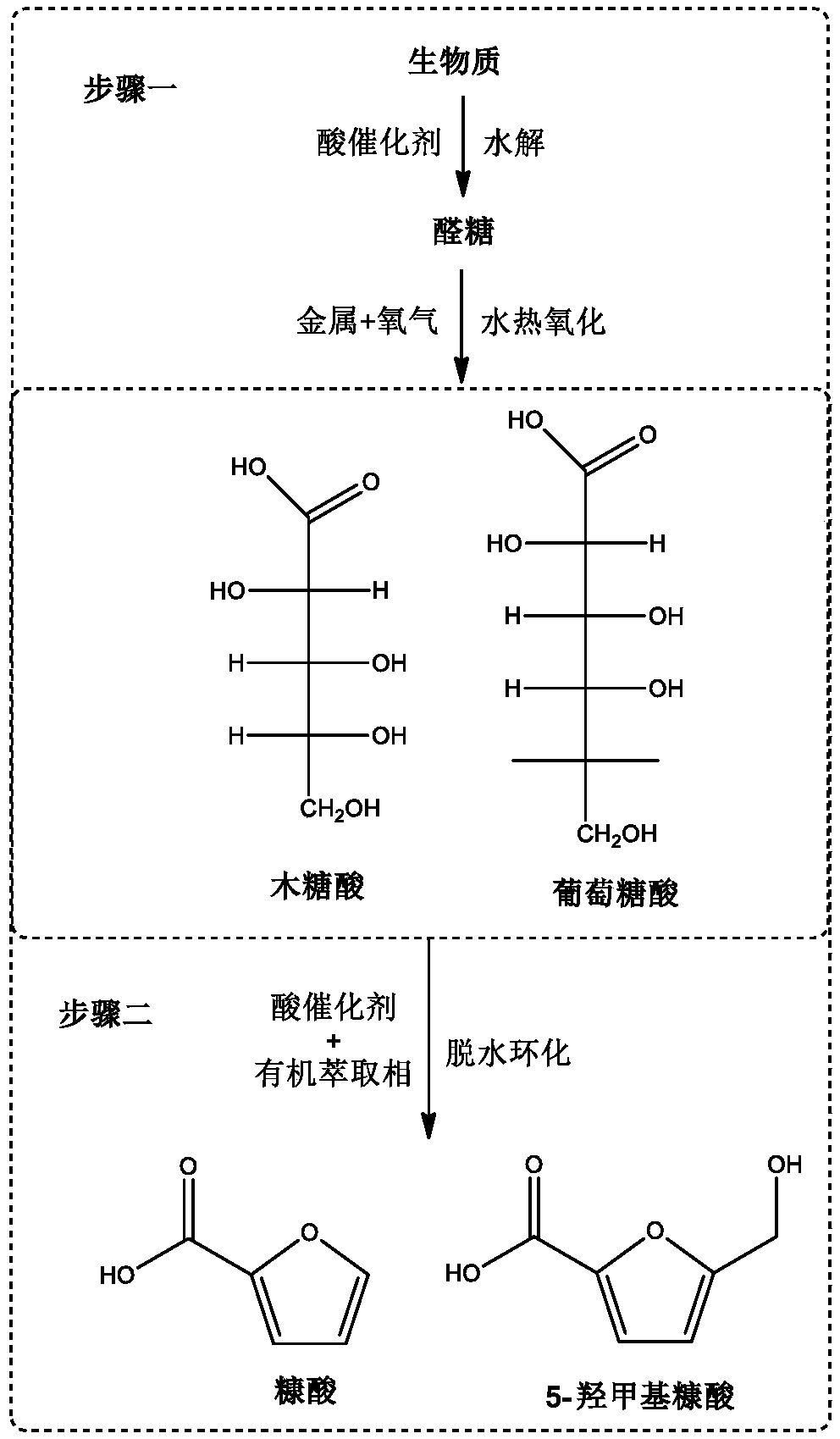
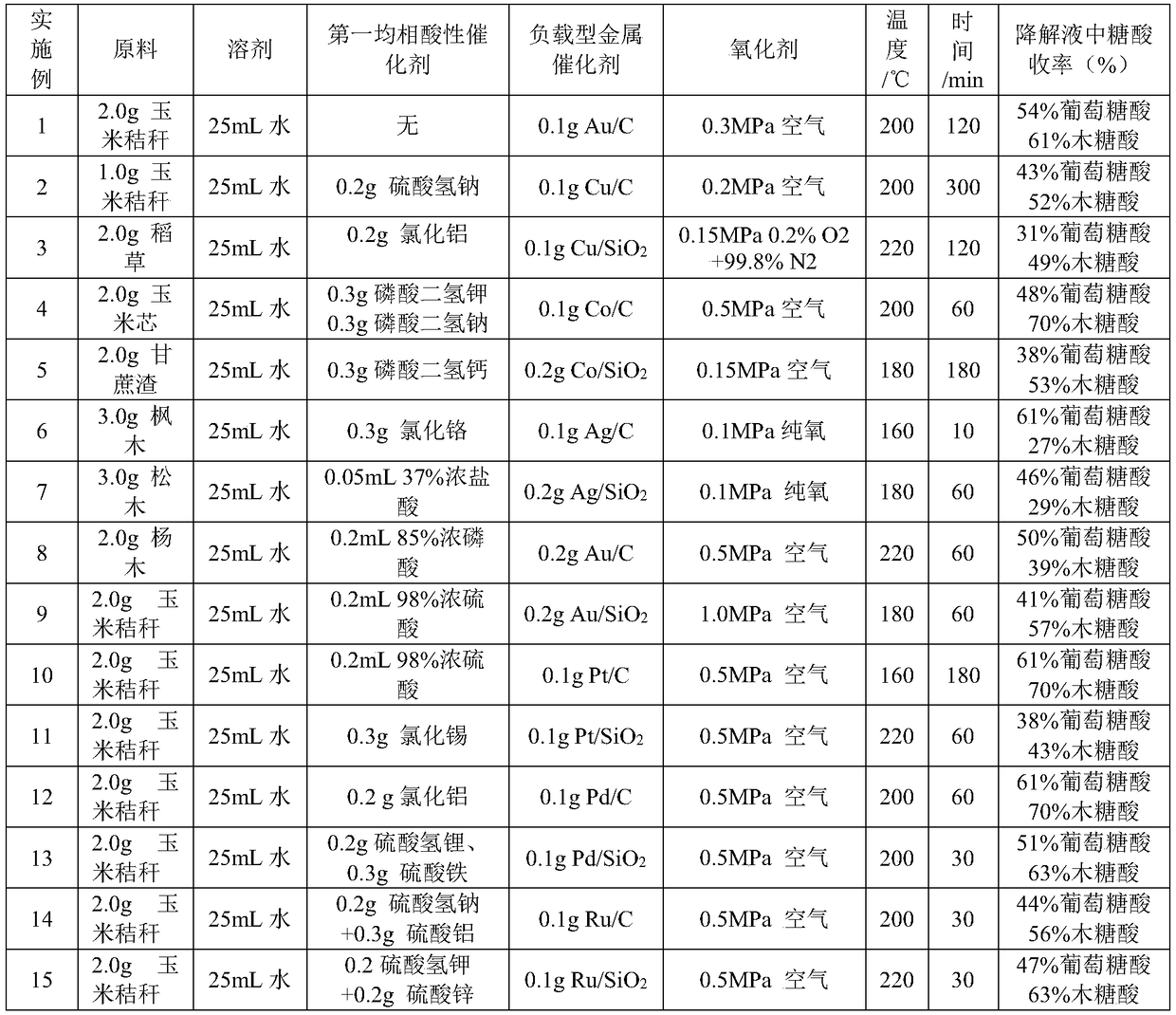
Method for preparing furoic acid and 5-hydroxymethyl furoic acid by biomasses
The invention discloses a method for preparing furoic acid and 5-hydroxymethyl furoic acid by biomasses. The method includes the steps: (1) taking oxygen-containing gas as an oxidizing agent, and converting the biomasses into degradation liquid containing gluconic acid and xylonic acid under hydrothermal reaction conditions through combined actions of a first homogeneous acid catalyst and a supported metal catalyst; (2) placing the degradation liquid containing the gluconic acid and the xylonic acid into an inert atmosphere, sequentially adding an organic solvent and a second homogeneous acidcatalyst to obtain mixed liquid, performing reaction on the mixed liquid to obtain the furoic acid and the 5-hydroxymethyl furoic acid. According to the method, a small amount of solid residue is generated in the whole reaction process, and the method avoids the problems of low target products, carbon deposition on device wall surface, pipeline blockage and the like caused by a lot of solid residue generated in traditional biomass degradation process.
Owner:GUIZHOU INST OF TECH
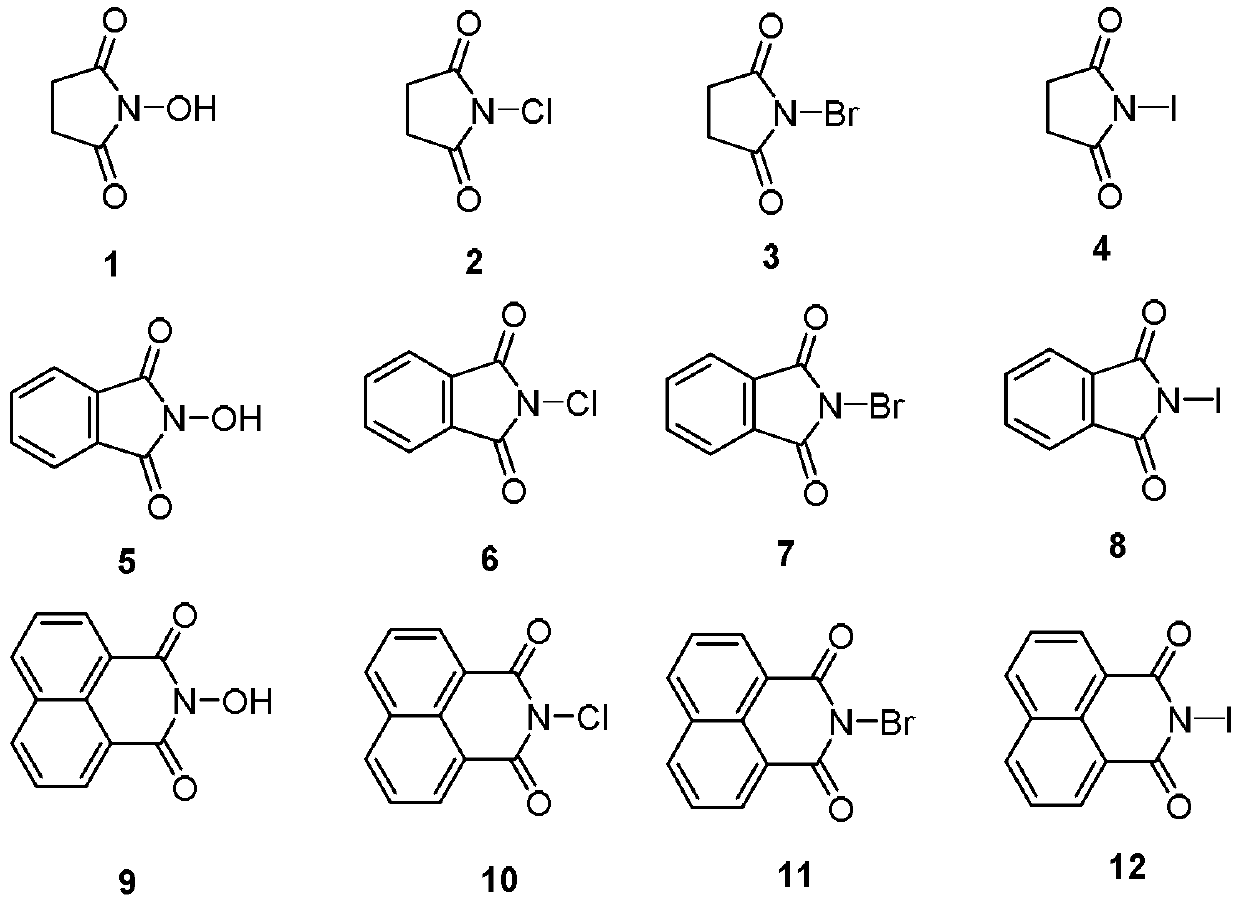
17-(5-ethyl-6-methylheptan-2-yl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl furan-3-carboxylate and extraction method and medicine application thereof
The invention relates to 17-(5-ethyl-6-methylheptan-2-yl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl furan-3-carboxylate as well as an extraction method and medicine application thereof, and belongs to a new compound and medicine application thereof. Ginseng stem and leaves and artemisias tolonifera are taken as raw materials and are subjected to pulverization, high temperature high pressure treatment, drying, chloroform extraction, silica-gel column chromatography and recrystallization, and then the 17-(5-ethyl-6-methylheptan-2-yl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl furan-3-carboxylate is obtained. The 17-(5-ethyl-6-methylheptan-2-yl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl furan-3-carboxylate is applied to preparation of medicines for treating the hypertension.
Owner:JILIN AGRICULTURAL UNIV
Fungal production of FDCA
The invention relates to fungal cells for the production of FDCA. The fungal cell is genetically modified to have at least one of a) a genetic modification that confers to or increases in the cell the ability to oxidize 5-hydroxymethyl-2-furancarboxylic acid to 5-formyl-2-furoic acid; and, b) a genetic modification that reduces catabolism of 2,5-furandicarboxylic acid in the cell. The fungal cell can further be genetically modified to increase the cell's ability to oxidize furanic aldehydes to the corresponding furanic carboxylic acids. The invention also relates to a process for the production of 2,5-furan-dicarboxylic acid (FDCA) wherein the cells of the invention are used for oxidation of a furanic precursors of FDCA.
Owner:PURAC BIOCHEM
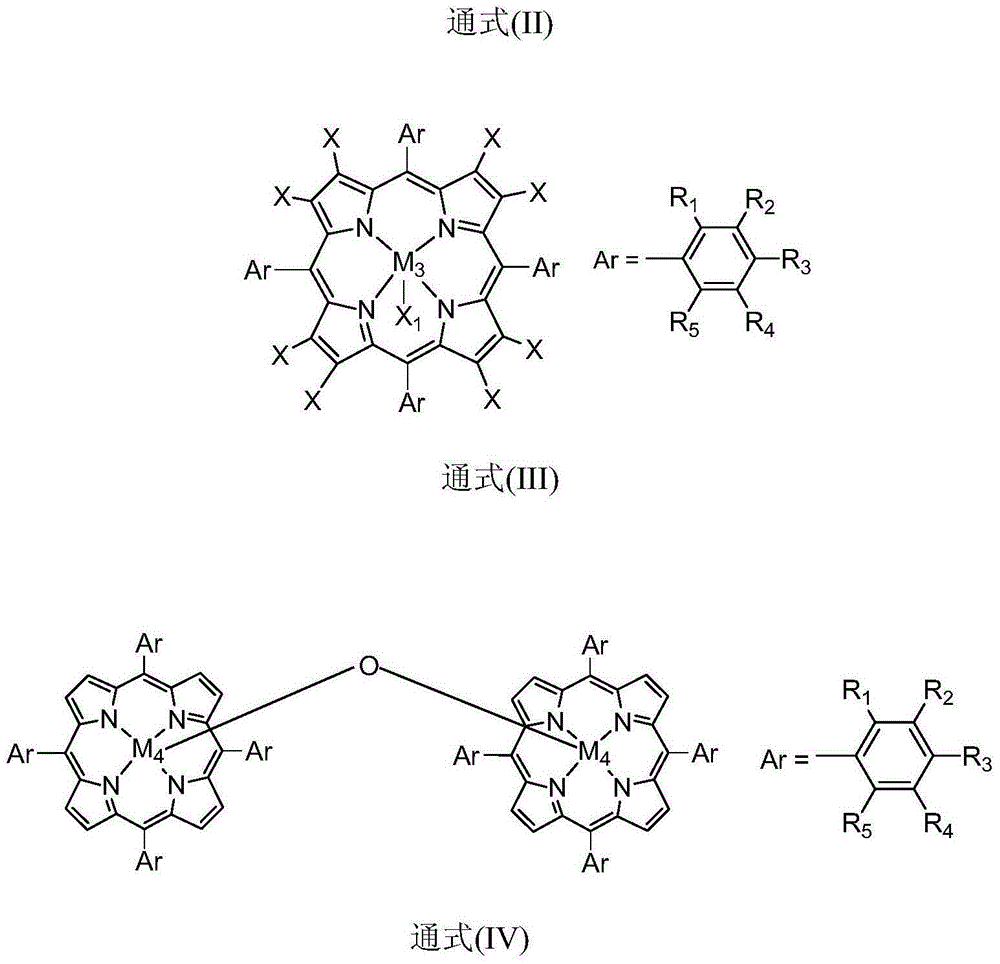
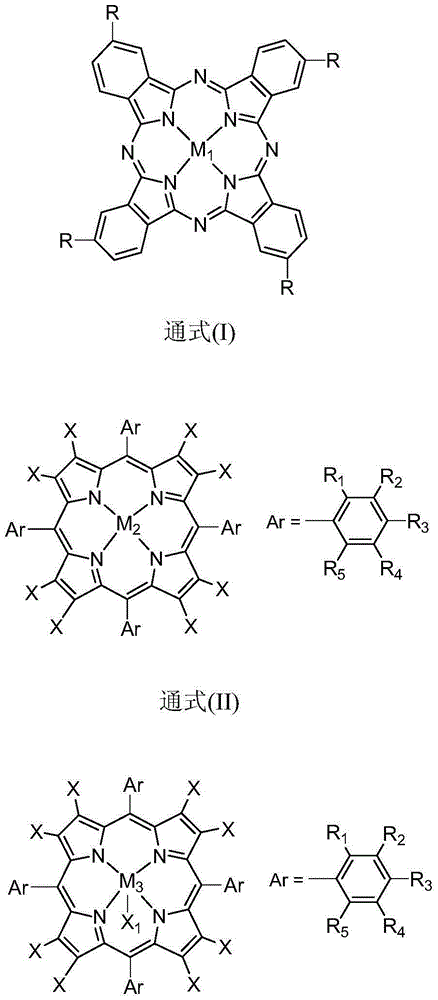
Method for co-producing epoxypropane and furoic acid
ActiveCN105001183AAvoid the disadvantages of traditional preparation processesHigh selectivityOrganic chemistryBulk chemical productionPorphyrinReaction temperature
The invention discloses a method for co-producing epoxypropane and furoic acid. According to the method, propylene and furfural serve as raw materials, oxygen serves as an oxidizing agent, organic solvent is added, metal phthalocyanine or metallloporphyrin compound serves as a catalyst, and catalytic reaction is controlled to be performed under the conditions that the reaction temperature is 30-140 DEG C and the reaction pressure is 0.1-3.0 MPa to obtain the epoxypropane and the furoic acid. The method has the advantages of being mild in reaction condition, high in efficiency, high in selectivity of the epoxypropane and the furoic acid, simple in technology and the like.
Owner:HUIZHOU RES INST OF SUN YAT SEN UNIV
Method for preparation of furoic acid from furfural
ActiveCN111217775AHigh yieldHigh selectivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystCombinatorial chemistry
The invention discloses a method for preparation of furoic acid from furfural. The method comprises the following steps of: uniformly mixing furfural, an oxidation catalyst and a solvent, and carryingout reaction in the presence of an oxygen source under certain temperature and pressure for a period of time to obtain furoic acid. The method has the advantages of high selectivity, few byproducts and mild reaction conditions, avoids the use of noble metal catalysts, greatly reduces the production cost, and has certain industrial application prospect.
Owner:UNIV OF SCI & TECH OF CHINA
Skin percutaneous absorption drug of adjuvant-containing mometasone furoate and adjuvant-containing water
InactiveCN102552287AGuaranteed stabilityReduce wasteOrganic active ingredientsSolution deliveryAdjuvantPercutaneous absorption
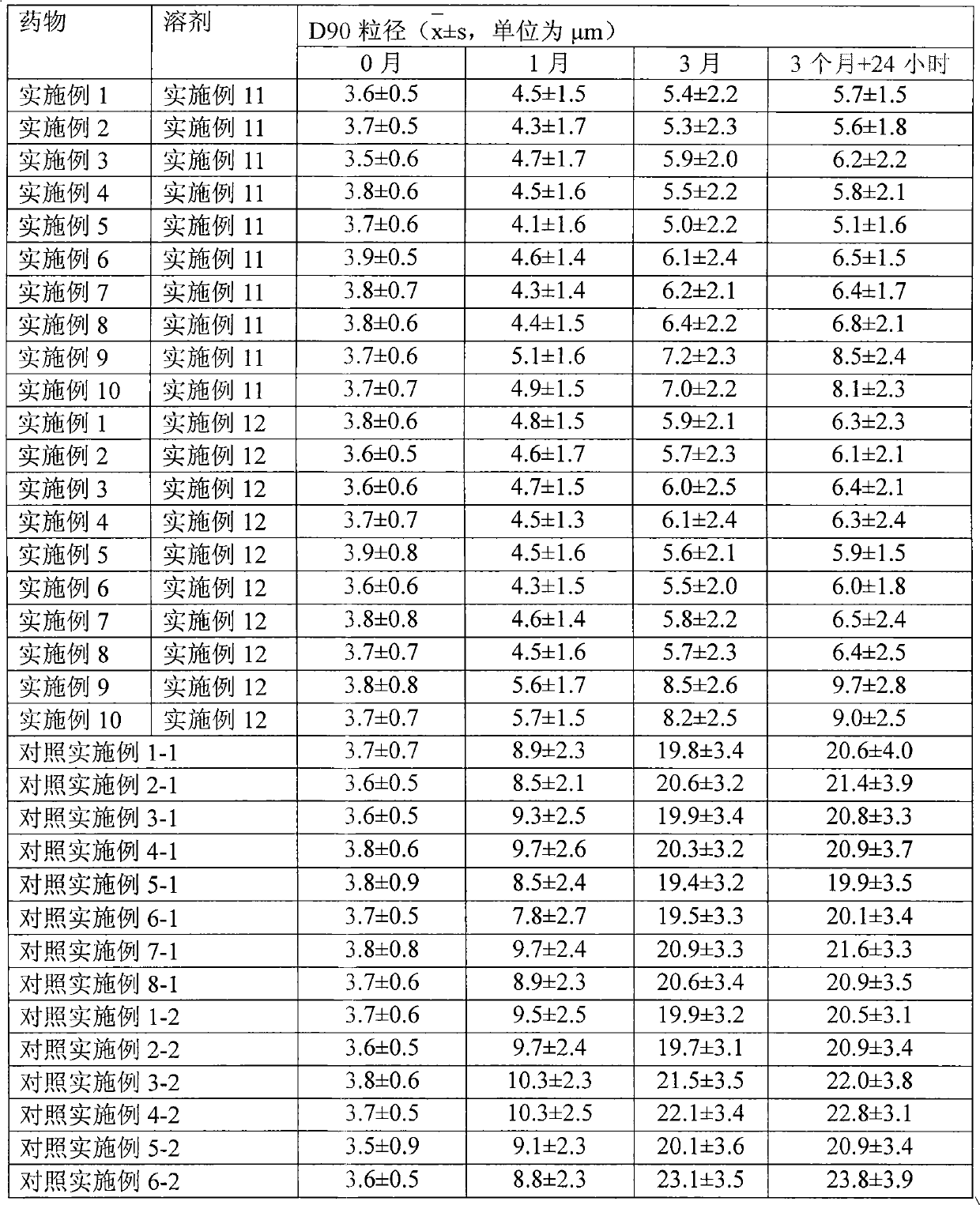
The invention relates to a skin percutaneous absorption drug of adjuvant-containing mometasone furoate and adjuvant-containing water. The skin percutaneous absorption drug comprises mometasone furoate and water, wherein the packing manner of the mometasone furoate adopts the individual packing, the mometasone furoate contains one or a plurality of solid medicine adjuvants, and is insoluble, the D90 particle size is 0.1-10 mum, the packing manner of the water adopts the individual packing, and the water contains one or a plurality of soluble medicinal adjuvants.
Owner:TIANJIN JINYAO GRP
A method for preparing furoic acid by oxidation of furfural on a microchannel reactor
ActiveCN110845456BImprove mixing efficiencyEfficient mixingOrganic chemistryChemical/physical/physico-chemical microreactorsIce waterPhysical chemistry
The present invention relates to a method for preparing furoic acid by oxidation of furfural on a microchannel reactor. The reaction solution is obtained at the outlet; (2) Acidification, crystal growth, filtration and rinsing with ice water are performed to obtain a wet product of furoic acid; (3) vacuum drying is performed to obtain solid furoic acid. The present invention uses a microchannel reactor to better control the heat of reaction and realize the continuous production of furoic acid, with fast reaction speed and short residence time, the purity of the prepared furoic acid is 99.5% and above, and the yield is 97.6% and above .
Owner:浙江清和新材料科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com























![17-(5-ethyl-6-methylheptan-2-yl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl furan-3-carboxylate and extraction method and medicine application thereof 17-(5-ethyl-6-methylheptan-2-yl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl furan-3-carboxylate and extraction method and medicine application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b5245e08-5652-4113-8b01-d76c3bfd324e/2014107721379100002DEST_PATH_IMAGE001.PNG)
![17-(5-ethyl-6-methylheptan-2-yl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl furan-3-carboxylate and extraction method and medicine application thereof 17-(5-ethyl-6-methylheptan-2-yl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl furan-3-carboxylate and extraction method and medicine application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b5245e08-5652-4113-8b01-d76c3bfd324e/309262DEST_PATH_IMAGE002.PNG)
![17-(5-ethyl-6-methylheptan-2-yl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl furan-3-carboxylate and extraction method and medicine application thereof 17-(5-ethyl-6-methylheptan-2-yl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl furan-3-carboxylate and extraction method and medicine application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b5245e08-5652-4113-8b01-d76c3bfd324e/657249DEST_PATH_IMAGE001.PNG)