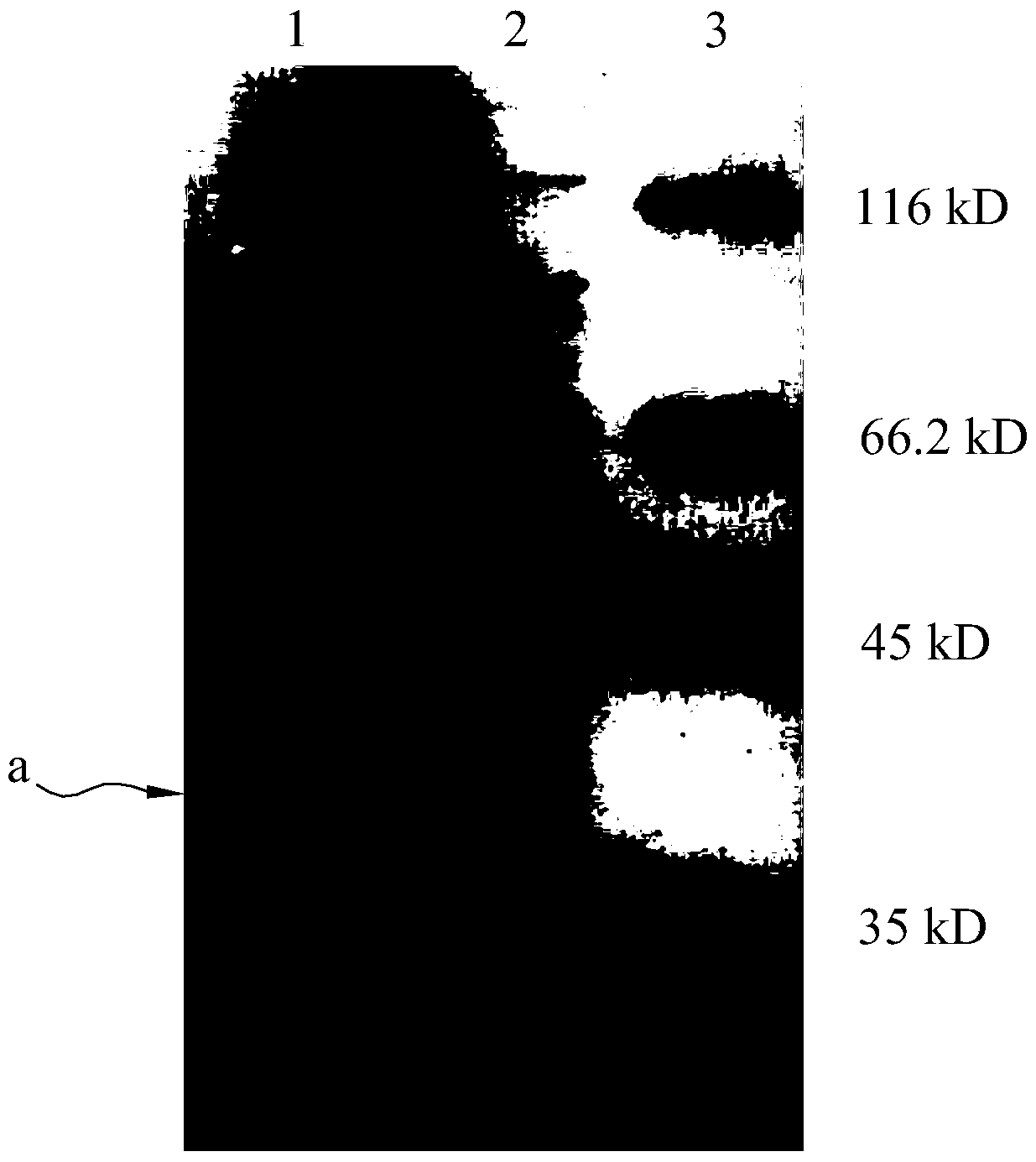Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
46results about How to "Simplify the cultivation procedure" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Potato isolated culture one-step seedling culture medium and optimization method and seedling method thereof
InactiveCN101790935AShort regeneration periodHigh reproductive coefficientHorticulture methodsPlant tissue cultureBud6-benzyladenine
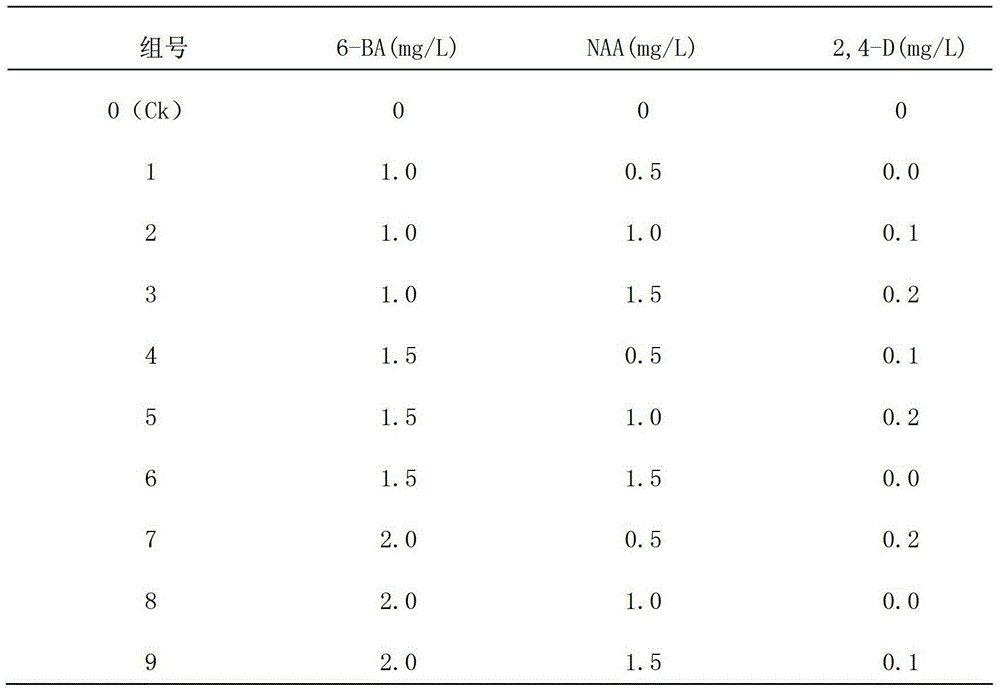
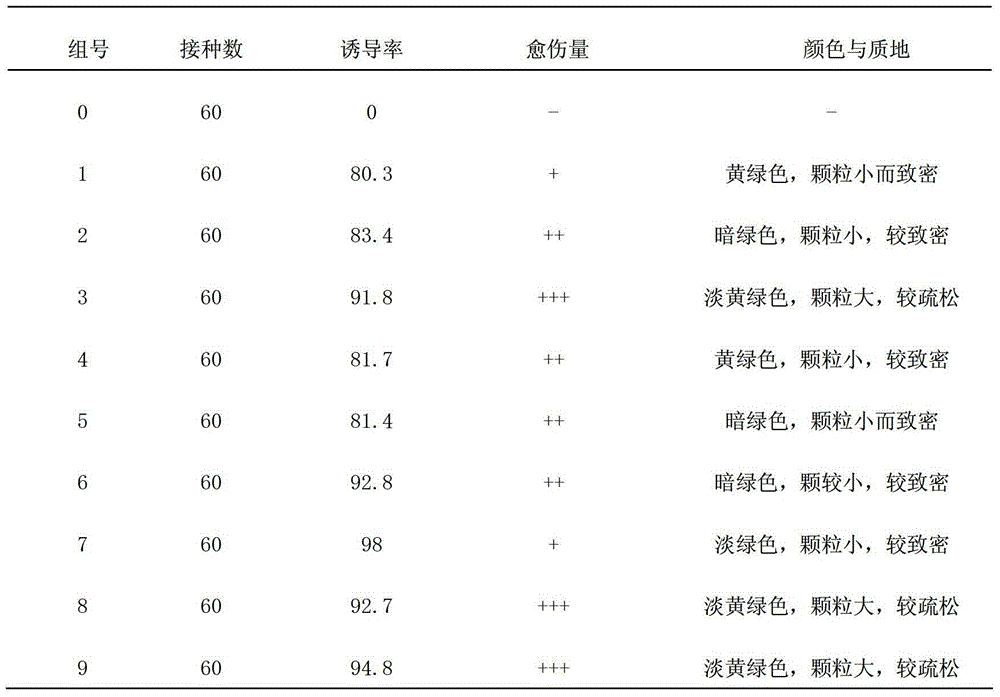
The invention discloses a method for optimizing a potato isolated culture one-step seedling culture medium. The culture medium makes leaves of a potato test tube plantlet undergo isolated culture to form a seedling in one step. The one-step seedling culture medium takes an MS culture medium as a basal medium, and 6-benzyladenine, naphthyl acetic acid and 2,4-dichlorphenoxyacetic acid with different concentrations and combinations are supplied in 1L of the MS culture medium; adventitious buds and adventitious roots are directly differentiated through callus induction; and the potato regenerated seedling is obtained in one step. The callus obtained by induction in a primary culture medium is unnecessarily inoculated to a differential medium for regenerating the seedling. Compared with an isolated culture multi-step regenerated seedling method, the method of the invention has the advantages of simplified steps, short culture period, high repeatability, and high seedling survival rate.
Owner:SICHUAN AGRI UNIV
Method for cultivating seedlings of polygonatum kingianum
InactiveCN107182780ARemove browningSimplify the cultivation procedurePlant tissue cultureHorticulture methodsBudRhizome
The method for raising seedlings of Rhizoma Polygonatum, the steps include: preparation of explants of Rhizoma Rhizoma Diannensis, culture for eliminating browning, callus induction, adventitious bud generation and proliferation culture, dark culture for promoting rhizome growth, rejuvenation culture of test-tube plantlets, rooting culture, hardening of seedlings Cultivation; the beneficial effect is: the Yunnan gold explants are carried out in the substratum A in the transfer culture of every 7 days, and the effect of removing the browning phenomenon occurring in the tissue culture process is remarkable; simultaneously carry out callus induction, cluster bud generation and Proliferation culture improves the reproduction efficiency, the reproduction coefficient can be increased to more than 8.67, and the tissue culture time can be shortened to 45 days; in the dark culture stage, the rhizome of Polygonatum yunnanensis is greatly improved, and the survival rate can reach more than 95%; the invention is divided into stages Four kinds of media are used for transfer culture, which overcomes the problem of easy breeding of fungi and accumulation of viruses when Polygonatum yunnanensis is used for group cultivation of seedlings. The cultivated seedlings have strong adaptability and stress resistance, which reduces the pollution rate of group cultured seedlings and improves To ensure the stability of species.
Owner:玉溪市祥馨农业技术开发有限公司
Coccidian oocyst culture preservative fluid and application method thereof
InactiveCN103374526AStrong in killing bacteriaViruses are strongProtozoaMicroorganism based processesAdditive ingredientCulture fluid
The invention belongs to the field of veterinary parasites, and relates to coccidian oocyst culture preservative fluid and an application method thereof. The invention is characterized in that the culture preservative fluid takes polyhaxemethylenguanidine hydrochloride as a main ingredient. The application method of the coccidian oocyst culture preservative fluid comprises the following steps of: separating and purifying fresh coccidian oocysts from fowl dung or intestinal tract tissues containing the coccidian oocysts; adding an appropriate amount of a polyhaxemethylenguanidine hydrochloride solution; adjusting the density of the oocysts; controlling the depth of a culture medium; putting the culture medium in a shaking table to perform shaking culture; performing microscopic examination; stopping culture when the sporulation rate of the oocysts exceeds 85%; collecting the oocysts; adding an appropriate amount of the polyhaxemethylenguanidine hydrochloride solution again; and preserving in a refrigerator of which the temperature is 4 DEG C. The coccidian oocyst culture preservative fluid is efficient, long-acting and safe, pollution-free to the environment, simple to prepare and low in cost, and is ideal coccidian oocyst culture preservative fluid. By applying the application method provided by the invention, the operation is simple and convenient, the efficiency is high, the automation can be realized, and the coccidian oocyst culture preservative fluid is suitable for large-scale culture preservation of coccidian oocysts.
Owner:SHANGHAI ACAD OF AGRI SCI
Open type simplified culture medium for taxus cuspidata
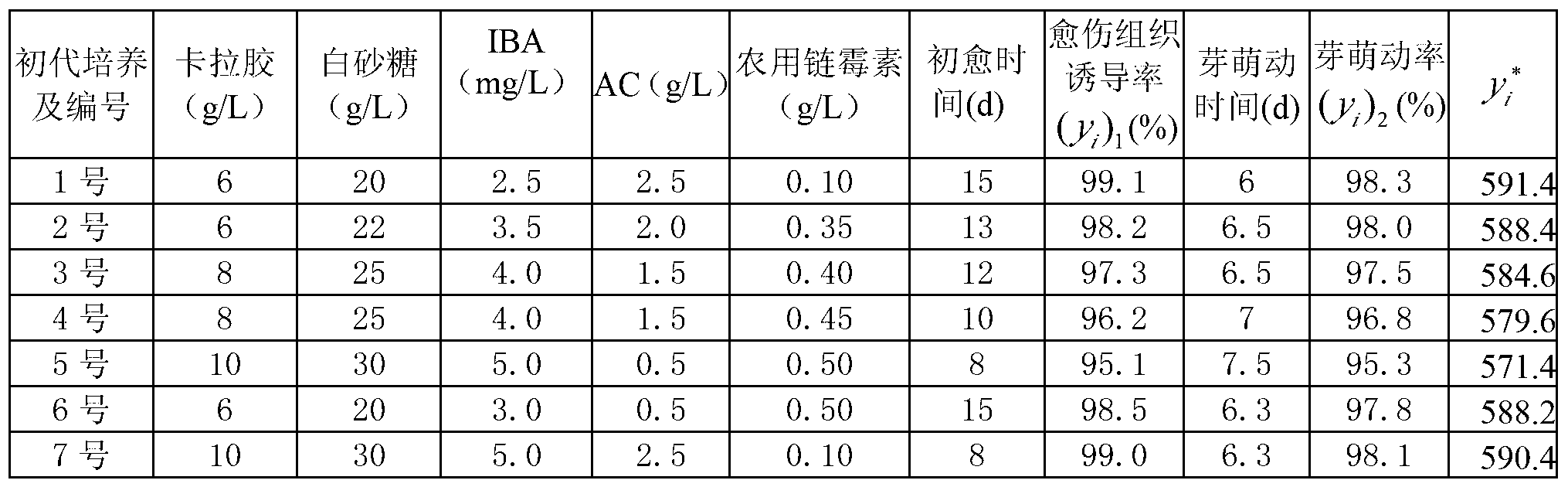
InactiveCN103238522AGood inhibitory effectEasy to produceHorticulture methodsPlant tissue cultureCarrageenanHigh pressure
The invention relates to an open type simplified culture medium for taxus cuspidate. The culture medium comprises a primary culture medium and a subculture medium. The primary culture medium is prepared by adding and mixing carrageenan, white granulated sugar, indolebutyric acid IBA, active carbon AC and agricultural streptomycin into optimized B5 basic mother liquor. The subculture is prepared by adding and mixing carrageenan, white granulated sugar, indolebutyric acid IBA, 6-benzyl amino adenine BA, active carbon AC and agricultural streptomycin into optimized MS basic mother liquor. According to the culture medium provided by the invention, with the adoption of open type simplified tissue culture, the cost and the inoculating time of the culture medium are remarkably saved, the autoclaved sterilization program is cancelled, and the production flow is simplified by replacing autoclaved sterilization and strict enclosed environment operation by a bacteriostatic agent, so that the labor and equipment investment in industrialized seedling production can be reduced, and the fund is saved by 30-50%.
Owner:JILIN UNIV
Method for directly inducing plant regeneration by using Vernicia fordii hypocotyl as explant
InactiveCN105613300ALittle researchImprove featuresHorticulture methodsPlant tissue cultureHypocotylVernicia fordii
The invention discloses a method for directly inducing plant regeneration by using Vernicia fordii hypocotyl as an explant, comprising the following steps: using hypocotyl of a Vernicia fordii seed aseptic seedling as an explant, directly inducing an adventitious bud, proliferating and elongation culturing the adventitious bud, and performing rooting culturing, domestication and transplanting to obtain a robust regenerated plant of Vernicia fordii. Compared with the prior art, the method has the advantages such as regeneration process simplicity, hormone addition singleness, operation convenience and relatively short regeneration cycle. A new technical means to rapidly propagate Vernicia fordii is provided, and also certain technical support is provided for genetically improving Vernicia fordii by genetic engineering means in future.
Owner:HEFEI INSTITUTES OF PHYSICAL SCIENCE - CHINESE ACAD OF SCI
Out-of-bottle bag rooting method for tissue culture seedlings of Europe and America hybrid aspens
InactiveCN102919128AReduce manufacturing costReduce overall man-hoursPlant tissue cultureHorticulture methodsGreenhousePeat
The invention relates to an out-of-bottle bag rooting method for tissue culture seedlings of Europe and America hybrid aspens. The method is to the problem that the cultivation cost is high, the production phase is long and the survival rate of transplanting of tissue culture seedlings is low in a tissue culture process of the Europe and America hybrid aspens. The method comprises the following steps: 1, plastically packaging the bottom of polypropylene plastic bags with a sealing machine to manufacture rooting culture bags; 2, mixing peat soils with sands, sterilizing at high speed to obtain composts and packing the composts into the rooting culture bags; 3, burrowing in the composts in the culture bags and wetting each hole with rooting culture solution; and 4, taking out the tissue culture seedlings of the Europe and America hybrid aspens from culture mediums, planting the tissue culture seedlings into the holes of the culture bags, sealing the top of the culture bags with the sealing machine, placing in greenhouses to culture for 5-7 days, gashing the top of the culture bags and then culturing for 8-10 days to obtain the rooting seedlings of the Europe and America hybrid aspens. The method is used in the field of asexual propagation of the Europe and America hybrid aspens.
Owner:NORTHEAST FORESTRY UNIVERSITY
One-step seedling and efficient in-vitro propagation method with gynura bicolor leaves
InactiveCN103202228APromote growthAuthentic qualityPlant tissue cultureHorticulture methodsBegoniaceaeGynura bicolor
The invention relates to a one-step seedling and efficient in-vitro propagation method for Begoniaceae gynura bicolor, which is an endangered plant with homology of medicine and food. The method provided by the invention comprises the following steps of: preparing a culture medium, and adjusting the culture medium through preparing, subpackaging and sterilizing; carrying out sterile inoculating directly on gynura bicolor aseptic seedling leaves which are taken as explants; culturing the inoculated culture medium in a culture chamber for 70-80 days under appropriate conditions, wherein the number of a single explant induced seedlings is 20 above; and decapping, transplanting regenerating plants to appropriate conditions, and culturing without hardening-seedling, wherein the commodity seedling survival rate achieves 100%. According to the method provided by the invention, the gynura bicolor tissue culture seedling regeneration period is short; the original seed property is kept effectively; the vegetative propagation coefficient is improved obviously; the operation is simple and convenient; the culture program is simple; the seedling cost is lowered obviously; and the method can act as an effective technology of industrialized production of high-quality seedlings of Begoniaceae gynura bicolor.
Owner:ZHAOQING UNIV
One-step plantlet formation medium and method for isolated culture of lamiophlomis rotata
InactiveCN103461138AShort regeneration periodHigh reproductive coefficientPlant tissue cultureHorticulture methodsBudMicrobiology
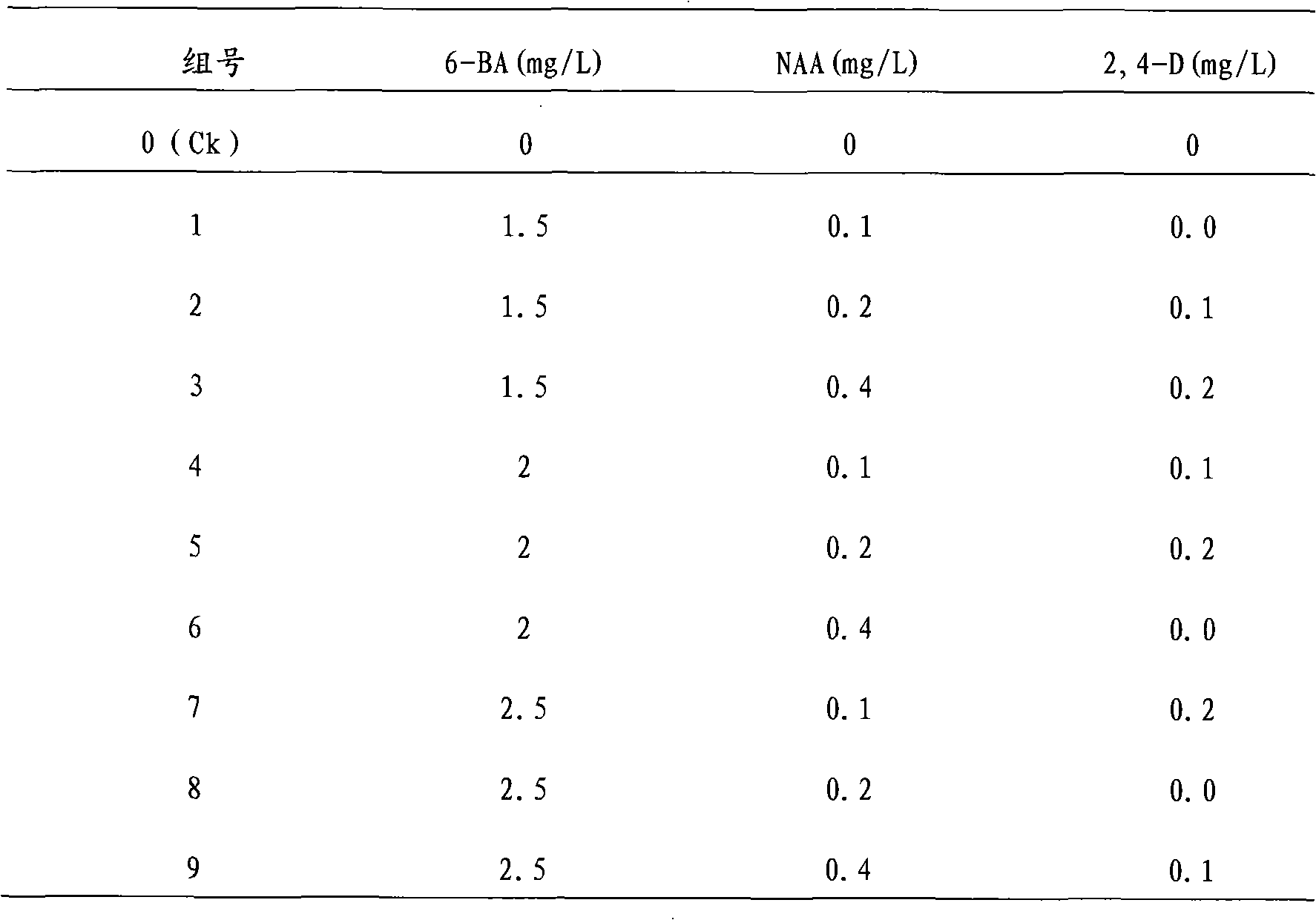
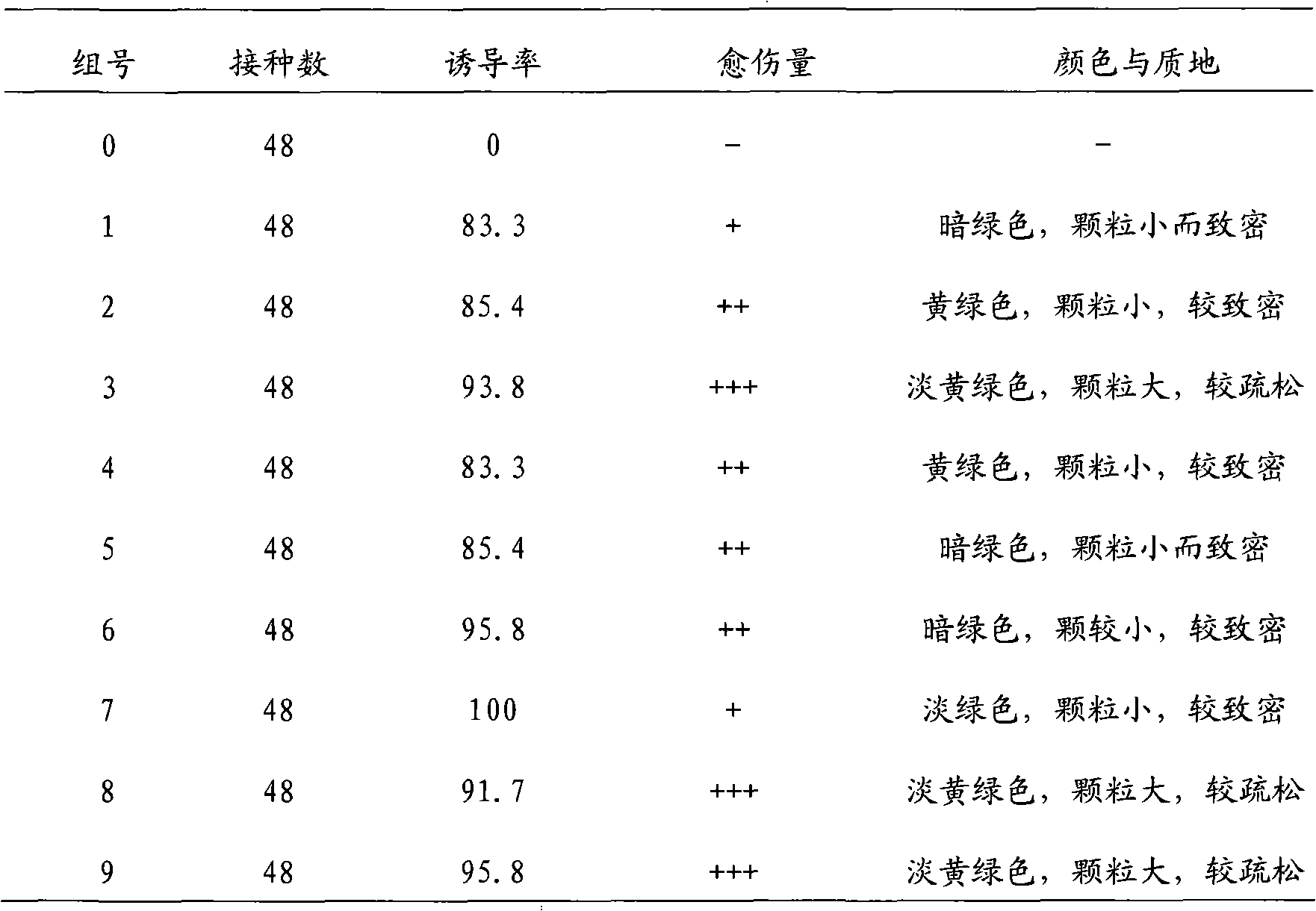
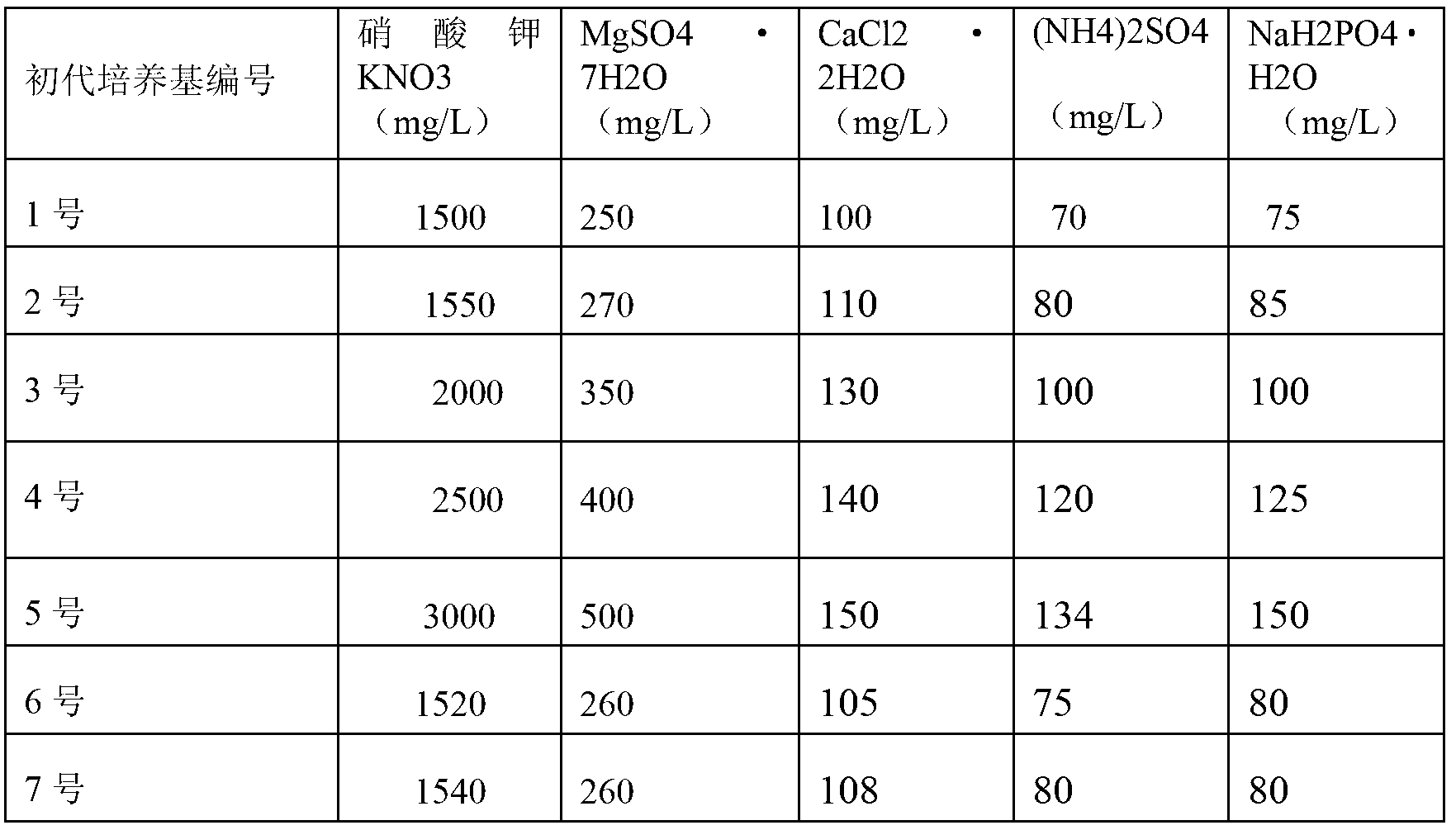
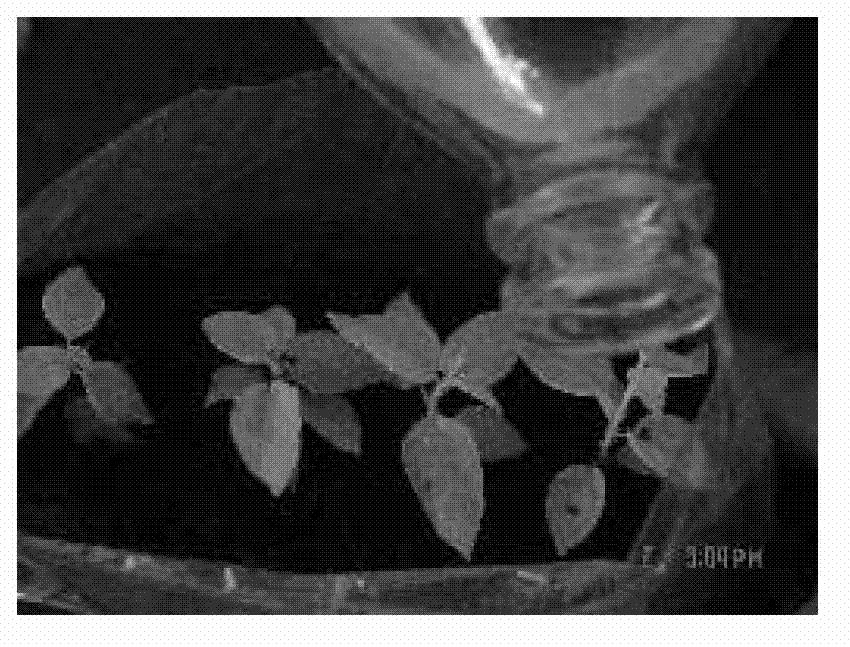
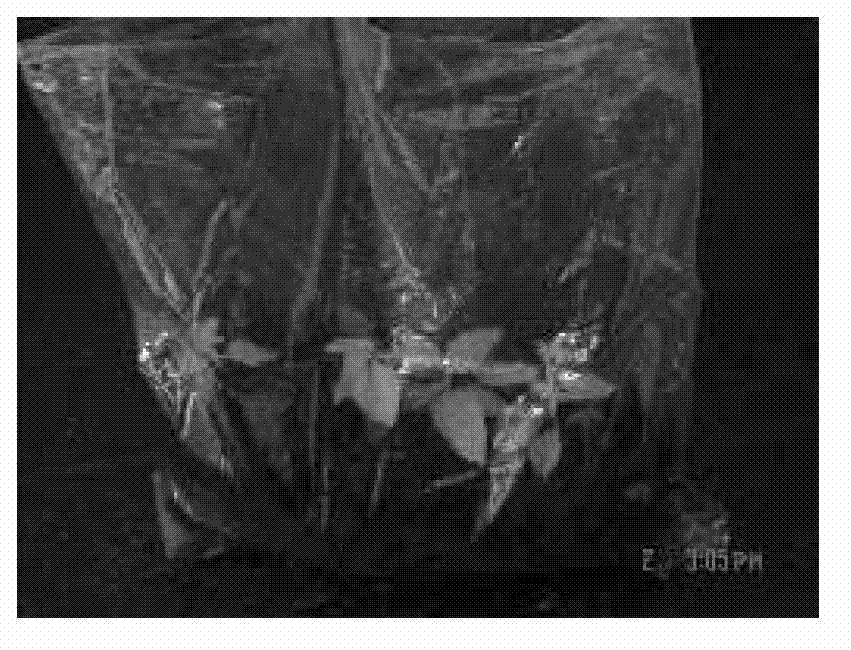
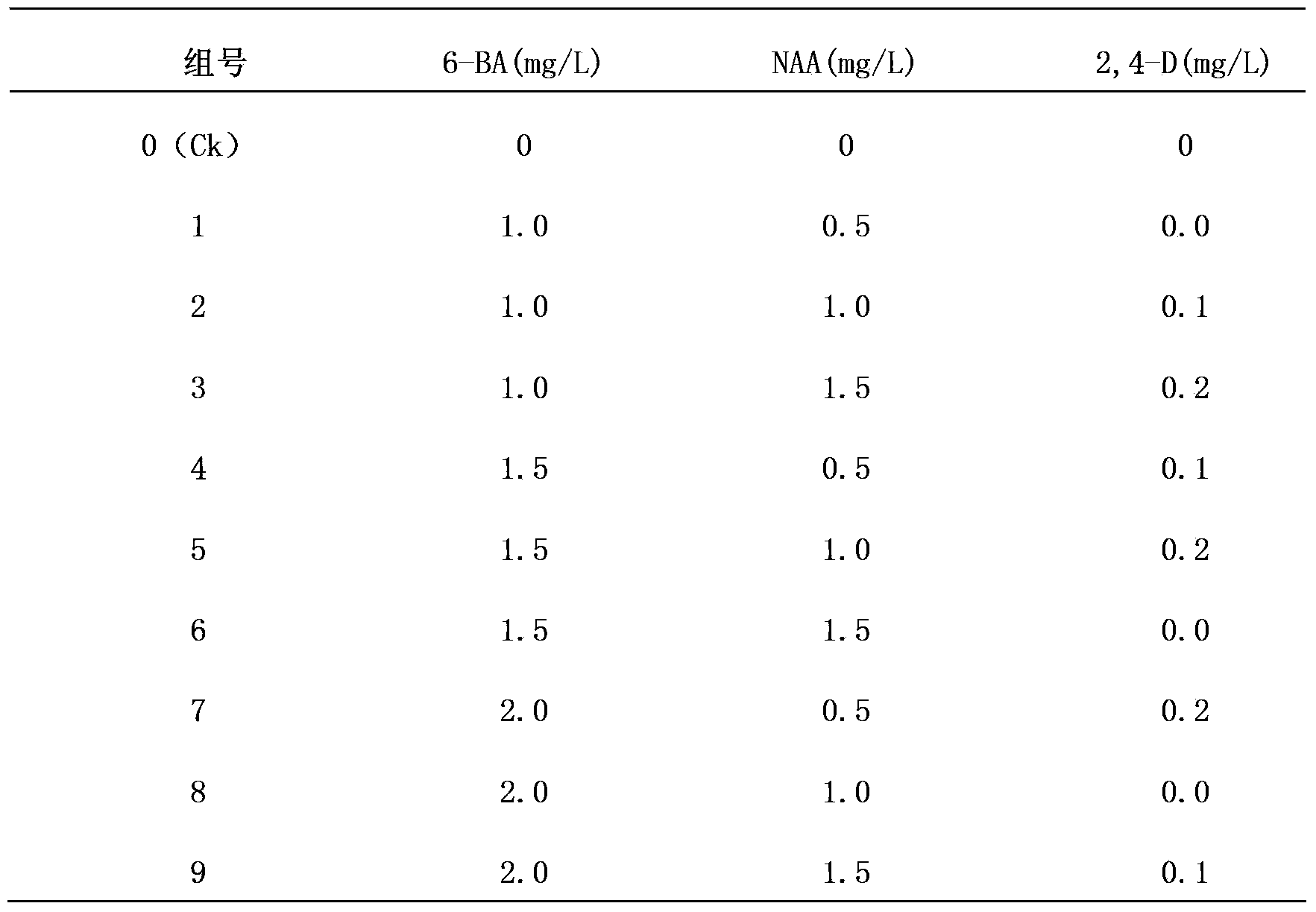
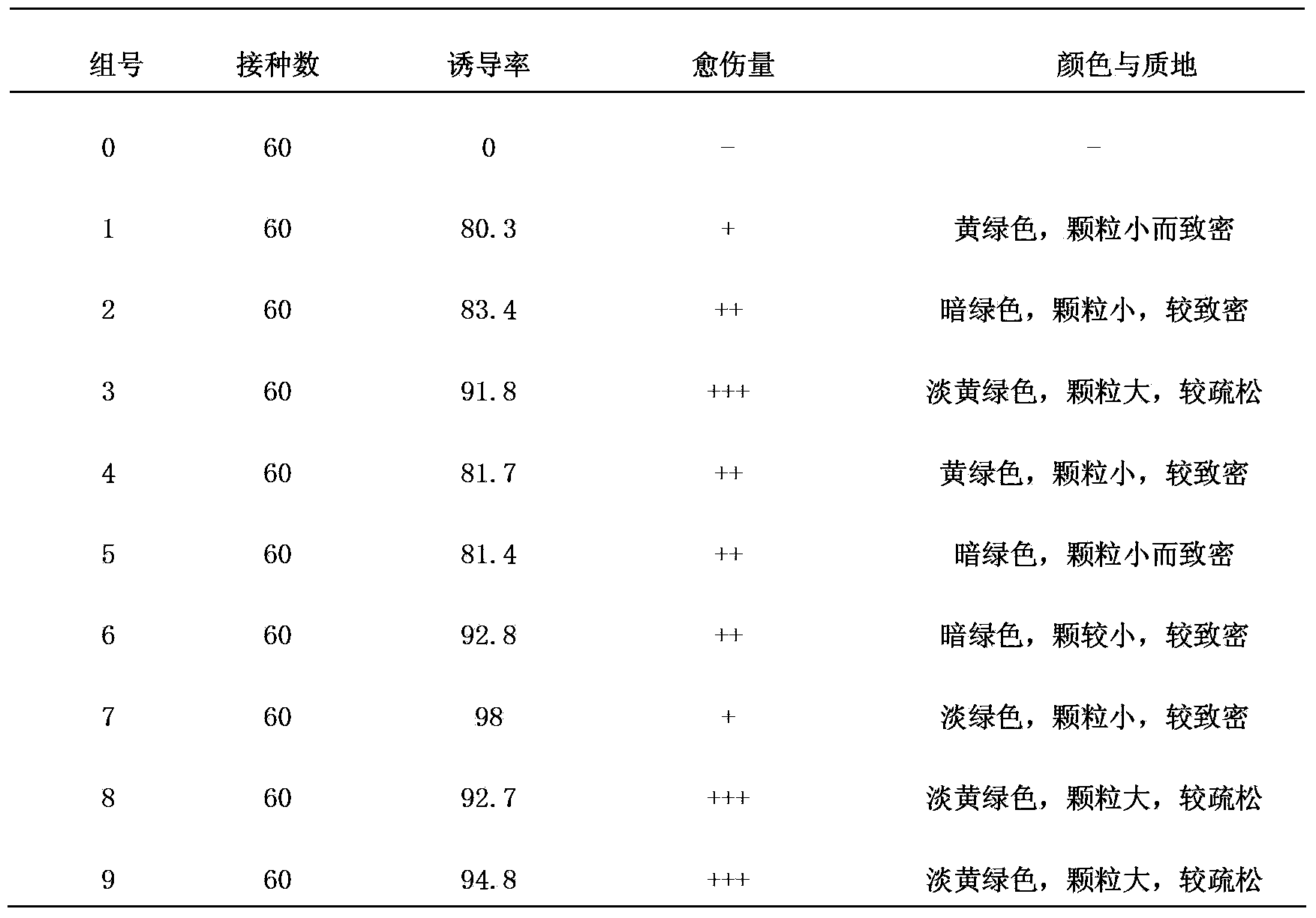
The invention discloses a one-step plantlet formation medium and method for the isolated culture of lamiophlomis rotata. By adopting the one-step plantlet formation medium, a lamiophlomis rotata test-tube plantlet leaf forms a plantlet in one step through isolated culture. The one-step plantlet formation culture medium is characterized in that a MS (Murashige and Skoog) medium is used as a basic medium, and 6-benzylaminopurine (6-BA), naphthylacetic acid (NAA) and 2,4-dichlorphenoxyacetic acid (2,4-D) which are different in concentrations and combinations are added to the base medium. According to the one-step plantlet formation method, the test-tube plantlet leaf serving as an explant is inoculated, then is induced by a callus and is directly differentiated into an adventitious bud and an adventitious root, so that the lamiophlomis rotata regenerated plantlet is obtained by one step. By adopting the one-step plantlet formation method, the callus obtained by induction in an initial medium does not need to be transferred into a differential medium for regenerating the plantlet. Compared with an isolated culture multi-step regeneration plantlet formation method, the one-step plantlet formation method has the advantages that the steps are simplified, the culture period is short, the repeatability is good, and the planting percent is high.
Owner:SICHUAN AGRI UNIV
Method for polyploidy induction and efficient artificial seedling culture of polyploidy plants of campanumoea lancifolia
ActiveCN113519394AShorten the cultivation cycleEnhanced Physiological and Biochemical ProcessesHorticulture methodsPlant tissue cultureBiotechnologyColchicine
The invention provides a method for polyploidy induction and efficient artificial seedling culture of polyploidy plants of campanumoea lancifolia, which comprises the following steps: (1) inoculating disinfected and sterilized seeds into a culture medium A for sterile germination; (2) soaking stem tips with leaves of sterile seedlings in a sterilized colchicine solution, then transferring the stem tips into a culture medium B for culture, and then transferring the stem tips into a culture medium C; and (3) completing proliferative rooting of polyploidy plants in the culture medium C in one step. According to the method, the in-vitro rapid propagation process of the campanumoea lancifolia polyploidy plant is optimized and adjusted, proliferation and rooting are synchronously carried out, the tissue culture process is simplified, the whole culture process is carried out in one culture medium at the same time, and the artificial in-vitro propagation efficiency is greatly improved. In addition, a campanumoea lancifolia homologous polyploidy induction system is established; under the condition of delayed polyploidy growth, a direct organ generation mode is adopted for proliferation, meanwhile, a high propagation coefficient is kept, the culture period is greatly shortened, the cost is low, the period is short, and the quality and the survival rate are high.
Owner:YUNNAN UNIV OF TRADITIONAL CHINESE MEDICINE
Culture medium for culturing antrodia camphorata by vessel culturing and preparation method thereof
ActiveCN106866235AImprove controllabilityReduce pollutionMagnesium fertilisersAlkali orthophosphate fertiliserPhosphateMonopotassium phosphate
The invention discloses a culture medium for culturing antrodia camphorata by vessel culturing and a preparation method thereof. The culture medium is prepared from the following raw materials in parts by weight: 200 to 300 parts of potato leaching powder, 20 to 40 parts of glucose, 0 to 20 parts of soluble soybean meal, 1 to 20 parts of malt extractant, 0.5 to 1.5 parts of magnesium sulfate, 10 to 60 parts of a mixture of a cinnamomum plant, 0.5 to 1.5 parts of potassium dihydrogen phosphate, 0.01 to 0.05 part of vitamin B1, 0 to 0.1 part of chloramphenicol, 15 to 25 parts of agar and 1,000 parts of ultrapure water. According to the culture medium disclosed by the invention, a culturing vessel is used for culturing the antrodia camphorata; the culturing condition has strong controllability; the culturing procedure is simplified; the sundry bacteria contamination can be reduced; the heavy metal pollution is avoided too; the growth increment of antrodia camphorata mycelium or sporocarp is increased as well; and the production cost is lowered.
Owner:SUBTROPICAL CROPS INST OF FUJIAN PROVINCE
Artificial efficient propagation method of aristolochia tuberosa
ActiveCN112167060AProliferate fastSimplify the cultivation procedureClimate change adaptationPlant tissue cultureAxillary budGenotype
The invention discloses an artificial efficient propagation method of aristolochia tuberosa. The method comprises the following steps of inoculating a disinfected and sterilized explant with a bud stem segments of the aristolochia tuberosa into a culture medium A for culture, starting propagation culture under the conditions that the illuminance is 1500-2000lx, the illumination time is 10h / d and the temperature is controlled to be 22 + / -1 DEG C, and obtaining a propagation coefficient of 3.57 after 35 days. A material is shorn into the stem segments containing 2-3 joints, the stem segments areinoculated into a culture medium B to be cultured, a clustered cluster system with base adventitious cluster buds and upper axillary buds growing is formed after 45 days, and the propagation coefficient reaches 10.0 or above. When the height of proliferated cluster buds is 3-4cm, single seedlings are inoculated into the culture medium B to be cultured for 45d to obtain rooted seedlings with strong seedlings and thick roots, and then the rooted seedlings are transplanted. The method is low in cost, short in period and high in test-tube plantlet quality and survival rate, provides technical support for protecting wild resources and breeding high-quality plantlets, and can be used for producing excellent plantlets with consistent genotype backgrounds in a short time.
Owner:YUNNAN UNIV OF TRADITIONAL CHINESE MEDICINE
Method and application for inducing direct somatic embryogenesis
ActiveCN109652424AEasy to operateSimplify the cultivation procedureOxidoreductasesPlant peptidesSomatic embryogenesisGermplasm
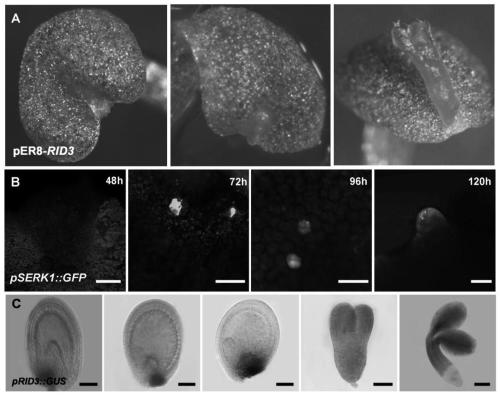
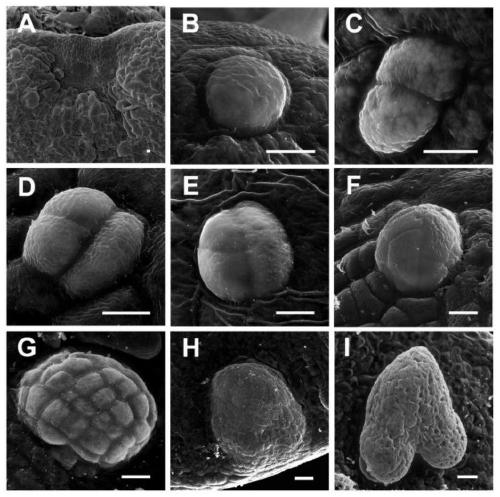
The invention discloses a method and application for inducing direct somatic embryogenesis. According to the method and application, function genes RID3(AT3G49180) and YUC10(AT1G48910) capable of promoting direct somatic embryogenesis are found. Through ectopic expression of the function genes, inducing can be directly conducted on arabidopsis cotyledons to form the somatic embryo without any callus. The method for inducing direct somatic embryogenesis has the advantages of being simple in culture, short in inducing period, high in inducing frequency and the like, and can be used for study onaspects of differentiation, development and totipotency expression of botanical cells, crop variety improvement, production of artificial seeds, storage of high-quality germplasm resources, mutant screening and the like.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
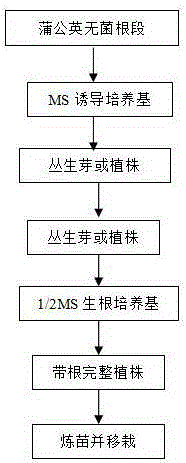
Method for establishing efficient regeneration system by taking dandelion roots as explants
The invention relates to a method for establishing an efficient regeneration system by taking medical and edible plant dandelion roots as explants. The method comprises the following steps: (1) putting the dandelion roots in an MS medium to induce one-step culture; (2) putting robust cluster buds or plants into a 1 / 2MS rooting medium, and performing cultivation to obtain complete seedlings with roots; (3) exercising the complete seedlings with the roots, putting the complete seedlings with the roots into a substrate to grow for 20 to 30d, and then transplanting the complete seedlings with the roots to a large field. The seedlings obtained by using the method are robust and high in survival rate, and a great number of high-quality seedlings of dandelion can be provided within a short time.
Owner:NINGXIA ACADEMY OF AGRI & FORESTRY SCI
Serum-free culture solution for in-vitro culture of sheep ovarian cortex tissue
InactiveCN112458041AMeet basic growth needsInhibition of proliferation rateCell dissociation methodsCulture processPenicillinGranular cell
The invention discloses a serum-free culture solution for in-vitro culture of sheep ovarian cortex tissue. The culture solution comprises a basic culture solution and an additive, wherein the basic culture medium is a mixed solution of alpha-MEM and DMEM; the additive comprises the following components: 3-10 micrograms / mL bovine serum albumin, 0.10-0.50mM sodium pyruvate, 1-5mM glutamine, 1-5mM hypoxanthine, 3-10 micrograms / mL insulin, 1-5 micrograms / mL transferrin, 2-8 nanograms / mL sodium selenite, 10-200 micrograms / mL VC, 50-200IU / mL penicillin and 50-200IU / mL streptomycin, and the pH valueis 6.9-7.5. Through the synergistic effect of the components, the proliferation speed of interstitial cells is inhibited, the proliferation of granular cells is promoted, the defect that the three-dimensional environment of original follicle development cannot be maintained due to tissue collapse caused by excessive proliferation of the interstitial cells in the culture process of the ovarian cortex tissue is overcome, the growth and development condition and survival time of original follicles in the ovarian cortex tissue in vitro are effectively improved, meanwhile, the influence of unknowncomponents in serum on research is avoided, and the serum-free culture solution has a good application prospect.
Owner:SHIHEZI UNIVERSITY
Rapid reproduction method of schlumbergera seedlings
InactiveCN101940160AOvercome the difficulty of not being able to carry out production on an annual basisSave land resourcesPlant tissue cultureHorticulture methodsSchlumbergeraCallus formation
The invention provides a rapid reproduction method of schlumbergera seedlings. The method comprises the following steps: inoculating a selected, disinfected and sterilized schlumbergera explant onto a culture medium for induction and carrying out synchronous induction and proliferation culture until the explant sprouts and differentiates out a bud; sub-culturing until being proliferated to 3-4 folds, and then transferring to a rooting medium for rooting culture until 3-5 fine roots are grown at the base of a plant; conventionally hardening and then transplanting to a mixed substrate of humus soil, red soil and pearlite; and watering and fertilizing conventionally for growing to obtain the schlumbergera seedlings. In the method, key links in the whole production technical flow of the schlumbergera seedlings are optimized and integrated, the culture medium for induction is blended and bud induction and proliferation culture are carried out synchronously, thus simplifying tissue culture procedure and shortening breeding cycle; and only by adopting two types of the culture media, the bud can be directly formed without callus formation, thus reducing probability of variation occurred on an adventitious bud, effectively controlling generation of a variant strain, being capable of fixing and maintaining fine variety of the schlumbergera seedlings rapidly, improving seedling quality and solving the problems of complicated parental source and unstable quality of the seedlings produced by the conventional breeding system.
Owner:FLOWER RES INST OF YUNNAN ACAD OF AGRI SCI
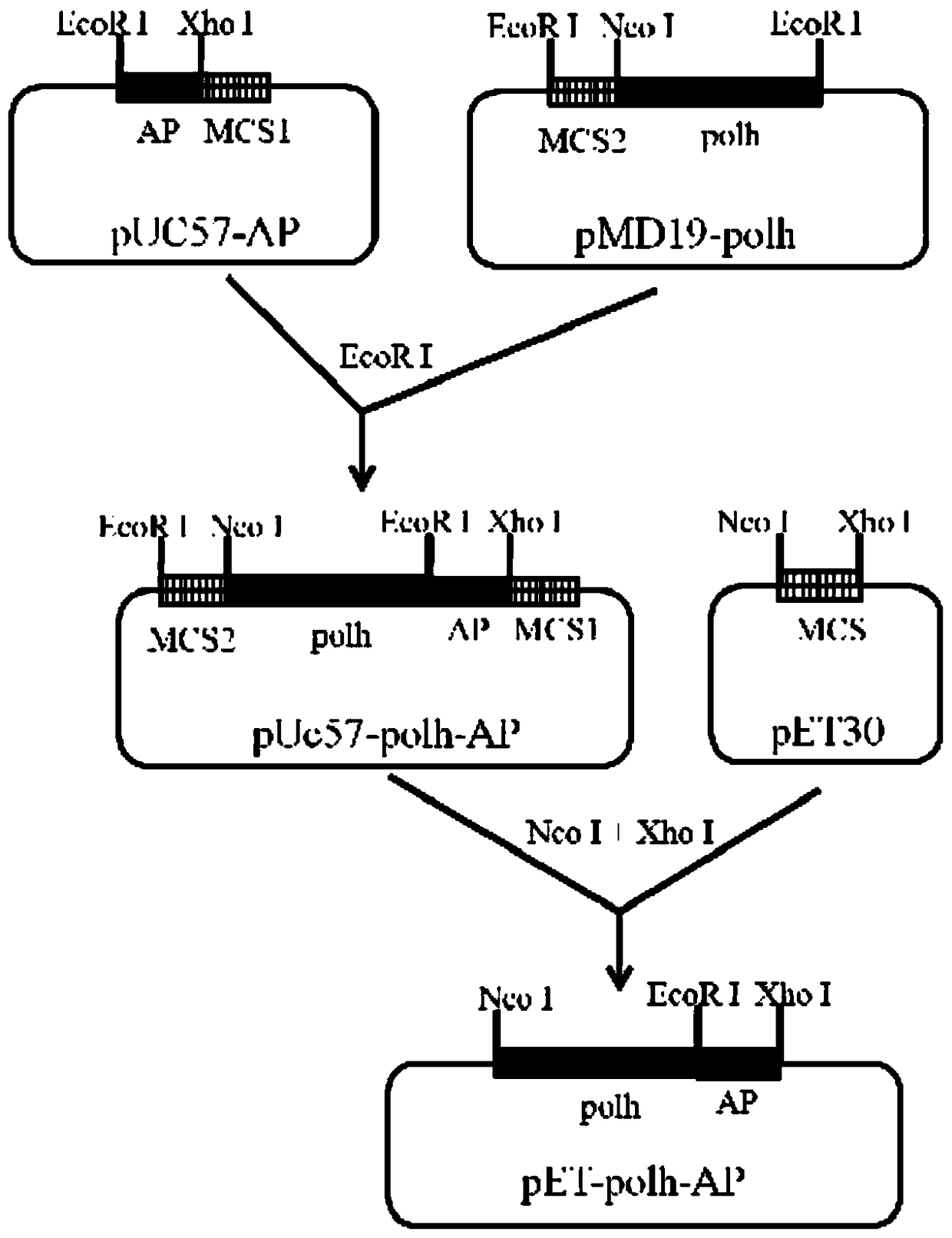
Method for expressing antimicrobial peptide apidaecin and preparing antimicrobial peptide apidaecin using Escherichia coli
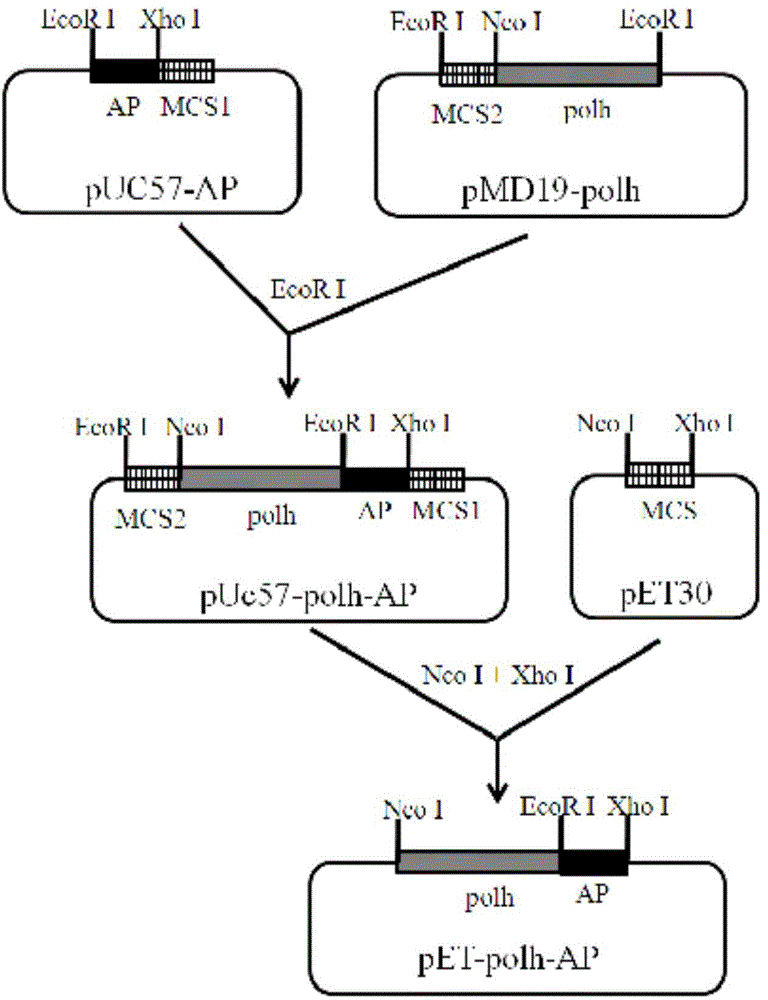
ActiveCN104805109BSimplify the cultivation procedureReduce manufacturing costHybrid peptidesVector-based foreign material introductionEscherichia coliInclusion bodies
The invention relates to a method for expressing an antibacterial peptide apidaecin by using Escherichia coli and for preparing the antibacterial peptide apidaecin. The method comprises the following steps: cloning AP2 gene and a polyhedron gene sequence polh into a recombinant vector in order to form a recombinant vector with a polh-AP fusion gene fragment; cloning the polh-AP fusion gene fragment into an expression vector, converting the expression vector to an expression host cell, and culturing the expression host cell to express a fusion protein with an antibacterial peptide AP2; and culturing the expression host cell to induce the expression of the recombinant protein polh-AP, centrifuging induced bacterial strains, collecting, re-suspending, carrying out ultrasonic fragmentation, centrifuging, collecting the above obtained insoluble inclusion body, purifying, and collecting a recombinant protein sample. An expression system has the characteristics of simple expression system culture program, low production cost, efficient expression of the antibacterial peptide apidaecin, and realization of AP2 and polh fusion expression, so compared with the prior art, the method has the advantages of effective reduction of toxicity of the AP2 to Escherichia coli, improvement of the stability of the antibacterial peptide, realization of high level expression of the antibacterial peptide, convenient purification of the active antibacterial peptide, and improvement of the output of the antibacterial peptide apidaecin.
Owner:福建旭牧联生物科技有限公司
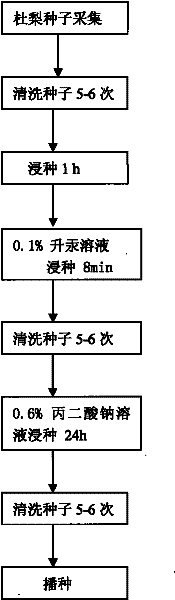
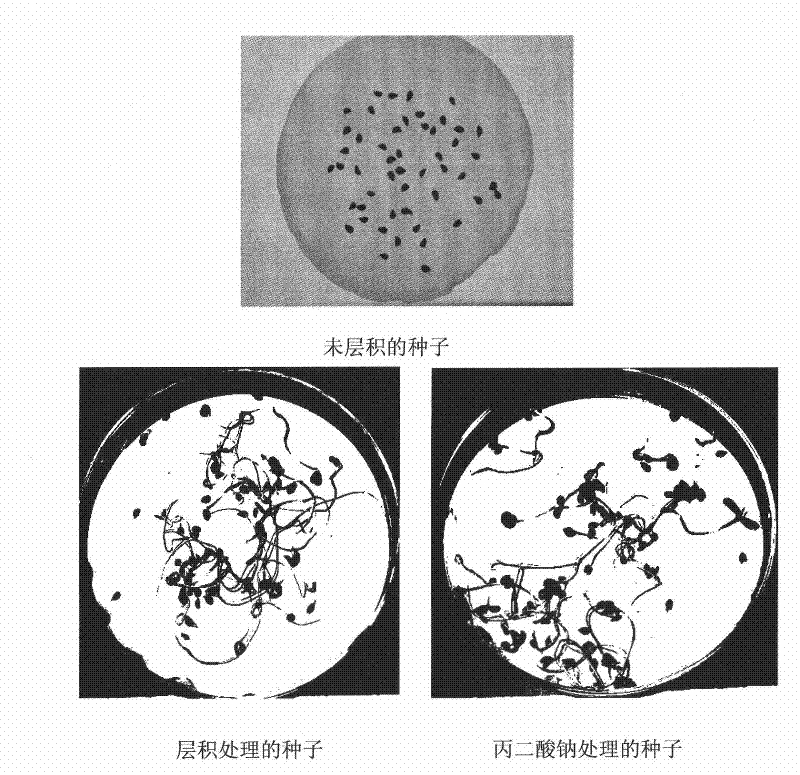
Method for promoting germination through release of pyrus betulaefolia seed dormancy by sodium malonate
InactiveCN101589666BShorten seedling timeSimplify the cultivation procedureBiocideAnimal repellantsPyrus betulaefoliaSeed dormancy
Owner:NANJING AGRICULTURAL UNIVERSITY
Method for expressing antibacterial peptide apidaecin by using Escherichia coli and for preparing antibacterial peptide apidaecin
ActiveCN104805109ASimplify the cultivation procedureReduce manufacturing costHybrid peptidesVector-based foreign material introductionEscherichia coliInclusion bodies
The invention relates to a method for expressing an antibacterial peptide apidaecin by using Escherichia coli and for preparing the antibacterial peptide apidaecin. The method comprises the following steps: cloning AP2 gene and a polyhedron gene sequence polh into a recombinant vector in order to form a recombinant vector with a polh-AP fusion gene fragment; cloning the polh-AP fusion gene fragment into an expression vector, converting the expression vector to an expression host cell, and culturing the expression host cell to express a fusion protein with an antibacterial peptide AP2; and culturing the expression host cell to induce the expression of the recombinant protein polh-AP, centrifuging induced bacterial strains, collecting, re-suspending, carrying out ultrasonic fragmentation, centrifuging, collecting the above obtained insoluble inclusion body, purifying, and collecting a recombinant protein sample. An expression system has the characteristics of simple expression system culture program, low production cost, efficient expression of the antibacterial peptide apidaecin, and realization of AP2 and polh fusion expression, so compared with the prior art, the method has the advantages of effective reduction of toxicity of the AP2 to Escherichia coli, improvement of the stability of the antibacterial peptide, realization of high level expression of the antibacterial peptide, convenient purification of the active antibacterial peptide, and improvement of the output of the antibacterial peptide apidaecin.
Owner:福建旭牧联生物科技有限公司
A kind of culture method of Rhodiola rosea aseptic seedling with well-developed root system
InactiveCN103858756BReduce experiment costSimplify the cultivation procedureHorticulture methodsPlant tissue cultureBiotechnologyRHODIOLA ROSEA ROOT
The invention relates to a method for cultivating Rhodiola rosea aseptic seedlings with well-developed root system, and belongs to the field of modern biological methods. The method comprises the following steps: (1), collecting wild Rhodiola rosea seedlings; (2), disinfecting the collected stems of Rhodiola rosea wild seedlings; (3), collecting young rhodiola rosea alpine stems (4) preparation of culture medium; (5) cultivation of aseptic seedlings of Rhodiola rosea with well-developed root system. The present invention overcomes the disadvantages and deficiencies such as weak seedling growth, difficult rooting, less root number, and short seedling age in the existing Rhodiola rosea tissue culture process, and provides a method for cultivating sterile seedlings of Rhodiola rosea with well-developed root system, In order to obtain high-quality regenerated plants of Rhodiola rosea.
Owner:JILIN NORMAL UNIV
A kind of dish culture medium of Antrodia camphorata and preparation method thereof
ActiveCN106866235BImprove controllabilityReduce pollutionMagnesium fertilisersAlkali orthophosphate fertiliserBiotechnologyMonopotassium phosphate
Owner:SUBTROPICAL CROPS INST OF FUJIAN PROVINCE
A method and application for inducing direct generation of somatic embryos
ActiveCN109652424BEasy to operateSimplify the cultivation procedureOxidoreductasesPlant peptidesSomatic embryogenesisGermplasm
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Tissue cultivaition method of vetiveria zizanioides transplant
InactiveCN102197786BOvercome the difficulty of not being able to carry out production on an annual basisSave land resourcesHorticulture methodsPlant tissue cultureChrysopogon zizanioidesPlantlet
Owner:FLOWER RES INST OF YUNNAN ACAD OF AGRI SCI
One-step seedling and efficient in-vitro propagation method with gynura bicolor leaves
InactiveCN103202228BPromote growthAuthentic qualityHorticulture methodsPlant tissue cultureBegoniaceaeGynura bicolor
The invention relates to a high-efficiency in vitro propagation method for one-step seedling formation of an endangered plant of the begoniaceae begonia family. The method of the present invention comprises: medium preparation, adopting the adjusted medium, including preparation, packaging and sterilization; adopting the leaves of aseptic seedlings of G. purple-backed aseptic seedlings as explants, directly carrying out aseptic inoculation; The culture bottles of good seeds are moved into the culture room, cultivated under suitable conditions for 70-80 days, and the number of seedlings induced by a single explant is more than 20; without hardening, the regenerated plants are opened and transplanted to suitable conditions for culture. The survival rate of commercial seedlings reaches 100%. According to the method of the present invention, the tissue culture of G. purple-backed seedlings has a short regeneration period, effectively maintains the characteristics of the original species, significantly improves the asexual reproduction coefficient, and is easy to operate, simple in the training procedure, and obviously reduces the cost of raising seedlings. An effective technology for the industrial production of high-quality seedlings of Heliotrope chinensis.
Owner:ZHAOQING UNIV
Culture method of aseptic Rhodiola sachalinensis A.Bor. seedling having developed roots
InactiveCN103858756AReduce experiment costSimplify the cultivation procedurePlant tissue cultureHorticulture methodsRhodiola sachalinensisPlantlet
The invention relates to a culture method of an aseptic Rhodiola sachalinensis A.Bor. seedling having developed roots and belongs to the field of modern biological methods. The culture method comprises the following steps of 1, acquiring a wild Rhodiola sachalinensis A.Bor. seedling, 2, carrying out disinfection treatment on stem of the acquired wild Rhodiola sachalinensis A.Bor. seedling, 3, carrying out cutting treatment on the young stem of Rhodiola sachalinensis A.Bor. seedling, 4, preparing a medium, and 5, carrying out culture of the aseptic Rhodiola sachalinensis A.Bor. seedling having developed roots. The culture method solves the problem that in the existing Rhodiola sachalinensis A.Bor. tissue culture, Rhodiola sachalinensis A.Bor. seedling growth is weak, root production is difficult, the number of roots is less and seedling age is short. Through the culture method of the aseptic Rhodiola sachalinensis A.Bor. seedling having developed roots, a high-quality Rhodiola sachalinensis A.Bor. regenerated plant is obtained.
Owner:JILIN NORMAL UNIV
A kind of one-step seedling growth medium and method for in vitro culture of unique flavor
InactiveCN103461138BShort regeneration periodHigh reproductive coefficientPlant tissue cultureHorticulture methodsLamiophlomis rotataBud
The invention discloses a one-step plantlet formation medium and method for the isolated culture of lamiophlomis rotata. By adopting the one-step plantlet formation medium, a lamiophlomis rotata test-tube plantlet leaf forms a plantlet in one step through isolated culture. The one-step plantlet formation culture medium is characterized in that a MS (Murashige and Skoog) medium is used as a basic medium, and 6-benzylaminopurine (6-BA), naphthylacetic acid (NAA) and 2,4-dichlorphenoxyacetic acid (2,4-D) which are different in concentrations and combinations are added to the base medium. According to the one-step plantlet formation method, the test-tube plantlet leaf serving as an explant is inoculated, then is induced by a callus and is directly differentiated into an adventitious bud and an adventitious root, so that the lamiophlomis rotata regenerated plantlet is obtained by one step. By adopting the one-step plantlet formation method, the callus obtained by induction in an initial medium does not need to be transferred into a differential medium for regenerating the plantlet. Compared with an isolated culture multi-step regeneration plantlet formation method, the one-step plantlet formation method has the advantages that the steps are simplified, the culture period is short, the repeatability is good, and the planting percent is high.
Owner:SICHUAN AGRI UNIV
A kind of high-efficiency artificial seedling raising method of Passiflora high-quality hybrid
ActiveCN110521598BOvercome the difficulty of not being able to carry out production on an annual basisSave land resourcesHorticulture methodsPlant tissue culturePassiflora serratifoliaPlantlet
The invention provides a high-efficiency artificial seedling-raising method of high-quality passionflower hybrids, which comprises the steps of: inoculating the sterilized leaf explants in a culture medium, inducing callus, forming clustered buds and proliferating , rooting induction, seedling hardening and transplanting. The present invention optimizes and adjusts the production process of hybrid seedlings of passionflower, synchronously carries out callus growth, cluster bud generation and cluster bud proliferation, simplifies the tissue culture process, and carries out three culture processes in one culture medium at the same time , greatly improving the reproductive efficiency. In addition, the invention solves the phenomena such as plant yellowing and leaf defoliation that are easy to occur in the tissue culture process of Passiflora plants, and has low cost, short cycle, high quality and high survival rate.
Owner:YUNNAN UNIV OF TRADITIONAL CHINESE MEDICINE
One-step seedling rapid propagation method for tissue culture of Chinese cabbage
ActiveCN104255532BShort regeneration periodHigh reproductive coefficientHorticulture methodsPlant tissue culturePeatBottle
The invention discloses a one-step seedling rapid propagation method for tissue culture of Chinese cabbage, which belongs to the technical field of plant tissue culture. The invention solves the problems of low coefficient, slow growth and difficulty in large-scale production in conventional propagation methods of the cabbage. The method is carried out according to the following steps: Step 1, disinfection and sterilization; Step 2, one-step seedling cultivation; Step 3, domestication and transplanting of test-tube seedlings: take out the rooted seedlings and put them in a seedling hardening room with sufficient light After 3 to 5 days, open the bottle mouth and continue to harden the seedlings for 2 to 3 days; then take out the test tube seedlings, wash the root culture medium, and transplant them into the substrate. The substrate is composed of peat soil and vermiculite, and the weight of peat soil and vermiculite The ratio of parts is 1:1, and the seedlings are sprayed 2-3 times a day for the first 7 days. The invention is used for tissue culture of Chinese cabbage.
Owner:RIZHAO POLYTECHNIC
A kind of method for tissue culture rapid propagation of dendrobium nobile
ActiveCN103598101BOvercome the difficulty of not being able to carry out production on an annual basisSave land resourcesHorticulture methodsPlant tissue cultureBudObserved Survival
Owner:FLOWER RES INST OF YUNNAN ACAD OF AGRI SCI
A kind of culture medium for cultivating the mother species of wild reticulata fringe
InactiveCN109566271BEarly germinationFast growthCultivating equipmentsMushroom cultivationBiotechnologyPaxillus involutus
The invention discloses a medium for cultivating a wild paxillus involutus stock culture. The medium is prepared from, by mass, 15-25g of soluble starch, 2-6g of peptone, 1-5g of KH2PO4, 0.5-4.5g of MgSO4, 1L of water and 2-6 of pH. The medium has the advantages of early germination, rapid growth, good growth, high mycelial biomass, short production cycle and the like, the biological characteristics of the bacterium are mastered, and a foundation is laid for further development of an application value and artificial domestication and cultivation. The successful cultivation of the stock culturealso provides new germplasm resources for the development and utilization of medicinal ingredients and teaching and researching, and has great economic and social benefits.
Owner:YANGTZE NORMAL UNIVERSITY
A kind of tissue culture breeding method of P.
ActiveCN106962204BReproduce fastStrong seedlingsHorticulture methodsPlant tissue cultureReproduction speedZoology
The invention discloses a tissue culture breeding method for acorus gramineus. The tissue culture breeding method comprises the steps of explant disinfection, induction, enrichment culture, subculture, rooting culture, seedling hardening and transplanting. According to the method, the acorus gramineus is high in reproduction speed, and roots and seedlings are robust; the cost and the production time are greatly saved; the problems that the source of parents of seedlings produced by a conventional breeding system is complicated and the quality of the seedlings is unstable; the tissue culture breeding method is suitable for standard, industrial and large-scale production; high-quality seedlings of the uniform standard are provided for the market; the problem that acorus gramineus seedlings cannot be yearly produced is solved.
Owner:FLOWER RES INST OF YUNNAN ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com