Method for expressing antibacterial peptide apidaecin by using Escherichia coli and for preparing antibacterial peptide apidaecin
A technology of Escherichia coli and antibacterial peptides, applied in the field of genetic engineering, can solve the problems of low expression, high cost, and low absolute yield, and achieve the effects of simple culture procedures, improved stability, and low production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: Construction of pET-polh-AP expression vector
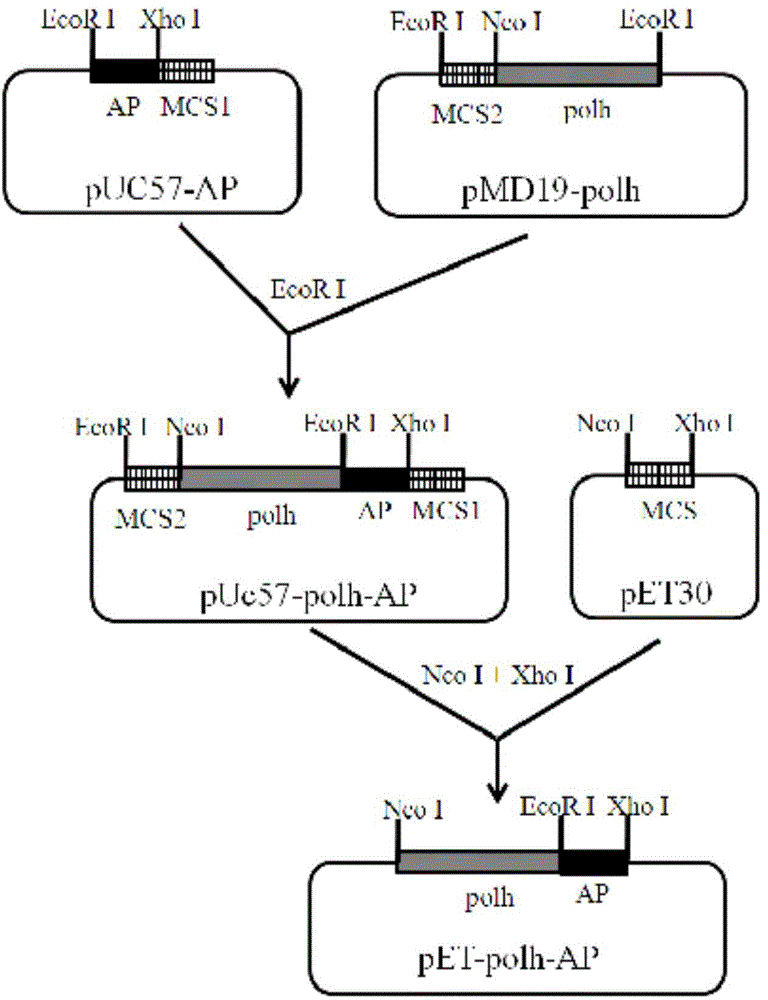
[0025] Chemically synthesize the AP2 gene fragment, its nucleotide sequence is as described in SEQ ID NO.1, the amino acid sequence of the AP2 protein expressed by the gene is as described in SEQ ID NO.2, and an EcoR I site is added at its 5' end during synthesis And hydroxylamine cleavage site coding sequence (Asn-Gly), 3' end added Xho I site. like figure 1 As shown, the above fragment was inserted into pUC57 to obtain the recombinant plasmid pUC57-AP containing the AP gene (provided by Shenggong). The polyhedron gene sequence polh (Gene ID: 7872889) was obtained by PCR cloning from the BmNPV genome, and the forward primer for PCR cloning was 5'-AAC GAATTC ATATGCCGGATTATTCATAC-3' (Nco I), reverse primer is 5'-TCGT GAATTC ATACGCCGGACCAGTG-3' (EcoR I).
[0026] The amplification system is:
[0027]
[0028] The amplification conditions are: pre-denaturation at 94°C for 5 minutes, and then enter the...
Embodiment 2
[0032] Embodiment 2: Expression of polh-AP fusion protein
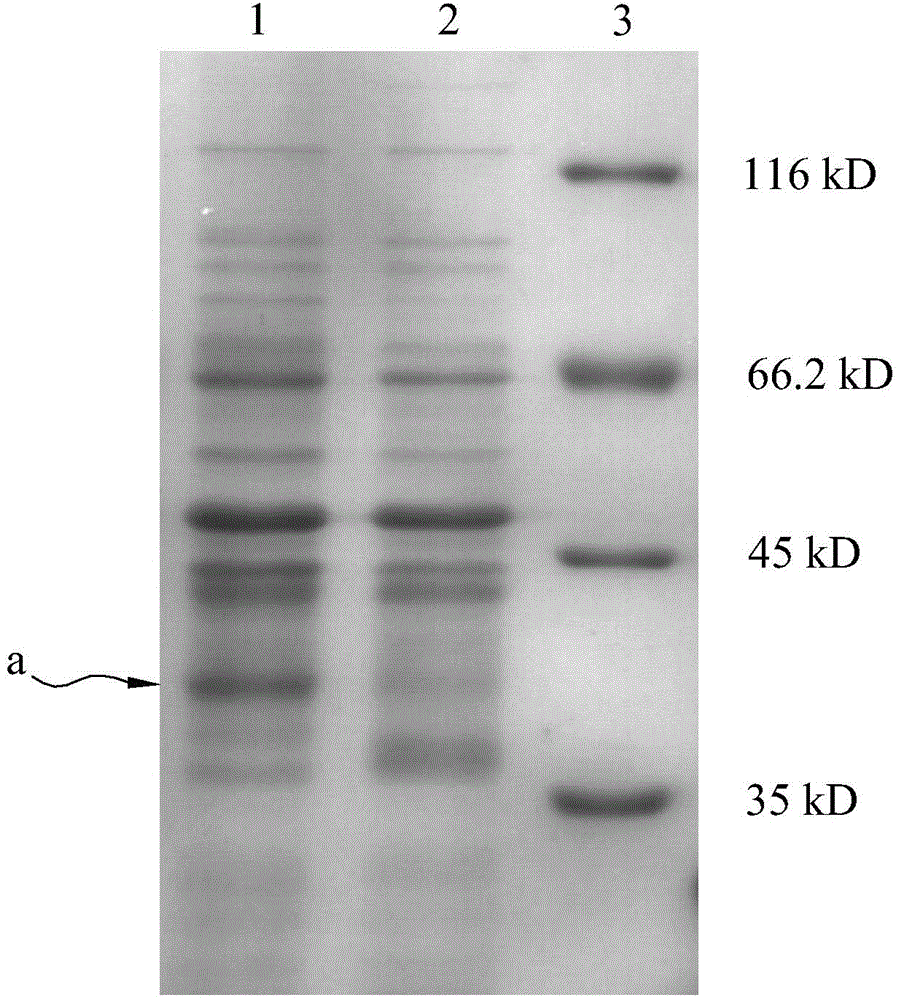
[0033] The expression vector pET-polh-AP obtained in Example 1 was transformed into E. coli BL21 competent cells, and cultured overnight at 37°C on LB culture plates containing kanamycin (50 μg / ml). Pick monoclonal plaques, place them in 5ml LB culture tubes, and culture them with shaking at 37°C for 10h, as the seeds of expression culture. According to the scale-up culture of 1:100, the conditions were 37°C and 150rpm shaking culture. When the OD600 reached 0.6, IPTG with a final concentration of 1mM was added to induce the expression of the recombinant protein polh-AP. 37°C, 150rpm shaking culture for 5h to end the expression, and SDS detection, the results are as follows figure 2 As shown, lane 1 is the expression profile 5h after IPTG induction, lane 2 is the expression profile without induction, and lane 3 is the expression profile of Mark protein, where a is the polh-AP fusion protein.
Embodiment 3
[0034] Embodiment 3: Purification and cracking of polh-AP fusion protein
[0035] Centrifuge at 5000rpm for 10min at 4°C to collect the E.coliBL21 cells expressing polh-AP fusion protein obtained in Example 2, resuspend in PBS (pH7.8), sonicate and centrifuge at high speed (15000rpm, 20min) to separate insoluble inclusion bodies . The resulting precipitate was then resuspended with PBS (pH9), shaken on ice to dissolve for 30 minutes, and the insoluble inclusion bodies were collected by high-speed centrifugation (15,000 rpm, 20 minutes). Finally, adjust the pH of the protein solution to pH 7.8 with 1M hydrochloric acid, let it stand for 2 hours, and centrifuge (15000rpm, 20min) to collect the precipitated protein.
[0036] Dissolve the obtained recombinant protein sample with PBS (pH12) to a final concentration of 15mg / ml, mix the recombinant protein solution with 3 times the volume of hydroxylamine lysis reaction buffer, and incubate at 55°C for 24h. Under these conditions, P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com