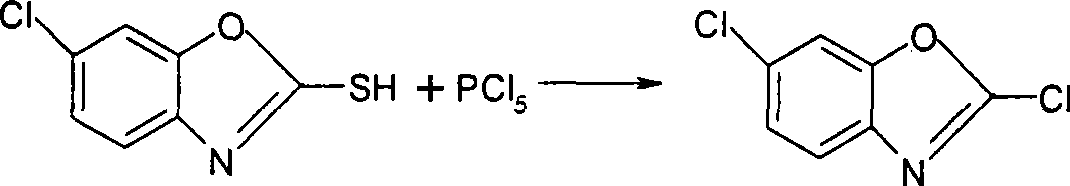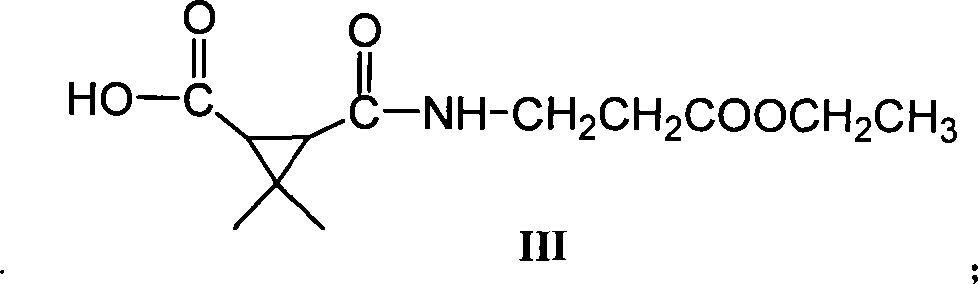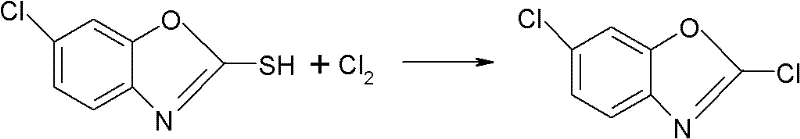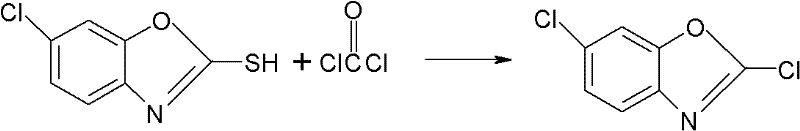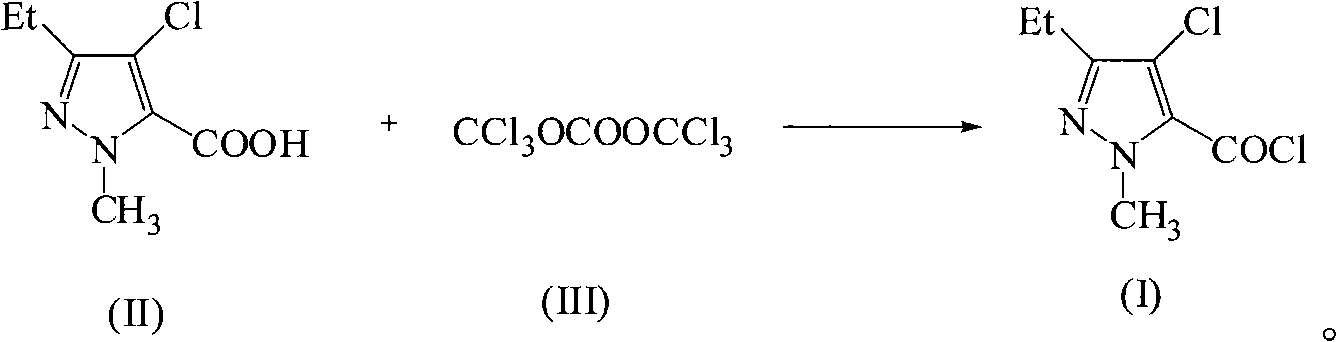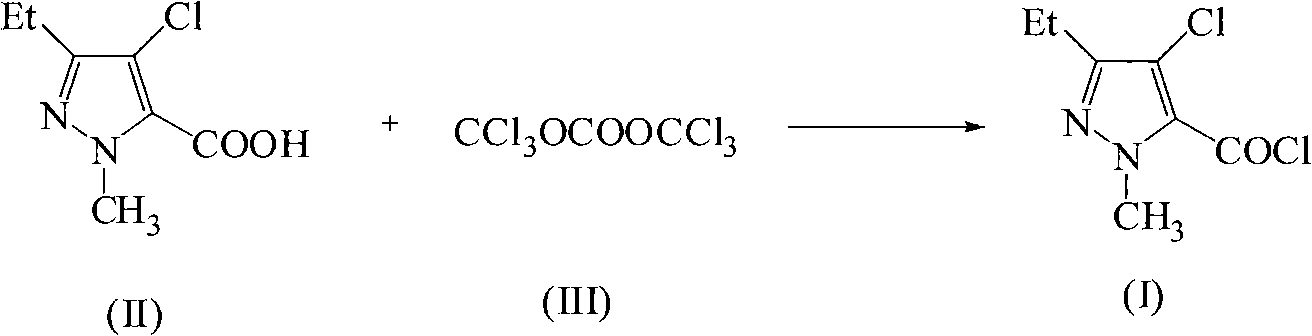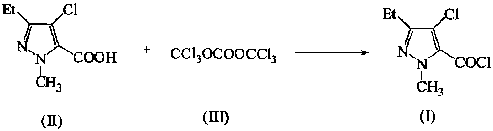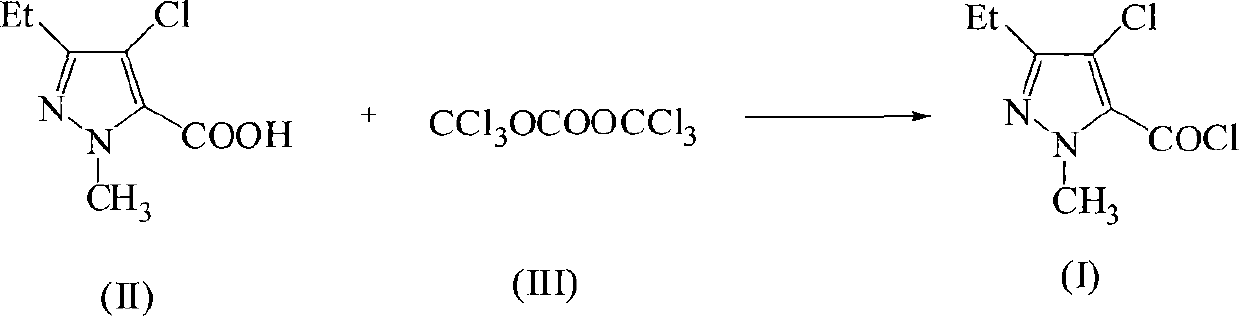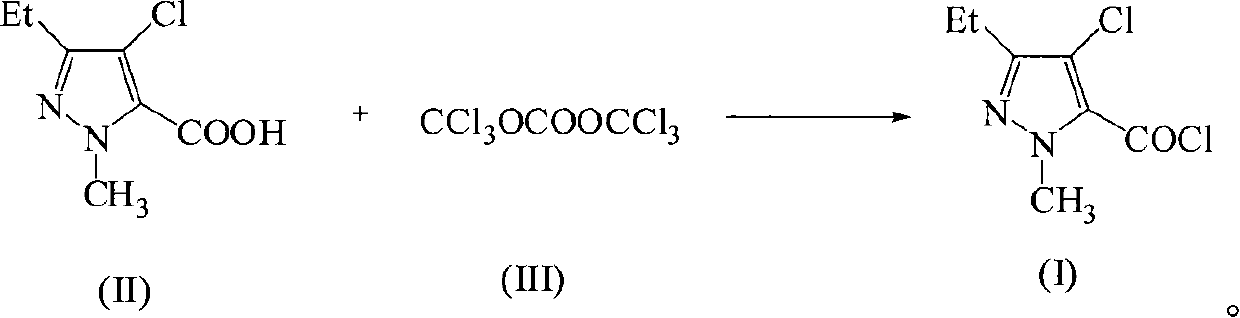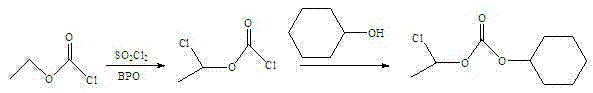Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Trichloromethyl chloroformate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Trichloromethyl chloroformate. Identifiers CAS Number. ... Diphosgene is a chemical compound with the formula ClCO 2 CCl 3. This colorless liquid is a valuable reagent in the synthesis of organic compounds. Diphosgene is related to phosgene and has comparable toxicity, ...
Method for synthesizing palonosetron hydrochloride
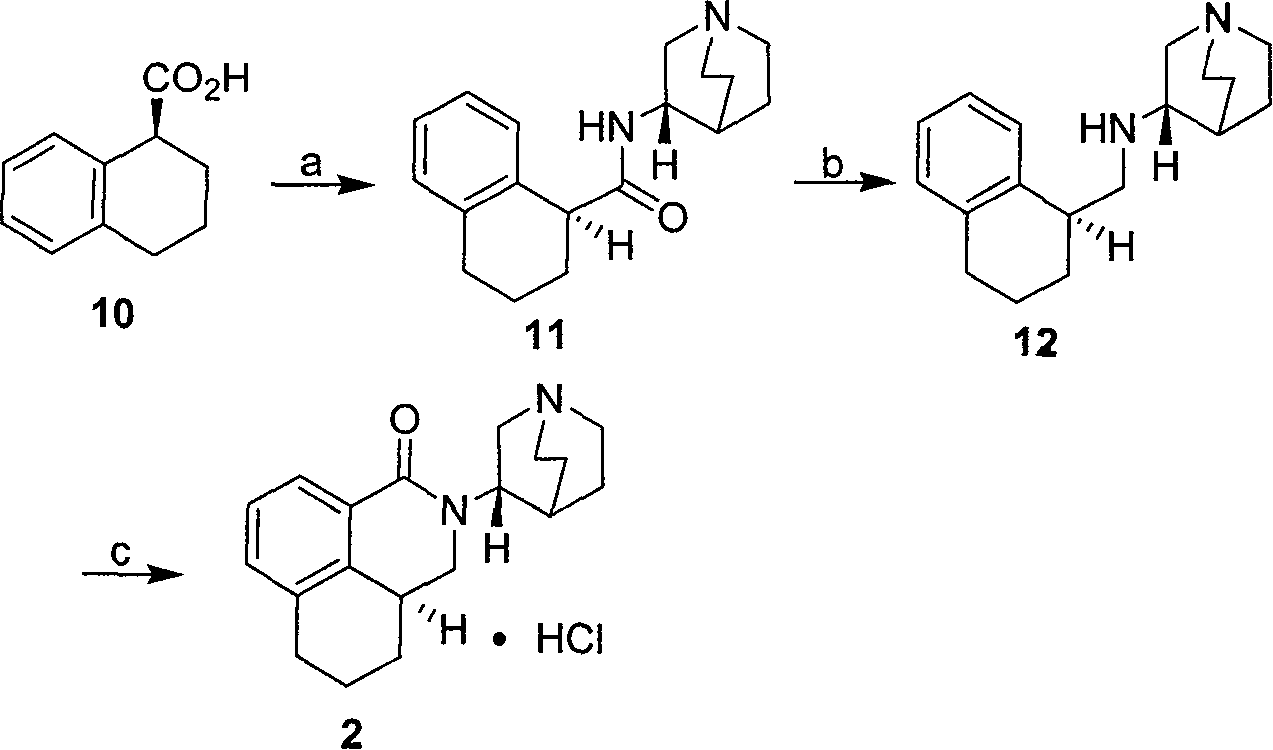
The invention discloses a novel synthesis method of palonosetron hydrochloride, which comprises that (1) (S)-tetralin formic acid is reacted with thionyl chloride and (S)-3-amido-quinine cyclic amine, to obtain (S, S)-quinuclidine tetralin formamide, (2), (S, S)-quinuclidine tetralin formamide is reacted with reductant and boron trifluoride diethyl etherate, to obtain (S, S)-tetralin methyl quinine cyclic amine, (3), (S, S)-tetralin methyl quinine cyclic amine is reacted with diphosgene to be added and reacted in boron trifluoride diethyl etherate solution, while the product is added and reacted with alcaine and water, to obtain palonosetron hydrochloride. And the synthesis route is represented as above: a: SOCI2, (S)-3-aminoquinuclidine, b: NaBH4, BF3OEt2, c: BF3OEt2, CICO2CCI3.
Owner:SHENZHEN NEPTUNUS PHARM CO LTD
Method for preparing 4,4'-dicyclohexyl methane diisocyanate
InactiveCN102093259AAccurate measurementControl the feeding ratioIsocyanic acid derivatives preparationOrganic compound preparationSolventMethane
The invention belongs to the technical field of organic compounds and in particular relates to a method for preparing dicyclohexyl methane diisocyanate by a non-phosgene method. In the method, 4,4'-diamino dicyclohexyl methane or an isomer mixture thereof or salt thereof or a mixture of amine and salt thereof, serving as a raw material, reacts with di(trichloromethyl)carbonic ester or trichloromethyl chloroformate or a mixture of the di(trichloromethyl)carbonic ester and the trichloromethyl chloroformate in an inert solvent. By the method, defects of long process route, complex technology, low safety and the like in the preparation of the 4,4'-dicyclohexyl methane diisocyanate by the traditional phosgene method are overcome. The method has the advantages of convenience of operation, high safety and environmental friendliness. In the method, reactants are charged and metered accurately and rate of charge is reduced, so production safety coefficient is improved. Tail gas is simple in treatment; hydrogen chloride gas generated by the reaction is absorbed by water; high-purity hydrochloric acid can be prepared; chloride ions can be utilized completely; and environmental friendliness is contributed.
Owner:UPCHEM CHINA
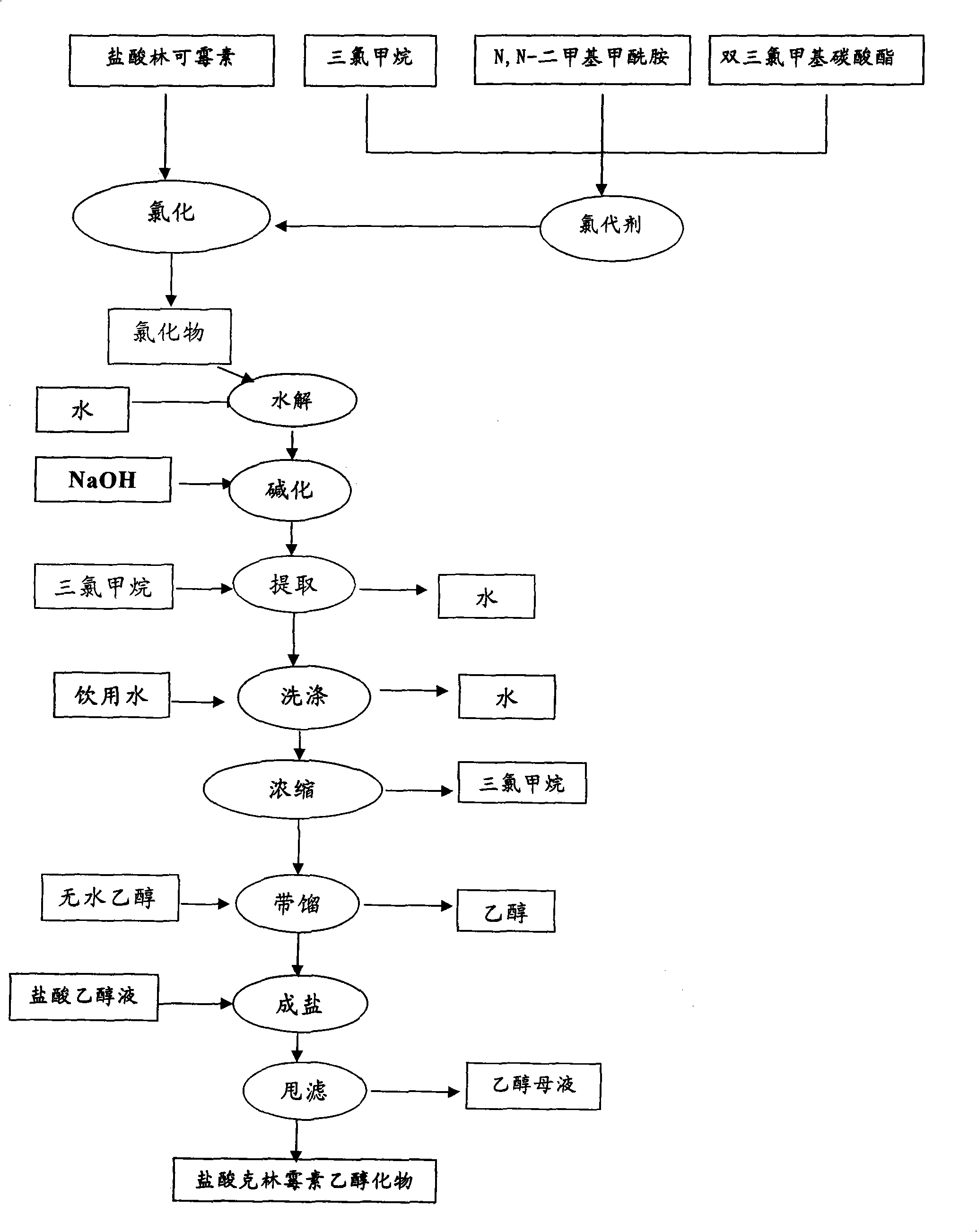
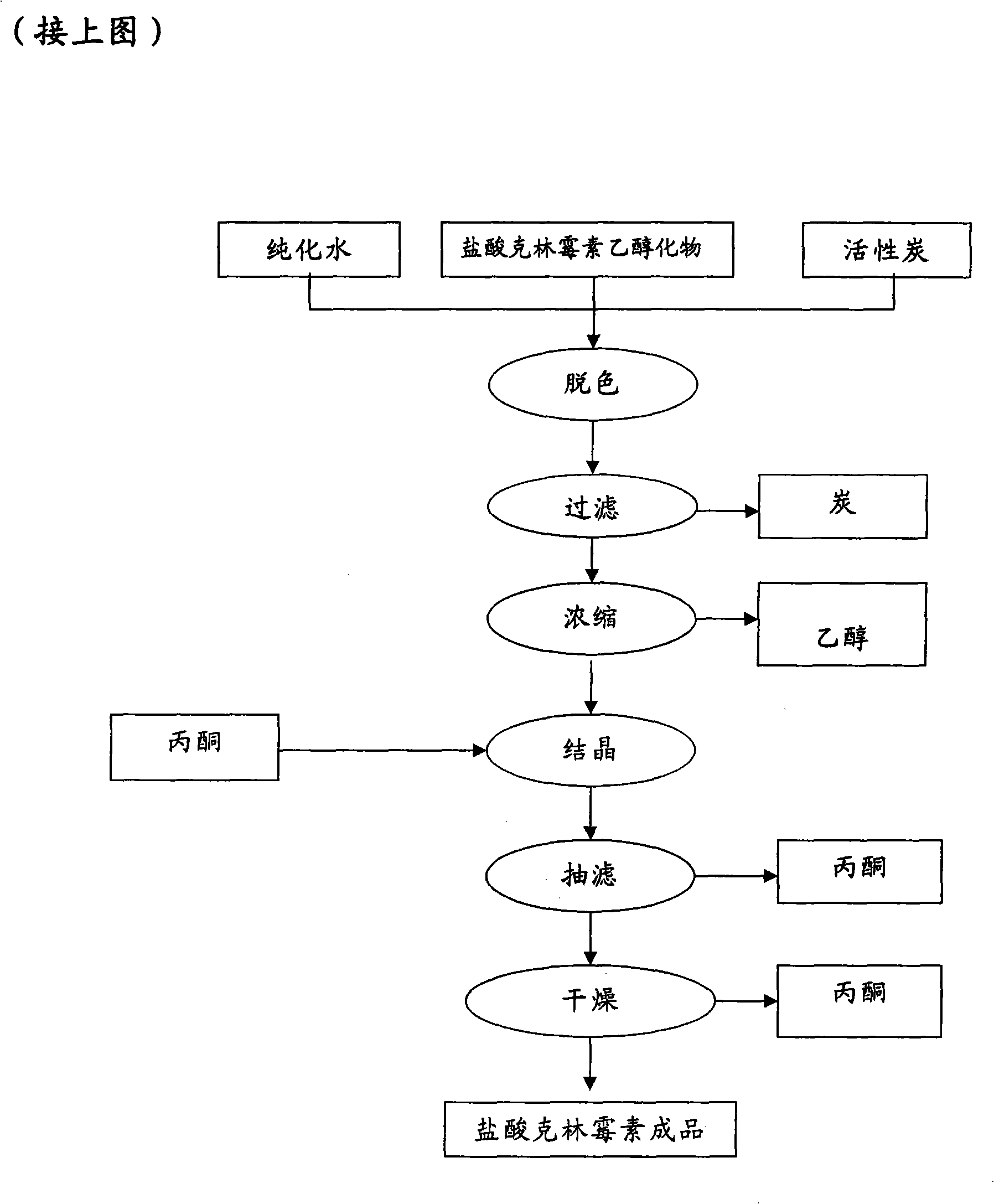
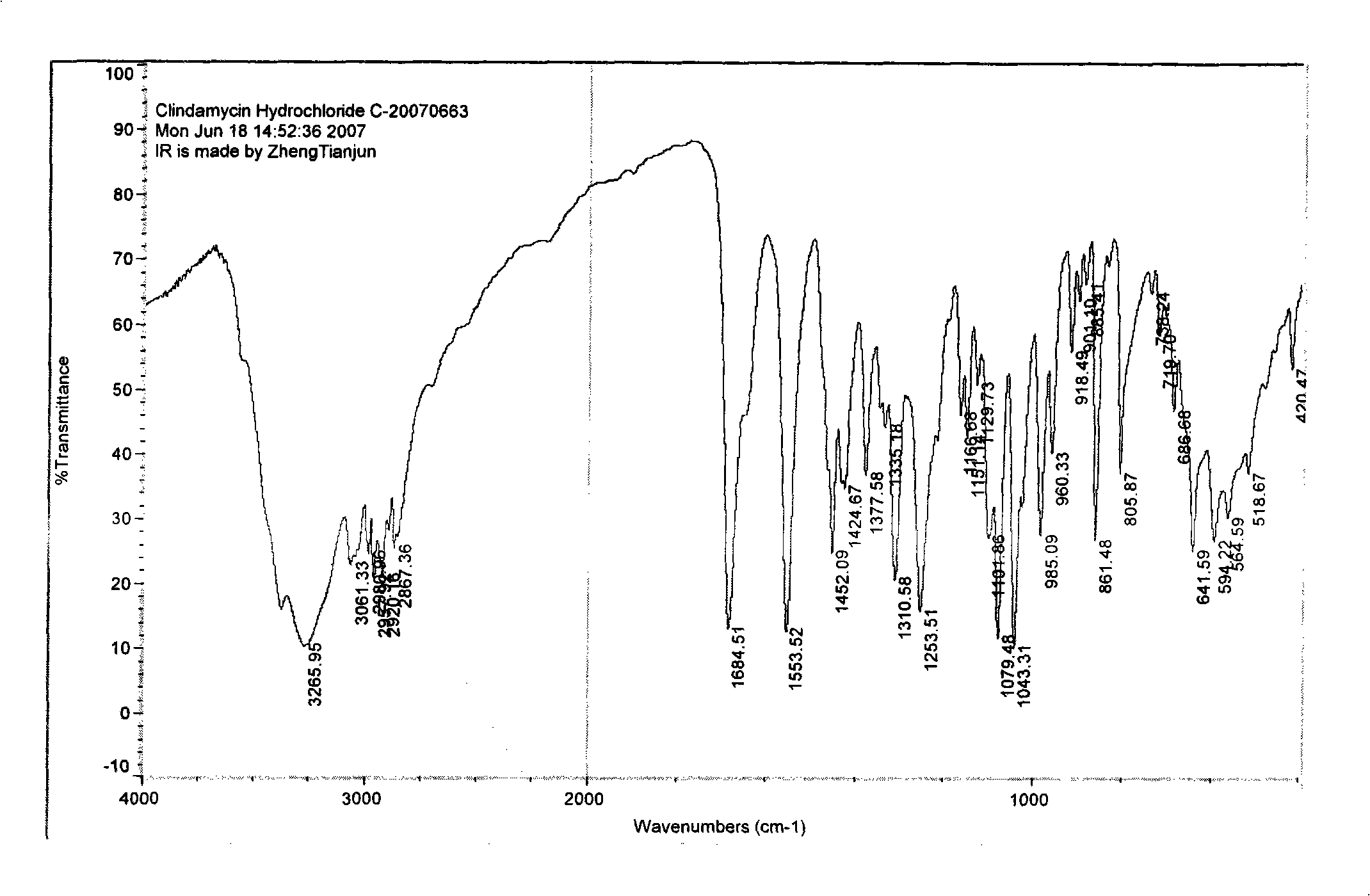
Industrial production method for clindamycin or salts thereof
The invention relates to an industrial production method for producing clindamycin or the salts of clindamycin. The method uses dual-trichloromethyl carbonate or trichloromethyl methyl chloroformate and amide to prepare the intermediate chlorophthalic agent. The method of the invention has high product purity, high yield, easier post-processing and other technical advantages, compared with the prior art.
Owner:重庆凯林制药有限公司 +1
Method for preparing methylcyclohexyl diisocyanate
InactiveCN102086162AAccurate measurementControl the feeding ratioOrganic compound preparationPreparation from carbamatesSimple Organic CompoundsSolvent
The invention belongs to the technical field of organic compounds and in particular relates to a method for preparing methylcyclohexyl diisocyanate by non-phosgene. The method comprises the following step of: reacting methylcyclohexyl diamine or an isomer mixture thereof or salt thereof or a mixture of amine and salt thereof as a raw material with bis(trichloromethyl)carbonate or trichloromethyl chloroformate or a mixture of the bis(trichloromethyl)carbonate and the trichloromethyl chloroformate in an inert solvent. In the method, the phosgene is replaced by the bis(trichloromethyl)carbonate or the trichloromethyl chloroformate or the mixture of the bis(trichloromethyl)carbonate and the trichloromethyl chloroformate; and the bis(trichloromethyl)carbonate or the trichloromethyl chloroformate or the mixture of the bis(trichloromethyl)carbonate and the trichloromethyl chloroformate reacts with methylcyclohexyl diamine or salt thereof to prepare the methylcyclohexyl diisocyanate. Compared with the traditional phosgene method, the method provided by the invention has convenience for operation, high safety, environment friendliness and mild reaction condition; and because the charging and the reaction are performed in normal pressure, the requirements on production equipment are reduced, and the process is simplified.
Owner:UPCHEM CHINA
Isocyanate preparing process
InactiveCN1436772AImprove protectionEasy to storeIsocyanic acid derivatives preparationOrganic compound preparationOrganic solventReaction temperature
The isocyanate preparing process is one reaction between proper primary amine and trichloromethyl chloroformate and features that at reaction temperature of -5 to 180 and normal pressure, primary amine is first dissolved in inert organic solvent and then reaction with liquid trichloromethyl chloroformate in the molar ratio to primary amine of 0.2-1. The present invention has the advantages caused by replacing trichloromethyl chloroformate for phosgene, including easy storage and transportation, decrease of toxic matter, easy operation, one half shortened reaction time and being ever suitable for industrial production.
Owner:SHANDONG XINHUA PHARMA CO LTD
Halogen-free environment-friendly polyurethane oil paint and preparation method thereof
ActiveCN104610525ASolve the problem of difficult flame retardantHigh oxygen indexFireproof paintsPolyurea/polyurethane coatingsHigh pressureSolvent
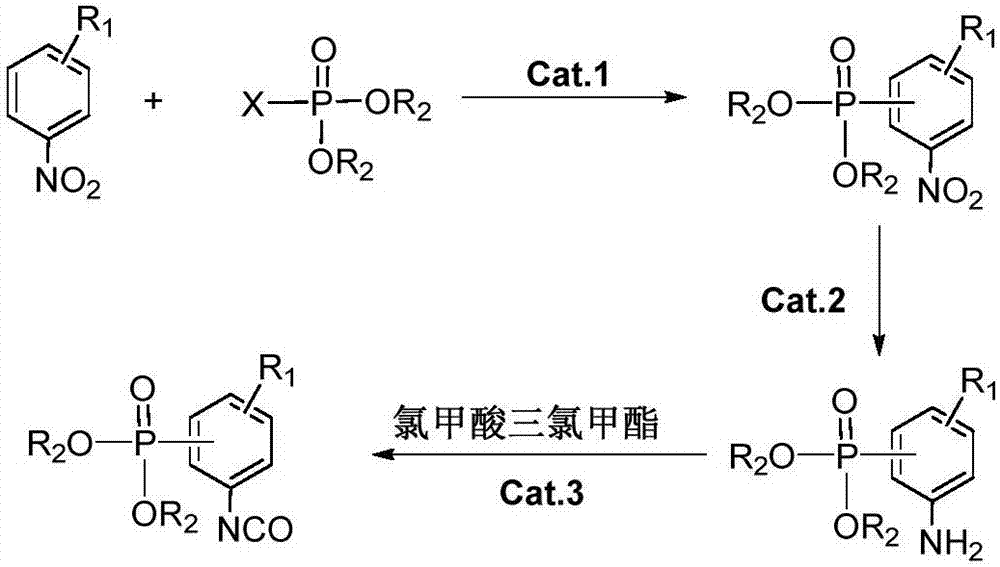
The invention discloses a preparation method of a halogen-free environment-friendly polyurethane oil paint. The preparation method comprises the following steps: performing refluxing catalytic hydrogenation reduction on a phosphatizing agent and a nitrobenzene derivative in an organic solvent with the presence of a metal catalyst to obtain a phosphatized aniline derivative, dropwise adding trichloromethyl chloroformate, mixing and stirring uniformly at room temperature, reacting continuously for 2-4 hours to obtain phosphatized phenyl isocyanate, stirring the phosphatized phenyl isocyanate and an alcoholic compound for 3 hours with the presence of a polymerization catalyst, adding an amine compound, stirring continuously for 2 hours, performing vacuum dewatering on a reaction mixture to obtain flame-retardant polyurethane resin, adding a curing agent and a solvent, and reacting to obtain the halogen-free environment-friendly polyurethane oil paint. The invention further discloses the halogen-free environment-friendly polyurethane oil paint. The halogen-free environment-friendly polyurethane oil paint is an expansive environment-friendly flame-retardant polyurethane oil paint with a phosphorous-nitrogen synergistic effect. In the production process of a flame retardant, raw materials are cheap and easily obtained; the preparation method is free of a high-heat and high-pressure process, and simple to operate, and energy consumption in the production process is low; the whole production process is saves energy and is environment-friendly.
Owner:安徽科赛富新材料科技有限公司
Method for synthesizing 4-nitrobenzyl chloroformate
InactiveCN1803758AEliminate production safety hazardsThe amount of three wastes is lessOrganic chemistryOrganic compound preparationOrganic solventChloroformate
The invention provides a process for synthesizing 4-Nitrobenzyl chloroformate, which comprises reacting trichloromethyl chloroformate or bis(trichloromethyl)carbonate with p-nitrobenzyl alcohol at -40-20 deg C under the action of acid-binding agent, finally carrying out post-treatment to obtain the outcome yield.
Owner:ZHEJIANG UNIV OF TECH
Method for synthesizing 2,6-dichlorobenzoxazole
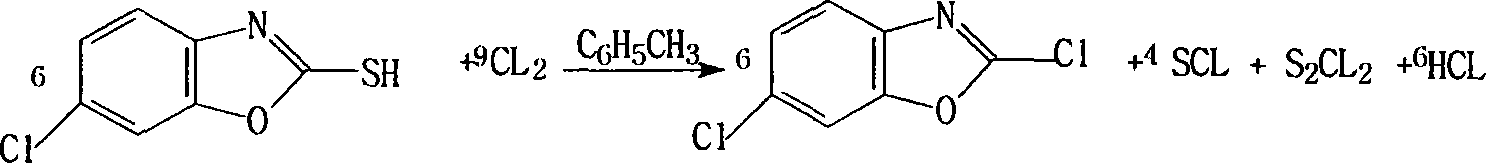
InactiveCN101372480AHigh yieldQuality improvementOrganic chemistryTrichloromethyl chloroformateRaw material
The invention discloses a method for synthesizing 2, 6-dichlorobenzoxazole, which takes 2-hydrosulphonyl-6-dichlorobenzoxazole as raw material and trichloromethyl chloroformate or dicarbamate (trichloromethyl) as a chlorinating agent, and the raw material and the chlorinating agent react at the temperature of 65-120 DEG C to prepare the 2, 6-dichlorobenzoxazole; wherein, the mol ratio between the raw material and the chlorinating agent ranges from 1:0.2 to 2. The method has simple technique, safe use and high yield.
Owner:JIANGSU TIANRONG GROUP +1
Production process of 1, 4-phenylene diisocyanate
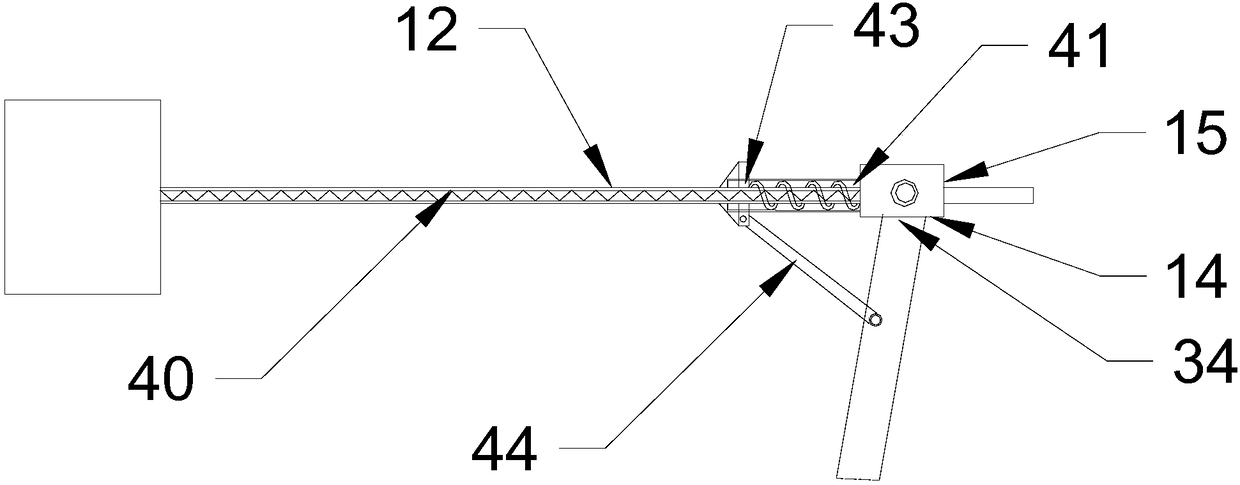
ActiveCN107011215ARealize synchronous rotationReal-time samplingIsocyanic acid derivatives preparationOrganic compound preparationRefluxChlorobenzene
The invention discloses a production process of 1, 4-phenylene diisocyanate. The production process includes: heating a solution of p-phenylenediamine and triethylamine to 100 DEG C, adding the heated solution and the chlorobenzene solution of di-(trichloromethyl) carbonate or the chlorobenzene solution of trichloromethyl chlorocarbonate into a reaction kettle, heating until reflux occurs, continuing adding the chlorobenzene solution of di-(trichloromethyl) carbonate or the chlorobenzene solution of trichloromethyl chlorocarbonate, rectifying, and collecting a 130-136 DEG C / 8mmHg rectifying product, wherein the reaction kettle comprises a drive motor, a stirring shaft and stirring blades, an automatic sampling device is mounted on the stirring shaft and comprises a sleeve and a sampling component, the sleeve is provided with a liquid storage cavity inside, and the sleeve comprises an annular fixed component located on the upper portion and an annular rotatable component located on the lower portion. The production process has the advantages that the sleeve, the sampling component and the stirring shaft can rotate synchronously by the annular fixed component and the annular rotatable component, real-time sampling during reaction is achieved, and sampling efficiency is increased.
Owner:UPCHEM CHINA
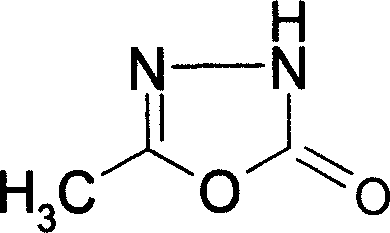
5-Methyl-1,3,4-oxadiazole-2(3H)-ones and preparing method thereof
The invention discloses a 5-methyl-1, 3, 4-oxadiazole-2 (3H)-ketone and making method, which is characterized by the following: looping acetydrazide and trichloromethyl chloroformate or di (trichloromethyl) carbonate to obtain the product; adopting pyridine or 4-dimethylamino pyridine as catalyst; using 1, 2-dichloroethane as dielectric solvent.
Owner:YANCHENG SHUANGNING AGRI CHEM
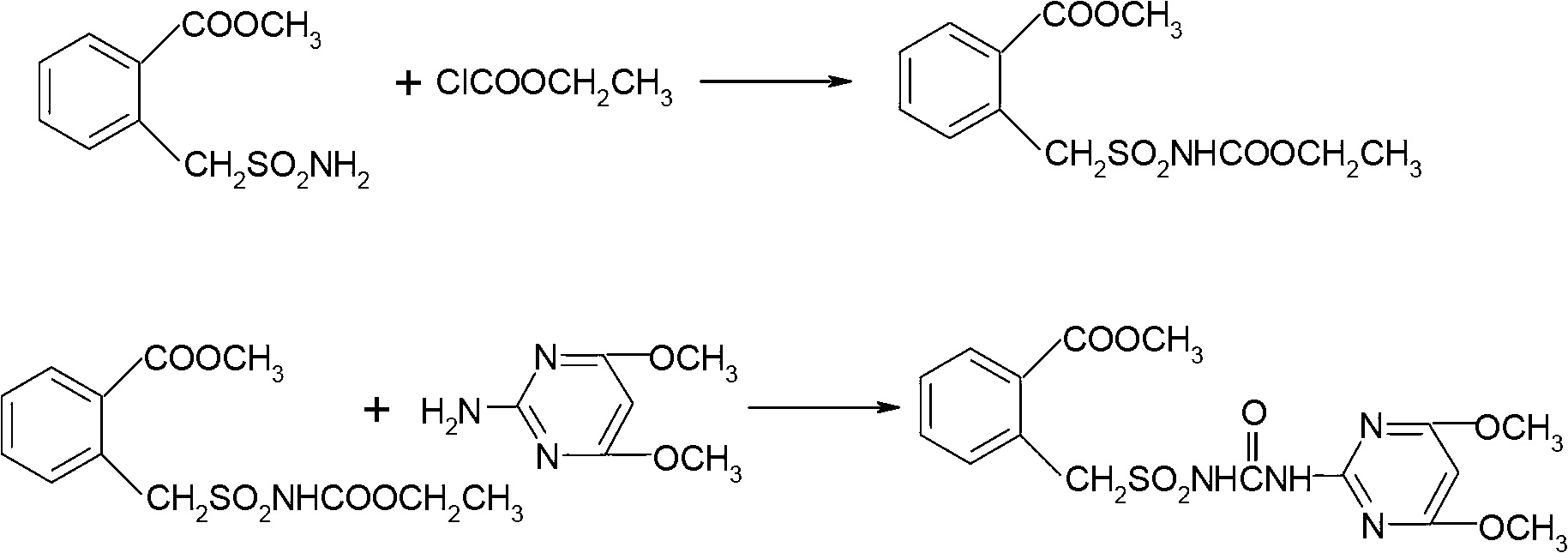
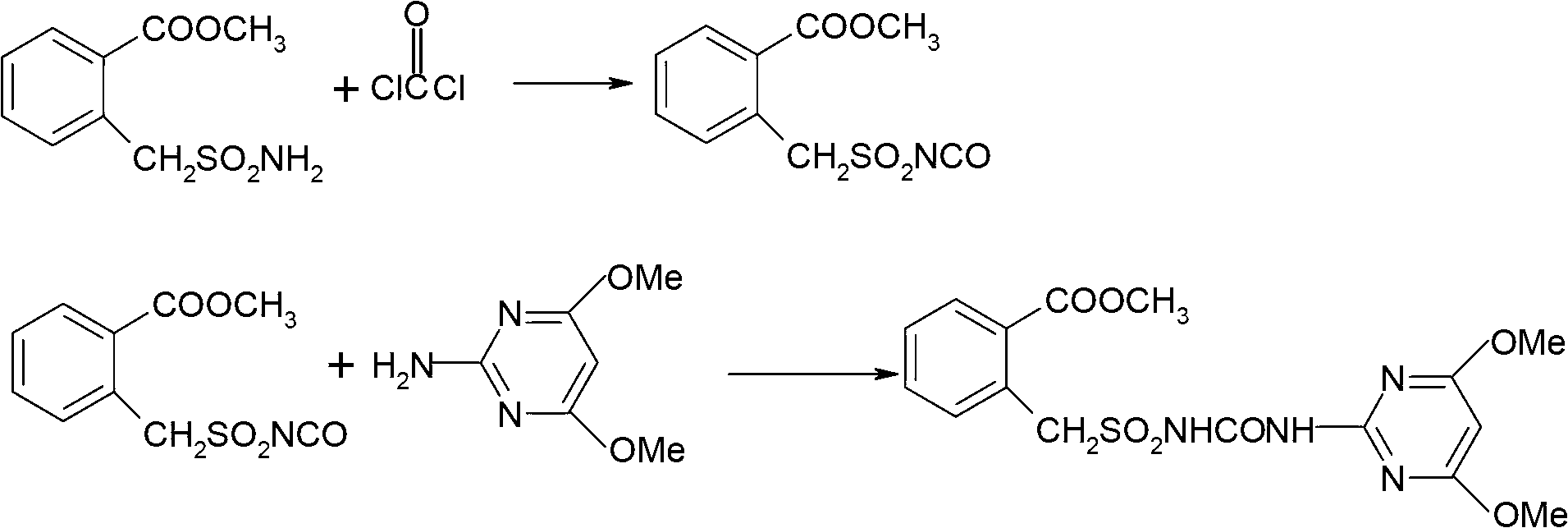
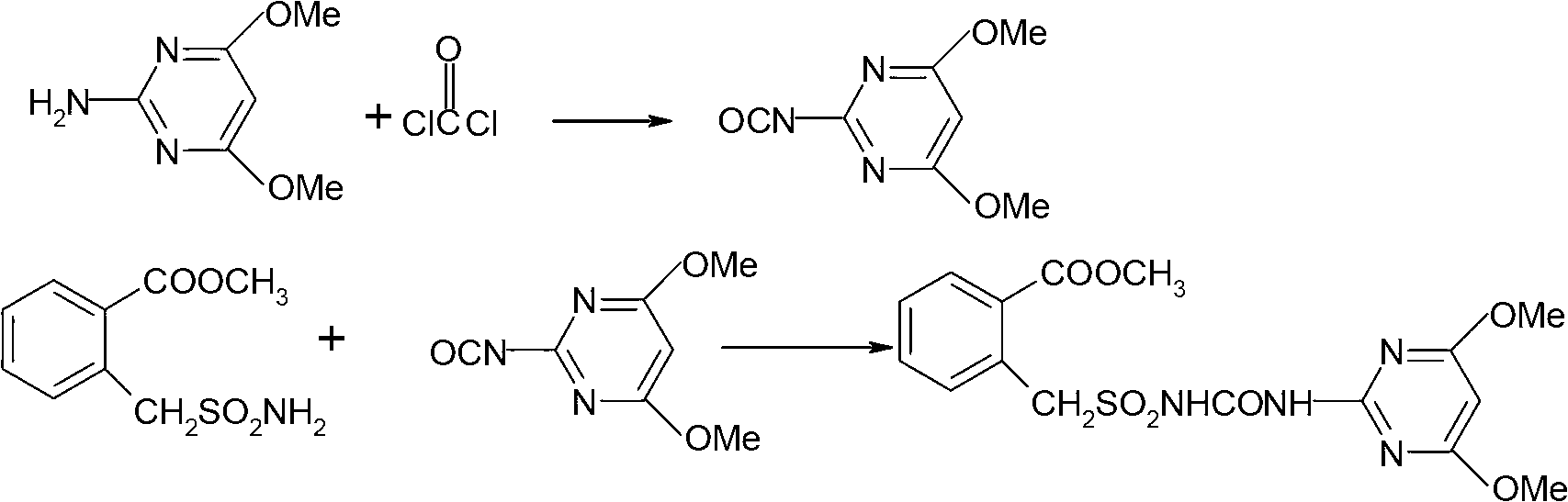
Method for preparing bensulfuron methyl
The invention discloses a method for preparing bensulfuron methyl. The method comprises the following steps of: mixing o-methyl formate benzyl sulfamide, bis(trichloromethyl) carbonate and normal-butyl isocyanate serving as a catalyst in dimethylbenzene, heating to the temperature of between 70 and 85 DEG C, heating to the temperature of between 110 and 130 DEG C slowly, adding trichloromethyl chloroformate slowly at the temperature of between 110 and 130 DEG C, performing esterification reaction at the temperature of between 110 and 130 DEG C, and removing a solvent and the catalyst after reaction; and adding o(methyl formate) benzyl sulfonyl isocyanate obtained by the esterification reaction into a condensation reaction solvent, stirring, cooling to the temperature of between 30 and 50 DEG C, continuing to add 2-amino-4,6-dimethoxy pyrimidine, performing condensation reaction at the temperature of between 50 and 90 DEG C, cooling, and drying to obtain the bensulfuron methyl. By the method, the bensulfuron methyl of which the content is more than 97 percent can be obtained, and the total yield is more than 90 percent.
Owner:JIANGSU TIANRONG GROUP
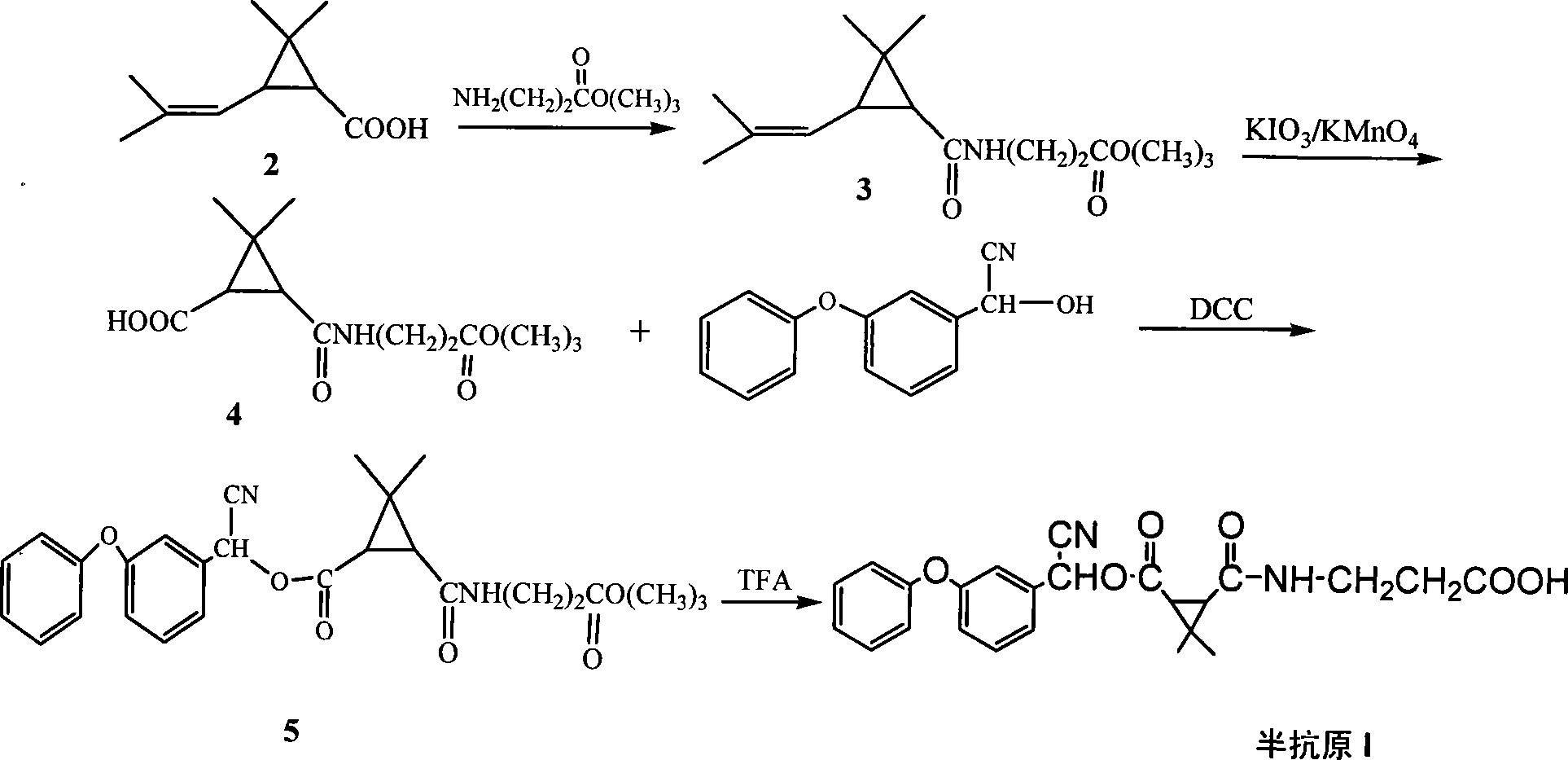
Method for synthesizing pyrethroid hapten compounds
InactiveCN101215247AQuick checkCarboxylic acid nitrile preparationOrganic compound preparationGamma-Aminobutyric acidAcetonitrile
The invention discloses a synthesis process of pyrethroids hapten compound by using dimethyl trichloromethyl chloroformate, gamma-aminobutyric acid and as main material, which comprises following steps that firstly generating 3-(2, 2-dimethyl-3-(2'-methacryloyl) cyclopropyl carbonyl amino) ethyl propionate, secondly generating 3-(3-ethyoxyl-3-oxopropanecarbonic acyl radical)-2, 2-dimethyl cyclopropane aminic acid, thirdly generating 2-hydroxy-2-(3-pheonexyphenyl) acetonitrile, fourthly generating cyano-(3-phenoxy phenyl) methyl N-2-ethyoxylcarbonylethyl-2, 2-dimethylcypromethyl carbonate, fifthly generating 3-(3-(( cyano(3-phenoxyphenyl) methoxyl) carbonic acyl radical)-2, 2-phenoxy phenylcypro carbamoyl) ethylformic acid. Hapten which is prepared by the invention comprises three similarity structures of most pyrethroids pesticides, ester with-CN and three-membered ring structure.
Owner:ZHEJIANG UNIV
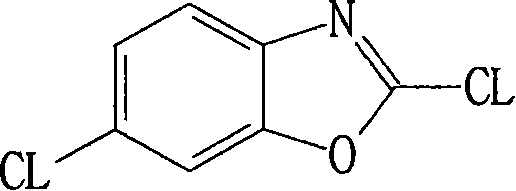
Method for preparing 2,6-dichloro benzoxazole
The invention relates to a method for preparing 2, 6-dichlorobenzoxazole, adopting 6-chlorine-2-mercaptobenzoxazole and trichloromethyl chloroformate to synthesize 2, 6-dichlorobenzoxazole under the action of catalyst. The method comprises the following concrete steps that: the 6-chlorine-2-mercaptobenzoxazole, dimethylformamide as catalyst and toluene as medium solvent are added to a reaction vessel with the temperature controlled to be between 40 and 70 DEG C; trichlorome-thylchloroformate is added in drops for 4-8 hours; after drop adding is completed, the materials are heated to between 60 and 100 DEG C, react for 1-4 hours and are cooled, and then a toluene solution of the 2, 6-dichlorobenzoxazole is obtained. The method replaces gaseous phosgene, chlorine or phosphorus pentachloridewith the trichloromethyl chloroformate; during reaction, the trichloromethyl chloroformate is decomposed into single phosgene to participate in the reaction, and then the target object 2, 6-dichlorobenzoxazole is obtained through the reaction. The method does not need to separate the product from the solvent, is simple and convenient to operate, high in product yield, which can reach over 98 percent, pale in product color, excellent in product quality and low in equipment requirements, and does not need tail gas treatment.
Owner:童渝
Preparation method of 4,4'-dicyclohexylmethane diisocyanate
ActiveCN103553969ALower requirementAccurate feeding and meteringIsocyanic acid derivatives preparationOrganic compound preparationSimple Organic CompoundsSolvent
The invention belongs to the technical field of an organic compound, and particularly relates to a method for preparing 4,4'-dicyclohexylmethane diisocyanate by utilizing a non-phosgene process. The method comprises the following step: reacting 4,4'-dicyclohexylmethane diisocyanate or an isomer mixture or salt thereof, or a mixture of amine and salt thereof which are as raw materials with bis(trichloromethyl) carbonate or trichloromethyl chlorocarbonate or a mixture thereof in an inert solvent. By adopting the method disclosed by the invention, the defects that preparation of the 4,4'-dicyclohexylmethane diisocyanate by utilizing the traditional phosgene process is long in process route, complicated in technique, poor in safety and the like are overcome. The method disclosed by the invention has the advantages of being convenient to operate, high in safety, environment-friendly, and accurate in reactant feeding and metering, and the feeding ratio is reduced, so that the safety coefficient of production is improved. In addition, exhaust treatment is simple; high-purity hydrochloric acid can be prepared from a hydrogen chloride gas generated by water absorption reaction; chloridion can be fully utilized; environmental conservation is facilitated.
Owner:UPCHEM CHINA
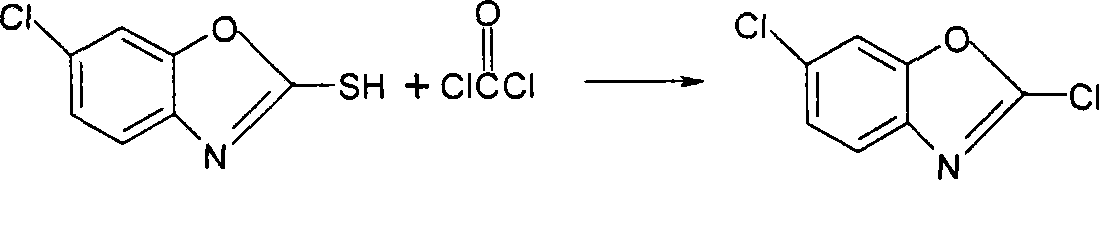
Synthesizing method for preparing high-purity 2,6-dichloro benzoxazole
The invention discloses a synthesizing method for preparing high-purity 2,6-dichloro benzoxazole. The method comprises the following steps of: mixing 2-sulfydryl-6-dichloro benzoxazole and di(trichloromethyl) carbonic ester in a medium solvent; heating to 45-55 DEG C; slowly heating to 80-110 DEG C stage by stage; perserving heat for reacting at the temperature of 80-110 DEG C for 0.5-2 hours; slowly adding trichloromethyl chloroformate; then preserving heat for 0.5-1 hours; removing a part of the solvent after reacting; cooling and devitrifying; and separating a solid out. According to the synthesizing method, a catalyst is not used, only a stage heating method and light solidification are adopted, and a small amount of trichloromethyl chloroformate is added at a last stage, so that a reaction can be undergone completely approximately, and a high-purity finished product is directly obtained. The reaction condition and process of the synthesizing method are mild, over 99 percent by mass of 2,6-dichloro benzoxazole can be obtained directly under the condition that complicated treatment is not required, and the yield of the high-purity 2,6-dichloro benzoxazole reaches above 98 percent.
Owner:JIANGSU TIANRONG GROUP
Production process of p-phenylene diisocyanate
ActiveCN107011215BReal-time samplingImprove sampling efficiencyIsocyanic acid derivatives preparationOrganic compound preparationRefluxDrive motor
The invention discloses a production process of 1, 4-phenylene diisocyanate. The production process includes: heating a solution of p-phenylenediamine and triethylamine to 100 DEG C, adding the heated solution and the chlorobenzene solution of di-(trichloromethyl) carbonate or the chlorobenzene solution of trichloromethyl chlorocarbonate into a reaction kettle, heating until reflux occurs, continuing adding the chlorobenzene solution of di-(trichloromethyl) carbonate or the chlorobenzene solution of trichloromethyl chlorocarbonate, rectifying, and collecting a 130-136 DEG C / 8mmHg rectifying product, wherein the reaction kettle comprises a drive motor, a stirring shaft and stirring blades, an automatic sampling device is mounted on the stirring shaft and comprises a sleeve and a sampling component, the sleeve is provided with a liquid storage cavity inside, and the sleeve comprises an annular fixed component located on the upper portion and an annular rotatable component located on the lower portion. The production process has the advantages that the sleeve, the sampling component and the stirring shaft can rotate synchronously by the annular fixed component and the annular rotatable component, real-time sampling during reaction is achieved, and sampling efficiency is increased.
Owner:UPCHEM CHINA
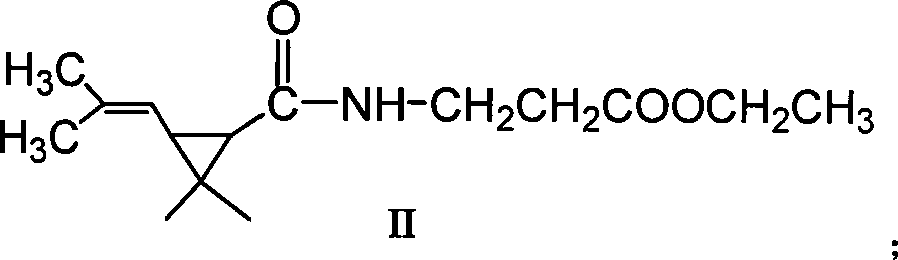
Green synthetic method for methyl-3-ethyl-4-chlorin-5-pyrazol formyl chloride
InactiveCN101307022AAdvanced process routeReasonable process conditionsOrganic chemistryState of artOrganic solvent
The invention discloses an environmental-friendly method for synthesizing 1-methyl-3-ethide-4-chlorine-5-pyrazole formyl chloride. The synthesizing method takes 1- methyl-3-ethide-4-chlorine-5- pyrazole formic acid which is shown as a formula (II) and bis (trichloromethyl) carbonate which is shown as a formula (III) as raw materials and takes organic amine as a catalyst. The raw materials and the catalyst react sufficiently in an organic solvent at a temperature of between 20 and 80 DEG C; and the 1-methyl-3-ethide-4-chlorine-5-pyrazole formyl chloride which is shown as the formula (I) is obtained by processing the reaction fluid. Compared with prior art, the method has advanced process flow, reasonable process conditions, and cheap and available raw materials, avoids the phosgene, the Cloruro de tionilo and the trichloromethyl chloroformate, and has simple and safe operation, high reaction yield coefficient, low production cost, great implementation value and social and economical benefits and basically does not have 'three wastes'.
Owner:ZHEJIANG UNIV OF TECH
Preparation method of trichlorosaccharose-6-ester
InactiveCN1911948ALow toxicityEasy to handleEsterified saccharide compoundsSugar derivativesOrganic solventSucrose
The present invention relates to preparation process of trichloro sucrose-6-ester, and is especially preparation process of trichloro sucrose-6-ester with trichloromethyl chloroformate as chlorinating reagent. After trichloromethyl chloroformate is dropped into organic amine solution of sucrose-6-ester at the temperature of -10 deg.c to +20 deg.c, the mixed solution is heated slowly to 80-120 deg.c and reacted for 1-3 hr; and the reacted liquid is further treated through neutralization with alkali solution, decompression stilling to eliminate solvent, dissolving in water, extracting, decolorizing and concentrating to obtain trichloro sucrose-6-ester. Of the material, the weight ratio between sucrose-6-ester and trichloromethyl chloroformate is 1 to 1.5-5. Using trichloromethyl chloroformate with low toxicity as chlorinating reagent results in simple post-treatment, basically no pollution and low cost. The organic amine solvent may be recovered for reuse, and the present invention has high yield.
Owner:ZHEJIANG HISOAR PHARMA
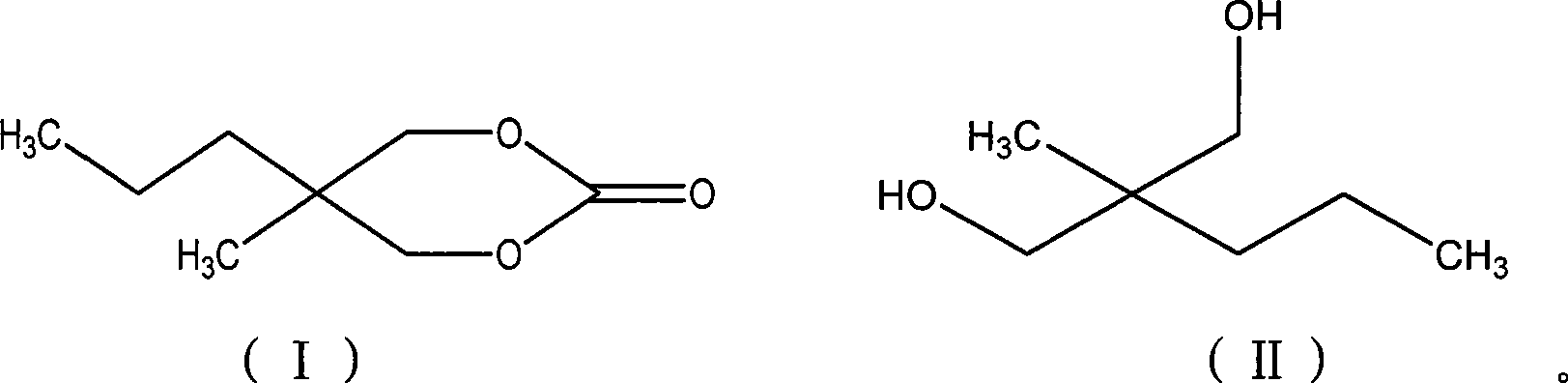
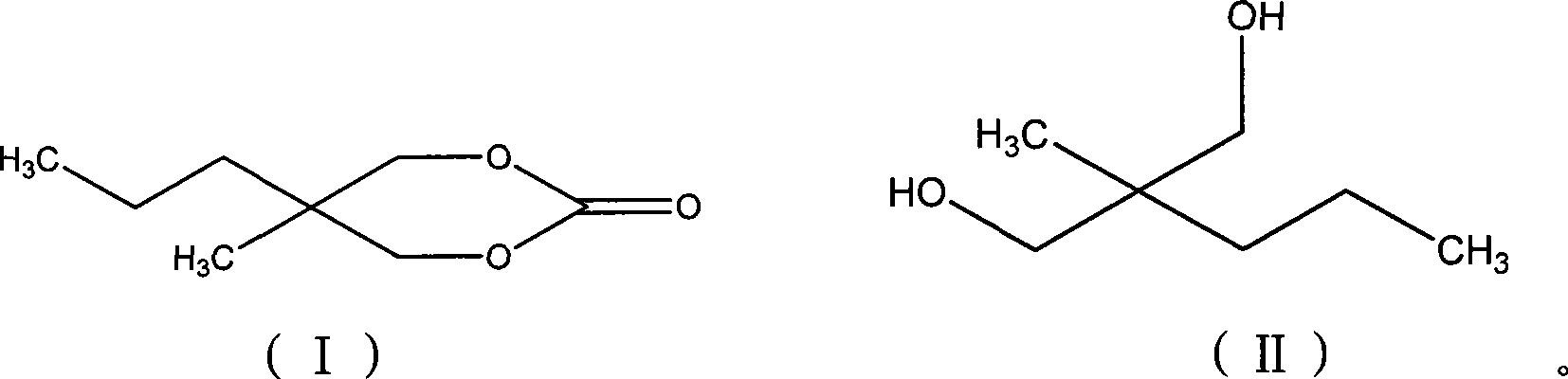
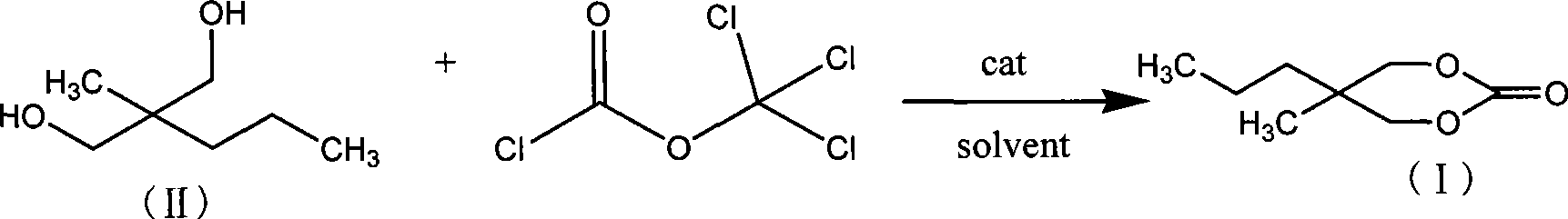
Method for synthesizing 2-methyl-2-propyl-1,3-propanediol dimethyl carbonate compound
InactiveCN101412705AEliminate security concernsEliminate pollutionOrganic chemistryOrganic solventMethyl carbonate
The invention relates to a method for synthesizing a 2-methyl-2-propyl-1, 3-propanediol diester carbonate compound by using trichloromethyl chloroformate, which comprises the following steps: using 2-methyl-2-propyl 1, 3-propanediol and the trichloromethyl chloroformate as raw materials to completely react with each other under the action of an organic amine catalyst in an organic solvent at a temperature of between 5 DEG C below zero and 50 DEG C, and separating and purifying reaction solution to obtain the 2-methyl-2-propyl-1, 3-propanediol diester carbonate, wherein the molar ratio of the 2-methyl-2-propyl 1, 3-propanediol to the trichloromethyl chloroformate to the organic amine catalyst is 1 to 0.5-2.5 to 0.2-2.5. The method adopts the trichloromethyl chloroformate to prepare the key intermediate 2-methyl-2-propyl-1, 3-propanediol diester carbonate of carisoprodol, has high yield, safety, no pollution, and simple operation and is a synthetic route with broad industrialization prospect.
Owner:ZHEJIANG UNIV OF TECH +1
Synthesis method of surfactant
PendingCN111004142AReduce selectionEmission reductionOrganic compound preparationTransportation and packagingActive agentWater chlorination
The invention discloses a synthetic method of a surfactant. The synthetic method comprises the following steps: (1) adding fatty alcohol, a catalyst and amino acid ester into a reactor; (2) closing the reactor, and slowly heating to 200-300 DEG C; (3) carrying out a heat preservation reaction for 2-10 hours; (4) degassing; and (5) treating to obtain a finished product. According to the invention,fatty alcohol is directly selected as an acylation reagent to perform a reaction, so that the step of fatty acid acylation is omitted, the selection of dangerous substance acylation reagents such as phosgene, thionyl chloride, trichloromethyl chloroformate, phosphorus trichloride, phosphorus pentachloride and phosphorus oxychloride is reduced, the emission of byproducts such as phosphorous acid and the like is reduced, and the method is feasible from the aspect of reaction mechanism; and fatty alcohol and amino acid ester are selected to be subjected to a direct reaction, so that impurities such as chlorine ions and the like are not introduced in the process, and the target product is obtained through the direct reaction so as to eliminate the steps of acidification, salification or waterwashing desalination and reduce the use and discharge of salt-containing wastewater or an organic solvent.
Owner:ZHANGJIAGANG GREAT CHEM
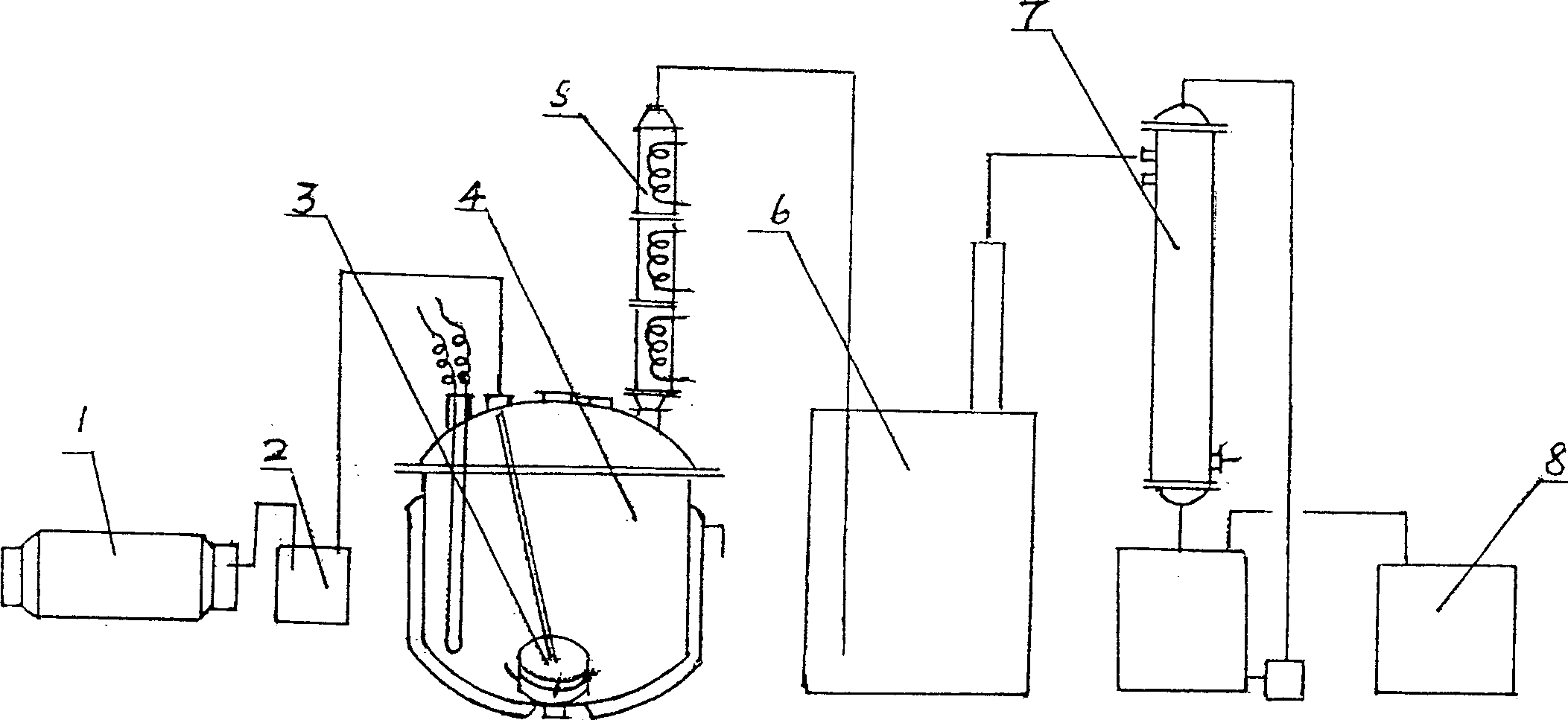
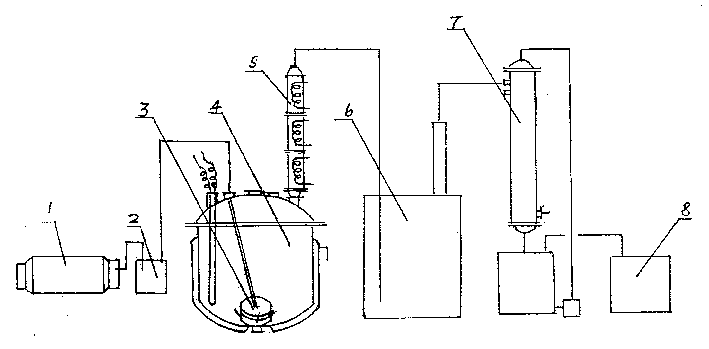
Prepn. method and apparatus of trichloromethy chloroformate
InactiveCN1389453ASafe recyclingReasonable principlePreparation from phosgene or haloformatesReaction temperatureChloroformate
The present invention relates to a preparation method of trichloromethyl chloroformate and its special-purpose equipment. It includes the following steps: placing methyl chlorocarbonate in an enamel reactor still; under the double catalytic action of liught and phosphorus pentachloride and under the conditino of that the reactor still temp. is 50-80 deg.C continuously introducing chlorine gas into the reactor still in speed of 8-15 kg / hr. to make chlorination reaction to produce trichloromethyl chloroformate and hydrogen chloride; reaction time is 48-70 hr., detecting specific weight of material d1(35 deg.C)=1.61-1.62, after the reaction reaches to end point, stopping introduction of chlorine gas, cooling and discharging to obtain the invented finished product trichloromethyl chloroformate whose purity is greater than or equal to 98%.
Owner:邵益丰
Technique for preparing trichloromethyl chloroformate
The invention relates to a method for preparing intermediate compound diphosgene for pesticide and medicine. It comprises following steps: taking methyl chloroformate as raw material, toluene as disslovant, injecting chlorine gas into pump with sepecial glass injecting pump, the proportion among methyl chloroformate, chlorine and toluene is: 1: 3.05- 3.5: 1- 2, stopping injecting chlorine gas when the reaction temperature reduces rapidly, heating to remove chlorine gas and getting product. The product content is between 50%- 70%.
Owner:梁建忠
Green synthetic method for methyl-3-ethyl-4-chlorin-5-pyrazol formyl chloride
InactiveCN101307022BAdvanced process routeReasonable process conditionsOrganic chemistryMethyl groupPyrazole
The invention discloses an environmental-friendly method for synthesizing 1-methyl-3-ethide-4-chlorine-5-pyrazole formyl chloride. The synthesizing method takes 1- methyl-3-ethide-4-chlorine-5- pyrazole formic acid which is shown as a formula (II) and bis (trichloromethyl) carbonate which is shown as a formula (III) as raw materials and takes organic amine as a catalyst. The raw materials and thecatalyst react sufficiently in an organic solvent at a temperature of between 20 and 80 DEG C; and the 1-methyl-3-ethide-4-chlorine-5-pyrazole formyl chloride which is shown as the formula (I) is obtained by processing the reaction fluid. Compared with prior art, the method has advanced process flow, reasonable process conditions, and cheap and available raw materials, avoids the phosgene, the Cloruro de tionilo and the trichloromethyl chloroformate, and has simple and safe operation, high reaction yield coefficient, low production cost, great implementation value and social and economical benefits and basically does not have 'three wastes'.
Owner:ZHEJIANG UNIV OF TECH
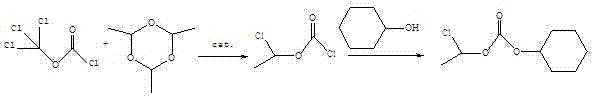
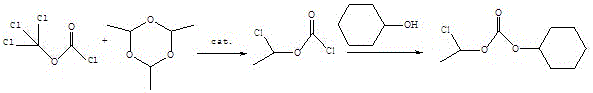
Preparation method of 1-chloroethyl cyclohexyl propyl carbonate
ActiveCN104496822AIncrease productionHigh purityCarbonic/haloformic acid esters purification/separationPreparation from phosgene or haloformatesDimethylaniline N-oxideCyclohexanol
The invention relates to a preparation method of 1-chloroethyl cyclohexyl propyl carbonate and belongs to the field of chemicals. The preparation method of 1-chloroethyl cyclohexyl propyl carbonate comprises the following steps of: adding triethylamine or pyridine or N,N-dimethylaniline into trichloromethyl chloroformate at the temperature between 10 DEG C below zero and 0 DEG C, and dropwise adding paraldehyde to carry out reaction; adding triethylamine or pyridine or N,N-dimethylaniline after reaction, dropwise adding cyclohexanol under the condition that the temperature in a reactor is below 20 DEG C, and stirring to carry out reaction; taking a product of reaction, washing with water, and distilling by reducing pressure to obtain an organic phase which is 1-chloroethyl cyclohexyl propyl carbonate. The synthesized 1-chloroethyl cyclohexyl propyl carbonate is high in yield and purity and low in cost, and the preparation method of 1-chloroethyl cyclohexyl propyl carbonate is easy to control in the preparation process, is easy to operate, is low in risk and is particularly suitable for industrial production.
Owner:扬州三友合成化工有限公司
Preparation method of 4,4'-dicyclohexylmethane diisocyanate
ActiveCN103553969BLower requirementAccurate feeding and meteringIsocyanic acid derivatives preparationOrganic compound preparationSimple Organic CompoundsSolvent
The invention belongs to the technical field of an organic compound, and particularly relates to a method for preparing 4,4'-dicyclohexylmethane diisocyanate by utilizing a non-phosgene process. The method comprises the following step: reacting 4,4'-dicyclohexylmethane diisocyanate or an isomer mixture or salt thereof, or a mixture of amine and salt thereof which are as raw materials with bis(trichloromethyl) carbonate or trichloromethyl chlorocarbonate or a mixture thereof in an inert solvent. By adopting the method disclosed by the invention, the defects that preparation of the 4,4'-dicyclohexylmethane diisocyanate by utilizing the traditional phosgene process is long in process route, complicated in technique, poor in safety and the like are overcome. The method disclosed by the invention has the advantages of being convenient to operate, high in safety, environment-friendly, and accurate in reactant feeding and metering, and the feeding ratio is reduced, so that the safety coefficient of production is improved. In addition, exhaust treatment is simple; high-purity hydrochloric acid can be prepared from a hydrogen chloride gas generated by water absorption reaction; chloridion can be fully utilized; environmental conservation is facilitated.
Owner:UPCHEM CHINA
Process for preparing isocyanate by phosgenation reaction
InactiveCN113105365AHighly corrosiveLess economic valueIsocyanic acid derivatives preparationOrganic compound preparationDistillationCarbonate ester
The invention relates to the field of preparation methods of isocyanate, and concretely relates to a process for preparing isocyanate by phosgenation reaction. The process comprises the following steps: mixing and stirring di(trichloromethyl) carbonate and a solvent in a preparation kettle to obtain trichloromethyl chloroformate; dropwise adding hydrochloric acid into the amine solution, heating, and condensing to remove water; mixing the amine solution and trichloromethyl chloroformate in a reactor to obtain crude isocyanate; introducing inert gas into the reactor, discharging hydrogen chloride generated by reaction from the reactor into a tail gas collecting box, and treating gas in the tail gas collecting box; and purifying the crude isocyanate through a distillation tower to obtain the isocyanate product. The preparation process adopted by the invention is more environment-friendly and safer, and can bring higher economic benefits.
Owner:新沂市永诚化工有限公司
Method for synthesizing 4-nitrobenzyl chloroformate
InactiveCN100439322CEliminate production safety hazardsThe amount of three wastes is lessOrganic chemistryOrganic compound preparationOrganic solventChloroformate
Owner:ZHEJIANG UNIV OF TECH
A kind of preparation method of 1-chloroethyl cyclohexyl propyl carbonate
ActiveCN104496822BIncrease productionHigh purityCarbonic/haloformic acid esters purification/separationPreparation from phosgene or haloformatesDimethylaniline N-oxideCyclohexanol
The invention relates to a preparation method of 1-chloroethyl cyclohexyl propyl carbonate and belongs to the field of chemicals. The preparation method of 1-chloroethyl cyclohexyl propyl carbonate comprises the following steps of: adding triethylamine or pyridine or N,N-dimethylaniline into trichloromethyl chloroformate at the temperature between 10 DEG C below zero and 0 DEG C, and dropwise adding paraldehyde to carry out reaction; adding triethylamine or pyridine or N,N-dimethylaniline after reaction, dropwise adding cyclohexanol under the condition that the temperature in a reactor is below 20 DEG C, and stirring to carry out reaction; taking a product of reaction, washing with water, and distilling by reducing pressure to obtain an organic phase which is 1-chloroethyl cyclohexyl propyl carbonate. The synthesized 1-chloroethyl cyclohexyl propyl carbonate is high in yield and purity and low in cost, and the preparation method of 1-chloroethyl cyclohexyl propyl carbonate is easy to control in the preparation process, is easy to operate, is low in risk and is particularly suitable for industrial production.
Owner:扬州三友合成化工有限公司
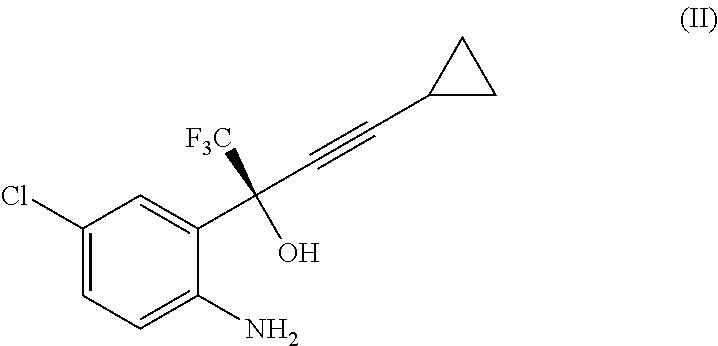
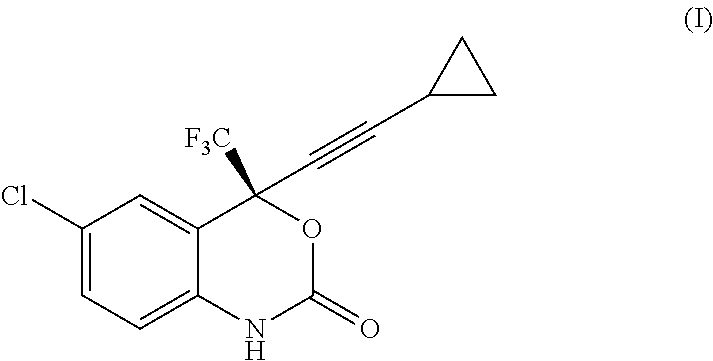
Process for the preparation of efavirenz
The present invention is directed to a process for the preparation of Efavirenz, (4S)-6-chloro-4-(cyclopropy-lethynyl)-4-(trifluoromethyl)-1,4-dihydro-2H-3,1-benzoxazin-2-one, of formula (I) comprising reacting the intermediate of formula (II) [0045] as a free base or a salt thereof, with diphosgene (TCMCF, trichlormethylchloroformate) Cl3CO—COCl in an organic solvent or in a biphasic medium comprised of an organic solvent and water, preferably but not mandatorily in the presence of a weak base in an amount sufficient to neutralise the reaction mixture or in an up to 30% molar excess of such amount.
Owner:F I S FAB ILTALIANA SINTETICI SPA
A kind of halogen-free environment-friendly polyurethane oil-based paint and preparation method thereof
ActiveCN104610525BSolve the problem of difficult flame retardantHigh oxygen indexFireproof paintsPolyurea/polyurethane coatingsAnilineSolvent
The invention discloses a preparation method of a halogen-free environment-friendly polyurethane oil paint. The preparation method comprises the following steps: performing refluxing catalytic hydrogenation reduction on a phosphatizing agent and a nitrobenzene derivative in an organic solvent with the presence of a metal catalyst to obtain a phosphatized aniline derivative, dropwise adding trichloromethyl chloroformate, mixing and stirring uniformly at room temperature, reacting continuously for 2-4 hours to obtain phosphatized phenyl isocyanate, stirring the phosphatized phenyl isocyanate and an alcoholic compound for 3 hours with the presence of a polymerization catalyst, adding an amine compound, stirring continuously for 2 hours, performing vacuum dewatering on a reaction mixture to obtain flame-retardant polyurethane resin, adding a curing agent and a solvent, and reacting to obtain the halogen-free environment-friendly polyurethane oil paint. The invention further discloses the halogen-free environment-friendly polyurethane oil paint. The halogen-free environment-friendly polyurethane oil paint is an expansive environment-friendly flame-retardant polyurethane oil paint with a phosphorous-nitrogen synergistic effect. In the production process of a flame retardant, raw materials are cheap and easily obtained; the preparation method is free of a high-heat and high-pressure process, and simple to operate, and energy consumption in the production process is low; the whole production process is saves energy and is environment-friendly.
Owner:安徽科赛富新材料科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com