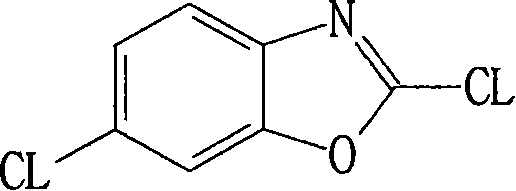
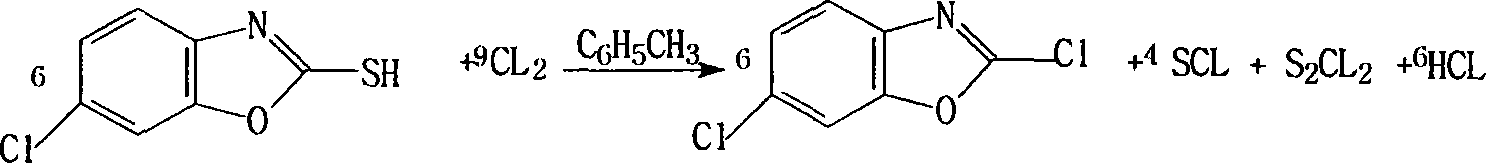
Method for preparing 2,6-dichloro benzoxazole
A technology of dichlorobenzoxazole and mercaptobenzoxazole, applied in directions such as organic chemistry, can solve the problems of producing phosphorus oxychloride by-products, high requirements for high vacuum equipment, and increasing the difficulty of separation, and achieves product yield. High rate, low equipment requirements, and excellent quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025] Example 1: Set up a stirrer, thermometer, dropping funnel and condenser in a 500ml reaction vessel, add 150ml of toluene, 50g of 6-chloro-2-mercaptobenzoxazole and 0.8g of dimethylformamide, stir, and heat to 60°C , Trichloromethyl chloroformate was added dropwise, after the addition, the temperature was raised to 80°C for reaction, and after 3 hours, the product was cooled to obtain 152g with a content of 30.3% and a yield of 90.9%.
[0026] Example 2: Set up a stirrer, thermometer, dropping funnel and condenser in a 500ml reaction vessel, add 150ml of toluene, 50g of 6-chloro-2-mercaptobenzoxazole and 1g of dimethylformamide, stir, and heat to 60°C, Trichloromethyl chloroformate was added dropwise, and after the addition, the temperature was raised to 80° C. to react for 3 hours, and the product was cooled to obtain 152 g with a content of 30.6% and a yield of 91.8%.
[0027] Example 3: Set up a stirrer, thermometer, dropping funnel and condenser in a 500ml reaction vessel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com