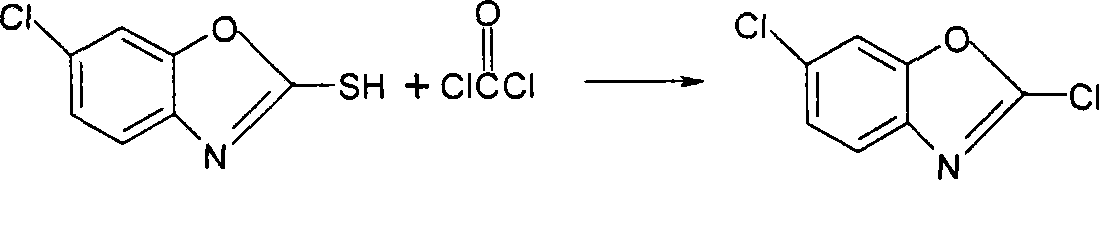
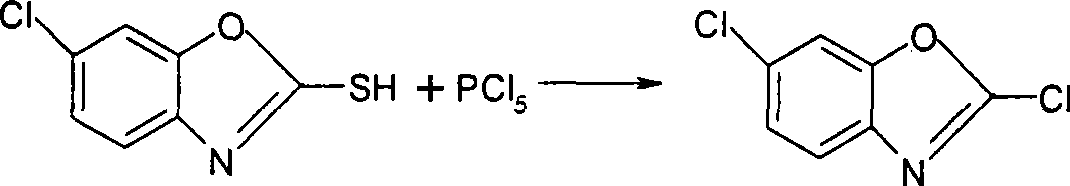Method for synthesizing 2,6-dichlorobenzoxazole
A technology of dichlorobenzoxazole and chlorobenzoxazole, which is applied in the field of pesticide synthesis, can solve the problem that phosgene is not allowed to be stored, and achieve the effects of production cost advantage, good quality and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Put 50 grams (100 percent) of 2-mercapto-6-chlorobenzoxazole, 250 ml toluene and 5 g DMF into a 500 ml four-necked reaction flask, stir and raise the temperature to 70-80 ° C, and start adding the weighed trichlorochloroformate dropwise. 30 grams of methyl ester (100 percent), the dropwise addition time is 7-8 hours, and the reflux reaction is 1.5 hours after the dropwise addition.
[0024] Carry out vacuum decompression precipitation, first remove toluene, then carry out high vacuum decompression distillation, and collect 85-95°C / -0.098~-0.095mpa distillate. The weight is 49 grams, the content is 98.5%, and the molar yield is 95.4%.
[0025] mp: 47~49℃.
[0026] Data:IR(KBr):v 3271.4,3105.9,3036.1,1880.2,1781.5,1731.7,1613.8,1602.2,1528.8,1479.5,1455.8,1422.9,1392.4,1340.3,1322.4,1303.4,1259.7,1242.5,1218.3,1170.4,1143.7, 1114.3, 1110.5, 1050.8, 1026.9, 941.2, 913.7, 867.1, 827.9, 815.0, 805.1, 756.3, 743.1, 730.8, 712.5, 701.8, 667.9, 626.5, 593.5, 554.0, 497.2, 47 ...
Embodiment 2
[0028] Put 50 grams (100 percent) of 2-mercapto-6-chlorobenzoxazole, 250 ml of toluene and 5 grams of DMF into a 500 ml four-necked reaction flask, stir and raise the temperature to 70-80 ° C, and start adding the weighed chloroformic acid dropwise Trichloromethyl ester 55 grams (100 percent), dropwise addition time 6-7h, add flow reaction 1.5 hours after the end of dropwise addition.
[0029] Carry out vacuum decompression precipitation, first remove toluene, then carry out high vacuum decompression distillation, and collect 85-95°C / -0.098~-0.095mpa distillate. The weight is 49.2 grams, the content is 98.49%, and the molar yield is 95.8%.
Embodiment 3
[0031] Put 50 grams (100 percent) of 2-mercapto-6-chlorobenzoxazole, 250 ml of toluene and 5 grams of DMF into a 500 ml four-necked reaction flask, stir and raise the temperature to 70-80 ° C, and start adding the weighed chloroformic acid dropwise Trichloromethyl ester 80 grams (100 percent), dropwise time 5-6h, reflux reaction for 2 hours after the dropwise addition.
[0032] Carry out vacuum decompression precipitation, first remove toluene, then carry out high vacuum decompression distillation, and collect 85-95°C / -0.095~-0.098mpa distillate. The weight is 49 grams, the content is 98.38%, and the molar yield is 95.32%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com