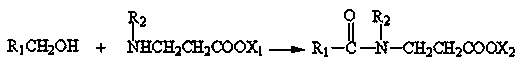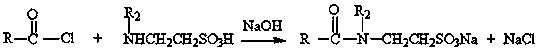Synthesis method of surfactant
A surfactant and synthesis method technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of insufficient green raw materials, "three wastes" discharge, low yield, etc., and improve the convenience of application Advantages, reduced use and emissions, and ease of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The synthetic method of the surfactant of the present embodiment is as follows:
[0042] Add 0.1 mol of tetradecyl alcohol, 0.15 mol of alanine ethyl ester, 0.05 mol of alanine methyl ester, and 0.02 g of manganese acetate into the reactor, close the reactor, slowly heat up to 210°C, and keep warm for 4 hours, connect to vacuum, vacuum degree -0.08~-0.09MPa, degas for 2 hours, adjust the pH value at 6.5~7.5, filter to obtain a light yellow solid product, and the conversion rate of lauryl alcohol is 81.4%.
Embodiment 2
[0044] The synthetic method of the surfactant of the present embodiment is as follows:
[0045] Add 0.1 mol of dodecyl alcohol, 0.14 mol of ethyl alanine, 0.03 g of manganese acetate, and 0.01 g of ferric oxide into the reactor, seal the reactor, slowly raise the temperature to 260°C, keep it warm for 3 hours, and insert Vacuum, vacuum degree -0.08~-0.09MPa, degas for 2 hours, adjust the pH value at 6.5~7.5, add water to adjust the solid content to 30.5%, filter to obtain a light yellow transparent liquid product, the conversion rate of lauryl alcohol is 91.2%.
Embodiment 3
[0047] The synthetic method of the surfactant of the present embodiment is as follows:
[0048]0.1mol lauryl alcohol, 0.1mol tetradecyl alcohol, 0.08mol methyl alanine, 0.1mol ethyl alanine, 0.025 gram of manganese acetate are added to the reactor, the reactor is closed, and the temperature is slowly raised to 200°C, heat preservation reaction for 6 hours, connect to vacuum, vacuum degree -0.08~-0.09MPa, degas for 2 hours, adjust the pH value at 6.5~7.5, filter to obtain a light yellow solid product, and the conversion rate of fatty alcohol is 91.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com