Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
99 results about "Pericardium tissue" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
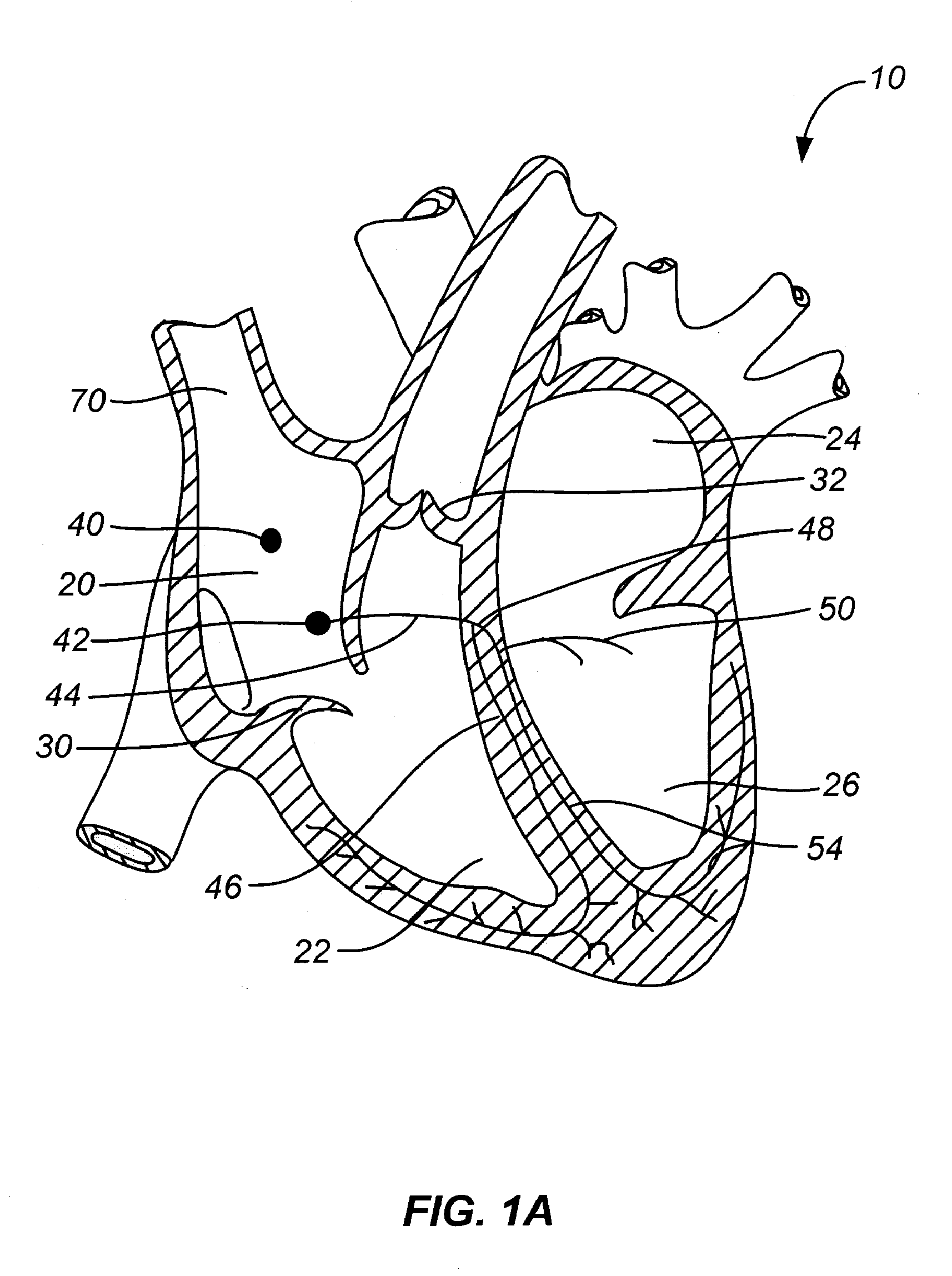
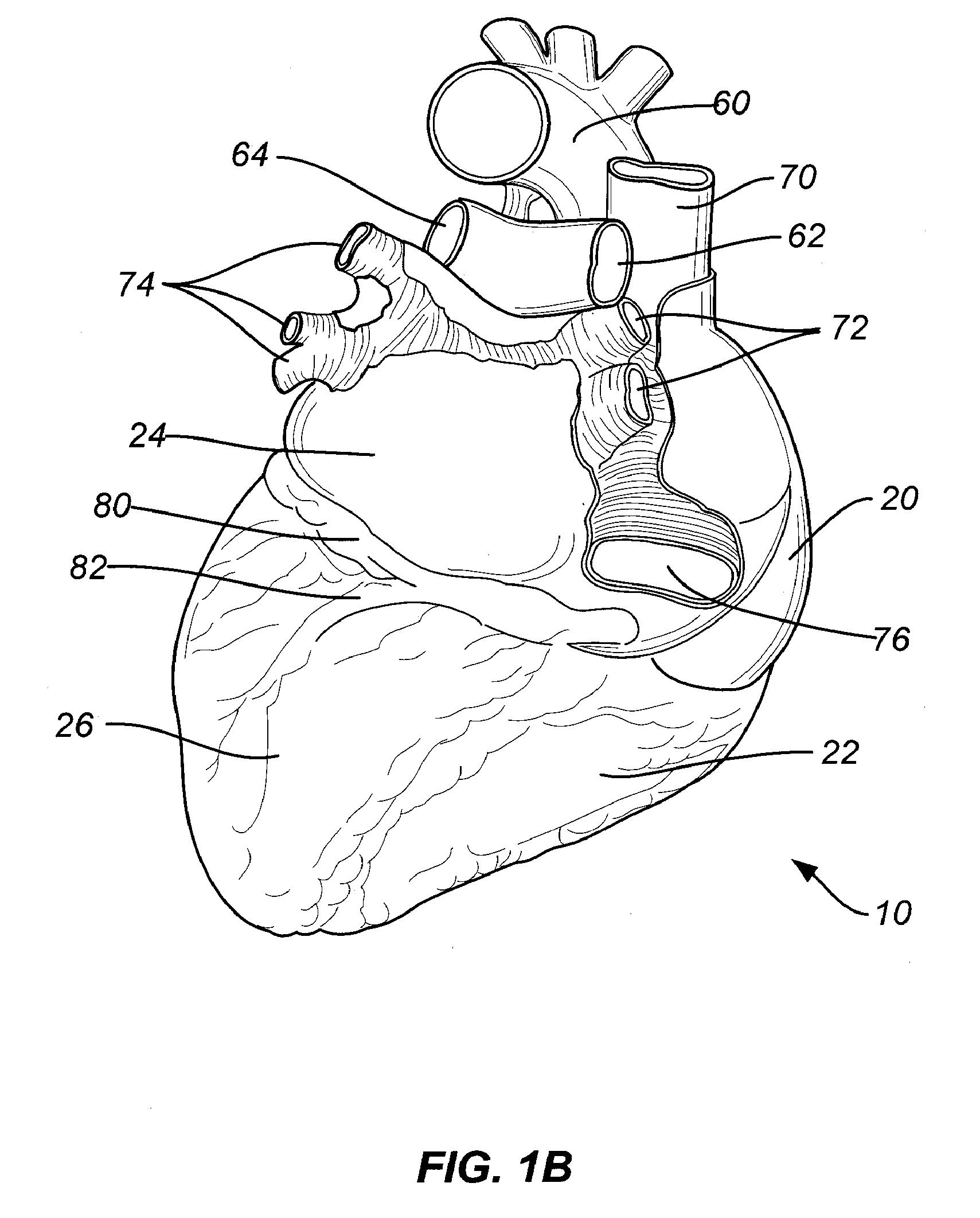
Fibrous pericardium. The fibrous pericardium is the most superficial layer of the pericardium. It is made up of dense and loose connective tissue, which acts to protect the heart, anchoring it to the surrounding walls, and preventing it from overfilling with blood.
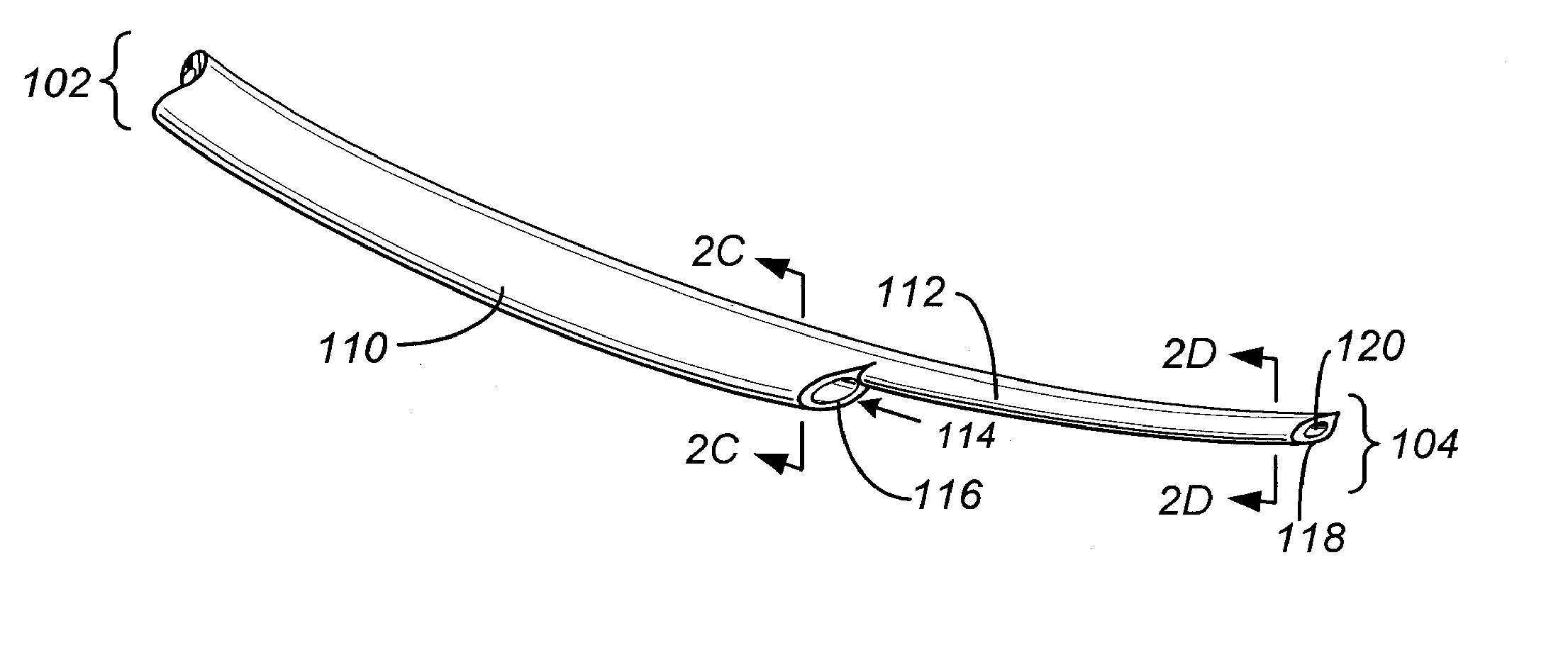
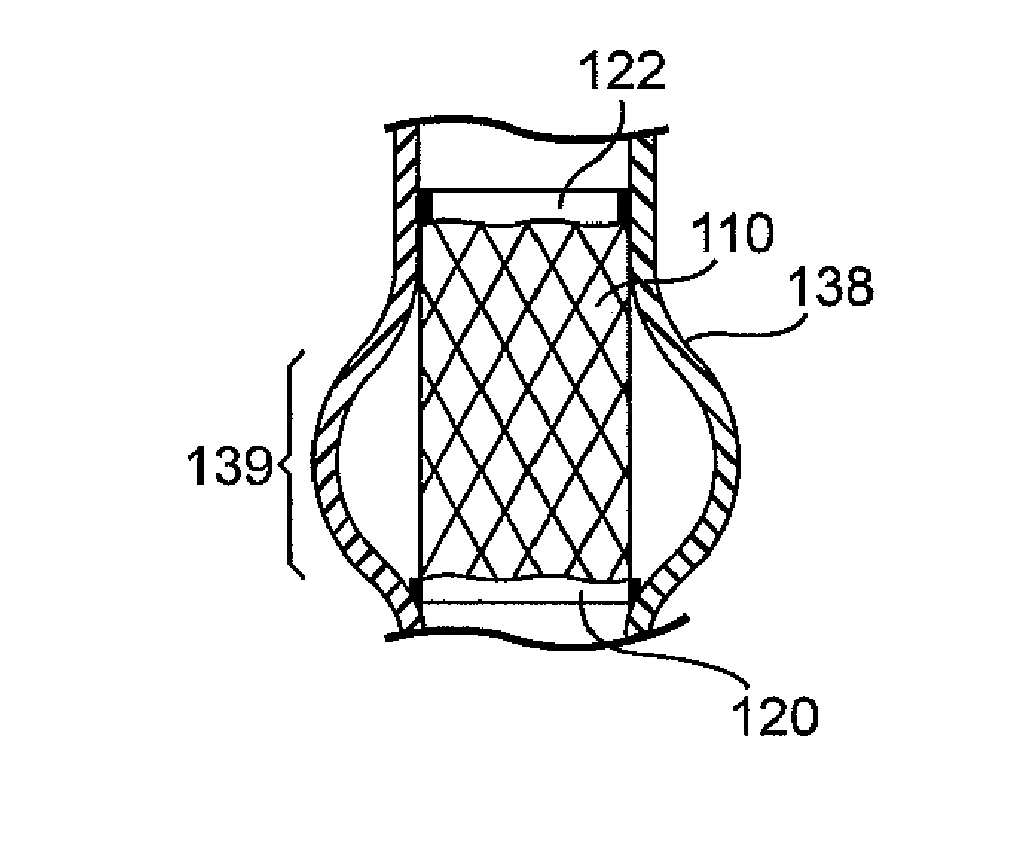
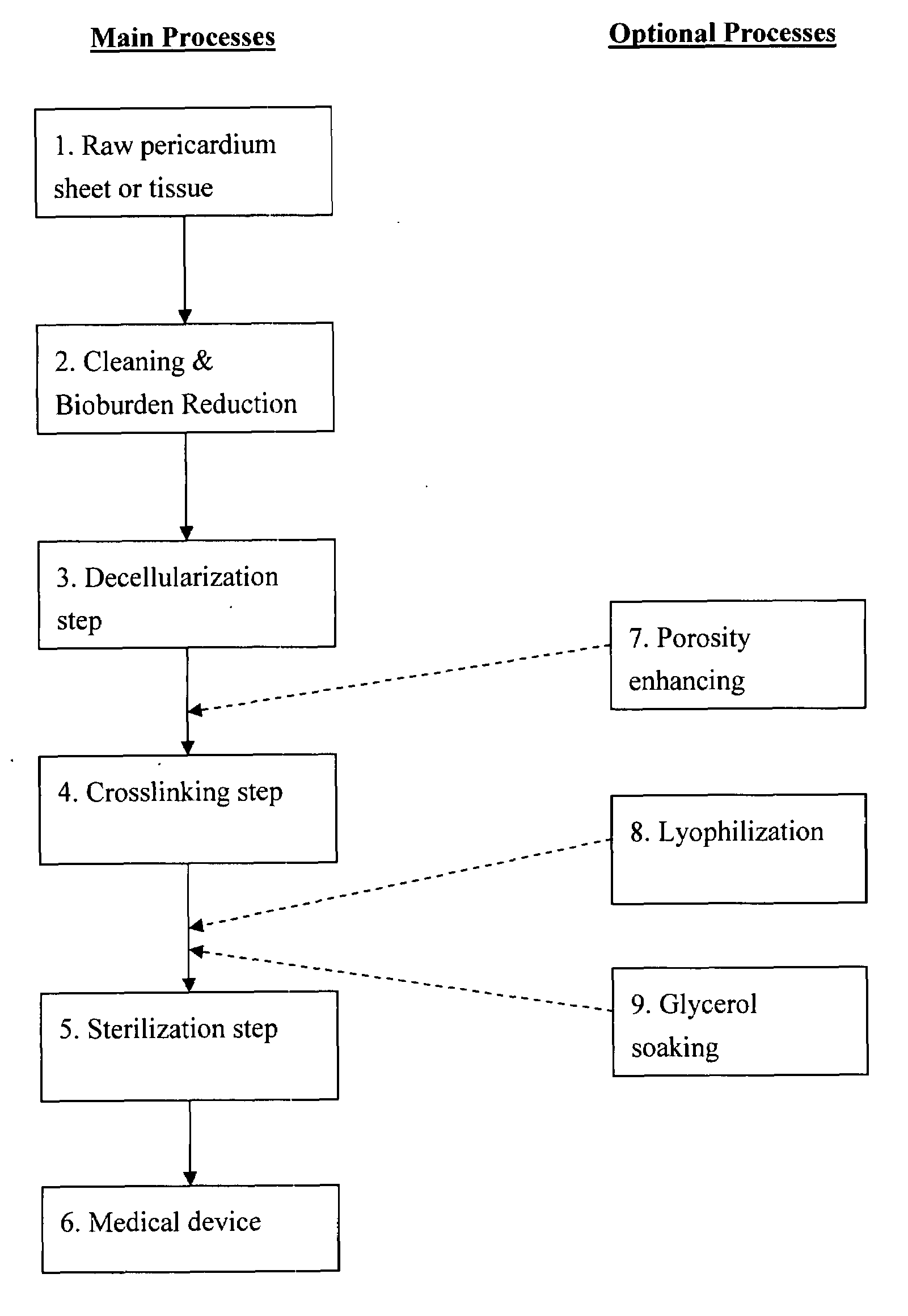
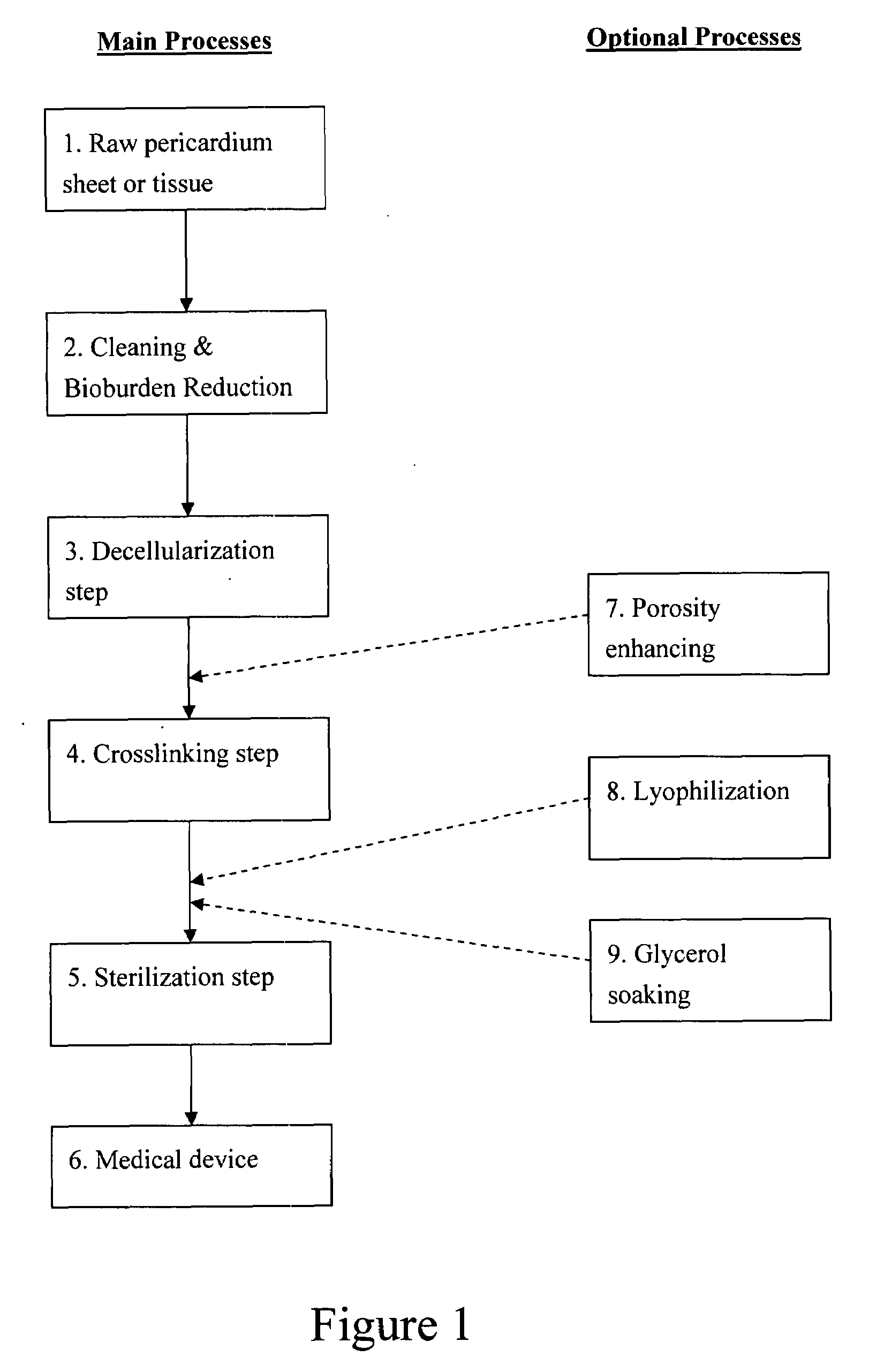
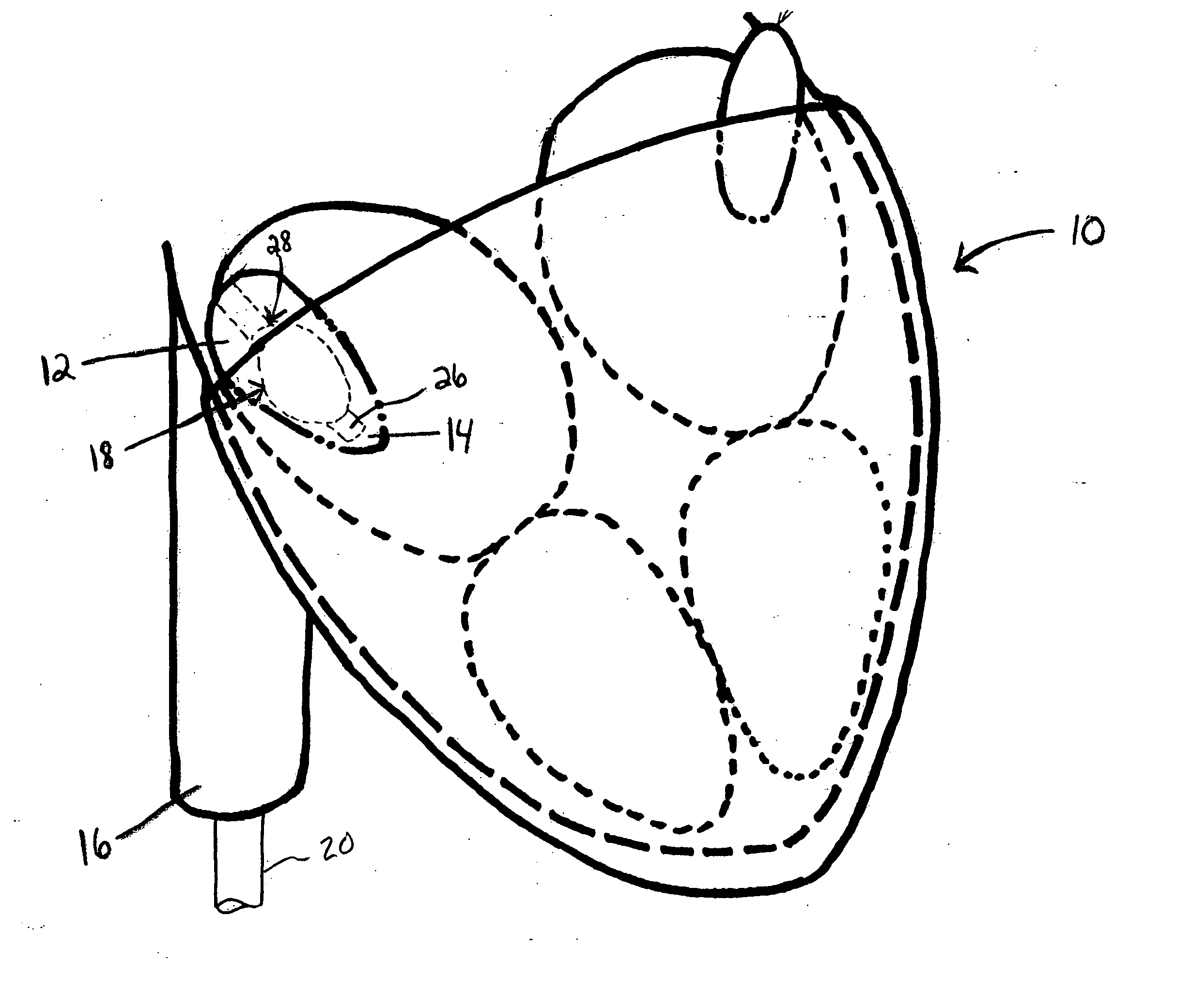
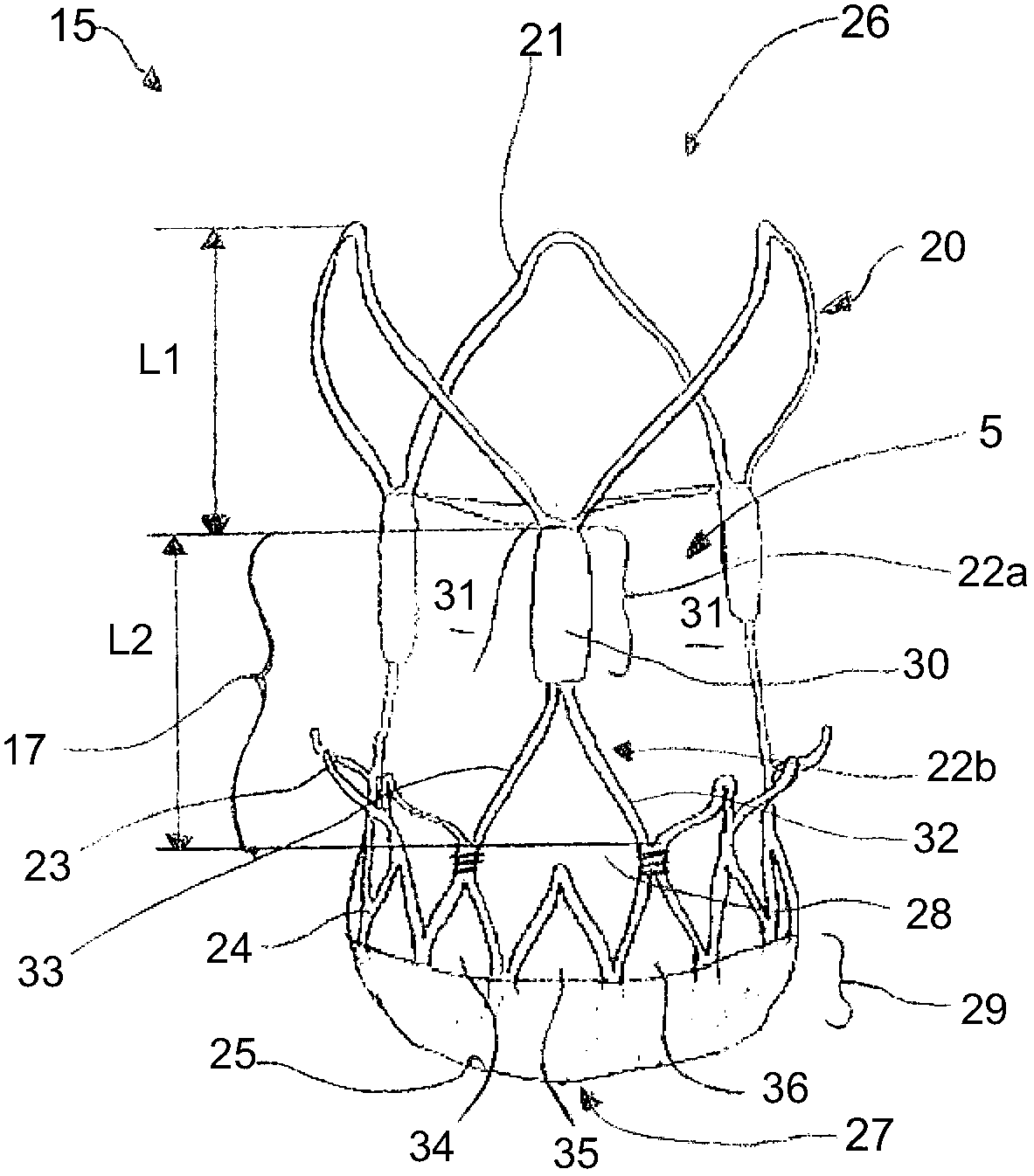
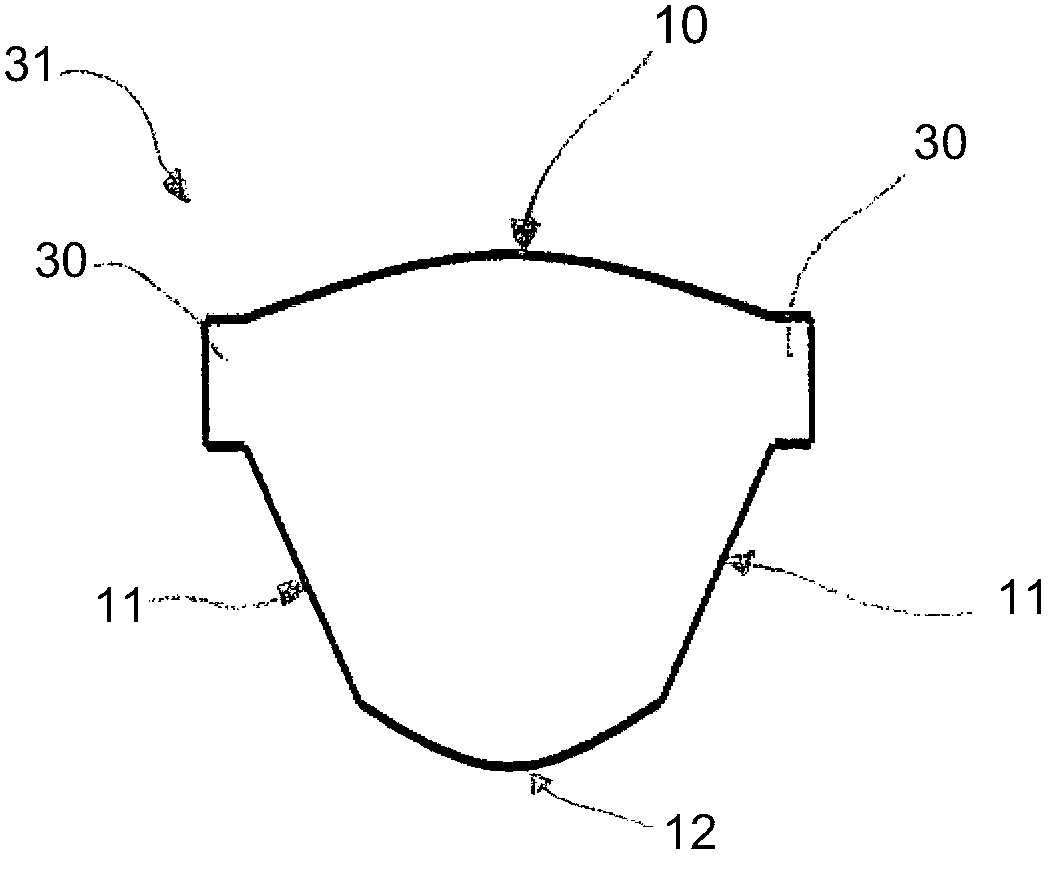
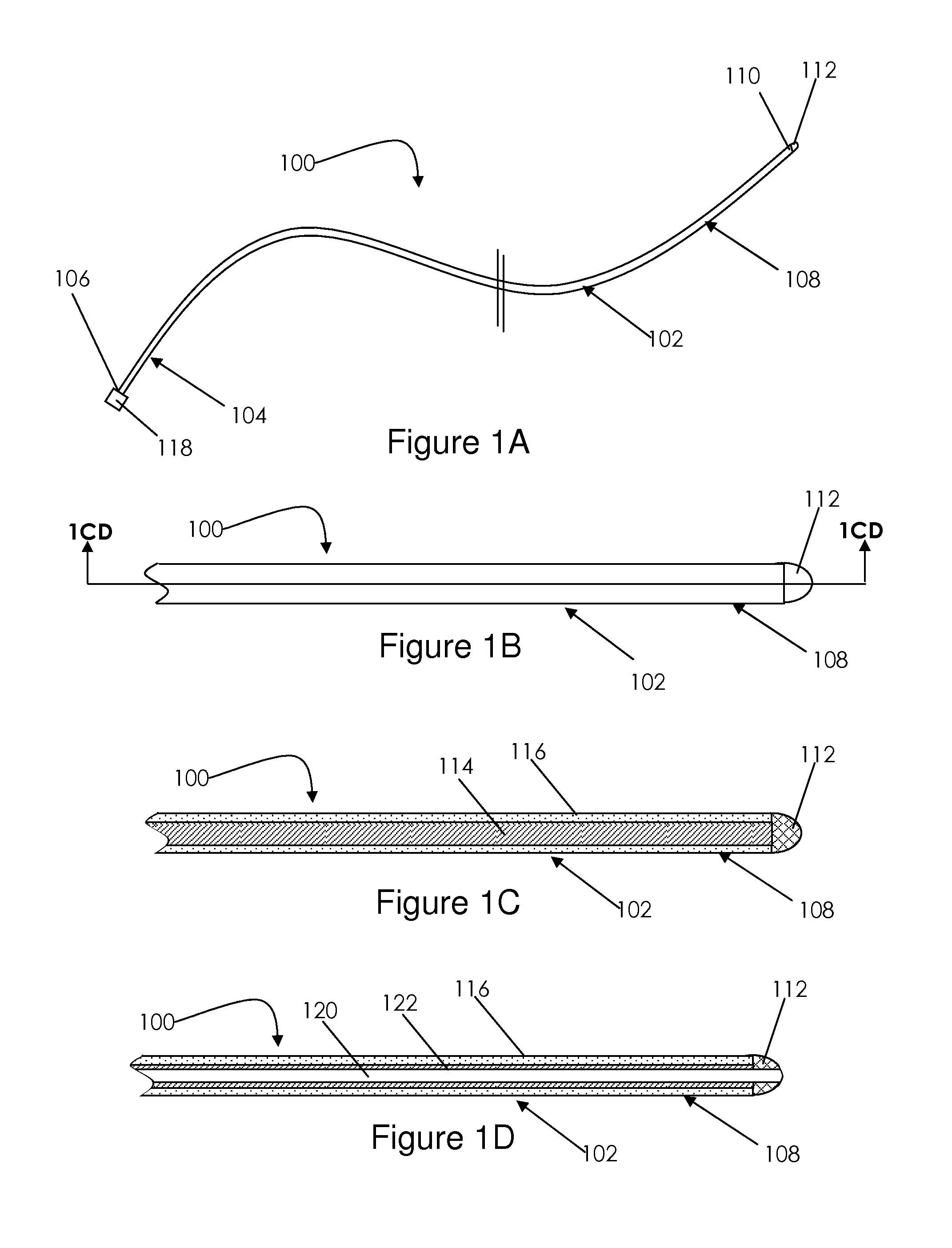
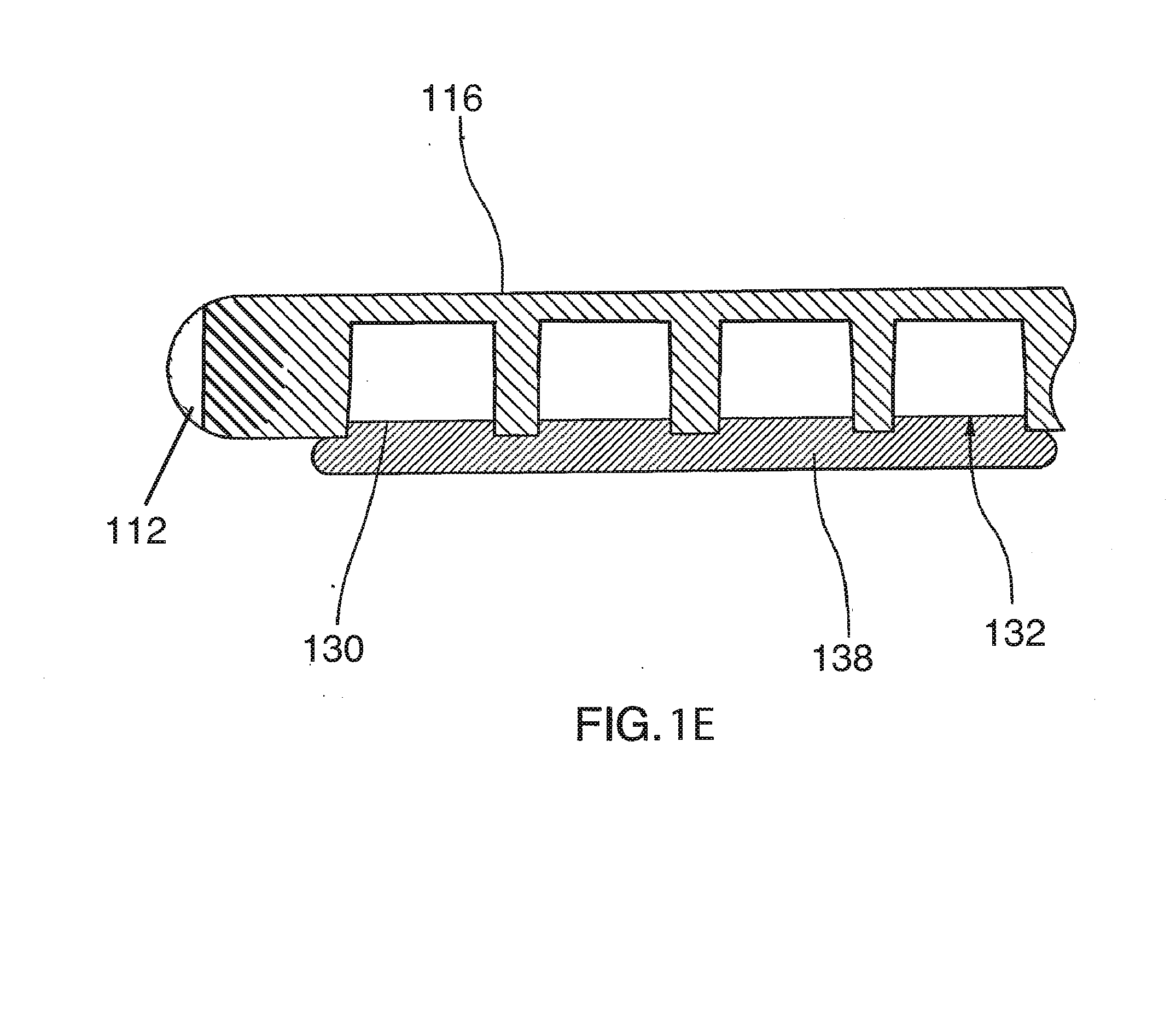
Prosthetic cardiac valve formed from pericardium material and methods of making same
ActiveUS20070233228A1Barrier to undesired abrasionPrevent and minimize valve leakageHeart valvesFinal product manufactureProsthetic valveProsthesis
Owner:MEDTRONIC INC
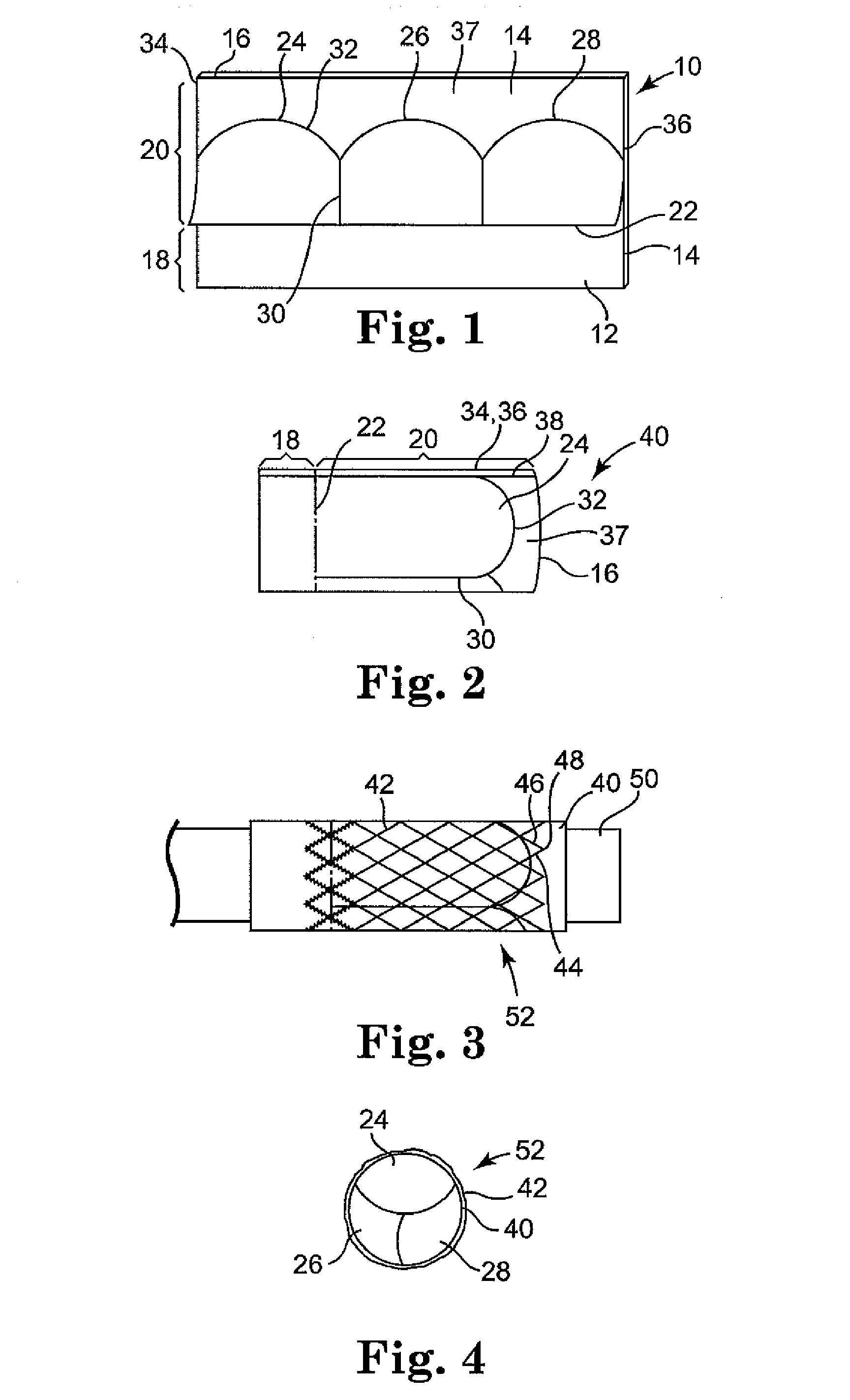
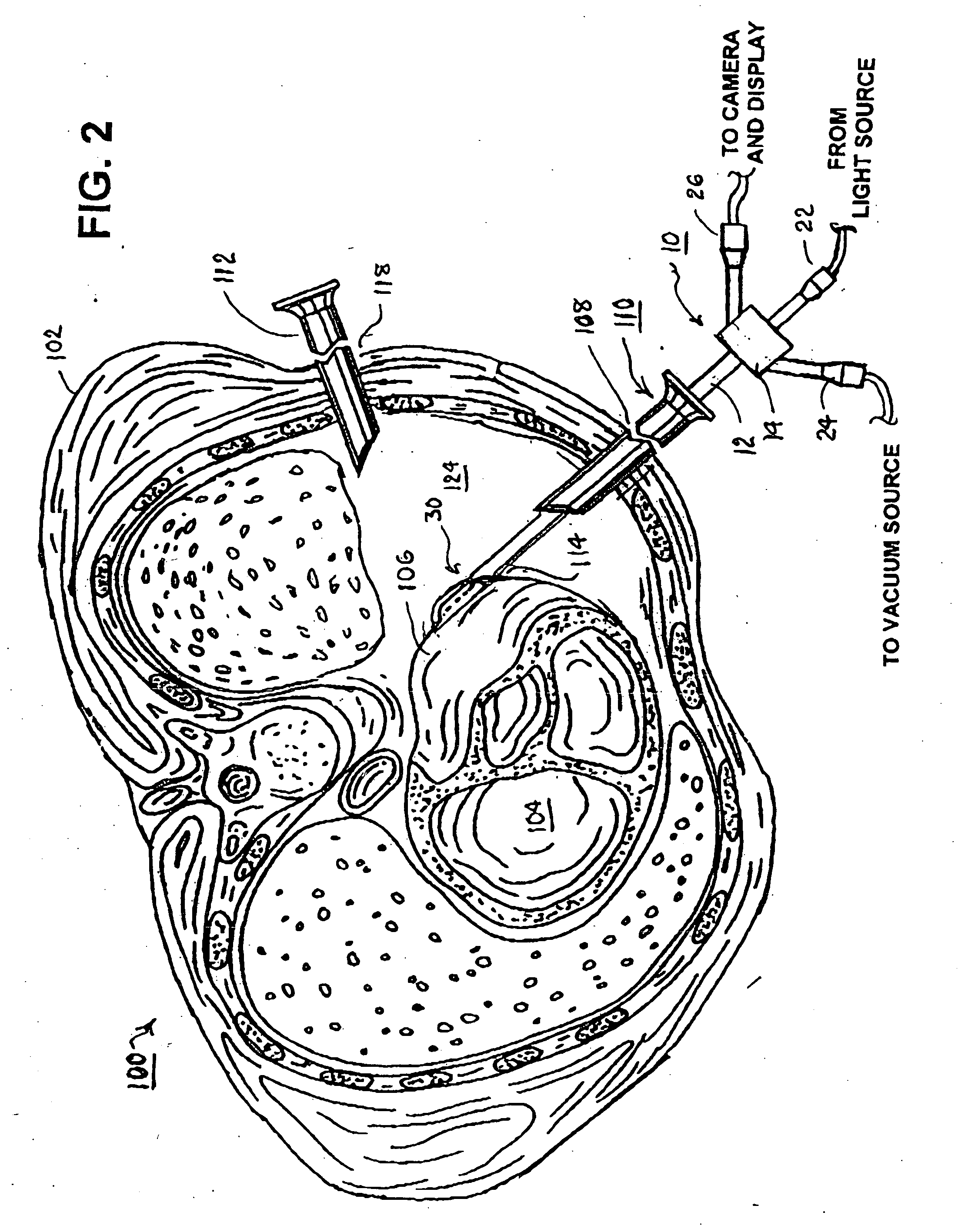
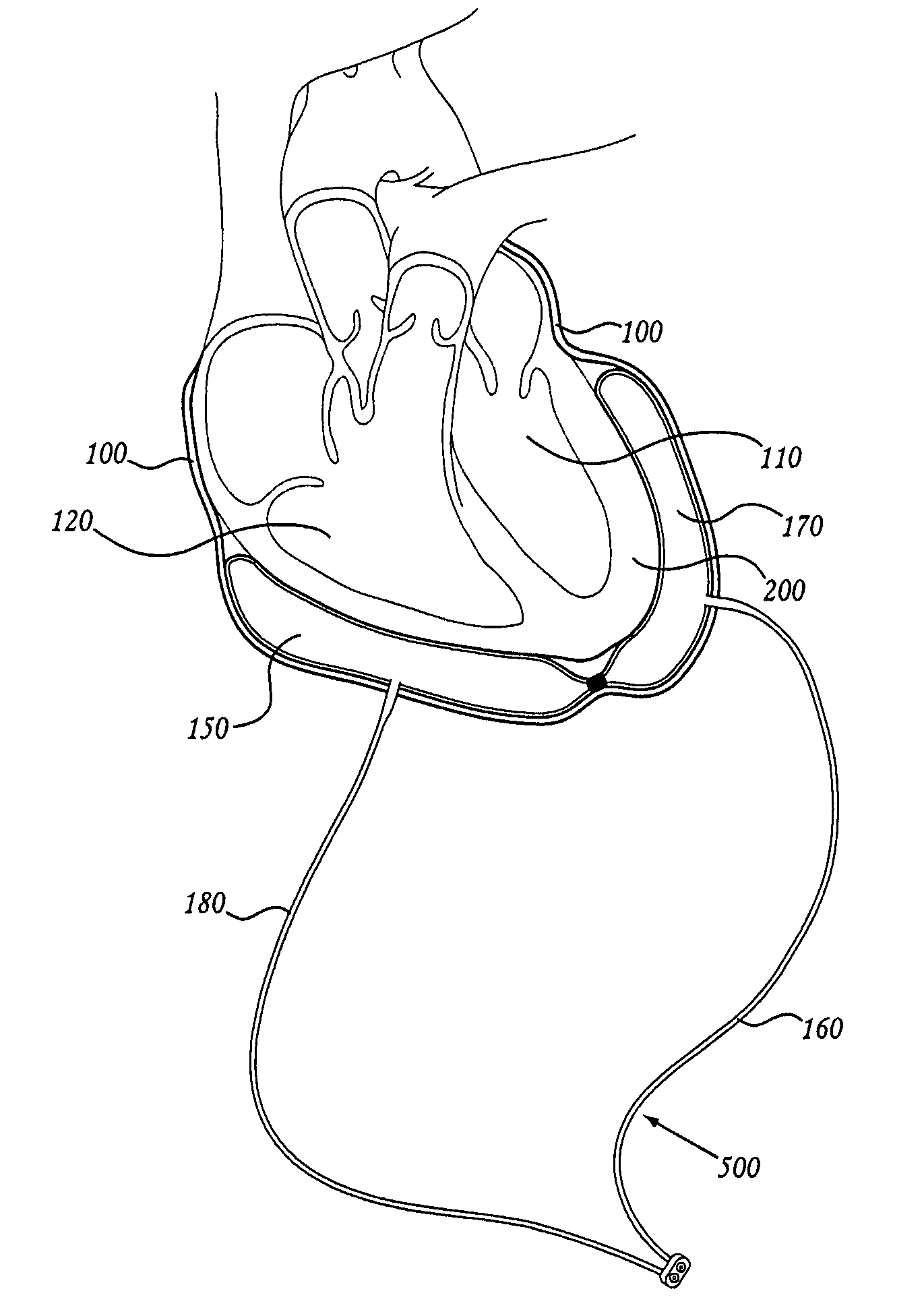
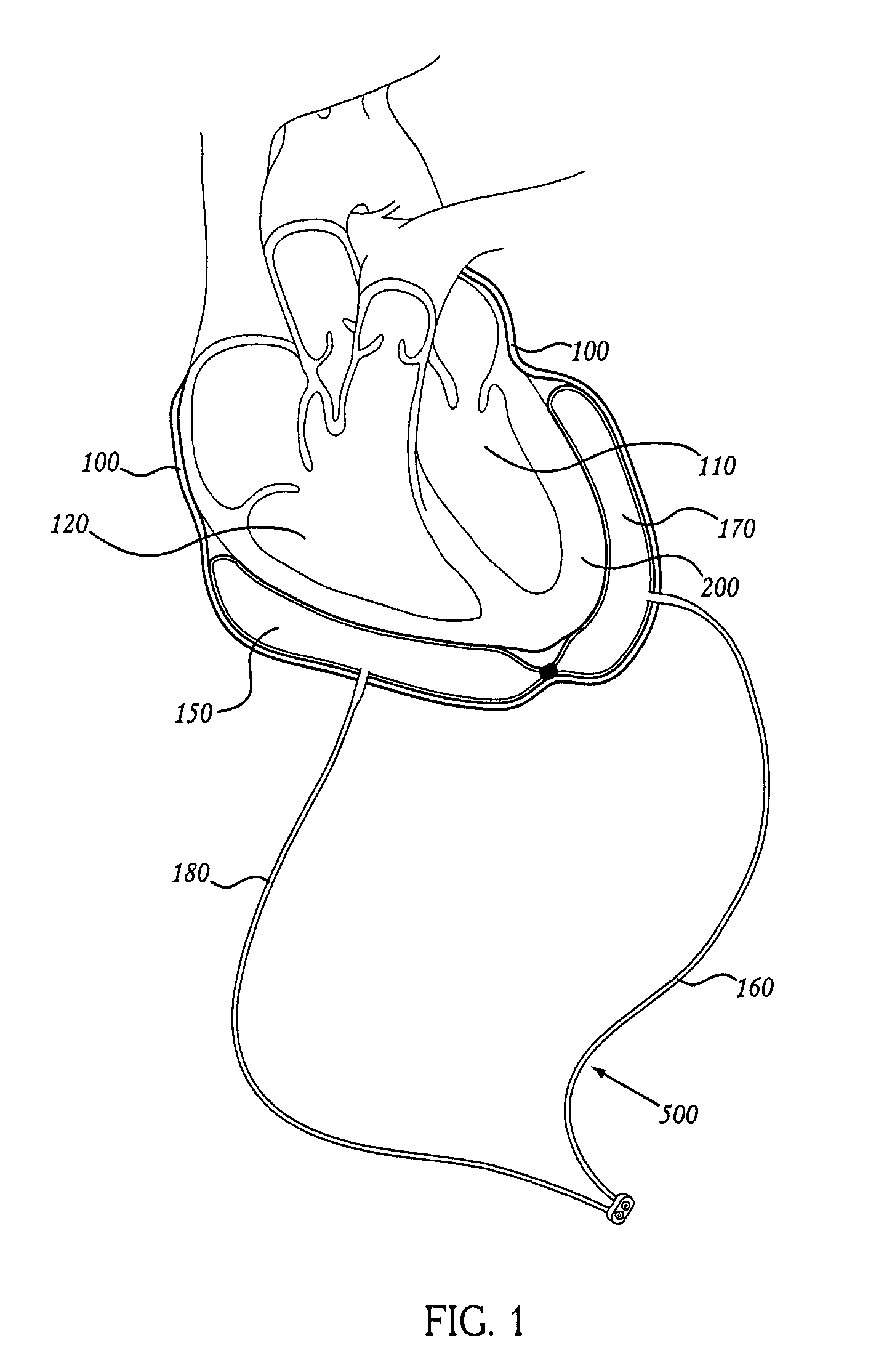
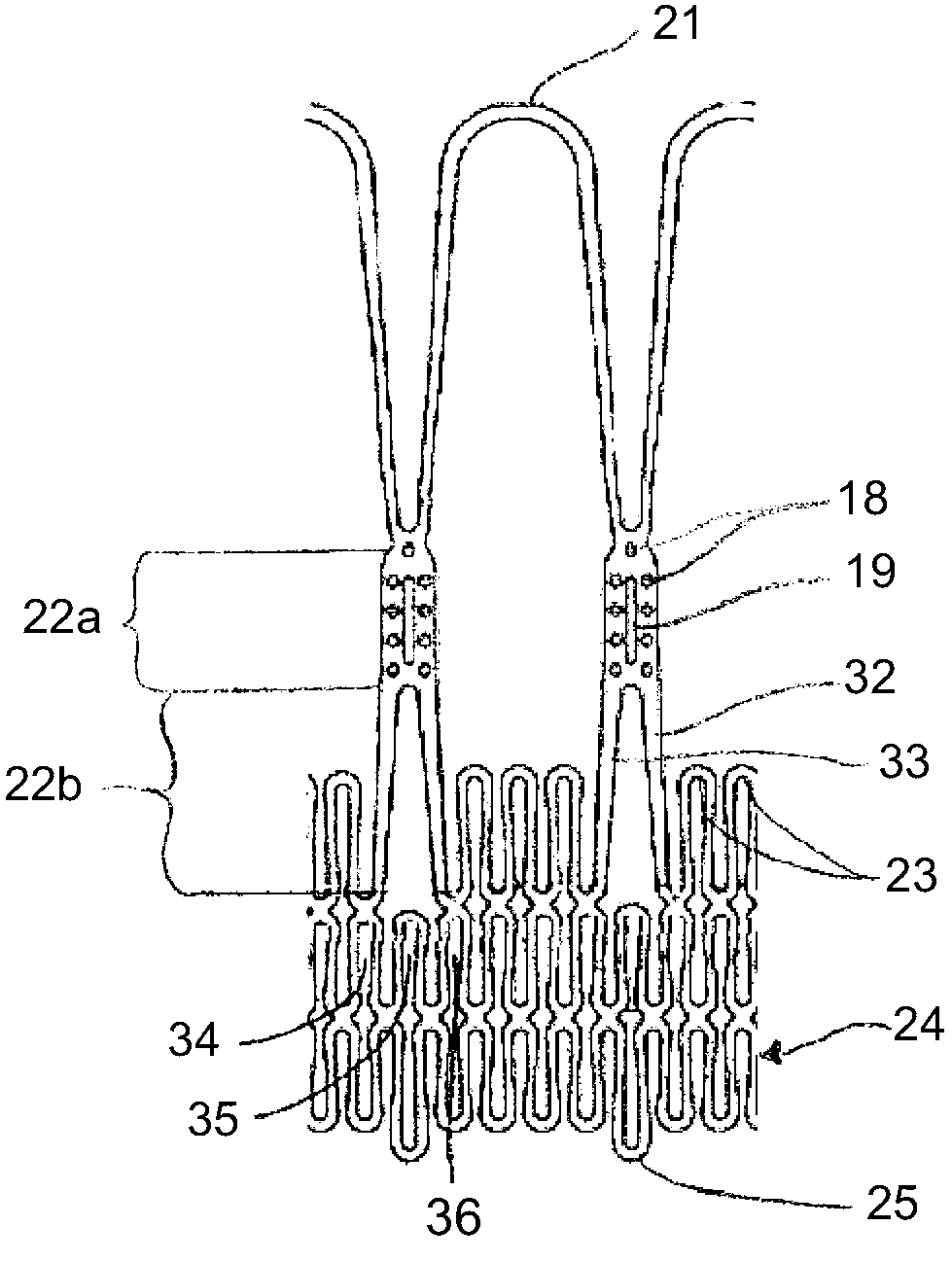
Methods and apparatus for accessing and stabilizing an area of the heart
Owner:MEDTRONIC INC
Methods and apparatus for accessing and stabilizing an area of the heart
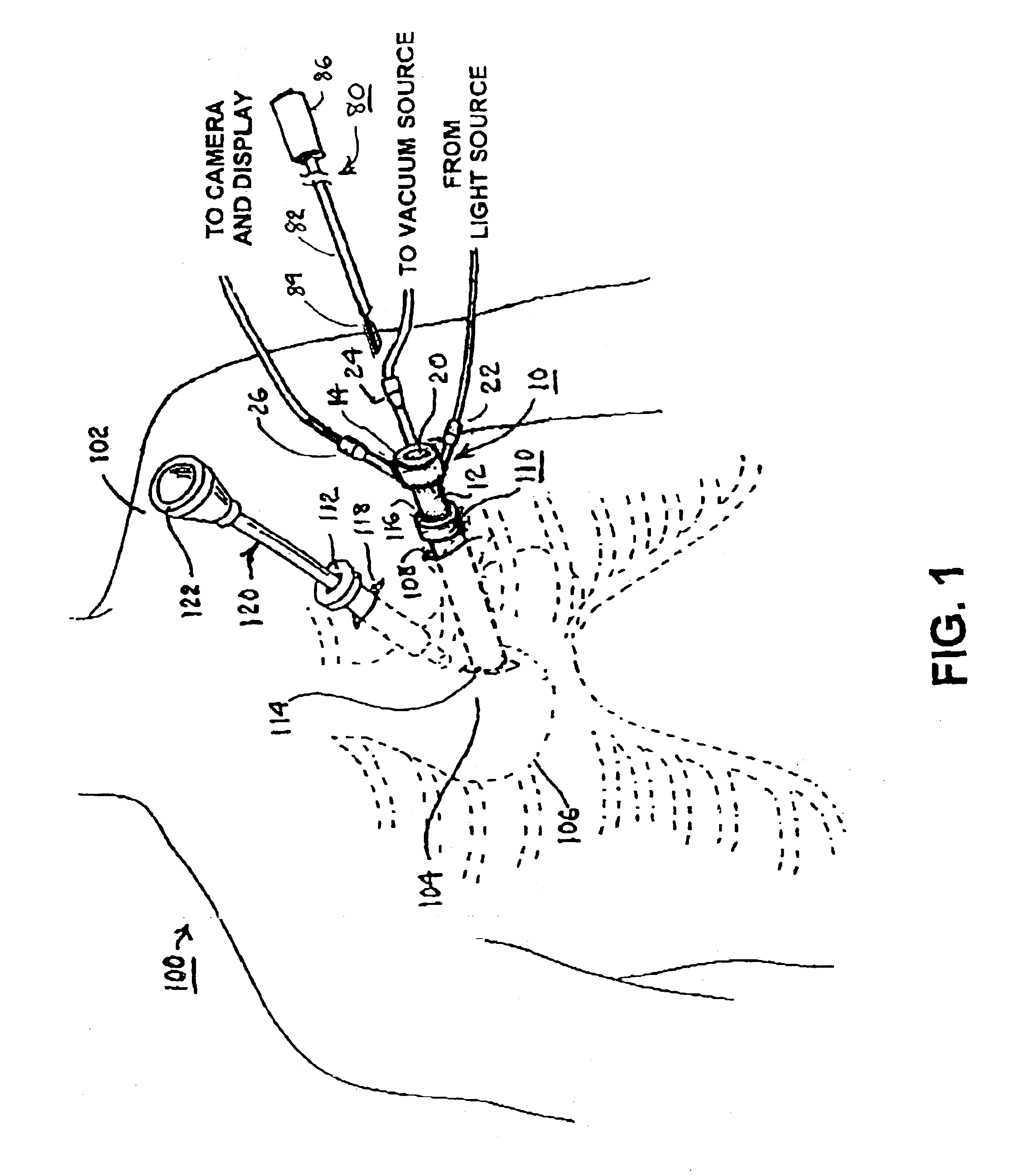
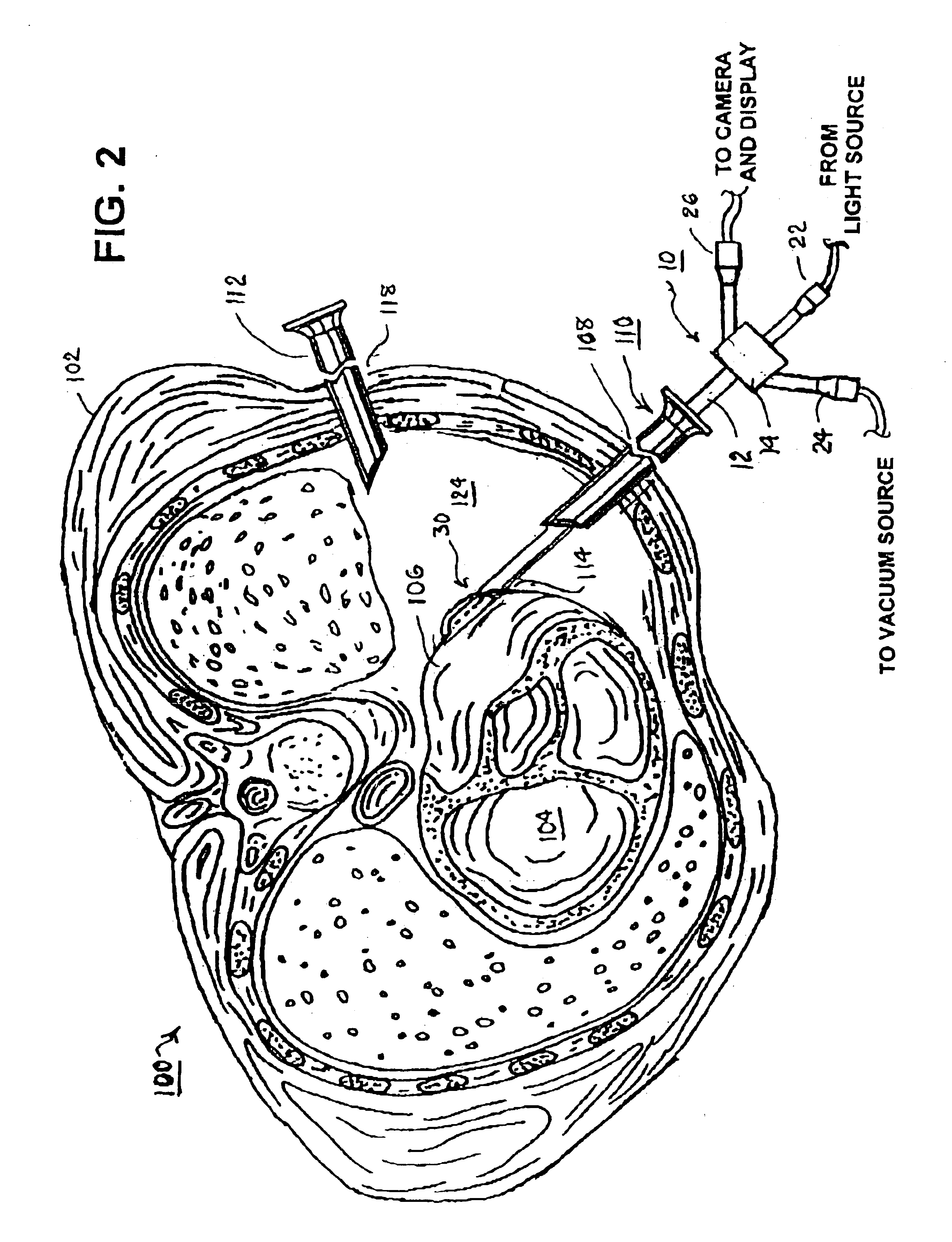
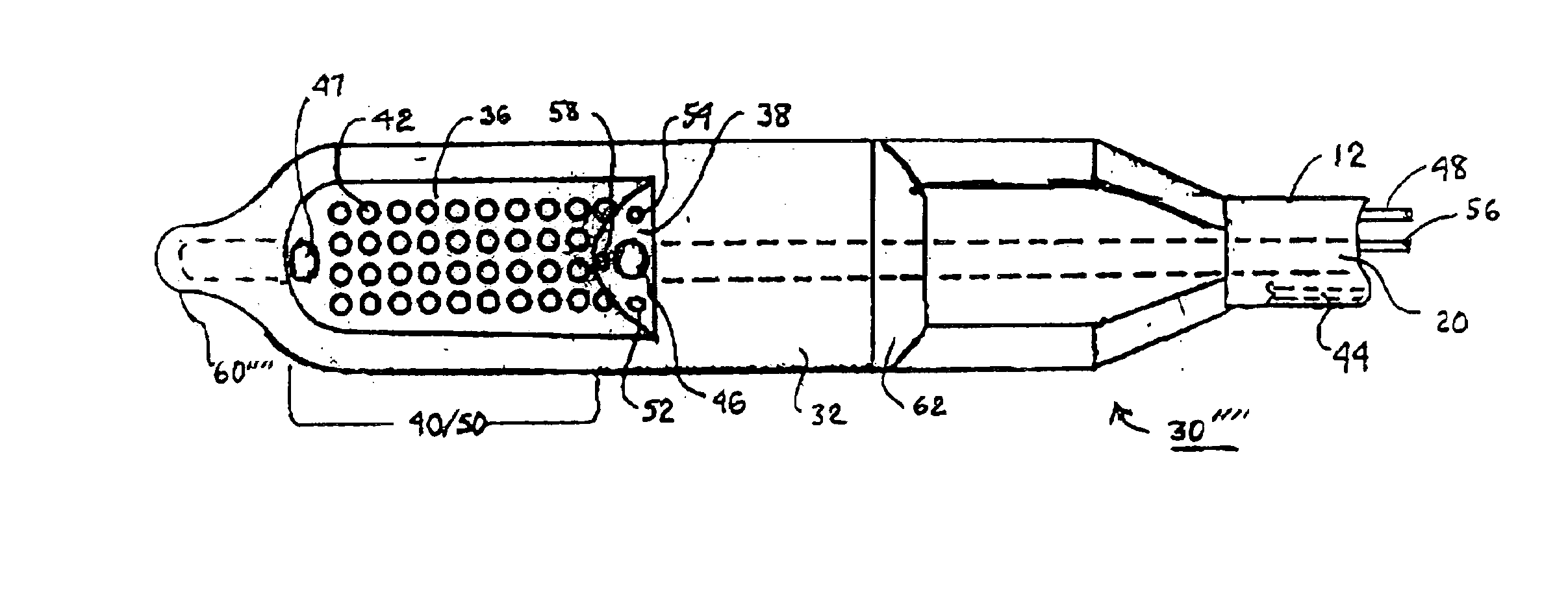
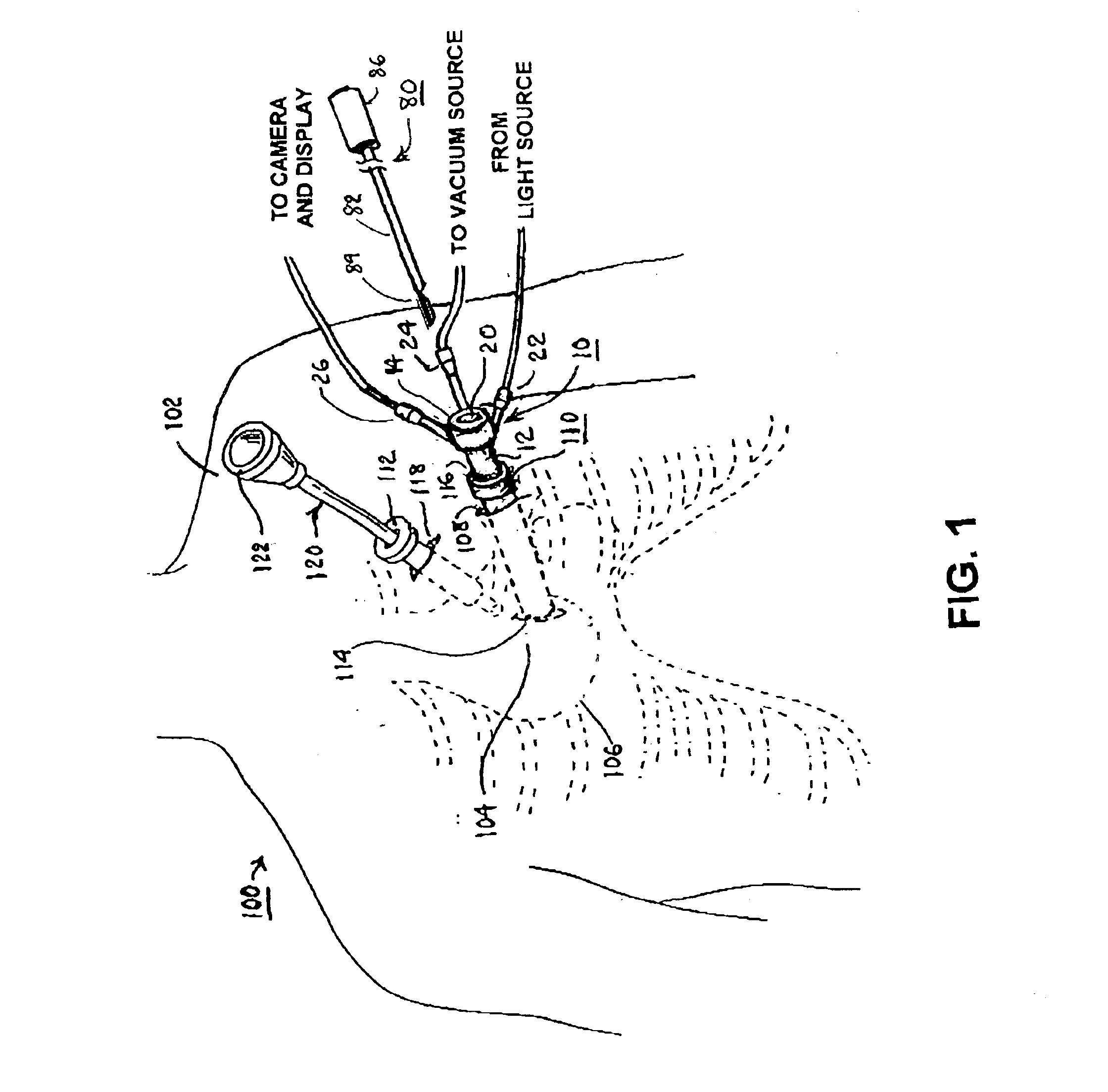
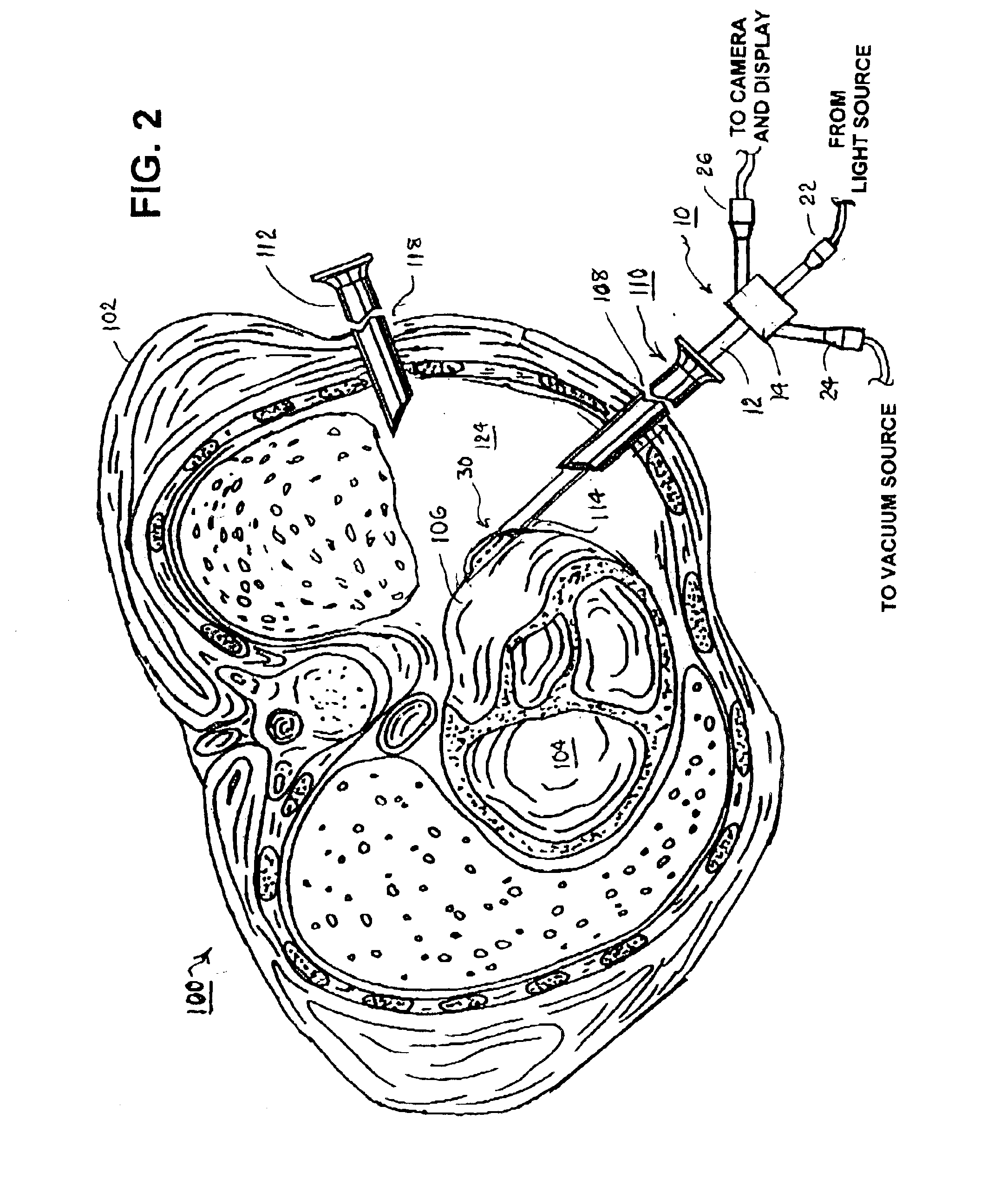
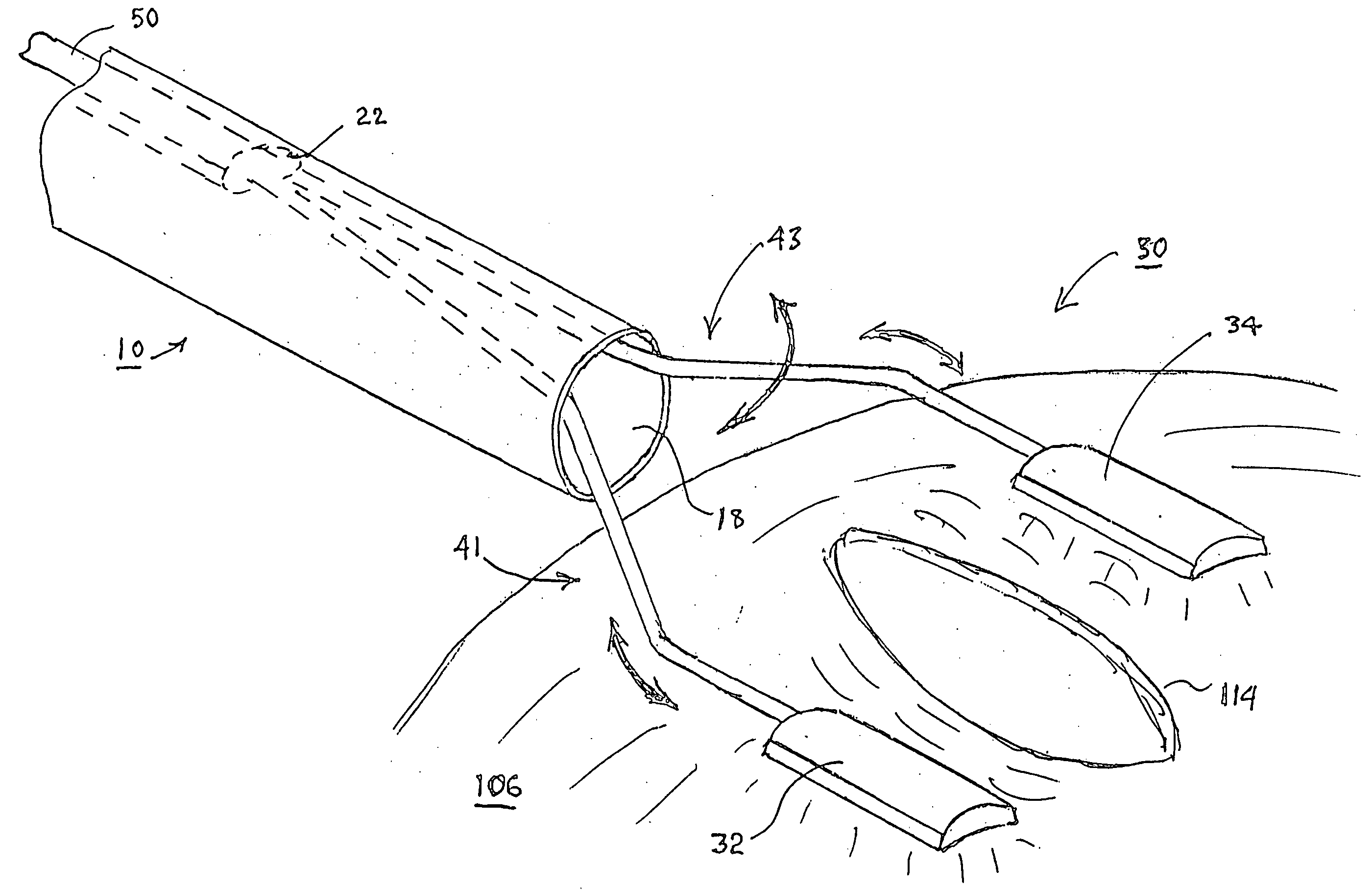
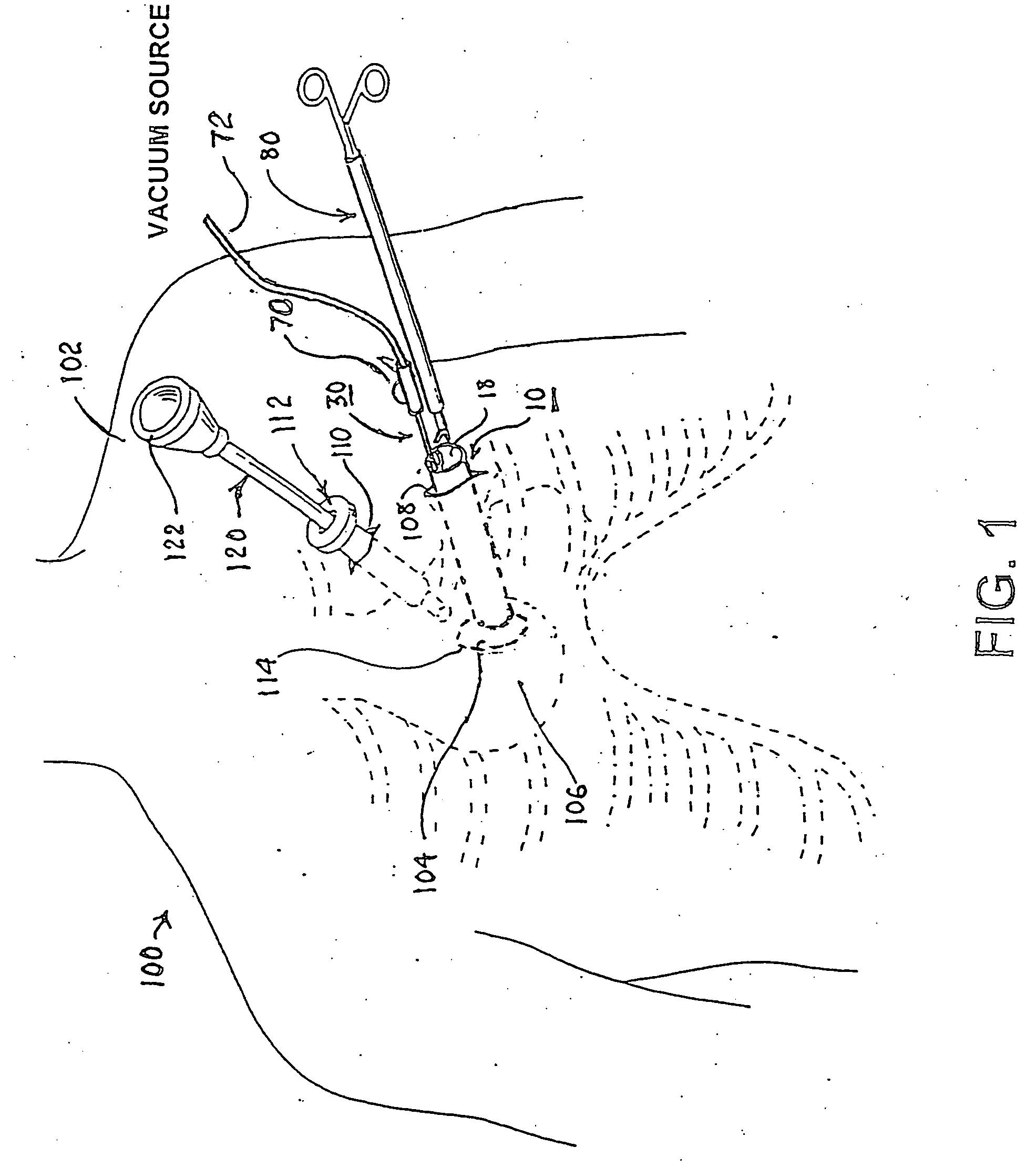
A tubular suction tool for accessing an anatomic surface or anatomic space and particularly the pericardium to access pericardial space and the epicardial surface of the heart to implant cardiac leads in a minimally invasive manner are disclosed. The suction tool incorporates a suction pad concave wall defining a suction cavity, a plurality of suction ports arrayed about the concave wall, and a suction lumen, to form a bleb of tissue into the suction cavity when suction is applied. The suction cavity extends along one side of the suction pad, so that the suction pad and suction cavity can be applied tangentially against a tissue site. The suction tool can incorporate light emission and video imaging of tissue adjacent the suction pad. A working lumen terminating in a working lumen port into the suction cavity enables introduction of tools, cardiac leads, and other instruments, cells, drugs or materials into or through the tissue bleb drawn into the suction cavity.
Owner:MEDTRONIC INC
Methods and tools for accessing an anatomic space
ActiveUS20060200002A1Reduce layeringRobust fixationSurgical instrument detailsSurgical pincettesPericardial spacePericardium tissue
Owner:MEDTRONIC INC
Transcoronary Sinus Pacing System, LV Summit Pacing, Early Mitral Closure Pacing, and Methods Therefor
ActiveUS20080082136A1Optimize early closureShorten the timeUltrasonic/sonic/infrasonic diagnosticsMulti-lumen catheterCoronary sinusPericardium tissue
A transcoronary sinus pacing system comprising a sheath having a lumen, a pacing catheter having a pacing needle, wherein the catheter can be advanced within the lumen and placed in the LV summit, and a right ventricular pacing device. A LV summit pacing device. An early mitral valve closure pacing device configured to operate with a right ventricular apex pacing device. A method for implanting a pacing device at a target coronary sinus tissue location, wherein the target can be the posterior LV summit. A method for achieving early closure of a mitral valve. A method for using visualization devices such as fluoroscopy or ultrasound and / or catheter features such as a radiopaque marker to locate a target location for LV pacing and to avoid piercing an artery or the pericardium when anchoring the LV pacing electrode.
Owner:GAUDIANI VINCENT
Prosthetic Cardiac Valve from Pericardium Material and Methods of Making Same
ActiveUS20120083879A1Barrier to undesired abrasionEasy to insertFinal product manufactureCell electrodesPericardium tissueProsthesis
A prosthetic stented heart valve which includes a compressible and expandable stent structure having first and second opposite ends, an expanded outer periphery, and a compressed outer periphery that is at least slightly smaller than the expanded outer periphery when subjected to an external radial force. The valve further includes a valve segment comprising a dual-layer sheet formed into a generally tubular shape having at least one longitudinally extending seam, and a plurality of leaflets formed by attachment of an outer layer of the dual-layer sheet to an inner layer of the dual-layer sheet in a leaflet defining pattern. The valve segment is at least partially positioned within the stent structure. The valve may further include at least one opening in the outer layer of the dual-layer sheet that is spaced from both the first and second ends of the stent structure.
Owner:MEDTRONIC INC
Methods and apparatus for accessing and stabilizing an area of the heart
InactiveUS20050261673A1Easy piercingSuture equipmentsSurgical furniturePericardial spacePericardium tissue
A tubular suction tool for accessing an anatomic surface or anatomic space and particularly the pericardium to access pericardial space and the epicardial surface of the heart to implant cardiac leads in a minimally invasive manner are disclosed. The suction tool incorporates a suction pad concave wall defining a suction cavity, a plurality of suction ports arrayed about the concave wall, and a suction lumen, to form a bleb of tissue into the suction cavity when suction is applied. The suction cavity extends along one side of the suction pad, so that the suction pad and suction cavity can be applied tangentially against a tissue site. The suction tool can incorporate light emission and video imaging of tissue adjacent the suction pad. A working lumen terminating in a working lumen port into the suction cavity enables introduction of tools, cardiac leads, and other instruments, cells, drugs or materials into or through the tissue bleb drawn into the suction cavity.
Owner:MEDTRONIC INC
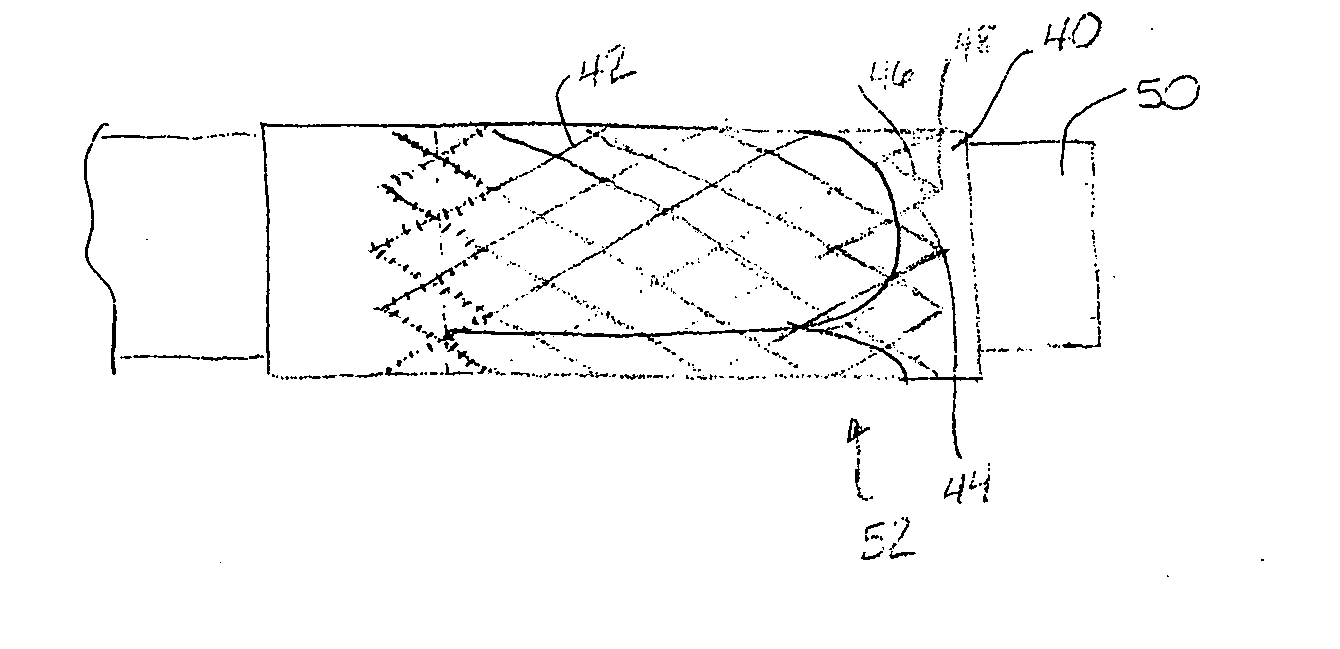
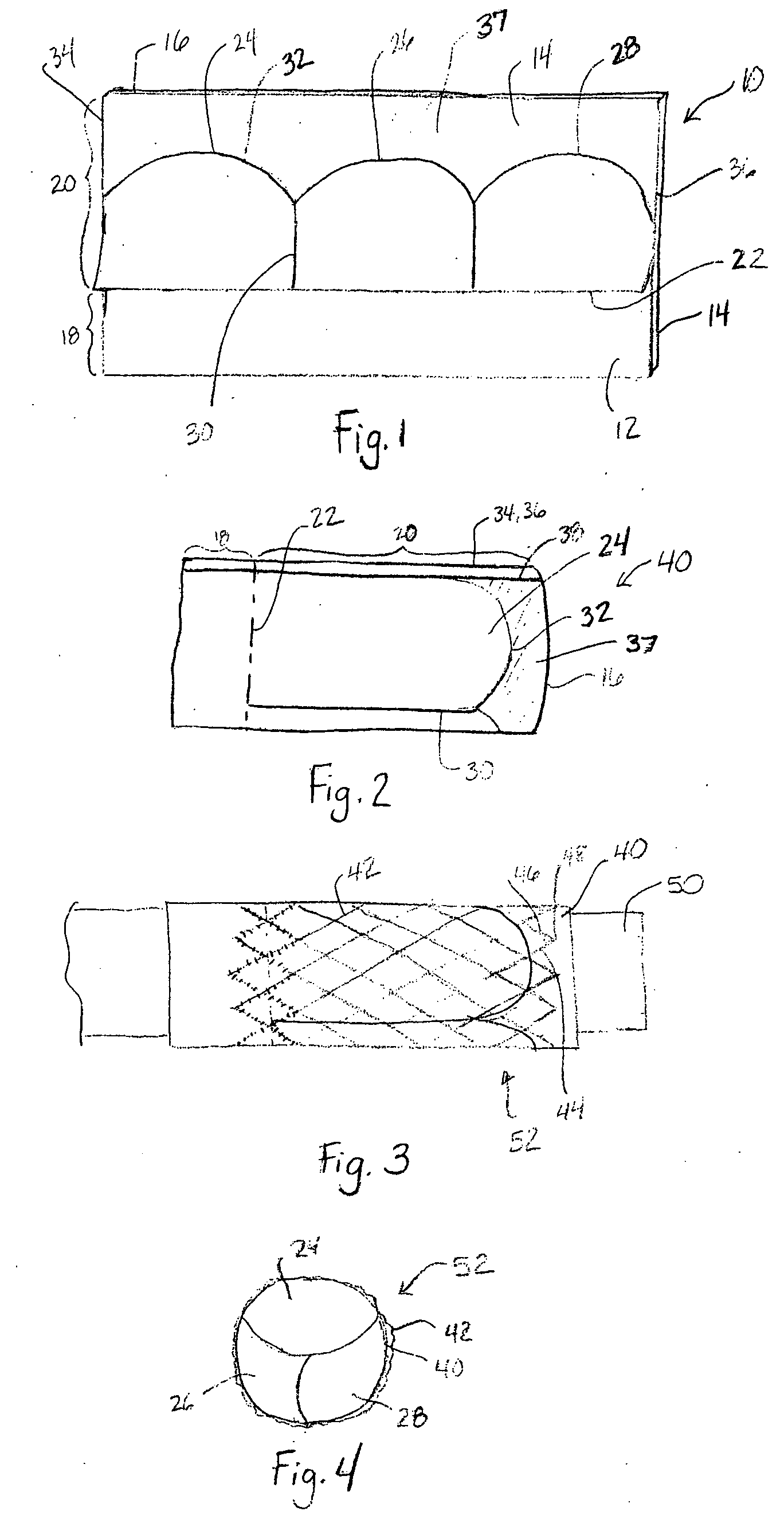
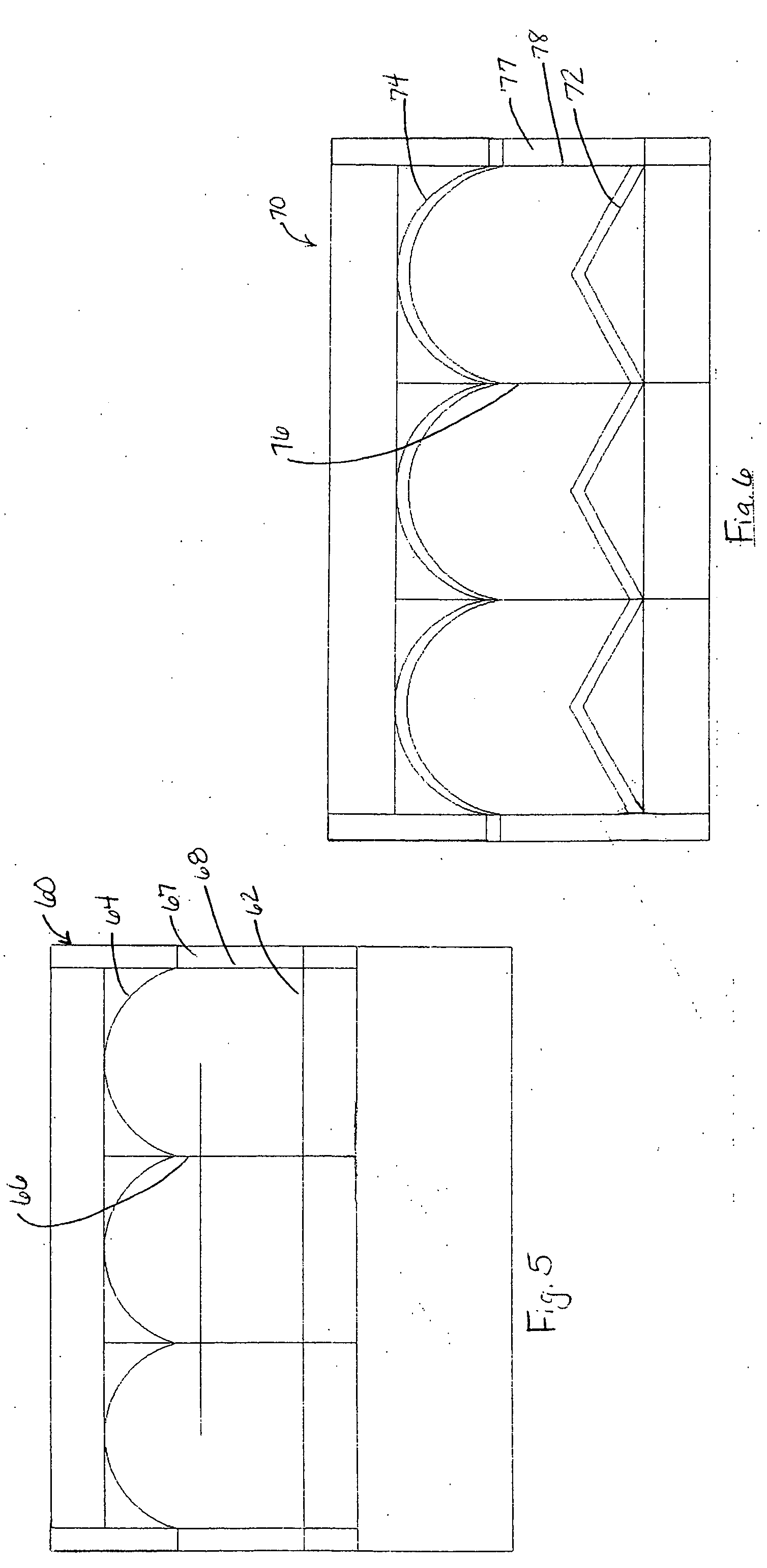
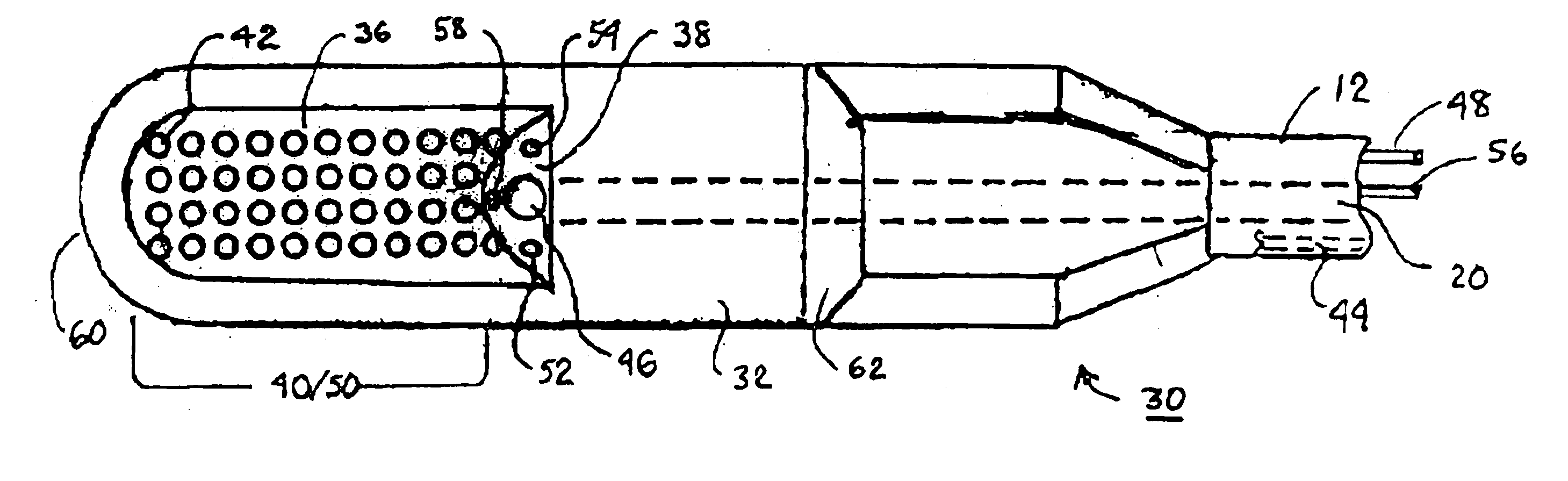
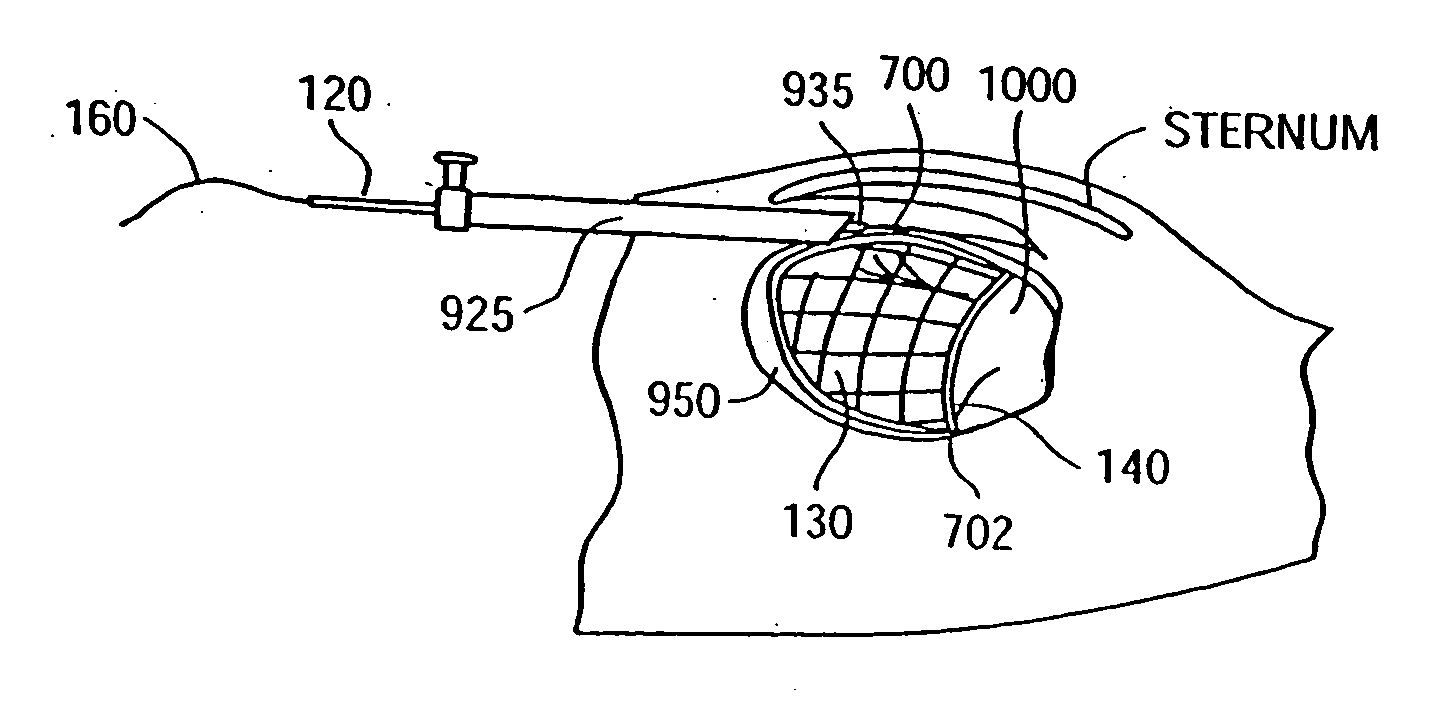
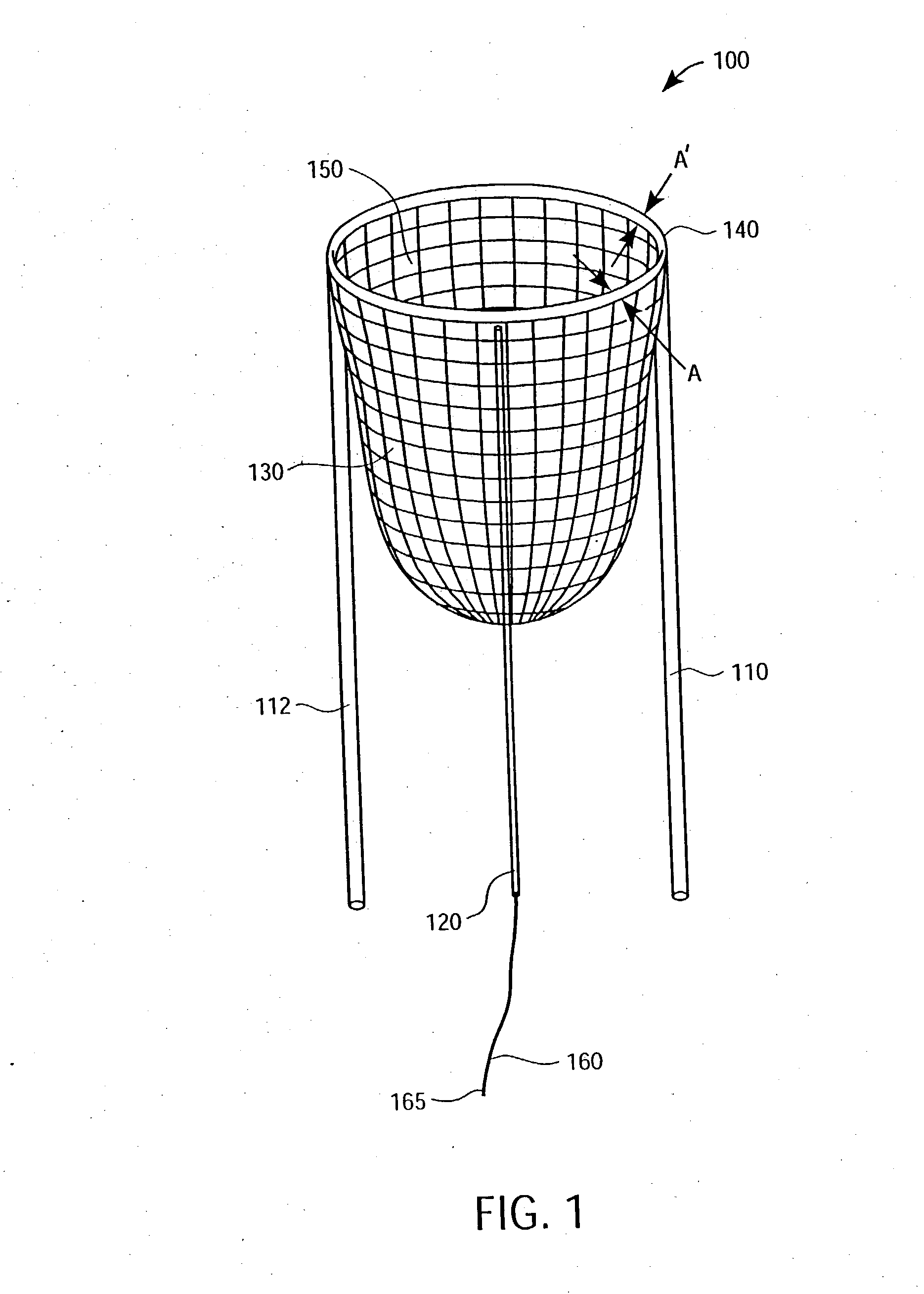
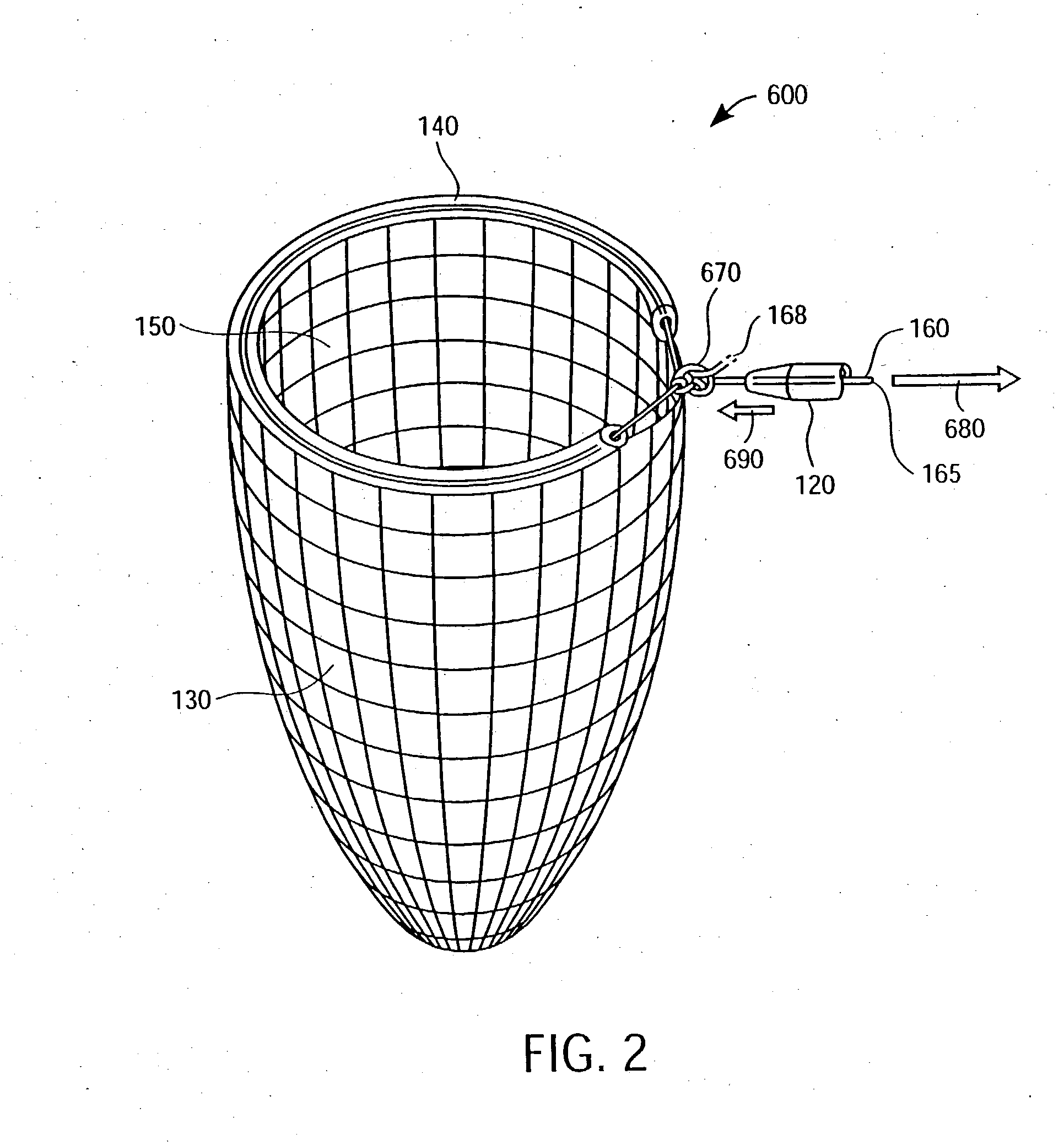
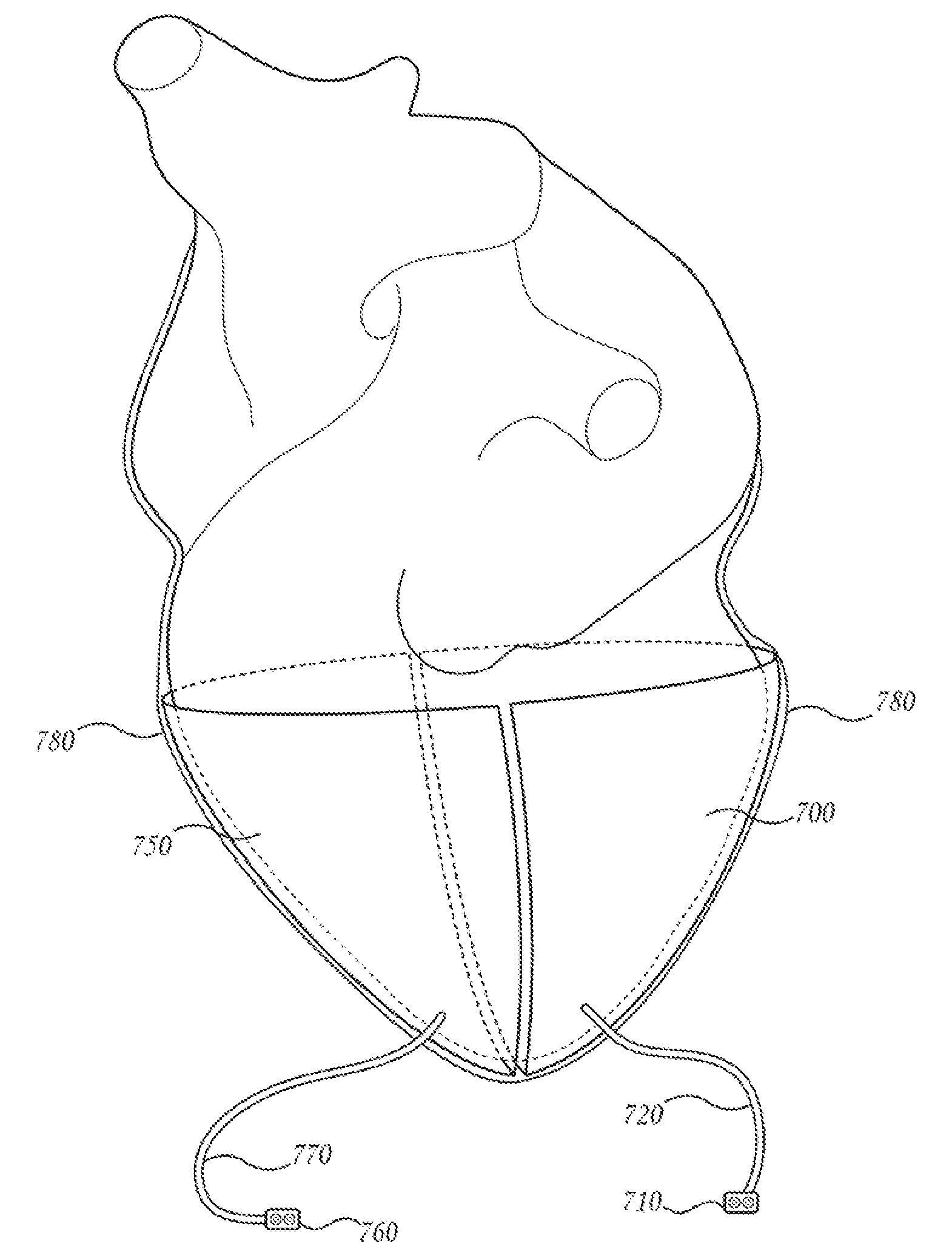
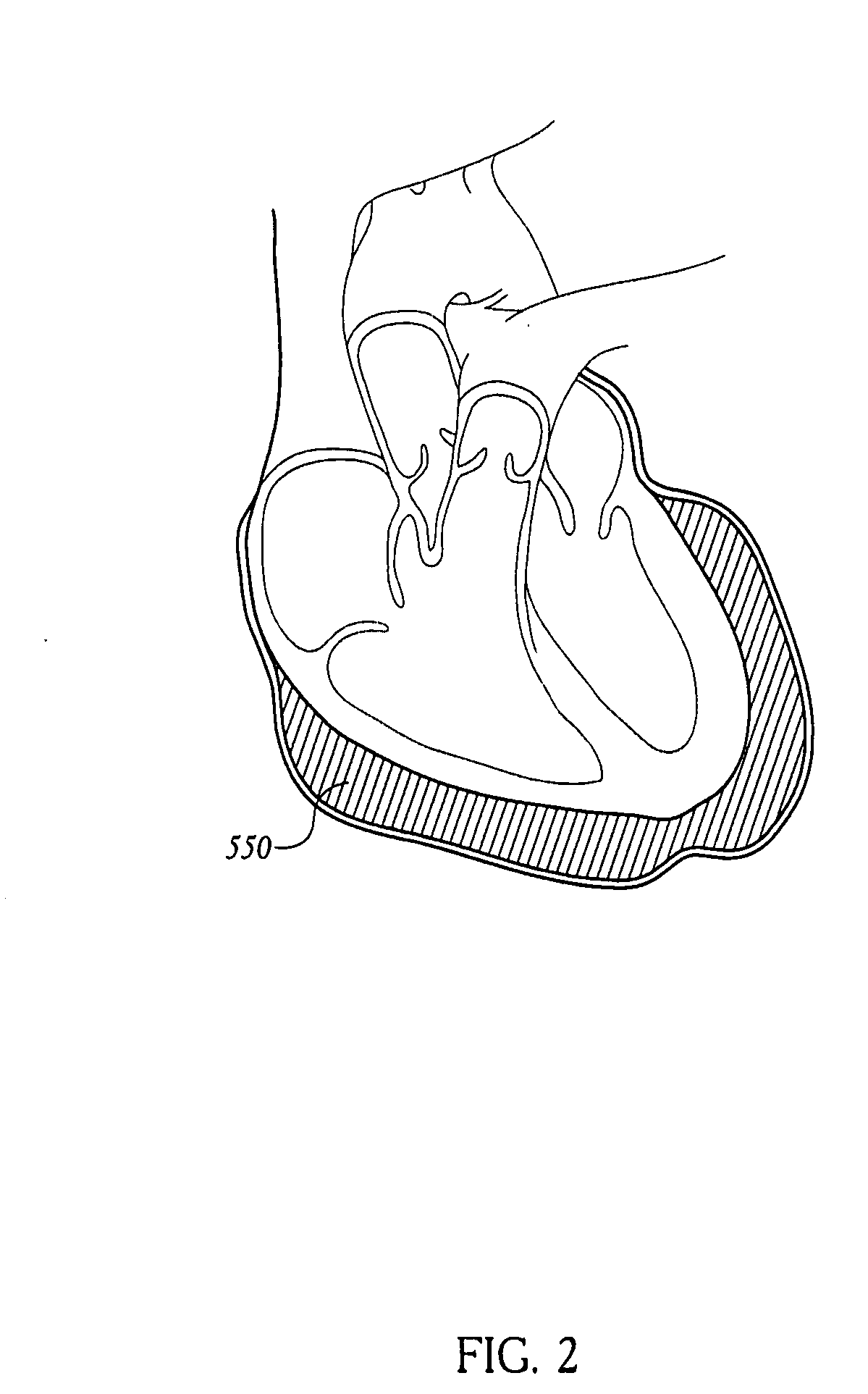
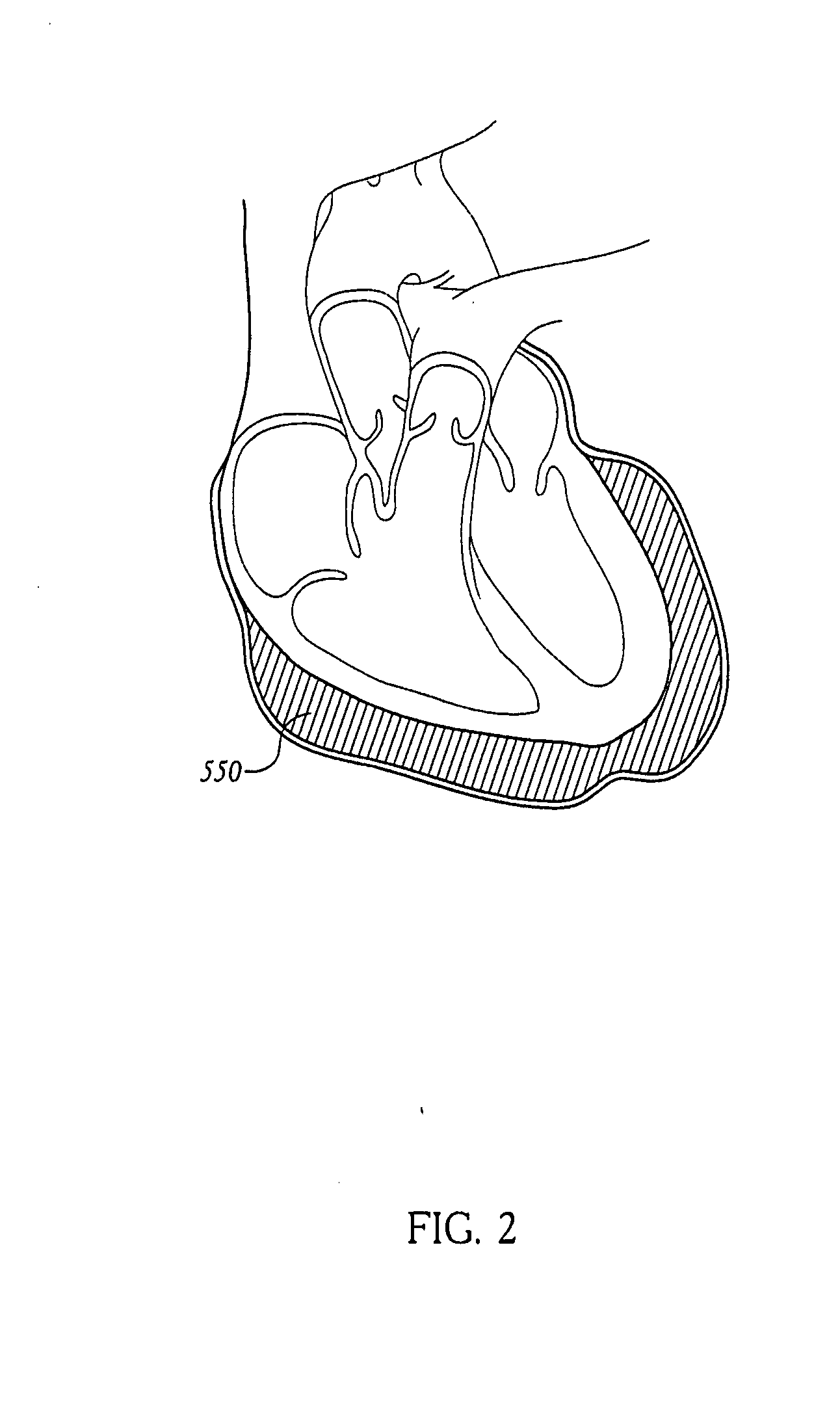
Apparatus and methods for cardiac restraint
Apparatus and methods for using the apparatus are disclosed for cardiac restraint. More specifically, the apparatus and methods are directed to accessing the pericardium, accessing the heart within the pericardium, and restraining the heart by at least partially enclosing the heart with the apparatus. An embodiment of a cardiac restraint apparatus according to the present invention comprises a jacket, the jacket having a rim which defines an opening for receiving a heart. The apparatus also comprises a knot pusher that has a hollow elongate body, as well as a strand that extends around the rim of the jacket and is tied into a slipknot. The strand is positioned such that at least one end portion of the strand extends through the knot pusher such that a distal end of the knot pusher can be moved into engagement with the slipknot, whereby pulling the end portion of the strand away from the heart while pushing the knot pusher against the slipknot and reducing the diameter of the opening defined by the rim. In addition, the apparatus comprises one or more guide elements that are attached to the jacket.
Owner:MAQUET CARDIOVASCULAR LLC
Method for cardiac restaint
Apparatus and methods for using the apparatus are disclosed for cardiac restraint. More specifically, the apparatus and methods are directed to accessing the pericardium, accessing the heart within the pericardium, and restraining the heart by at least partially enclosing the heart with the apparatus. An embodiment of a cardiac restraint apparatus according to the present invention comprises a jacket, the jacket having a rim which defines an opening for receiving a heart. The apparatus also comprises a knot pusher that has a hollow elongate body, as well as a strand that extends around the rim of the jacket and is tied into a slipknot. The strand is positioned such that at least one end portion of the strand extends through the knot pusher such that a distal end of the knot pusher can be moved into engagement with the slipknot, whereby pulling the end portion of the strand away from the heart while pushing the knot pusher against the slipknot and reducing the diameter of the opening defined by the rim. In addition, the apparatus comprises one or more guide elements that are attached to the jacket.
Owner:MAQUET CARDIOVASCULAR LLC
Pericardial tissue sheet
InactiveUS20080195230A1Low antigenicityLow immunogenicityFluid jet surgical cuttersSurgical instruments for heatingThermal energyTissue material
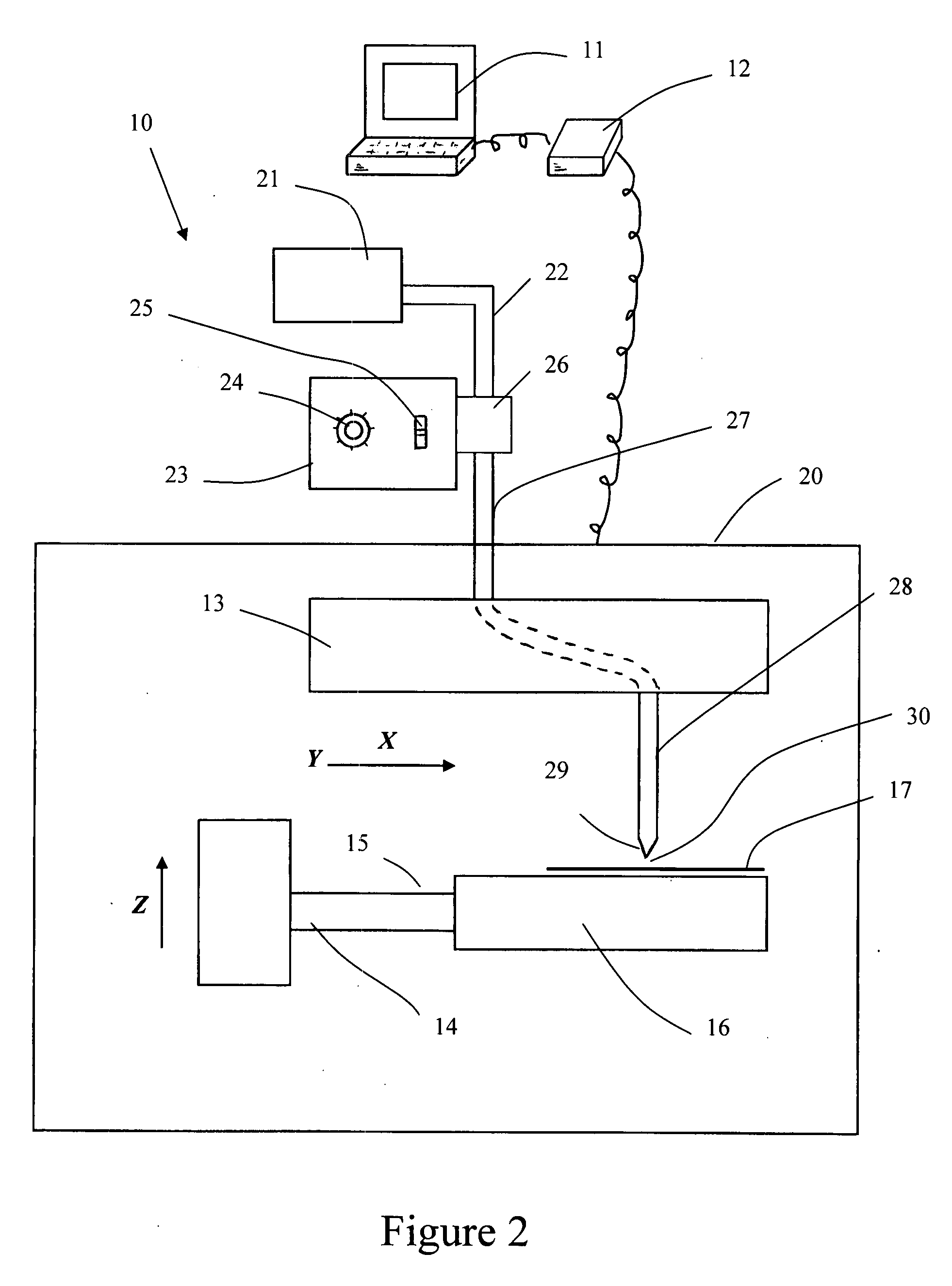
A method of cutting tissue material of biology origin employs a plotted water-jet or RF cutting system. The cutting system is computer controlled and includes a water-jet or RF cutting means combined with a motion system. The cutting energy is selected so that communication of thermal energy into the segment beyond the edge is minimized to avoid damaging the segment adjacent the edge.
Owner:QUIJANO RODOLFO C +1
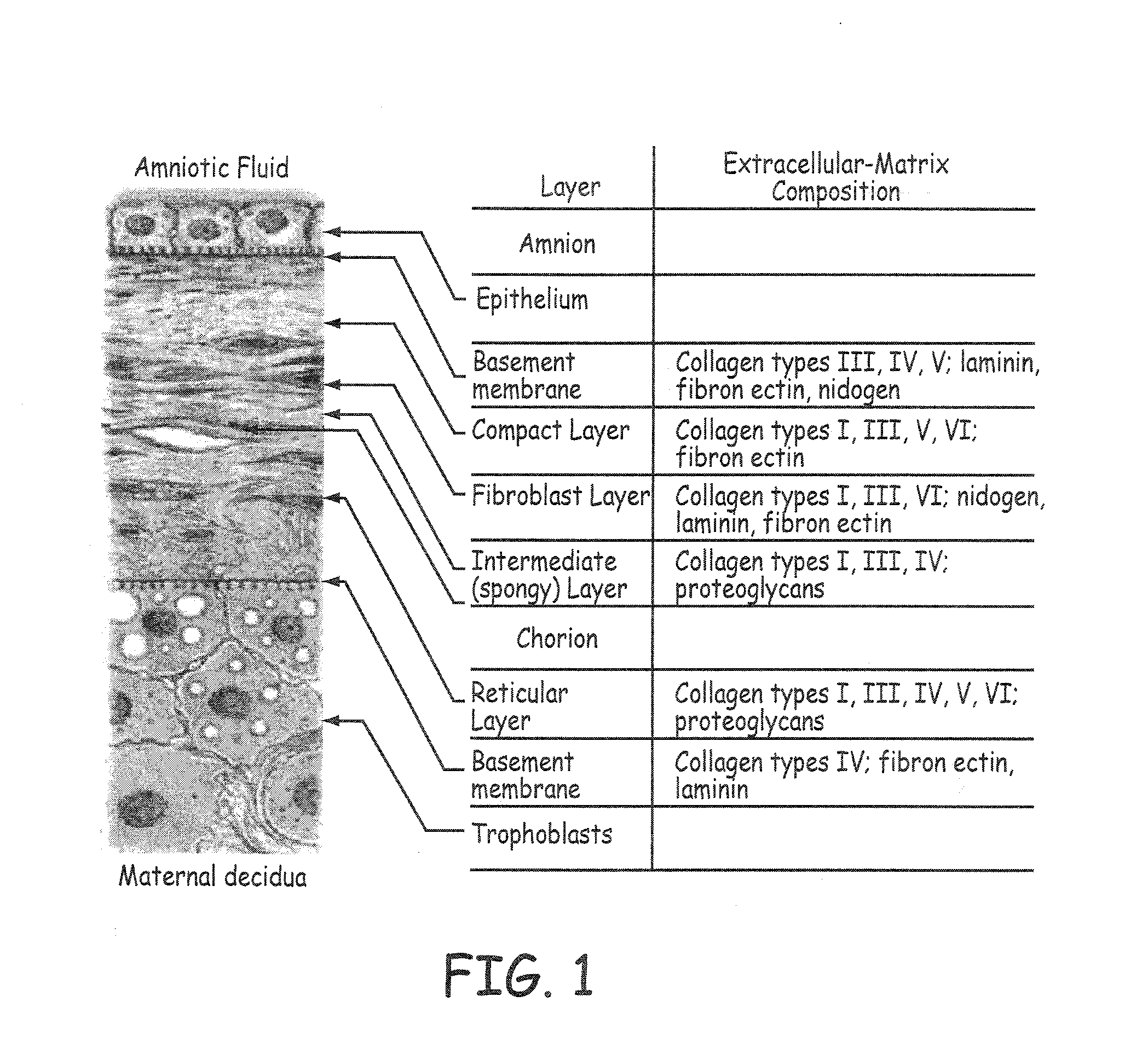
Stents modified with material comprising amnion tissue and corresponding processes
A stent scaffold combined with amniotic tissue provides for a biocompatible stent that has improved biocompatibility and hemocompatibility. The amnion tissue can be variously modified or unmodified form of amnion tissue such as non-cryo amnion tissue, solubilized amnion tissue, amnion tissue fabric, chemically modified amnion tissue, amnion tissue treated with radiation, amnion tissue treated with heat, or a combination thereof. Materials such as polymer, placental tissue, pericardium tissue, small intestine submucosa can be used in combination with the amnion tissue. The amnion tissue can be attached to the inside, the outside, both inside and outside, or complete encapsulation of the stent scaffold. In some embodiments, at least part of the covering or lining comprises a plurality of layers of amnion tissue. The method of making the biocompatible stent and its delivery and deployment are also discussed.
Owner:PEYTANT SOLUTIONS INC
Protecting biological structures, including the great vessels, particularly during spinal surgery
InactiveUS20050126576A1Improve barrier propertiesAvoid formingRestraining devicesSurgeryIntracranial surgeryThoracic bone
Natural and / or synthetic materials to form a strong barrier between the skeletal system and the great vessels. In the preferred embodiments, a natural or synthetic material is used to prevent scar tissue from forming around the vessels and / or to act as barrier placed between the vessels and the skeletal system, including the spine. Devices according to the invention may also be used over the dura and nerves following laminectomy procedures, between the sternum and the pericardium or heart following cardiac procedures, in intra-abdominal procedures such as intestinal or vascular surgery, over the brain in intra-cranial surgery, over the ovaries or other organs or tissues in the female genitourinary system, over the prostate or other organ or tissues in the male genitourinary system, or in other surgeries on humans or animals.
Owner:FERREE BRET A
Continuous compartment pressure monitoring device
Described herein is a compartment pressure monitoring device that enables the continuous monitoring of pressure in a physiological compartment, such as those found in the hand, forearm, upper arm, abdomen, pericardium, thigh and leg. In various embodiments, the device includes a tube connected at a first end to a balloon, and at a second end to a pressure gauge. A trocar sleeve may also be connected to the tube at or near its first end, such that a compatible trocar may be used to facilitate the insertion and positioning of the device within the compartment. The pressure gauge includes an alarm device configured to sound or display an alarm when a predetermined pressure threshold on the balloon has been met or exceeded. Also described is a method of continuously monitoring compartment pressure, using the compartment pressure monitoring device of the invention.
Owner:CEDARS SINAI MEDICAL CENT
Devices and methods for access through a tissue wall
A method of accessing the pericardial space comprises providing a primary catheter having an inflatable balloon, inserting the primary catheter into the vascular system, forwarding the primary catheter through the vascular system to an atrial appendage, inflating the balloon to engage the wall of the appendage sufficient to separate opposing wall portions of the appendage between the cardiac tissue and the pericardium. The inflated balloon increases the distance of the pericardium from the cardiac tissue to permit easier access into the pericardial space via the appendage. The inflated balloon further can be configured to create a seal of the balloon exterior surface relative to an inner wall of the appendage adjacent to an apex of the appendage. The balloon seal isolates a portion of the interior of the appendage adjacent the apex allowing the removal of blood from the isolated portion. This prevents cross-contamination of fluid between the pericardial space and the appendage. An implantable valve positionable in the apex of the appendage permits repeatable access into the pericardial space.
Owner:CARDIA ACCESS
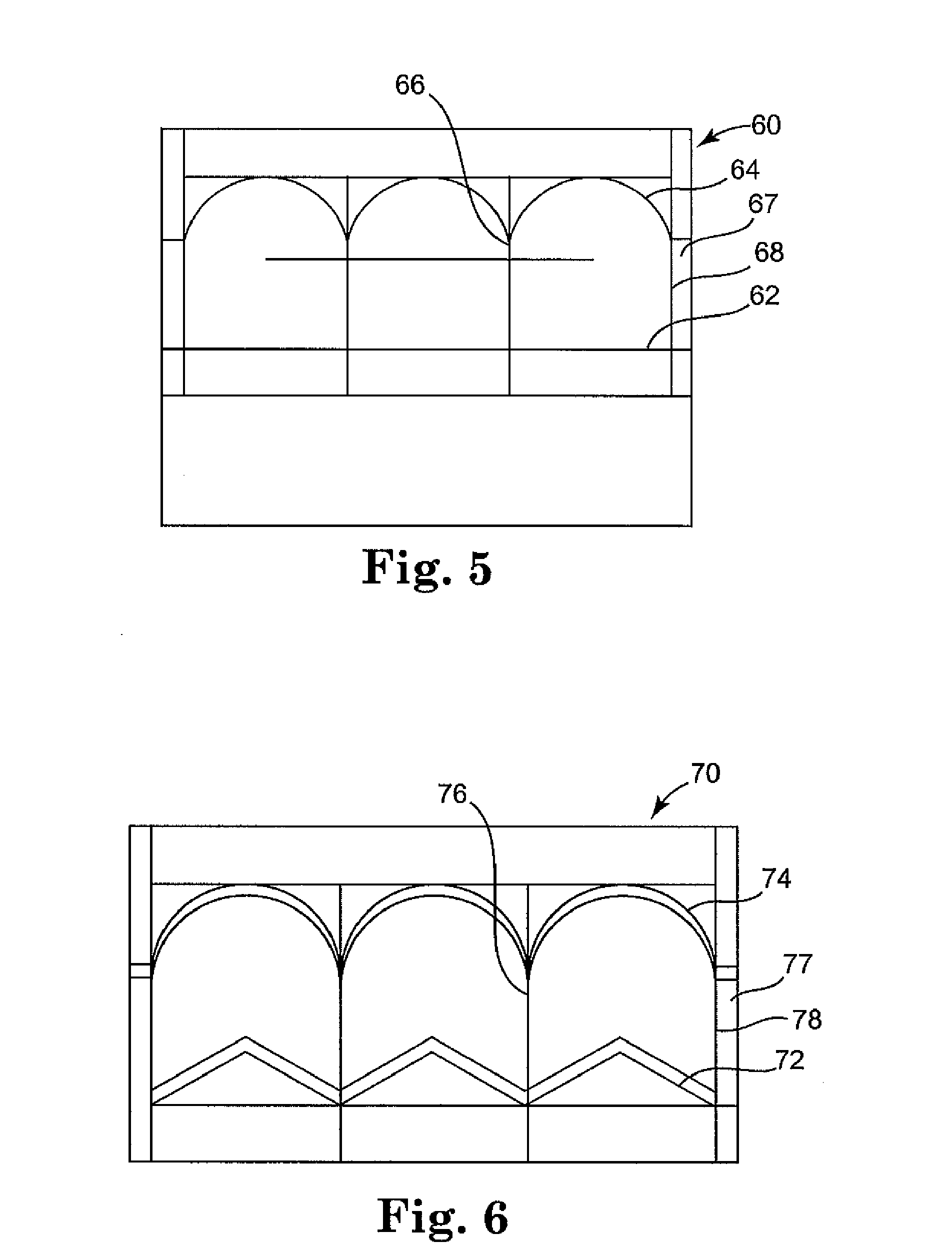
Pericardial inserts
InactiveUS20080275294A1Expansion is limitedGood biocompatibilityEpicardial electrodesHeart valvesPericardium tissueCardiology
Devices, systems and methods are provided which are capable of applying pressure and constraint to the heart and use the pericardium to assist in the application of the pressure and force to the heart.
Owner:GERTNER MICHAEL
Methods of using pericardial inserts
InactiveUS20080027269A1Expansion is limitedGood biocompatibilityEpicardial electrodesHeart valvesPericardium tissue
Devices, systems and methods are provided which are capable of applying pressure and constraint to the heart and use the pericardium to assist in the application of the pressure and force to the heart.
Owner:GERTNER MICHAEL
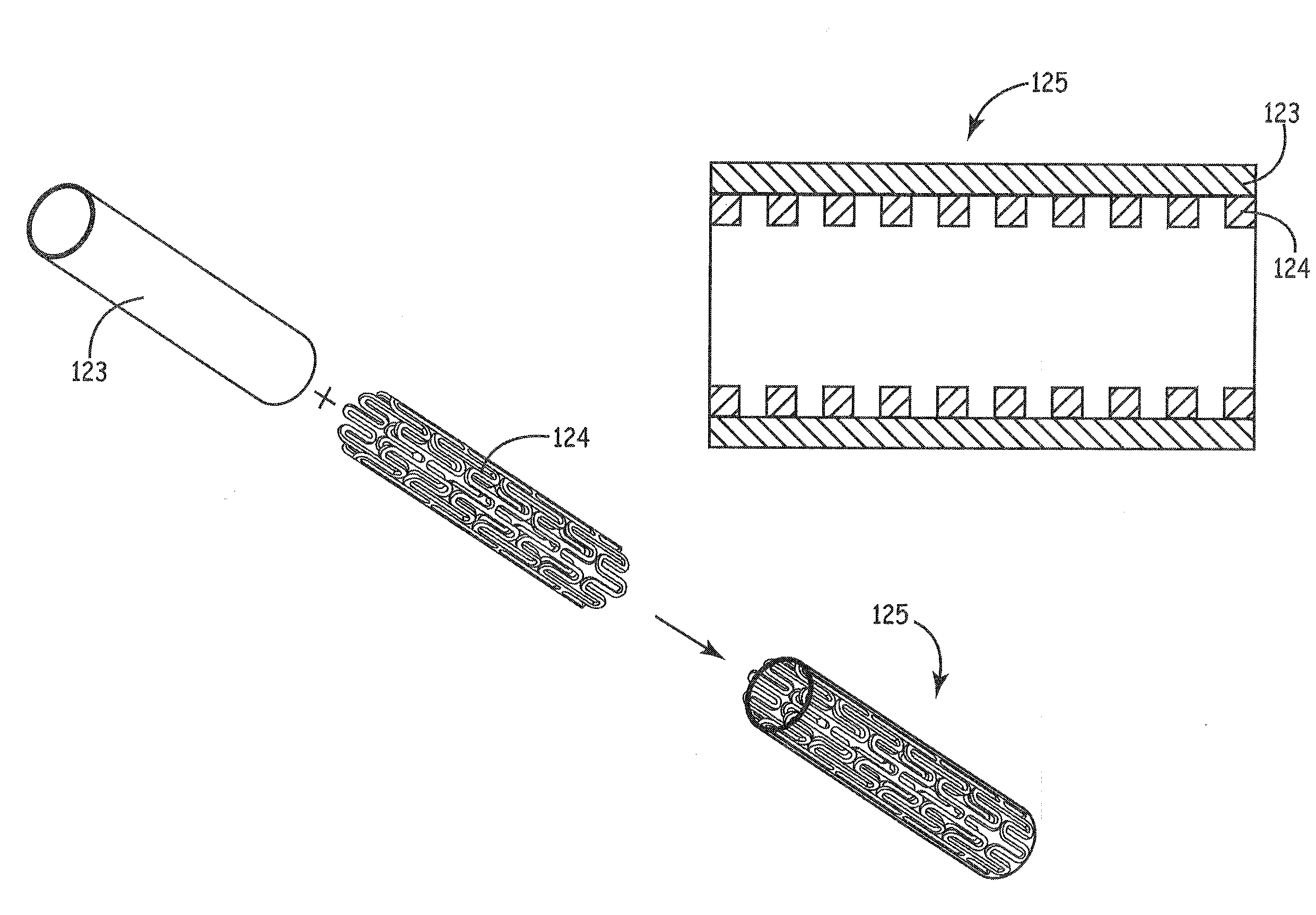
Recyclable and adjustable interventional stent for intravascular constriction
InactiveUS20140303710A1Easy to achieve satisfactoryEasy to stable parameterStentsBlood vesselsCalcificationBlood vessel
A recyclable and adjustable interventional stent for intravascular constriction. The stent main body is divided into three parts and shaped like a waist drum with expansion parts (1, 4) being arranged on the upper and lower parts of the stent main body respectively for supporting and positioning. A variable aperture part (2) is arranged in the middle of the stent main body. The upper expansion part (1) is or is not provided with a coating; the middle variable aperture part (2) and the upper half part of the lower expansion part (4) are covered with a pericardium (3) subjected to anti-calcification treatment; and a metal wire ring (5) is passed through the lowermost edge of the stent. A compound conveying guide pipe is composed of an outer sheath (6) and a core (7). The core (7) is a hollow pipe and a wire hanging groove is arranged on the outer side wall of the tip of the pipe to hang the metal wire ring (5) of the lowermost edge of the stent. A fixing bolt (8) on the outer sheath (6) is used for fixing the relative position between the outer sheath (6) and the core (7). The stent is used to replace conventional pulmonary artery banding as, adhesion not being formed around the heart and major vessels and pulmonary stenosis not being formed, difficulties during radical surgery are not increased.
Owner:JIANGSU PROVINCE HOSPITAL
Valve replacement devices, delivery device for a valve replacement device and method of production of a valve replacement device
InactiveCN103108611ADoes not prevent compressionSmall sizeStentsHeart valvesPericardium tissueHeart valve replacement
A device for heart valve replacement comprises a valve component having at least two valve leaflets preferably made of pericardium tissue. Each valve leaflet includes at least two tabs. The device further includes a stent component configured to be radially compressible into a compressed state and expandable into a functional state. The stent component comprises a first end, a second end and at least one intermediate section arranged between said first and said second end. The intermediate section has at least two commissural posts generally aligned parallel to an axis spanning from the first end to the second end. The commissural posts are formed in the shape of a wishbone.
Owner:SYMETIS
Pericardial inserts
InactiveUS20070232849A1Good biocompatibilityPromote ingrowthEpicardial electrodesHeart valvesPericardium tissue
Devices, systems and methods are provided which are capable of applying pressure and constraint to the heart and use the pericardium to assist in the application of the pressure and force to the heart.
Owner:GERTNER MICHAEL
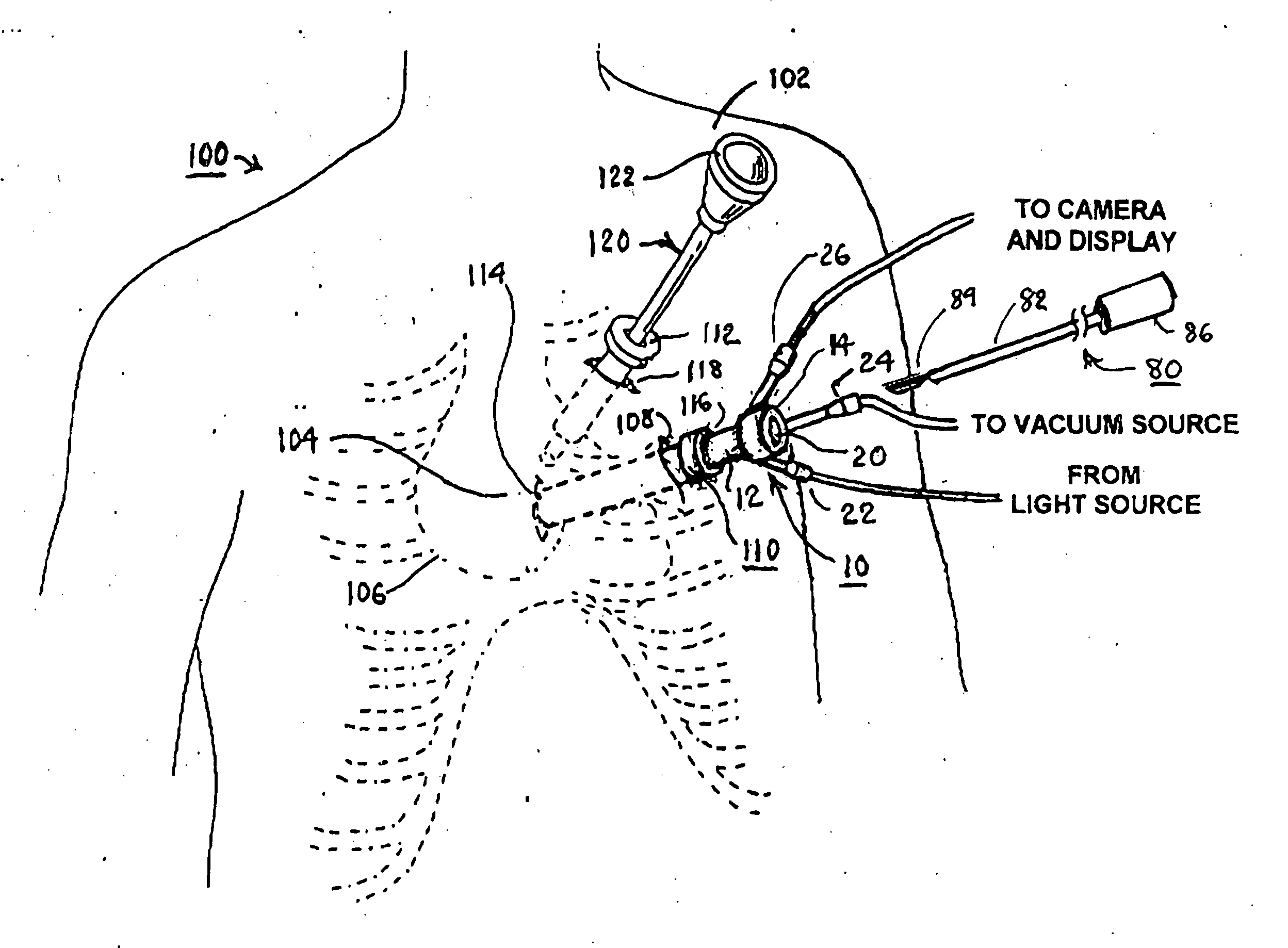
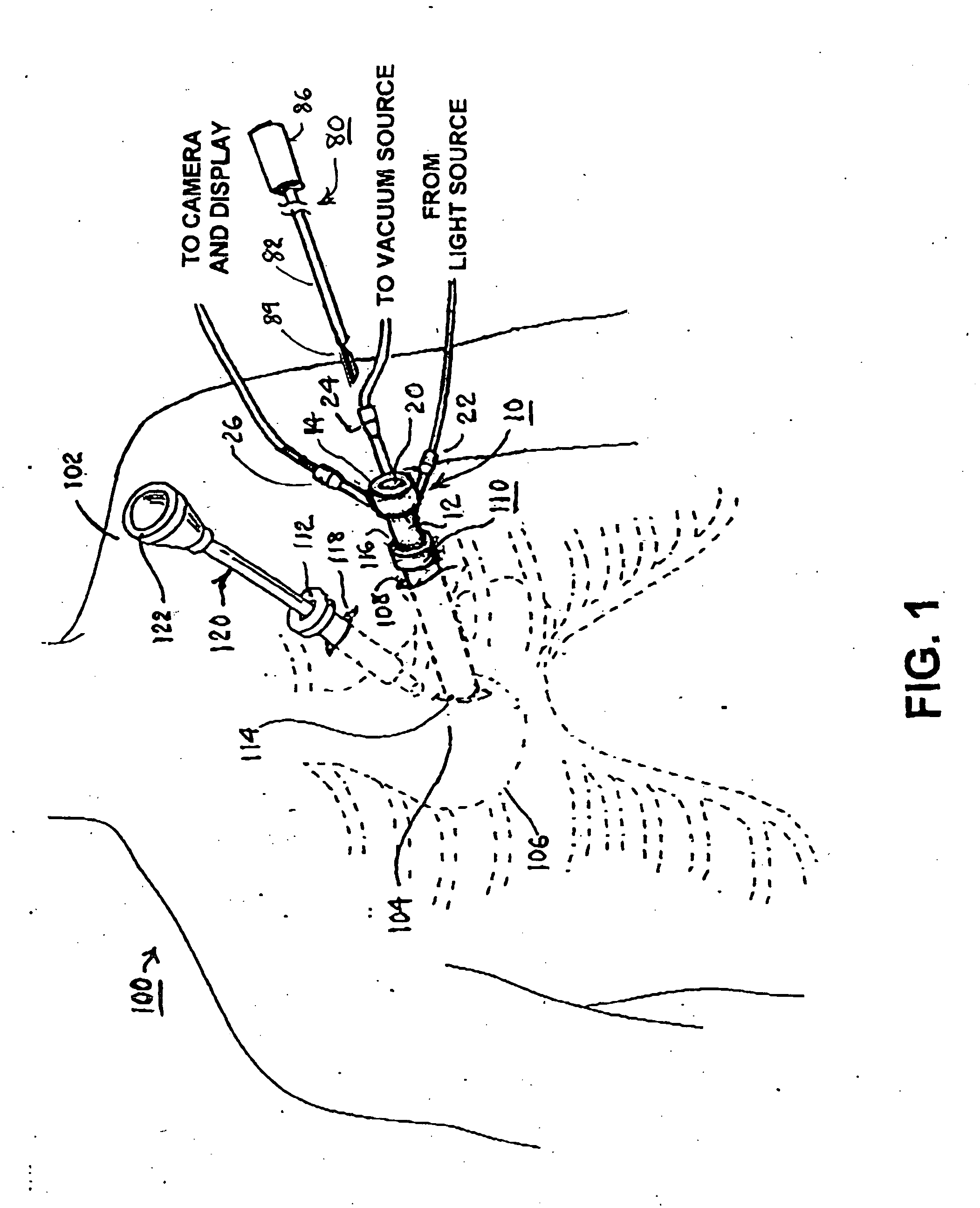
Surgical device and method for pericardium retraction
A surgical device and method for retracting a pericardium tissue, the surgical device having a tissue-engaging member for providing a negative pressure suction force to the pericardium tissue which is sufficient to retract same. The surgical device may be positioned in a retracting configuration wherein it provides a tensile load on the contacted portion of pericardium tissue thereby also positioning the patient's heart, that is anatomically attached to the pericardium tissue, within the thoracic cavity of the patient. The surgical device may be temporarily coupled to a surgical platform in its retracting configuration.
Owner:PAOLITTO ANTHONY +2
Ultrasound ablation catheter and method for its use
ActiveUS20050015079A1Facilitates longitudinal movementSure easyUltrasonic/sonic/infrasonic diagnosticsCatheterEpicardial ablationPericardium tissue
Catheters and methods for epicardial ablation are provided. A suitable catheter comprises an elongated catheter body and an ultrasound transducer mounted at or near the distal end of the catheter body. The transducer has a front surface and an opposing back surface, wherein the transducer is positioned to transmit ultrasound energy toward tissue facing the front surface but not toward tissue facing the back surface. A sensor is mounted within the catheter near the ultrasound transducer for sensing a location and an orientation of the ultrasound transducer within a patient. A suitable method involves introducing the distal end of the catheter introducing into the pericardium of a patient. The transducer's front surface is positioned so that it generally faces tissue to be ablated, and the tissue is ablated with ultrasound energy generated by the transducer.
Owner:BIOSENSE WEBSTER INC
Apparatus and method for auto-retroperfusion of a coronary vein
An apparatus for automatically retroperfusing a coronary vein includes an intraluminal cannula having a main body portion extending between a proximal end portion and a distal end portion. The proximal end portion is for connecting to an artery outside of the pericardium to automatically supply oxygenated blood from the artery for retroperfusion. The main body portion and the distal end portion are insertable through a vein that is fluidly connected with the coronary vein and into the coronary vein. An expandable stent is attached to the distal end portion for expanding radially into engagement with the interior wall of the coronary vein to secure the distal end portion at a desired location within the coronary vein. Occluding structure for at least partially occluding the coronary vein is provided at the distal end to decrease the back-flow of blood into the right atrium during retroperfusion. The cannula includes passages that allow a limited amount of blood to bypass the occluding structure.
Owner:THE CLEVELAND CLINIC FOUND
Cardiac Electrosurgery
ActiveUS20140228841A1Easy accessDiagnosticsSurgical needlesElectrical resistance and conductancePericardium tissue
Devices and methods are disclosed for providing access to the pericardial cavity while reducing risk of myocardial damage. One method includes advancing a puncture device towards a heart, the puncture device including an energy delivery device; measuring an electrical impedance at the energy delivery device; delivering energy from the energy delivery device to at least partially puncture a pericardium; and repeating one or more of the above steps, if necessary, until the energy delivery device is located at least partially within the pericardial cavity. Some embodiments of the method include using supplemental means of monitoring, including measuring voltage to plot an ECG, medical imaging and using contrast fluid, using tactile feedback, and aspirating fluid.
Owner:BOSTON SCI MEDICAL DEVICE LTD
Recombinant lubricin molecules and uses thereof
InactiveUS7642236B2Promote generationOrganic active ingredientsPeptide/protein ingredientsPericardium tissueSynovial joints
Recombinant lubricin molecules and uses thereof. Novel recombinant lubricin molecules and their uses as lubricants anti-adhesive agents and / or intra-articular supplements for, e.g., synovial joints, meniscus, tendon, peritoneum, pericardium and pleura are provided.
Owner:WYETH LLC
Ultrasound ablation catheter and method for its use
ActiveUS20060122591A1Sure easyEasy to moveUltrasonic/sonic/infrasonic diagnosticsCatheterEpicardial ablationTransducer
Owner:BIOSENSE WEBSTER INC
Minimally-Invasive Method and Device for Permanently Compressing Tissues within the Body
InactiveUS20100114152A1Avoid formingMinimally invasiveHeart valvesSurgeryElastomerCompression device
The present invention is method for permanently compressing tissues in the body. The method employs a compression device made of a spring and a flexible sheet that cooperate to form a compressive envelope around the desired tissue. The spring is preferably Z-shaped or a coil. The sheet is made of a flexible material and the material is preferably elastic. The sheet is preferably a biocompatible elastic material, such as a mesh made of stainless steel or a woven or non-woven elastomer. The method is minimally invasive because it deploys the compression device through the patient's skin directly to the tissue, as opposed to through catheterization or open invasive surgery, such as open-heart surgery. The preferred use is for compressing the left atrial appendage to prevent clots from forming and circulating, thereby preventing strokes. The device is deployed by making an incision in a patient's chest, inserting the compression device through the incision into the pericardium without piercing the heart, and deploying it around the entire appendage. The device remains in place by its own compressive nature: either the spring, the sheet, or both components compress the appendage and cause the device to stay in place due to friction. Preferably the entire compression device is left in place, but the spring or the sheet may be removed, leaving the remaining component to compress the appendage.
Owner:SHUKLA HIMANSHU
Stents modified with material comprising amnion tissue and corresponding processes
A stent scaffold combined with amniotic tissue provides for a biocompatible stent that has improved biocompatibility and hemocompatibility. The amnion tissue can be variously modified or unmodified form of amnion tissue such as non-cryo amnion tissue, solubilized amnion tissue, amnion tissue fabric, chemically modified amnion tissue, amnion tissue treated with radiation, amnion tissue treated with heat, or a combination thereof. Materials such as polymer, placental tissue, pericardium tissue, small intestine submucosa can be used in combination with the amnion tissue. The amnion tissue can be attached to the inside, the outside, both inside and outside, or complete encapsulation of the stent scaffold. In some embodiments, at least part of the covering or lining comprises a plurality of layers of amnion tissue. The method of making the biocompatible stent and its delivery and deployment are also discussed.
Owner:PEYTANT SOLUTIONS INC
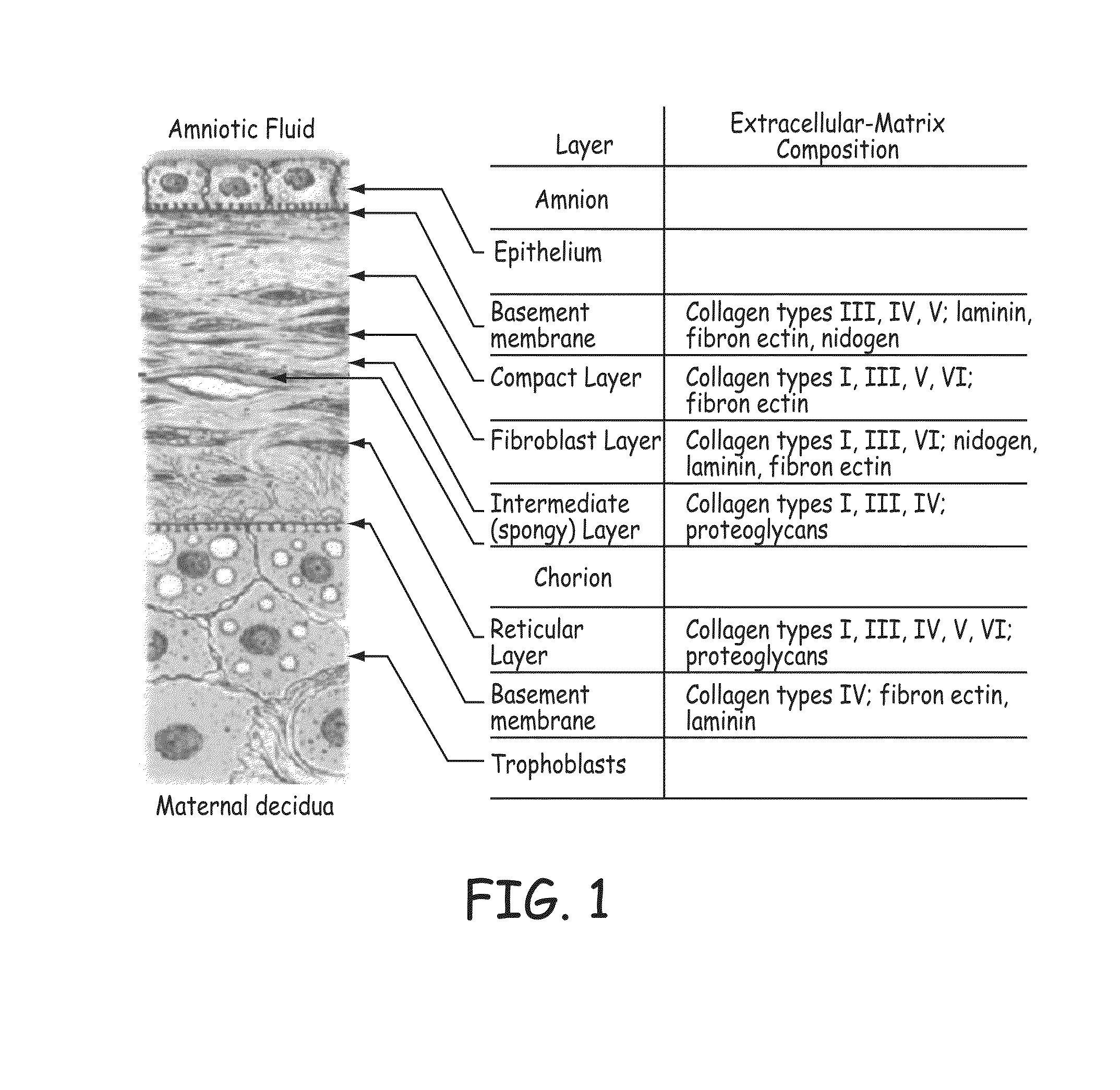
Ocular surface biomembrane and preparation method thereof
The invention provides a preparation method of an ocular surface biofilm, which uses animal pericardium, intestinal membrane and adipose omentum as raw materials through degreasing, decellularization, multi-directional antigen removal, epoxy fixation, curved surface molding, surface Modification, cobalt-60γ-ray irradiation sterilization and other process steps. The ocular surface biofilm obtained by the method of the present invention has a curved surface similar to that of the eyeball, can be close to the eyeball, has no antigenicity, good histocompatibility, can participate in the healing process of ocular surface wounds, and is beneficial to wound healing; Good air permeability and bacterial filtration can play a good role in the protection and treatment of the ocular surface; it has good strength, can be pasted and sewed, and is easy to use. In addition, through the surface modification of drug sustained release, it can also be made into a therapeutic ocular surface biofilm that can release anti-infective drugs slowly.
Owner:SUMMIT GD BIOTECH
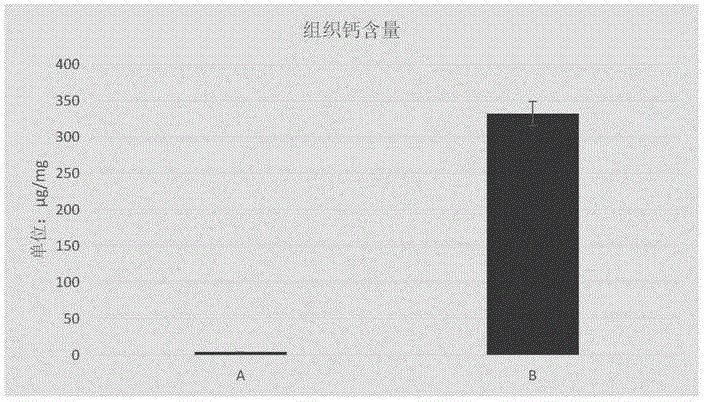
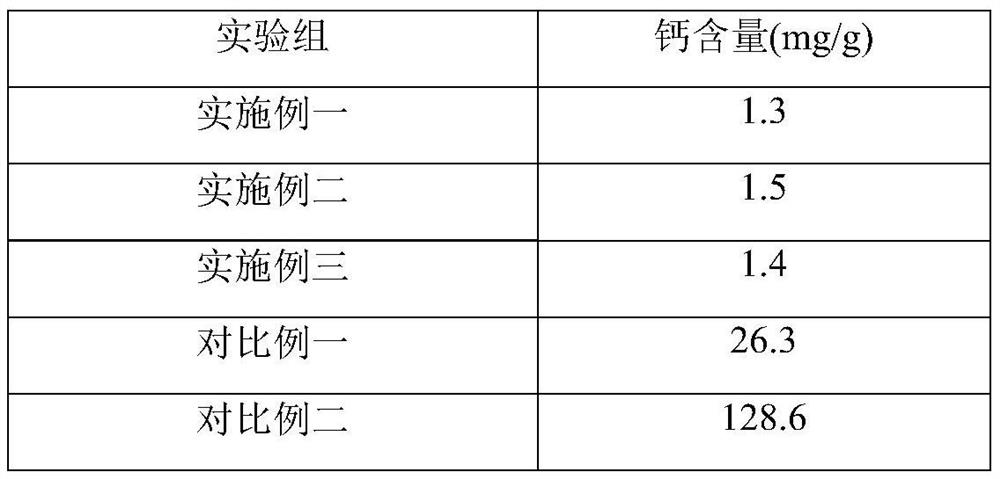
Decellularized anti-calcification heart patch and preparation method thereof
ActiveCN104998299ASuitable degradation cycleSlightly altered collagen structureProsthesisActive agentHeart chamber
The invention relates to a preparation method of a decellularized anti-calcification heart patch. The method mainly comprises the steps that raw material pericardial tissue is degreased through an organic reagent and processed by a mixed solution of a high salt, a surface active agent and alkali, and finally sterilization treatment is performed to obtain the decellularized anti-calcification heart patch. According to the obtained heart patch, due to the special decellularized anti-calcification technology, the collagenous fiber three-dimensional pore structure of the raw materials is reserved, meanwhile, cells contained in the materials are effectively removed, and the immunogenicity is lowered; the materials can guide cells to grow in the materials, scar tissue generation is reduced, and the good anti-calcification ability is achieved; the excellent mechanical property is achieved, arterio-venous pressure difference between heart chambers can be resisted, and the repair effect is ensured. The preparation method is applicable to atrial septal defects, ventricular septal defects, aortic stenosis and the like caused by the congenital heart disease.
Owner:SHAANXI BOYU REGENERATIVE MEDICINE CO LTD
Bioprosthetic valve and processing method thereof
PendingCN111701079AFully fixedAvoid degradationTissue regenerationProsthesisBioprosthetic valveTissue material
The invention discloses a bioprosthetic valve and a processing method thereof. The processing method comprises the following steps: flattening a flat pericardial tissue material and fixing the edge; sequentially contacting a pericardial tissue material with the fixed edge with an acellular solution, a cleaning solution, a fixing solution, a capping reagent, a deloading solution and a cleaning solution, forming a pressure difference between the two sides of the pericardial tissue material in the solution contact process, and enabling various solutions to pass through the interior of the pericardial tissue material under the action of the pressure difference so as to finish the treatment of the pericardial tissue material. According to the treatment method disclosed by the invention, phospholipid, cell components, immunogenicity, calcification sites, toxic reagent residues and the like in tissues can be effectively removed, the anti-calcification and anti-coagulation properties of the treated tissue are remarkably enhanced, and the pericardial material can be extruded under the action of pressure, so that the treated pericardial material has excellent mechanical properties.
Owner:SICHUAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com