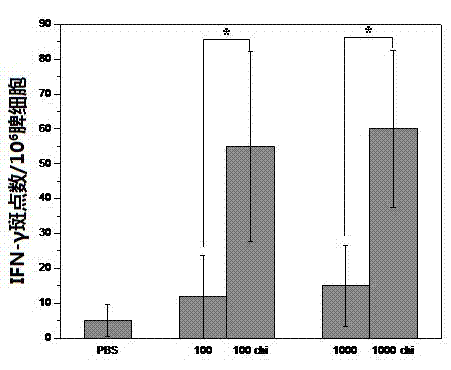
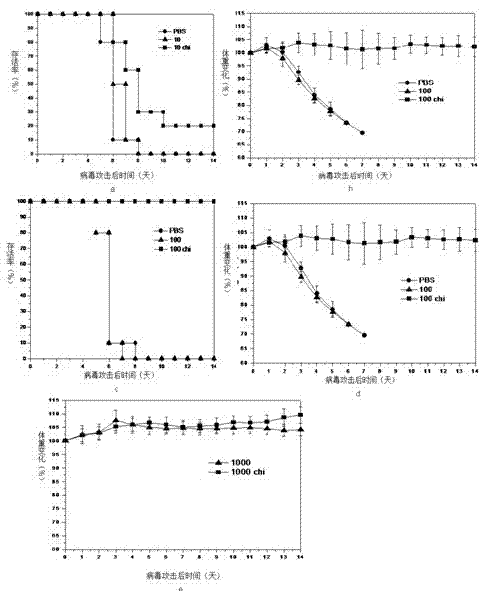
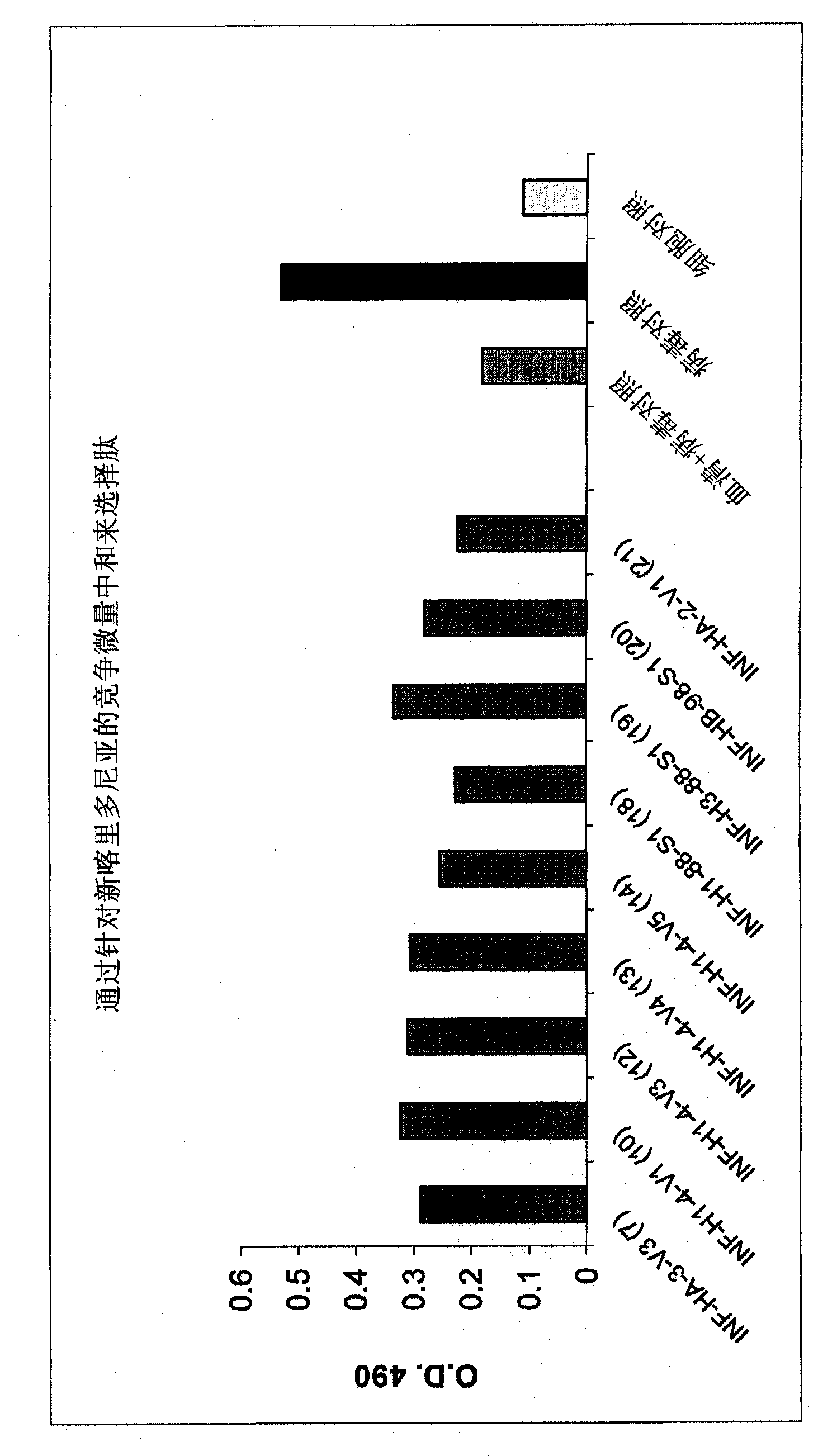
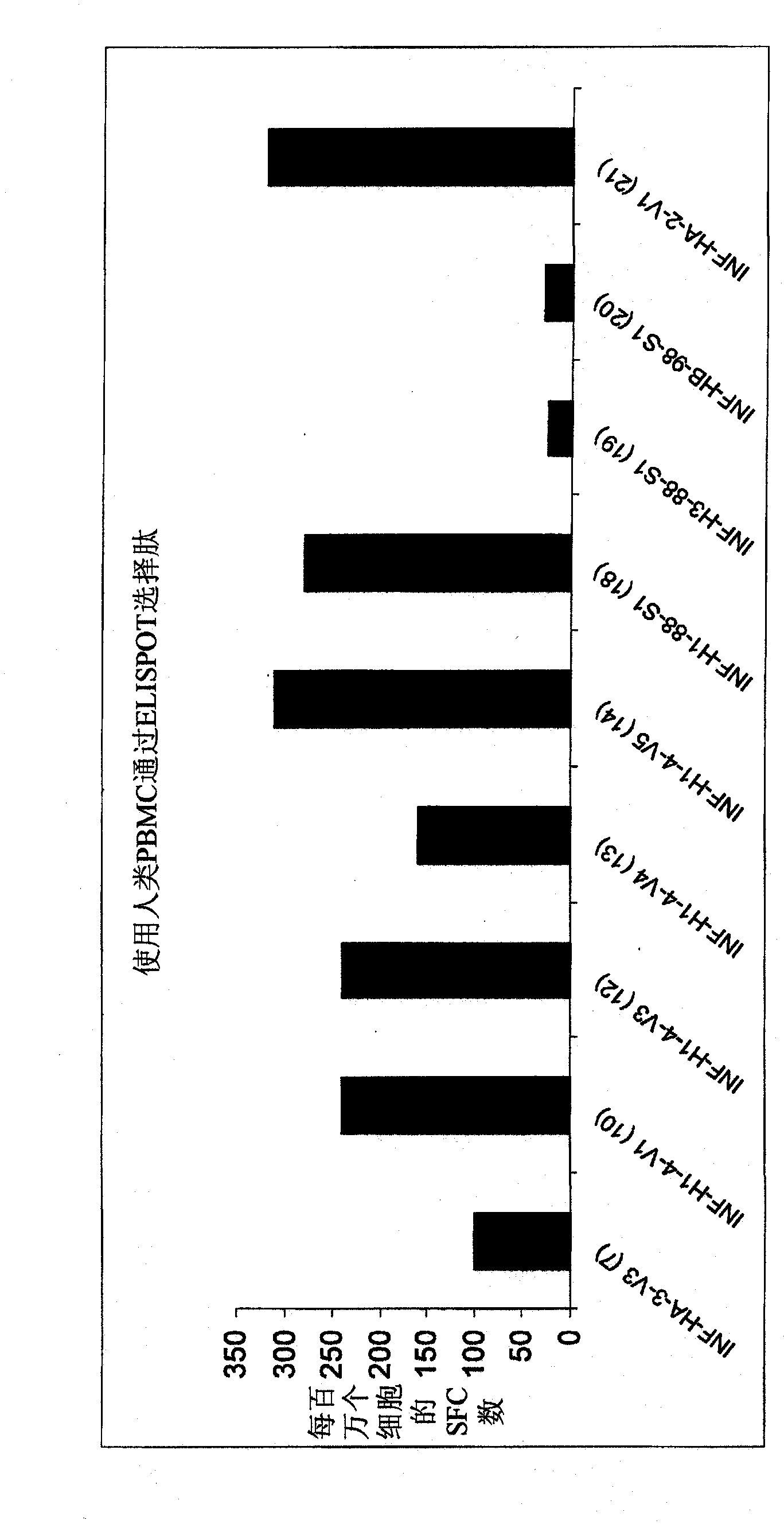
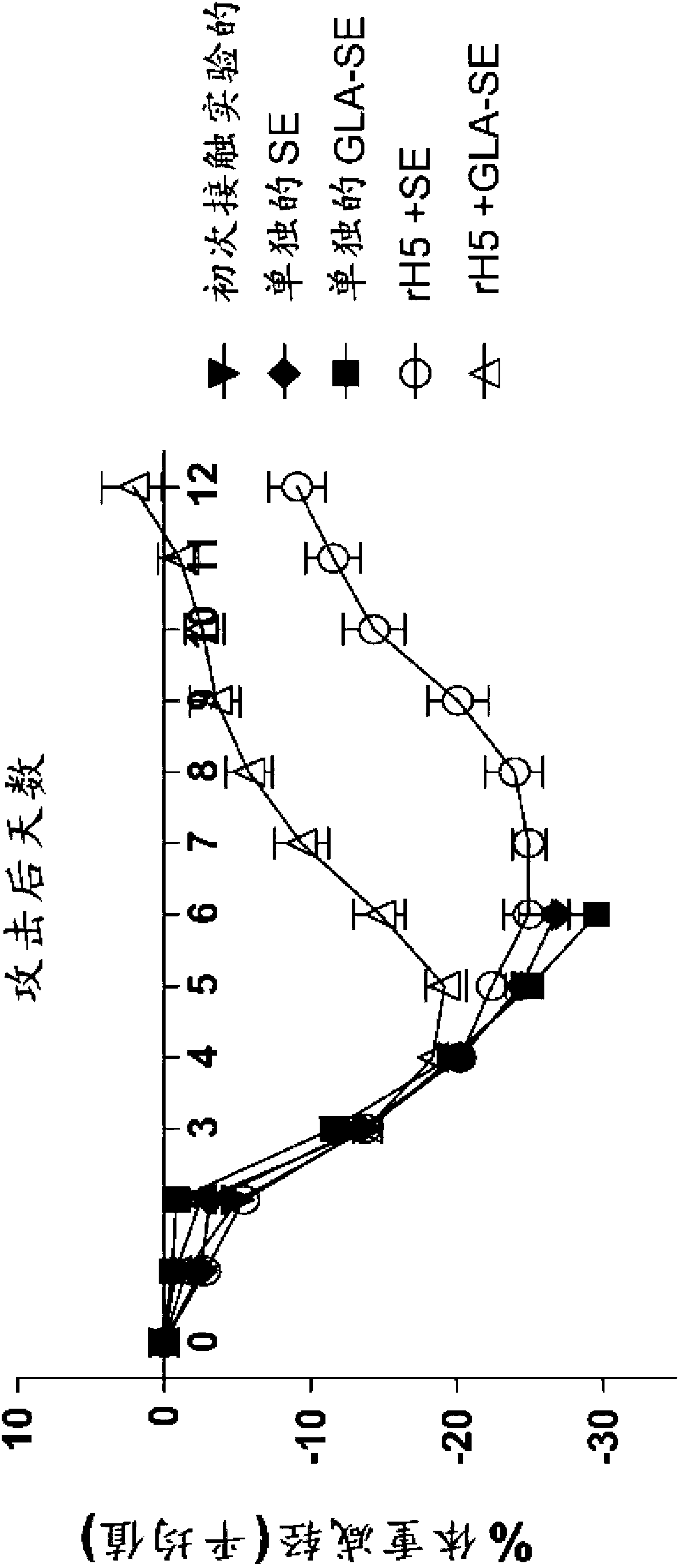
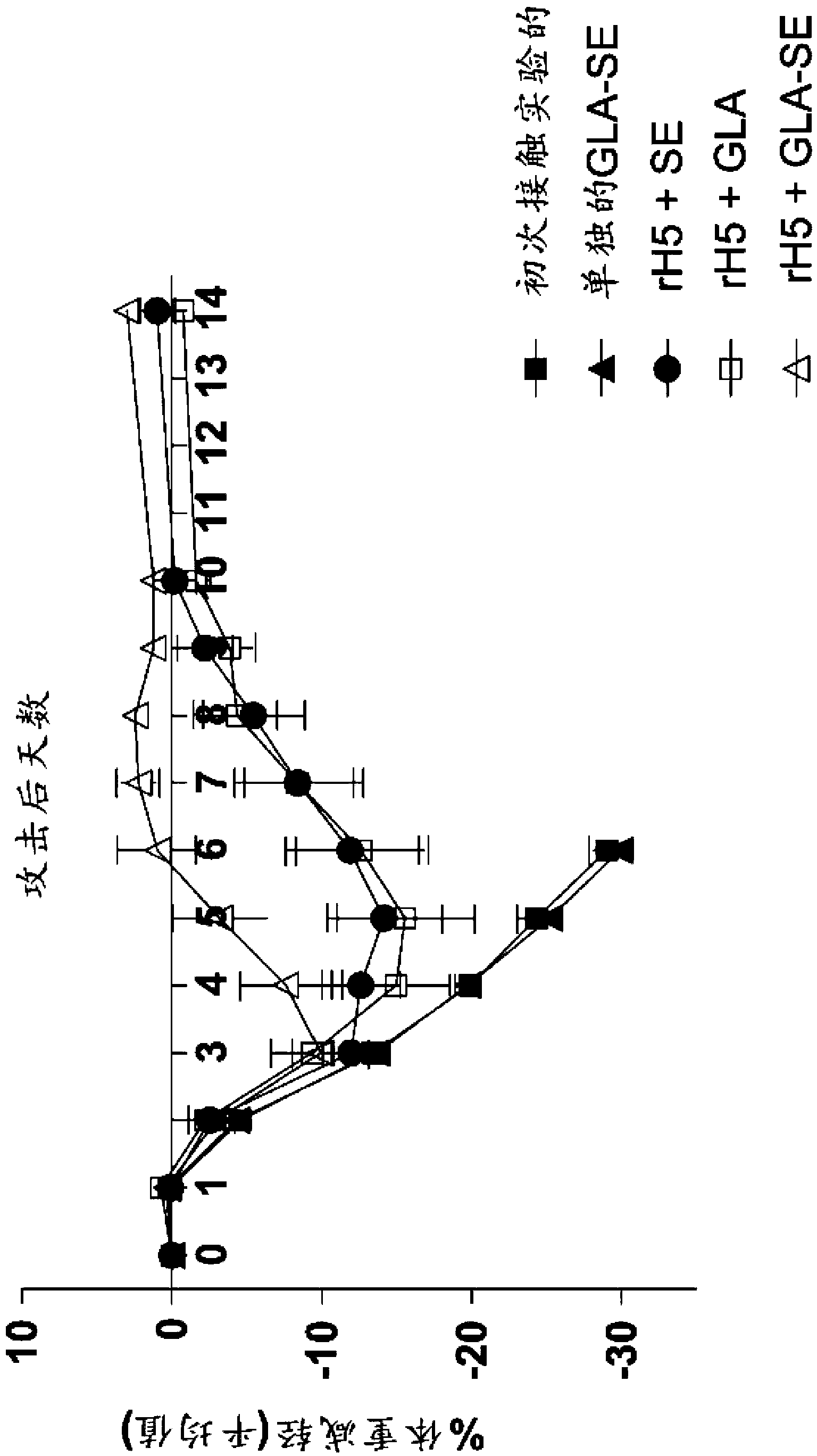
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
40 results about "Pandemic influenza" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Influenza vaccines
InactiveUS20080299151A1Less protectionBroad and efficient protective immunitySsRNA viruses negative-senseOrganic active ingredientsHemagglutininMammal
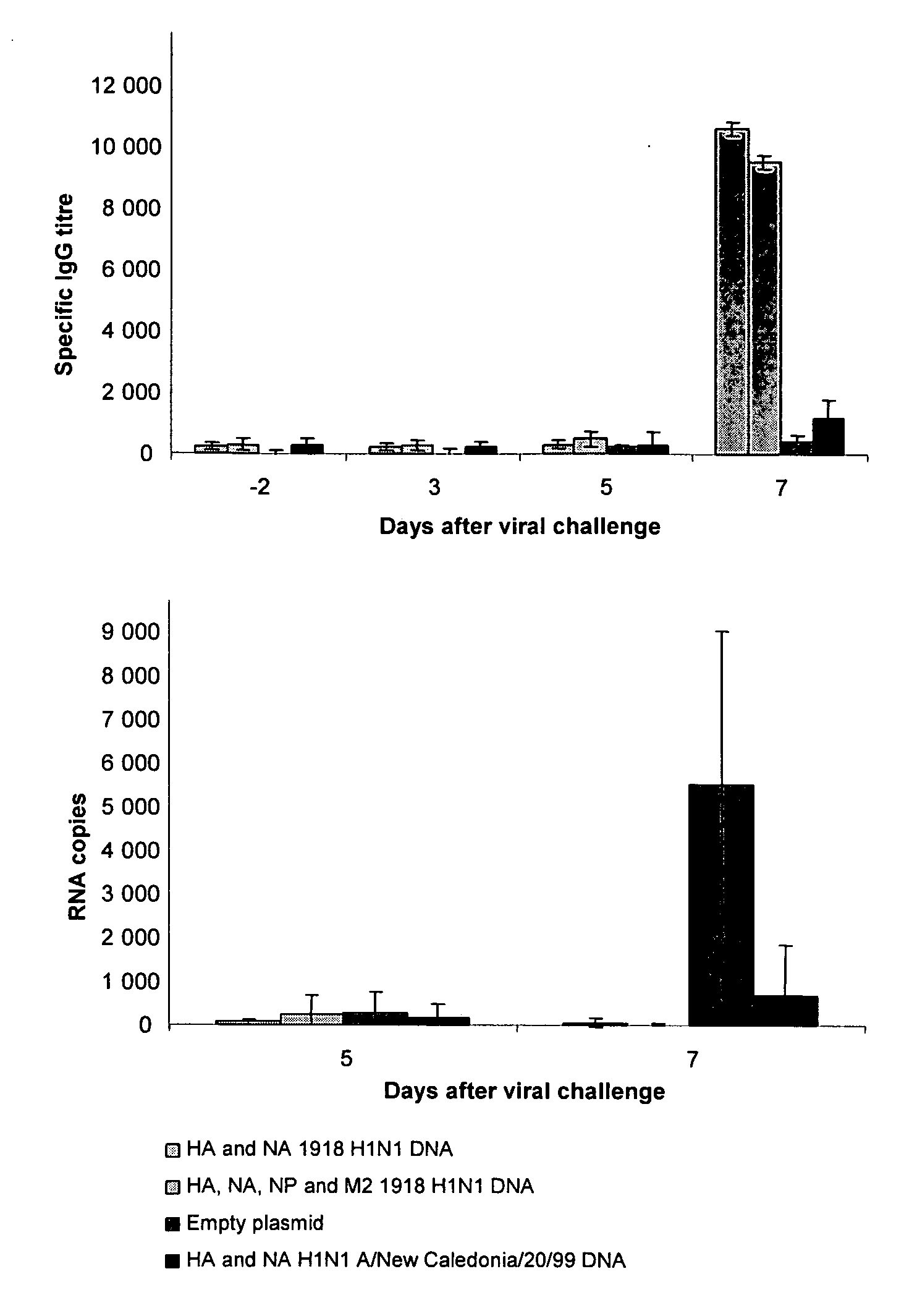
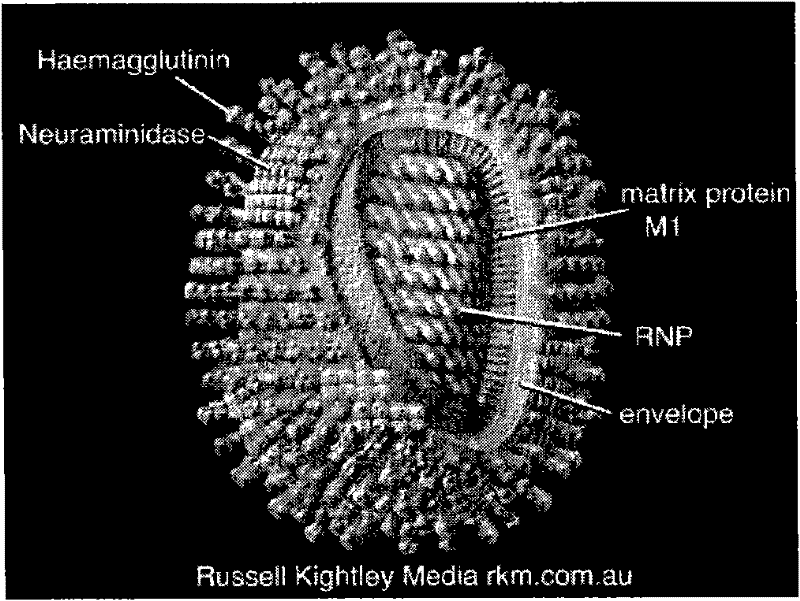
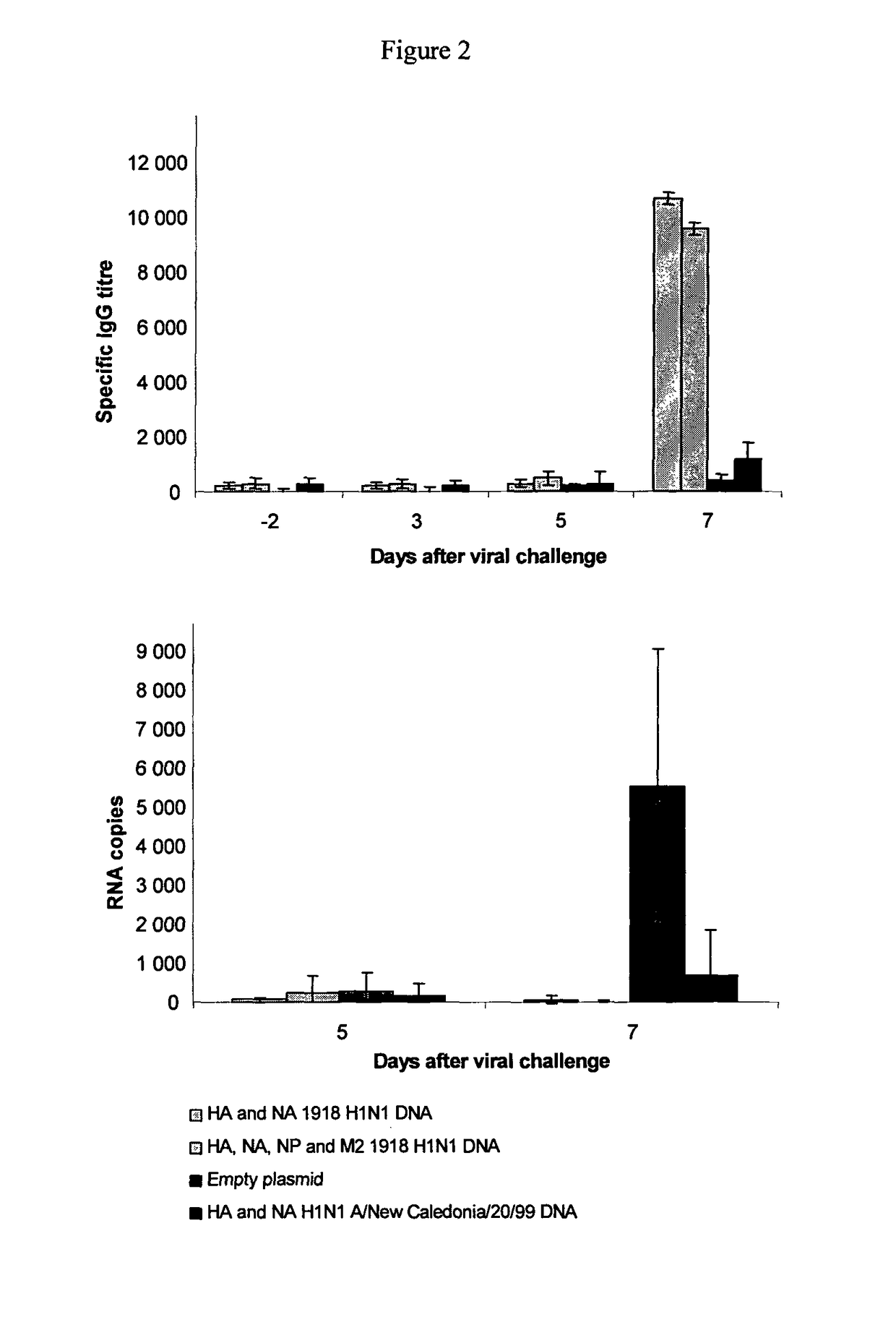
Described herein are vaccines and the use of naked DNA and / or RNA encoding hemagglutinin (HA) from pandemic influenza, e.g., the 1918 H1N1 and / or the 1957 H2N2 and / or the 1968 H3N2 influenza A virus, as a vaccine component against present day and coming H1, H2, H3, H5, N1, N2 containing influenza A infections in humans and swine optionally with the naked DNA and / or RNA encoding Neuraminidase (NA) and / or matrix protein (M) and / or the nucleoprotein (NP) from pandemic influenza virus included. If the vaccine components are used as DNA or RNA vaccines with or without the corresponding protein, the codons can optionally be “humanized” using preferred codons from highly expressed mammalian genes and the administration of this DNA vaccine can be by saline or buffered saline injection of naked DNA or RNA, or injection of DNA plasmid or linear gene expressing DNA fragments coupled to particles. Addition of the matrix protein (M) and / or the nucleoprotein (NP) from the 1918 influenza strain is also disclosed.
Owner:STATENS SERUM INST
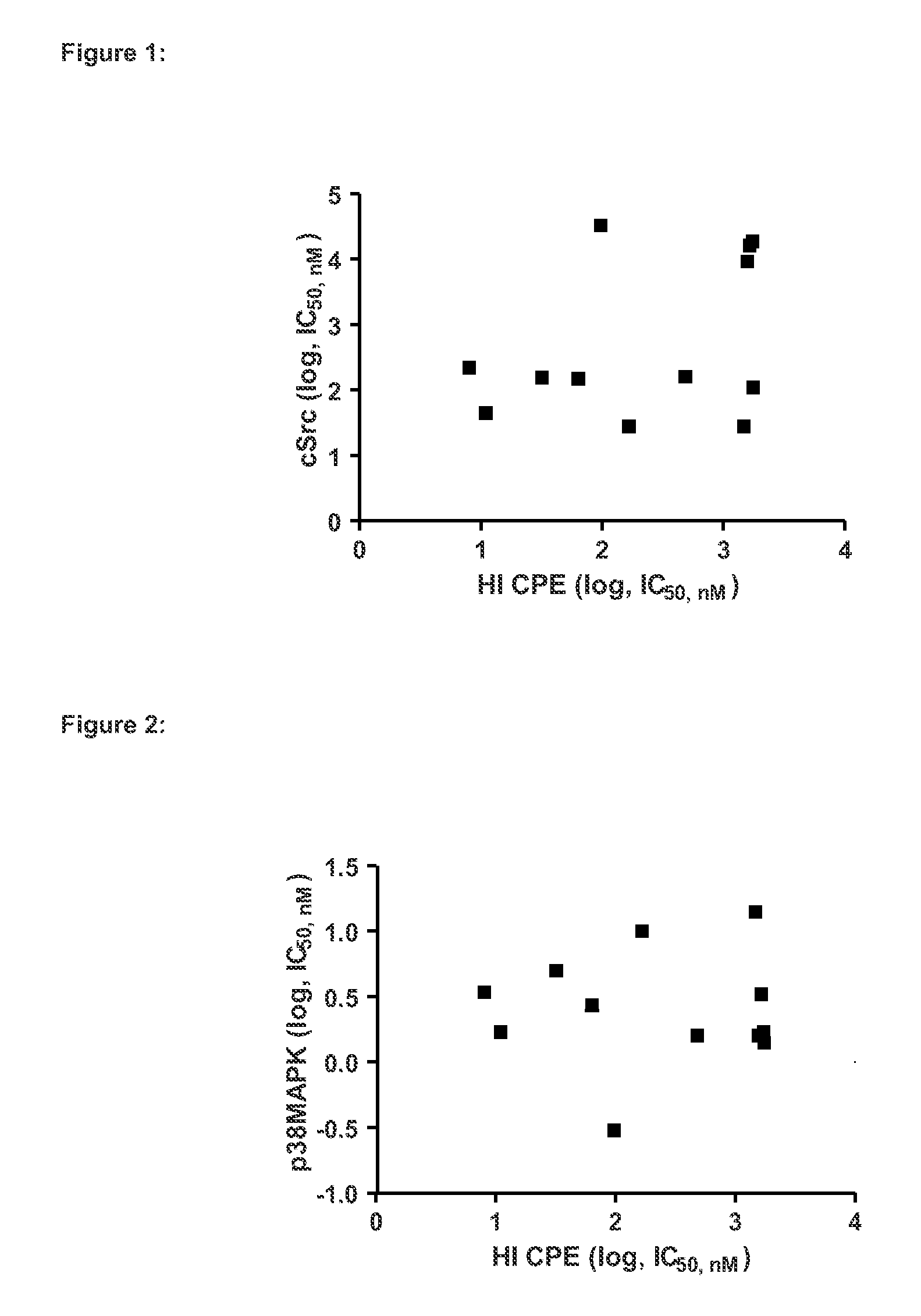
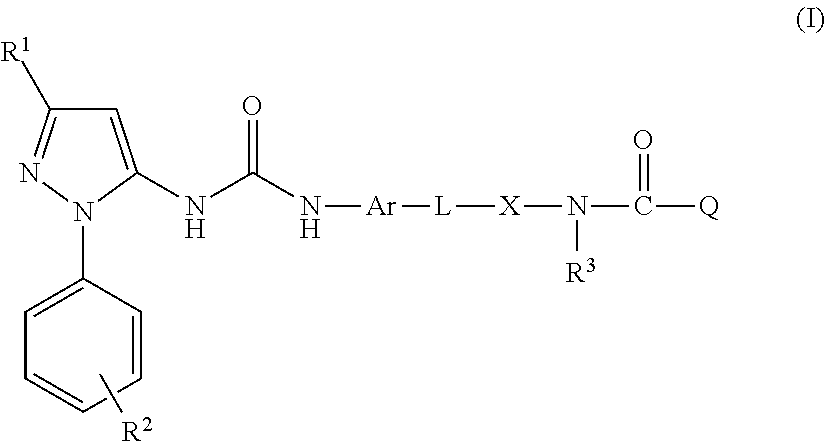
Inhibitors of hemopoietic cell kinase (p59-hck) and their use in the treatment of influenza infection
InactiveUS20120244120A1Efficient methodRisk minimizationBiocideCompound screeningHEMOPOIETIC CELL KINASEViral infection
The present invention relates inter alia to the treatment or prevention of influenza virus infection (including subtypes influenza A virus, influenza B virus, avian strain H5N1, A / H1N1, H3N2 and / or pandemic influenza) using compounds which inhibit the activity of p59-HCK and to a method of screening for a candidate drug substance intended to prevent or treat influenza virus infection in a subject, said method comprising identifying a test substance capable of inhibiting p59-HCK activity.
Owner:RESPIVERT
Avian influenza virus live attenuated vaccine and uses thereof
ActiveUS20110150912A1Poor replicationLess virulentSsRNA viruses negative-senseBacteriaHeterologousDonor strain
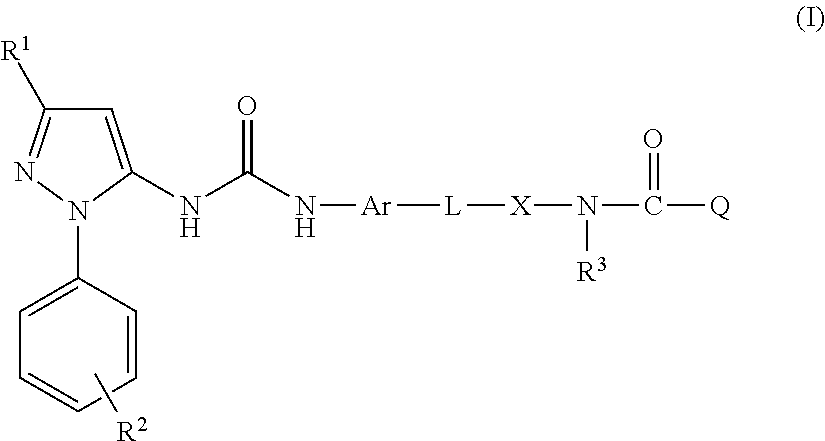
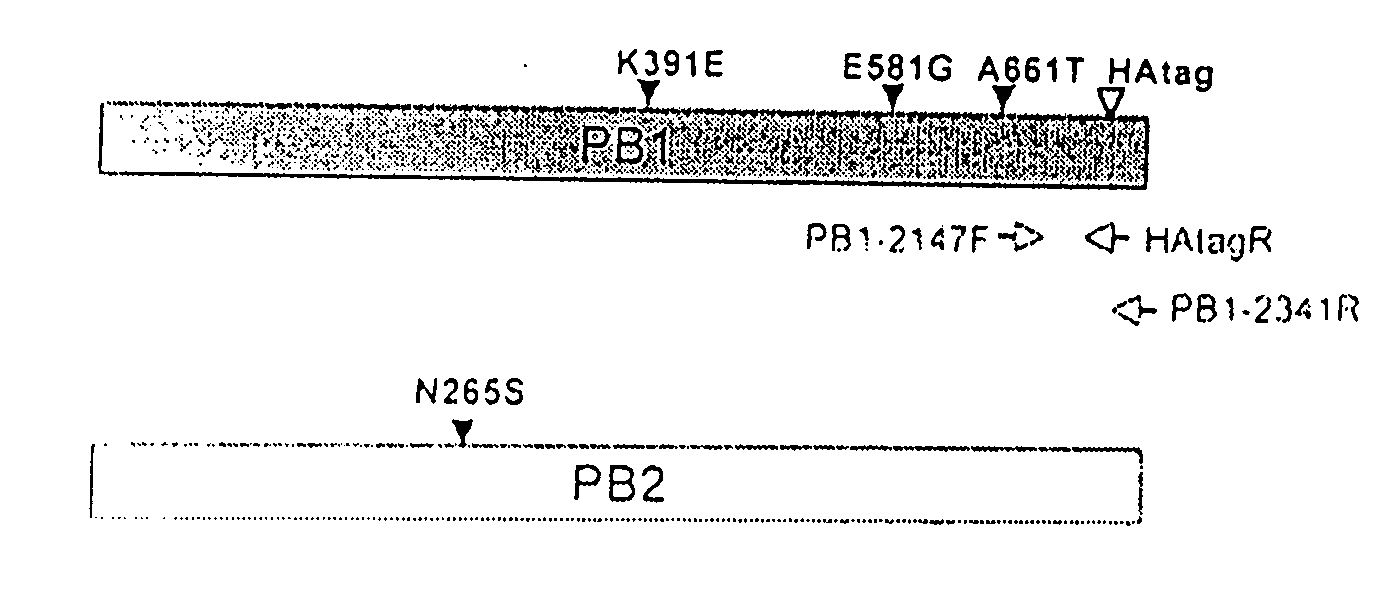
Described in this application are attenuated strains of avian influenza virus containing temperature sensitive mutations in addition to a genetic tag in the PB1 gene. The attenuated viruses are useful as avian and mammalian vaccine for protective immunity against homologous and heterologous lethal challenges with influenza virus. A genetically modified avian influenza virus backbone is described which can be used as a master donor strain for the generation of live attenuated vaccines for epidemic and pandemic influenza.
Owner:UNIV OF MARYLAND
Avian influenza virus live attenuated vaccine and uses thereof
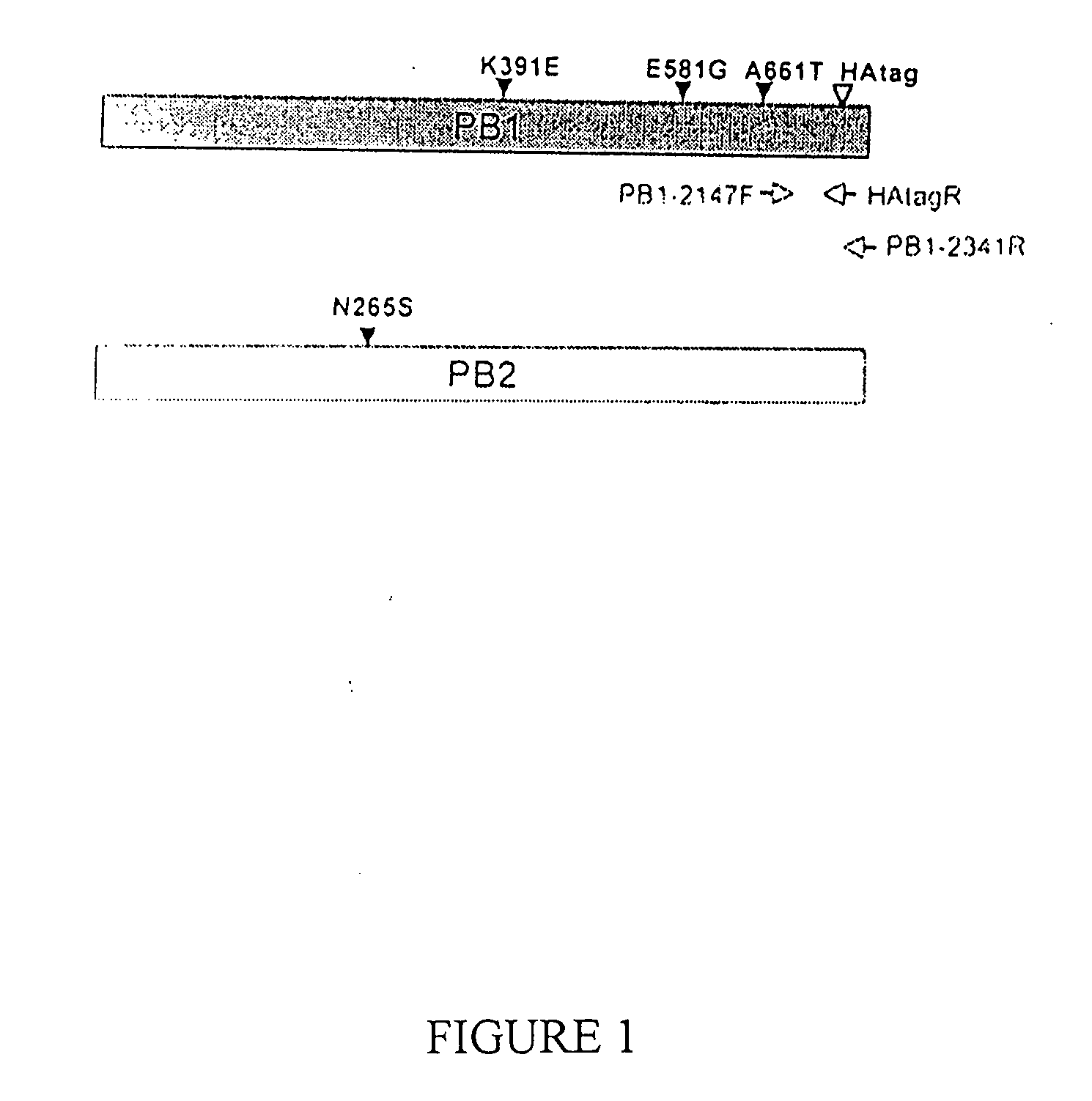
Described in this application are attenuated strains of avian influenza virus containing temperature sensitive mutations in addition to a genetic tag in the PB1 gene. The attenuated viruses are useful as avian and mammalian vaccine for protective immunity against homologous and heterologous lethal challenges with influenza virus. A genetically modified avian influenza virus backbone is described which can be used as a master donor strain for the generation of live attenuated vaccines for epidemic and pandemic influenza.
Owner:UNIV OF MARYLAND
Inhibitors of Hemopoietic Cell Kinase (P59-HCK) and Their Use in the Treatment of Influenza Infection
The present invention relates inter alia to the treatment or prevention of influenza virus infection (including subtypes influenza A virus, influenza B virus, avian strain H5N1, A / H1N1, H3N2 and / or pandemic influenza) using compounds which inhibit the activity of p59-HCK and to a method of screening for a candidate drug substance intended to prevent or treat influenza virus infection in a subject, said method comprising identifying a test substance capable of inhibiting p59-HCK activity.
Owner:RESPIVERT
A kind of lipid microsphere composition
The invention relates to a lipid microsphere composition. The lipid microsphere composition is characterized by comprising a lipid microsphere emulsifying agent and an immunogenic composition, wherein the lipid microsphere emulsifying agent comprises 1 to 30 weight percent of pharmaceutical grade grease, 0.1 to 10 weight percent of zwitterionic / non-ionic surfactant composition, 0.01 to 5 weight percent of stabilizing agent and the balance of aqueous solution; and the immunogenic composition is a univalent to multivalent composition and usually a trivalent composition, and contains a virus antigen or antigenic preparation from one or more pandemic influenza epidemic related influenza virus strains or with one or more pandemic influenza epidemic related influenza virus strains. The lipid microsphere composition not only can serve as an excellent aid of a vaccine, but also can serve as a conveying system of the vaccine. The lipid microsphere composition is applicable to different administration routes of the vaccine, improves compliance, diversity and selectivity of clinical application, and achieves wide, safe and effective immune effect.
Owner:LIAONING CHENGDA BIOTECH
Method for preparing Vero cell influenza virus vaccine
ActiveCN102078605ASimple purification processQuality standardsAntiviralsAntibody medical ingredientsInfluenza virus vaccineIon exchange
The invention provides a method for preparing a Vero cell influenza virus vaccine. Seasonal trivalent influenza vaccines and pandemic influenza storage vaccines with qualified quality can be prepared by improving a vaccine purification process, combining hydrophobic chromatography with ion exchange and auxiliarily adopting other purification methods.
Owner:吉林亚泰生物药业股份有限公司
Influenza vaccines
ActiveUS20100160421A1Less protectionBroad and efficient immunitySsRNA viruses negative-senseOrganic active ingredientsHemagglutininSaline injection
Described herein are vaccines and the use of naked DNA and / or RNA encoding hemagglutinin (HA) from pandemic influenza, e.g., the 1918 H1N1 and / or the 1957 H2N2 and / or the 1968 H3N2 influenza A virus, as a vaccine component against present day and coming H1, H2, H3, H5, N1, N2 containing influenza A infections in humans and swine optionally with the naked DNA and / or RNA encoding Neuraminidase (NA) and / or matrix protein (M) and / or the nucleoprotein (NP) from pandemic influenza virus included. If the vaccine components are used as DNA or RNA vaccines with or without the corresponding protein, the codons can optionally be “humanized” using preferred codons from highly expressed mammalian genes and the administration of this DNA vaccine can be by saline or buffered saline injection of naked DNA or RNA, or injection of DNA plasmid or linear gene expressing DNA fragments coupled to particles. Addition of the matrix protein (M) and / or the nucleoprotein (NP) from the 1918 influenza strain is also disclosed.
Owner:STATENS SERUM INST
Vaccines for Pandemic Influenza
InactiveUS20110305748A1High potencyReduce doseSsRNA viruses negative-senseViral antigen ingredientsHemagglutininAdjuvant
Owner:IMMUNE DESIGN CORP
Influenza A virus Vero cell adapted strain and application thereof
ActiveCN101560503ATo satisfy the market's needsAvoid reduced vaccine efficacyInactivation/attenuationMicroorganism based processesHemagglutininReassortment
The invention discloses an influenza A virus Vero cell adapted strain and application thereof. The influenza A virus Vero cell adapted strain is named as A / Yunnan / 1 / 2005Va(H3N2), and the preserving number of the strain is CCTCC NO:V200514. The strain comprises an H3 subgroup hemagglutinin gene, an N2 subgroup neuraminidase gene and 6 internal genes for high yield of characteristic influenza viruses on Vero cells. The blood coagulation titer can be maintained at more than 1,024 by continuous subculturing of the strain on the Vero cells. The strain can be taken as a donor strain and subjected to genetic reassortment with an epidemic strain, or reverse genetics technology is adopted to perform genetic reassortment on the 6 internal genes and 2 surface protein genes of the epidemic strain to obtain the Vero cell adapted strain of the epidemic strain, and finally the adapted strain is used for preparing a Vero cell influenza vaccine or a Vero cell pandemic influenza vaccine.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Optimized influenza vaccines
ActiveUS20110229518A1Broad cross-reactivityPrevent/limit infection and spread of infectionSsRNA viruses negative-senseSugar derivativesInfluenza aHemagglutination
The invention concerns nucleotides vaccines encoding influenza proteins with few or no glycosylation sites. Since these first introductions of pandemic influenzas the viruses have drifted, accumulating mutations at antigenic sites, but also the N-glycosylation pattern has changed during the drifted years, accumulating N-linked glycosylation sequons that help mask the antigenic sites for recognition by the host immune system. These “naked” initial haemagglutinins induce a broad cross reactivity against widely drifted influenza subtypes. The origin of the DNA or RNA can be both pandemic influenza strains, which codes for proteins which have a naturally low content of glycosylation sites and / or DNA or RNA from non-pandemic influenza strains where the nucleotides have been mutated or changed so it encodes for proteins with less or no glycosylation sites. The invention also discloses DNA or RNA encoding the haemagglutinin (HA) from pandemic influenza A, e.g. the 1918 H1N1 and / or the 1957 H2N2 and / or the 1968 H3N2 influenza A virus, optionally with the Neuraminidase (NA) and / or matrix protein (M) and / or the nucleoprotein (NP) from these pandemic influenza virus included, mixed together with DNA or RNA from non-pandemic influenza A as a vaccine against present day and future influenza A viruses.
Owner:STATENS SERUM INST
Application of chitosan as adjuvant in preparation of influenza virus attenuated live vaccine
InactiveCN102824635AImproving immunogenicityReduce dosageAntiviralsAntibody medical ingredientsInfluenza pandemicImmunogenicity
The invention discloses an application of chitosan in preparation of influenza virus attenuated live vaccines, and provides an adjuvant which can reduce the using amount of the influenza virus attenuated live vaccine. According to the invention, chitosan is adopted as an influenza virus attenuated live vaccine adjuvant for the first time, which enhances the immunogenicity of the vaccine, induces human body to generate stronger immune response, effectively reduces the using amount of the vaccine, effectively enlarges immunization population within existing production capacity and technology, and has important social meaning and application value for pandemic influenza.
Owner:SHANGHAI INST OF BIOLOGICAL PROD CO LTD
Method for preparing nasal-spray type influenza virus vaccine containing CpG ODN and poly I:G adjuvant
InactiveCN101745109AImmunity-boosting propertiesLess antigenAerosol deliveryAntiviralsInfluenza virus vaccineLysis
The invention provides a method for preparing nasal-spray type influenza virus vaccine containing CpG ODN and poly I:G adjuvant, which belongs to the technical field of biology. The method comprises the following steps: adopting chicken embryo to culture proliferative influenza virus; obtaining purified influenza virus through ultrafiltration concentration and column chromatography; obtaining influenza vaccine stock solution through lysis; and proportioning the influenza vaccine stock solution of a required type to CpG ODN adjuvant or I:G adjuvant. The invention provides the method for preparing novel nasal-spray type influenza virus vaccine, which can reduce the amount of influenza virus antigen, provides convenience for immunization and vaccination, and is suitable for rapidly improving the capacity of supplying influenza vaccine under threat of global pandemic influenza.
Owner:云南沃森生物技术股份有限公司
Influenza-pandemic influenza bivalent combined vaccine and preparation method thereof
InactiveCN101524538AFlu preventionReduce the number of vaccinationsAntiviralsAntibody medical ingredientsAntigenBird flu
The invention provides a high-efficiency low-cost influenza-pandemic influenza bivalent combined vaccine which can be produced in large amount and a preparation method thereof, relating to a novel influenza-pandemic influenza vaccine preparation used for injection and a preparation process thereof and comprising preparation of virus seed banks and production and preparation of vaccine stock solution. The preparation method refers to that of whole-virus inactivated vaccines, split vaccines and subunit vaccines of the influenza and split vaccines of the pandemic influenza. The influenza-pandemic influenza bivalent combined vaccine preparation provided by the invention comprises two antigens for influenza and bird flu and can conveniently prevent viral epidemics and outbreak of the influenza and bird flu.
Owner:CHENGDU KANGHUA BIOLOGICAL PROD
Lipid microsphere composition
The invention relates to a lipid microsphere composition. The lipid microsphere composition is characterized by comprising a lipid microsphere emulsifying agent and an immunogenic composition, wherein the lipid microsphere emulsifying agent comprises 1 to 30 weight percent of pharmaceutical grade grease, 0.1 to 10 weight percent of zwitterionic / non-ionic surfactant composition, 0.01 to 5 weight percent of stabilizing agent and the balance of aqueous solution; and the immunogenic composition is a univalent to multivalent composition and usually a trivalent composition, and contains a virus antigen or antigenic preparation from one or more pandemic influenza epidemic related influenza virus strains or with one or more pandemic influenza epidemic related influenza virus strains. The lipid microsphere composition not only can serve as an excellent aid of a vaccine, but also can serve as a conveying system of the vaccine. The lipid microsphere composition is applicable to different administration routes of the vaccine, improves compliance, diversity and selectivity of clinical application, and achieves wide, safe and effective immune effect.
Owner:LIAONING CHENGDA BIOTECH
Combined vaccine of seasonal influenza and pandemic influenza for people and preparation method
ActiveCN103285391ANo local irritationSolve the difficult problem of large-scale vaccinationAntiviralsAntibody medical ingredientsHemagglutininHuman influenza
The invention discloses a combined vaccine of seasonal influenza and pandemic influenza for people. The hemagglutinin concentration of each vaccine is 10-60 mug / ml of H1N1 human influenza vaccine,10-60 mug / ml of H3N2 human influenza vaccine, 10-60 mug / ml of B human influenza vaccine, and 10-60 mug / ml of H5N1 human influenza vaccine respectively. The invention also discloses a preparation method of the combined vaccine. The combined vaccine disclosed by the invention has high safety; the problem that the traditional avian influenza vaccine is difficultly vaccinated at a large scale is solved; explosive epidemics of the H5N1 human influenza vaccine is effectively prevented and controlled when the seasonal influenza is prevented; the combined vaccine has great social and economic benefits.
Owner:WUHAN INST OF BIOLOGICAL PROD CO LTD
Megaribavirin alone or combination of other antiviral, antioxidant and a perflubron emulsion for treatment of viral disease
InactiveUS20100297033A1Less side effectsImprove toleranceBiocideDispersion deliveryPeroxynitriteAnti oxidant
The present invention is a novel method using a MegaRibavirin aerosol or a MegaRibavirin combination of therapeutics for the treatment of viral disease particularly the pandemic influenza strains “swine” 2009 H1N1 and H5N1. This invention utilizes Ribavirin in an aerosol Mega Dose (61-161 mg / ml) alone or combined with or without other antivirals, a perfluorocarbon emulsion and anti-inflammatory / anti-oxidants. Where applicable, the perfluorocarbon emulsion may dissolve these agents enabling a depot effect and possible protracted delivery. In addition perfluorocarbon emulsions have the possible added benefit of oxygen carrying capacity and alveolar nitric oxide sequestration, which may reduce peroxynitrite formation and decrease Influenza severity.
Owner:MTM RES
Method of eliciting an immune response against pandemic influenza virus
ActiveUS20100291146A1SsRNA viruses negative-senseViral antigen ingredientsAdjuvantImmunostimulating Complexes
A method for eliciting or inducing a protective immune response in a subject against a pandemic subtype of influenza virus comprises administering to the subject a composition comprising (i) at least one immunogen of an endemic influenza subtype, and (ii) an immunogen-free immunostimulating complex as adjuvant.
Owner:CSL LTD
Reagents and methods for detecting influenza virus proteins
ActiveUS8669046B2Simplify the linkImprove solubilitySsRNA viruses negative-sensePeptide/protein ingredientsInfluenza virus nucleoproteinVaccine Potency
Antibodies that specifically bind to a peptide having an amino acid sequence as found at the N-terminus of the HA2 fusion peptide of the influenza A virus may be raised by inoculating a mammal with a conjugate of the peptide. In one embodiment, the conjugate comprises the peptide linked to a spacer (e.g. 6-aminocaproic acid) and a carrier protein (e.g. KLH). The antibodies may be used as a universal reagent for detecting HA proteins of influenza viruses. The antibodies are useful as versatile reagents for laboratory research and vaccine potency determination, especially in the event of pandemic influenza outbreaks.
Owner:HER MAJESTY THE QUEEN & RIGHT OF CANADA REPRESENTED BY THE MIN OF HEALTH
Influenza a and b virus replication-inhibiting peptides
A synthesized or isolated influenza virus replication-inhibiting peptide that competitively inhibits protein-protein interaction of the PA and PB1 of both influenza Virus Types A and B and novel in vitro binding screen to identify peptides with antiviral activity against influenza viruses of both type A and B is disclosed. In addition to the well-known pandemic influenza A viruses (such as the 1918 ''Spanish'' flu or H5N1), both type A and B viruses contribute greatly to the annual recurring epidemics that cause the vast majority of human cases and medical cost. Surprisingly, it was found that the novel virus replication-inhibiting, are able to inhibit protein-protein interaction of the PA and PB1 subunits of the heterotrimeric viral RNA polymerase complex of both influenza virus types A and B. The viral polymerase sub- unit interaction domain turned out as an effective target for the new antivirals, as correct assembly of the three viral polymerase subunits PB1, PB2 and PA is required for viral RNA synthesis and infectivity.
Owner:PIKE PHARMA
Influenza-activated constructs and methods of use thereof
ActiveUS20150376622A1Prevent sepsisPrevent cytokine stormMicrobiological testing/measurementFermentationHuman influenzaSubject matter
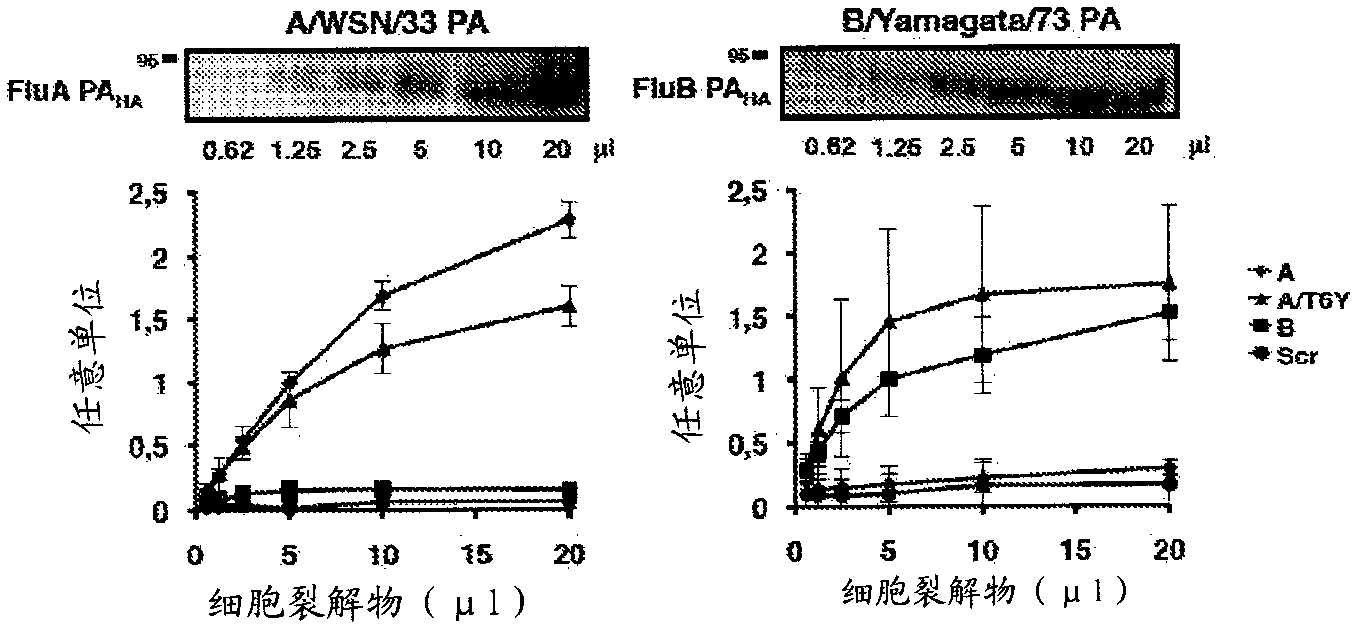
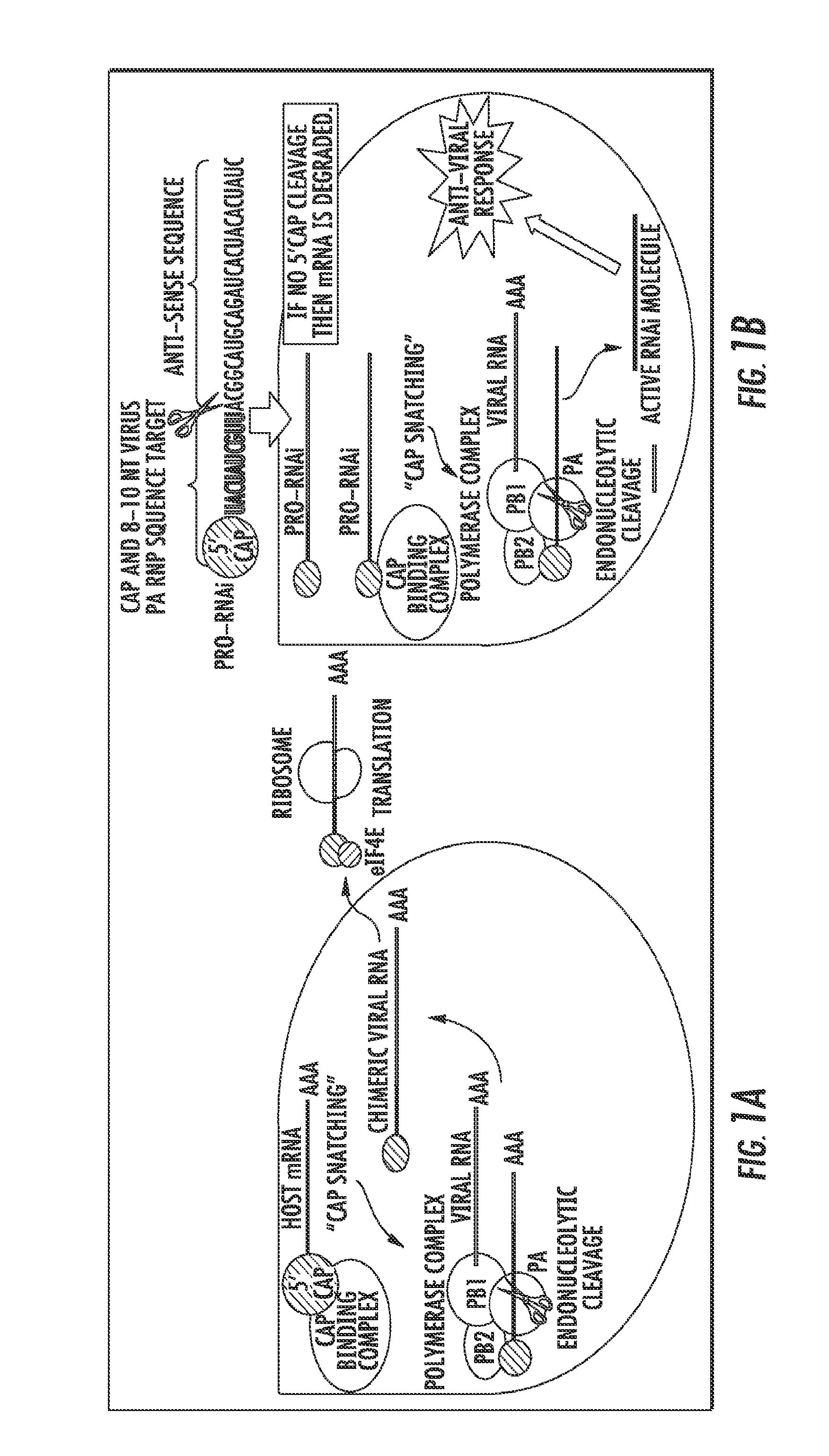
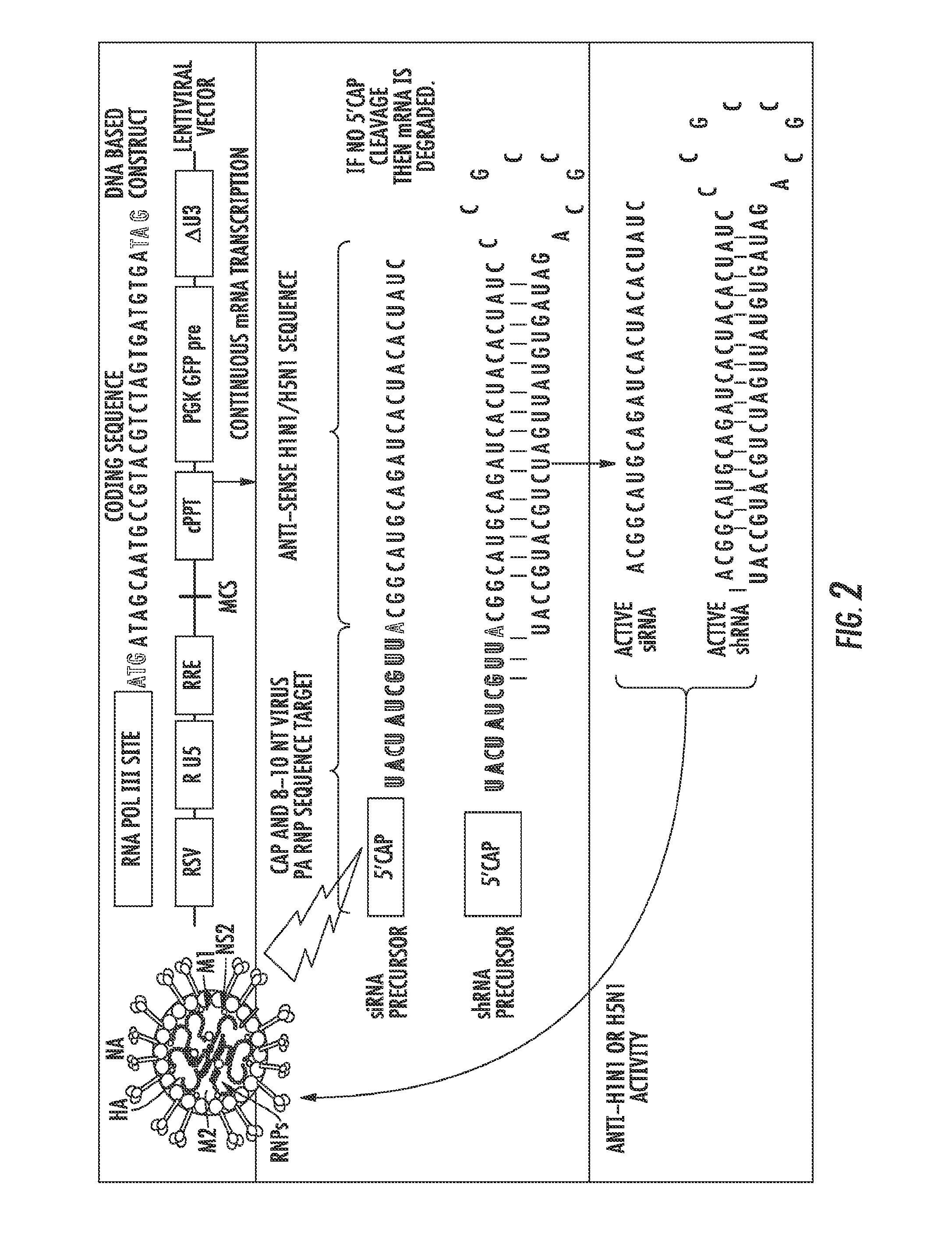
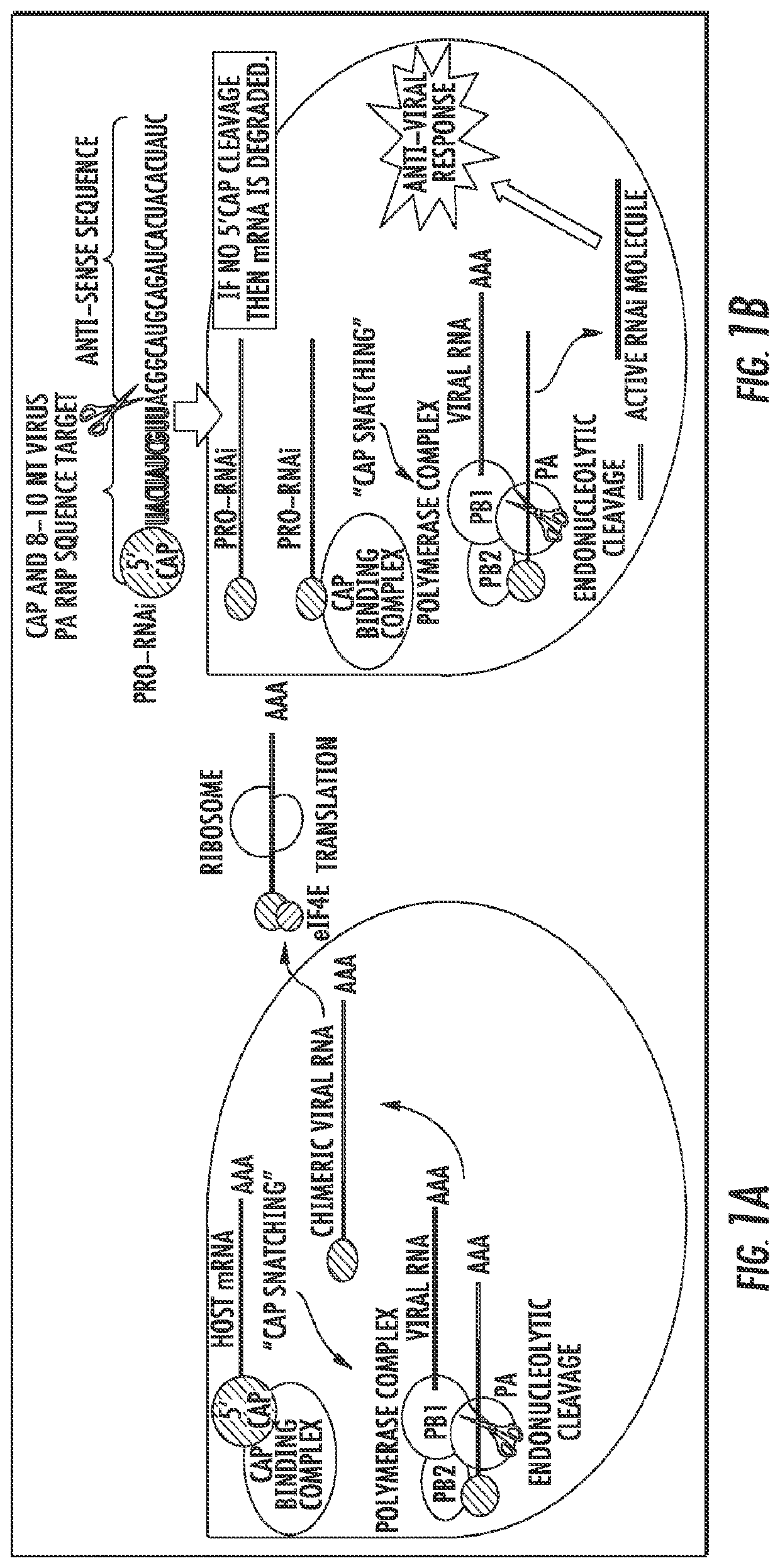
The presently disclosed subject matter provides a novel approach for the treatment, prevention, and diagnosis of Cap-Snatching virus infections, particularly all classes of human influenza, including pandemic influenza. The methods involve the use of constructs for RNA-interference (RNAi).
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Device for preventing pandemic influenza
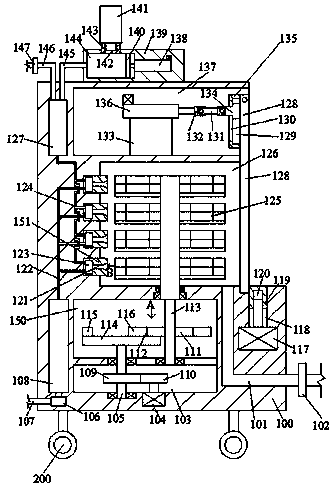
InactiveCN108543091AImprove precisionImprove securityLavatory sanitoryChemicalsEngineeringPandemic influenza
The invention discloses a device for preventing pandemic influenza, wherein the device includes a body; the lower end face of the body is provided with a number of universal wheels, and the body is internally fixed with a transmission cavity; a placement cavity located in the body is arranged above the transmission cavity; the left side of the placement cavity is provided with a liquid storage cavity located in the body; a sterilizing cavity located in the body and having an end port facing the right is arranged above the placement cavity; a push cavity located in the body and having an end port facing the right is arranged above the sterilizing cavity; the left side of the push cavity is provided with a return cavity located in the body; a first power source is fixed in the lower end wallof the transmission cavity. The device has simple structure, is convenient to use, realizes the process of automatic sterilization of objects, and effectively prevents the pandemic influenza.
Owner:王裕迪
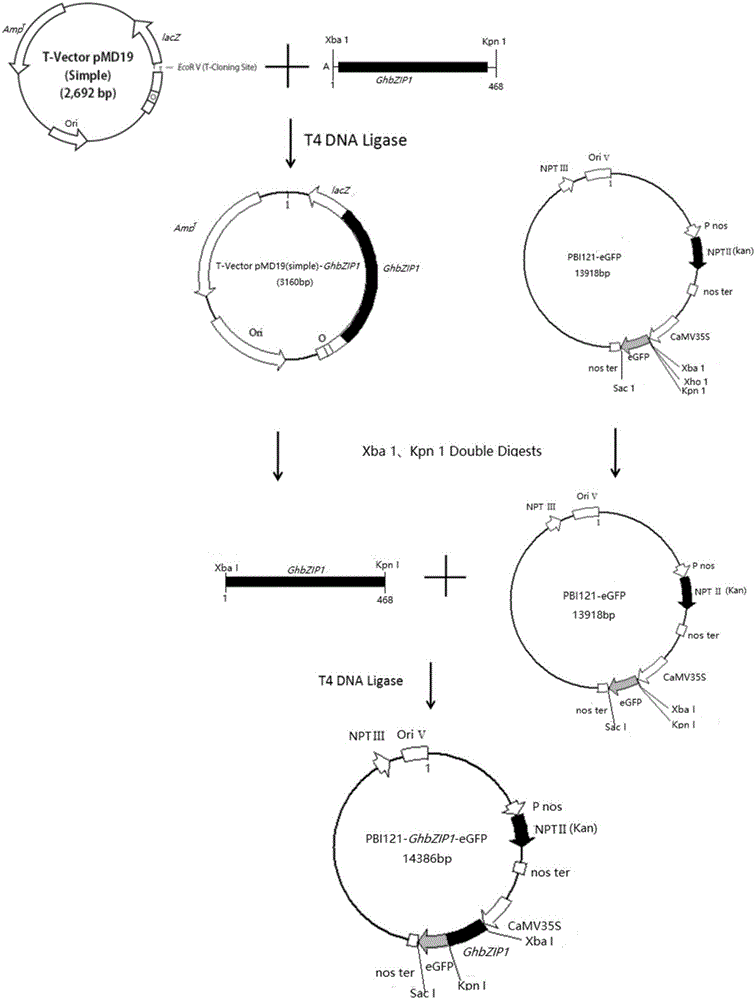
Method for creating cytoplasmic male sterile line by utilizing cotton transgenosis
The invention provides a method for creating a cytoplasmic male sterile line by utilizing cotton transgenosis. The method comprises the following steps: taking GhbZIP1 found in upland cotton as a target gene; adopting a transgenosis method for acquiring a cytoplasmic male sterility germplasm; and performing nuclear replacement backcross with homologous or heterological maintainer line or variety (line), thereby breeding a homologous or heterological nucleation male sterile line. The cell nucleus of the male sterile line acquired according to the invention is sourced from a non-transgenosis maintainer line or variety (line), so that a transgenosis-free plasmid vector is as same as a transgenosis plant and is free from safety risk and market risk; the cytoplasm of the acquired male sterile line is sourced from cultivated variety (line), so that the composition of high-advantage cross combination is benefited; various cytoplasmic male sterile germplasms can be created, so that the potential risk in pandemic influenza of a certain disease possibly caused by the wide popularization and long-term use of single cytoplasm coeno-species in the agricultural production can be avoided; the application prospect is ultra-wide.
Owner:GUANGXI UNIV
Multimeric multiepitope polypeptides in improved seasonal and pandemic influenza vaccines
ActiveUS9303070B2Improve the protective effectEnhance immune responseSsRNA viruses negative-senseViral antigen ingredientsInfluenza aPeptide
The present invention relates to use of multimeric multi-epitope peptide-based compositions for immunizing subjects against influenza by administering the compositions to the subject prior to or together with seasonal or pandemic influenza vaccines. The present invention also relates to compositions that include a multimeric multi-epitope polypeptide and a seasonal or pandemic preparation against influenza.
Owner:BIONDVAX PHARMA
Compositions and methods for treating influenza
The present application provides compositions and methods useful for treating influenza. As described herein, the compositions and methods are based on the development of peptides and peptide combinations which exhibit immunogenic properties against influenza. In some embodiments, the peptide combinations induce a protective response against multiple strains of influenza, e.g., seasonal strains of influenza or even the new pandemic influenza A (H1N1) virus of swine origin.
Owner:VARIATION BIOTECHNOLOGIES INC
Vaccines for pandemic influenza
InactiveCN102946900ARegain weightImproving immunogenicitySsRNA viruses negative-senseViral antigen ingredientsHemagglutininAdjuvant
Owner:免疫设计公司
Ribavirin perflubron emulsion composition for treating viral diseases
InactiveUS20210069098A1Less side effectsImprove toleranceOrganic active ingredientsDispersion deliveryDiseaseAlveolus pulmonis
The present invention is a novel method using a MegaRibavirin aerosol or a MegaRibavirin combination of therapeutics for the treatment of viral disease particularly the pandemic influenza strains “swine” 2009 HI N1 and H5N1. This invention utilizes Ribavirin in an aerosol Mega Dose (61-161 mg / ml) alone or combined with or without other antivirals, a perfluorocarbon emulsion and anti-inflammatory / anti-oxidants. Where applicable, the perfluorocarbon emulsion may dissolve these agents enabling a depot effect and possible protracted delivery. In addition, perfluorocarbon emulsions have the possible added benefit of oxygen carrying capacity and alveolar nitric oxide sequestration, which may reduce peroxynitrite formation and decrease Influenza severity.
Owner:MTM RES
Optimized influenza vaccines
ActiveUS9764024B2Prevent/limit infection and spread of infectionImprove the level ofSsRNA viruses negative-senseSugar derivativesInfluenza aHemagglutination
The invention concerns nucleotides vaccines encoding influenza proteins with few or no glycosylation sites. Since these first introductions of pandemic influenzas the viruses have drifted, accumulating mutations at antigenic sites, but also the N-glycosylation pattern has changed during the drifted years, accumulating N-linked glycosylation sequons that help mask the antigenic sites for recognition by the host immune system. These “naked” initial haemagglutinins induce a broad cross reactivity against widely drifted influenza subtypes. The origin of the DNA or RNA can be both pandemic influenza strains, which codes for proteins which have a naturally low content of glycosylation sites and / or DNA or RNA from non-pandemic influenza strains where the nucleotides have been mutated or changed so it encodes for proteins with less or no glycosylation sites. The invention also discloses DNA or RNA encoding the haemagglutinin (HA) from pandemic influenza A, e.g. the 1918 H1N1 and / or the 1957 H2N2 and / or the 1968 H3N2 influenza A virus, optionally with the Neuraminidase (NA) and / or matrix protein (M) and / or the nucleoprotein (NP) from these pandemic influenza virus included, mixed together with DNA or RNA from non-pandemic influenza A as a vaccine against present day and future influenza A viruses.
Owner:STATENS SERUM INST
Influenza-activated constructs and methods of use thereof
The presently disclosed subject matter provides a novel approach for the treatment, prevention, and diagnosis of Cap-Snatching virus infections, particularly all classes of human influenza, including pandemic influenza. The methods involve the use of constructs for RNA-interference (RNAi).
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Method for detecting emerging pandemic influenza
A method for detecting emerging pandemic influenza strains is provided. RT-PCR is used to detect HPAI followed by pyrosequencing to detect codons defining human or avian influenza signatures. This method screens for avian influenza viruses containing mutations suspected of making the virus more infective or virulent to humans.
Owner:MRIGLOBAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com