Vaccines for pandemic influenza
A technology of epidemic, influenza virus, applied in the field of vaccine for pandemic influenza
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] A. Preparation of hemagglutinin
[0024] 1. The source of HA
[0025] At least 4 different influenza A viruses are currently of pre-pandemic or pandemic concern: H5N1, H1N1, H7N7, and H9N2.
[0026] Influenza A virus subtype H5N1 is a subtype of influenza A virus that can cause disease in humans and many other animal species. A highly pathogenic strain of H5N1 (HPAI H5N1) is the cause of "bird flu" or "bird flu". It is currently a disease of poultry and although it can infect humans, most or all of it has had extensive physical contact with infected birds. H5N1 is classified as a pre-pandemic virus because not all the conditions for a pandemic have been met, most notably the inability of the virus to spread easily and sustainably between humans.
[0027] Avian influenza of the H5N1 type (referred to herein as "H5N1") first emerged in Asia and has spread globally. H5N1 viruses continue to evolve and can now be classified into different clades and subclades based on t...
Embodiment 1
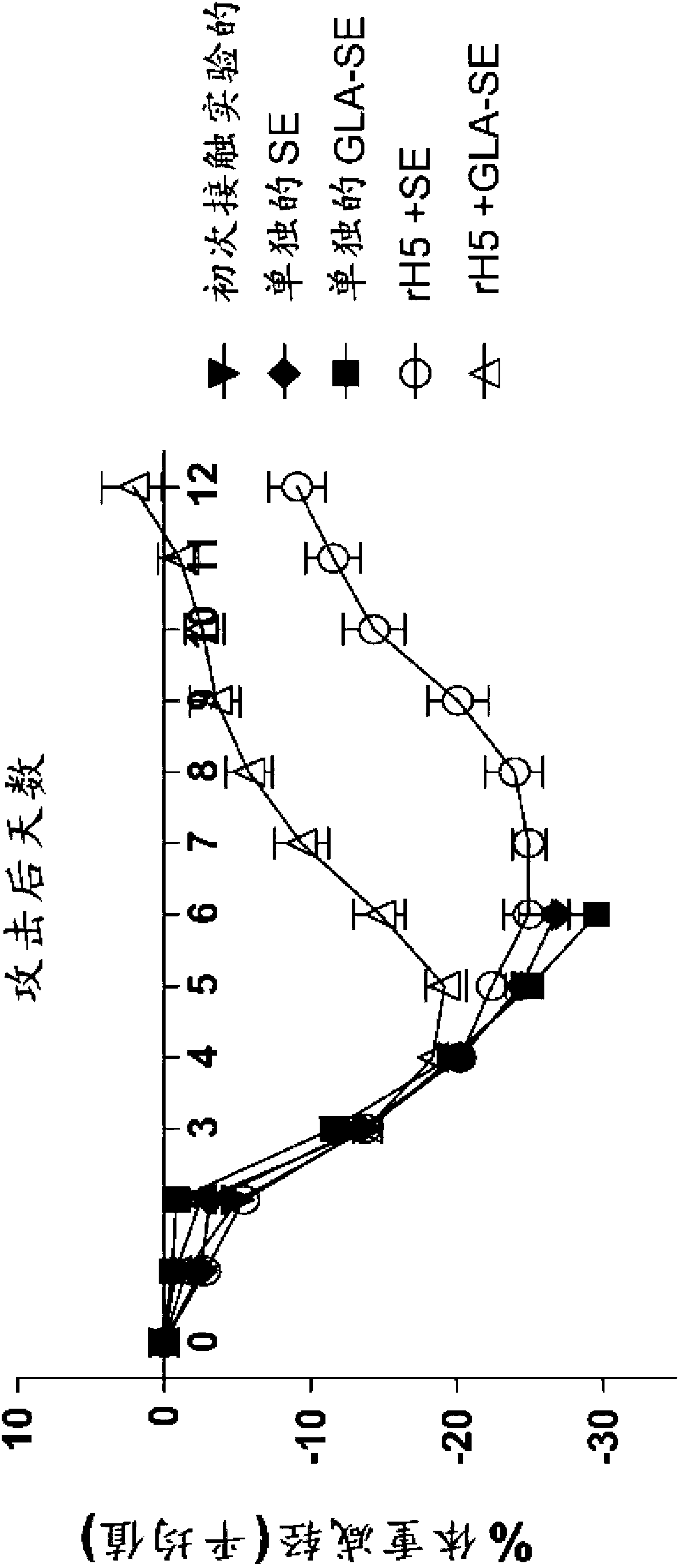
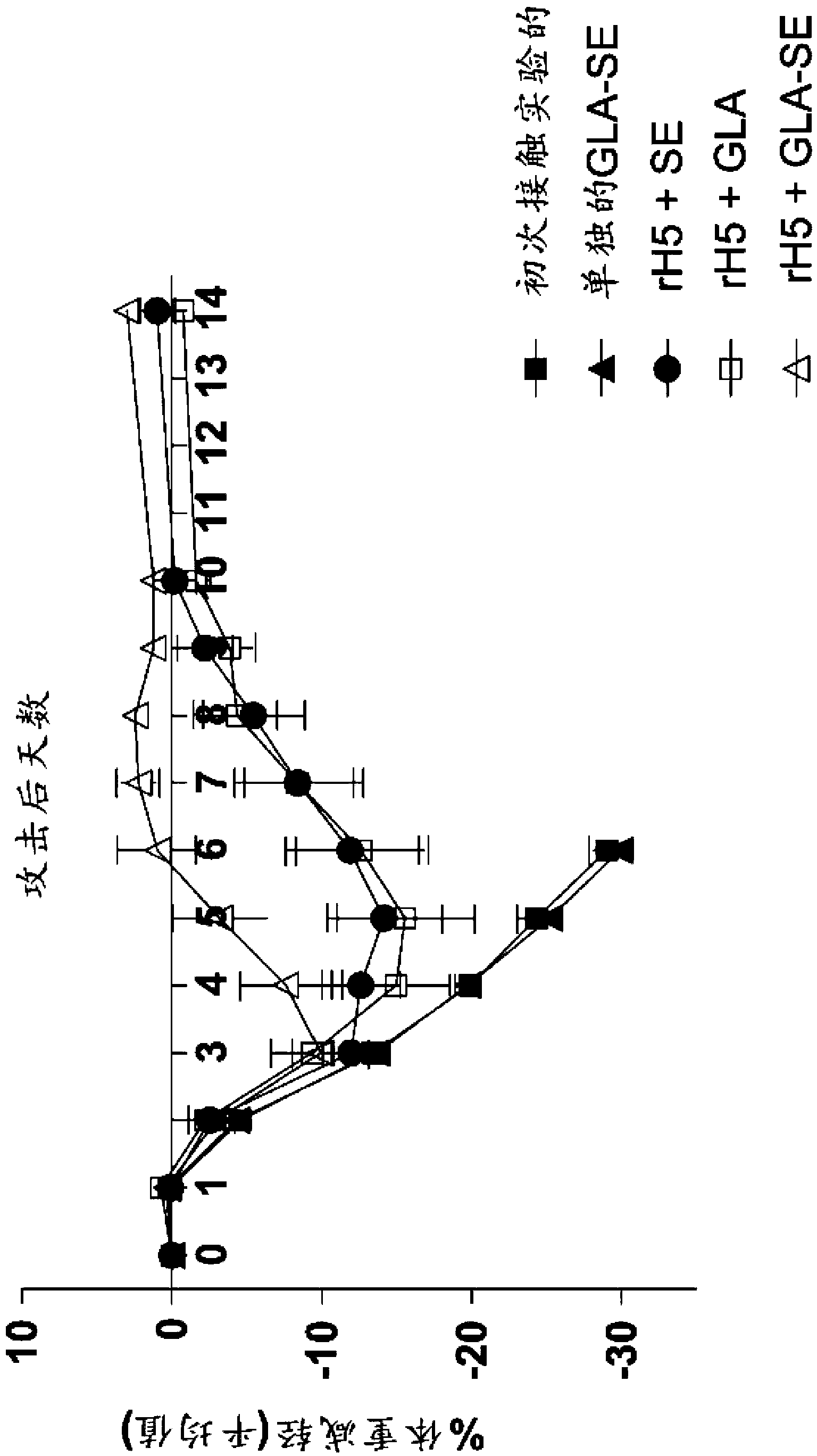
[0124] Efficacy of a single injection of rH5 / GLA-SE influenza vaccine in mice
[0125] This example shows that a single vaccination with recombinant influenza virus H5 (rH5) protein is effective in eliciting a protective antiviral immune response in mice challenged with high titer H5N1 virus. rH5 protein (derived from H5N1 Viet Nam 1203; available from Protein Sciences, Meriden, CT) alone or formulated with GLA-SE adjuvant in increasing amounts (0, 50, 150, 450, 900 or 2700 ng) Balb / c mice (5 / group) were injected intramuscularly (IM) once with (20 μg of GLA in 2% SE). Pass H5N1Viet Nam 1203 after 14 days (1000xLD 50 ) to challenge mice with intranasal administration. Mice were monitored daily for weight loss and euthanized if the weight loss exceeded 20-30%. Vaccination with rH5 protein alone did not provide protective immunity, as all mice injected with rH5 in the absence of adjuvant died spontaneously after virus challenge (18 / 25 animals) or showed overt morbidity and Eu...
Embodiment 2
[0131] rH5 / GLA-SE influenza vaccine confers heterologous immunity in mice when administered as a single injection
[0132] In this example, the protective efficacy of recombinant vaccines formulated with GLA adjuvant against heterologous virus challenge is demonstrated. For these experiments, mice were immunized with a single injection of rH5 protein isolated from H5N1 Indonesia (clade 2.3), and then challenged with H5N1 VN virus, as described above. As a positive control, mice were inoculated with the homologous rH5 protein from H5N1 Vietnam, while as a negative control, mice were inoculated with an irrelevant HSV-2 viral protein (rG013). As shown in Table 1, all mice vaccinated with HSV-2 died regardless of the protein-adjuvant formulation. All mice vaccinated with 50 ng of homologous rH5VN protein alone died, whereas all mice vaccinated with rH5VN formulated with GLA-SE adjuvant survived, consistent with previous findings. Importantly, all mice receiving 50ng or 200ng of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com