Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
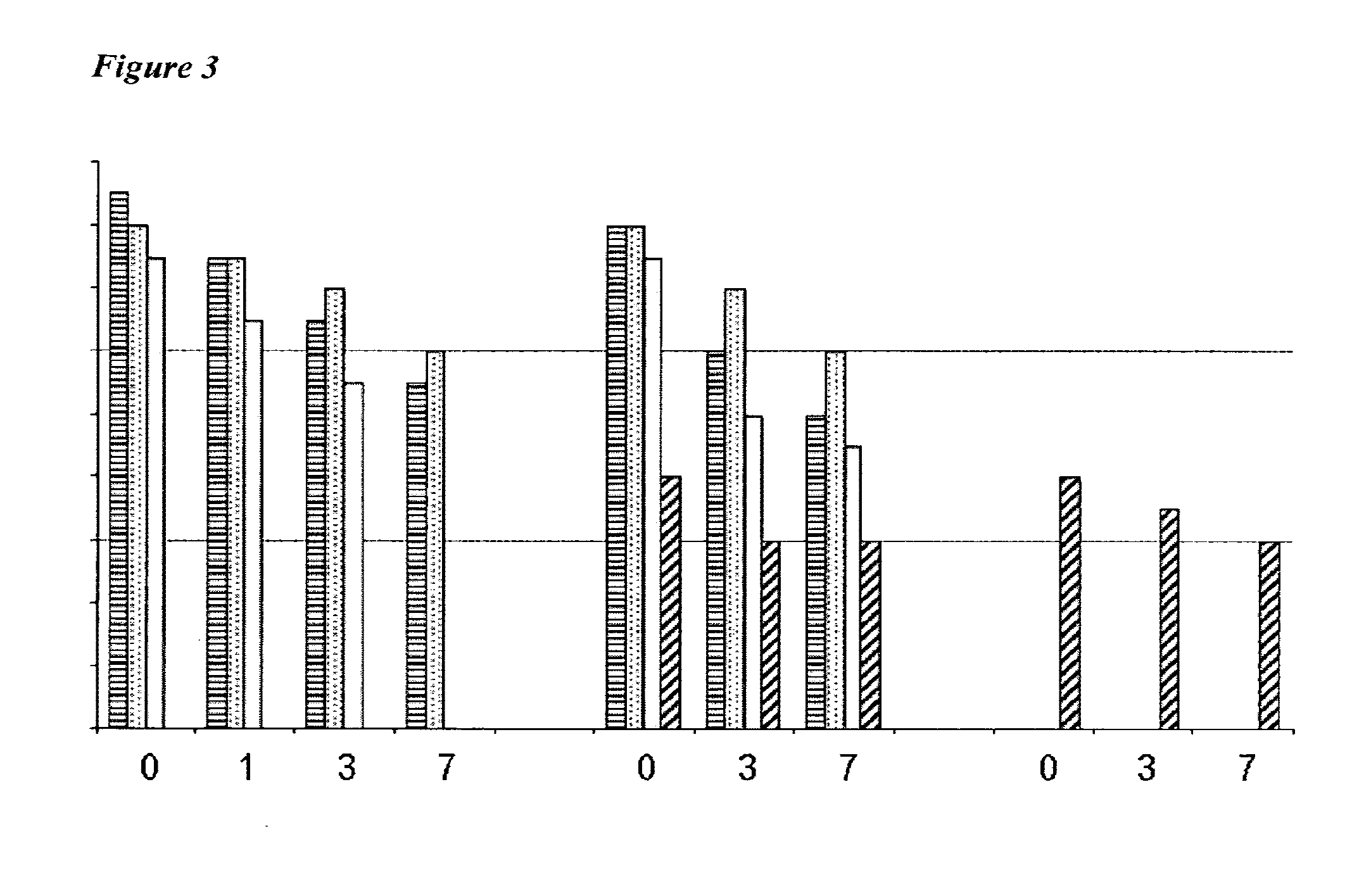
179 results about "Hemagglutinin (influenza)" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
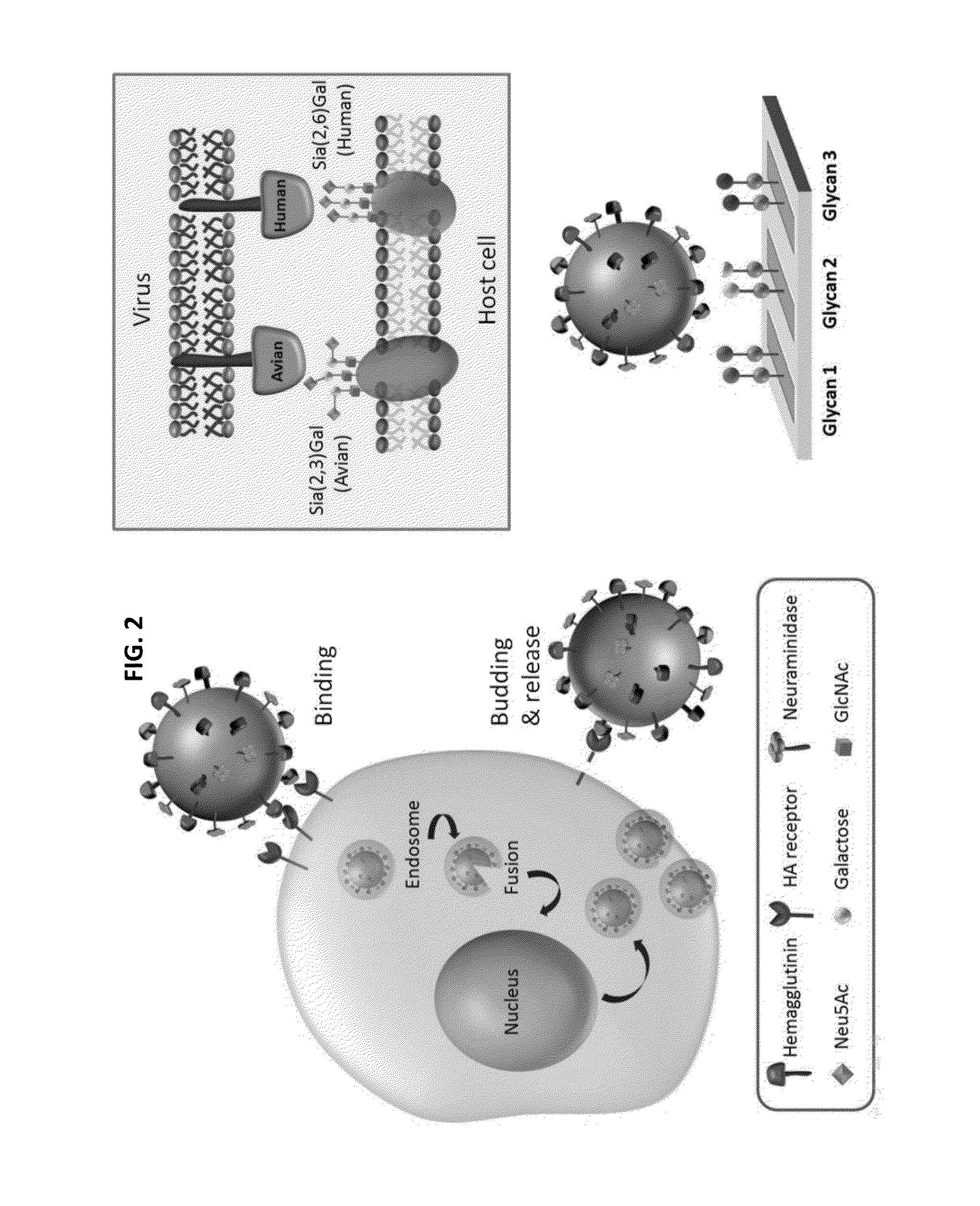
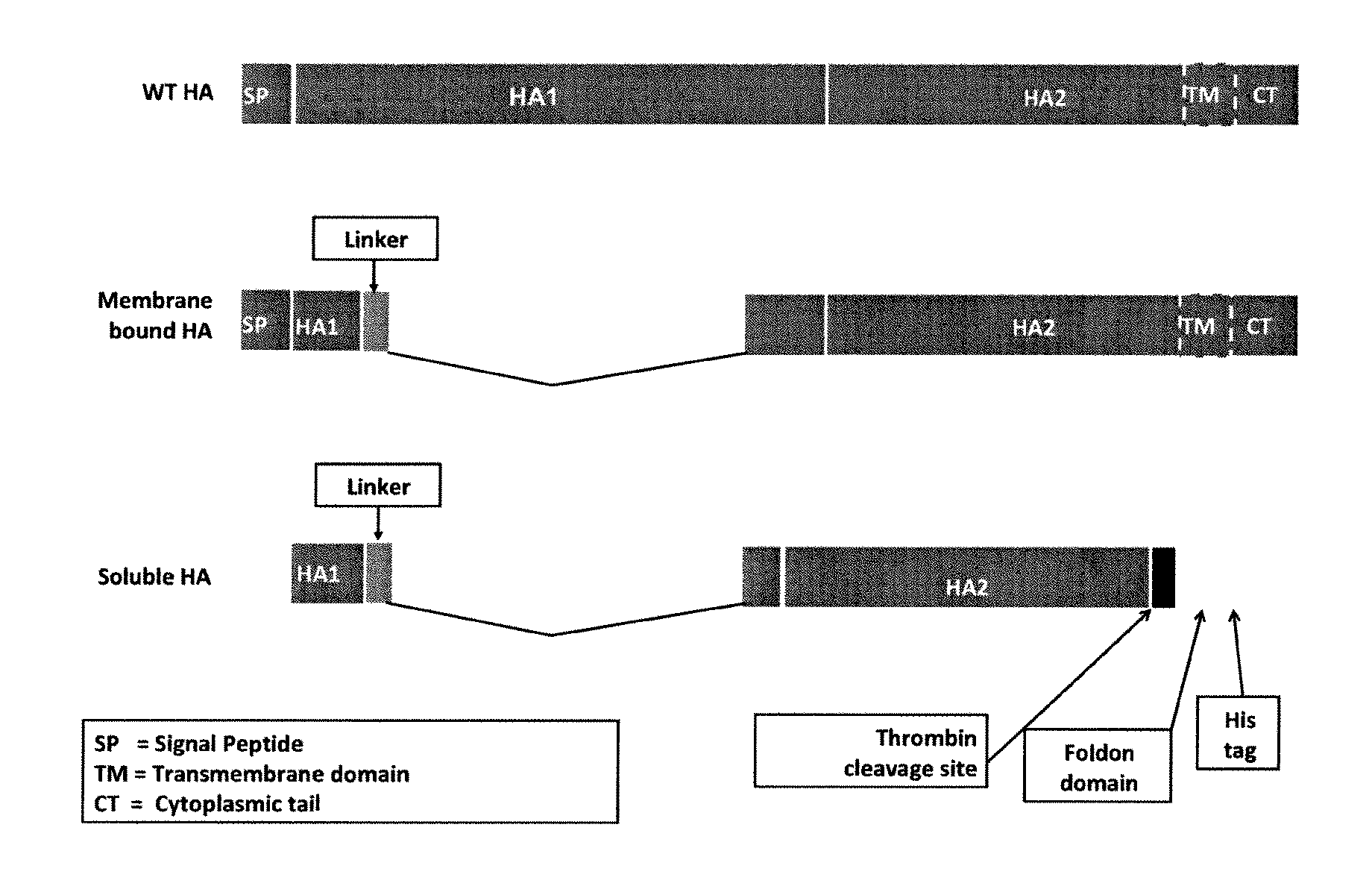
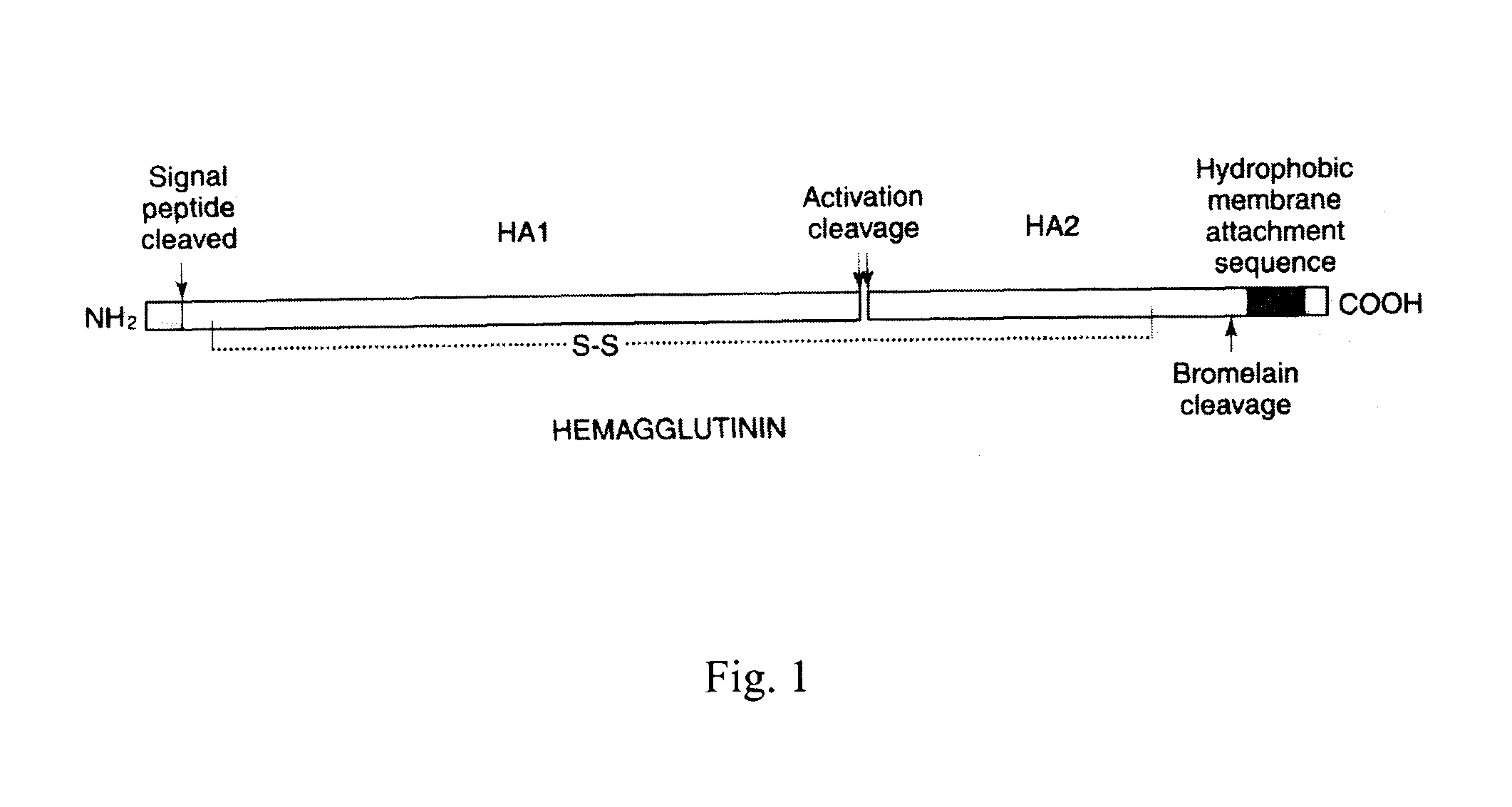
Influenza hemagglutinin (HA) or haemagglutinin (British English) is a homotrimeric glycoprotein found on the surface of influenza viruses and is integral to its infectivity. Hemagglutinin is a Class I Fusion Protein, having multifunctional activity as both an attachment factor and membrane fusion protein. Therefore, HA is responsible for binding Influenza virus to sialic acid on the surface of target cells, such as cells in the upper respiratory tract or erythrocytes, following which event the virus is internalised. Secondarily, HA is responsible for the fusion of the viral envelope with the late endosomal membrane once exposed to low pH (5.0-5.5).
Influenza virus vaccines and uses thereof
ActiveUS20100297174A1Reduce severityImprove survivalSsRNA viruses negative-sensePeptide/protein ingredientsHemagglutininInfluenza virus vaccine
Owner:MT SINAI SCHOOL OF MEDICINE
Influenza Hemagglutinin And Neuraminidase Variants
InactiveUS20080069821A1Extended half-lifeReducing/increasing polypeptide antigenicityFungiVirusesHemagglutininNeuraminidase
Owner:MEDIMMUNE LLC +1
Peptide-based vaccine for influenza
A human synthetic peptide-based influenza vaccine for intranasal administration comprises a mixture of flagella containing at least four epitopes of influenza virus reactive with human cells, each expressed individually in Salmonella flagellin, said influenza virus epitopes being selected from the group consisting of: (i) one B-cell hemagglutinin (HA) epitope; (ii) one T-helper hemagglutinin (HA) or nucleo-protein (NP) epitope that can bind to many HLA molecules; and (iii) at least two cytotoxic lymphocyte (CTL) nucleoprotein (NP) or matrix protein (M) epitopes that are restricted to the most prevalent HLA molecules in different human populations.
Owner:YEDA RES & DEV CO LTD
Influenza hemagglutinin and neuraminidase variants
InactiveUS20060008473A1SsRNA viruses negative-senseViral antigen ingredientsHemagglutininNeuraminidase
Polypeptides, polynucleotides, methods, compositions, and vaccines comprising (avian pandemic) influenza hemagglutinin and neuraminidase variants are provided.
Owner:GOVERNMENT OF THE US SEC THE DEPT OF HEALTH SERVICES NAT INSTITTUTE OF HEALTH
Vaccines Including Antigen From Four Strains of Influenza Virus
InactiveUS20140178429A1Reduce in quantityFacilitate matterSsRNA viruses negative-senseViral antigen ingredientsHemagglutininAdjuvant
Owner:SEQIRUS UK LTD
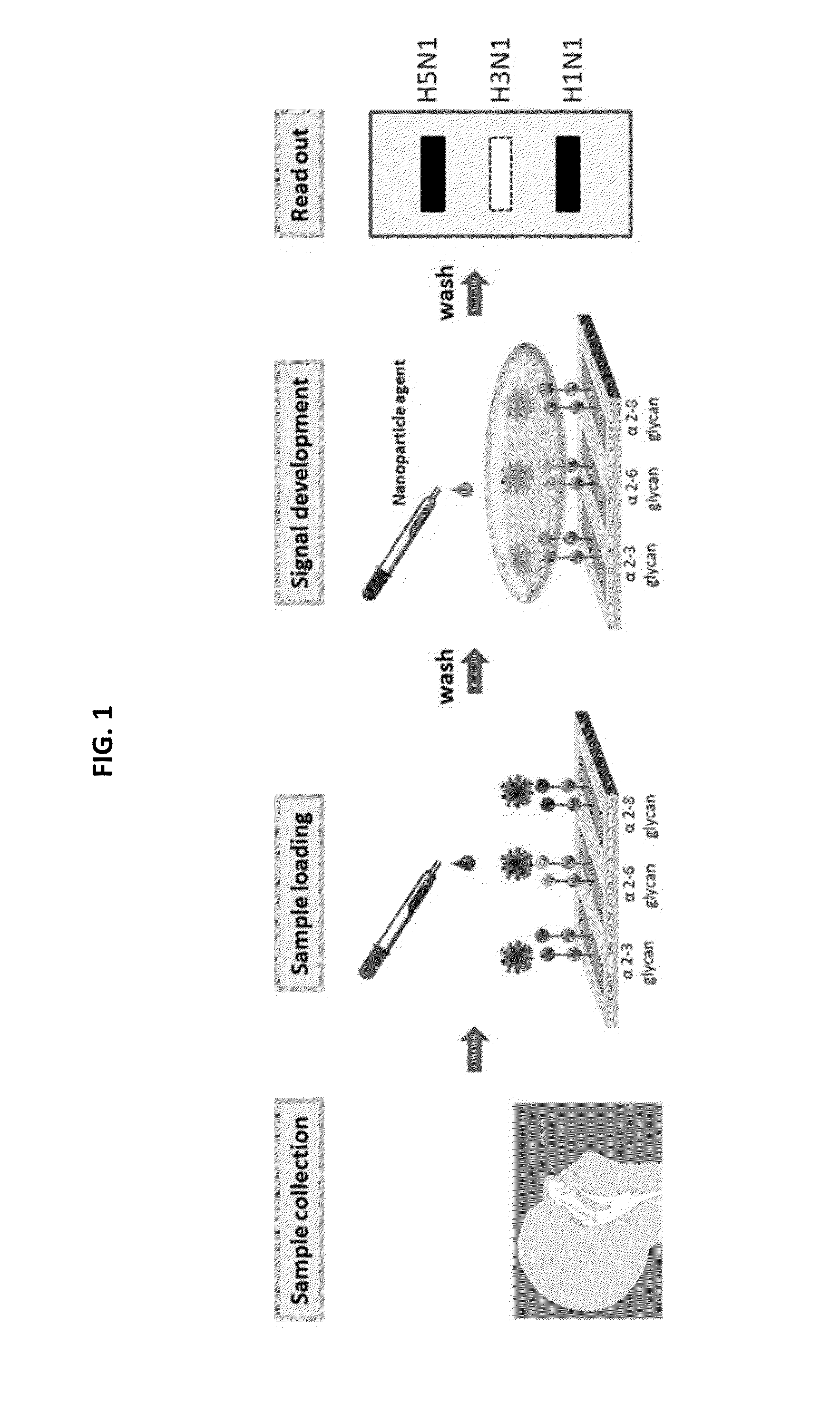
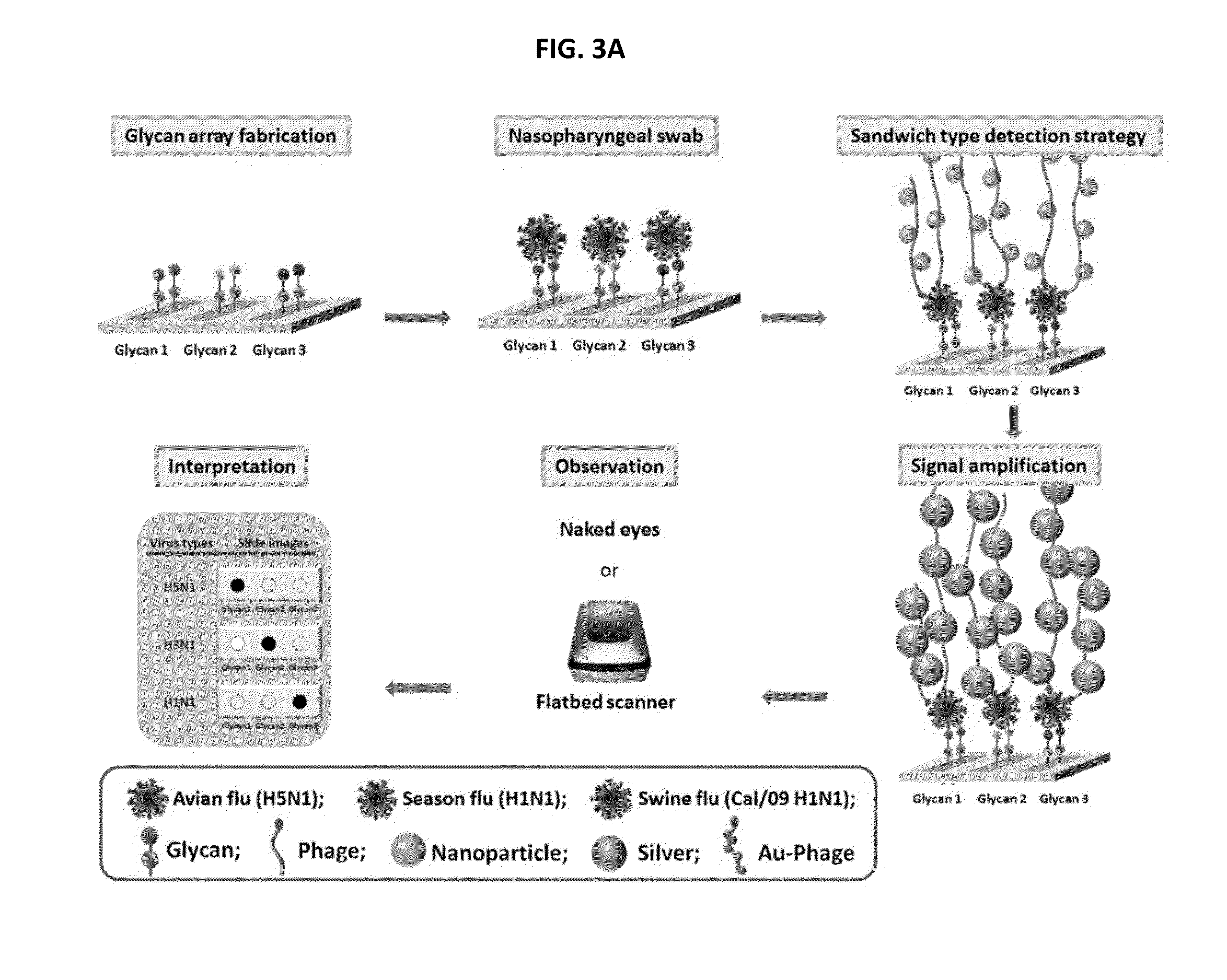
Glycan arrays for high throughput screening of viruses
ActiveUS20150160217A1Improve signal-to-noise ratioThe process is simple and fastSugar derivativesMicrobiological testing/measurementHemagglutininHigh-Throughput Screening Methods
Glycan arrays that can detect and distinguish between various sub-types and strains of influenza virus are provided. Methods for using the glycan arrays with assays using nanoparticle amplification technique are disclosed. Sandwich assays using gold nanoparticles conjugated to phage particles comprising influenza virus-specific antibodies for detecting multiple serotypes using a single reaction are provided. Plurality of glycans directed to specific target HA of influenza virus comprises the array. Detector molecules comprising noble metals conjugated to (a) phage display particles expressing antibodies against hemagglutinin and (b) neuraminidase binding agents are disclosed.
Owner:ACAD SINIC
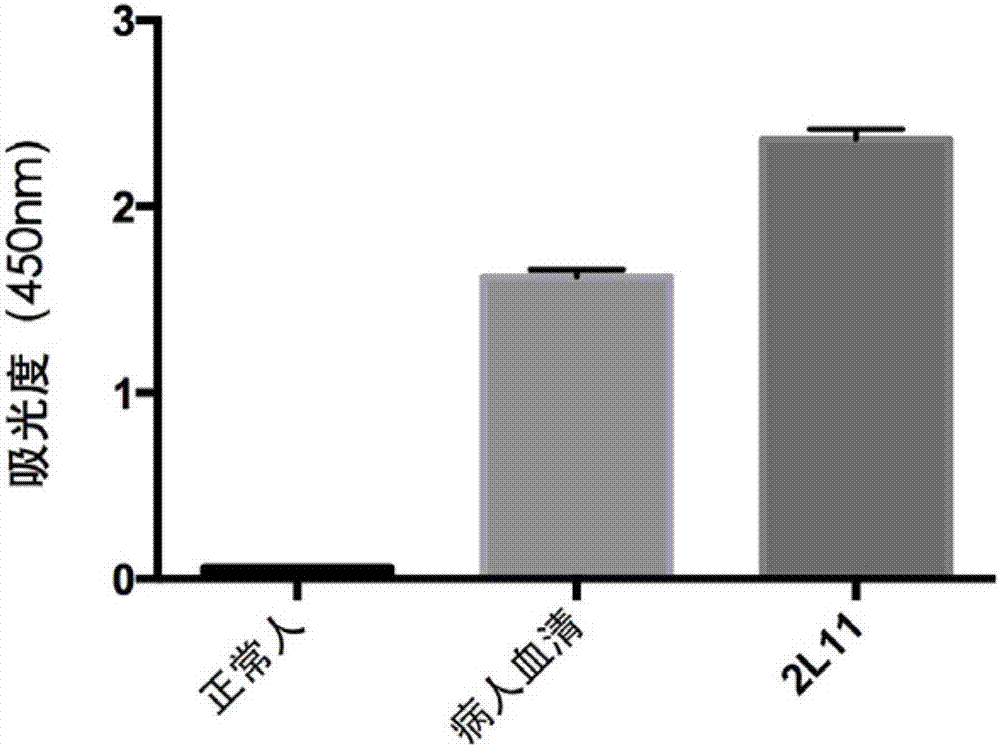
An anti-H7N9 completely humanized monoclonal antibody 2L11, and a preparing method and applications thereof
ActiveCN107353340AHigh affinityImprove featuresImmunoglobulins against virusesAntiviralsHemagglutininHeavy chain
The invention relates to an anti-H7N9 completely humanized monoclonal antibody 2L11, and a preparing method and applications thereof. The amino acid sequence of the heavy chain variable region of the antibody is shown as SEQ ID NO:2, or an amino acid sequence having similar functions and obtained by subjecting the sequence shown as the SEQ ID NO:2 to replacement, deletion or addition of one or more amino acids; and / or the amino acid sequence of the light chain variable region of the antibody is shown as SEQ ID NO:4, or an amino acid sequence having similar functions and obtained by subjecting the sequence shown as the SEQ ID NO:4 to replacement, deletion or addition of one or more amino acids. The antibody 2L11 can be bonded in a targeted manner with hemagglutinin HA of H7N9 viruses. Compared with mouse-sourced antibodies, genes of the completely humanized antibody are completely derived from human genes and do not comprise components of other species, and therefore no side or toxic effect such as anti-mouse antibodies is generated in a human body, and the antibody 2L11 has better biocompatibility, and is more suitable and has higher potential as a macro-molecule medicine treating influenza viruses.
Owner:SHENZHEN INST OF ADVANCED TECH
Influenza nucleic acid molecules and vaccines made therefrom
ActiveUS20110182938A1SsRNA viruses negative-senseOrganic active ingredientsHemagglutininInfluenza A antigen
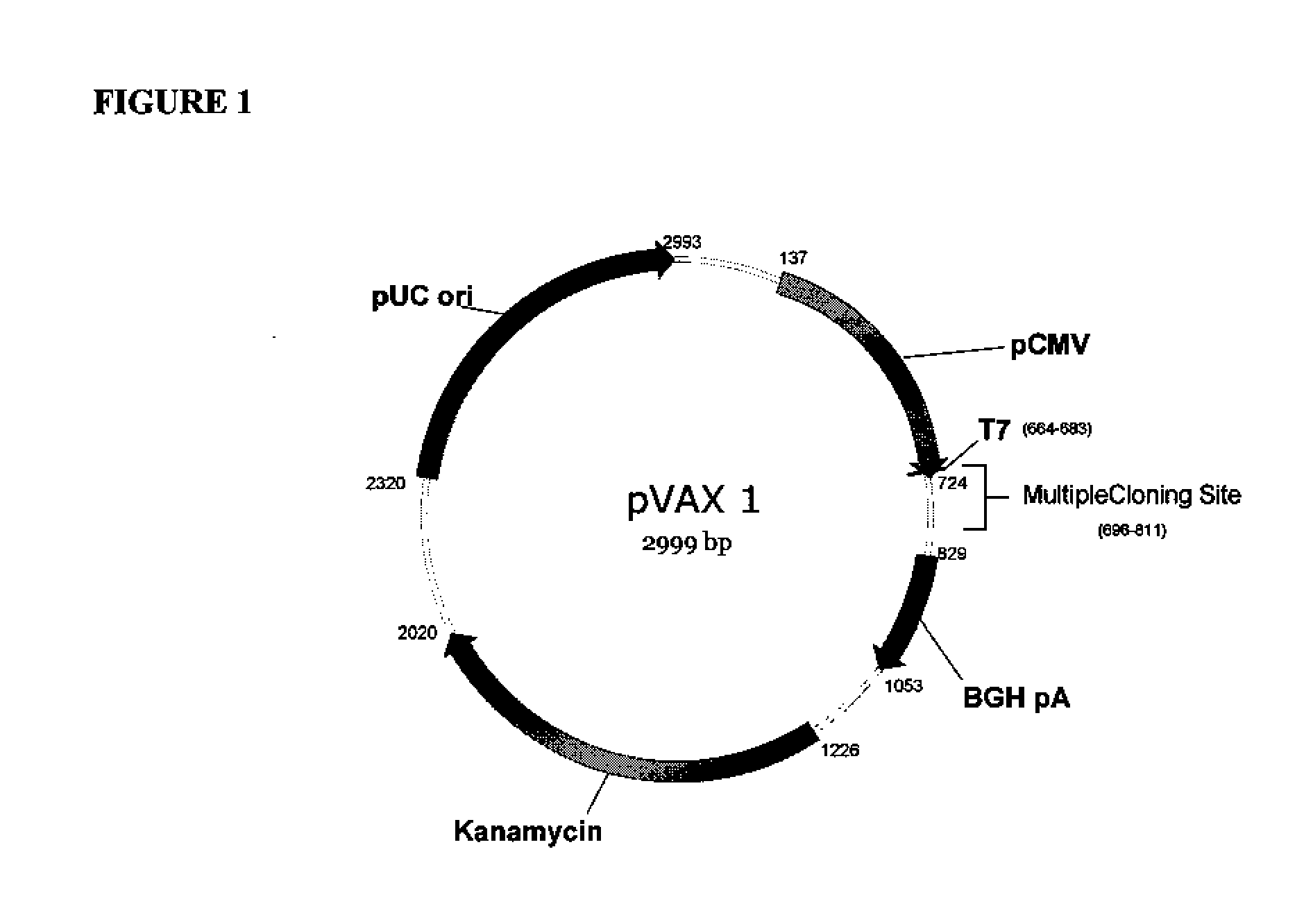
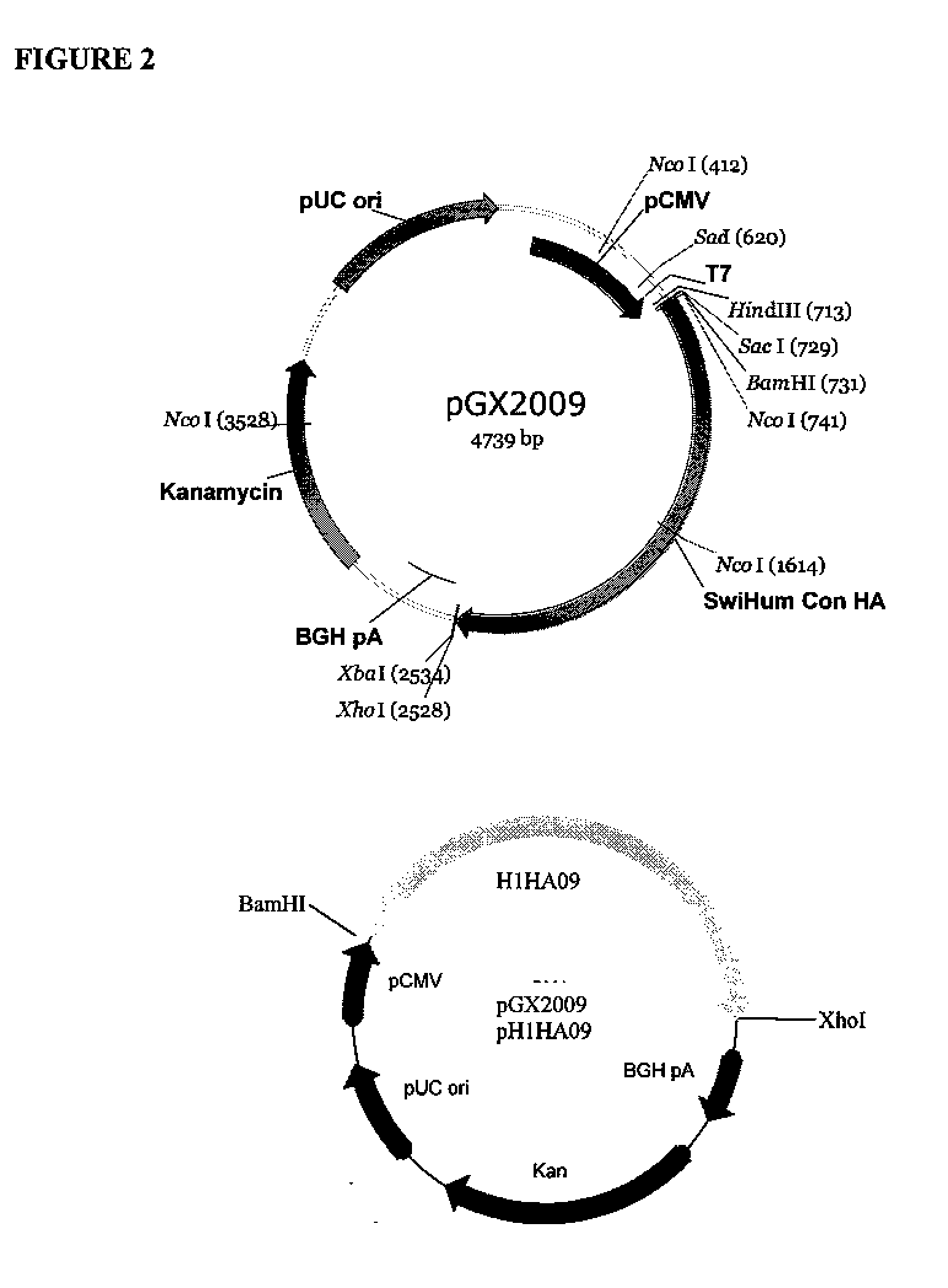
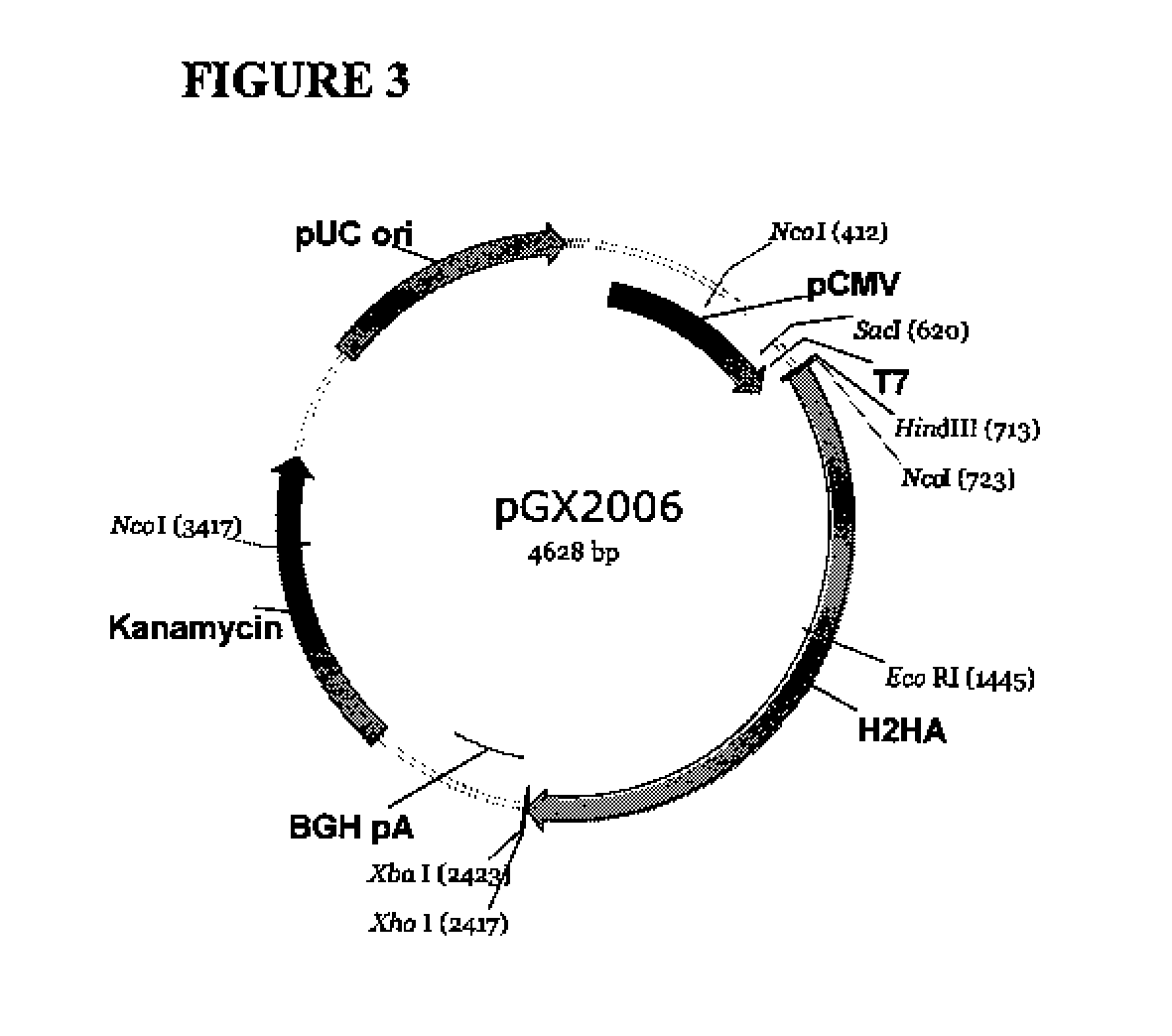
Provided herein are nucleic acid sequences that encode novel consensus amino acid sequences of HA hemagglutinin, as well as genetic constructs / vectors and vaccines expressing the sequences. Also provided herein are methods for generating an immune response against one or more Influenza A serotypes using the vaccines that are provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Human Anti-Human Influenza Virus Antibody
InactiveUS20110319600A1Effect survival rateEffect weight lossSugar derivativesImmunoglobulins against virusesHemagglutininHuman Influenza A Virus
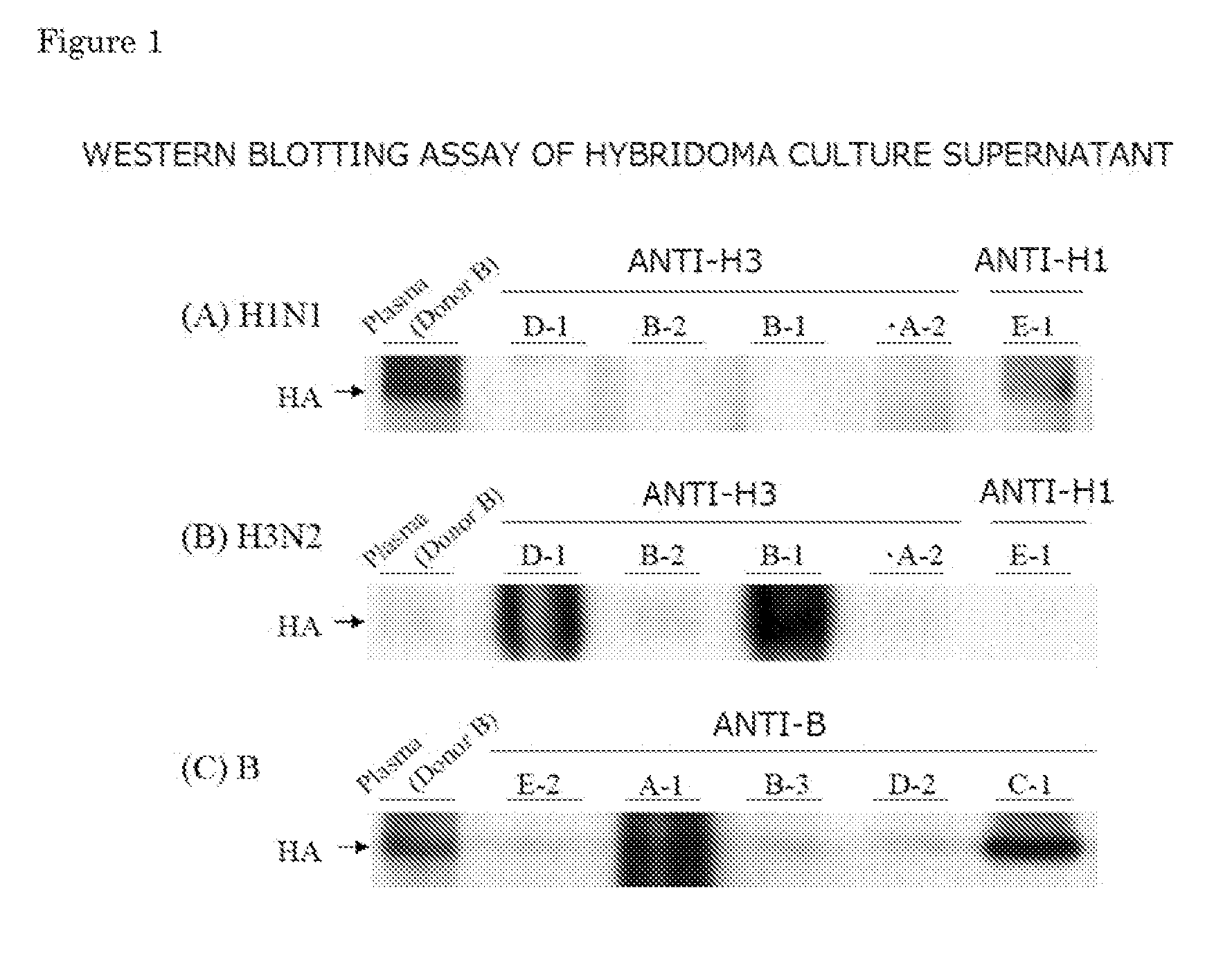
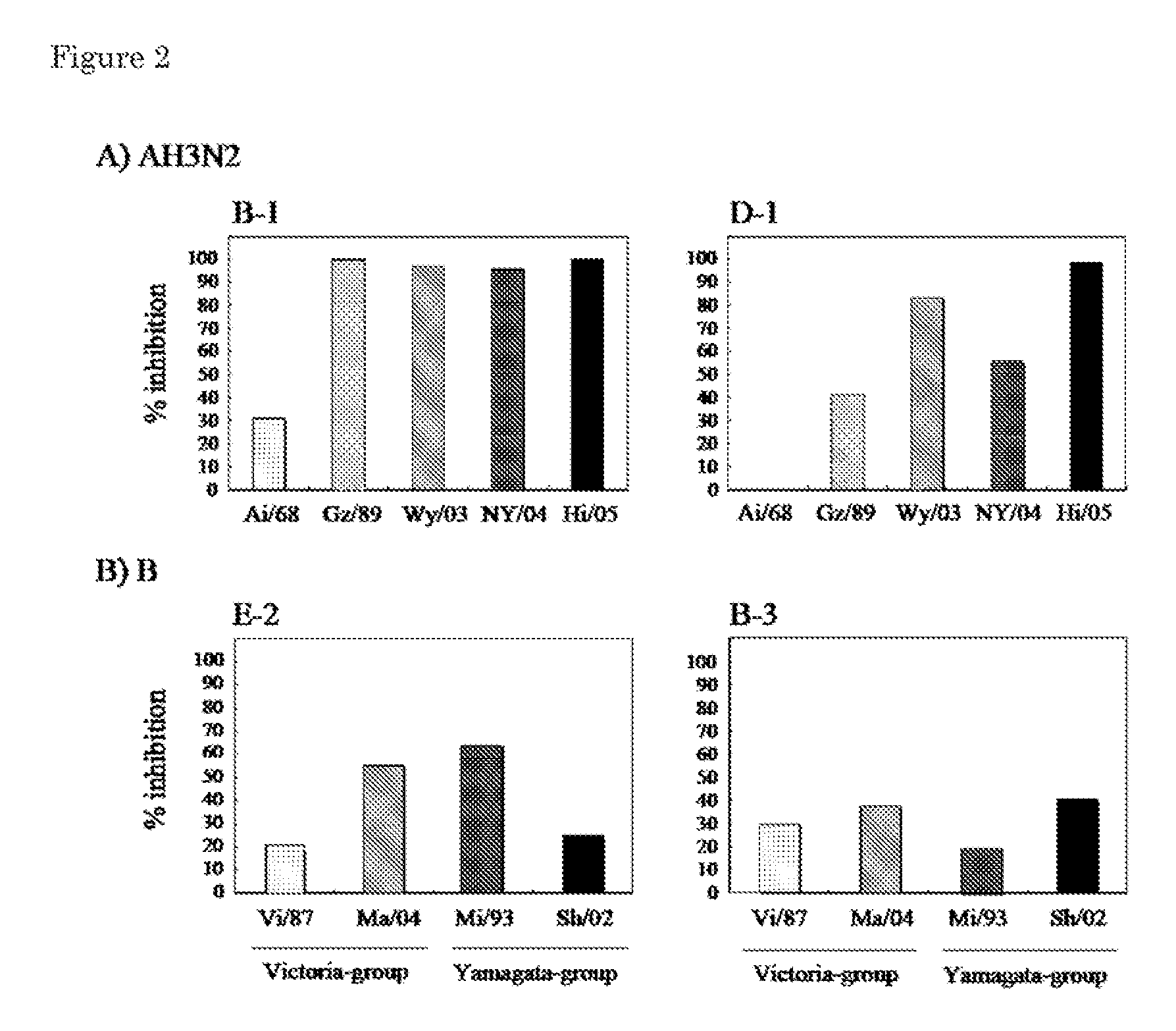
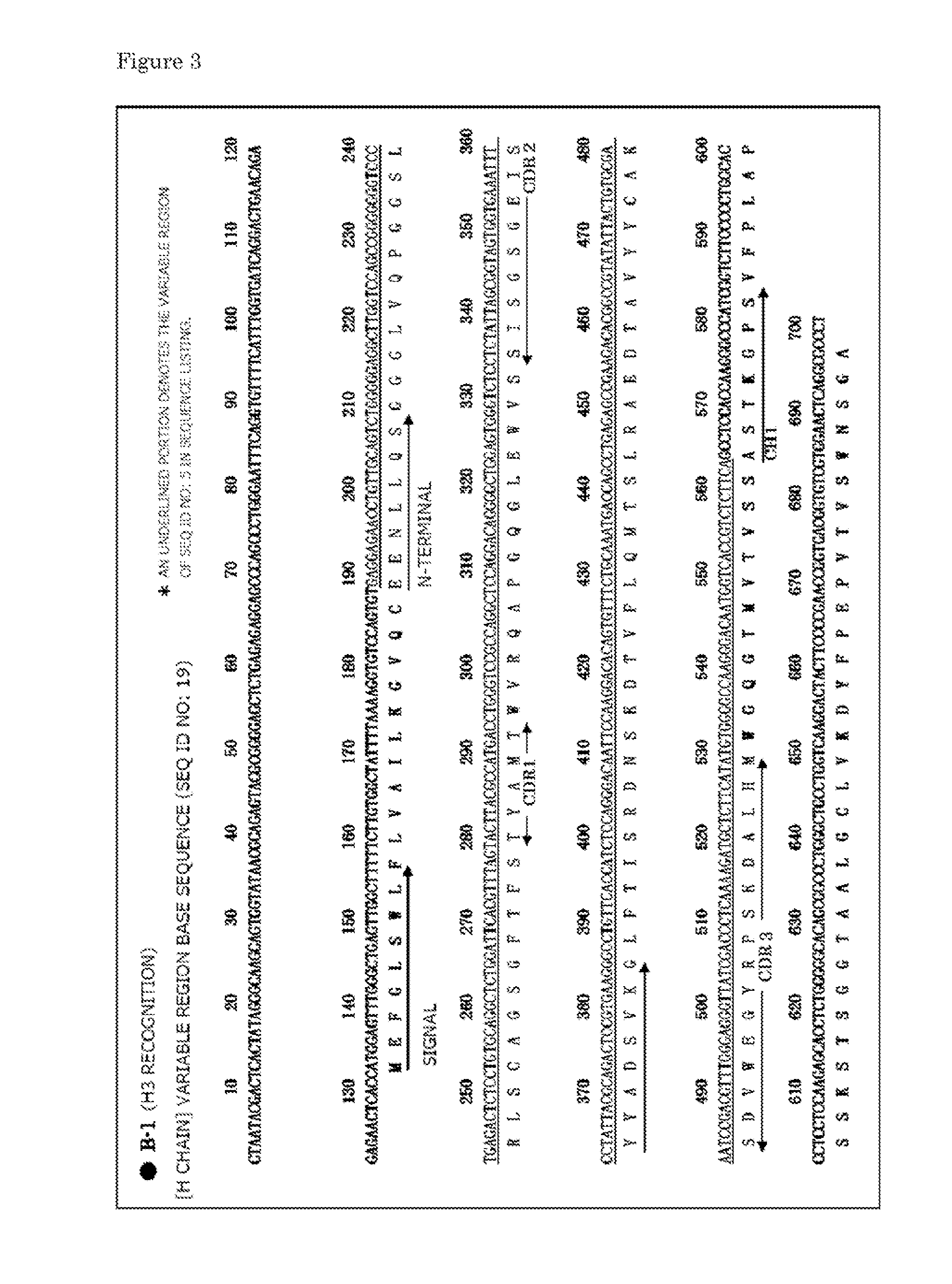
Provided is a human antibody having a neutralization activity against a human influenza virus. More specifically, provided is a human antibody which recognizes a highly conserved region in a human influenza A virus subtype H3N2 or a human influenza B virus and has a neutralization activity against the virus. The human antibody is a human anti-human influenza virus antibody, which has a neutralization activity against a human influenza A virus subtype H3N2 and binds to a hemagglutinin HA1 region of the human influenza A virus subtype H3N2, or which has a neutralization activity against a human influenza B virus, and includes, as a base sequence of a DNA encoding a variable region of the antibody, a sequence set forth in any one of SEQ ID NOS: 5 to 12.
Owner:OSAKA UNIV +1
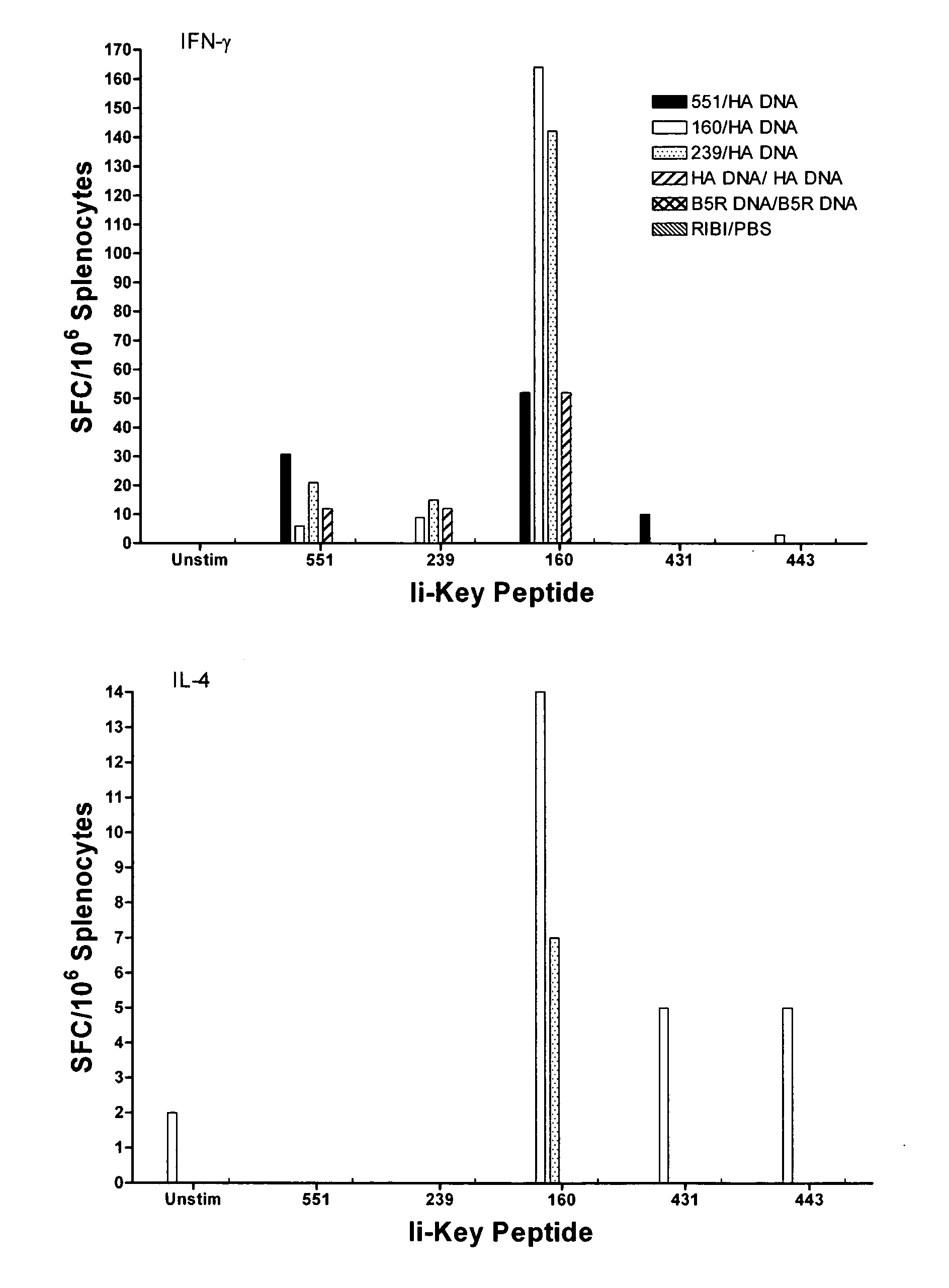
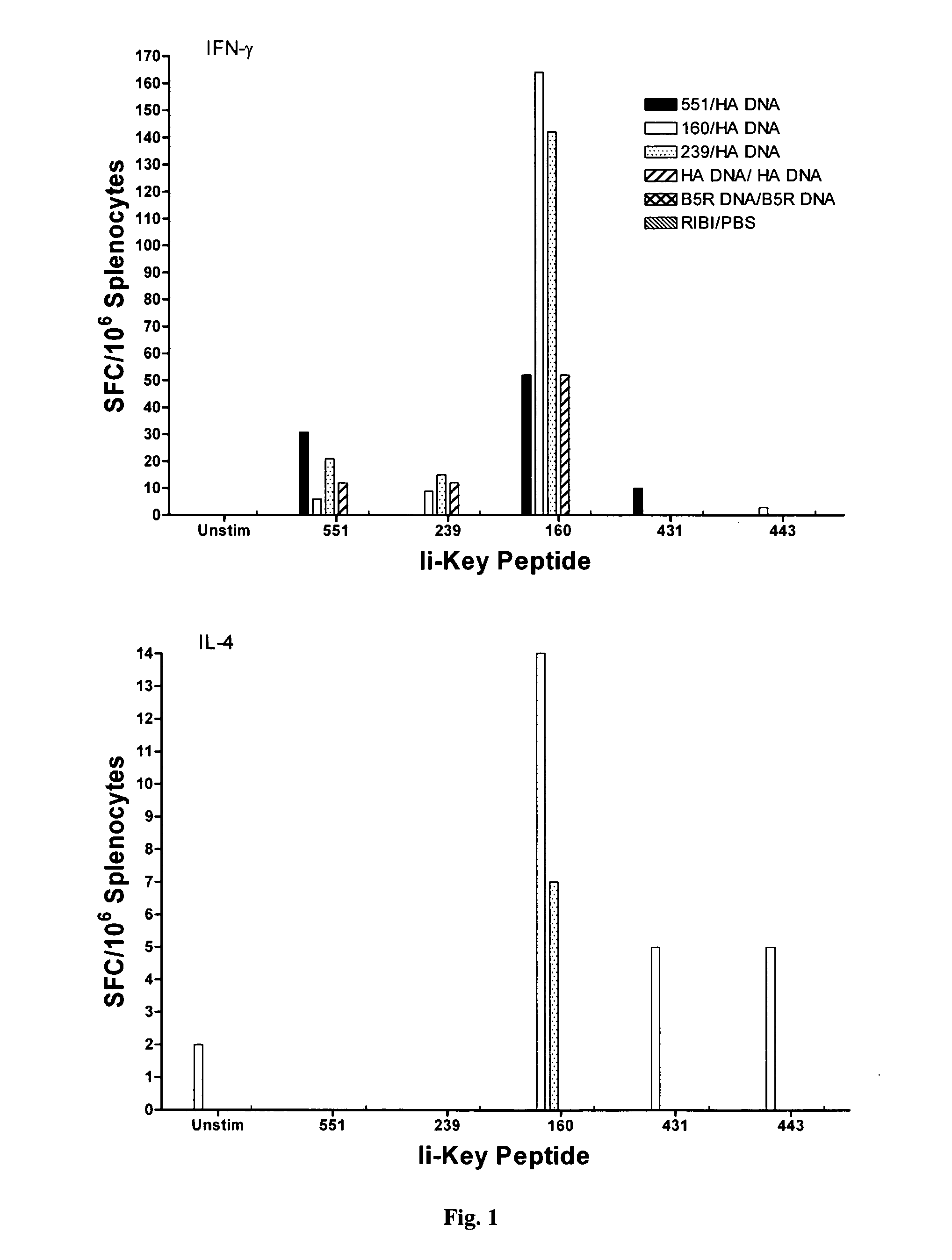
Ii-key enhanced vaccine potency
InactiveUS20080095798A1Improve effectivenessEasy to manageAntibacterial agentsSsRNA viruses negative-senseHemagglutininVaccine Potency
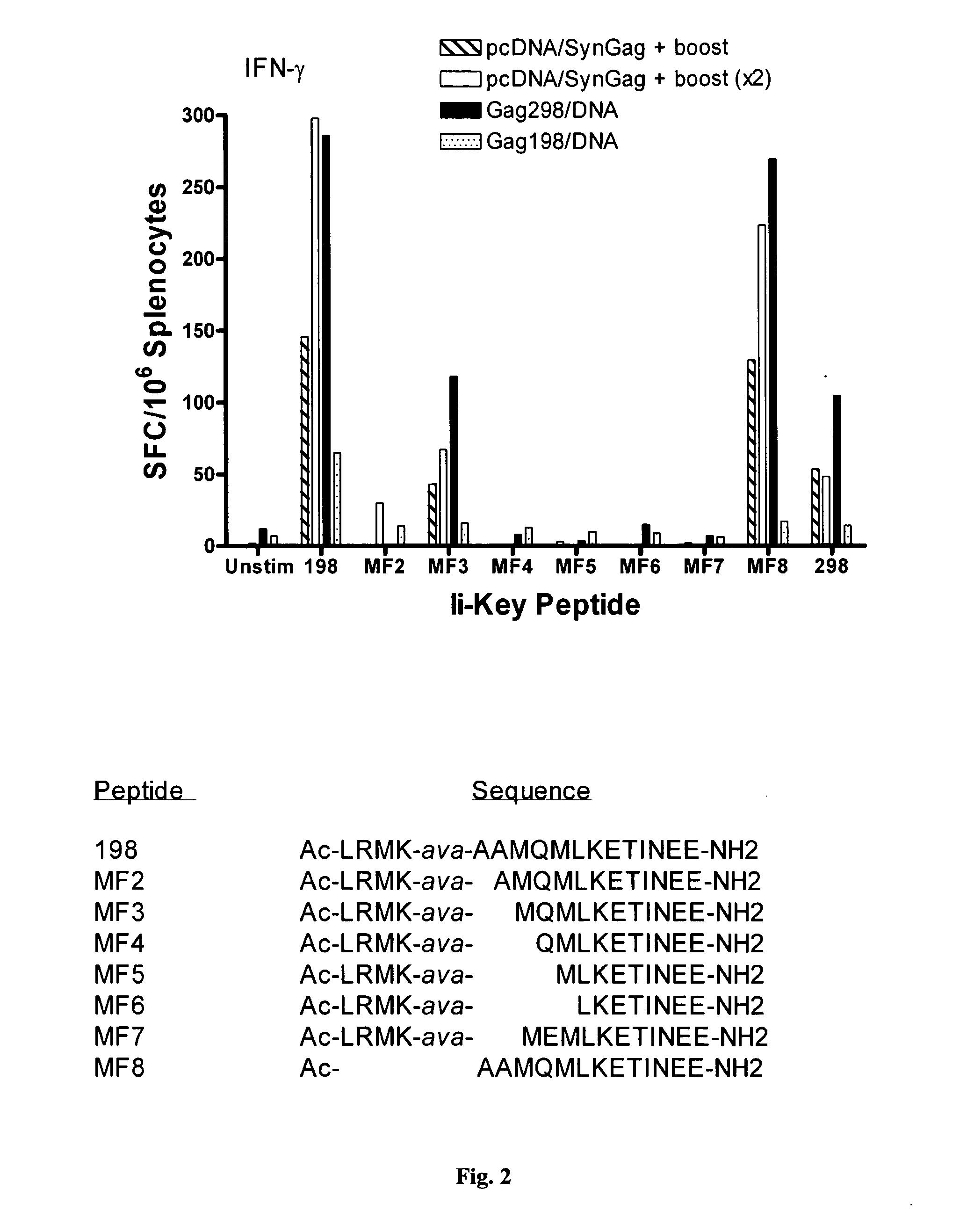
Disclosed is a method for increasing vaccine potency whereby a subject's immune system is first primed with an Ii-Key hybrid peptide construct before the subject subsequently receives a vaccine for a pathogen of interest. The vaccine may be comprised of a protein or portion thereof that is encoded by the genome of the pathogen. The vaccine may also be a DNA vaccine comprised of DNA encoding a protein of the pathogen. The Ii-Key hybrid peptide construct includes the LRMK residues of Ii-Key protein and an MHC Class II epitope of the protein or portion thereof which is used in the vaccine. The Ii-Key construct may be administered in the form of a nucleic acid construct encoding the Ii-Key hybrid peptide. Priming with Ii-Key peptides enhances the immunogenicity of rHA protein and HA and HIV DNA vaccines. Methods are described relating to the use of Ii-Key hybrid constructs in vaccine protocols wherein the pathogen is HIV or Influenza A, including H5N1. Methods and compositions are described wherein the MHC Class II epitope of the Ii-Key hybrid is hemagglutinin encoded by Influenza A or the Gag protein encoded by HIV.
Owner:ANTIGEN EXPRESS
Anti-(influenza a virus subtype h5 hemagglutinin) monoclonal antibody
InactiveUS20110065095A1Prevention of prevalenceFaster assayMicrobiological testing/measurementBiological material analysisHemagglutininEpitope
A method of immunoassay of H5 subtype influenza A virus by which the virus can be accurately assayed even in cases where a certain level of mutation has occurred in the H5 subtype influenza A virus, and a kit therefor, and a novel anti-H5 subtype influenza A virus monoclonal antibody which can be used for the immunoassay are disclosed. The antibody or an antigen-binding fragment thereof of the present invention undergoes antigen-antibody reaction with hemagglutinin of H5 subtype influenza A virus, and the corresponding epitope of the antibody or an antigen-binding fragment thereof is located in a region other than the receptor subdomain (excluding C-terminal region thereof consisting of 11 amino acids), which antibody or an antigen-binding fragment thereof does not have neutralizing activity against the influenza A virus.
Owner:FUJIREBIO CO LTD +1
Chimeric Influenza Virus-Like Particles Comprising Hemagglutinin
ActiveUS20120189658A1Easy to captureStrong immune responseSsRNA viruses negative-senseAntibody mimetics/scaffoldsHemagglutininVirus-like particle
A method for synthesizing chimeric influenza virus-like particles (VLPs) within a plant or a portion of a plant is provided. The method involves expression of chimeric influenza HA in a plant or a portion of a plant. The invention is also directed towards a VLP comprising chimeric influenza HA protein and plants lipids. The invention is also directed to a nucleic acid encoding chimeric influenza HA as well as vectors. The VLPs may be used to formulate influenza vaccines, or may be used to enrich existing vaccines.
Owner:MEDICAGO INC
Human binding molecules capable of binding to and neutralizing influenza b viruses and uses thereof
ActiveUS20130243792A1High economic impactSugar derivativesMicrobiological testing/measurementHemagglutininMonoclonal antibody
Described are binding molecules, such as human monoclonal antibodies, that bind to hemagglutinin of influenza B viruses, and have a broad neutralizing activity against such influenza viruses. These binding molecules do not bind to hemagglutinin of influenza A viruses. Further provided are nucleic acid molecules encoding the binding molecules, and compositions comprising the binding molecules. The binding molecules can be used in the diagnosis of, prophylaxis against, and / or treatment of influenza B virus infections.
Owner:JANSSEN VACCINES & PREVENTION BV
Conserved Hemagglutinin Epitope, Antibodies to the Epitope and Methods of Use
InactiveUS20120128684A1Prevention and reduction of severitySsRNA viruses negative-sensePeptide/protein ingredientsHemagglutininEpitope
Disclosed are antibodies that bind to the stem region of influenza hemagglutinin in the neutral pH conformation, hemagglutinin epitopes in the stem region, and methods of making and using both.
Owner:BURNHAM INST FOR MEDICAL RES +1
Influenza virus vaccines and uses thereof
ActiveUS20140328875A1Highly potent and broadly neutralizing antibodiesStimulate immune responseSsRNA viruses negative-senseBacteriaHemagglutininInfluenza virus vaccine
Provided herein are chimeric influenza hemagglutinin (HA) polypeptides, compositions comprising the same, vaccines comprising the same, and methods of their use.
Owner:MT SINAI SCHOOL OF MEDICINE
Anti-H7N9 full-human-derived monoclonal antibody 5J13 and preparation method and application thereof
ActiveCN106519027AHigh affinityImprove featuresImmunoglobulins against virusesAntiviralsHemagglutininSide effect
The invention relates to an anti-H7N9 full-human-derived monoclonal antibody 5J13 and a preparation method and application thereof. Amino acid sequences of heavy and light chain CDR1, CDR2 and CDR3 of the antibody are GFSFSNYG in the heavy chain CDR1 area, ISYDGTNK in the heavy chain CDR2 area, AKGRGPYCSSSICYHGMDV in the heavy chain CDR3 area, QSVLSGSINMNY in the light chain CDR1 area, WAS in the light chain CDR2 area and QQYYSTPLT in the light chain CDR3 area correspondingly. The antibody can be combined with hemagglutinin A of the H7N9virus in a targeted mode and has remarkable neutralization activity in resisting H7N9 virus infection. Compared with a murine antibody, genes of the full-human-derived antibody are completely from human genes and have no components of other species, toxic and side effects such as the anti-mouse anti-antibody are avoided, the biocompatibility is better, and the anti-H7N9 full-human-derived monoclonal antibody 5J13 is more suitable and has more potential in becoming a macromolecular drug for treating influenza virus.
Owner:SHENZHEN INST OF ADVANCED TECH
Influenza virus vaccines and uses thereof
ActiveUS9051359B2Elimination of glycosylationReduce severitySsRNA viruses negative-sensePeptide/protein ingredientsHemagglutininInfluenza virus vaccine
Provided herein are influenza hemagglutinin stem domain polypeptides, compositions comprising the same, vaccines comprising the same and methods of their use.
Owner:MT SINAI SCHOOL OF MEDICINE
Influenza Hemagglutinin And Neuraminidase Variants
InactiveUS20080057081A1Efficient productionExtended half-lifeSsRNA viruses negative-senseViral antigen ingredientsHemagglutininNeuraminidase
Polypeptides, polynucleotides, methods, compositions, and vaccines comprising (avian pandemic) influenza hemagglutinin and neuraminidase variants are provided.
Owner:MEDIMMUNE LLC
Antiviral agents and vaccines against influenza
InactiveUS20090208531A1SsRNA viruses negative-senseOrganic active ingredientsHemagglutininPrime boost
These vaccines target H5N1, H1, H3 and other subtypes of influenza and are designed to elicit neutralizing antibodies, as well as cellular immunity. The DNA vaccines express hemagglutinin (HA) or nucleoprotein (NP) proteins from influenza which are codon optimized and / or contain modifications to protease cleavage sites of HA which affect the normal function of the protein. Adenoviral constructs expressing the same inserts have been engineered for prime boost strategies. Protein-based vaccines based on protein production from insect or mammalian cells using foldon trimerization stabilization domains with or without cleavage sites to assist in purification of such proteins have been developed. Another embodiment of this invention is the work with HA pseudotyped lentiviral vectors which would be used to screen for neutralizing antibodies in patients and to screen for diagnostic and therapeutic antivirals such as monoclonal antibodies.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Influenza virus reassortment
ActiveUS20130216573A1Reduce transcriptionReduce translationSsRNA viruses negative-senseAnimal cellsHemagglutininNeuraminidase
Methods for producing reassortant viruses are provided wherein the transcription and / or translation of the hemagglutinin and / or neuraminidase genes are suppressed.
Owner:SEQIRUS UK LTD
Microparticles with adsorbed polypeptide-containing molecules
ActiveUS7501134B2Easy to produceSsRNA viruses negative-senseAntibacterial agentsHemagglutininLactide
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Arrayed detector system for measurement of influenza immune response
ActiveUS20090275016A1Faster and reliable detectionWide dynamic rangeSsRNA viruses negative-senseBioreactor/fermenter combinationsHemagglutininEpitope
A sensor chip for detecting an immune response against an influenza virus, the sensor chip including a substrate having a surface and a plurality of hemagglutinin polypeptides bound to discrete locations on the surface of the substrate, each hemagglutinin polypeptide having a hemagglutinin epitope. Detection devices containing the sensor chip and methods of detecting influenza immune responses are also described herein.
Owner:UNIVERSITY OF ROCHESTER
Influenza vaccines with reduced amount of emulsion adjuvant
InactiveUS20090304742A1Eliminate needAvoid the needSsRNA viruses negative-senseViral antigen ingredientsHemagglutininInfluenza vaccine
Influenza vaccines with oil-in-water emulsion adjuvants are known. The amount of emulsion adjuvant required for an influenza vaccine can be reduced, thereby allowing more vaccines to be made from a given amount of emulsion, and / or minimizing the amount of emulsion that has to be produced for a given number of vaccine doses. These vaccines can conveniently be made by mixing (i) an oil-in-water emulsion and (ii) an aqueous preparation of an influenza virus antigen. In one aspect, substantially equal volumes of components (i) and (ii) are used; in another aspect, an excess volume of component (ii) is used. When using substantially equal volumes, component (ii) has a hemagglutinin concentration of more than 60 μg influenza virus strain per ml. Components (i) and (ii) can be presented in kit form.
Owner:NOVARTIS AG
Soluble recombinant influenza antigens
ActiveUS8771703B2Reduce complexityExpression level and yieldSsRNA viruses negative-senseAntibody mimetics/scaffoldsHemagglutininReticulum cell
Owner:MEDICAGO INC
Anti-H7N9 full human derived monoclonal antibody hIg311 and preparation method and application thereof
ActiveCN110746503AGood neutralizing activityGood biocompatibilityImmunoglobulins against virusesAntiviralsHemagglutininAntiendomysial antibodies
The application relates to an anti-H7N9 full human derived monoclonal antibody hIg311 and a preparation method and application thereof. A memory B cell PCR method is used for quickly screening a fullhuman derived monoclonal antibody hIg311, and the anti-H7N9 human derived monoclonal antibody hIg311 is free from any mouse derived components. The antibody disclosed by the application can realize target combination of hemagglutinin HA of H7N9 viruses, and has notable anti H7N9 viral infection neutralization activity. The antibody disclosed by the application does not generate toxic and side effects for resisting mouse resisting antibodies, has good biocompatibility, and is suitable for becoming and has potential to become macromolecular drugs for treating influenza viruses.
Owner:SHENZHEN INST OF ADVANCED TECH
Influenza virus vaccines and uses thereof
ActiveUS20160136262A1SsRNA viruses negative-senseAntibody mimetics/scaffoldsHemagglutininInfluenza virus vaccine
Provided are influenza hemagglutinin stem domain polypeptides comprising (a) an influenza hemagglutinin HA1 domain that comprises an HA1 N-terminal stem segment comprising the amino acids from position 1 to position x, preferably from position p to position x, of the HA1 domain, covalently linked by a linking sequence of 0-50 amino acid residues to an HA1 C-terminal stem segment, comprising the amino acids from position y to and including the C-terminal amino acid of the HA1 domain; and (b) an influenza hemagglutinin HA2 domain, wherein the hemagglutinin stem domain polypeptide is resistant to protease cleavage at the junction between HA1 and HA2, and wherein one or more amino acid of the amino acids at positions 337, 340, 352, 353, 402, 406, 409, 413 and / or 416 have been mutated, as compared to the corresponding positions in wild-type influenza HA.
Owner:JANSSEN VACCINES & PREVENTION BV
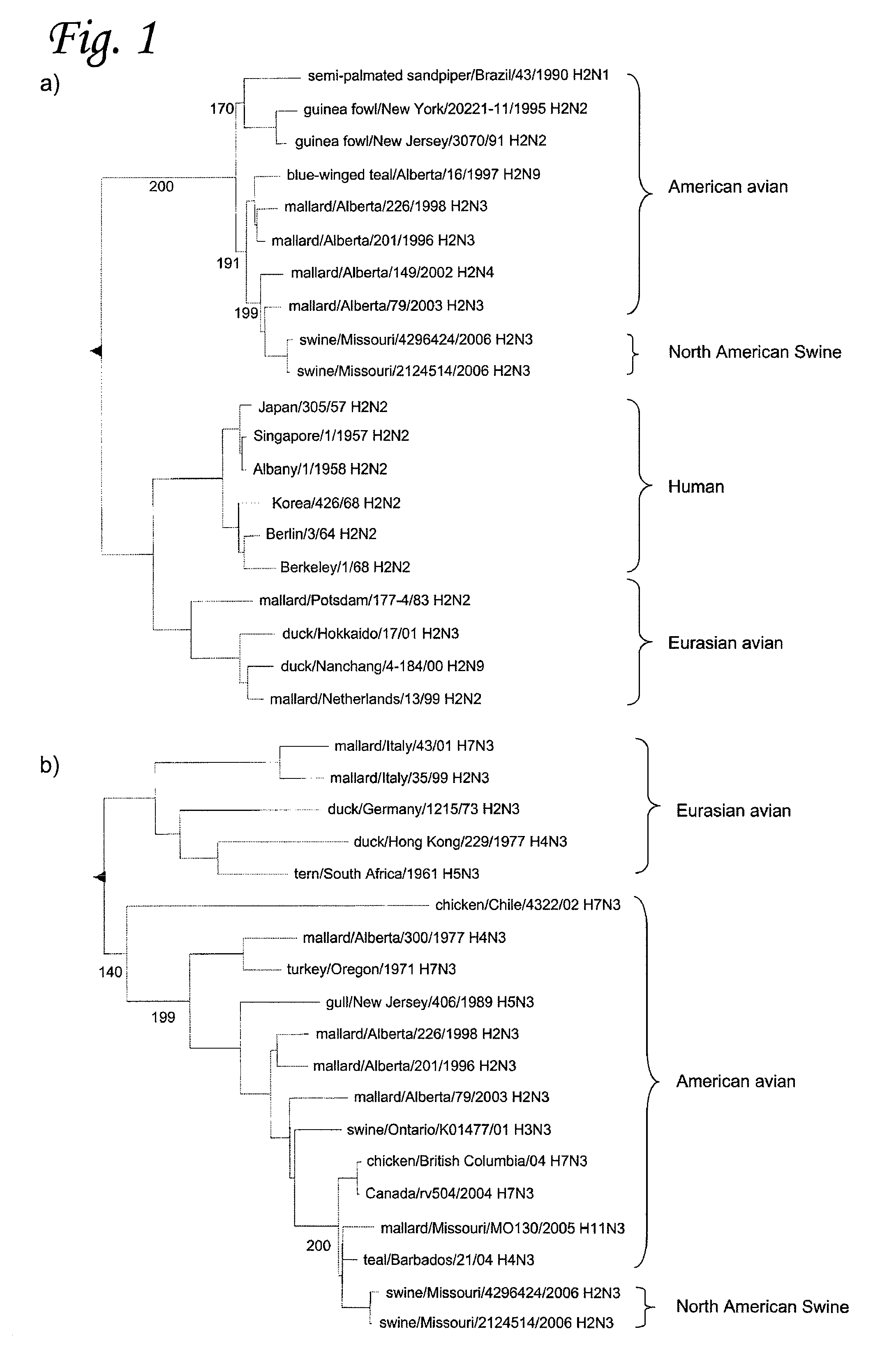
H2N3 influenza A viruses and methods of use
Owner:RGT UNIV OF MINNESOTA +2
Lectin compositions and methods for modulating an immune response to an antigen
InactiveCN101119745APolypeptide with localisation/targeting motifSsRNA viruses negative-senseHemagglutininLectin
The present invention relates to a fusion polypeptide comprising at least about 10 contiguous amino acid residues of an influenza virus hemagglutinin and at least about 5 contiguous amino acids of a naturally occurring GM-CSF molecule.
Owner:GENITRIX LLC
Recombinant avian influenza vaccine and uses thereof
ActiveUS20100189731A1Highly immunogenicImprove protectionSsRNA viruses negative-sensePeptide/protein ingredientsHemagglutininEpitope
The present invention encompasses influenza vaccines, in particular avian influenza vaccines. The vaccine may be a subunit vaccine based on the hemagglutinin of influenza. The hemagglutinin may be expressed in plants including duckweed. The invention also encompasses recombinant vectors encoding and expressing influenza antigens, epitopes or immunogens which can be used to protect animals against influenza. It encompasses also a vaccination regimen compatible with the DIVA strategy, including a prime-boost scheme using vector and subunit vaccines.
Owner:MERIAL INC
Multivalent immunogenic composition
ActiveCN103394082AImprove securitySave the number of seedsBacterial antigen ingredientsViral antigen ingredientsHemagglutininTetanus toxoids
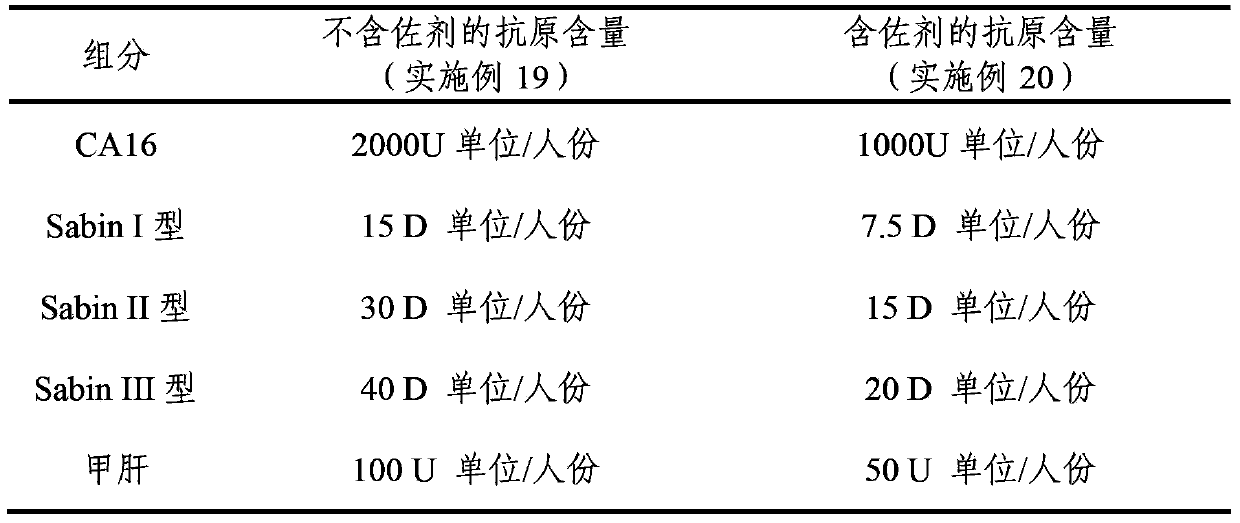
The invention provides a multivalent immunogenic composition, which includes an inactivated hepatitis A antigen and an inactivated poliovirus. The composition also can further include over one or two of a purified pertussis antigen, diphtheria toxoid, tetanus toxoid, filamentous hemagglutinin, Haemophilus influenzae type b polysaccharide, Neisseria meningitidis capsular polysaccharide, a hepatitis B virus antigen, enterovirus 71 and a coxsackievirus A16 antigen, and a physiologically acceptable carrier. The composition involved in the invention is employed to immunize the inoculated population in the form of a bivalent vaccine or more combined vaccines. Without reducing the immune effects of each immunizing antigen, the inoculation number of times can be reduced at the same time, and the time and human resources can also be saved.
Owner:SINOVAC BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com