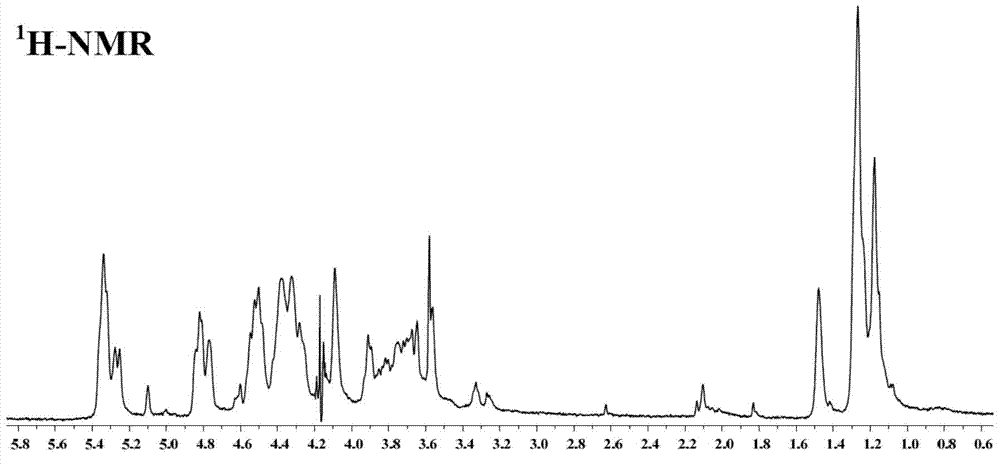
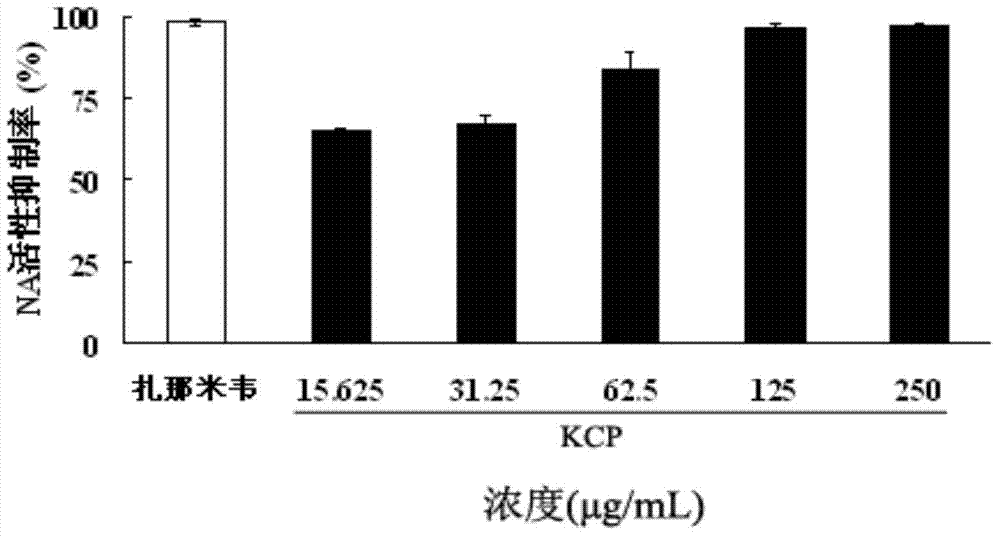
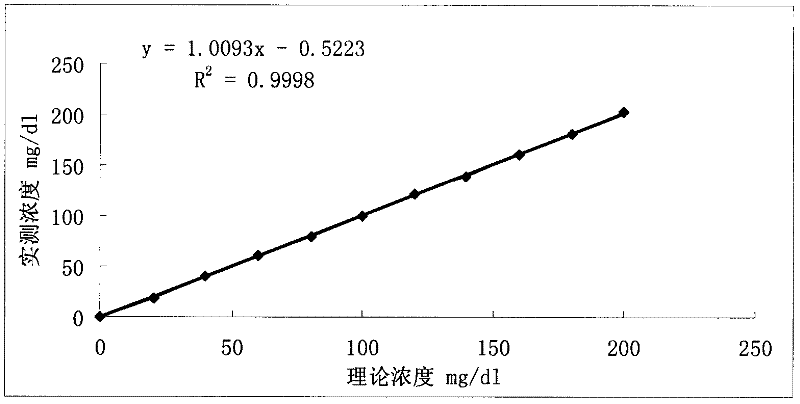
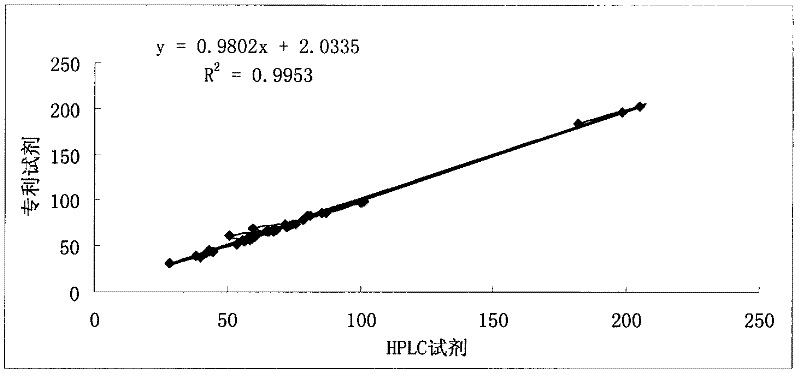
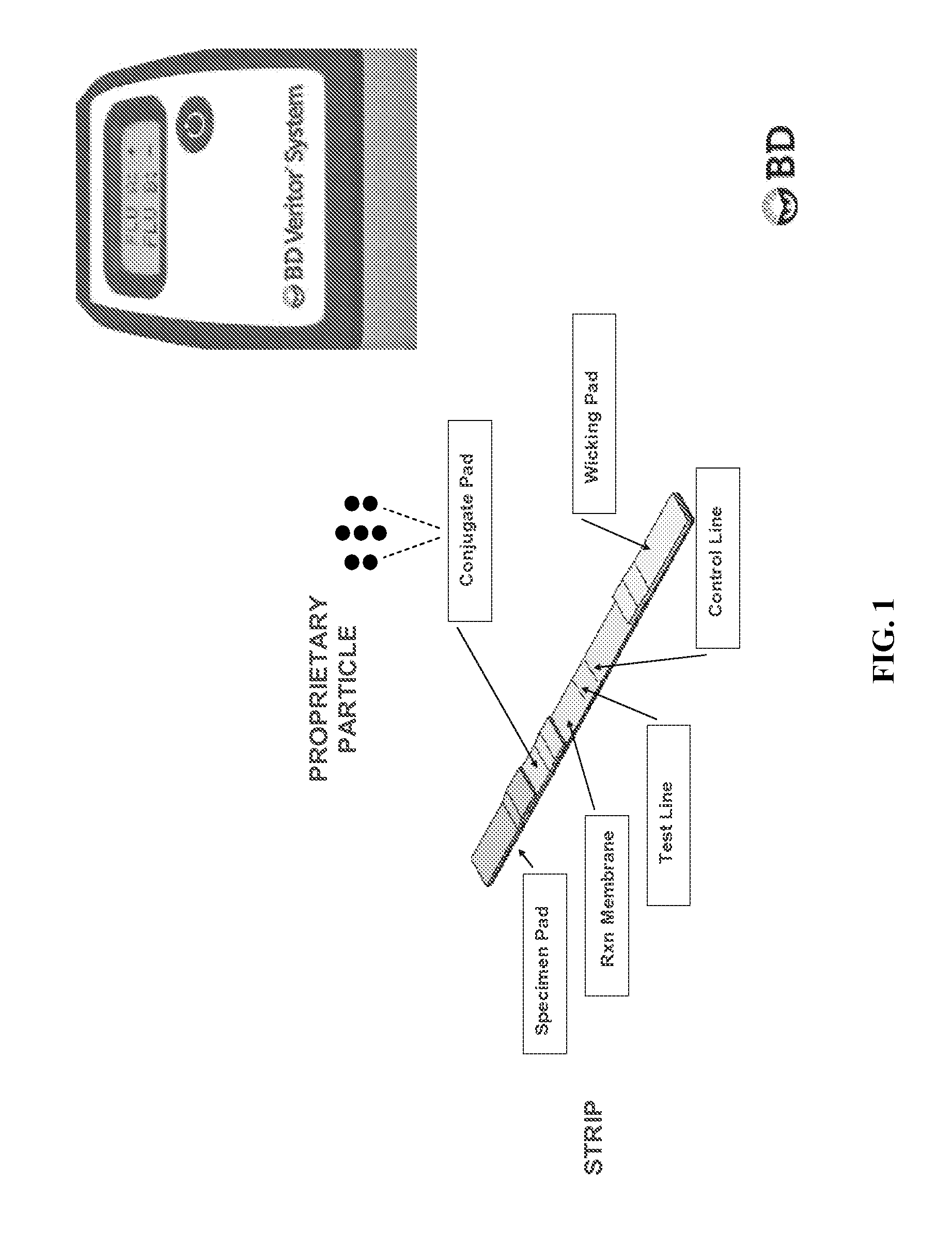
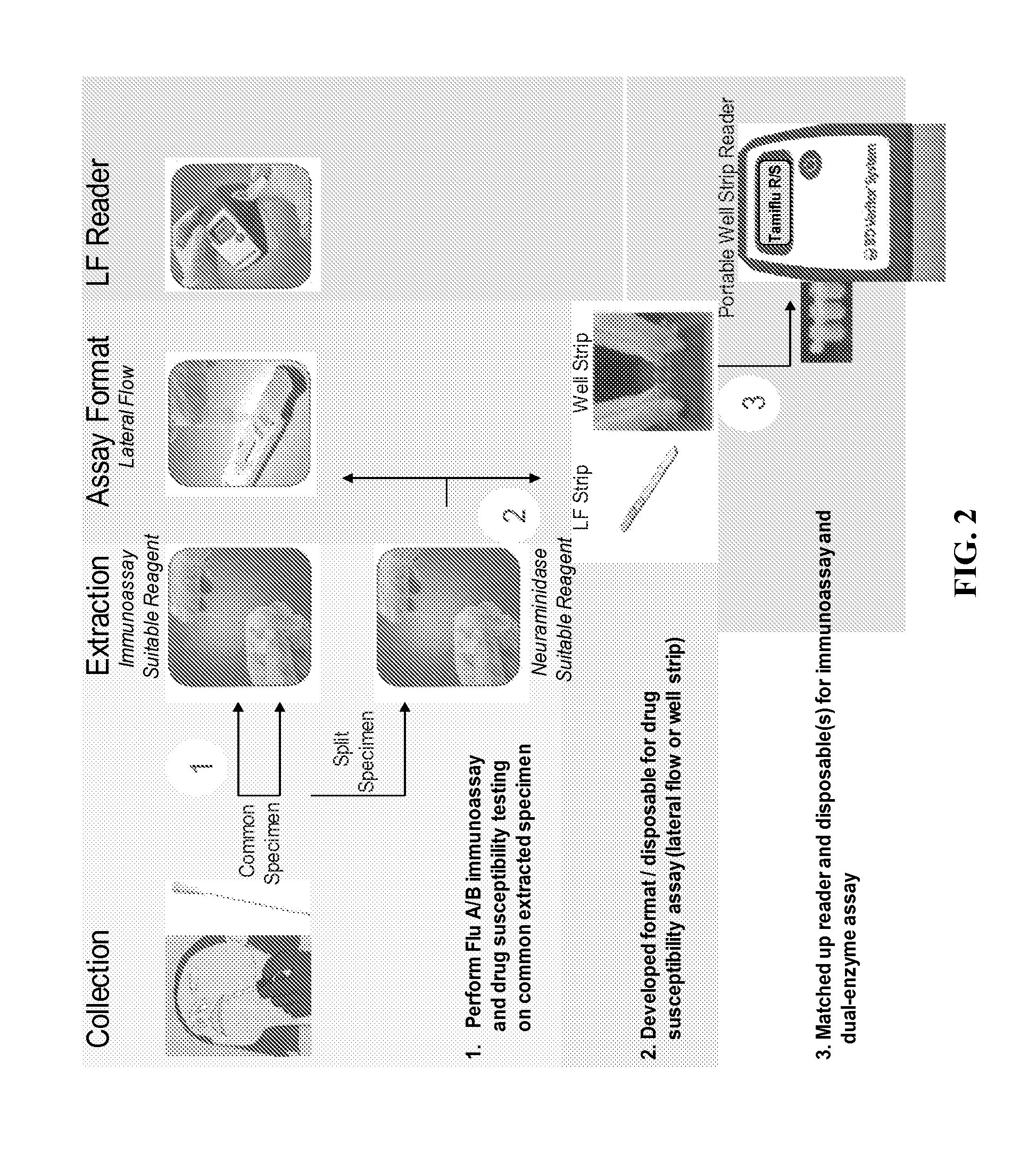
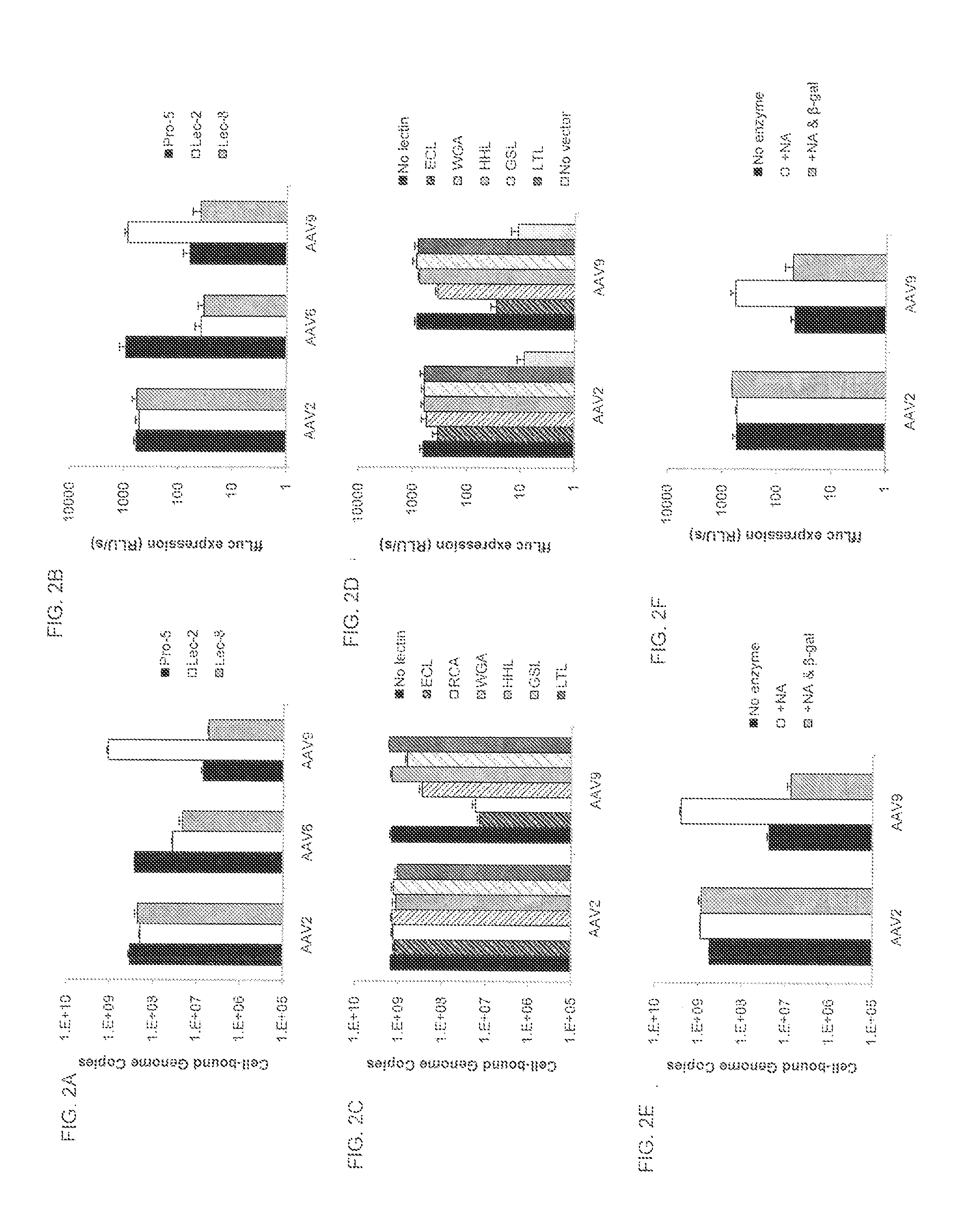Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
278 results about "Influenza A neuraminidase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Neuraminidase enzymes are a large family, found in a range of organisms. The best-known neuraminidase is the viral neuraminidase, a drug target for the prevention of the spread of influenza infection. The viral neuraminidases are frequently used as antigenic determinants found on the surface of the influenza virus.
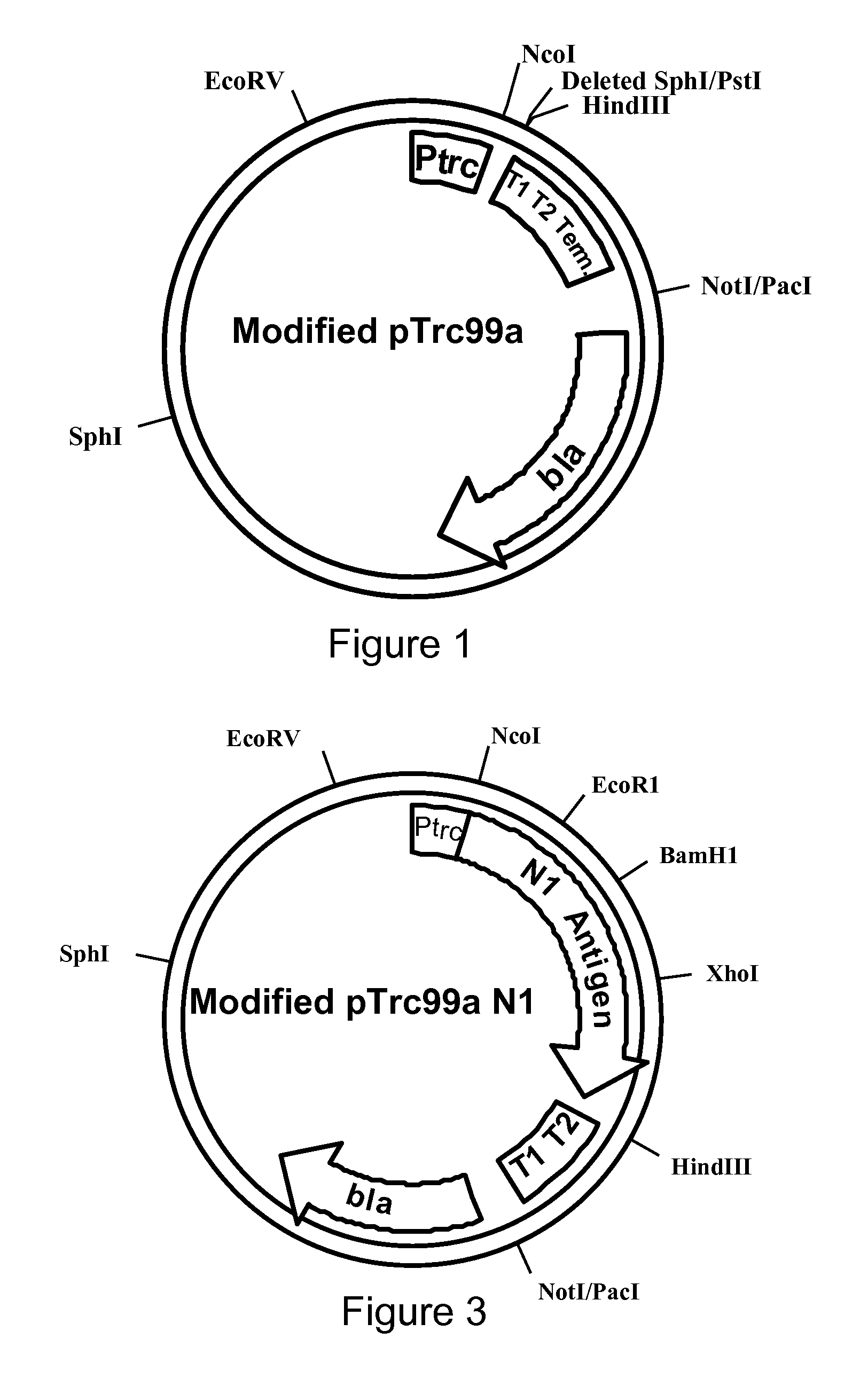
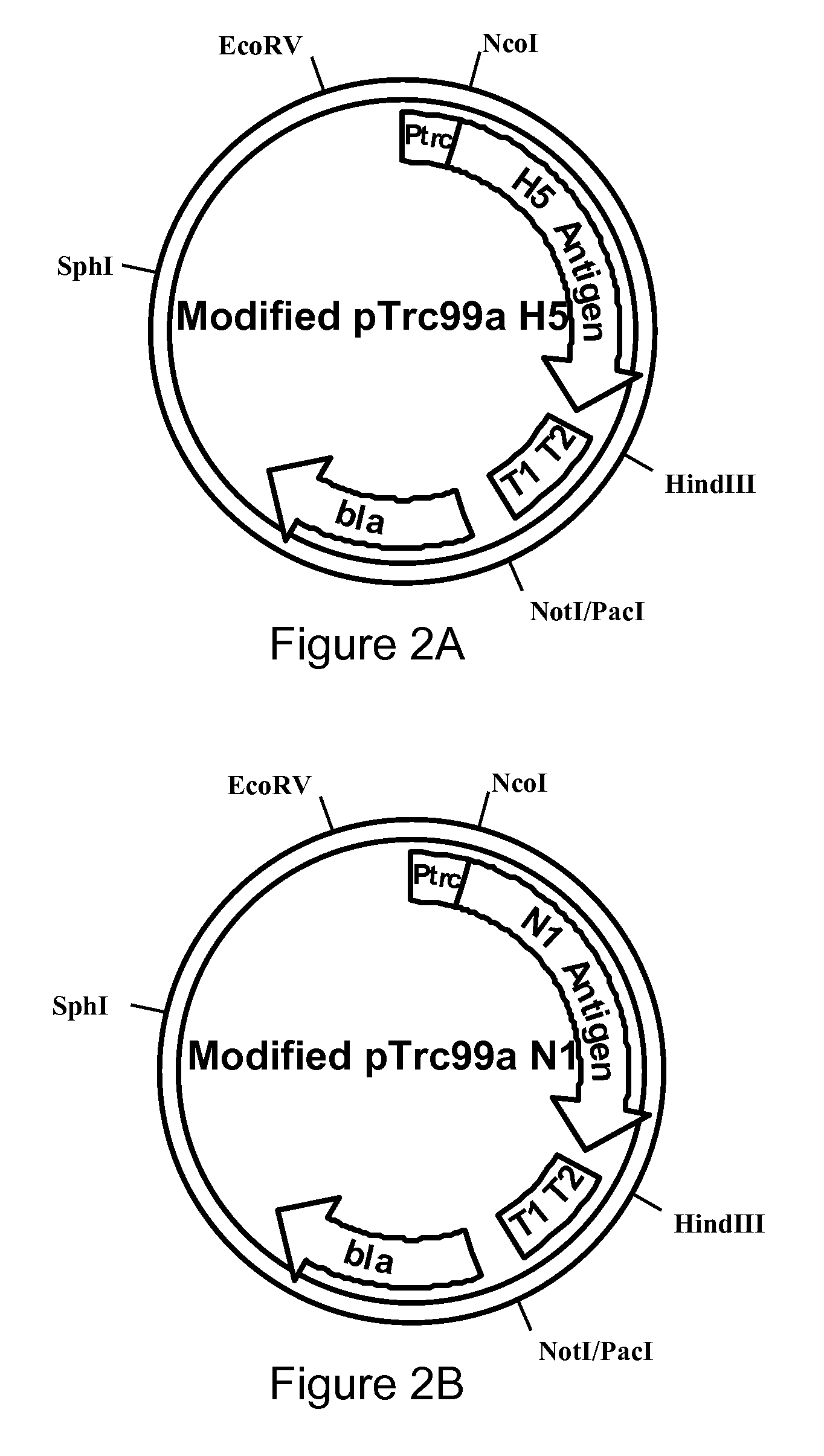
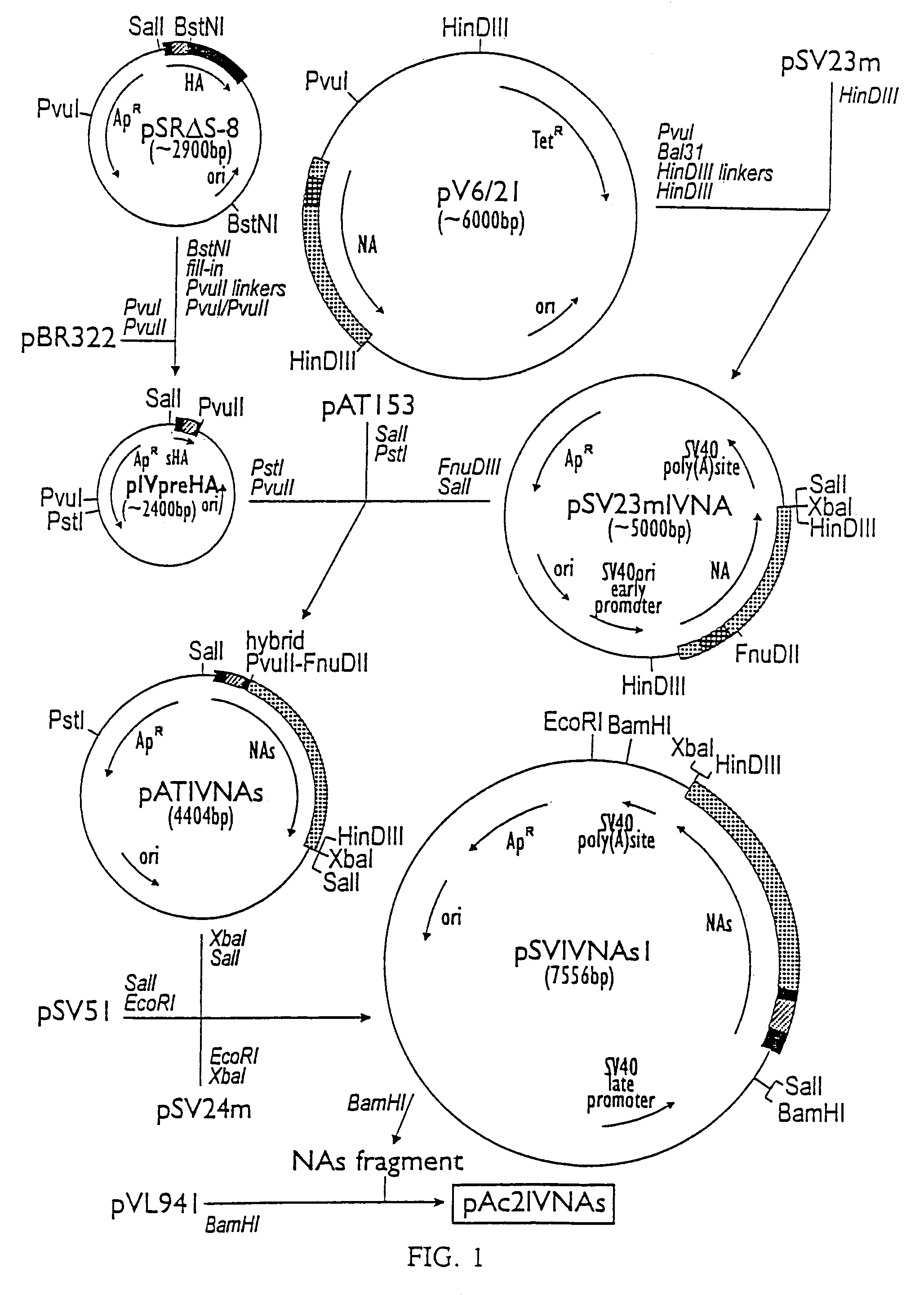
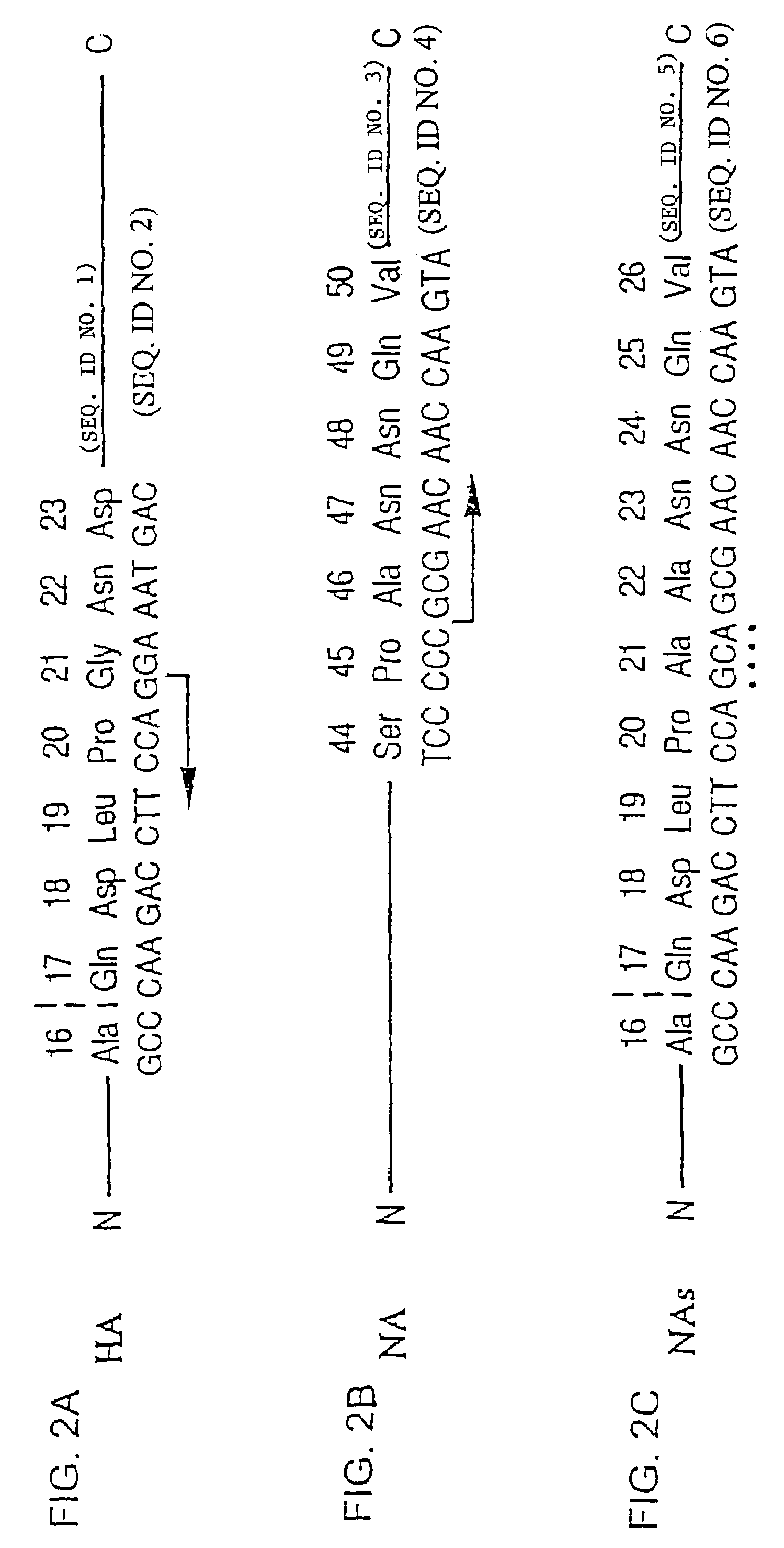
Influenza hemagglutinin and neuraminidase variants
InactiveUS20050042229A1Efficient productionSsRNA viruses negative-senseHydrolasesHemagglutininNeuraminidase
Polypeptides, polynucleotides, methods, compositions, and vaccines comprising influenza hemagglutinin and neuraminidase variants are provided.
Owner:MEDIMMUNE LLC
DNA transfection system for the generation of infectious influenza virus
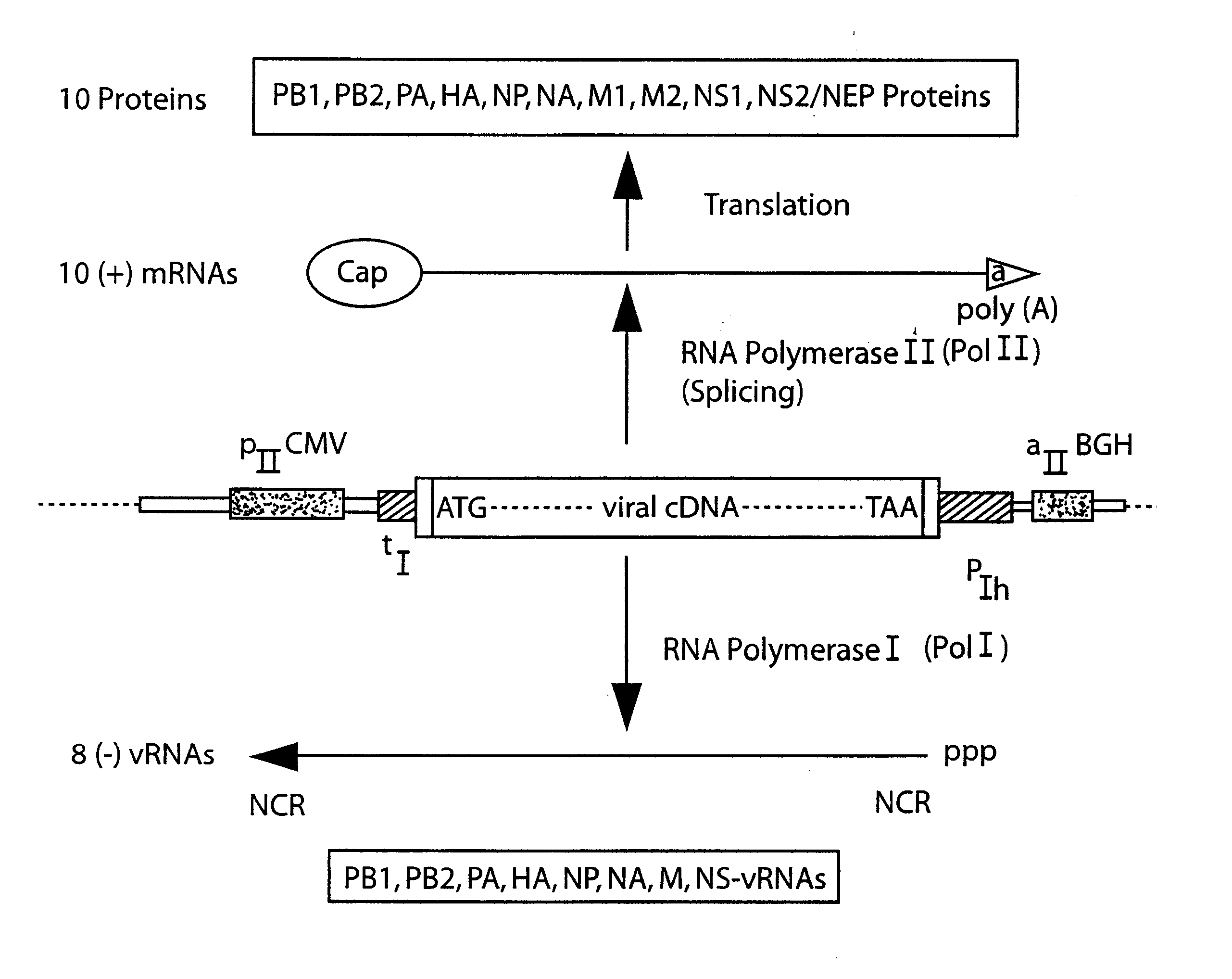
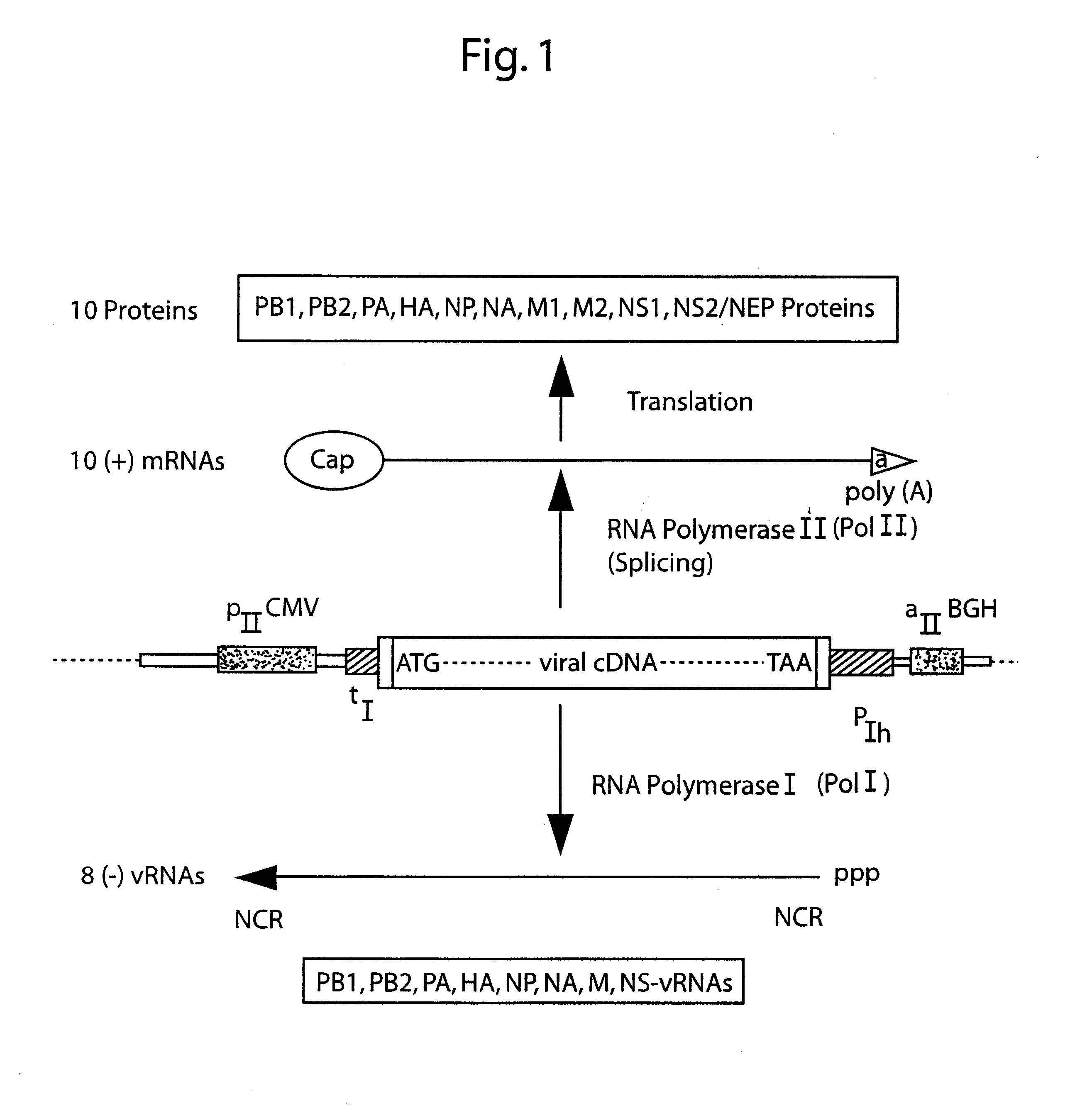
The present invention is based on the development of a dual promoter system (preferably a RNA pol I-pol II system) for the efficient intracellular synthesis of viral RNA. The resultant minimal plasmid-based system may be used to synthesize any RNA virus, preferably viruses with a negative single stranded RNA genome. The viral product of the system is produced when the plasmids of the system are introduced into a suitable host cell. One application of the system is production of attenuated, reassortant influenza viruses for use as antigens in vaccines. The reassortant viruses generated by cotransfection of plasmids may comprise genes encoding the surface glycoproteins hemagglutinin and neuramimidase from an influenza virus currently infecting the population and the internal genes from an attenuated influenza virus. An advantageous property of the present invention is its versatility; the system may be quickly and easily adapted to synthesize an attenuated version of any RNA virus. Attenuated or inactivated RNA viruses produced by the present invention may be administered to a patient in need of vaccination by any of several routes including intranasally or intramuscularly.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
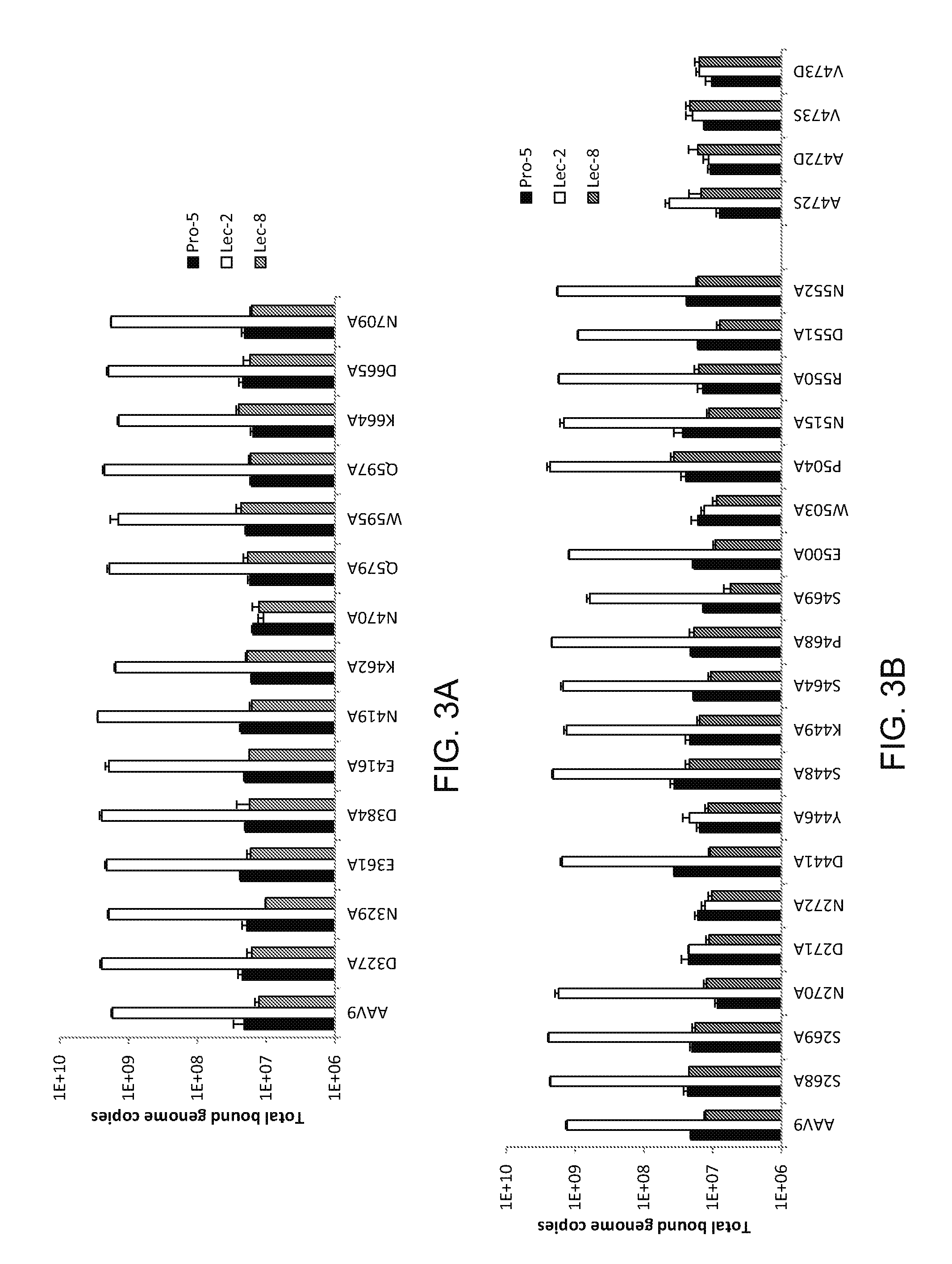
Compositions and Methods for Altering Tissue Specificity and Improving AAV9-Mediated Gene Transfer
ActiveUS20130323226A1Improve efficiencyImprove usabilityOrganic active ingredientsVectorsViral vectorFhit gene
A method of altering the targeting and / or cellular uptake efficiency of an adeno-associated virus (AAV) viral vector having a capsid containing an AAV9 cell surface binding domain is described. The method involves modifying a clade F cell surface receptor which comprises a glycan having a terminal sialic acid residue and a penultimate β-galactose residue. The modification may involve retargeting the vector by temporarily functionally ablate AAV9 binding in a subset of cells, thereby redirecting the vector to another subset of cells. Alternatively, the modification may involve increasing cellular update efficiency by treating the cells with a neuraminidase to expose cell surface β-galactose. Also provided are compositions containing the AAV9 vector and a neuraminidase. Also provided is a method for purifying AAV9 using β-galactose linked to solid support. Also provided are mutant vectors which have been modified to alter their targeting specificity, including mutant AAV9 in which the galactose binding domain is mutated and AAV in which an AAV9 galactose binding domain is engineered.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Neuraminidase-supplemented compositions
InactiveUS6485729B1Less importancePrevents and lessens HA immunodominanceSsRNA viruses negative-senseAntibody mimetics/scaffoldsNasal cavityAdjuvant
An anti-influenza vaccine composition wherein the improvement is that the vaccine includes, as an additive, neuraminidase (NA). The base anti-influenza vaccine can be any commercially available anti-influenza vaccine. The composition can include and be administered with an adjuvant. The vaccine composition provides protection in a host, animal or human, against influenza infection, including viral replication and systemic infection. Oral, nasal or other mucosal or per needle administration, including intracutaneous, intradermal, intramuscular, intravascular, and intravenous, are included.
Owner:PROTEIN SCI
DNA transfection system for the generation of infectious influenza virus
InactiveUS20050186563A1Improve effectivenessElicit protective immunitySsRNA viruses negative-senseFungiDual promoterSingle-Stranded RNA
The present invention is based on the development of a dual promoter system (preferably a RNA pol I-pol II system) for the efficient intracellular synthesis of viral RNA. The resultant minimal plasmid-based system may be used to synthesize any RNA virus, preferably viruses with a negative single stranded RNA genome. The viral product of the system is produced when the plasmids of the system are introduced into a suitable host cell. One application of the system is production of attenuated, reassortant influenza viruses for use as antigens in vaccines. The reassortant viruses generated by cotransfection of plasmids may comprise genes encoding the surface glycoproteins hemagglutinin and neuraminidase from an influenza virus currently infecting the population and the internal genes from an attenuated influenza virus. An advantageous property of the present invention is its versatility; the system may be quickly and easily adapted to synthesize an attenuated version of any RNA virus. Attenuated or inactivated RNA viruses produced by the present invention may be administered to a patient in need of vaccination by any of several routes including intranasally or intramuscularly.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Influenza hemagglutinin and neuraminidase variants
Polypeptides, polynucleotides, methods, compositions, and vaccines comprising influenza hemagglutinin and neuraminidase variants are provided.
Owner:MEDIMMUNE LLC
Influenza vaccines
InactiveUS20080299151A1Less protectionBroad and efficient protective immunitySsRNA viruses negative-senseOrganic active ingredientsHemagglutininMammal
Described herein are vaccines and the use of naked DNA and / or RNA encoding hemagglutinin (HA) from pandemic influenza, e.g., the 1918 H1N1 and / or the 1957 H2N2 and / or the 1968 H3N2 influenza A virus, as a vaccine component against present day and coming H1, H2, H3, H5, N1, N2 containing influenza A infections in humans and swine optionally with the naked DNA and / or RNA encoding Neuraminidase (NA) and / or matrix protein (M) and / or the nucleoprotein (NP) from pandemic influenza virus included. If the vaccine components are used as DNA or RNA vaccines with or without the corresponding protein, the codons can optionally be “humanized” using preferred codons from highly expressed mammalian genes and the administration of this DNA vaccine can be by saline or buffered saline injection of naked DNA or RNA, or injection of DNA plasmid or linear gene expressing DNA fragments coupled to particles. Addition of the matrix protein (M) and / or the nucleoprotein (NP) from the 1918 influenza strain is also disclosed.
Owner:STATENS SERUM INST
Live bacterial vaccines for viral infection prophylaxis or treatment
InactiveUS20080124355A1Enhance immune responseSsRNA viruses negative-senseBacterial antigen ingredientsHemagglutininBacteroides
The present invention provides a vaccine, method of use, and kit employing genetically isolated and stabilized, live attenuated bacterial strains including Salmonella that express one or more avian influenza antigens for use in live vaccine compositions that can be orally administered to an individual to protect against avian influenza. Genetic stabilization may be achieved through deletion of IS200 elements and bacteria phage and prophage elements. The bacterial strains may be genetically isolated from external phage infection by constitutive expression of a P22 phage repressor. Nucleic acid sequences encoding antigenic hemagglutinin and neuraminidase avian influenza proteins, having at least one modified codon for optimum expression when transferred into a prokaryotic microorganism for improved immunogenicity
Owner:AVIEX TECH
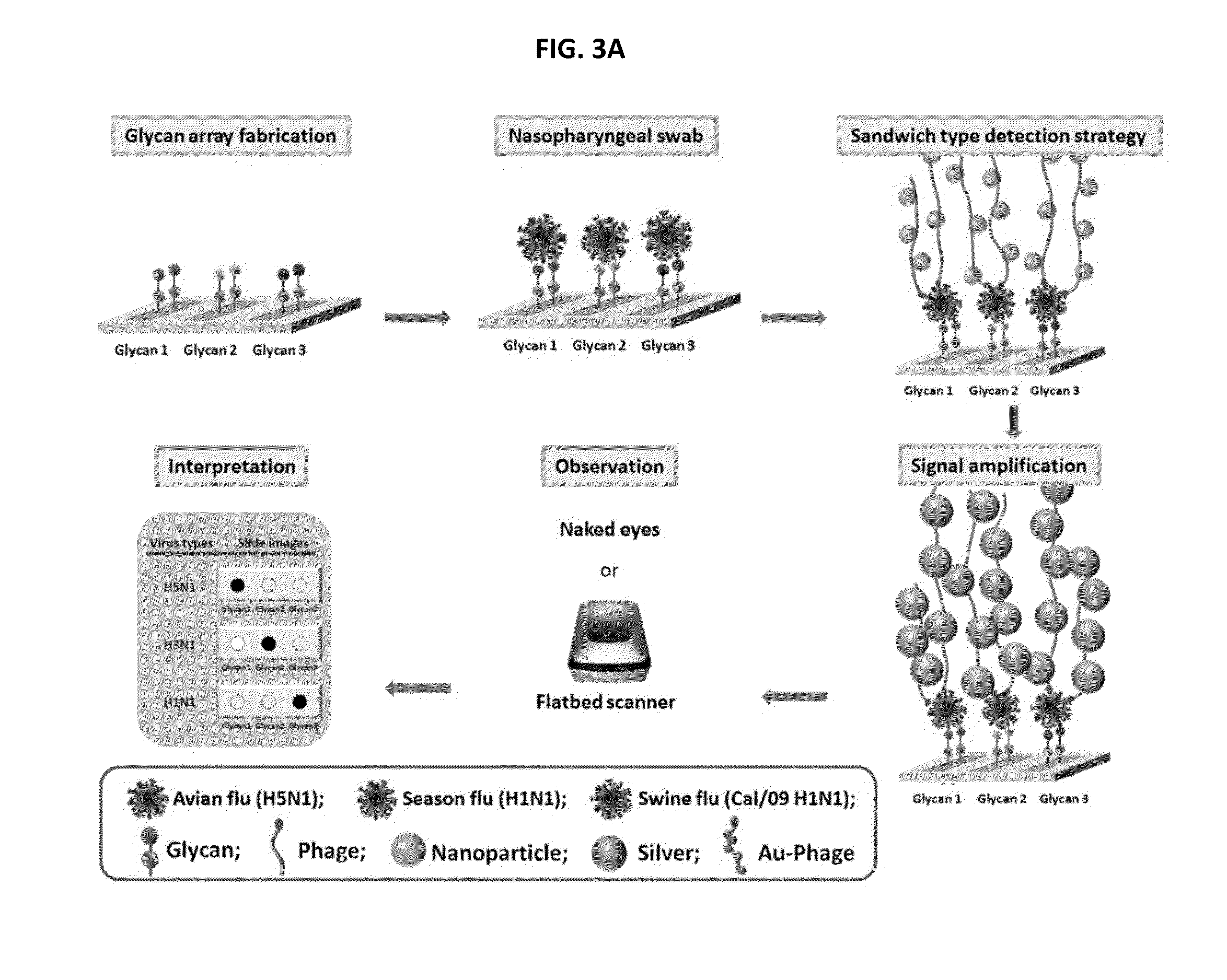
Glycan arrays for high throughput screening of viruses
ActiveUS20150160217A1Improve signal-to-noise ratioThe process is simple and fastSugar derivativesMicrobiological testing/measurementHemagglutininHigh-Throughput Screening Methods
Glycan arrays that can detect and distinguish between various sub-types and strains of influenza virus are provided. Methods for using the glycan arrays with assays using nanoparticle amplification technique are disclosed. Sandwich assays using gold nanoparticles conjugated to phage particles comprising influenza virus-specific antibodies for detecting multiple serotypes using a single reaction are provided. Plurality of glycans directed to specific target HA of influenza virus comprises the array. Detector molecules comprising noble metals conjugated to (a) phage display particles expressing antibodies against hemagglutinin and (b) neuraminidase binding agents are disclosed.
Owner:ACAD SINIC
Multivalent neuraminidase inhibitor conjugates
InactiveUS7205333B2High binding affinityReduce capacityBiocideMicrobiological testing/measurementReactive siteActive site
The invention relates to a multimeric compound or a pharmaceutically acceptable salt or derivative thereof which comprises three or more neuraminidase-binding groups attached to a spacer or linking group, in which the neuraminidase-binding group is a compound which binds to the active site of influenza virus neuraminidase, but is not cleaved by the neuraminidase. The invention also relates to processes for the preparation of the multimeric compound defined above, pharmaceutical compositions containing them or methods for the treatment and / or prophylaxis of a viral infection involving them.
Owner:BIOTA SCI MANAGEMENT PTI LTD
Influenza sensor
InactiveUS6893814B2Bioreactor/fermenter combinationsBiological substance pretreatmentsNeuraminidaseFluorescence
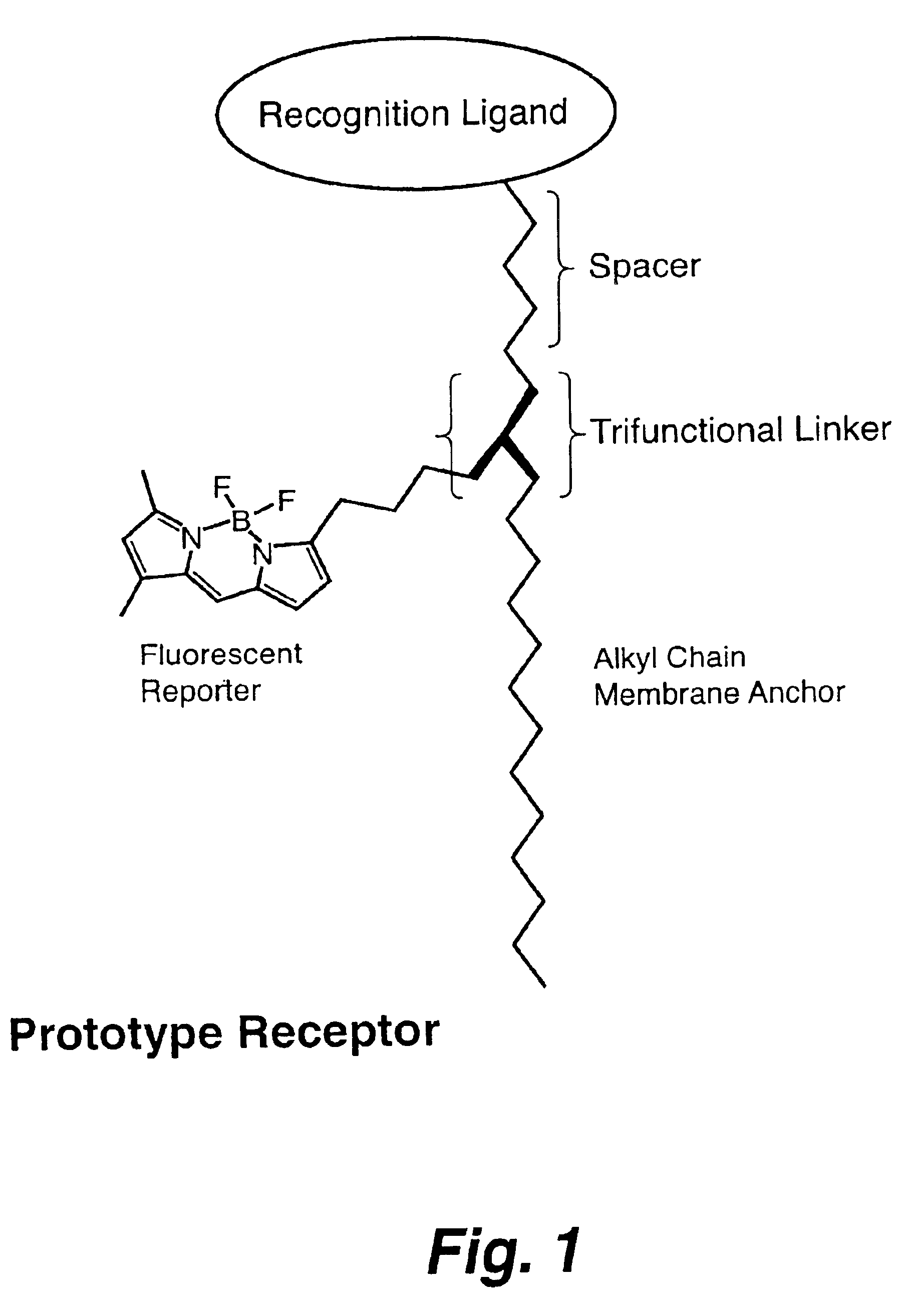
A sensor for the detection of tetrameric multivalent neuraminidase within a sample is disclosed, where a positive detection indicates the presence of a target virus within the sample. Also disclosed is a trifunctional composition of matter including a trifunctional linker moiety with groups bonded thereto including (a) an alkyl chain adapted for attachment to a substrate, (b) a fluorescent moiety capable of generating a fluorescent signal, and (c) a recognition moiety having a spacer group of a defined length thereon, the recognition moiety capable of binding with tetrameric multivalent neuraminidase.
Owner:LOS ALAMOS NATIONAL SECURITY
Gynaecologic multi-item dry chemical united detection test paper strip and its measuring method
InactiveCN101320040AReflect cleanlinessComprehensive detectionMaterial analysis by observing effect on chemical indicatorBiological testingWhite blood cellPaper tape
The invention relates to a test paper tape which can be used in the gynecological multiprogramming dry-chemistry joint detection. The test paper tape provided by the utility model comprises a dry-chemistry multiprogramming detection test paper tape which consists of a plastic substrate tape and various solidified regent blocks, and sample diluent. The dry regent blocks include combinations formed by increasing or decreasing at least three or more regent blocks of a Ph test regent block, a lactic acid regent block, a hydrogen peroxide concentration regent block, a leukocyte esterase concentration regent block, a neuraminidase activity regent block, an amine test regent block, a proline aminopeptidase substrate reagent block, an oxidase substrate reagent block, a N-acetylamine hexosidase substrate reagent block, a trichomonas specific protease substrate hydrolysis reagent block. the gynecological multiprogramming dry-chemistry joint detection test paper test provided by the utility model can detect Ph, lactic acid, hydrogen peroxide, leukocyte esterase, neuraminidase, amine test, proline aminopeptidase, oxidase, N-acetylamine hexosidase and trichomonas specific protease which are contained in leucorrhea sample at the same time, and can accurately reflect the microorganism environment of a women reproductive tract, the cleanness of leucorrhea secretion and the conditions of Candida albicans, trichomonas, gonococcus and the pathogens of bacterial vaginosis, thereby making the gynecological trichomonas detection more comprehensive; besides, the test paper tape provided by the utility model can be used easily and conveniently, and results can be obtained quickly. If the test paper tape is used together with a gynecological dry-chemistry analyzer, the operation can become easier and the result can be obtained more quickly.
Owner:杭州健宝医疗器械有限公司
Prevention and treatment of secondary infections following viral infection
InactiveCN101909615ALow efficacyReduce the ratioAntibacterial agentsOrganic chemistryViral infectionSecondary infection
This invention relates to a synthetic nutritional composition suitable for use in the prevention of secondary infections following a viral infection characterised by neuraminidase activitycomprising a sialylated oligosaccharide and N-acetyl-lactosamine and / or an oligosaccharide containing N-acetyl-lactosamineThe invention further extends to the use of such a composition in the prevention of secondary infections such as otitis media.
Owner:NESTEC SA
Fucosan sulphate, preparation method thereof, and application of fucosan sulphate in preparing anti-influenza virus medicine
The invention belongs to the field of marine medicines, and relates to a fucosan sulphate, a preparation method thereof and application of the fucosan sulphate in preparing an anti-influenza virus medicine. Polysaccharide with a main chain taking alpha-1,2-D-mannose and beta-1,4-D-glucuronic acid as repetitive units, and with a branched chain of alpha-1,3-L-fucosan sulphate is obtained by virtue of hot-water extraction, calcium chloride precipitation and DEAE-Cellulose chromatographic column purification. The fucosan sulphate prepared by the invention is high in inhibition effect on the influenza virus neuraminidase activities of A H1N1, H5N1 and H3N2, and obvious in protection effect on the dog kidney epithelial cells infected by A H1N1. The fucosan sulphate provided by the invention has the advantages of being rich in raw material source, simple in preparation process, easy to industrialize, high in product water solubility, high in stability, free from toxic and side effects, and the like, and has the prospect of being developed to the anti-influenza virus medicine.
Owner:威海人生药业有限公司
Reagents and kits for detection of influenza virus and the like
ActiveUS20080286758A1Simple and rapid and specific and sensitive detectionHigh detection sensitivityOrganic chemistryMicrobiological testing/measurementNeuraminidaseFluorescein
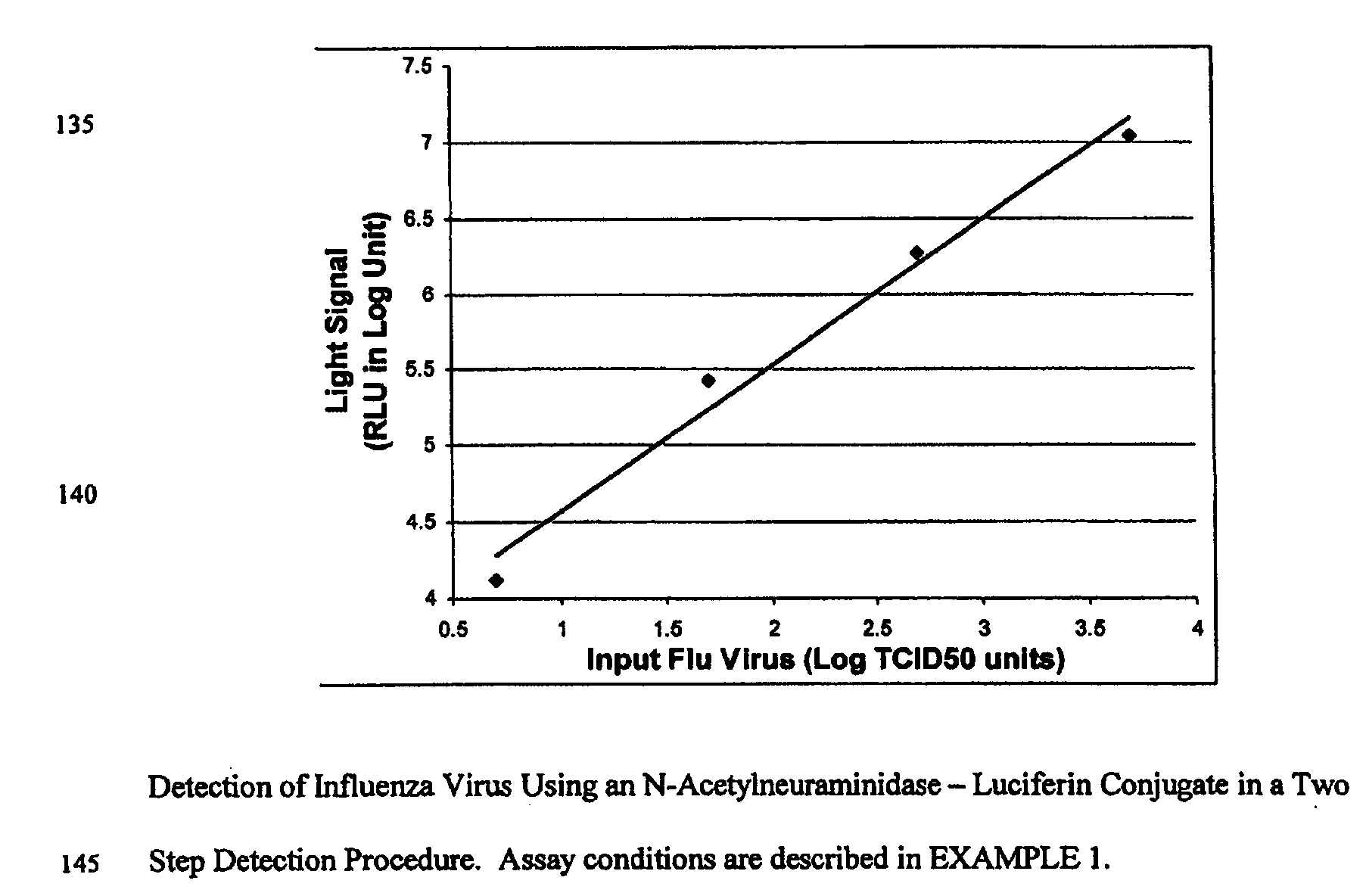
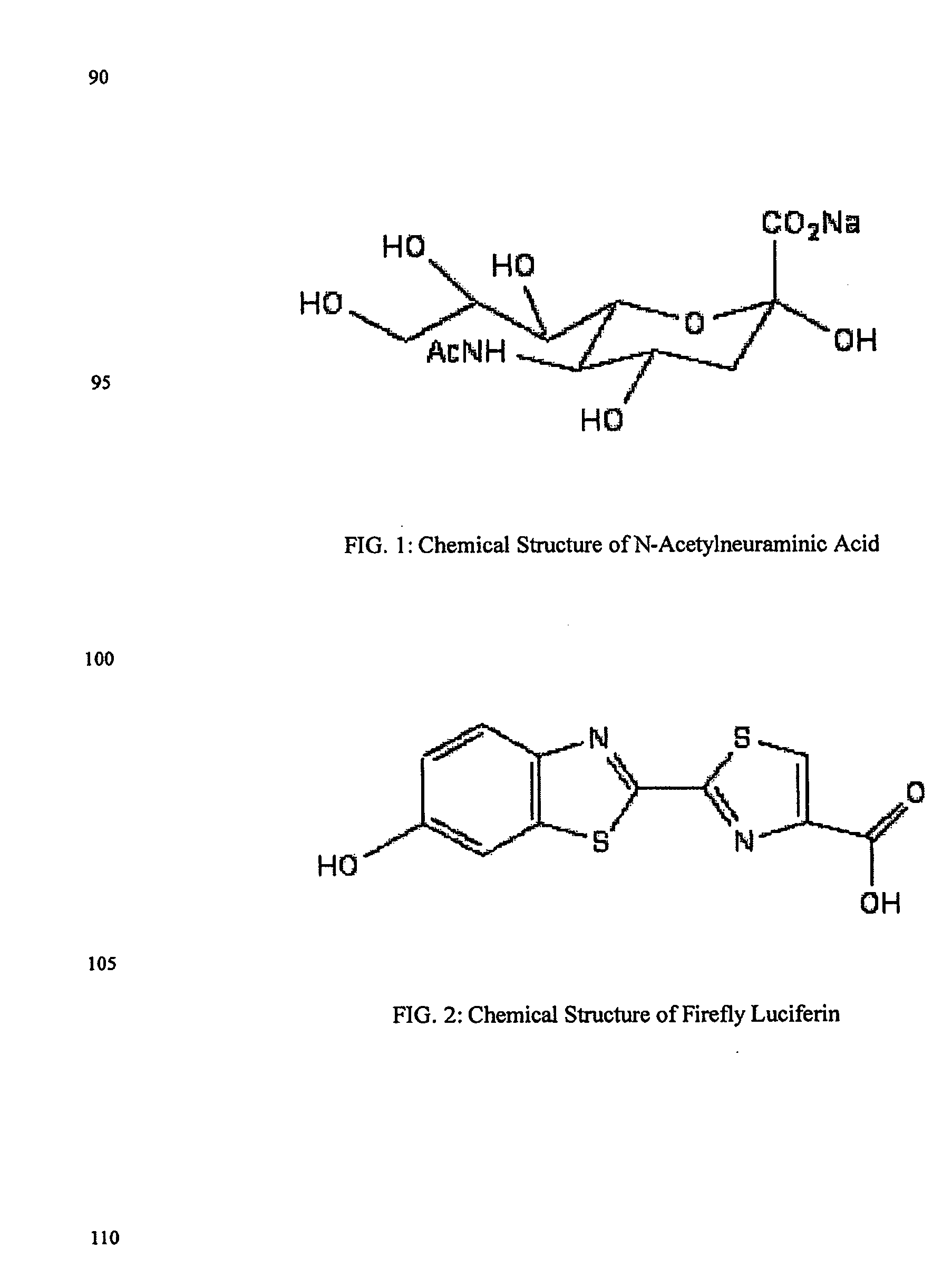
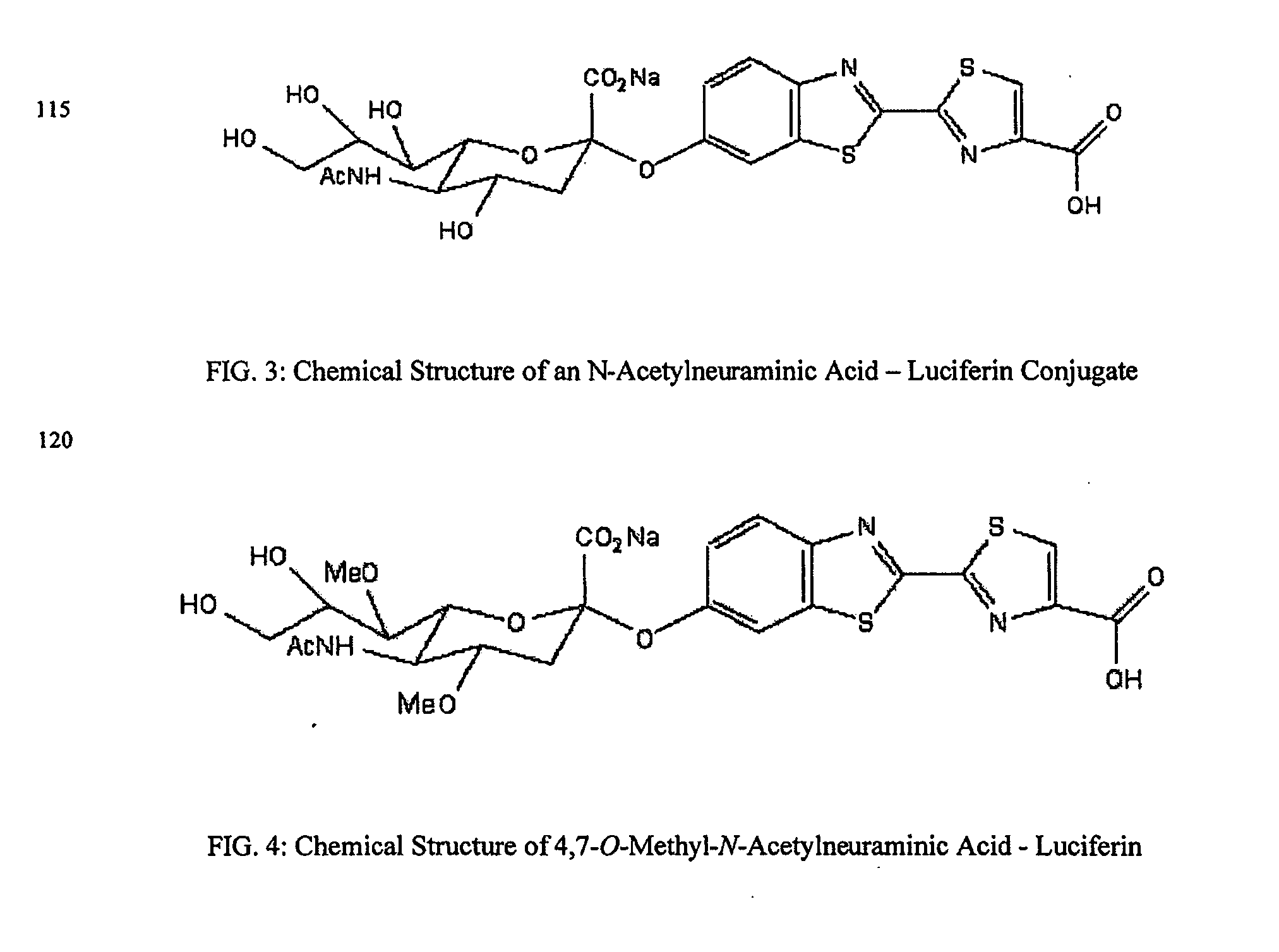
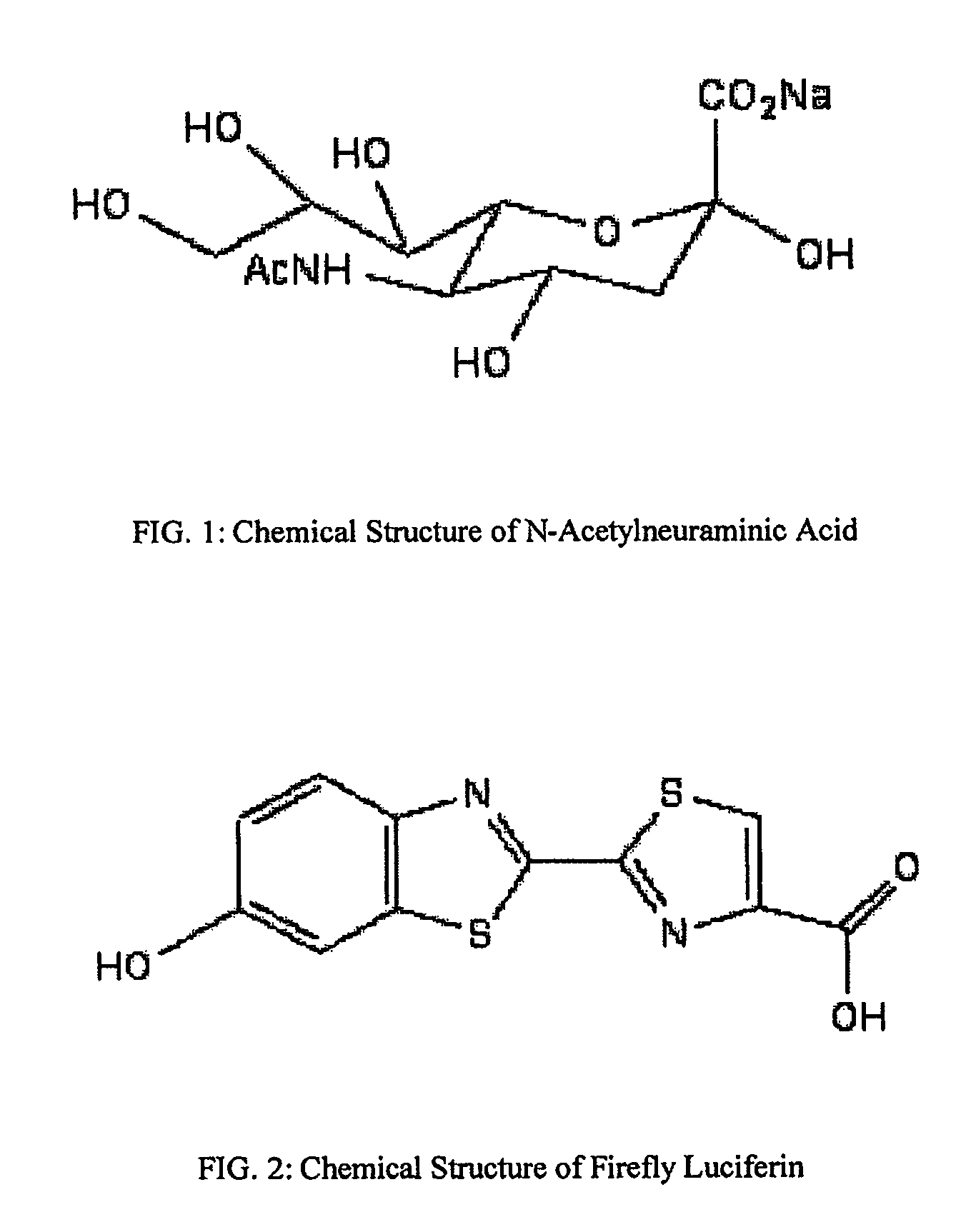
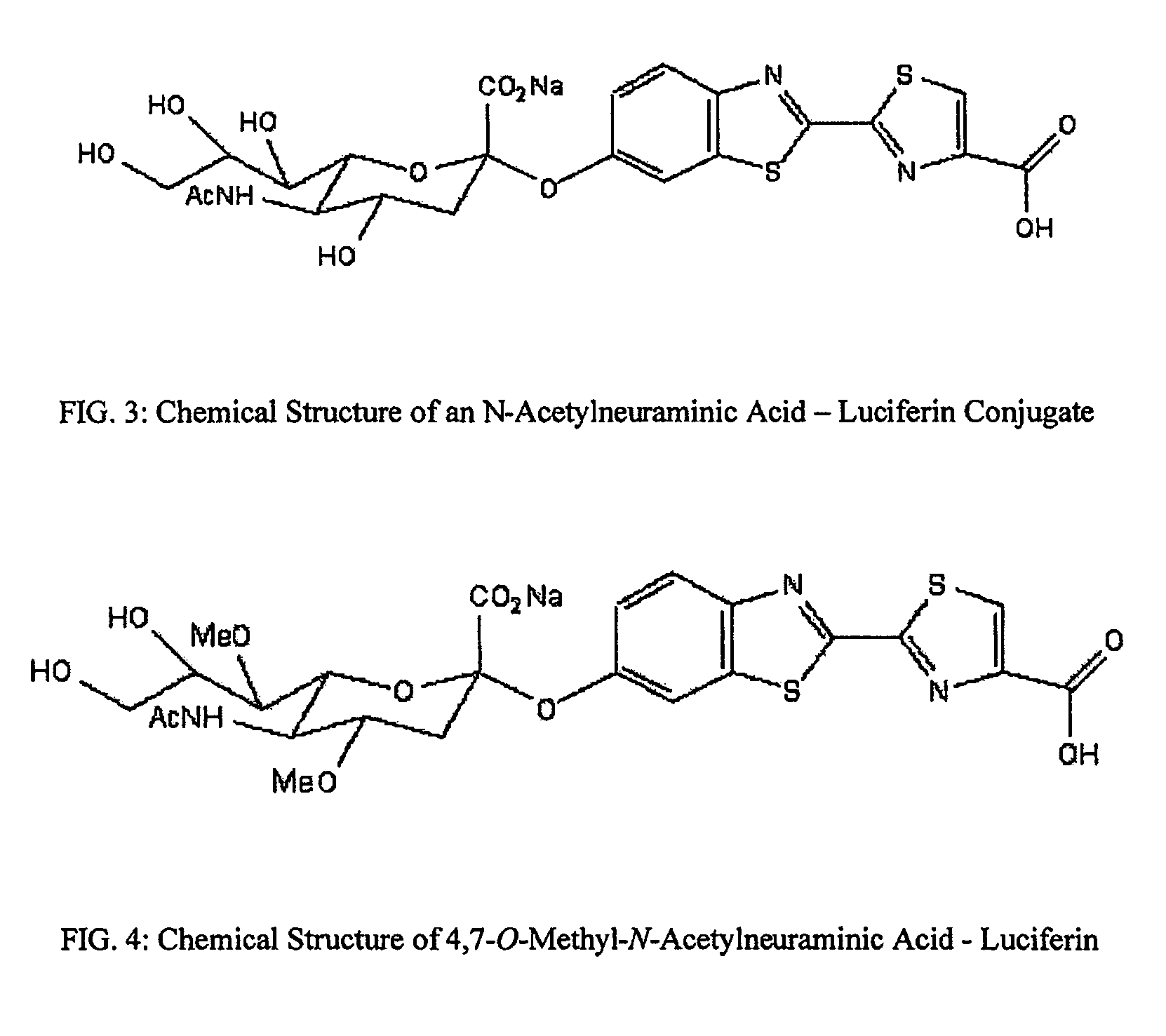
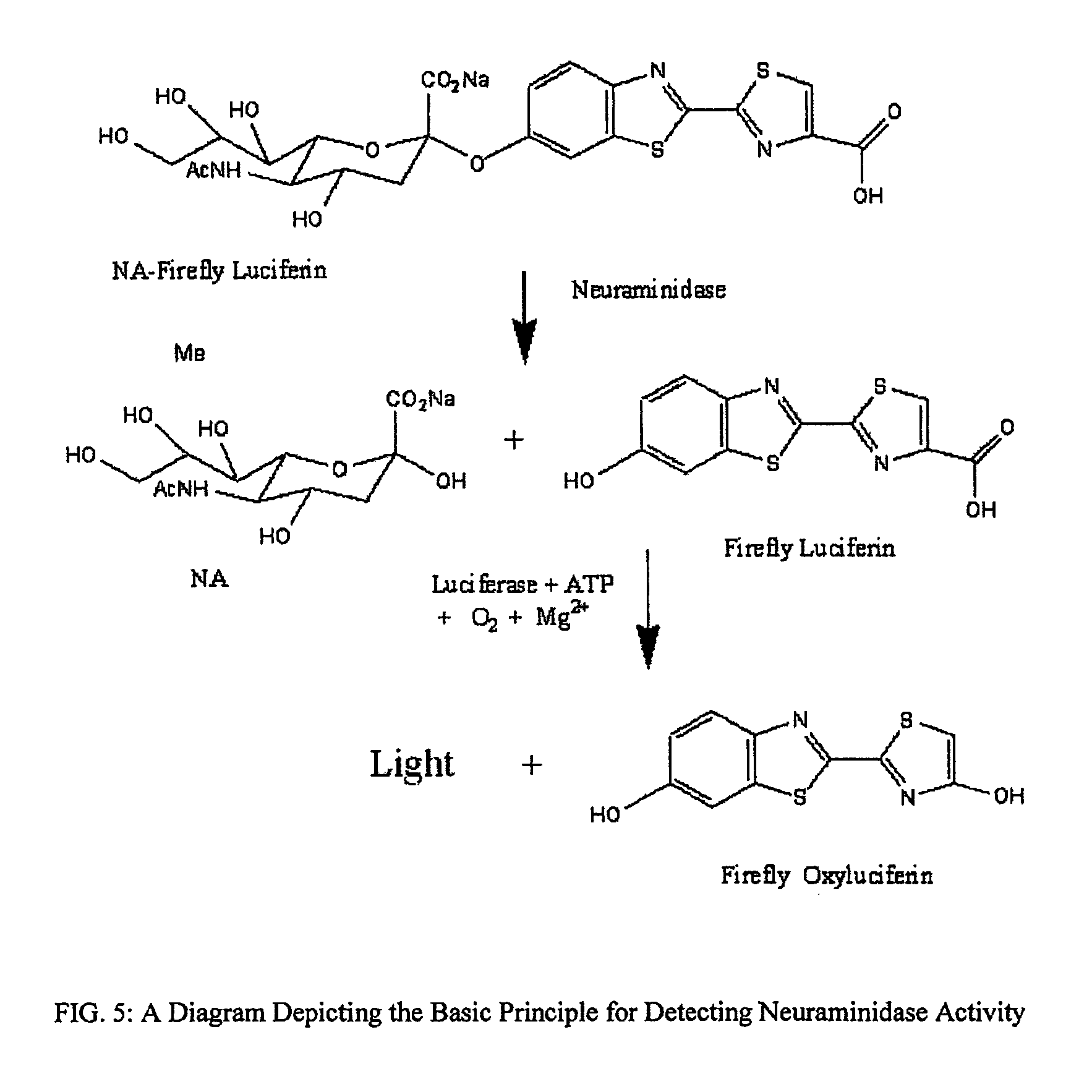
The present invention relates to reagents and methods for influenza virus detection. These reagents and methods disclosed in the present invention enable simple, rapid, specific and sensitive detection of influenza virus types A and B. These reagents are N-acetylneuraminic acid-firefly luciferin conjugates which can be cleaved by influenza virus neuraminidase.
Owner:CELLEX BIOLOGICAL TECH SUZHOU CO LTD
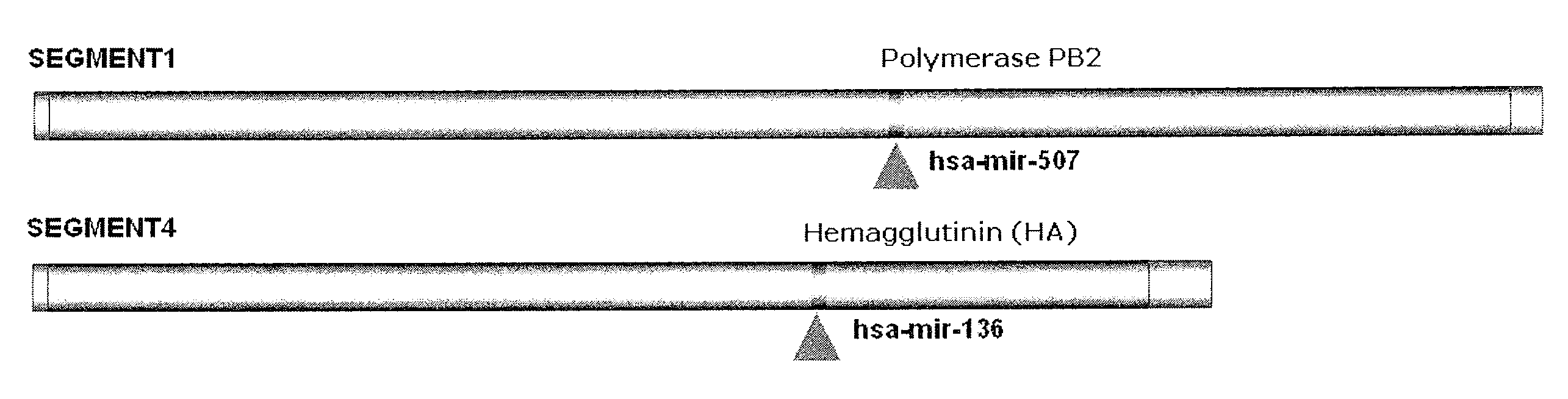
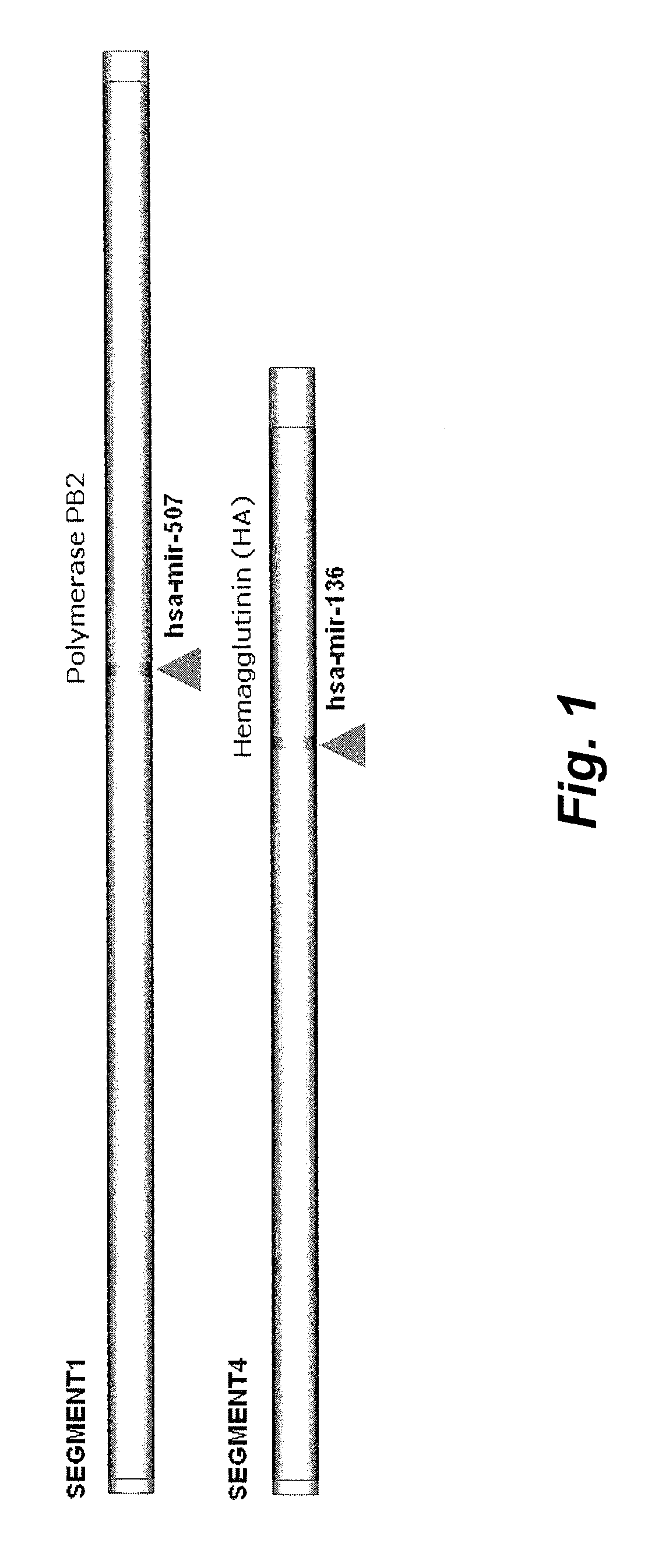
Targets for human micro rnas in avian influenza virus (H5N1) genome
The present invention relates to targets for Human microRNAs in Avian Influenza Virus (H5N1) Genome and provides specific miRNA targets against H5N1 virus. Existing therapies for Avian flu are of limited use primarily due to genetic re-assortment of the viral genome, generating novel proteins, and thus escaping immune response. In animal models, baculovirus-derived recombinant H5 vaccines were immunogenic and protective, but results in humans were disappointing even when using high doses. Currently, two classes of drugs are available with antiviral activity against influenza viruses: inhibitors of the M2 ion channel, amantadine and rimantadine, and inhibitors of neuraminidase, oseltamivir, and zanamivir. There is paucity of information regarding effectiveness of these drugs in H5N1 infection. These drugs are also well known to have side effects like neurotoxicity. Thus there exists a need to develop alternate therapy for targeting the Avian flu virus (H5N1). The present invention addresses this need in the field.
Owner:COUNCIL OF SCI & IND RES
Influenza hemagglutinin and neuraminidase variants
Polypeptides, polynucleotides, methods, compositions, and vaccines comprising influenza hemagglutinin and neuraminidase variants are provided.
Owner:MEDIMMUNE LLC
Influenza Hemagglutinin And Neuraminidase Variants
InactiveUS20090175898A1Efficient productionSsRNA viruses negative-senseViral antigen ingredientsHemagglutininNeuraminidase
Polypeptides, polynucleotides, methods, compositions, and vaccines comprising influenza hemagglutinin and neuraminidase variants are provided.
Owner:MEDIMMUNE LLC
Influenza vaccine
InactiveUS7468259B2High protection levelSsRNA viruses negative-senseFungiNeuraminidaseMembrane anchor
A method for manufacturing recombinant neuraminidase by culturing in a suitable culture medium host cells which are transformed with a neuraminidase expression vector or infected with a virus which is transformed with a neuraminidase expression vector, wherein the expression vector comprises at least a part of the coding region of a neuraminidase gene of an influenza virus minus the region which codes for the membrane anchor, or a modified version thereof, preceded in phase by a signal sequence; and isolating the expression product neuraminidase from the culture medium. The invention further relates to vectors expressing the neuraminidase.
Owner:VLAAMS INTERUNIVERSITAIR INST VOOR BIOTECHNOLOGIE VZW
Novel vaccines against multiple subtypes of influenza virus
ActiveUS20090169505A1Elicit immune responseSsRNA viruses negative-sensePeptide/protein ingredientsHemagglutininMammal
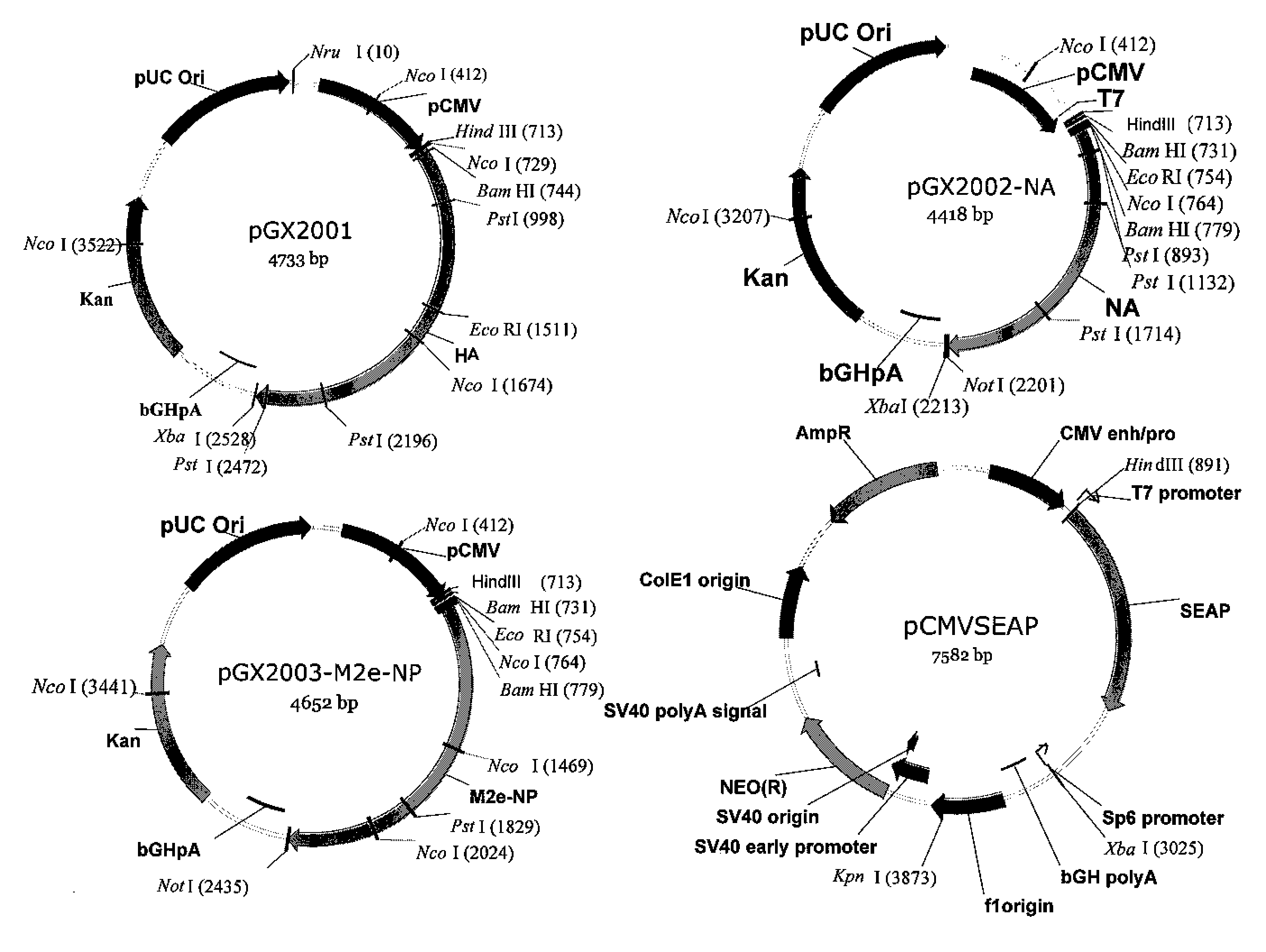
An aspect of the present invention is directed towards DNA plasmid vaccines capable of generating in a mammal an immune response against a plurality of influenza virus subtypes, comprising a DNA plasmid and a pharmaceutically acceptable excipient. The DNA plasmid is capable of expressing a consensus influenza antigen in a cell of the mammal in a quantity effective to elicit an immune response in the mammal, wherein the consensus influenza antigen comprises consensus hemagglutinin (HA), neuraminidase (NA), matrix protein, nucleoprotein, M2 ectodomain-nucleo-protein (M2e-NP), or a combination thereof. Preferably the consensus influenza antigen comprises HA, NA, M2e-NP, or a combination thereof. The DNA plasmid comprises a promoter operably linked to a coding sequence that encodes the consensus influenza antigen. Additionally, an aspect of the present invention includes methods of eliciting an immune response against a plurality of influenza virus subtypes in a mammal using the DNA plasmid vaccines provided.
Owner:VGX PHARMA +1
Influenza Hemagglutinin And Neuraminidase Variants
InactiveUS20080057081A1Efficient productionExtended half-lifeSsRNA viruses negative-senseViral antigen ingredientsHemagglutininNeuraminidase
Polypeptides, polynucleotides, methods, compositions, and vaccines comprising (avian pandemic) influenza hemagglutinin and neuraminidase variants are provided.
Owner:MEDIMMUNE LLC
Influenza hemagglutinin and neuraminidase variants
InactiveUS20050287172A1Efficient productionSsRNA viruses negative-senseViral antigen ingredientsHemagglutininNeuraminidase
Polypeptides, polynucleotides, methods, compositions, and vaccines comprising (avian pandemic) influenza hemagglutinin and neuraminidase variants are provided.
Owner:MEDIMMUNE LLC
Method and kit for stably detecting sialic acid by enzyme method
The invention relates to a method and a kit for stably detecting sialic acid by an enzyme method. The method comprises the following steps of: generating N-acetylneuraminic acid from bound sialic acid under the catalysis of neuraminic acid aldolase; transforming the N-acetylneuraminic acid into N-acetylmannosamine under the catalysis of neuraminidase; reacting the product with mannosamine dehydrogenase and nicotinamide adenine dinucleotide (NAD) to generate reduced nicotinamide adenine dinucleotide (NADH); and measuring the sialic acid content by detecting the amount of the generated NADH. The invention also relates to the kit prepared according to the method. The method and the kit are conveniently and quickly used, the sialic acid is not required to be redissolved by dissolving liquid, is stable for a long time and has small inter-bottle variation, the method and the kit are suitable for various biochemical analyzers, secondary pollution is avoided, and a production process method is simplified.
Owner:BEIJING STRONG BIOTECH INC
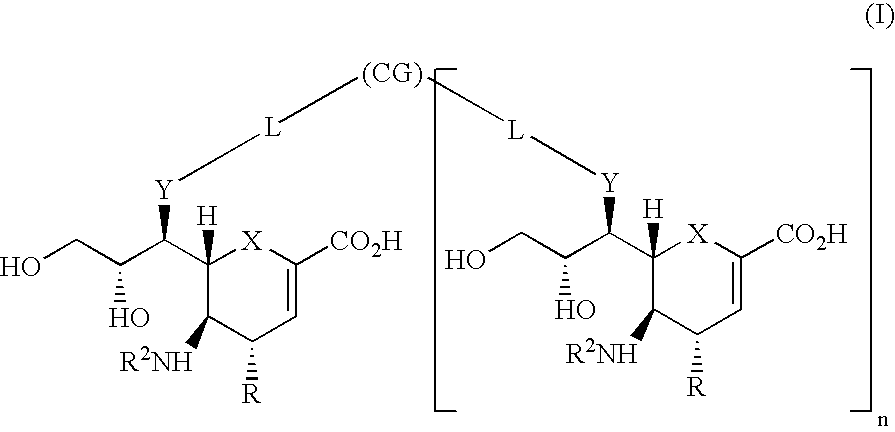
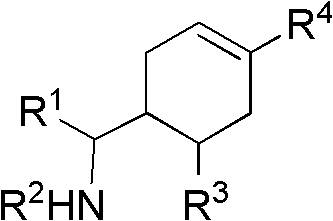
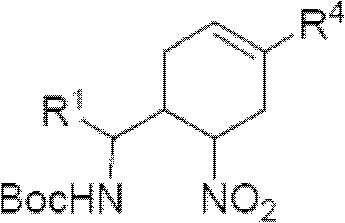
Cyclohexene compound having influenza virus neuraminidase inhibition activity, preparation method and application
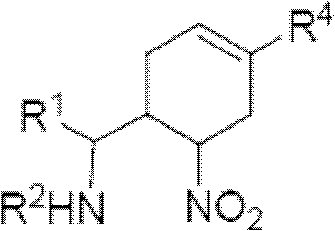
InactiveCN102964267AHas neuraminidase inhibitory activityOvercoming drug resistanceCarbamic acid derivatives preparationCarboxylic acid nitrile preparationCyclohexeneEnantiomer
The invention provides a compound expressed by a general formula I, or its pharmaceutically acceptable salt, and an analyzed enantiomer and a purified diastereomer, wherein R<1>is H or C1-12 alkyl groups; R<2> is H, -C(O)R<1a>, -C(O)OR<1a> or -S(O)2R<1b>, wherein R<1a> is H or C1-6 alkyl groups, R<1b> is selected from H or C1-6 alkyl groups halogenated hydrocarbon, phenyl or aryl group; R<3> is selected from C1-4 alkyl groups substituted amino, halogen, hydroxyl, sulfydryl, guanidyl, nitryl or cyano group; and R<4> is -(CH2)nCO2H, -(CH2)nP(O)(OH)2, -(CH2)nCO2R<1a> and -(CH2)nP(O)(OR<1a>)2, wherein R<1a> is H or C1-6 alkyl groups, n is an integer between 0 and 4, or a salt of the above groups. The invention also provides a preparation method of the cyclohexene compound expressed by the general formula I, and an application of the cyclohexene compound taken as an influenza virus neuraminidase inhibitor for preparing the medicines to prevent or treat the influenza diseases.
Owner:SUN YAT SEN UNIV
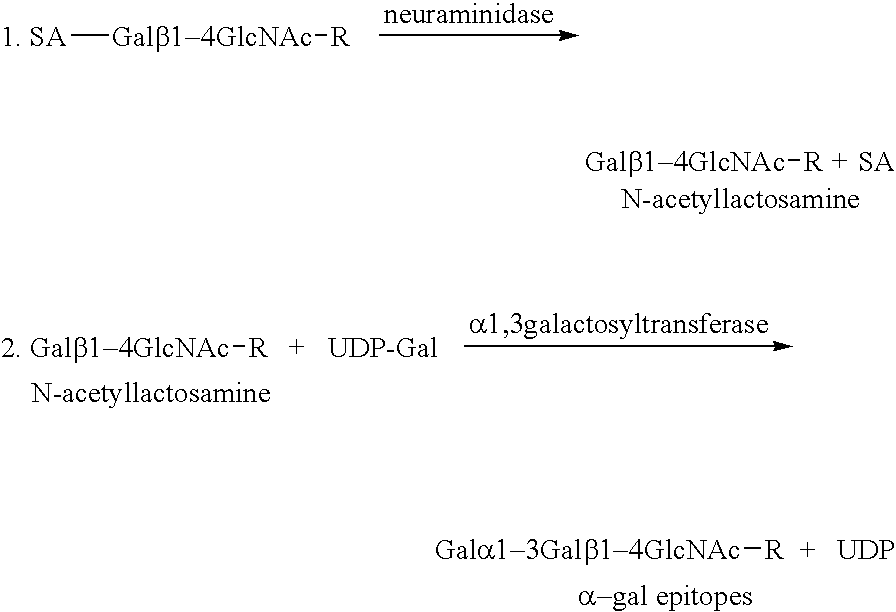
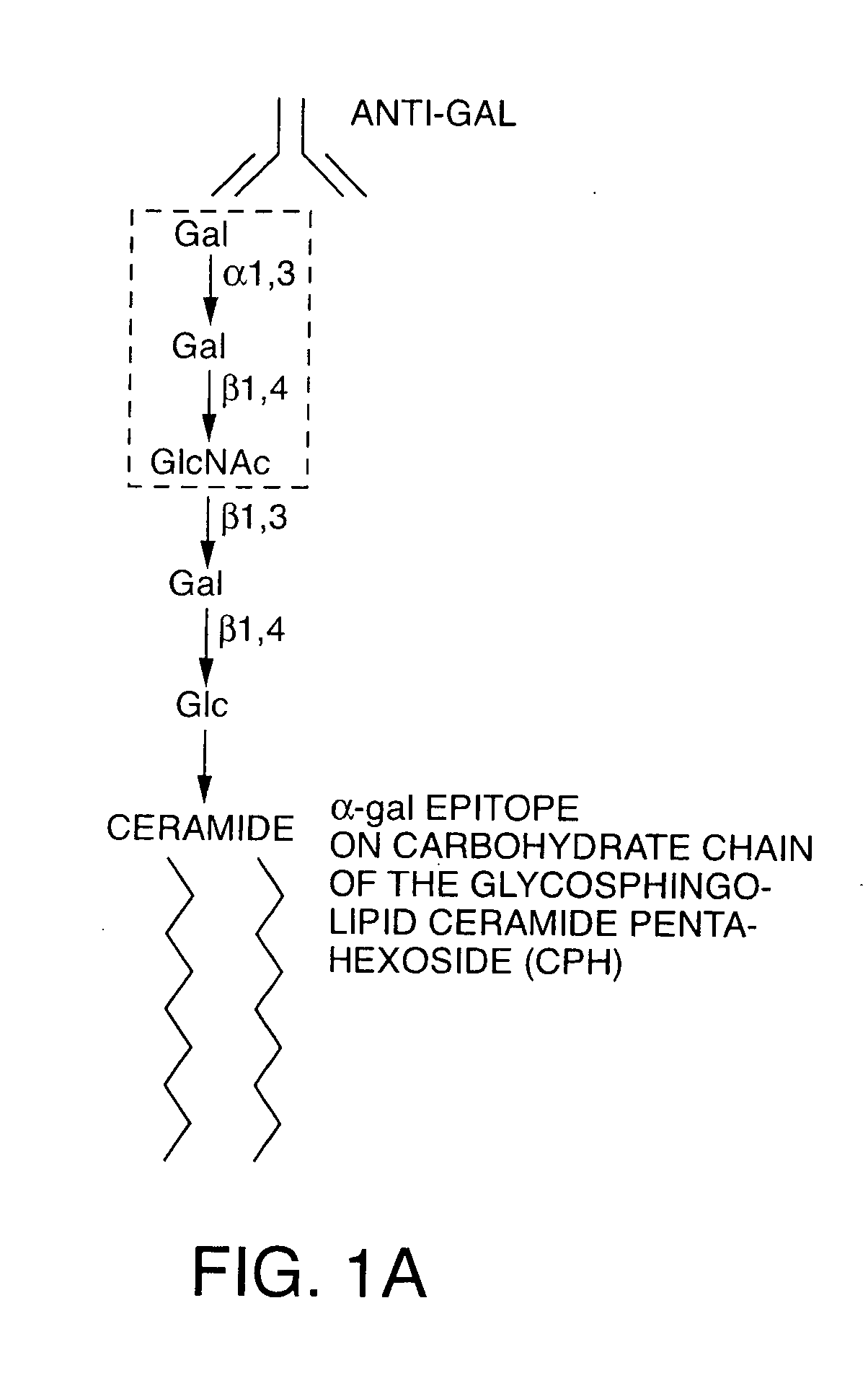
Tumor lesion regression and conversion in situ into autologous tumor vaccines by compositions that result in anti-Gal Antibody Binding
InactiveUS20060251661A1Reduce the overall heightBiocideSugar derivativesAntigenAbnormal tissue growth
The present invention discloses that an intratumoral injection of: i) glycolipids with α-gal epitope; ii) gene vectors comprising an α1,3galactosyltransferase gene; or iii) a mixture of α1,3galactosyltransferase, neuraminidase, and uridine diphosphate galactose results in tumor regression and / or destruction. Binding of the natural anti-Gal antibody to de novo expressed tumoral α-gal epitopes induces inflammation resulting in an anti-Gal antibody mediated opsonization of tumor cells and their uptake by antigen presenting cells. These antigen presenting cells migrate to draining lymph nodes and activate tumor specific T cells thereby converting the treated tumor lesions into in situ autologous tumor vaccines. This therapy can be applied to patients with multiple lesions and in neo-adjuvant therapy to patients before tumor resection. In addition to the regression and / or destruction of the treated tumor, such a vaccine will help in the immune mediated destruction of micrometastases that are not detectable during the removal of the treated tumor.
Owner:UNIV OF MASSACHUSETTS MEDICAL SCHOOL
Reagents and kits for detection of influenza virus and the like
ActiveUS7893272B2Simple and rapid and specific and sensitive detectionHigh detection sensitivityOrganic active ingredientsBiocideNeuraminidaseFluorescein
The present invention relates to reagents and methods for influenza virus detection. These reagents and methods disclosed in the present invention enable simple, rapid, specific and sensitive detection of influenza virus types A and B. These reagents are N-acetylneuraminic acid-firefly luciferin conjugates which can be cleaved by influenza virus neuraminidase.
Owner:CELLEX BIOLOGICAL TECH SUZHOU CO LTD
Quality control product of AV/BV combined test kit and preparation method thereof
ActiveCN102864208AEasy to prepareEasy to operateMicrobiological testing/measurementBULK ACTIVE INGREDIENTHydrogen peroxide
The invention provides a five-item quality control product of an AV / BV combined test kit and a preparation method thereof, belonging to quality control products for controlling the quality of kits used during female gynecological clinical detection. The quality control product comprises a vaginal discharge five-item positive quality control product and a vaginal discharge five-item negative quality control product, and the two types of quality control products are stored in the states of dry powder. The quality control product is used to carry out quality detection of a vaginitis test kit and can carry out quality control on five biochemical indexes including hydrogen peroxide, neuraminidase, leucocyte esterase, beta-glucuronidase and coagulase. By means of taking biological enzymes as active ingredients of the quality control product and preparing a dry powder quality control product by adopting a freezing-drying technology, the deactivation of a liquid quality control product in transportation and storage processes is avoided. The quality control product does not contain any human vaginal discharge, and does not cause infection to the environment and operators.
Owner:北京中生金域诊断技术股份有限公司
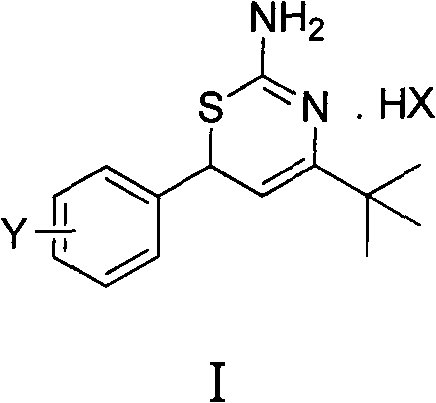
Preparation method and medical application of 4-tert-butyl-6-phenyl-2-amino-6H-1,3-thiazine salt
The invention discloses a preparation method and medical application of 4-tert-butyl-6-phenyl-2-amino-6H-1,3-thiazine salt. The preparation method of the 4-tert-butyl-6-phenyl-2-amino-6H-1,3-thiazine salt comprises the steps of: stirring 0.010 mol of 1-phenyl-4,4-dimethyl pentene-3-ketone, 0.012 mol of thiourea and 0.011 mol of acid in an organic solvent at a temperature of between 30 and 80 DEG C, performing TLC detection on reaction process, completing reaction, filtering, washing and drying the obtained product and obtaining the 4-tert-butyl-6-phenyl-2-amino-6H-1,3-thiazine salt (I). The 4-tert-butyl-6-phenyl-2-amino-6H-1,3-thiazine salt (I) can be medically used for preparing influenza virus neuraminidase inhibitors. In a formula, HX is hydrochloric acid, phosphoric acid, hydrobromic acid, sulphuric acid, trifluoroacetic acid or methanesulfonic acid, and Y is Cl, Br, CH3, Et, CF3, OCH3 or OEt.
Owner:HUNAN UNIV
Sialidase detection kit
InactiveCN102399851ASolve complexityFix stability issuesMicrobiological testing/measurementSialidasePreservative
The invention provides a sialidase detection kit. The kit comprises a reagent 1 and a reagent 2, wherein the reagent 1 comprises the components of buffer solution, neuraminidase, lactic dehydrogenase, surfactant, stabilizer and preservative; and the reagent 2 comprises the components of buffer solution, reducing coenzyme, neuraminic acid aldolase, stabilizer and preservative. The kit has accurate detection and low cost and is convenient, applicable, stable and reliable.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
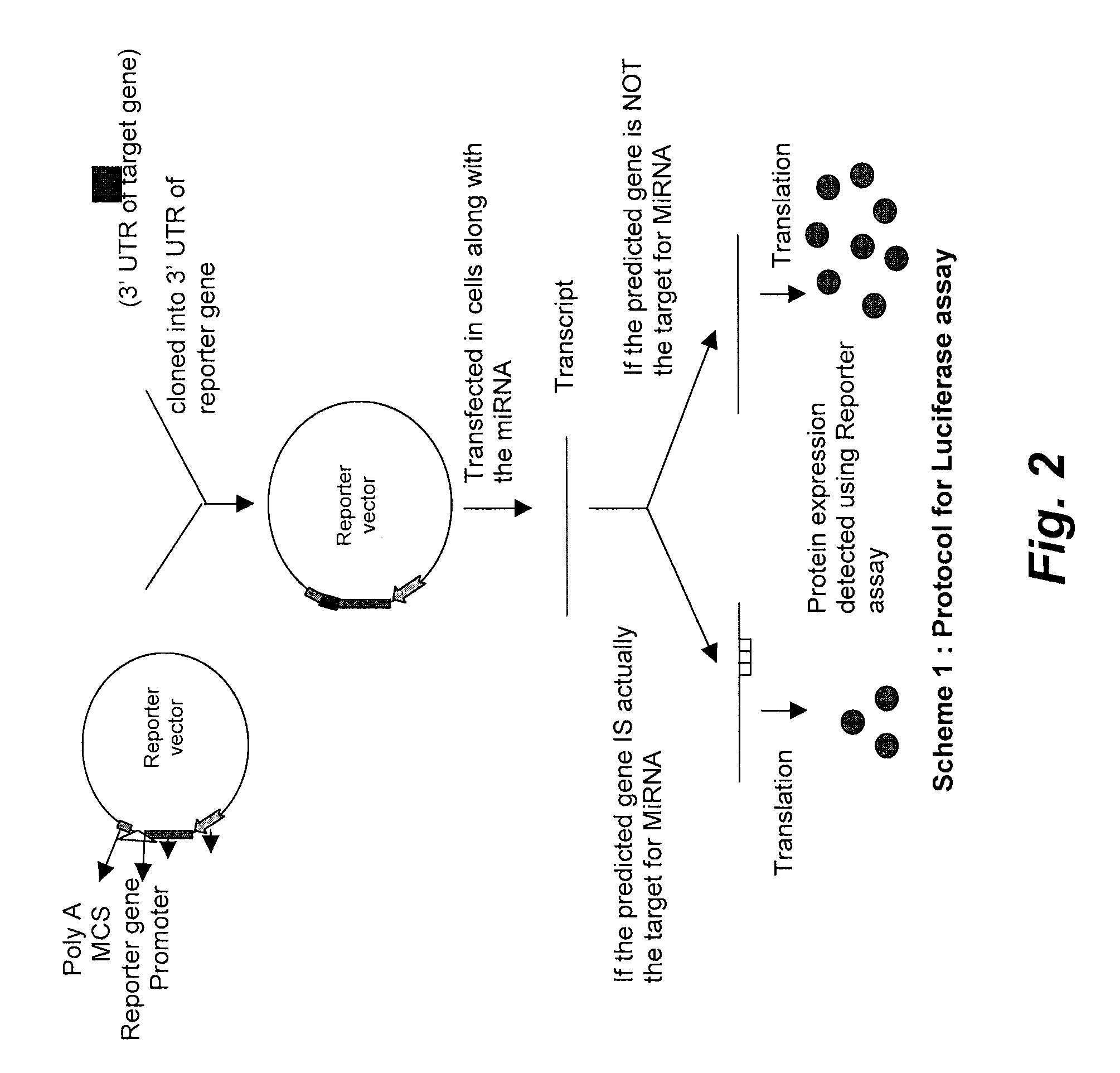
Methods and kits for the diagnosis of influenza
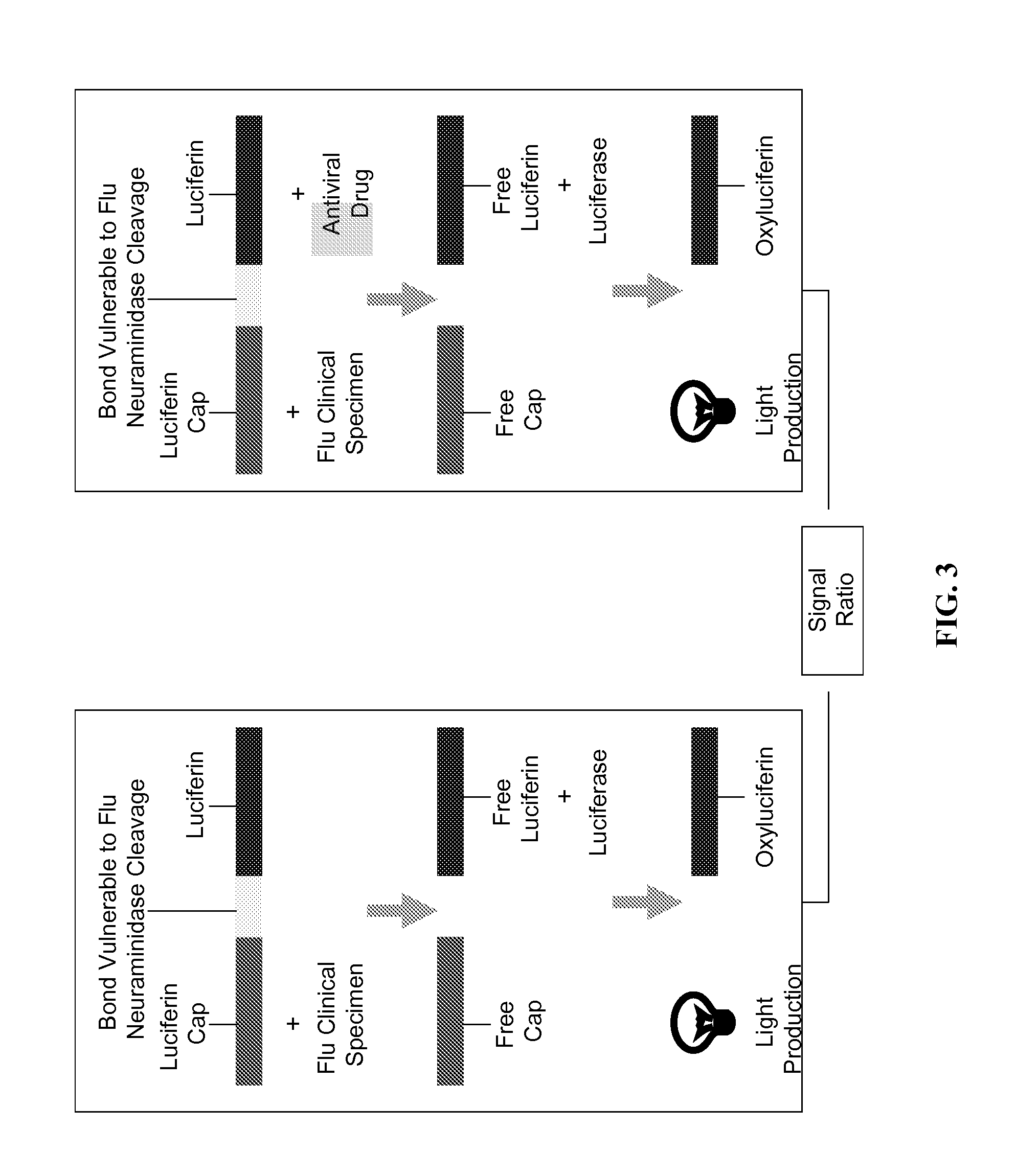
ActiveUS20160041167A1Inhibits neuraminidase activityBioreactor/fermenter combinationsCompound screeningNeuraminidaseAntiviral drug
Some embodiments provided herein relate to combined assays. In some embodiments, an assay for identifying influenza type A or influenza type B is combined with an assay for determining the sensitivity of an influenza neuraminidase to an antiviral drug.
Owner:BECTON DICKINSON & CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com