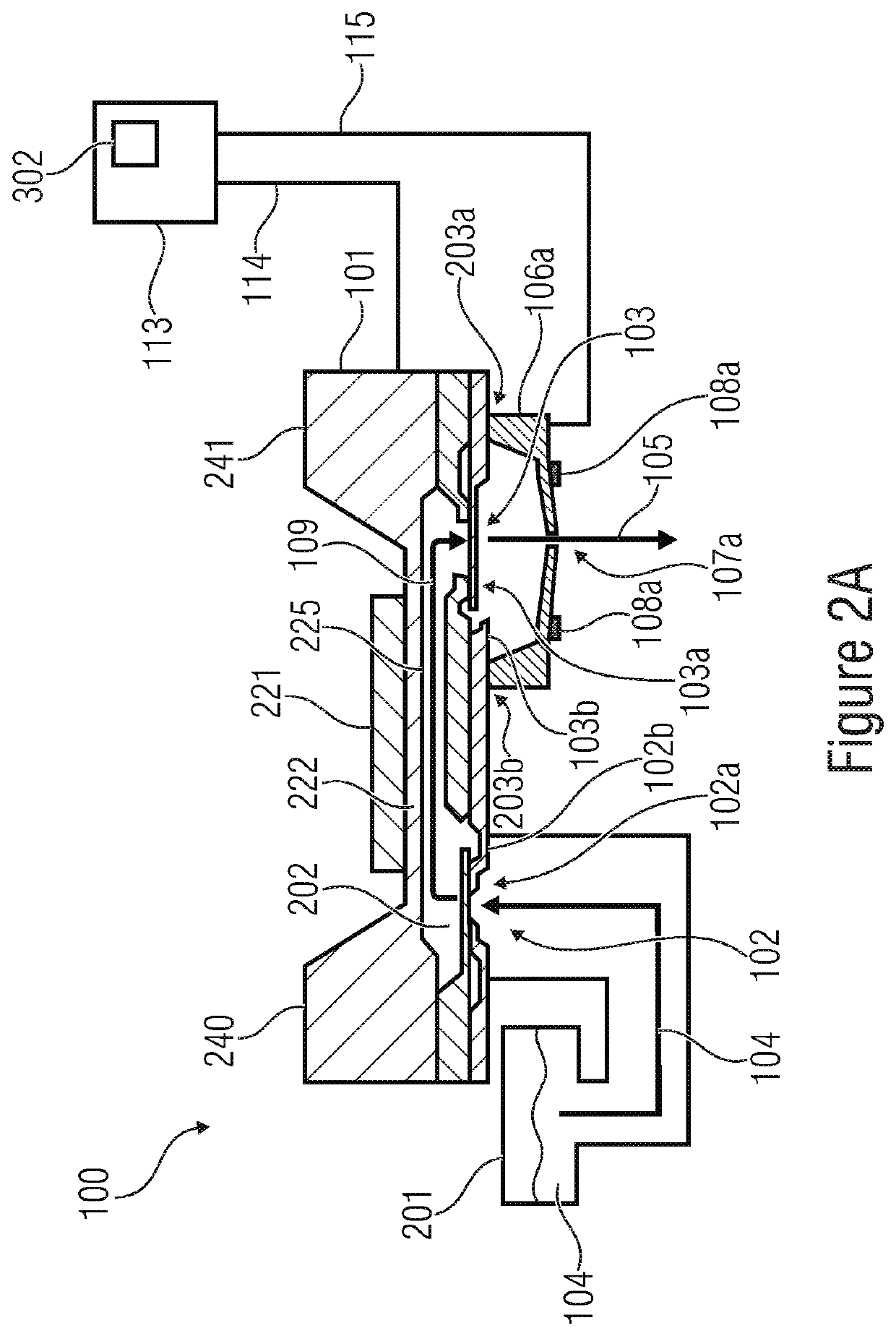
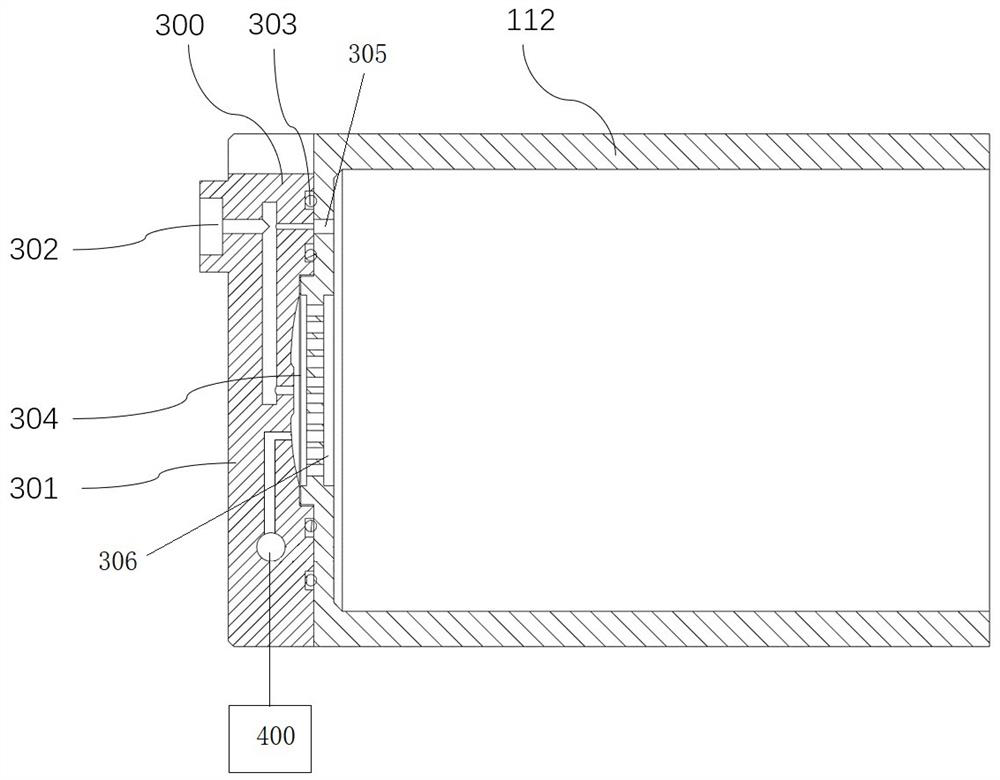
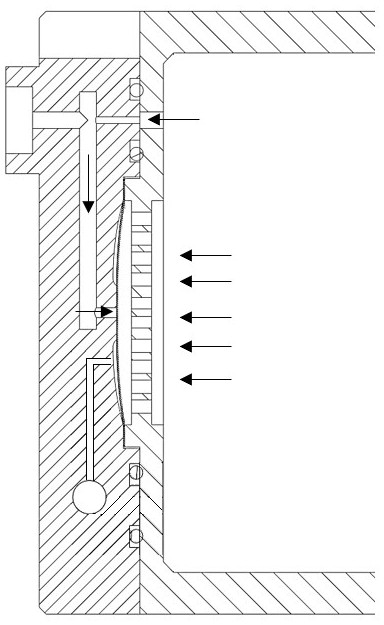
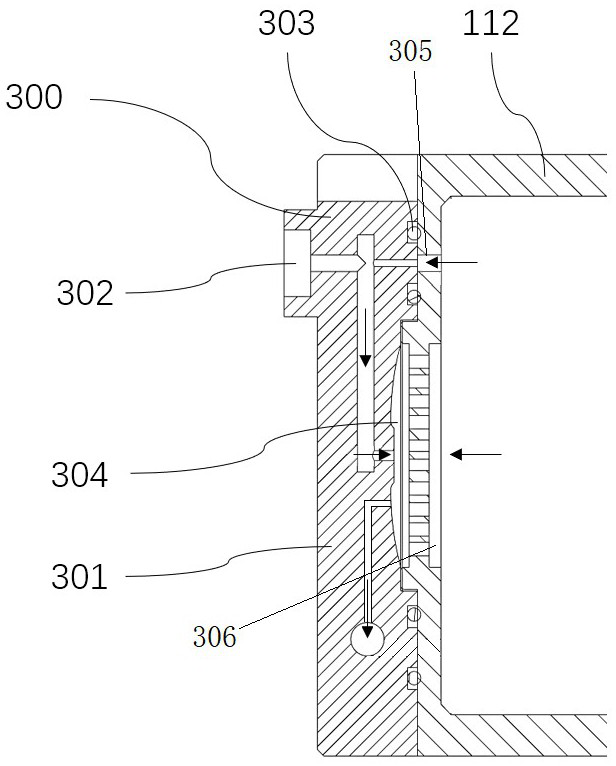
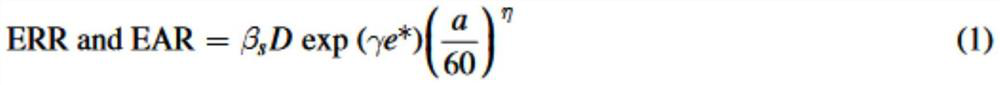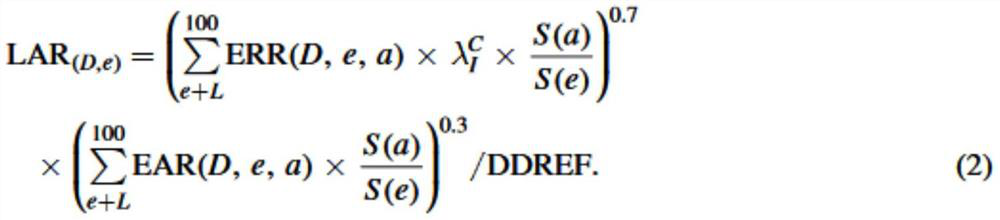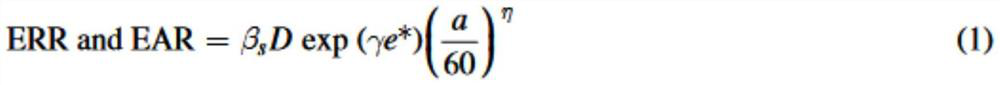Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34 results about "Microdosing" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
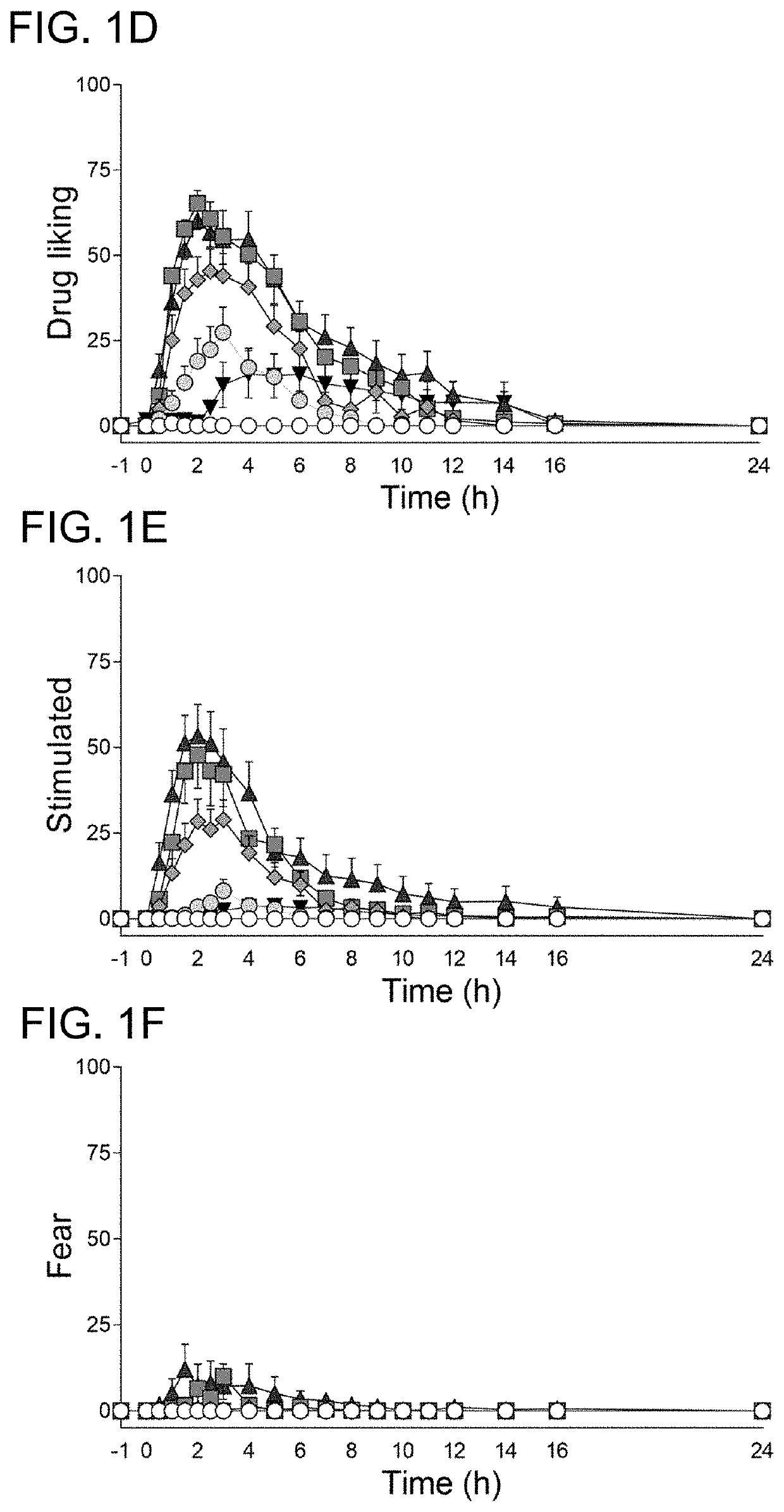
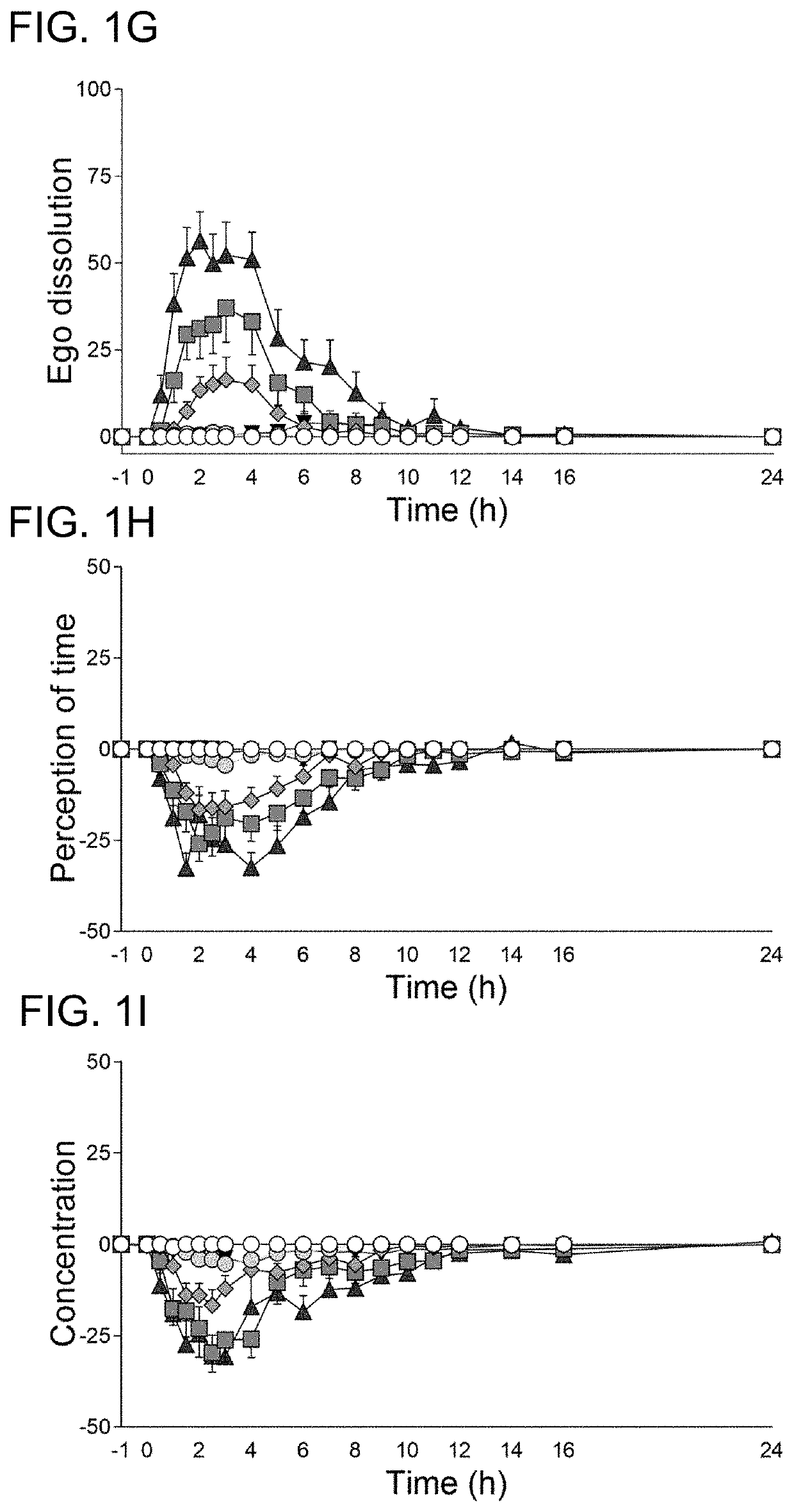
Microdosing, or micro-dosing, is a technique for studying the behaviour of drugs in humans through the administration of doses so low ("sub-therapeutic") they are unlikely to produce whole-body effects, but high enough to allow the cellular response to be studied. This is called a "Phase 0 study" and is usually conducted before clinical Phase I to predict whether a drug is viable for the next phase of testing. Human microdosing aims to reduce the resources spent on non-viable drugs and the amount of testing done on animals.
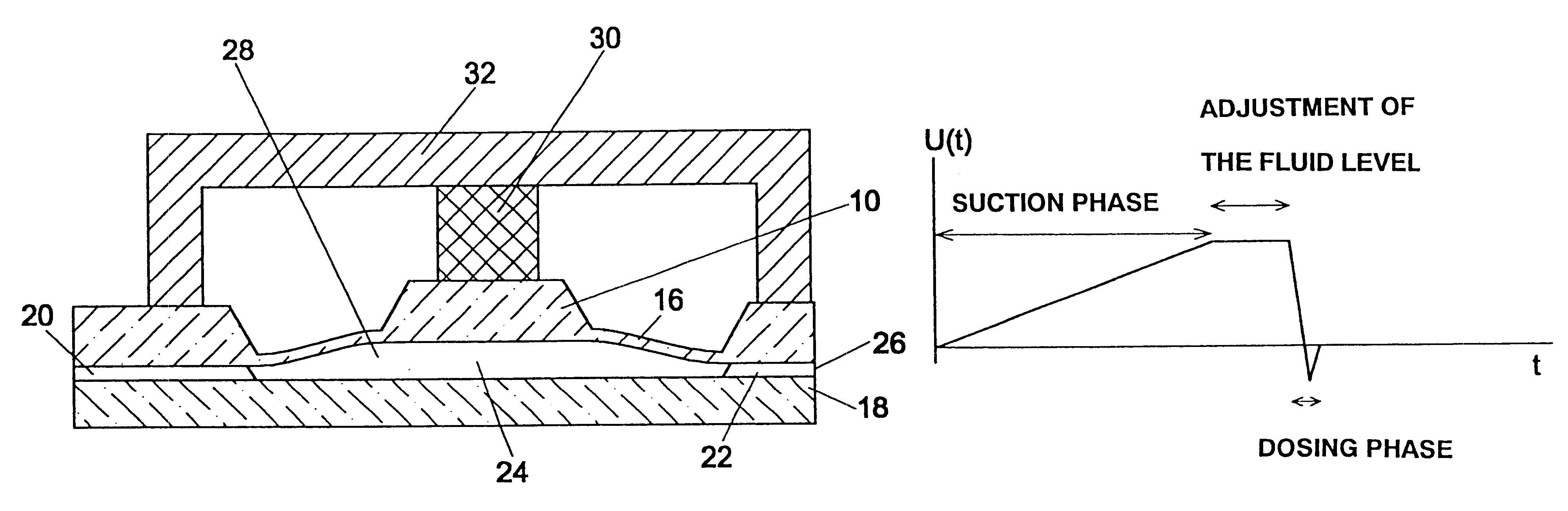
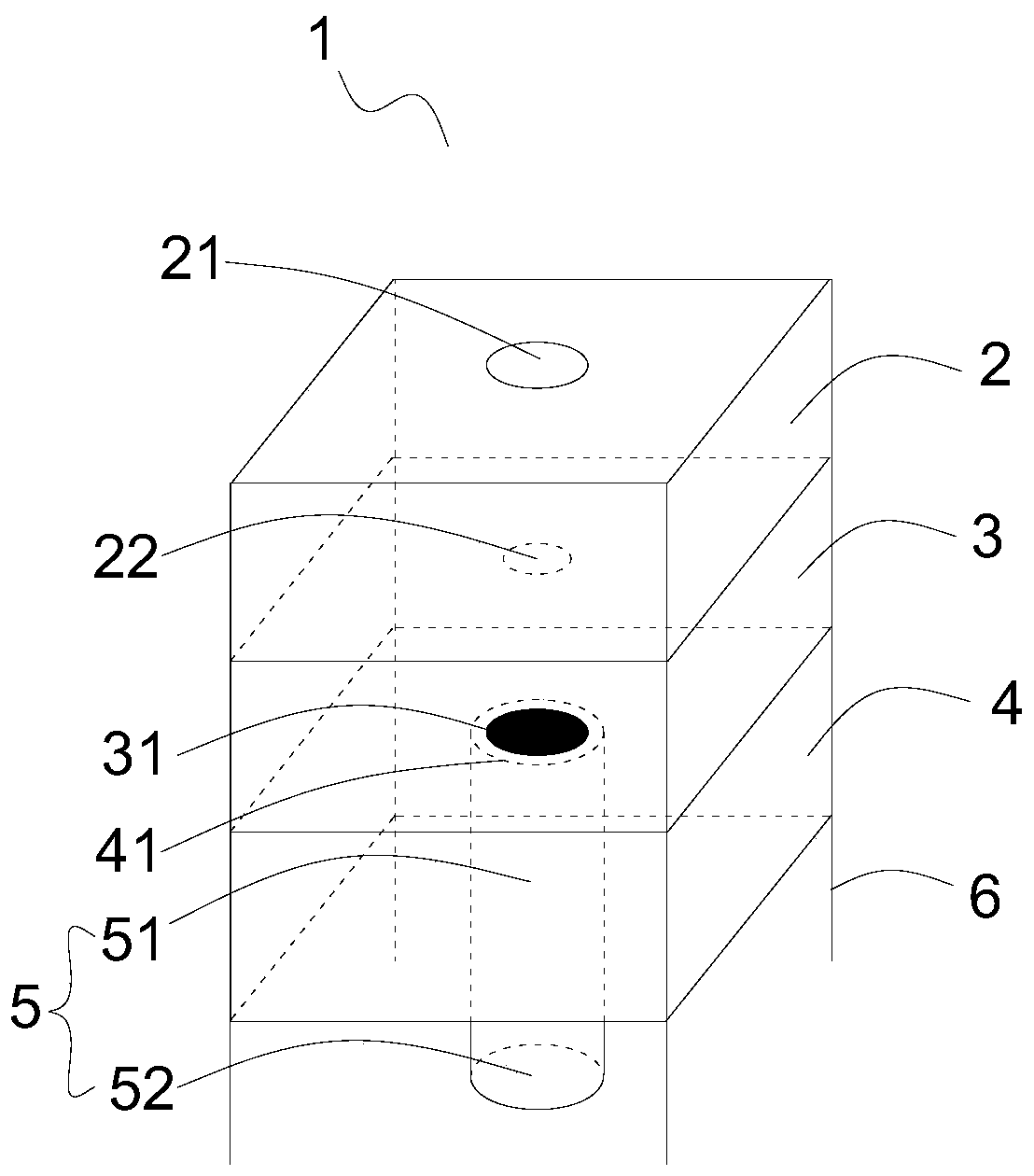
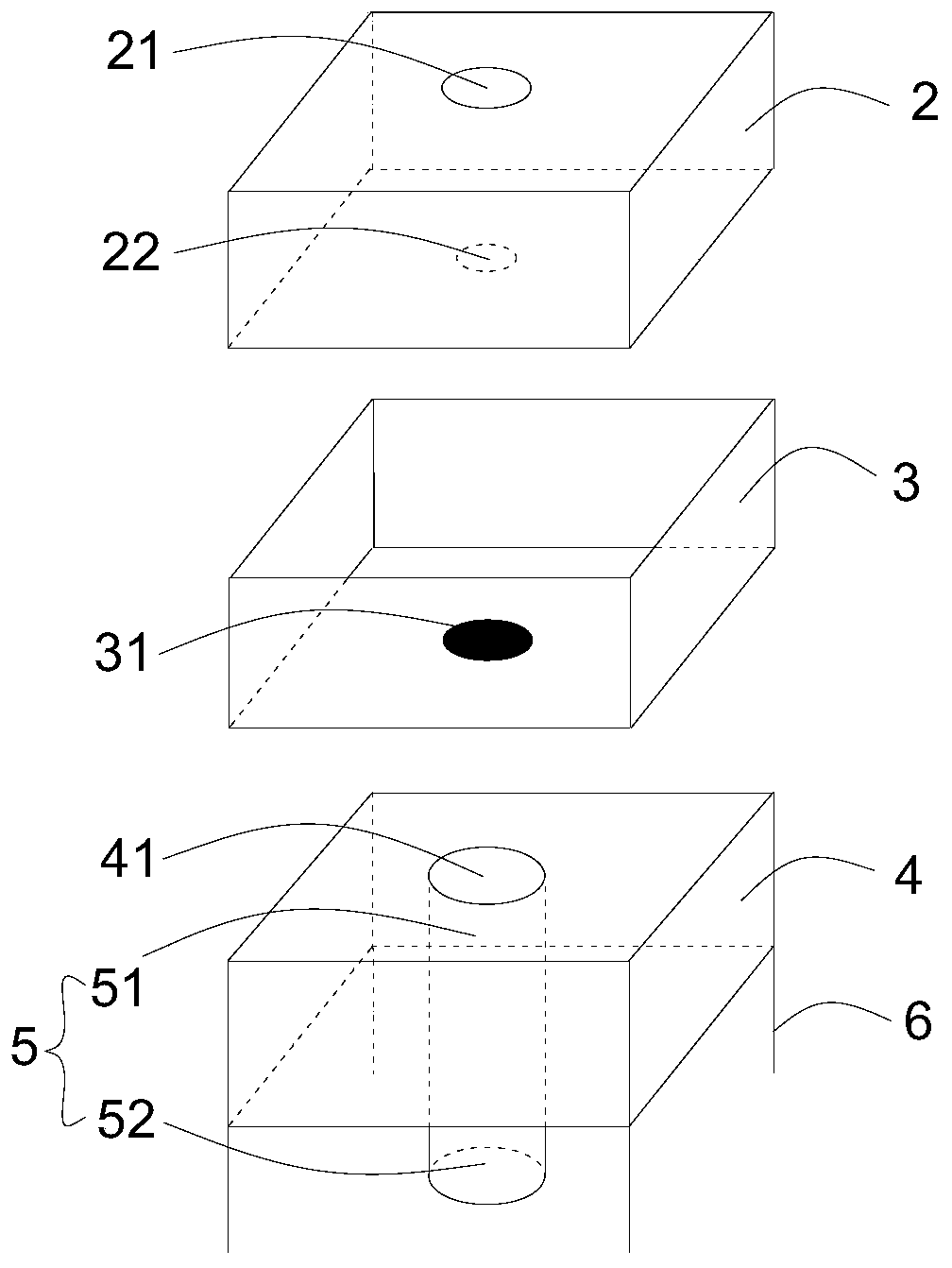
Microdosing device
InactiveUS6416294B1Easy dischargeContracting/expanding measuring chambersChemical microanalysisEngineeringMechanical engineering
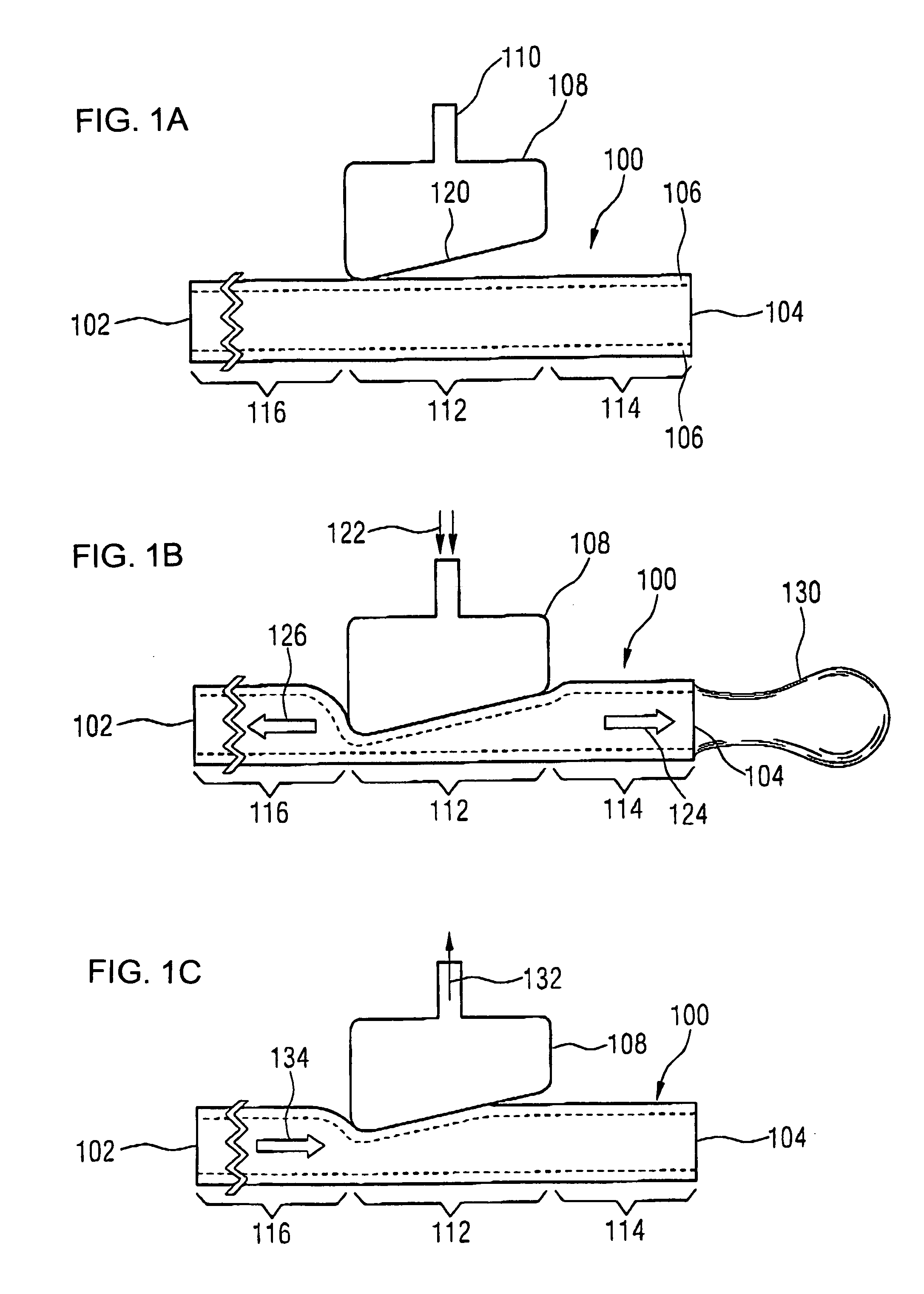
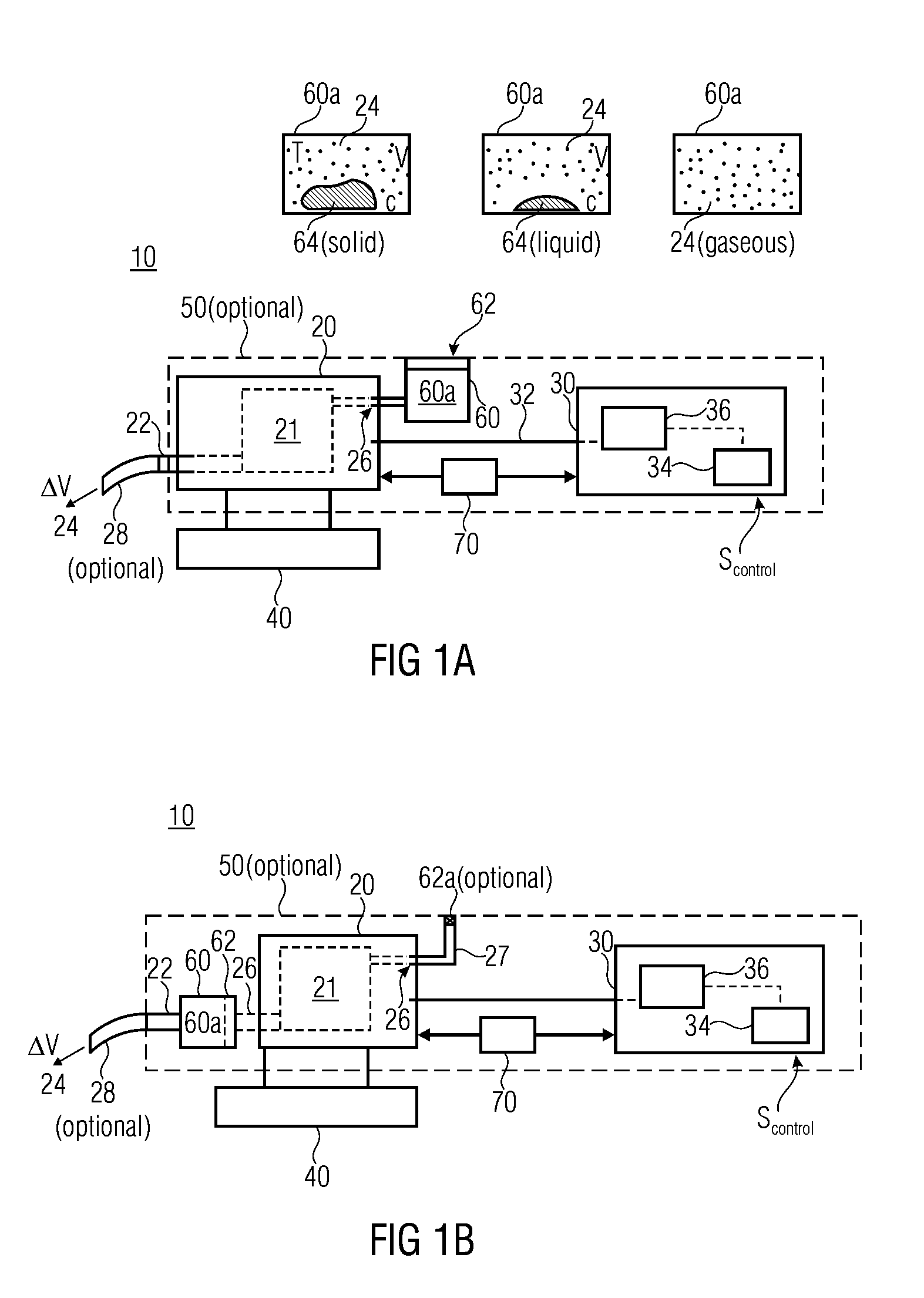
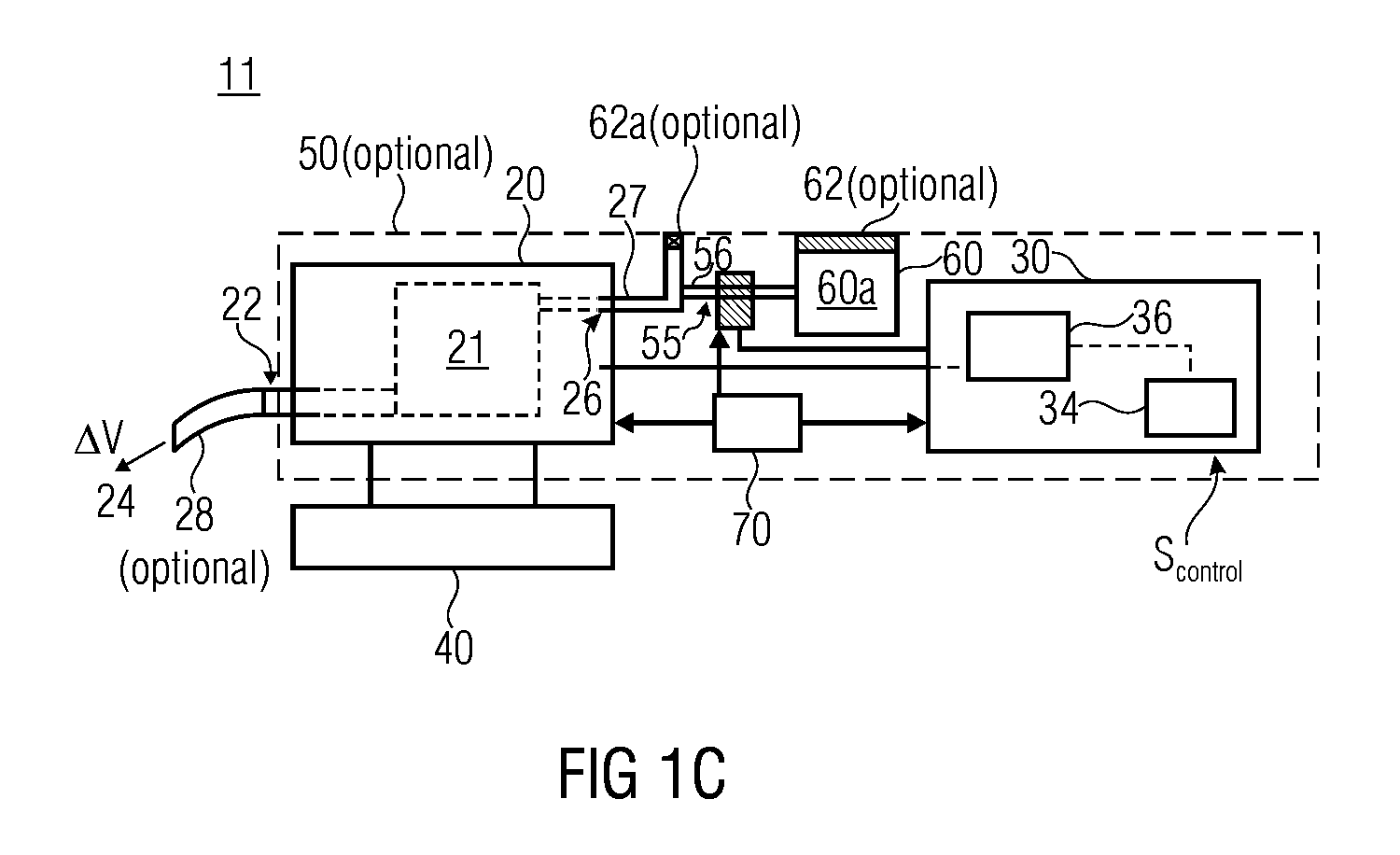
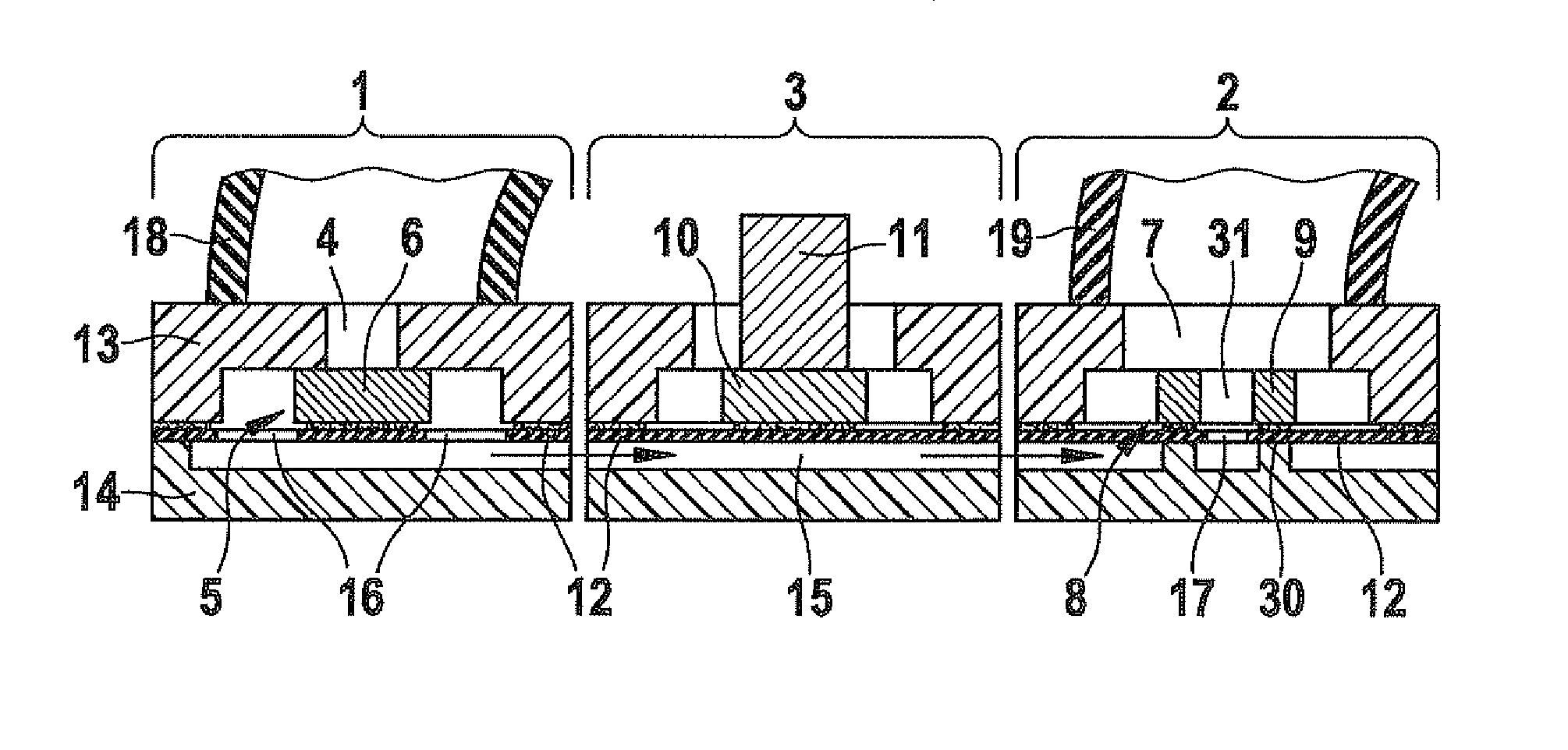
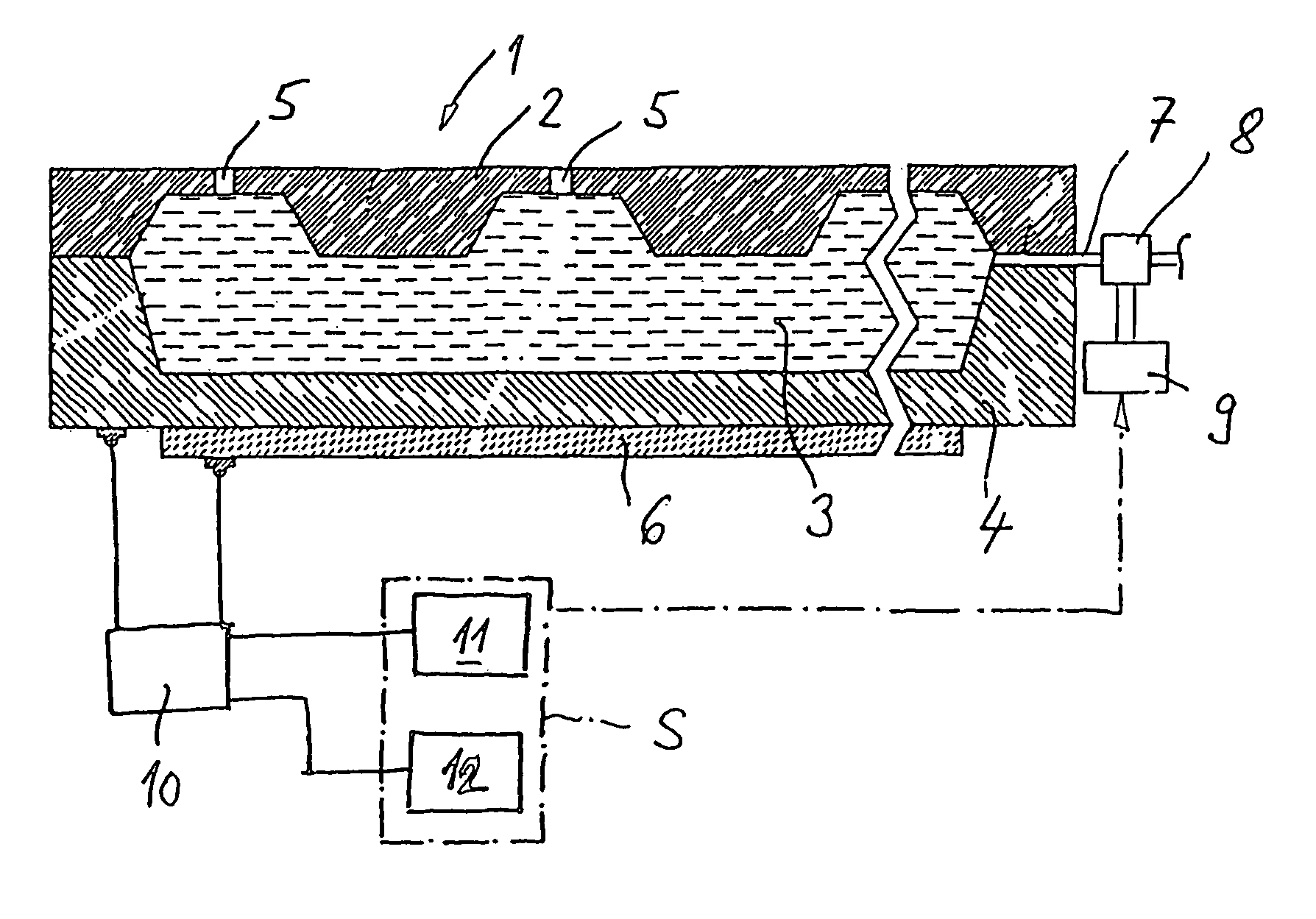
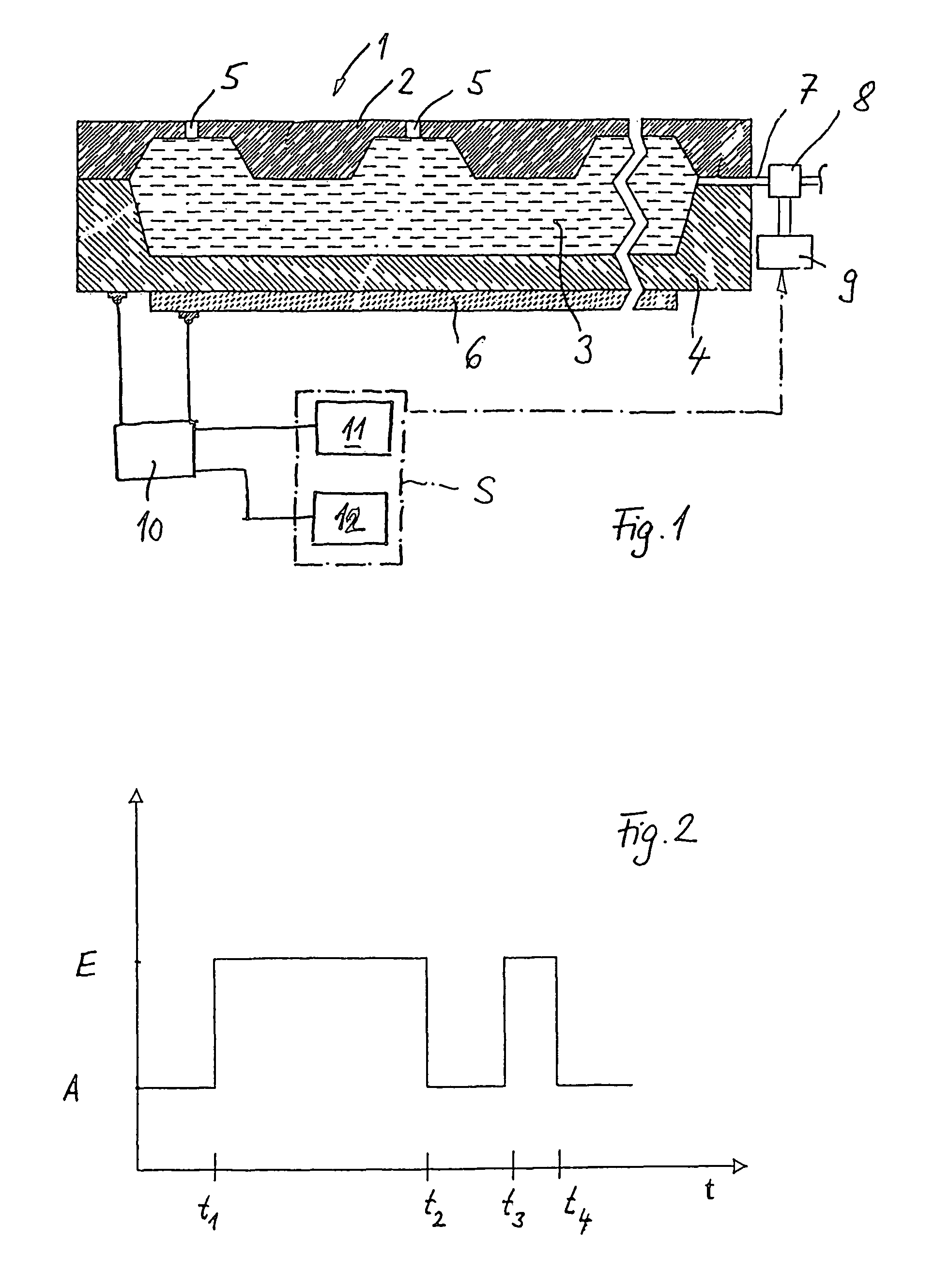
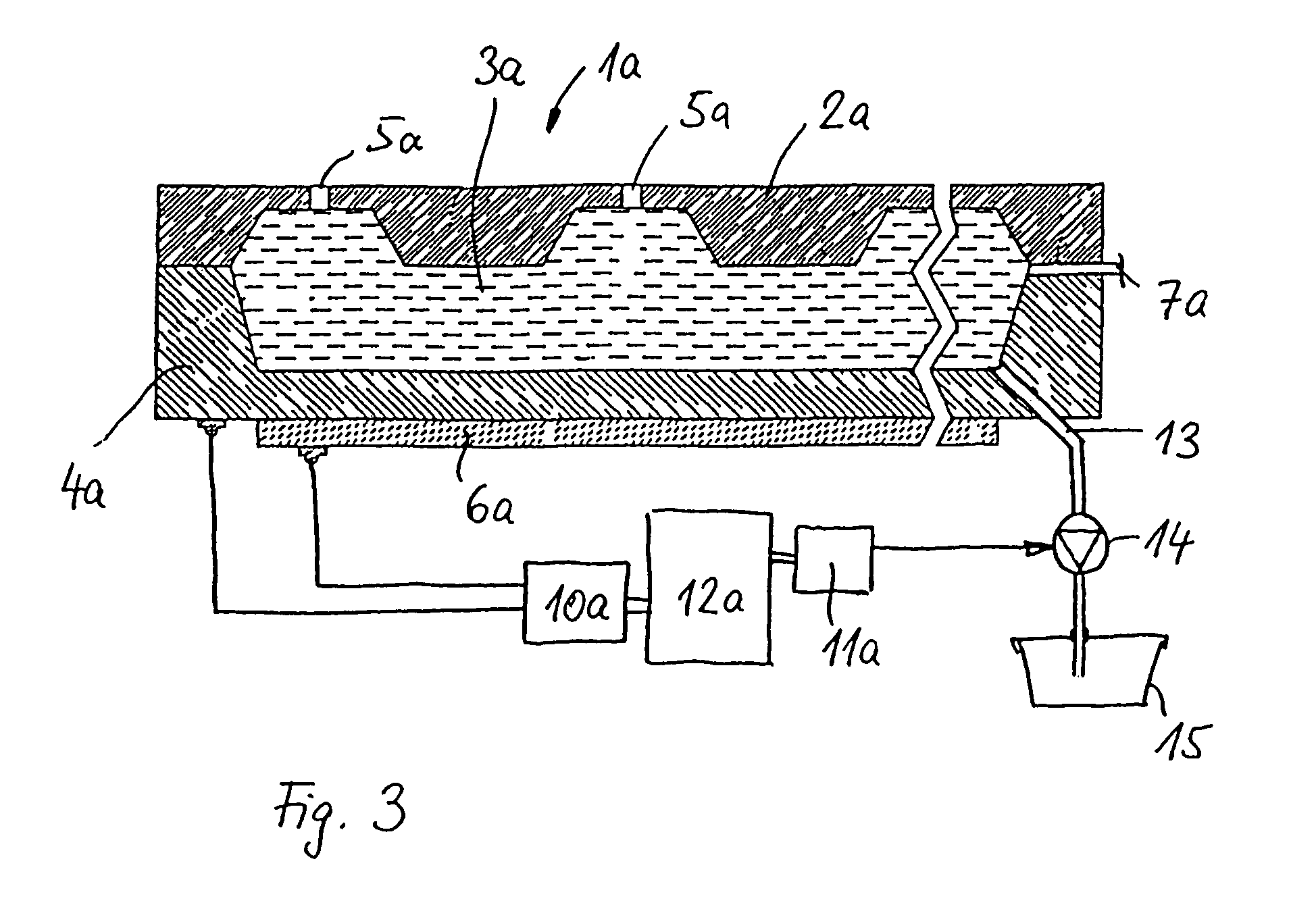
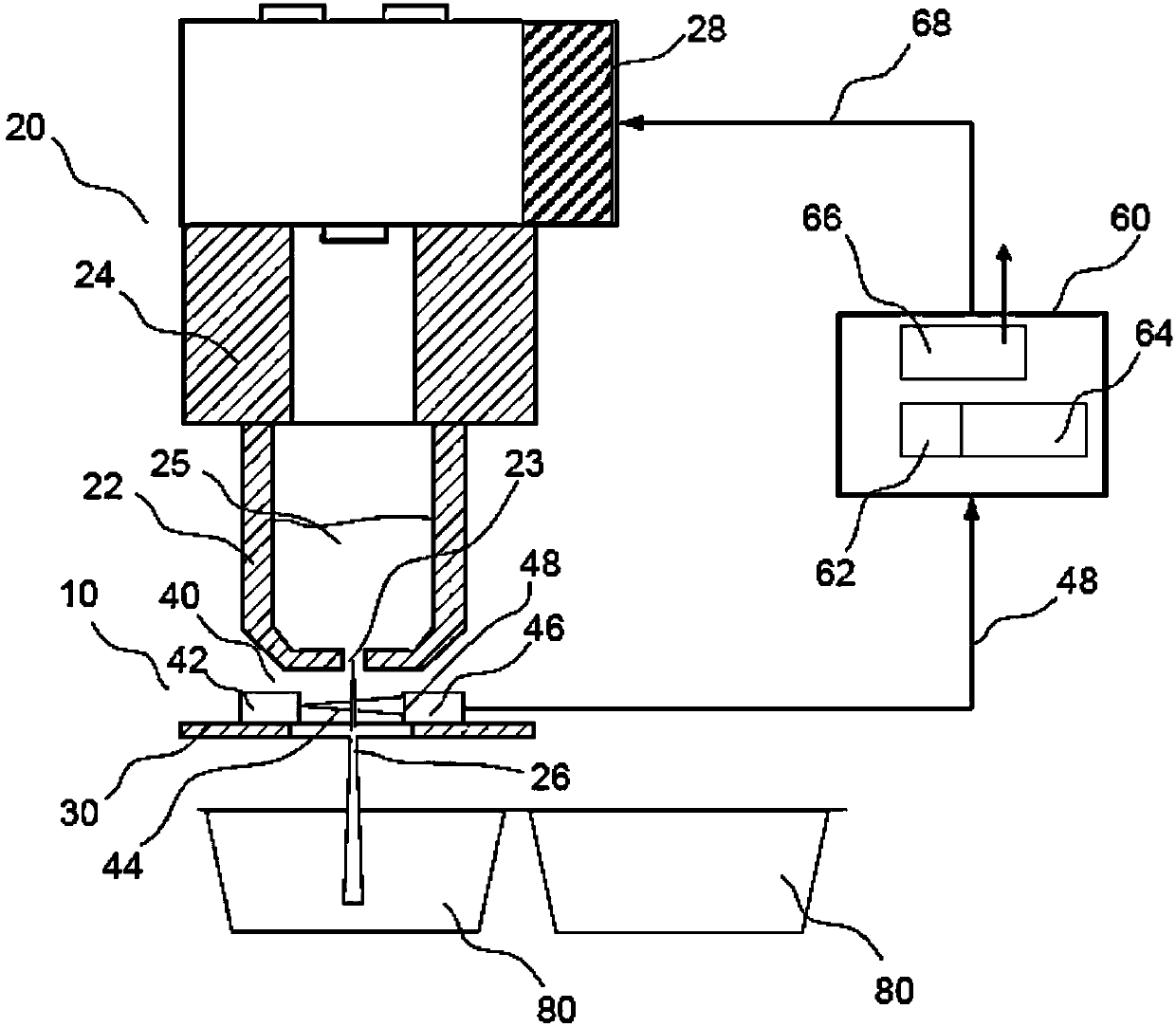
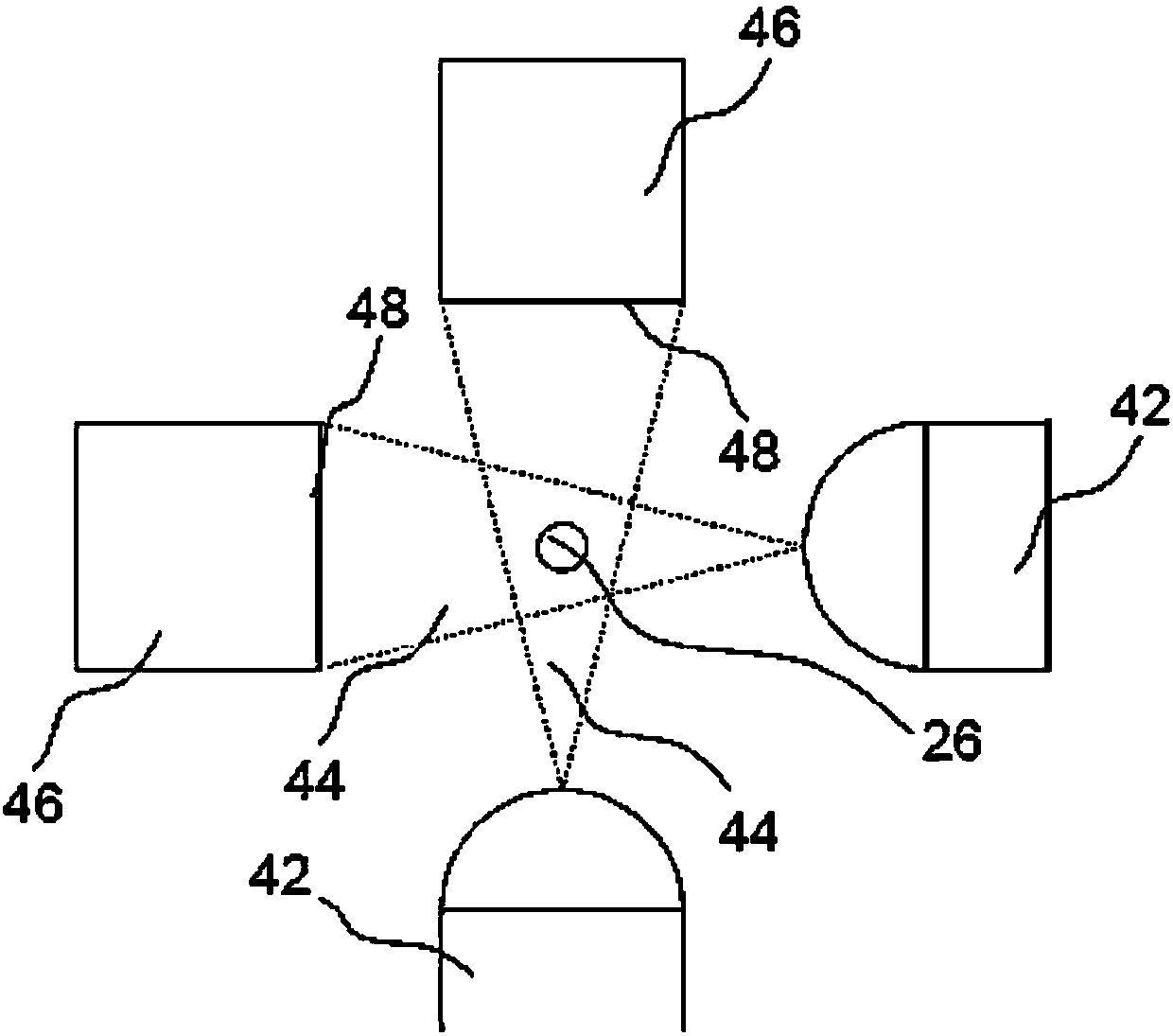
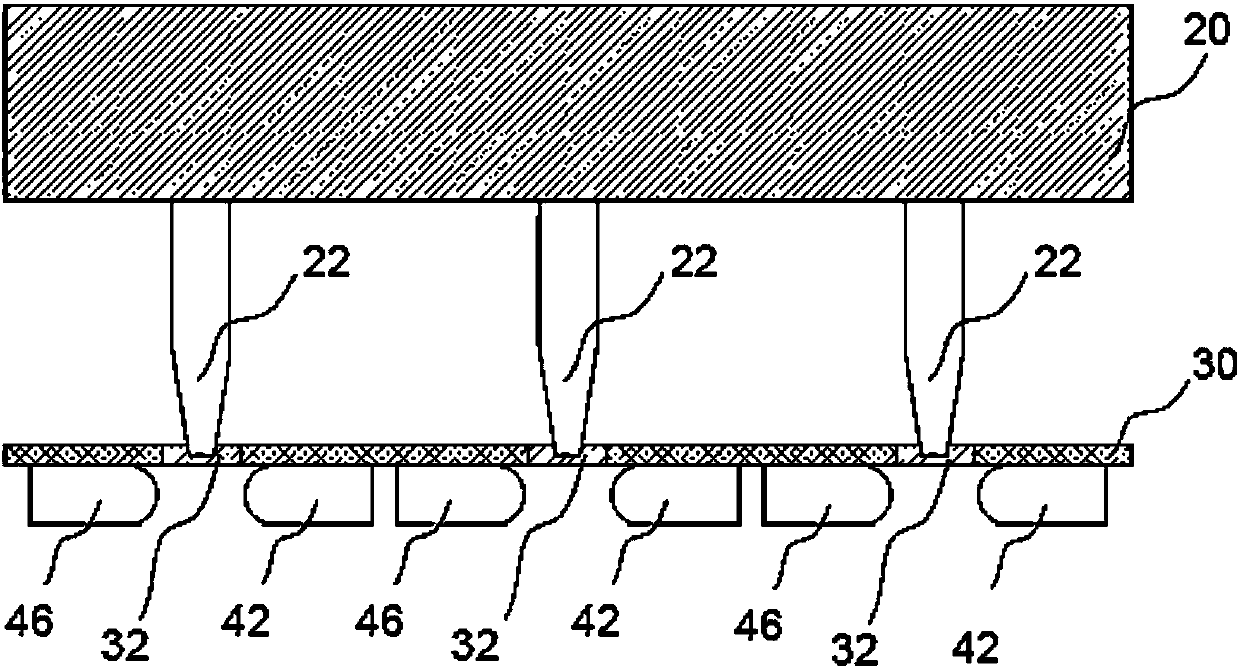
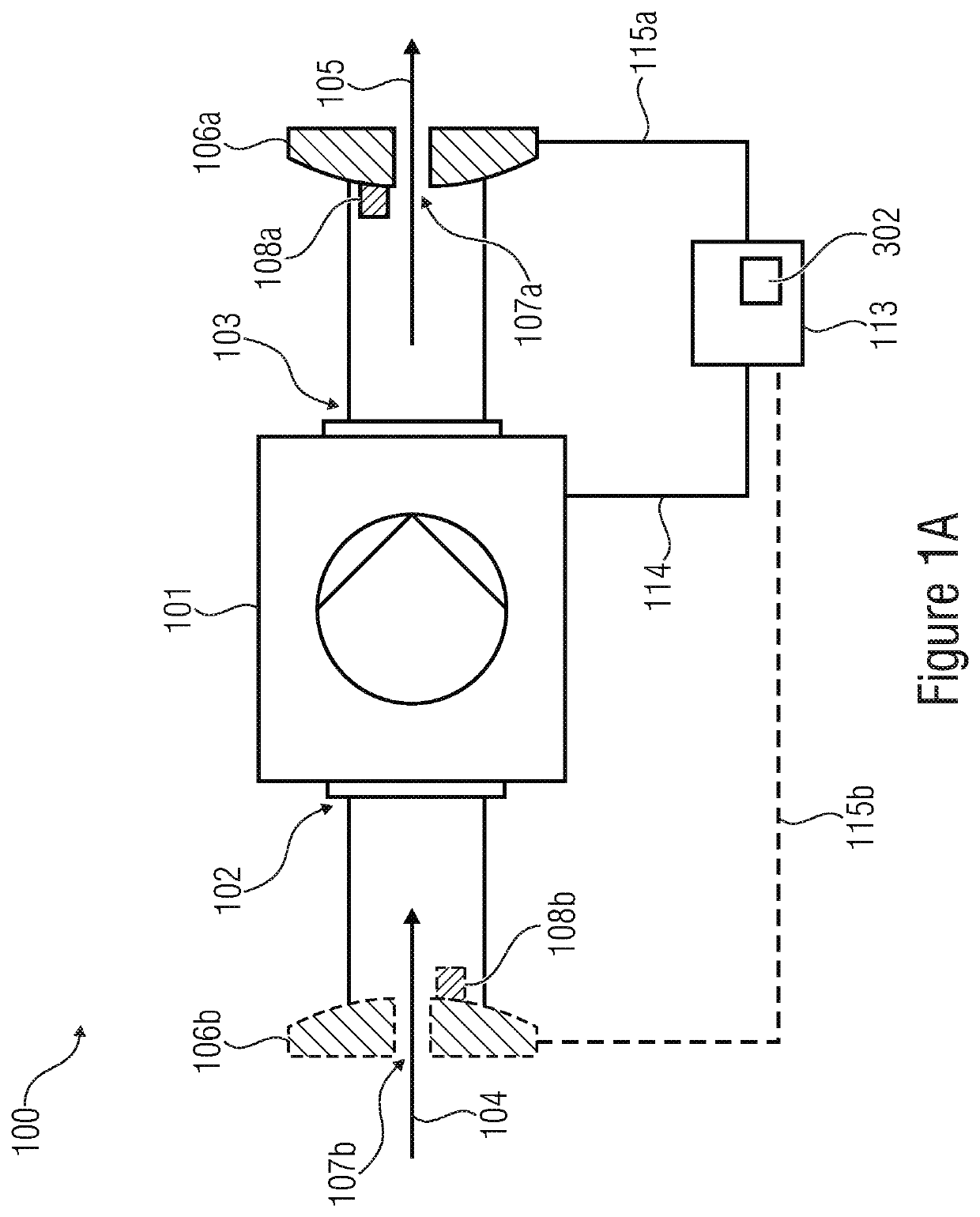
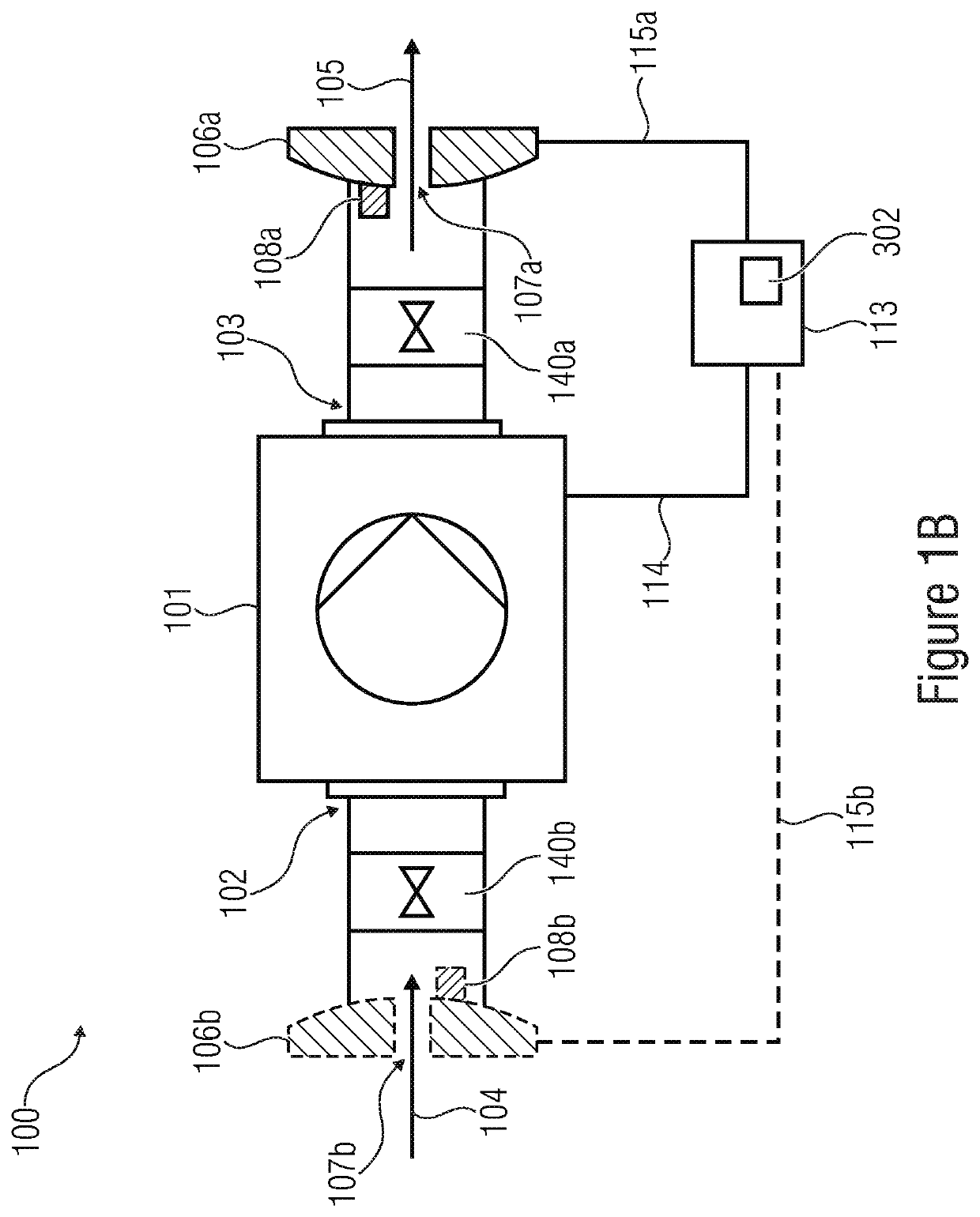
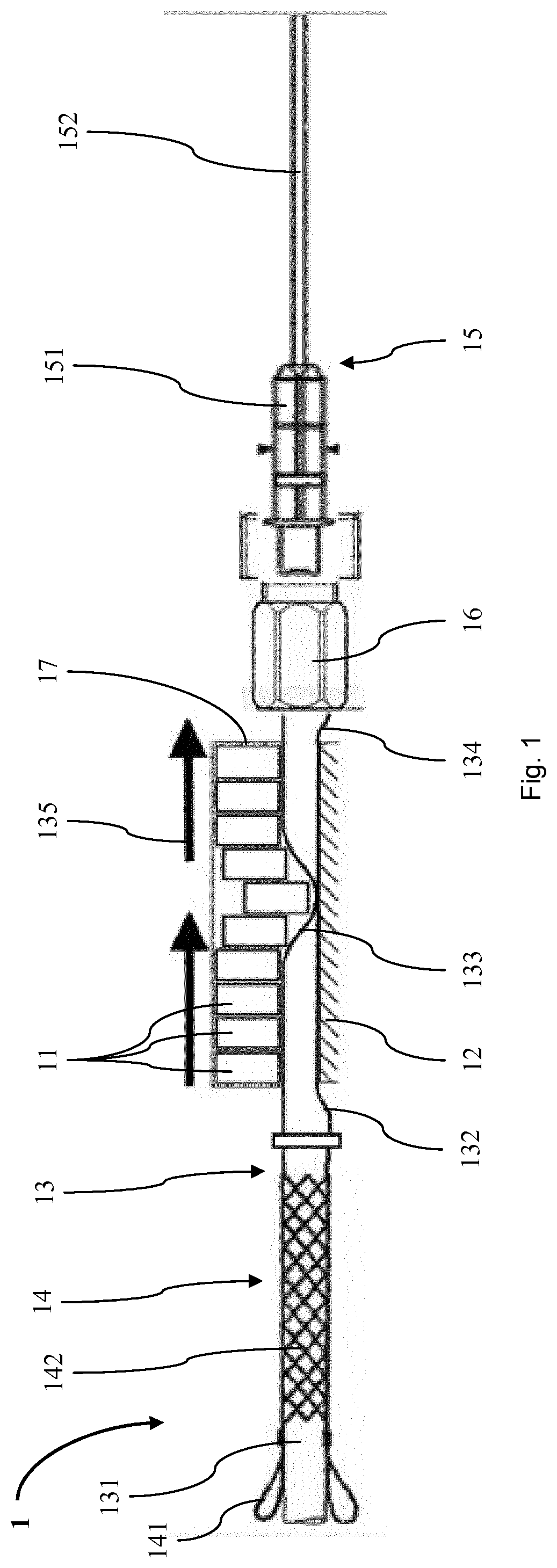
A volume sensor-free microdosing device comprises a pressure chamber which is at least partly delimited by a displacer, an actuating device for actuating the displacer, the volume of the pressure chamber being adapted to the changed by actuating the displacer, a media reservoir which is in fluid communication with the pressure chamber, an outlet opening which is in fluid communication with the pressure chamber, and a control mechanism. The control mechanism drives the microdosing device in such a way that a small change of volume of the pressure chamber volume is effected per unit time by a movement of the displacer from a first position to a predetermined second position, the second position of the displacer defining a larger volume of the pressure chamber than the first position, so as to suck a fluid volume into the pressure chamber, and that, in a second phase, a large volume change of the pressure chamber volume is effected per unit time by a movement of the displacer from the second position to the first position, so as to eject a defined fluid volume from the outlet opening in this way.
Owner:ZYRUS BET GMBH & CO PATENTE I KG +1
Microdosing apparatus and method for dosed dispensing of liquids
ActiveUS20060147313A1Simple structureEasy to changeSpraying apparatusFlexible member pumpsEngineeringElectrical impedance
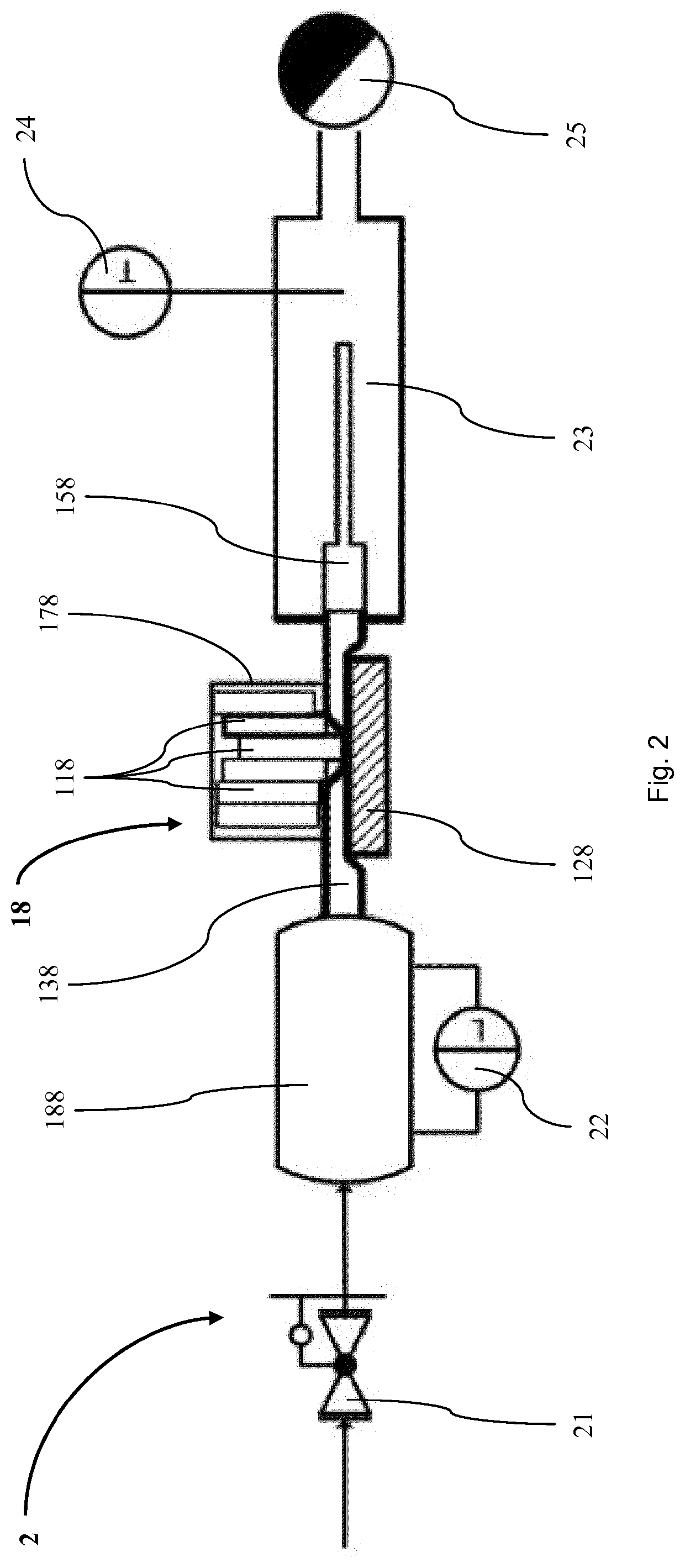
A microdosing apparatus comprises a fluid conduit having a flexible tube with a first end for connecting to a fluid reservoir and a second end where an outlet opening is located. An actuating device with a displacer with adjustable hub is provided, by which the volume of a portion of the flexible hub can be changed to thereby dispense liquid as free flying droplets or as free flying jet at the outlet opening by moving the displacer between a first end position and a second end position, whereby the tube is partly compressed in the first or the second end position. In other words, a microdosing apparatus comprises fluid conduit having a portion along which a cross section of the fluid conduit is variable by an actuating device to effect a change of the volume of the fluid conduit. A ratio of a fluidic impedance between the position of the actuating device and the outlet opening to a fluidic impedance between a first end of the fluid conduit and the position of the actuating device is variable by changing the position of the actuating device, so that a dosing volume dispensed at the outlet opening is variable thereby by at least 10%.
Owner:BIOFLUIDIX
Device for detection of a change of pressure in a canal of a microdosing device
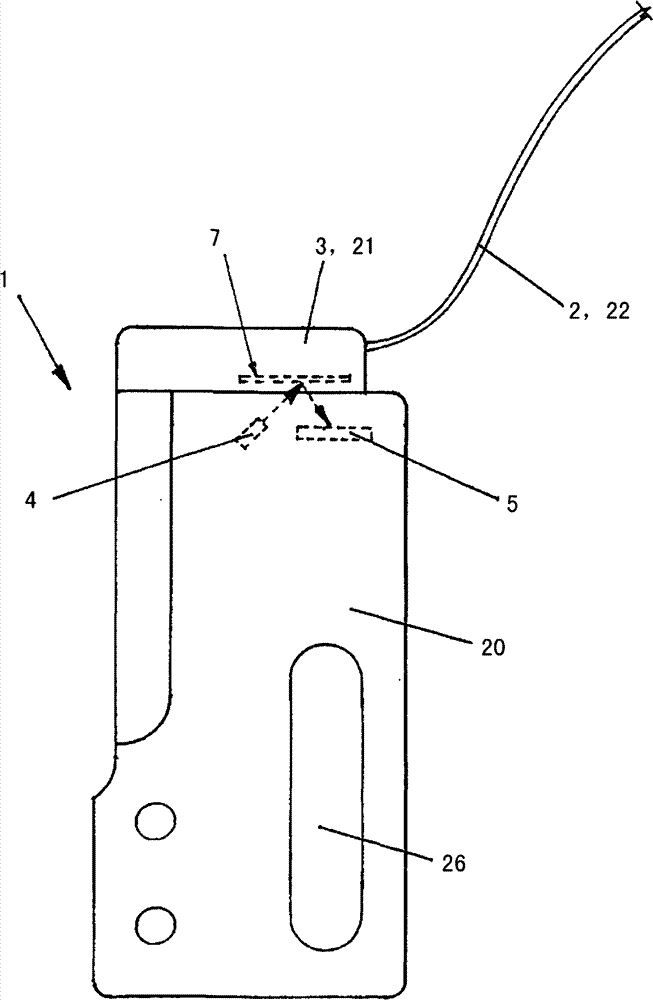
InactiveCN101371127AReduce inaccuracyHigh measurement accuracyFluid pressure measurement by electric/magnetic elementsMedical devicesMedicineInfusion set
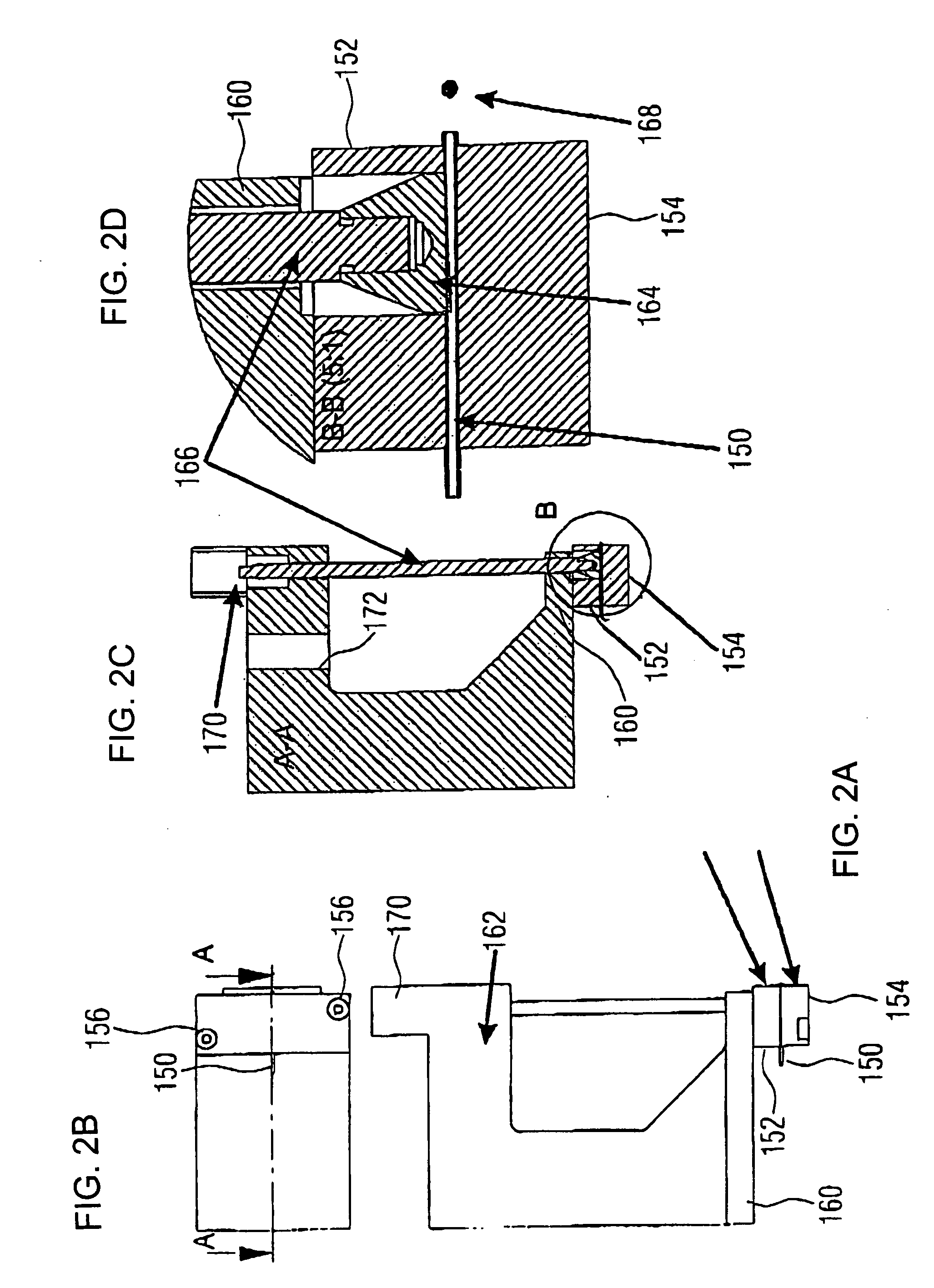
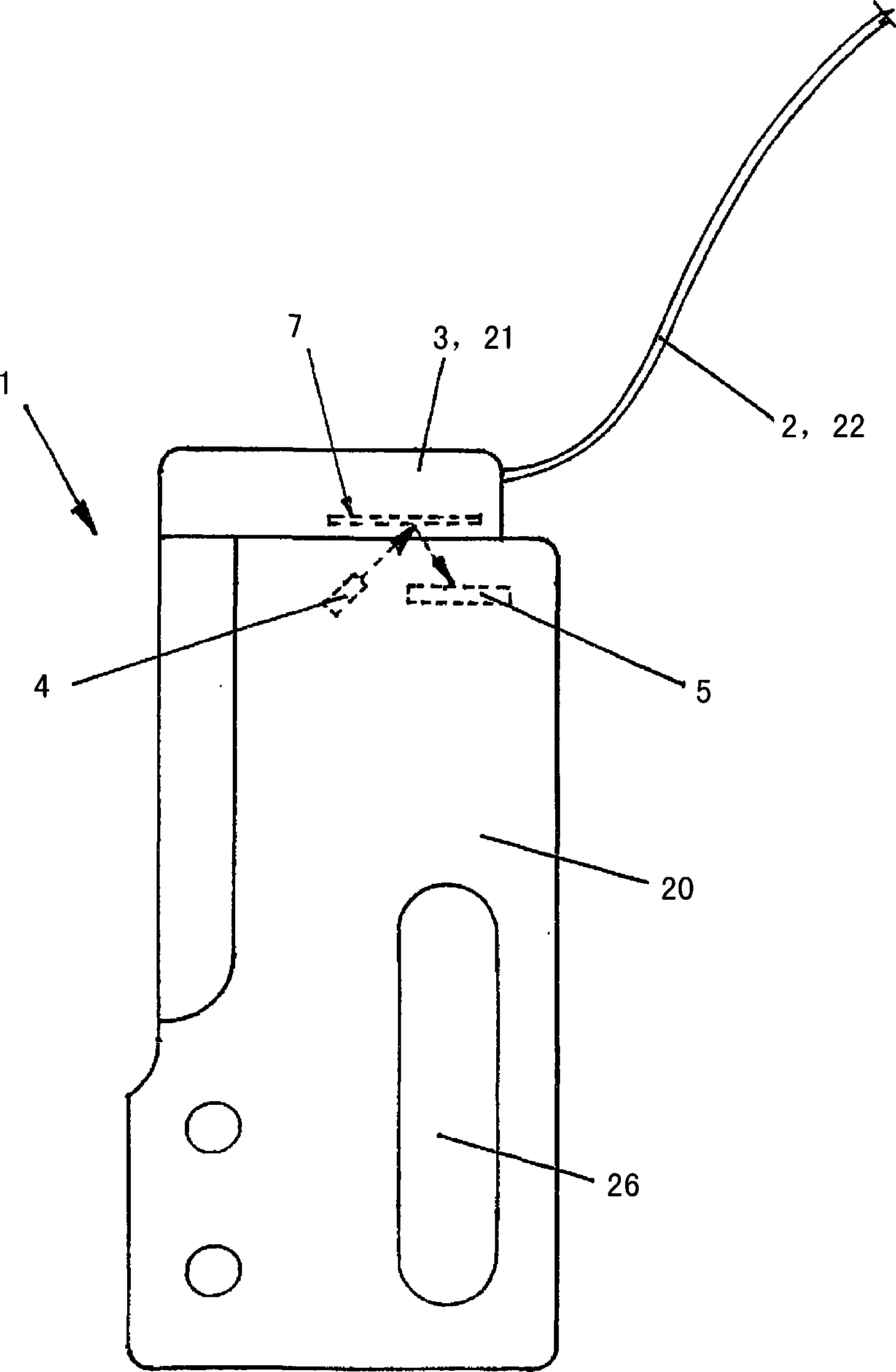
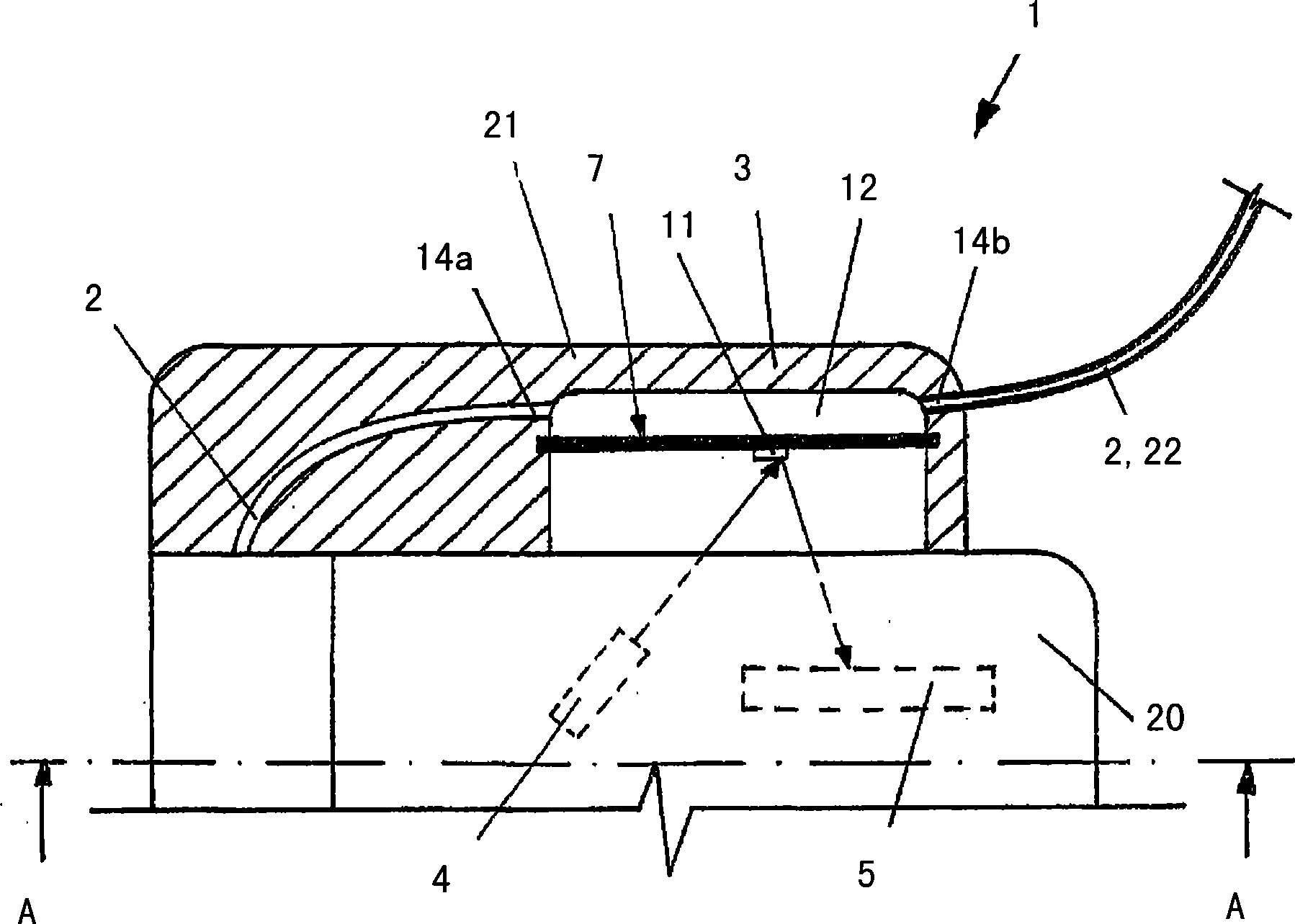
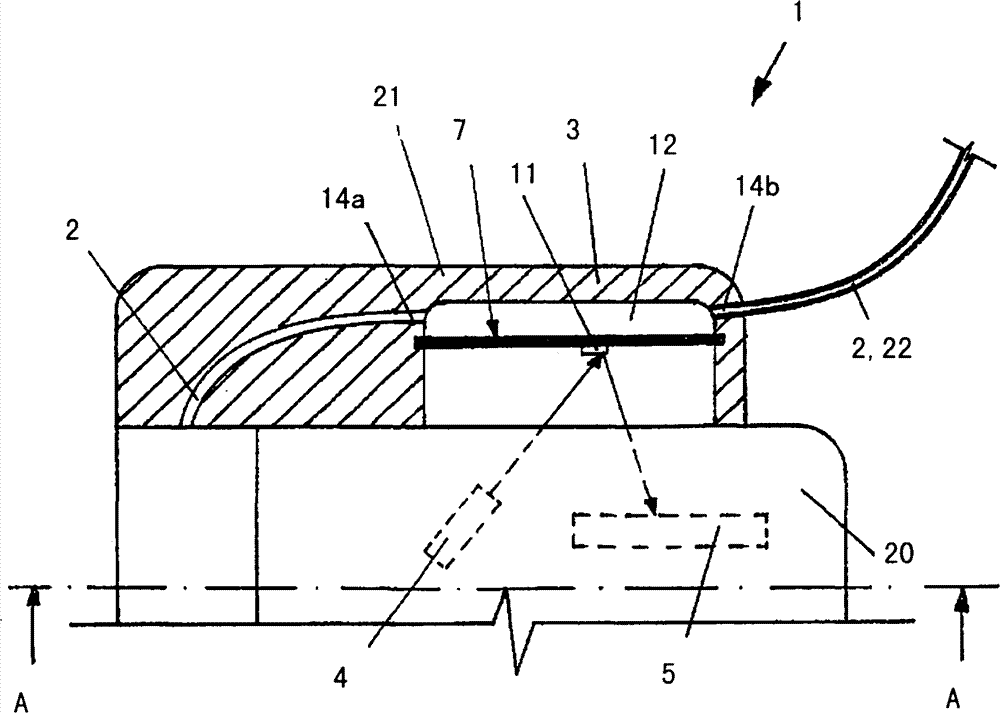
The invention relates to an insulin pump (1) with an infusion set adapter (21) having a diaphragm (7) exposed to the liquid path (2). Arranged in the housing (20) of the insulin pump (1) is a measurement arrangement comprising emitter (4) and sensor (5), by means of which a bowing of the diaphragm (7) following a change in the liquid pressure in the liquid path (2) can be detected without contact, for recognizing an occlusion in the liquid path (2). This yields the advantage that an accurate and delay-free occlusion recognition with low system cost becomes possible.
Owner:F HOFFMANN LA ROCHE & CO AG
Accelerated opiate dependence detoxification process
The present invention provides accelerated detoxification methods for the treatment of a substance abuse-related condition in a subject. A method of the present invention may comprise administering to the subject an effective amount of at least one sedative (e.g, clonidine, diazepam, or olanzapine) and a micro-dose of an opioid antagonist (e.g., naltrexone or naloxone) for at least one day; optionally administering a small dose of an opiate, and administering to the subject a detoxifying amount of a second opioid antagonist (e.g., naloxone); and may further comprise administering to the subject a third opioid antagonist (such as, naltrexone) for an extended period of time (e.g., 12 months).
Owner:COLEMAN PETER R
Microdosing device
ActiveUS7584903B2Improve dose accuracyEasy to fillLiquid surface applicatorsMovable spraying apparatusBiomedical engineeringMicrodosing
A microdosing device includes a dosing chamber which at least temporarily receives a quantity of liquid, and to which at least one dispensing opening is assigned. A vibration unit is operatively connected to at least one boundary surface of the dosing chamber in order to cause this boundary surface to oscillate for the purpose of a dispensing operation.At least two admission channels spaced apart from one another are provided on the dosing chamber.
Owner:APTAR RADOLFZELL
Implantable devices and methods for evaluation of active agents
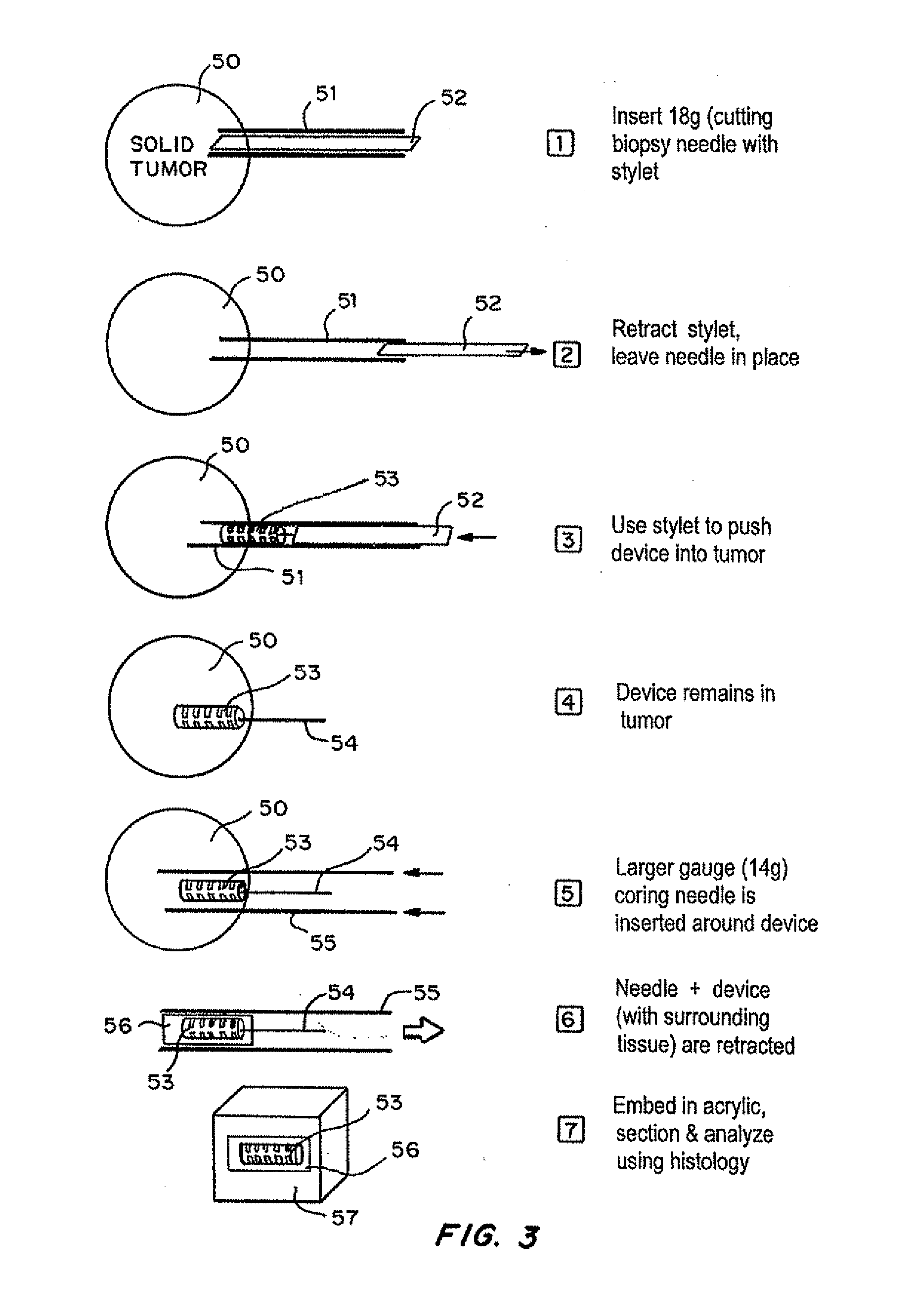
InactiveUS20150005595A1Accurate predictionRemove overlapGuide wiresSurgical needlesActive agentImplanted device
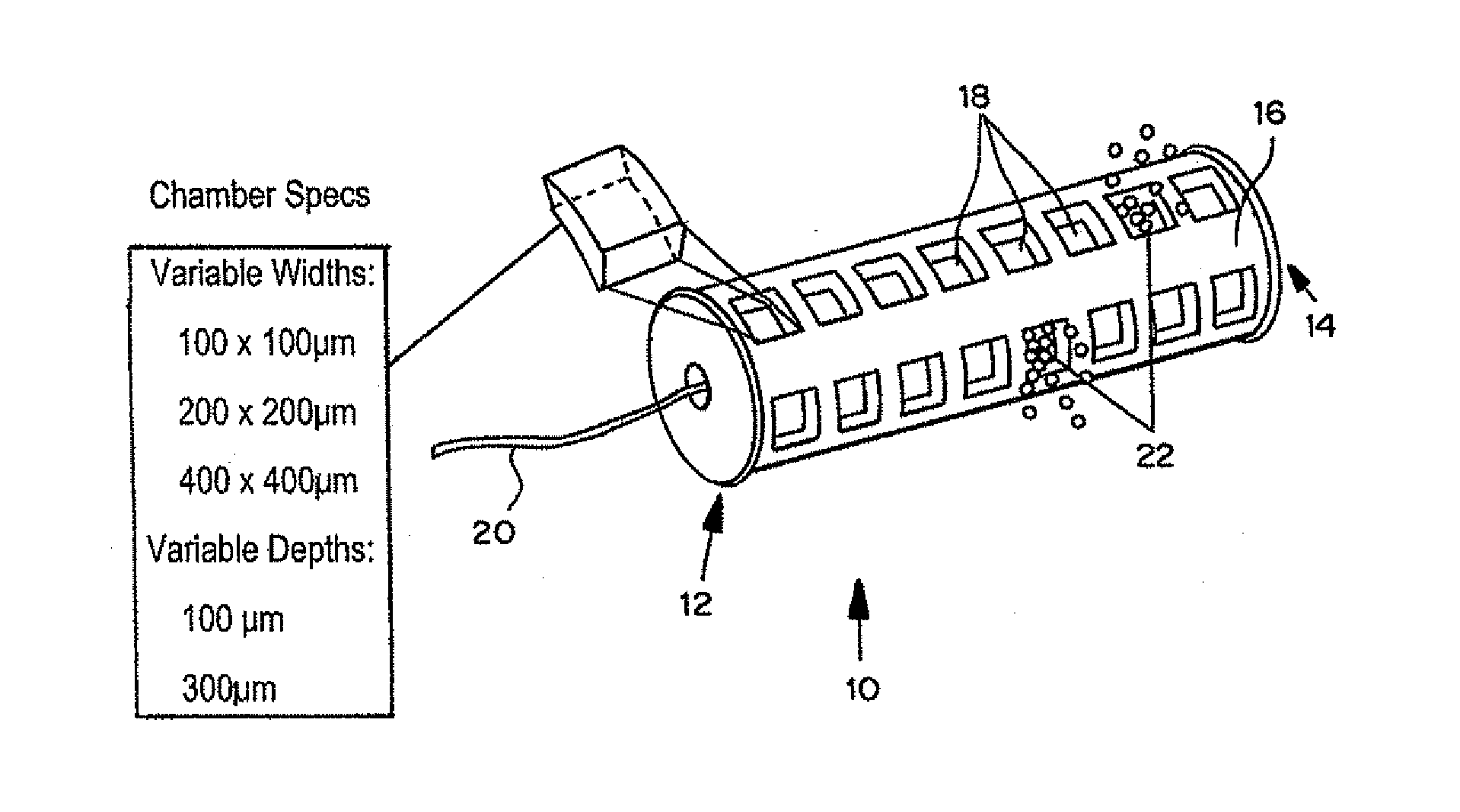
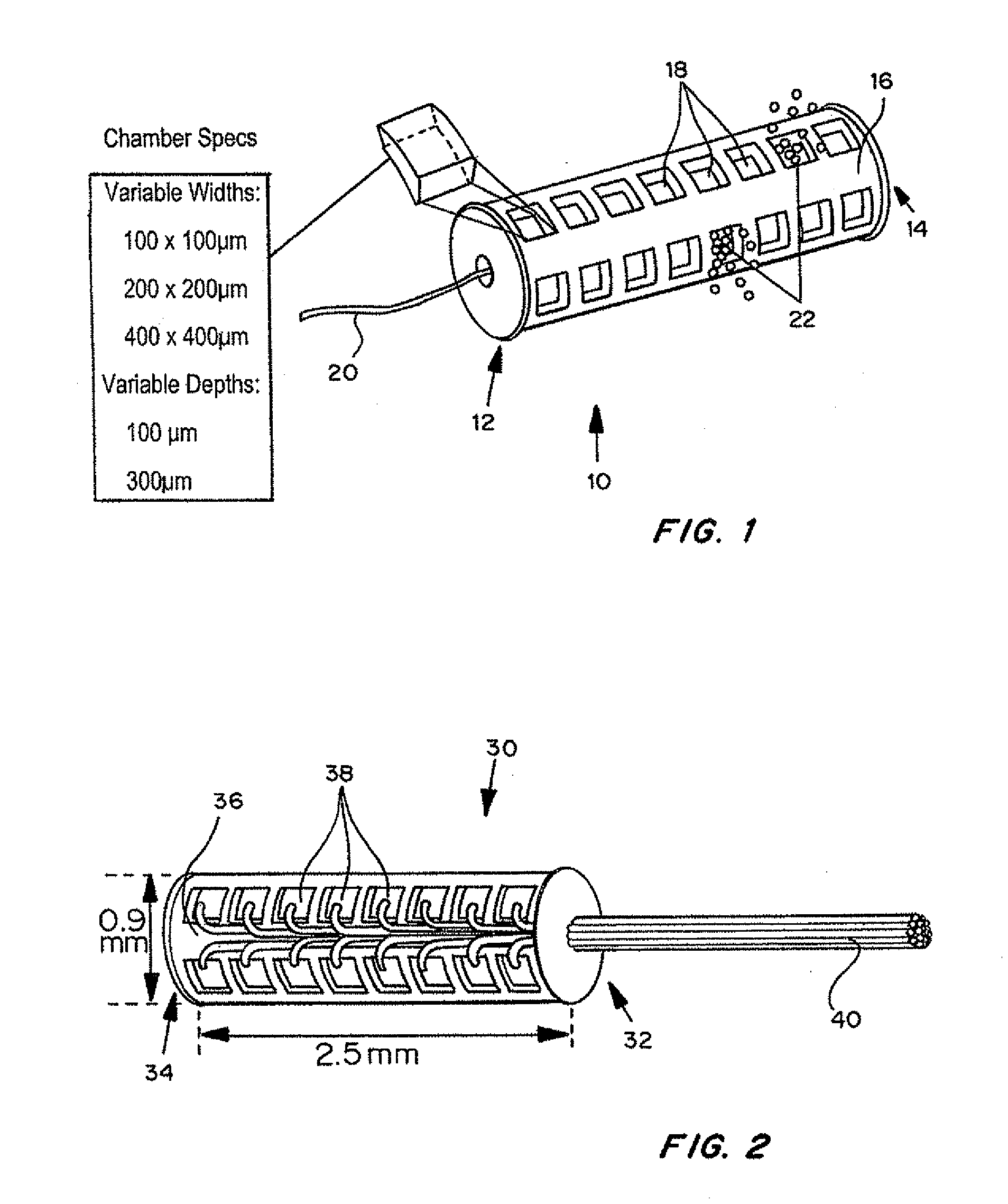
Devices for the local delivery of microdose amounts of one or more active agents, alone or in combination, in one or more dosages, to selected tissue of a patient are described. The devices generally include multiple microwells arranged on or within a support structure and contain one or more active agents, alone or in combination, in one or more dosages and / or release pharmacokinetics. In an exemplary embodiment, the device has a cylindrical shape, having symmetrical wells on the outside of the device, each well containing one or more drugs, at one or more concentrations, sized to permit placement using a catheter, cannula, or stylet. Optionally, the device has a guidewire, and fiber optics, sensors and / or interactive features such as remote accessibility to provide for in situ retrieval of information and modification of device release properties. In a preferred embodiment, the fiber optics and / or sensors are individually accessible to discrete wells.
Owner:KIBUR MEDICAL INC +1
Controllable fluid sample dispenser and methods using the same
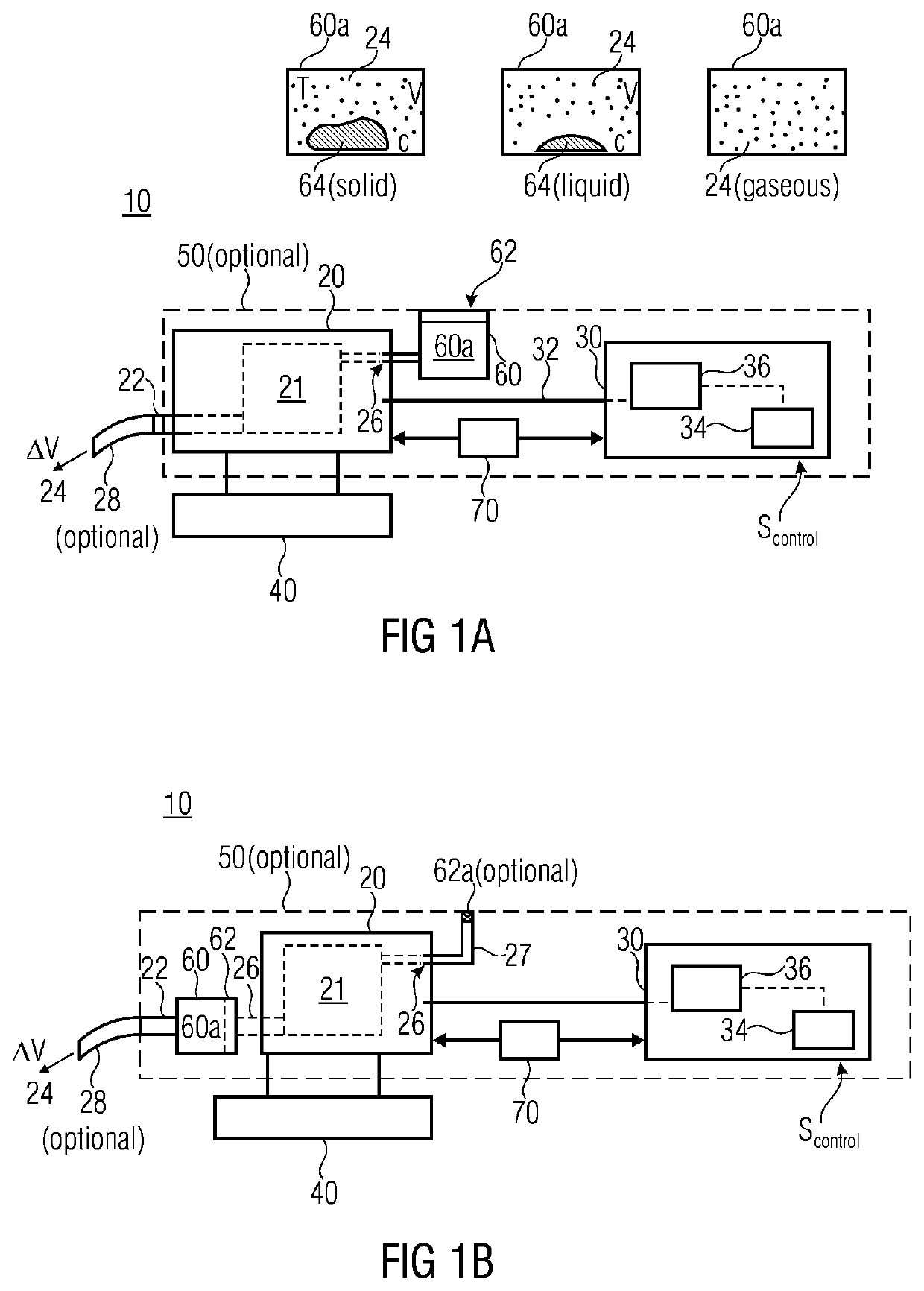
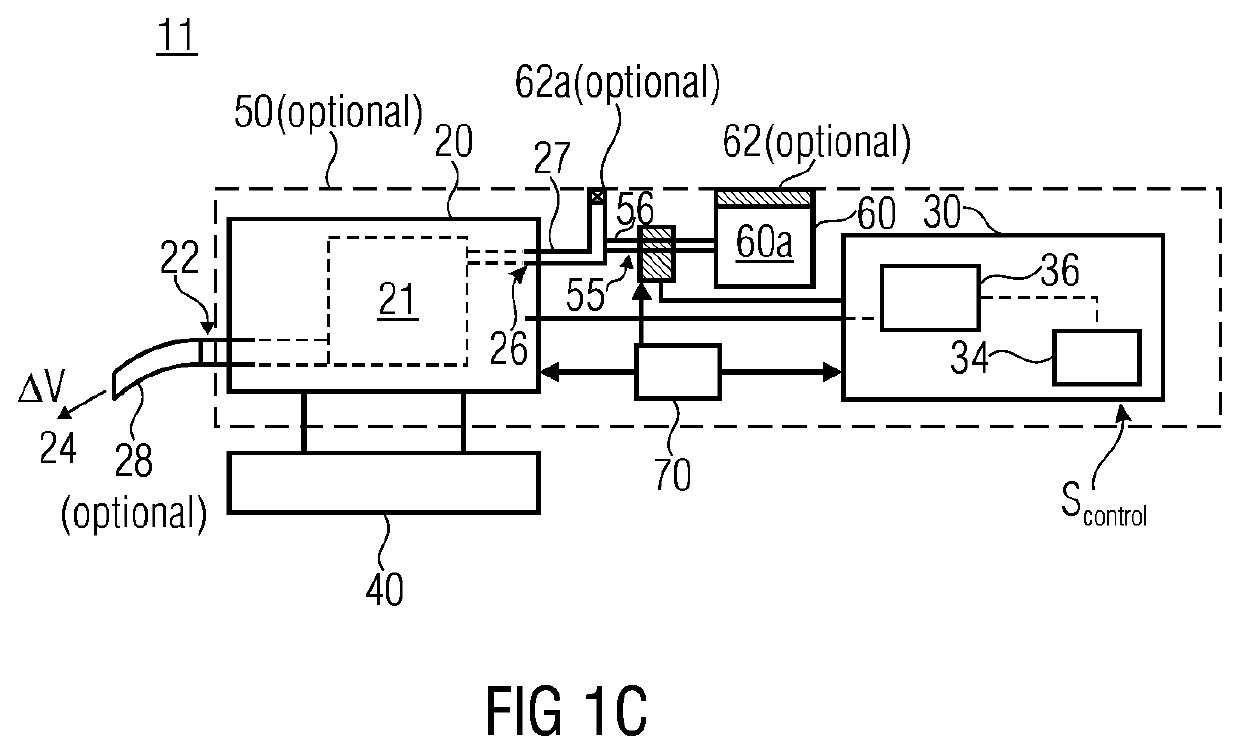
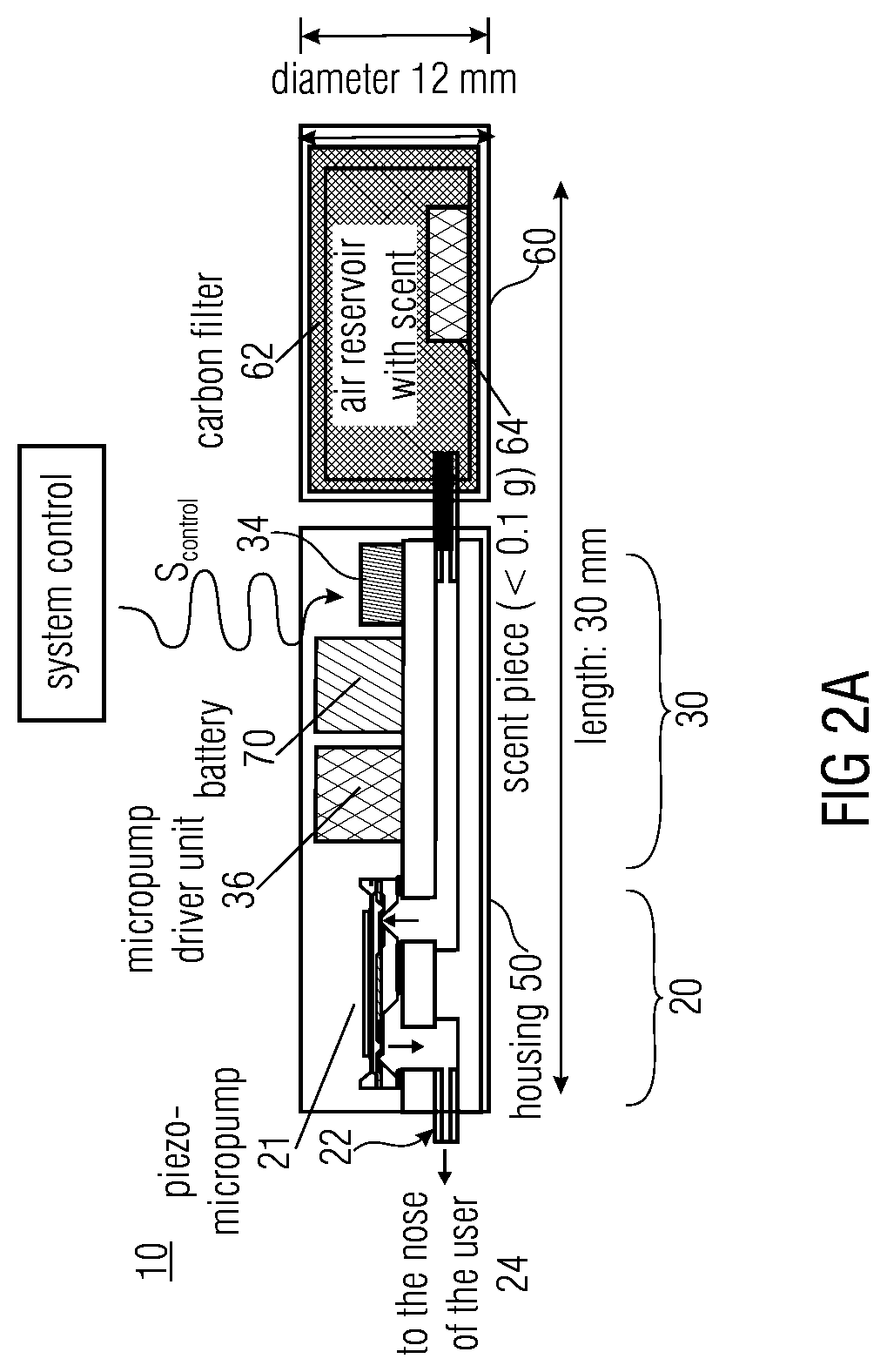
ActiveUS20140069420A1High resultHigh acceptanceMedical devicesDiagnostic recording/measuringNoseDose rate
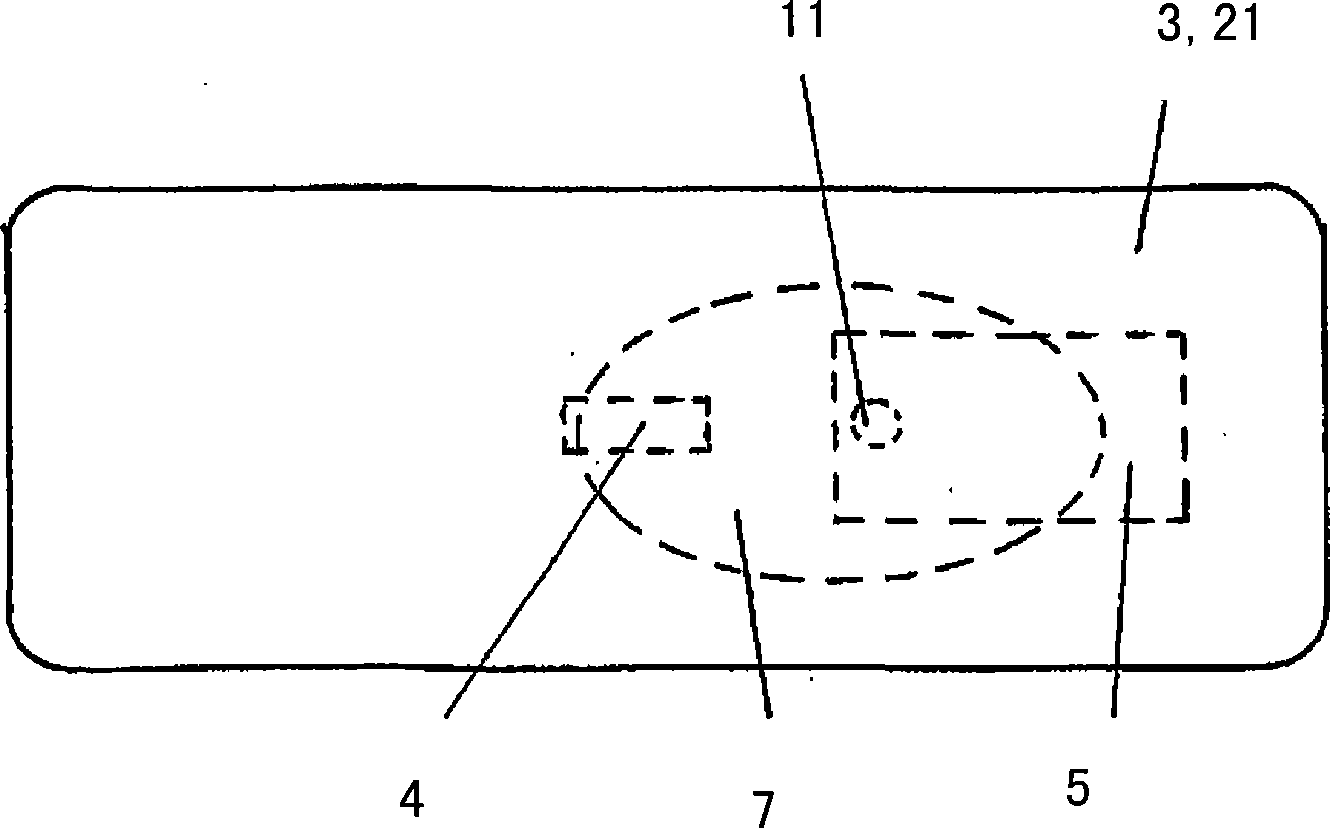
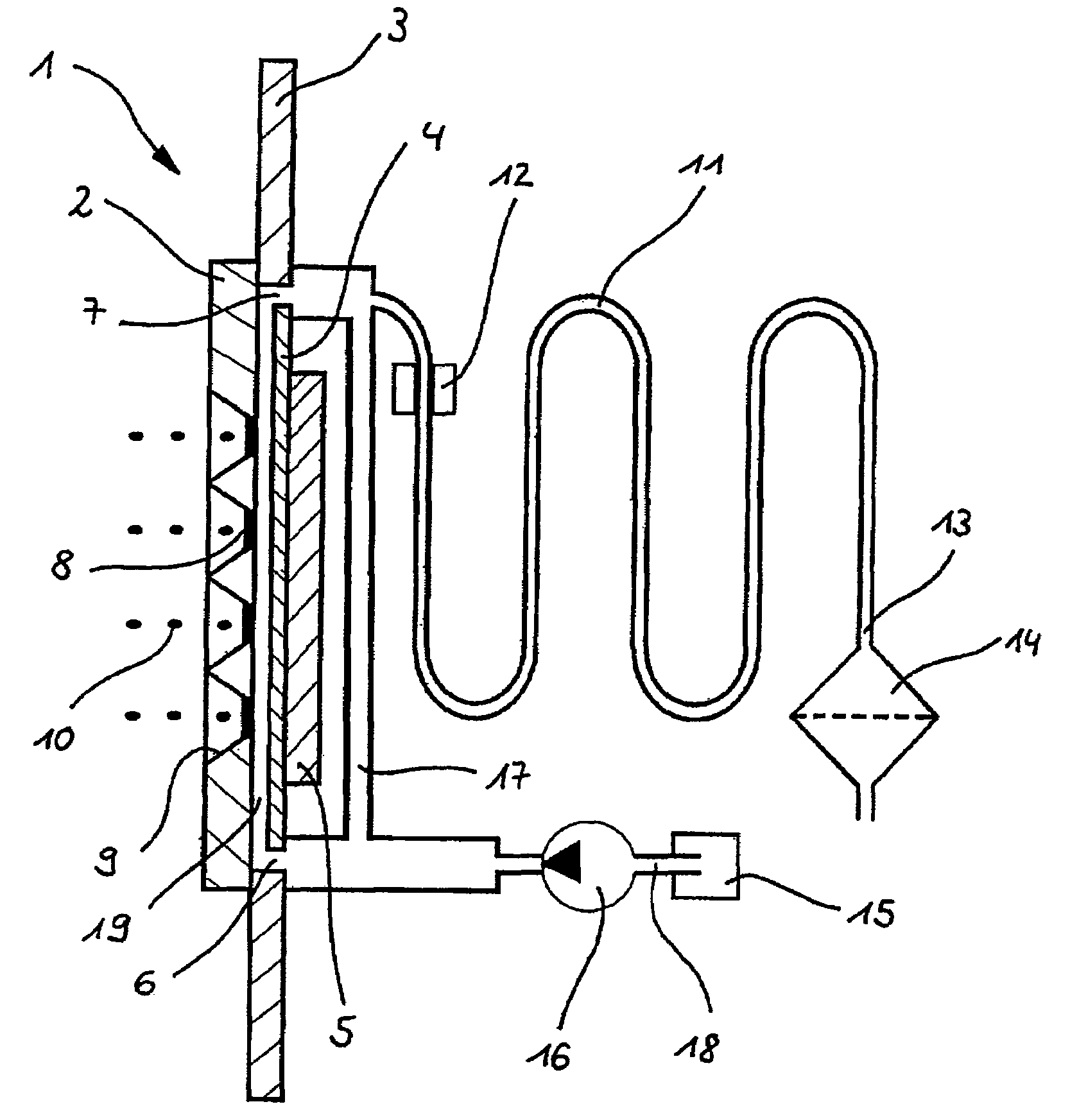
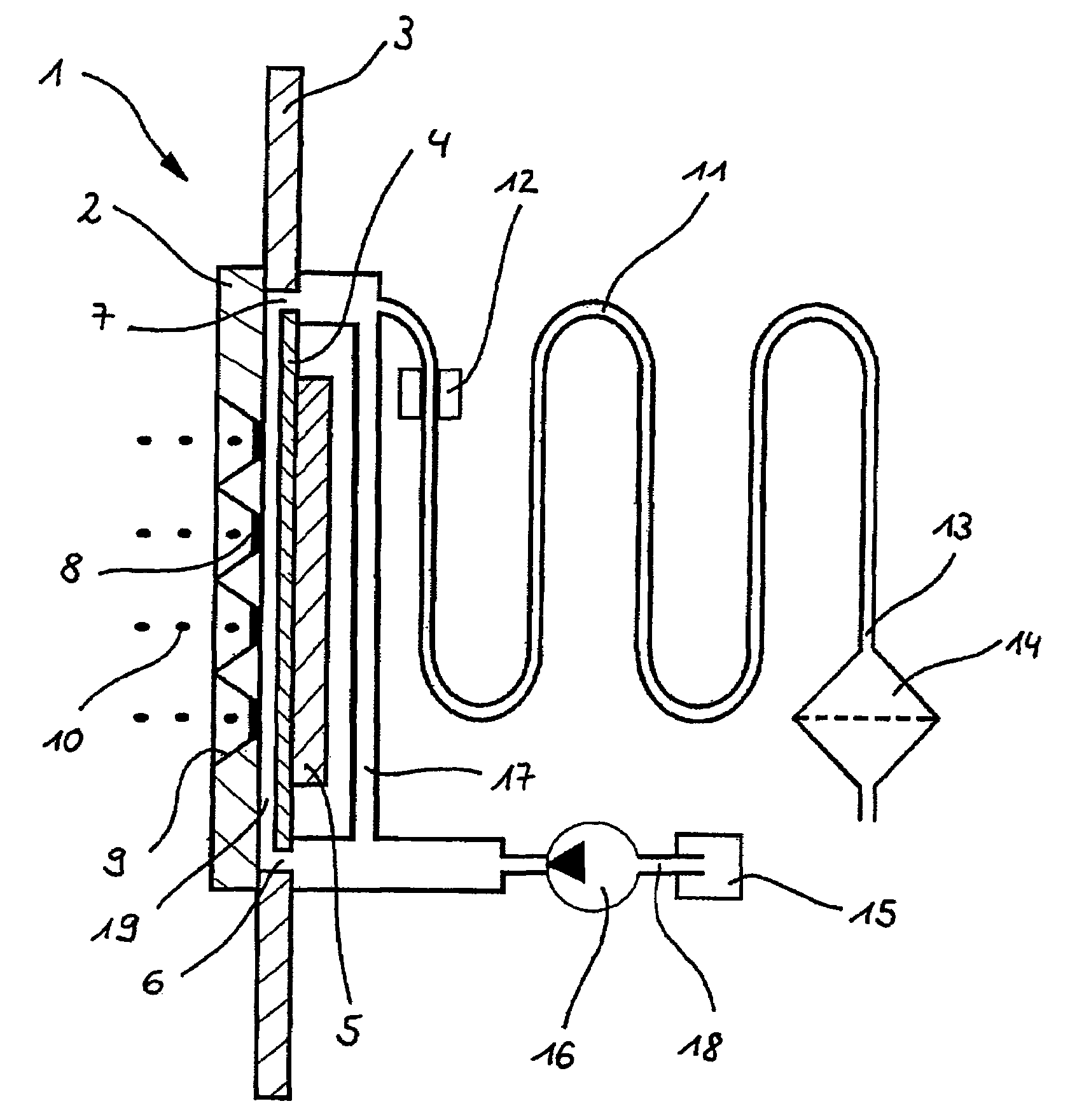
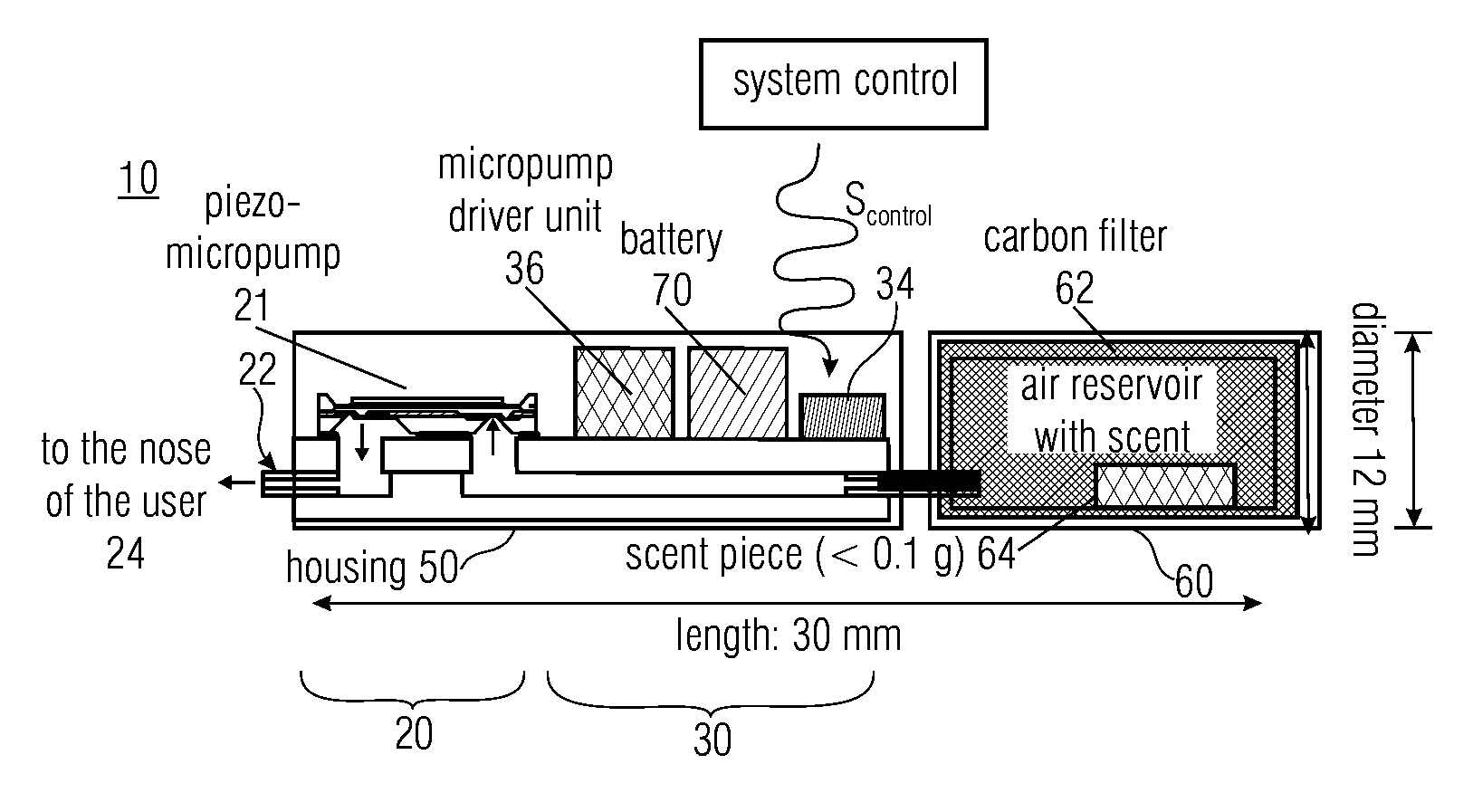
A fluid sample dispenser includes a microdosing device for outputting, during an activation state, a fluid sample at a fluid sample outlet to the environment, wherein the microdosing device is placeable adjacent to a person's nose so that a distance between the outlet of the microdosing device and a nostril of the person's nose is within a predefined range, and a microdosing driver unit for adjusting a dosing rate of the scent sample output at the scent sample outlet by selectively activating the microdosing device.
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV
Implantable Devices And Methods For The Evaluation of Active Agents
InactiveUS20130184593A1Accurate predictionSurgical needlesVaccination/ovulation diagnosticsFiberActive agent
Devices for the local delivery of microdose amounts of one or more active agents, alone or in combination, in one or more dosages, to selected tissue of a patient are described. The devices generally include multiple microwells arranged on or within a support structure. The microwells contain one or more active agents, alone or in combination, in one or more dosages and / or release pharmacokinetics. In an exemplary embodiment, the device has a cylindrical shape, having symmetrical wells on the outside of the device, each well containing one or more drugs, at one or more concentrations, sized to permit placement using a catheter, cannula, or stylet. Optionally, the device has a guidewire, and fiber optics, sensors and / or interactive features such as remote accessibility (such as WiFi) to provide for in situ retrieval of information and modification of device release properties. In the most preferred embodiment, the fiber optics and / or sensors are individually accessible to discrete wells.
Owner:KIBUR MEDICAL INC
Microdosing Device for Dosing of Smallest Quantities of a Medium
InactiveUS20100166585A1Manufactured simply and cost-effectivelyCompact designPositive displacement pump componentsFlexible member pumpsPistonMicrodosing
A microdosing device for dosing smallest quantities of a medium, having an inlet device, an outlet device, a pump device and a media duct that leads from the inlet device to the outlet device, the inlet device having an inlet opening and an inlet valve having an inlet valve piston, and the outlet device having an outlet opening and an outlet valve having an outlet valve piston, and the pump device having a pump piston. The inlet valve piston, the outlet valve piston and the pump piston are situated on a common diaphragm layer. The microdosing device has the advantage that it is able to be produced in a simple and cost-effective manner.
Owner:ROBERT BOSCH GMBH
Method of Inducing Dendritic and Synaptic Genesis in Neurodegenerative Chronic Diseases
The present invention discloses a method to recover and restore dendritic and synaptic neuron connections that have been degraded or destroyed by neurodegenerative diseases. In the present invention tryptamines are used to induce neuro plasticity and restore both dendritic density and synaptic connections of neurons in the brain. In the preferred embodiment LSD given in micro doses can induce dendritic and synaptic genesis in neuronal networks and improve the quality of life of people with neurodegenerative diseases such as Alzheimer's, Huntington's, Multiple Sclerosis, Parkinson's and Frontotemporal dementia.
Owner:PETCAVICH ROBERT JOSEPH
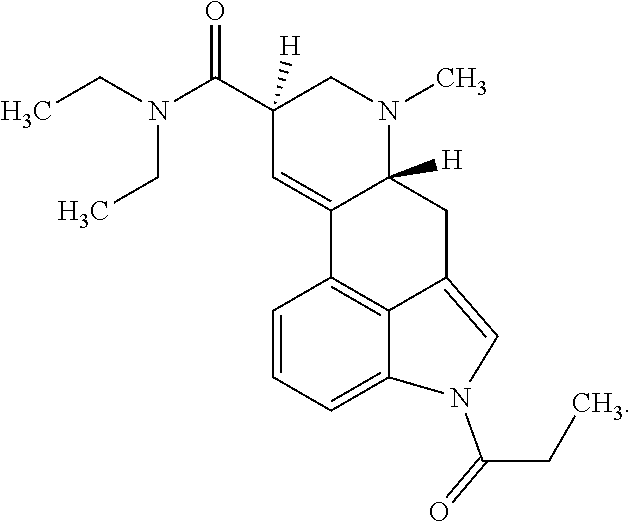
Transdermal micro-dosing delivery of psychedelics derivatives
PendingUS20210322447A1Less drugLess adverse effect effectDigestive systemSheet deliveryCompulsive disordersHeadaches
The present disclosure relates to the transdermal administration of psychedelics, such as psilocybin, psilocin, lysergic acid diethylamine (LSD), and / or ibogaine, and derivatives of these compounds, for the treatment and / or prevention and / or control of severe depression (treatment resistant), major depressive disorder, obsessive-compulsive disorder, quitting smoking, alcohol addiction, cocaine addiction, opioid addiction, anxiety (stress), adult ADHD, cluster headaches, and cancer related or other end-of-life psychological distress.
Owner:PIKE THERAPEUTICS INC
Lsd dose identification
ActiveUS20210315884A1Maximum positive subjective acute effectMinimizing negative acute effectNervous disorderDisease diagnosisDosage adjustmentAfter treatment
Owner:UNIVSSPITAL BASEL
Microdosing device
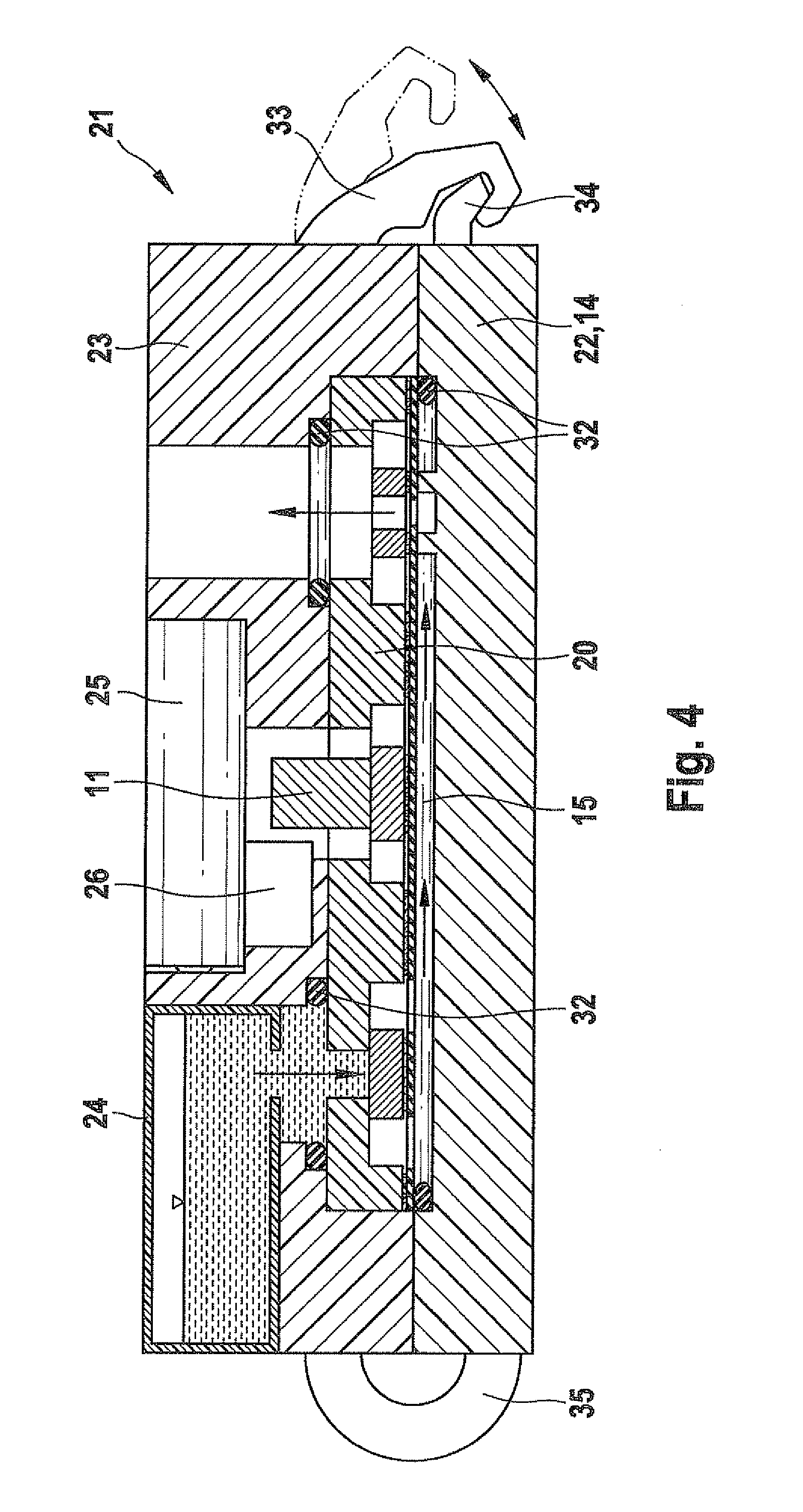
ActiveUS8113204B2Accurate and time-defined dosingAvoid pollutionLiquid surface applicatorsOther heat production devicesBiomedical engineeringMicrodosing
A microdosing device with a dosing chamber for the at least temporary reception of a liquid quantity, and with which is associated at least one discharge opening is provided. A vibrating unit in operative connection with at least one boundary surface of the dosing chamber is provided in order to vibrate the same for a discharge process, and with a delivery function unit connected to the vibrating unit for activating the latter during a delivery time period. A drying function unit is additionally provided and can be activated in time-separated manner with respect to the delivery function unit in order to free the dosing chamber from liquid residues.
Owner:APTAR RADOLFZELL
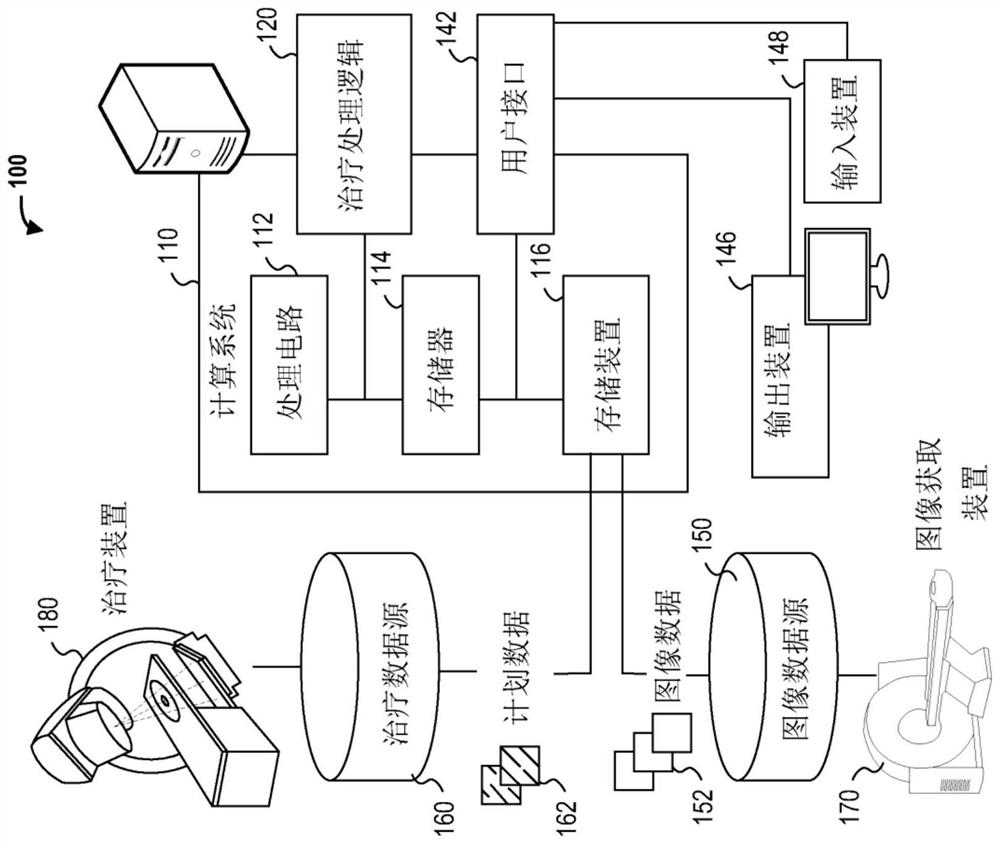
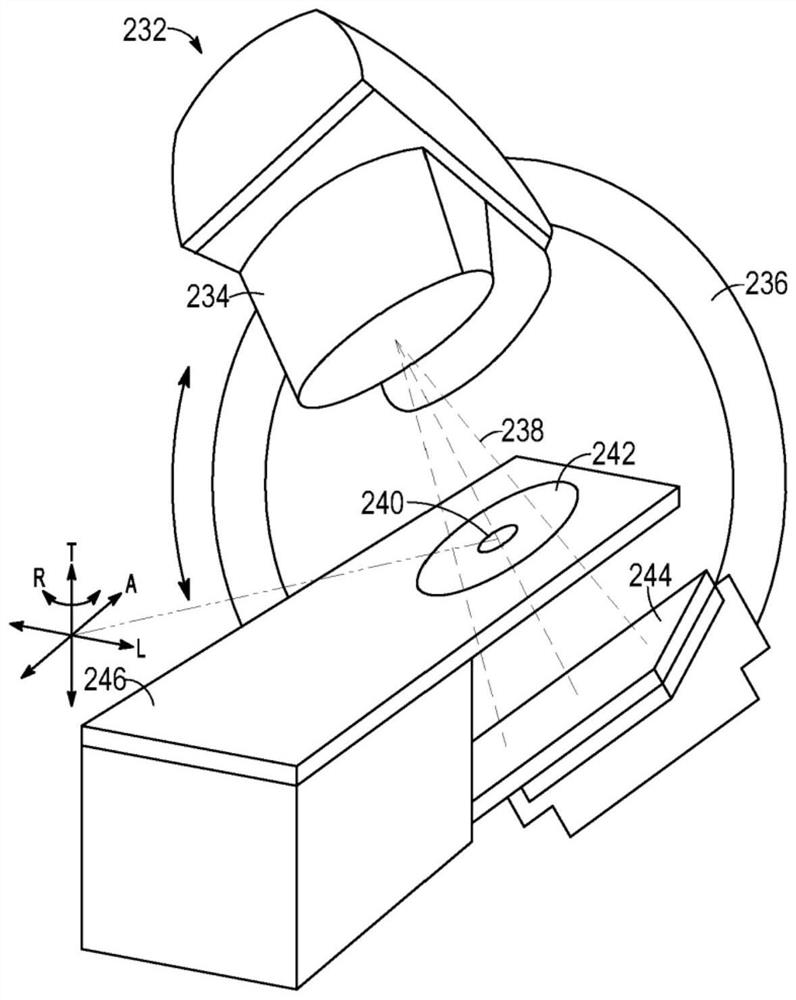
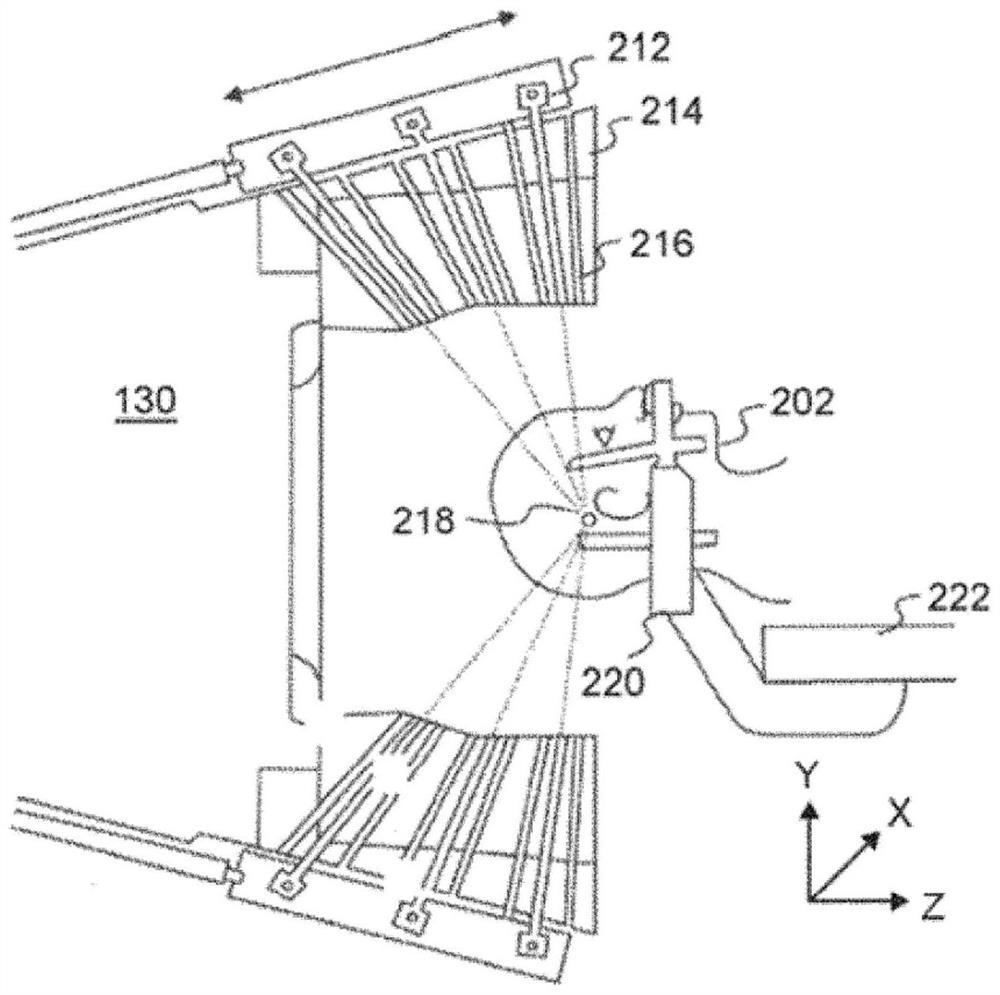
Radiotherapy treatment planning using microdose functions
ActiveCN114423494AMechanical/radiation/invasive therapiesMedical automated diagnosisNuclear medicineTherapy planning
Techniques for generating radiotherapy treatment plan parameters are provided. The technology comprises the following steps: receiving radiotherapy treatment plan information; processing the radiotherapy treatment plan information to estimate one or more radiotherapy treatment plan parameters based on processing of an output of the sub-processing dependent on the derivative of the estimated dose calculation; and generating a radiotherapy treatment plan using the estimated one or more radiotherapy treatment plan parameters.
Owner:医科达股份有限公司
Microdosing device and automatic microdosing method
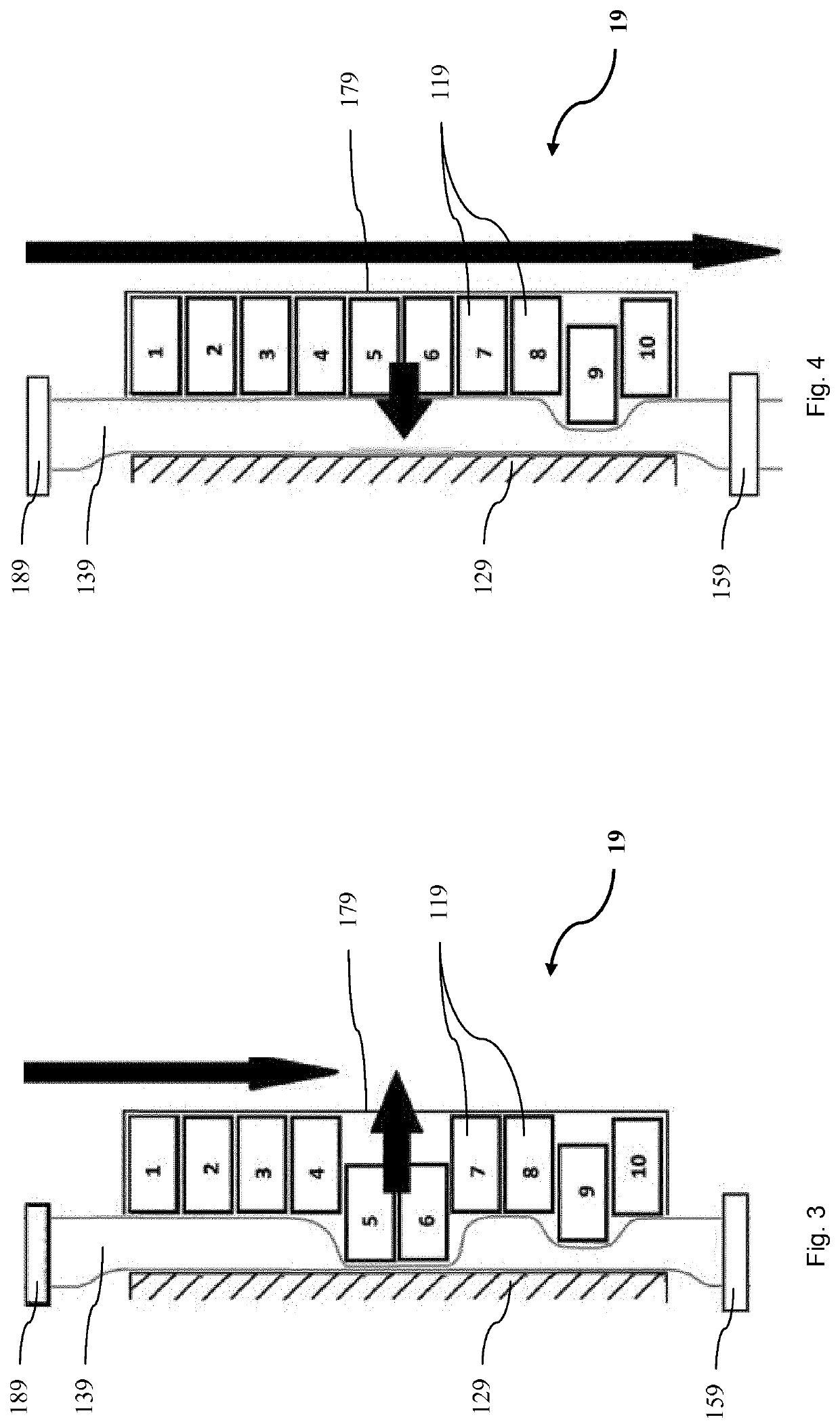
ActiveCN107765022ASimple structureEasy to cleanBioreactor/fermenter combinationsBiological substance pretreatmentsEngineeringOptical control
Means and methods for dispensing small amounts of liquid from multi-channel microdispensing devices, suitable for use in automatic processing in biological assays and for the cultivation of cells andtissues, by means of optical control of the dosed liquid by specific light barrier units.
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV
Methods and devices for delivering pilocarpine to the eye as a micro-dose stream of droplets
The present disclosure provides methods and devices for delivering pilocarpine to the eye. In certain aspects, the disclosure provides method for delivering a composition comprising pilocarpine to an eye of a subject in need thereof as a micro-dose stream of droplets. In certain embodiments, the method treats or alleviates one or more symptoms of presbyopia, in particular presbyopia in adults 40 years of age or older, in the subject in need thereof. In certain embodiments, the present disclosure utilizes relatively high concentrations of pilocarpine in the solution to be administered and as a single active agent.
Owner:EYENOVIA
Biological dose calculation method for boron neutron capture therapy
ActiveCN112618970AX-ray/gamma-ray/particle-irradiation therapyMedical imaging dataImaging Procedures
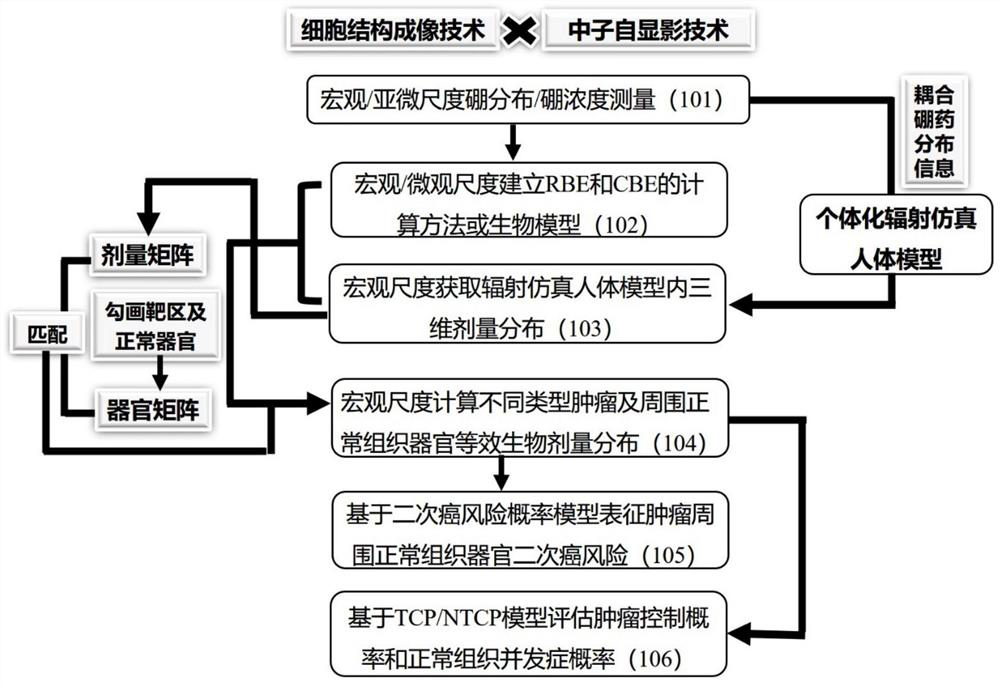
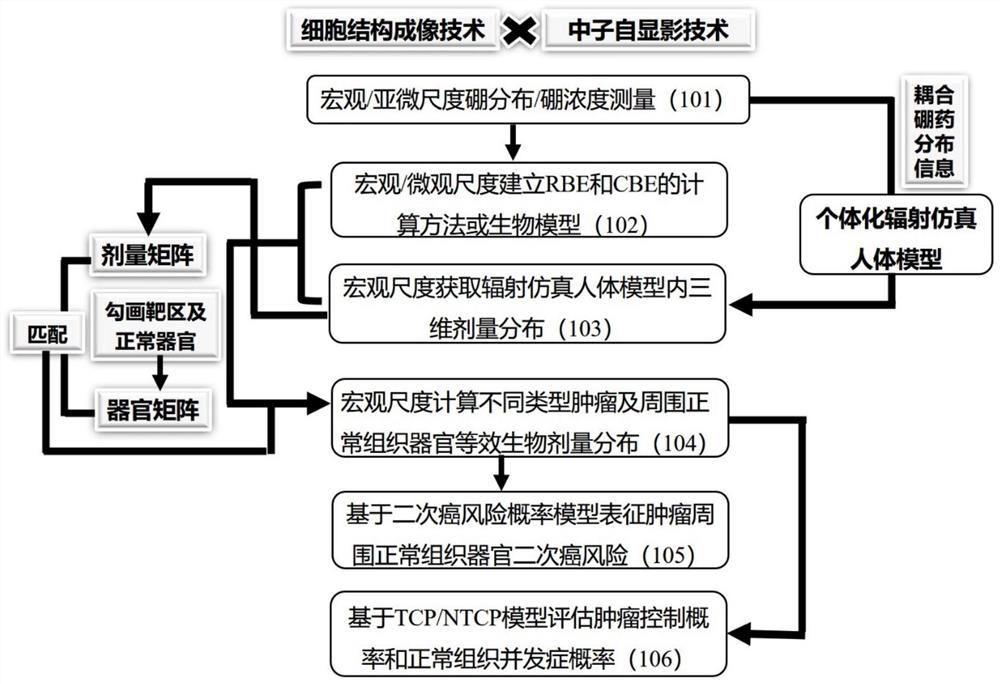
The invention relates to a biological dose calculation method for boron neutron capture therapy (BNCT). According to the method disclosed by the invention, accurate measurement of the tumor cell scale and tumor / normal tissue vessel boron concentration distribution is realized based on a neutron autography technology; according to different boron drug distribution characteristics, neutron energy spectra and tissue material characteristics, the corresponding relationship between boron distribution and neutron energy and the relative biological effect (RBE) or the complex biological effect (CBE) of all ray components of the BNCT is constructed through Monte Carlo simulation and a microdosimetry physical model; a radiation simulation human body model of patient individualization is constructed based on the medical image data, and then, three-dimensional dose distribution is obtained; and according to contouring of a gross tumor volume and normal structures, the equivalent biological dose is acquired by combining three-dimensional physical dose distribution and biological effect factors, and the dose volume histogram (DVH) of tumors and normal structures are obtained by calculation, so that a theoretical basis is provided for the design of novel targeted drugs and biological conversion of the accurate BNCT clinical treatment plan.
Owner:NANJING UNIV OF AERONAUTICS & ASTRONAUTICS
Microdosing system
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV
Safety valve assembly suitable for micro-dose infusion, micro-dose secretion pump and insulin pump
ActiveCN113786554ASolve the problem of injecting medium too fastPressure infusionValvesMedicineInsulin pump
The invention provides a safety valve assembly suitable for micro-dose infusion, a micro-dose secretion pump and an insulin pump. The safety valve assembly comprises a valve body, a flexible body and a needle withdrawing unit. The valve body is connected with a medium output device, the flexible body is connected between the valve body and the medium output device, and the needle withdrawing unit is connected with the valve body; the medium output device is provided with a medium inlet and outlet and a flexible body driving port, a first pipeline is arranged in the valve body, the input end of the first pipeline is connected with the medium inlet and outlet, the output end of the first pipeline is located on one side of the flexible body, and the flexible body driving port is located on the other side of the flexible body; and the sectional area of the medium inlet and outlet is smaller than that of the flexible body driving port. The problem that the medium injection speed is too high in the output failure process of medium output device can be solved, and under certain application conditions such as insulin or medicament injection, excessive injection can cause life danger.
Owner:IMOTION SHANGHAI PROD DESIGN +1
Controllable fluid sample dispenser and methods using the same
ActiveUS10639432B2Improve accuracyImprove reliabilityMedical devicesDiagnostic recording/measuringNostrilPhysical therapy
A fluid sample dispenser includes a microdosing device for outputting, during an activation state, a fluid sample at a fluid sample outlet to the environment, wherein the microdosing device is placeable adjacent to a person's nose so that a distance between the outlet of the microdosing device and a nostril of the person's nose is within a predefined range, and a microdosing driver unit for adjusting a dosing rate of the scent sample output at the scent sample outlet by selectively activating the microdosing device.
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV
Device for detection of a change of pressure in a canal of a microdosing device
InactiveCN101371127BHigh measurement accuracyImprove measurement reliabilityFluid pressure measurement by electric/magnetic elementsMedical devicesMedicineInsulin pump
Owner:F HOFFMANN LA ROCHE & CO AG
Treatment of post-bariatric hypoglycemia using mini-dose stable glucagon
Post-bariatric hypoglycemia (PBH) is an increasingly-recognized complication of gastric bypass surgery. Current therapeutic options have suboptimal efficacy. Small doses of stable liquid glucagon canbe used to treat or prevent post-bariatric hypoglycemia.
Owner:XERIS PHARMA INC +1
A Tissue Equivalent Correction Method for Microdose Detectors
ActiveCN114034721BImprove accuracyImprove detection accuracyMaterial analysis using wave/particle radiationRadiation measurementBiological organism
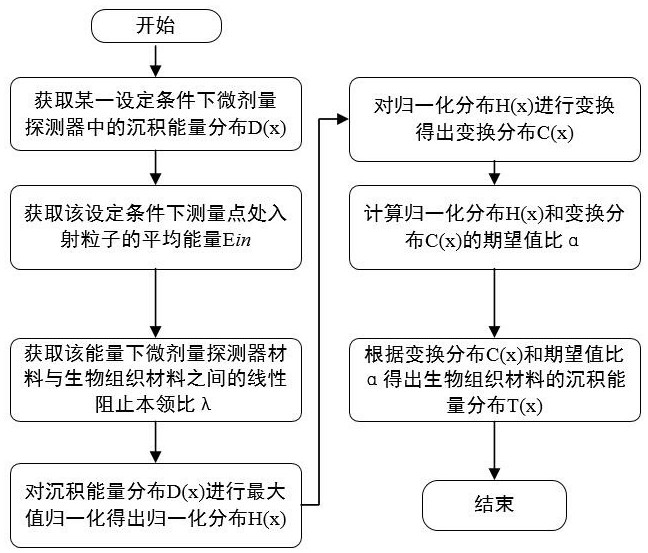
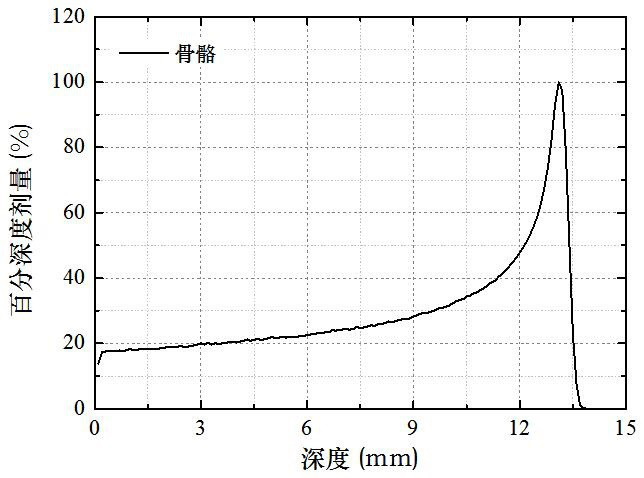
The invention discloses a tissue equivalent correction method for a micro-dose detector, which belongs to the technical field of radiation measurement and radiation protection. The average incident energy of particles entering the micro-dose detector under certain conditions; calculate the linear stopping power ratio of the biological tissue material and the micro-dose detector material under the current incident energy; normalize the deposition energy distribution; Transform the normalized distribution; calculate the expected value ratio of the normalized distribution and the transformed distribution; calculate the deposition energy distribution of the biological tissue material according to the transformed distribution and the expected value ratio 。 The invention can accurately convert the deposition energy distribution measured by the detector into the deposition energy distribution in the biological tissue under the same condition.
Owner:CHENGDU UNIV
Methods and Compositions for Microdosing, Simulating Extended Release Formulations
InactiveUS20220168221A1Easy to spreadEasy to manageOrganic active ingredientsDispersion deliveryBlood levelPhysiology
This application is directed to a method of microdosing by dissolving drug in water and permitting a human or animal to consume the drug during waking hours. Metering for an animal is the amount of water they drink, in the pattern as consumed. By setting drug concentration so that a target dose will be consumed in a typical amount of daily drinking water, the dose is spread out into a series of smaller doses over time. For a human, this can be metered more specifically and thoughtfully. A mechanism to make this convenient for humans is provided. A model of blood levels is described. The clinical benefits are greater than predicted from extrapolating from a daily dose to dosing twice a day to a projected 6-12 times a day with the microdosing method.
Owner:LARWOOD DAVID J
Dose-controllable medium conveying structure, medium conveying method and micro-dose secretion pump
PendingCN112569426AMedia output is stableMedium output precisionMedical devicesPressure infusionMedicineDose delivery
The invention provides a dose-controllable medium conveying structure, a medium conveying method and a micro-dose secretion pump. The dose-controllable medium conveying structure comprises a dose output assembly, the dose output assembly comprises a first multi-way valve, a second multi-way valve and a cavity, a sealing piece is connected in the cavity, a first cavity space on one side of the sealing piece and a second cavity space on the other side of the sealing piece are independent of each other through the sealing piece, and the size of the first cavity space and the size of the second cavity change along with movement or deformation of the sealing piece. The direction of an external medium entering the dose output assembly is controlled by controlling the conduction directions of thefirst multi-way valve and the second multi-way valve; and through the pressure of the medium entering the first cavity space / the second cavity space, the sealing piece moves or deforms, the second cavity space / the first cavity space is extruded, and the medium in the second cavity space / the first cavity space is discharged. According to the invention, the effect of one-time dose delivery can be accurate, and the dose delivered for one time cannot be changed due to the influence of external factors.
Owner:IMOTION SHANGHAI PROD DESIGN +1
High-flux medicine decoction and collection device and method
InactiveCN111135088ARealize the collectionEnsure sufficient separationPharmaceutical product form changeSimultaneous control of multiple variablesMedicineHigh flux
The invention discloses a high-flux medicine decoction and collection device and method. The device comprises a plurality of decoction units, wherein the decoction units are arranged in an array mode;each decoction unit comprises a pressurizing chamber, a decoction, a heater and a collection capillary pipeline; air resistors are arranged on the walls of the pressurizing chambers; the air resistors are connected with the walls of the pressurizing chambers through return springs; the decoction chambers are connected with the pressurizing chambers through the air resistors; filtering membranes are arranged on the walls of the decoction chambers; the heaters are used for heating the decoction chambers; one end of each collection capillary pipeline is connected with the filtering membrane of each decoction chamber through a switch part. As multiple decoction units of an identical structure are arranged in the array mode, traditional Chinese medicines of micro dosages can be simultaneouslydecocted in a large-scale mode, and a decoction function of high fluxes and micro reactions can be achieved; and in addition, a decoction collection mode of pressurization and filtering membranes ensures that a decoction can be sufficiently collected even in case of a micro dosage, and the problem that the decoction cannot be sufficiently separated from medicine residues can be solved.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
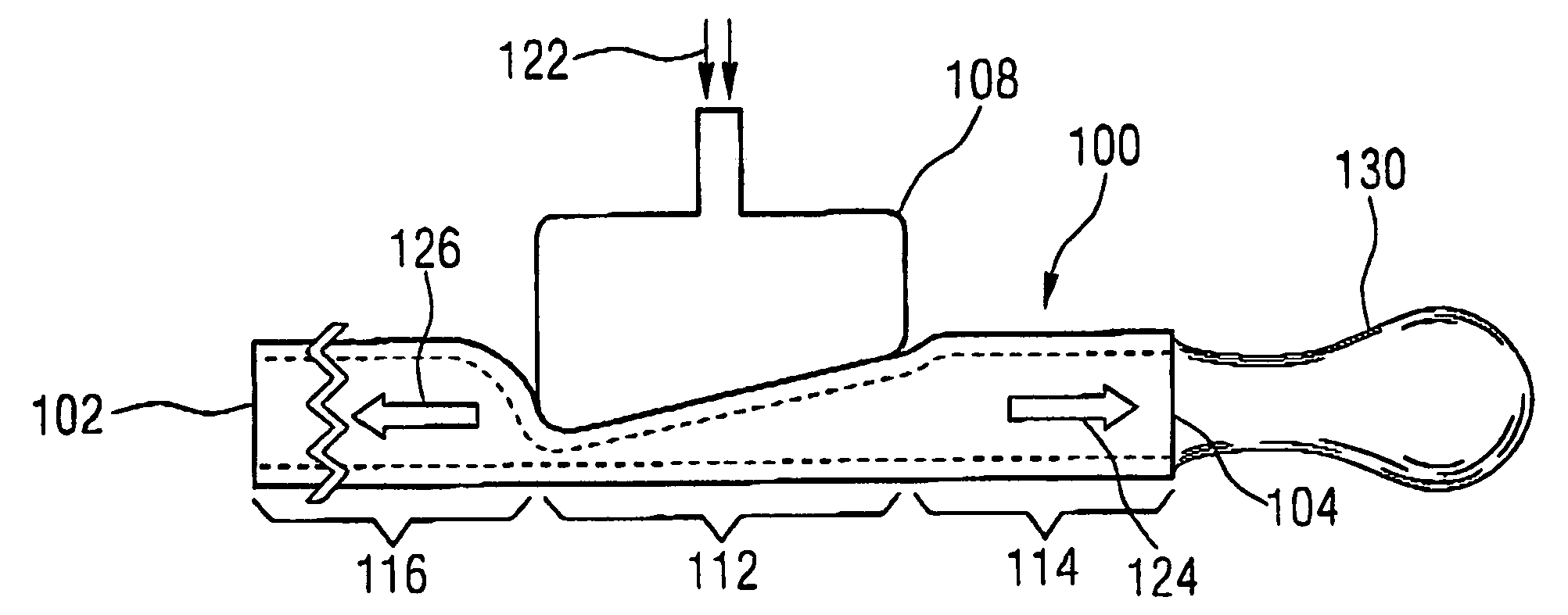
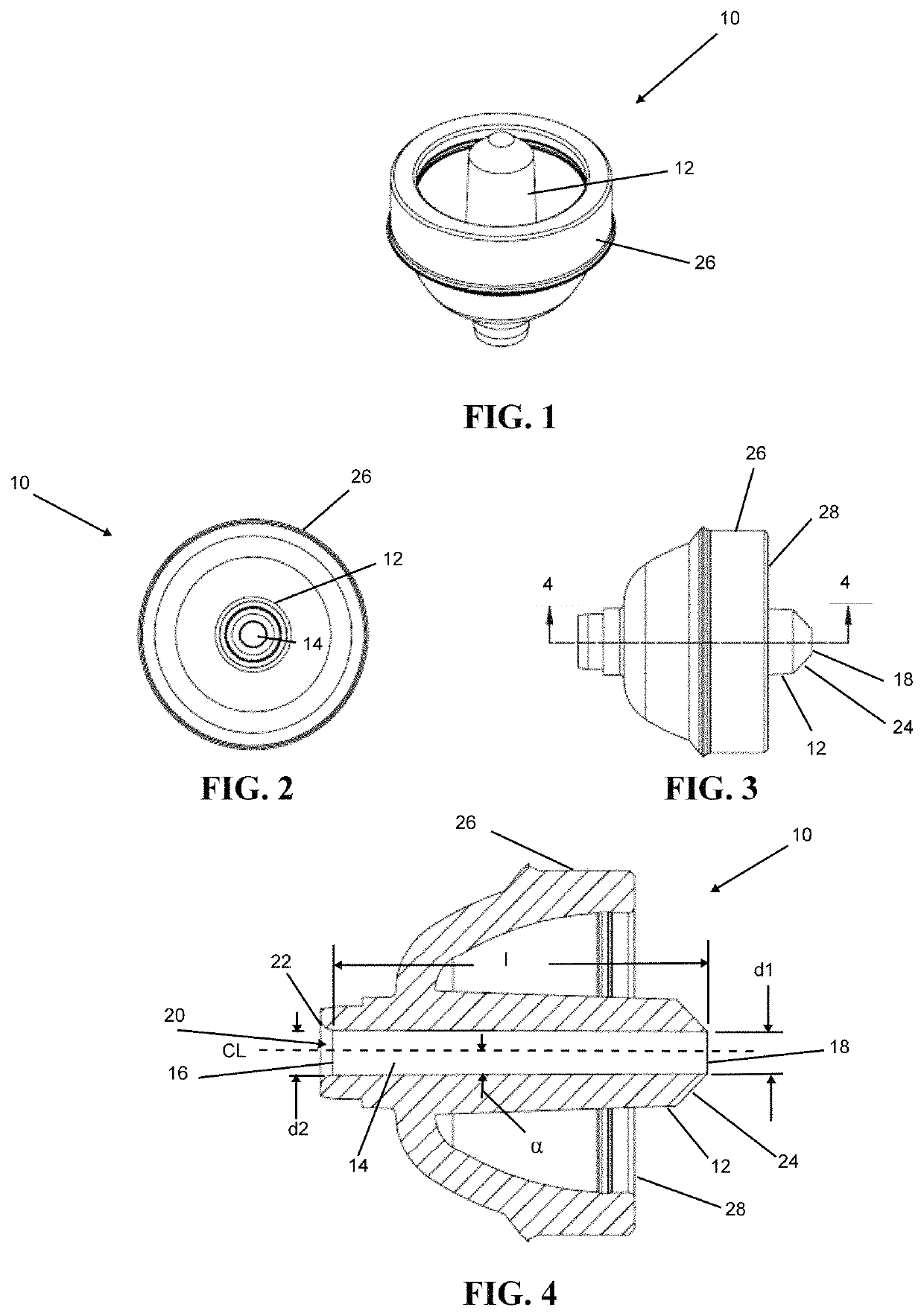
Nozzle
A nozzle is provided herein for ophthalmic dispensers which is configured to accommodate microdosing. The nozzle includes a converging pathway. Preferably, the pathway converges so as to impart momentum to liquid passing therethrough through an increase of velocity. A tapered portion may be provided flared openly from the inlet to best accept liquid flow thereinto and provide a funneling effect into the flowpath. Preferably, the flowpath terminates at an outlet which is internally un-radiused and circumscribed by a chamfered surface.
Owner:COHEN
Microdosing
PendingUS20210346598A1Reduce surface tensionInhibit aggregationMedical devicesPressure infusionPeristaltic pumpBiomedical engineering
A dosing system for transferring an aseptic fluid in dosages into a container, comprising a peristaltic pump configured such that the filling accuracy for fill volumes of the aseptic fluid <100 μL is ±3 μL.
Owner:F HOFFMANN LA ROCHE & CO AG
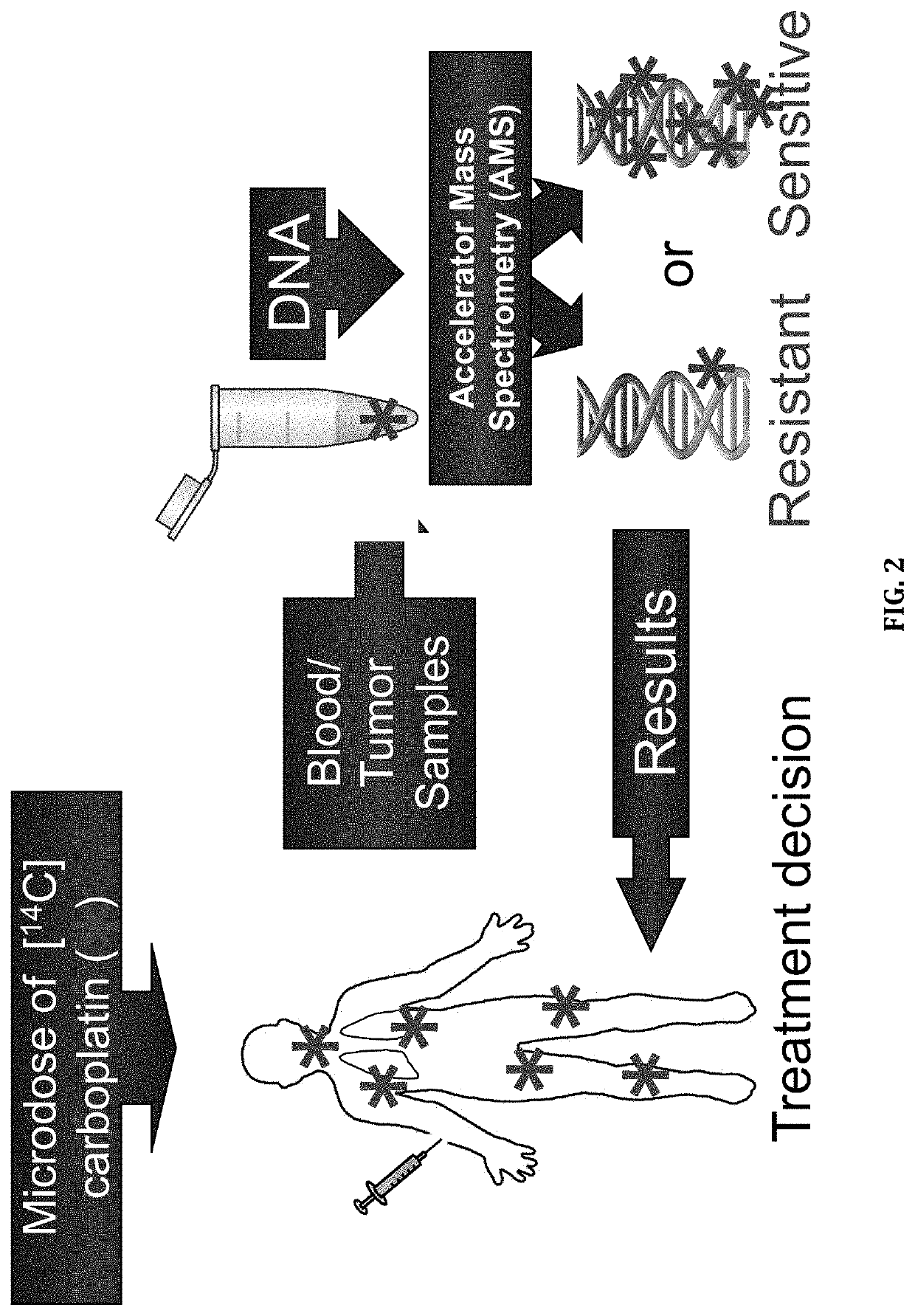
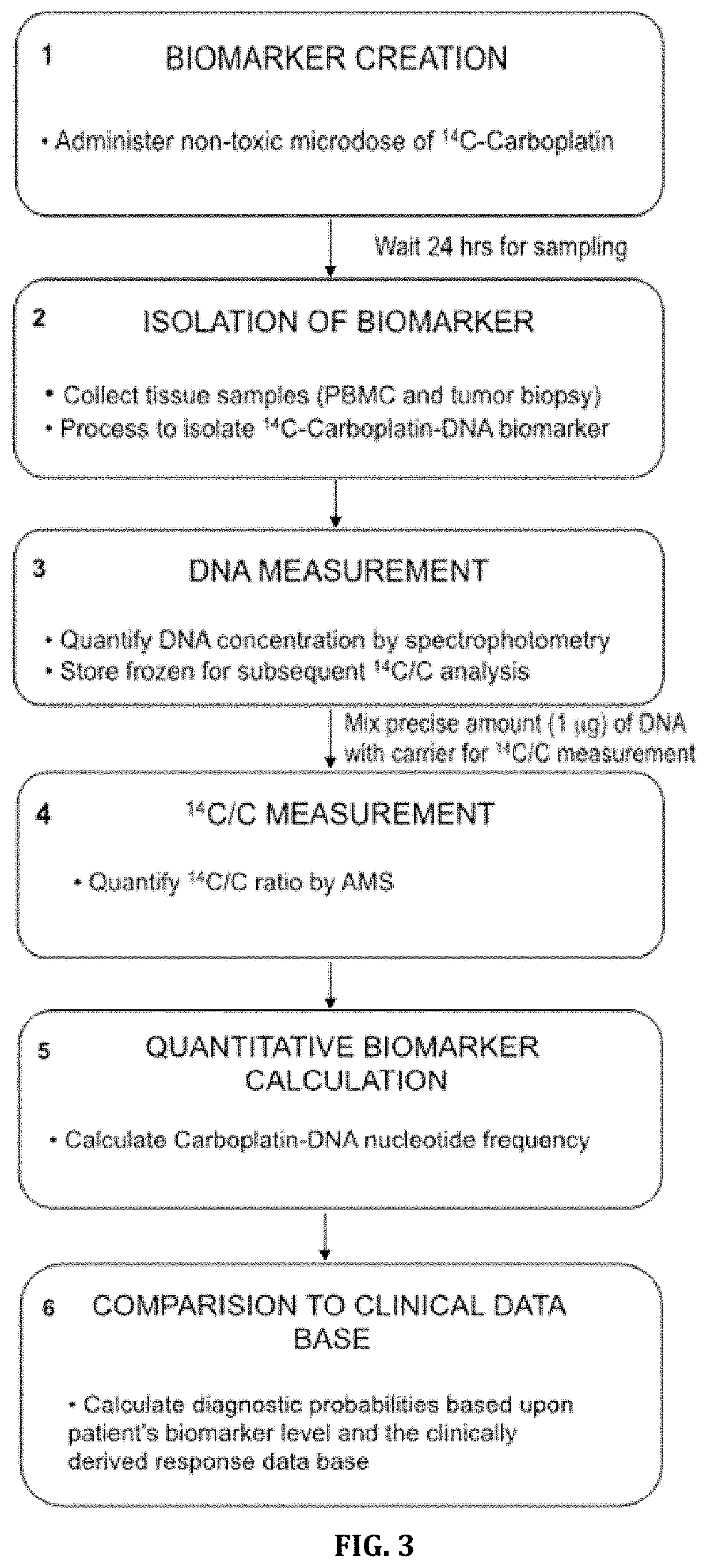
Cytotoxic Chemotherapy-Based Predictive Assays for Acute Myeloid Leukemia
InactiveUS20200010910A1Organic active ingredientsOrganic chemistry methodsMicroDoseTherapeutic effect
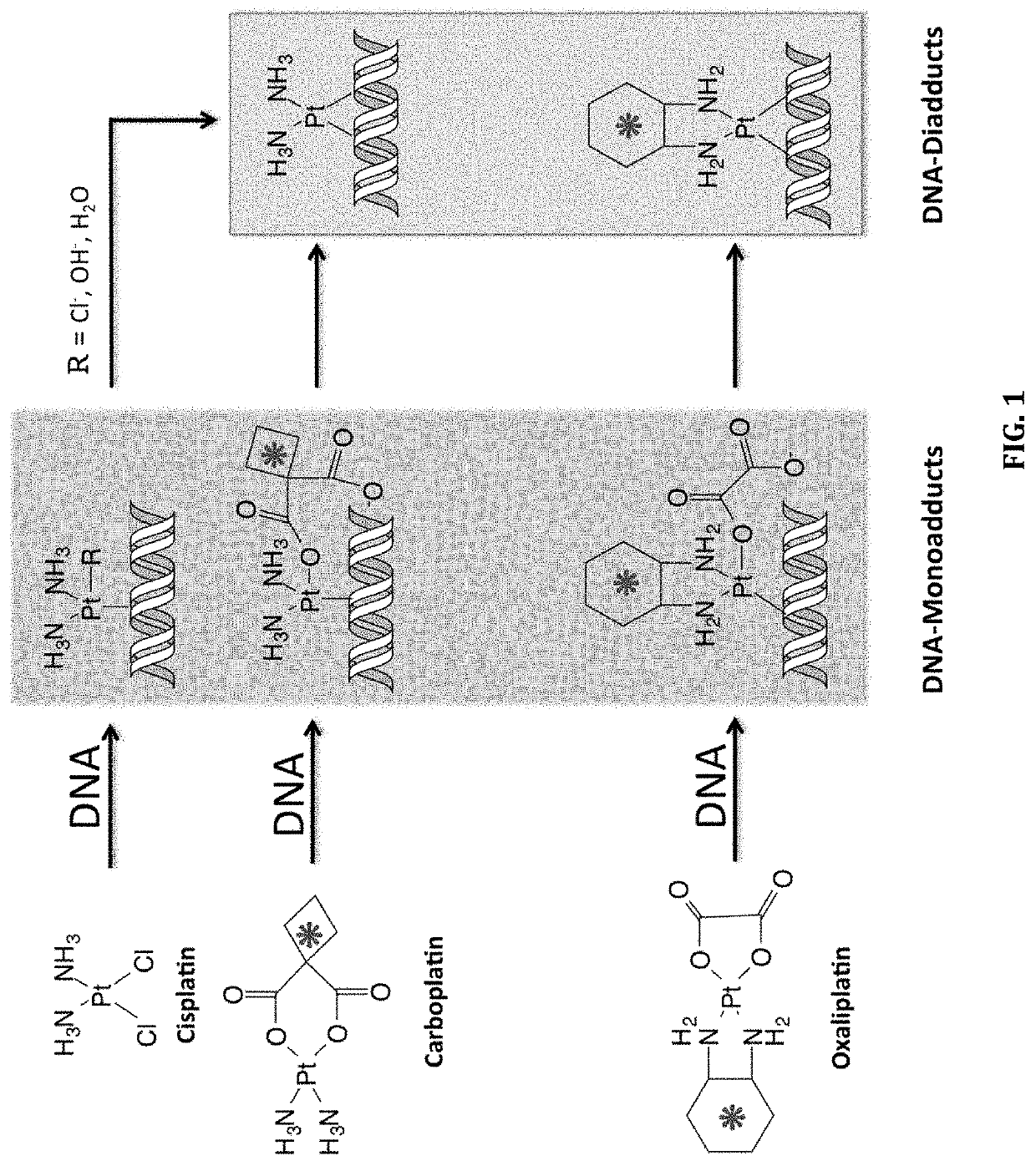
The invention relates to methods, systems and kits for determining therapeutic effectiveness or toxicity of cancer-treating compounds that incorporate into or bind to DNA. In particular, the invention is directed to methods, systems and kits for predicting a patient's treatment outcome after administration of a microdose of therapeutic composition to the patient or a sample from the patient. The methods provides physicians with a diagnostic tool to segregate cancer patients into differential populations that have a higher or lower chance of responding to a particular therapeutic treatment.
Owner:LAWRENCE LIVERMORE NAT SECURITY LLC
A kind of device and medium used in boron neutron capture therapy biological dose calculation method
ActiveCN112618970BX-ray/gamma-ray/particle-irradiation therapyMedical imaging dataImaging Procedures
The invention relates to a biological dose calculation method for boron neutron capture therapy (BNCT). The method of the present invention is based on neutron autoradiography technology to realize accurate measurement of tumor cell scale and tumor / normal tissue vascular boron concentration distribution; according to the distribution characteristics of different boron drugs, neutron energy spectrum and tissue material characteristics, through Monte Carlo simulation and The microdosimetry biological model constructs the corresponding relationship between boron distribution and neutron energy and the relative biological effect (RBE) or composite biological effect (CBE) of each BNCT ray component; constructs a patient's individualized radiation simulation human body model based on medical image data, and then The three-dimensional dose distribution is obtained; according to the contours of the tumor target area and normal organs, and combined with the three-dimensional physical dose distribution and biological effect factors to obtain the equivalent biological dose, the dose-volume histogram (DVH map) of the tumor and normal tissues and organs is calculated, which is The theoretical basis is provided for the design of new targeted drugs and the biological transformation of precise BNCT clinical treatment plans.
Owner:NANJING UNIV OF AERONAUTICS & ASTRONAUTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com