Implantable Devices And Methods For The Evaluation of Active Agents
a technology of active agents and implantable devices, which is applied in the field of implantable devices and methods for the evaluation of active agents, can solve problems such as failure to account for patient-specific factors, and achieve the effect of accurately predicting the systemic drug respons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Prototype Testing in Mouse Model
[0104]Materials and Methods
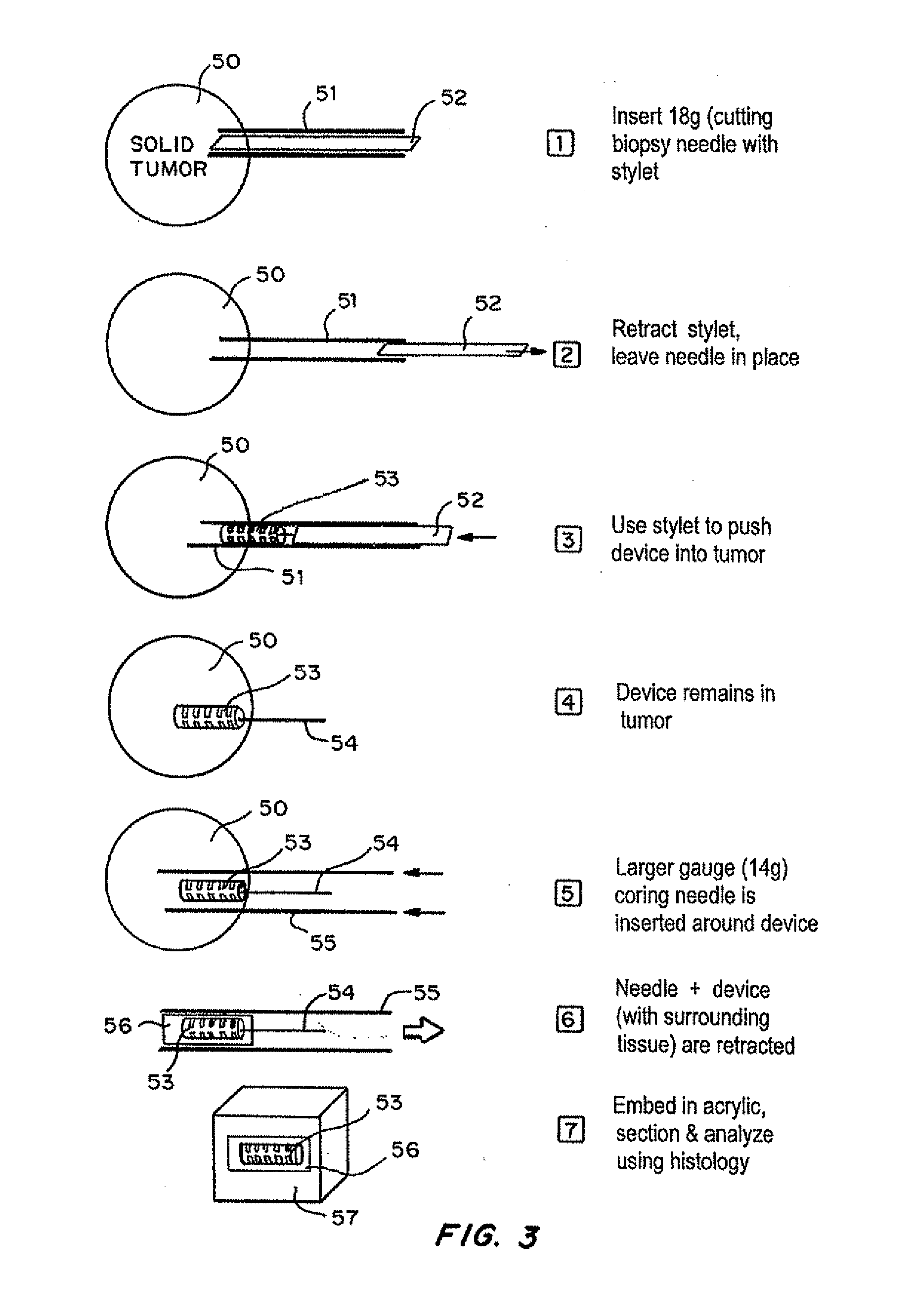
[0105]As shown in FIG. 5, a mouse model for a human cancer cell line is prepared by injection of human cancer cells such as MDA MB-231 into the mammary fat pad of an immunodeficient mouse. Tumors are allowed to implant and proliferate to approximately 150-170 mm3.
[0106]Individual drugs are administered systemically by injection to the mice to establish local pharmacokinetics for the drugs. For breast cancer cells, representative drugs to be tested include docetaxel, doxorubicin, irinotecan, transtuzumab, and bevacizumab.
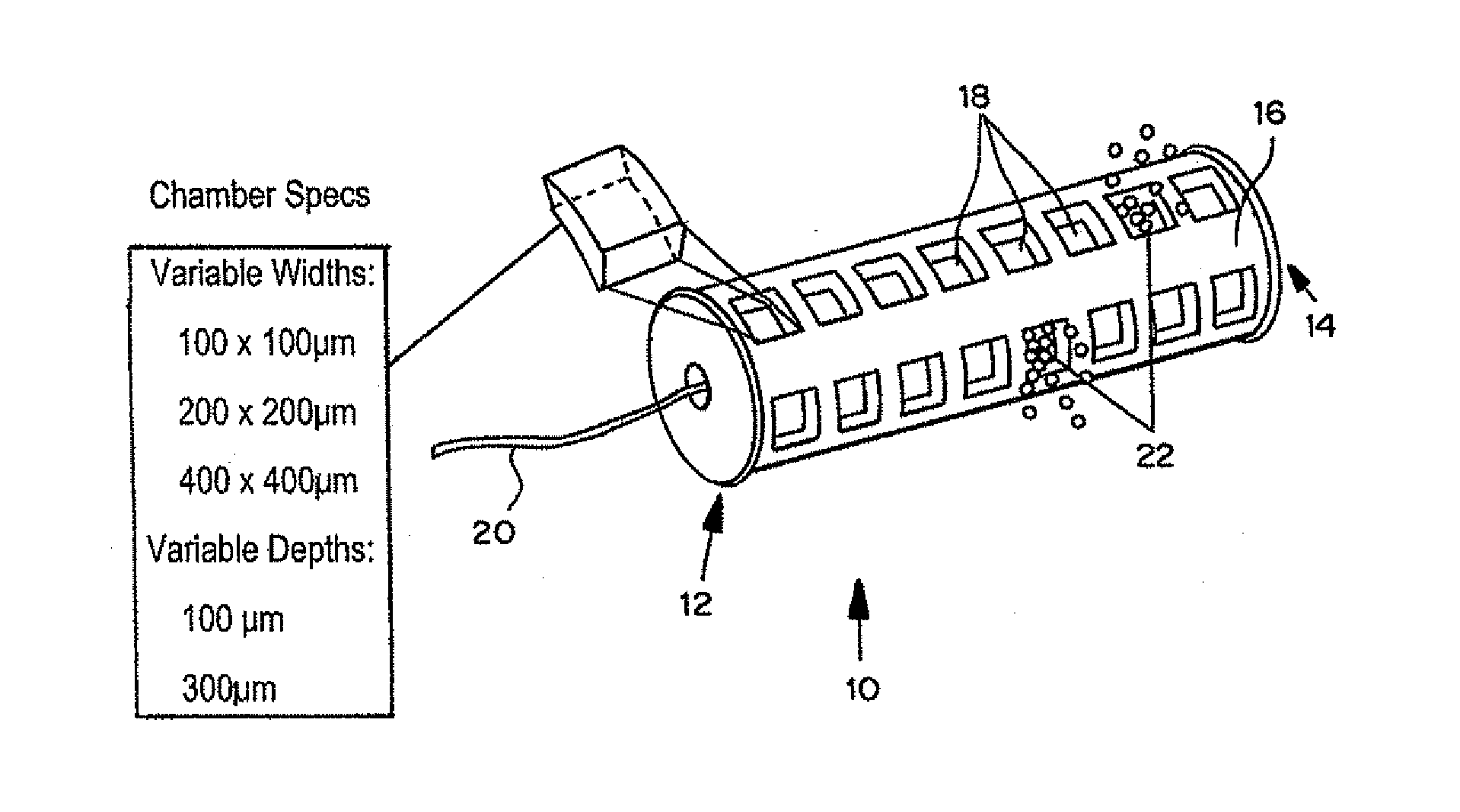
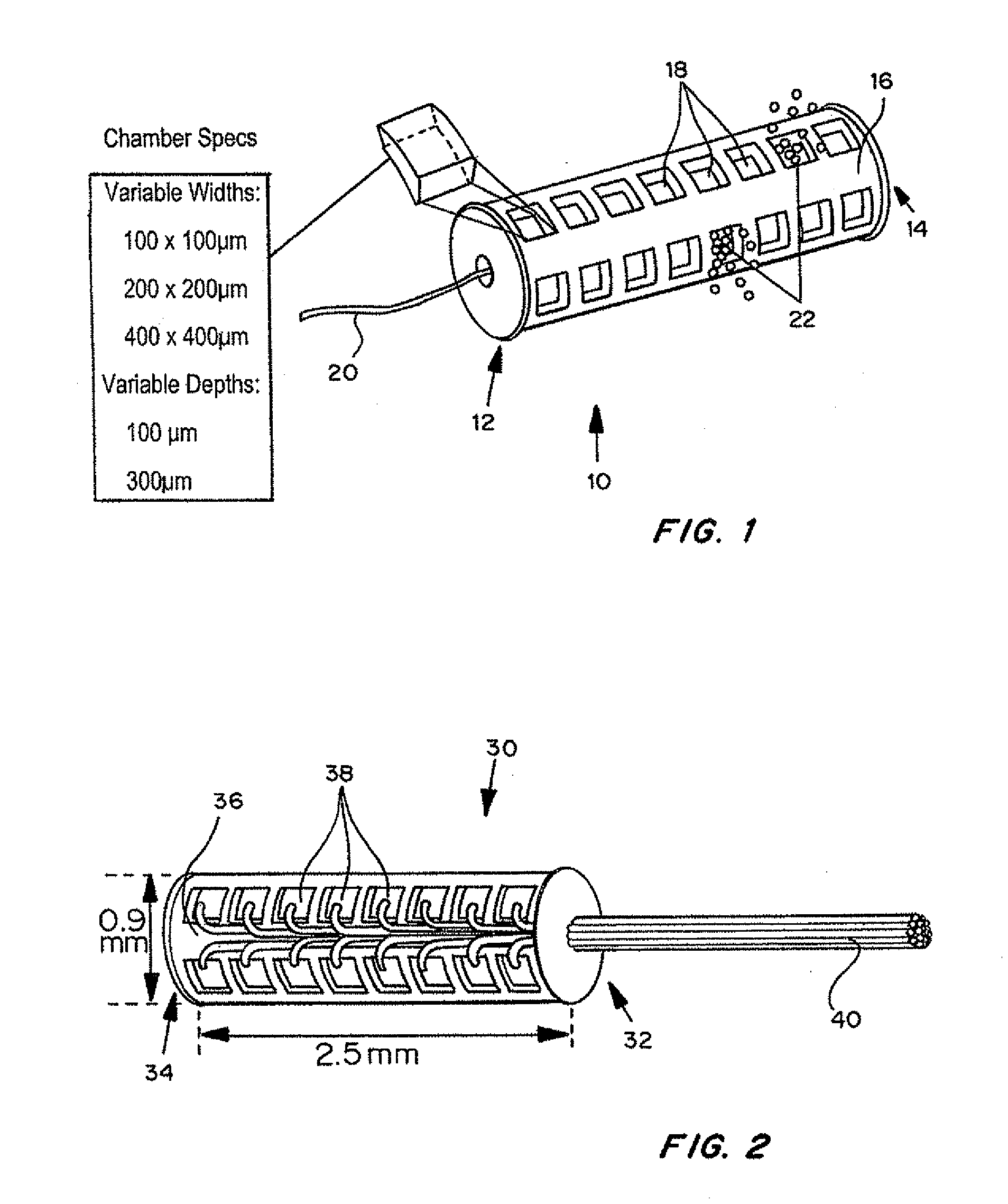
[0107]Devices were tested in approximately 50 animals for biocompatibility and integration with tissue. Data was obtained by computed tomography, magnetic resonance and histopathology.
[0108]A device with 14 reservoirs was loaded with approximately 1.5 microgram doxorubicin (crystalline powder) per reservoir. The device can be loaded with the same drugs based on the results of the systemic testing. Each drug i...
example 2
Methods for Controlled Local Release of Drugs into Tissue
[0114]Materials and Methods
[0115]Several methods for controlling the release / diffusion of compounds into tissue, including precise spatial placement of reservoirs along device mantle; geometry and size of reservoirs; and formulation of released compounds, were developed. In this manner, the device reservoirs from which the compounds diffuse are engineered to expose only regions of tissue that are directly adjacent to the reservoir opening, to the released compound. This creates distinct local regions in which the effect of compounds can be assessed without interference of other compounds released from different reservoirs.
[0116]Results
[0117]Cross-section of device shows release of two compounds. Drug A was released upward and diffused into a larger region, while Drug B was released downward into a relatively smaller region.
[0118]The precise control over the transport time as a function of distance from reservoirs is shown in F...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com