Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
67 results about "Medication abuse" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Abuse-resistant opioid solid dosage form
InactiveUS20060073102A1Prevent and discourage abuseGood curative effectOrganic active ingredientsPill deliveryN-methyl-D-aspartate Receptor AntagonistsOpioid abuse
The present invention pertains to a solid dosage form comprising an analgesically effective amount of opioid analgesic and an opioid abuse-deterring amount of a nontoxic N-methyl-D-aspartate receptor antagonist contained in a carrier which isolates, or separates, the antagonist from the opioid analgesic. The nontoxic N-methyl-D-aspartate receptor antagonist is released and made available only when the dosage form is misused, as would be the case when the dosage form is crushed or dissolved and thereafter administered in a manner other than that indicated, e.g., by injection or intranasally.
Owner:ENDO PHARMA INC
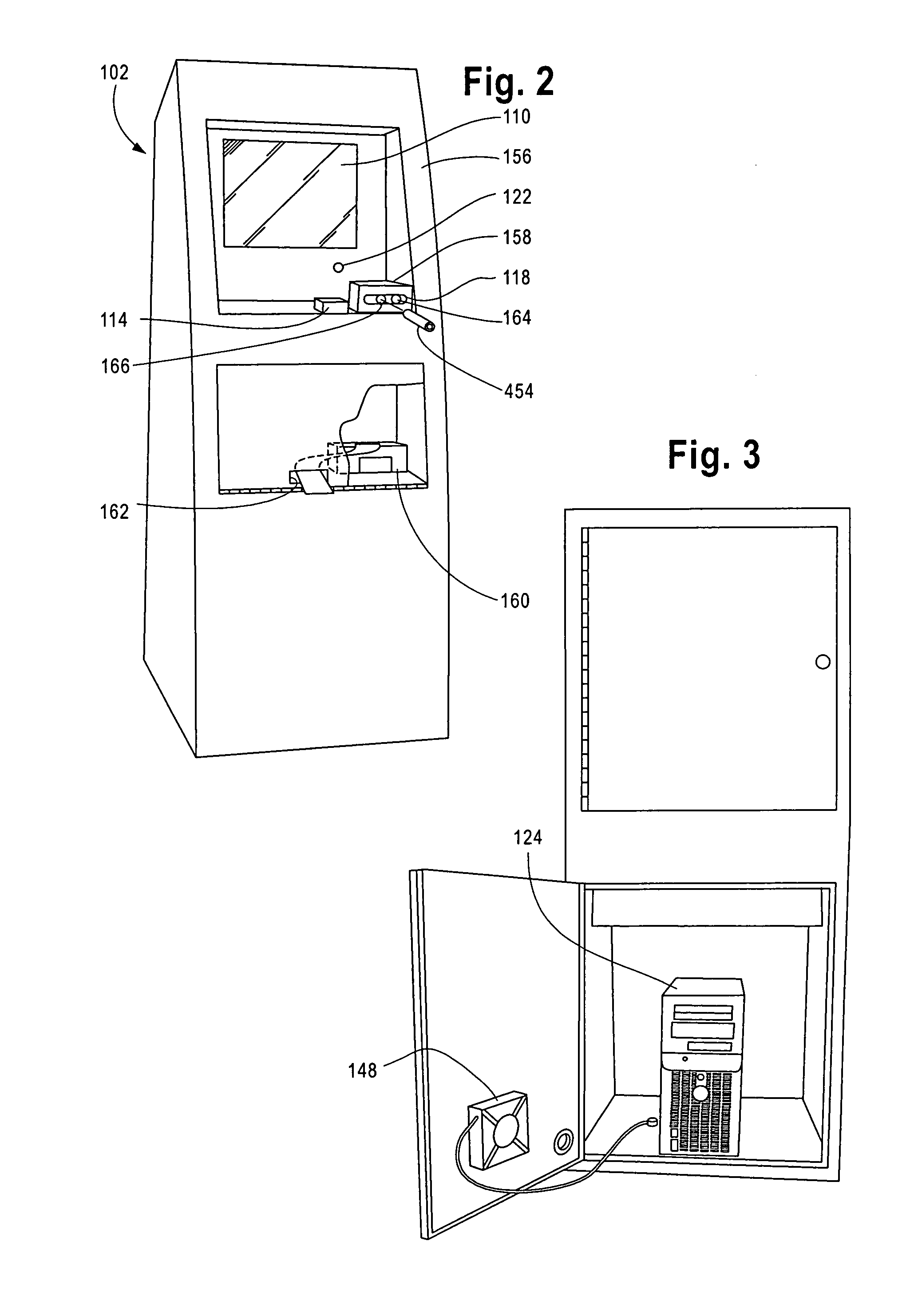
Apparatus and method for passive testing of alcohol and drug abuse
InactiveUS20100204600A1Digital data processing detailsPerson identificationCompound organicAlcohol and drug
An automated system and method for passive testing of alcohol and drug abuse. The system enters a participant or subject into the system who is to be monitored during a probationary or other program for alcohol or drug abuse offenders. The system provides a drug testing home device or a drug testing kiosk device for use by the participant. The system enrolls the biometrics information of the participant into the computer system (e.g., finger print, voice, image, volatile compound organic gas level, and pH level). When the participant is to be tested in accordance with a testing schedule, the system validates these same biometrics of the participant, conducts the test, and then analyzes the test information for determining if the participant has been using alcohol or other drugs and should be subjected to a confirming urinalysis exam.
Owner:JUSTICE EZ TRAC
Oral dosage form comprising an antimisuse system
InactiveUS20080008659A1Fast absorptionAvoid misuseHeavy metal active ingredientsNervous disorderSolventDrug abuser
Owner:FLAMEL IRELAND
Preventing or reducing drug abuse and overdose events
ActiveUS20130034503A1Reducing drug abuseEliminate orNervous disorderIn-vivo testing preparationsDrug abuse testDrug
A method and compositions for treating a patient that prevent or reduce drug abuse and overdose events.
Owner:KAVESH SHELDON
Oral Film Containing Opiate Enteric-Release Beads
A control release and abuse-resistant opiate drug delivery oral wafer or edible oral film dosage to treat pain and substance abuse is provided. The drug delivery oral wafer or edible oral film dosage includes a controlled release layer containing enteric-release beads dispersed in a polymer matrix. The enteric-release beads are formed from a therapeutic amount of an opioid agonist and / or pharmaceutically acceptable salt thereof and a sub-therapuetic amount of opioid antagonist and / or pharmaceutically acceptable salt thereof coated or encapsulated in an enteric-release polymer. The controlled release polymer matrix dissolves or disintegrates following administration or consumption of the oral wafer or edible oral film, releasing the enteric-release beads to be swallowed, with subsequent absorption of the active ingredients within the patient's intestines.
Owner:LTS LOHMANN THERAPIE-SYST AG
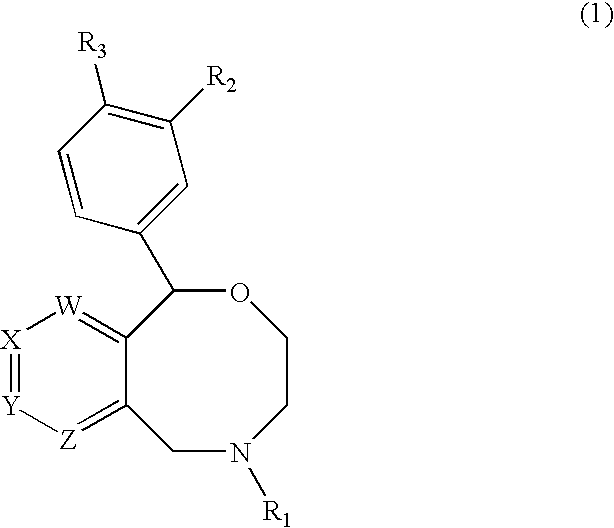
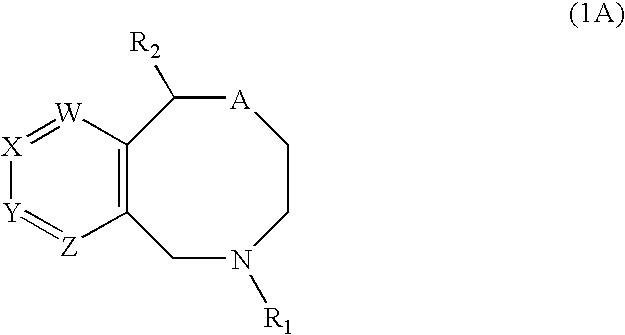
Novel benzoxazocines and their therapeutic use
Compounds of the general formula (1), wherein one of W, X, Y and Z is N or CR4 and the others are each CH; and R4 is a specified substituent. These compounds inhibit monocamine reuptake, and are useful in the treatment of pain, emesis depression, post traumatic stress disorders, attention deficit disorders, obsessive compulsive disorders, pre-menstrual syndrome, substance abuse and sexual dysfunction.
Owner:SOSEI R&D LIMITED
Methods and compositions for preventing opioid abuse
ActiveUS20160326182A1Preventing opioid abuseOrganic active ingredientsOrganic chemistryChemical MoietyOpioid abuse
Abuse-resistant opioid compounds, drug delivery systems, pharmaceutical compositions comprising an opioid covalently bound to a chemical moiety are provided. Methods of delivering an active ingredient to a subject and methods of preventing opioid abuse are also provided.
Owner:3ST RES LLC +1
19-nor c3, 3-disubstituted c21-c-bound heteroaryl steroids and methods of use thereof
ActiveUS20160083417A1Eliminate potential for oxidationInhibit metabolismOrganic active ingredientsNervous disorderDiseaseSubstance abuser
Provided herein are 19-nor C3,3-disubstituted steroids of Formula (I): and pharmaceutically acceptable salts thereof; wherein, , R1, R2, R3a, R3b, R4a, and R4b are as defined herein, and A is a carbon bound substituted or unsubstituted 5- to 6-membered heteroaryl ring as defined herein. Such compounds are contemplated useful for the prevention and treatment of a variety of CNS-related conditions, for example, treatment of sleep disorders, mood disorders, schizophrenia spectrum disorders, convulsive disorders, disorders of memory and / or cognition, movement disorders, personality disorders, autism spectrum disorders, pain, traumatic brain injury, vascular diseases, substance abuse disorders and / or withdrawal syndromes, and tinnitus.
Owner:SAGE THERAPEUTICS
Multimediator transporter inhibitors for use in treatment of central nervous system disorders
InactiveUS20100093706A1Improve functionalityTreatment for depressionBiocideNervous disorderDiseaseSubstance abuser
The invention provides a class of inhibitors, packaged pharmaceuticals comprising such inhibitors, and uses of the inhibitors in treating, or the manufacturing medicaments for treating central nervous system disorders, including depression, anxiety, sleep disorders, obesity, attention deficit disorder (ADD), attention deficit hyperactivity disorder (ADHD), sexual dysfunction, substance abuse, and movement disorders. Related business methods, such as methods for conducting a pharmaceutical business and methods for conducting a medical assistance reimbursement program, are also provided.
Owner:PREXA PHARMA
Oral dosage form comprising an antimisuse system
InactiveUS8895063B2Fast absorptionAvoid misuseCosmetic preparationsHeavy metal active ingredientsDosage formCombinatorial chemistry
An oral solid dosage form containing one or several active principle(s) having analgesic properties, the composition of said dosage form being such that it prevents the misuse of said dosage form through the liquid extraction of the active principle(s) contained therein, using commonly available solvents.Said oral solid dosage form containing at least one salt of at least one analgesic active principle, and an anti-misuse system comprising at least one quenching agent, said quenching agent being suitable for inducing complexation of said analgesic active principle salt when the analgesic active principle salt is improperly extracted, notably by a drug abuser, in vitro in solution from said oral solid dosage form.
Owner:FLAMEL IRELAND
Chimeric hybrid analgesics
InactiveUS6881829B2Little and no developmentUndesirable side-effectNervous disorderAntipyreticTolerabilityWhole body
The present invention provides composition of matter for and methods of treating pain and drug abuse using novel chimeric hybrid molecules containing an opioid moiety of chemically modified morphine (3) that binds to and activates the human mu (μ) opioid receptor, with the opioid moiety linked through a novel linker-hinge (4) to a substance P peptide fragment moiety (5) that binds to and activates the human substance P receptor. The hybrid alkaloid / peptide chimeric molecules produce clinically efficacious opioid analgesia with little or no development of opioid tolerance or formation of opioid dependence. The hybrid alkaloid / peptide analgesics may be administered intrathecally, systemically or orally.
Owner:CHIMERACOM LLC
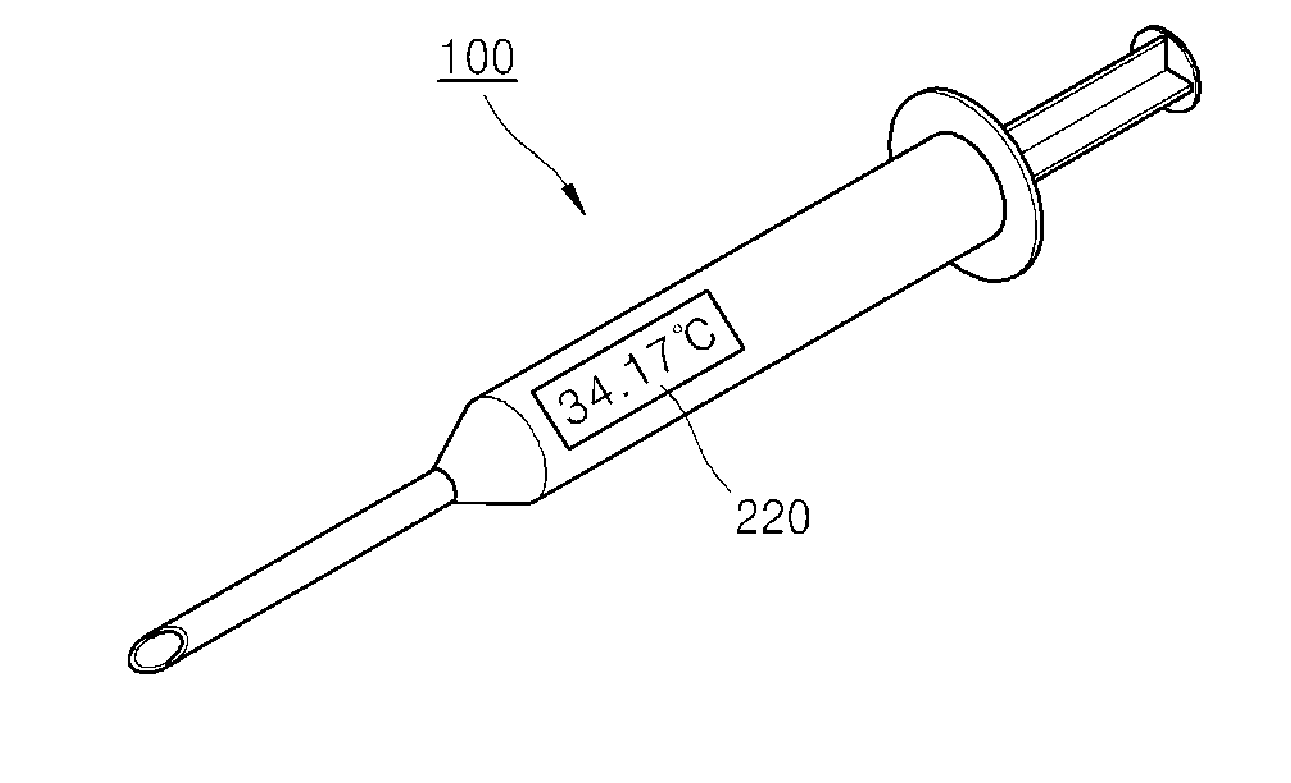
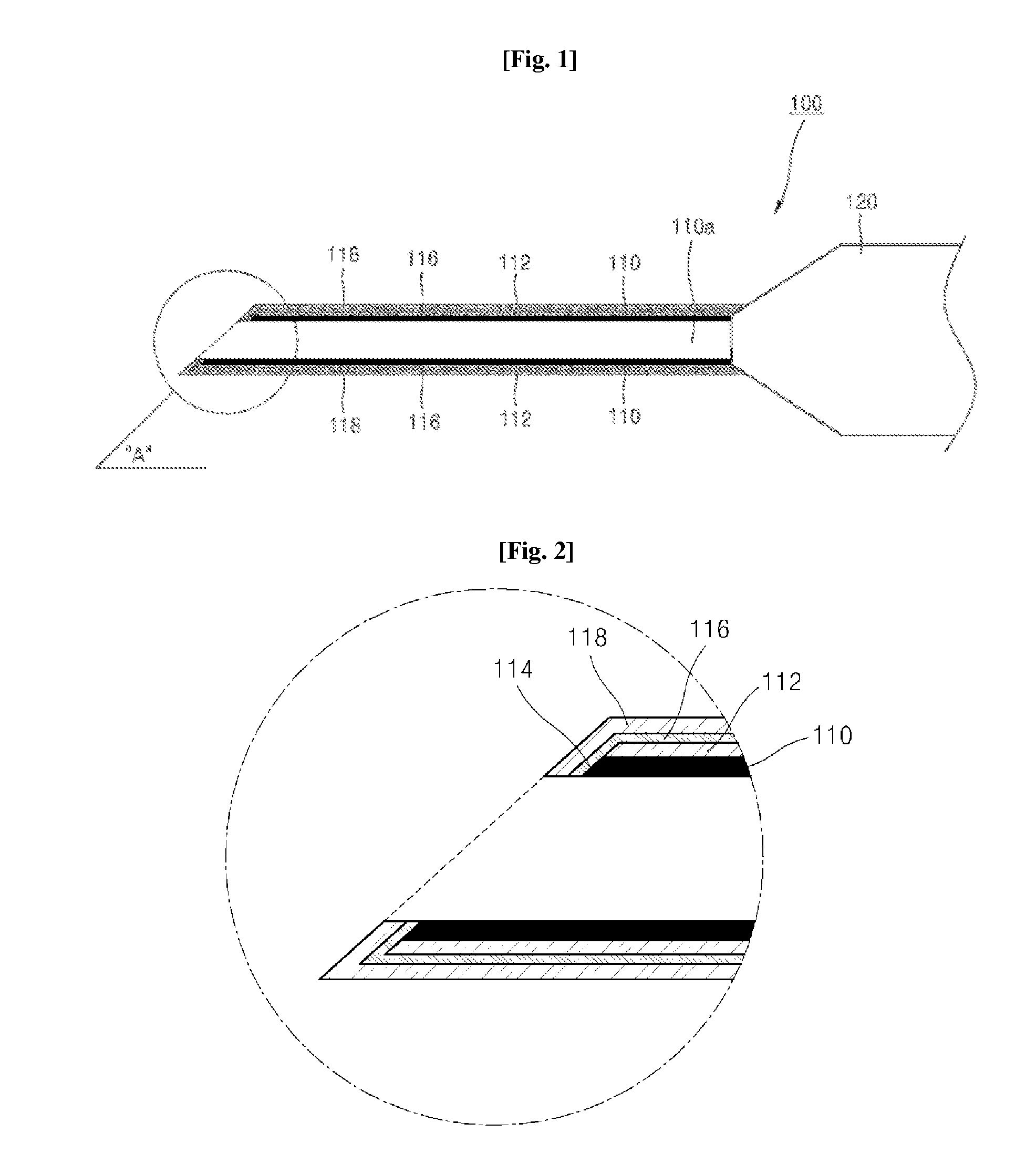
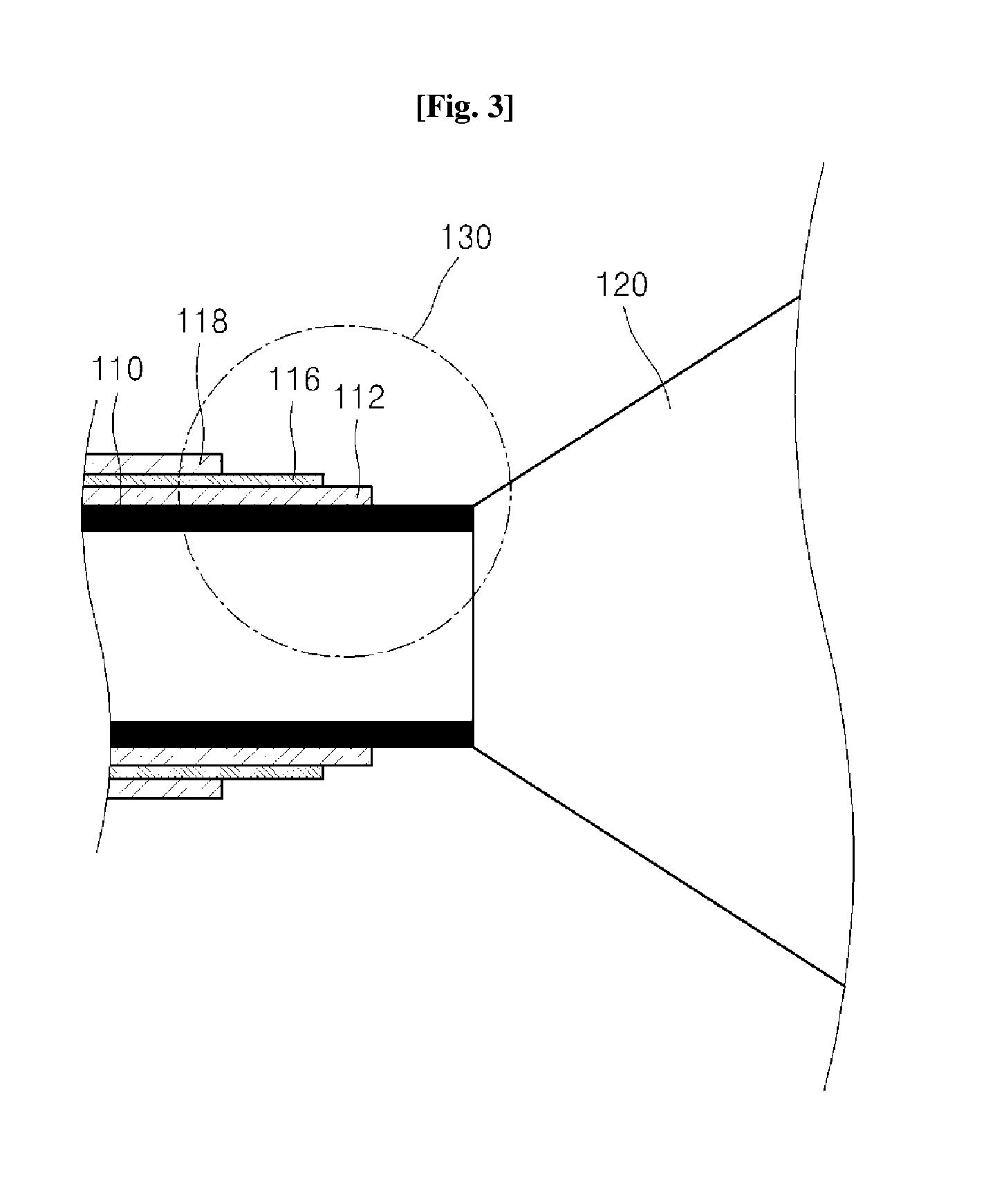
Syringe capable of measuring temperature of a patient body and method of manufacturing the same
InactiveUS20140031758A1Easy to measureDrug and medicationsInfusion syringesSubstance abuseSyringe needle
Disclosed are a syringe capable of measuring an inner temperature of a patient body and a method of manufacturing the same. In the syringe, a different metal from that of a syringe needle is deposited on an inclined surface of a tip portion of the syringe needle to form a thermocouple junction with the metal of the syringe needle, whereby the region causing pain in a patient body can be diagnosed while allowing administration of medicine thereto, thereby enabling efficient treatment and significantly reducing potential harm due to drug abuse.
Owner:GWANGJU INST OF SCI & TECH
Oral film containing opiate enteric-release beads
A control release and abuse-resistant opiate drug delivery oral wafer or edible oral film dosage to treat pain and substance abuse is provided. The drug delivery oral wafer or edible oral film dosage includes a controlled release layer containing enteric-release beads dispersed in a polymer matrix. The enteric-release beads are formed from a therapeutic amount of an opioid agonist and / or pharmaceutically acceptable salt thereof and a sub-therapeutic amount of opioid antagonist and / or pharmaceutically acceptable salt thereof coated or encapsulated in an enteric-release polymer. The controlled release polymer matrix dissolves or disintegrates following administration or consumption of the oral wafer or edible oral film, releasing the enteric-release beads to be swallowed, with subsequent absorption of the active ingredients within the patient's intestines.
Owner:LTS LOHMANN THERAPIE-SYST AG
Propellane compounds, preparation method and application thereof
InactiveCN101402636ANo side effectsOrganic active ingredientsNervous disorderPropellaneSubstance abuse
The invention discloses propellane compounds, a preparation method and application thereof, in particular aromatic or fat thick heterocyclic propellane compounds, a preparation method thereof and applications for treating drug abuse as an opioid receptor excitant or an antagonist and for using as a novel analgesic agent and so on. The structures of the compounds are shown in a general formula (I).
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
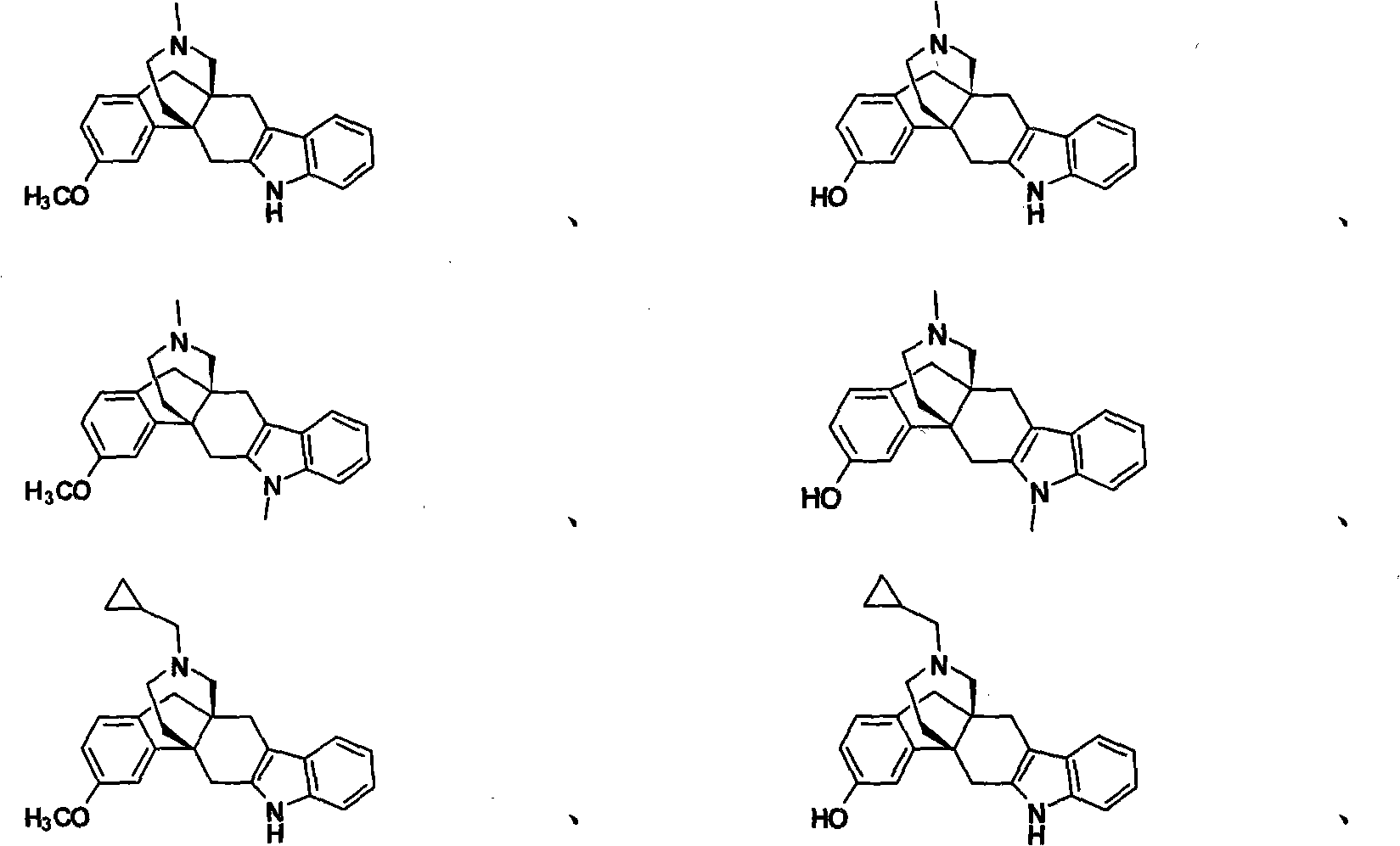
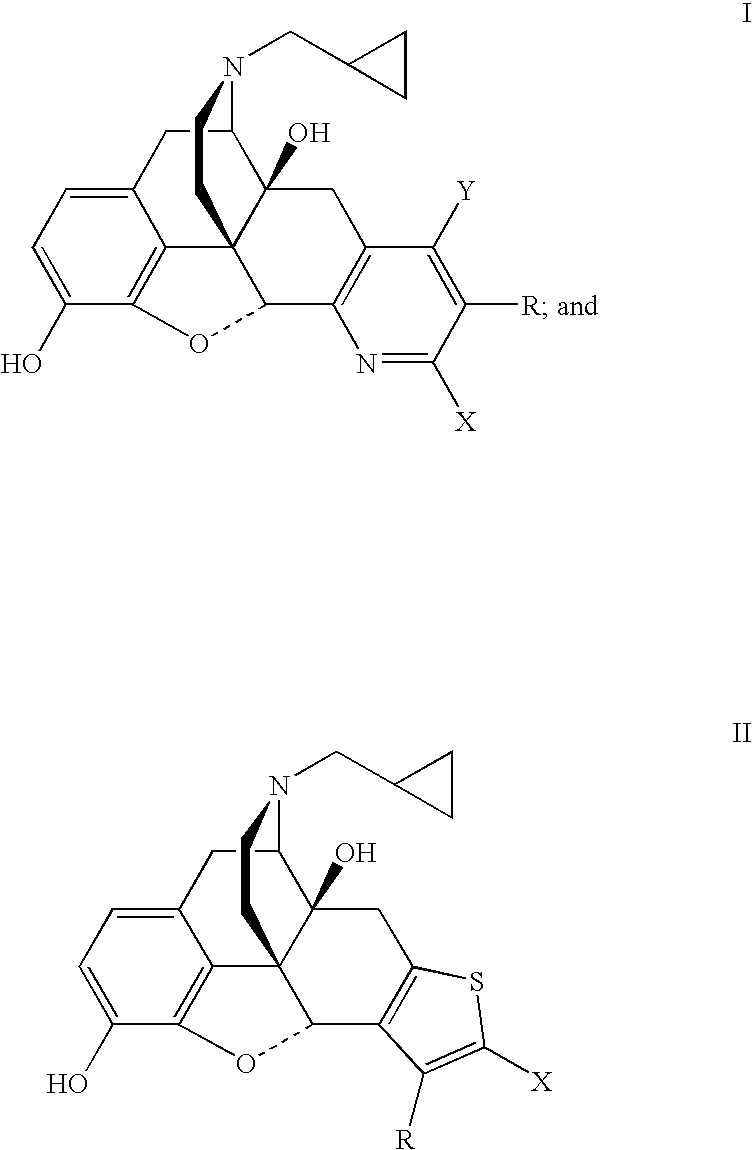
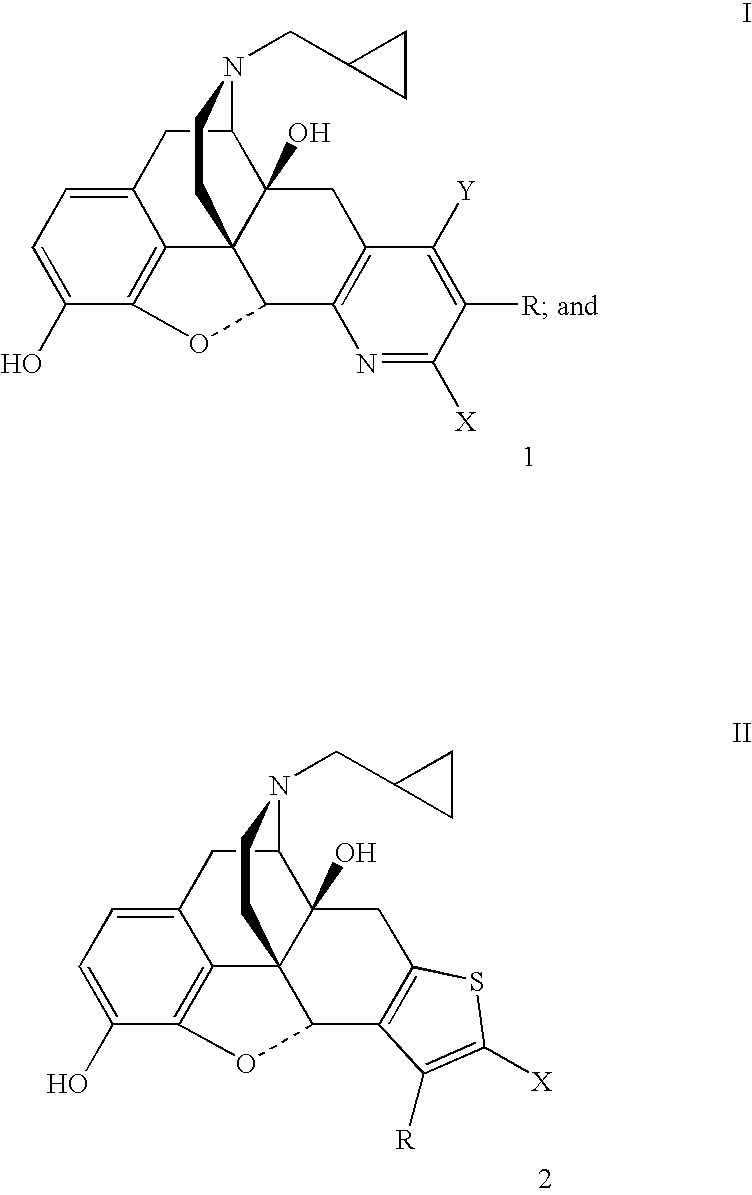
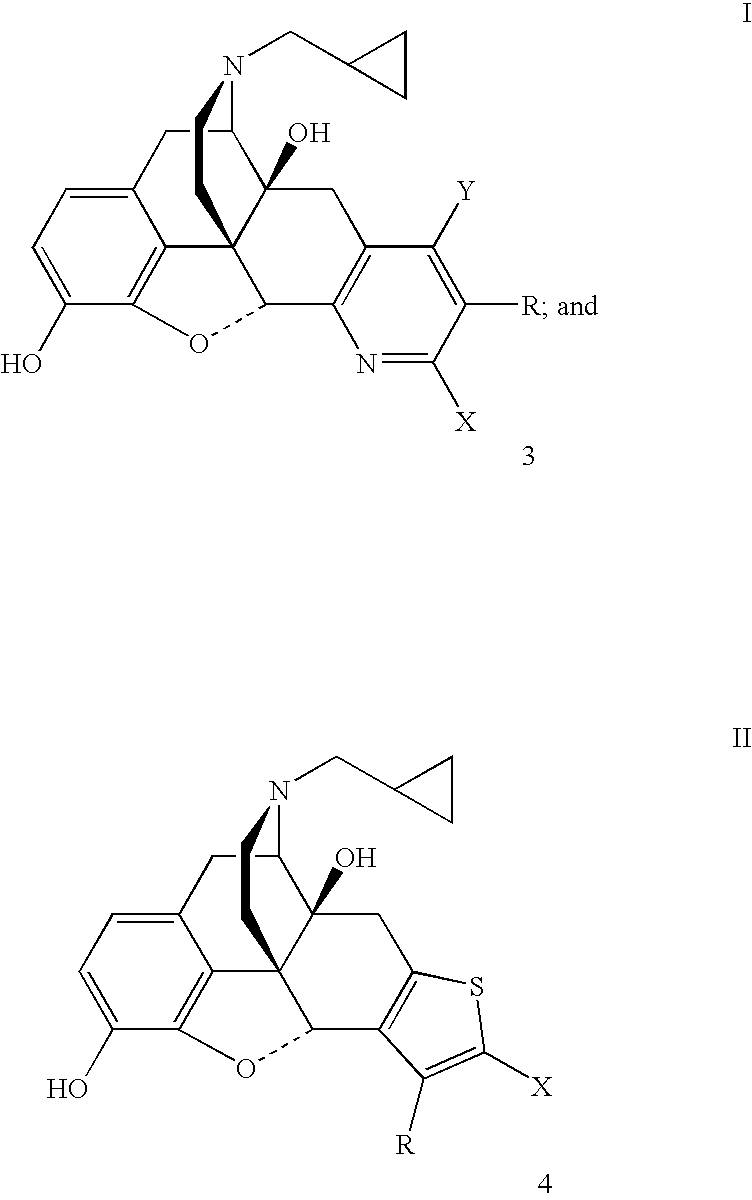
Pyridomorphinans, thienomorphinans and use thereof
InactiveUS7015326B1Modulate toleranceEfficient modulationOrganic chemistryHydrogenMedicinal chemistry
Compounds represented by the formulae: wherein each of Y, X and R individually is selected from the group consisting of hydrogen hydroxy, halo, CF3, NO2, CN, NH2, COR1 and CO2R2 wherein R1 is selected from the group consisting of alkyl, aryl, alkaryl, and NH2, and R2 is selected from the group consisting of alkyl aryl, and aralkyl, and provided that at least one of Y, X and R in formula I is other than hydrogen; and pharmaceutically acceptable salts thereof are provided along with uses as immunodulaters and / or treating for drug abuse and / or as analegesics for treating pain.
Owner:SOUTHERN RES INST & IP
Mobile phone software for behavior intervention aiming at MSM
The invention relates to mobile phone software for behavior intervention aiming at MSM. The mobile phone software for MSM behavior intervention comprises modules of health assessment, health education, behavior change planning, forum and the like. Behavior assessment aiming at MSM can be performed, a pertinent behavior change plan and a personal incentive plan can be formulated, thus daily behavior feedback and weekly behavior assessment can be performed, education of knowledge about AIDS can be performed, sexual behaviors, drug abuse, medication compliance for antiviral therapy of HIV positive patients and the like can be intervened, and thus the purposes of preventing AIDS virus infection and promoting physical and psychological health can be achieved.
Owner:严谨
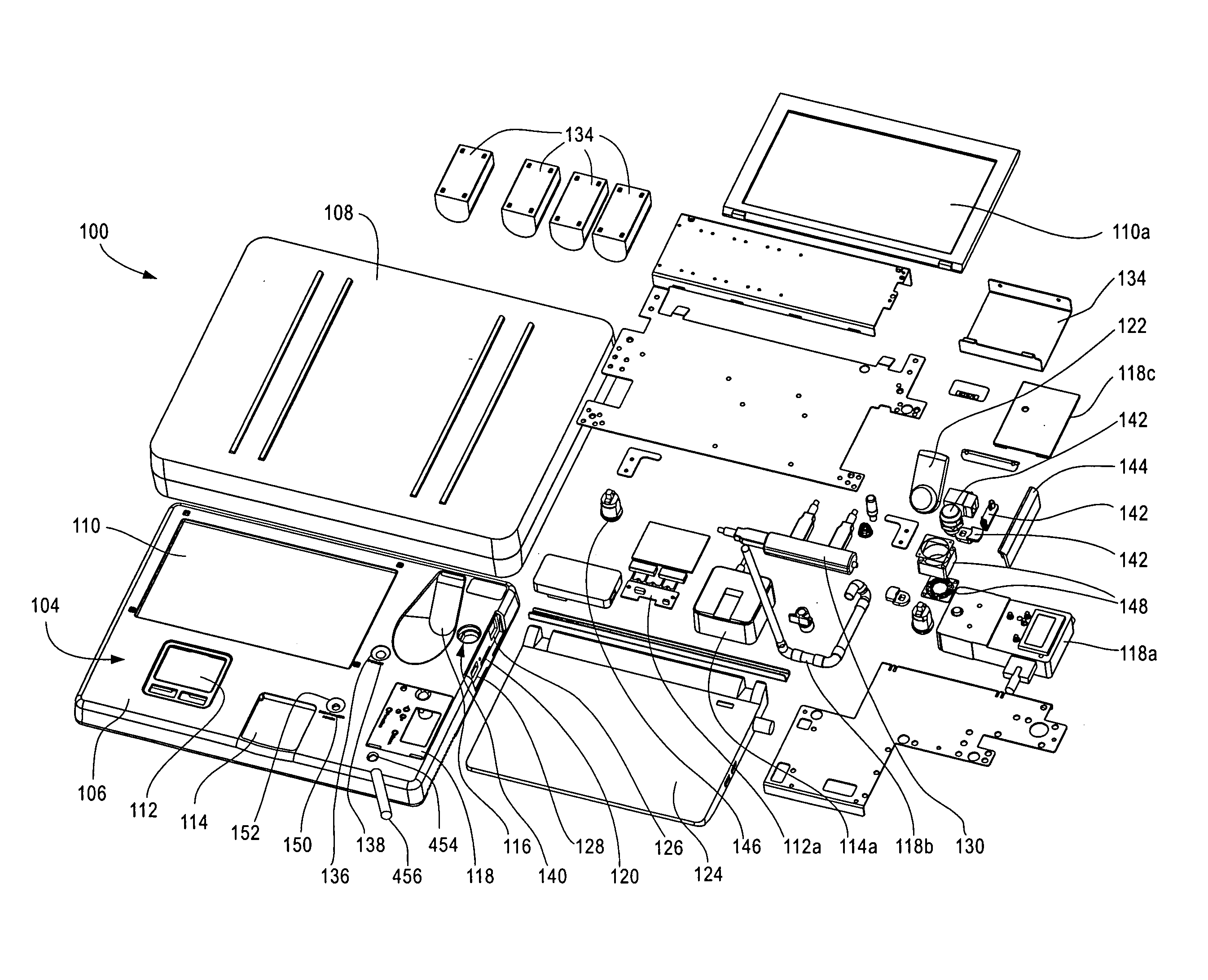
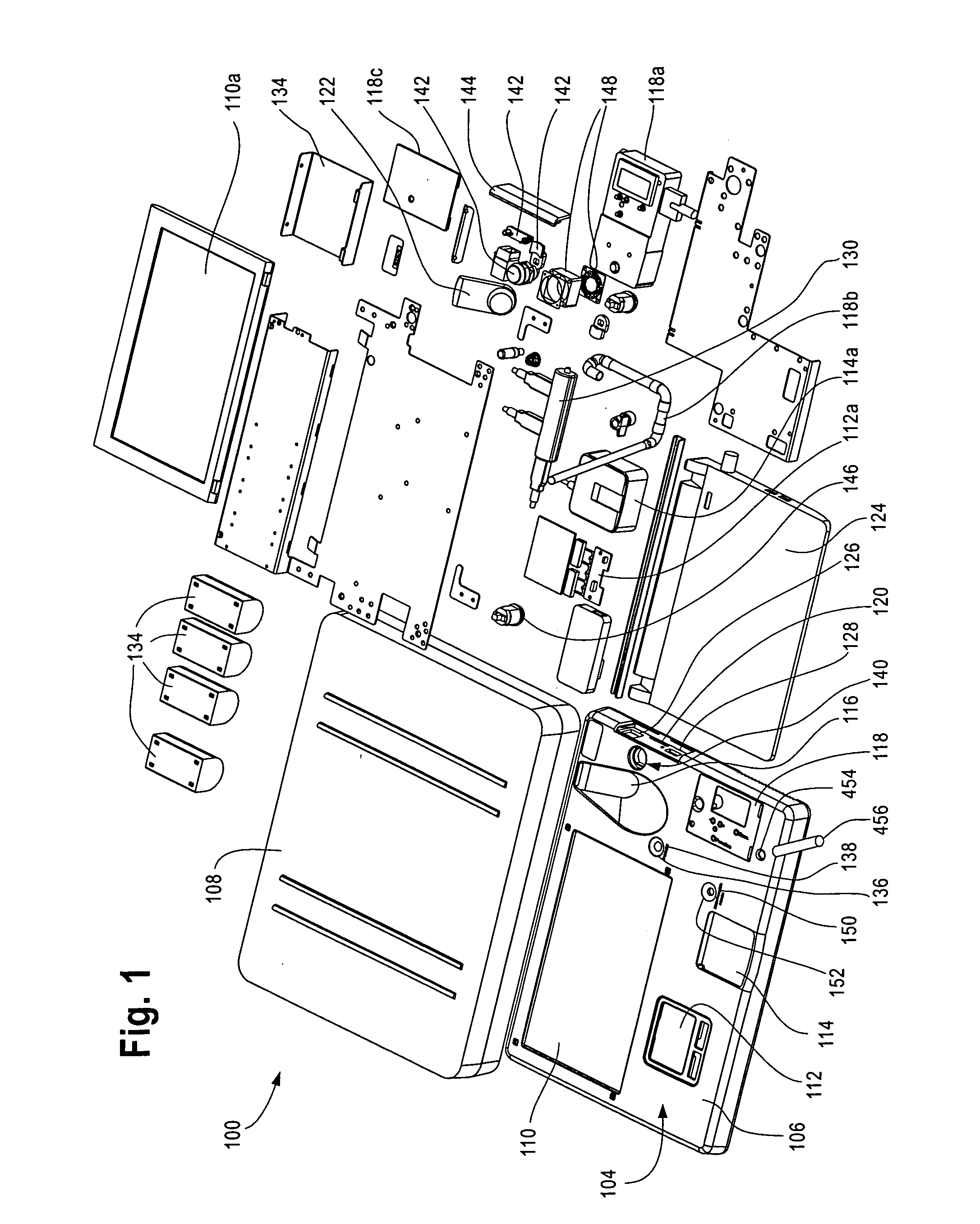
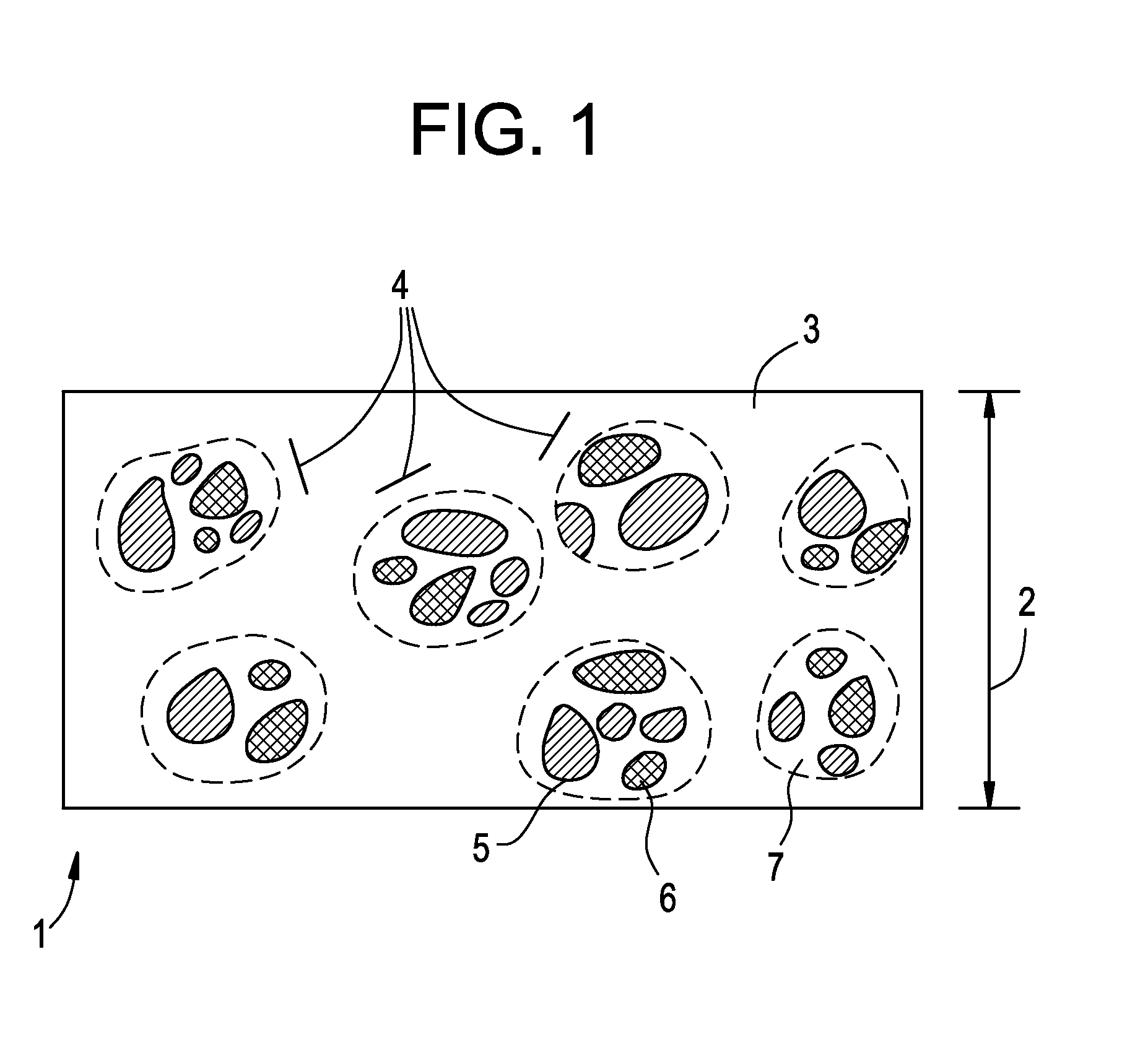
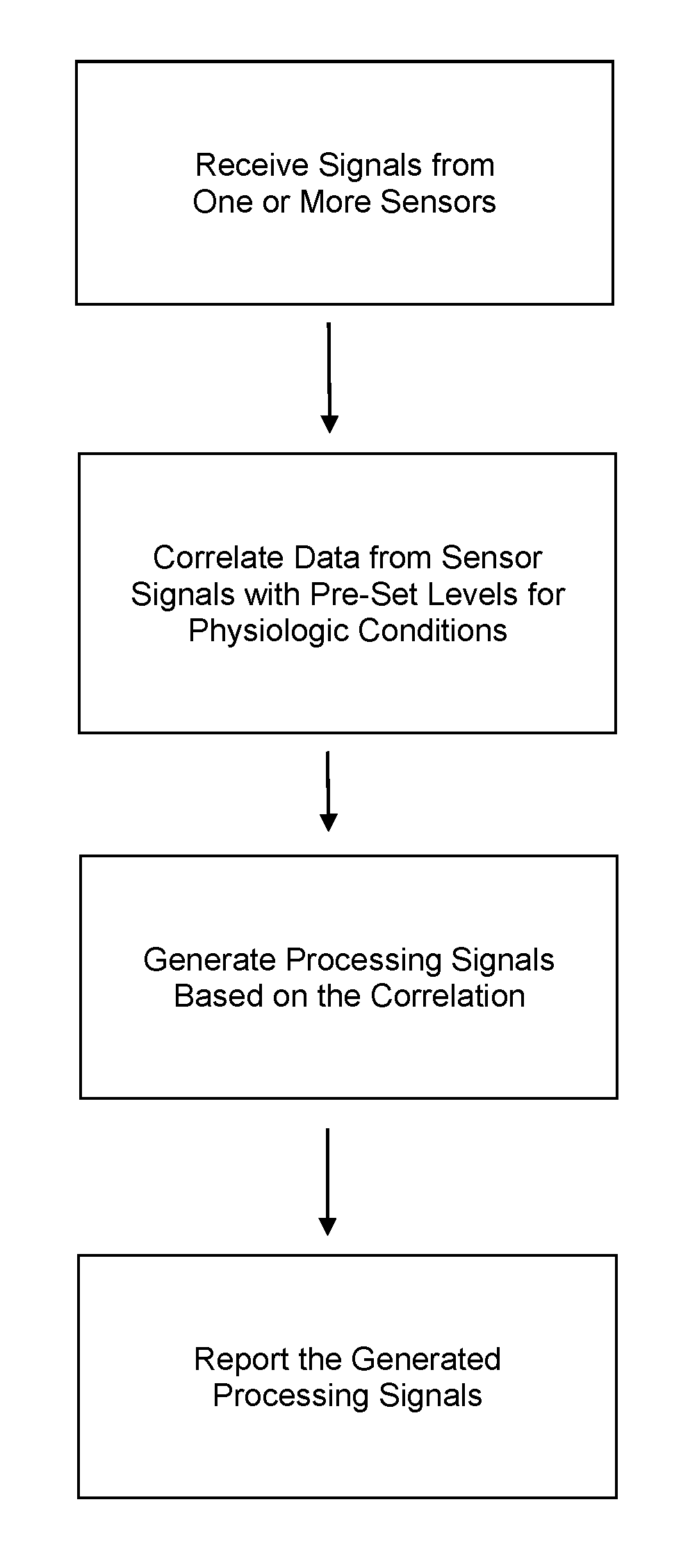
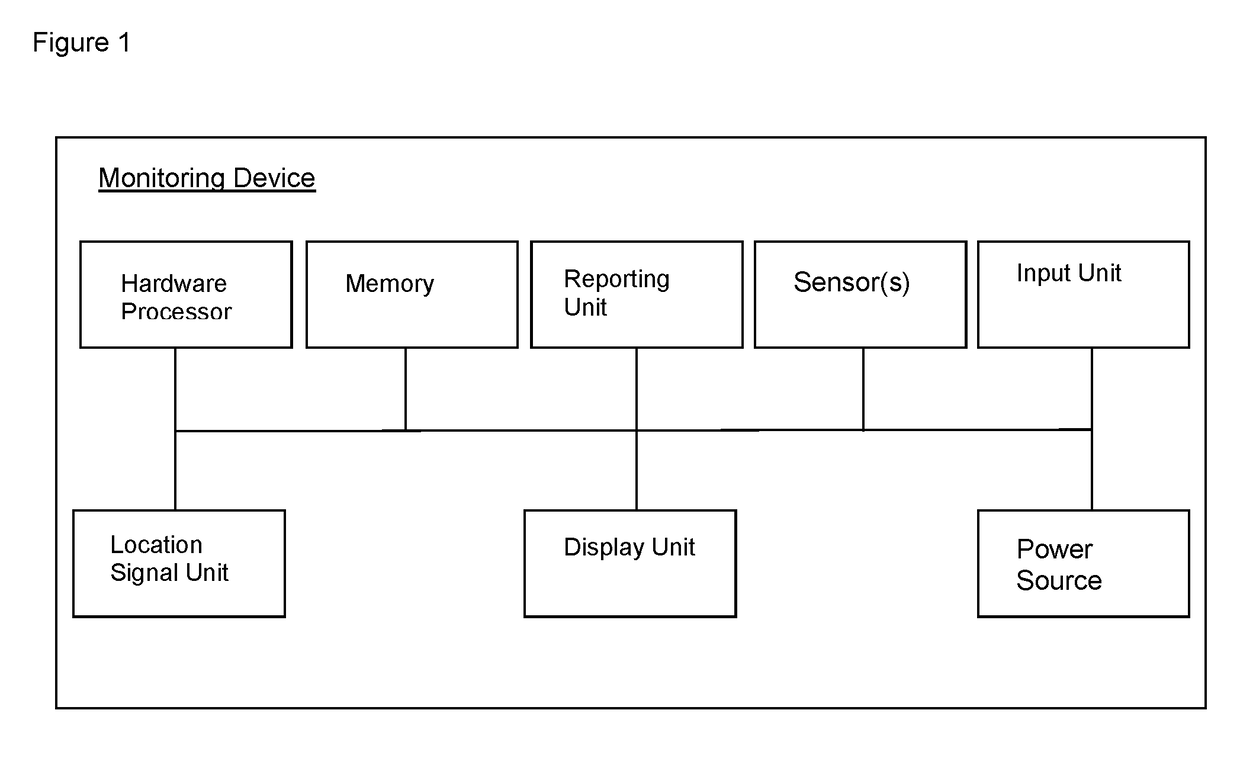
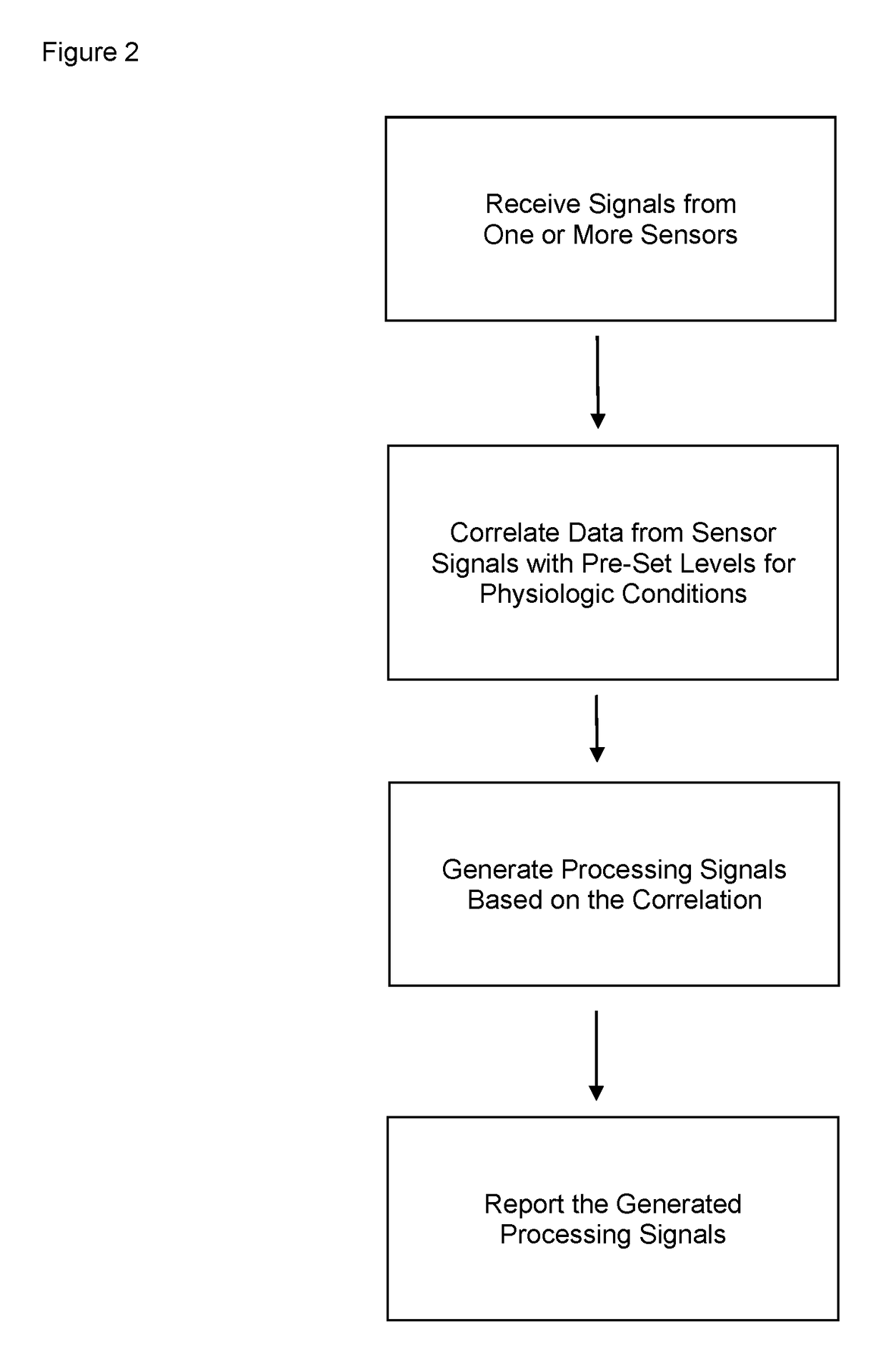
Device and methods for monitoring or preventing misuse or abuse of analgesics
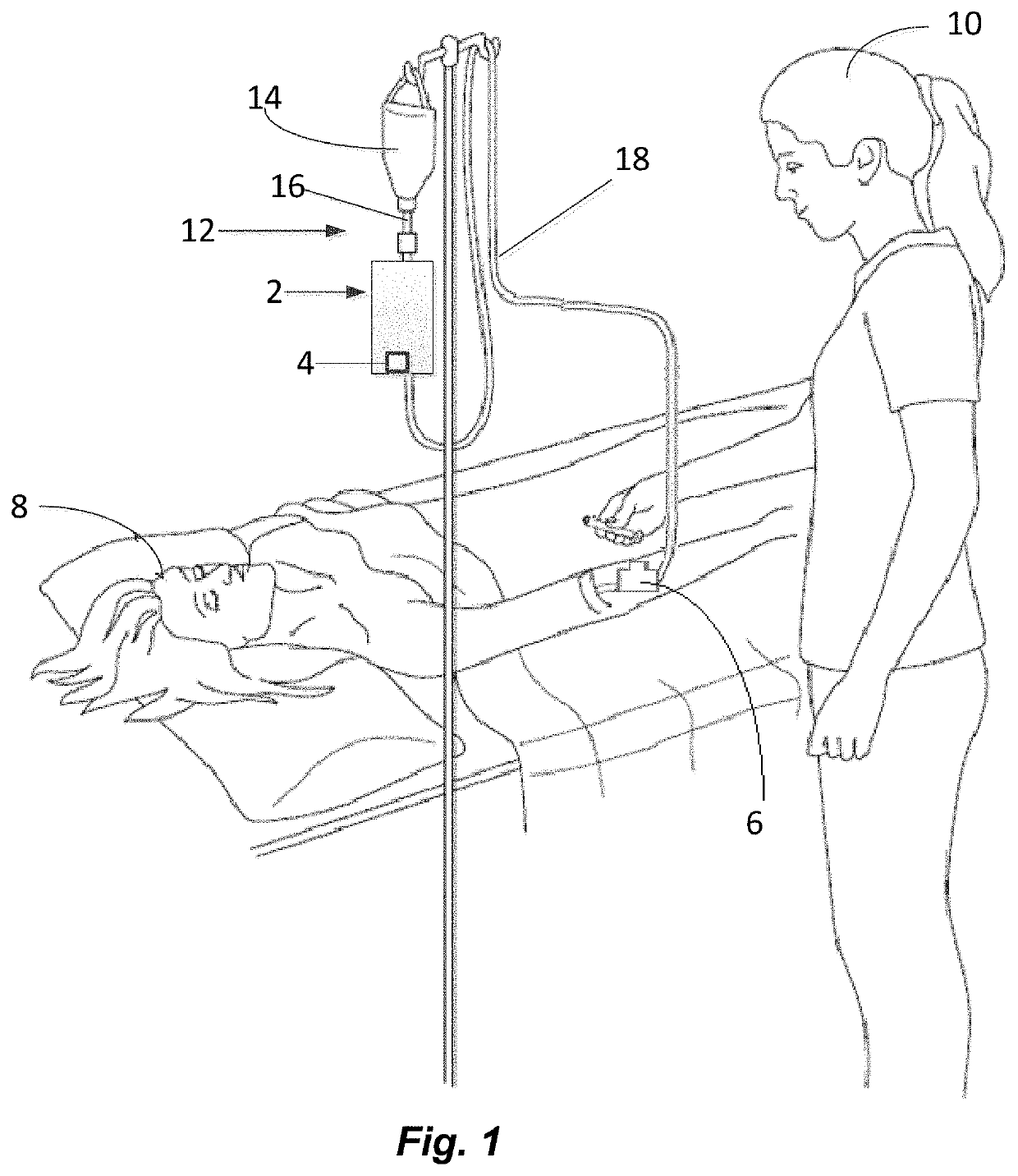
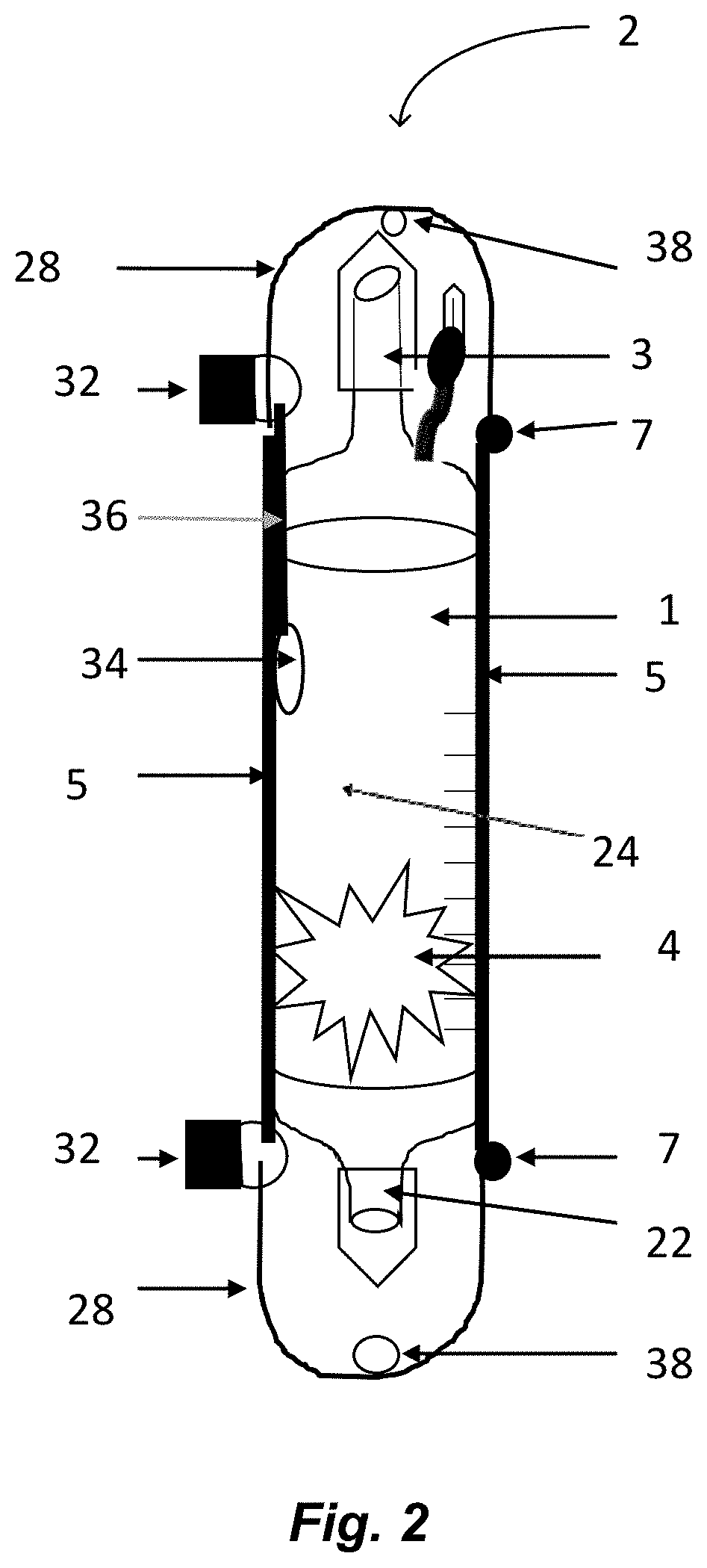
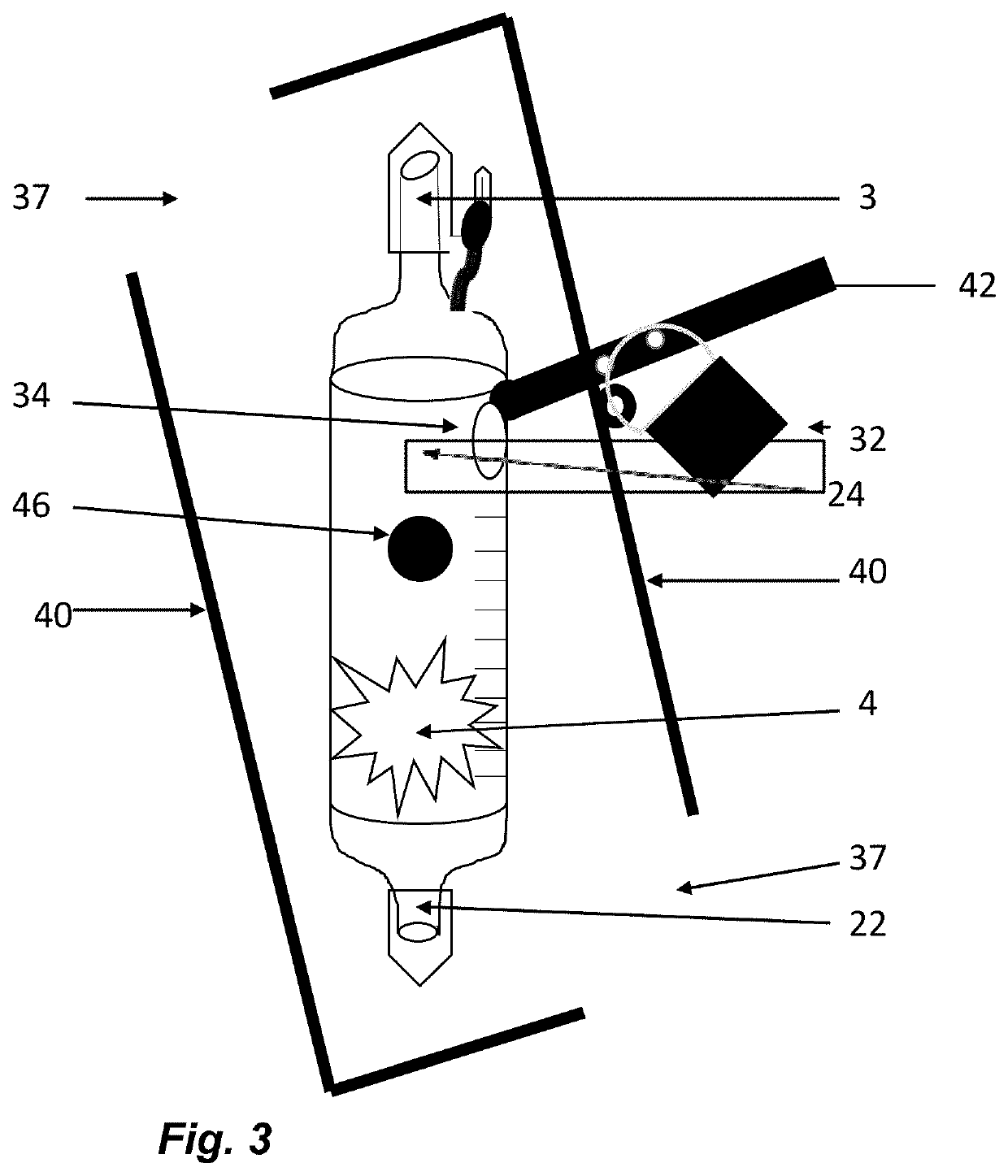
The invention relates to methods and devices for measuring or monitoring a subject undergoing one or more therapeutic treatments in real time to prevent drug abuse or misuse. The devices of the invention are intended to be worn or carried by the subjects, and they can thus be defined as portable or wearable devices. The devices are further configured to monitor the subjects and to prevent potential drug abuse or drug overdose.
Owner:ISVIAL LLC
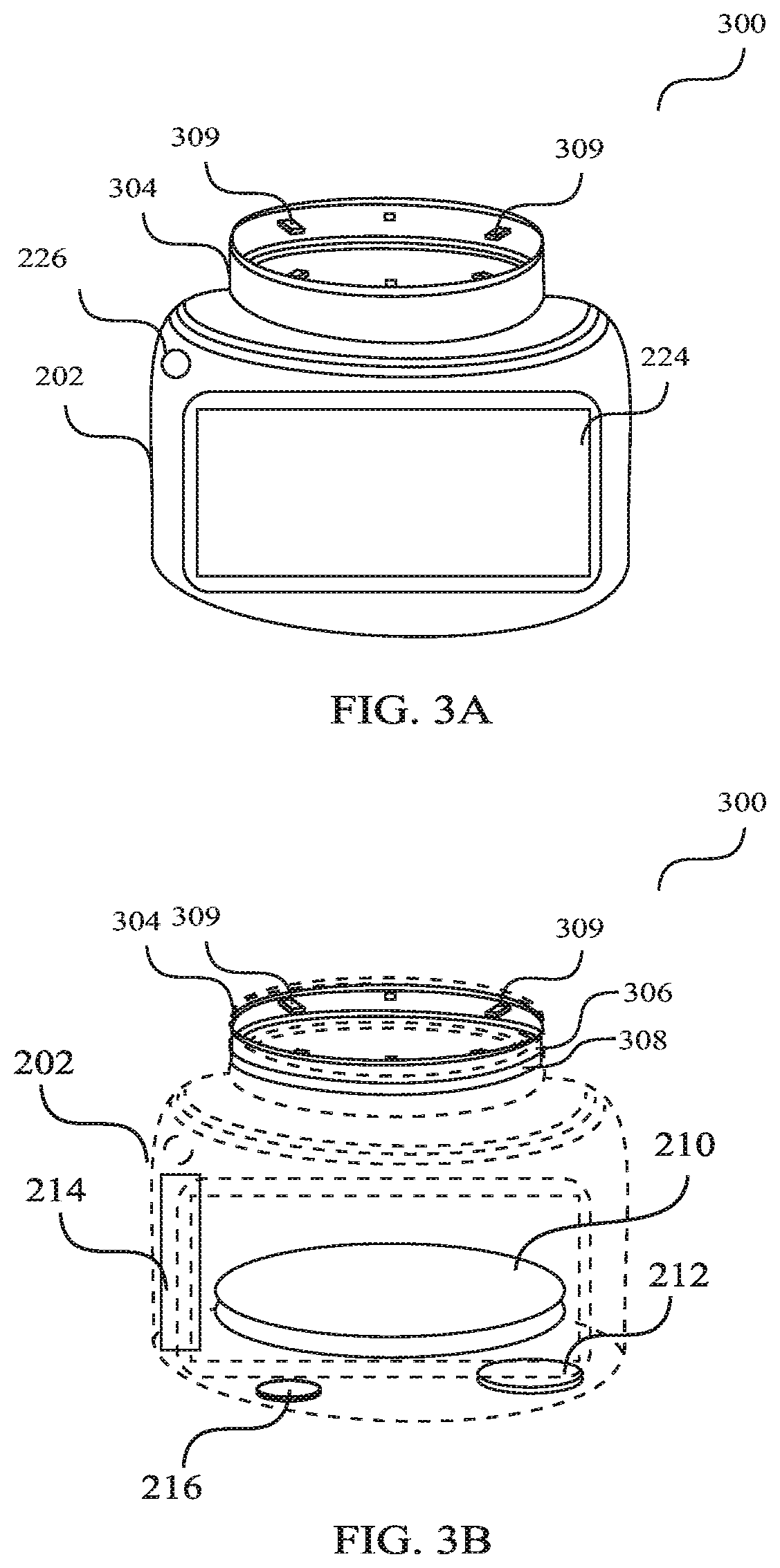
Device for deterring abuse of drugs
A device for deterring drug abuse includes a housing configured to contain a drug accessible through an access port. The device includes a physical deterrent configured to move from an unsecured position, wherein the access port of the housing is normally accessible, to a secure position, wherein the access port of the housing is normally inaccessible. The device further includes a locking mechanism that is coupled with the physical deterrent. The locking mechanism has a locked mode, wherein the physical deterrent does not normally move from the secure position to the unsecured position. The locking mechanism is further coupled with a deterrent container having a deterrent substance. The device is configured such that attempts to access the drug when the physical deterrent is in the secure position, and when the locking mechanism is in the locked mode, activate the release of the deterrent substance to the drug.
Owner:EIGHTY EIGHT PHARMA INC
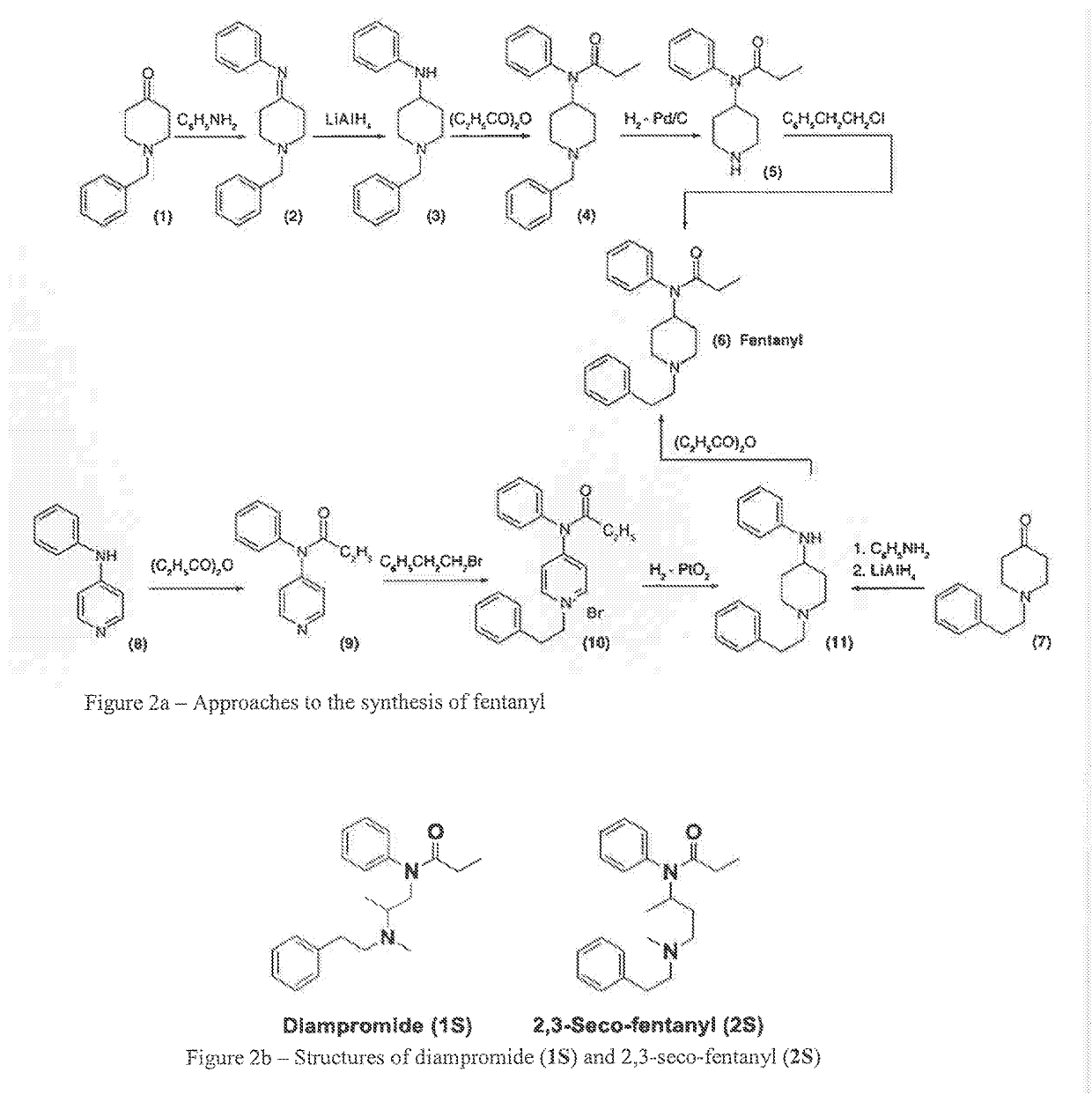
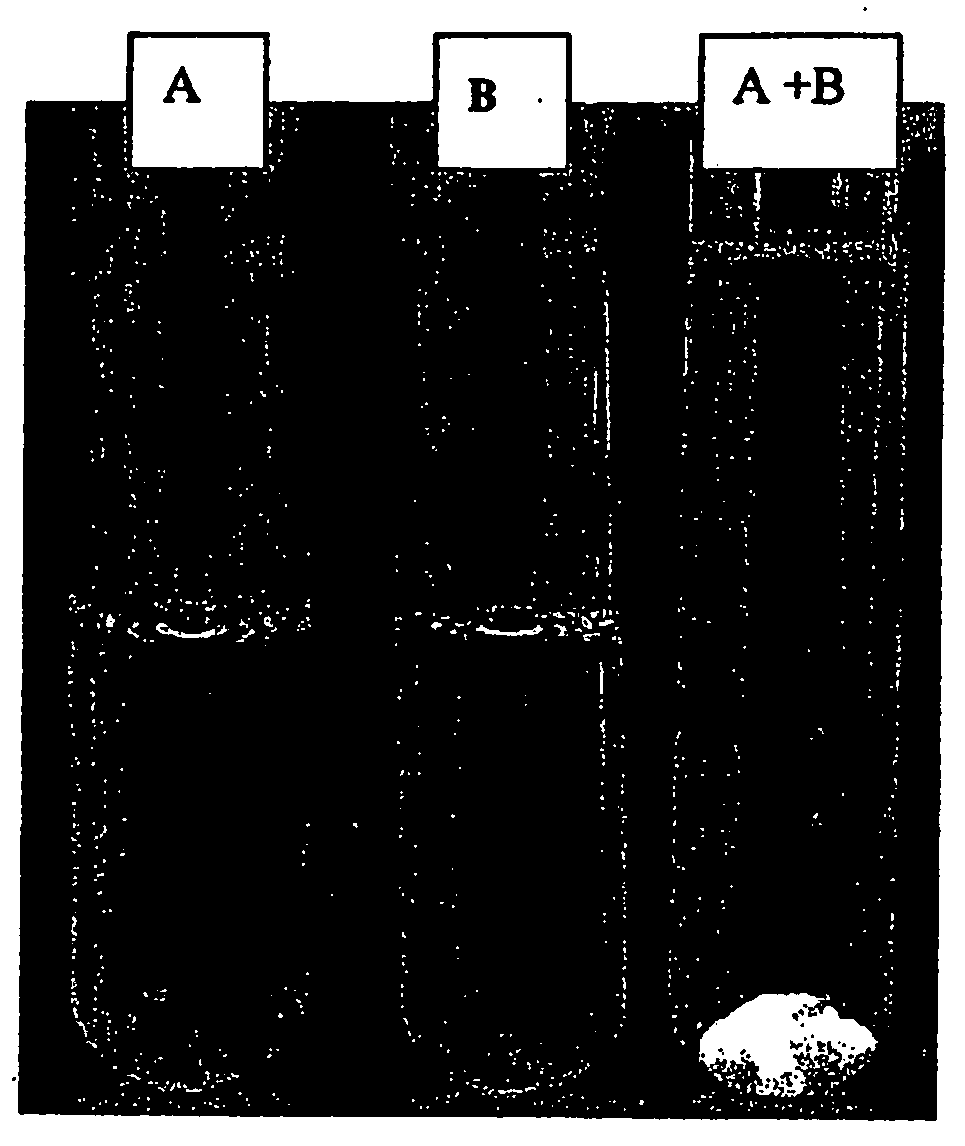
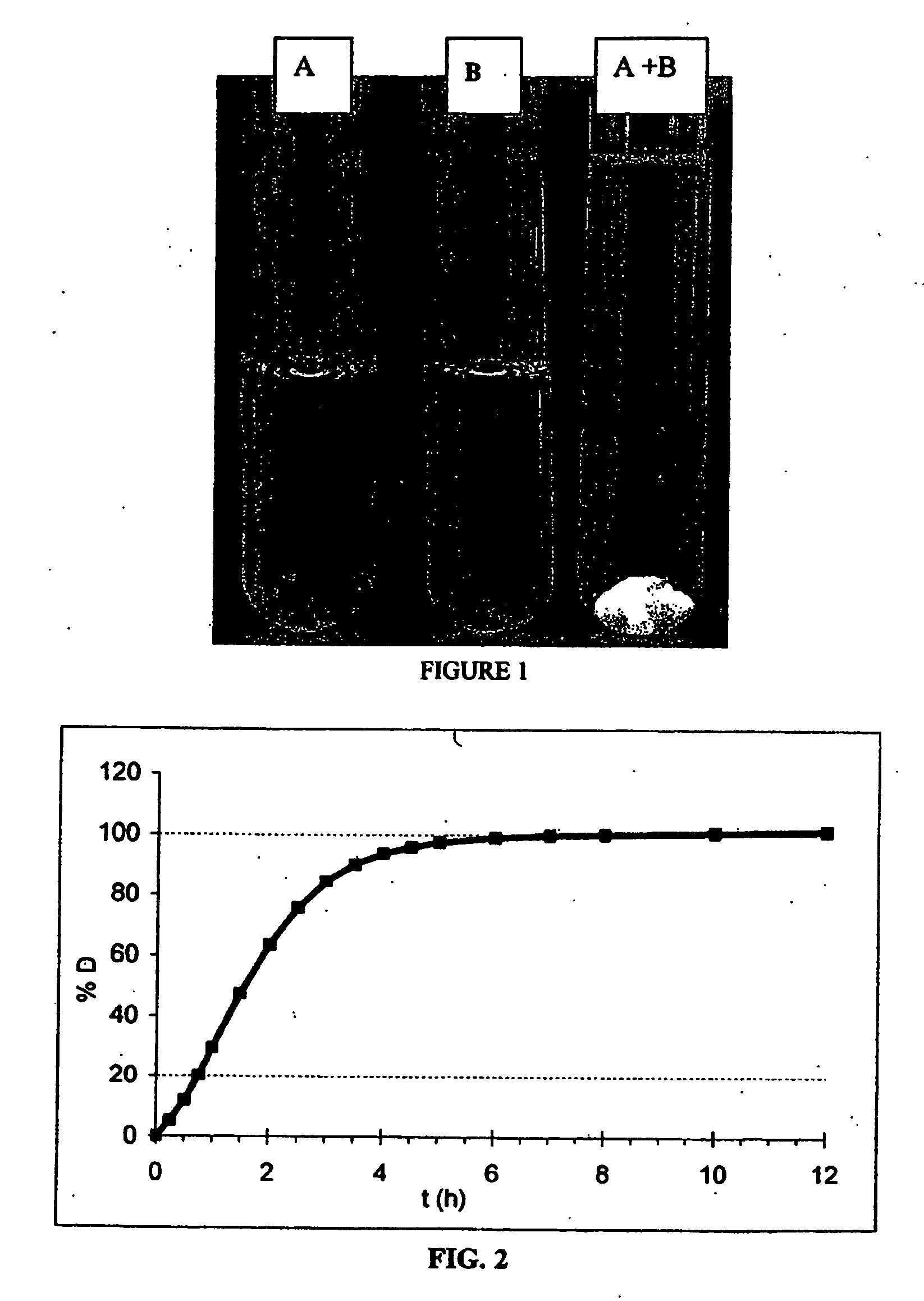
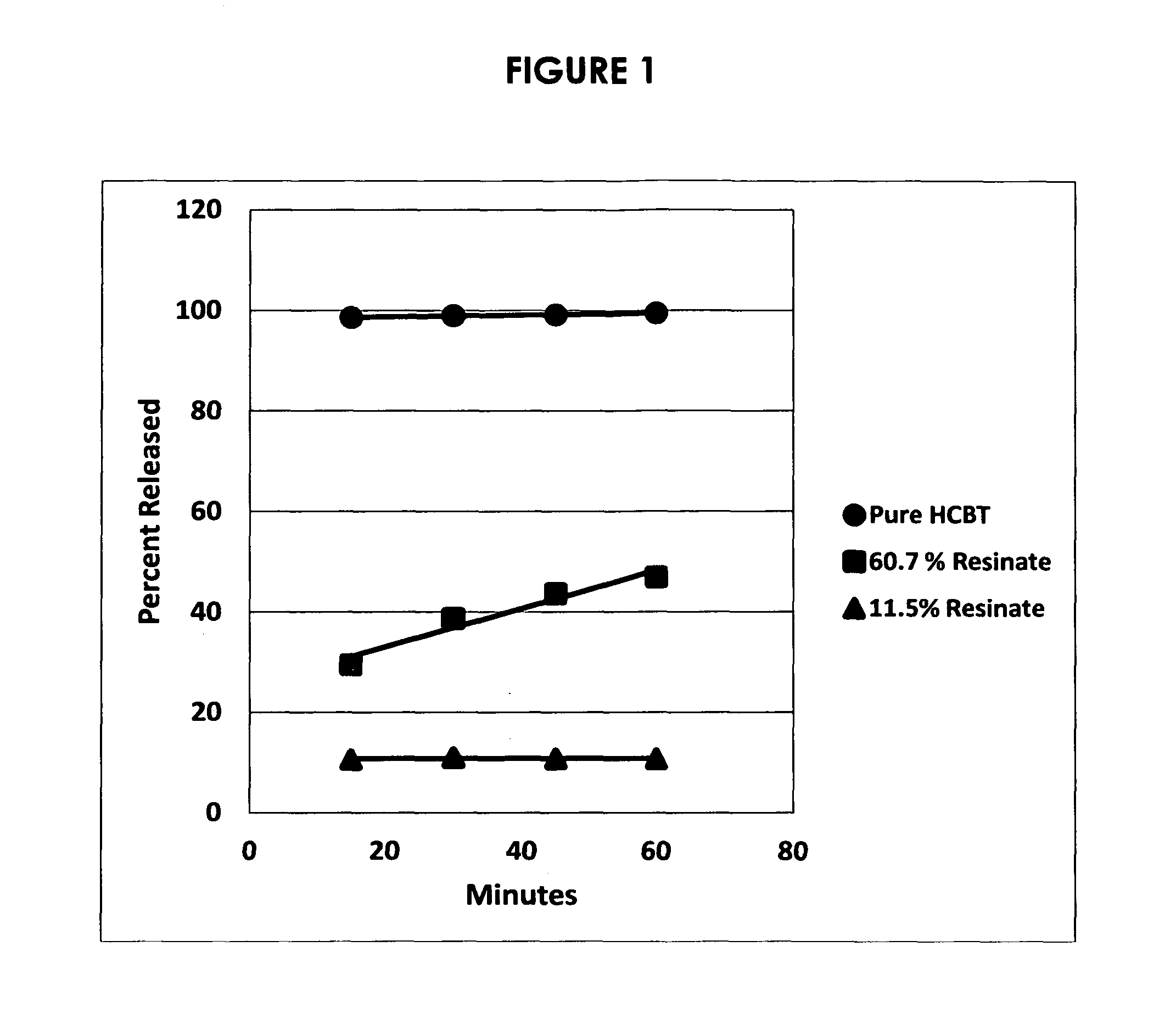
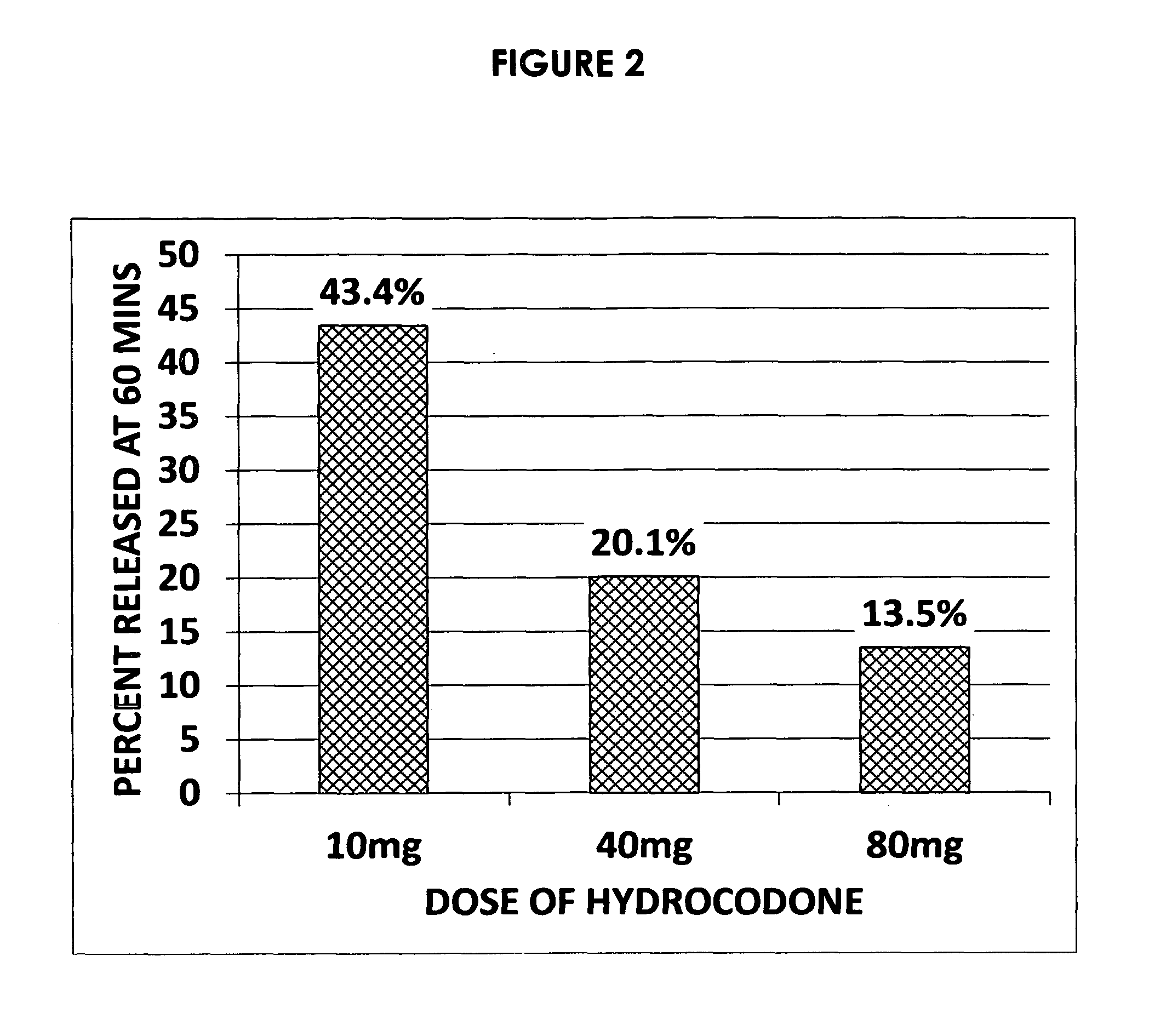
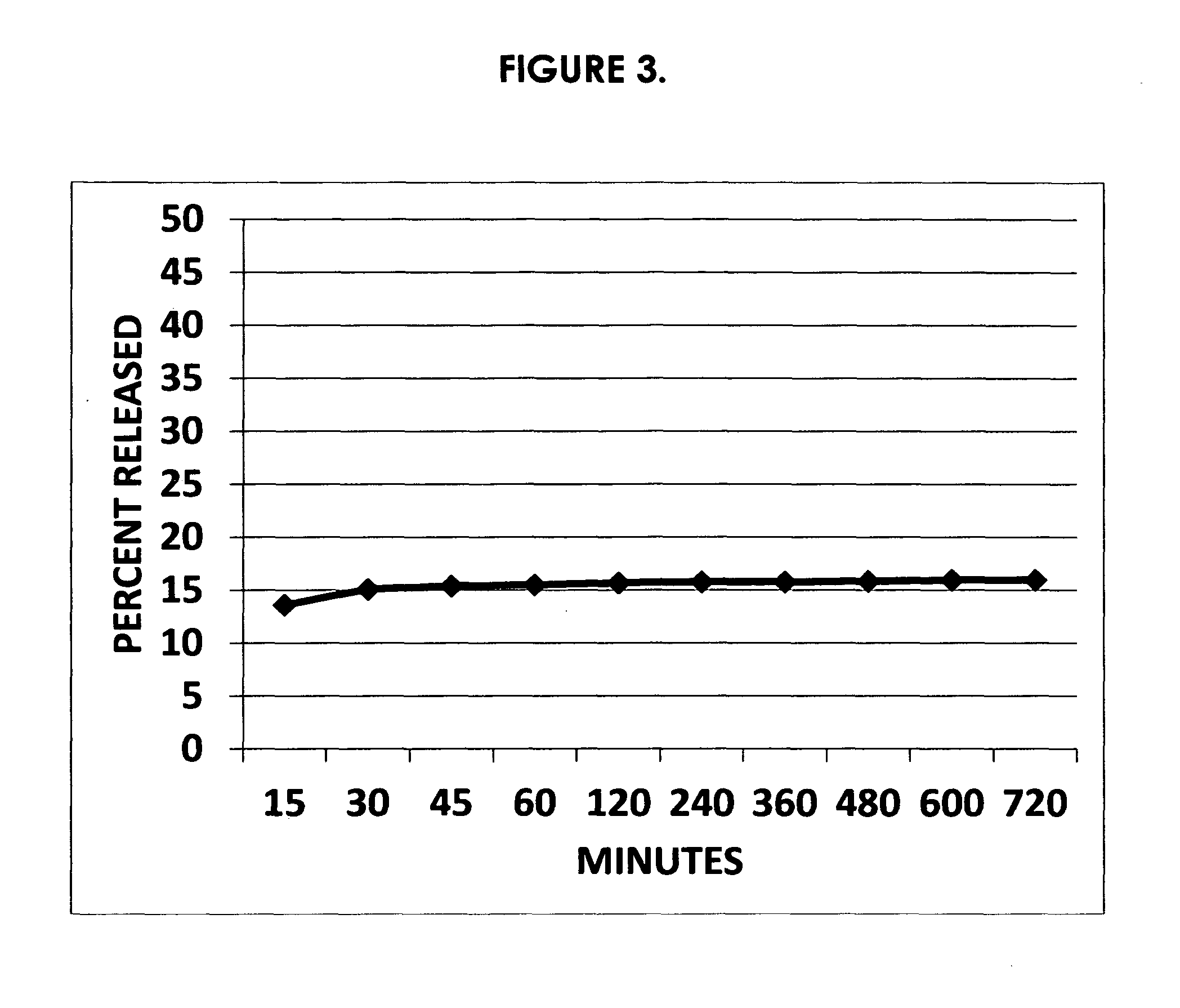
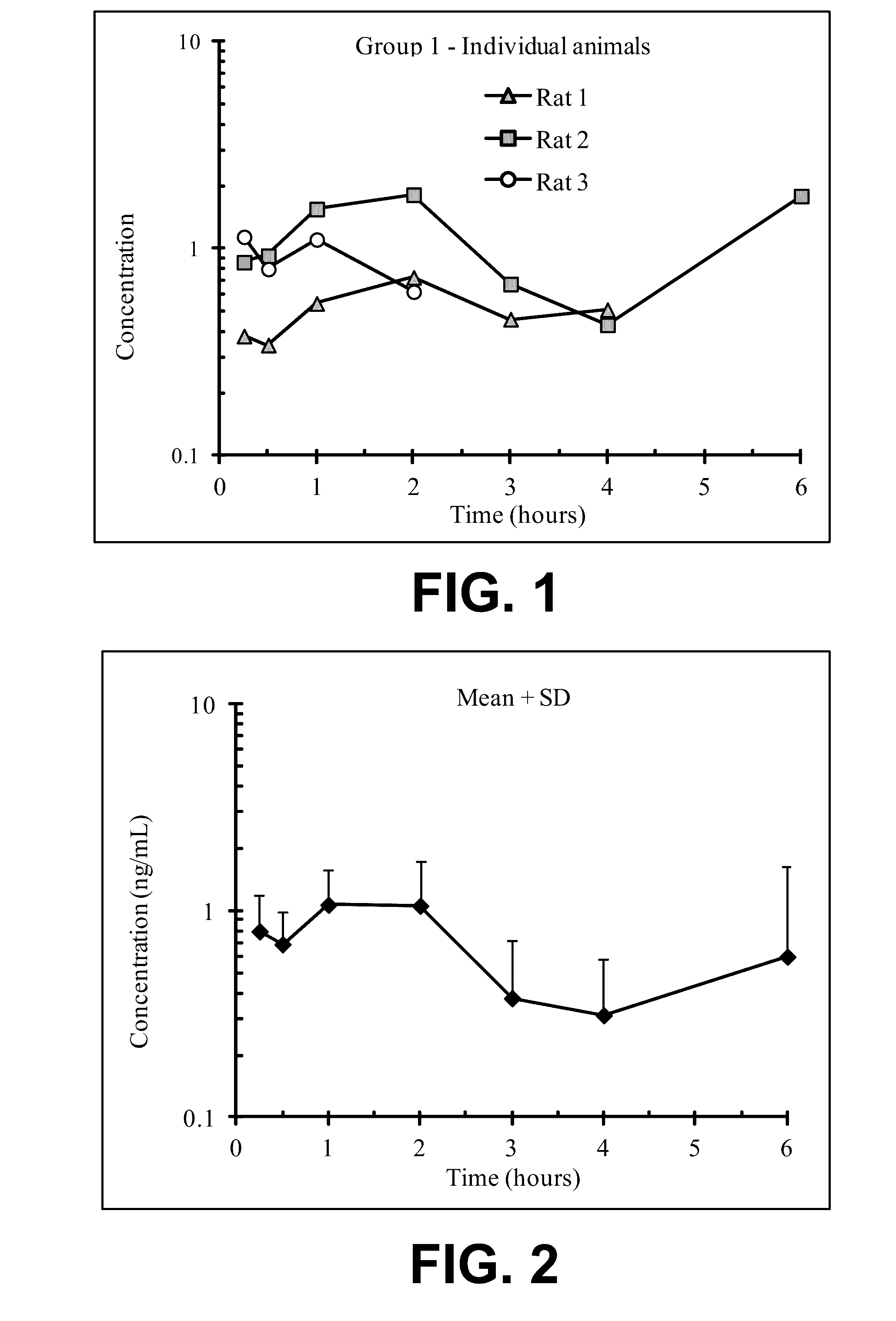
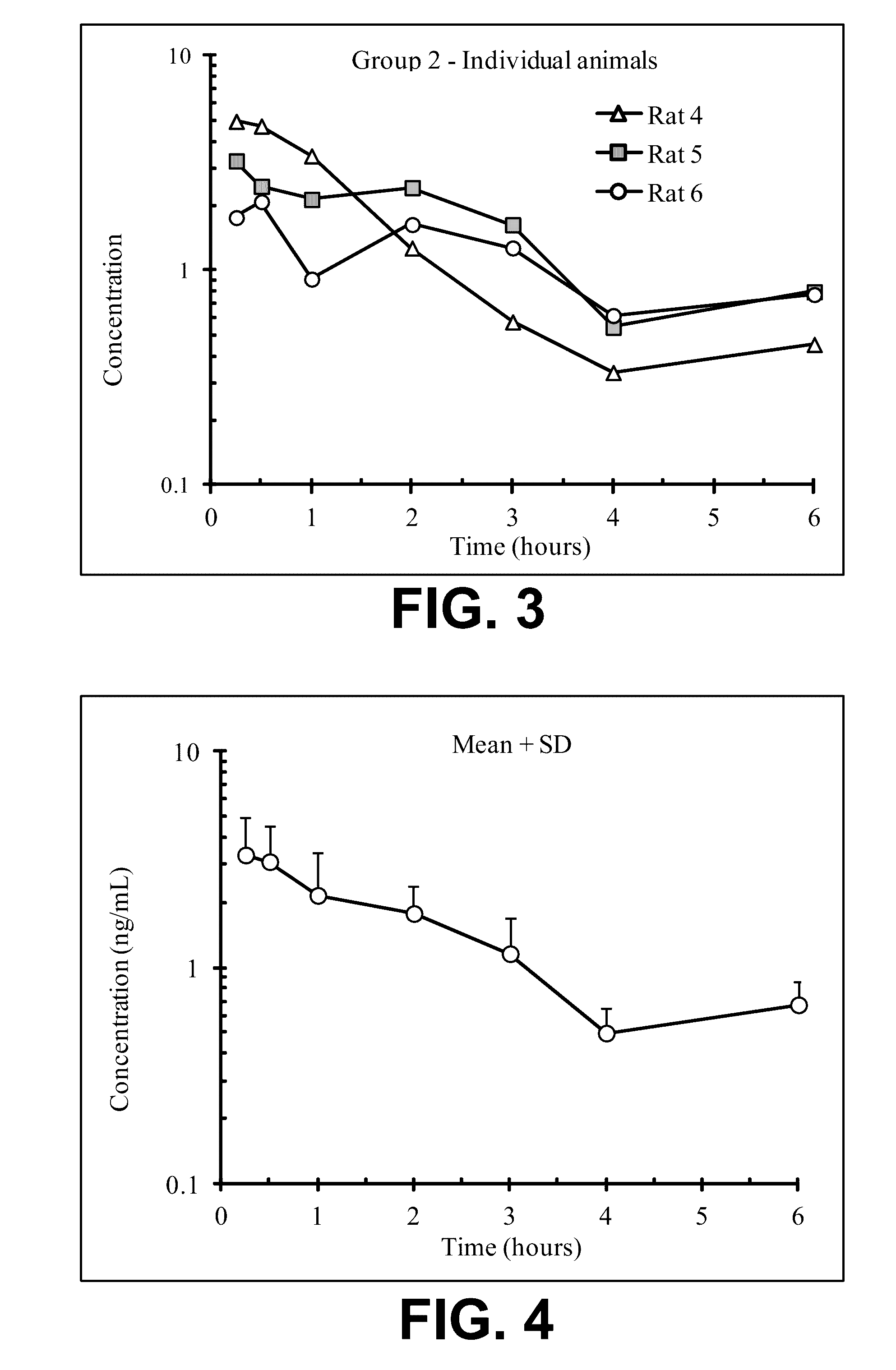
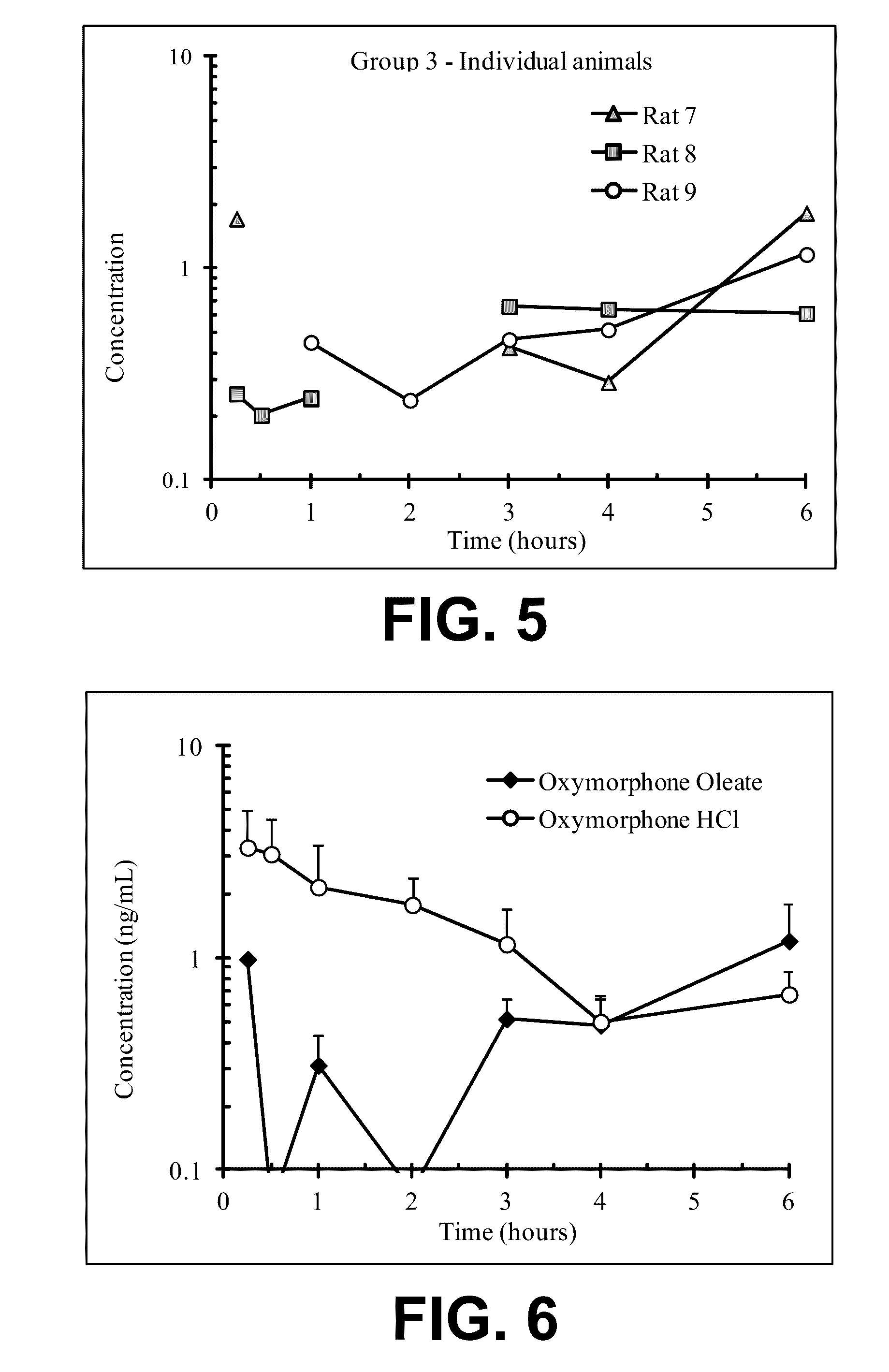
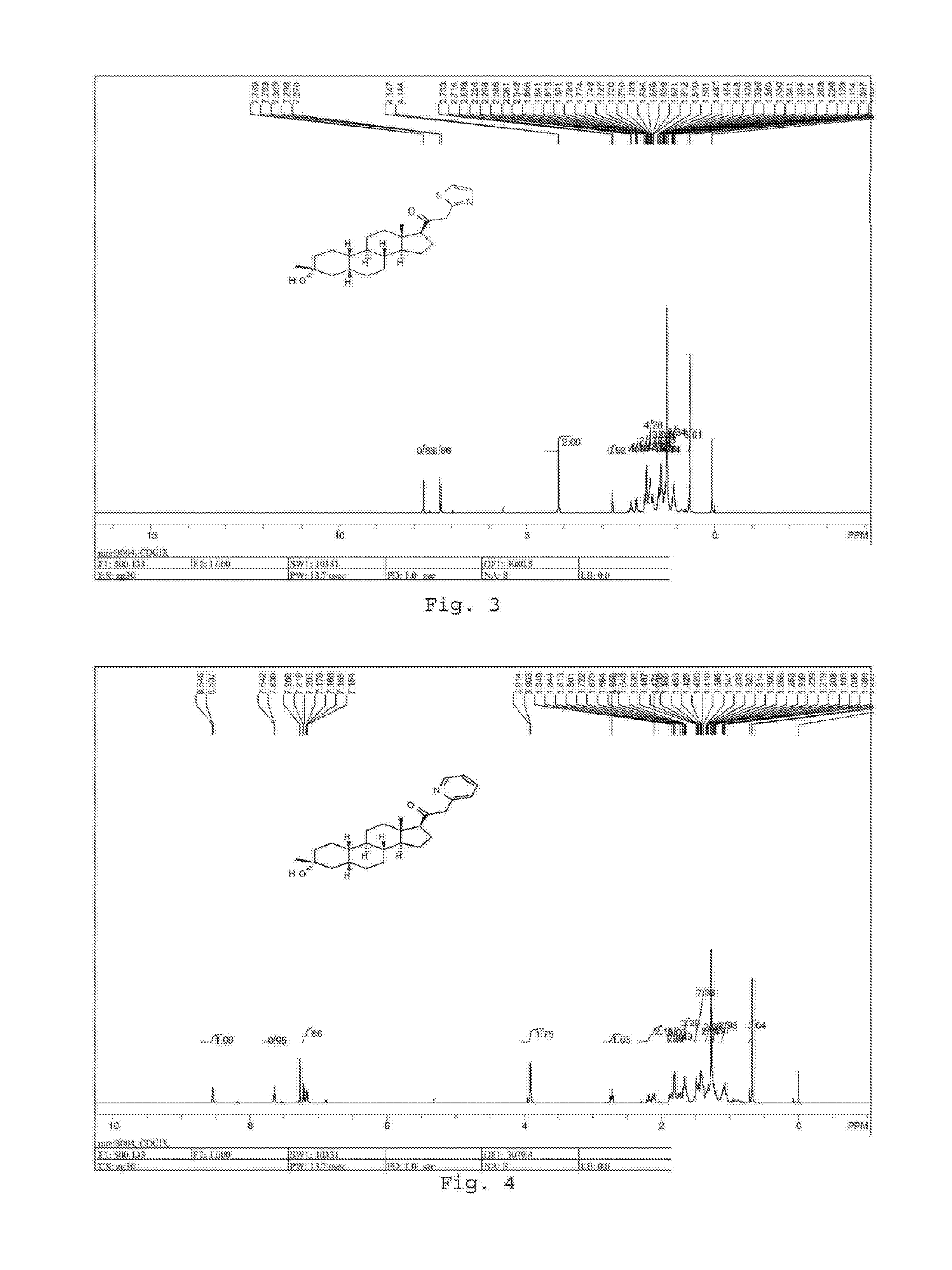
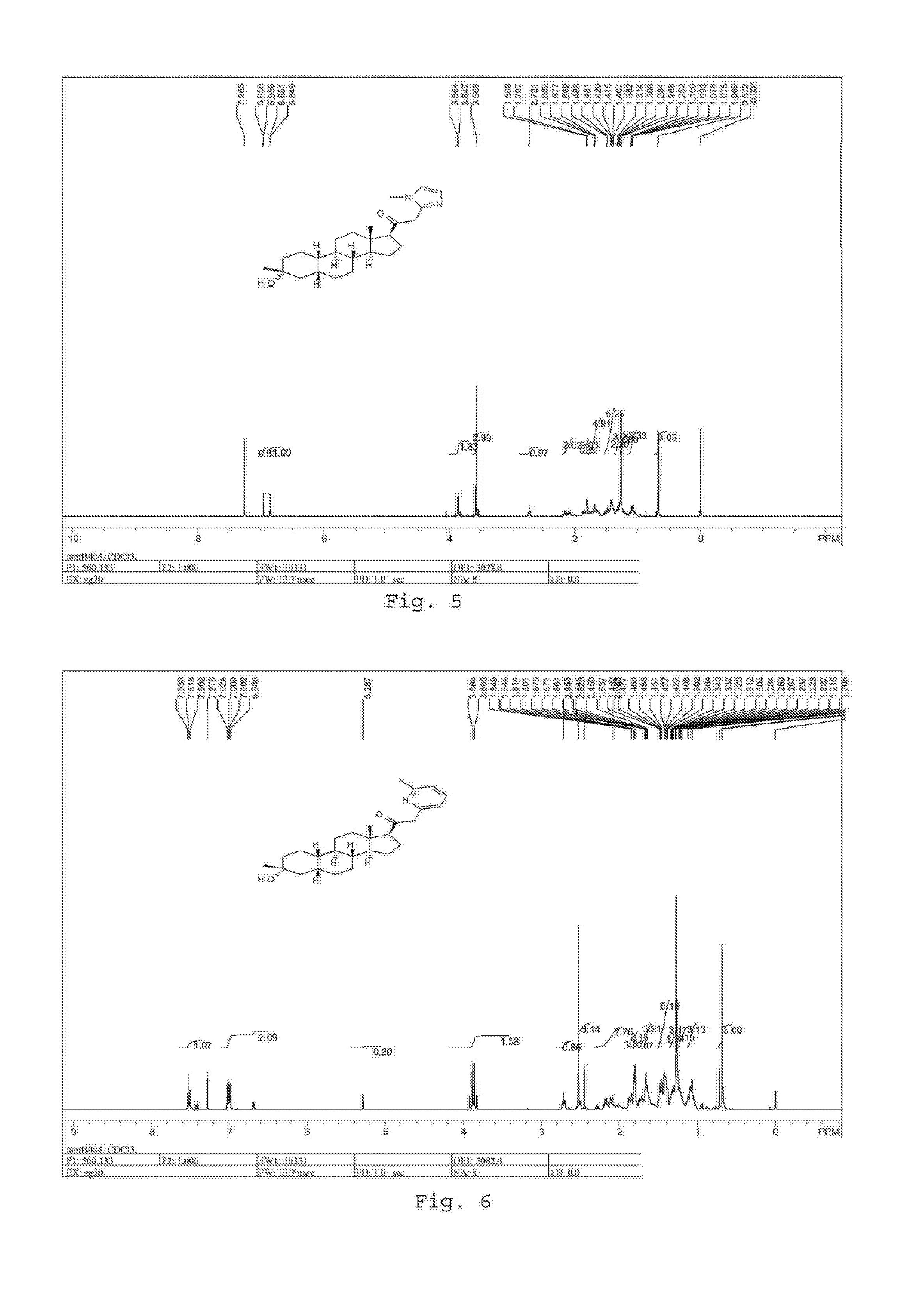
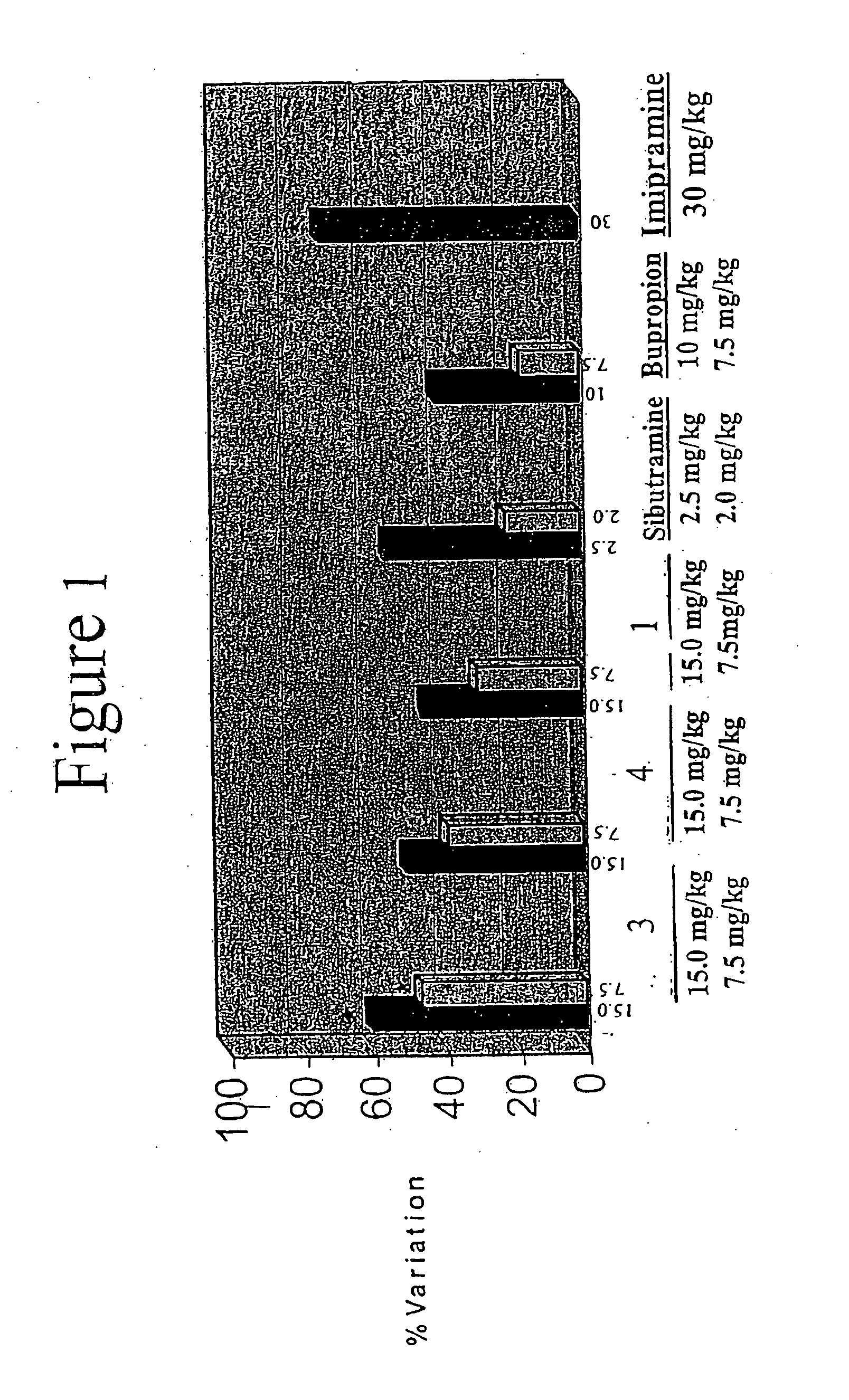
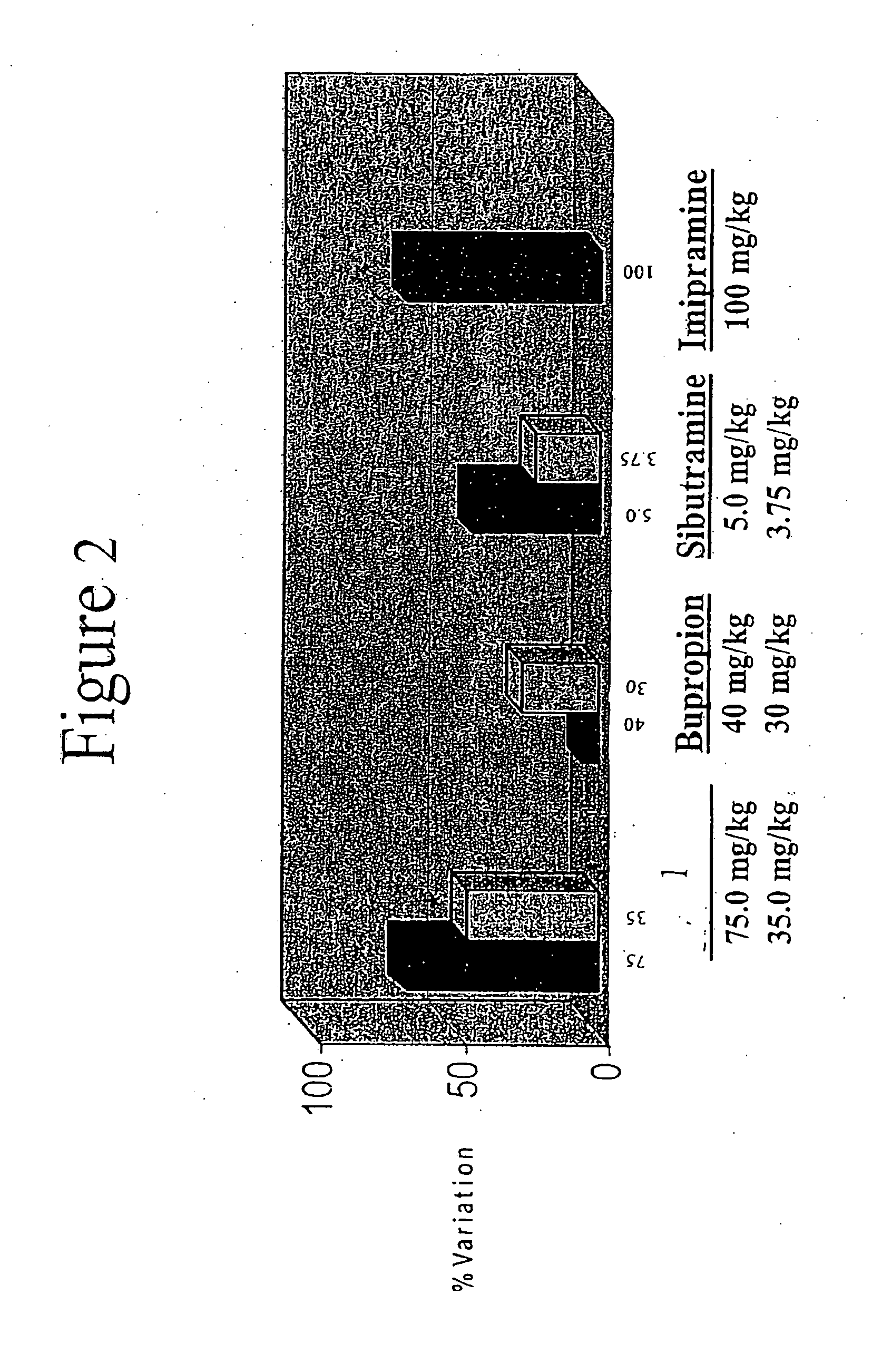
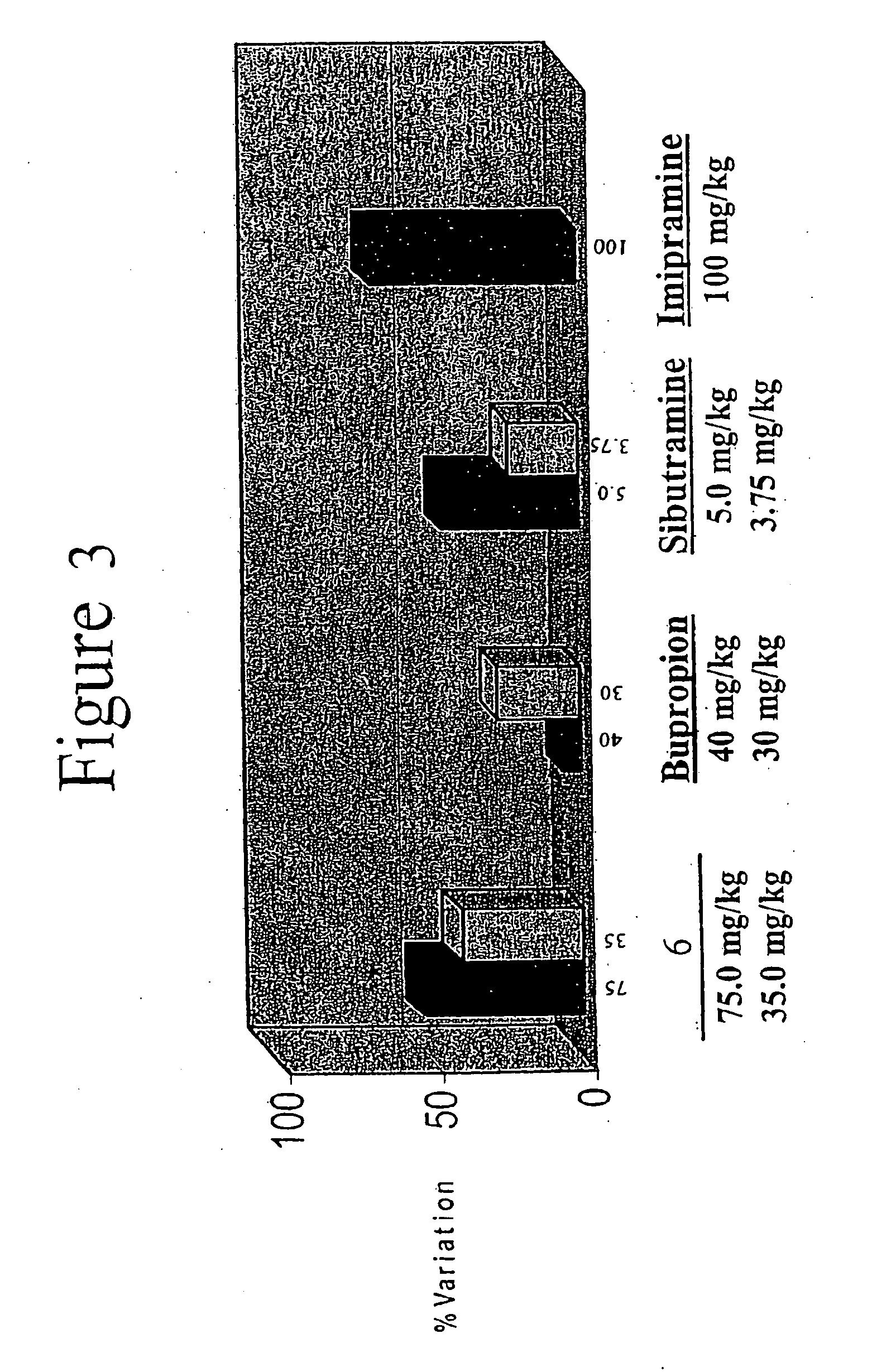
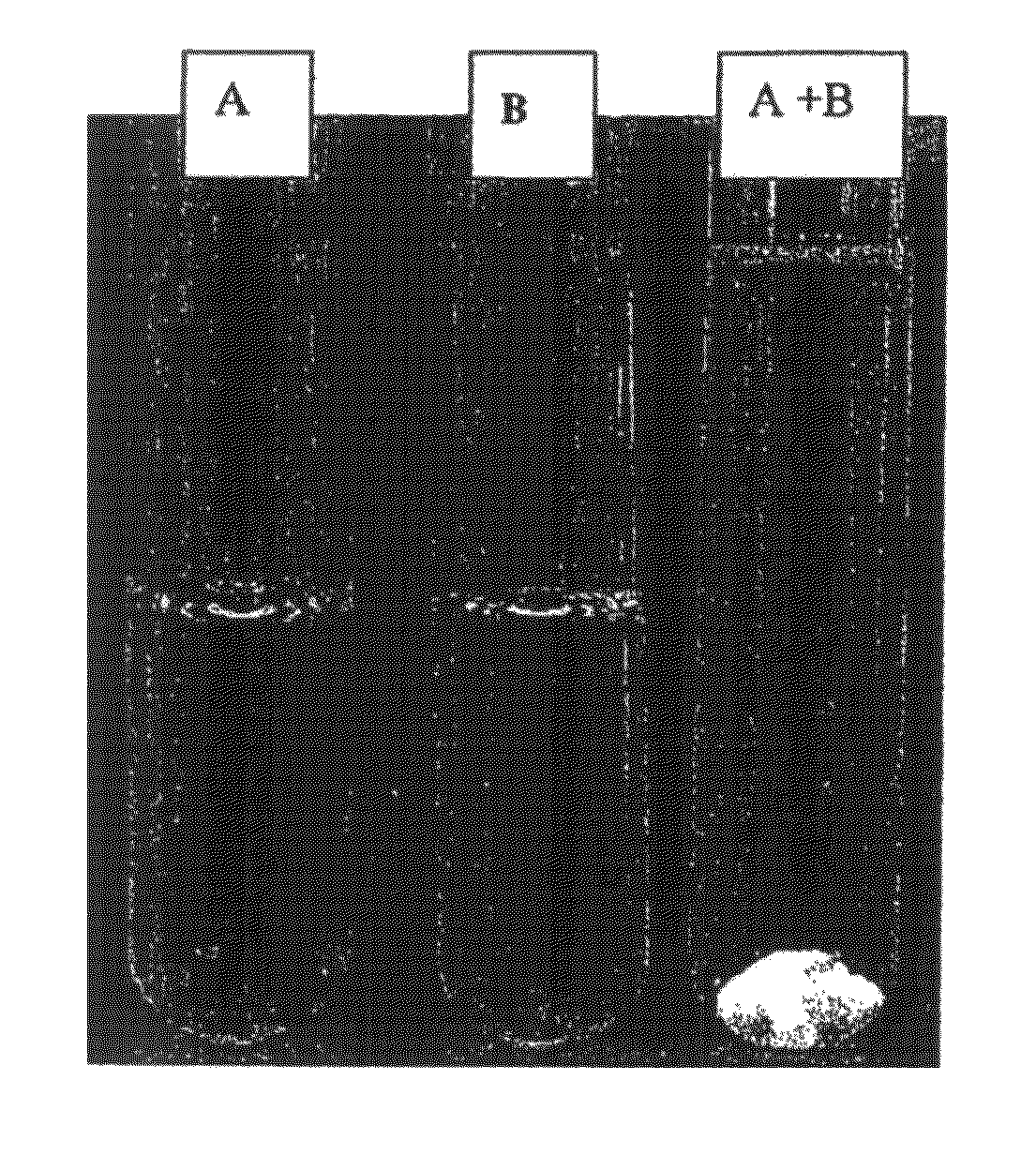
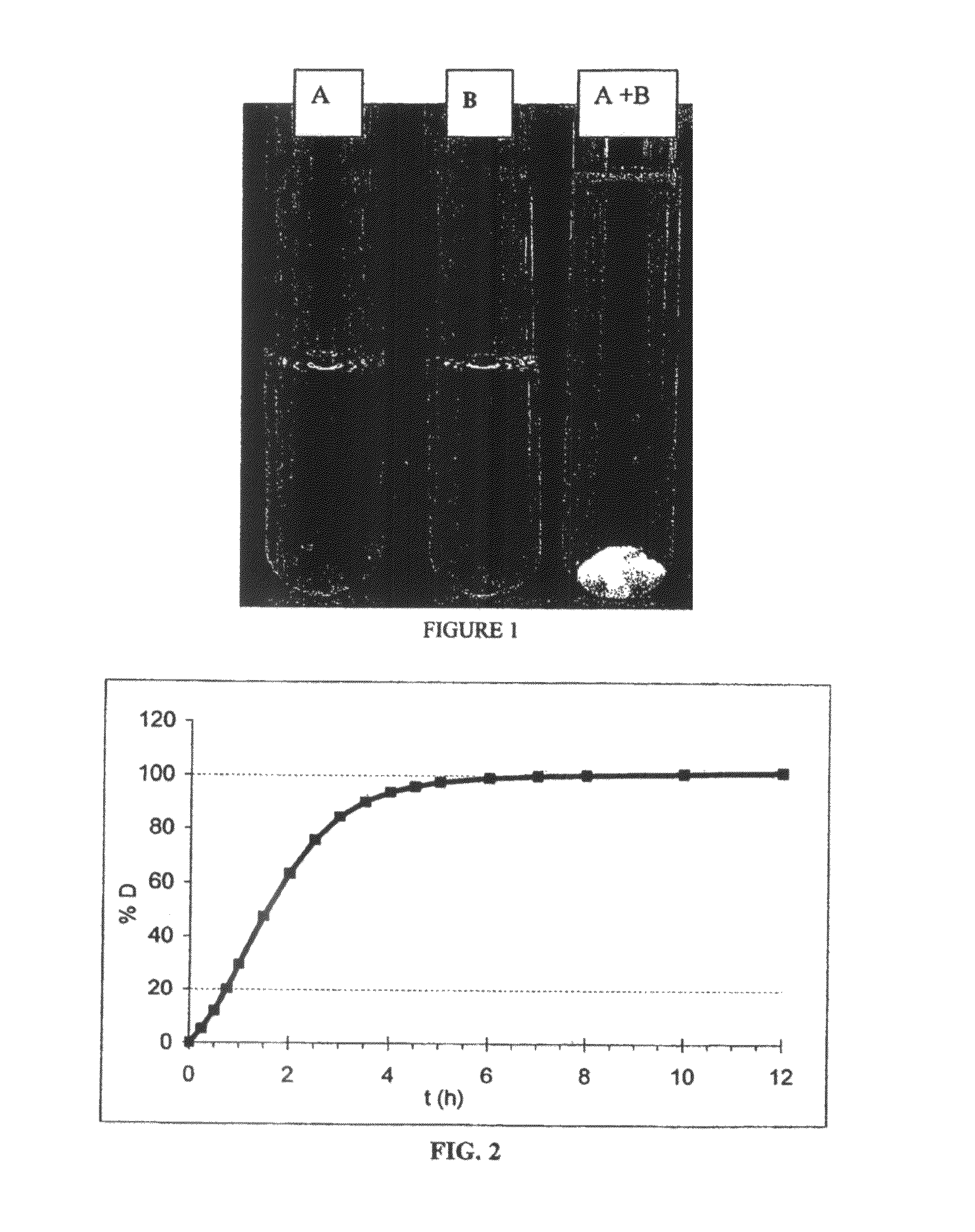
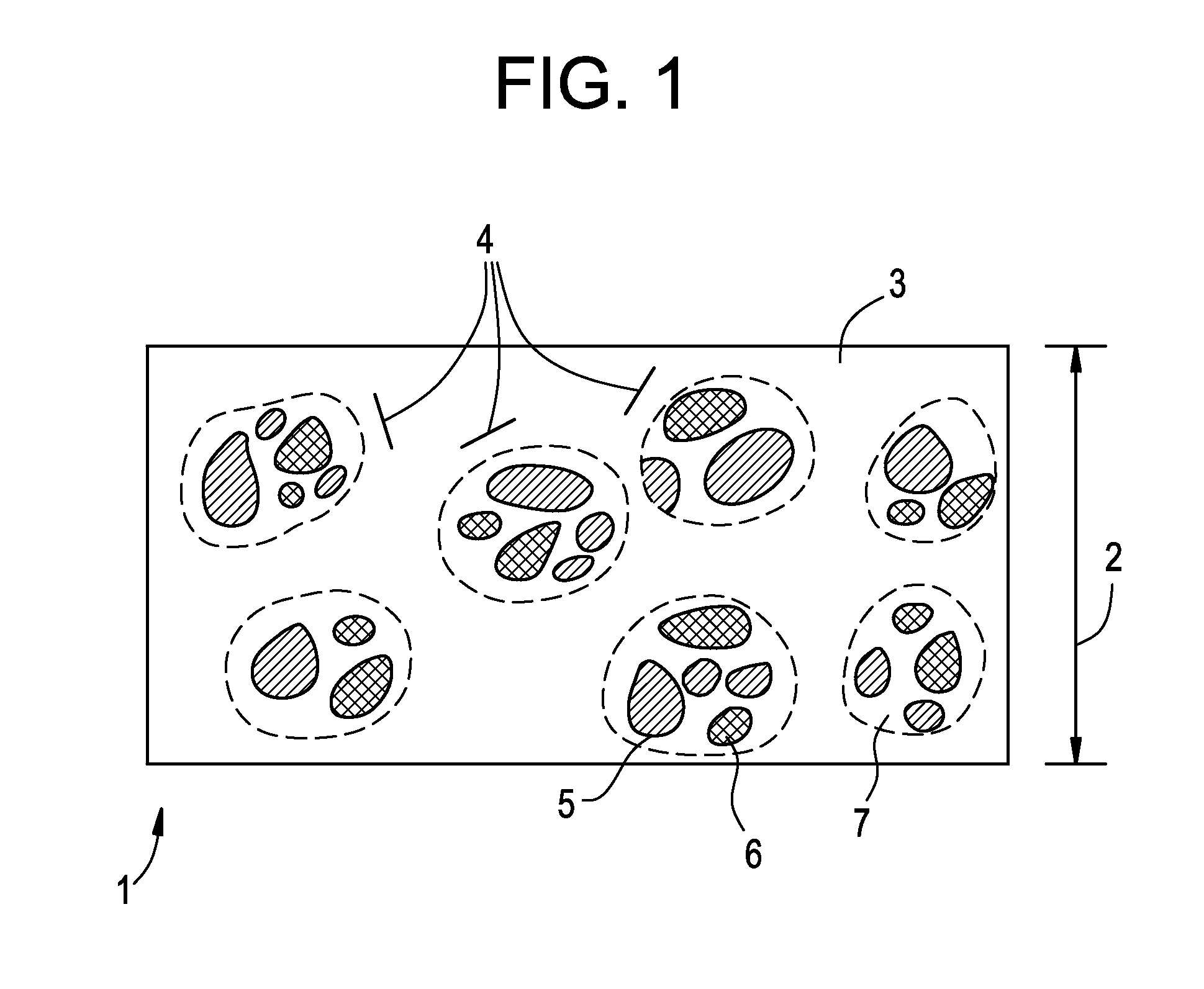
Acyclic cucurbit[n]uril type molecular containers to treat intoxication and decrease relapse rate in substance abuse disorders
ActiveUS20170246180A1Reduce the impactOrganic active ingredientsNervous disorderSubstance abuserRelapse rate
Provided are methods for reversing the effects of drugs of abuse. The method involves administering acyclic CB[n]-type compounds to a mammal in need of the reversal of the effects from a drug of abuse.
Owner:THE GENERAL HOSPITAL CORP +1
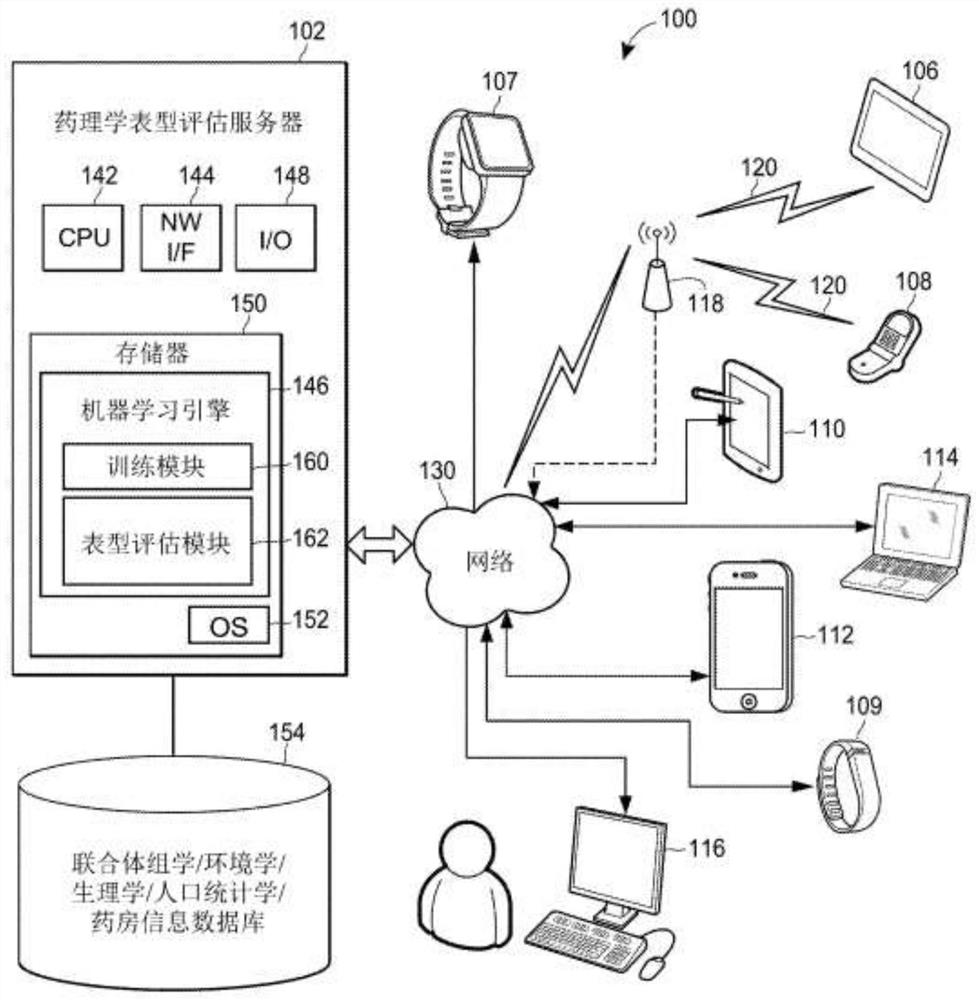
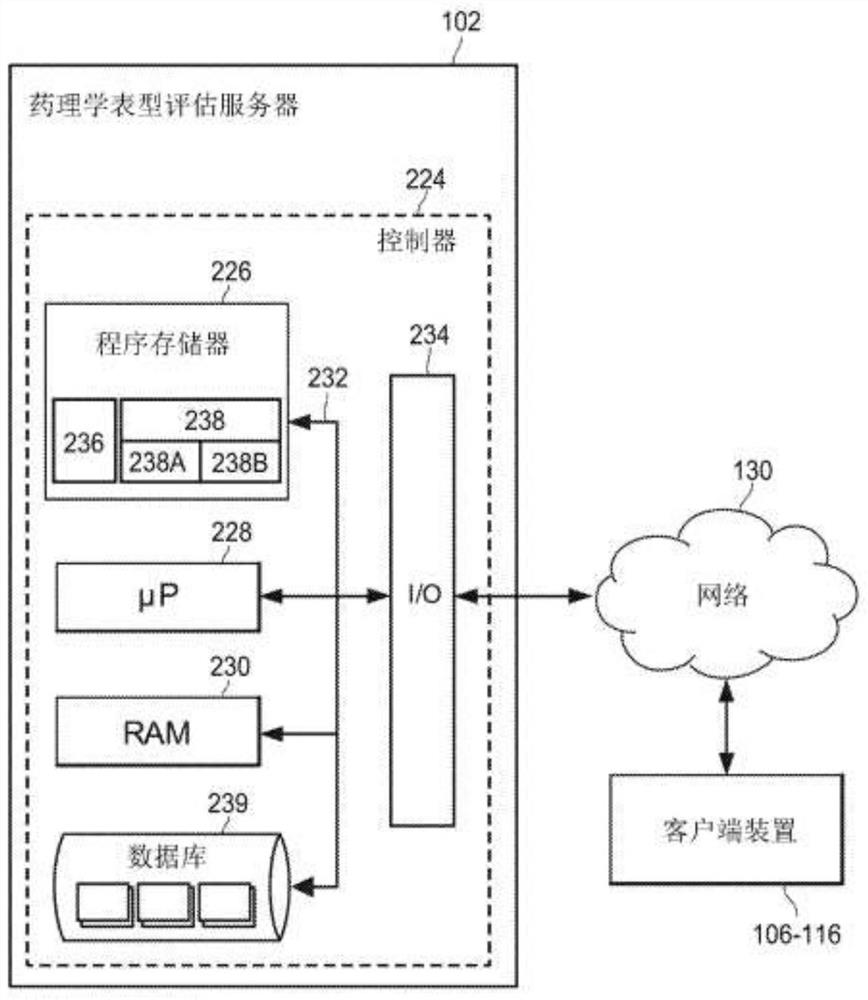
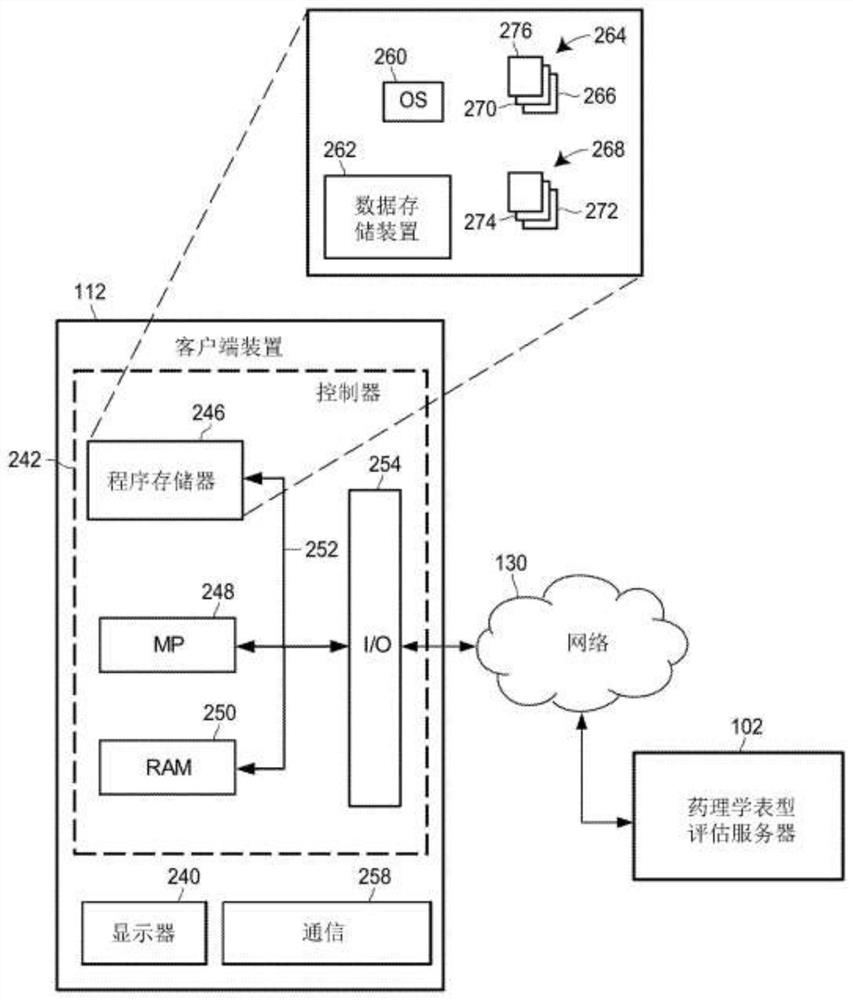
Individual and cohort pharmacological phenotype prediction platform
PendingCN111742370AIdentification is accurate and validMedical data miningHealth-index calculationSubstance abuserIdiopathic disease
For patients who exhibit or may exhibit primary or comorbid disease, pharmacological phenotypes may be predicted through the collection of panomic, physiomic, environmental, sociomic, demographic, andoutcome phenotype data over a period of time. A machine learning engine may generate a statistical model based on training data from training patients to predict pharmacological phenotypes, includingdrug response and dosing, drug adverse events, disease and comorbid disease risk, drug-gene, drug-drug, and polypharmacy interactions. Then the model may be applied to data for new patients to predict their pharmacological phenotypes, and enable decision making in clinical and research contexts, including drug selection and dosage, changes in drug regimens, polypharmacy optimization, monitoring,etc., to benefit from additional predictive power, resulting in adverse event and substance abuse avoidance, improved drug response, better patient outcomes, lower treatment costs, public health benefits, and increases in the effectiveness of research in pharmacology and other biomedical fields.
Owner:RGT UNIV OF MICHIGAN
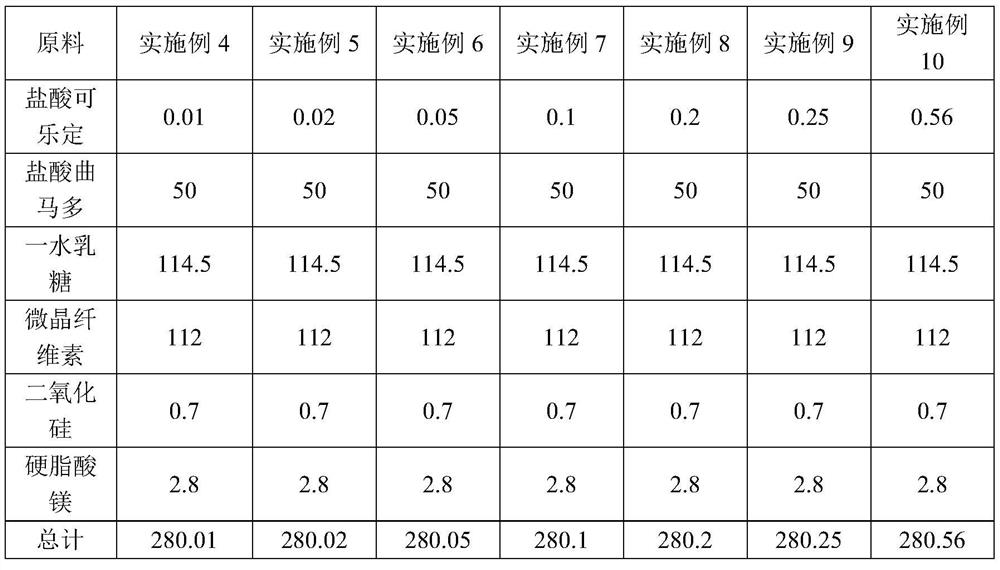
Pharmaceutical composition for relieving or eliminating protracted opioid abstinence syndrome and application of pharmaceutical composition
ActiveCN112494486AImprove Medication AdherenceImprove experienceOrganic active ingredientsNervous disorderWithdrawal syndromeUse medication
The invention discloses a pharmaceutical composition for relieving or eliminating protracted opioid abstinence syndrome and application of the pharmaceutical composition. The pharmaceutical composition comprises clonidine hydrochloride and tramadol hydrochloride in a mass ratio of (0.01-0.8):(40-60), and the application of the pharmaceutical composition in preparation of drugs for relieving or eliminating the protracted opioid abstinence syndrome is also disclosed. The pharmaceutical composition is more suitable for detoxification of opioid abuse patients, has the advantages of good effect, short time, completion in 3-5 days, safety, effectiveness and convenience in operation, has obvious clinical advantages and curative effects, can improve the medication compliance and medication experience of the patients, and prevents abuse.
Owner:SHENZHEN SCIENCARE MEDICAL INDUSTRIES CO. LTD.
Preventing or reducing drug abuse and overdose events
ActiveUS9211292B2Reducing drug abuseEliminate orNervous disorderIn-vivo testing preparationsDrugMedication abuse
A method and compositions for treating a patient that prevent or reduce drug abuse and overdose events.
Owner:KAVESH SHELDON
Methods and kits for diagnosing, assessing or quantitating drug use, drug abuse and narcosis, internuclear ophthalmoplegia, attention deficit hyperactivity disorder (ADHD), chronic traumatic encephalopathy, schizophrenia spectrum disorders and alcohol consumption
The invention provides methods for diagnosing, assessing or quantitating drug use, drug abuse or narcosis or for differentiating drug use, drug abuse or narcosis from brain injury in a subject by tracking eye movement of at least one eye of the subject, analyzing eye movement of at least one eye of the subject, comparing eye movement of at least one eye of the subject the normal or mean eye movement; and, optionally calculating a standard deviation or p value for eye movement of at least one eye of the subject as compared to the normal or mean eye movement.
Owner:NEW YORK UNIV
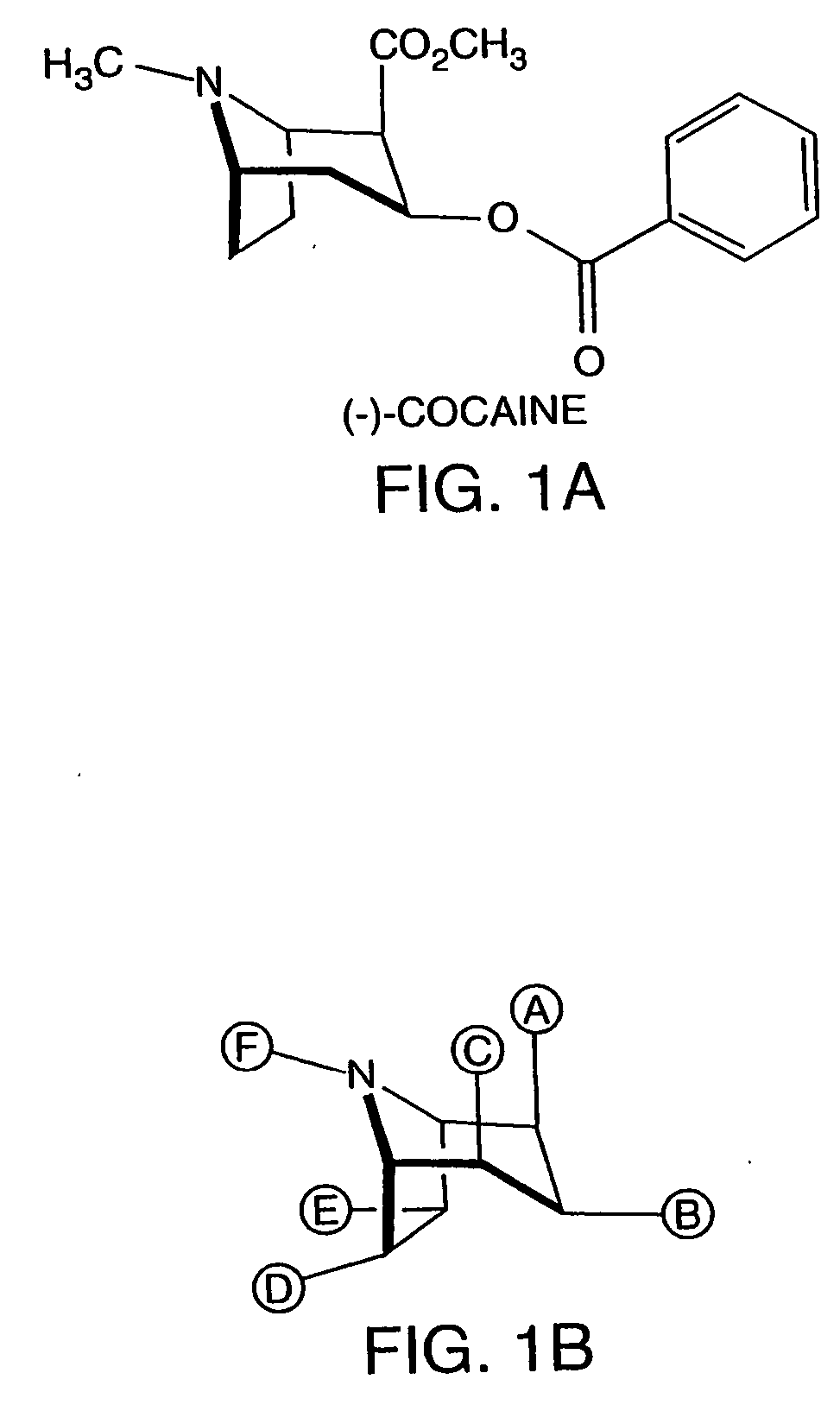
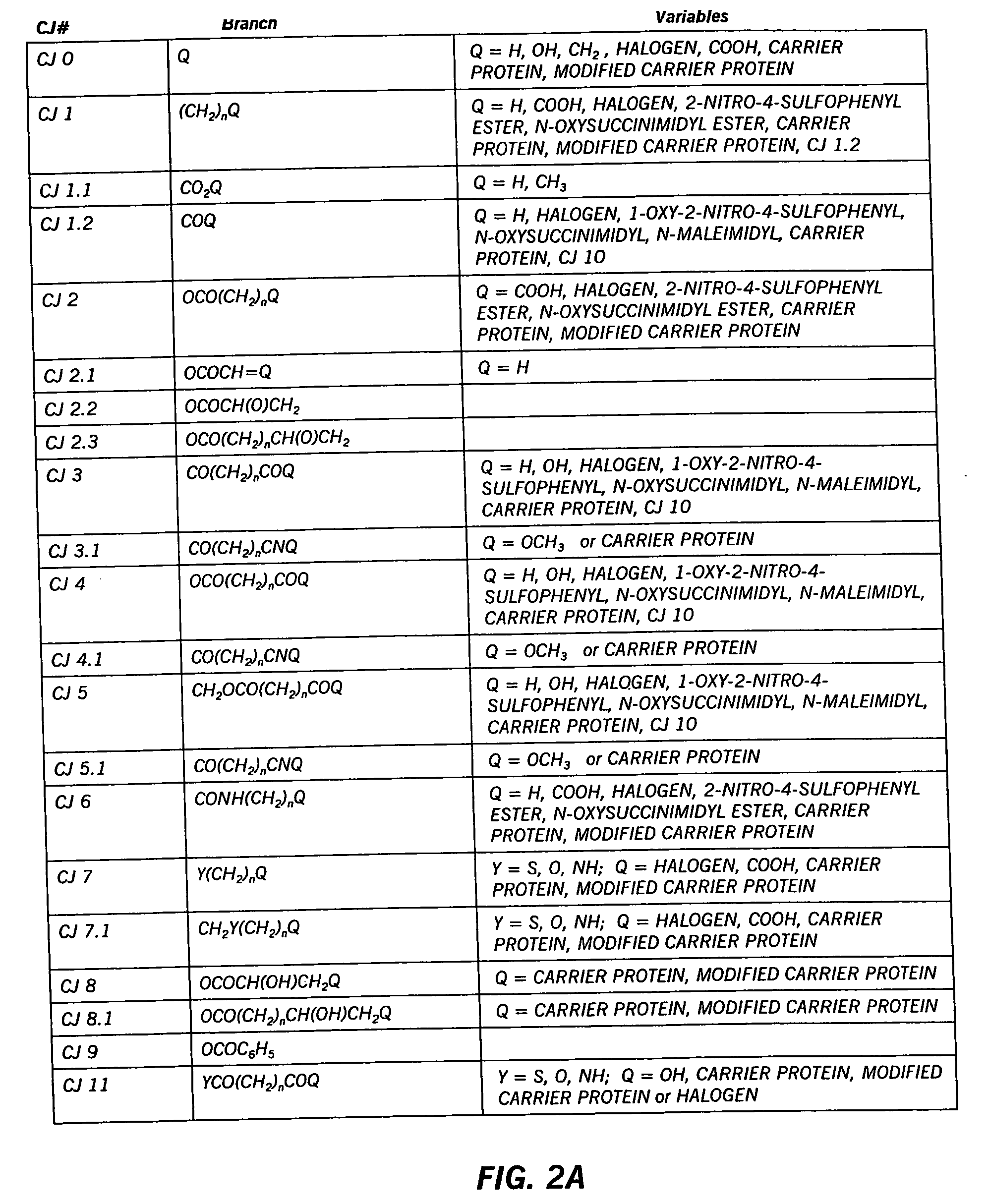
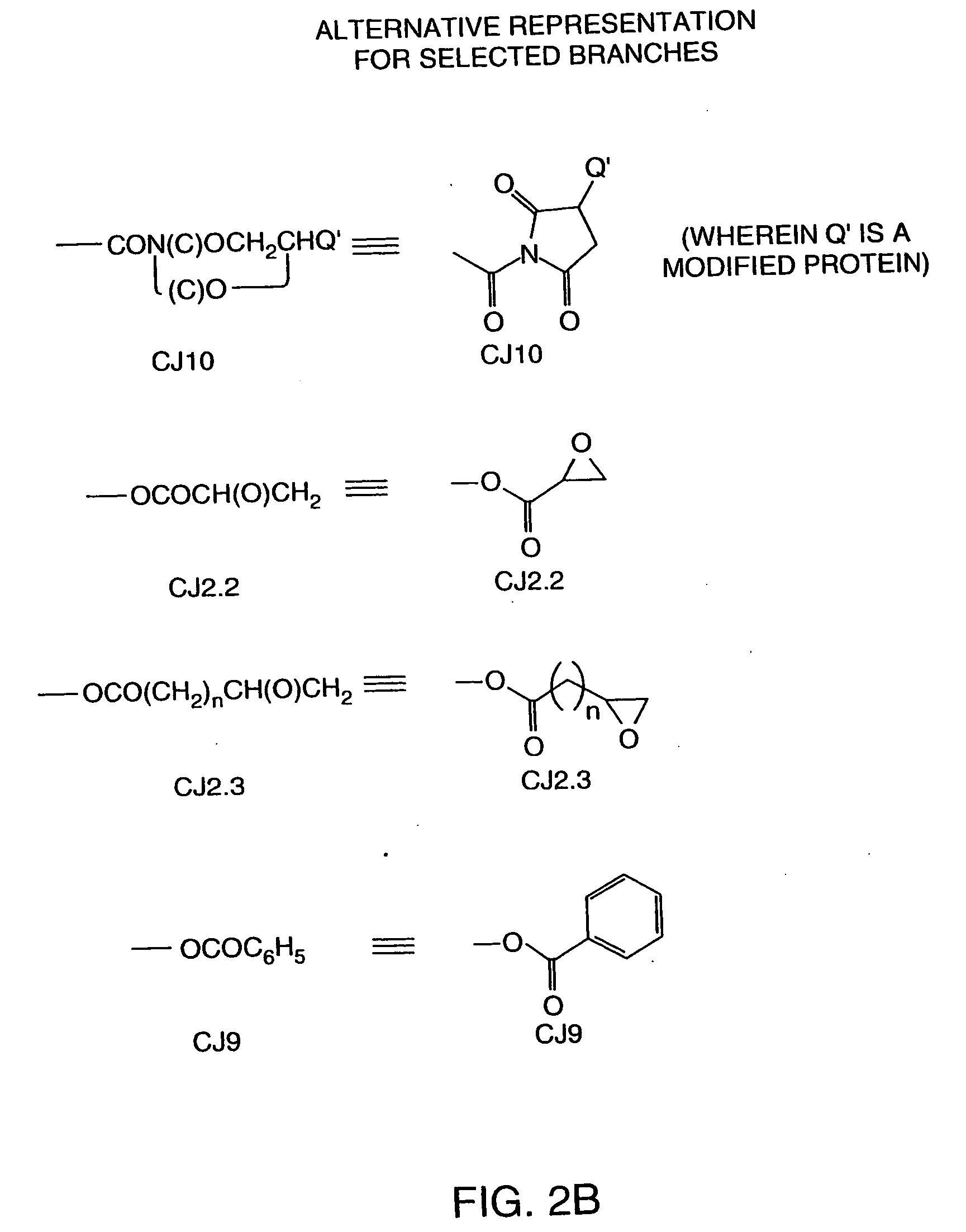
Hapten-carrier conjugates for use in drug-abuse therapy and methods for preparation of same
InactiveUS20050124061A1Easy to producePharmacological effect is diminishedAnimal cellsNervous disorderPassive ImmunizationsTherapy drug addiction
Hapten-carrier conjugates capable of eliciting anti-hapten antibodies in vivo by administering, in a therapeutic composition, are disclosed. Methods of preparing said conjugates and therapeutic compositions are also disclosed. Where the hapten is a drug of abuse, a therapeutic composition containing the hapten-carrier conjugate is particularly useful in the treatment of drug addiction, more particularly, cocaine addiction. Passive immunization using antibodies raised against conjugates of the instant invention is also disclosed. The therapeutic composition is suitable for co-therapy with other conventional drugs.
Owner:XENOVA
Deuterated morphine derivatives
ActiveUS20160052931A1Broad utilityReduce riskHeavy metal active ingredientsBiocideNormorphineΜ-opioid receptor
The invention relates to new morphine derivatives deuterated at the 7,8-position of the morphine ring, furthermore to a process for the preparation thereof, and to pharmaceutical compositions comprising them. The new deuterated morphine derivatives show high and selective μ-opioid receptor binding activity leading to the benefit of higher analgesic activity at lower dosages inducing thereby reduced adverse effects compared to the hydrogenated derivatives. The compounds of the invention are useful for example in the treatment of pain or can be used as antitussive agents with a reduced risk of the possibility of drug abuse.
Owner:SZEGEDI TUDOMANYEGYETEM
Pharmaceutical composition for treating diabetes and preparation method thereof
PendingCN113842452AAvoid harmRestore immunityPowder deliveryPeptide/protein ingredientsMalpighia emarginataPsyllium Seed Husk
The invention provides a pharmaceutical composition for treating diabetes and a preparation method thereof, and relates to the technical field of medicines. The pharmaceutical composition for treating the diabetes comprises cereal dietary fibers, whey protein, milk powder, acerola cherries, Chinese wolfberry fruits, silybum marianum, a white kidney bean extract, chia seeds, psyllium seed husks, cordyceps militaris, cordyceps sinensis, oyster peptides, anoectochilus formosanus, lecithin, dendrobium officinale, selenium-enriched yeast powder, natto powder, vitamins, a sweetening agent, prebiotics and a calcium agent. Through mutual matching of specific components, the pharmaceutical composition can effectively control blood sugar and restore immunity. Moreover, all the components are medicinal and edible raw materials, so that the effects of purifying blood and reducing blood sugar can be achieved for patients through dietetic invigoration, and the pharmaceutical composition is green, safe and free of toxic and side effects, so that injury caused by abuse of medicines is effectively avoided.
Owner:甘小云
Medicated spray for treatment of substance abuse, overdose, addiction and impulse control disorders
PendingUS20200352917A1Organic active ingredientsDispersion deliverySubstance abuserImpulse control disorder
A method to treat carfentanyl overdose comprising administrating a pharmaceutical formulation in the form of liquid solution for spray administration by the nasal and / or buccal route containing naltrexone as active ingredient in amounts greater than 1%.
Owner:GOOBERMAN LANCE L
System and method for medication compliance and drug abuse prevention
PendingUS20220192927A1Facilitates userInput/output for user-computer interactionDrug and medicationsSubstance use preventionOfficinal
A medication compliance and drug abuse prevention device for use as a cap with conventional medication container. The neck of the cap is adapted for mating with the neck of any conventional medication container. The mating of the cap neck with the container neck forms a seal therebetween and the seal can be maintained in a locked or unlocked state as per requirement. The cap has a weighment module configured to measure a mass of a medicinal substance contained inside the container. A control module determines a difference in the mass of the medicinal substance measured before and after the unlocked state and initiate one or more alert actions if the difference in mass of the medicinal substance is found to be less than or more than a prescribed mass of medicinal substance.
Owner:SANGHAVI NIVEDITA LAKSHMI MEHUL
Pharmaceutical formulation
ActiveUS20200246253A1Reduce riskDesirable balance of propertyOrganic active ingredientsNervous disorderOpioidergicOpioid overdose
The present invention relates to a film comprising an alginate salt of a monovalent cation or a mixture of alginate salts containing at least one alginate salt of a monovalent cation, and an antagonist of an opioid receptor, an inverse agonist of an opioid receptor, or a prodrug thereof. The present invention further relates to methods for manufacturing such a film, and the use of such a film in the treatment of a human patient, in particular the use of such a film in the treatment of the effects of acute opioid overdose, or the use of such a film in reducing the risk of opioid abuse.
Owner:KLARIA PHARMA HLDG AB
Deuterated morphine derivatives
ActiveUS9447108B2Wide range of usesHigh and selective μ-opioid receptor activityOrganic active ingredientsNervous disorderΜ-opioid receptorSubstance abuse
The invention relates to new morphine derivatives deuterated at the 7,8-position of the morphine ring, furthermore to a process for the preparation thereof, and to pharmaceutical compositions comprising them. The new deuterated morphine derivatives show high and selective μ-opioid receptor binding activity leading to the benefit of higher analgesic activity at lower dosages inducing thereby reduced adverse effects compared to the hydrogenated derivatives. The compounds of the invention are useful for example in the treatment of pain or can be used as antitussive agents with a reduced risk of the possibility of drug abuse.
Owner:SZEGEDI TUDOMANYEGYETEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com






![Acyclic cucurbit[n]uril type molecular containers to treat intoxication and decrease relapse rate in substance abuse disorders Acyclic cucurbit[n]uril type molecular containers to treat intoxication and decrease relapse rate in substance abuse disorders](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/866e14d6-fcc3-41c7-a3a5-6aac17e0f222/US20170246180A1-20170831-D00000.png)
![Acyclic cucurbit[n]uril type molecular containers to treat intoxication and decrease relapse rate in substance abuse disorders Acyclic cucurbit[n]uril type molecular containers to treat intoxication and decrease relapse rate in substance abuse disorders](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/866e14d6-fcc3-41c7-a3a5-6aac17e0f222/US20170246180A1-20170831-D00001.png)
![Acyclic cucurbit[n]uril type molecular containers to treat intoxication and decrease relapse rate in substance abuse disorders Acyclic cucurbit[n]uril type molecular containers to treat intoxication and decrease relapse rate in substance abuse disorders](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/866e14d6-fcc3-41c7-a3a5-6aac17e0f222/US20170246180A1-20170831-D00002.png)