Pharmaceutical composition for relieving or eliminating protracted opioid abstinence syndrome and application of pharmaceutical composition
A composition and syndrome technology, applied in the fields of addiction medicine and neurobiology, can solve the problems of long withdrawal time, poor medication compliance and medication experience of patients, and poor clinical application effect, and achieve the elimination of withdrawal symptoms, Obvious clinical advantages and curative effects, and the effect of improving medication experience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
[0037] Embodiment 1-3 A kind of compound tablet for alleviating or eliminating opioid withdrawal syndrome
[0038] Table 1 shows the prescription of a compound tablet for alleviating or eliminating the opioid withdrawal syndrome of Examples 1-3.
[0039] Table 1 A kind of prescription of compound tablet for alleviating or eliminating opioid withdrawal syndrome (each tablet contains / mg)
[0040]
[0041]
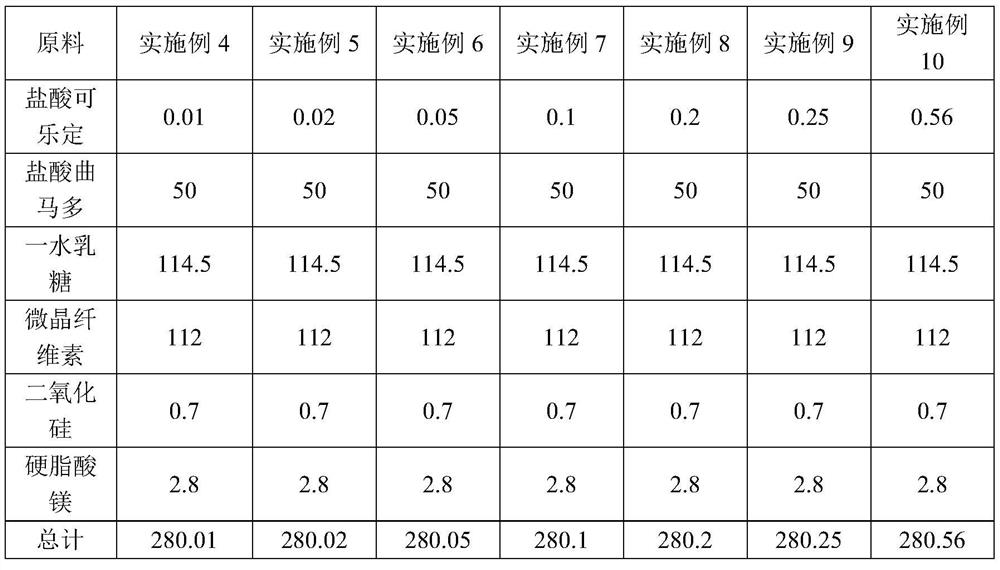
Embodiment 4-10
[0042] Embodiment 4-10 A kind of compound tablet for alleviating or eliminating opioid withdrawal syndrome
[0043] Table 2 shows the prescription of a compound tablet for alleviating or eliminating the opioid withdrawal syndrome of Examples 4-10.
[0044] Table 2 A kind of prescription of compound tablet for alleviating or eliminating opioid withdrawal syndrome (each tablet contains / mg)
[0045]
[0046] The preparation method of the compound tablet comprises the following steps:
[0047] (1) the clonidine hydrochloride of recipe quantity is dissolved in water, sieve after granulation with recipe quantity microcrystalline cellulose, oven dry, obtain granule;
[0048] (2) the granule obtained in step (1) is mixed with the tramadol hydrochloride and lactose monohydrate of recipe quantity, then silicon dioxide and the magnesium stearate of recipe quantity are added and mixed, and the coating is compressed into a tablet, to obtain the The compound tablet.
experiment example 2
[0086] Experimental example 2. The main pharmacodynamics study of drugs inhibiting morphine withdrawal symptoms
[0087] (1) Mouse jumping test
[0088] The mice were randomly divided into blank control group and drug group 1-12 by body weight, wherein, drug group 1-12 (respectively administered compound tablets prepared in Example 1-10 and single tablets prepared in Comparative Example 1-2), Divided into low-dose group, middle-dose group and high-dose group, 10 in each group. All groups were intraperitoneally injected with morphine, 6 times a day, with a dose of 10 mg / kg / time on the first day, 10 mg / kg / time on the 2nd-3rd day, 50 minutes after the last injection of morphine, the low-dose group of the drug , middle-dose group and high-dose group were administered by intragastric administration of 36 mg / kg, 72 mg / kg and 143 mg / kg respectively, and the blank control group was administered an equal volume of normal saline. After 30 minutes of intragastric administration, each gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com