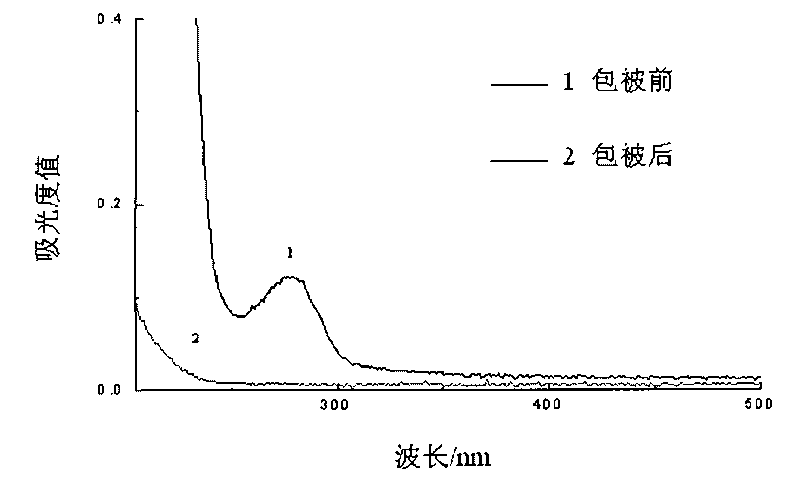
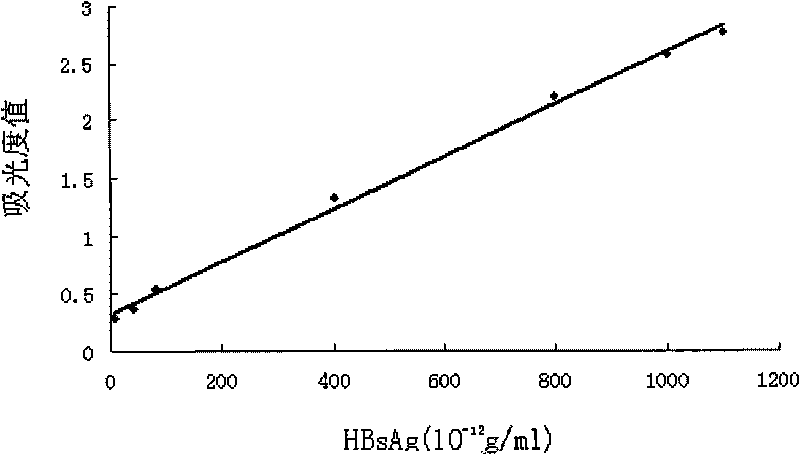
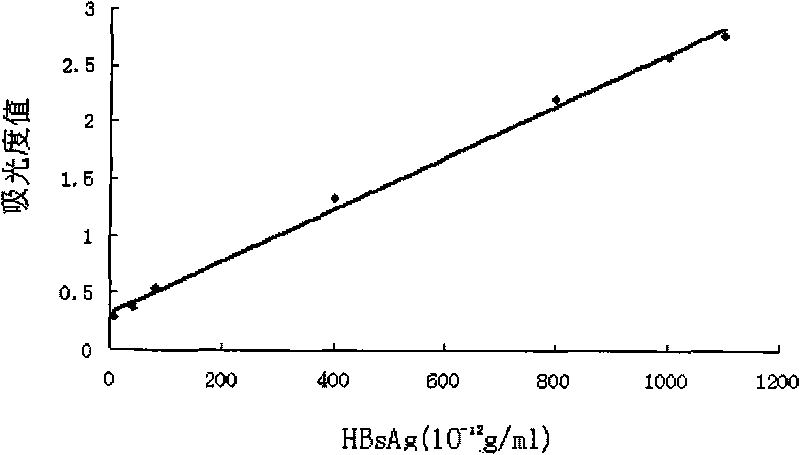
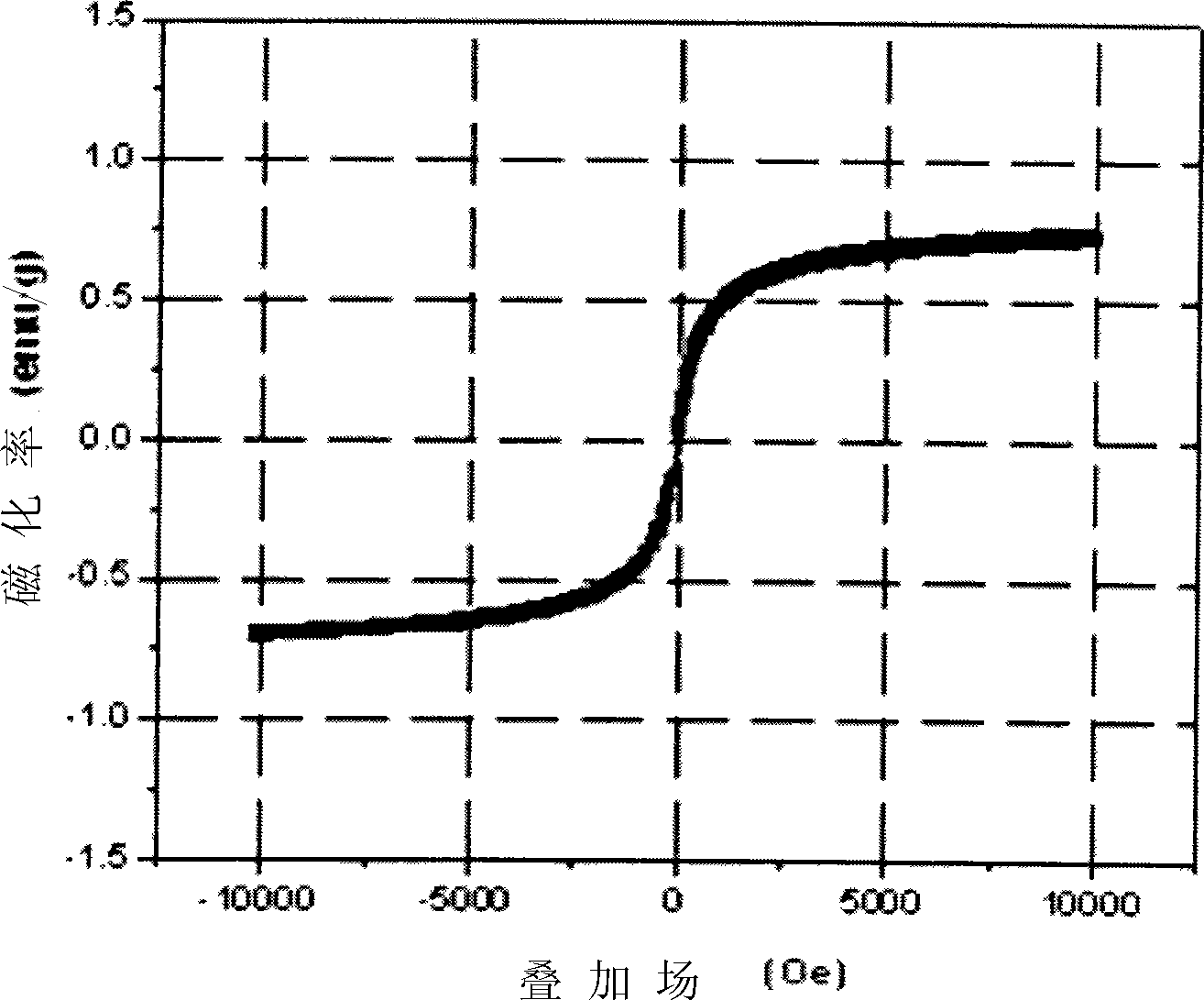
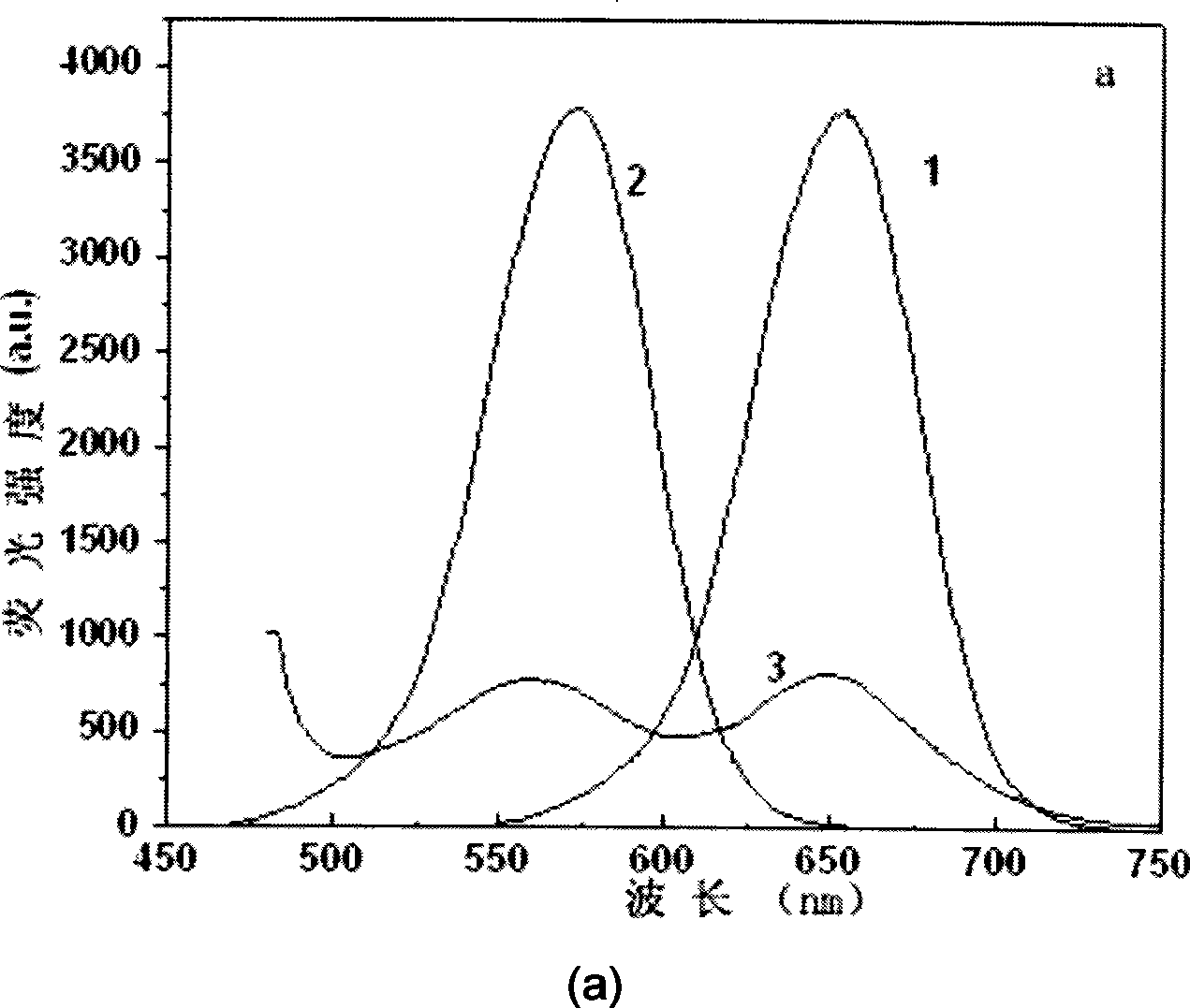
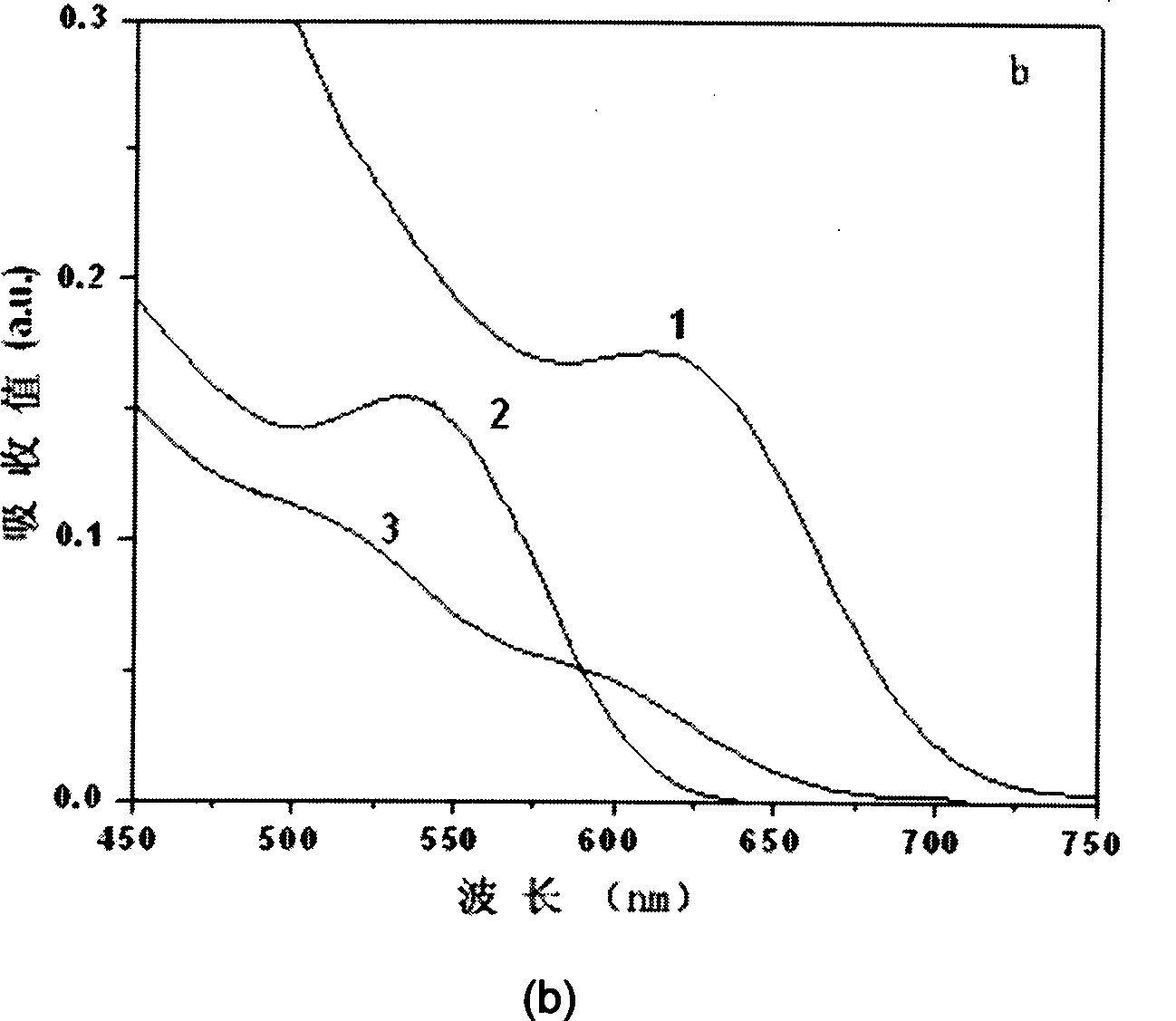
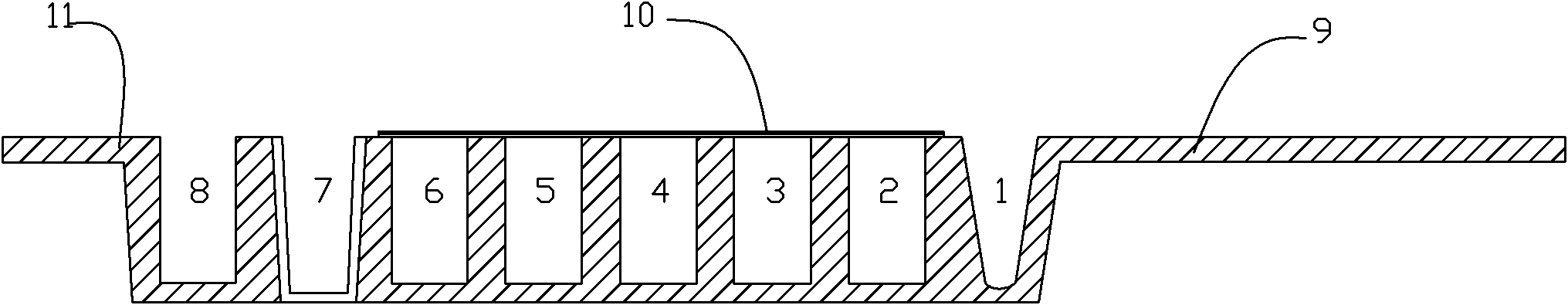
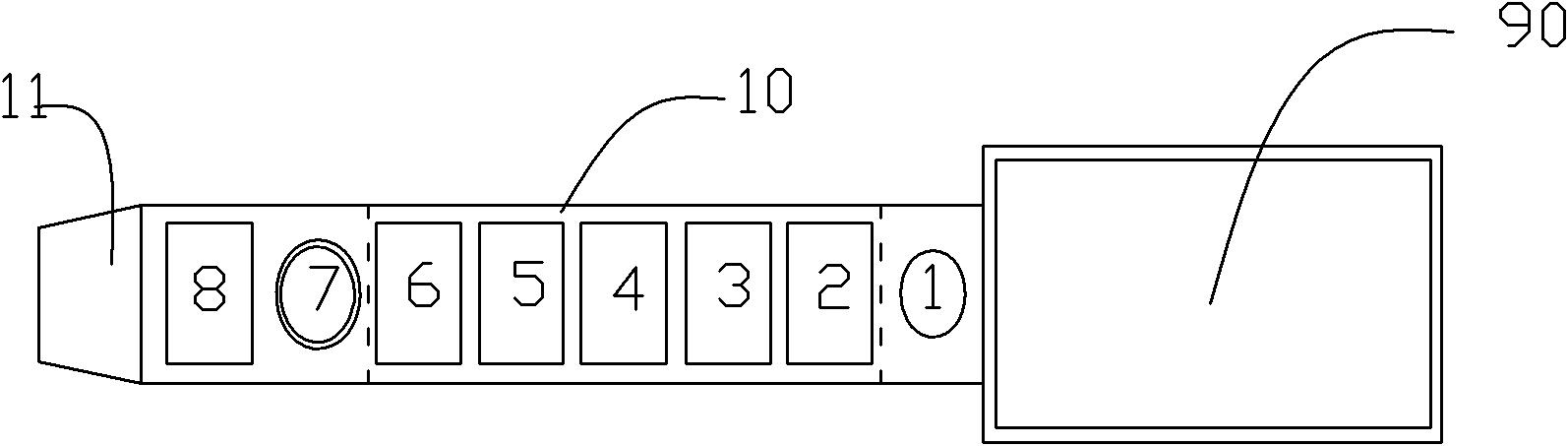
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
79 results about "Immunological tests" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
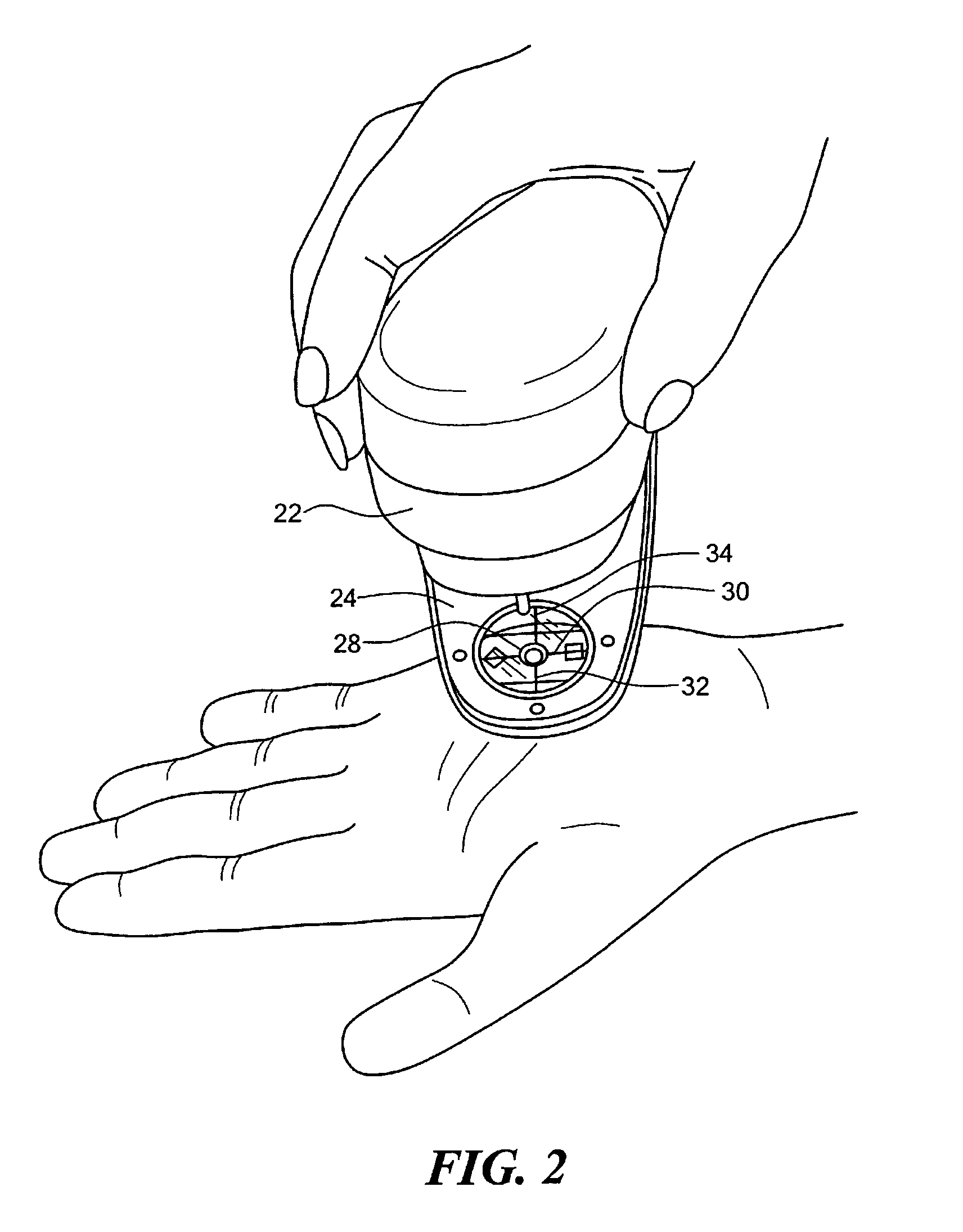
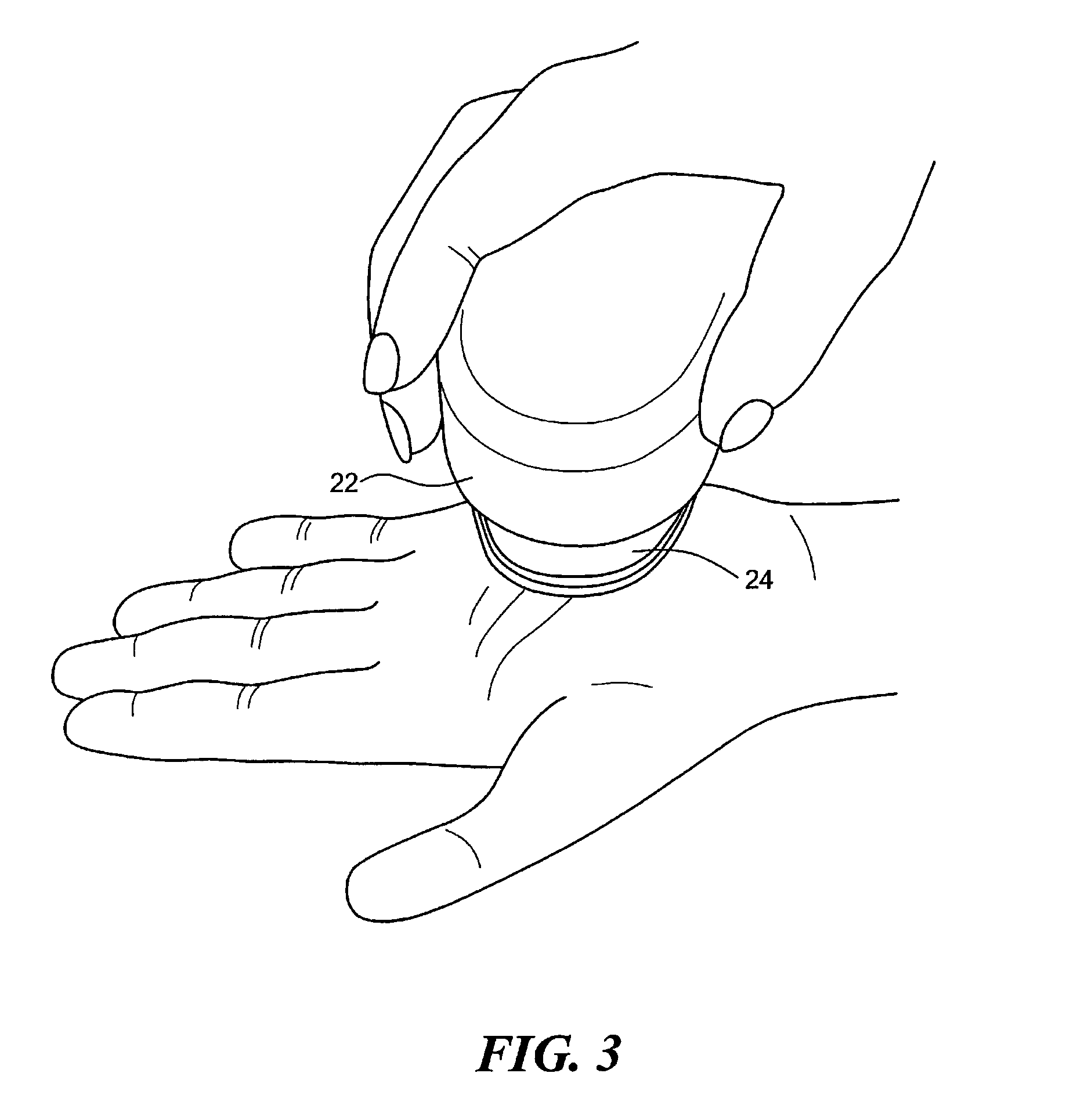
Method for performing immunological test on biomolecules by avidin/streptavidin magnetic composite particles
ActiveCN101713779ALarge coupling capacityImprove suspension stabilityMaterial analysisBiotin-streptavidin complexStrong binding
The invention relates to the immune analysis field, in particular relating to a method for performing an immunological test on biomolecules by avidin / streptavidin magnetic composite particles, which takes avidin / streptavidin magnetic composite particles as carriers, and comprises the following steps:, coating biotinylated antibody or antigen, adding a specimen to be tested and enzyme labeled antibody / enzyme labeled antigen / enzyme labeled anti-antibody, adding chromogenic substrate and testing.Biotin and avidin / streptavidin has strong binding capacity, can fix the antigen / antibody labeled by the biotin through the avidin / streptavidin labeled on the surface of the magnetic composite particles so as to use the system to test specific antibody / antigen. The test system has the advantages of high sensitivity, high specificity, high stability, strong adaptability and the like.
Owner:XIAN GOLDMAG NANOBIOTECH
Method for preparing amino functional magnetic fluorescent coding microsphere with double-nucleocapsid structure
InactiveCN101530766AImprove stabilityMagnetically responsiveInorganic material magnetismMicroballoon preparationMicrosphereDual core
The invention belongs to the technical field of biomolecular labeling, in particular to a method for preparing an amino functional magnetic fluorescent coding microsphere with double-nucleocapsid structure. Two semiconductor quantum dots with different fluorescence colors and superparamagnetic ferric oxide nano-particles are embedded in a same silicon dioxide nano-particle in inverse microemulsion to form an amino functional magnetic fluorescent coding microsphere which has the granularity between 40nm and 100nm, high fluorescence intensity and high stability. The invention adjusts the fluorescence position and the intensity of the microsphere by changing the sort and the proportion of the quantum dots of different fluorescence colors and realizes different fluorescent coding. The addition of superparamagnetic ferroferric oxide ensures that the microsphere can gather and move fast in a targeted way under the action of an externally-applied magnetic field. Through amido modification on the surface of the microsphere, the invention can be widely applied to the fields of immunological test, nucleic acid hybridization, gene analysis, cell classification and imaging, etc.
Owner:JILIN UNIV
Method for testing anti-SmD1 antibody IgG and reagent device
The invention provides a method for immunologically detecting an anti-SmD1 antibody IgG on the basis of an enzyme-linked immunosorbent assay principle and a reagent device , which relate to an analysis method used for enzyme-linked immunosorbent assay of the anti-SmD1 antibody IgG independently, individually and disposably, a reagent device and a matched reagent. In the method, a plurality of reagents required in the enzyme-linked immunosorbent assay of the anti-SmD1 antibody IgG can be contained in one analyzing device; and according to the method, relevant immunological tests can be conveniently conducted according to using requirements on the tested project, and better basis is provided for clinical practice.
Owner:SHENZHEN YHLO BIOTECH
Immunological test element with improved control zone
The invention concerns a test element for carrying out an immunological sandwich test for determining an analyte from a liquid sample containing a reagent zone or conjugate zone which contains a conjugate of an analyte binding partner and a label which can be detected directly or indirectly by visual, optical or electrochemical means (e.g., an enzyme, fluorescent or direct label, etc.) wherein the conjugate can be dissolved by the liquid sample, a detection zone which contains a permanently immobilized (i.e., which cannot be detached by the liquid sample) binding partner for the analyte or for complexes containing the analyte; and a control zone which contains a permanently immobilized binding partner for the conjugate of analyte binding partner and label characterized in that the control zone additionally contains one or more permanently immobilized binding partner(s) for the analyte or for complexes containing the analyte.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Analytical sandwich test for determining NT-proBNP
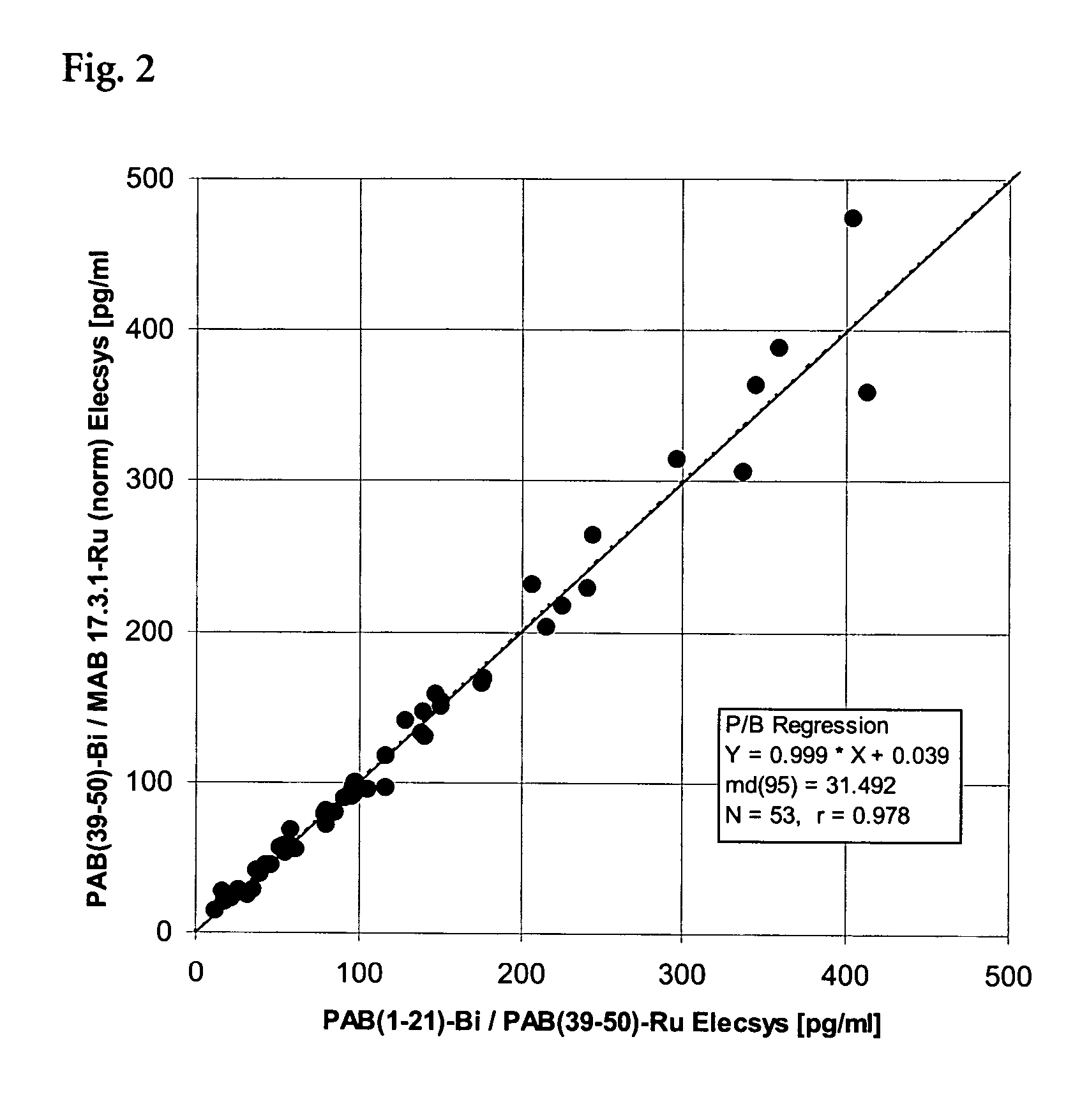
ActiveUS20050118662A1Good correlationBioreactor/fermenter combinationsBiological substance pretreatmentsEpitopeAmino acid
The present invention concerns an immunological test for determining NT-proBNP comprising at least two antibodies to NT-proBNP, wherein at least one of the antibodies to NT-proBNP is a monoclonal antibody. One of these antibodies is directed at least against parts of the epitope of NT-proBNP comprising the amino acids 38 to 50. In addition, one of these antibodies is directed at least against parts of the epitope of NT-proBNP comprising the amino acids 1 to 37 or 43 to 76. The epitope recognized by the antibodies can slightly overlap.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
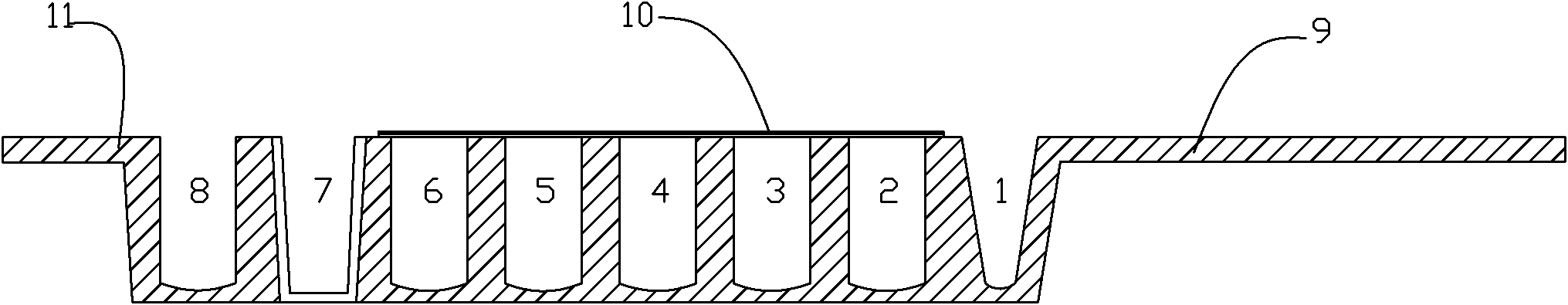
Single column immunological test elements
ActiveUS8076126B2Maximum versatilityImprove throughputBioreactor/fermenter combinationsSequential/parallel process reactionsEngineeringTest material
A plurality of individual single column test elements are provided for use in a clinical testing apparatus. Each test element is defined by a single test column that includes a quantity of a test material, such as gel material or a bead matrix, including a cover strip used to access the contents of the test column. Individual test elements can be stored, retained and dispensed for testing patient samples.
Owner:ORTHO-CLINICAL DIAGNOSTICS
Method for Increasing the Dynamic Measuring Range of Test Elements Based on Specific Binding Reactions
The invention concerns a method for increasing the dynamic measuring range of especially immunological test elements in particular immunological chromatography test strips that can be evaluated optically that are based on specific binding reactions. The invention enables the dynamic measuring range of test elements based on specific binding reagents, especially of immunological test elements to be shifted towards higher analyte concentrations without impairing the lower detection limit. For this purpose it is proposed according to the invention that at least two zones are provided in or on the test element which contain reagents that generate detectable signals of different strengths due to different affinities for the analyte (for example in the case of antibodies that have different affinities for the analyte) or due to different principles of interaction with the analyte or with other reagents involved in the analyte detection (for example antibodies directed against the analyte in one zone and an analyte analogue in another zone). The signals in the at least two zones are used to evaluate the analyte concentration-signal strength relationship and are used to determine the analyte by means of a suitable method (correlation).
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Ultrasensitive immunoassays
InactiveUS20050233351A1Reduce the numberMicrobiological testing/measurementEnzymologyImmobilized AntibodiesOligonucleotide
The present invention relates to an immunological test kit and immunoassay using a first immobilized antibody having affinity for a specific antigen. The invention is characterized by a second and third antibody being specific for different determinants of the antigen and modified with cross-linkable oligonucleotides. For detection, the oligonucleotides are amplified, whereby only such oligonucleotides will be amplified which have been cross-linked to each other. In this way unspecific background is avoided and detection is possible down to single molecules.
Owner:LANDEGREN ULF
Truncated-form streptococcus hemolyticus bacteriolysin O and detection kit using same
ActiveCN102964435ASpecific immunogenicityIncrease productionDepsipeptidesBiological testingEscherichia coliBacteroides
The invention provides a truncated-form streptococcus hemolyticus bacteriolysin O gene and an ASO detection kit. According to the invention, part of SLO gene fragments are cloned, and the part of SLO genes are cloned into an expression vector of escherichia coli and the like so as to realize mass expression and facilitate purification. In particular, the invention provides a DNA sequence which has a base sequence as shown in SEQ ID NO:1. According to the invention, the truncated-form SLO sequence can greatly improve the yield of SLO protein; more than 150 mg of SLO protein can be obtained through purification of one liter of bacterial culture, and the purity is more than 90%; immunological tests prove that the sectionally expressed SLO still maintains the specific immunogenicity.
Owner:BEIJING LEADMAN BIOCHEM
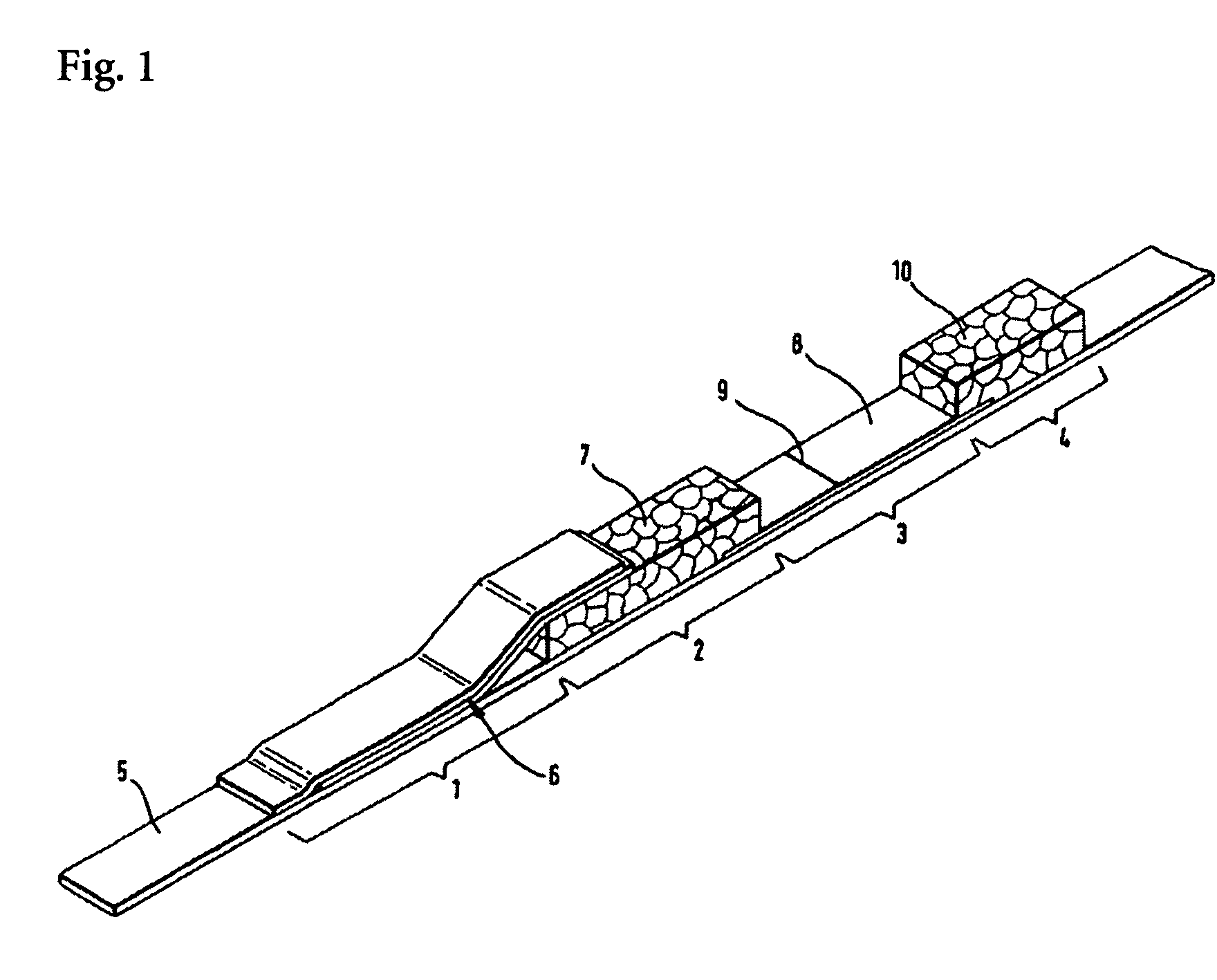
Test kit and use thereof
PCT No. PCT / CH97 / 00121 Sec. 371 Date Sep. 4, 1998 Sec. 102(e) Date Sep. 4, 1998 PCT Filed Mar. 21, 1997 PCT Pub. No. WO97 / 35663 PCT Pub. Date Oct. 2, 1997The test kit includes a substrate and, welded or bonded thereto, a plastic sheet having blisters. One of the blisters is shaped as a siphon. The other blisters act as reaction vessels and also serve to receive and store a reagent. The test kit may contain a test strip in one of the blisters. The test kit is particularly suited for carrying out immunological tests, whereby the siphon markedly facilitates the procedure during the washing step.
Owner:INTEX PHARMA PROD
Sample pretreatment solution for immunological test and method for using the same
InactiveCN1576842APreparing sample for investigationBiological material analysisPretreatment methodPharynx
Owner:SYSMEX CORP
Blood coagulant for blood collection container
ActiveCN102212514ALong storage timeCoagulation rate is less affected by temperatureEnzyme stabilisationBiological testingBlood Collection TubeHemolysis
Owner:SHANGHAI KEHUADIAGNOSITIC MEDICAL PRODS
Test Element Having Combined Control and Calibration Zone
ActiveUS20110244598A1Analysis by electrical excitationAnalysis by subjecting material to chemical reactionAnalyteEngineering
A test element, for example in the form of an immunological test strip functioning according to the sandwich principle, for the fluorophoric detection of one or more analytes in a sample comprising an analyte detection zone and a combined control and calibration zone. The combined control and calibration zone include a fluorophore and binding partners for the specific binding of reagents labelled with a fluorophore. Furthermore, the invention concerns a method for calibrating an analyte-specific measurement signal, a method for determining the concentration of an analyte in a sample and the use of the test element for calibrating a signal generated in the analyte detection zone of a test element.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Method for holding multiple types of diagnostic test consumables in a random access single container
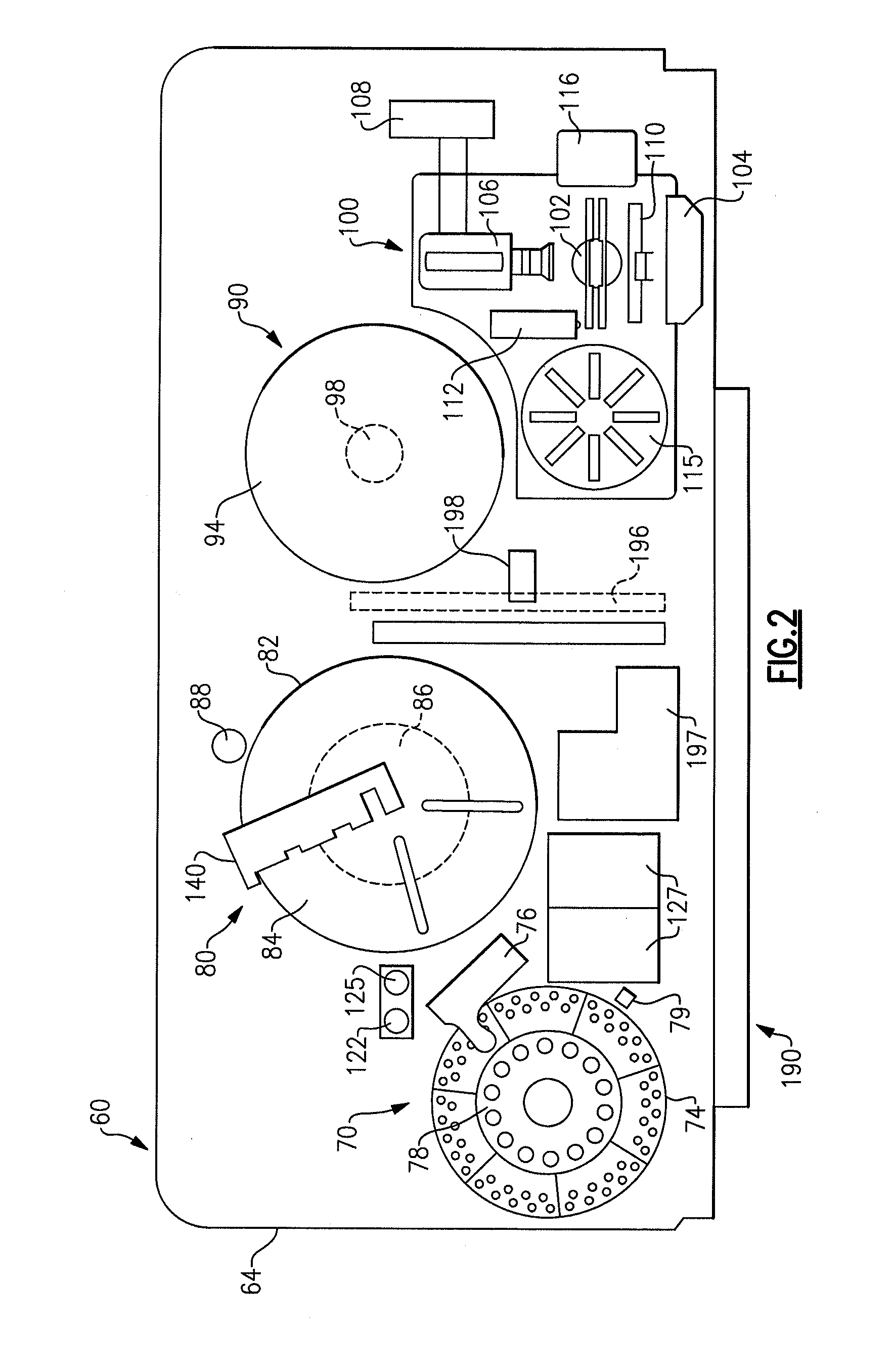
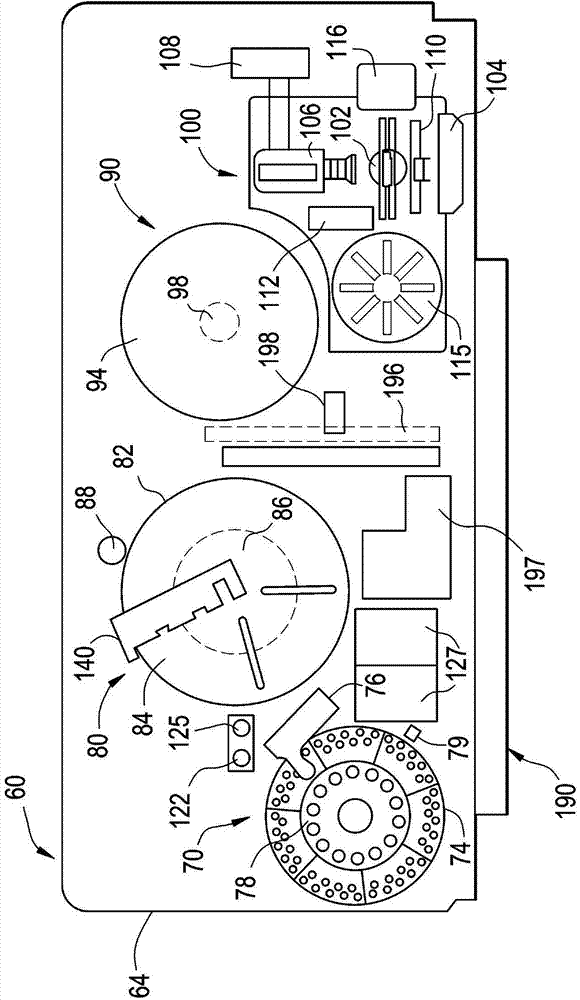
ActiveUS20140163920A1Test may changeConsiderable timeMeasurement arrangements for variableDigital computer detailsImmunodiagnosticsDiagnostic test
An immunodiagnostic test method includes holding a selection of immunological test elements or consumables in one or more containers attached to or positioned in the analyzer and providing random access to any test element therein. The container can hold multiple types of test elements in compartments or slots. Through sensing of a test element position in its slot, the detection mechanism of the invention provides for random access to multiple types of test elements in any sleeve and within a single sleeve, and provides efficient inventory control. The method increases the number of test element types that may be loaded onto an analyzer and maintains fast determination of inventory.
Owner:ORTHO-CLINICAL DIAGNOSTICS
Application of flavonoid
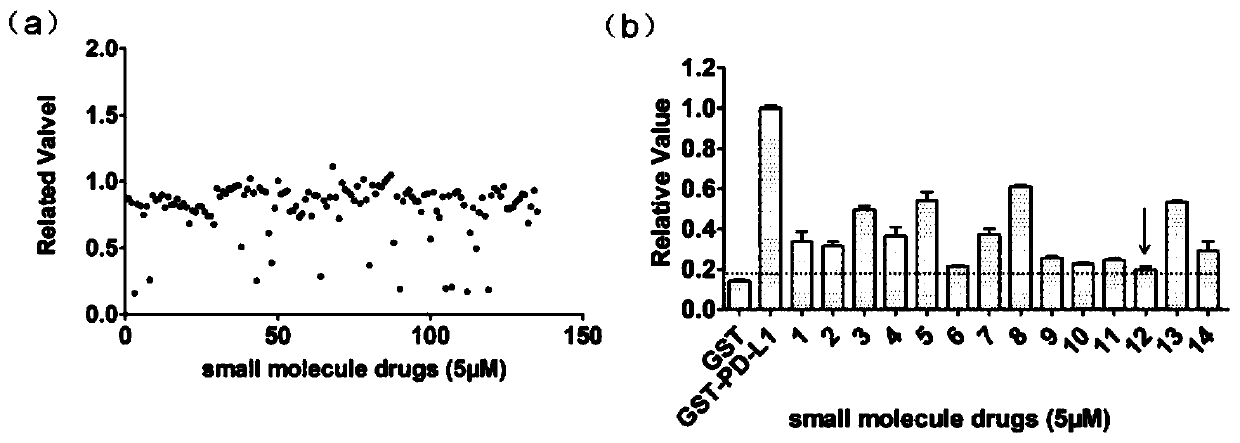
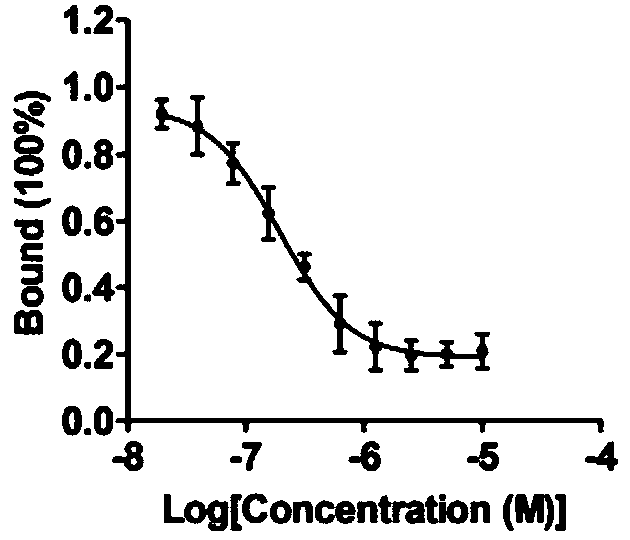
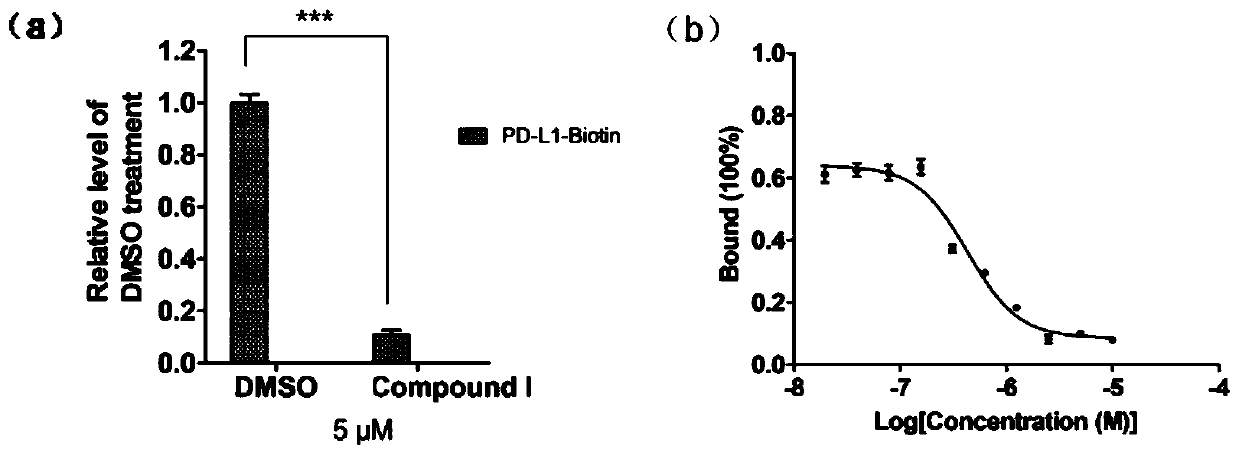
InactiveCN110200959AAvoid interactionHigh affinityOrganic active ingredientsAntineoplastic agentsMda mb 231PD-L1
The invention relates to an application of a flavonoid compound, the flavonoid compound is 3, 3', 4', 5, 7-pentahydroxyflavone dihydrate, the ELISA and protein fluorescence quenching experiments provethat the compound can effectively inhibit interaction of PD-1 / PD-L1 proteins, and has a strong affinity for PD-L1 protein. Cell clone number formation and ELISA detection of IL-2 secretion assay prove that the compound can effectively enhance the tumor killing activity of T lymphocyte Jurkat cells against MDA-MB-231 and NCI-H460 tumor cells as well as the expression level of IL-2, and then reverse T cellular immunosuppression. The research results of the application provide a drug for tumor immunotherapy targeting the PD-1 / PD-L1 immunological test site.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Determining cholesterol directly on skin surface
InactiveUS8236516B2High sensitivityStrong specificityColor measuring devicesMaterial analysis by observing effect on chemical indicatorThroatAnalyte
A process is provided for analyzing a specimen of biological material in any of a number of biochemical or immunological tests for an analyte which involves subjecting the specimen to treatment which develops a color correlating to the amount of analyte in the specimen. According to the invention at least one defined color characteristic selected from hue angle, chroma, saturation and lightness of the developed color is measured and the results of that measurement analyzed to determine the presence or concentration of the analyte in the specimen. Particular applications are to the detection of cancerous or pre-cancerous abnormalities from the analysis of lung mucus, throat mucus, cervical mucus or seminal fluid.
Owner:MIRACULINS
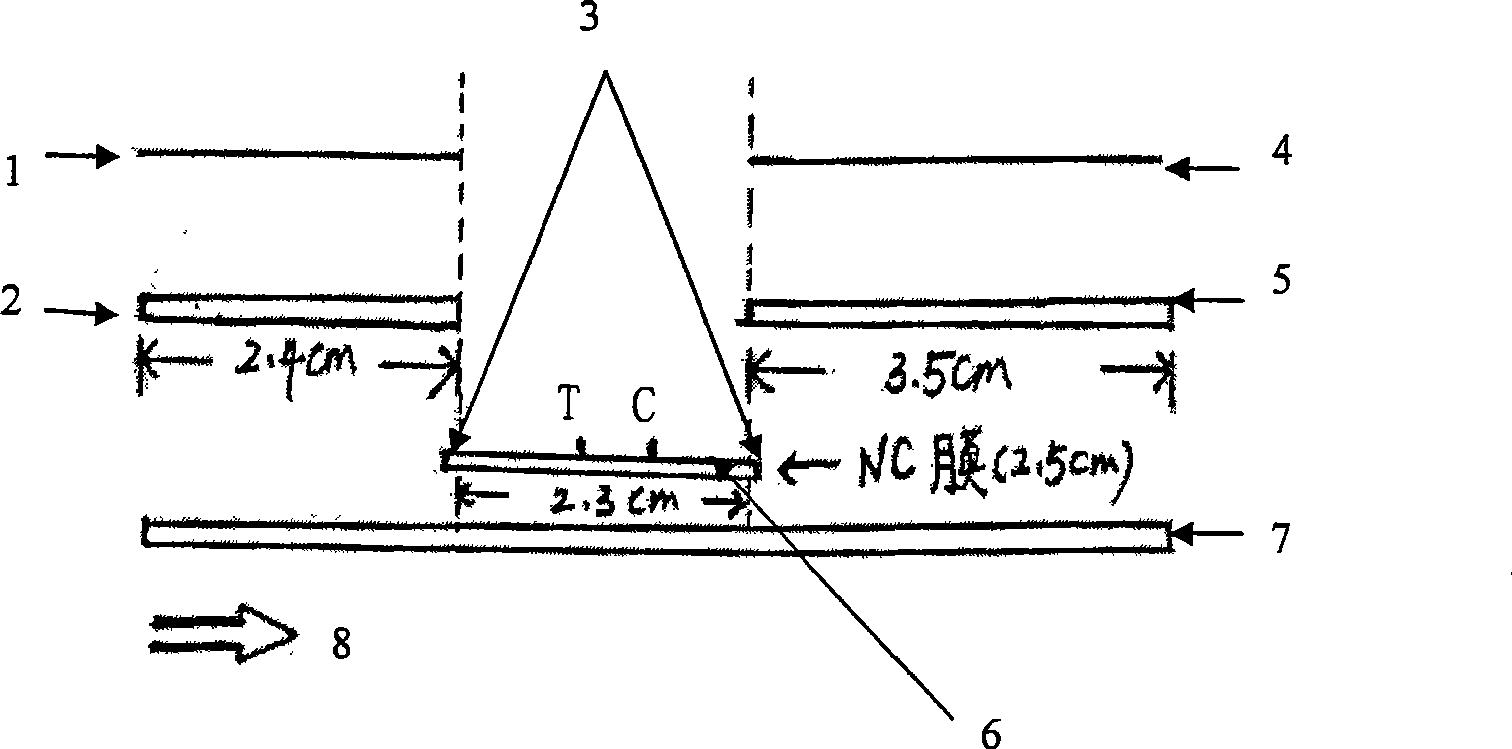
Rabbit hemorrhagic disease virus colloidal gold test strip
ActiveCN101520459AStrong specificityLow specificityMaterial analysisHemorrhagic diseasesScreening test
The invention relates to a rabbit hemorrhagic disease virus(RHDV) colloidal gold test strip, and belongs to the technical field of immune tests of the colloidal gold. One end of a bottom plate of the test strip is a colloidal gold-RHDV polyclonal antibody combination glass fiber film, and an antibody solid-phase cellulose nitrate film is arranged on the middle part of the bottom plate; a RHDV single-resistance test line, namely T line and a goat anti-rabbit IgG control line, namely C line are arranged on the bottom plate; and the other end of the bottom plate is absorbent paper. When a tested sample is carried with the RHDV, the RHDV can specifically combine the anti-RHDV polyclonal antibody labeled by the colloid so as to be identified by the anti-RHDV monoclonal antibody, namely the double-antibody sandwich specific combination happens, so that whether the sample is carried with the RHDV can be tested. The invention also provides a RHDV immunological test method established by utilizing the monoclonal antibody and the polyclonal antibody, which has the advantages of quickness, accuracy, convenience and the like, and the method is particularly suitable for the on-the-spot test and preliminary screening test and has bright prospect.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Sample pretreatment solution for immunological test and method for using the same
InactiveUS20090269735A1Good backgroundSure easyMicrobiological testing/measurementBiological material analysisPara-influenza virusesImmunological tests
Sample pretreatment solutions for influenza virus tests by immunochromatography are described.Methods detecting influenza virus by immunochromatography using the sample pretreatment solutions and Kits comprising the sample pretreatment solution and an immunochromatographic device are also described.
Owner:SYSMEX CORP
Analytical sandwich test for determining NT-proBNP
ActiveUS7507550B2Bioreactor/fermenter combinationsBiological substance pretreatmentsEpitopeMonoclonal antibody
The present invention concerns an immunological test for determining NT-proBNP comprising at least two antibodies to NT-proBNP, wherein at least one of the antibodies to NT-proBNP is a monoclonal antibody. One of these antibodies is directed at least against parts of the epitope of NT-proBNP comprising the amino acids 38 to 50. In addition, one of these antibodies is directed at least against parts of the epitope of NT-proBNP comprising the amino acids 1 to 37 or 43 to 76. The epitope recognized by the antibodies can slightly overlap.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Preparation method and application of ractopamine monoclonal antibody
InactiveCN102286103AStrong specificityLow cross-reactivityImmunoglobulinsMaterial analysisMonoclonal antibodyApplication specific
The invention discloses a method for preparing a specific monoclonal antibody and its application, and relates to the technical field of detection. By modifying the structure of ractopamine hydrochloride and synthesizing an artificial antigen of ractopamine, a specific monoclonal antibody of ractopamine is prepared. Ractopamine monoclonal antibody is used as the core reagent to prepare colloidal gold immunological test strips for detecting ractopamine. The ractopamine antibody prepared by the present invention is a highly specific monoclonal antibody, which has the characteristics of strong specificity, high sensitivity, and high accuracy; the present invention utilizes the test strip prepared on the basis of the highly specific monoclonal antibody for detecting Residual ractopamine has the characteristics of simplicity, rapidity, sensitivity, accuracy and low cost, and its application has broad prospects.
Owner:安徽缘远博爱生物技术有限公司
Single column immunological test elements
ActiveUS20100015726A1Reduce storage capacityImprove throughputBioreactor/fermenter combinationsSequential/parallel process reactionsEngineeringTest material
A plurality of individual single column test elements are provided for use in a clinical testing apparatus. Each test element is defined by a single test column that includes a quantity of a test material, such as gel material or a bead matrix, including a cover strip used to access the contents of the test column. Individual test elements can be stored, retained and dispensed for testing patient samples.
Owner:ORTHO-CLINICAL DIAGNOSTICS
Method for holding multiple types of diagnostic test consumables in a random access single container
ActiveCN103869089AShorten the timeIncrease throughputCounting mechanisms/objectsBiological testingEngineeringTest element
An immunodiagnostic test method includes holding a selection of immunological test elements or consumables in one or more containers attached to or positioned in the analyzer and providing random access to any test element therein. The container can hold multiple types of test elements in compartments or slots. Through sensing of a test element position in its slot, the detection mechanism of the invention provides for random access to multiple types of test elements in any sleeve and within a single sleeve, and provides efficient inventory control. The method increases the number of test element types that may be loaded onto an analyzer and maintains fast determination of inventory.
Owner:ORTHO-CLINICAL DIAGNOSTICS
Method for increasing the dynamic measuring range of test elements based on specific binding reactions
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Sudan 1 capable of being directly marked and preparation method and application thereof
The invention belongs to the technical field of immunological test, and in particular relates to a method for preparing Sudan 1 capable of being directly marked and application of the Sudan 1. In the method, a technical route of denovo synthesis is mainly utilized, amino active group is introduced successfully on the premise of not changing an antigenic determinant in a host molecular structure of the Sudan 1, and a trim capable of directly being connected with fluorescent substances and enzymes is provided for the Sudan 1; and an important problem of competitive antigen preparation is solved for establishing a Sudan 1 competitive method enzyme-linked immuno sorbent assay (ELISA) detection system. The whole method is simple and practicable, easy to operate, and high in efficiency of connecting the fluorescent substances and the enzymes.
Owner:NANJING UNIV
Method for diagnosing Lyme disease using a cellular immunological test
The present invention relates to a method for diagnosing Lyme disease in a subject, the method comprising the steps of: (a) obtaining a sample from said subject, (b) contacting said sample with a source of Borrelia antigens and (c) determining the expression level of a pro-inflammatory cytokine in said sample at the end of step (b).
Owner:STICHTING RADBOUD UNIVERSITAIR MEDISCH CENT
Spectrophotometric measurement in color-based biochemical and immunological assays
InactiveUS20050003468A1Remove uncertaintyHigh sensitivityColor measuring devicesMaterial analysis by observing effect on chemical indicatorThroatAnalyte
A process is provided for analyzing a specimen of biological material in any of a number of biochemical or immunological tests for an analyte which involves subjecting the specimen to treatment which develops a color correlating to the amount of analyte in the specimen. According to the invention at least one defined color characteristic selected from hue angle, chroma, saturation and lightness of the developed color is measured and the results of that measurement analyzed to determine the presence or concentration of the analyte in the specimen. Particular applications are to the detection of cancerous or pre-cancerous abnormalities from the analysis of lung mucus, throat mucus, cervical mucus or seminal fluid.
Owner:MIRACULINS
Method for production of antibody
InactiveCN101883862ASuitable for screeningIncrease production capacityImmunoglobulins against animals/humansAntibody ingredientsAntigenStimulant
Disclosed is a method for producing an antibody, which comprises the steps of: immunizing a tissue containing a cell to be immunized in vitro in a liquid culture medium containing an antigen and a stimulant; selecting an immunized cell; and producing the antibody from the selected immunized cell. The method enables to produce an antibody in large quantity within a short period, and therefore can contribute to the widespread use of an immunological test.
Owner:ALLIED COLLOIDS LTD
Brucella abortus immune marking recombinant strain and application thereof in preparation of vaccine
InactiveCN101629151AHigh toxicityPromote eradicationAntibacterial agentsBacterial antigen ingredientsSerum igeBacterial strain
The invention discloses brucella abortus immune marking recombinant strain and the application thereof in preparation of vaccine. The recombinant strain is obtained by inactivating glycosyltransferase gene in the brucella abortus. The recombinant bacterial strain has weaker toxicity, can be taken as vaccine to immunize animals without inactivation, and can distinguish immune vaccinated animals from clinically diseased animals by means of a serological technology. By being immunized with the strain of the invention, a mouse generates antibody and standard bacterial strain which are different from the existing strain, so that the diseased animals can be distinguished from the immunized animals by the immunological test of animal sera. The invention is applicable to improving the brucella abortus vaccine, researching diagnosis identification agent, and developing the brucella abortus vaccine and the matched diagnosis identification agent, and has significant meaning for eradicating and cleaning the brucella abortus of the animals in the world.
Owner:CHINA AGRI UNIV
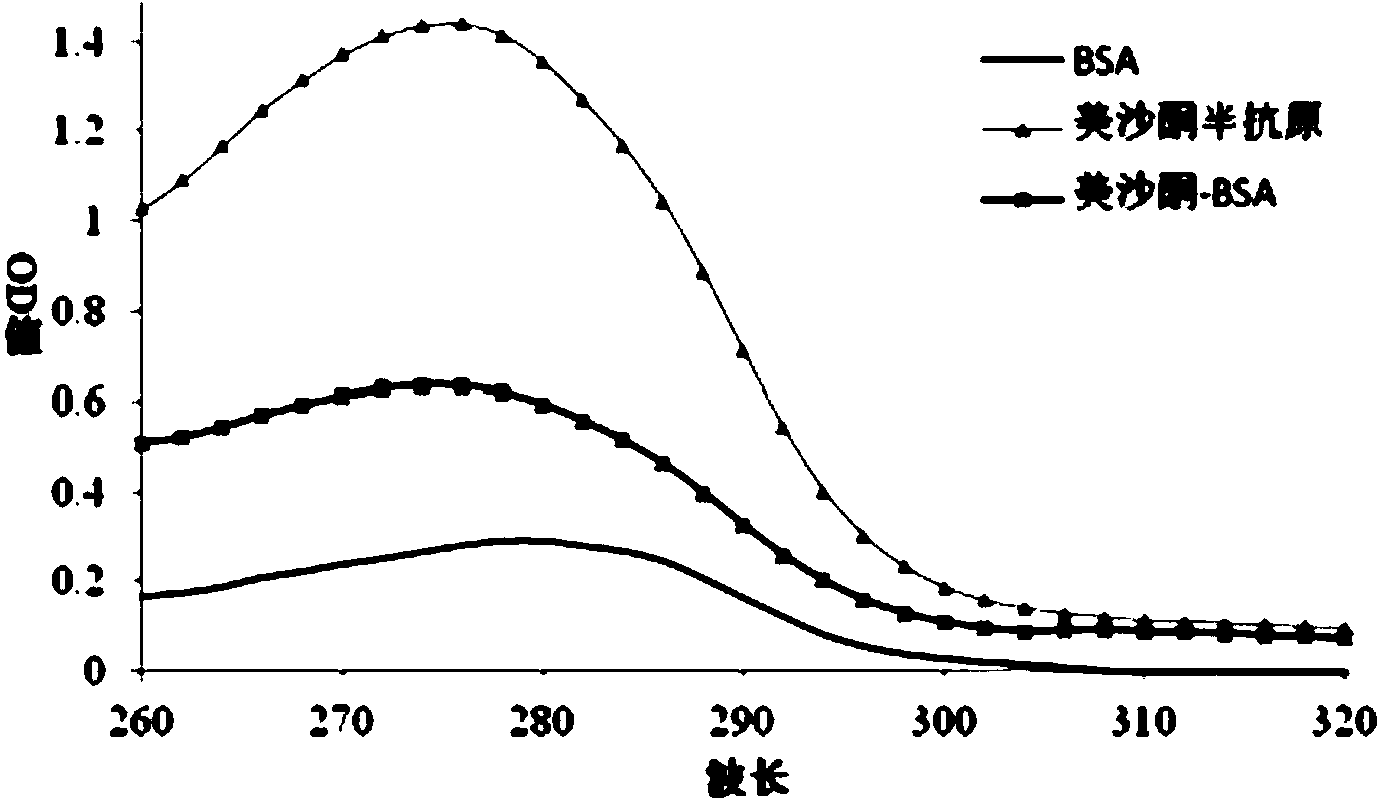
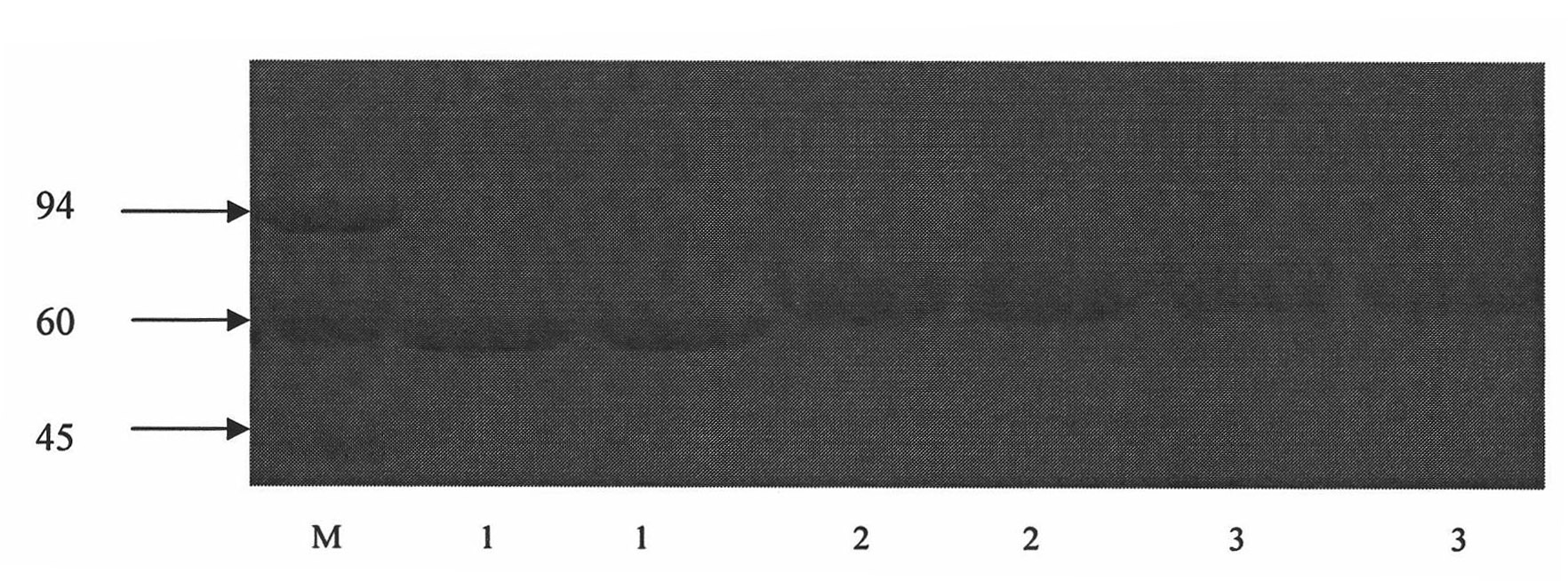
Methadone hapten, preparation method of methadone hapten, methadone antigen, methadone monoclonal antibody and application of methadone monoclonal antibody
ActiveCN103360271AEasy to prepareEasy to operateOrganic compound preparationOvalbuminImmunogenicityRecombinant DNA
The invention discloses a methadone hapten, a preparation method of the methadone hapten, a methadone antigen, a methadone monoclonal antibody and application of the methadone monoclonal antibody. The methadone hapten has a structure represented by a formula I shown in a drawing; according to the preparation method of the methadone hapten, methadone is reduced into hydroxyl methadone, the hydroxyl methadone is subjected to esterification reaction with succinic anhydride, and then, the methadone hapten is prepared; the methadone antigen is prepared through connecting the methadone hapten with a protein carrier; the methadone monoclonal antibody is prepared through applying the methadone antigen to an animal for immunization and then using a hybridoma technique or DNA (Deoxyribonucleic Acid) recombination method; and the methadone monoclonal antibody is applied to the preparation of reagents, reagent strips or kits for detecting samples. The methadone hapten, the methadone antigen and the methadone monoclonal antibody have the advantages that the antigen disclosed by the invention has high immunogenicity, and the immunoreactivity of methadone is preserved; the methadone monoclonal antibody is excellent in specificity and high in potency; and a colloidal gold-labeled methadone monoclonal antibody immunological test strip is high in sensitivity and strong in specificity, is simple, convenient and quick, and can be applied to site detection, and the like.
Owner:GUANGZHOU WONDFO BIOTECH
Monoclonal antibody capable of resisting heavy metal copper and preparation method thereof
InactiveCN102071170AHigh potencyImprove efficiencySerum albuminMicroorganism based processesCopperHybridoma cell
The invention discloses a monoclonal antibody capable of resisting heavy metal copper. The monoclonal antibody is secreted by the hybridoma cell strain C9D11 with the CCTCC NO of C201087. The monoclonal antibody capable of resisting heavy metal copper has high potency. The antibody of the invention can be used for the immunological test of the residual copper ions. The advantages of the antibody, namely high speed, low price, sensitivity and high specificity can be utilized to develop a convenient field testing method, thus the method is suitable for the sampling test of agricultural and livestock products and the rapid test of customs clearance. The monoclonal antibody has practical significance in increasing the efficiency and quality of risk assessment and ensuring the food safety.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com