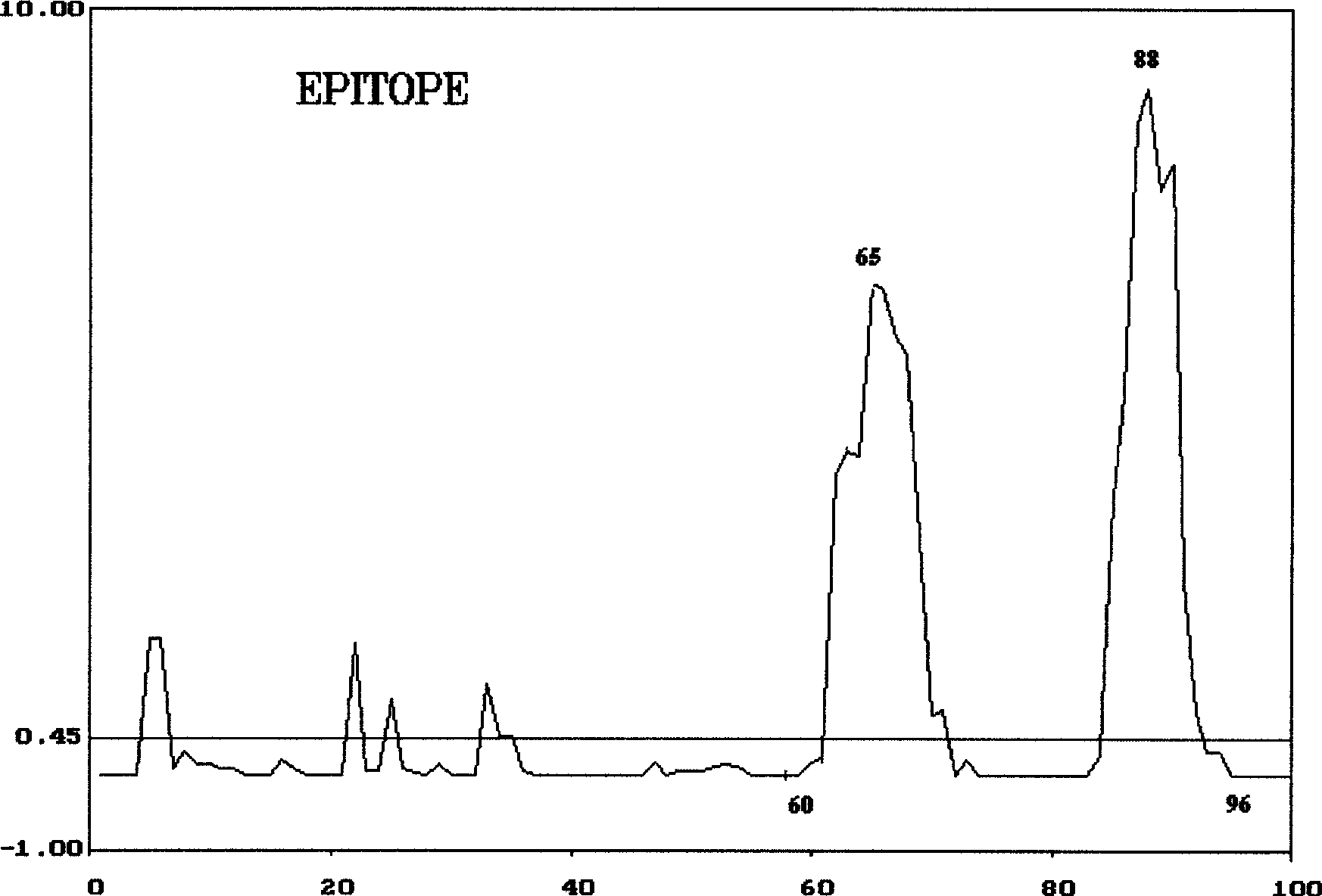
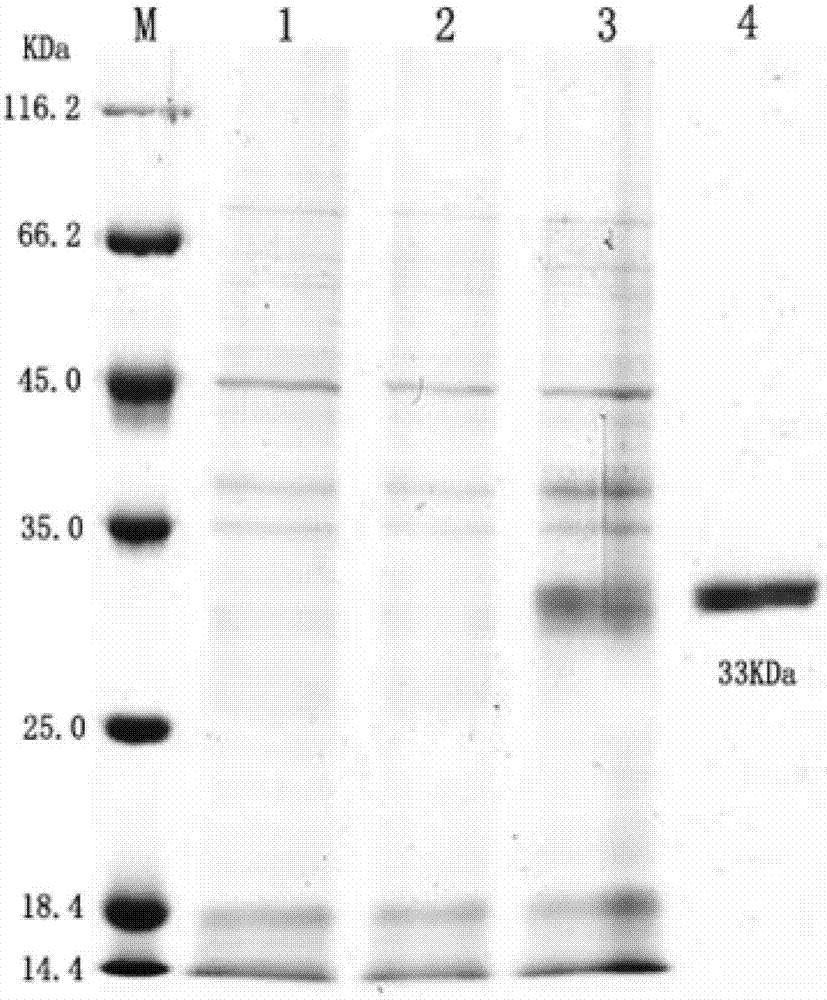
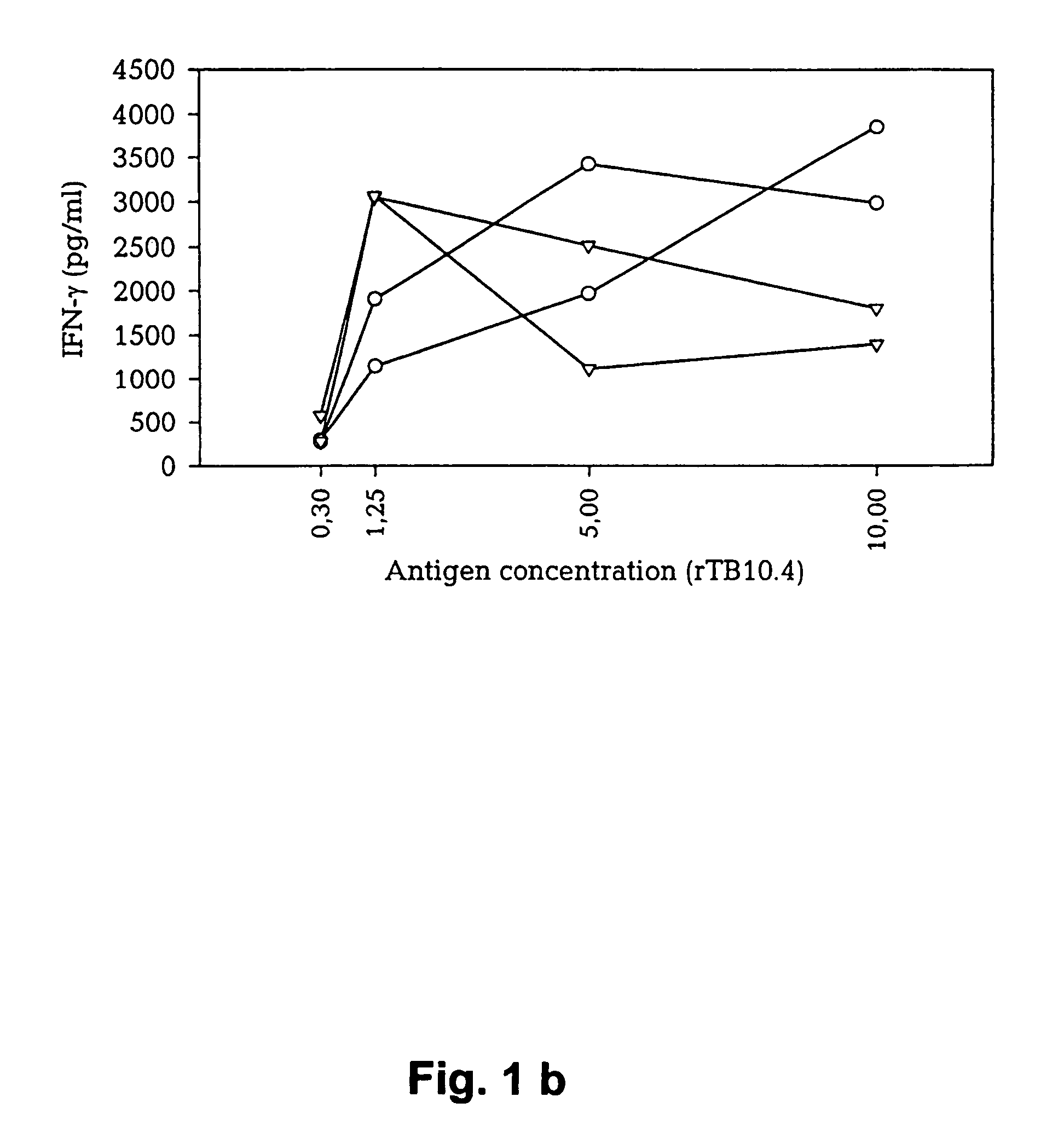
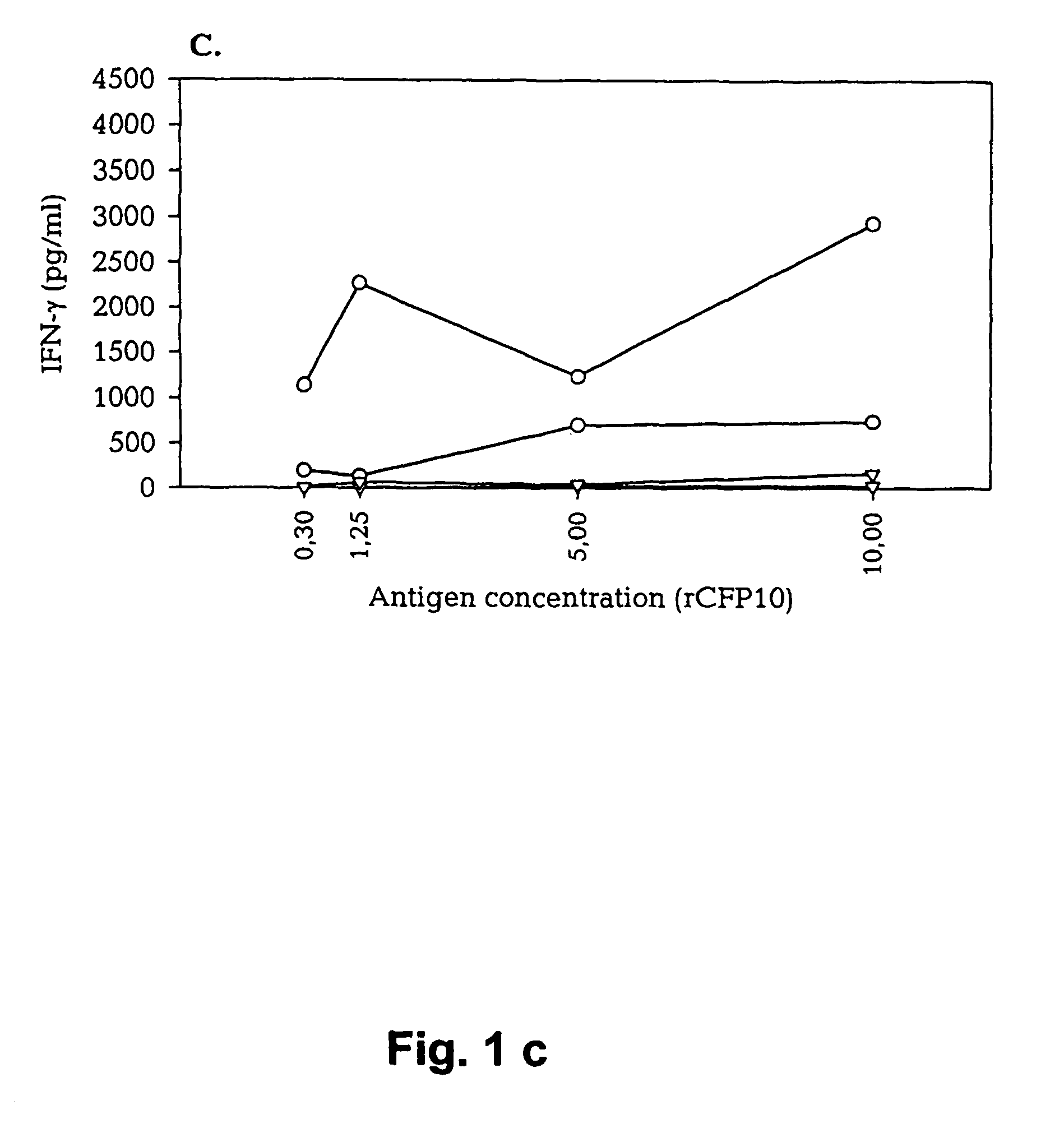
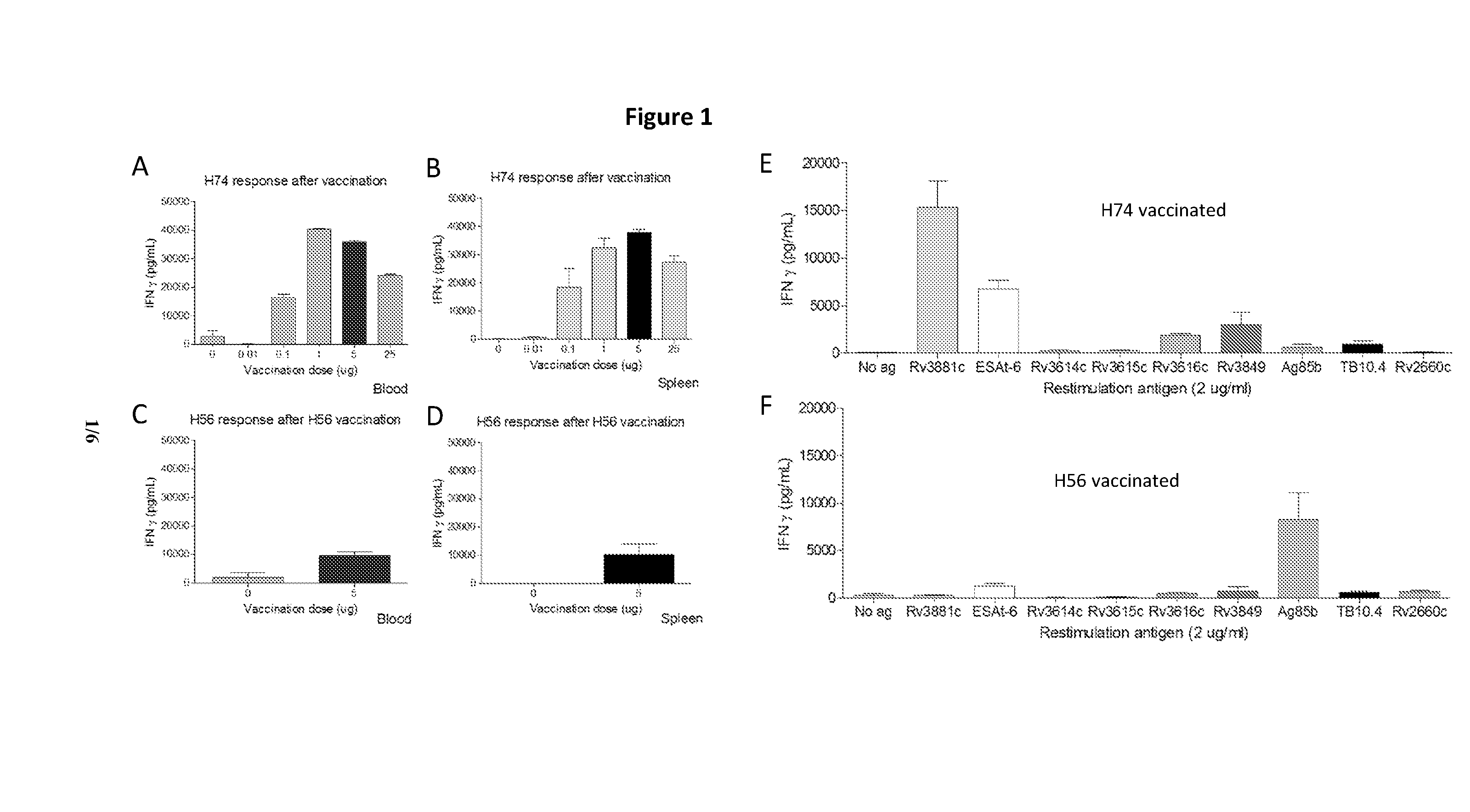
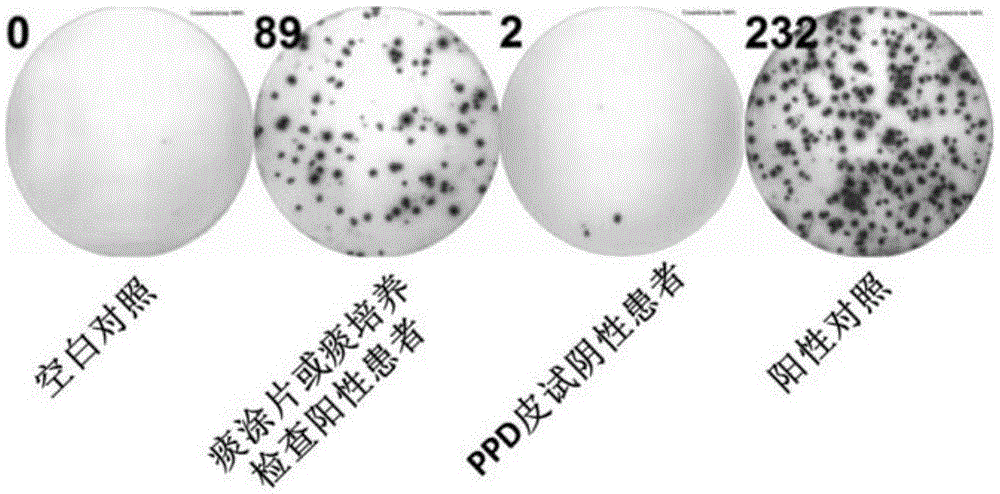
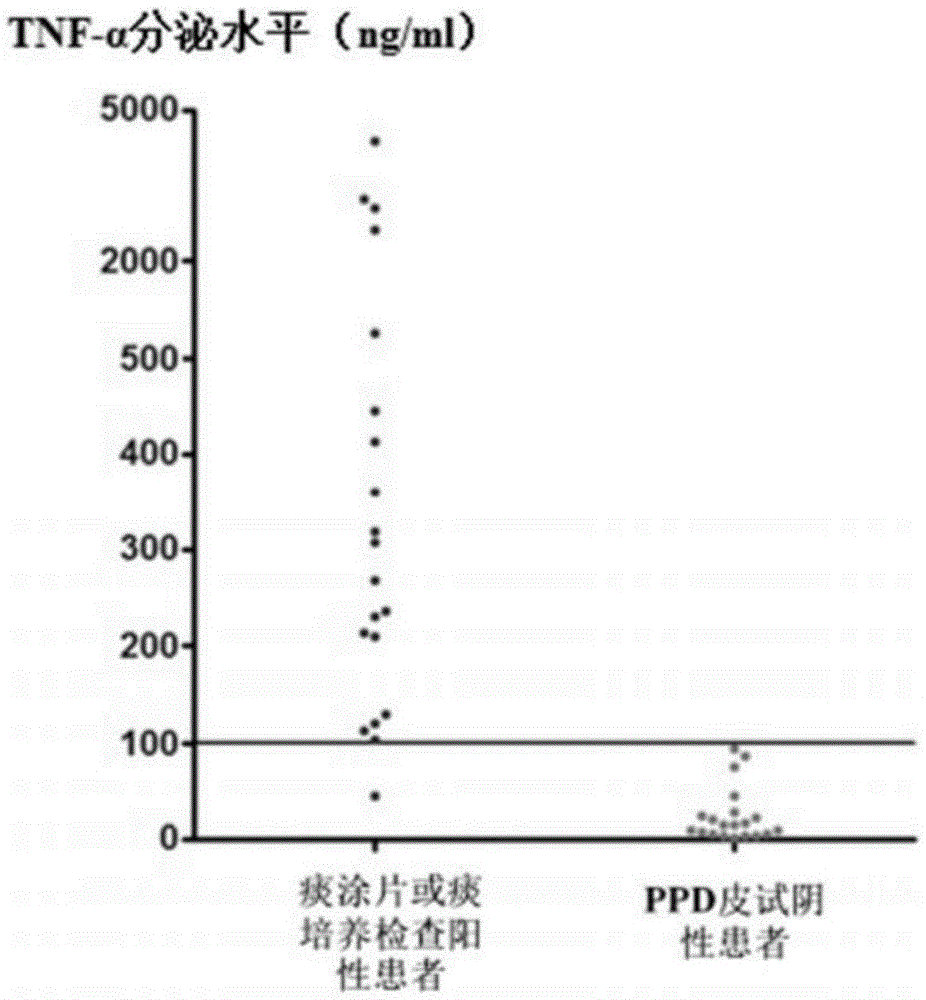
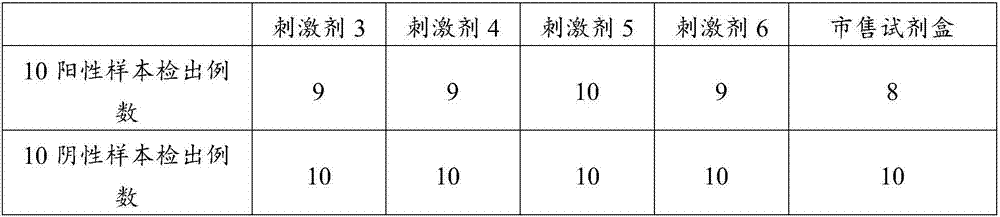
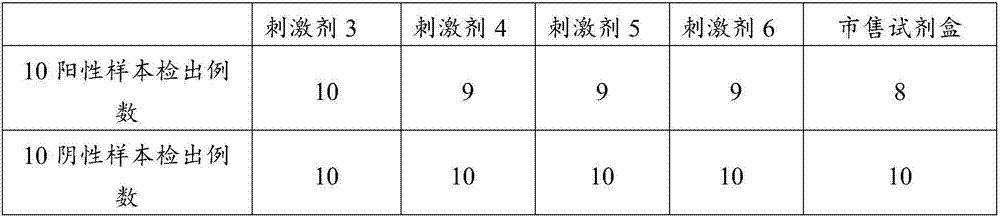
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
69 results about "ESAT-6" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
ESAT-6, the 6 kDa early secretory antigenic target produced by Mycobacterium tuberculosis, is a secretory protein and potent T cell antigen. It is used in tuberculosis diagnosis by the whole blood interferon γ test QuantiFERON-TB Gold, in conjunction with CFP-10 and TB7.7.
Nucleic acids fragments and polypeptide fragments derived from M. tuberculosis
InactiveUS6641814B1Bioreactor/fermenter combinationsBiological substance pretreatmentsSkin testADAMTS Proteins
The present invention is based on the identification and characterization of a number of M. tuberculosis derived novel proteins and protein fragments (SEQ ID NOs: 2, 4, 6, 8, 10, 12, 14, 16, 17-23, 42, 48, 50, 52, 54, 56, 58, 60, 62, 64, 66, 68, 70, 72-86, 88, 90, 92, 94, 141, 143, 145, 147, 149, 151, 153, and 168-171). The invention is directed to the polypeptides and immunologically active fragments thereof, the genes encoding them, immunological compositions such as vaccines and skin test reagents containing the polypeptides. Another part of the invention is based on the surprising discovery that fusions between ESAT-6 and MPT59 are superior immunogens compared to each of the unfused proteins, respectively.
Owner:STATENS SERUM INST
Nucleic acid fragments and polypeptide fragments derived from M. tuberculosis
InactiveUS20020094336A1Bacterial antigen ingredientsPeptide/protein ingredientsSkin testADAMTS Proteins
The present invention is based on the identification and characterization of a number of M. tuberculosis derived novel proteins and protein fragments (SEQ ID NOs: 2, 4, 6, 8, 10, 12, 14, 16, 17-23, 42, 48, 50, 52, 54, 56, 58, 60, 62, 64, 66, 68, 70, 72-86, 88, 90, 92, 94, 141, 143, 145, 147, 149, 151, 153, and 168-171). The invention is directed to the polypeptides and immunologically active fragments thereof, the genes encoding them, immunological compositions such as vaccines and skin test reagents containing the polypeptides. Another part of the invention is based on the surprising discovery that fusions between ESAT-6 and MPT59 are superior immunogens compared to each of the unfused proteins, respectively.
Owner:STATENS SERUM INST
Kit for detecting active tuberculosis based on antigen-specific TNF-alpha-ELISA (enzyme linked immunosorbent assay) and application thereof
InactiveCN103604933AGood performance parametersStrong specificityDisease diagnosisBiological testingPeripheral blood mononuclear cellHorseradish peroxidase
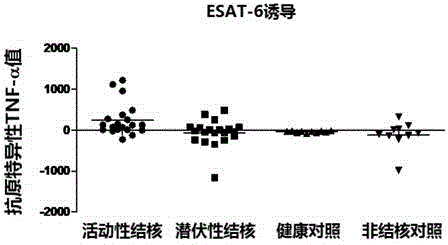
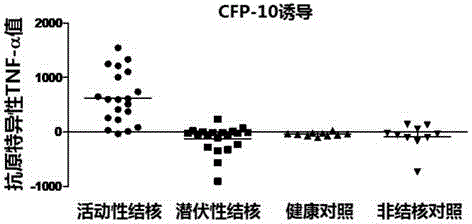
The invention discloses a kit for detecting active tuberculosis based on antigen-specific TNF-alpha-ELISA (enzyme linked immunosorbent assay) and an application thereof. The kit comprises an ELISA plate coating a TNF-alpha antibody, a TNF-alpha protein standard, protein liquid of ESAT-6 and CFP-10, a biotin-marked TNF-alpha antibody and avidin-marked horseradish peroxidase. By adopting the kit for detecting active tuberculosis based on TNF-alpha-ELISA, a method for distinguishing active tuberculosis infection from latent tuberculosis infection is established; the TNF-alpha released by the peripheral blood mononuclear cell under the stimulus of tuberculosis-specific antigens ESAT-6 and CFP-10 and the background TNF-alpha released by the peripheral blood mononuclear cell without stimulus are quantitatively detected through the ELISA technology, and the value of the antigen-specific TNF-alpha is obtained by subtracting the two so as to perform direct diagnosis on the active tuberculosis.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
Tuberculosis diagnostic test
InactiveUS7632646B1High sensitivityPeptide/protein ingredientsGenetic material ingredientsIn vivoMycobacterium
A method of diagnosing in a host infection by or exposure to a mycobacterium which expresses ESAT-6 comprising (i) contacting a population of T cells from the host with one or more peptides or analogues selected from the peptides represented by SEQ ID NO:1 to 11 and analogues thereof which can bind a T cell receptor which recognises any of the said peptides, and (ii) determining whether the T cells of said T cell population recognise the peptide(s) and / or analogue(s). The method may be performed in vivo. Peptides and a kit which enable the method to be carried out are provided.
Owner:OXFORD IMMUNOTEC
Polyepitope tuberculosis gene vaccine and its prepn process
InactiveCN101088559ARaise the level of immune responseStimulate immune responseAntibacterial agentsBacterial antigen ingredientsEpitopeGenetic vaccine
The present invention discloses one kind of polyepitope tuberculosis gene vaccine comprising one kind of carrier and one inserted segment of target gene, which has in the 5' end one whole length HSP65 gene, and in the 3' end serially arranged selected epitope genes, including ESAT-6Á1-20 positions genes and 61-81 positions genes of ESAT-6, 62-84 positions genes of Ag85A, 121-155 positions genes of Ag85B, 143-166 positions genes of Ag85A, 234-256 positions genes of Ag85B and 177-228 positions genes of MPT64C. The present invention discloses the application of the polyepitope tuberculosis gene vaccine. Mouse immunizing experiment shows that the gene vaccine can well induce humoral immunity response. The gene vaccine of the present invention can well induce Th1 type immunity response to mycobacterium tuberculosis and enhance the anti-mycobacterium tuberculosis immunity response level.
Owner:上海欣安基因免疫与疫苗研究开发有限公司
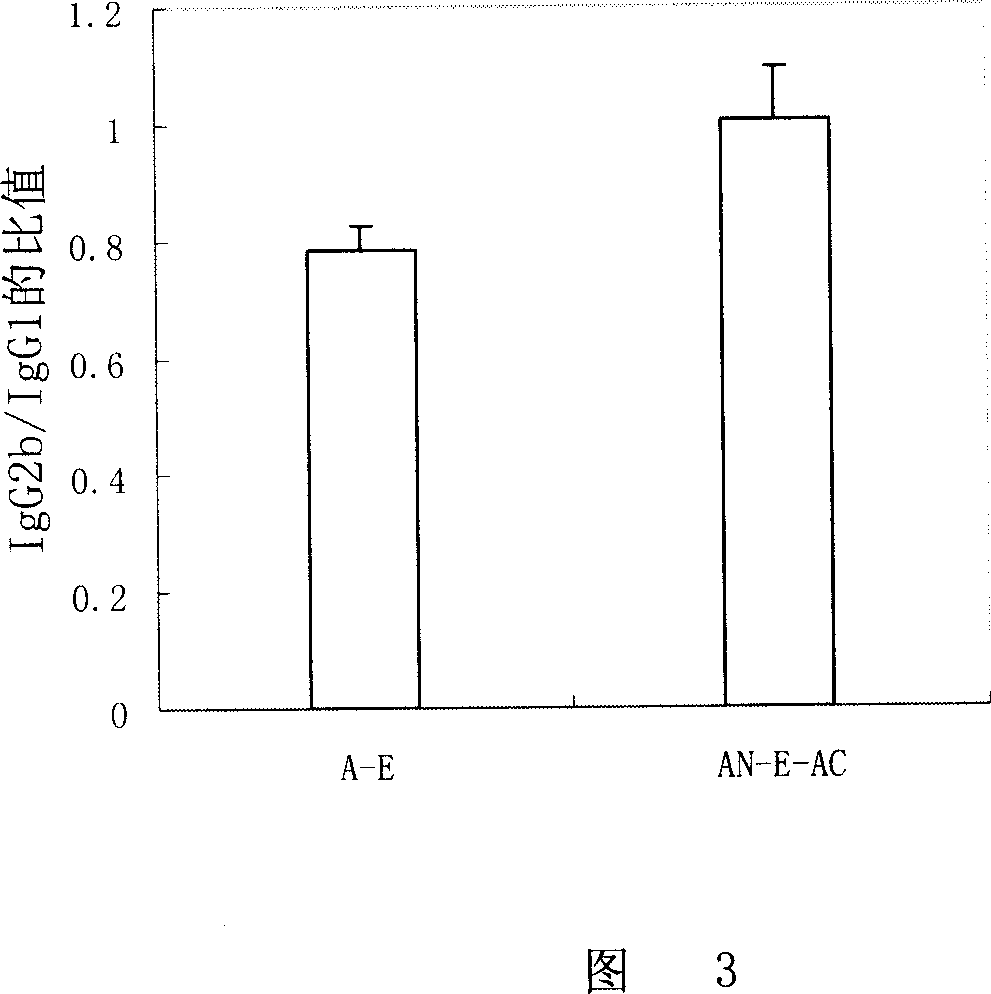
Mosaic vaccine of Ag85B and ESAT-6
The invention relates to Ag85B-ESAT-6 embedded protein vaccine, which is matched with agent MPL-TDM to immunize mouse, to make the IgG2a / IgG1 and IFN- gamma discharge higher then Ag85B-ESAT-6 fusion protein, while later one can generate same protective effect as BCG after infected by bacillus tubercle.
Owner:FUDAN UNIV
TB diagnostic based on antigens from M. tuberculosis
The present invention is based on the identification and characterization of a number of M. tuberculosis derived novel proteins and protein fragments (SEQ ID NOs: 2, 4, 6, 8, 10, 12, 14, 16, 17-23, 42, 48, 50, 52, 54, 56, 58, 60, 62, 64, 66, 68, 70, 72-86, 88, 90, 92, 94, 141, 143, 145, 147, 149, 151, 153, and 168-171). The invention is directed to the polypeptides and immunologically active fragments thereof, the genes encoding them, immunological compositions such as vaccines and skin test reagents containing the polypeptides. Another part of the invention is based on the surprising discovery that fusions between ESAT-6 and MPT59 are superior immunogens compared to each of the unfused proteins, respectively.
Owner:STATENS SERUM INST
ELISA fleck diagnosis kit for tubercle bacillus infect and method for preparing specific antigen
The invention relates to a reagent kit for diagnosing enzyme linked immune spots infected by tubercle bacillus and a method for preparing a specific antigen. The reagent kit comprises a reagent kit body and a detection reagent arranged in the kit body; the detection reagent comprises a positive standard solution, a chromogenic agent, a concentrated detergent and a diluent; the detection reagent also comprises a specific antigen of mycobacterium tuberculosis, a coated INF-gamma resistant antibody and an enzyme label secondary antibody of a vector protein conjugate; the specific antigen of mycobacterium tuberculosis is fusion protein expressed by an ESAT-6 gene and an EIS gene of the mycobacterium tuberculosis; and the INF-gamma resistant antibody is a murine IgG antibody. The method for preparing the specific antigen comprises the following steps: proper tubercle bacillus special antigen ESAT-6 and EIS are combined; and the prepared recombining fusion protein contains main antigenic determinants of two antigens. The recombining fusion protein definitely contains a T cell epitope suitable for the limitation of different HLA, and does not influence results by different groups and different HLA distribution. Clinical tests on tuberculosis patients and healthy people of different groups show that the reagent kit has more excellent specificity and sensitivity than a current similar product.
Owner:THE THIRD PEOPLES HOSPITAL OF SHENZHEN
Composition based on tubercle bacillus antigenic polypeptide for diagnosing tuberculosis
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA +1
Eukaryotic expression CFP10-ESAT-6 fusion protein for diagnosis of bovine tuberculosis
InactiveCN103204945AGenetic manipulation is simpleGrow fastMicroorganism based processesBiological testingPichia pastorisAllergic reaction
The invention provides a eukaryotic expression CFP10-ESAT-6 fusion protein for diagnosis of bovine tuberculosis, which is applied to clinical diagnosis of the bovine tuberculosis. A cfp10-esat6 fusion gene is obtained by PCR (polymerase chain reaction) amplification, a recombinant plasmid pPICZ alpha A-(cfp10-esat6) is constructed, the transfer into Pichia pastoris GS115 is performed, and the high-purity CFP10-ESAT-6 fusion protein is obtained by methanol induction and affinity chromatography. The eukaryotic expression CFP10-ESAT-6 fusion protein is used for an intradermal allergic reaction which is used for diagnosis of the bovine tuberculosis and an antigen-specific bovine IFN-gamma test as a stimulus, the former has the sensitivity of 47.4% and the specificity of 83.3% in comparison with the results of a comparison allergic reaction, and the later has the positive coincidence rate of 82.8% and the negative coincidence rate of 87.4 in comparison with a bovine IFN-gamma kit, thereby showing greater value in diagnosis of the bovine tuberculosis.
Owner:YANGZHOU UNIV
Tuberculosis vaccine and diagnostics based on the Mycobacterium tuberculosis sat-6 gene family
InactiveUS7867502B1Improve featuresFacilitate export of the polypeptideAntibacterial agentsBacteriaGene productMycobacterium
This invention relates to a polypeptide fragment which comprises an amino acid sequence encoded by a member of the esat-6 gene. A member of the esat-6 gene family is defined as gene encoding a small protein and that two such genes are arranged next to each other on the genome and that at least one of the gene products has an amino acid sequence identity to either Rv3874, Rv3875, or Rv0288 of at least 15%.
Owner:STATENS SERUM INST
Immuno-fluorescent staining method for detecting mycobacterium tuberculosis in leukocytes and kit
The invention discloses a method for rapidly detecting mycobacterium tuberculosis in blood (blood plasma and blood cells) through fluorescent staining and a kit.ESAT-6 and CFP-10 antigens of the mycobacterium tuberculosis in the blood are specifically detected by using monoclonal antibodies of RD-1 zone ESAT-6 and CFP-10 antigens of the mycobacterium and are used for etiologic detection and early-stage and rapid clinical diagnosis of tuberculosis diseases.According to the method, the blood is divided into the blood plasma and the blood cells, the mycobacterium tuberculosis enriched in the blood plasma is separated by using a micro-fluid device based on a membrane, meanwhile a slide is coated with hemocytes (leukocytes), and the mycobacterium tuberculosis in the blood plasma and the leukocytes are detected by using the monoclonal antibodies of the ESAT-6 and CFP-10 antigens and adopting direct and indirect fluorescent staining methods.The method is simple in operation, high in sensitivity and strong in specificity and is a definite tuberculosis etiology diagnosis method, and the kit for detecting the mycobacterium tuberculosis in the blood is developed.In addition, by means of the method, pathogenic mycobacterium tuberculosis infection can be detected, and theoretically non-pathogenic mycobacterium tuberculosis produced in blood due to bacillus calmette guerin vaccine inoculation can be also distinguished.
Owner:肖乐义
Recombinant bacillus Calmette-Guerin vaccine and its preparation method
InactiveCN1737153AIncrease secretionGood effectBacterial antigen ingredientsVector-based foreign material introductionEscherichia coliTuberculosis mycobacterium
The invention relates to a recombinant bacillus Calmette-Guerin vaccine and its preparation method, wherein tuberculosis mycobacterium ag85b, esat-6 and IFN-gamma gene sequences are inserted into colibacillus-tuberculosis mycobacterium shuttle plasmids to form recombinant plasmid, and are transformed into BCG to form recombinant tuberculosis vaccine. The recombinant vaccine can be used for prevention and treatment of tuberculosis with better immunological effects than BCG.
Owner:FUDAN UNIV
Mycobacterium tuberculosis fusion protein and application thereof in induction of peripheral blood mononuclear cells to generate cytokines
ActiveCN105601747AIncreased sensitivityStrong specificityAntibacterial agentsBacterial antigen ingredientsAntigenPeripheral blood mononuclear cell
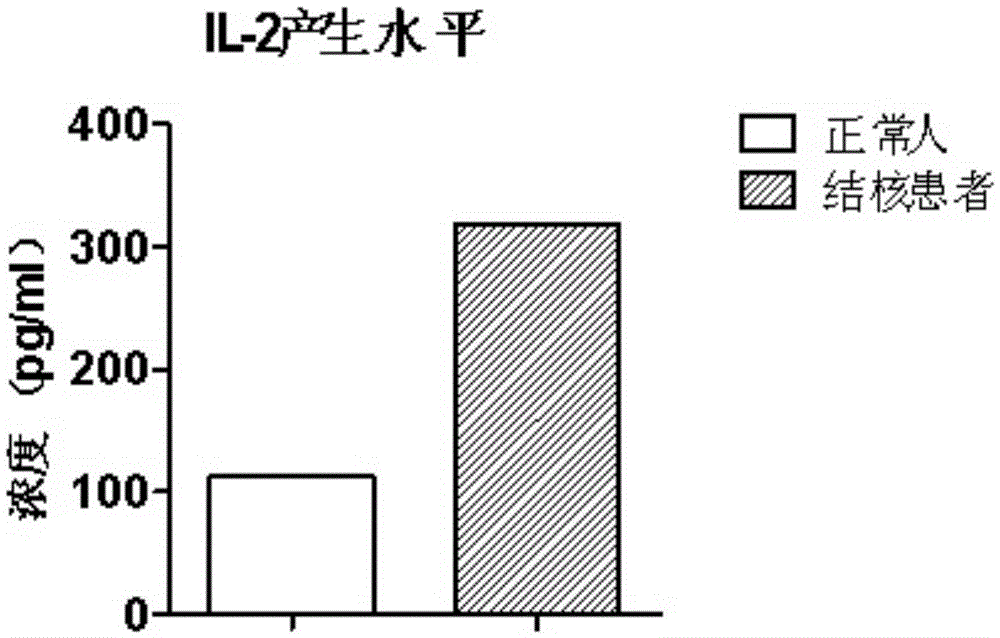
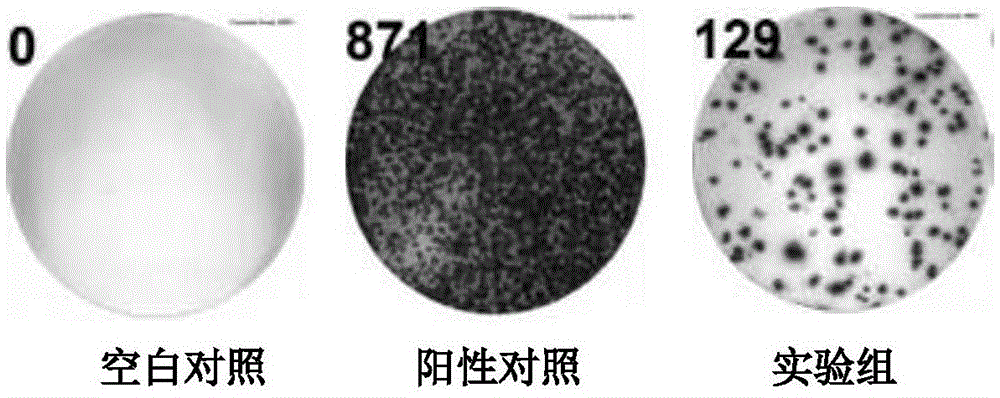
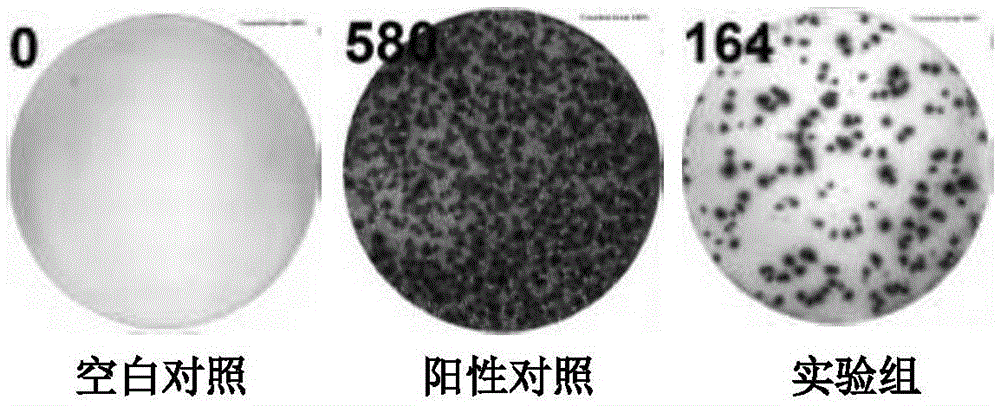
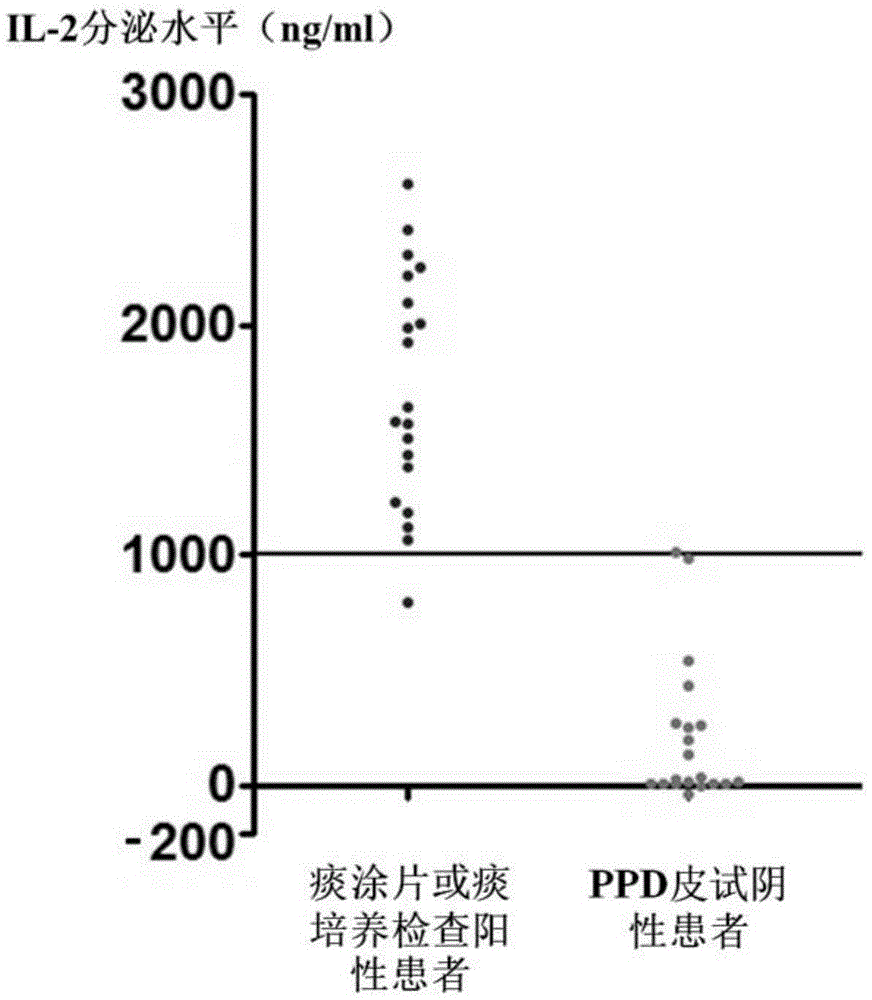
The invention discloses a mycobacterium tuberculosis fusion protein and an application thereof in induction of peripheral blood mononuclear cells (PBMCs) to generate cytokines. The fusion protein includes three proteins PPE41, ESAT-6 and PE25, and the proteins are connected through connecting peptides. Compared with present stimulants, the fusion protein provided by the invention has the advantages of efficient effect, strong sensitivity, high specificity and good stimulation effect. The fusion protein stimulates the PBMCs to generate a large amount of mycobacterium tuberculosis antigen specific IFN-gamma, IL-2, TNF-alpha and other tuberculosis related factors, and the above stimulation induction reaction is free from BCG vaccine interference. The fusion protein can effectively improve the tuberculosis detection rate and is of positive significance to control the tuberculosis. The fusion protein can be applied in researches of the tuberculosis pathopoiesis and immunoprophylaxis mechanisms and control of the tuberculosis as a stimulant.
Owner:SUN YAT SEN UNIV
Mycobacterium tuberculosis ESAT-6 recombinant dipolymer, preparation method and application thereof
InactiveCN101805397AIncreased antigen specificityIncreased sensitivityMicroorganism based processesDepsipeptidesSerodiagnosesSkin test
The invention belongs to the field of biological engineering and diagnostic medicine, in particular to a mycobacterium tuberculosis ESAT-6 recombinant dipolymer, a preparation method thereof, an antigenicity analysis and an application thereof in the serodiagnosis of tuberculosis. The invention uses the DNA of a chimeric gene nucleic acid vaccine HG856A plasmid containing 2 * esat-6 copies as the template, obtains 2 * esat-6 gene segments by PCR amplification, and clones, expresses and purifies an ESAT-6 recombinant dipolymer protein in E.coli. The protein is connected with the two ESAT-6 monomers through amino acid Tyr and Val which are coded by the AccI restriction enzyme cutting site, i.e. GTC TAC, carries six* His tags at the N end, and can be purified by nickel-chelate affinity chromatography. Experiments confirm that the purified recombinant dipolymer has favorable reactogenicity and antigenic specificity. The recombinant dipolymer can be used as an antigen for the early diagnosis of the tuberculosis, including the serodiagnosis of ELISA, ELISPOT or the like, or can be used as an antigen for a skin test.
Owner:SHANGHAI PUBLIC HEALTH CLINICAL CENT
Recombinant mycobacterium smegmatis strain capable of expressing mycobacterium tuberculosis Ag 85B and ESAT-6 fusion protein and application thereof
InactiveCN101875913AStrong immune responseGood immune protectionAntibacterial agentsBacterial antigen ingredientsMycobacterium smegmatisEukaryotic plasmids
The invention relates to a recombinant mycobacterium smegmatis strain capable of expressing mycobacterium tuberculosis Ag 85B and ESAT-6 fusion proteins and application thereof. Recombinant plasmids containing the Ag 85B and ESAT-6 fusion protein genes are turned into mycobacterium smegmatis (AE-MS for short) through electrotransformation, and the preservation serial number thereof is CCTCC M2010097. When used for immunizing mice, the recombinant mycobacterium smegmatis strain AE-MS obtained through screening can induce the immune response level which is stronger than that of the mycobacterium smegmatis. The invention also relates to the application of the structured mycobacterium smegmatis strain to the preparation of preparations or medicaments used for preventing and treating tuberculosis. The recombinant mycobacterium smegmatis expressing the Ag 85B and ESAT-6 fusion genes integrates the advantages of target antigens and live carriers; and after immunization, the recombinant mycobacterium smegmatis can increase the immune protection of an organism by simulating the stronger immune response of the organism, thereby having good prospect of application.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
New m.tuberculosis vaccines
InactiveUS20170043003A1High expressionReduce bacterial loadAntibacterial agentsBacterial antigen ingredientsAntigenMycobacterium microti
The present invention is directed to a fusion protein, antigen cocktails and immunological compositions such as vaccines against infections caused by virulent mycobacteria, e.g. by Mycobacterium tuberculosis, Mycobacterium africanum, Mycobacterium bovis, Mycobacterium microti, Mycobacterium canettii, Mycobacterium pinnipedii or Mycobacterium mungi. The fusion protein, antigen cocktails and immunological compositions are based on proteins secreted by the ESAT-6 secretion system 1 (ESX-1) and are among the most immunodominant M. tuberculosis (MTB) antigens.
Owner:STATENS SERUM INST
Production process of high-titer tuberculin skin test diagnostic reagent PPD (Purified Protein Derivative)
ActiveCN109182167AHigh potencyComply with labeling requirementsBacteriaMicroorganism based processesAntigenSkin test
The invention discloses a production process of a high-titer tuberculin skin test diagnostic reagent PPD (Purified Protein Derivative). The production process comprises bacterial culture, inactivationand precipitation. The bacterial culture specifically comprises the following process steps: culturing and developing a working seed culture into dry agglomerated light-yellow bacterial lawn, performing microscopic examination on the bacterial lawn as bacillus brevis that is micro bent and round in two ends; and performing two-generation culture to obtain wrinkled light yellow mycoderm, performing passage culture for three generations when the mycoderm overspreads the surface of the culture medium and the culture solution is clarified and transparent, so as to form multi-wrinkled light yellowmycoderm, and performing four-generation culture for 8-10 weeks. According to the production process of the high-titer PPD capable of enriching two antigens such as ESAT-6 and CFP10, the PPD preparedby the process disclosed by the invention has high titer, and the content of the ESAT-6 and CFP10 is high.
Owner:BEIJING SANROAD BIOLOGICAL PROD CO LTD
Tuberculosis diagnostic composition and application thereof
ActiveCN102608333BReduce false negativesReduce irritation holesAntibacterial agentsFungiAntigenMycobacterium Infections
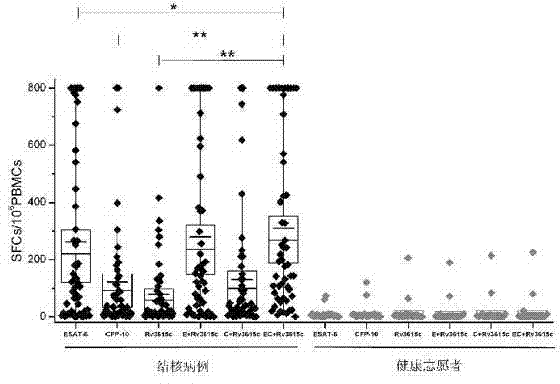
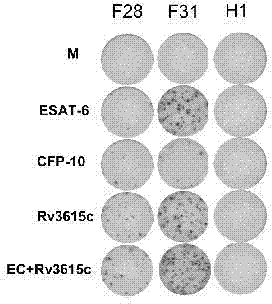
The invention relates to a tuberculosis diagnostic composition and application of the tuberculosis diagnostic composition, in particular provides the tuberculosis diagnostic composition which consists of optional two or three of antigen ESAT-6 derived polypeptide, antigen CFP-10 derived polypeptide and antigen Rv3615c derived polypeptide. The invention also provides application of the tuberculosis diagnostic composition in a specific T cellular immune reaction caused by the detection of mycobacterium tuberculosis infection in vitro and application of the preparation of a tuberculosis diagnostic agent. Compared with the existing diagnostic technology, the diagnostic cost is reduced, the detection design and the operation process are simplified, and the detection sensitivity is obviously improved.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI +2
Monoclonal antibody TBEF3 of tubercle bacillus-resistant ESAT-6 and application thereof
InactiveCN101985473AStable secretionNo decline in secretionImmunoglobulins against bacteriaMicroorganism based processesAntibody affinityCongenic
The invention provides a monoclonal antibody TBEF3 of tubercle bacillus-resistant secretory antigen target protein in early stage. The monoclonal antibody TBEF3 is prepared by the following steps: selecting a specific antigen peptide mouse; collecting sensitized mouse B lymphocyte and mouse myeloma cell to fuse; judging positive clones by using an enzyme linked immunosorbent assayelisa and an immunoblotting testing method; establishing a hybridoma cell line TBEF3 for secreting monoclonal antibody of tubercle bacillus-resistant secretory ESAT-6 in early stage; carrying out multiplication culturing on the positive cells and injecting to congenic mouse enterocoelia for induction of generation of ascitic fluid containing antibody; and carrying out collection and antibody affinity purification to obtain the monoclonal antibody TBEF3 of tubercle bacillus-resistant secretory antigen target protein in early stage. The invention also provides an application of the monoclonal antibody in testing the secretory antigen target protein of mycobacterium tuberculosis in early stage.
Owner:ZHEJIANG UNIV +1
Mycobacterium tuberculosis ESAT-6 protein detection kit, as well as preparation method and use method
InactiveCN104807993AHigh molar extinction coefficientIncrease contact areaBiological material analysisMaterial analysis by optical meansLow noiseProtein detection
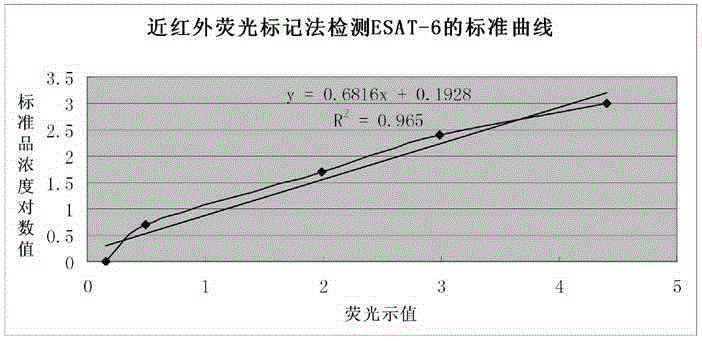
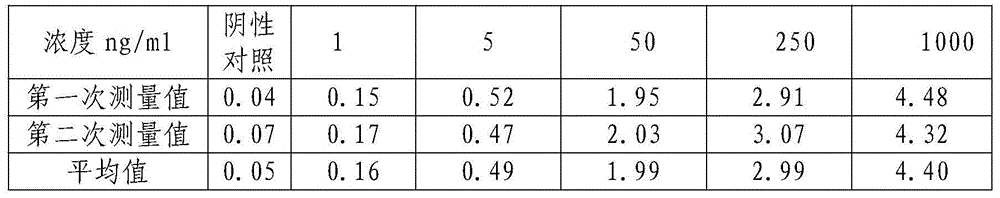
The invention relates to a mycobacterium tuberculosis ESAT-6 protein detection kit based on the nano-immunomagnetic bead-near infrared fluorescence labeling method. In the detection kit, a monoclonal antibody targeting the ESAT-6 is labeled with a near infrared fluorescence dye; the surface of a nano-magnetic bead is coated with a polyclonal antibody targeting the ESAT-6. Based on the double-antibody sandwiching principle, combined substances and free substances are separated from each other magnetically; a portable high-sensitivity low-noise stimulating fluorescence detection instrument is adopted for detection of the fluorescence intensity of a magnetic combined substance, so as to detect the ESAT-6 protein content in a sample to be detected. The detection kit is suitable for ESAT-6 detection of a clinical pathological sample, and has the advantages of being instant, sensitive, stray fluorescence excitation-resistant, and long in emitted fluorescence saving time.
Owner:广东国际旅行卫生保健中心(广东出入境检验检疫局口岸门诊部) +1
Mixed polypeptide for producing tuberculosis associated cell factors by inducing peripheral blood mononuclear cell
InactiveCN105541975AIncreased sensitivityStrong specificityDepsipeptidesBlood/immune system cellsPeripheral blood mononuclear cellCentral Memory T-Cell
The invention discloses a mixed polypeptide for producing tuberculosis associated cell factors by inducing a peripheral blood mononuclear cell. The mixed polypeptide has polypeptide sequences represented by SEQ ID NO:1-18. Compared with common stimulating proteins CFP-10, ESAT-6 and Rv1985c or a mixture of the three proteins, the mixed polypeptide has relatively efficient induction performance and the relatively good specificity. The mixed polypeptide only reacts with immunologic memory T cells produced after human body is infected by mycobacterium tuberculosis, the produced cell factors are tuberculosis associated factors including IFN-gamma, IL-2, TNF-alpha and the like with mycobacterium tuberculosis antigen specificity, and meanwhile, the stimulus response is not interfered by BCG. The specific antigen polypeptide provided by the invention is significant for the research of tuberculosis pathopoiesia and immunoprophylaxis mechanisms and therefore has positive significance to the control of tuberculosis.
Owner:GUANGZHOU DEAOU MEDICAL TECH CO LTD
Antigen and kit for detecting tuberculosis-infected T cells and application of kit
ActiveCN107144694AStop transmissionStop the epidemicChemiluminescene/bioluminescenceDisease diagnosisTrue positive rateT cell
The invention discloses an antigen for detecting tuberculosis-infected T cells. The amino acid sequence of the antigen is shown as SEQ ID NO.1-SEQ ID NO.26; eight polypeptides are randomly selected from the SEQ ID NO.1-SEQ ID NO.26 and are collaborated with ESAT-6 and CFP-10 polypeptides and derivatives thereof, and specifically-stimulated mycobacterium tuberculosis-infected T cells can specifically secrete IFN-gamma, so that the detection sensitivity is increased. The invention provides a novel kit applicable to detection of the tuberculosis-infected T cells. The kit is used for directly performing antigenic stimulation on peripheral blood, without needing separating mononuclear cells from the peripheral blood; the kit is relatively high in detection sensitivity and detection specificity, easy and convenient to operate, low in detection cost and relatively high in clinical application value.
Owner:武汉海吉力生物科技有限公司
Method for modifying bird flu virus through interferon inducible protein ESAT6
InactiveCN101792775ASignificant progressProminentlyMicroorganism based processesVector-based foreign material introductionBird fluVirus Protein
The invention relates to a method for modifying a bird flu virus through interferon inducible protein ESAT6, which adopts an early secretory antigen target ESAT-6 gene of mycobacterium tuberculosis to modify the bird flu virus and is a method for splicing into an exogenous DNA on the basis of establishing a bird flu virus reverse genetic operating system. The selected ESAT-6 protein is secretory protein with a T cell multifunctional epiposition and has the main function of stimulating an organism to generate IFN, which has uniqueness in researching a virus anti-infective immune mechanism path. After a bird flu virus gene is modified through the DNA, when the modified virus is rescued, not only the transcription, the translation and the virus packaging of bird flu virus protein are not influenced, but also the ESAT-6 protein can be simultaneously generated.
Owner:GUANGDONG LAB ANIMALS MONITORING INST +1
Whole blood INF-gamma specific antigen protein and preparation method and application thereof
ActiveCN102174114ARapid diagnosisSpecific diagnosisBacteriaMicroorganism based processesNucleotideImmunogenicity
The invention relates to a whole blood interferon-gamma (INF-gamma) specific antigen protein prepared by a gene engineering method and a preparation method and application thereof, a nucleotide sequence for coding the protein, a recombinant vector and a recon. The antigen protein is a fusion protein obtained by connecting early secreting antigen target 6 (ESAT-6), cultufiltrate protein 10 (CFP-10) and MPB64 in any order through a connecting peptide, wherein the connecting peptide is a short peptide which does not influence the immunogenicity of the ESAT-6, the CFP-10 and the MPB64. The tuberculosis specific antigen quickly diagnoses tuberculosis, has high sensitivity and specificity, and has great significance for the early diagnosis of the tuberculosis.
Owner:武汉海吉力生物科技有限公司
Fusion expression method of mycobacterium tuberculosis ESAT-6 protein in pichia
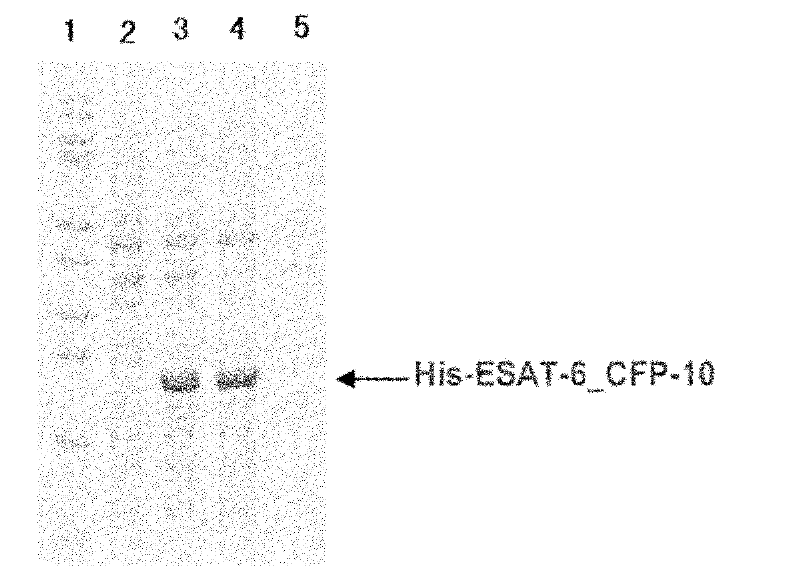
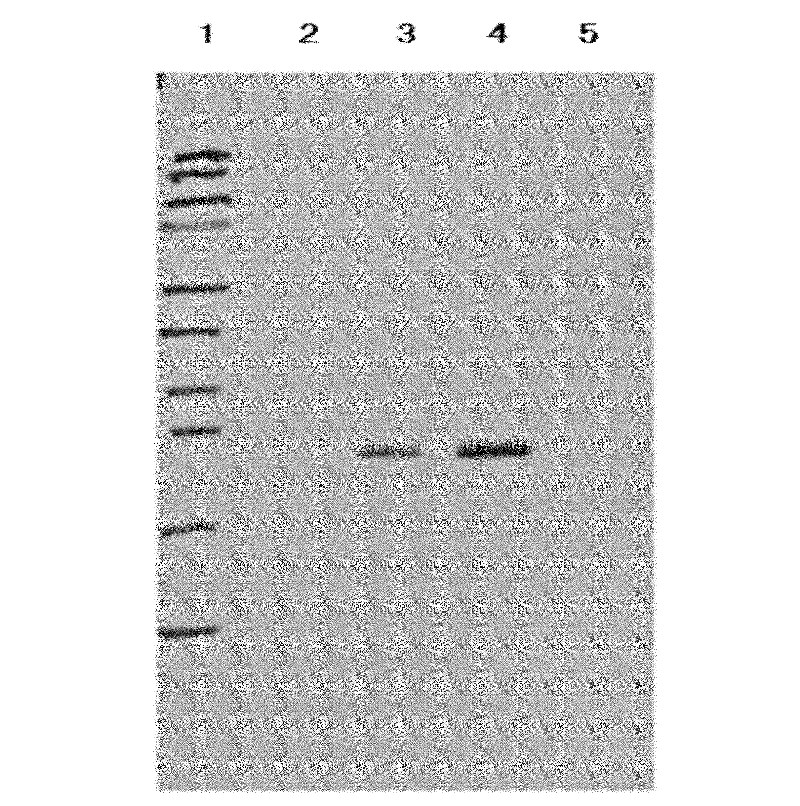
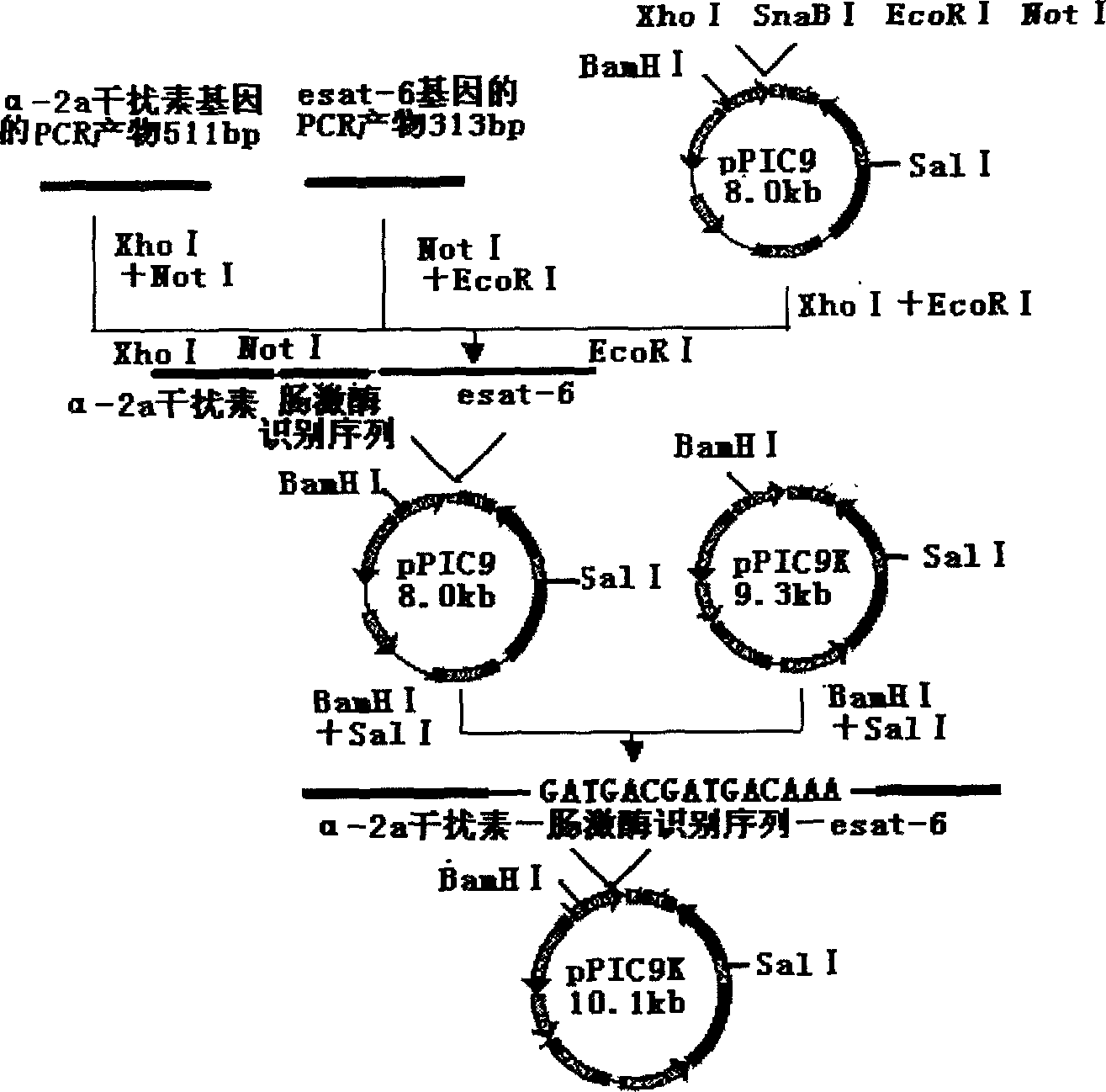
The invention belongs to the biological technology domain, is concrete is in one tuberculosis difference bacillus Esat-6 protein in Bi Chishi yeast fusion expression method. He (Arab League sends) the esat-6 gene and person the -2a disturbance factorConnects, two linker manner enterokinase recognition sequence, will reorganize the fusion gene the DNA fragment insertion to express carrier pPIC9K, the electric shock inducts Bi Chishi in yeast strain SMD1168, will realize the Esat-6 fusion protein highly effective secretion expression. Obtain pure Esat-6 after the sparse water chromatographic analysis and the ionic exchange.
Owner:FUDAN UNIV
Constructing a DNA Chimera for Vaccine Development Against Leishmaniasis and Tuberculosis
InactiveUS20110150932A1Antibacterial agentsBacterial antigen ingredientsSequence analysisImmunogenicity Study
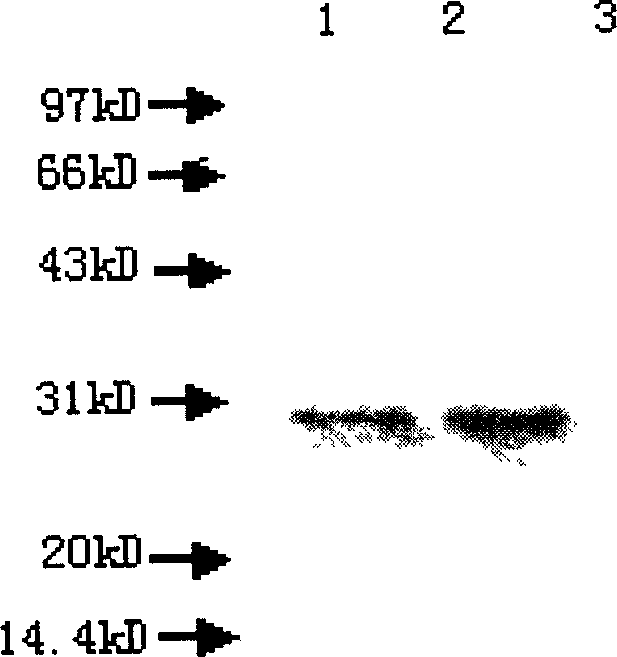
A novel recombinant chimera of DNA construct having esat-6 region of Mycobacterium tuberculosis and kinesin region of Leishmania donovani cloned together on two sides of self cleaving peptide in a DNA vaccine vector pVAX-1 wherein the chimeric construct is operatively linked to a transcriptional promoter thus capable of self replication and expression within the mammalian cell, and the process of preparation thereof comprising: analysis of the predicted protein sequence of kinesin motor domain and esat-6 domain using Promiscuous MHC Class-1 Binding Peptide Prediction Servers; amplification of gene coding for kinesin motor domain and esat-6 domain; cloning of kinesin esat-6 gene region in pGEM-T™ vector for sequence analysis; generation of chimeric construct by directional cloning in pVAX-1 vector. In-vitro expression analysis of kinesin motor domain and esat-6 domain from the clones using cell free translation system and immunogenicity studies; and splenocyte proliferation and cytokines studies using the above mentioned constructs.
Owner:DEPT OF BIOTECHNOLOGY MINIST OF SCI & TECH GOVERNMENT OF INDIA +1
M.tuberculosis vaccines
InactiveUS10004793B2Reduce bacterial loadProlong survival timeAntibacterial agentsBacterial antigen ingredientsAntigenMycobacterium microti
Owner:STATENS SERUM INST
Antigen for detecting tuberculosis infection T cells, kit and application
ActiveCN107219363AStop transmissionStop the epidemicChemiluminescene/bioluminescenceDisease diagnosisTrue positive rateAntigen stimulation
The invention discloses an antigen for detecting tuberculosis infection T cells. The amino acid sequence of the antigen is shown as SEQ ID NO.1 to SEQ ID NO.28; any eight polypeptides in SEQ ID NO.1 to SEQ ID NO.28 are coordinated with ESAT-6 and CFP-10 polypeptides and derivatives to have a common effect to specifically stimulate mycobacterium tuberculosis infected T cells to specifically secrete IFN-gamma, and the detection sensitivity is increased. The invention provides a novel detection kit for the tuberculosis infection T cells; when the kit is used, peripheral blood is directly subjected to antigen stimulation and mononuclear cells of the peripheral blood do not need to be separated; the detection sensitivity and the detection specificity are relatively high; the novel detection kit is simple and convenient to operate, has relatively low detection cost and has relatively high clinical application value.
Owner:武汉海吉力生物科技有限公司
Methods for detection of mycobacterium tuberculosis
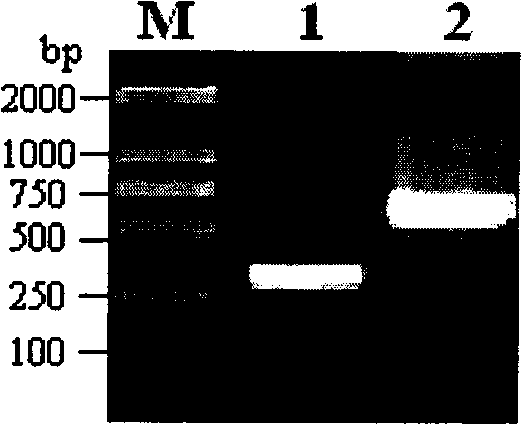
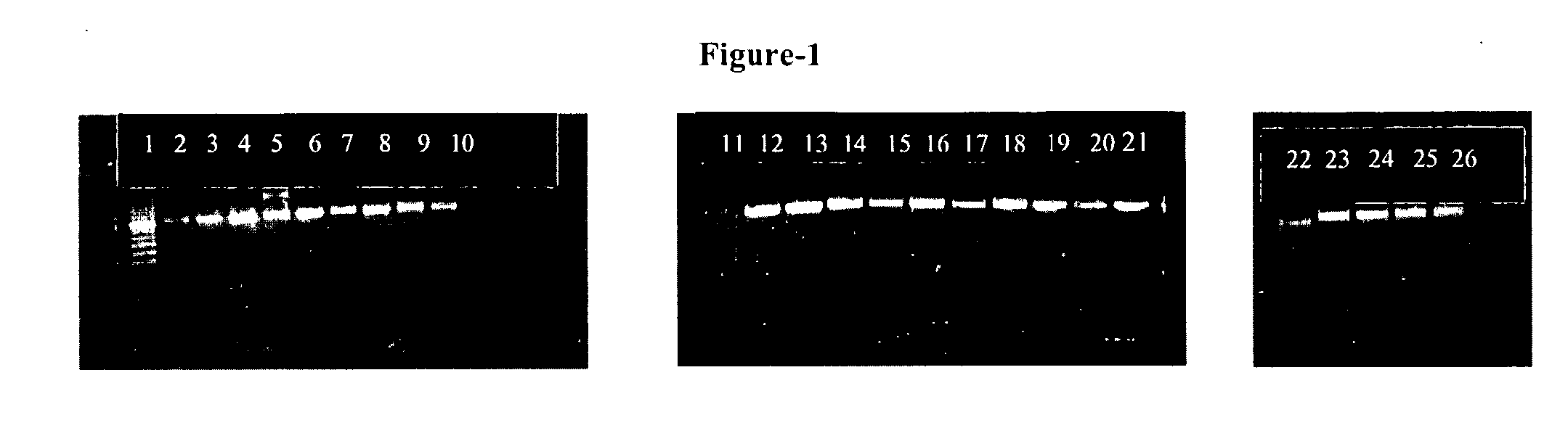
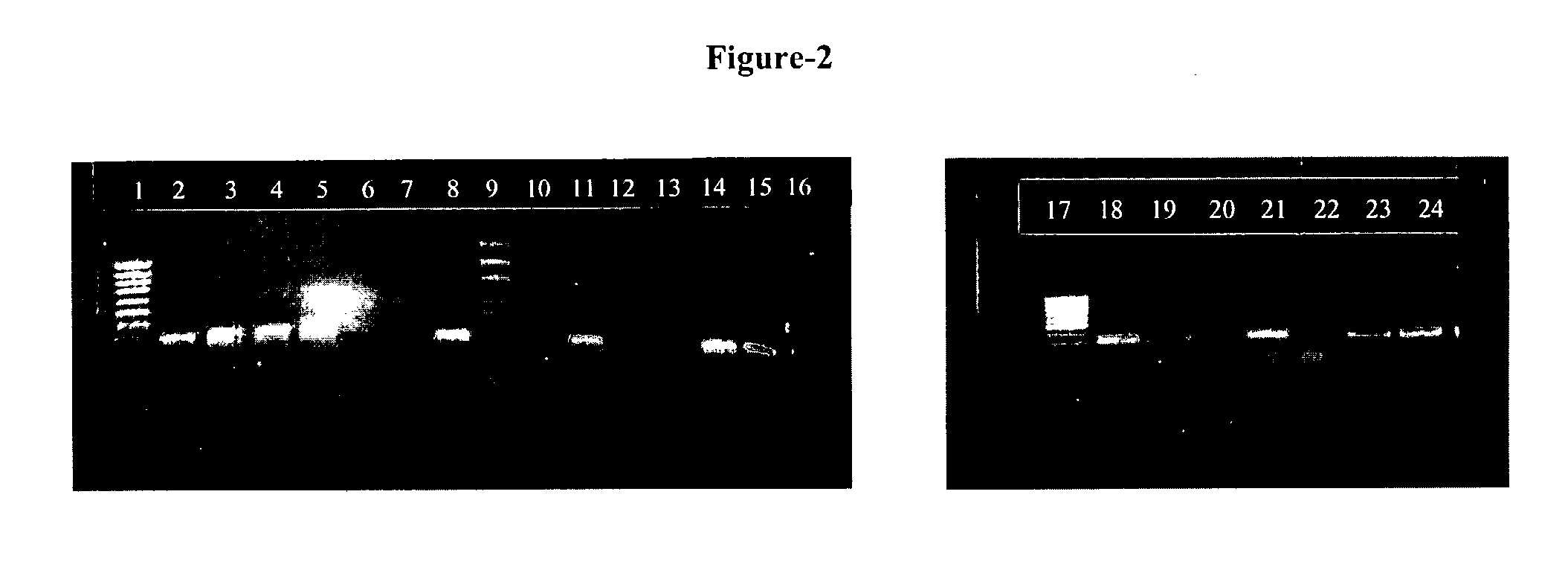
The present invention provides an oligonucleotide primer pair having SEQ ID NO: 3 and SEQ ID NO: 4 for amplification of Early Secretory Antigenic Target (esat)-6-gene of Mycobacterium species. The invention also provides a method for detecting M .tuberculosis in a sample based on the amplification of esat-6 gene, comprising isolating DNA template from the sample, amplifying with the above oligonucleotide primer pair and subjecting the amplified DNA product to separation and staining to detect the presence of amplified DNA product for identifying Mycobacterium tuberculosis in the sample. The invention further provides a diagnostic kit for detection of Mycobacterium tuberculosis. The invention also provides a method of detecting Mycobacterium tuberculosis from a sample by amplifying the 16s rRNA region from the isolated DNA template by conventional methods to detect Mycobacterium species and further amplifying the positive sample contains Mycobacterium species using primers positive for ESAT-6 region detection of Mycobacterium tuberculosis.
Owner:ALL INDIA INST OF MEDICAL SCI & DEPT OF BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com