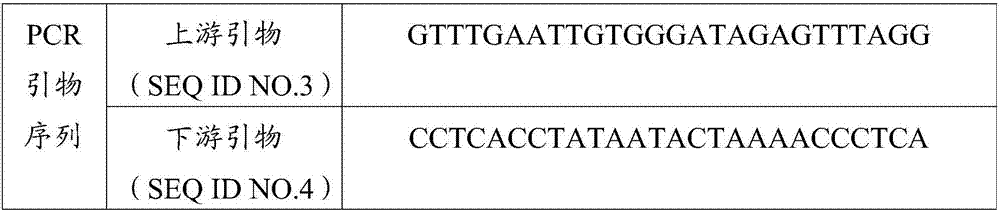
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
55results about How to "Specific diagnosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Primer, method and kit for detecting animal clonorchiasis sinensis specificity
InactiveCN101586161AQuick checkEasy to detectMicrobiological testing/measurementDNA/RNA fragmentationSequence databaseUltraviolet lights
The present invention discloses a primer, a method and a kit for detecting the animal clonorchiasis sinensis specificity, a nucleotide sequence of an upstream primer of the primer is represented by SEQ ID NO:1, a nucleotide sequence of a downstream primer is represented by SEQ ID NO:2. the present invention implements an PCR amplification to a detectingformwork DNA by the primer, an amplifying outcome yield is processed by an agarose gel electrophoresis and observed under an ultraviolet light, if it is a positive result, there will be a specificity amplifying band, otherwise there will not be a band. According to an ITS zone sequence database OF THE animal clonorchiasis sinensis, the invention designs the primer, builds a rapid, special and sensitive PCR method, and it is capable of authenticating the animal clonorchiasis sinensis accurately. The operation of the kit of the invention is simple and programmable, the method specificity is strong, the sensibility is high, the result judgement is objective, and the invention is capable of being used for diagnosing the animal clonorchiasis sinensis and inquiring epidemiology.
Owner:SOUTH CHINA AGRI UNIV
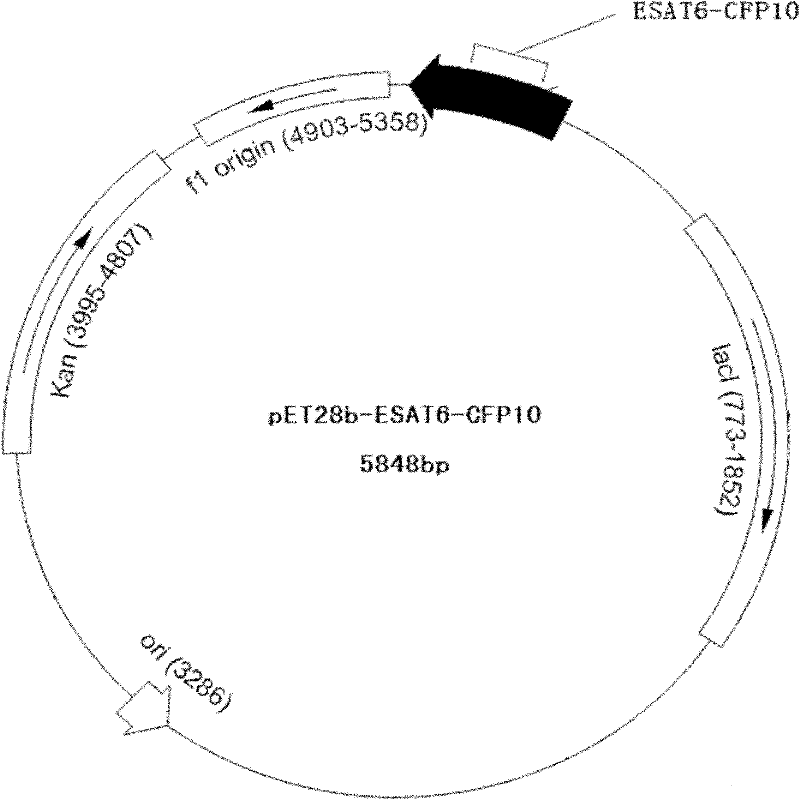
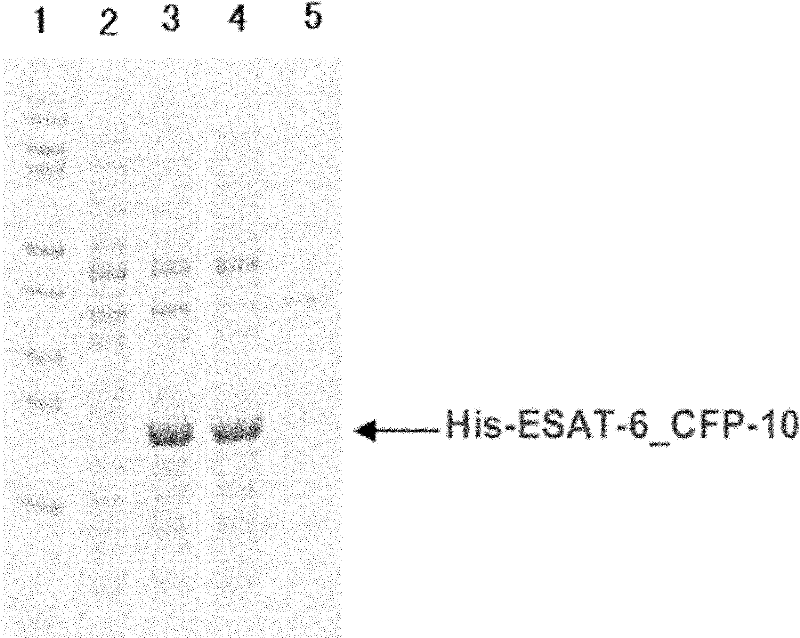
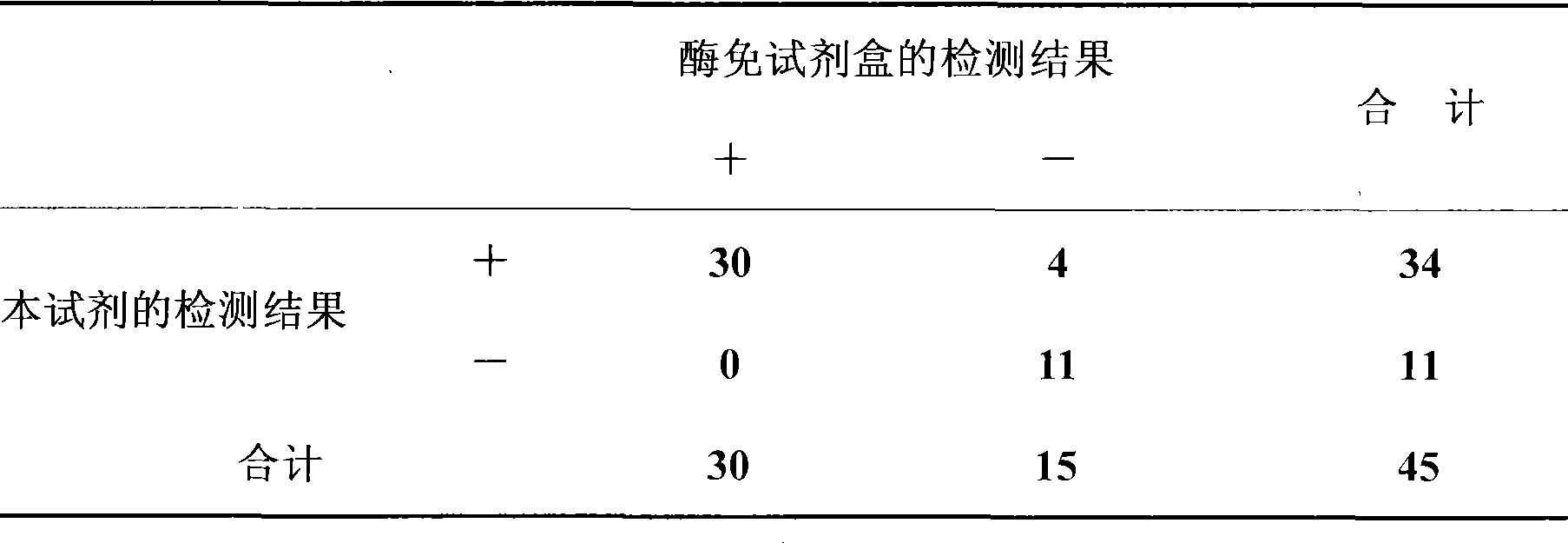
Detection kit for diagnosing tuberculosis
ActiveCN102175875ARapid diagnosisSpecific diagnosisBiological testingHybrid peptidesBiologyVaccine Immunogenicity
The invention relates to a detection kit for diagnosing tuberculosis, comprising a recombinant antigenic protein, wherein the recombinant antigenic protein is the fusion protein obtained by connecting ESAT-6 (early secreting antigen target 6KD), CFP-10 (cultufiltrate protein 10KD), and MPB64 (Mycobacterium bovis 64KD) by connecting peptides according to an arbitrary sequence, wherein the connecting peptides are short peptides which do not influence the immunogenicity of ESAT-6, CFP-10 and MPB64. Preferably, the recombinant antigenic protein in the detection kit for diagnosing tuberculosis is the antigenic protein with an amino acid sequence which is SEQ ID: No.1. When the detection kit in the invention is used for diagnosing tuberculosis, the diagnosis time is short, the diagnosis is fast, the sensitivity is strong, and the specificity is high; and the detection kit has an important significance on early diagnosis of tuberculosis.
Owner:武汉海吉力生物科技有限公司
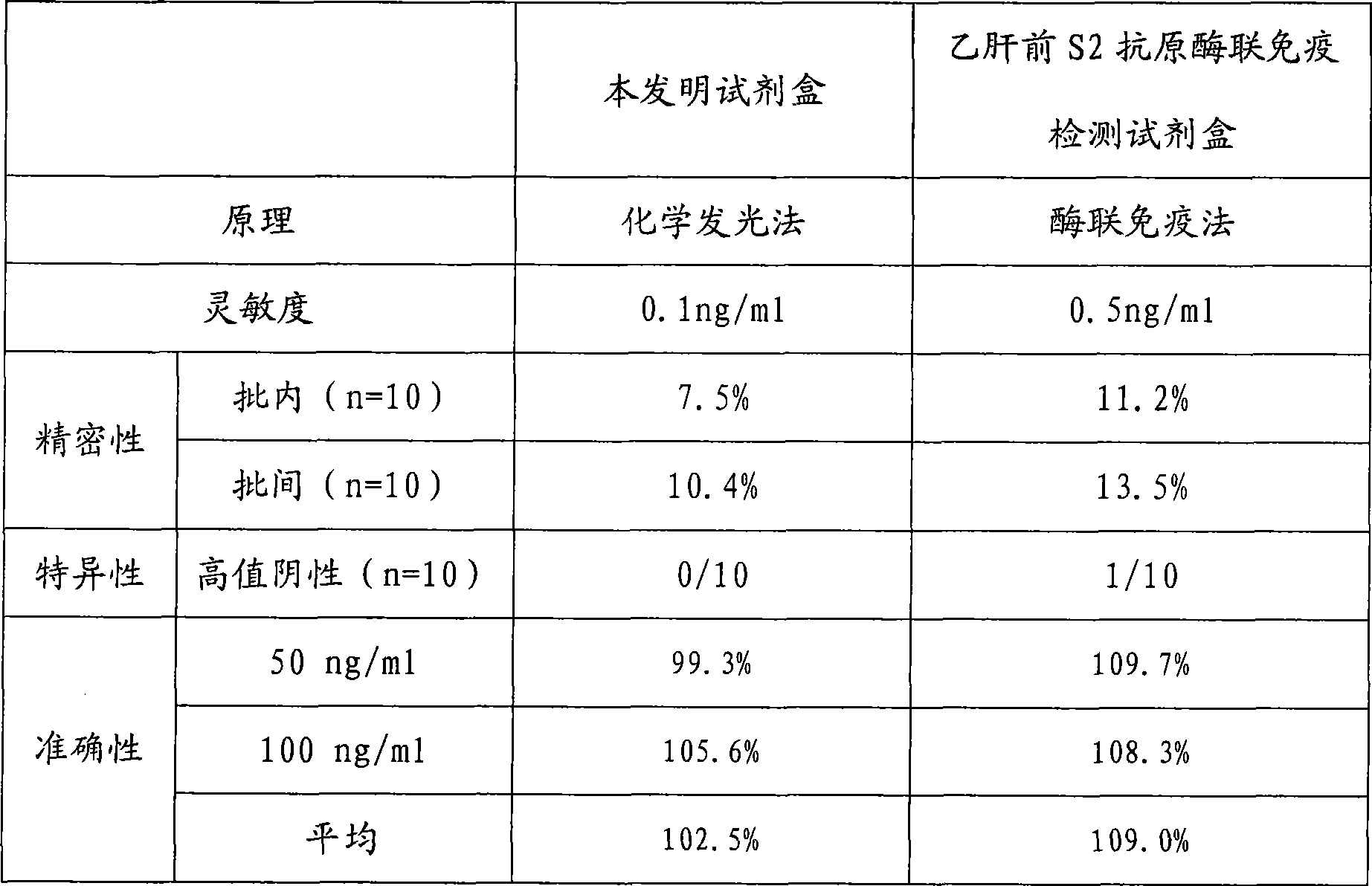
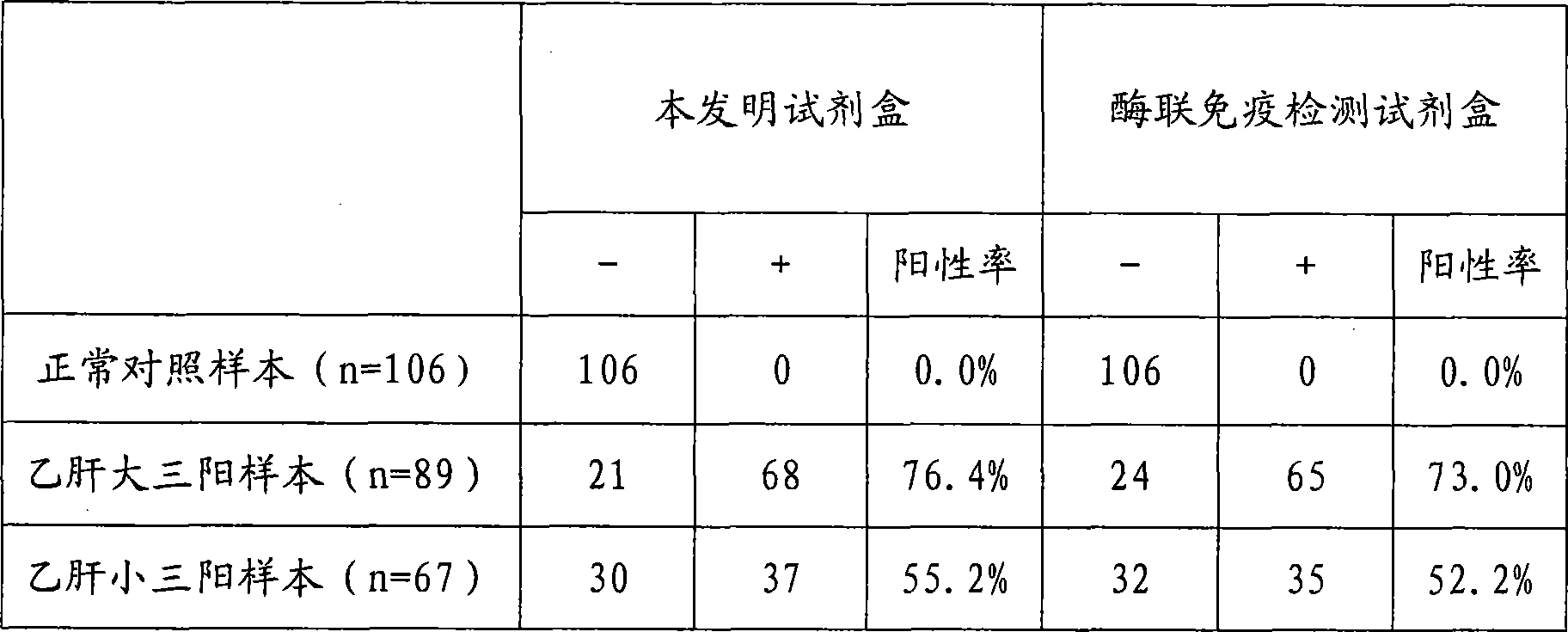
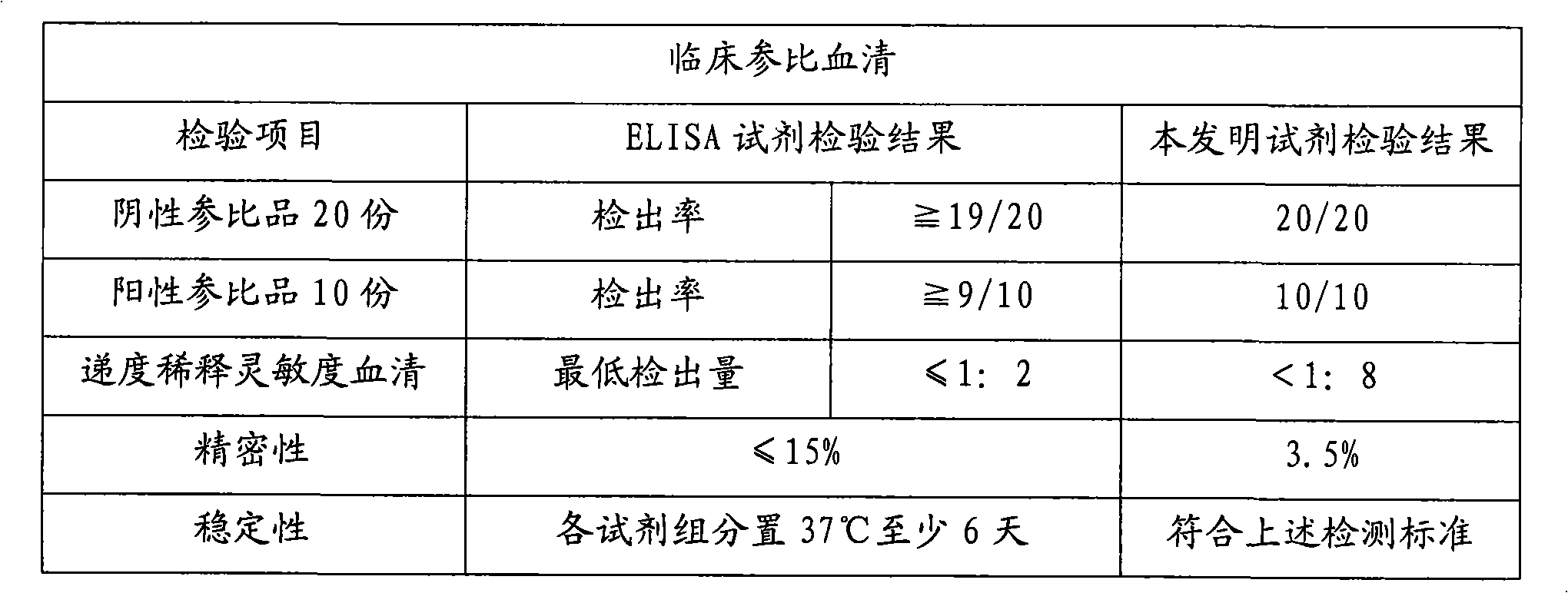
Large protein pre-S surface antigen for hepatitis B virus chemiluminescence immune assay kit and method for making same
InactiveCN101382553AHigh sensitivityNo cross reactionChemiluminescene/bioluminescenceBiological testingAntigenImmune profiling
The invention relates to the immunoassay medical field, concretely, the invention provides a determination kit of the front S area of the large protein (HBV-LP) of hepatitis B virus surface antigen, and a preparation method thereof. The kit based on the invention comprises: 1) a working calibrator of the front S area of the HBV-LP; 2) a streptavidin carrier; 3) a biotinylation monoclonal antibody of the front S area of the HBV-LP; 4) an alkaline phosphatase-marked monoclonal antibody of the front S area of the HBV-LP; and 5) a chemical luminescent substrate. Further, the preparation method of the kit based on the invention comprises the steps of: 1) preparing the working calibrator by the sterling product of the front S area of the HBV-LP; 2) enveloping the carrier by the streptavidin; 3) carrying out biotinylation to the monoclonal antibody of the front S area of the HBV-LP; 4) marking the monoclonal antibody of the front S area of the HBV-LP by the alkaline phosphatase; 5) preparing the chemical luminescent substrate; 6) subpackaging the working calibrator, an enzyme marker and the chemical luminescent substrate; and 7) assembling and installing for forming finished goods. The kit has the advantages of being simple and convenient, fast, sensitive, stable and the like.
Owner:北京科美东雅生物技术有限公司
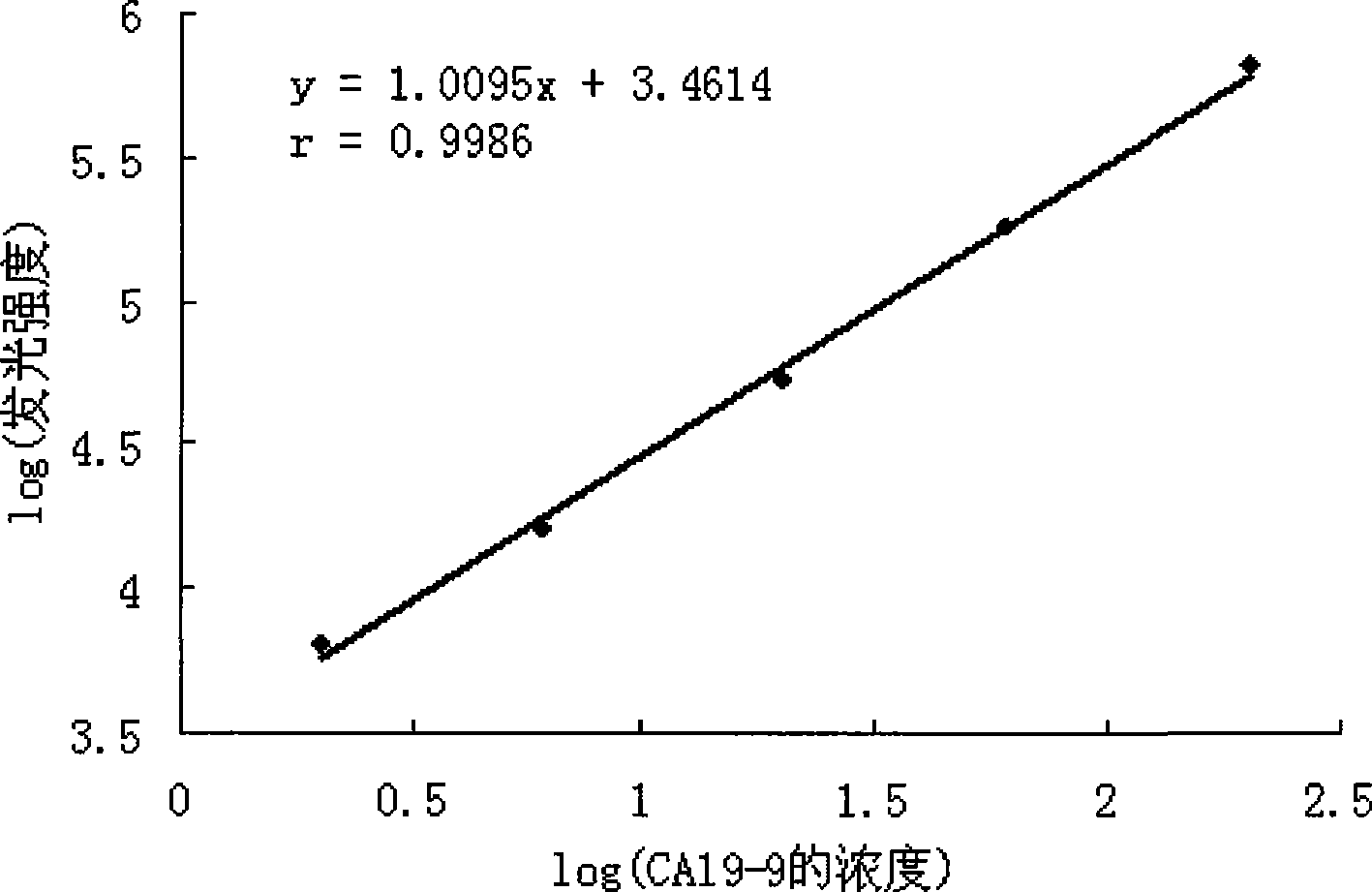
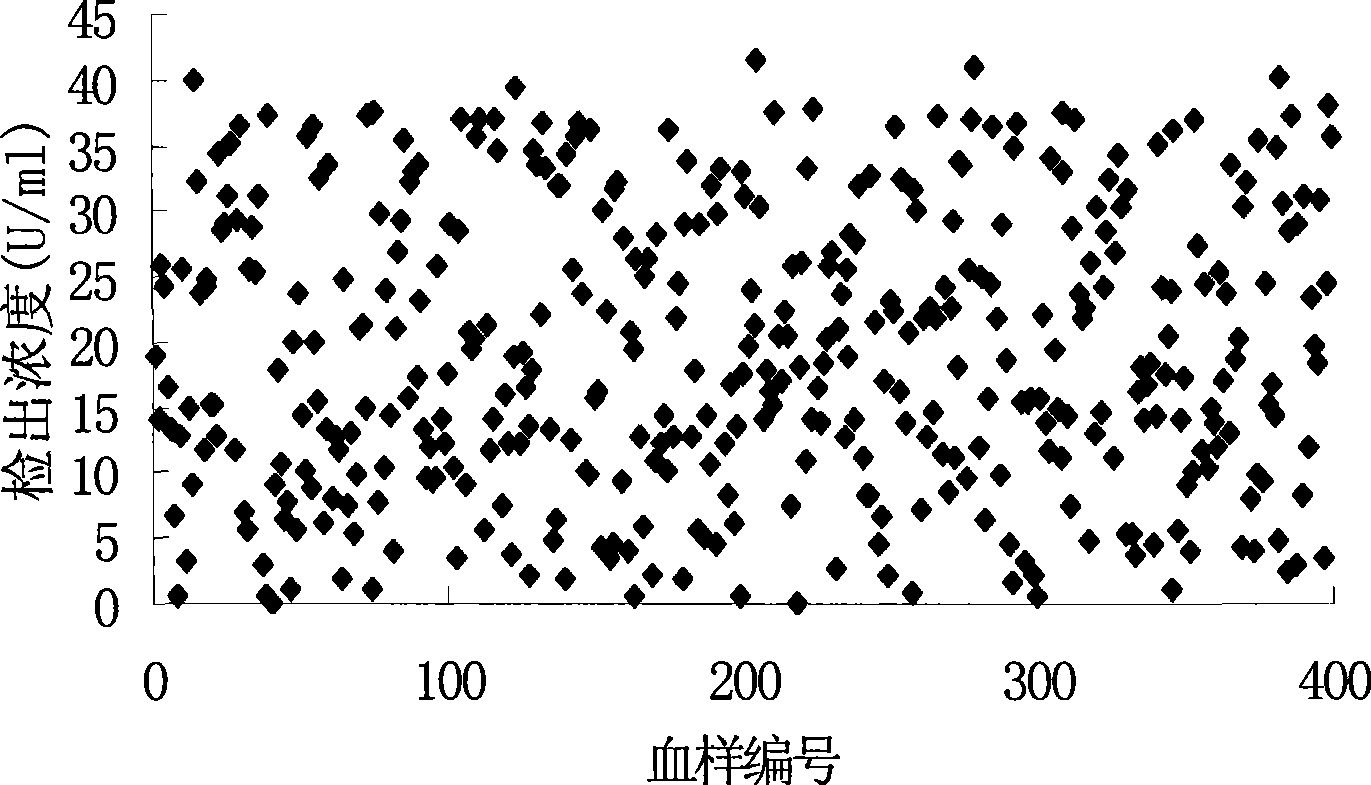
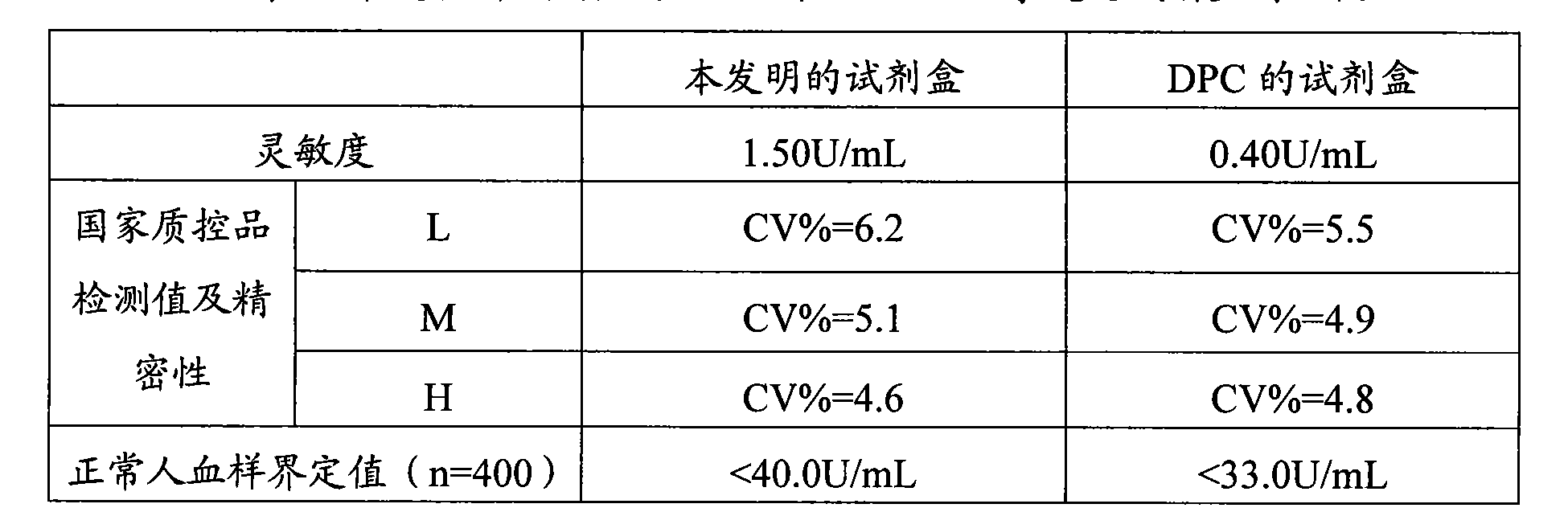
Tumor-associated antigen 19-9 chemical luminescence immune analytic determination reagent kit and preparation method thereof
InactiveCN101373188AGuaranteed sensitivityEasy to produce and operateChemiluminescene/bioluminescenceWilms' tumorPeroxiredoxin
The invention relates to the immune assay medical field, and in particular provides a tumor-associated antigen 19-9 chemiluminescence immune assay determination kit and a preparation method thereof. According to the invention, the kit comprises (1) a CA19-9 calibrator, (2) a CA19-9 monoclonal antibody envelop vector, (3) a horse radish peroxidase marker of a CA19-9 monoclonal antibody and (4) a chemiluminescence substrate. Furthermore, the preparation method of the invention comprises the following steps: (1) the calibrator is prepared with pure CA19-9; (2) the vector is enveloped by the CA19-9 monoclonal antibody; (3) the CA19-9 monoclonal antibody is marked by the horse radish peroxidase; (4) the hemiluminescence substrate is prepared; (5) the CA19-9 calibrator, the peroxidase marker and the chemiluminescence substrate are split-charged; and (6) the finished product is assembled. The kit has the advantages of convenience, quickness, sensitivity, specificity and stability, etc.
Owner:北京科美东雅生物技术有限公司
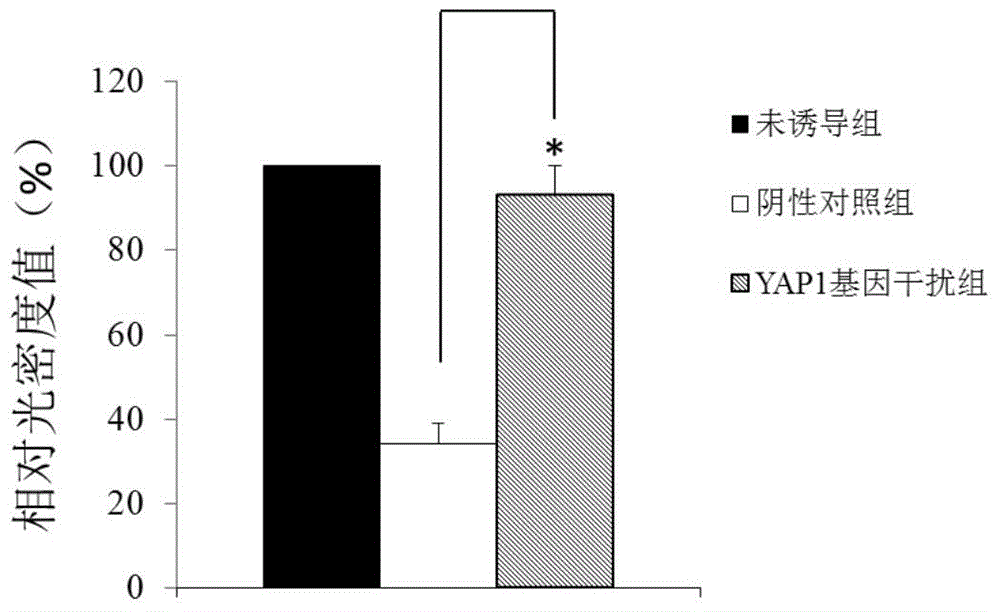
Application of YAP1 gene in diagnosing and treating Alzheimer's disease
ActiveCN104962657ATimely diagnosisSpecific diagnosisOrganic active ingredientsNervous disorderDisease patientYAP1
The invention discloses a YAP1 gene which can be used as a molecular marker for early diagnosis of Alzheimer's disease. The QPCR (quantitative polymerase chain reaction) experiment proves that compared with common people, the YAP1 gene expression in the blood of an Alzheimer's disease patient is obviously enhanced. The RNA (ribonucleic acid) interfering experiment proves that the YAP1 can influence the Abeta mediated neurotoxicity action. According to the research results, a drug capable of inhibiting YAP1 gene expression or inhibiting YAP1 gene expression product functions can be developed, thereby implementing clinical prevention and treatment of Alzheimer's disease.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
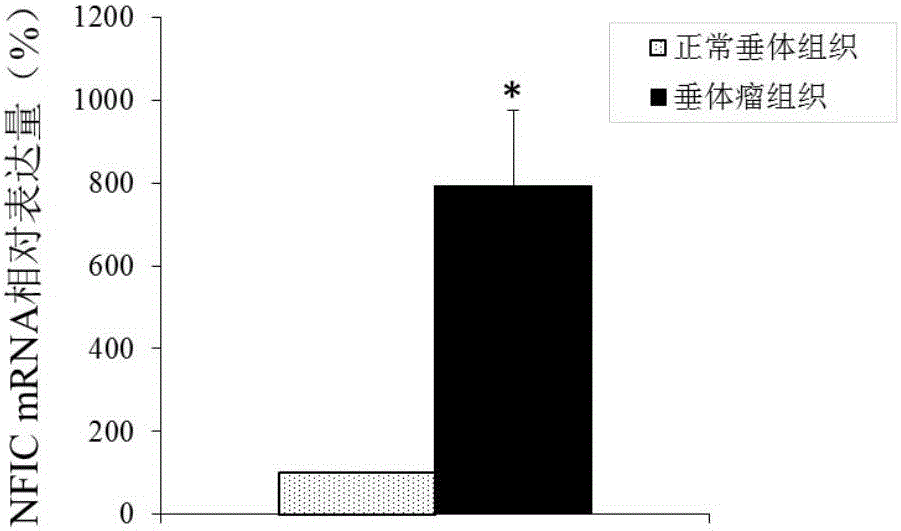
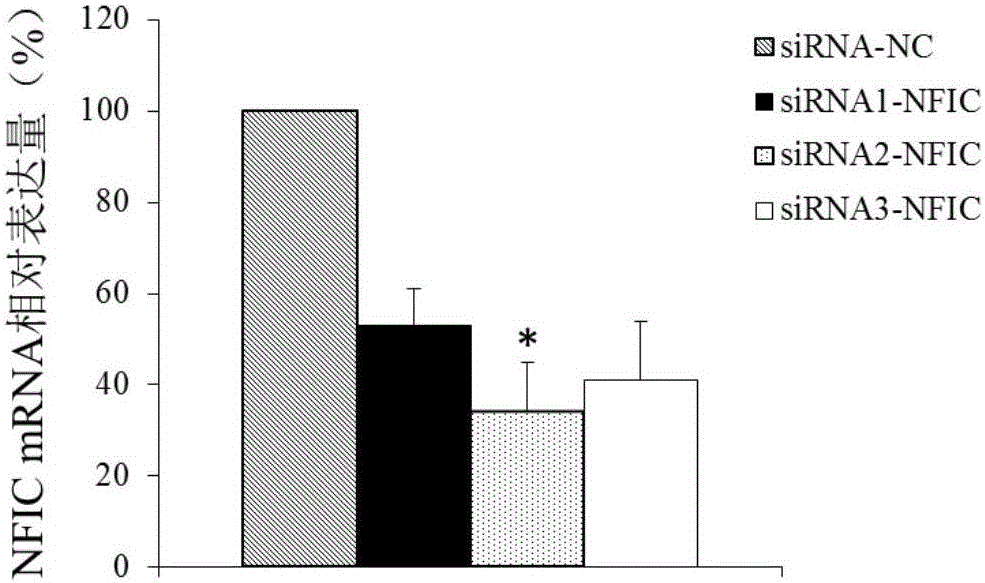
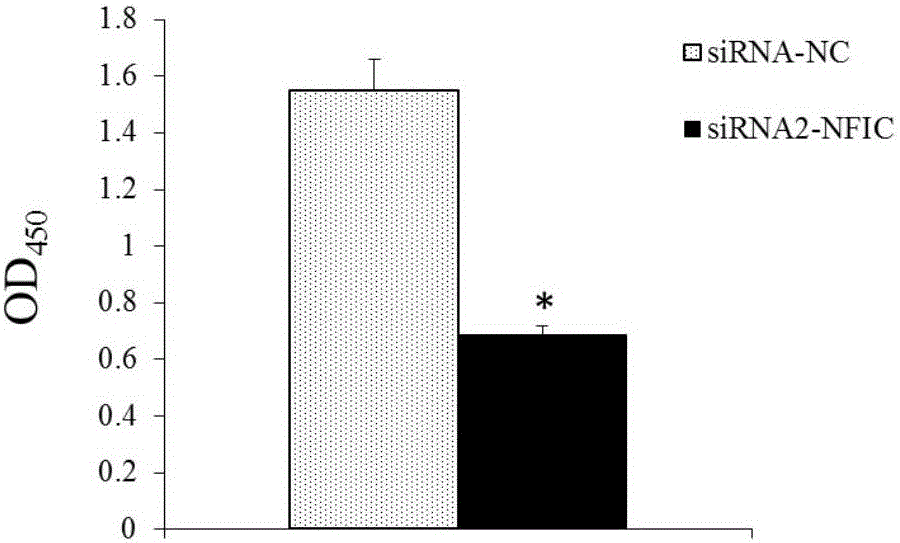
Application of NFIC gene in preparation of diagnostic and therapeutic products for pituitary adenomas
ActiveCN105838818ATimely diagnosisSpecific diagnosisOrganic active ingredientsMicrobiological testing/measurementFhit geneBiology
The invention discloses NFIC gene capable of serving as a molecular marker for early diagnosis of pituitary adenomas. Experiments of the invention prove that NFIC gene has significantly improved expression in pituitary adenomas tissues when compared to normal pituitary tissues; RNA interference experiments prove that NFIC can affect the proliferation of pituitary adenomas cells. According to the study results of the invention, it is possible to develop drugs capable of inhibiting NFIC gene expression, thus preventing and treating pituitary adenomas clinically.
Owner:BEIJING NEUROSURGICAL INST
Hepatitis b virus preS2 antigen chemiluminescence immune analysis determination reagent kit and preparing method thereof
InactiveCN101377503AHigh sensitivityStrong specificityChemiluminescene/bioluminescenceAntigenBiotin-binding proteins
The invention discloses a detection kit for detecting hepatitis B virus pre-S2 antigens. The kit provided by the invention comprises calibrators, solid-phase vectors which are coated by avidin, biotinylation antibodies, anti-HBs markers, chemiluminescent substrates and concentrated washing solutions. The preparation of the kit comprises the following steps:1) preparing the calibrators with pure hepatitis B virus pre-S2 antigens, 2) coating the solid-phase vectors with the avidin, 3) biotinylating of hepatitis B virus pre-S2 antigen monoclonal antibodies, 4) marking the anti-HBs monoclonal antibodies with markers, 5) preparing the chemiluminescent substrates, 6) preparing the concentrated washing solutions, 7) packaging the calibrators, the markers, the chemiluminescent substrates and the washing solutions and 8) assembling finished products. The kit of the invention has the advantages of high sensitivity, strong specificity, fast detection speed, simple and convent operation, good repeatability and the like, can specially detect the content of the hepatitis B virus pre-S2 antigens in the human body after being affected by hepatitis B virus, and can be used as an auxiliary means for the diagnosis and the prognosis of hepatitis B.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
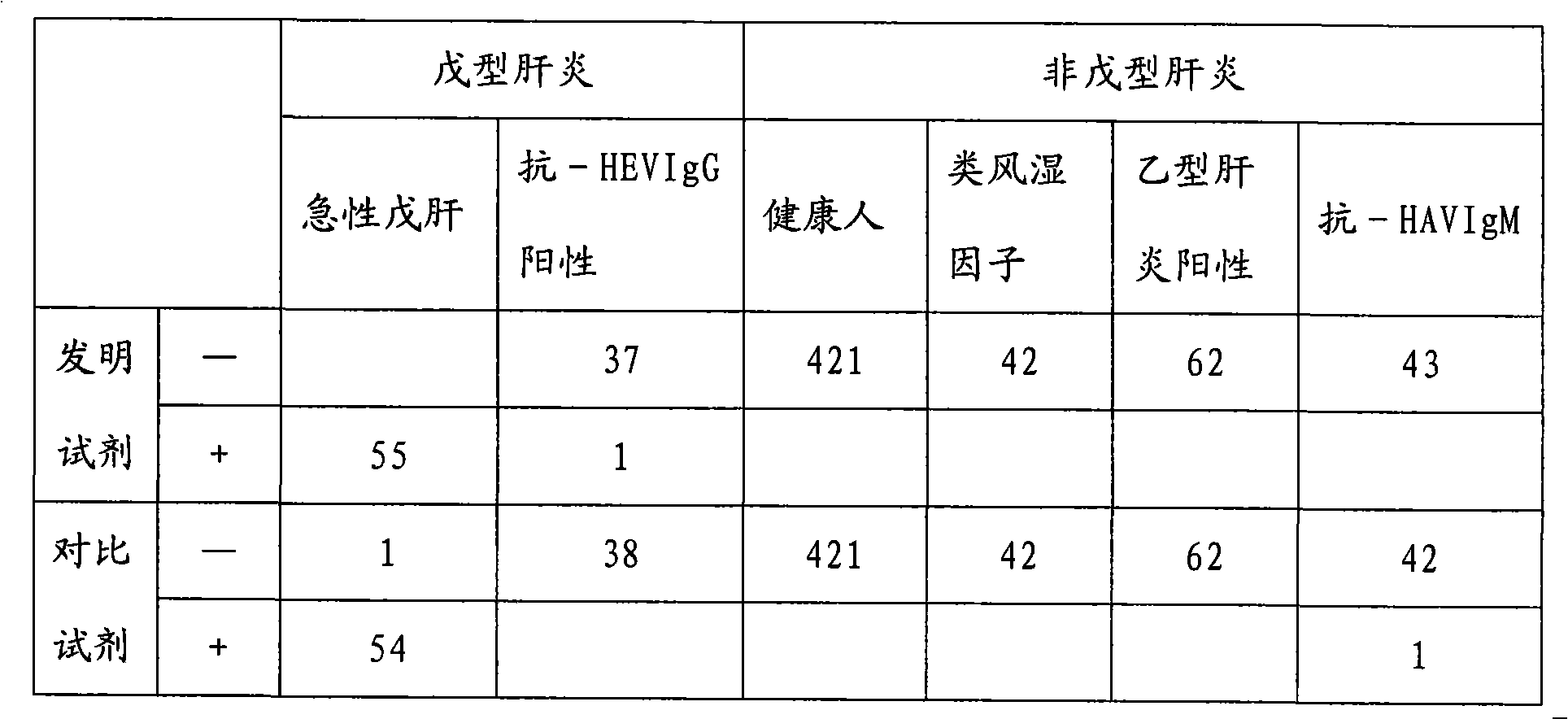
Chemiluminescence immunoassay kit of hepatitis E virus IgM antibody and preparation method thereof
InactiveCN101551395AEfficient use ofGuaranteed SensitivityChemiluminescene/bioluminescenceIgm antibodyPerformance index
The invention relates to a chemiluminescence immunoassay kit of a hepatitis E virus IgM antibody and a preparation method thereof, which belongs to the technical field of clinical in-vitro diagnosis immunoassay. The preparation method of the kit comprises the following steps: the reference substance is prepared according to the negative and the positive serums of the hepatitis E virus IgM antibody; a solid phase carrier is coated by an anti-human-mu chain anti-body (monoclonal antibody or polyclonal antibody); the recombinant antigen of the hepatitis E virus is labeled by enzyme; a chemiluminescent substrate solution acted by the enzyme is prepared; a concentrated cleaning solution is prepared; the negative reference substance, the positive reference substance, the labeled combination, the chemiluminescent substrate and the concentrated cleaning solution of the hepatitis E virus IgM antibody are subpackaged; and all the components are assembled into finished products. The prepared kit has stable performance indexes (specificity, sensitivity and stability) and can be applied to the early diagnosis of the hepatitis E.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Marker GP50 for coenuriasis, as well as coenuriasis diagnosing kit for diagnosing coenuriasis
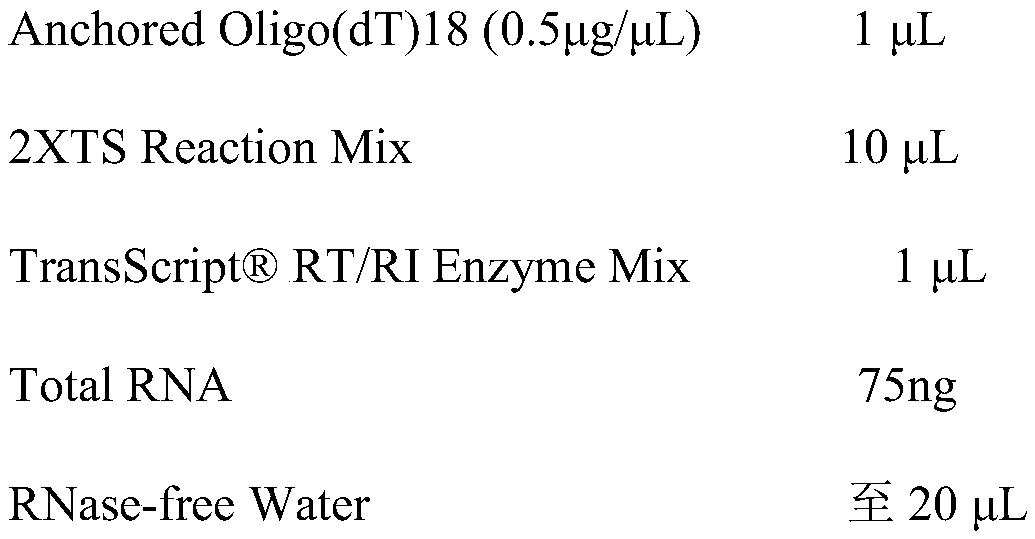
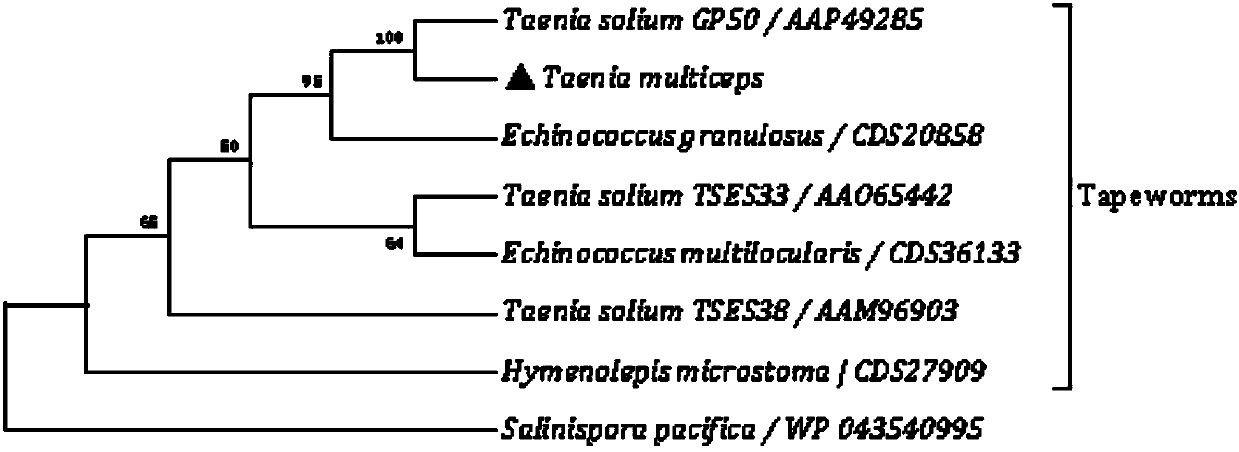
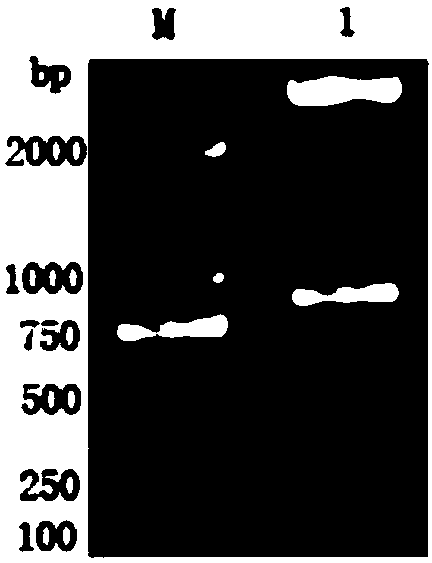
The invention belongs to the field of animal disease diagnosis, and particularly relates to a marker GP50 for coenuriasis, as well as a coenuriasis diagnosing kit for diagnosing coenuriasis. According to a coenuriasis indirect ELISA diagnosing method, the sensibility reaches 95%, and the specificity reaches 92.6%. Monitoring of a serum antibody of a goat artificially infected with taenia multiceps discovers that the GP50 antibody values in the whole detection period after infection are all positive. Therefore, the GP50 recombinant protein can be used as the coenuriasis marker, and is used for diagnosing coenuriasis. According to the coenuriasis indirect ELISA diagnosing kit, the coenuriasis can be quickly, sensitively and specifically used for diagnosing coenuriasis, and a foundation is laid for early diagnosis and the treatment effect evaluation of coenuriasis infection.
Owner:SICHUAN AGRI UNIV
Application of non-coding RNA relevant to occurrence and development of laryngeal squamous cell carcinoma
InactiveCN110157808ATimely diagnosisSensitive diagnosisMicrobiological testing/measurementDNA/RNA fragmentationNon-coding RNALaryngeal squamous cell carcinoma
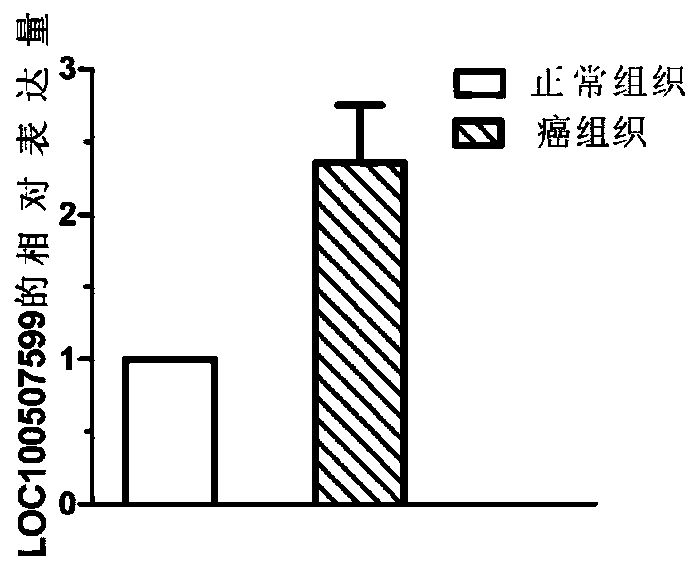
The invention discloses application of a non-coding RNA relevant to occurrence and development of laryngeal squamous cell carcinoma. The non-coding RNA is LOC100507599. According to the application, through combination of a high throughput sequencing technology with bioinformation analysis, it is found for the first time that expression of the LOC100507599 in a patient suffering from the laryngealsquamous cell carcinoma is up-regulated, and the conclusion is verified through further QPCR, which indicates that the LOC100507599 can serve as a molecular target to be applied to diagnosis and treatment of the laryngeal squamous cell carcinoma.
Owner:THE SECOND HOSPITAL OF HEBEI MEDICAL UNIV
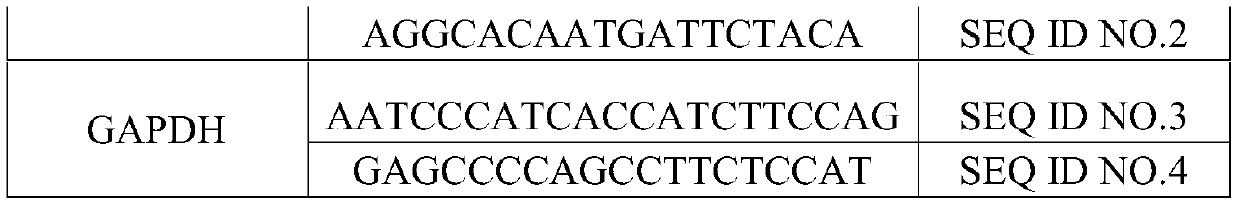
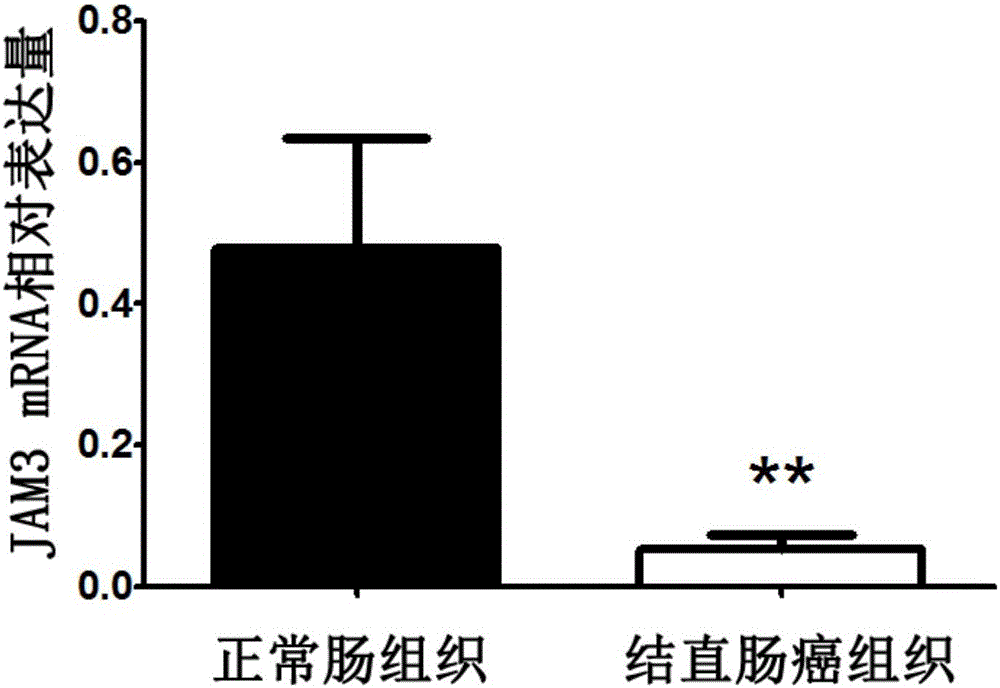
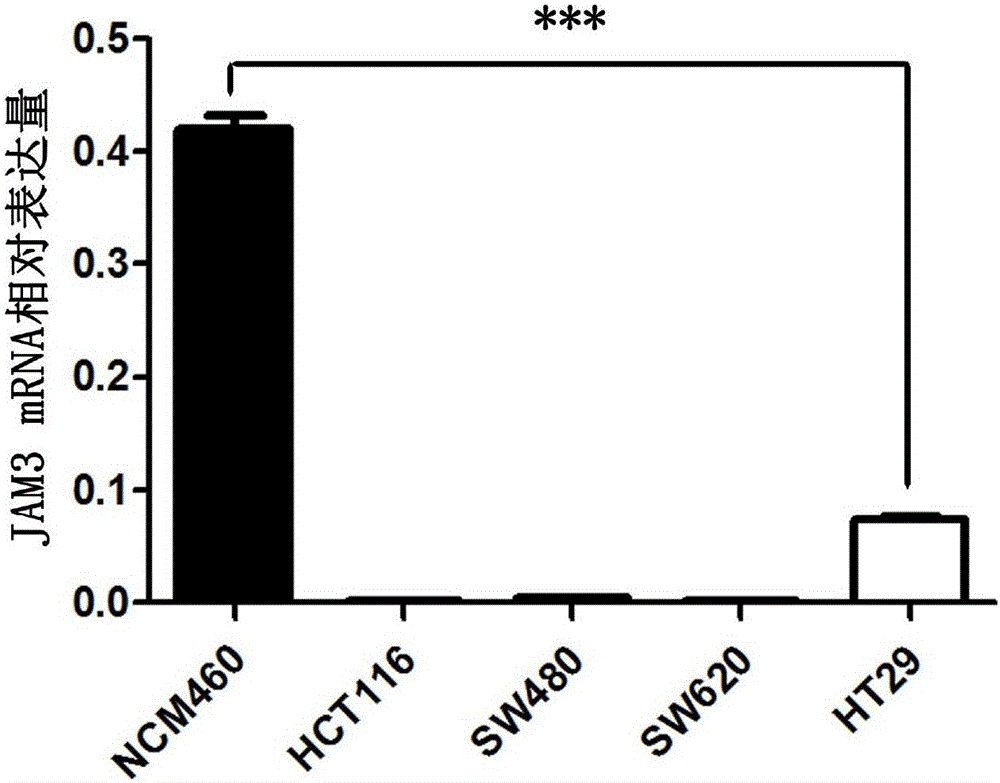
Application of JAM3 gene to preparation of colorectal cancer diagnosis kit and kit
InactiveCN105755152ATimely diagnosisSpecific diagnosisMicrobiological testing/measurementBiological testing5 year survival rateMortality rate
The invention provides application of a colorectal cancer diagnosis molecular marker, namely, a JAM3 gene which is good in specificity and sensitivity in preparing a colorectal cancer diagnosis kit, and the colorectal cancer diagnosis kit. The application has the main benefits that a novel molecular marker of colorectal cancer is discovered, application of the JAM3 gene to preparation of the colorectal cancer diagnosis kit and the colorectal cancer diagnosis kit are provided, and compared with a conventional detection method, the molecular marker is relatively timely and specific in diagnosis, so that the five-year survival rate of patients suffering from colorectal cancer can be increased, the death rate is reduced, and wide application prospects can be achieved.
Owner:XIAMEN INST OF RARE EARTH MATERIALS
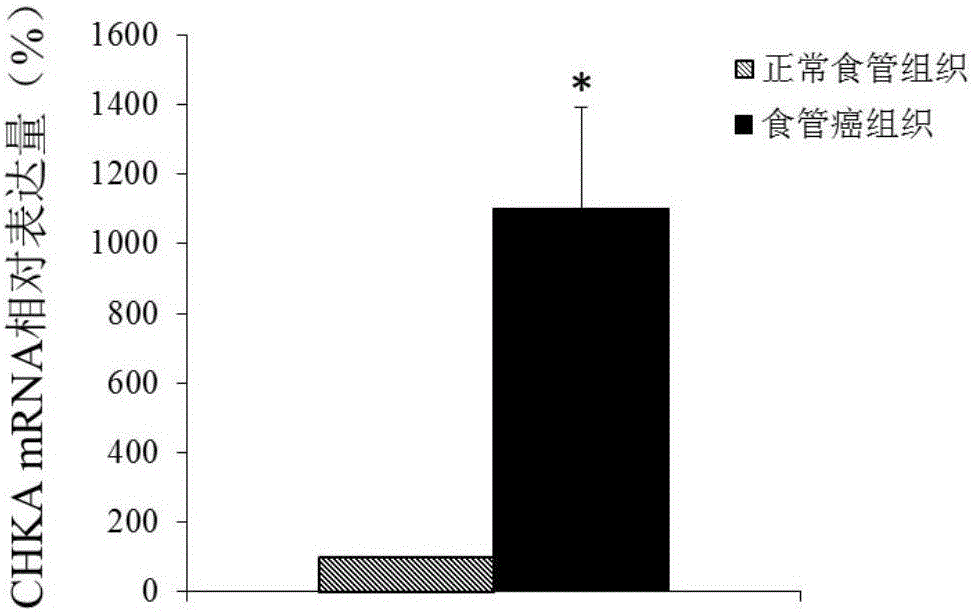
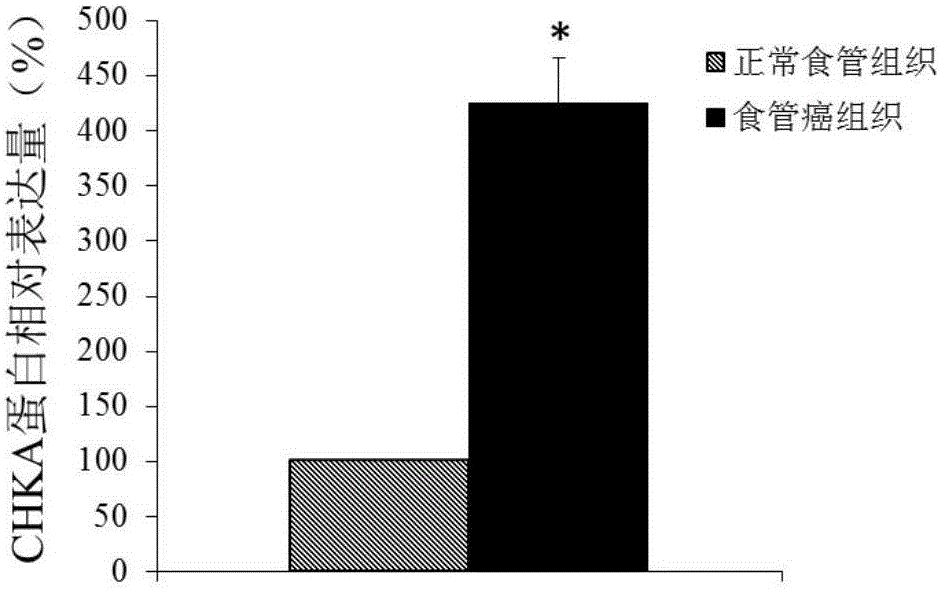
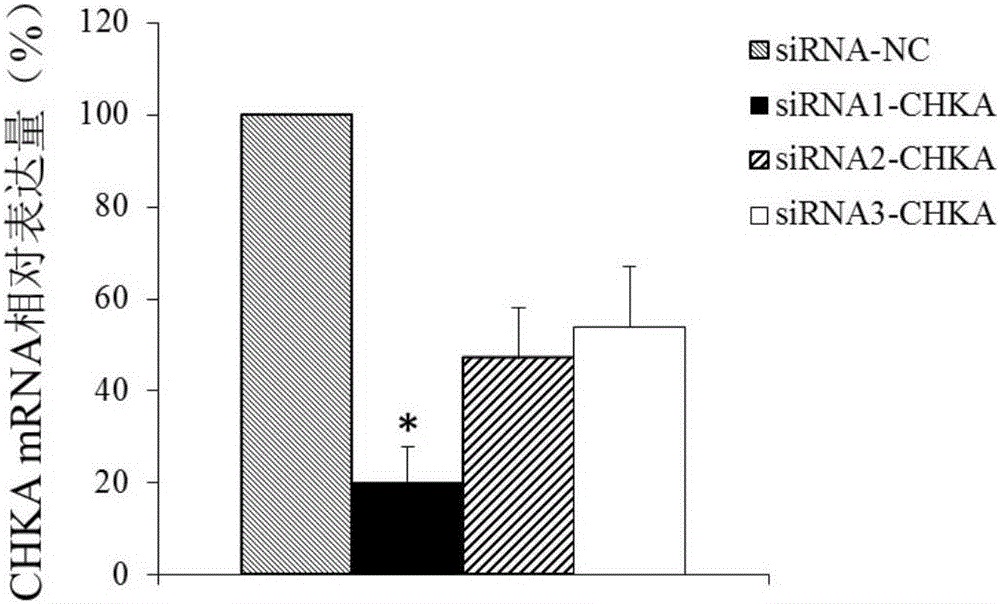
Application of CHKA gene to preparation of esophageal cancer diagnosis and treatment product
ActiveCN105886625ATimely diagnosisSpecific diagnosisMicrobiological testing/measurementBiological testingEsophageal cancerCancer research
The invention discloses a CHKA gene that can function as a molecular marker for early diagnosis of esophageal cancer. Experiments prove that compared with normal esophageal tissues, the CHKA gene in esophageal cancer tissues has significantly increased expression, and RNA interference experiments prove that CHKA can affect proliferation of esophageal cancer cells. According to study results herein, it is possible to develop medicine to inhibit CHKA gene expression so as to clinically prevent and treat the esophageal cancer.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
Multiple PCR detecting reagent case for diagnosis of oesophagostomiasis in pig
InactiveCN101230382ARapid diagnosisSpecific diagnosisMicrobiological testing/measurementPositive controlEpidemiologic survey
The invention discloses a multiple PCR detection kit which can be used in the diagnosis of the swine esophagostomiasis. The detection kit comprises DNA lysis solution, PCR reaction solution, as well as the DNA positive control of toothed Oesophagostomum and the positive control of four-ratched Oesophagostomum. By using the ITS repeat DNA fragments of swine toothed Oesophagostomum and four-ratched Oesophagostomum as genetic markers, the invention builds up a fast, specific and sensitive multiple PCR methods which can be used for diagnosing swine esophagostomiasis. With simple and programmed operation and objective result judgments, the invention can be used for diagnosing swine esophagostomiasis, for investigating epidemiology, and for identifying and diagnosing toothed Oesophagostomum and four-ratched Oesophagostomum.
Owner:SOUTH CHINA AGRI UNIV
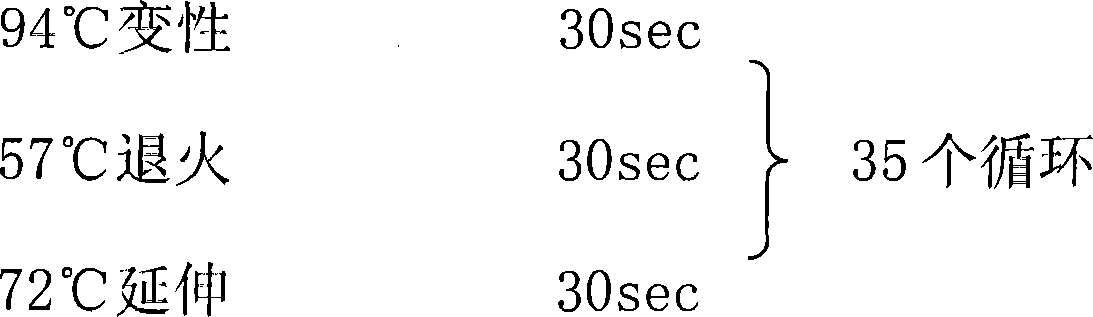
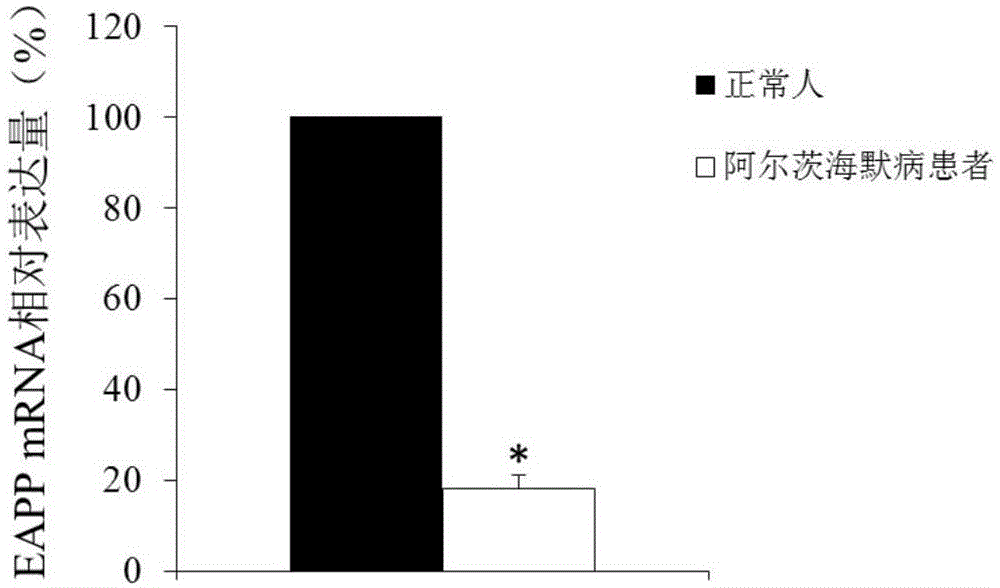
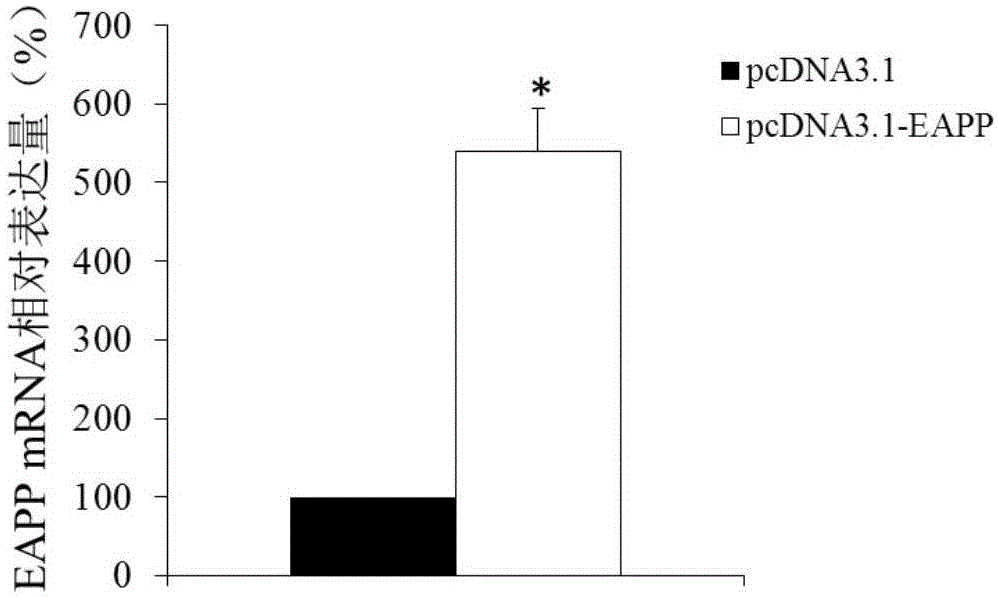
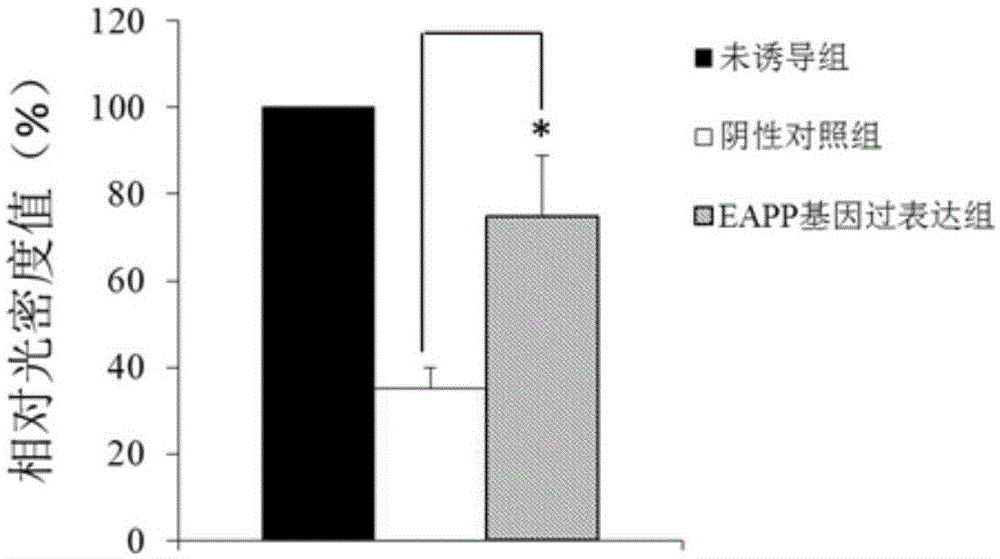
Application of reagent for detecting EAPP genetic expression in Alzheimer disease diagnosis and treatment
ActiveCN105002172ATimely diagnosisSpecific diagnosisPeptide librariesNervous disorderDisease riskDiagnosis early
The invention discloses a molecular marker-EAPP gene for early diagnosis of the Alzheimer disease. An experimental result shows that the mRNA level of the EAPP gene in blood of the patient suffering from the Alzheimer disease is remarkably lower than that of normal people, so that whether a tested person has the risk of the Alzheimer disease or not or whether the tested person has suffered from the Alzheimer disease or not is judged by determining the expression level of the EAPP gene in the blood of the tested person. The EAPP gene can be used for preparation and application for the patient suffering from the Alzheimer disease or the crowd with the high Alzheimer disease risk so as to be used for treating the Alzheimer disease or preventing the Alzheimer disease. The new diagnosis method is provided for diagnosing the Alzheimer disease clinically and the new candidate medicine is provided for treating the Alzheimer disease.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
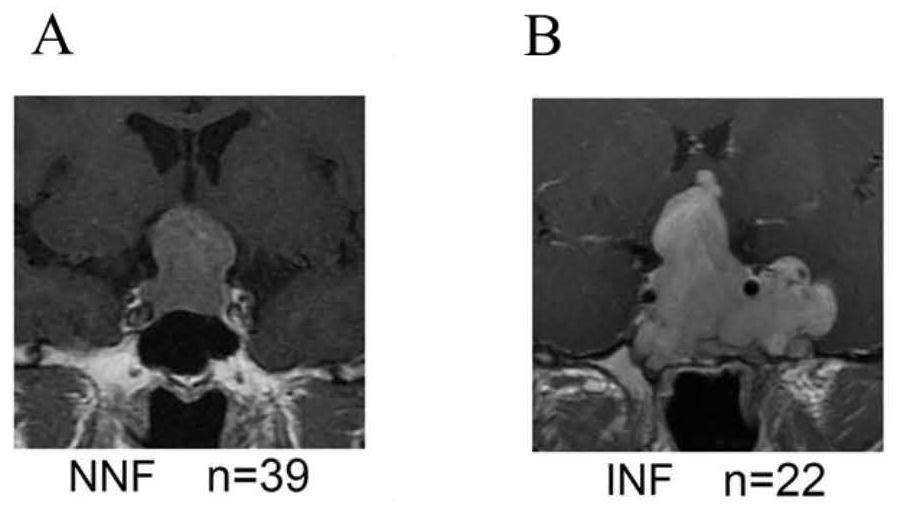
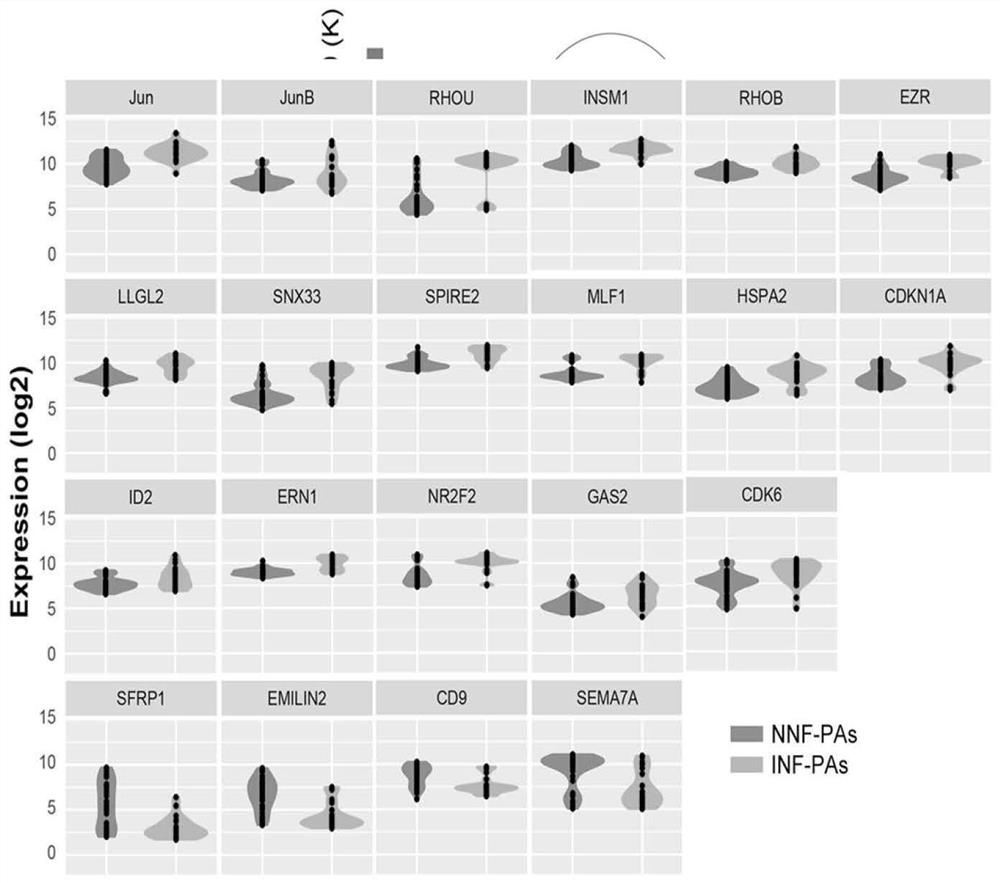
Gene combination for distinguishing non-invasive and invasive non-functional pituitary adenomas
ActiveCN112626207ASpecific diagnosisSensitive diagnosisMicrobiological testing/measurementMaterial analysisPhysiologyRHOB
The invention discloses a gene combination for distinguishing non-invasive and invasive non-functional pituitary adenomas. The gene combination consists of the following genes of INSM1, Jun, JunB, RHOU, RHOB, EZR, LLGL2, SNX33, SPIRE2, MLF1, HSPA2, CDKN1A, ERN1, NR2F2, GAS2, CDK6, SFRP1, EMILIN2, CD9 and SEMA7A. By detecting the expression level of the gene combination, the non-invasive and invasive non-functional pituitary adenomas can be distinguished.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI +1
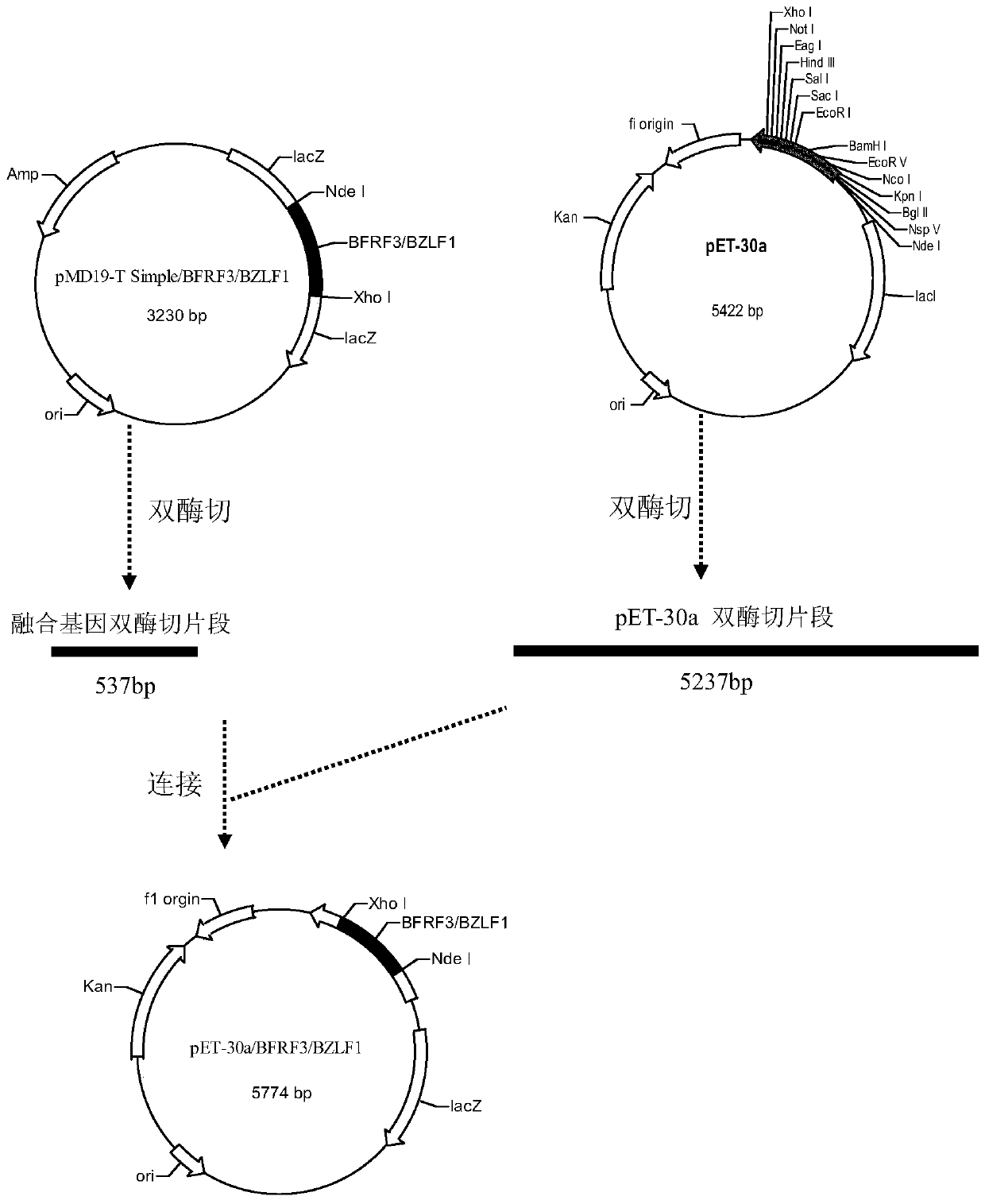
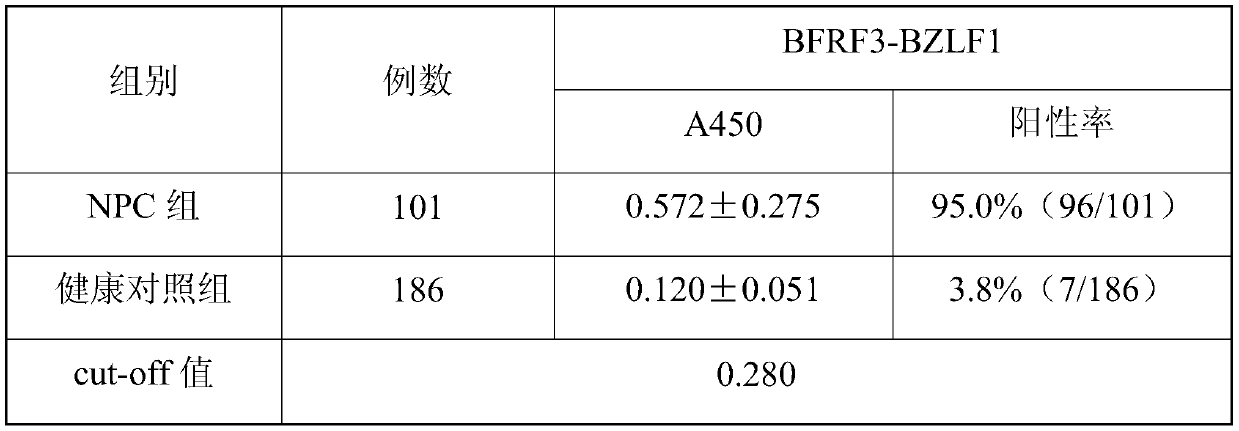
EB virus BFRF3-BZLF1 fusion protein, gene, vector containing same, host cell, test strip and production method and application thereof
ActiveCN109929040AHigh sensitivityHigh diagnostic sensitivityBacteriaMicroorganism based processesMononucleosisTarget antigen
The invention provides an EB virus BFRF3-BZLF1 fusion protein, a gene, a vector containing the BZLF1 fusion protein, a host cell, a test strip, a production method and application thereof. The EB virus BFRF3-BZLF1 fusion protein is obtained by connecting the EB virus BFRF3 protein or an EB virus BFRF3 protein fragment with an EB virus BZLF1 protein or EB virus BZLF1 protein fragment through a connecting peptide, and preferably, the EB virus BFRF3-BZLF1 fusion protein has an amino acid sequence shown in SEQ ID NO:1. The EB virus BFRF3-BZLF1 fusion protein can be used as a target antigen for detecting an EB virus, provides a basis for screening and early diagnosing nasopharyngeal carcinoma, infectious mononucleosis and Burkitt lymphoma, and has the advantages of simplicity, rapidness, sensitivity and specificity.
Owner:北京贝思泰生物科技有限公司
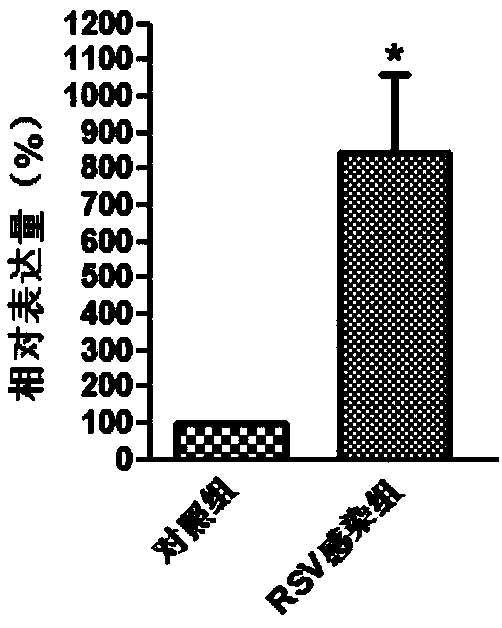
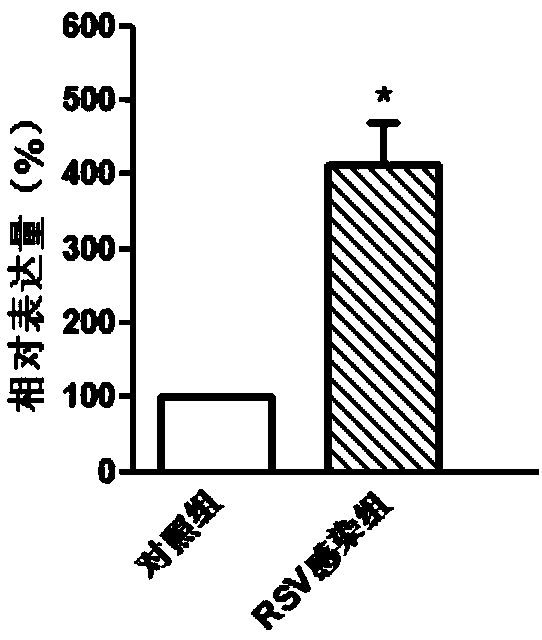
Molecular marker in blood serving as diagnosis index of respiratory syncytial virus infection
InactiveCN109321652ATimely diagnosisSpecific diagnosisMicrobiological testing/measurementDNA/RNA fragmentationViral antibodyNormal people
The invention discloses a molecular marker in blood serving as a diagnosis index of respiratory syncytial virus infection. The molecular marker in blood is TYMS. Experiments prove that the content ofTYMS gene and TYMS protein in the blood of people infected with respiratory syncytial virus obviously increases compared with that of normal people. Due to the expression difference between TYMS geneand TYMS protein, the content increase can serve as an index for distinguishing the people infected with respiratory syncytial virus from the non-infected people. Compared with traditional virus antibody detection method and virus antigen detection method, the non-virus index in the blood is adopted for virus detection by the invention, and high-sensitivity detection can be realized.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
Molecular marker for diagnosing and treating hysteromyoma
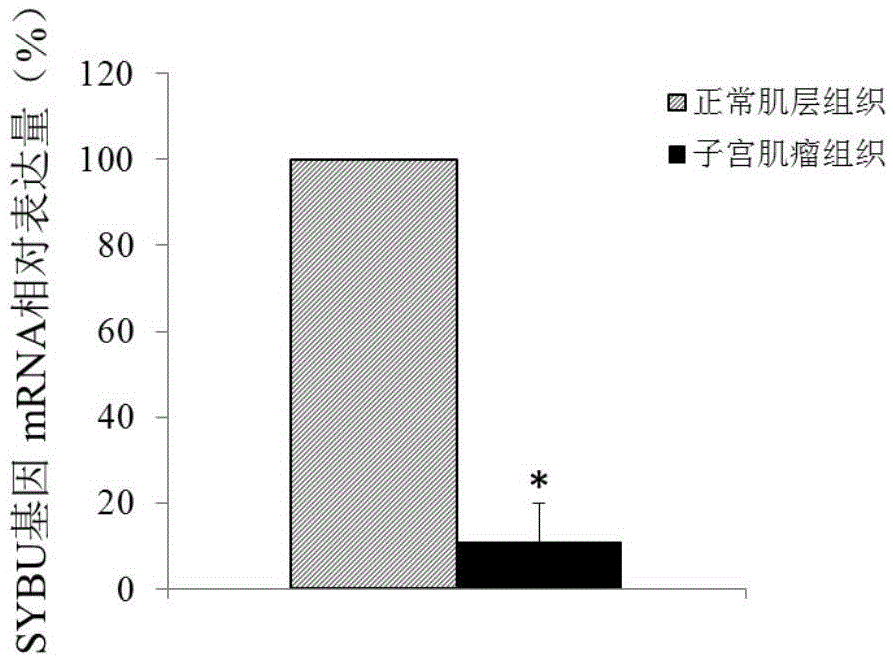
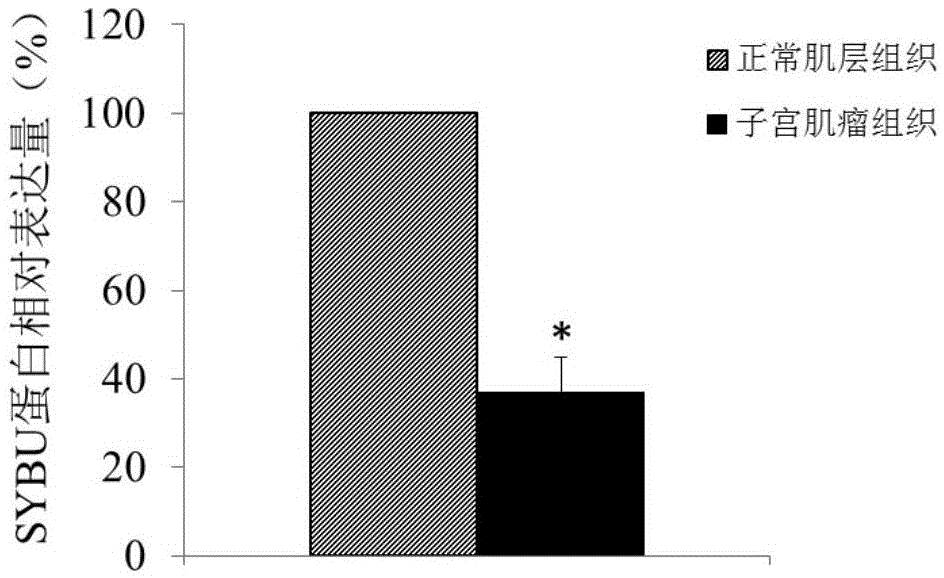
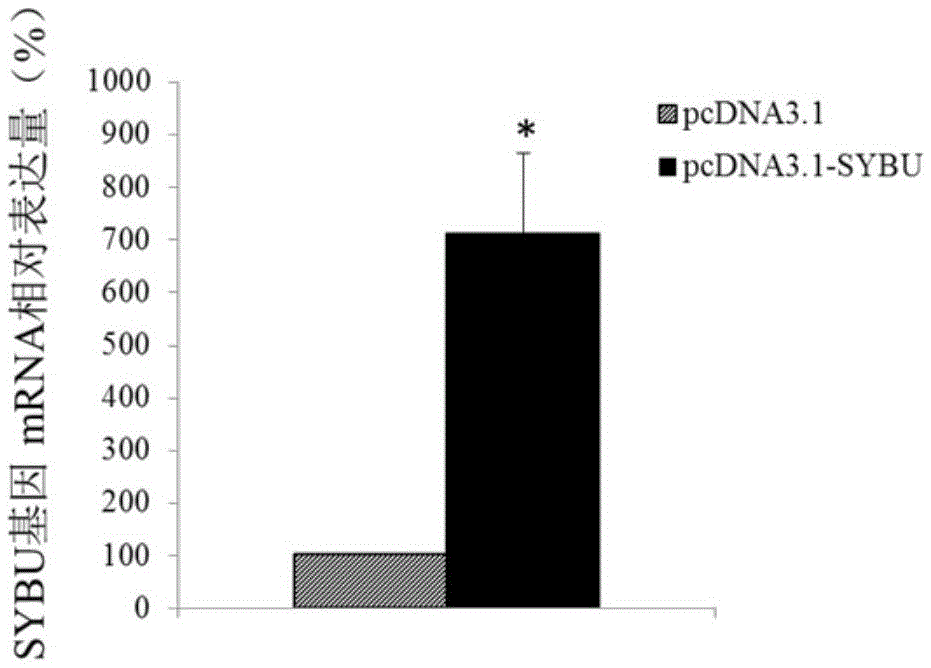
ActiveCN105603116ATimely diagnosisSpecific diagnosisPeptide/protein ingredientsMicrobiological testing/measurementWestern blotApoptosis
The invention discloses a molecular marker for diagnosing and treating hysteromyoma. QPCR and Western blot methods prove remarkable difference of expressions of SYBU genes in the hysteromyoma tissue and normal tissues. Moreover, in-vitro cell culture experiments of the invention prove that the expression of the SYBU genes can inhibit the proliferation of hysteromyoma cells while promoting apoptosis, so that the SYBU genes and expression products thereof can be used for preparing a medicine for treating hysteromyoma and are widely and clinically applied.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
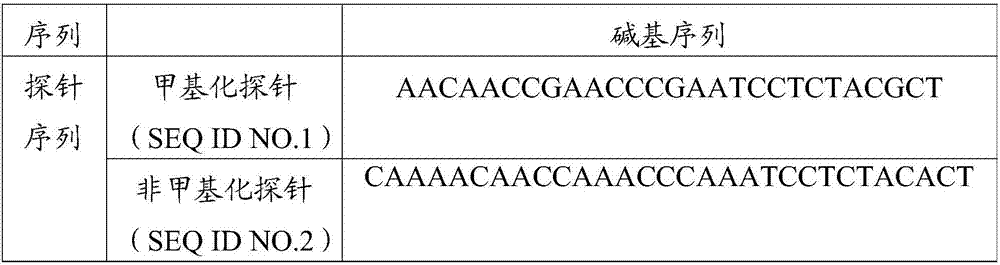
DKK-3 gene methylation diagnostic reagent system and kit, and applications thereof
ActiveCN106967792AAchieve early diagnosisReduce mortalityMicrobiological testing/measurementDNA/RNA fragmentationMolecular diagnosticsMethylation
The invention belongs to the field of molecular diagnosis, and more specifically relates to a DKK-3 gene promoter region methylation diagnostic reagent system and a kit, and applications thereof. The DKK-3 gene promoter region methylation diagnostic reagent system comprises a probe sequence SEQ ID NO.1 used for detecting DKK-3 gene promoter region methylation, and a probe sequence SEQ ID NO.2 used for detecting DKK-3 gene promoter region non-methylation. The invention also provides the DKK-3 gene promoter region methylation diagnostic kit containing the DKK-3 gene promoter region methylation diagnostic reagent system, and applications of the DKK-3 gene promoter region methylation diagnostic reagent system or the DKK-3 gene promoter region methylation diagnostic kit in preparing reagents used for cervical carcinoma detection and / or diagnosis. Detection of DKK-3 gene promoter region methylation level is capable of guiding early stage diagnosis, treatment, and evaluating prognosis of cervical carcinoma effectively, and realizing in-time specific convenient diagnosis.
Owner:天津知因生物科技有限公司
New diagnostic function of MAEA gene in blood
ActiveCN108796067ATimely diagnosisSpecific diagnosisMicrobiological testing/measurementBiological testingMedicineMyocardial infarction diagnosis
The invention discloses an MAEA gene and an application of an expression product of the MAEA gene to preparation of a diagnostic product. The diagnostic product can be used for diagnosing myocardial infarction. The risk of myocardial infarction of a testee in the future can be judged or the state of myocardial infarction is confirmed by detecting the level of the MAEA gene and the expression product thereof. The diagnostic product takes peripheral blood of the testee as a detection object, and can achieve noninvasive, rapid, sensitive and accurate diagnostic effects.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
Marker gp50 of cerebral polycephaly and a kit for diagnosing cerebral polycephalic
The invention belongs to the field of animal disease diagnosis, and particularly relates to a marker GP50 for coenuriasis, as well as a coenuriasis diagnosing kit for diagnosing coenuriasis. According to a coenuriasis indirect ELISA diagnosing method, the sensibility reaches 95%, and the specificity reaches 92.6%. Monitoring of a serum antibody of a goat artificially infected with taenia multiceps discovers that the GP50 antibody values in the whole detection period after infection are all positive. Therefore, the GP50 recombinant protein can be used as the coenuriasis marker, and is used for diagnosing coenuriasis. According to the coenuriasis indirect ELISA diagnosing kit, the coenuriasis can be quickly, sensitively and specifically used for diagnosing coenuriasis, and a foundation is laid for early diagnosis and the treatment effect evaluation of coenuriasis infection.
Owner:SICHUAN AGRI UNIV
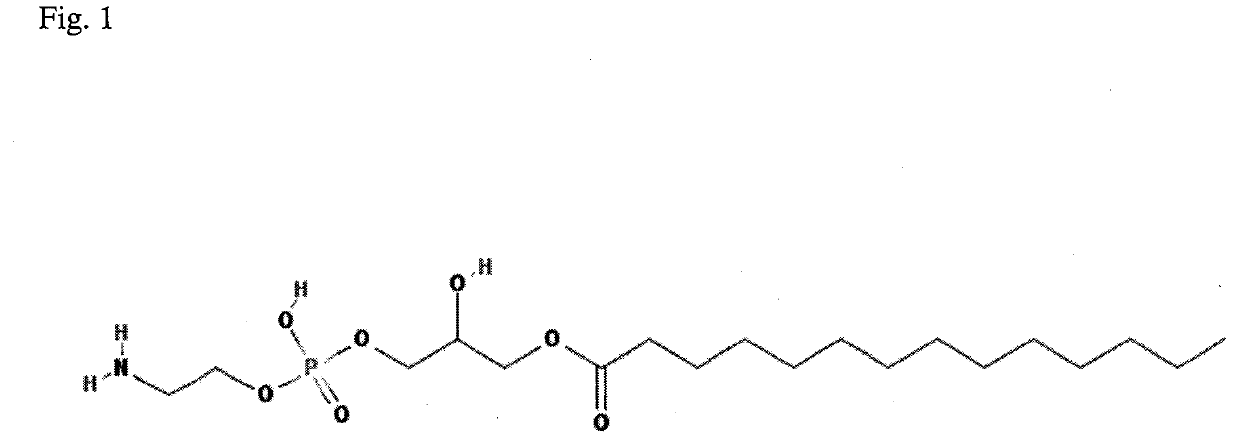
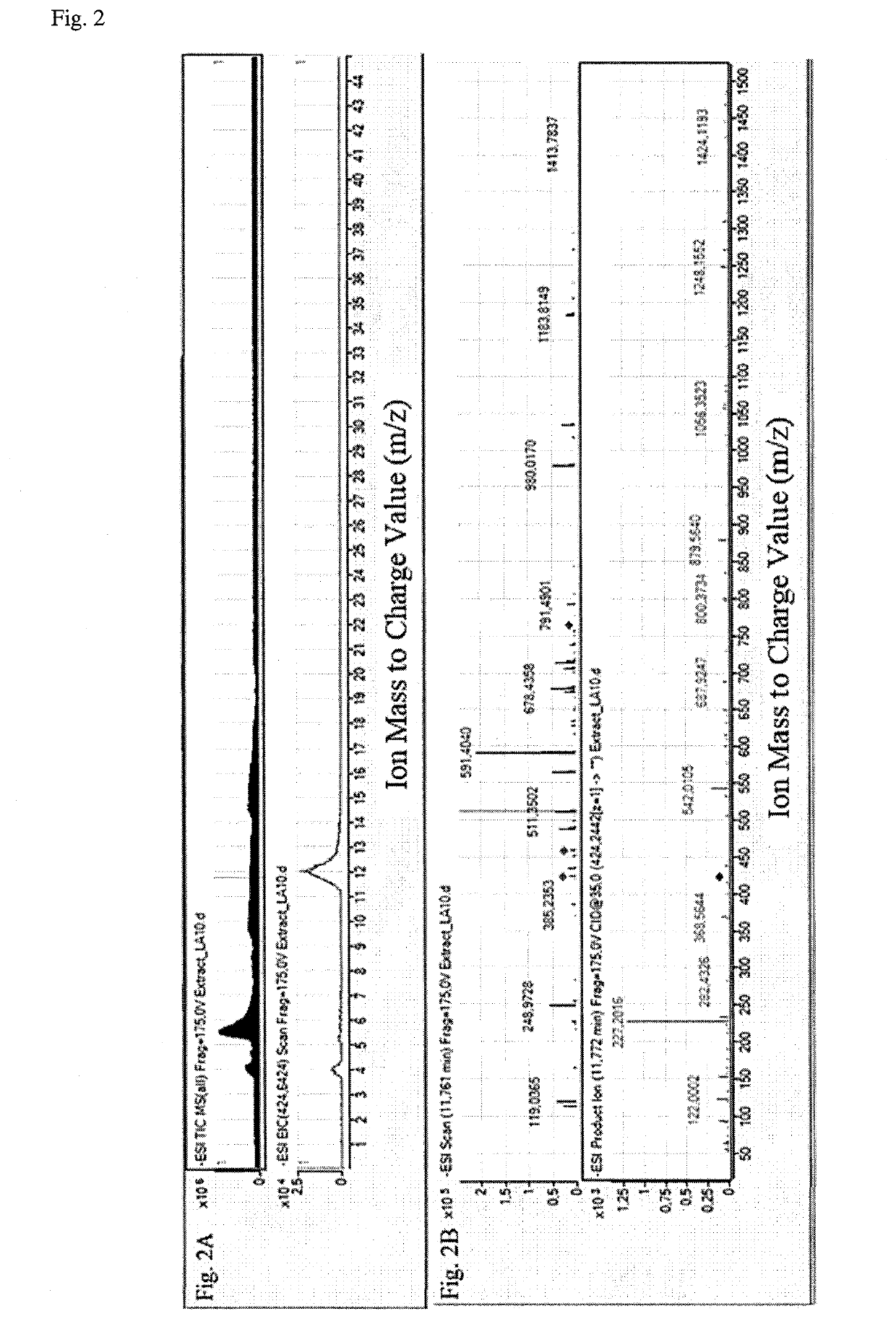
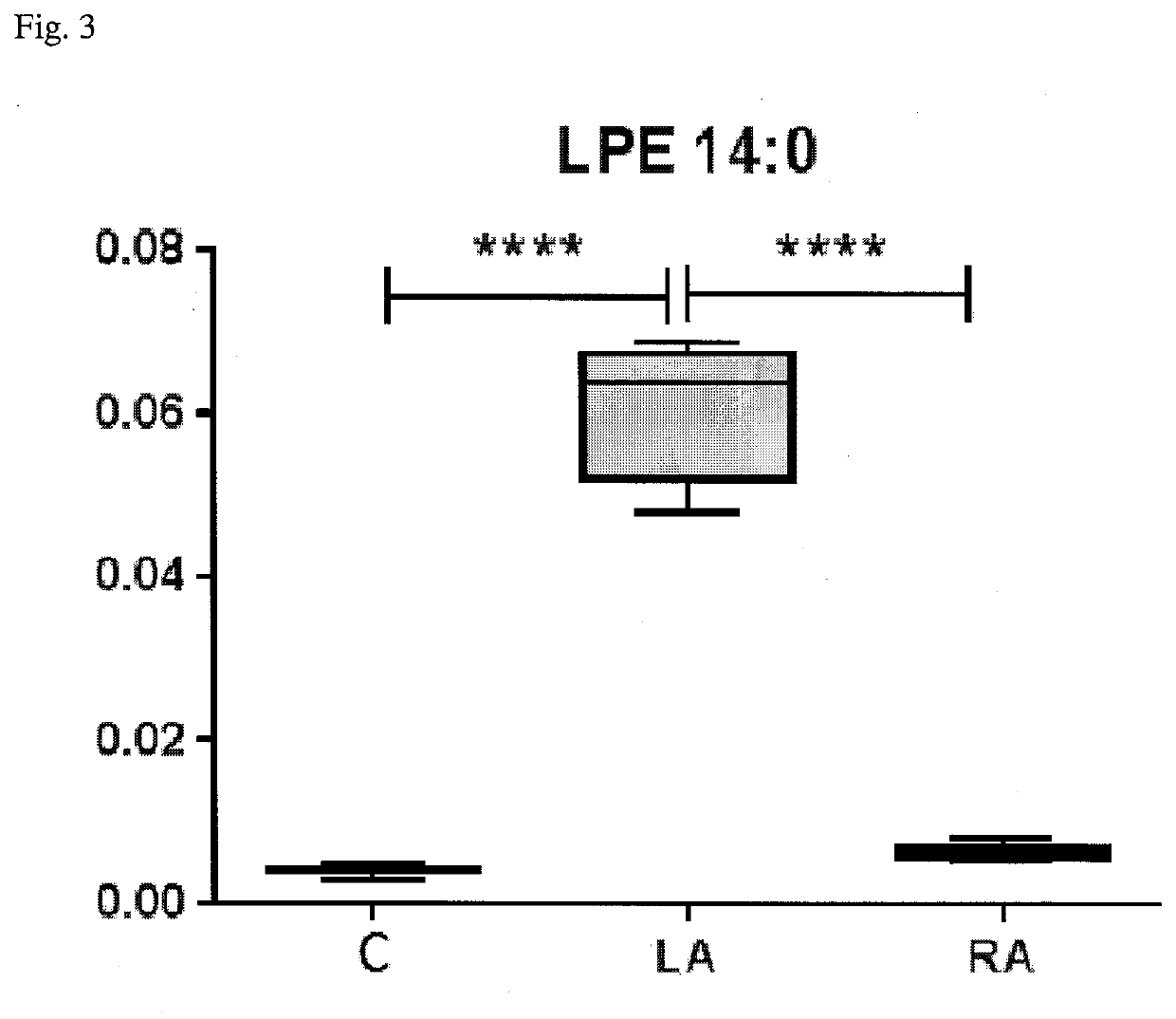
Method for diagnosis of lyme arthritis, method for differential diagnosis of lyme arthritis, lysophosphatidylethanolamine for use as biomarker, kit for diagnosis of lyme arthritis and kit for differential diagnosis of lyme arthritis
PendingUS20210255209A1Quick and precise and specific and sensitive diagnosisPrecise and easyComponent separationDisease diagnosisReference sampleSacroiliitis
The subject matter of the invention relates to a method for in vitro diagnosis of Lyme disease and a method for in vitro differential diagnosis of Lyme arthritis versus rheumatoid arthritis, in which methods, in a sample from a subject, the level of lysophosphatidylethanolamine comprising myristic acid (LysoPE(14:0)) is determined and such determined level of lysophosphatidylethanolamine is compared with the level of lysophosphatidylethanolamine comprising myristic acid in a reference sample; in wherein the level of lysophosphatidylethanolamine comprising myristic acid which is higher than the level in the said reference sample indicates that the subject suffers from Lyme disease. The subject matter of the invention further relates to lysophosphatidylethanolamine comprising myristic for use as a biomarker of Lyme disease, as a biomarker of Lyme arthritis, as a biomarker for differential diagnosis of Lyme arthritis versus rheumatoid arthritis, as a biomarker of neuroborreliosis. The subject matter of the invention also relates to a kit for in vitro diagnosis of Lyme disease and a kit for in vitro differential diagnosis of Lyme arthritis, which kits comprise a means for determining the level of lysophosphatidylethanolamine comprising myristic acid and instructions for carrying out the methods for diagnosis according to the invention.
Owner:UNIV MEDYCZNY W BIAYMSTOKU
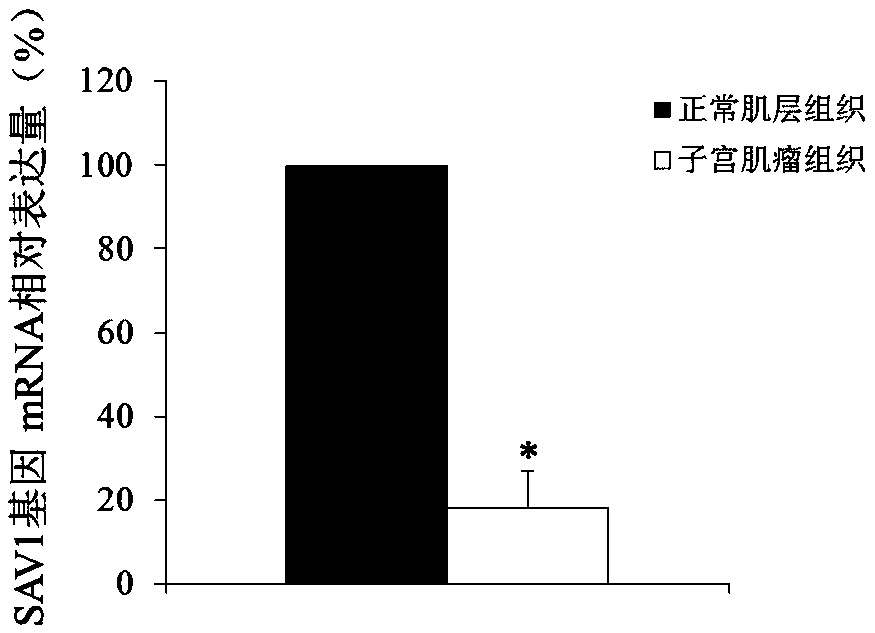
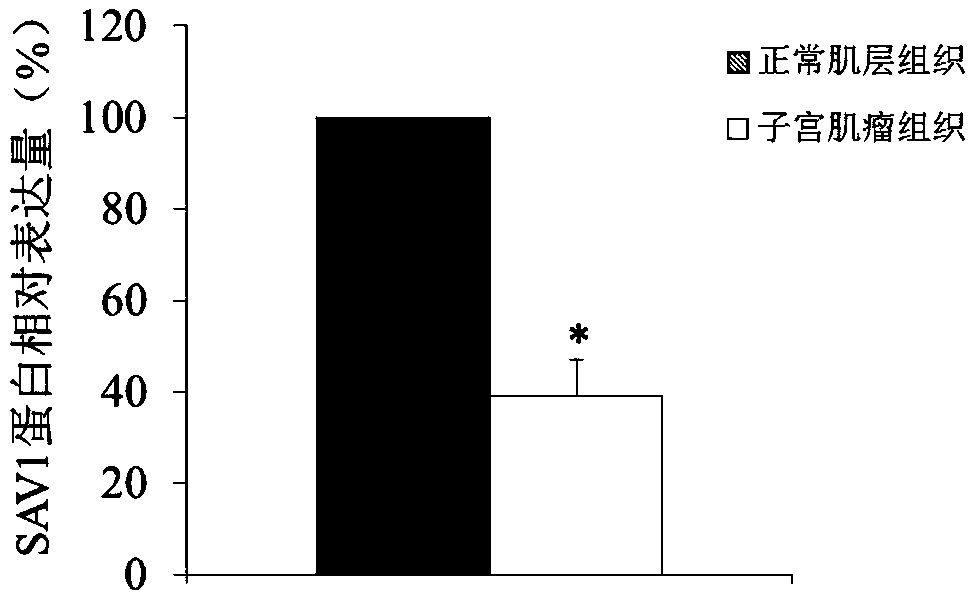
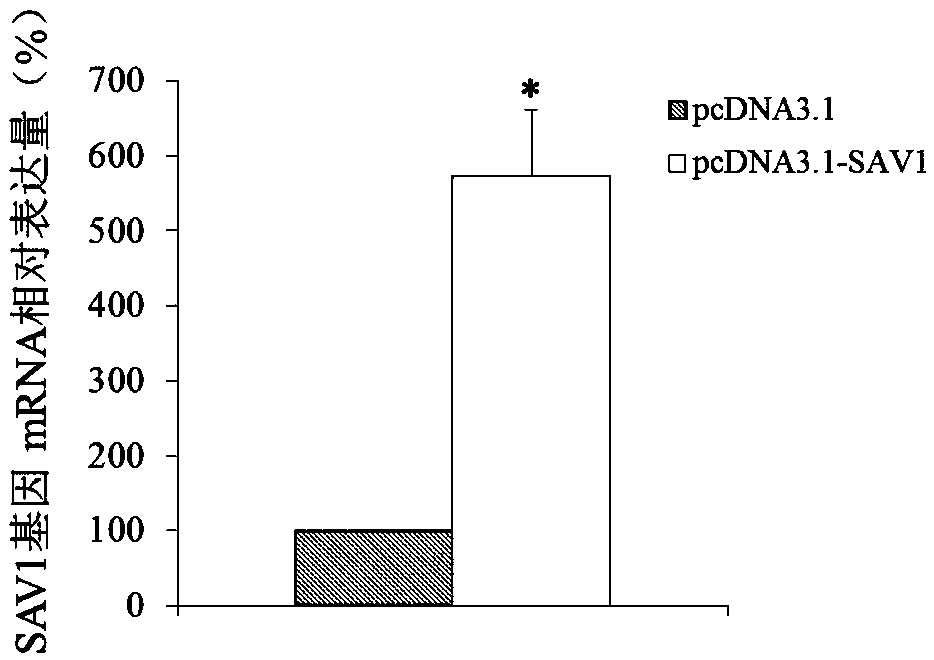
Use of SAV1 (Salvador family WW domain-containing protein 1) gene as hysteromyoma diagnostics and treatment marker
ActiveCN105506170ATimely diagnosisSpecific diagnosisMicrobiological testing/measurementBiological testingWestern blotNormal tissue
The invention discloses a molecular marker, SAV1 (Salvador family WW domain-containing protein 1) gene, for early diagnostics of hysteromyoma and an expression product thereof. By using QPCR (quantitative polymerase chain reaction) and Western blot methods, it is proved that the SAV1 gene has differential expression in hysteromyoma tissues and normal tissues and can be used as an index for the early diagnostics of hysteromyoma. In addition, the invention also discloses the SAV1 gene and the expression product thereof which may be used as targets for treating hysteromyoma and used for guiding the development of new drugs.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
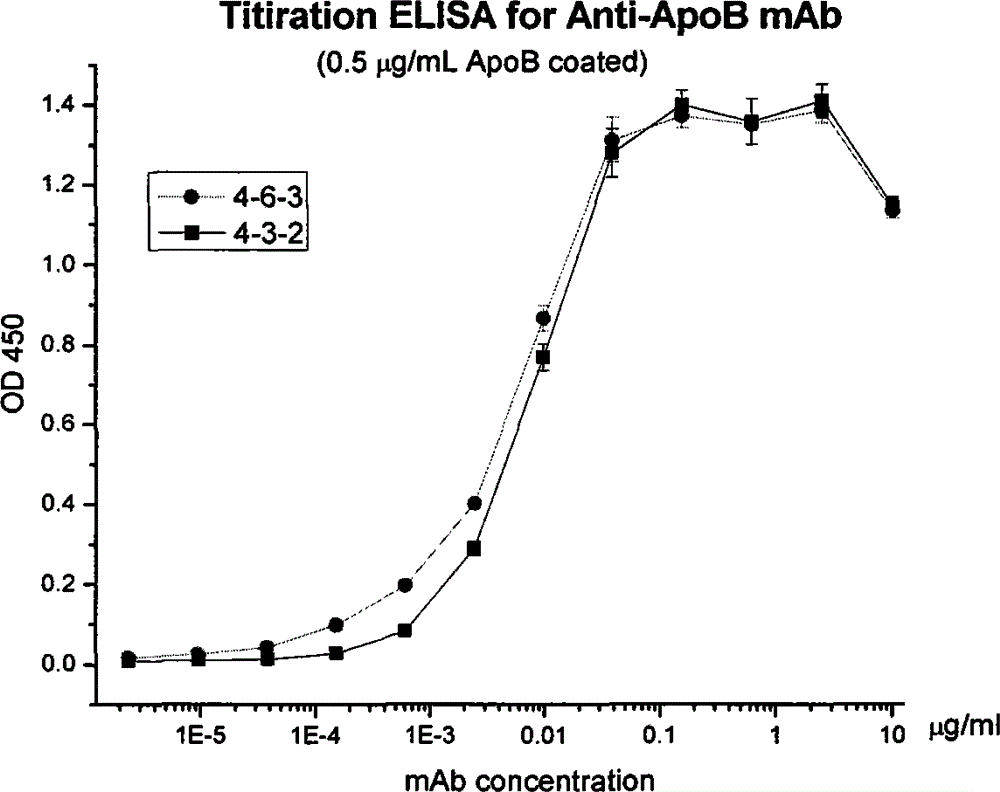
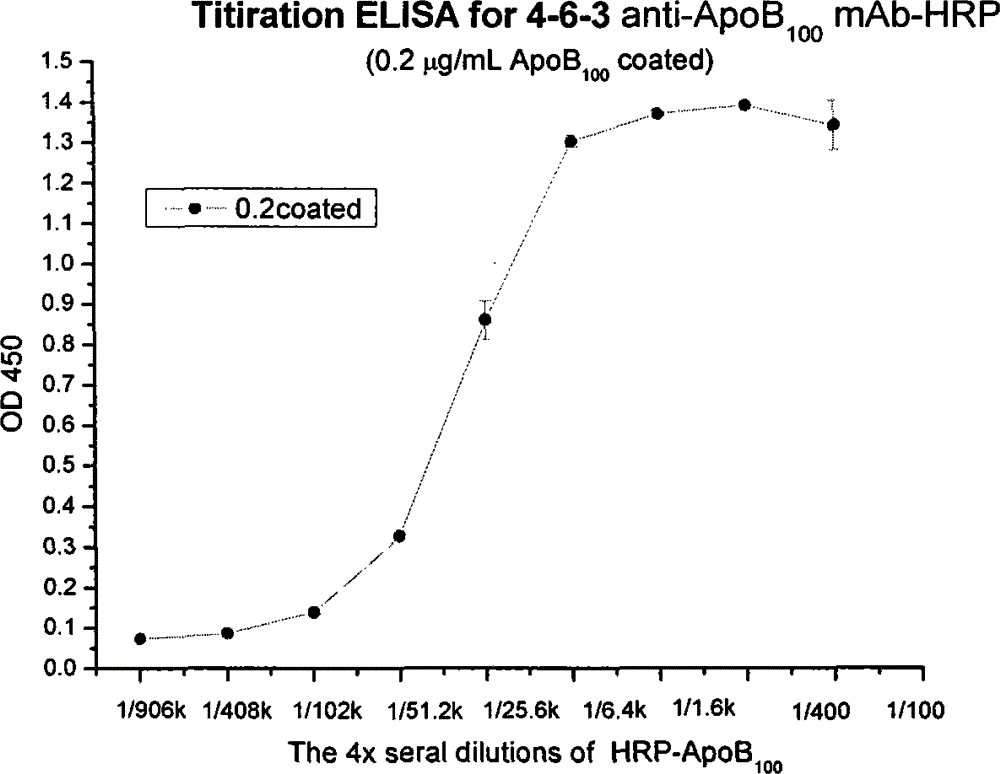
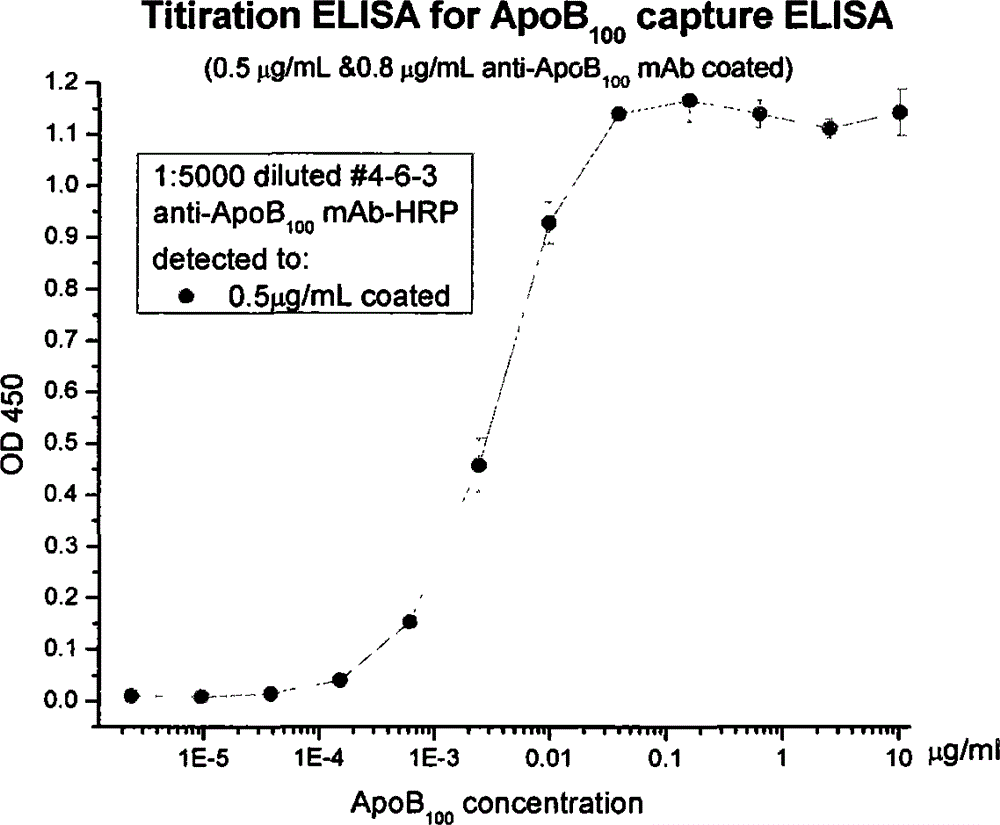
Human apolipoprotein B100 (ApoB100) monoclonal antibody and chemiluminescence immune assay determination kit adopting the human ApoB100 monoclonal antibody
ActiveCN102943066BRapid diagnosisSpecific diagnosisImmunoglobulins against animals/humansMicroorganism based processesHuman apolipoproteinMonoclonal antibody
The invention provides a chemiluminescence immune assay determination kit adopting a human apolipoprotein B100 (ApoB100) monoclonal antibody and a preparation method thereof. The invention also provides the human ApoB100 monoclonal antibody which can specifically recognize human ApoB100, a hybrid tumor for producing the human ApoB100 monoclonal antibody, and a method for high-sensitivity detection of human ApoB100 by the human ApoB100 monoclonal antibody. The chemiluminescence immune assay determination kit has high sensitivity, a wide detection scope and a low cost and is convenient for operation.
Owner:大庆麦伯康生物技术有限公司 +2
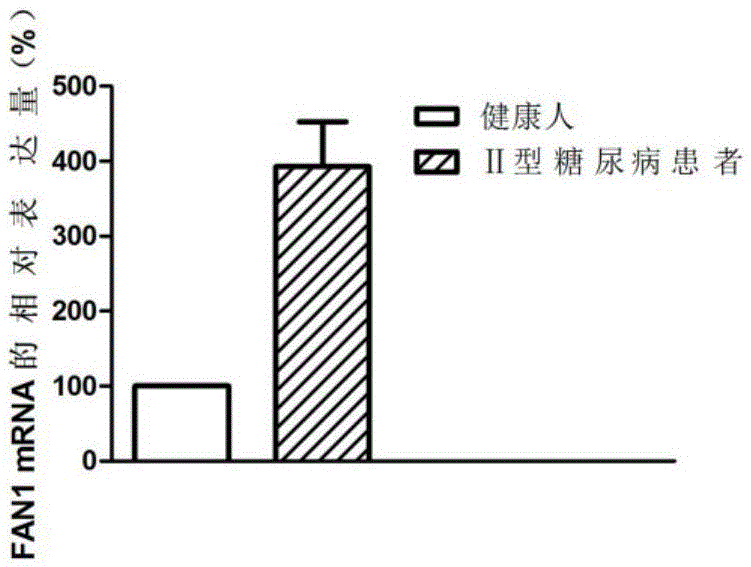
Application of FAN1 genes in II-type diabetes diagnosis
ActiveCN105648074AAchieve early diagnosisTimely diagnosisMicrobiological testing/measurementDisease diagnosisDiabetic patientGene
The invention discloses an application of FAN1 genes in II-type diabetes diagnosis. The expression level of the FAN1 genes in blood of a patient suffering from II-type diabetes is higher than that of the FAN1 genes in blood of healthy people, whether a patient suffers from diabetes can be diagnosed by detecting the expression level of the FAN1 genes in the blood of the patient, and accordingly, early treatment of the patients can be realized.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
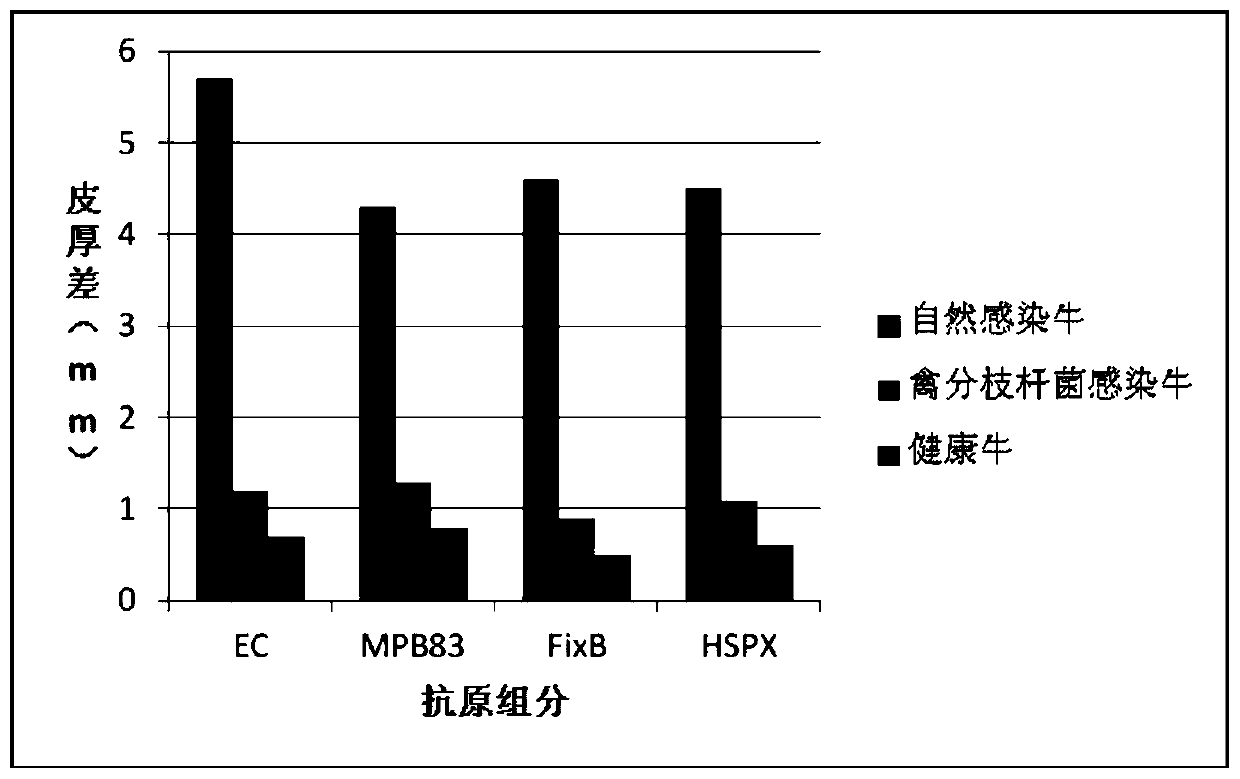
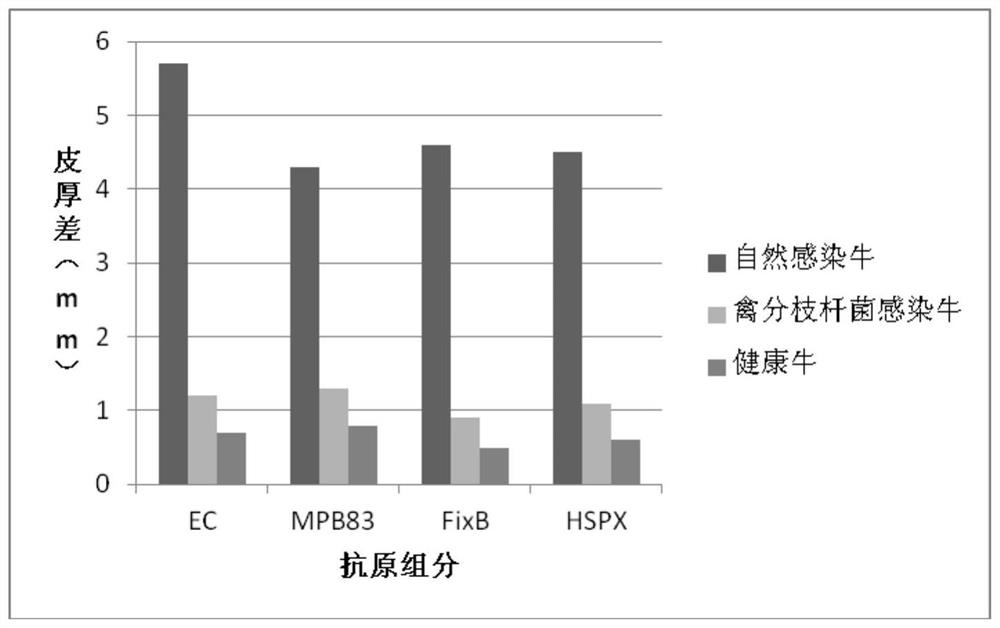
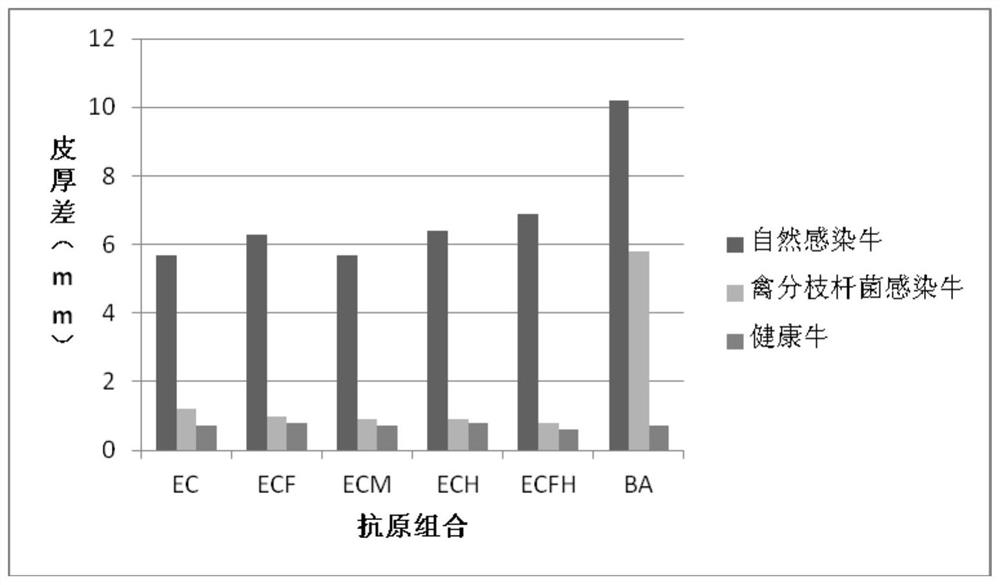
Cocktail antigen for detection of allergic reaction of bovine tuberculosis
ActiveCN109776662AEliminate infectionEasy to detectDepsipeptidesBiological testingAntigenAllergic transfusion reaction
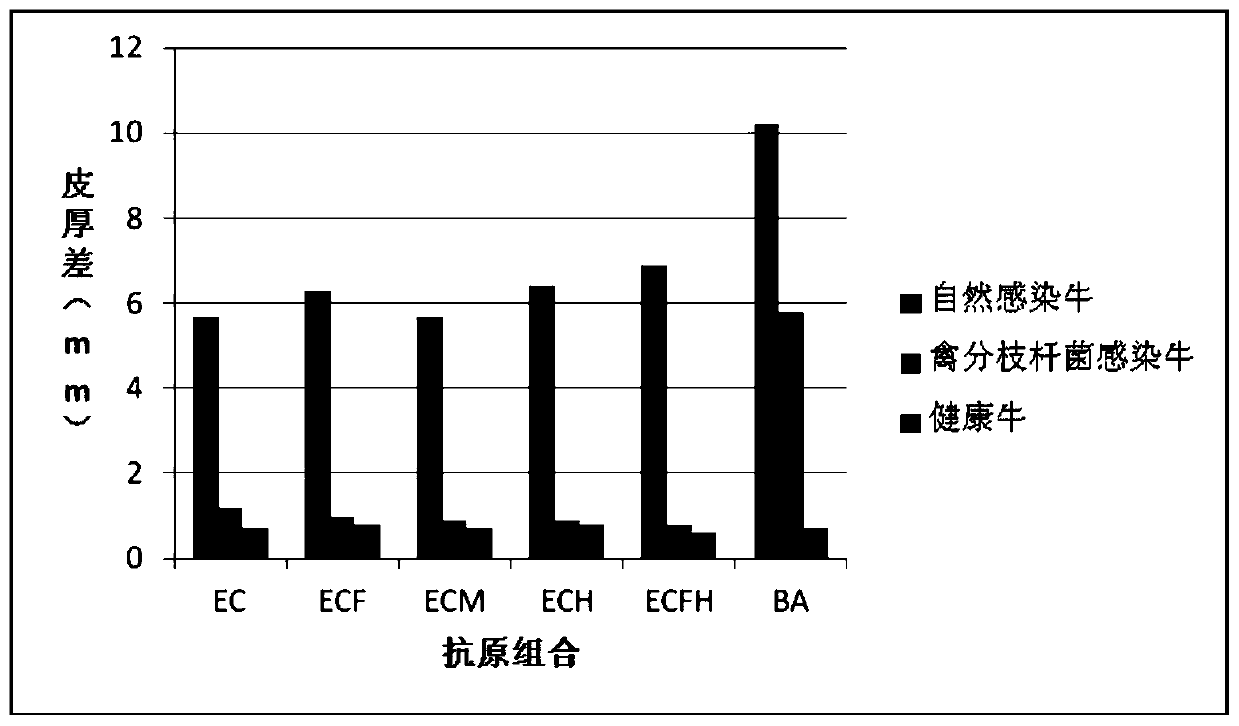
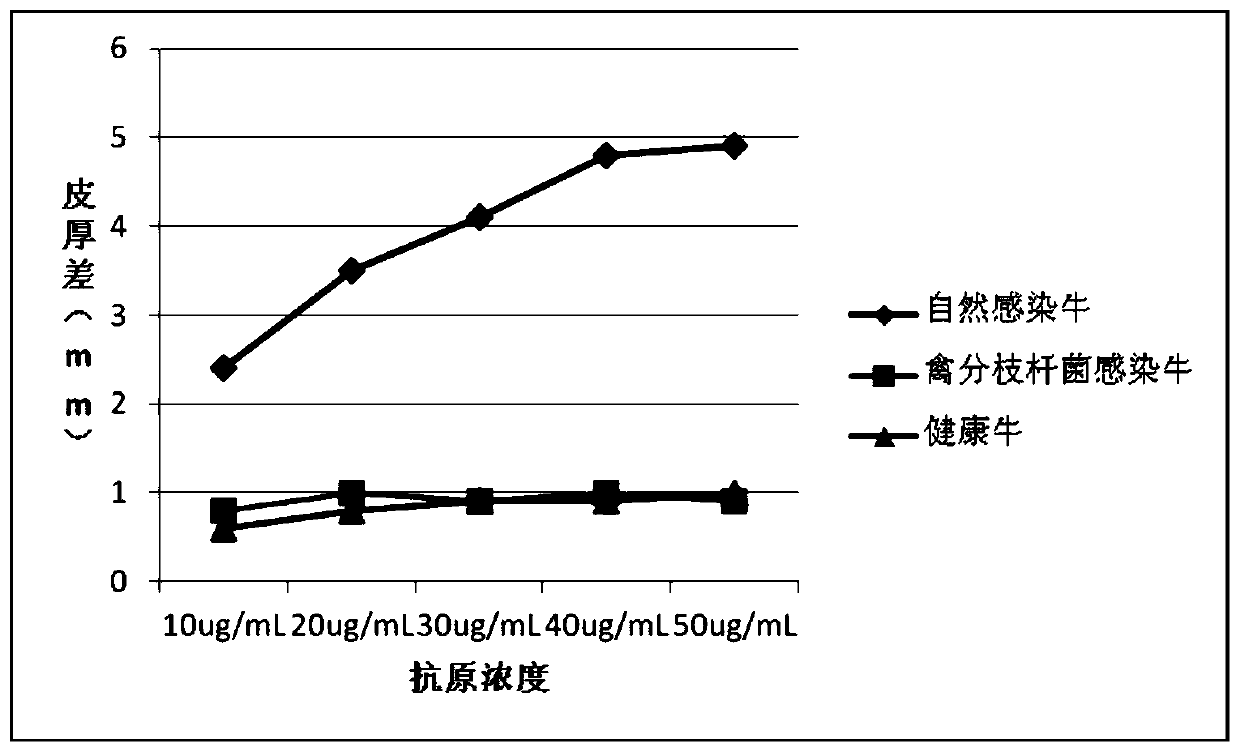
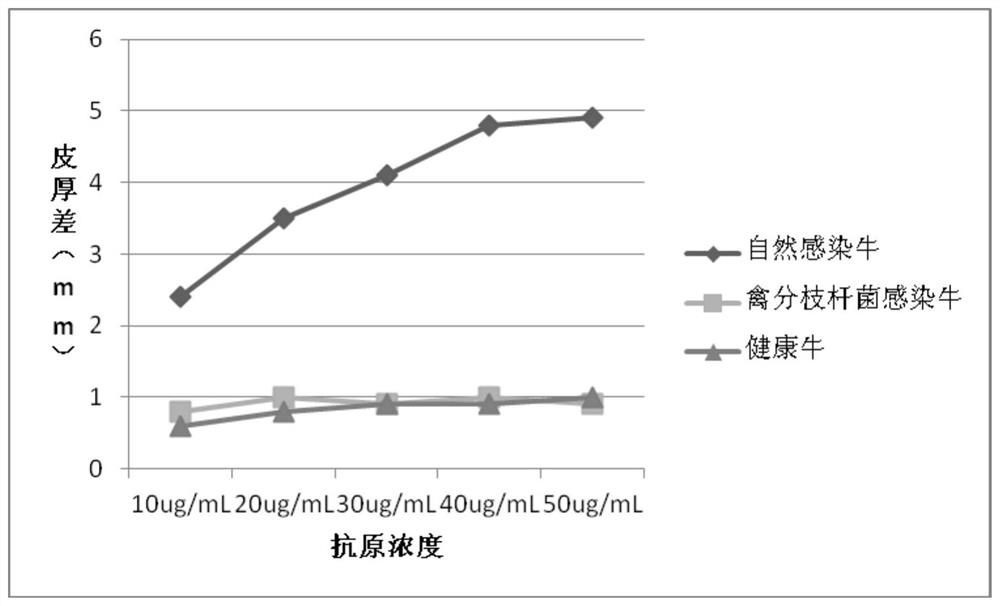
The invention provides a cocktail antigen for detection of an allergic reaction of bovine tuberculosis. The cocktail antigen comprises an ESAT6 antigen, a CFP10 antigen, an FixB antigen and an HSPX antigen, wherein the mass ratio of the ESAT6 antigen to the CFP10 antigen to the FixB antigen to the HSPX antigen is 2:2:1:5:1.5. The cocktail antigen is used for preparing a detection antigen of the allergic reaction of the bovine tuberculosis. The cocktail antigen can replace bovine type PPD and poultry type PPD used for comparing the allergic reaction, a single intradermal allergic reaction is carried out by using the cocktail antigen to achieve the effect of comparing the allergic reaction, a series of operation such as two-point shearing, measurement of the skin thickness, injection, re-testing of the skin thickness after 72 hours and calculation of a skin thickness difference are simplified into single-point operation and do not need calculation, the whole detection process is simplerand more convenient; meanwhile, the infection of poultry type mycobacteria and other non-specific mycobacteria can be excluded, and the bovine tuberculosis is diagnosed more specially.
Owner:CHINA ANIMAL HEALTH & EPIDEMIOLOGY CENT
miRNA markers related to auxiliary diagnosis of myocardial fibrosis diseases and application of miRNA markers
ActiveCN112813158ATimely diagnosisSpecific diagnosisMicrobiological testing/measurementPharmaceutical active ingredientsDiseaseDrug target
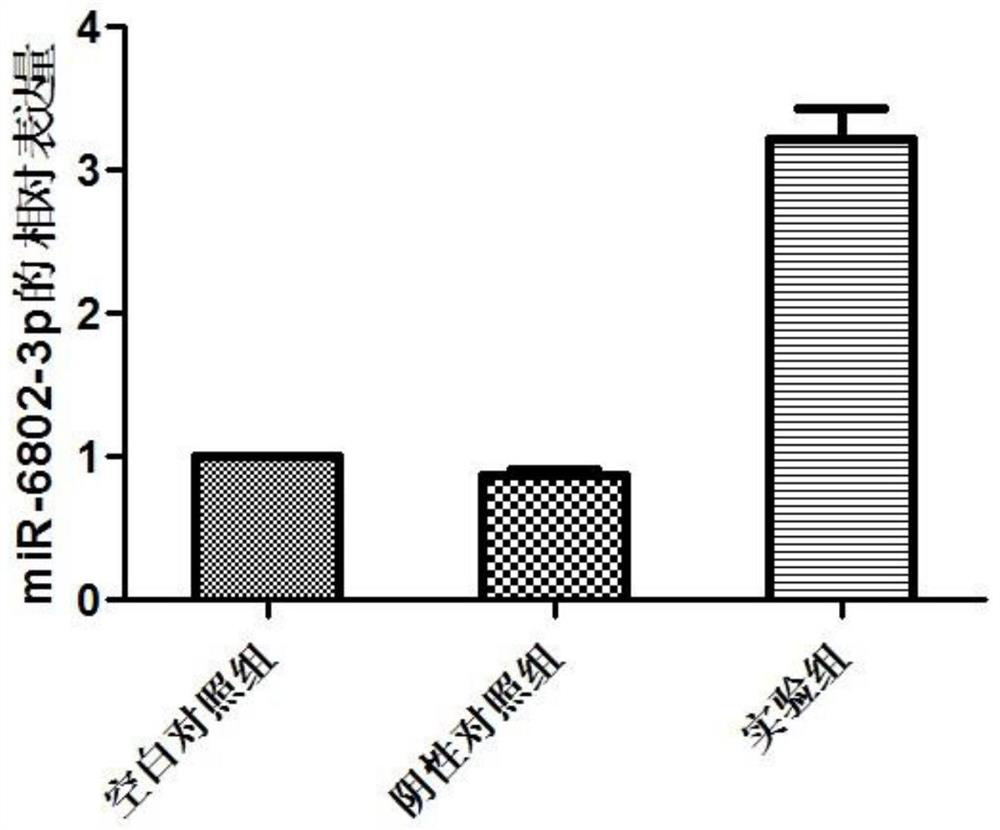
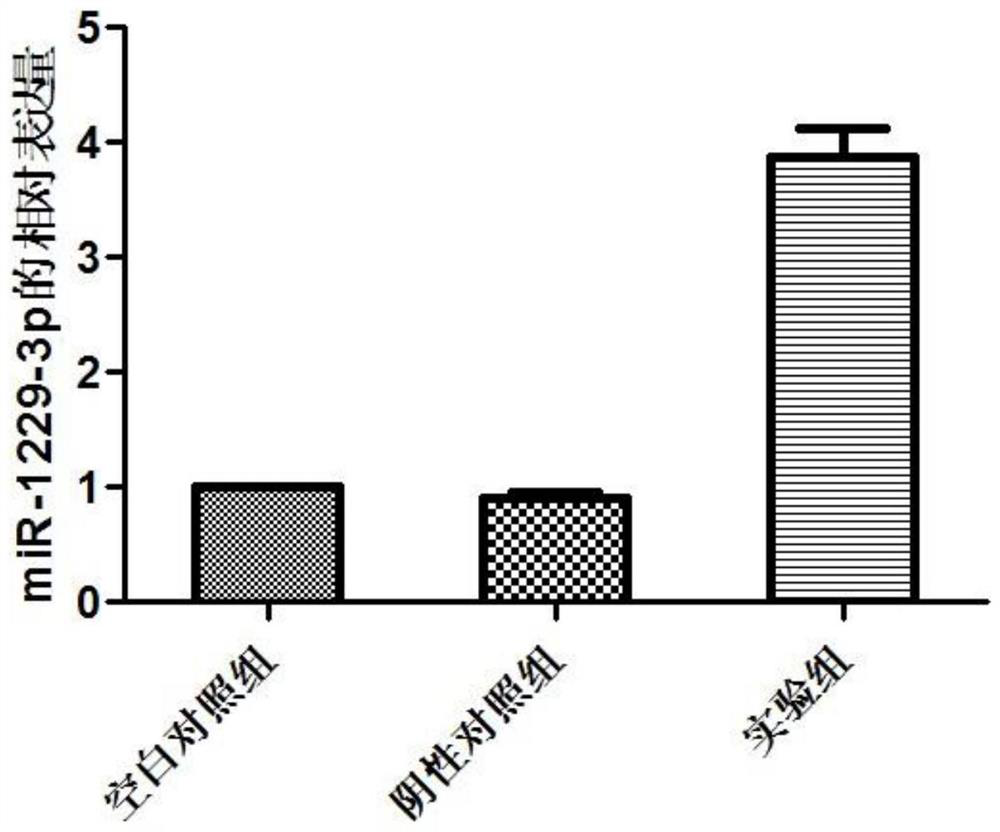
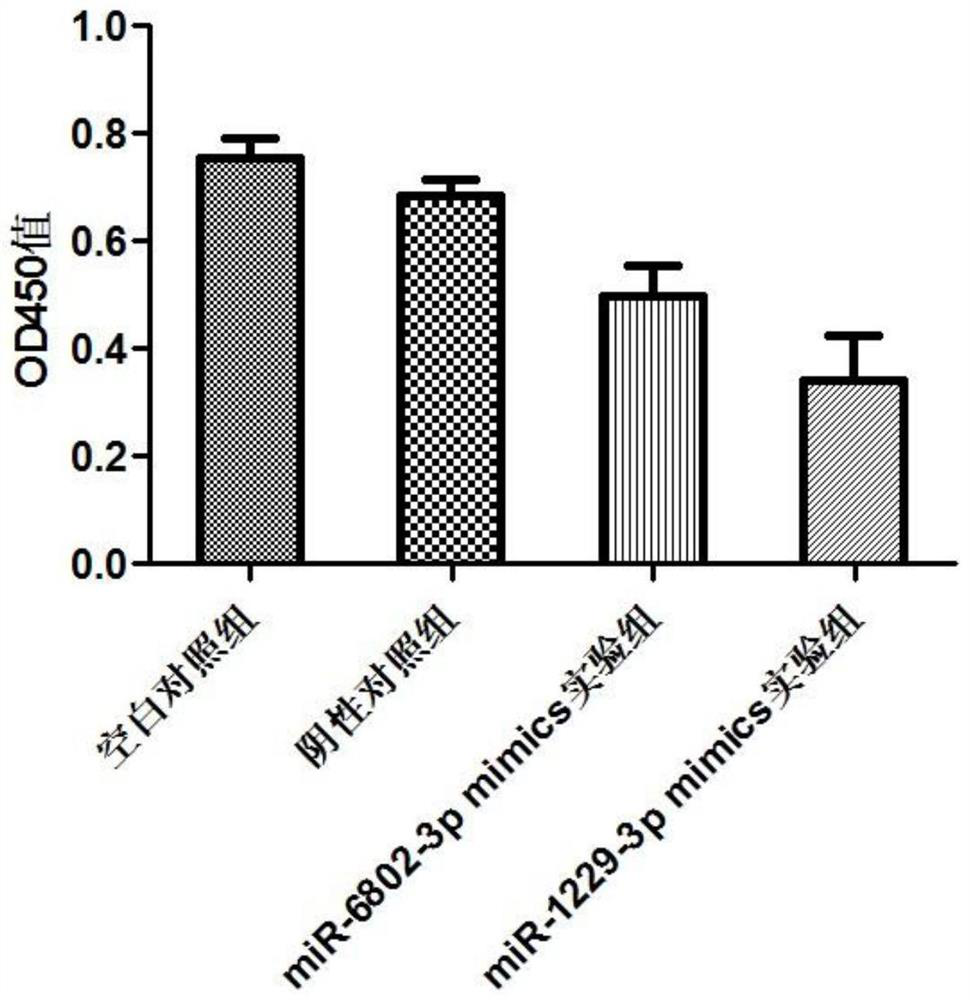
The invention discloses miRNA markers related to auxiliary diagnosis of myocardial fibrosis diseases and application of the miRNA markers. Hsa-miR-6802-3p and hsa-miR-1229-3p which are differentially expressed in patients suffering from myocardial fibrosis diseases are screened out by a high-throughput sequencing analysis technology, further cell experiments verify that the miRNA biomarkers hsa-miR-6802-3p and hsa-miR-1229-3p can be used in clinical auxiliary diagnosis of myocardial fibrosis, have important significance in early diagnosis of myocardial fibrosis, provide drug targets for clinical preparation of drugs for myocardial fibrosis, and are helpful for realizing targeted therapy of myocardial fibrosis.
Owner:SHIJIAZHUANG PEOPLES HOSPITAL
Application of PCDH18 gene to preparation of colorectal cancer diagnosis kit and kit
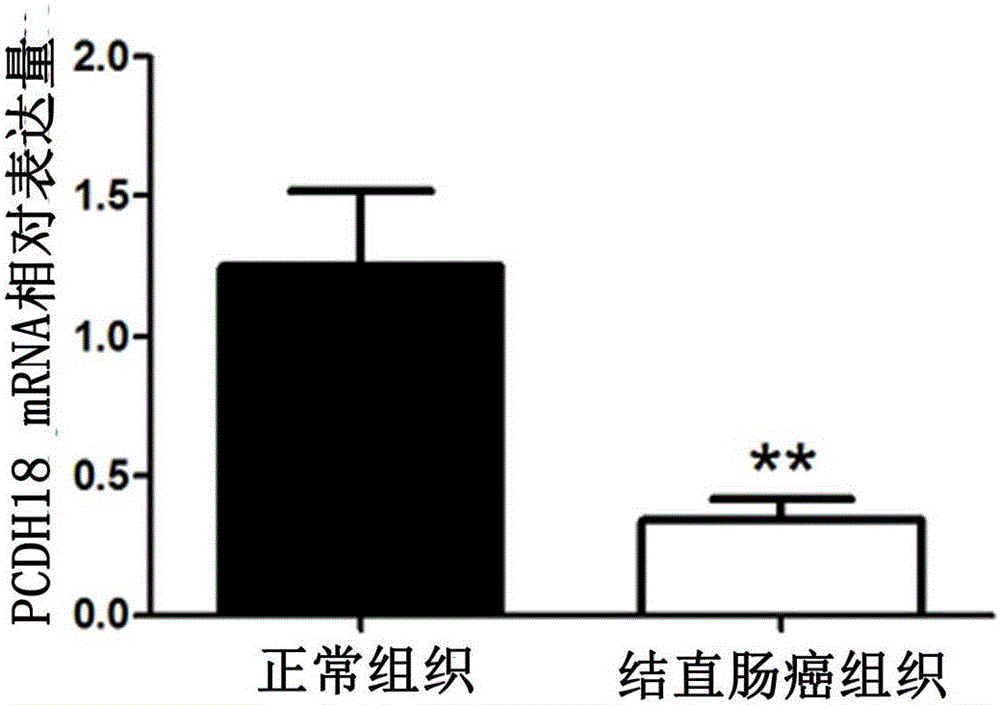
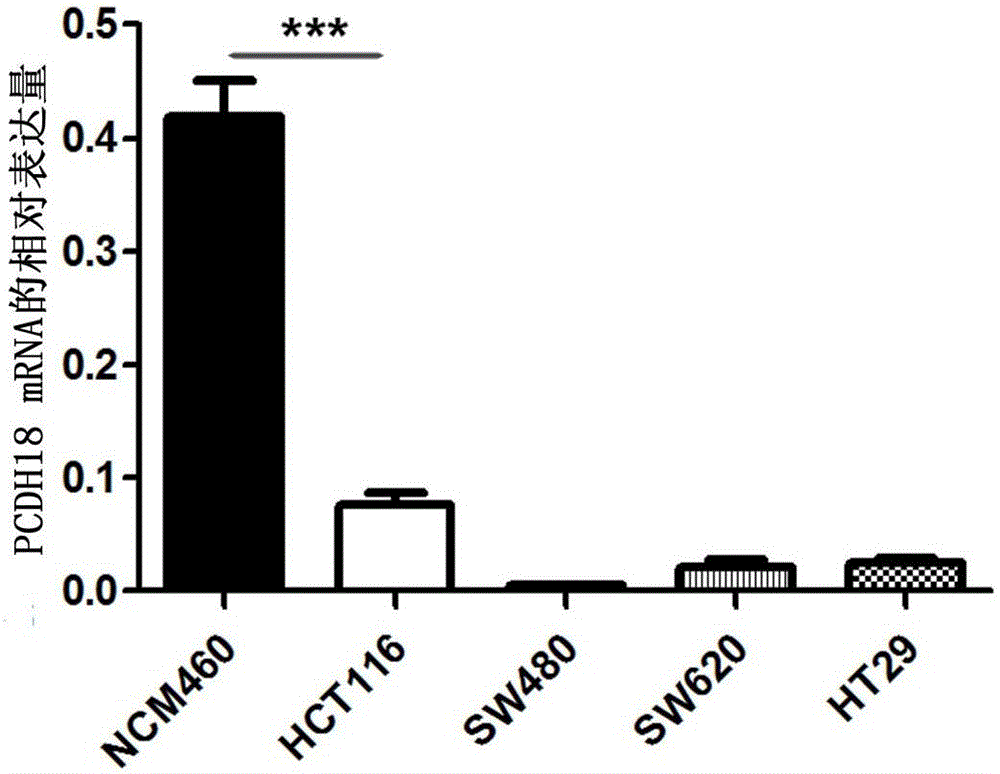
ActiveCN105755153ATimely diagnosisImprove 5-year survival rateMicrobiological testing/measurementBiological material analysisFive-year survival rateColorectal cancer
The invention provides application of a colorectal cancer diagnosis molecular marker, namely, a PCDH18 gene which is good in specificity and sensitivity in preparing a colorectal cancer diagnosis kit, and the colorectal cancer diagnosis kit. The application has the main benefits that a novel molecular marker of colorectal cancer is discovered, application of the PCDH18 gene to preparation of the colorectal cancer diagnosis kit and the colorectal cancer diagnosis kit are provided, and compared with a conventional detection method, the molecular marker is relatively timely and specific in diagnosis, so that the five-year survival rate of patients suffering from colorectal cancer can be increased, the death rate is reduced, and wide application prospects can be achieved.
Owner:XIAMEN INST OF RARE EARTH MATERIALS
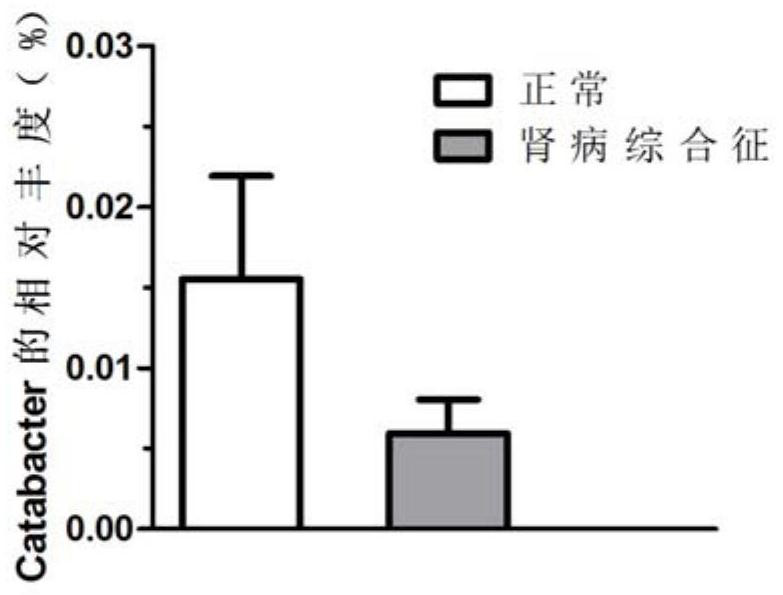
Enterobacteriaeae Catabacter pertinent to nephrotic syndromes, and application of enterobacteriaeae Catabacter pertinent to nephrotic syndromes
PendingCN111647652ADownregulation of abundanceImprove the quality of lifeMicrobiological testing/measurementDNA/RNA fragmentationNephrosisCatabacter
The invention discloses enterobacteriaeae Catabacter pertinent to nephrotic syndromes. The inventor of the invention finds that the enterobacteriaeae Catabacter is pertinent to the nephrotic syndromesfor the first time, the abundance of the enterobacteriaeae Catabacter in the nephrotic syndromes is notably reduced, which indicates that the enterobacteriaeae Catabacter can be used as a microorganism marker for diagnosing the nephrotic syndromes, and further individualized treatment is realized.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
A Cocktail Antigen Used in Detection of Bovine Tuberculosis Allergic Reaction
ActiveCN109776662BEliminate infectionEasy to detectDepsipeptidesBiological testingAntigenAllergic transfusion reaction
Owner:CHINA ANIMAL HEALTH & EPIDEMIOLOGY CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com