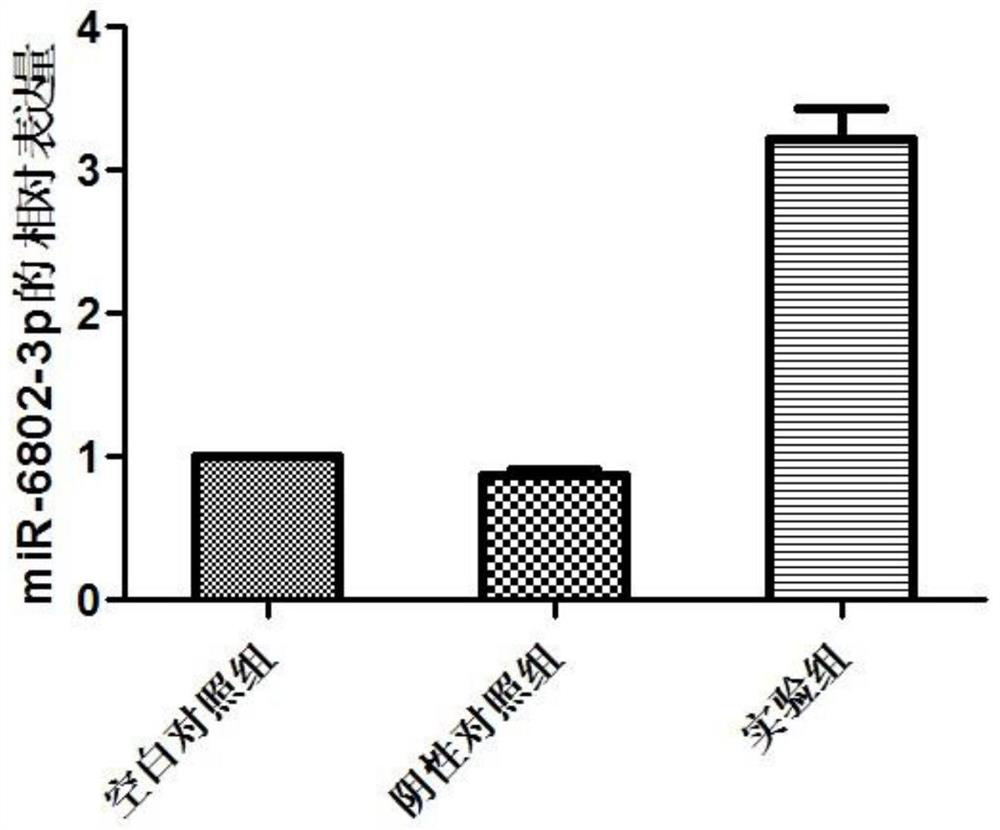
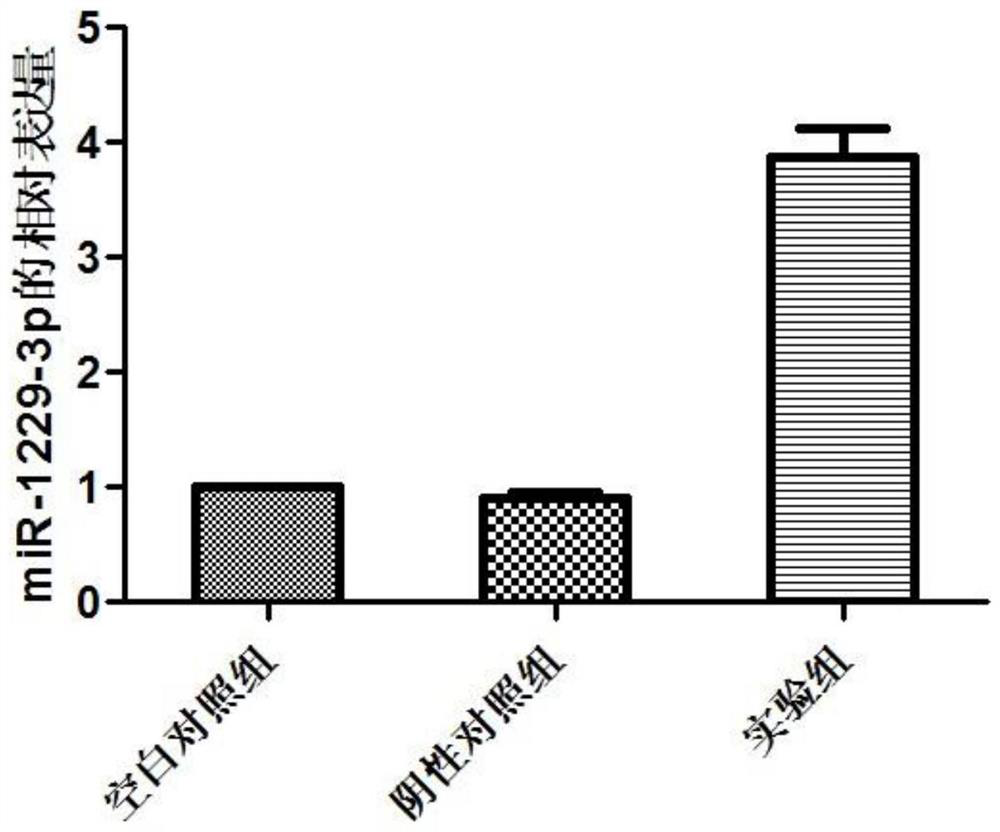
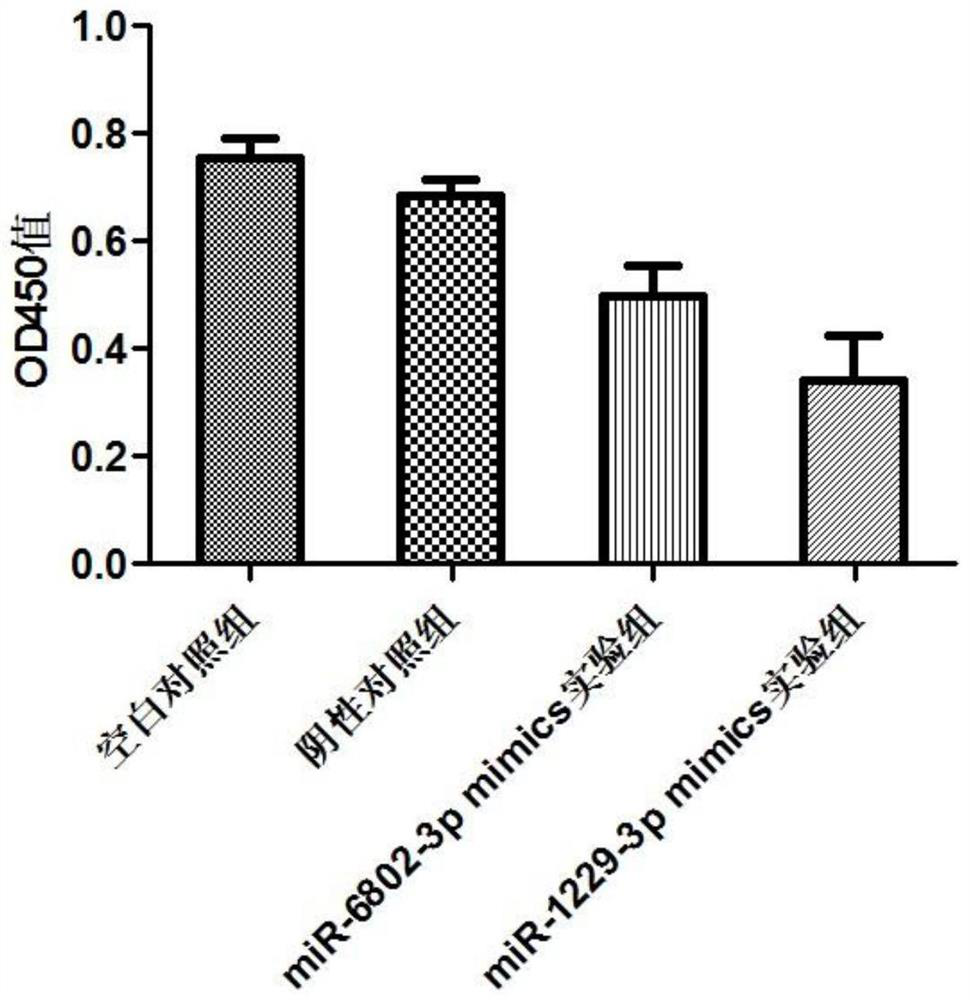
miRNA markers related to auxiliary diagnosis of myocardial fibrosis diseases and application of miRNA markers
A myocardial fibrosis and drug technology, applied in the field of biomedicine, can solve the problem of myocardial fibrosis disease correlation that has not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1 Screening biomarkers related to myocardial fibrosis
[0074] 1. Research object
[0075] Three cases of patients with myocardial fibrosis provided by the cardiology department of the hospital were collected, and their blood samples were collected respectively. At the same time, three cases of healthy people were collected as healthy controls, and their blood samples were collected respectively. All patients were excluded from malignant tumors, acute infection, trauma and severe liver and kidney diseases, cerebral embolism, pulmonary embolism, lower extremity venous thrombosis, DIC and acute renal insufficiency and other diseases.
[0076] All research subjects were informed about this study and signed the informed consent form, which was approved by the organizational ethics committee of the hospital.
[0077] 2. Sequencing experiments
[0078] The sequencing experiments used the Illumina TruseqTM RNA sample prep Kit method for strand-specific library construc...
Embodiment 2
[0117] Example 2 Detection of the effects of miRNA on the proliferation and migration of cardiac fibroblasts
[0118] 1. Cell culture
[0119] The human primary cardiac fibroblasts (HCF) preserved in liquid nitrogen were taken out and resuscitated, inoculated in DMEM medium containing 10% fetal bovine serum, placed at 37°C, 95% humidity and 5% CO 2 Cells in the logarithmic growth phase were subcultured in a cell incubator for experiments.
[0120] 2. Digestion and passage of primary cardiac fibroblasts
[0121] When the fusion rate of the cells reaches about 80%-90% of the bottom area of the full culture dish and the culture medium turns yellow, suck up the liquid in the culture dish, add 4mL trypsin, put it in the incubator for digestion for 2min, take it out and put it in Observe under a microscope, after the cells retract and the intercellular space widens, quickly absorb the trypsin and add 2 mL of culture medium containing 10% fetal bovine serum to stop the digestion;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com