Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
186 results about "Epinephrine synthesis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
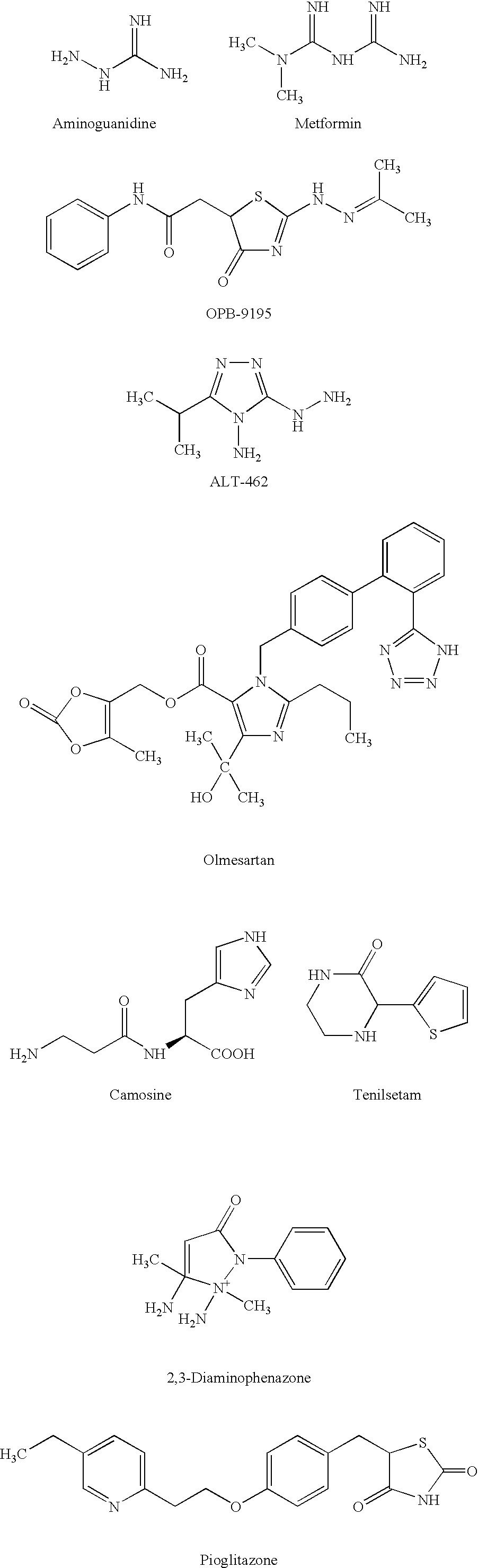
Epinephrine is synthesized in the medulla of the adrenal gland in an enzymatic pathway that converts the amino acid tyrosine into a series of intermediates and, ultimately, epinephrine. Tyrosine is first oxidized to L-DOPA, which is subsequently decarboxylated to give dopamine.
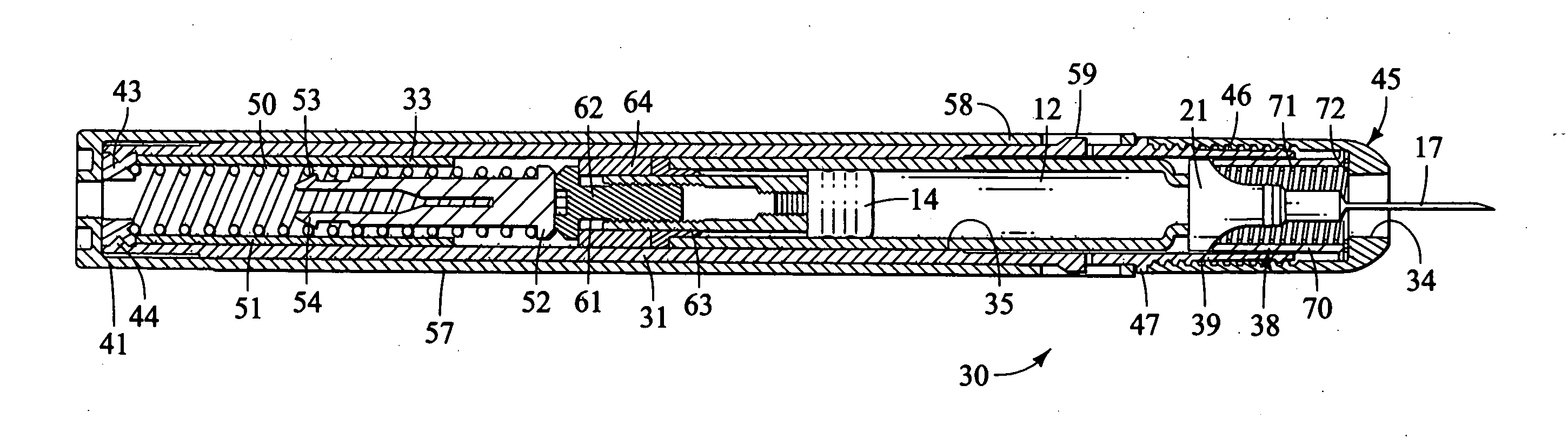
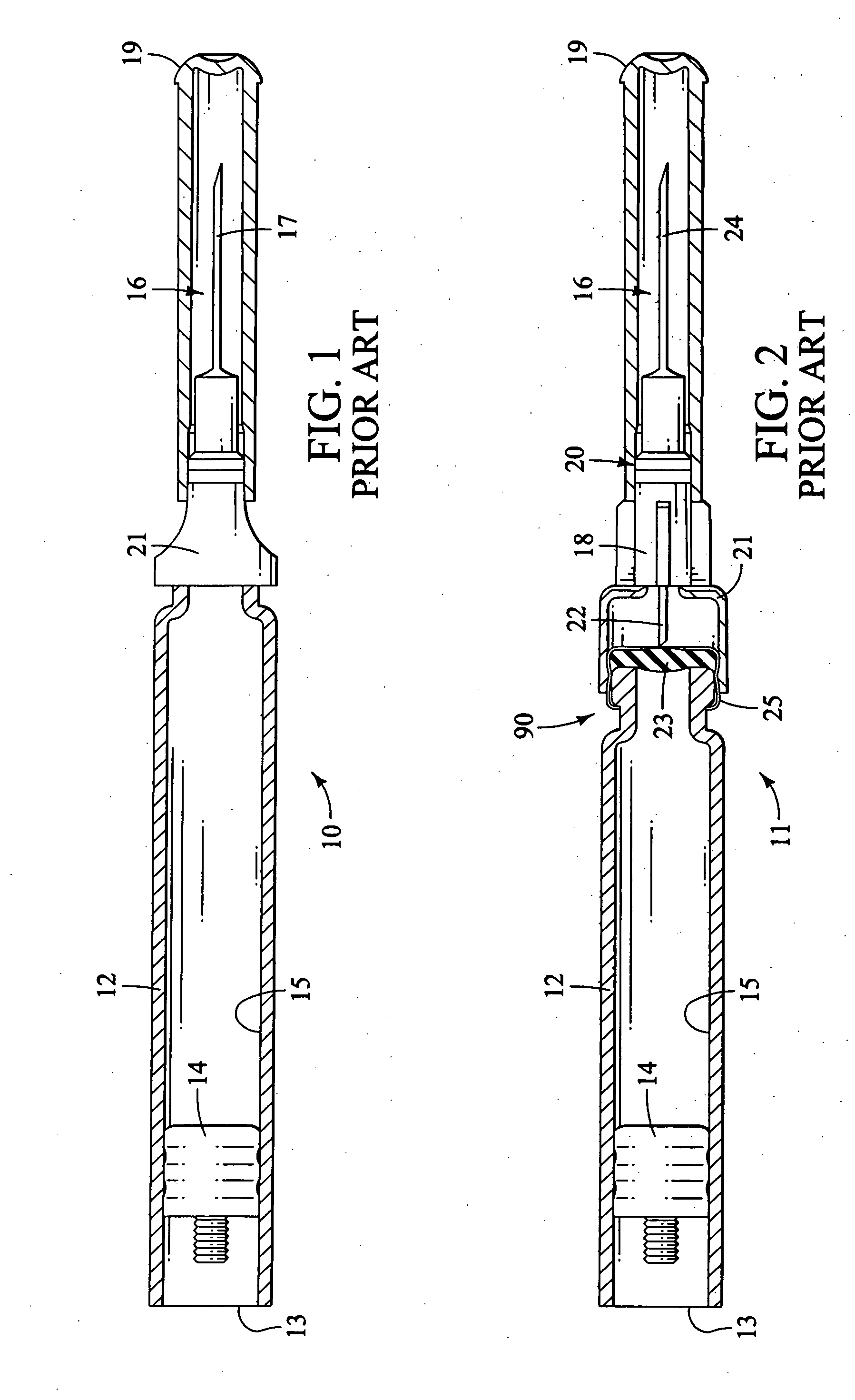
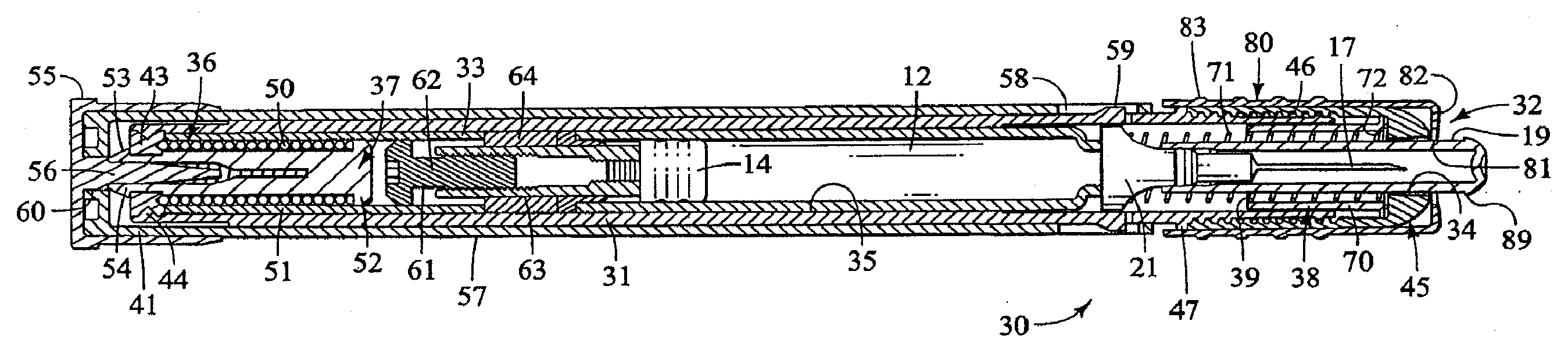
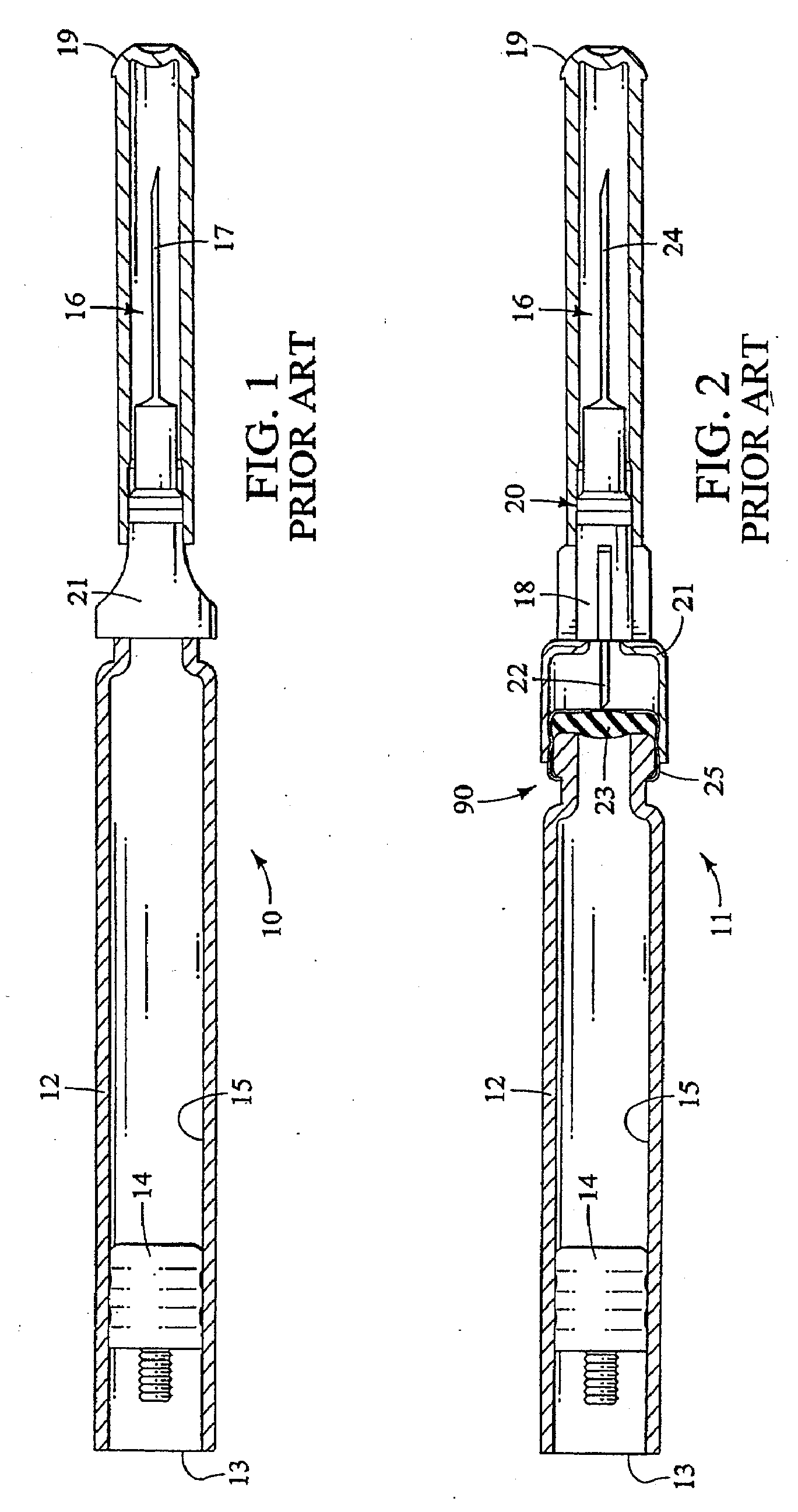
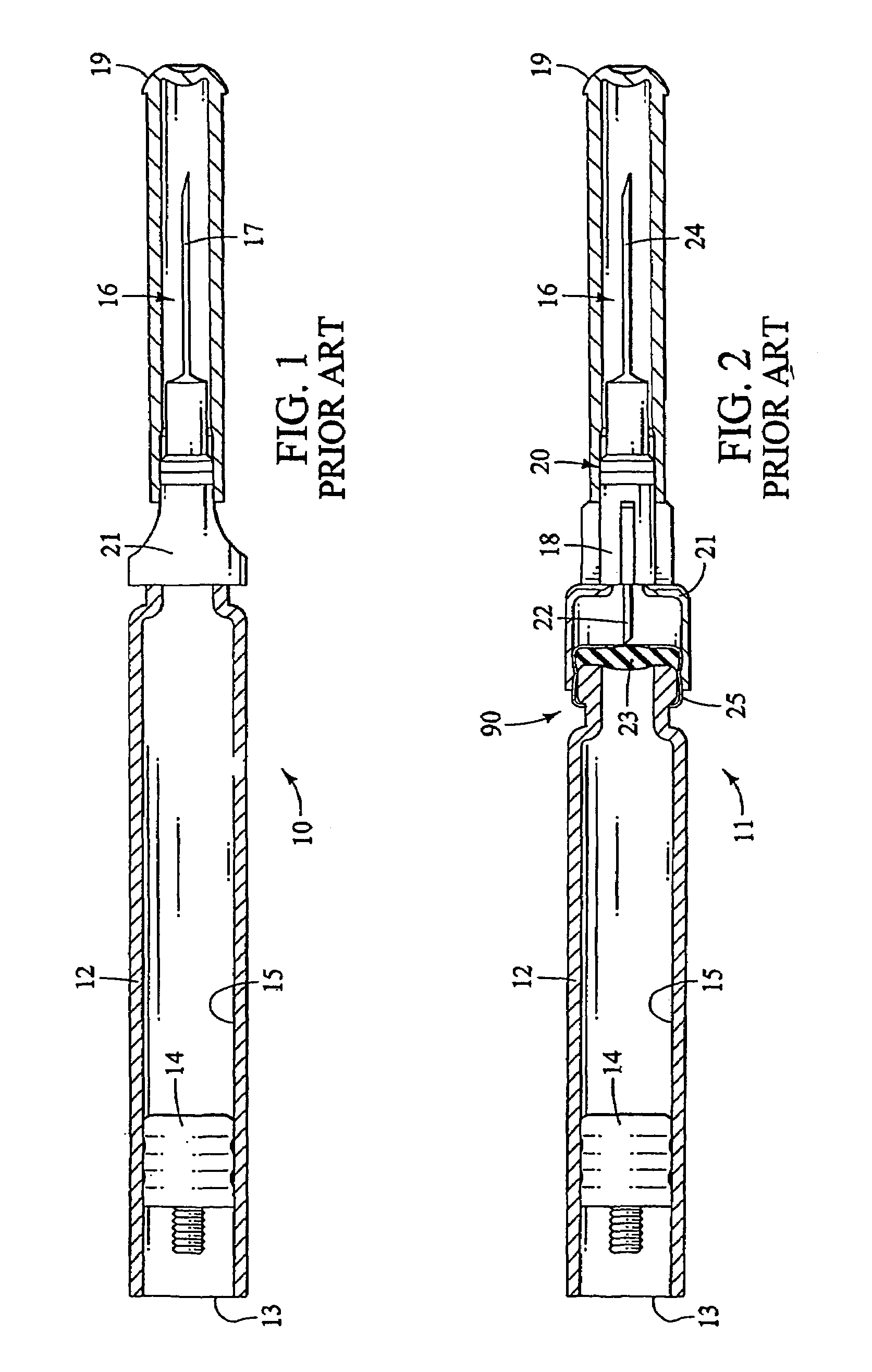
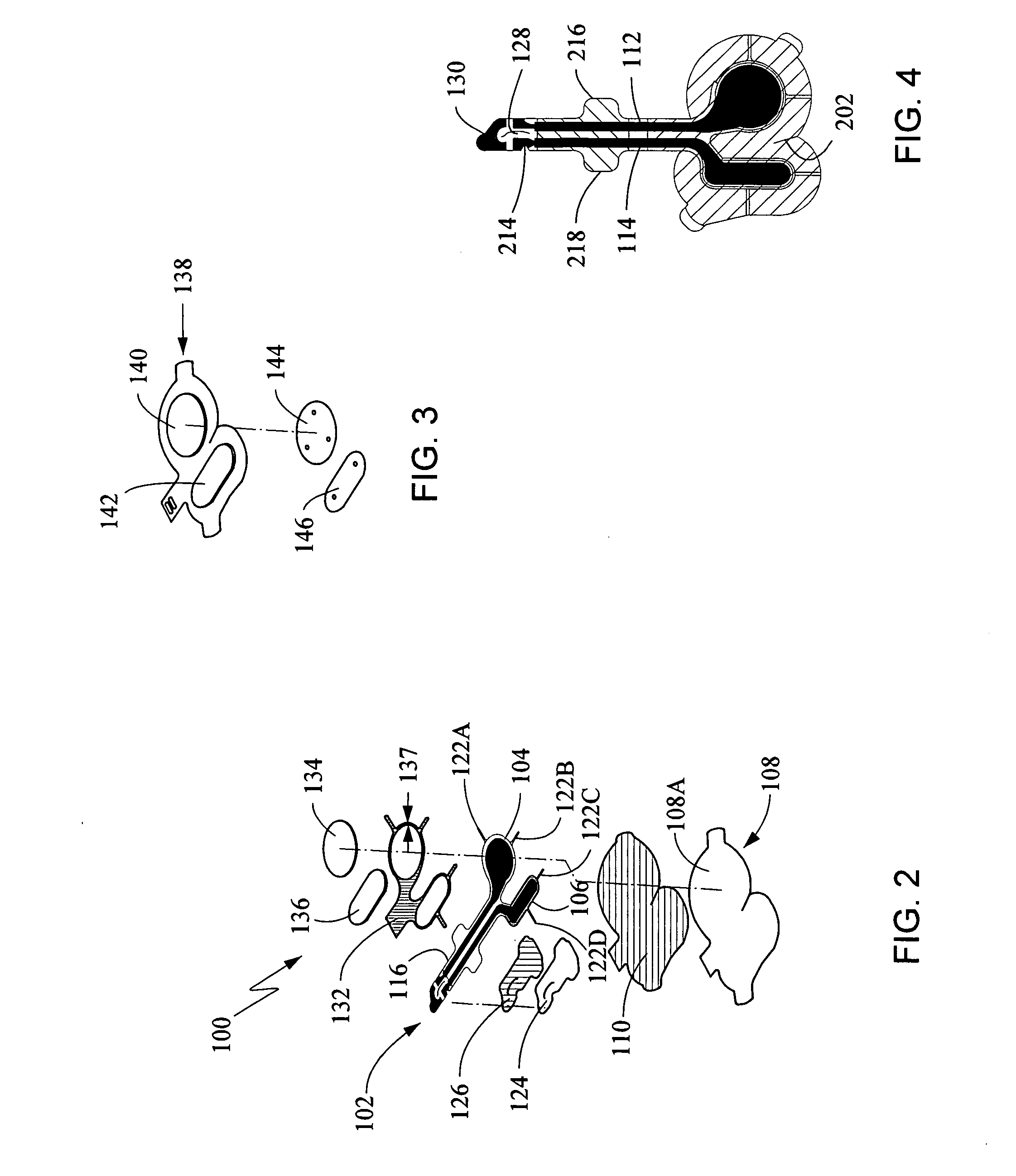
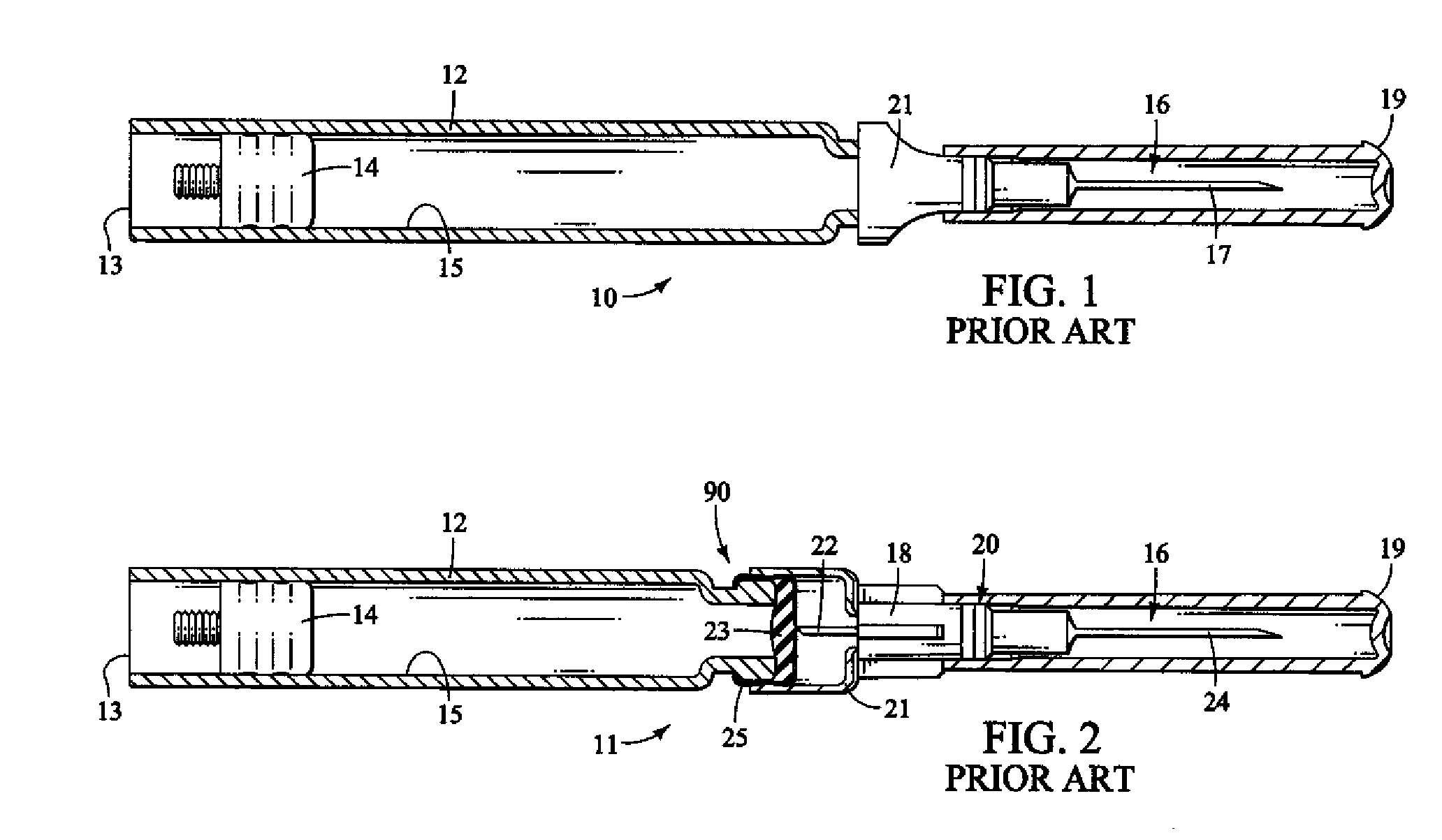
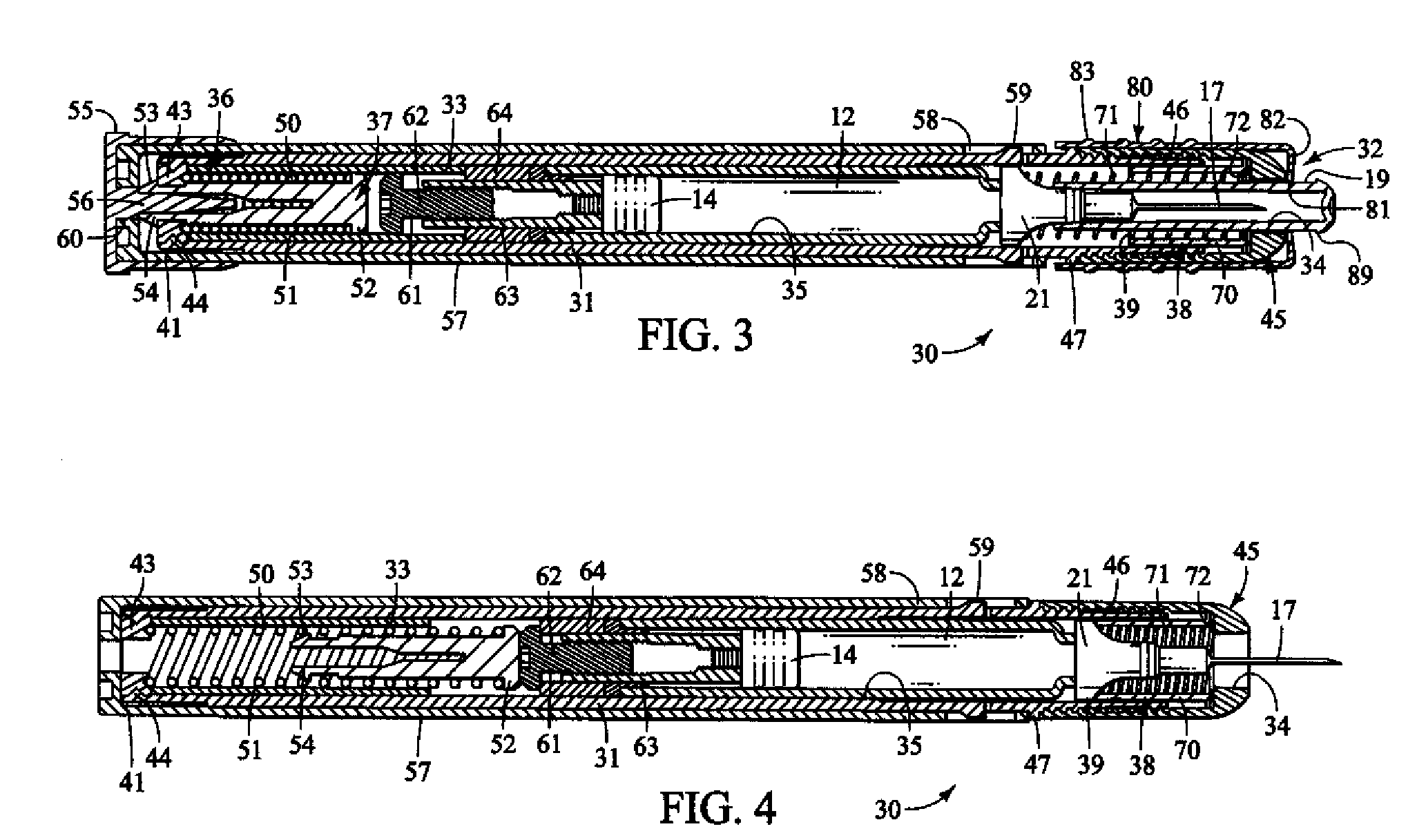
Method and apparatus for delivering epinephrine
A method of administering epinephrine to a patient includes administering a first dose by automatic injection followed by administering a second dose by manual injection. The first and second injections are administered using the same syringe. In some embodiments, the first and second injections provide the same volume of medicine to the patient. In particular embodiments, the first and second injections have volumes of 0.15 or 0.3 ml.
Owner:VERUS PHARMA +1
Method and apparatus for delivering epinephrine
A method of administering liquid medicine such as epinephrine to a patient includes administering a first dose followed by administering an optional second dose is described herein. Also described herein are devices useful for carrying out the methods described and kits containing these devices.
Owner:WASHINGTON BIOTECH CORP
Injector device
An injector device for the percutaneous injection of fluids, such as medications, is disclosed. The device can have a mobile phone case. The mobile phone case can hold an auto-injector cartridge having a trigger, spring-loaded needle and reservoir holding a medication such as epinephrine or insulin. The device mobile phone case can communicate injection data to the phone. The phone can communicate the injection data to an emergency service provider.
Owner:FRIEDMAN STEVEN A
Epinephrine formulations
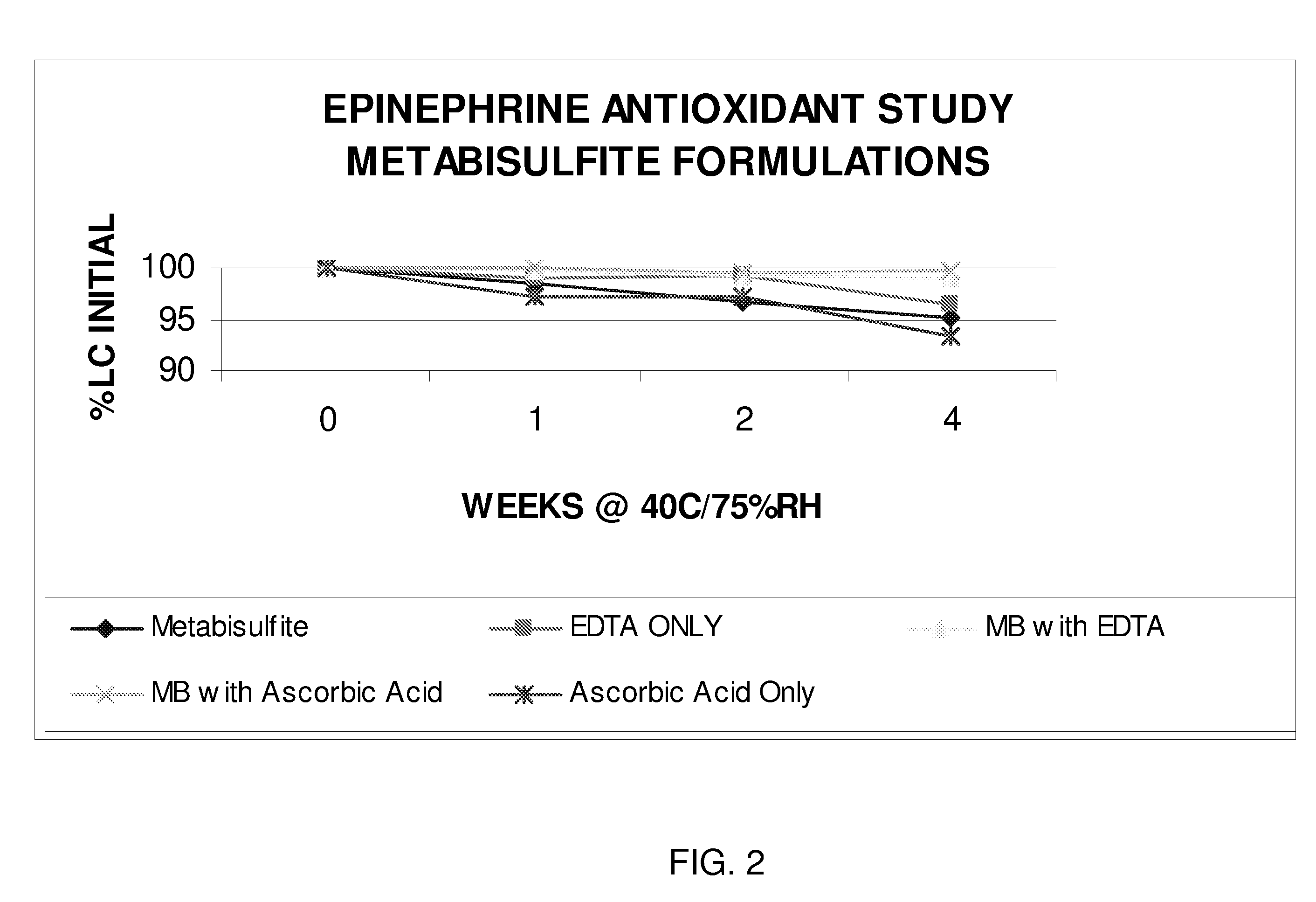
InactiveUS20080269347A1Improve stabilityBiocideOrganic active ingredientsCardiorespiratory arrestAntioxidant
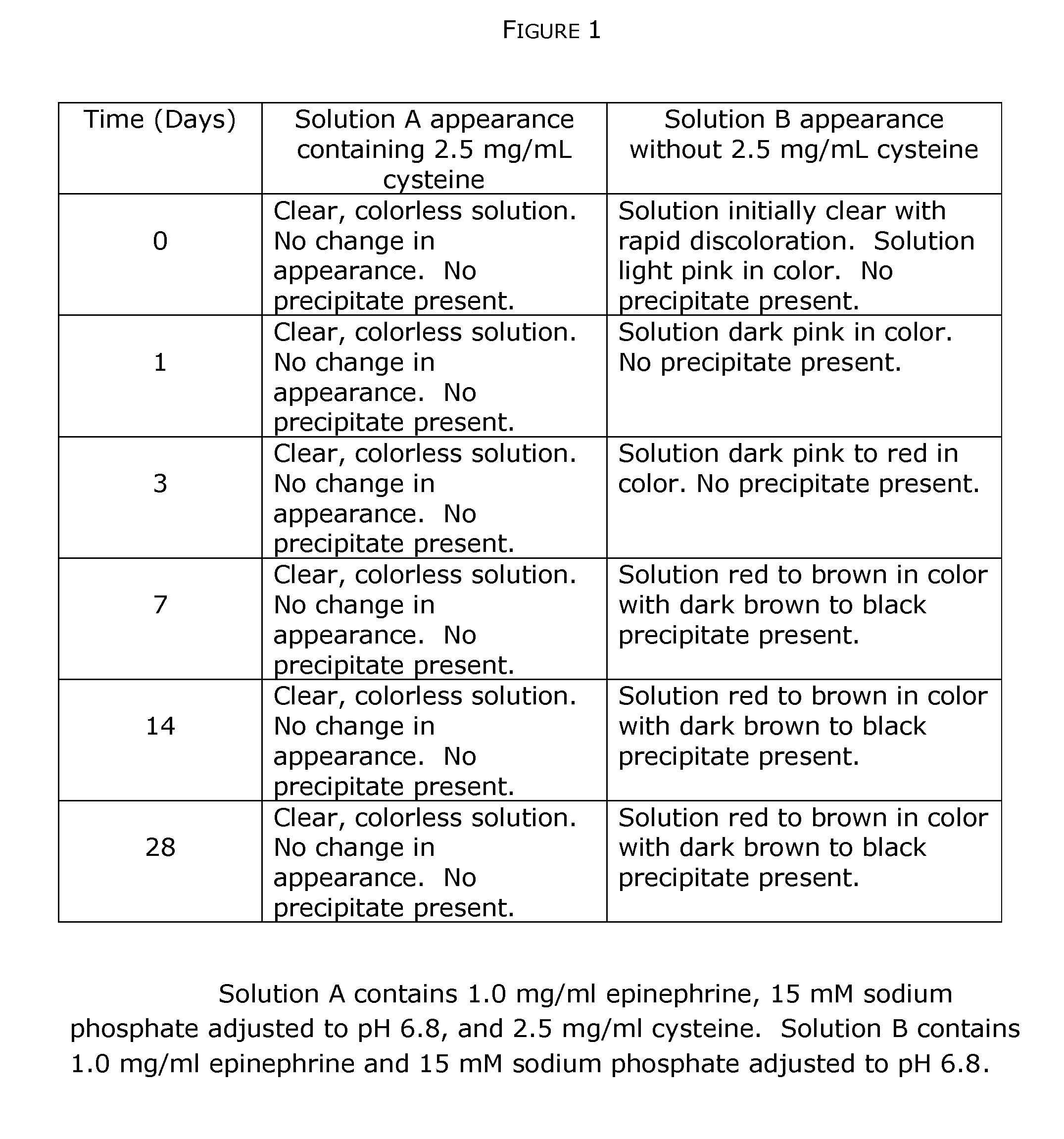
The present invention generally concerns an epinephrine formulation that has enhanced stability. In particular embodiments, the formulation is an injectable formulation. In specific aspects, the formulation comprises epinephrine, EDTA, and one or more of an antioxidant such as cysteine, citric acid, acetylcysteine, or thioglycerol. The formulations are suitable for any medical condition that is in need of epinephrine, although in specific embodiments the medical condition is anaphylaxis, asthma, or cardiac arrest.
Owner:UNION SPRINGS PHARMA
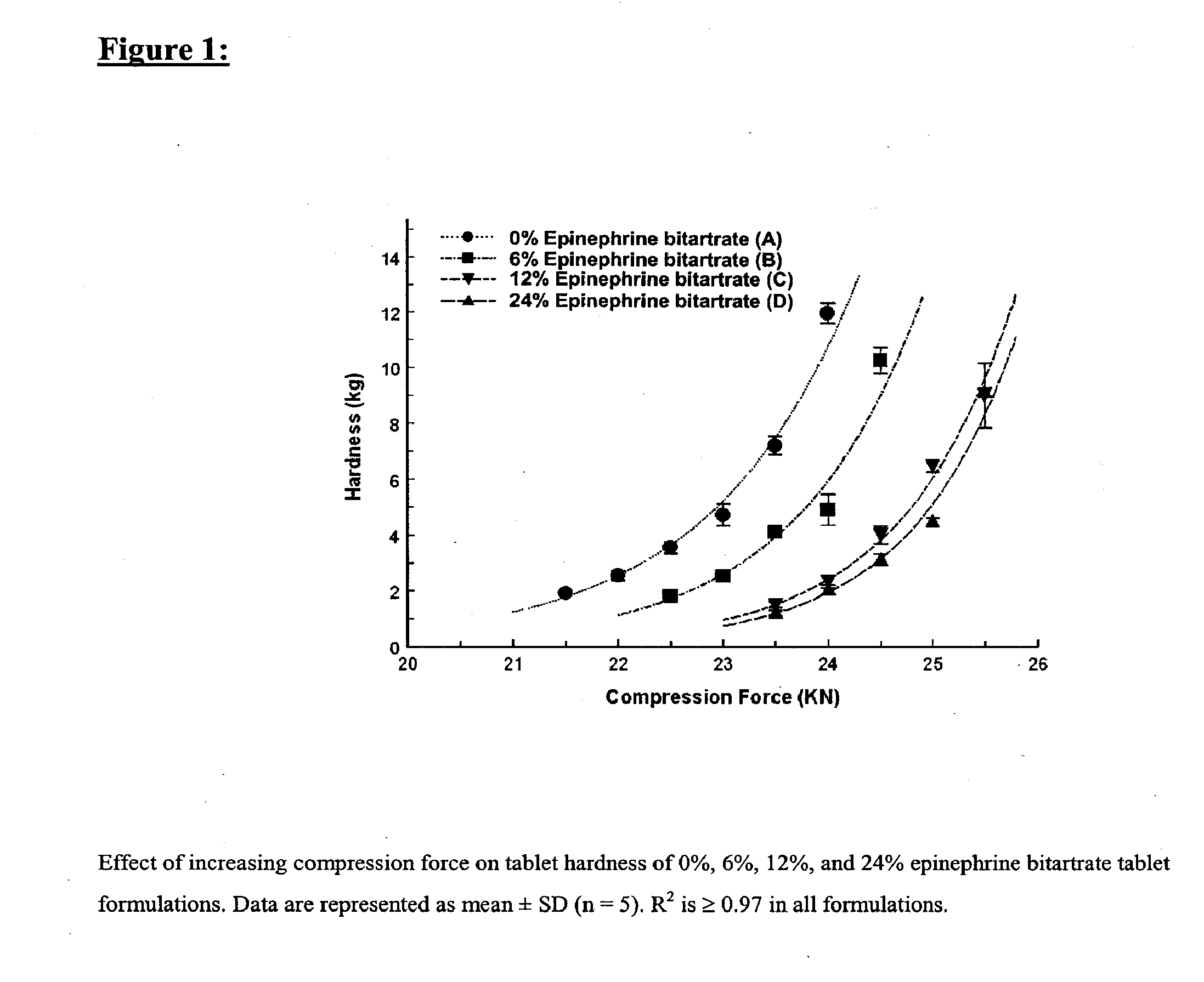
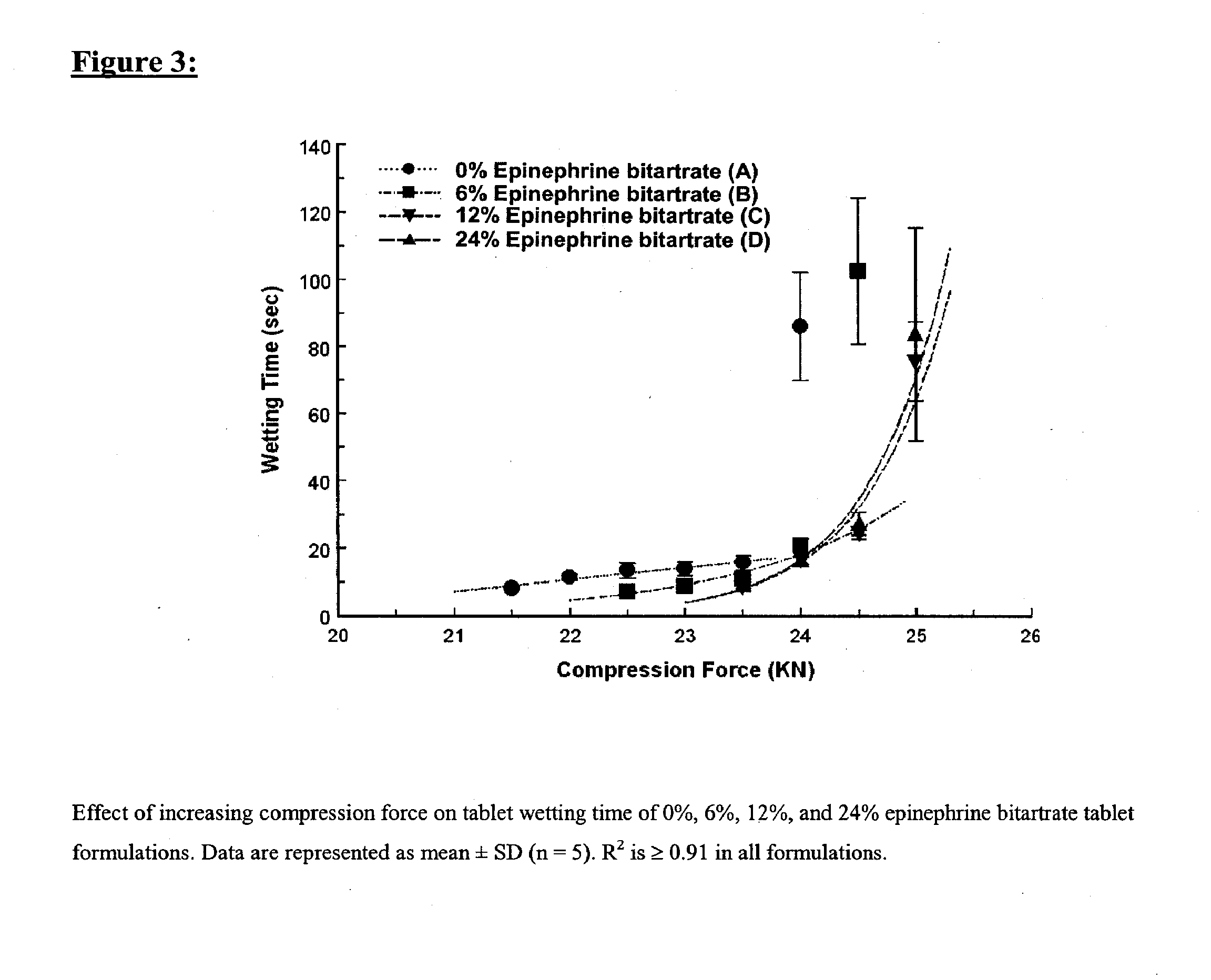
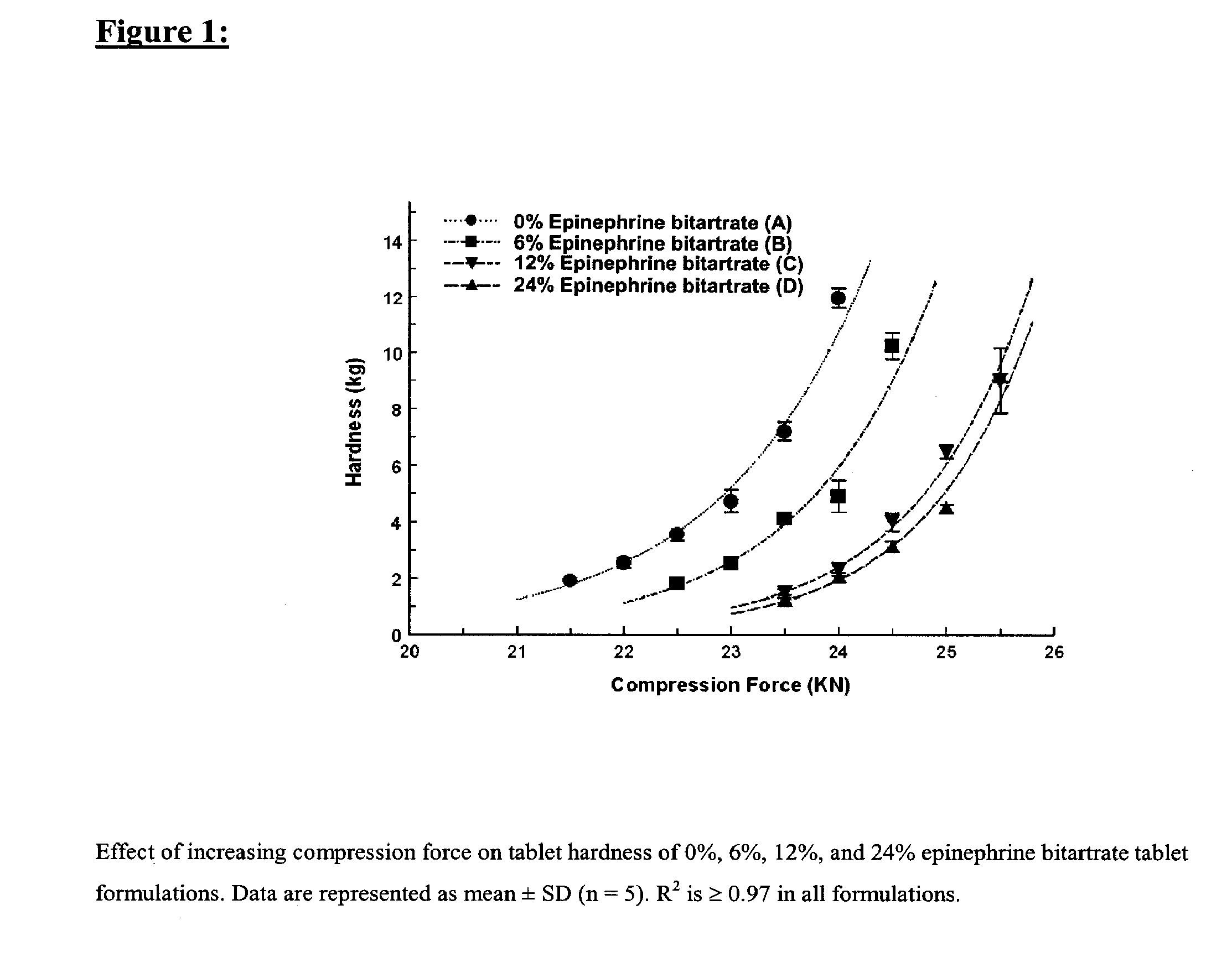
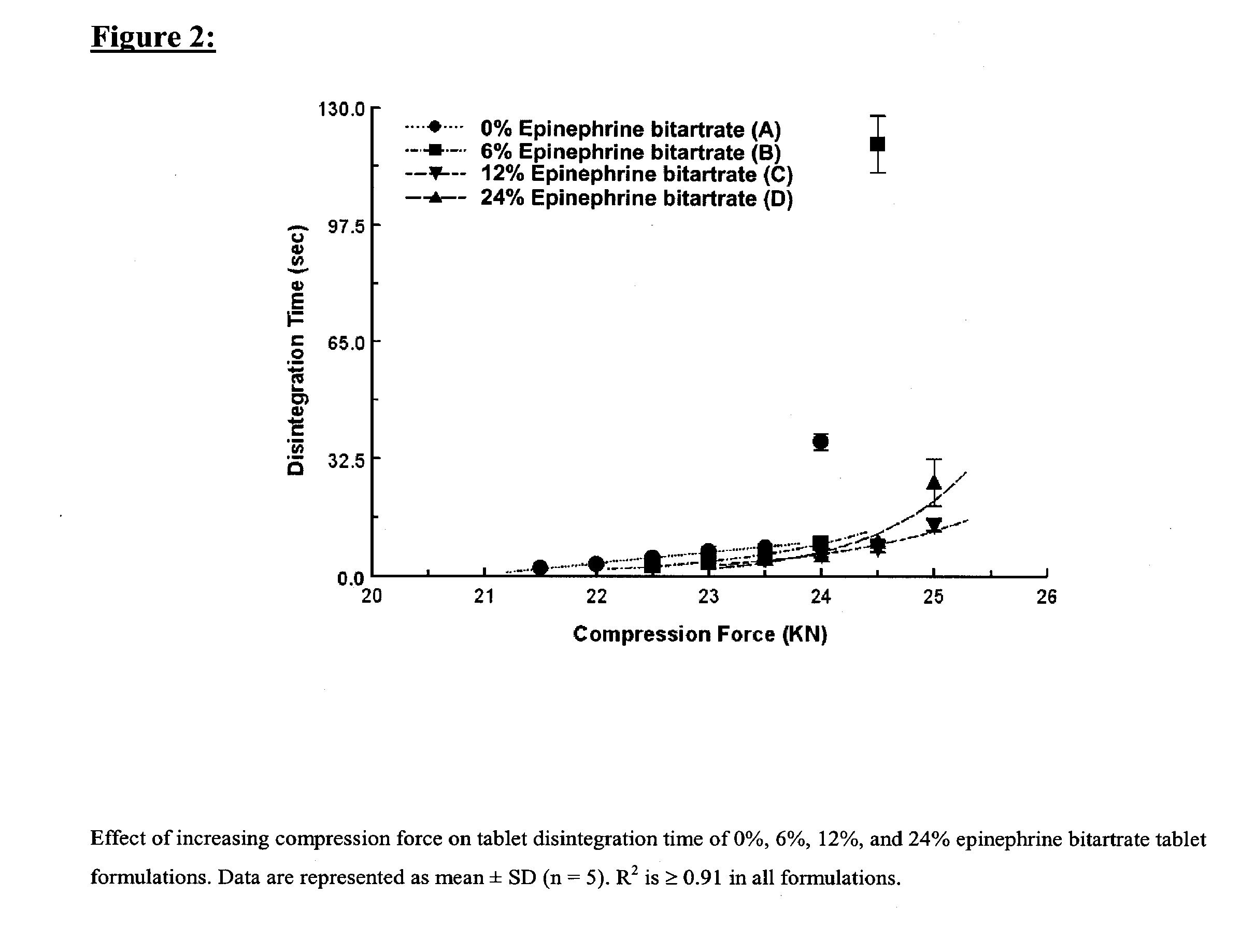
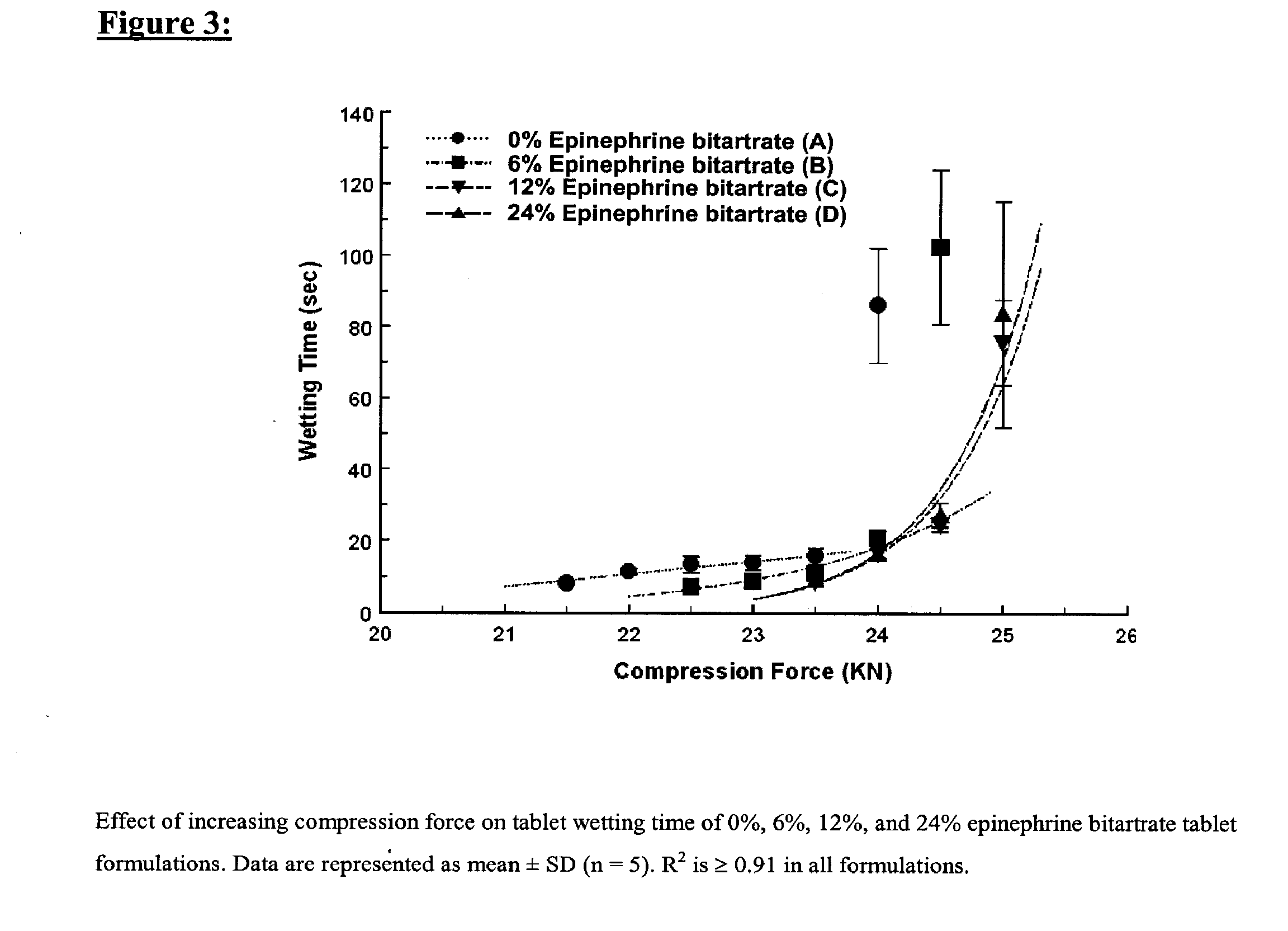
Fast-disintegrating epinephrine tablets for buccal or sublingual administration
Described herein are formulations for fast-disintegrating epinephrine tablets which can be prepared for buccal or sublingual administration, wherein the fast-disintegrating epinephrine tablets can produce plasma epinephrine concentrations substantially equivalent to those achieved by traditional injectable dosage forms comprising epinephrine injected either subcutaneously or intramuscularly.
Owner:UNIVERSITY OF MANITOBA
Method and apparatus for delivering epinephrine
A method of administering liquid medicine such as epinephrine to a patient includes administering a first dose followed by administering an optional second dose is described herein. Also described herein are devices useful for carrying out the methods described and kits containing these devices.
Owner:WASHINGTON BIOTECH CORP
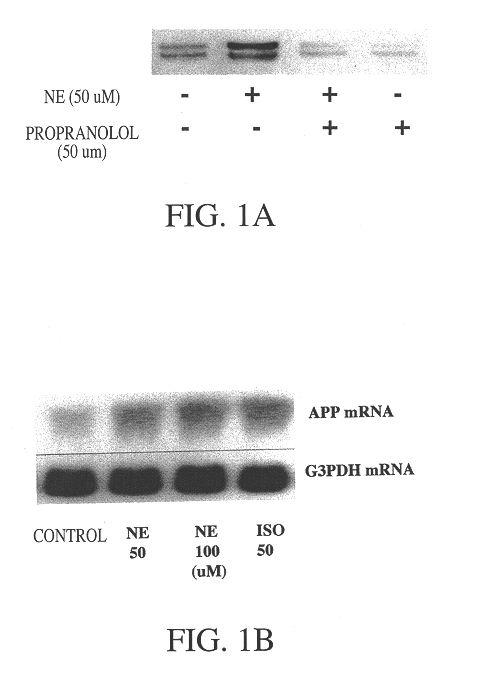
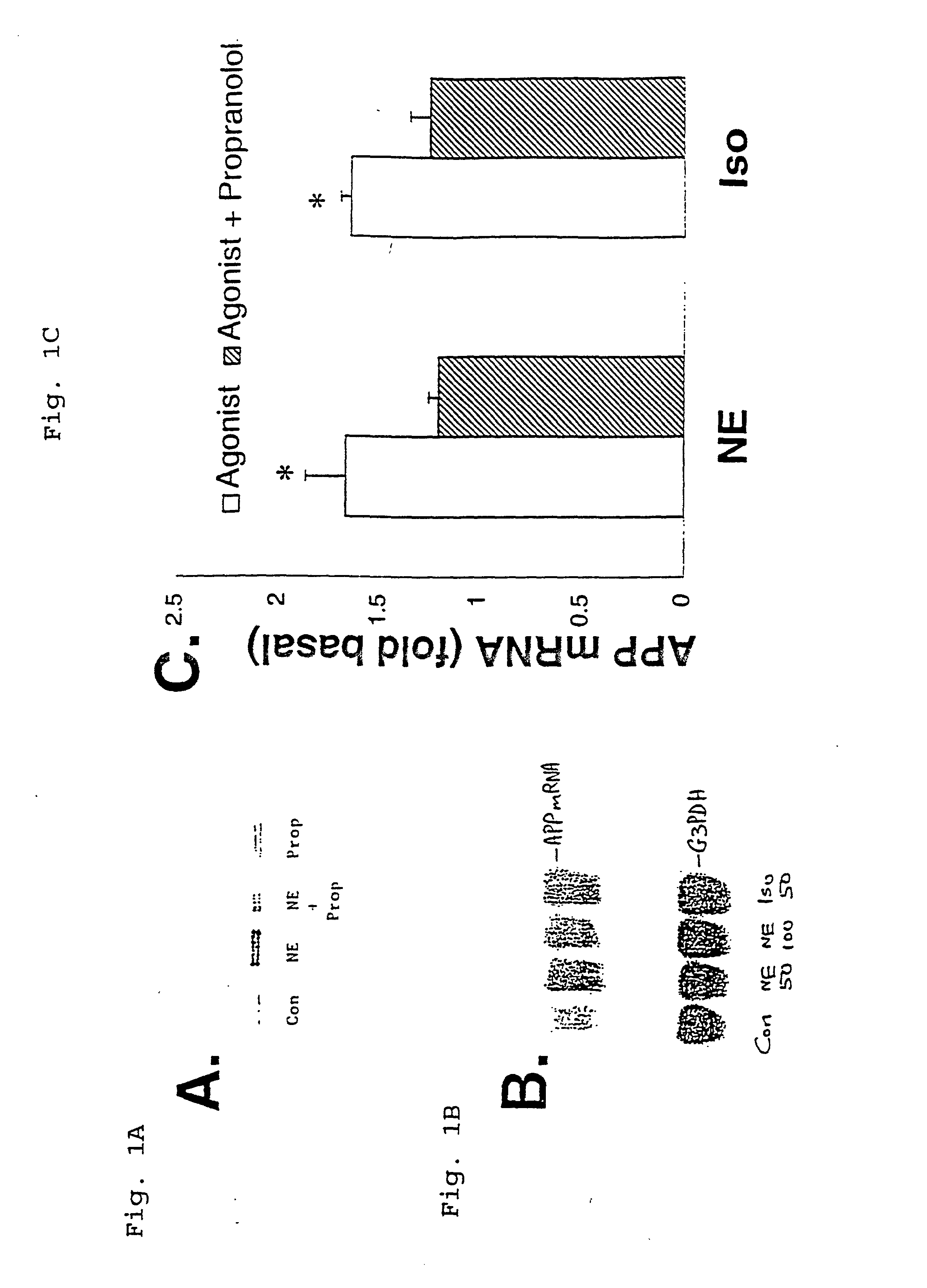
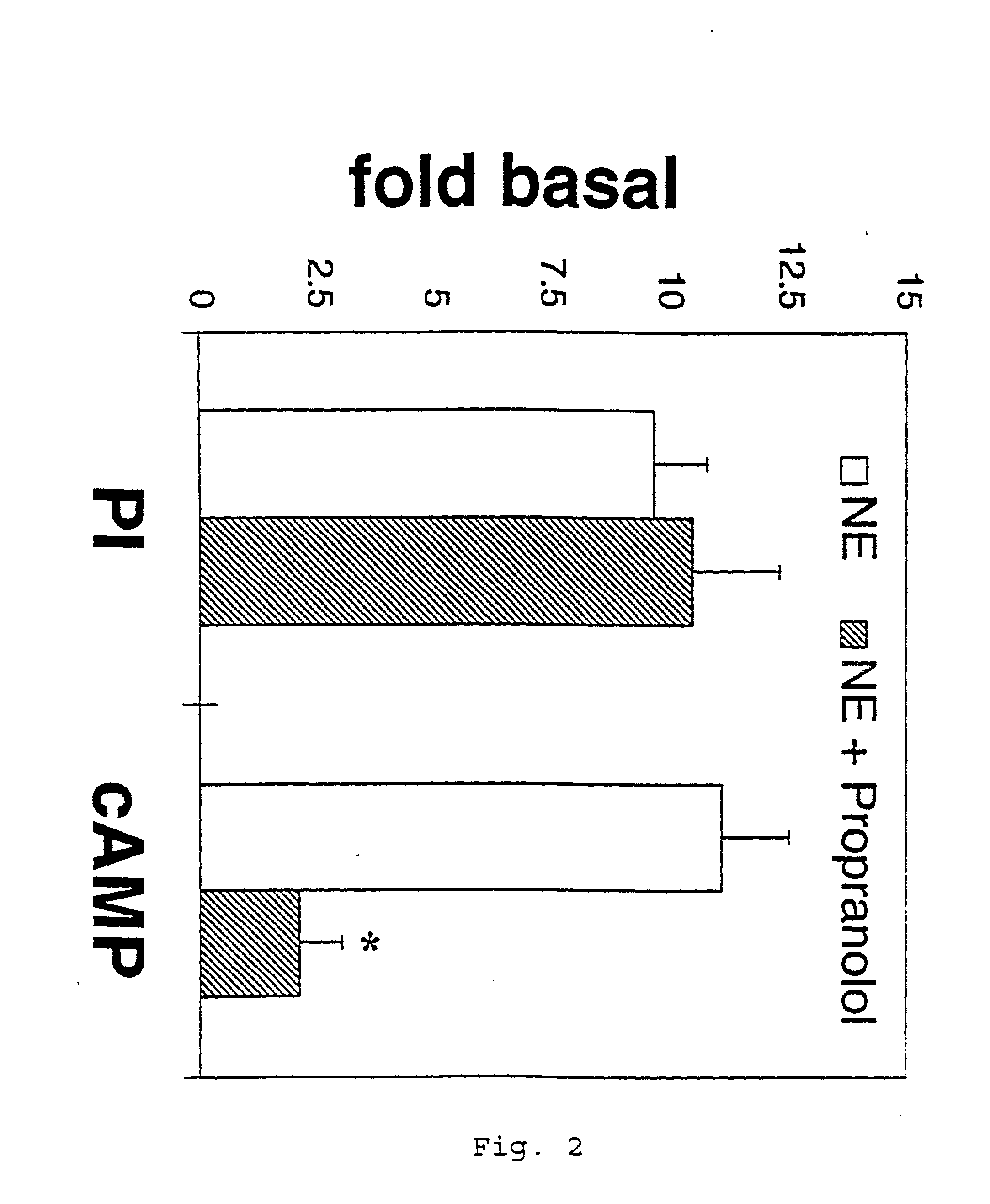
Compositions and methods for treatment of neurological disorders and neurodegenerative diseases
InactiveUS6469055B2Increased formationPromote activationBiocideNervous disorderGlial fibrillary acidic proteinDisease
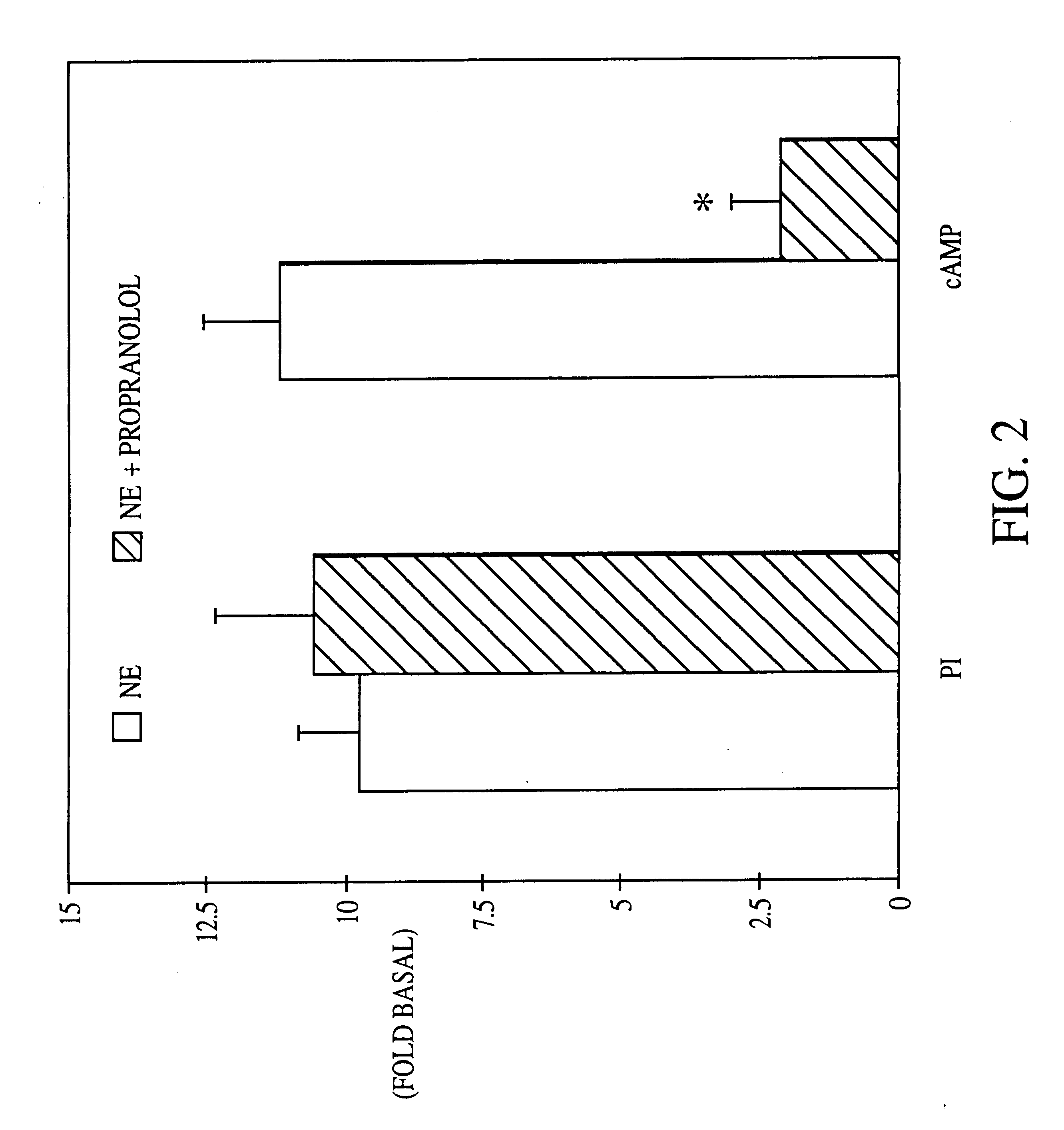
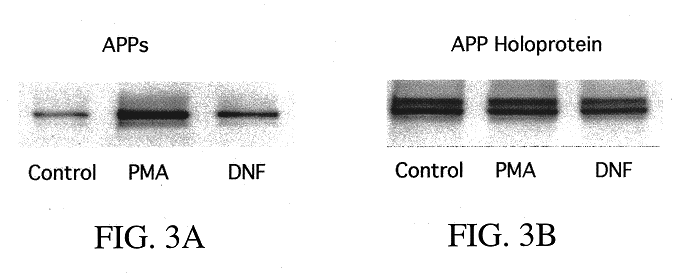
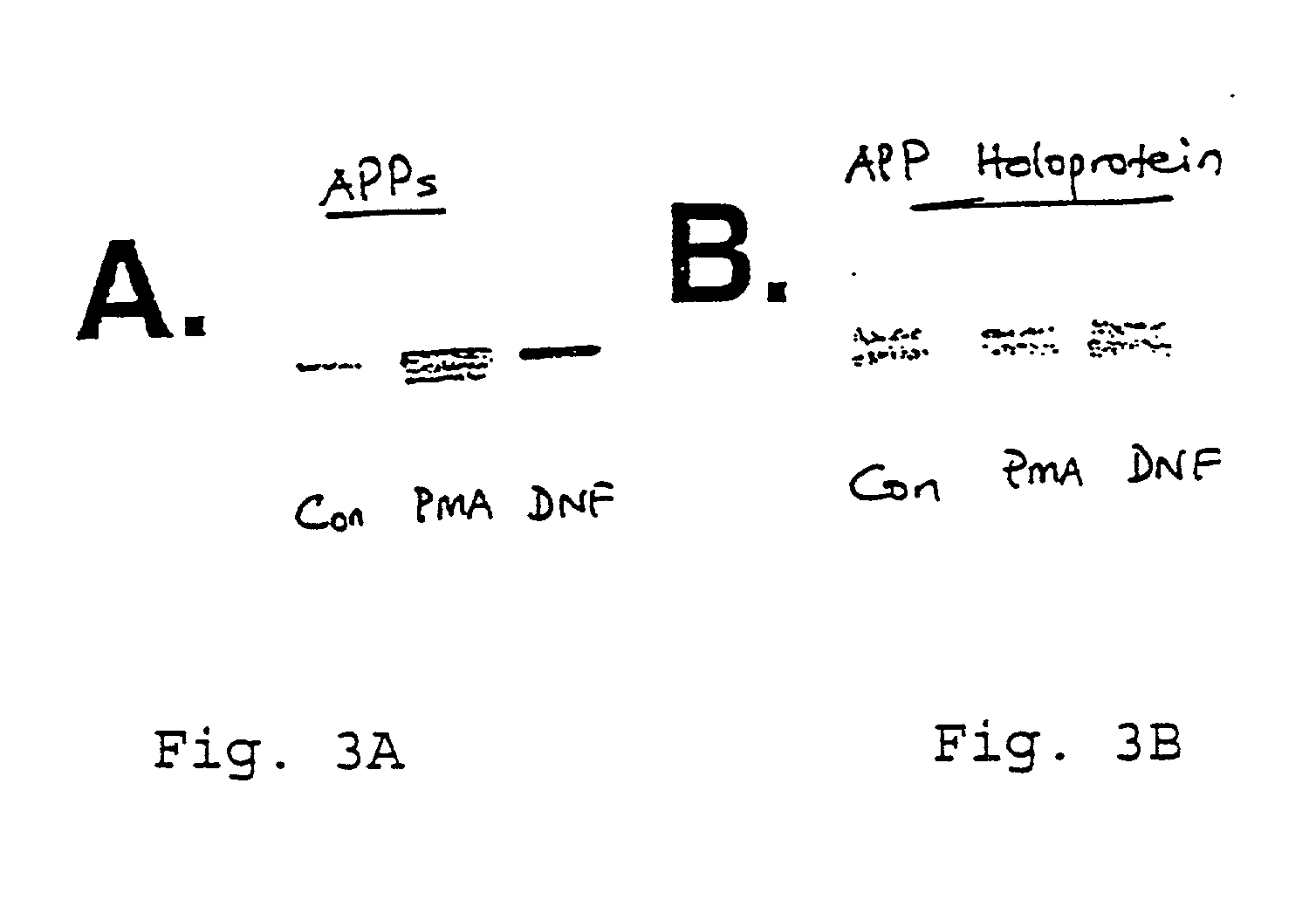
It has been discovered that the stimulation of beta-adrenergic receptors, which activate cAMP formation, give rise to increased APP and GFAP synthesis in astrocytes. Hence, the in vitro or in vivo exposure of neuronal cells to certain compositions comprising beta-adrenergic receptor ligands or agonists, including, e.g., norepinephrine, isoproterenol and the like, increases APP mRNA transcription and consequent APP overproduction. These increases are blocked by beta-adrenergic receptor antagonists, such as propranolol. The in vitro or in vivo treatment of these cells with 8Br-cAMP, prostaglandin E2 (PG E2), forskolin, and nicotine ditartrate also increased APP synthesis, including an increase in mRNA and holoprotein levels, as well as an increase in the expression of glial fibrillary acidic protein (GFAP). Compositions and methods are disclosed of regulating APP overexpression and mediating reactive astrogliosis through cAMP signaling or the activation of beta-adrenergic receptors. It has further been found that the increase in APP synthesis caused by 8Br-cAMP, PG E2, or forskolin is inhibited by immunosuppressants, immunophilin ligands, or anti-inflammatory agents, such as cyclosporin A, and FK-506 (tacrolimus), as well as ion-channel modulators, including ion chelating agents such as EGTA, or calcium / calmodulin kinase inhibitors, such as KN93. The present invention has broad implications in the alleviation, treatment, or prevention of neurological disorders and neurodegenerative diseases, including Alzheimer's Disease.
Owner:MASSACHUSETTS INST OF TECH
Epinephrine dosing regimens
The present invention relates to methods for administering a first dose of epinephrine solution and an optional second dose of epinephrine solution. Also provided herein are kits useful in these methods.
Owner:SCIELE PHARMA
Electrically assisted lidocaine and epinephrine delivery device having extended shelf-stability
InactiveUS20050228336A1Great confidenceFewer returnsOrganic active ingredientsElectrotherapyLidocaineAnesthetic
Highly shelf-stable electrically assisted transdermal drug delivery systems for delivering epinephrine, typically with an anesthetic such as lidocaine, are provided along with methods for making the highly shelf-stable epinephrine-containing transdermal delivery device. Highly shelf-stable packaged electrode assemblies for transdermal delivery of epinephrine also are provided.
Owner:VYTERIS
Intranasal Formulation of Epinephrine for the Treatment of Anaphylaxis
ActiveUS20150005356A1Fast onset timeSuitable for intranasal useBiocidePharmaceutical delivery mechanismBronchospasmInjection epinephrine
This invention relates to pharmaceutical compositions of epinephrine for delivery to the nasal mucosa and methods of treating a subject in acute severe anaphylaxis, bronchospasm or during cardiopulmonary resuscitation (CPR). The composition further comprising agents, that either prevent localized degradation of epinephrine or enhance its absorption in the nasal mucosa to counter anaphylactic effects, symptoms or complications in a subject.
Owner:G2B PHARMA
Fast-disintegrating epinephrine tablets for buccal or sublingual administration
Described herein are formulations for fast-disintegrating epinephrine tablets which can be prepared for buccal or sublingual administration, wherein the fast-disintegrating epinephrine tablets can produce plasma epinephrine concentrations similar to those achieved by an approximately 0.3 mg epinephrine dose in the thigh (Epi-Pen).
Owner:UNIVERSITY OF MANITOBA
Method and apparatus for delivering epinephrine
Owner:WASHINGTON BIOTECH CORP
Compositions and methods for treatment of neurological disorders and neurodegenerative diseases
InactiveUS20020052407A1Prevent APP over-expressionInhibit overexpressionBiocideNervous disorderGlial fibrillary acidic proteinDisease
It has been discovered that the stimulation of beta-adrenergic receptors, which activate cAMP formation, give rise to increased APP and GFAP synthesis in astrocytes. Hence, the in vitro or in vivo exposure of neuronal cells to certain compositions comprising beta-adrenergic receptor ligands or agonists, including, e.g., norepinephrine, isoproterenol and the like, increases APP mRNA transcription and consequent APP overproduction. These increases are blocked by beta-adrenergic receptor antagonists, such as propranolol. The in vitro or in vivo treatment of these cells with 8Br-cAMP, prostaglandin E2 (PG E2), forskolin, and nicotine ditartrate also increased APP synthesis, including an increase in mRNA and holoprotein levels, as well as an increase in the expression of glial fibrillary acidic protein (GFAP). Compositions and methods are disclosed of regulating APP overexpression and mediating reactive astrogliosis through cAMP signaling or the activation of beta-adrenergic receptors. It has further been found that the increase in APP synthesis caused by 8Br-cAMP, PG E2, or forskolin is inhibited by immunosuppressants, immunophilin ligands, or anti-inflammatory agents, such as cyclosporin A, and FK-506 (tacrolimus), as well as ion-channel modulators, including ion chelating agents such as EGTA, or calcium / calmodulin kinase inhibitors, such as KN93. The present invention has broad implications in the alleviation, treatment, or prevention of neurological disorders and neurodegenerative diseases, including Alzheimer's Disease.
Owner:MASSACHUSETTS INST OF TECH
Methods of treatment of patients at increased risk of development of ischemic events and compounds hereof
InactiveUS20130040898A1Impair thrombus formationIncreased riskElcosanoid active ingredientsInorganic active ingredientsBeta blockerPlatelet inhibitors
The present invention relates to compounds for treatment that protects the endothelium, prevents pathologic thrombus formation in the microcirculation and preserves platelet number and function and thus may be related to treatment or prevention of ischemic events in patients with cardiovascular disease. The present invention is particularly useful for patients having or being at increased risk of development of an ischemic event such as an acute myocardial infarction and / or no-reflow phenomena and / or ischemia-reperfusion injury by administration of agent(s) modulating and / or preserving endothelial integrity. The compounds may be administered in combination with standard treatment of acute cardiovascular ischemic events such as Platelet inhibitors such as aspirin (ASA), Thienopyridins, GPIIb / IIIa inhibitors), Parenteral anticoagulants such as unfractioned heparin (UFH), bivalirudin, enoxaparin, and fondaparinux, Verapamil, Adenosine, Sodium nitroprusside, Nitroglycerin, Epinephrine, Beta-blockers and surgical methods such as percutaneous coronary intervention (PCI), PCI with thrombus aspiration, PCI with stents.
Owner:THROMBOLOGIC
Inhalable epinephrine
The present invention is directed toward particles for delivery of epinephrine to the respiratory system and methods for treating a patient in need of epinephrine. The particles and respirable compositions comprising the particles of the present invention described herein comprise the bioactive agent epinephrine, or a salt thereof, as a therapeutic agent. The particles are preferably formed by spray drying. Preferably, the particles and the respirable compositions are substantially dry and are substantially free of propellents. In a preferred embodiment, the particles have aerodynamic characteristics that permit targeted delivery of epinephrine to the site(s) of action.
Owner:CIVITAS THERAPEUTICS
Stabilization of quinol composition such as catecholamine drugs
InactiveUS20120029085A1Stabilizing compoundReduced form requirementsBiocidePharmaceutical delivery mechanismCatecholaminePh buffering
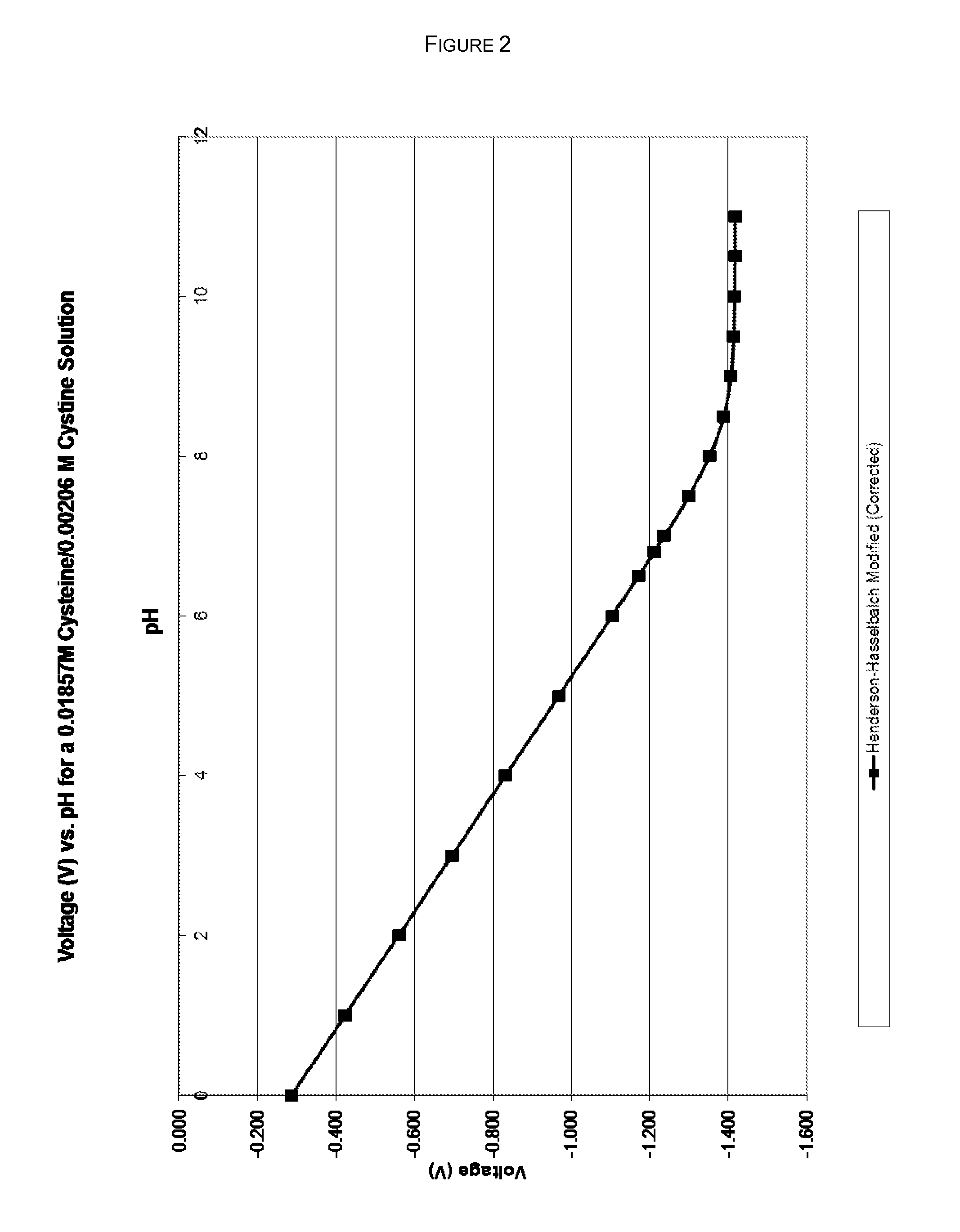
Compositions and methods are provided for obtaining stabilized quinol compositions, such as catecholamine drugs (e.g., epinephrine solutions), and also for obtaining stable pharmaceutical formulations that comprise a stabilized quinol composition and a second pharmacologically active component such as a local anesthetic or other active drug ingredient having a reversibly protonated amine group. Stability is achieved through the inclusion of an appropriately selected pH buffer and a thiol agent, based on redox and pH buffering principles including pKa of the buffer and of the reversibly protonated amine group.
Owner:MACKAY JON
Preparation for Iontophoresis
InactiveUS20090163597A1Low absorption rateStable materialBiocideAntipyreticElectrical driveBiomedical engineering
A preparation used for iontophoresis in order to absorb a physiologically active substance via the skin or mucosa using electrical driving force and the preparation containing a local anesthetic, epinephrine or its salt, water and chlorobutanol.
Owner:TEIKOKU SEIYAKU KK TEIKOKU SEIYAKU CO LTD
Novel prostamides for the treatment of glaucoma and related diseases
Disclosed herein are compositions comprising an amide related to a prostaglandin and an amine wherein the amine is selected from the group consisting of epinephrine, dopamine, serotonin, and analogs or prodrugs thereof. Also disclosed are certain chemical compounds, pharmaceutical compositions, and methods of treating glaucoma.
Owner:ALLERGAN INC
Anti-glycation agents for preventing age- diabetes- and smoking-related complications
InactiveUS20050043408A1Loss of mechanical propertyReduce usageBiocideNervous disorderChemical structureDiabetes mellitus
The invention provides new inhibitors of protein glycation, identified from compound libraries by a high throughput screening assay. The anti-glycation agents so identified are characterized by a variety of chemical structures and are useful for the prevention or treatment of age-, diabetes-, and smoking-related complications, including neuropathy, nephropathy, ocular pathologies, or the loss of mechanical properties of collagenous tissues. Among compounds identified as having the anti-glycation activity, of special interest are epinephrine and its analogs, in particular D-epinephrine and its analogs, which are particularly useful for the prevention or treatment of age-, diabetes-, and smoking-related ocular pathologies.
Owner:NAT RES COUNCIL OF CANADA
Inhalable epinephrine
The present invention is directed toward particles for delivery of epinephrine to the respiratory system and methods for treating a patient in need of epinephrine. The particles and respirable compositions comprising the particles of the present invention described herein comprise the bioactive agent epinephrine, or a salt thereof, as a therapeutic agent. The particles are preferably formed by spray drying. Preferably, the particles and the respirable compositions are substantially dry and are substantially free of propellents. In a preferred embodiment, the particles have aerodynamic characteristics that permit targeted delivery of epinephrine to the site(s) of action.
Owner:CIVITAS THERAPEUTICS
Epinephrine formulations
ActiveUS9119876B1Low level of oxidative degradantsLow impurity contentPowder deliveryOrganic active ingredientsPreservativeMedicine
Pharmaceutical compositions comprising epinephrine, methods of administration, and methods of making the same. Compositions may comprise at least one of an active agent, a pH raising agent, an antioxidant, a transition metal complexing agent, a pH lowering agent, a tonicity regulating agent, optionally a preservative, and optionally a solvent.
Owner:PAR PHARMA
Blocking induction of tetrahydrobioterin to block induction of nitric oxide synthesis
InactiveUS6153615AReduce inhibitionRestore sensitivityBiocidePeptide/protein ingredientsSide effectTreatment effect
Guanosine triphosphate pathway tetrahydrobiopterin synthesis antagonist and / or pterin salvage pathway tetrahydrobiopterin synthesis antagonists are administered to inhibit nitric oxide synthesis from arginine in vascular cells in a subject in need of such inhibition (e.g., for prophylactic or curative effect for endotoxin- or cytokine-induced hypotension or for restoration of vascular contractile sensitivity to pressor agents in the treatment of such hypotension). The tetrahydrobiopterin synthesis antagonist may be administered with alpha 1-adrenergic agonist or with nitric oxide synthase inhibitor. The tetrahydrobiopterin synthesis antagonists are also administered to attenuate inflammation caused by induced nitric oxide production in immune cells. Unwanted counterproductive or side effects can be eliminated or ameliorated by administration additionally of levodopa with or without carbidopa and L-5-hydroxytryptophane.
Owner:CORNELL RES FOUNDATION INC
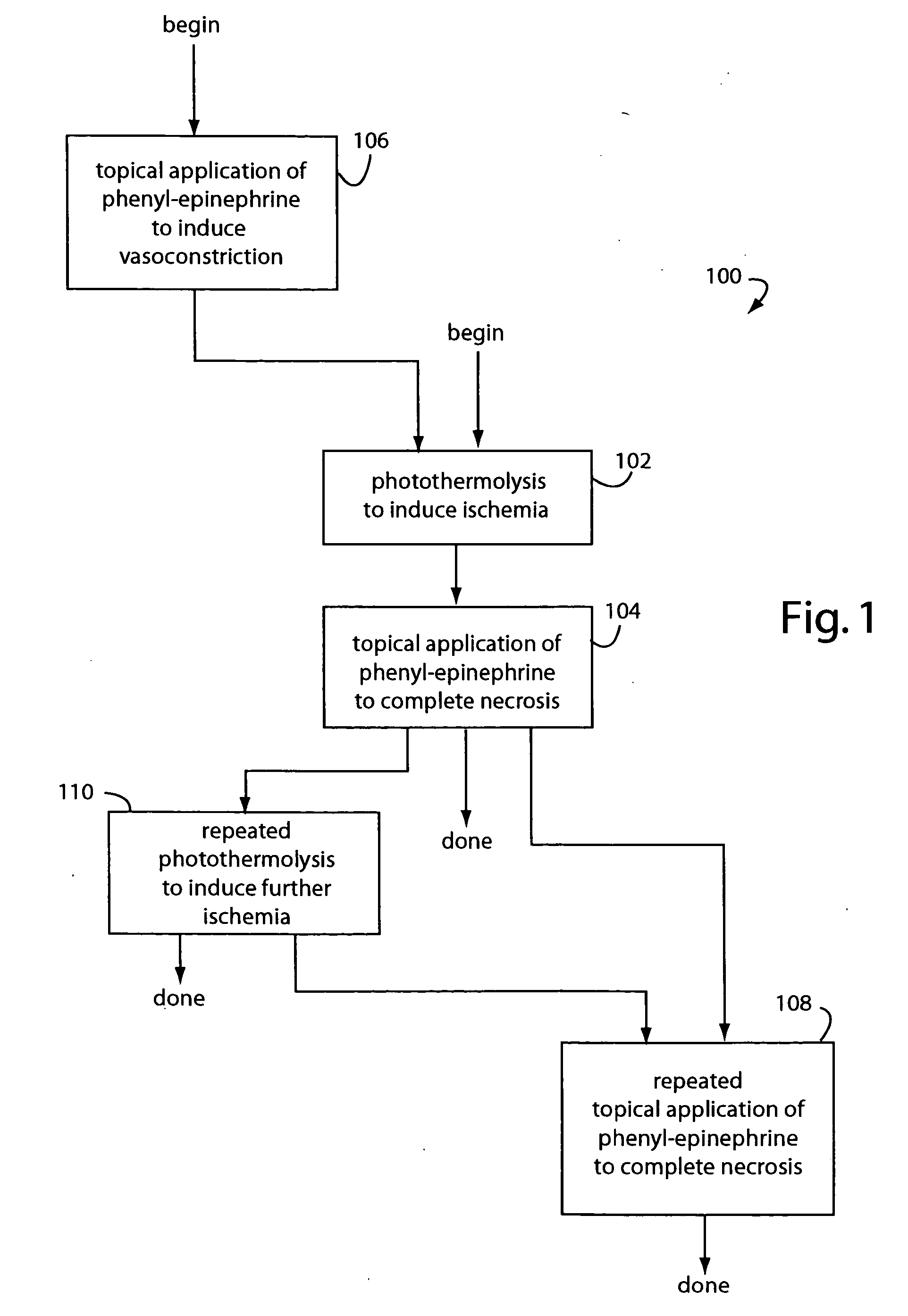
Topical phenyl-epinephrine Rosacea treatment
InactiveUS20050256204A1Safe and effectiveEffective temporary reliefBiocideOrganic active ingredientsArteriolar VasoconstrictionDisease
A near-permanent skin treatment includes photothermolysis of reddened facial skin to induce ischemia. Reperfusion of the photothermolysis treated skin is inhibited by following with regular applications of phenyl-epinephrine carried in a lotion until vascular necrosis is complete. Alternatively, a temporary treatment for reddened facial skin includes only cosmetic as-needed applications of phenyl-epinephrine carried in lotion to induce vasoconstriction in Rosacea and other similarly embarrassing skin disorders.
Owner:BITTER PATRICK H SR
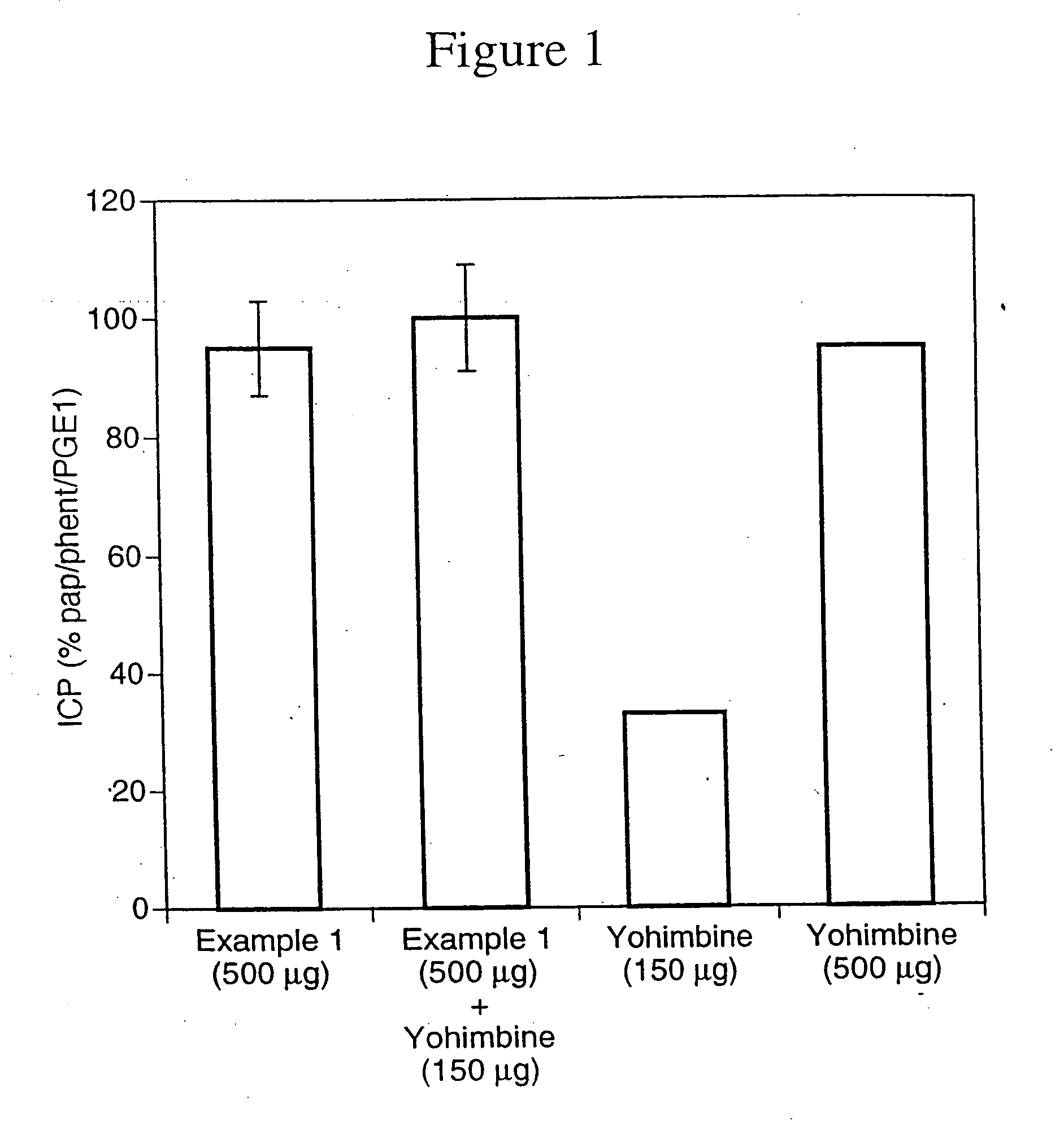
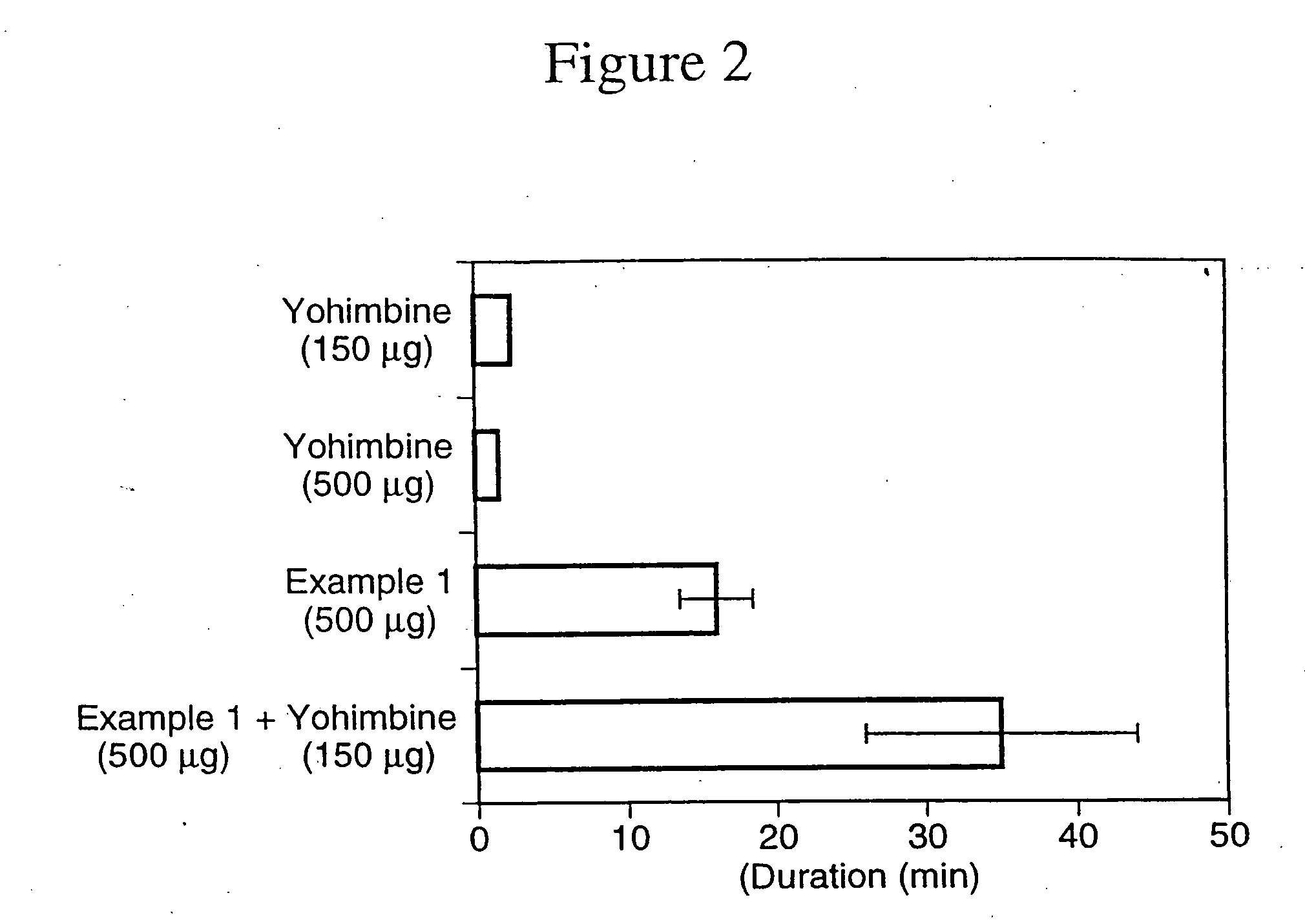
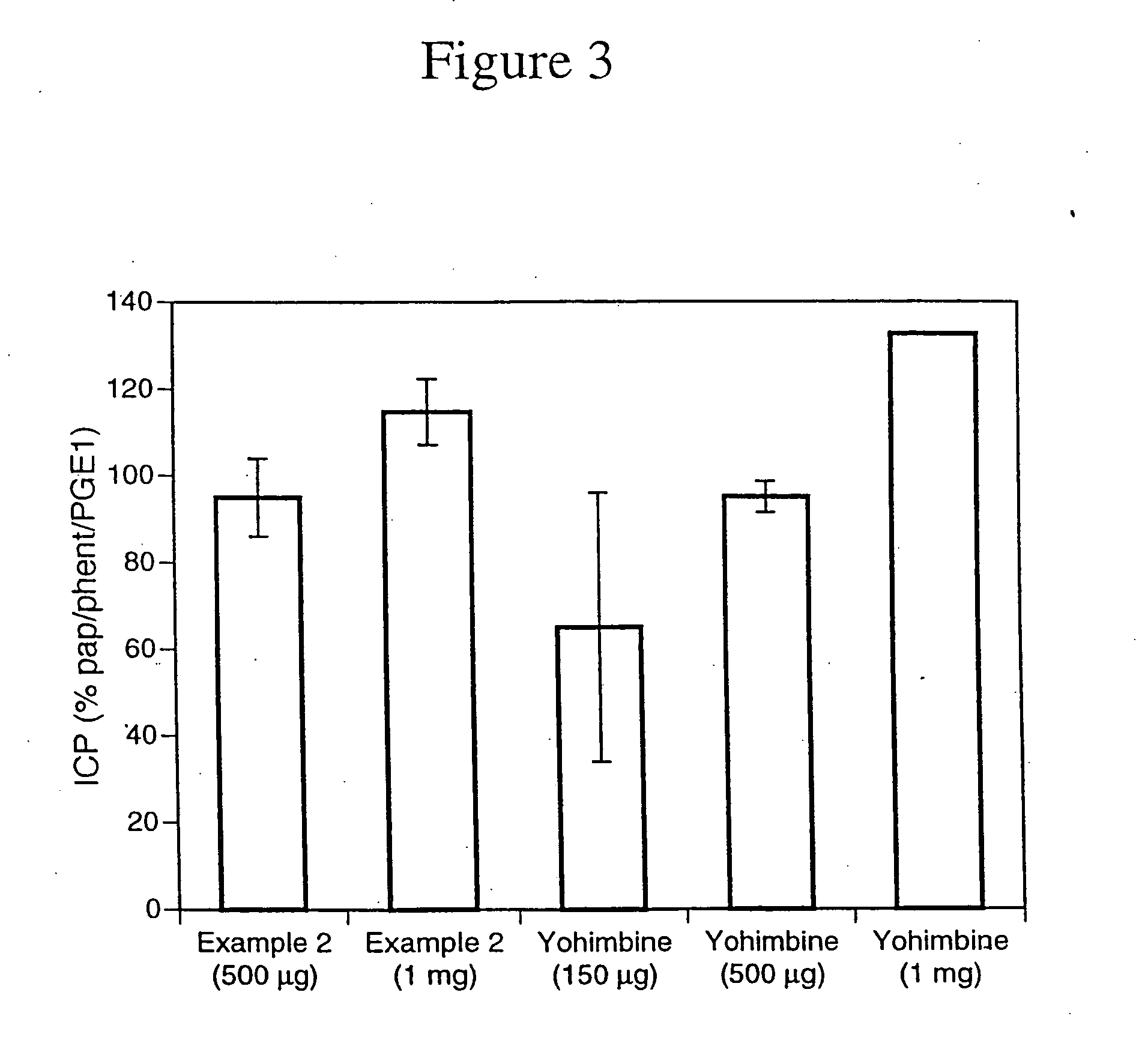
Nitrosated and nitrosylated alpha-adrenergic receptor antagonist compounds
The present invention describes novel nitrosated and / or nitrosylated α-adrenergic receptor antagonists, and novel compositions containing at least one nitrosated and / or nitrosylated α-adrenergic receptor antagonist, and, optionally, one or more compounds that donate, transfer or release nitric oxide, elevate endogenous levels of endothelium-derived relaxing factor, stimulate endogenous synthesis of nitric oxide or are a substrate for nitric oxide synthase, and / or one or more vasoactive agents. The present invention also provides novel compositions containing at least one α-adrenergic receptor antagonist, and one or more compounds that donate, transfer or release nitric oxide, elevate endogenous levels of endothelium-derived relaxing factor, stimulate endogenous synthesis of nitric oxide or is a substrate for nitric oxide synthase and / or one or more vasoactive agents. The present invention also provides methods for treating or preventing sexual dysfunctions in males and females, for enhancing sexual responses in males and females, and for treating or preventing benign prostatic hyperplasia, hypertension, congestive heart failure, variant (Printzmetal) angina, glaucoma, neurodegenerative disorders, vasospastic diseases, cognitive disorders, urge incontinence, or overactive bladder, and for reversing the state of anesthesia.
Owner:NITROMED
Intra-operative procedure for post-operative pain control
InactiveUS20050176823A1Eliminating post-operative painFree from painBiocideInorganic active ingredientsPost-Procedural PainSolution flow
The instant invention is directed towards an intra-operative method and kit for essentially eliminating pain associated with and resulting from surgical procedures, comprising the administration of a medical solution including a mixture of an injectable anesthetic, epinephrine, sodium chloride and an injectable anti-inflammatory agent to a plurality of sites within a surgical field utilizing a means for administration which, in a contemplated embodiment, may include a hollow shaft having a proximal end and a distal end, wherein upon administration, said medical solution flows within said shaft from said proximal end toward said distal end, which distal end is defined by a solid end having a plurality of circumferentially positioned apertures in said shaft for providing radially directed flow of the medicated solution circumferentially about said shaft.
Owner:DIAZ ROBERT L
Methods for Buccal, Lingual or Sublingual Dosing Regimens of Epinephrine for the Treatment of Allergic Emergencies
InactiveUS20070293581A1Patient compliance is goodReduce worriesBiocideOrganic active ingredientsDosing regimenDosage form
The present invention relates to methods of administering dosage forms which comprise epinephrine, including buccal, lingual, sublingual or transmucosal dosage forms comprising epinephrine for treatment of allergic emergencies, including anaphylaxis. Also provided herein are kits and packaging systems useful in these methods.
Owner:SCIELE PHARMA
Method and apparatus for providing therapeutically effective dosage formulations of lidocaine with and without epinephrine
An apparatus for providing therapeutically effective mixtures of buffered lidocaine (with and without epinephrine) while simultaneously increasing the shelf life, having a two-chambered medicine vial, in which one chamber isolates up to 20 ml of a lidocaine solution (1% with or without epinephrine) and the other chamber isolates up to 2 ml of sodium bicarbonate (8.4%), with a fracturable wall therebetween such that the wall is fractured by bending or twisting, for mixing the lidocaine and sodium bicarbonate to create a buffered lidocaine for administration in therapeutically effective amounts, and further comprising stopper means, like a rubber stopper, for the insertion of a syringe for extraction of the mixture for administration. Also shown is a method for isolating the components for shelf life and providing mixing for an effective composition.
Owner:GIRGIS AKRAM +1
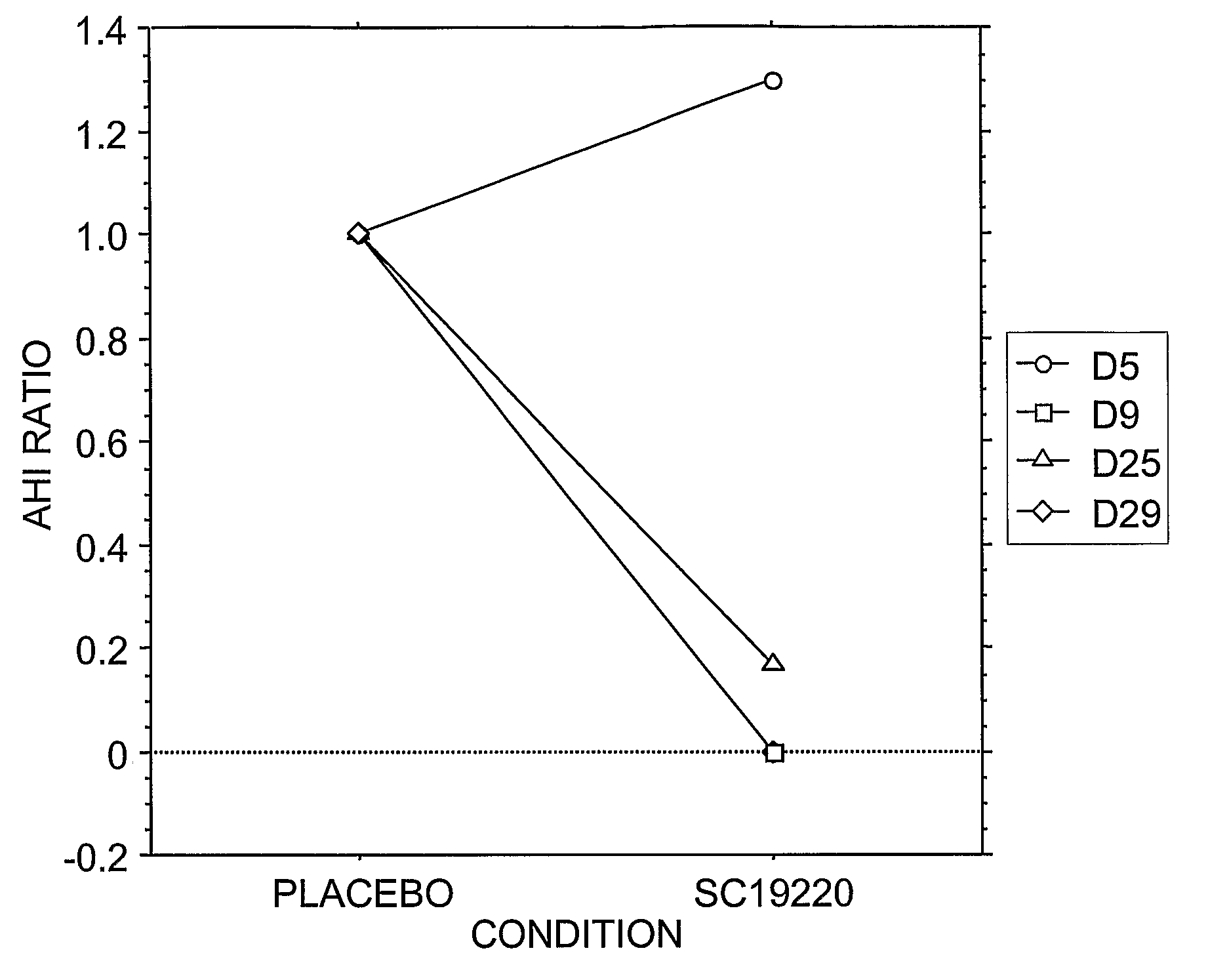
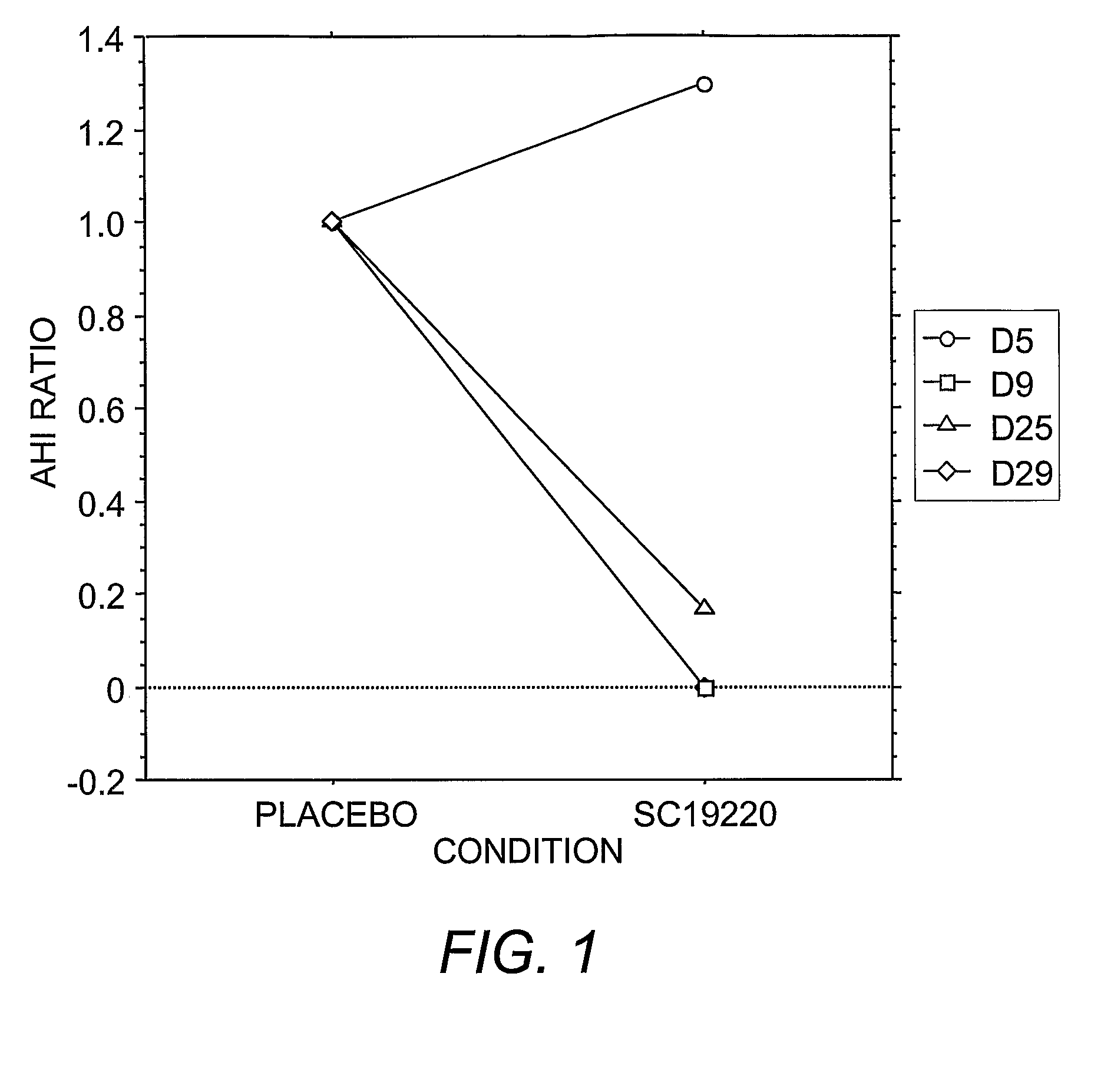
Pharmacological Treatments for Sleep Disorders (Apnoe) With Prostanoid Receptor Antagonists
InactiveUS20080261922A1Preventing and ameliorating sleep-related breathing disorderBiocideNervous disorderNorepinephrine reuptake inhibitorPhosphate
This invention is directed to methods for preventing or ameliorating sleep-related breathing disorders. The method comprises administration to a patient in need thereof an effective dose of one or a combination of prostanoid receptor antagonists (eg. ramatroban, ifetroban, diphloretin, phosphate, polyphloretin phosphate, seratrodast, SC19220). The prostanoid receptor antagonist or combination of prostanoid receptor antagonists can be administered in conjunction with one or more serotonin receptor agonists, one or more cannabinoids receptor agonists, one or more serotonin reuptake inhibitors, one or more noradrenalin reuptake inhibitors, a combination of reuptake inhibitors, an inhibitor of prostanoid synthesis, or any combination of the foregoing.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Intranasal formulation of epinephrine for the treatment of anaphylaxis
ActiveUS9789071B2Increases absolute nasal residency timeMaximal stability of unstable APIsPharmaceutical delivery mechanismPharmaceutical non-active ingredientsBronchospasmNose
This invention relates to pharmaceutical compositions of epinephrine for delivery to the nasal mucosa and methods of treating a subject in acute severe anaphylaxis, bronchospasm or during cardiopulmonary resuscitation (CPR). The composition further comprising agents, that either prevent localized degradation of epinephrine or enhance its absorption in the nasal mucosa to counter anaphylactic effects, symptoms or complications in a subject.
Owner:G2B PHARMA
Stable epinephrine suspension formulation with high inhalation delivery efficiency
ActiveUS8367734B1Efficient deliveryImprove efficacyBiocidePowder deliveryAntioxidantChlorofluorocarbon
A stable suspension aerosol formulation of epinephrine is suitable for administration through inhalation comprising a therapeutically effective amount of epinephrine, hydrofluorocarbon propellant, co-solvent, surfactant, and antioxidant. The suspension aerosol formulation further comprises [pre-] pre-micronized epinephrine suspended in an alcohol / surfactant solution with hydrofluoroalkane propellant. The suspension formulation provides a highly efficient delivery of drug microparticles into the respirable region of patients' lungs and has the following advantages: lower dosage requirement, minimum alcohol content, with less impurities generated during storage, improved efficacy and safety, and exhibits no ozone depleting potential compared to a formulation containing chlorofluorocarbon.
Owner:AMPHASTAR PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com