Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "Darunavir" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This drug is used with other HIV medications to help control HIV infection.
Combination therapy comprising tenofovir alafenamide hemifumarate and cobicistat for use in the treatment of viral infections
InactiveCN104105484AAntiviralsHeterocyclic compound active ingredientsEmtricitabineTenofovir alafenamide
The use of the hemifumarate form of {9-[(R)-2-[[(S)-[[(S)-l- (isopropoxycarbonyl)ethyl]amino]phenoxyphosphinyl]methoxy]propyl]adenine} (tenofovir alafenamide hemifumarate) in combination with cobicistat is disclosed. In addition, the combination of tenofovir alafenamide hemifumarate, cobicistat, emtricitabine, and elvitegravir, and the combination of tenofovir alafenamide hemifumarate, cobicistat, emtricitabine, and darunavir, are disclosed.
Owner:GILEAD SCI INC
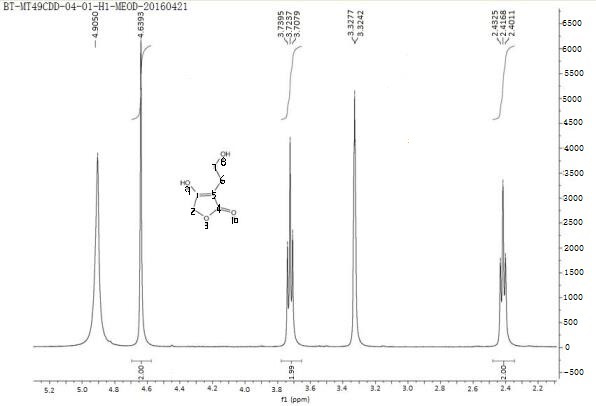
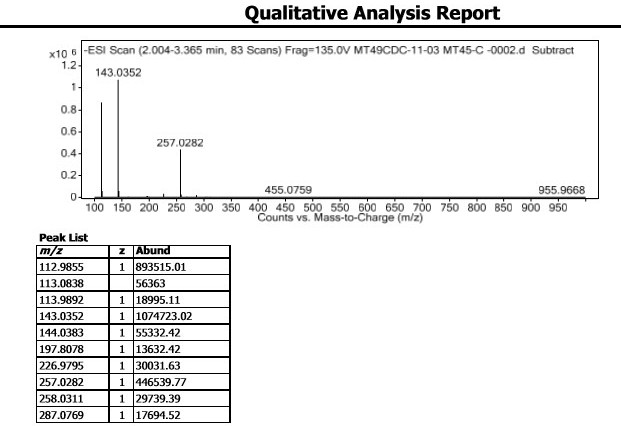
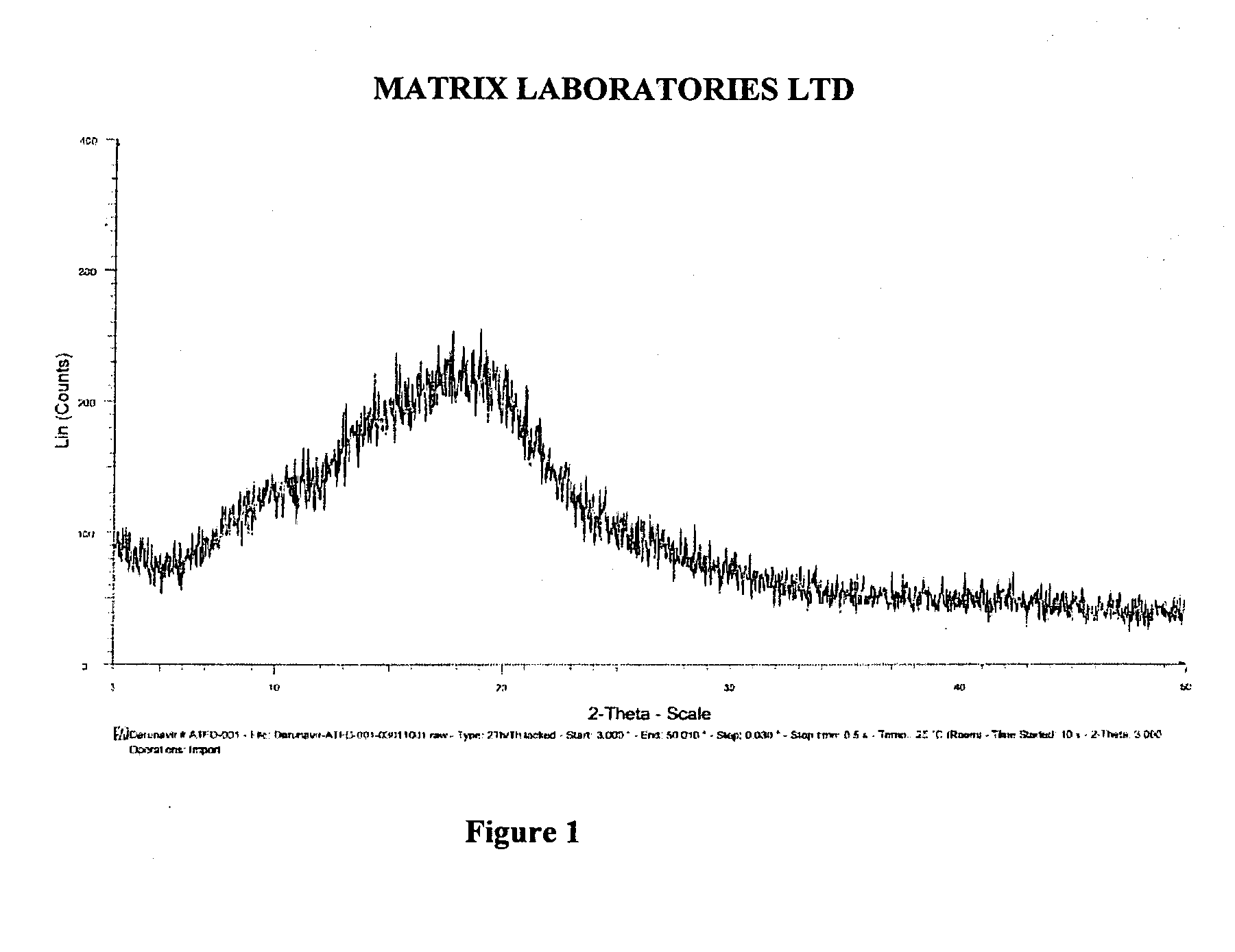
Synthetic methods of hexahydrofuro[2,3-b]furan-3-ol and enantiomer thereof
ActiveCN103864813ARaw materials are cheap and easy to getEasy to operateOrganic chemistryFuranEnantiomer
The invention discloses synthetic methods of hexahydrofuro[2,3-b]furan-3-ol and an enantiomer thereof. The synthetic method of hexahydrofuro[2,3-b]furan-3-ol comprises the step c or the step b to the step c or the step a to the step c in the following synthetic route as shown in the description. The synthetic method of the enantiomer (3R,3aS,6aR)hexahydrofuro[2,3-b]furan-3-ol comprises the step c to the step f or the step b to the step f or the step a to the step f in the following synthetic route as shown in the description, wherein R1 is selected from C1 to C4 alkyl or aralkyl, and R2 is selected from C1 to C4 alkyl. The synthetic methods provided by the invention have the advantages of cheap and easily available raw materials, simple operation, low cost and the like, are suitable for large-scale production, and have practical value for realization of industrialized production of darunavir.
Owner:SHANGHAI DESANO CHEM PHARMA +1
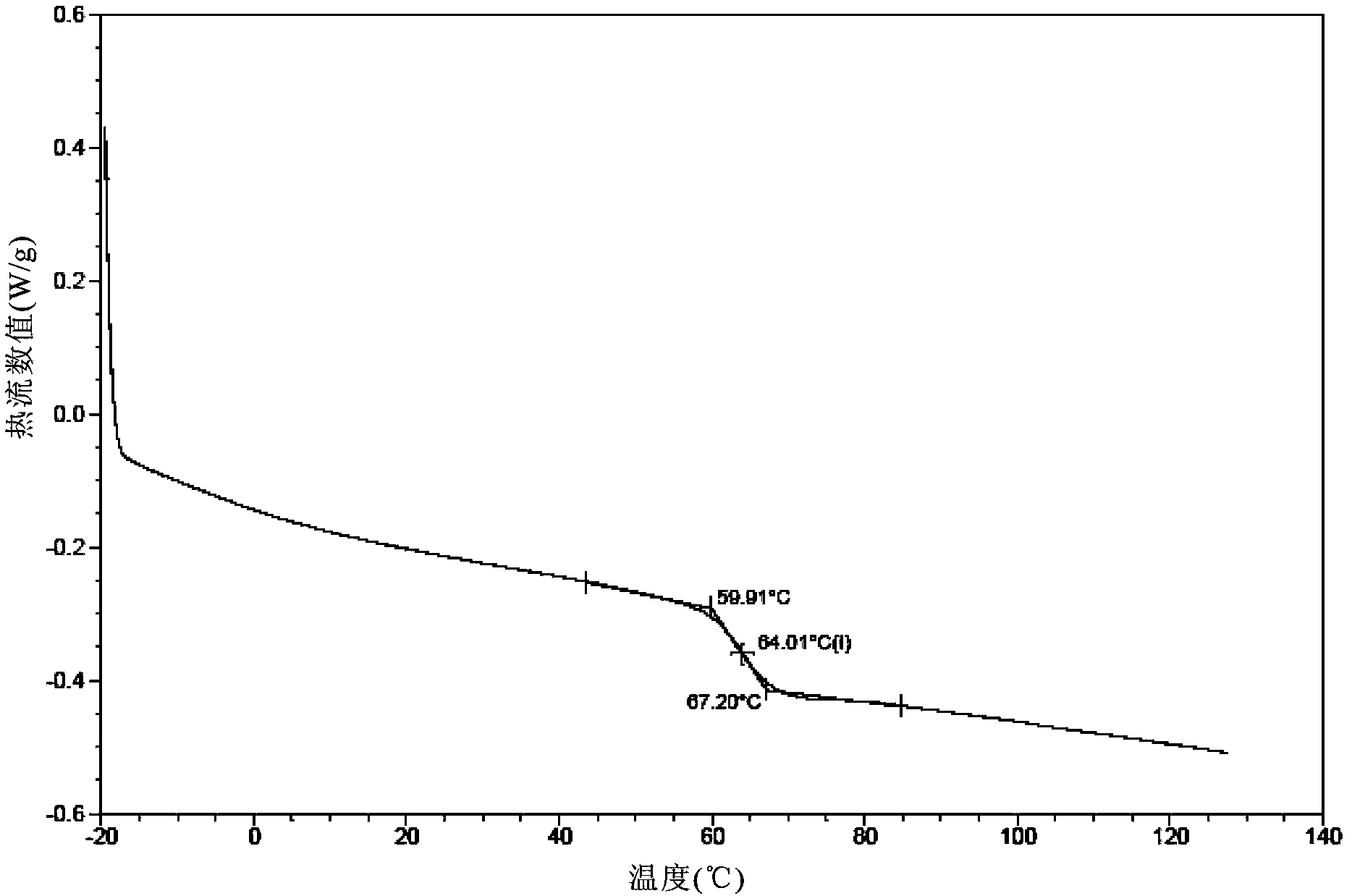
Preparation method of Darunavir amorphous matter
ActiveCN103509031AAddressing Purity IssuesFix stability issuesOrganic chemistrySolventAnalytical chemistry
The invention relates to a preparation method of a Darunavir amorphous matter. Specifically, the method disclosed by the invention comprises the steps of: mixing a Darunavir-containing first solution with a second solvent so as to obtain precipitated amorphous Darunavir. The product made by the method has high purity and good stability. The method has the advantages of simple operation, low production cost, environmental friendliness, and suitability for industrialized production.
Owner:SHANGHAI DESANO PHARMA INVESTMENT +1
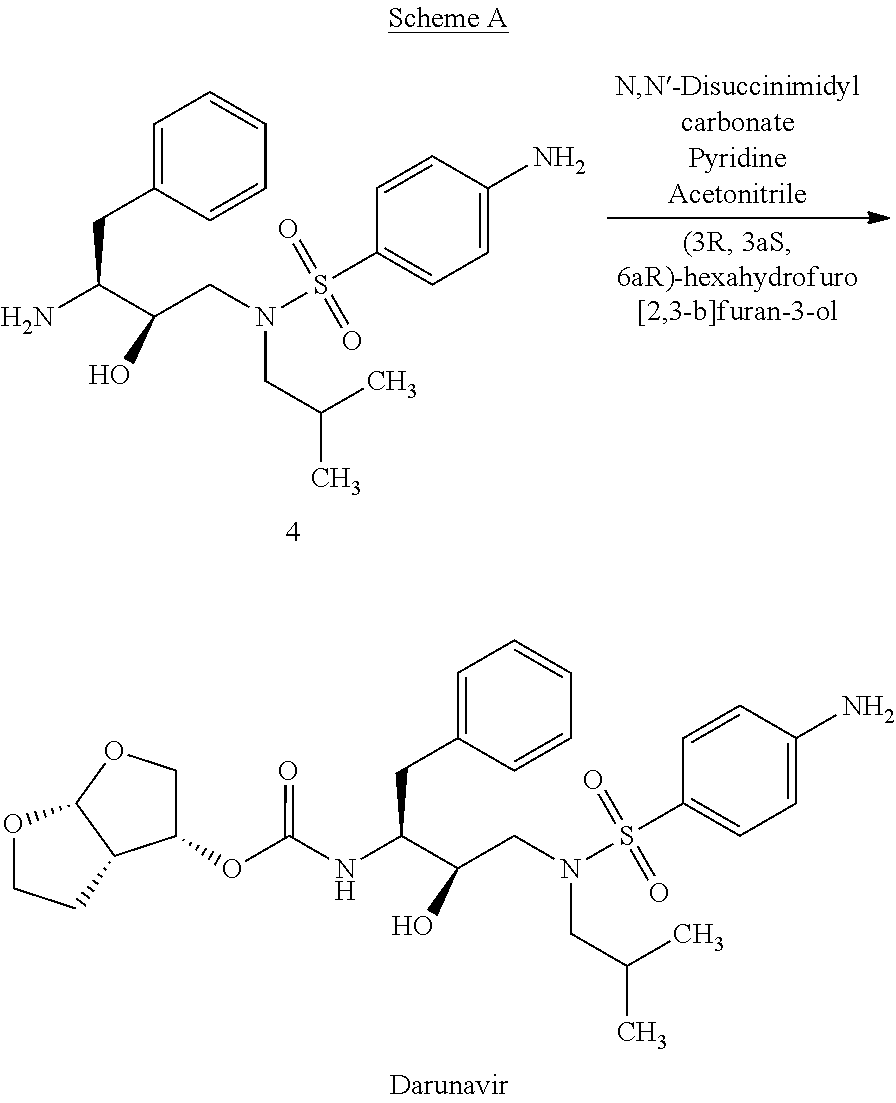
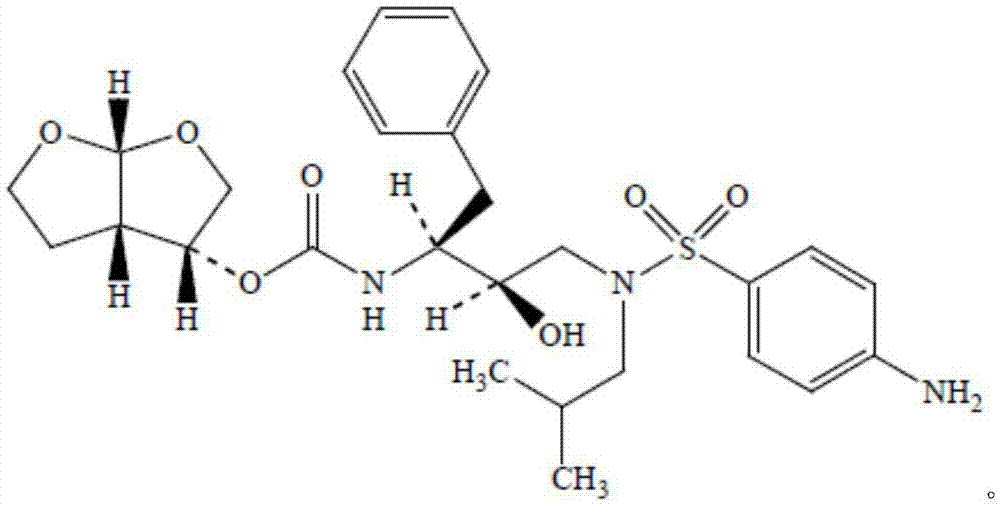
Process for the preparation of darunavir
A process for the preparation of Darunavir comprises the reacting of 4-amino-N-(2R,3S) (3-amino-2-hydroxy-4-phenylbutyl)-N-isobutyl-benzenesulfonamide with (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol derivative in N-methyl-2-pyrrolidinone and isolating the resulting Darunavir. The process yields Darunavir with a very low level of the difuranyl impurity.
Owner:MYLAN LAB
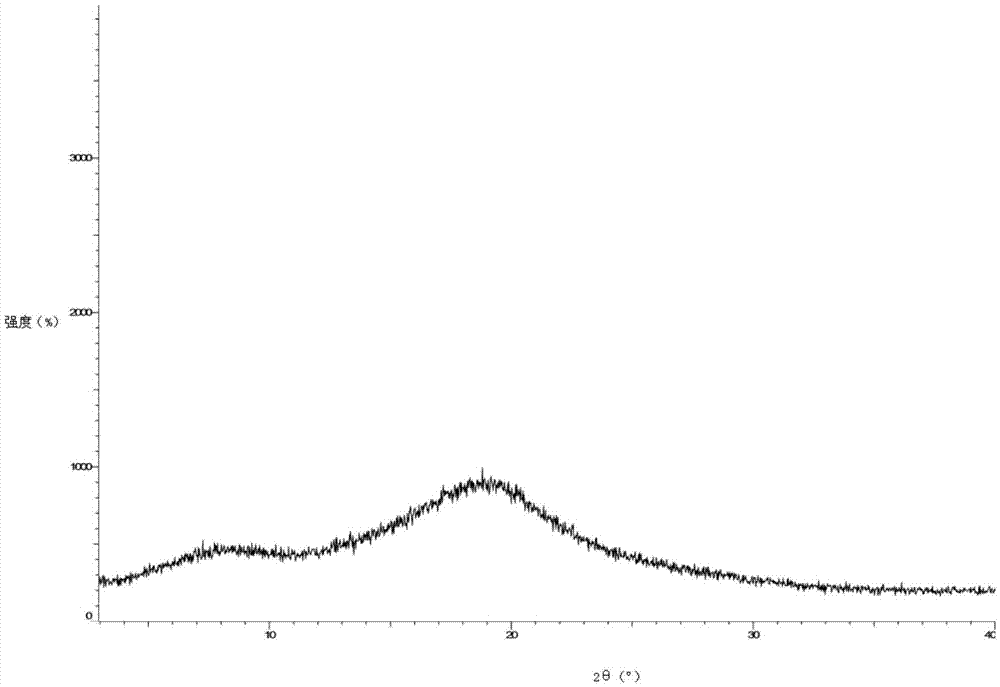
Darunavir amorphous form preparation method
InactiveCN106854212ASimple processShorten the production cycleOrganic chemistryAnti solventStereochemistry
The present invention relates to the technical field of pharmaceutical chemistry, particularly to a darunavir amorphous form preparation method. According to the present invention, the preparation method belongs to the anti-solvent method in crystal form preparation screening, wherein the crystal form is screened by using the anti-solvent in the prior art while the final product is the specific crystal form of darunavir such as WO2013114382 and is not the amorphous form; and the preparation method of the present invention is different from the method in the prior art, has the high production capacity, and is suitable for industrial production. The amorphous form of darunavir is defined in the specification.
Owner:ZHEJIANG JIUZHOU PHARM CO LTD
Medical intermediate and preparation method thereof
InactiveCN106957288ALow costRaw materials are cheap and easy to getOrganic chemistryChemical structureDarunavir+Ritonavir
The invention discloses a medical intermediate. The medical intermediate has the chemical structure shown as a formula II shown in the description, wherein the R is selected from methyl, ethyl, n-propyl, isopropyl, normal-butyl, isobutyl, tertiary butyl, sec-butyl, phenyl and naphthyl. The invention also discloses a preparation method of the intermediate; the operation is simple; the cost is low; the preparation method is suitable for scale production. The intermediate can be used for preparing a key intermediate of darunavir; important significance and practical values are realized on the industrial production of the darunavir.
Owner:YANCHENG DESANO PHARMA CO LTD
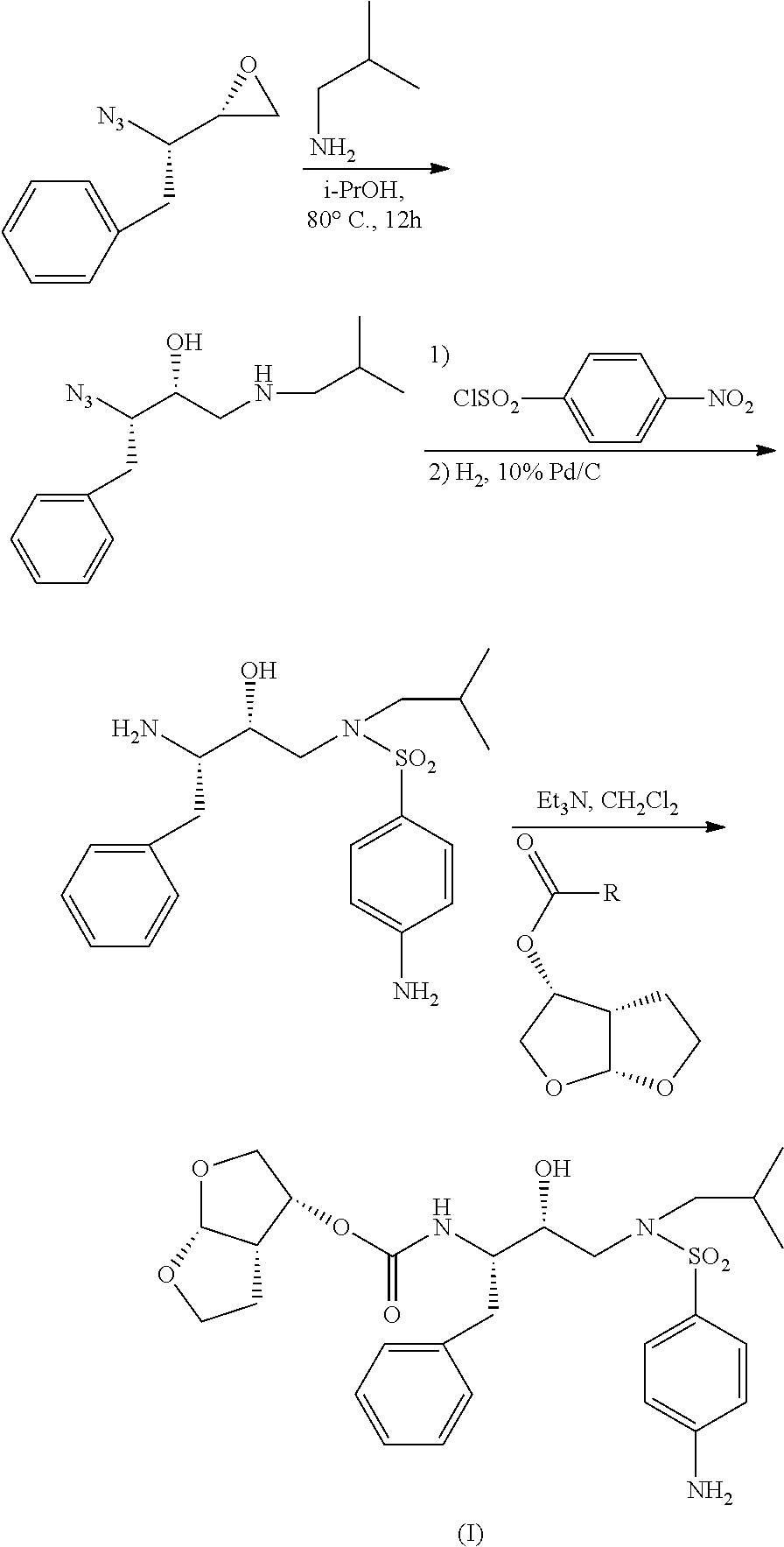
Process for the preparation of darunavir and darunavir intermediates
The present invention relates to a process for the preparation of darunavir, a nonpeptide protease inhibitor (PI), useful for the treatment of HIV / AIDS patients harboring multidrug-resistant HIV-1 variants that do not respond to previously existing HAART regimens. The present invention further relates to processes for the stereo-directed preparation of darunavir intermediates, in particular (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol and to certain novel intermediates obtained by such processes.
Owner:MAPI PHARMA
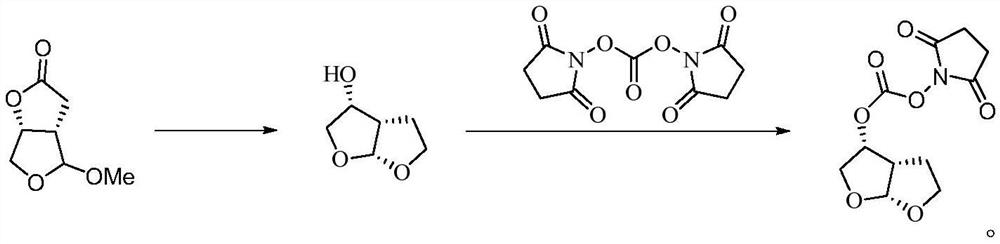
Method for preparing (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol
ActiveCN106928248ASuitable for industrial productionRaw materials are cheap and easy to getOrganic chemistryBulk chemical productionFuranChemical synthesis
The invention belongs to the field of chemical synthesis, and discloses a method for preparing a darunavir intermediate namely (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol. According to the method, (R)-3-hydroxyl-4-acetaldol derivatives (I) are used as raw materials, and under the action of a catalyst, the (R)-3-hydroxyl-4-acetaldol derivatives (I) stereoselectively react with an ethanediol derivative so as to obtain an intermediate (II); and the obtained intermediate (II) is subjected to protecting group removal, and under the acid condition, cyclization is performed, so that the (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol is obtained. According to the preparation method, the raw materials are low in cost and easy to obtain, the reaction stereoselectivity is high, the operation is simple, the route is short, the cost is low, and the method is suitable for industrialized production of darunavir.(As shown in the description).
Owner:QINGDAO UNIV OF SCI & TECH +1
Process for the preparation of darunavir
A process for the preparation of Darunavir comprises the reacting of 4-amino-N-(2R, 3S) (3-amino-2-hydroxy-4-phenylbutyl)-N-isobutyl-benzenesulfonamide with (3R, 3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol derivative in N-methyl-2-pyrrolidinone and isolating the resulting Darunavir. The process yields Darunavir with a very low level of the difuranyl impurity.
Owner:MYLAN LAB
Method for preparing darunavir amorphous
Owner:SHANGHAI DESANO PHARMA INVESTMENT +1
Method for preparing darunavir
The invention relates to the technical field of medicines and discloses a method for preparing darunavir. The method comprises the following step: preparing 20-30 parts of cobalt, 10-20 parts of triethylamine, 10-20 parts of ferrous oxide, 10-20 parts of zinc oxide, 3-5 parts of 1.2 equivalent of marina hydrogen methane, 20-30 parts of zinc, 50-80 parts of a ferric nitrite solution, 30-40 parts ofa metal base, 40-50 parts of alumina, 10-20 parts of silica, 3-5 parts of p-acetamide benzoyl sulfonyl chloride, 3-5 parts of a precipitant, 50-80 parts of an ethanol solution and 20-30 parts of nickel for later use. According the method for preparing the darunavir, 20-30 parts of cobalt, 10-20 parts of triethylamine, 10-20 parts of ferrous oxide, 10-20 parts of zinc oxide, 3-5 parts of 1.2 equivalent of marina hydrogen methane, 10-20 parts of silica, 3-5 parts of p-acetamide benzoyl sulfonyl chloride, 3-5 parts of the precipitant, 50-80 parts of the ethanol solution and 20-30 parts of nickelare adopted for later use, the reaction materials used in the method are low in price and low in reaction environment requirement, correspondingly, the preparation cost of the darunavir is greatly reduced, patients can afford the darunavir, and the darunavir can truly benefit the patients.
Owner:ABA CHEM CORP
Anti-retroviral compositions
The present invention relates to pharmaceutical antiretroviral compositions comprising a combination of antiretroviral agents (darunavir, dolutegravir and ritonavir), the manufacturing process thereof and use of the said compositions for the prevention, treatment or prophylaxis of HIV infection.
Owner:HETERO LABS LTD
Darunavir composition with improved dissolution speed
ActiveCN111821309AImprove dissolution rateDissolution rate is fastOrganic active ingredientsSuppositories deliveryDiseaseAcquired Immune Deficiency Syndrome Virus
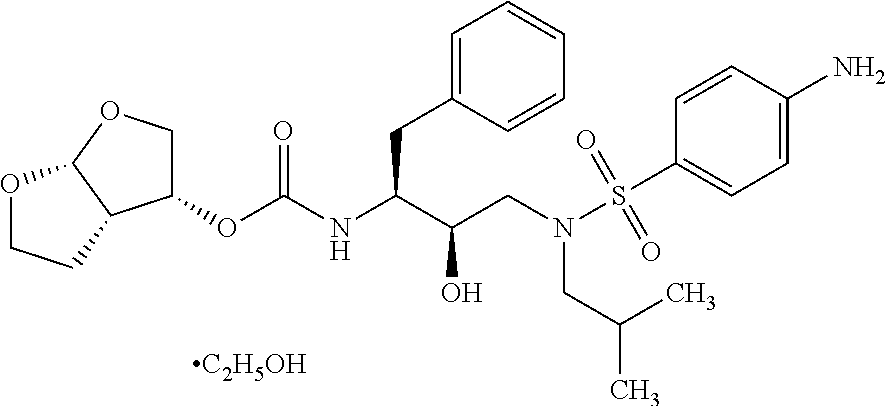
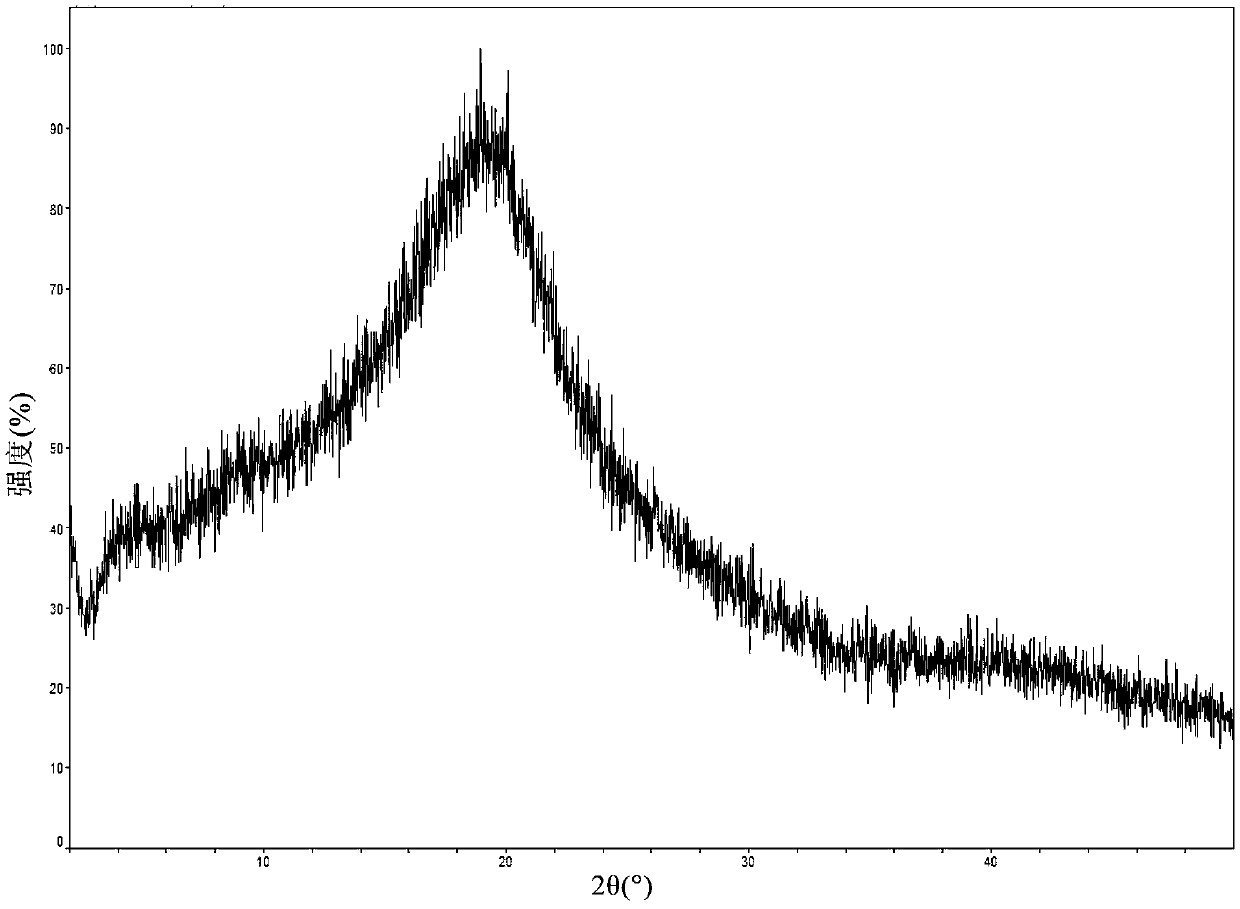
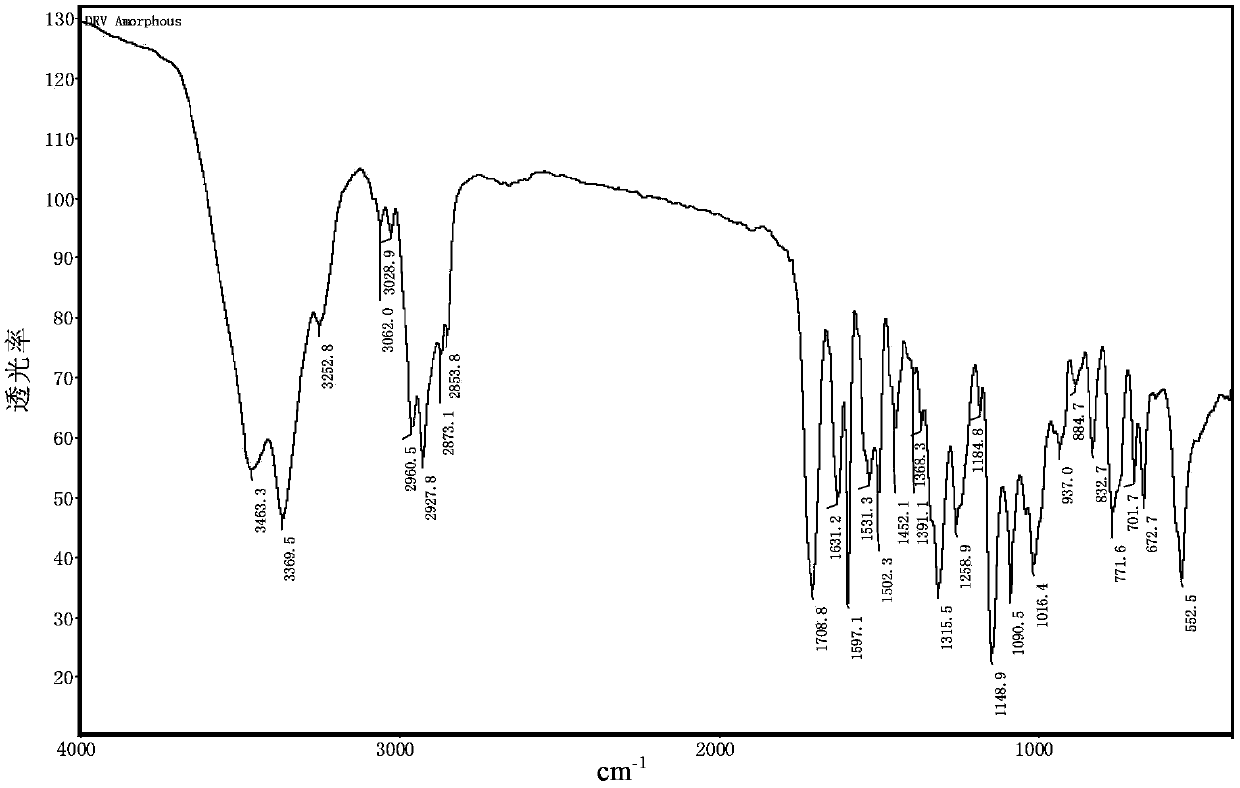
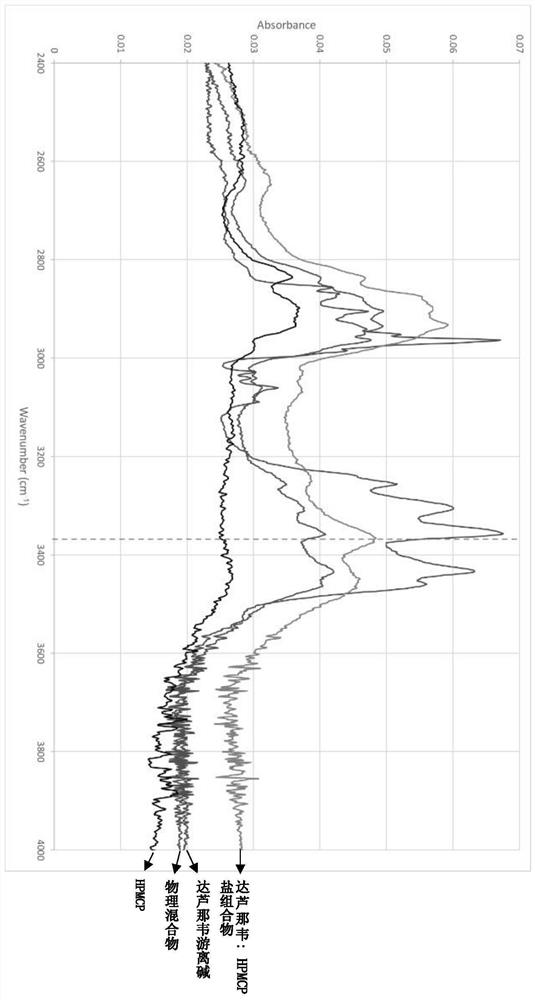
The invention relates to a composition with an improved dissolution rate. The composition comprises a salt formed by darunavir and an acidic polymer. The darunavir: acidic polymer salt composition hasa faster dissolution rate and better stability. The composition can be applied to the treatment of viral infection diseases, such as acquired immunodeficiency syndrome (HIV), viral hepatitis and coronavirus infection.
Owner:SHENZHEN NYCRIST TECH CO LTD
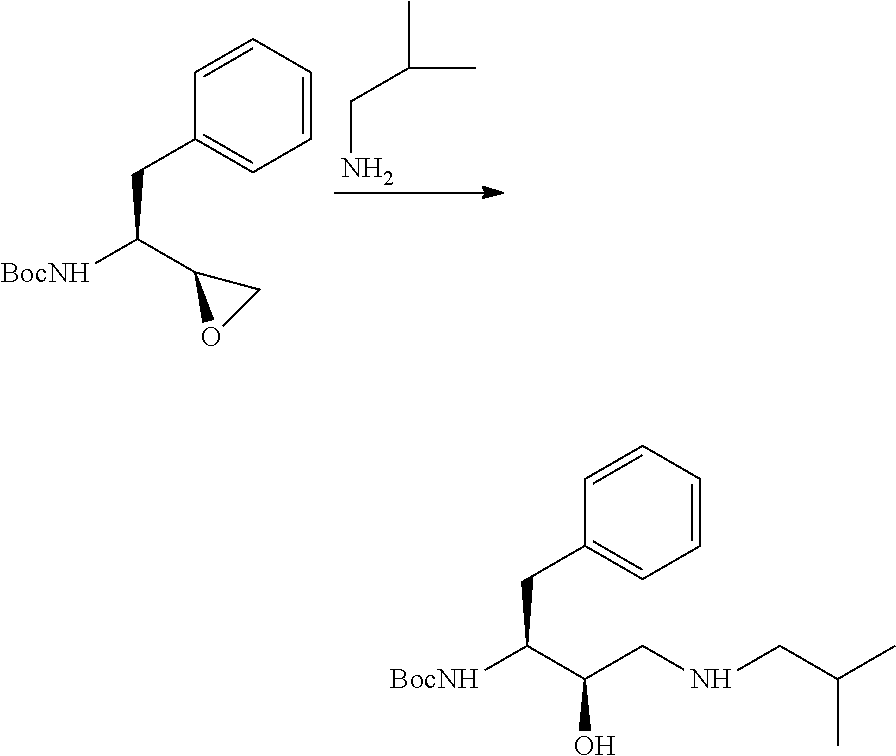
Novel process to prepare intermediates of HIV-protease inhibitors thereof
InactiveUS20160075643A1Conveniently to industrial scaleHigh yieldOrganic compound preparationSulfonic acid amide preparationDarunavir+RitonavirProteinase activity
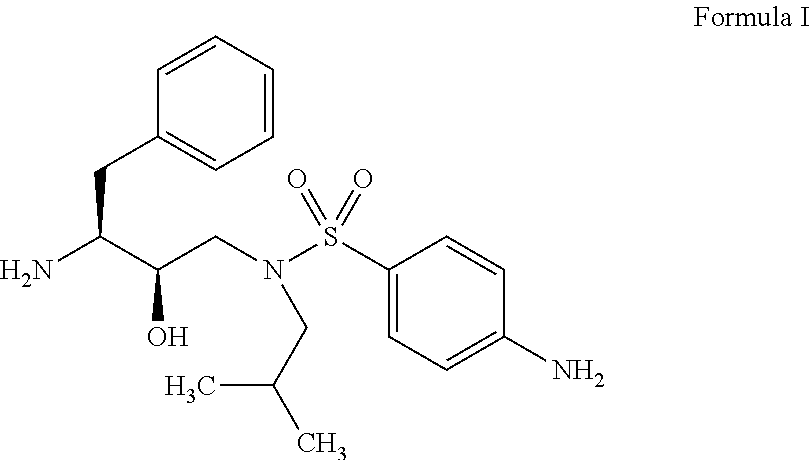
The present invention relates to an industrially feasible and economically viable process for the preparation of (1S,2R)-3-[[4-aminophenyl)-sulfonyl](2-methylpropyl)amino]-2-hydroxy-1-(phenyl-methyl)propyl]amine of formula I and its salt thereof and optionally converting it to HIV-protease inhibitors like Darunavir, Amprenavir or its prodrug Fosamprenavir.
Owner:ZCL CHEM
A kind of ketoreductase mutant used for producing darunavir intermediate
ActiveCN111662889BHigh alcohol dehydrogenase activityHigh coenzyme cycle timesBacteriaMicroorganism based processesTert-Butyloxycarbonyl protecting groupGenetics
The embodiment of the present invention discloses a ketoreductase mutant used to produce darunavir intermediates, which belongs to the field of biocatalytic methods and application technologies, and specifically relates to a method for producing (2S,3S)-1-chloro-3- tert-butoxycarbonylamino-4-phenyl-2-butanol converts 3S-1-chloro-3 ‑tert-butoxycarbonylamino‑4‑phenyl‑2‑butanone is converted to (2S,3S)‑1‑chloro‑3‑tert‑butoxycarbonylamino‑4‑phenyl‑2‑butanol, as compared to wild-type Compared with the sequence, it has higher alcohol dehydrogenase activity, has more than 90% similarity with SEQ ID NO.8 and has one or more mutations in the following characteristics: I54E, S85A, K182V, the ketoreductase mutant The sequence is SEQ ID NO.2. The present invention uses alcohols for coenzyme circulation, the substrate concentration is as high as 110g / L, the amount of substrate / NADP is as high as 1100:1, the number of coenzyme cycles is high, and the downstream application range is effectively expanded.
Owner:NANJING LANGEN BIOLOGICAL SCI & TECH
Anti-retroviral compositions
ActiveUS11045423B2Pharmaceutical product form changeAntiviralsPharmaceutical drugANTIRETROVIRAL AGENTS
The present invention relates to pharmaceutical antiretroviral compositions comprising a combination of antiretroviral agents (darunavir, dolutegravir and ritonavir), the manufacturing process thereof and use of the said compositions for the prevention, treatment or prophylaxis of HIV infection.
Owner:HETERO LABS LTD
A method for synthesizing hexahydrofuro[2,3-b]furan-3-alcohol and its enantiomers
ActiveCN103864813BRaw materials are cheap and easy to getEasy to operateOrganic chemistryFuranEnantiomer
The invention discloses synthetic methods of hexahydrofuro[2,3-b]furan-3-ol and an enantiomer thereof. The synthetic method of hexahydrofuro[2,3-b]furan-3-ol comprises the step c or the step b to the step c or the step a to the step c in the following synthetic route as shown in the description. The synthetic method of the enantiomer (3R,3aS,6aR)hexahydrofuro[2,3-b]furan-3-ol comprises the step c to the step f or the step b to the step f or the step a to the step f in the following synthetic route as shown in the description, wherein R1 is selected from C1 to C4 alkyl or aralkyl, and R2 is selected from C1 to C4 alkyl. The synthetic methods provided by the invention have the advantages of cheap and easily available raw materials, simple operation, low cost and the like, are suitable for large-scale production, and have practical value for realization of industrialized production of darunavir.
Owner:SHANGHAI DESANO CHEM PHARMA +1
A kind of method for preparing darunavir intermediate
The invention discloses a method for preparing a darunavir intermediate, in particular to a method for preparing a compound of formula I.
Owner:成都博腾药业有限公司
Method for preparing darunavir intermediate through biological catalysis
ActiveCN111690696AHigh alcohol dehydrogenase activityIncrease the number of cyclesBacteriaMicroorganism based processesAlcoholMutant
The embodiment of the invention discloses a method for preparing a darunavir intermediate through biological catalysis, and belongs to the technical field of a biological catalysis method and an application. 3S-1-chloro-3-tert-butyloxycarbonylamino-4-phenyl-2-butanol can be converted into (2S, 3S)-1-chloro-3-tert-butyloxycarbonylamino-4-phenyl-2-butanol through a ketoreductase mutant; the ketoreductase mutant is derived from wild type ketoreductase of Starmella magnoliae, has higher alcohol dehydrogenase activity compared with a wild type sequence, has 90% or above similarity with SEQ ID NO.8and has one or more of the following characteristics: I54E, S85A and K182V; and the sequence of the ketoreductase mutant is shown as SEQ ID NO. 4. Alcohols are used for coenzyme circulation, the substrate concentration is up to 110 g / L, the ratio of the substrate dosage to the NADP dosage is up to 1100: 1, the number of times of coenzyme circulation is high, and the downstream application range iseffectively widened.
Owner:NANJING LANGEN BIOLOGICAL SCI & TECH
Process for preparation of Darunavir
ActiveUS9475821B2Simple and cost-effective processHigh purityOrganic chemistry methodsAntiviralsPropionateMedicinal chemistry
Provided are a process for preparation of darunavir or solvates or a pharmaceutically acceptable salt thereof substantially free of bisfuranyl impurities and a process for preparation of amorphous darunavir using the darunavir propionate solvate.
Owner:LAURUS LABS
Method for synthesizing darunavir intermediate by using microchannel reactor
PendingCN113896658ALow impurity contentImprove mixing efficiencyCarbamic acid derivatives preparationOrganic compound preparationPhenylsulfonamidePhenyl group
The invention discloses a method for synthesizing a darunavir intermediate by using a microchannel reactor. The synthesis method comprises the following steps: taking 1-benzyl-2, 3 epoxy n-propyl-tert-butyl carbamate as an initial raw material, and carrying out amination, sulfonylation, Boc protecting group removal and microchannel hydrogenation reduction reaction to generate the darunavir intermediate 4-amino-N-(2R, 3S)-(3-amino-2-hydroxy-4-phenyl-butyl)-N-isobutyl-benzenesulfonamide. The method has the advantages of simplicity in operation, safety, environment friendliness, easiness in batch synthesis, low energy consumption and high purity.
Owner:LIVZON GROUP CHANGZHOU KONY PHARMA
Darunavir inhaled dry powder pharmaceutical composition and preparation method thereof
The present invention belongs to the technical field of medicines and discloses a darunavir inhaled dry powder pharmaceutical composition, and a preparation method and medical uses of the composition.
Owner:JIANGSU ALICORN PHARMATECH CO LTD
Process for preparation of darunavir
ActiveUS20160355525A1Simple and cost-effective processHigh purityOrganic chemistry methodsAntiviralsPropionateImpurity
The present invention provides a process for the preparation of darunavir or solvates or a pharmaceutically acceptable salt thereof substantially free of bisfuranyl impurities, particularly darunavir propionate solvate. The present invention also provides a process for preparation amorphous darunavir using the darunavir propionate solvate.
Owner:LAURUS LABS
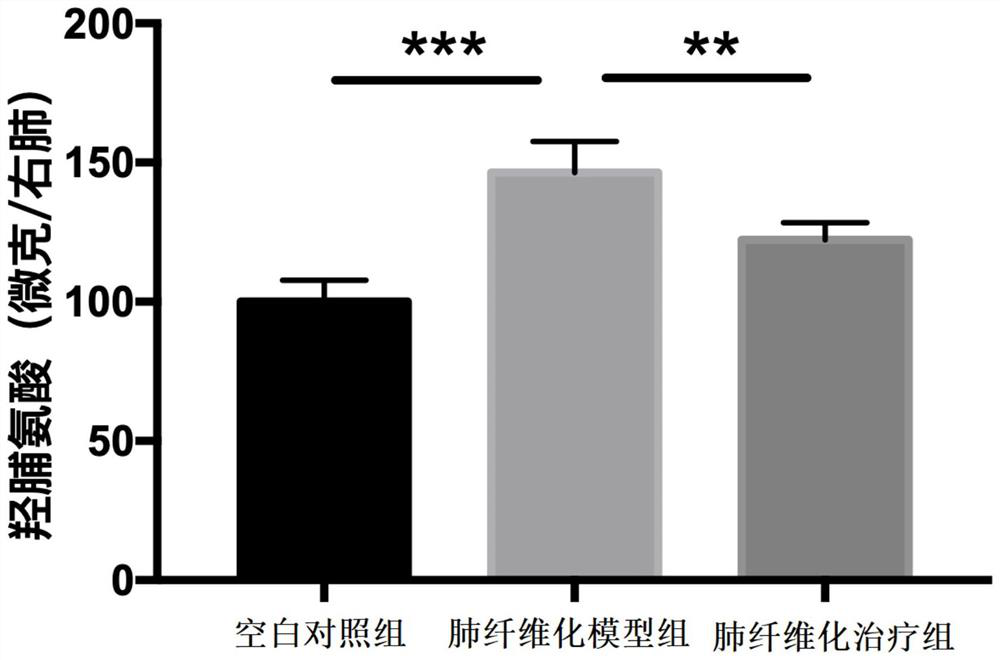
Application of darunavir in preparation of medicine for treating pulmonary fibrosis diseases
PendingCN114028409AGood effectNo adverse reactionOrganic active ingredientsRespiratory disorderDiseasePharmaceutical drug
The invention discloses application of darunavir in preparation of the medicine for treating the pulmonary fibrosis diseases, darunavir has a good effect on pulmonary fibrosis, has no adverse reaction, can slow down bleomycin-induced pulmonary fibrosis of mice, provides a new medicine for treating, relieving or improving the pulmonary fibrosis diseases, and has a good application prospect.
Owner:NANKAI UNIV
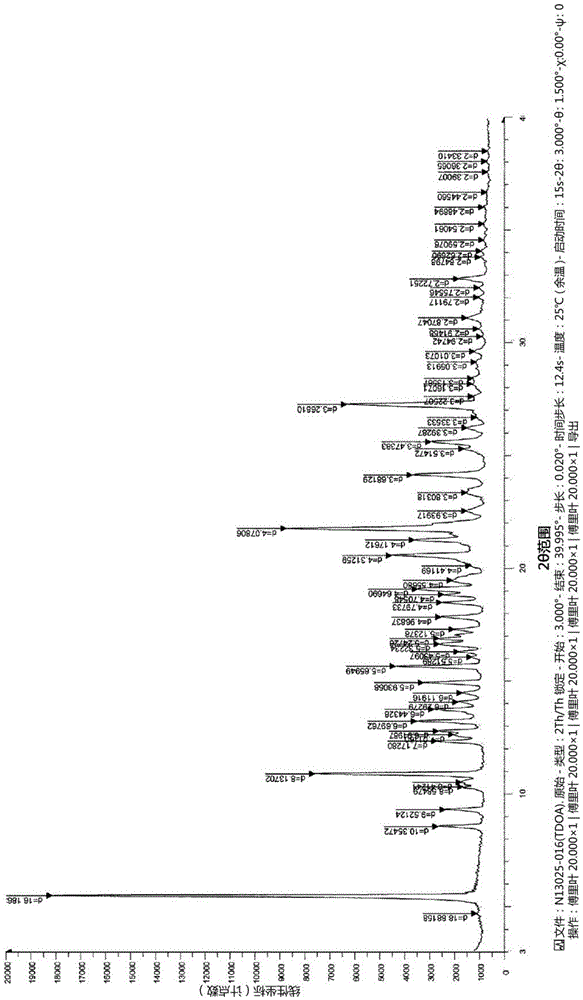
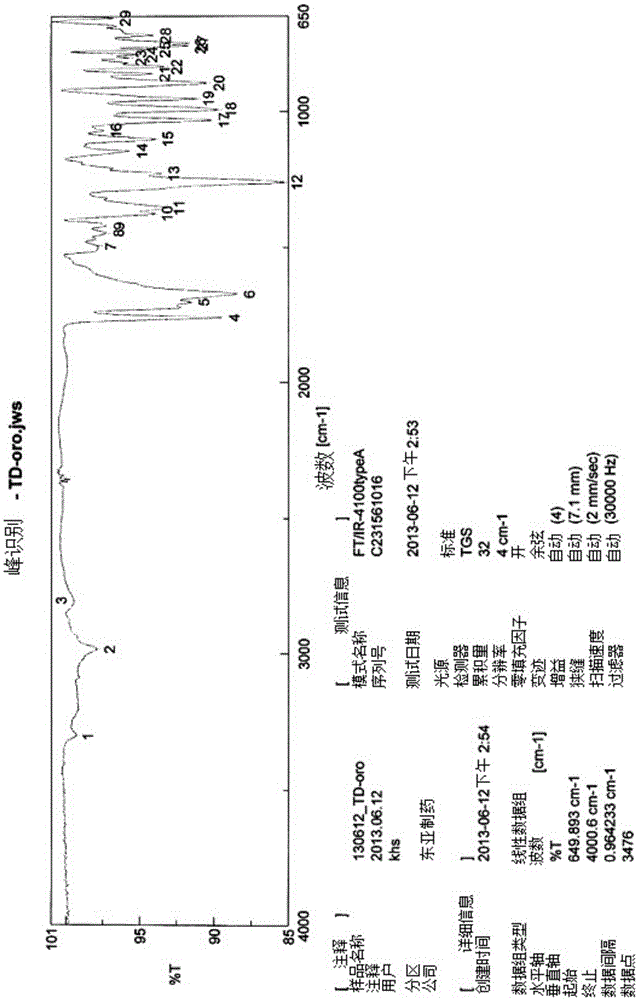
Novel tenofovir disoproxil salt and the preparation method thereof
InactiveCN105452249AImprove stabilityGood light stabilityOrganic chemistry methodsAntiviralsEmtricitabineBULK ACTIVE INGREDIENT
The present invention relates to a novel tenofovir disoproxil salt and the preparation method thereof. The crystalline form of tenofovir disoproxil orotate according to the present invention is an anhydride and it has superior physicochemical properties that are useful in pharmaceutical preparation as it resolves problems caused by physicochemical properties of tenofovir disoproxil fumarate. Namely, the crystalline form of tenofovir disoproxil orotate according to the present invention has a better than equal light stability compared to tenofovir disoproxil fumarate and has a superior stability especially under extreme stress conditions. Additionally, it is neither corrosive nor harmful to human body, thus making it safe for preparation and easy to manage. All of these characteristics make this invention useful for mass production. The present invention also provides a preparation method with and without using organic solvent, thus providing an industrially applicable and environment-friendly preparation method. Furthermore, tenofovir disoproxil orotate prepared according to the present invention can be used as an antiviral treating agent and can also be made into an anti-HIV treating composition if mixed with other drugs that have anti-HIV activity. The composition can be made into mixtures with one to six of the following other anti-HIV active ingredient medications that include Emtricitabine, Efavirenz, Rilpivirenz, Cobicistat, Elvitegravir, Raltegravir, Ritonavir, Lopinavir, Darunavir, Atazanavir and salts thereof for the treatment of HIV infection.
Owner:DONG A ST CO LTD
Preparation method of darunavir
The invention relates to the technical field of chemical engineering and discloses a preparation method of darunavir, which includes a step of preparing raw materials comprising: 20-30 mg of N-dibenzyl-L-methyl phenylalanine ester, 40-90 mg of a N-hydroxyl compound, 20-40 mg of Binqing methane in 1 equivalent, 100-150 mg of butyl lithium, 50-80 mg of a tetrahydrofuran solvent, 70-110 mg of amines,60-100 mg of (2S,3R)-2-amino-4-chloro-phenylbutane-3-ol, 40-60 mg of a halogenated aromatic ring compound, 10-20 mg of triethylamine and dichloromethane, 5-20 mg of di-tert butyl dicarbonate in 1.3 equivalents, and 20-40 mg of metal alkali; and preserving 20-30 mg of the N-dibenzyl-L-methyl phenylalanine ester, 20-40 mg of the Binqing methane in 1 equivalent, 100-150 mg of the butyl lithium and 70-110 mg of the amines at -20 DEG C for later use. The preparation method is low in demand on environment, avoids complex operations in conventional preparation methods, is easier to prepare the darunavir and is not liable to cause waste on consumables, so that the method is convenient to carry out.
Owner:ABA CHEM NANTONG
A kind of industrialized production method of high-purity darunavir intermediate
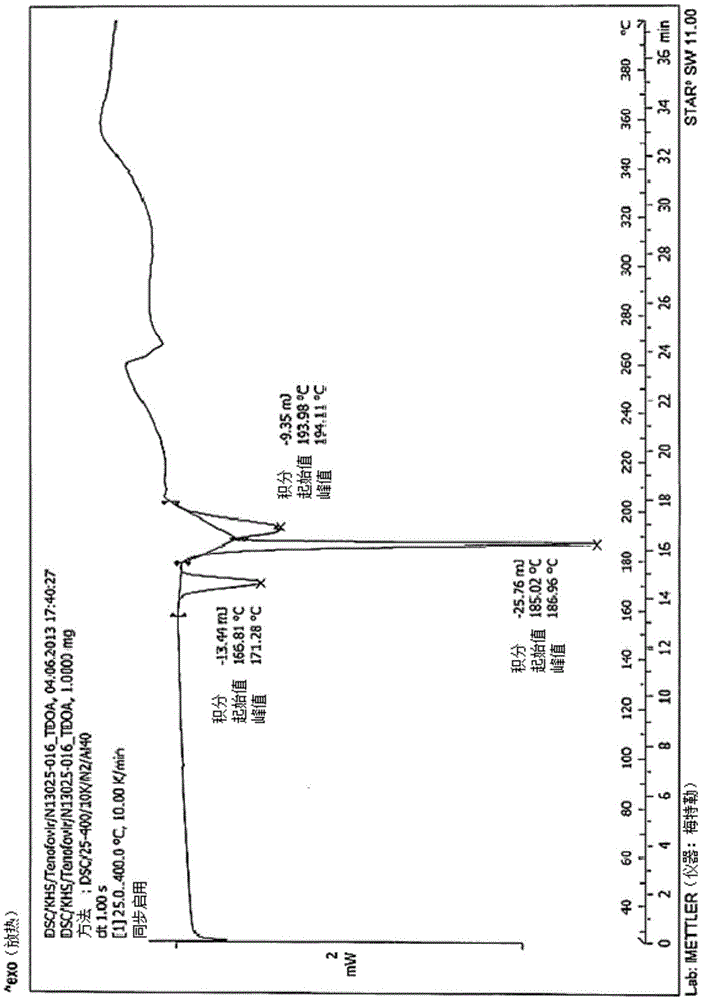
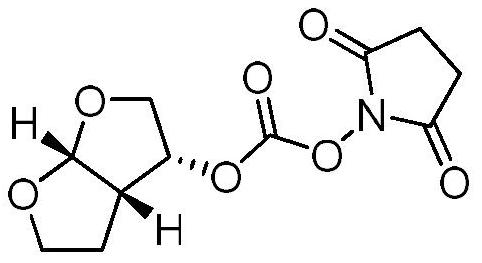
The invention relates to the field of biochemical technology, in particular to an industrial production method of a darunavir intermediate (3R, 3aS, 6aR)-hydroxyhexahydrofuro[2,3-β]furylsuccinimidyl carbonate. Compared with the existing technology, the industrial production method of (3R, 3AS, 6AR)-hydroxyhexahydrofuro[2,3-β]furylsuccinimide-based carbonate provided by the present invention can efficiently and stably produce High-quality (3R,3AS,6AR)-hydroxyhexahydrofuro[2,3-β]furylsuccinimide carbonate was produced with a purity greater than 99% and an unknown single impurity less than 0.1%.
Owner:GENCHEM & GENPHARM CHANGZHOU CO LTD
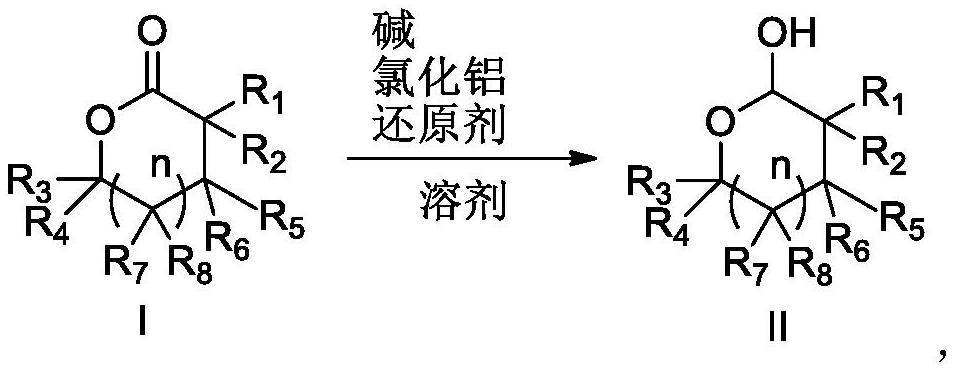
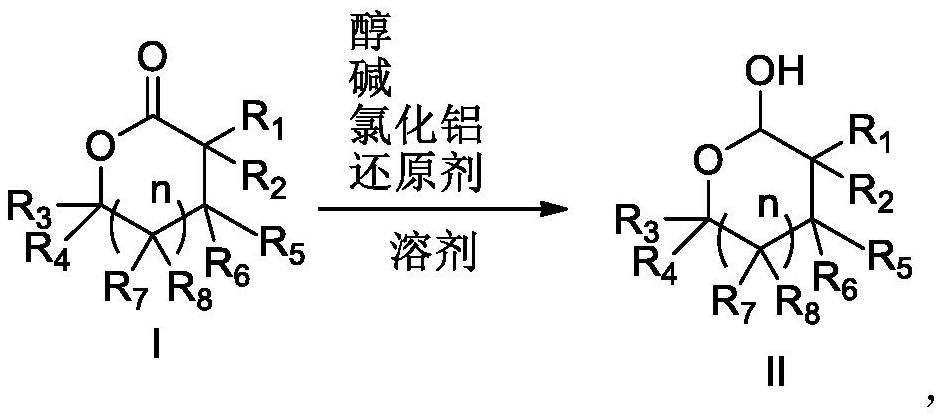
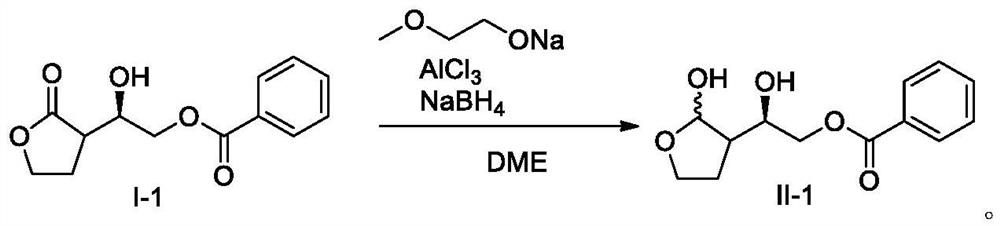
Preparation method for reducing lactone into hemiacetal
The invention relates to the field of medicine synthesis, particularly to a preparation method for reducing a lactone structure into hemiacetal, particularly to a preparation method for a darunavir intermediate. According to the method, a compound shown in a formula I is reduced under the conditions of alkali, aluminum trichloride, a reducing agent and a solvent to prepare a compound shown in a formula II, or the compound shown in the formula I is reduced under the conditions of alcohol, alkali, aluminum trichloride, a reducing agent and a solvent to prepare the compound shown in the formula II, wherein R1, R2, R3, R4, R5 and R7 are hydrogen, hydroxyl, amino, alkyl, aryl, fluorine, chlorine or bromine, R6 and R8 are hydrogen, hydroxyl, amino, benzoyloxy, alkyl, aryl, fluorine, chlorine orbromine, and n is 0 or 1.
Owner:ZHEJIANG JIUZHOU PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Synthetic methods of hexahydrofuro[2,3-b]furan-3-ol and enantiomer thereof Synthetic methods of hexahydrofuro[2,3-b]furan-3-ol and enantiomer thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a1da4192-7904-4c16-93c1-c43592e12b61/FDA00002610524400011.png)
![Synthetic methods of hexahydrofuro[2,3-b]furan-3-ol and enantiomer thereof Synthetic methods of hexahydrofuro[2,3-b]furan-3-ol and enantiomer thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a1da4192-7904-4c16-93c1-c43592e12b61/FDA00002610524400012.png)
![Synthetic methods of hexahydrofuro[2,3-b]furan-3-ol and enantiomer thereof Synthetic methods of hexahydrofuro[2,3-b]furan-3-ol and enantiomer thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a1da4192-7904-4c16-93c1-c43592e12b61/BDA00002610524500021.png)







![Method for preparing (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol Method for preparing (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/783db2a2-0a71-4d9c-adab-79549c237b61/BDA0001220355190000011.png)
![Method for preparing (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol Method for preparing (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/783db2a2-0a71-4d9c-adab-79549c237b61/BDA0001220355190000021.png)
![Method for preparing (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol Method for preparing (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/783db2a2-0a71-4d9c-adab-79549c237b61/BDA0001220355190000031.png)








![A method for synthesizing hexahydrofuro[2,3-b]furan-3-alcohol and its enantiomers A method for synthesizing hexahydrofuro[2,3-b]furan-3-alcohol and its enantiomers](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0d89e49d-5ff7-4dba-a82b-488f17d96f62/BDA00002610524500021.png)
![A method for synthesizing hexahydrofuro[2,3-b]furan-3-alcohol and its enantiomers A method for synthesizing hexahydrofuro[2,3-b]furan-3-alcohol and its enantiomers](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0d89e49d-5ff7-4dba-a82b-488f17d96f62/BDA00002610524500031.png)
![A method for synthesizing hexahydrofuro[2,3-b]furan-3-alcohol and its enantiomers A method for synthesizing hexahydrofuro[2,3-b]furan-3-alcohol and its enantiomers](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0d89e49d-5ff7-4dba-a82b-488f17d96f62/BDA00002610524500041.png)