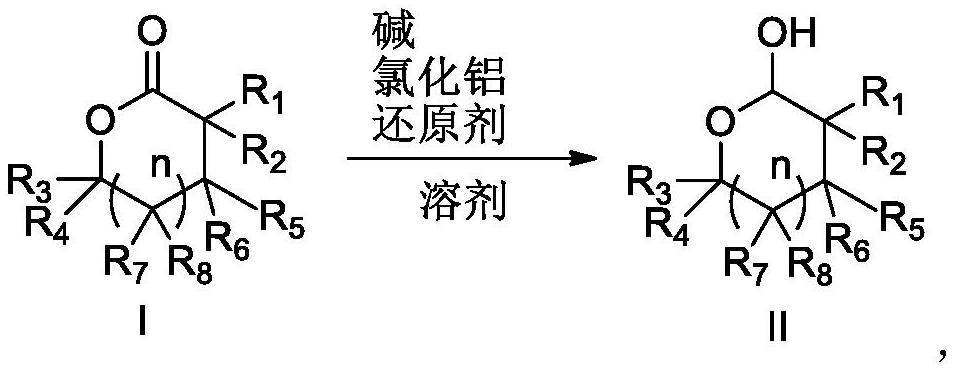
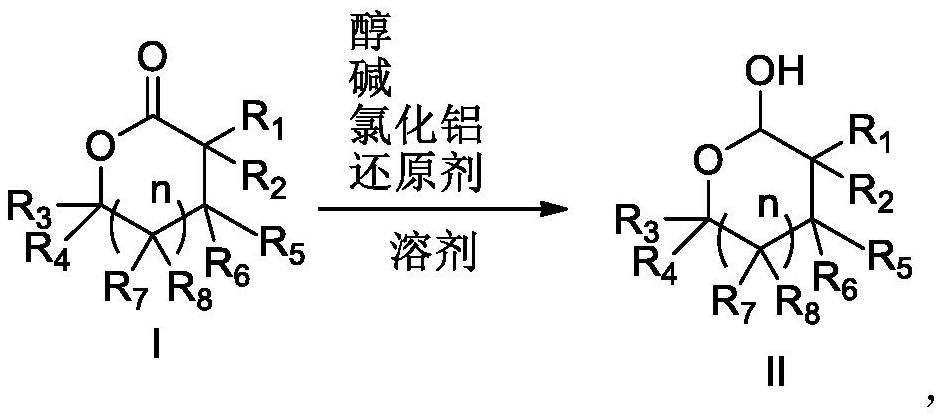
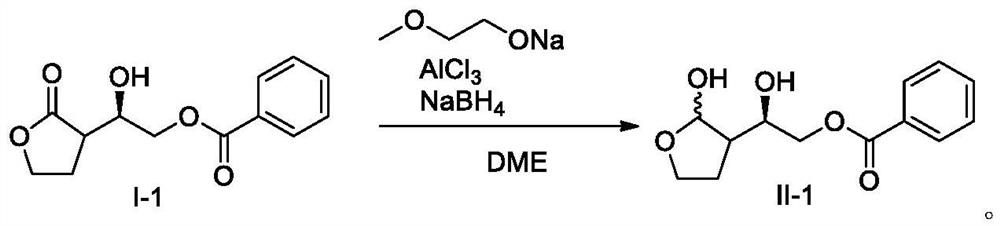
Preparation method for reducing lactone into hemiacetal
A technology of hemiacetal and lactone, applied in the direction of organic chemistry, etc., can solve the problems of not knowing the synthetic route of red aluminum, no disclosure, etc., and achieve the effects of high application value, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]
[0039] Drop into ethylene glycol dimethyl ether (50ml) in the 100ml three-necked flask, the ethylene glycol monomethyl ether solution (5.2g, 60mmol) of 50% sodium ethylene glycol monomethyl ether starts magnetic stirring, after stirring and dissolving, cool down to 0 -5°C, add aluminum trichloride (4.0g, 30mmol), stir for 10min, add sodium borohydride (1.1g, 30mmol) at 0-5°C, stir for 10min, then add compound I-1 at 0-5°C ( 5.0g, 20mmol) in 10ml of ethylene glycol dimethyl ether solution, stirred and reacted at 0-5°C for 4 hours; added 7.5g of concentrated sulfuric acid into 45g of water, cooled to 0-5°C, and then added the reaction system dropwise into the sulfuric acid aqueous solution, Extract the aqueous acid phase with 50ml*3 ethyl acetate, combine the organic phases, wash once with 50ml saturated aqueous sodium bicarbonate solution, and wash once with 50ml drinking water, and evaporate the organic phase to dryness under reduced pressure to obtain 4.5g of white...
Embodiment 2
[0041]
[0042] Drop into ethylene glycol dimethyl ether (70ml) in the 100ml three-necked flask, the ethylene glycol monomethyl ether solution (5.2g, 60mmol) of 50% sodium ethylene glycol monomethyl ether starts magnetic stirring, after stirring and dissolving, cool to 0 -5°C, add aluminum trichloride (4.0g, 30mmol), stir for 10min, add sodium borohydride (1.1g, 30mmol) at 0-5°C, stir for 10min, add compound I-2 at 0-5°C ( 7.44g, 20mmol) in 10ml of ethylene glycol dimethyl ether solution, stirred and reacted at 0-5°C for 4 hours; added 7.5g of concentrated sulfuric acid into 70g of water, cooled to 0-5°C, and then added the reaction system dropwise into the sulfuric acid aqueous solution, Extract the aqueous acid phase with 70ml*3 ethyl acetate, combine the organic phases, wash once with 70ml saturated aqueous sodium bicarbonate solution, and wash once with 70ml drinking water, and evaporate the organic phase to dryness under reduced pressure to obtain 6.8g of white solid, w...
Embodiment 3
[0044]
[0045] Put ethylene glycol dimethyl ether (100ml) into a 250ml three-necked flask, ethylene glycol monomethyl ether (4.55g, 60mmol) and start magnetic stirring, after stirring and dissolving, cool down to 0-5°C, add aluminum trichloride (4.0 g, 30mmol), stirred for 10min, triethylamine (6.0g, 60mmol) was added dropwise at 0-5°C, stirred for 10min, sodium borohydride (1.1g, 30mmol) was added at 0-5°C, and after stirring for 10min, 0- Add compound I-1 (5.0g, 20mmol) in 10ml of ethylene glycol dimethyl ether solution at 5°C, stir and react at 0-5°C for 2 hours; add 7.5g of concentrated sulfuric acid into 45g of water, cool to 0-5°C, and then Add the reaction system dropwise into aqueous sulfuric acid solution, extract the aqueous acid phase with 50ml*3 ethyl acetate, combine the organic phases, wash once with 50ml saturated aqueous sodium bicarbonate solution, and wash once with 50ml drinking water, and evaporate the organic phase to dryness under reduced pressure. 4....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com