Darunavir composition with improved dissolution speed
A technology for darunavir and dissolution rate, applied in the field of compositions with improved dissolution rate, can solve problems such as no darunavir involved, and achieve the effects of fast dissolution rate and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0060] Embodiment 2: the mensuration of darunavir content:
[0061] Agilent 1260 liquid chromatograph, Agilent C18 (15×0.46mm) chromatographic column, acetonitrile:water ratio of 1:1 as mobile phase, injection volume 20μL, flow rate 1mL / min, UV detection wavelength 267nm.
[0062] Take an appropriate amount of darunavir ethanol solvate, accurately weighed, add mobile phase to dissolve and quantitatively dilute to make a solution containing about 10 μg per 1 mL, as a reference solution, accurately measure 20 μL, inject it into a liquid chromatograph, and record the chromatogram picture.
[0063] In addition, get darunavir obtained in Example 1: an appropriate amount of acidic polymer salt composition, and measure with the same method, and calculate with the peak area by the external standard method, darunavir: darunavir in the acidic polymer salt composition The weight percentage of Wei is about 40%.
Embodiment 3
[0064] Embodiment 3: infrared spectrum measurement
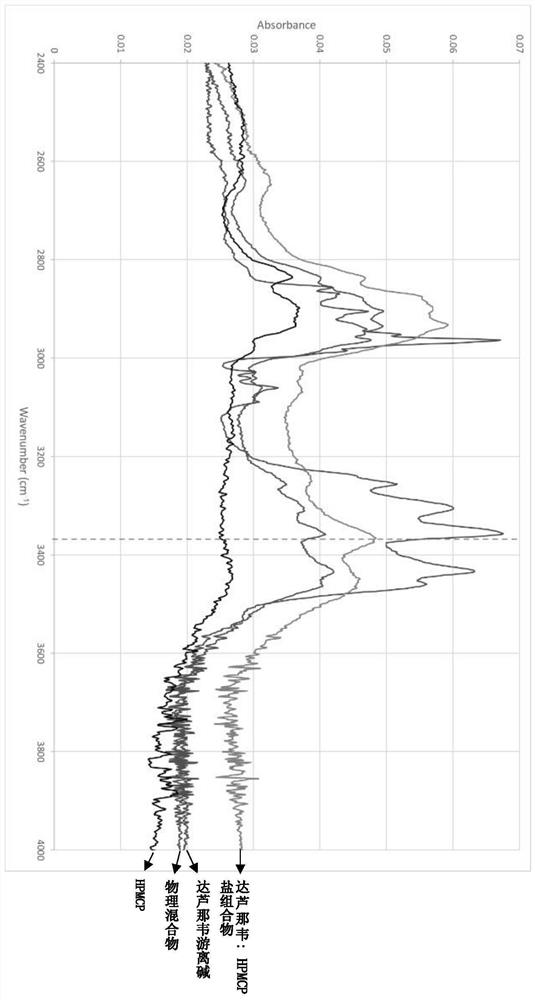
[0065] A Fourier transform infrared spectrometer (Shimadzu) was used with a matched attenuated internal reflectance accessory fitted with a diamond crystal.
[0066] The spectral acquisition range is 4000-650cm-1, 32 scans, and the spectral resolution is 4.0cm-1. Before recording the spectrum of each sample, the measurement was performed with air as a blank.
[0067] In this disclosure, the term "mixture" or "physical mixture" refers to a simple physical mixture of darunavir and HPMCP obtained by combining the dry components and then physically stirring them together.
[0068] As is known in the art, co-powdering does not substantially alter the physical form of the drug, eg its crystalline or amorphous character, co-powdering is not intended to produce an amorphous drug / polymer dispersion.
[0069] Darunavir obtained in Example 1: the acidic polymer salt composition is carried out infrared spectrum test, and with 40% daru...
Embodiment 4
[0083] Example 4: X-Ray Powder Diffraction (XRPD)
[0084] X-ray powder diffraction was carried out using the Holland PANalytical X’Pert sharp shadow X-ray powder diffractometer (PW3040 / 60), using Cu-Kα radiation, wavelength The divergence slit is 1 / 8°, the X-ray light tube voltage is 45kV, the X-ray light tube current is 40mA, the scanning range is 2-40° (2θ), the step size is 0.0260°, and the scanning time of each step is 78.7950s.
[0085] Spread the sample on the sample tray for testing, data collection software X’Pert Data Collector, data viewing software HighScore Plus.
[0086] Darunavir of the present invention: physical state and physical stability of acidic polymer salt are measured, choose darunavir: HPMCP salt. image 3 For darunavir: the X-ray powder diffraction pattern of the HPMCP salt separated and measured immediately after spray-drying preparation shows diffuse peaks in the figure and does not show sharp diffraction peaks, which confirms that the product is a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com