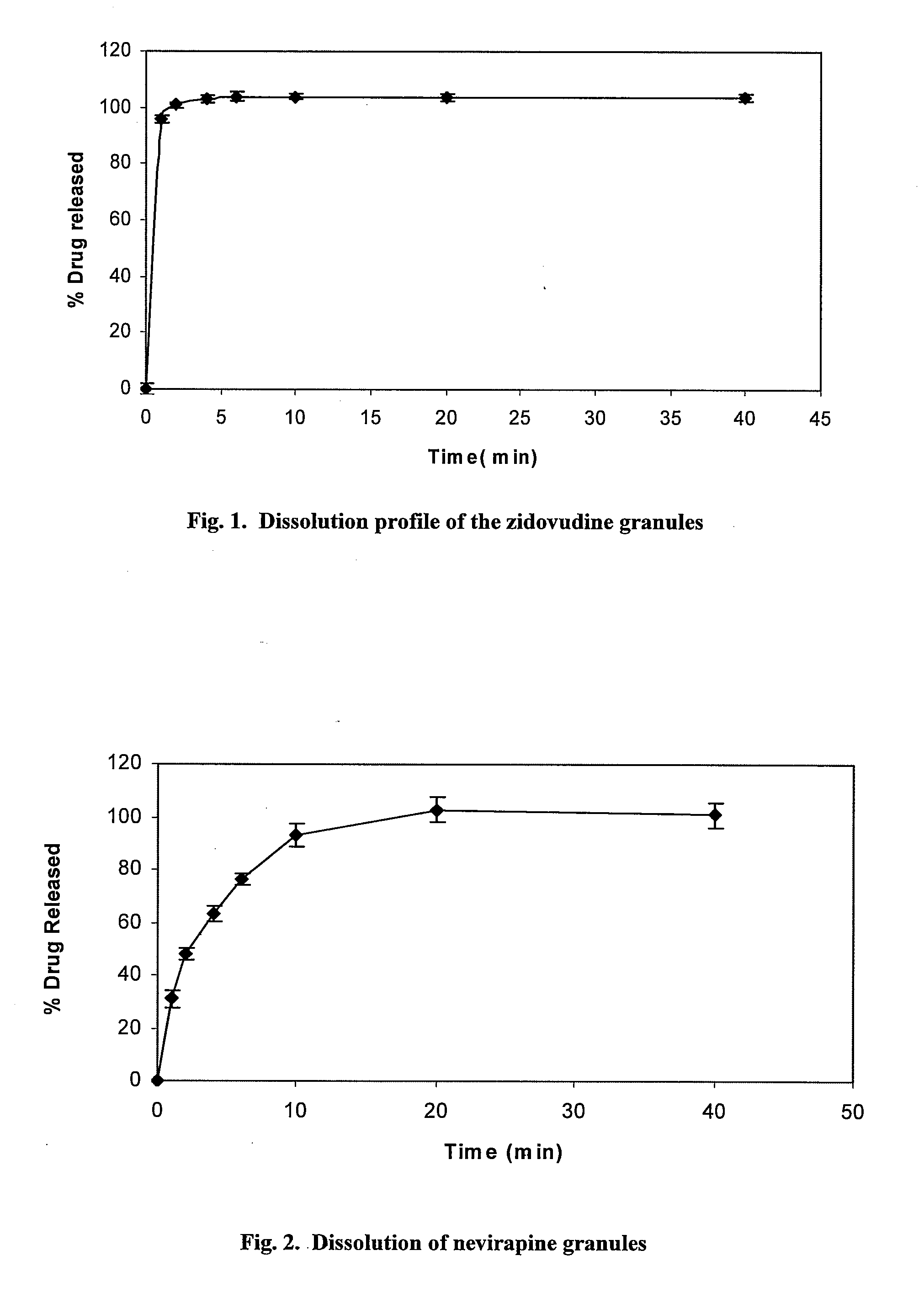
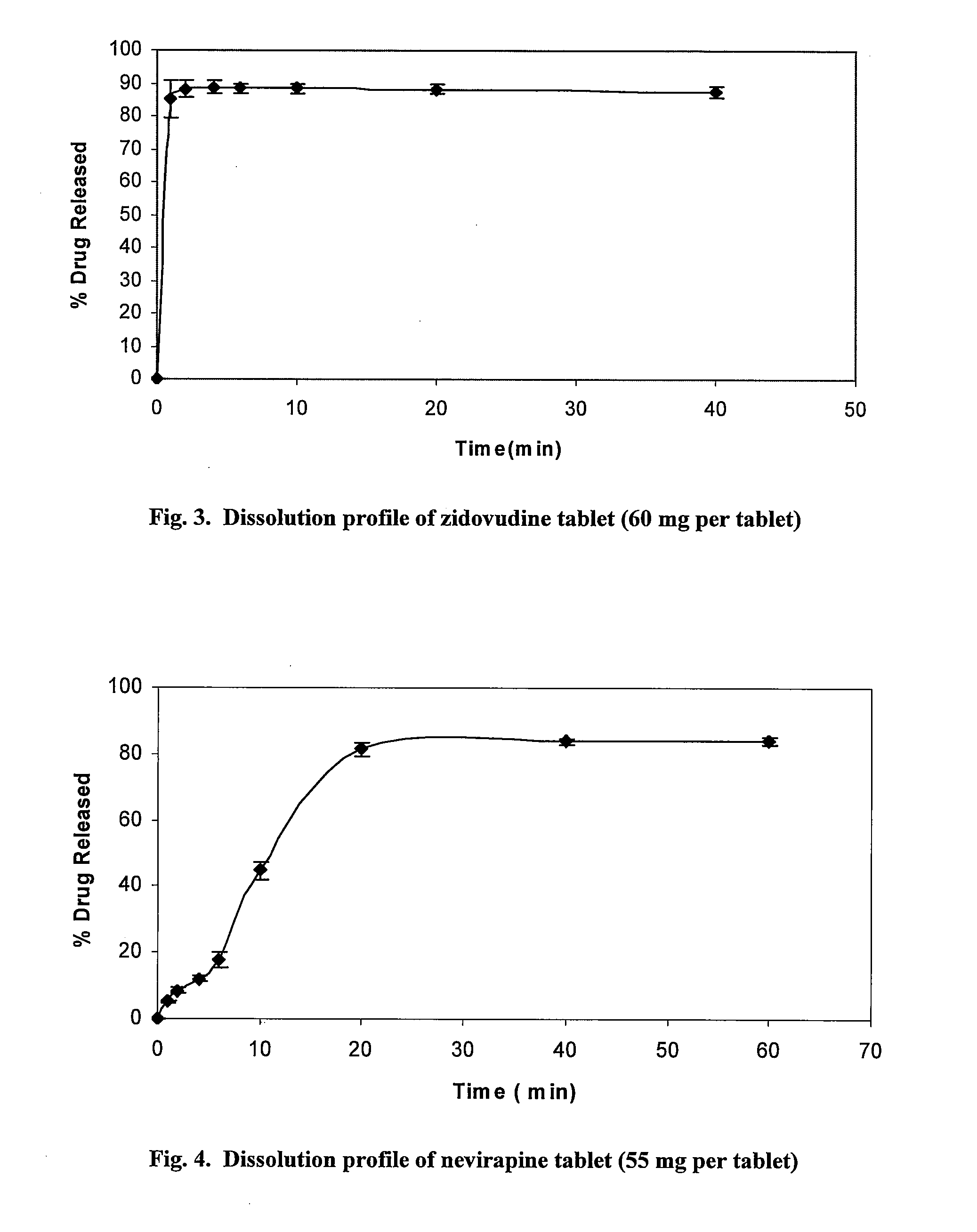
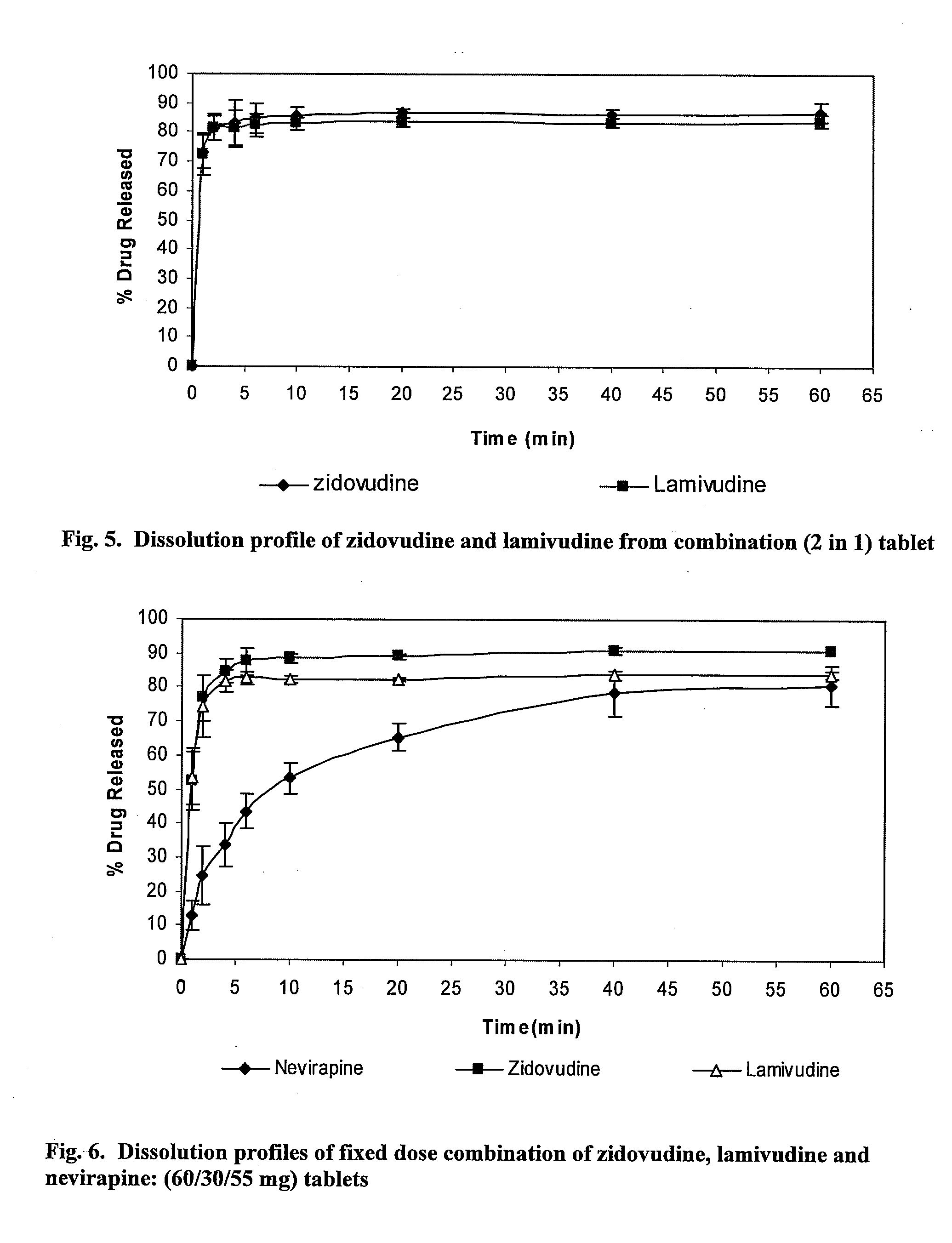
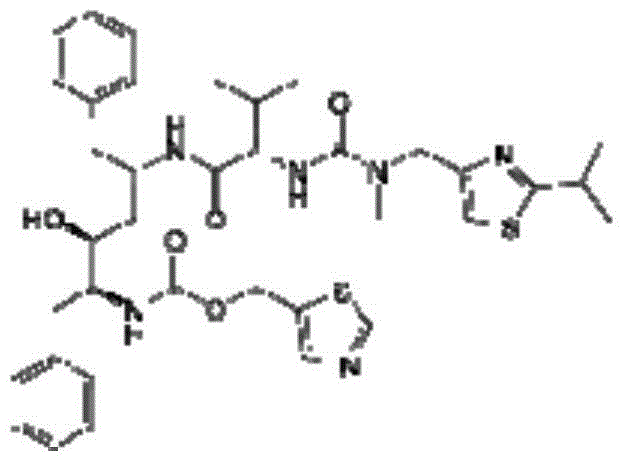
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
56 results about "ANTIRETROVIRAL AGENTS" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The management of HIV/AIDS normally includes the use of multiple antiretroviral drugs in an attempt to control HIV infection. There are several classes of antiretroviral agents that act on different stages of the HIV life-cycle. The use of multiple drugs that act on different viral targets is known as highly active antiretroviral therapy (HAART).
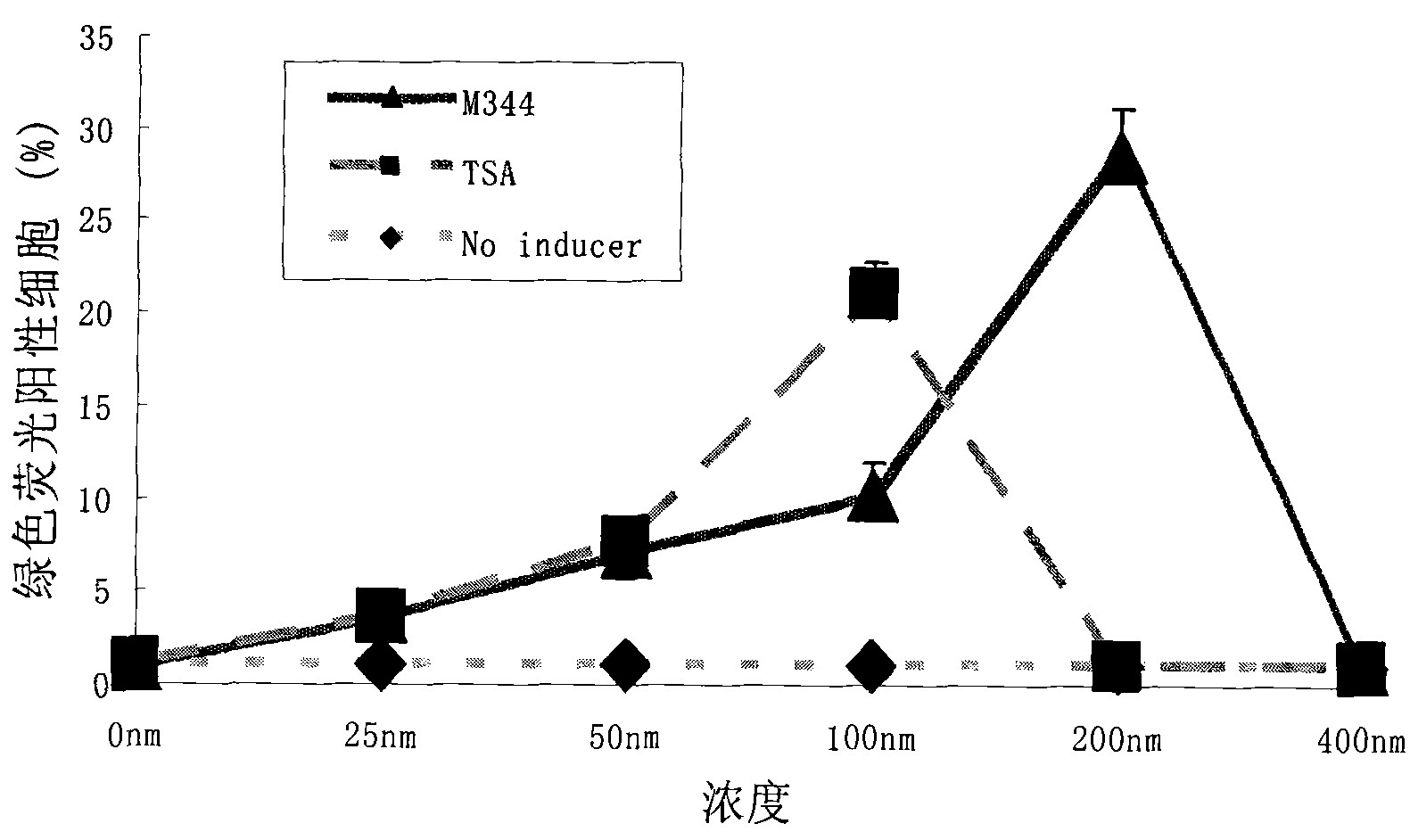
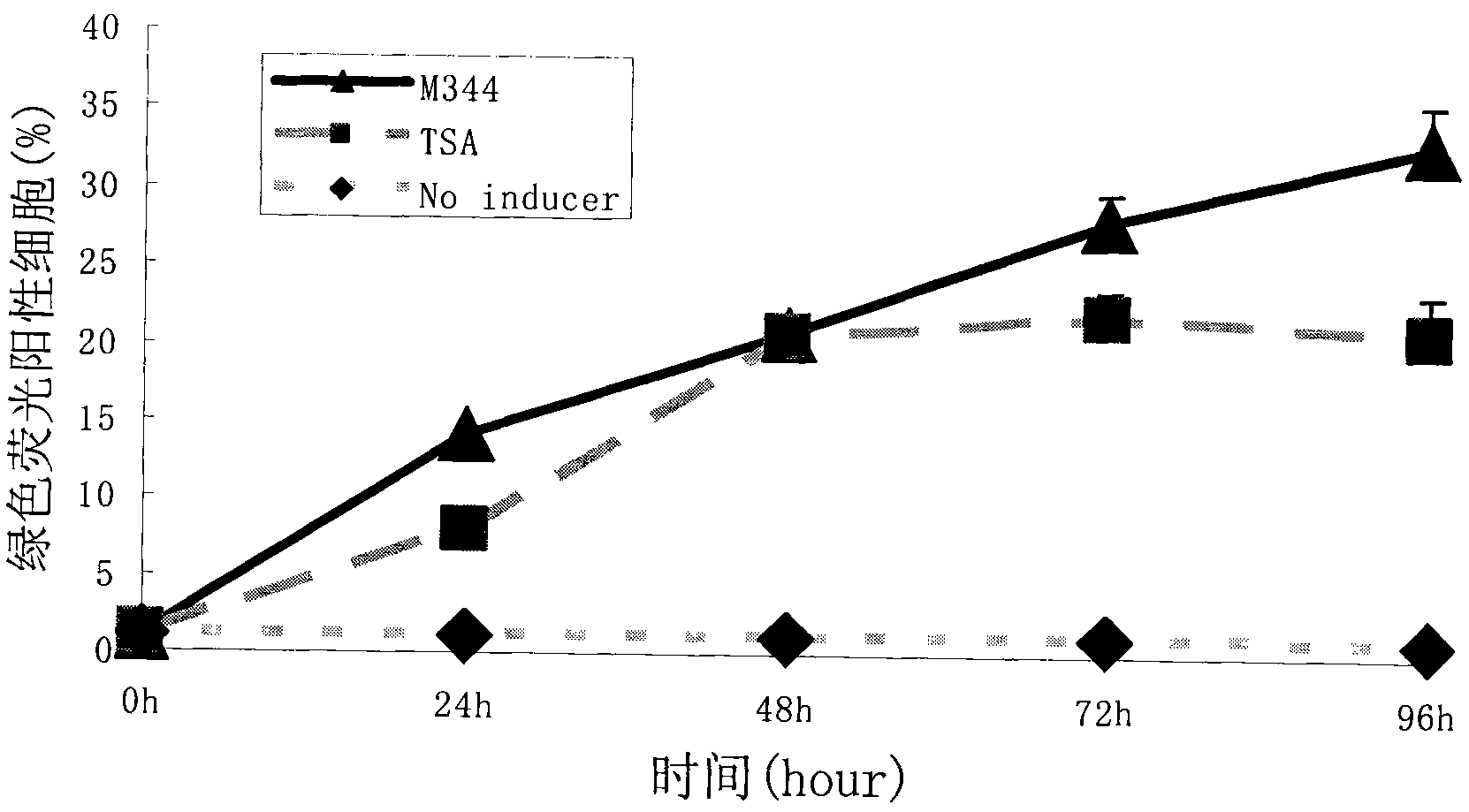
Combination therapy comprising the use of protein kinase C modulators and Histone Deacetylase inhibitors for treating HIV-1 latency
InactiveUS20100166806A1Adverse propertyPrevent HIV-1-induced cytotoxicityBiocideOrganic chemistryReverse transcriptaseHydroxamic acid
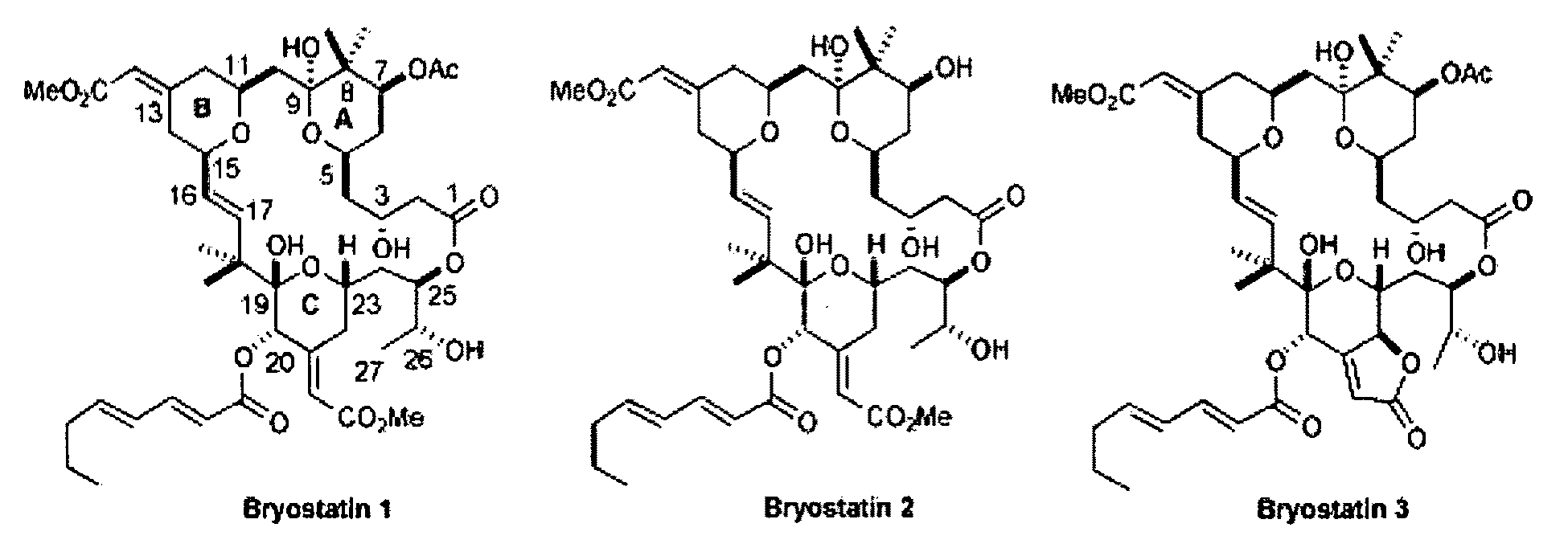
The invention relates to a combination of treatments, more particularly a combination treatment for HIV-1 infection. The present invention is directed to the use of bryostatin-1 and their natural and synthetic derivatives for AIDS therapy, in particular to the use of bryostatins in combination with other active drugs such as Histone Deacetylases (HDACs) inhibitors and anti-retrovirals, for the treatment of HIV-1 latency. According to the present invention, we provide a combination therapy for the treatment of HIV-1 latency which employs bryostatin-1 (and analogues) and one of the following HDAC inhibitors; valproic acid, butyrate derivatives, hydroxamic acids and benzamides. While HDACi can be used in continuous dosing protocol, bryostatins can be used following a cyclical dosing protocol. Bryostatins can be formulated in pharmaceutical acceptable carriers including nanoparticles, phospholipids nanosomes and / or biodegradable polymer nanospheres. This combination therapy needs to be used in patients treated with antiretroviral therapy (HIV-1 protease inhibitors, HIV-1 reverse transcriptase inhibitors, HIV-1 integrase inhibitors, CCR5 co-receptor inhibitors and fusion inhibitors).
Owner:APHIOS
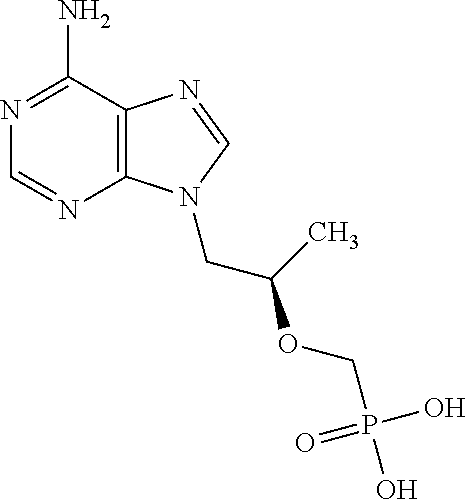
Organic thiophosphate antiretroviral agents
InactiveUS20090239817A1Reducing and preventing effectPromote repairBiocideSulfur/selenium/tellurium active ingredientsImmunodeficiency virusAmifostine
A method for the prevention or treatment of human immunodeficiency virus infection by administering an effective amount of amifostine, phosphonol, or similar compound to an individual in need is provided.
Owner:US DEPT OF HEALTH & HUMAN SERVICES +1
Application of bromine structural domain protein inhibitor to preparation of anti-HIV-1 latency drugs
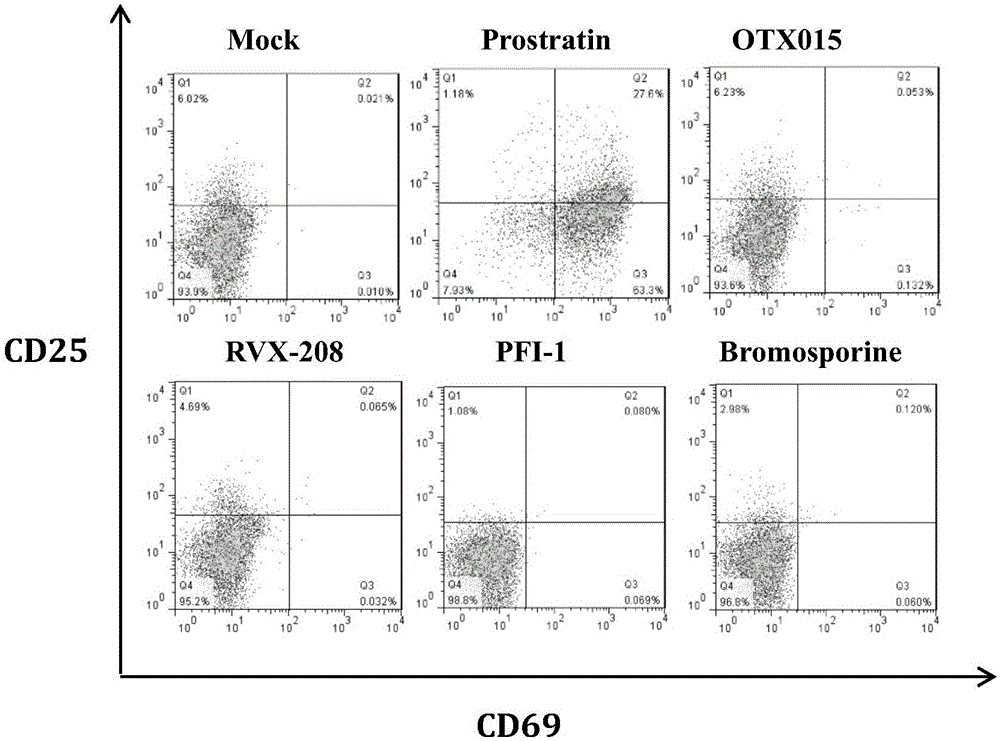
InactiveCN106265679AHigh induction activationDoes not cause systemic T cell activationAntiviralsEster active ingredientsWhole bodyFactor ii
The invention belongs to the medicine field and relates to the application of a bromine structural protein BET inhibitor to anti-HIV-1 latency treatment. Experiments prove that the chemical has an HIV-1 latent cell activation induction effect without causing activation of T cells of the whole body, can achieve synergetic activation after being combined with a protein kinase c agonist or cytokines, and can kill activated latent infected cells after being combined with antiretroviral drugs, so as to accelerate the elimination of a latent virus storage. Furthermore, the bromine structural domain protein inhibitor can be used for preparing anti-HIV-1 latency drugs and can provide a new interventional way and strategy for complete cure of AIDS.
Owner:FUDAN UNIV
Potent combinations of zidovudine and drugs that select for the K65R mutation in the HIV polymerase
InactiveCN101878032ALow toxicityImprove absolute antiviral effectAntiviralsCarbohydrate active ingredientsNucleoside Reverse Transcriptase InhibitorRetroviral infection
Owner:EMORY UNIVERSITY
Single cell analysis of HIV replication capacity and drug resistance
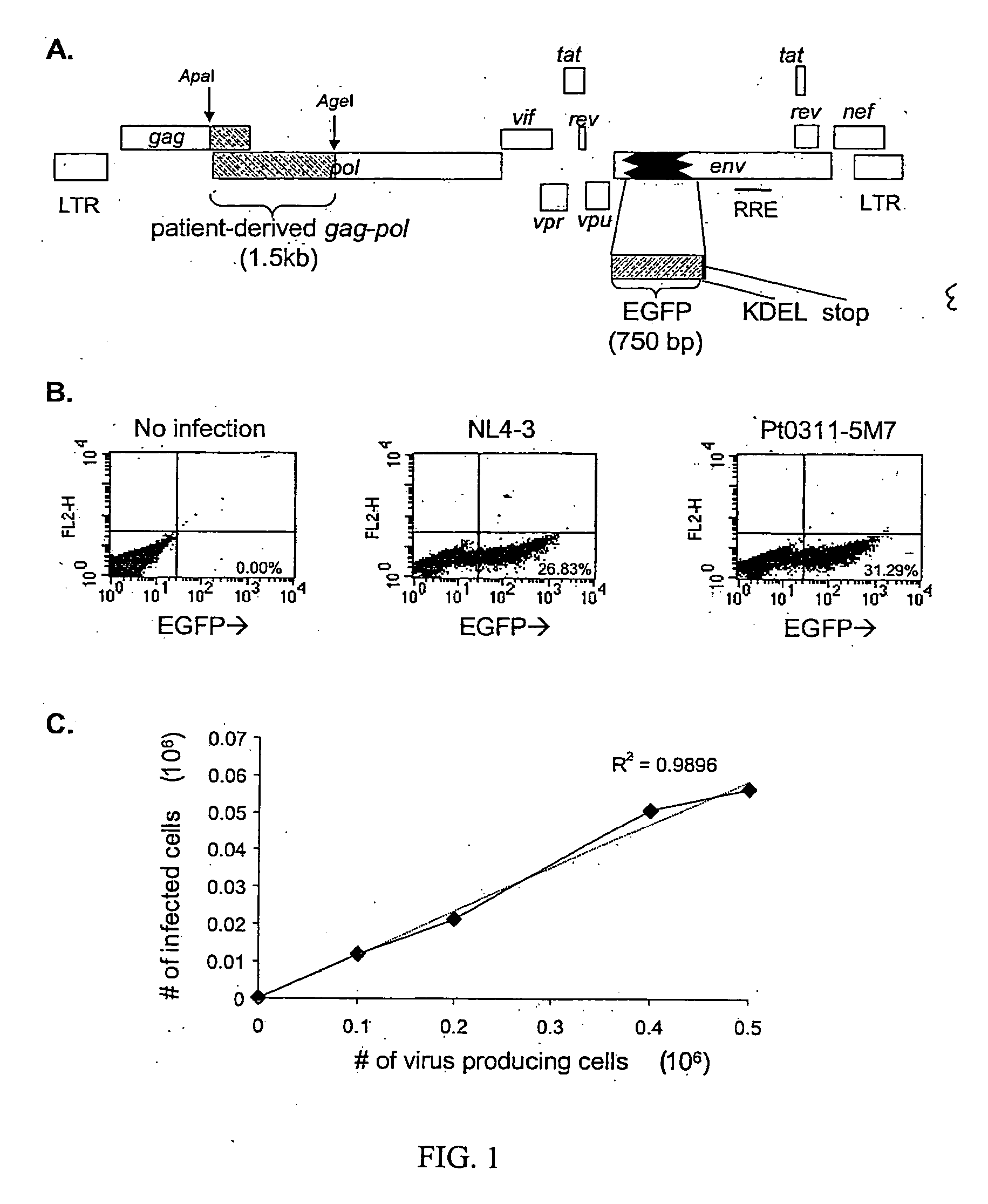
ActiveUS20050244818A1Less-effective in controllingAccurate countVectorsMicrobiological testing/measurementAssayResistant virus
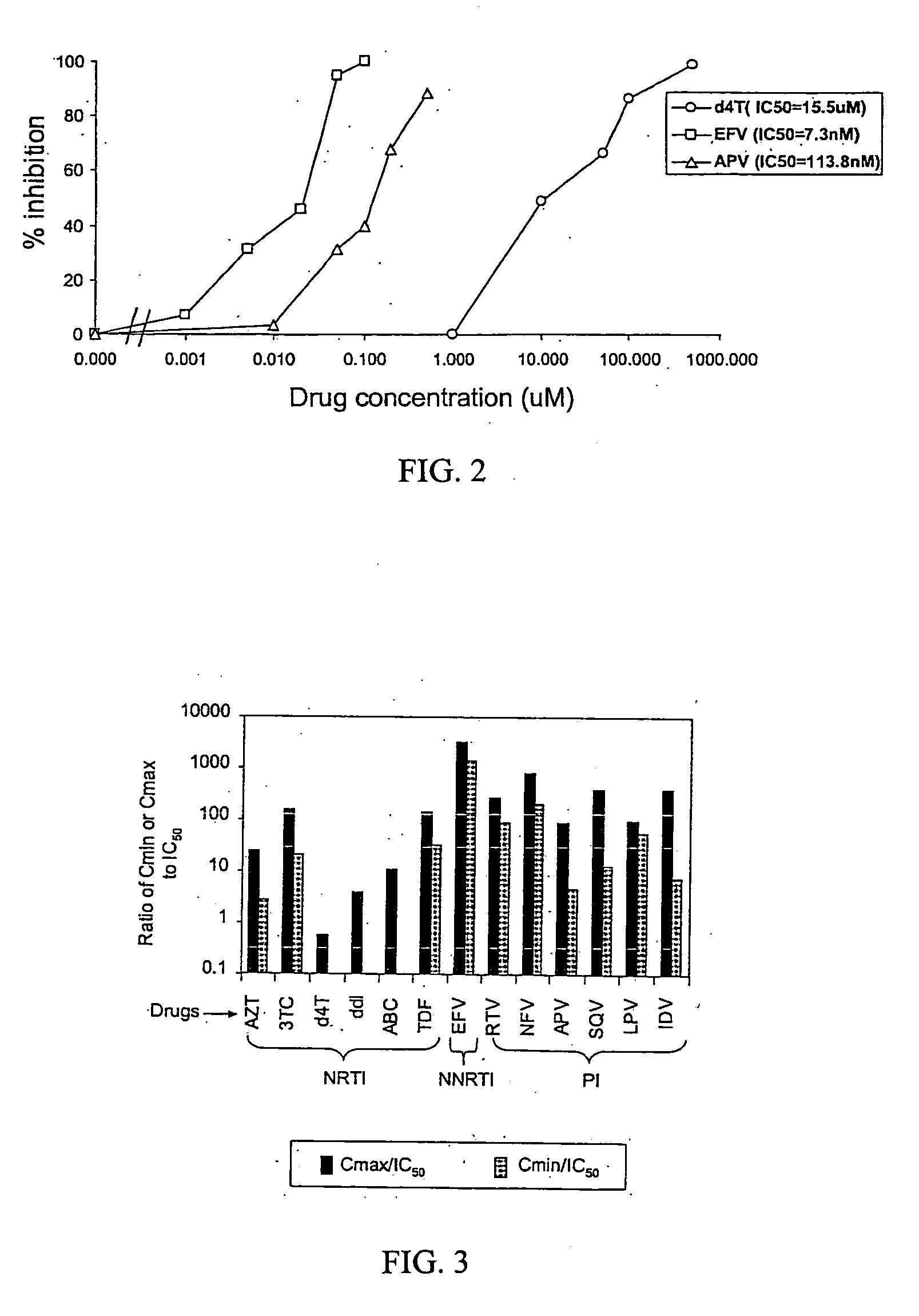
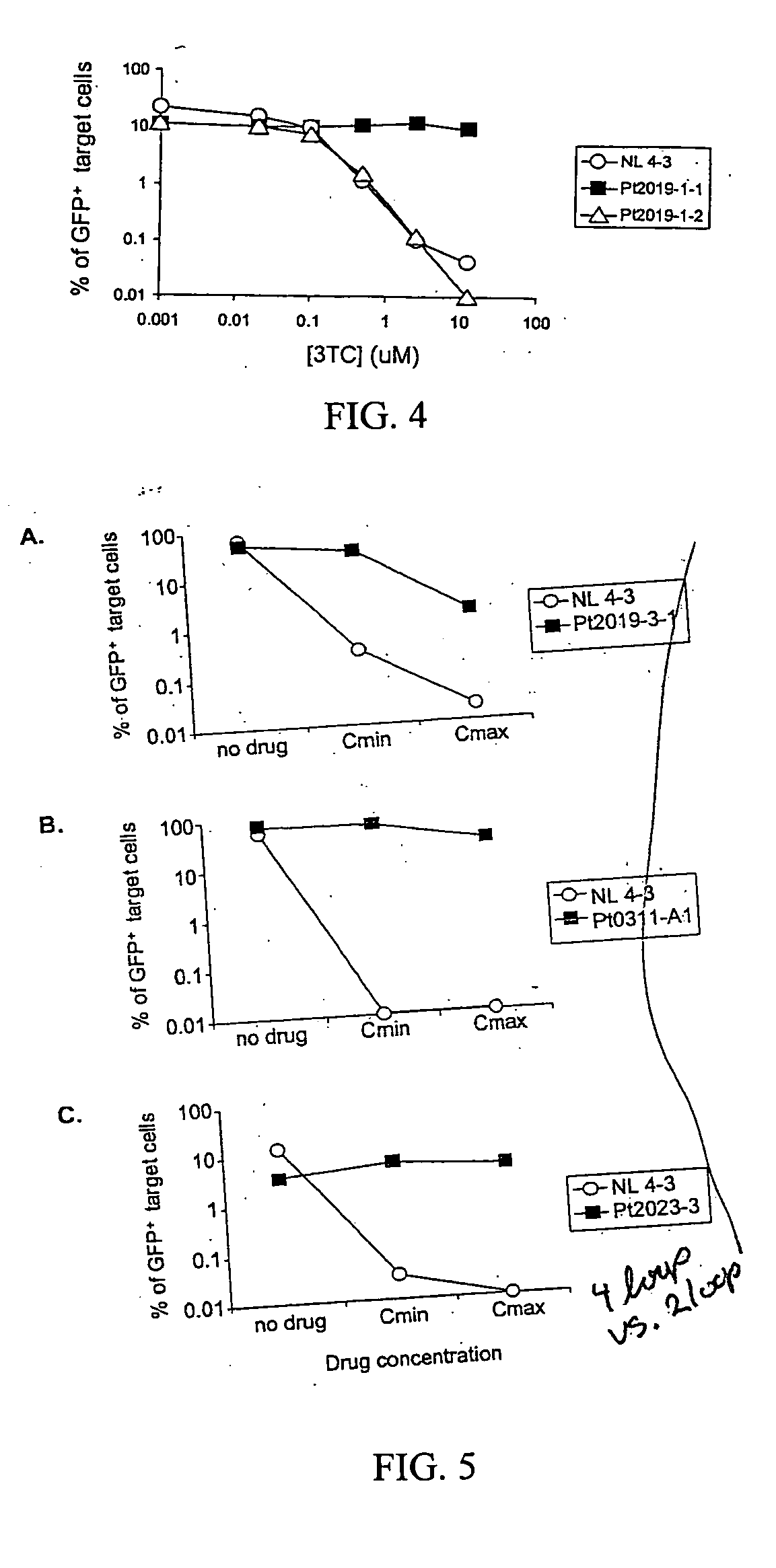
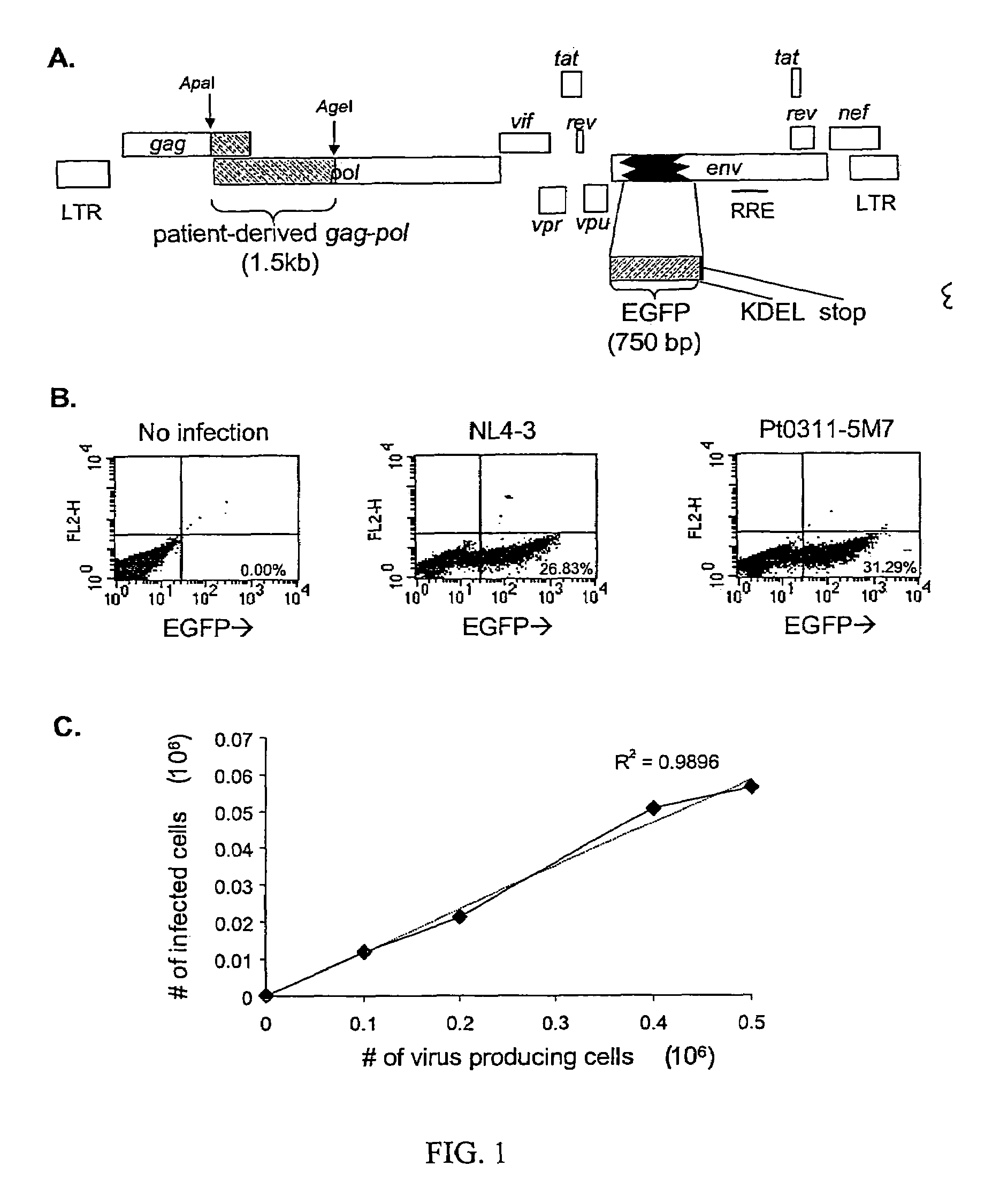
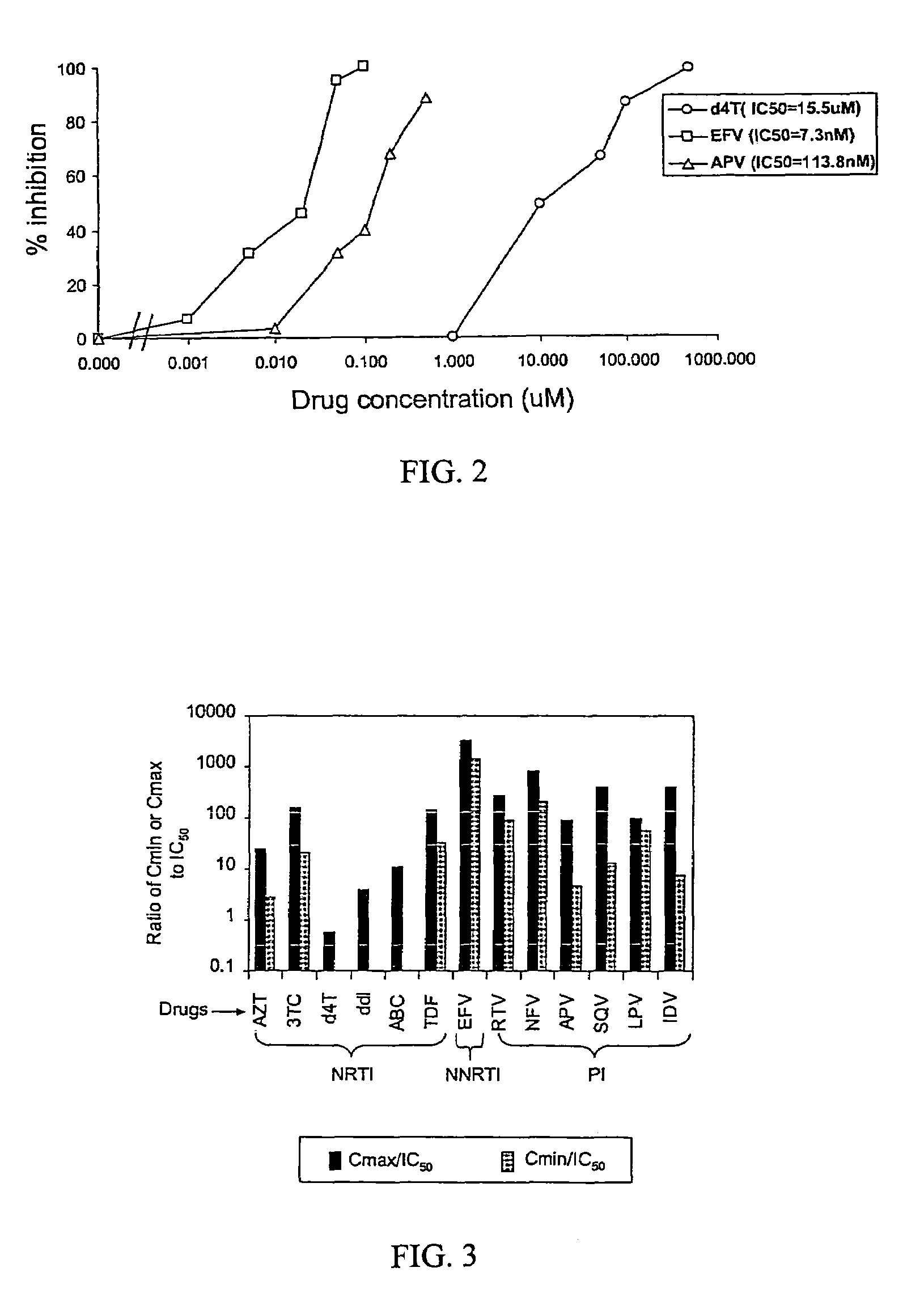
A novel single-cell-level phenotypic assay is described, which can simultaneously analyze HIV-1 drug susceptibility and intrinsic replication capacity. This allows quantitative dissection of the functions of antiretroviral drugs into suppression of viral replication and selection of resistant viruses with diminished replication capacities. The disclosed assay provides a tool for the rational evaluation of treatment decisions for patients failing antiretroviral therapy and is expected to be an important part in clinical management of HIV.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Single cell analysis of HIV replication capacity and drug resistance
ActiveUS7468274B2Less-effective in controllingAccurate countVectorsMicrobiological testing/measurementResistant virusViral replication
A novel single-cell-level phenotypic assay is described, which can simultaneously analyze HIV-1 drug susceptibility and intrinsic replication capacity. This allows quantitative dissection of the functions of antiretroviral drugs into suppression of viral replication and selection of resistant viruses with diminished replication capacities. The disclosed assay provides a tool for the rational evaluation of treatment decisions for patients failing antiretroviral therapy and is expected to be an important part in clinical management of HIV.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
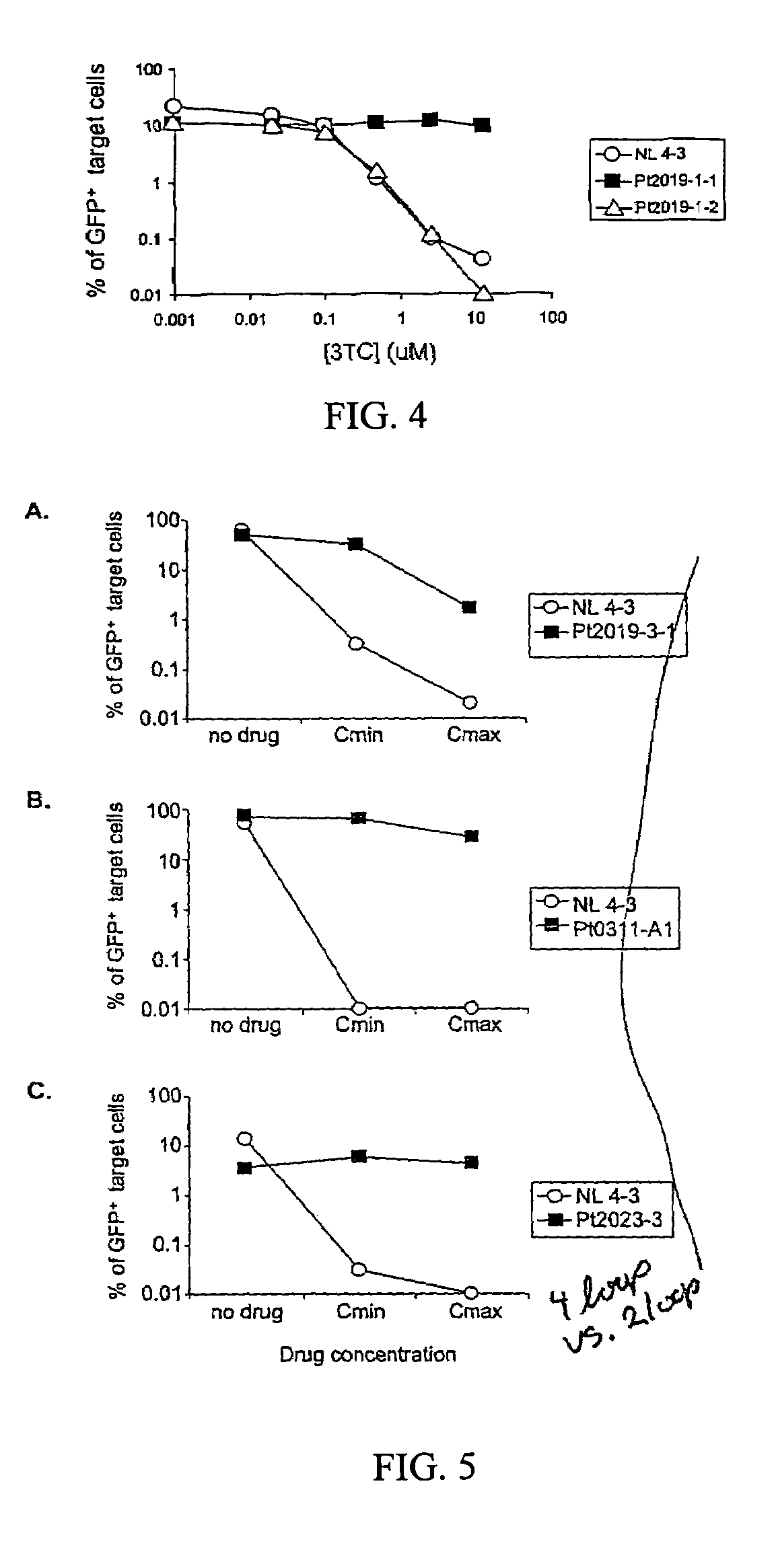
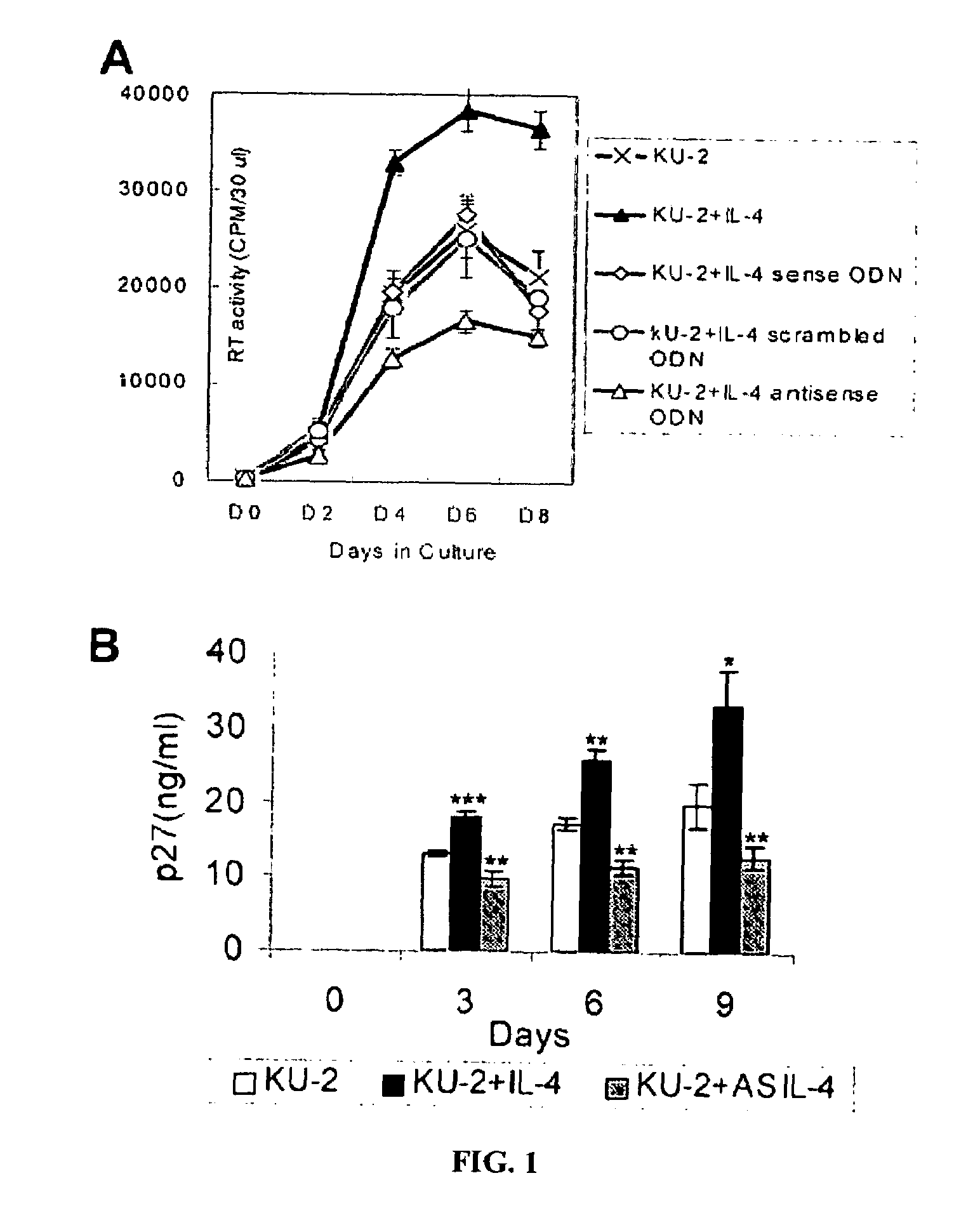
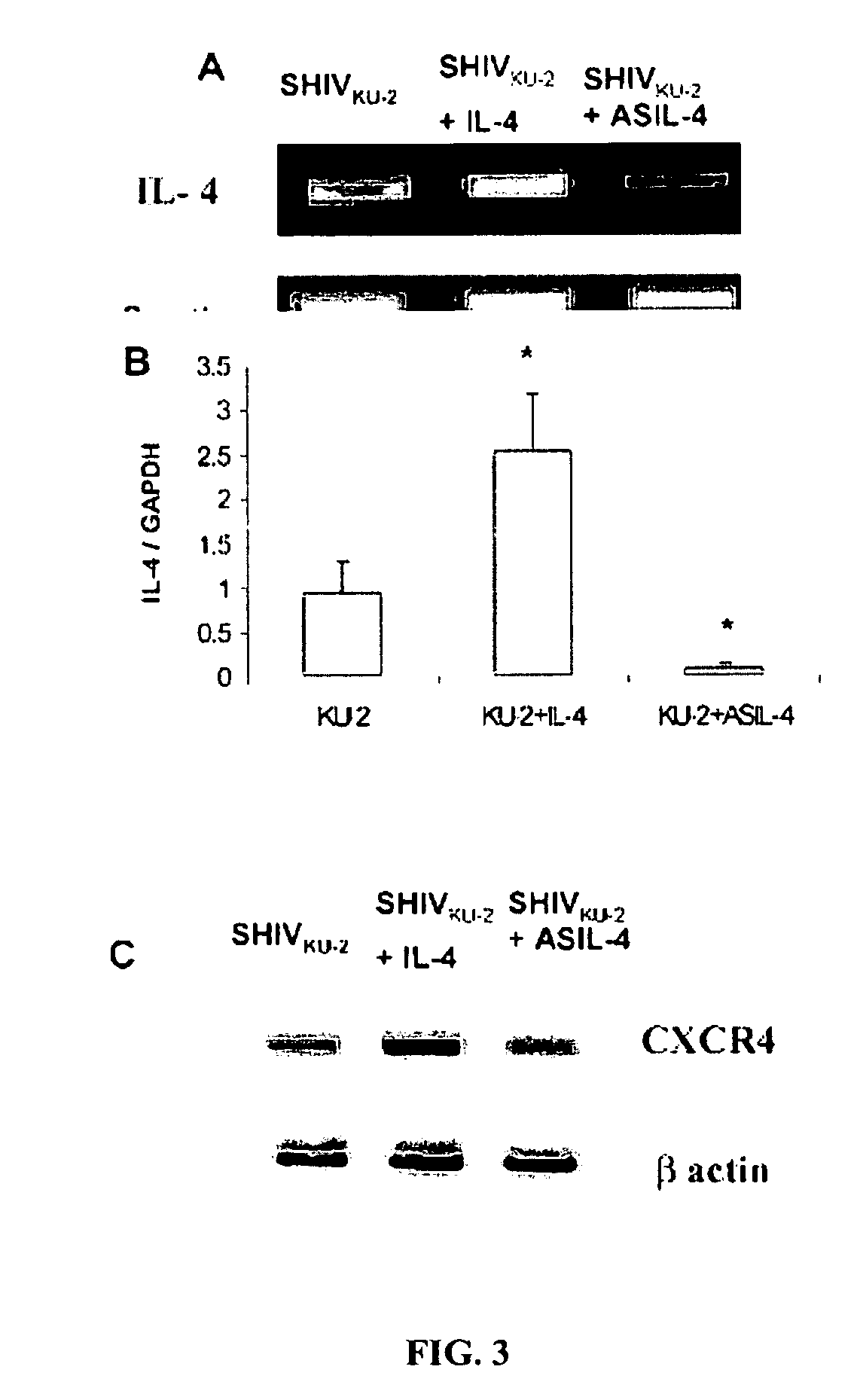
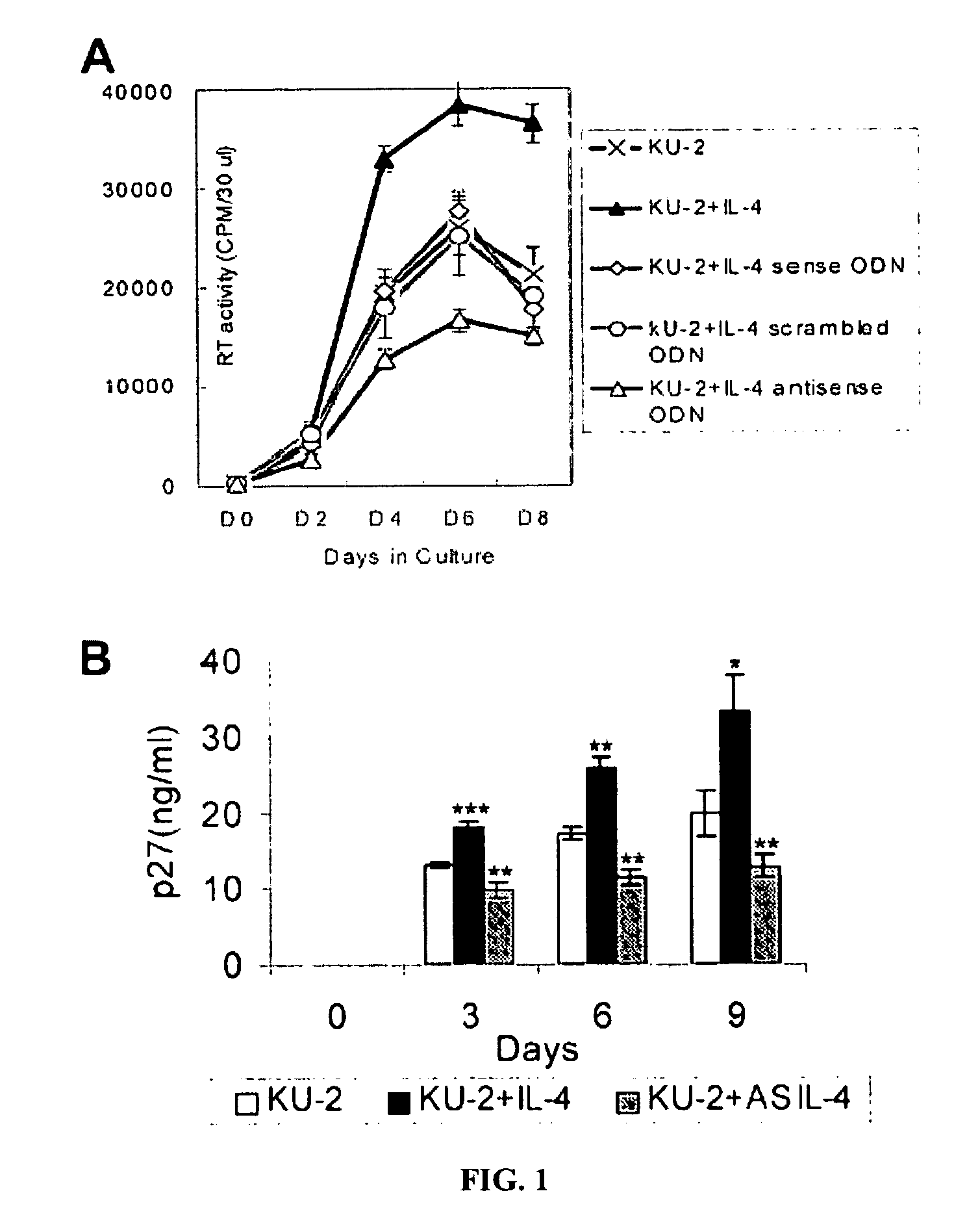
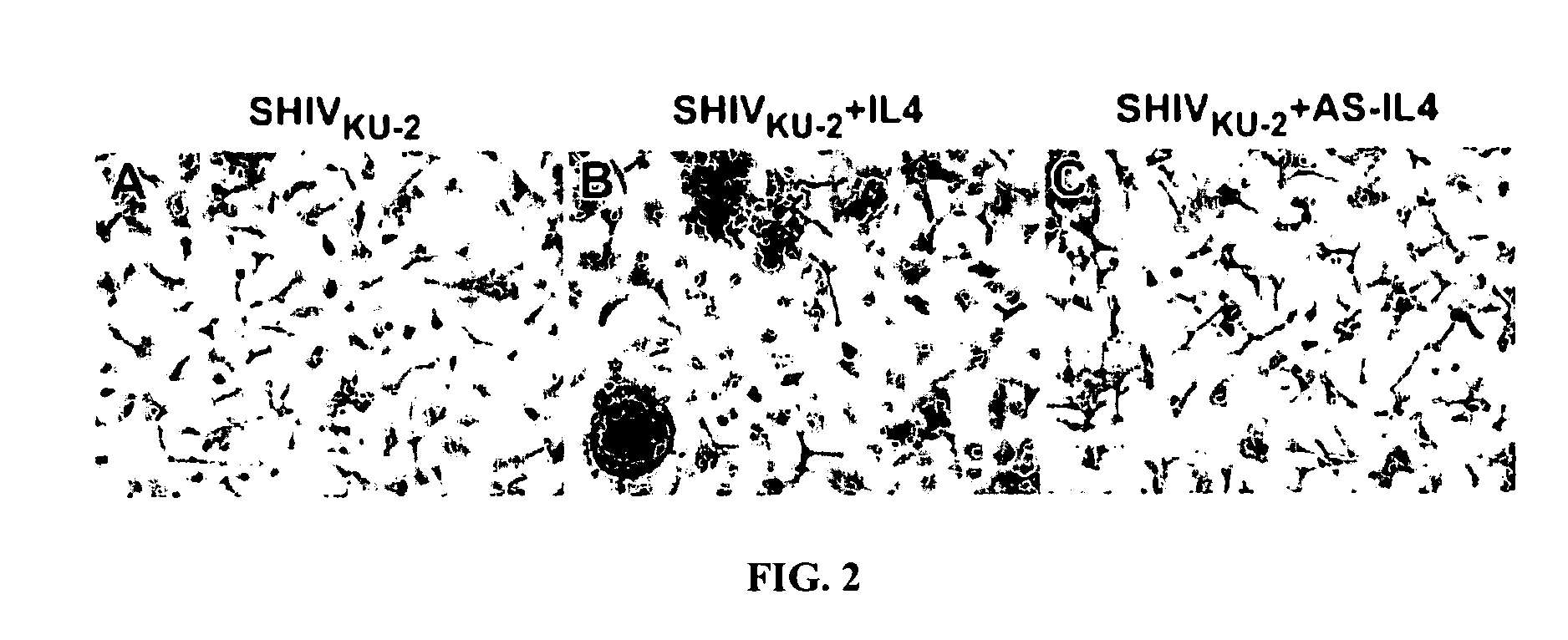
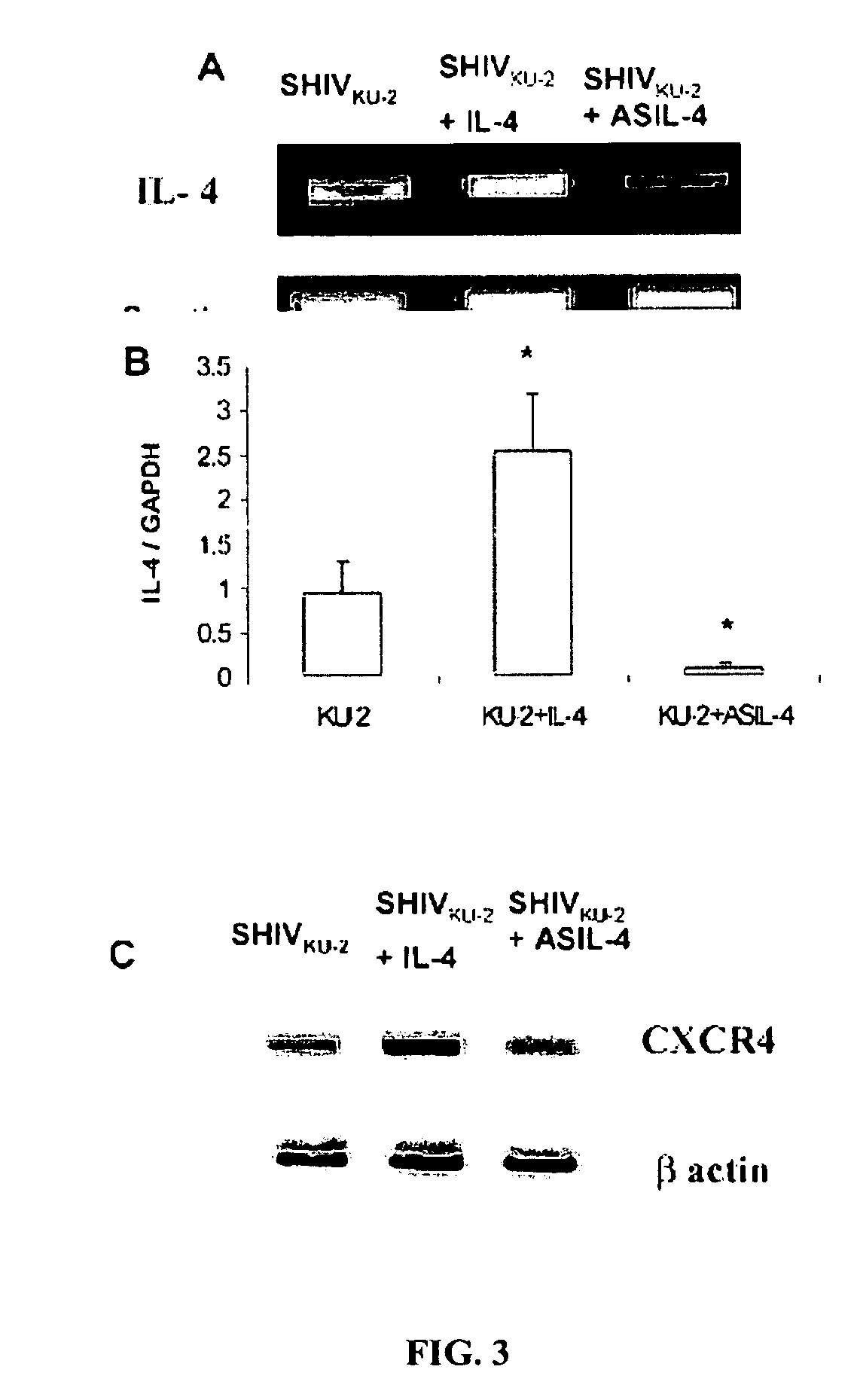
Inhibition of HIV and SHIV replication with antisense interleukin-4
A method of treating or preventing SHIV or HIV infection in a subject comprising administering a therapeutically effective amount of a antisense IL-4. The antisense IL-4 inhibits viral replication in the liver, lungs, spleen, and even the lymph nodes of the subject. Further, the antisense IL-4 can be used in combination with other antiretroviral agents or vaccines.
Owner:UNIV KANSAS MEDICAL CENT
Antiretroviral drug formulations for treatment of children exposed to hiv/aids
InactiveUS20110117193A1Reduce morbidityDissolve fastPowder deliveryBiocideImmunodeficiency virusMother to child transmission
The present disclosure provides fast disintegrating formulations for the treatment of human immunodeficiency virus (HIV) and acquired immune deficiency syndrome (AIDS) in patients such as neonatal, perinatal and pediatric children. Neonatal and perinatal formulations provide for the prevention or reduction of incidence of mother to child transmission of HIV. Also provided are formulations and methods for treating pediatric children having HIV / AIDS. The orally administered fast disintegrating formulations are in granule and tablet form and are specially formulation for children to increase adherence to treatment protocols.
Owner:DUQUESNE UNIVERSITY
Pharmaceutical compositions of antiretrovirals
The present invention relates to the stable pharmaceutical dosage forms of combination of antiretroviral agents. More particularly, the present invention relates to stable pharmaceutical dosage forms of Lamivudine, Zidovudine and Nevirapine, prepared by granulation technology.
Owner:AUROBINDO PHARMA LTD
Brefeldin A and derivative and application thereof
ActiveCN105566277AStrong anti-HIV latent activityEasy to removeOrganic active ingredientsOrganic chemistryInfected cellFermentation
The invention discloses brefeldin A (BFA) and a derivative and application thereof. The brefeldin A has the extremely high anti-HIV latency activity, and the series compound is obtained through a static culture fermentation method and chemical modification. The brefeldin A and the derivative thereof are of a structure shown as a formula (I) (please see the formula in the description), wherein R1-R5 represent H, OH, CH3, OCH3 and (OCH3)2 or OCOCH3 respectively, and R6 represents CH3. The brefeldin A and the derivative thereof can serve as anti-HIV latency drugs and can be applied to preparation of a drug for treating an AIDS jointly with an antiretroviral drug, when the brefeldin A and the derivative thereof are jointly applied with the antiretroviral drug, the brefeldin A and the derivative thereof can enable latent HIVs in infected cells to be expressed, have the effect of intervening HIV latency and can clear the activated and infection-latent cells to accelerate clearance of a latent virus storage, and the wide application prospect is achieved.
Owner:浙江美新控股有限公司
Topical pharmaceutical composition comprising tenofovir, an antibacterial agent and,optonally ciclopirox
The present invention relates in general to a topical pharmaceutical composition comprising an antiretroviral agent in combination with a bactericidal agent and optionally an antifungal agent, particularly for use as a contraceptive.
Owner:CIPLA LTD
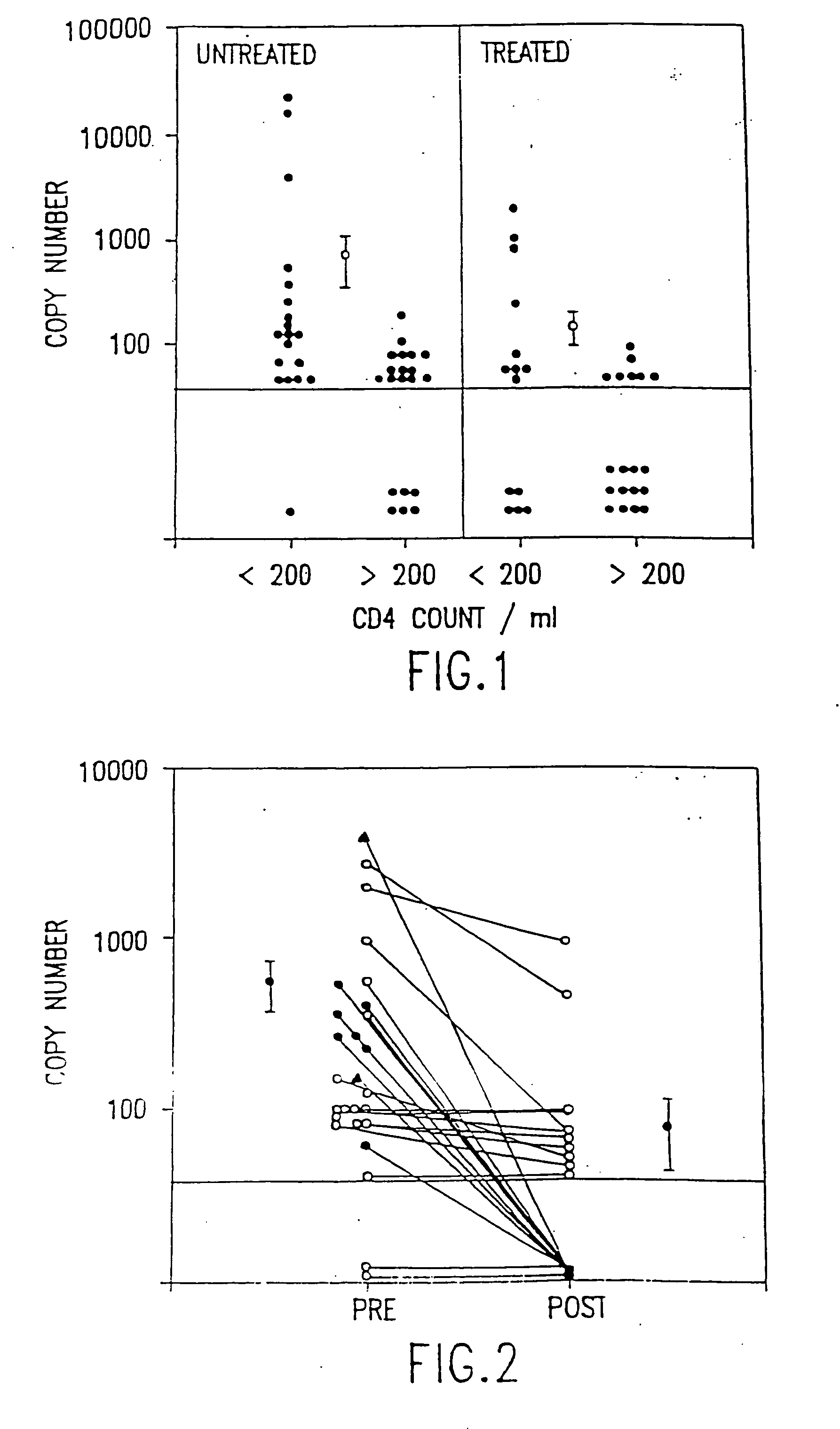
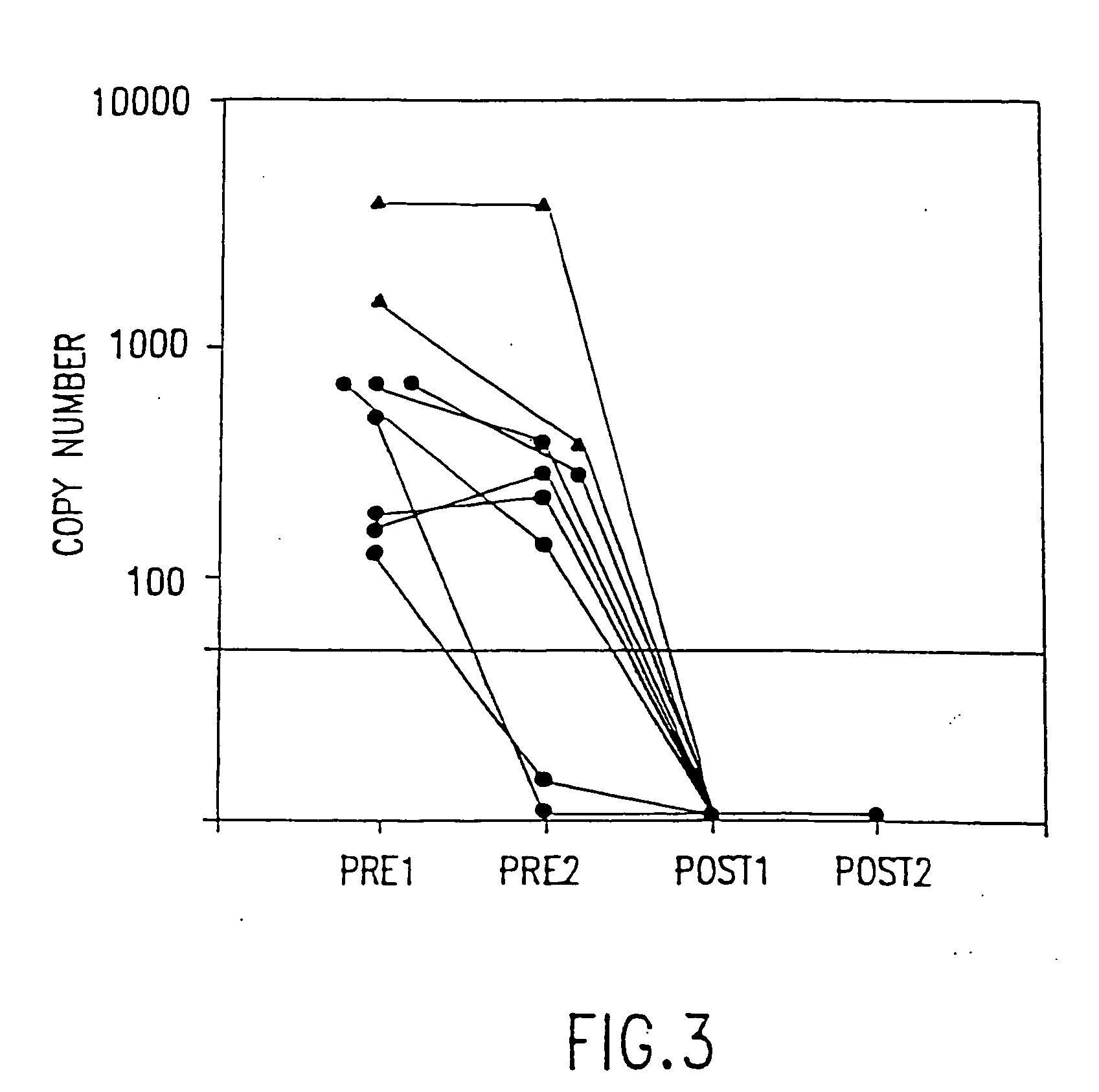
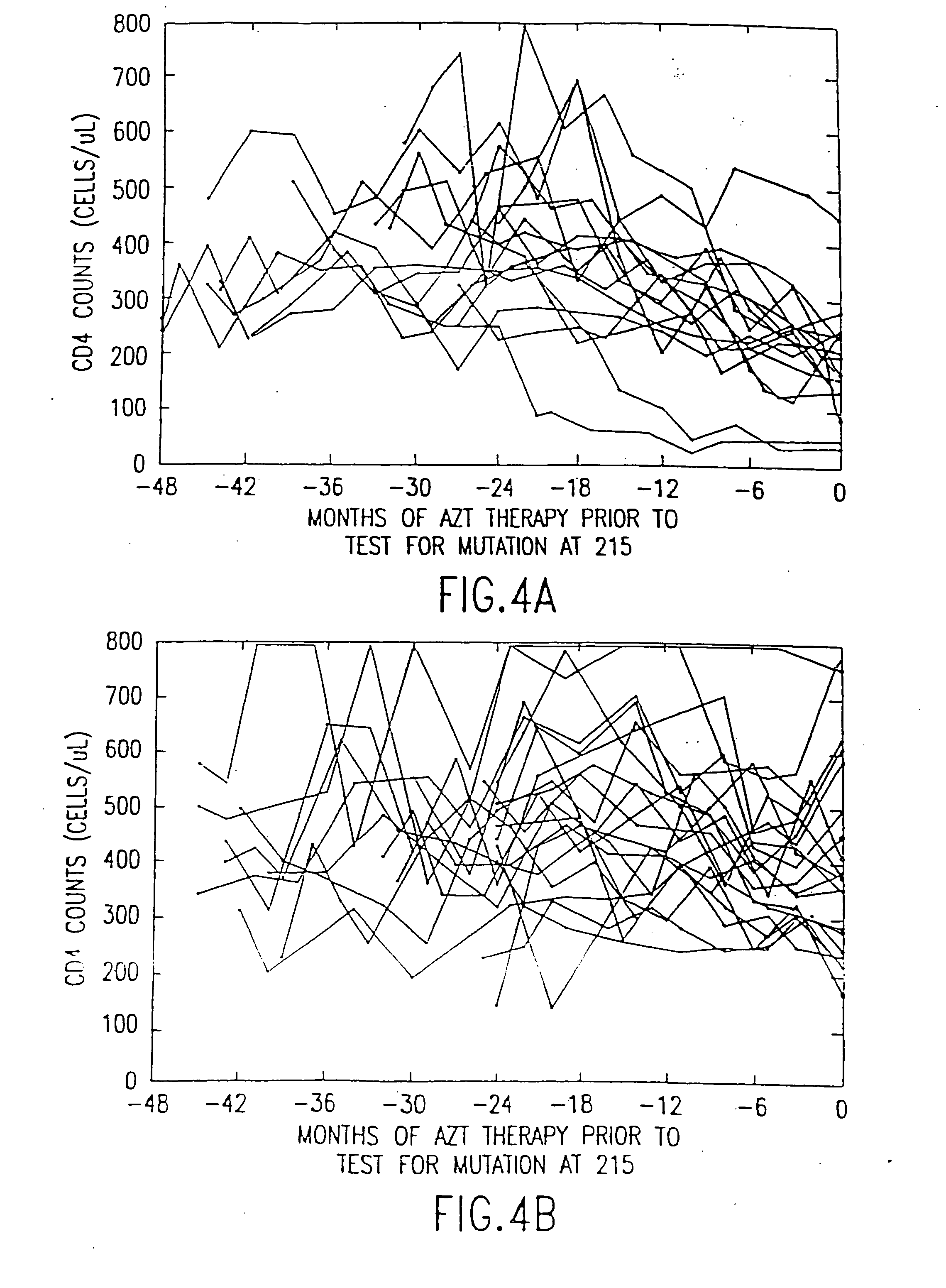
Polymerase chain reaction assays for monitoring antiviral therapy and making therapeutic decisions in the treatment of acquired immunodeficiency syndrome
InactiveUS20030118986A1Inhibit deteriorationConducive to survivalBioreactor/fermenter combinationsBiological substance pretreatmentsRegimenImmunodeficiency virus
The present invention relates to methods of monitoring, via polymerase chain reaction, the clinical progression of human immunodeficiency virus infection and its response to antiretroviral therapy. According to the invention, polymerase chain reaction assays may be used to predict immunological decline and to identify, at an early stage, patients whose infection has become resistant to a particular antiretroviral drug regimen.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Antiretroviral Drug Targeting Human Endogenous Retrovirus
ActiveUS20190263895A1Strong cytotoxicityImprove rendering capabilitiesOrganic active ingredientsNervous disorderReverse transcriptaseANTIRETROVIRAL AGENTS
The invention relates to an antibody, a fragment or a derivative thereof, for use as an antiretroviral drug targeting a virus belonging to human endogenous retroviruses type W (HERV-W), wherein said antibody, fragment or derivative thereof is directed against HERV-W Envelope protein (HERV-W Env). The invention also relates to a composition comprising said antibody and a retroviral reverse-transcriptase inhibitory drug, for use as an antiretroviral drug targeting a virus belonging to HERV-W.
Owner:GENEURO SA
Organic thiophosphate antiretroviral agents
InactiveUS20160374967A1Promote repairReduced mutagenesisBiocideSulfur/selenium/tellurium active ingredientsImmunodeficiency virusAmifostine
A method for the prevention or treatment of human immunodeficiency virus infection by administering an effective amount of amifostine, phosphonol, or similar compound to an individual in need is provided.
Owner:UNITED STATES OF AMERICA +1
Topical Pharmaceutical Composition
InactiveUS20150246065A1Improve efficacyAvoid spreadingBiocideOintment deliveryDermatologyANTIRETROVIRAL AGENTS
The present invention relates in general to a topical pharmaceutical composition comprising an antiretroviral agent in combination with a bactericidal agent and optionally an antifungal agent, particularly for use as a contraceptive.
Owner:CIPLA LTD
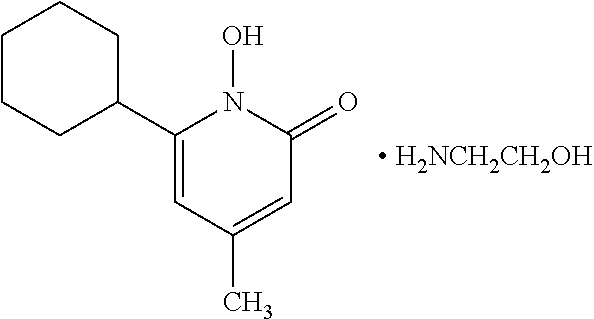
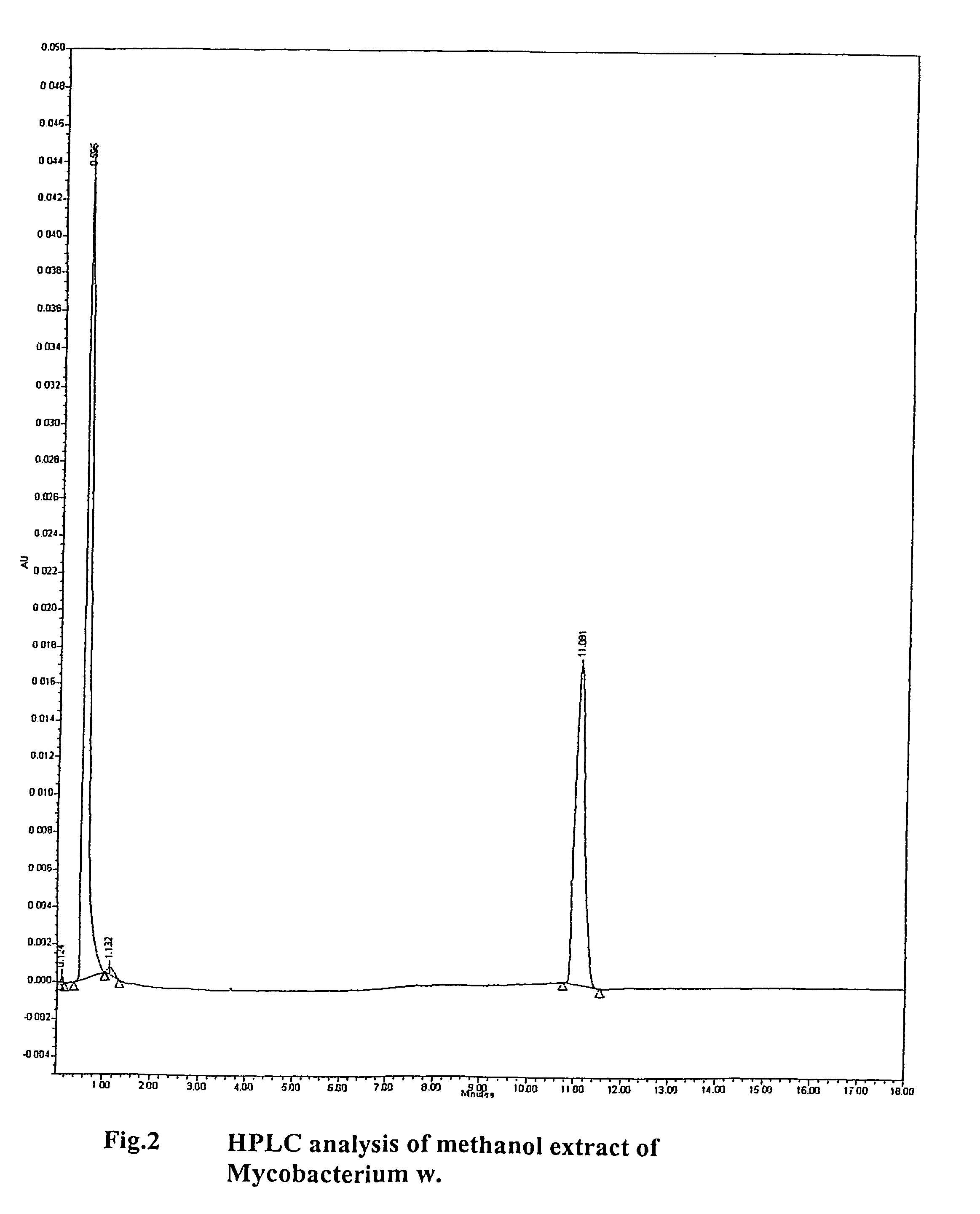
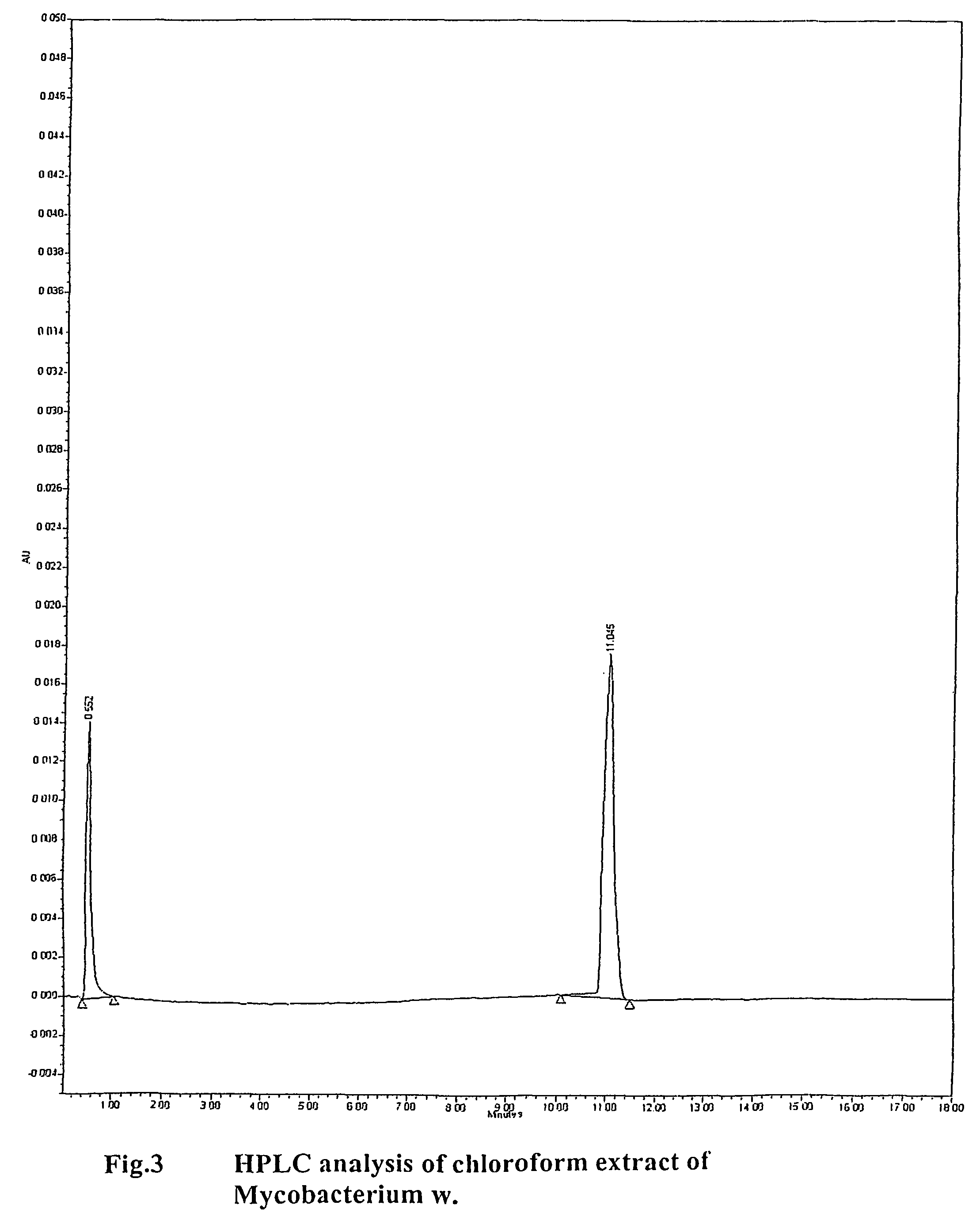
Method of treating human-immunodeficiency virus (HIV) disease infection
InactiveUS8110204B2Relieving symptoms of HIVReduce developmentBiocideBacterial antigen ingredientsFeline immunodeficiency virusSide effect
Human Immunodeficiency virus causes depletion of CD4 cells. The depletion of CD4 cells results in decrease in immunity of an infected individual. Due to decrease immunity various opportunistic infections occur. These infections are cause for morbidity and mortality in HIV infected individuals. The treatment of HIV these includes antiretroviral drugs. These drugs have their own side effects and immune reconstitution achieved is delayed and slow. Various attempts have been made to improve CD4 count, use of IL-2 is one of them. It is associated with systemic side effects during the period of its administration. The present invention provides method of using mycobacterium w for the management of HIV. According to present invention mycobacterium w when given intradermally is effective in prophylaxis and treatment of AIDS or AIDS related complex (ARC). It is found to improve immunity as well as CD4 count. It is found to eliminate symptoms like fever, diarrhea. The effect is seen even when no antiretrovirals are used.
Owner:CADILA PHARMA
High Drug Loaded Tablet Composition for Treating HIV
The present invention relates to pharmaceutical antiretroviral compositions comprising a combination of antiretroviral agents, the manufacturing process thereof and use of the said compositions for the prevention, treatment or prophylaxis of diseases caused by retro viruses, specifically acquired immune deficiency syndrome or an HIV infection.
Owner:HETERO LABS LTD
Methods for treatment of HIV and other infections using a T cell or viral activator and anti-retroviral combination therapy
InactiveUS20050208049A1BiocideOrganic active ingredientsPeripheral blood mononuclear cellCD4 antigen
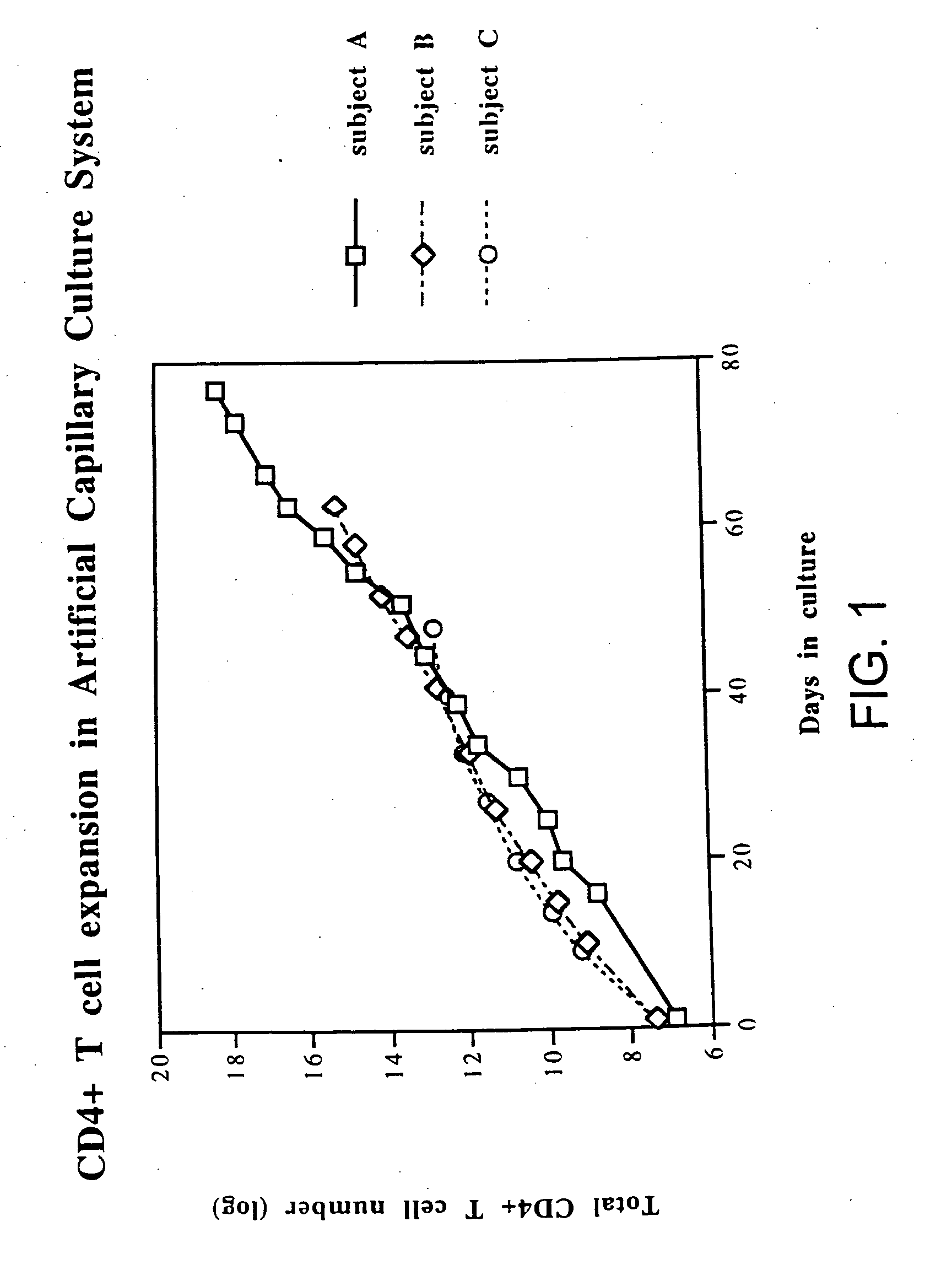
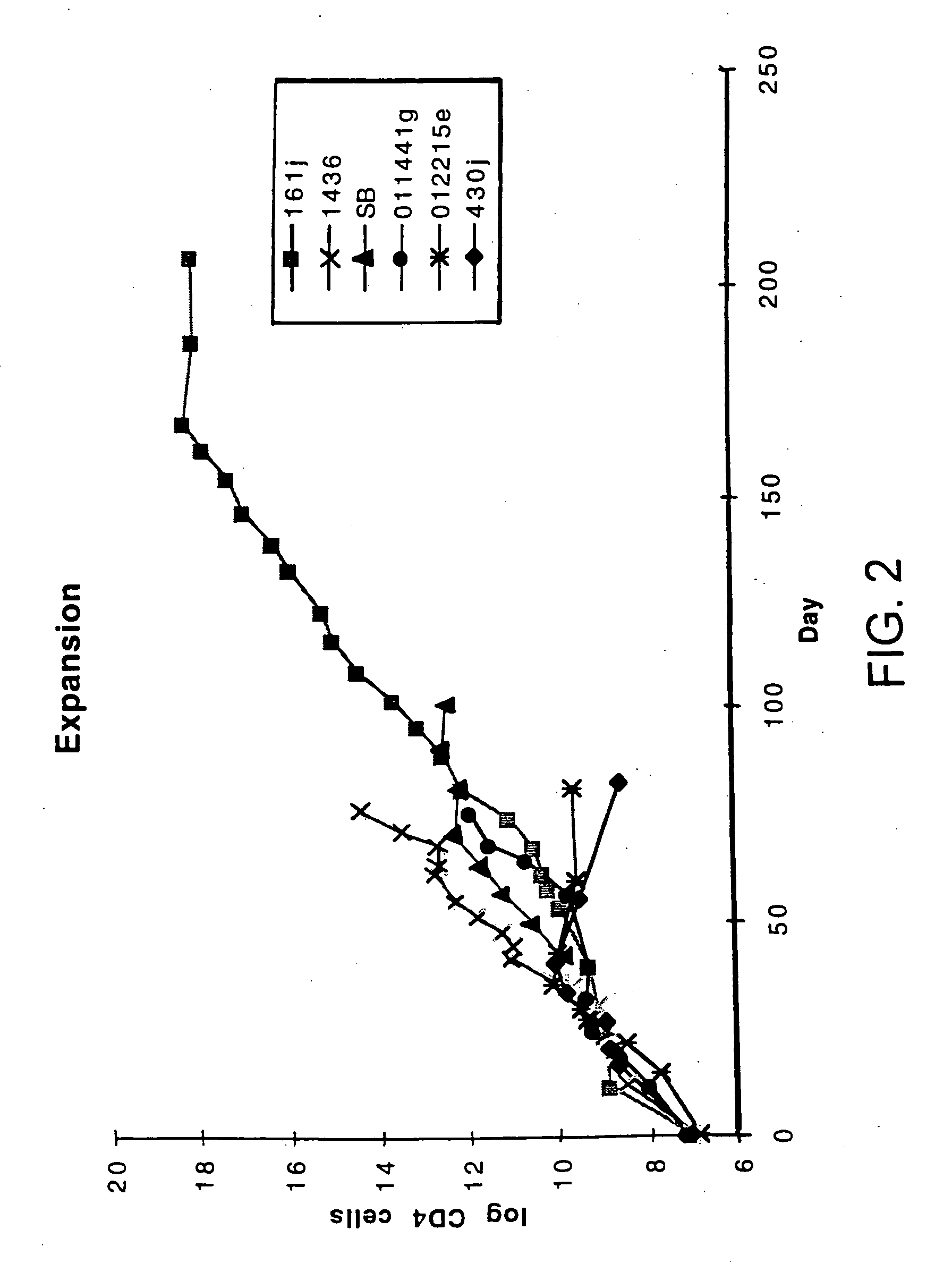
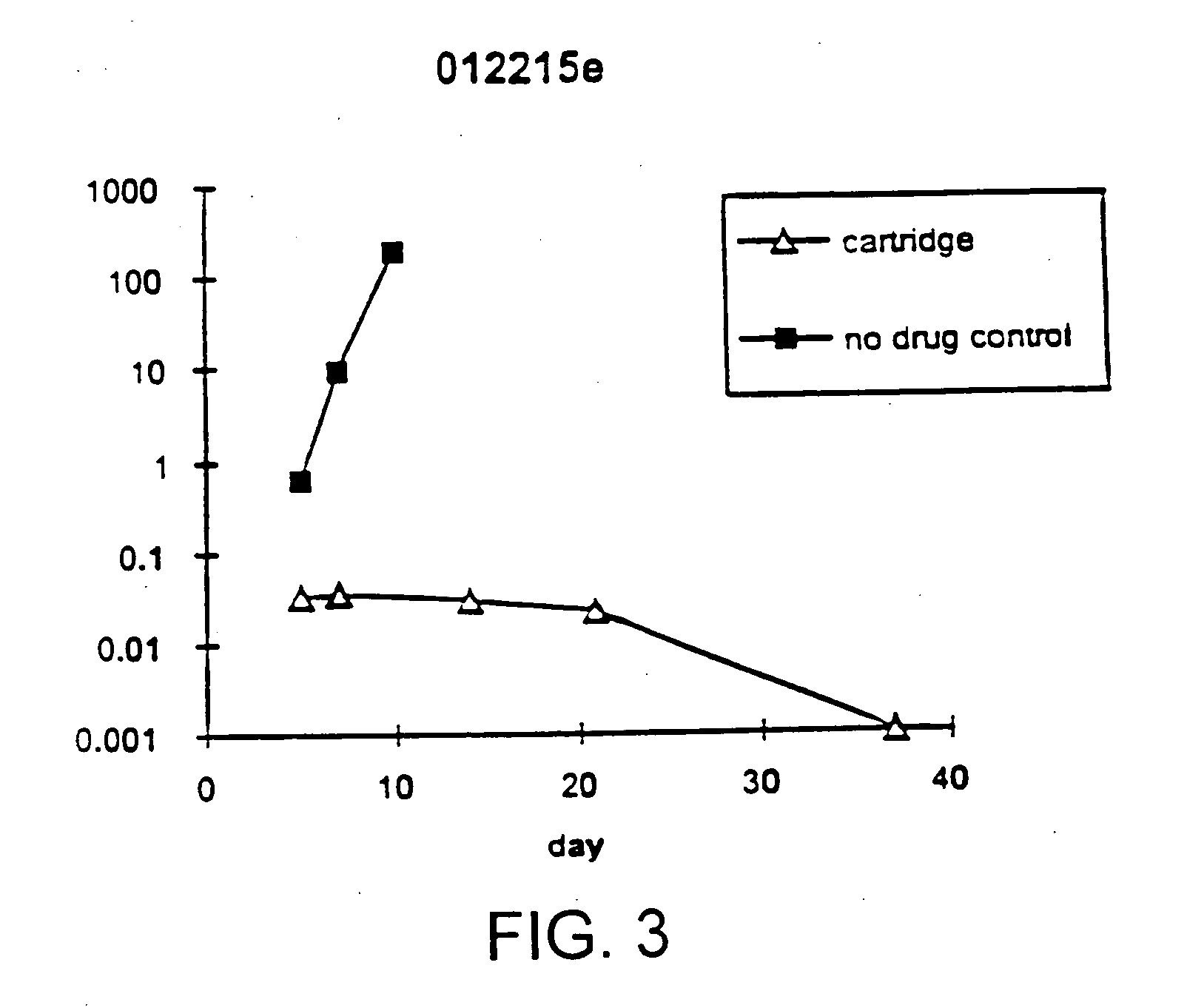
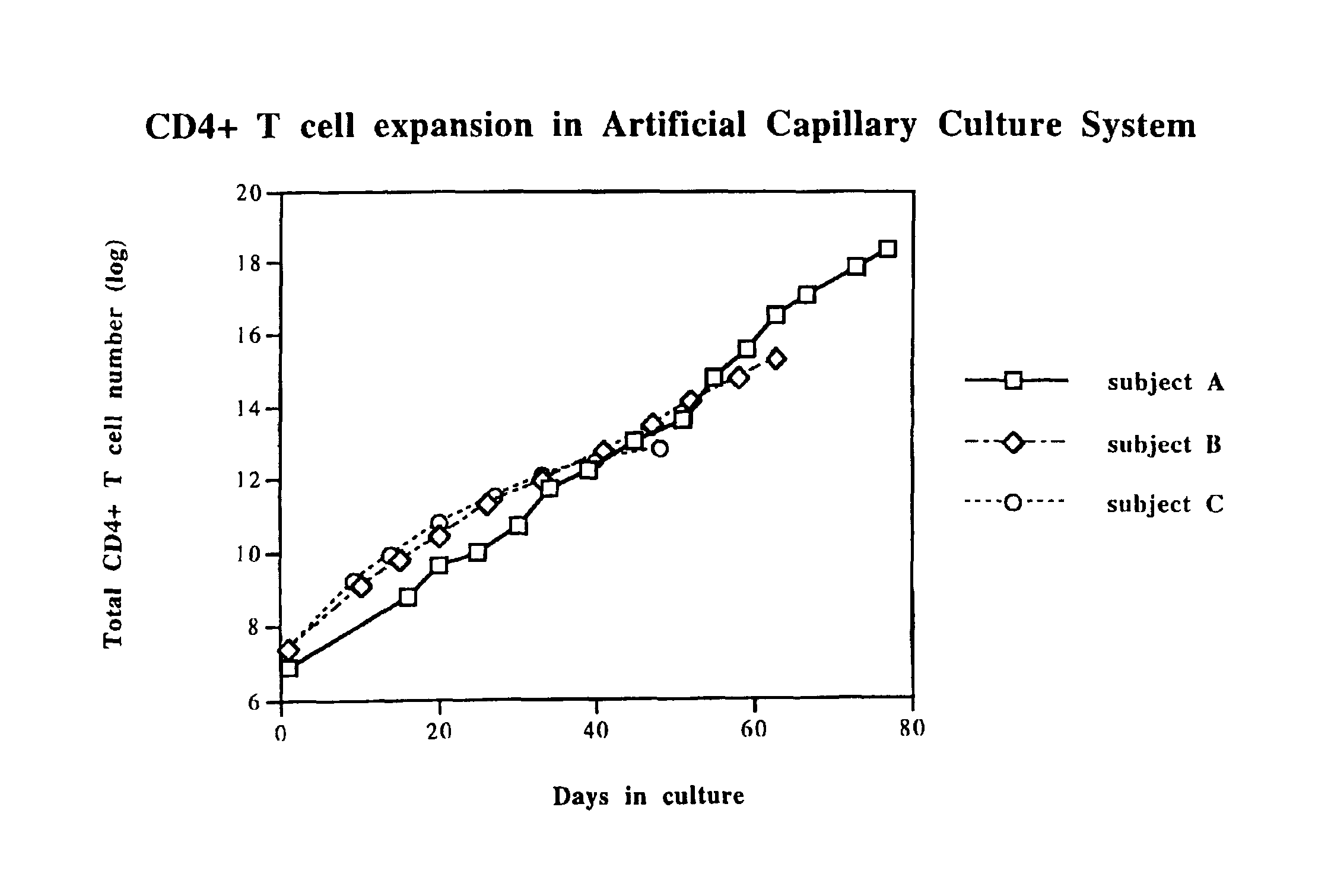
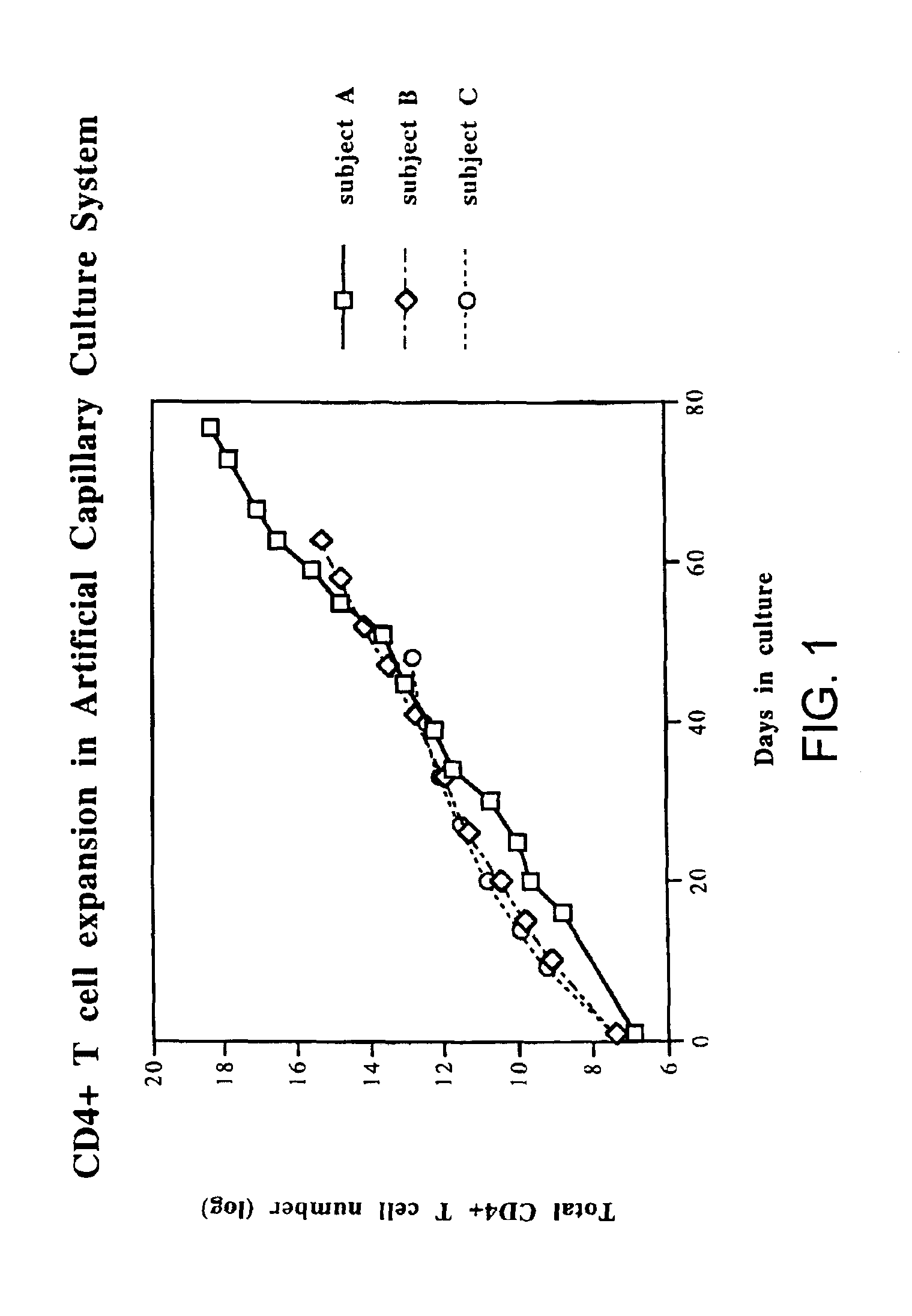
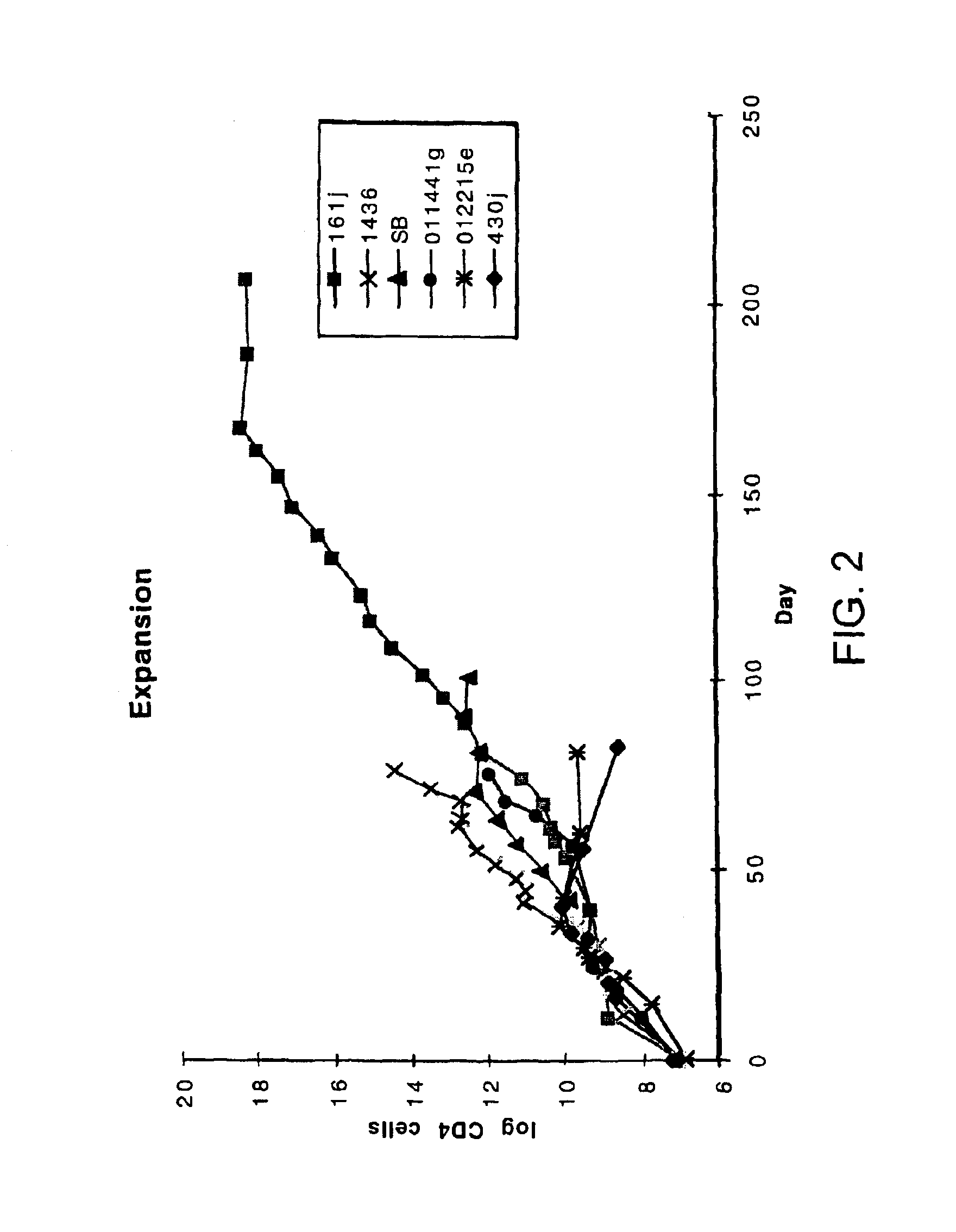
Disclosed is a method for treating infection with a pathogen. The method involves administration of: (1) a substance which induces active pathogen replication in a cell latently infected with HIV and (2) an anti-pathogen drug. Also disclosed are methods for expanding CD4+ T cells from peripheral blood mononuclear cells isolated from human subjects in the presence of an antiretroviral drug and for treating HIV infection by infusing the expanded CD4+ cells into HIV-infected patients.
Owner:WONG JOHNSON T
Nanoparticles and methods of use
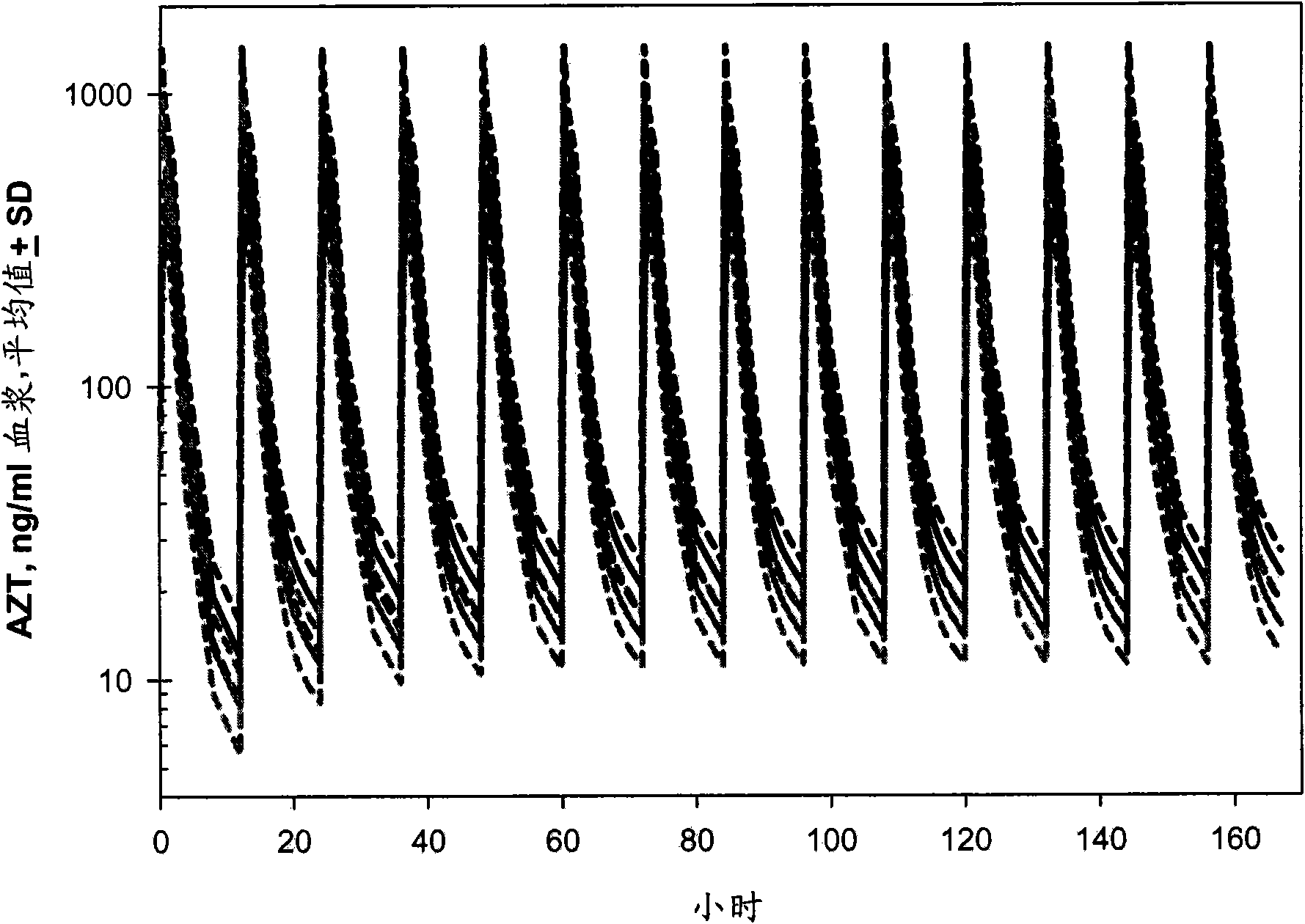
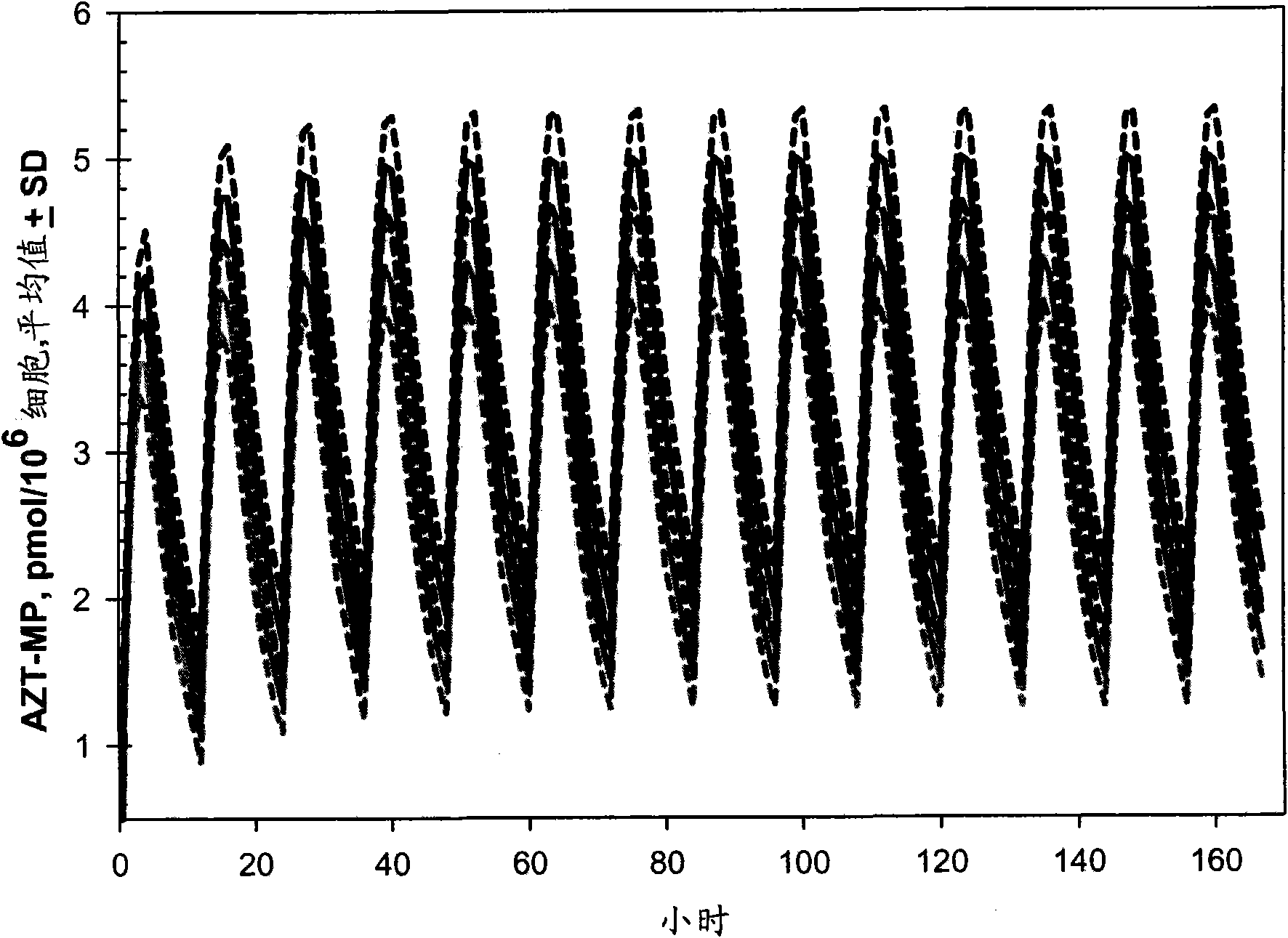
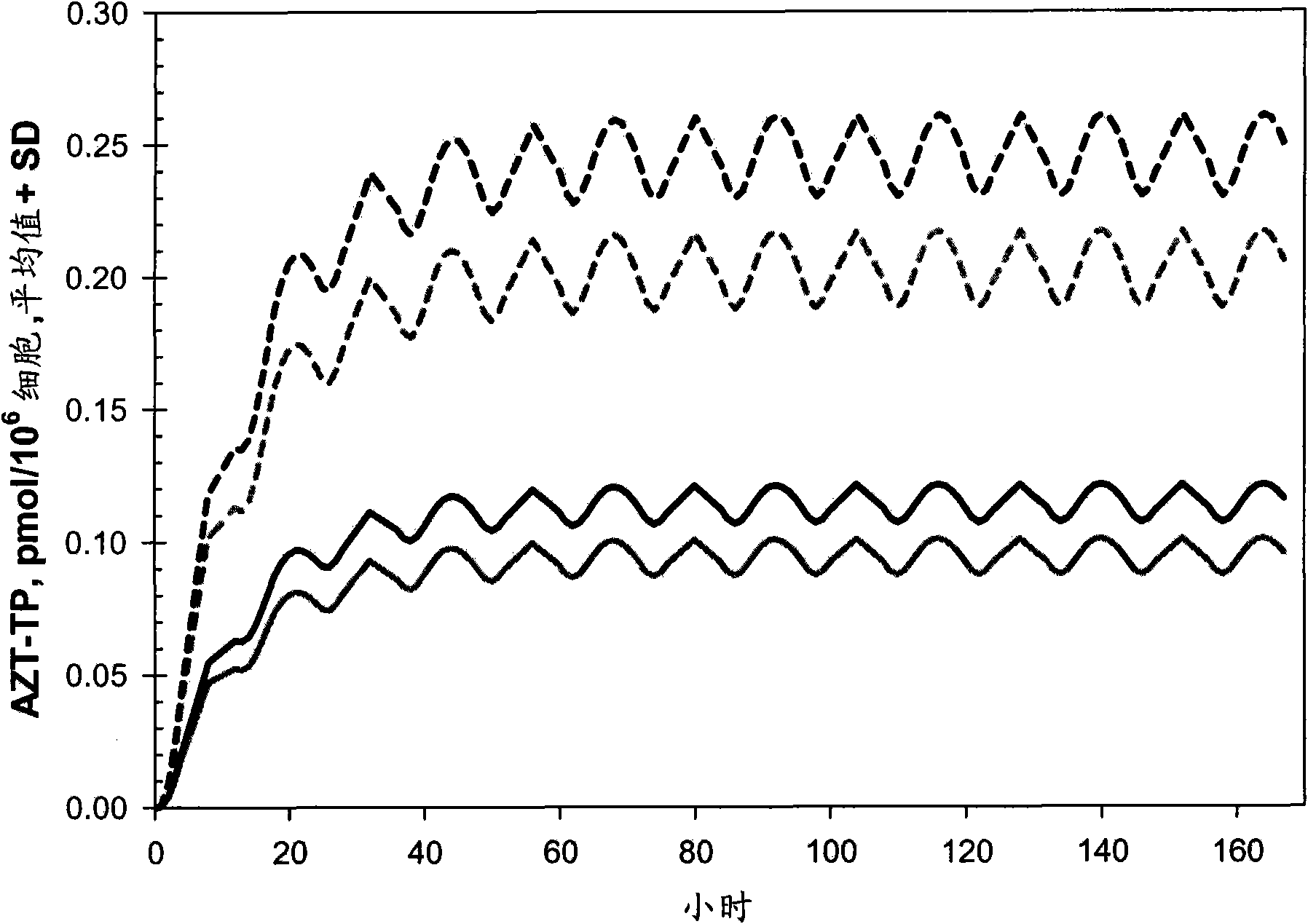
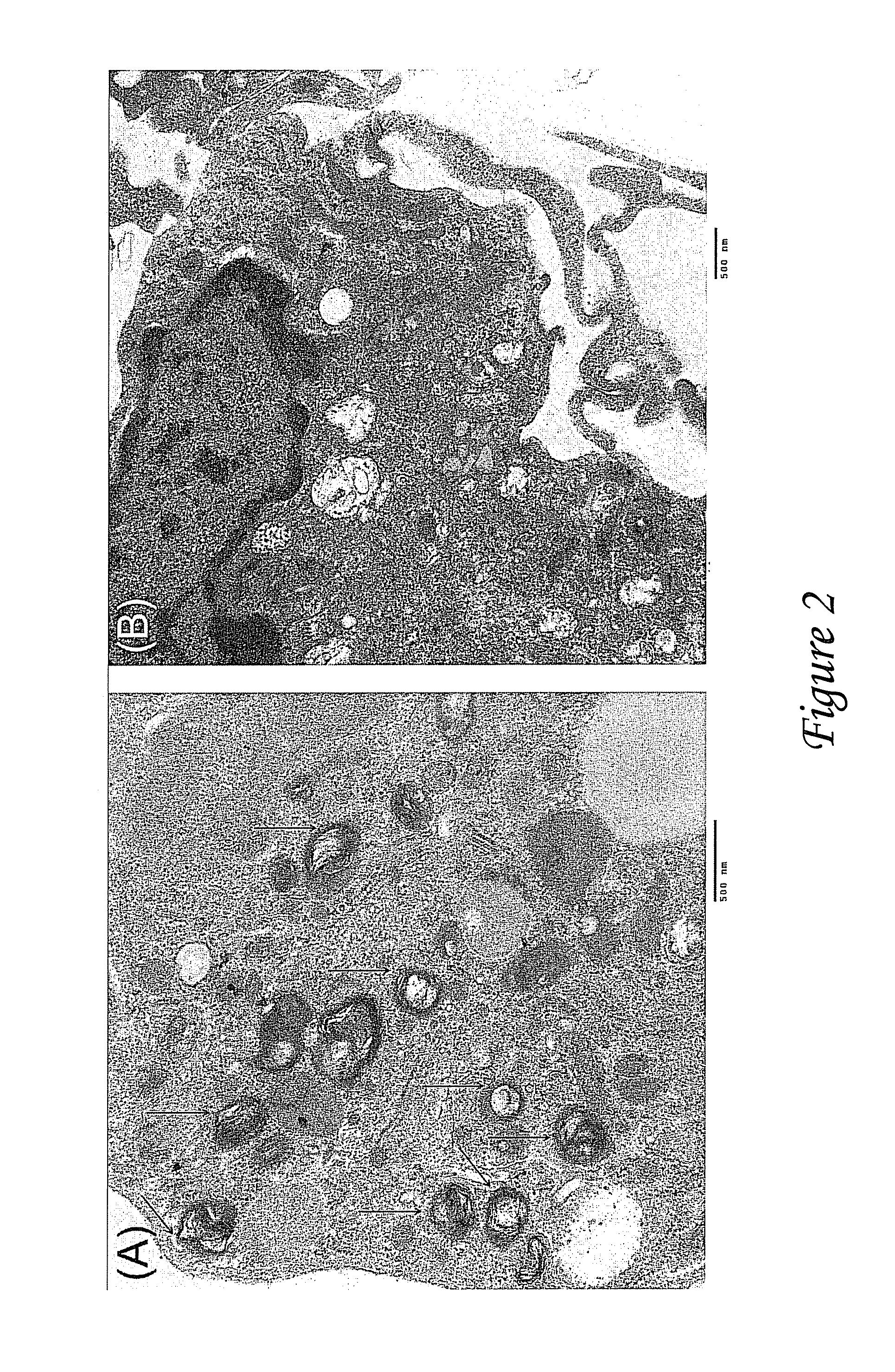
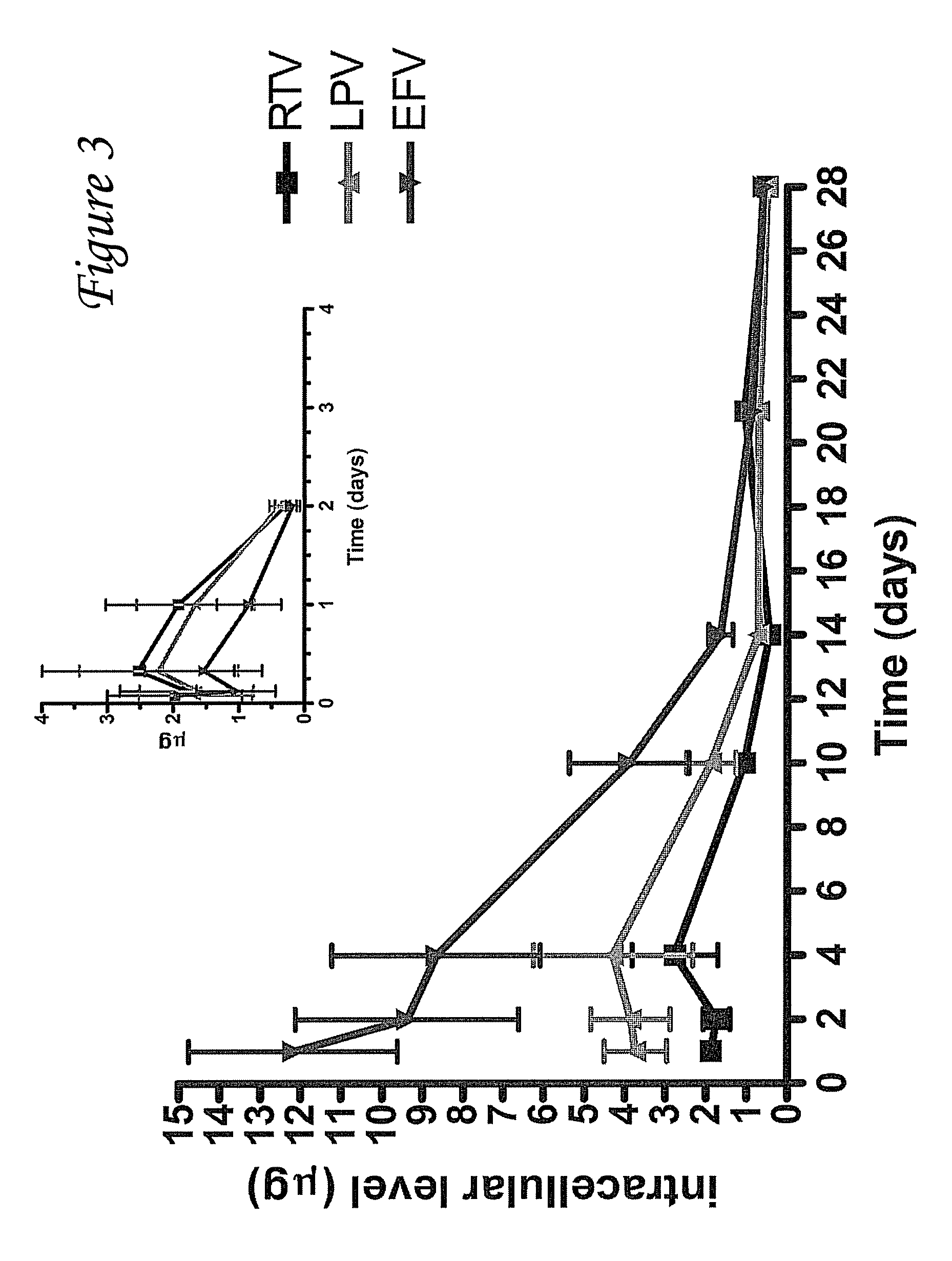
Provided herein are nanoparticles and methods for using nanoparticles. The nanoparticles include at least three antiretroviral agents. When introduced to cells the nanoparticles cause an increase in the intracellular concentration of the antiretroviral agents to a level that is at least the IC50 against HIV-I or HIV-2. This concentration may be maintained for at least 21 days after the cells are contacted with the nanoparticle. When administered to a subject the nanoparticles cause the concentration of the antiretroviral agents to increase to at least 100 ng / ml in the serum of the subject, at least 0.5 μg / gram tissue in an organ of the subject, or a combination thereof. Such a concentration may be maintained for at least 21 days after the administration.
Owner:CREIGHTON UNIVERSITY
Risk assessment for cutaneous adverse drug reactions from antiretroviral agent
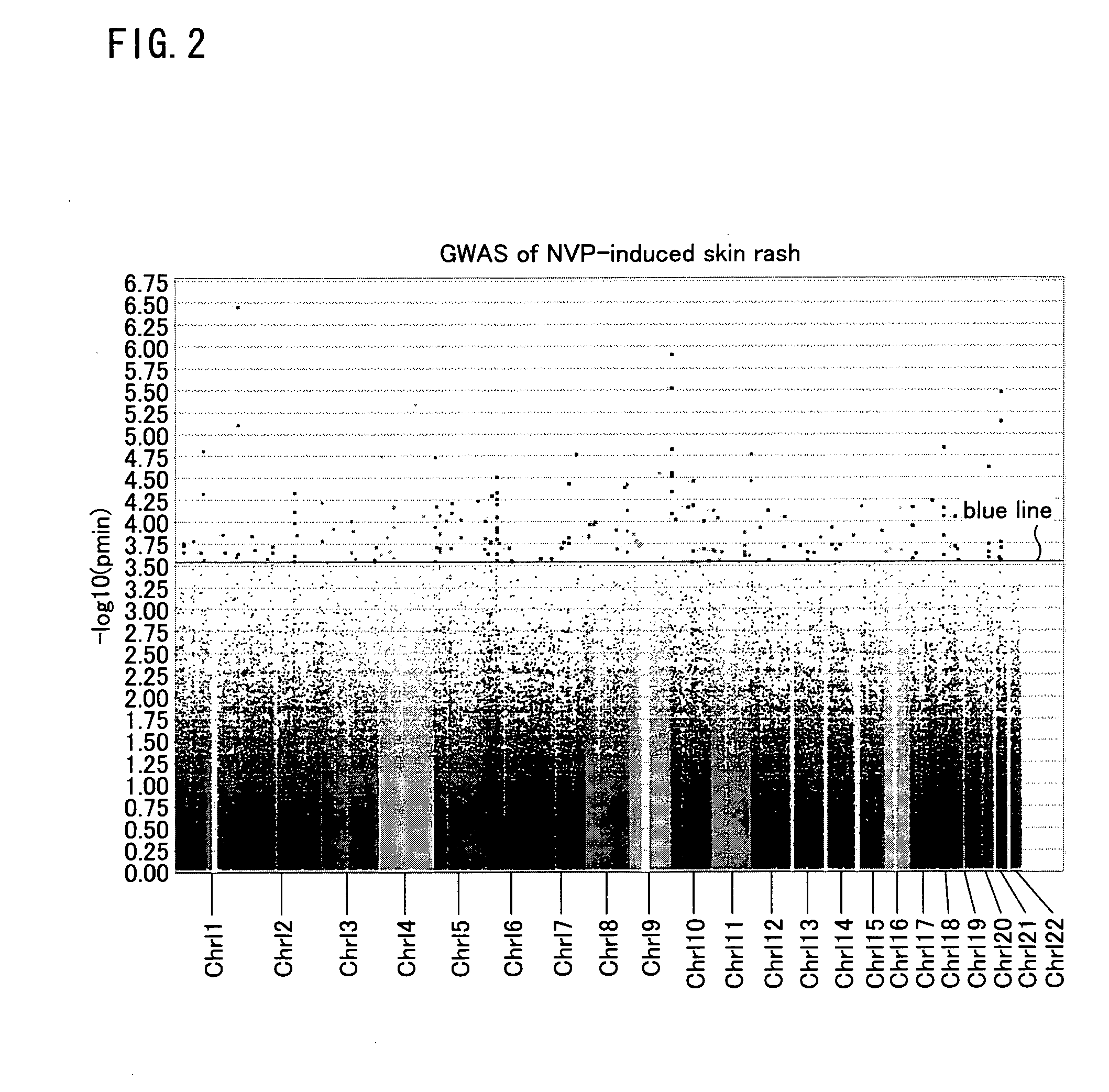
ActiveUS20110301043A1Microbiological testing/measurementDisease diagnosisNucleoside Reverse Transcriptase InhibitorHLA-B
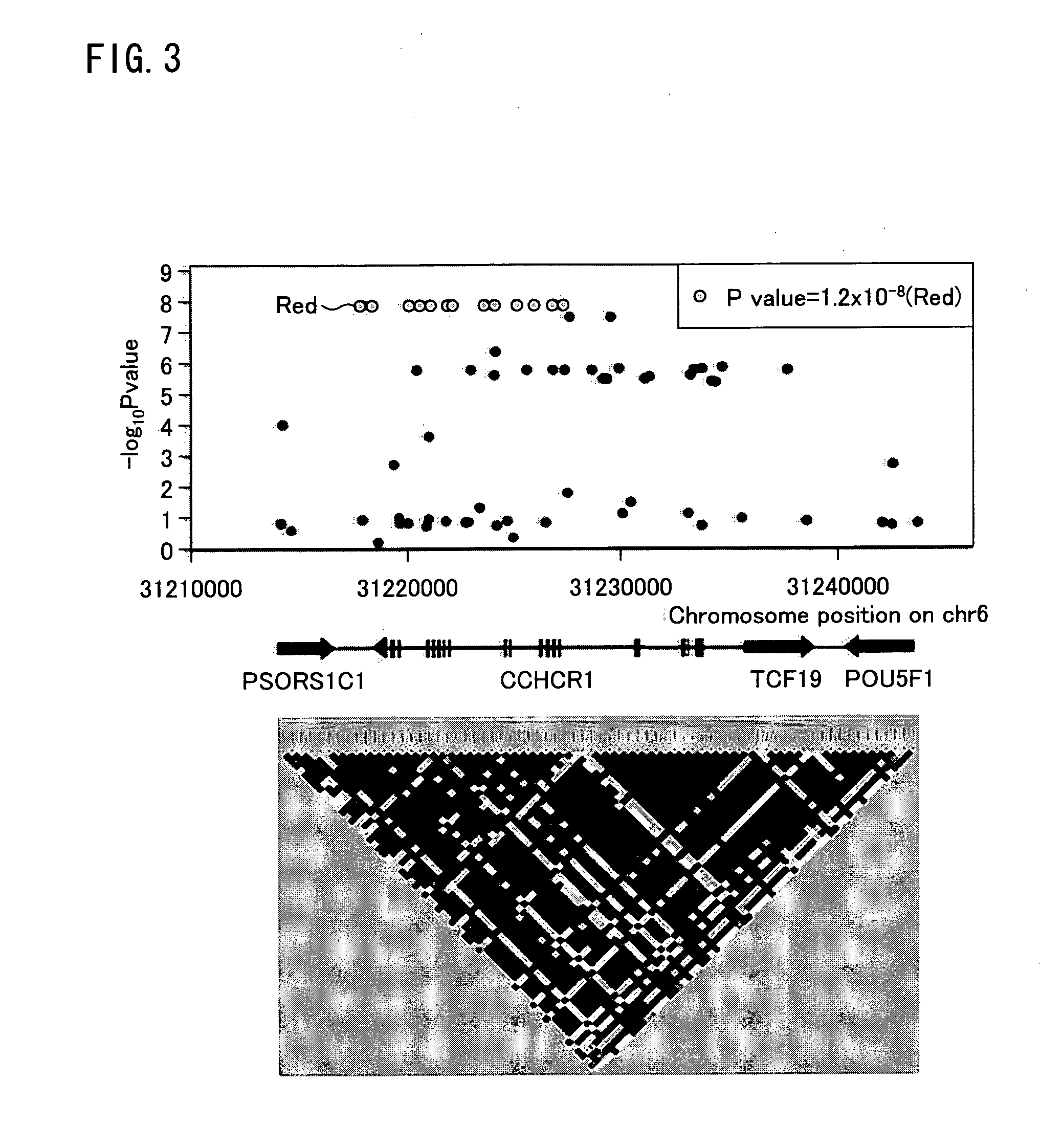
The present invention provides a method of predicting the risk of a patient for developing cutaneous adverse drug reaction to non-nucleoside reverse transcriptase inhibitors such as nevirapine by using HLA-B*3505 allele and / or polymorphisms in the CCHCR1 gene.
Owner:MAHIDOL UNIV +3
Application of 4-dimethyl amino nitrogen-5-hydroxy-formyl-hexyl amine for preparing anti-human immunodeficiency virus (HIV) latent medicine
InactiveCN102000048AEasy to passHigh induction activationNervous system cellsAntiviralsHuman immunodeficiencyBiological activation
The invention belongs to the medical field and relates to an application of 4-dimethyl amino nitrogen-5-hydroxy-formyl-hexyl amine for preparing an anti-human immunodeficiency virus (HIV) latent medicine. The compound has the effect on inducing activation of HIV latent cells, and the compound can be added into a cell culture fluid to induce activation of the HIV latent cells. The compound can be combined with a methyltransferase inhibitor or cell factors or an NF-kB signal activator to form an anti-HIV medicine composition; and the compound can also be combined with the existing HIV medicines to better inhibit and eliminate HIV viruses. Compared with the similar compounds, the compound of the invention has better effect on inducing activation of the HIV latent cells and lower cell toxicity. Especially, the compound can be combined with antiretroviral agents to kill the activated cells of latent infection, thereby accelerating the elimination of a latent virus reservoir. The invention provides a new method and way for thoroughly curing the acquired immune deficiency syndrome (AIDS).
Owner:FUDAN UNIV
Antiretroviral composition
InactiveCN104411300AImprove complianceOrganic active ingredientsAntiviralsMedicineANTIRETROVIRAL AGENTS
The present invention provides a pharmaceutical solid oral sprinkle composition comprising one or more antiretroviral drugs, and a method of manufacturing the same. The present invention is particularly useful for treatment of an HIV infection, AIDS related complex, or AIDS.
Owner:CIPLA LTD
Anti-retroviral compositions
The present invention relates to pharmaceutical antiretroviral compositions comprising a combination of antiretroviral agents (darunavir, dolutegravir and ritonavir), the manufacturing process thereof and use of the said compositions for the prevention, treatment or prophylaxis of HIV infection.
Owner:HETERO LABS LTD
Methods for treatment of HIV and other infections using a T-cell or viral activator and anti-retroviral combination therapy
Disclosed is a method for treating infection with a pathogen. The method involves administration of: (1) a substance which induces active pathogen replication in a cell latently infected with HIV and (2) an anti-pathogen drug. Also disclosed are methods for expanding CD4+ T cells from peripheral blood mononuclear cells isolated from human subjects in the presence of an antiretroviral drug and for treating HIV infection by infusing the expanded CD4+ cells into HIV-infected patients.
Owner:WONG JOHNSON T
Polymerase chain reaction assays for monitoring antiviral therapy and making therapeutic decisions in the treatment of acouired immunodeficiency syndrome
InactiveUS20060110726A1Inhibit deteriorationImprove the quality of lifeMicrobiological testing/measurementRegimenImmunologic Deficiency Syndromes
The present invention relates to methods of monitoring, via polymerase chain reaction, the clinical progression of human immunodeficiency virus infection and its response to antiretroviral therapy. According to the invention, polymerase chain reaction assays may be used to predict immunological decline and to identify, at an early stage, patients whose infection has become resistant to a particular antiretroviral drug regimen.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
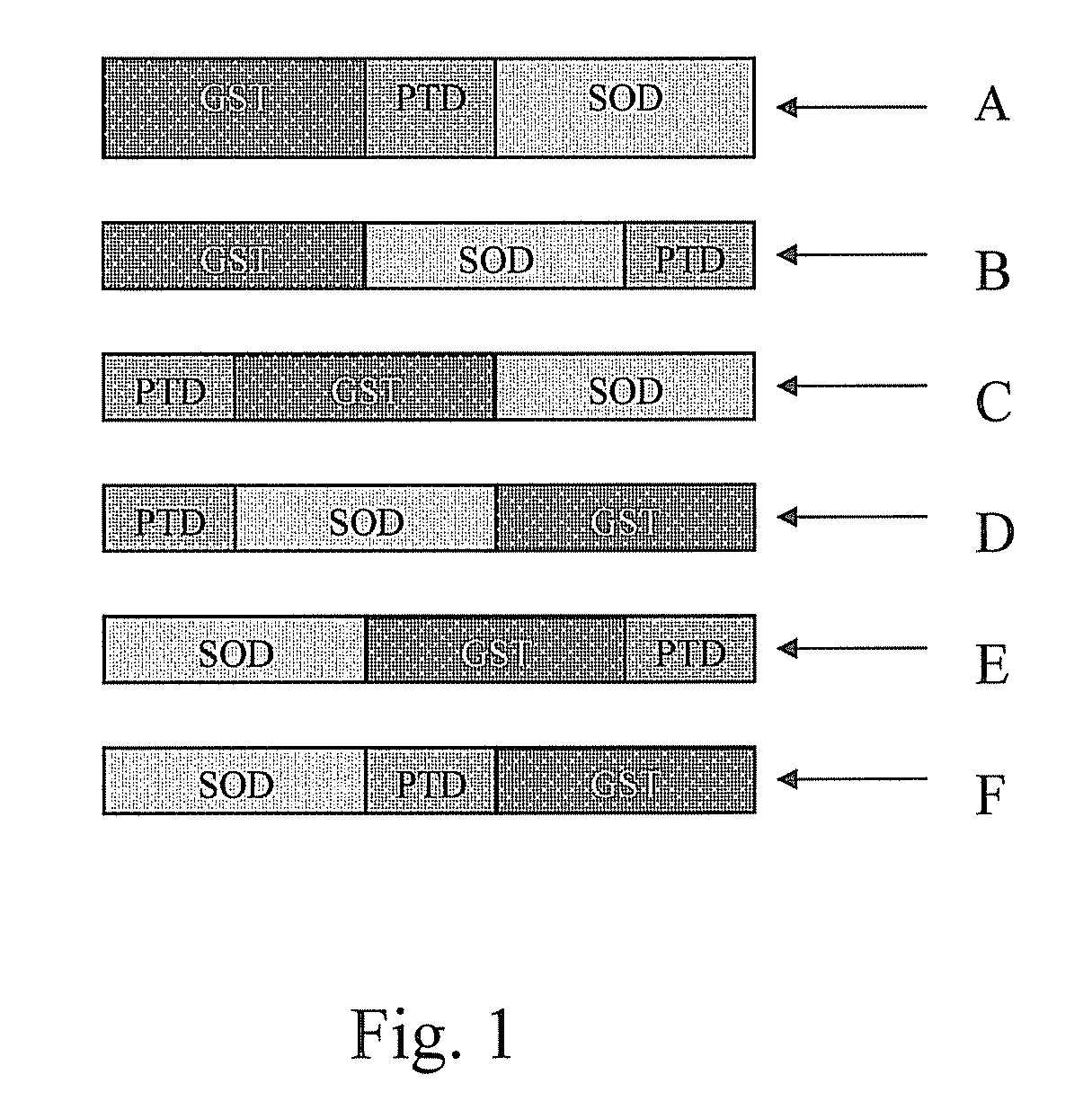
Fusion proteins and methods for treating HIV infection and aids related symptoms
ActiveUS20130336946A1Avoid virus infectionGrowth inhibitionPolypeptide with localisation/targeting motifOrganic active ingredientsTransit PeptideSuperoxide dismutase
The present invention discloses pharmaceutical compositions and methods for using a fusion protein comprising a superoxide dismutase and a transit peptide. The present invention also discloses pharmaceutical compositions and methods for using the fusion protein in combination with other antiretroviral agents for treating patients with AIDS or HTV infection.
Owner:TIANJIN XIJI BIOTECH
Anti-retroviral compositions
ActiveUS11045423B2Pharmaceutical product form changeAntiviralsPharmaceutical drugANTIRETROVIRAL AGENTS
The present invention relates to pharmaceutical antiretroviral compositions comprising a combination of antiretroviral agents (darunavir, dolutegravir and ritonavir), the manufacturing process thereof and use of the said compositions for the prevention, treatment or prophylaxis of HIV infection.
Owner:HETERO LABS LTD
Inhibition of HIV and SHIV replication with antisense interleukin-4
ActiveUS20070111958A1Inhibits viral replicationBiocideMicroencapsulation basedMedicineViral replication
A method of treating or preventing SHIV or HIV infection in a subject comprising administering a therapeutically effective amount of a antisense IL-4. The antisense IL-4 inhibits viral replication in the liver, lungs, spleen, and even the lymph nodes of the subject. Further, the antisense IL-4 can be used in combination with other antiretroviral agents or vaccines.
Owner:UNIV KANSAS MEDICAL CENT
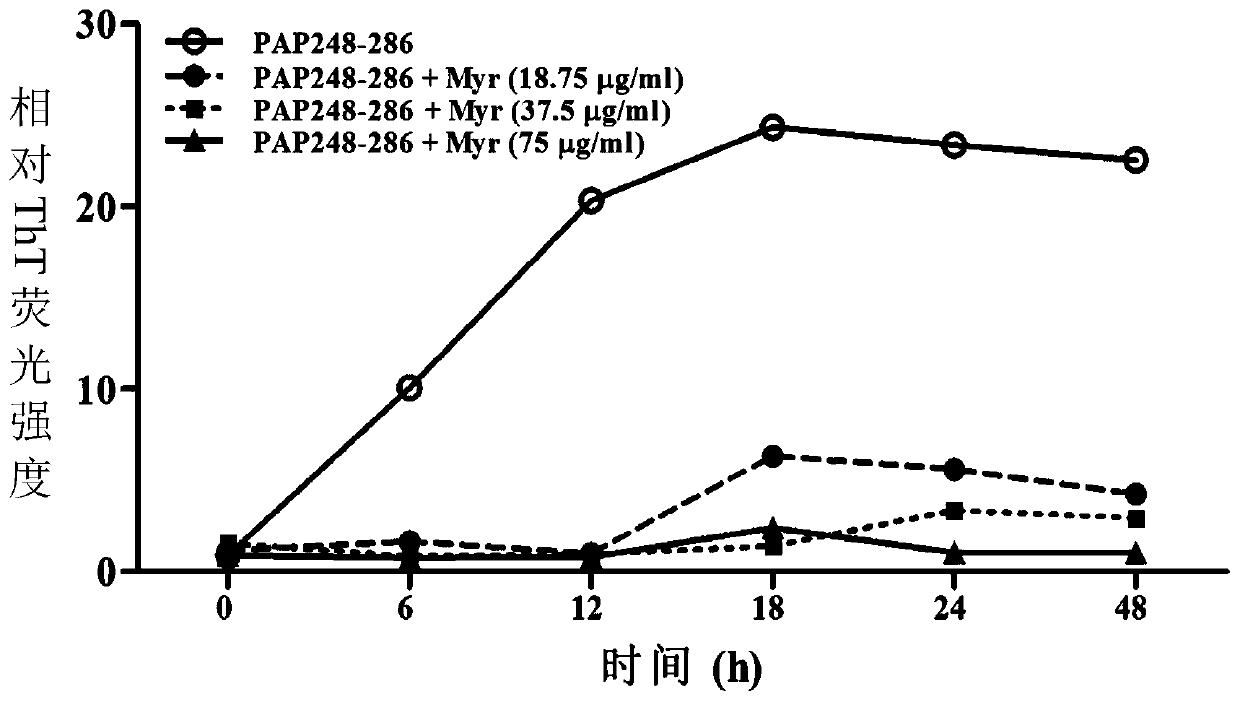
New uses of myricetin and its derivatives and a microbicide against HIV infection
ActiveCN106974904BGood anti-HIV activityInhibition formationAntiviralsHeterocyclic compound active ingredientsMaravirocClinical efficacy
The invention discloses a novel application of myricetin and derivatives thereof and an anti-HIV infection microbicide. The myricetin can inhibit formation of SEVI, destroy a mature SEVI structure and directly block off HIV infection enhancement caused by the SEVI; and in addition, the myricetin, which also has a good anti-HIV activity, can be developed into the dual-function microbicide capable of antagonizing the SEVI and resisting HIV, so as to prevent possibility that a poor clinical curative effect is caused due to existence of amyloid fibers in seminal fluid to the greatest extent; with the presence of the seminal fluid, the myricetin can coordinate with any one anti-retroviral agent, which is selected from azidothymidine, raltegravir, maraviroc, tenofovir, nevirapine and efavirenz, so as to resist the HIV; the multi-component anti-HIV infection microbicide, which contains the myricetin and the anti-retroviral agent, can be developed; and the microbicide can simultaneously act on two aspects, namely the seminal fluid and viruses, so that a better anti-HIV infection clinical curative effect can be developed.
Owner:广州南方医大科技园有限公司
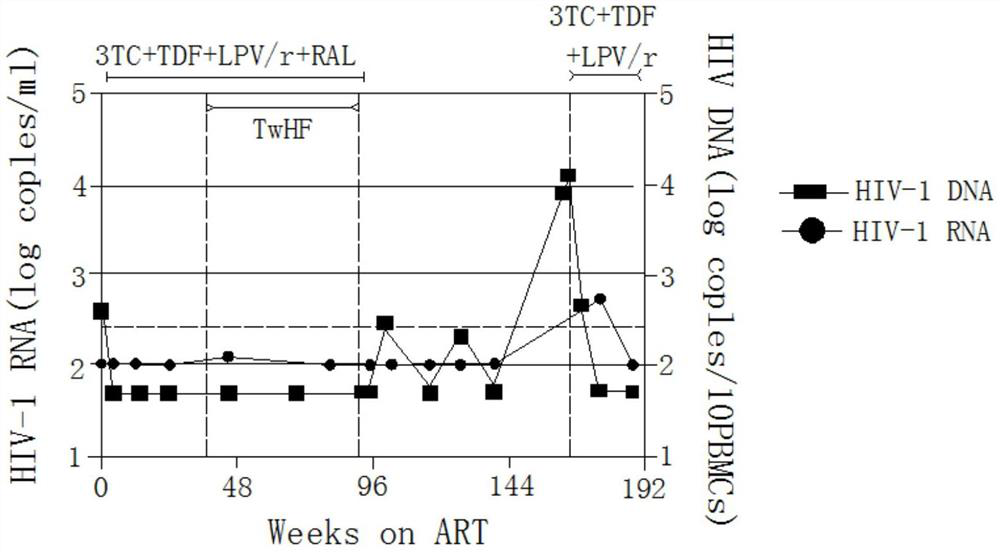
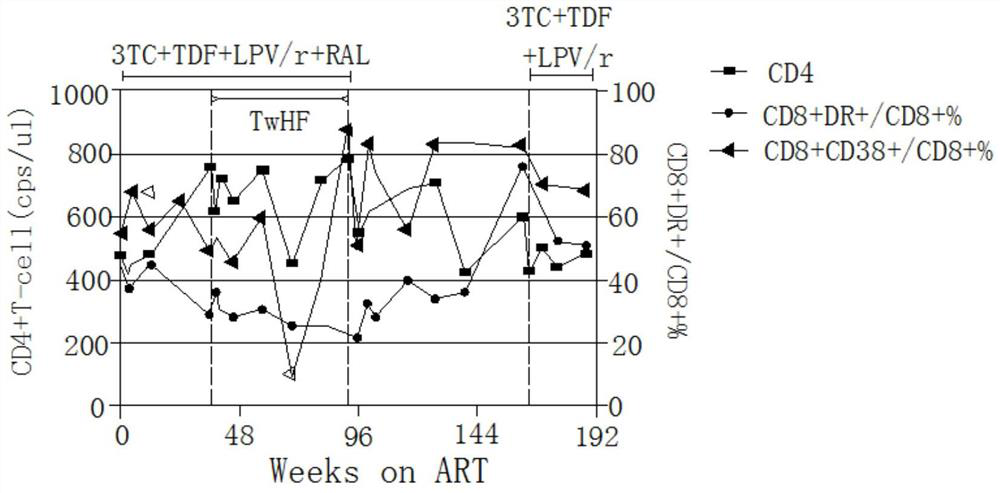
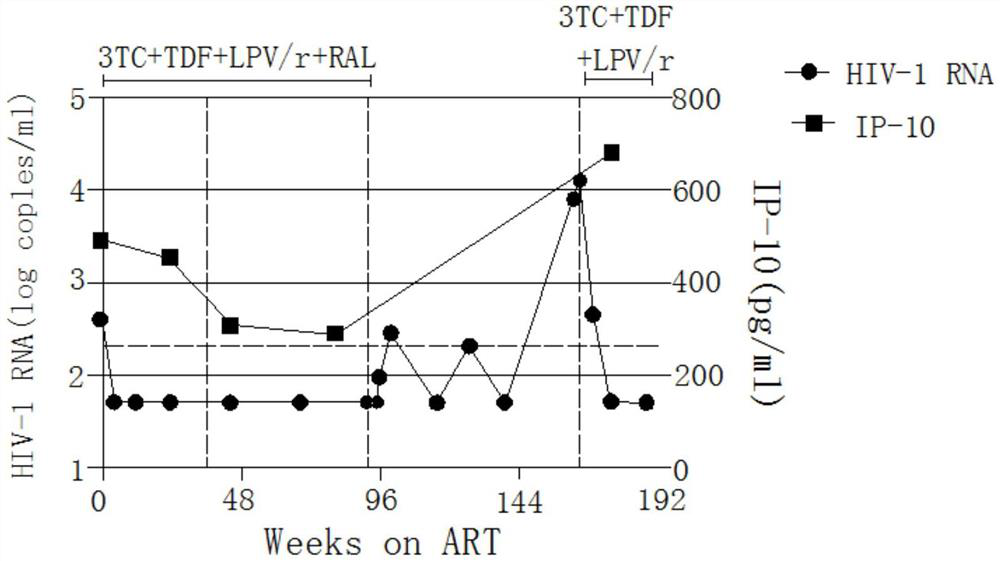
Application of Tripterygium wilfordii in the preparation of functional drugs for curing AIDS
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com