Brefeldin A and derivative and application thereof
A technology of brefeldia and feldspar, which is applied in the direction of medical preparations containing active ingredients, organic active ingredients, antiviral agents, etc., can solve the problems of large toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1F29-61A
[0086] The obtained fractions were separated by reverse-phase high-performance liquid chromatography (SepaxAmethystC-18 (5μm, 21.2×250mm) chromatographic column, detection wavelength 210nm), and the mobile phase used was a methanol-water system with a volume ratio of 55:45 and wasocratic at 10mL / min. Elution, collect the chromatographic peak of 36-39min, recover solvent, obtain compound F29-61A, such as Figure 1-4 and 1-5 Shown, according to nuclear magnetic resonance data, its structure is as follows, is formula I structure, R 1 is OH; R 2 for H; R 3 for H; R 4 is OH; R 5 for H; R 6 for CH 3 , the molecular formula is C 16 h 24 o 4 , The compound was identified as brefeldin A.
[0087]
[0088] C11 cells were planted in a 96-well plate at 2×10E4 cells per well, and 100 μL of 1640 medium (Gibco) containing 10% FBS (Gibco) was added to each well. After the cells were treated with F29-61A for 24 / 48h, the green fluorescent expression of the cells was observed under...
Embodiment 2F29-63F
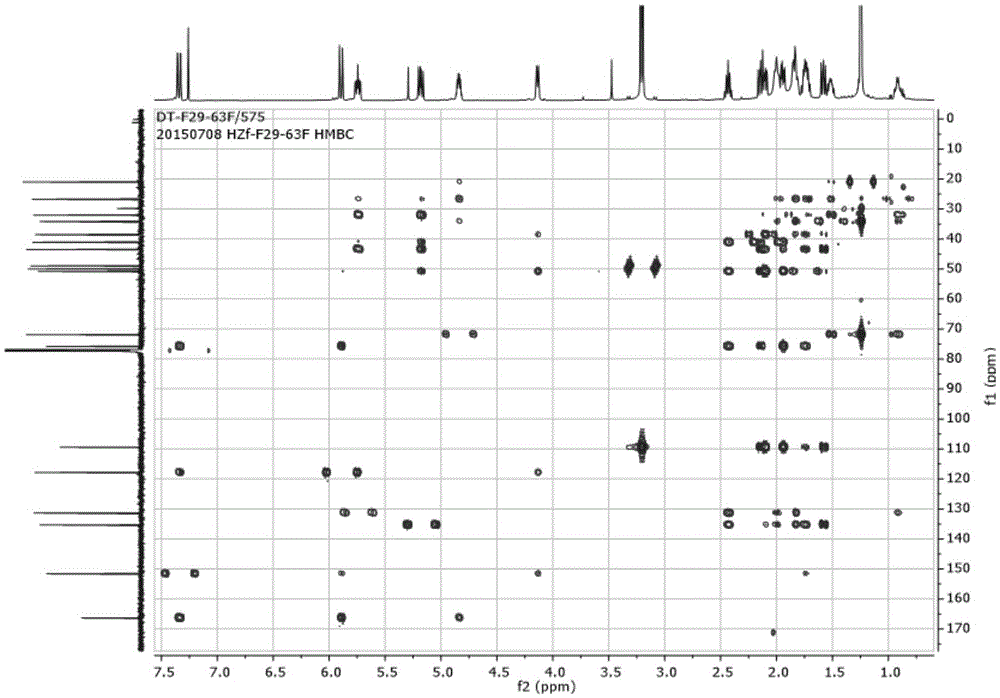
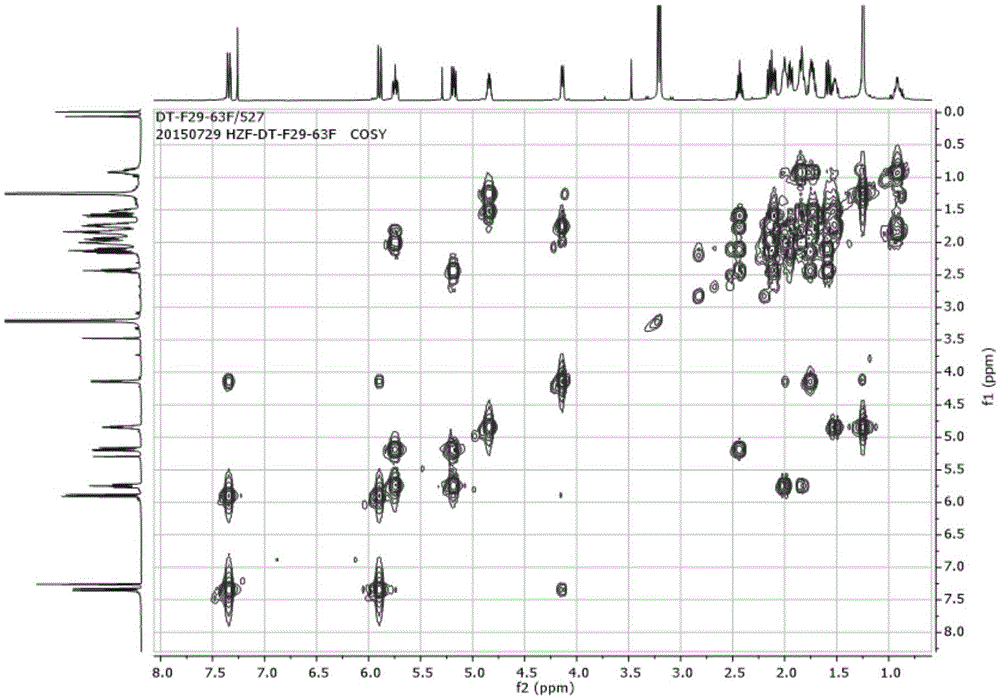
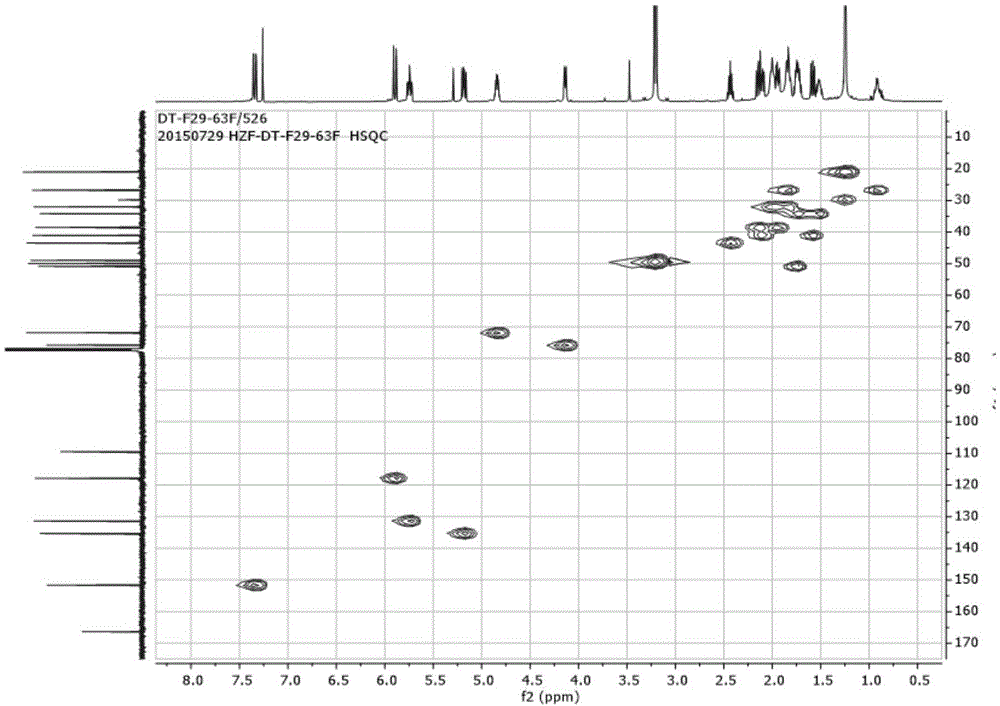
[0092] The obtained fractions were separated by reverse-phase high-performance liquid chromatography (SepaxAmethystC-18 (5μm, 21.2×250mm) chromatographic column, detection wavelength 210nm), the mobile phase used was a methanol-water system with a volume ratio of 65:35 and an isocratic flow rate of 12mL / min. After eluting, collecting the chromatographic peak at 36-40 min, and recovering the solvent, the compound F29-63F having anti-HIV latent activity of the present invention can be obtained. Such as diagram 2-1 , 2-2 , 2-3, 2-4, 2-5, 2-6, 2-7, according to the NMR data, the compound was identified as 7,7-dimethoxybrefeldinC.
[0093]
Embodiment 3F29-84
[0095] The obtained fractions were separated by reversed-phase high-performance liquid chromatography (SepaxAmethystC-18 (5μm, 21.2×250mm) chromatographic column, detection wavelength 210nm), the mobile phase used was a methanol-water system with a volume ratio of 50:50 and an isocratic flow rate of 10mL / min. After elution, the chromatographic peaks at 26-30 min were collected, and the solvent was recovered to obtain compound F29-84F. Such as Figure 3-1 , 3-2 , 3-3, 3-4, 3-5, 3-6, 3-7, according to the NMR data, the compound was identified as 6α-hydroxybrefeldinC.
[0096]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com