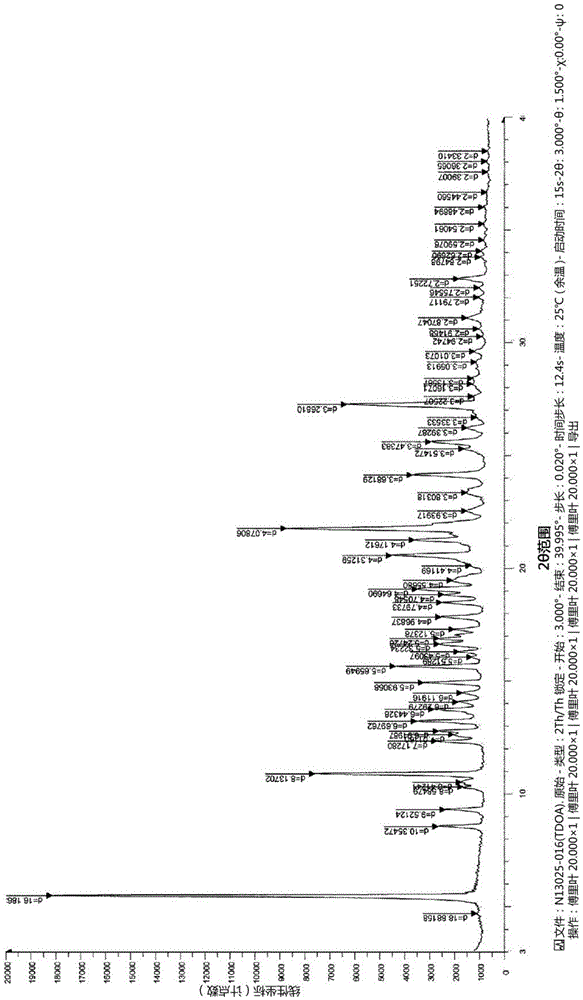
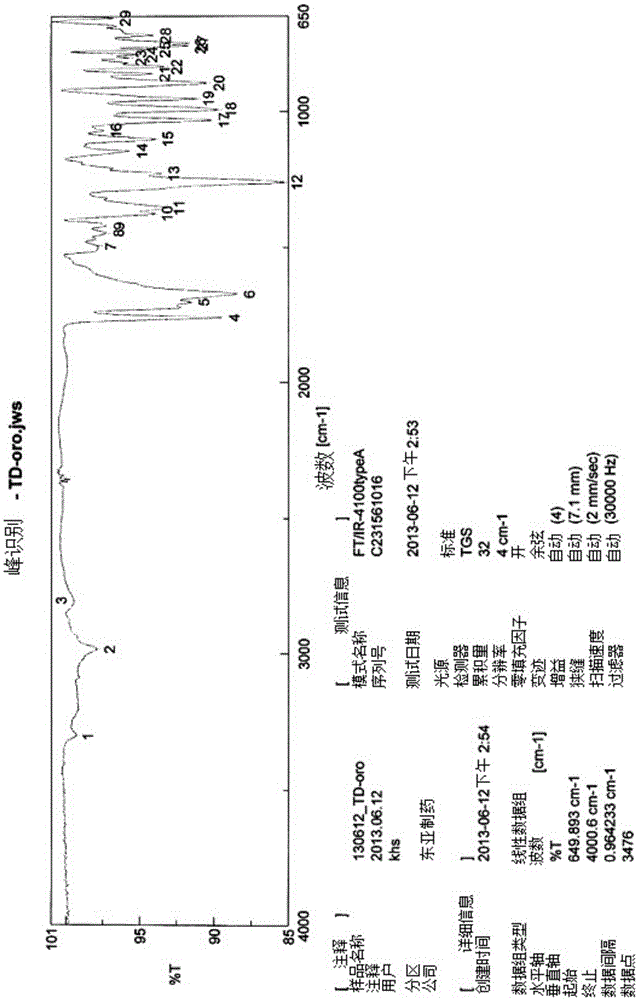
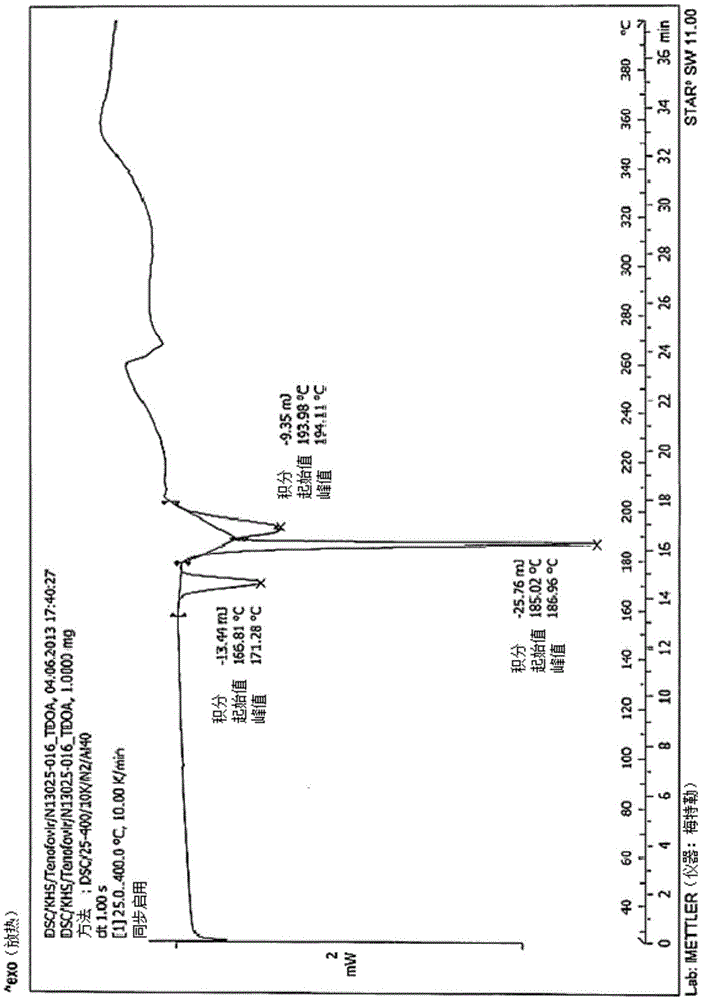
Novel tenofovir disoproxil salt and the preparation method thereof
A technology of tenofovir and tenofovir disoproxil, which is applied in the field of new tenofovir salt and its preparation, can solve the physical and chemical properties of tenofovir disoproxil fumarate and other problems, and achieve excellent preparation processing performance , stable treatment, good photostability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] preparation of tenofovir disoproxil orotate (1)
[0056] Tenofovir (25 g) was added to the reaction apparatus and dissolved with methanol (125 ml). Orotic acid (7.96 g) and purified water were added to the reaction solution, which was then suspended at room temperature for 10 minutes and heated at an internal temperature of 70° C. for 1 hour until all solids had dissolved. The reaction solution was reacted at an internal temperature of 20°C for 1-2 hours to obtain crystals. The obtained crystals were vacuum filtered, washed with methanol:water (100ml, 1:3), and dried. The dried crystals were vacuum dried at an internal temperature of 60° C. for 15 hours.
[0057] Purity (by HPLC): 99.3%; Yield: 27.69g (85%)
[0058] 1 HNMR(DMSO-d6)δppm11.29(s,1H,NH), 10.8(s,1H,NH), 8.14(s,1H,CH), 8.04(s,1H,CH), 7.36(s,2H, NH 2 ), 5.97(s,1H,CH), 5.48-5.56(m,4H,CH 2 ), 4.79-4.81 (m,2H,CH 2 ), 4.14-4.26 (ddd, 2H, CH 2 ), 3.92-4.01(m,3H,CH), 1.22(s,12H,CH 3 ), 1.04(s,3H,CH 3 ). ...
Embodiment 2
[0059] preparation of tenofovir disoproxil orotate (2)
[0060] Tenofovir (25 g) was added to the reaction apparatus and dissolved with methanol (125 ml). Orotic acid (7.96 g) and purified water were added to the reaction solution, which was then suspended at room temperature for 10 minutes and warmed at an internal temperature of 70° C. for 1 hour until all solids had dissolved. The reaction solution was reacted at an internal temperature of 20°C for 1-2 hours to obtain crystals. The obtained crystals were vacuum filtered, washed with ethanol:water (100ml, 1:3), and dried. The dried crystals were vacuum dried at an internal temperature of 60° C. for 15 hours.
[0061] Purity (by HPLC): 99.3%; Yield: 27.58g (84.7%)
[0062] 1 HNMR: same as Example 1.
Embodiment 3
[0063] preparation of tenofovir disoproxil orotate (3)
[0064] Tenofovir (25 g) was added to the reaction apparatus and suspended in purified water (750 ml). Orotic acid (7.96 g) was added to the reaction solution and warmed at an internal temperature of 70-80° C. for 1-2 hours until all solids had dissolved. The reaction solution was reacted at an internal temperature of 20°C for 1-2 hours to obtain crystals. The obtained crystals were subjected to vacuum filtration, washed with purified water (100 ml), and dried. The dried crystals were vacuum dried at an internal temperature of 60° C. for 15 hours. This example provides an environmentally friendly method for preparing crystalline tenofovir disoproxil orotate without using organic solvents.
[0065] Purity (tested by HPLC): 98%; Yield: 26g (79.8%)
[0066] 1 HNMR: same as Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com