Darunavir inhaled dry powder pharmaceutical composition and preparation method thereof
A technology of darunavir and its composition, which is applied in the field of preparation of inhalation dry powder pharmaceutical composition, which can solve the problems of increasing the risk of powder agglomeration or agglomeration, low solubility of darunavir, and large amount of dissolving solvent used
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
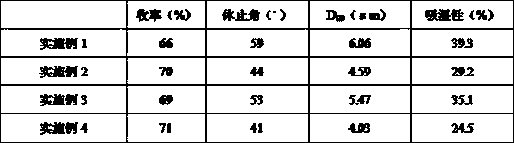
Embodiment 1
[0036] Embodiment 1: Preparation of amorphous darunavir
[0037] Dissolve darunavir in 50% ethanol solution, the solid content of the solution is 3% (w / v), spray dry according to the following process parameters, collect the obtained particles, and calculate the yield,
[0038] Inlet temperature: 120°C;
[0039] Spray flow: 6ml / min;
[0040] Atomization pressure: 180KPa;
[0041] Spray gas flow rate: 0.6 m 3 / min.
Embodiment 2
[0042] Example 2: Preparation of amorphous darunavir granules
[0043] Weigh Darunavir: Mannitol: Leucine in a ratio of 7:2:1 (w / w / w) and dissolve in 50% ethanol solution, the solid content of the solution is 3% (w / v), according to the implementation The process parameters in Example 1 were spray-dried, and the resulting particles were collected.
Embodiment 3
[0044] Example 3: Preparation of amorphous darunavir granules
[0045] According to the ratio of 7:2:1 (w / w / w), darunavir: lactose: leucine was dissolved in 50% ethanol solution, and the solid content of the solution was 3% (w / v), according to the example The process parameters in 1 are spray-dried, and the resulting particles are collected.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com