A kind of industrialized production method of high-purity darunavir intermediate
A technology of darunavir and production method, applied in the field of darunavir intermediates, can solve the problems of low product yield, low raw material reduction efficiency, and obtaining quality target products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
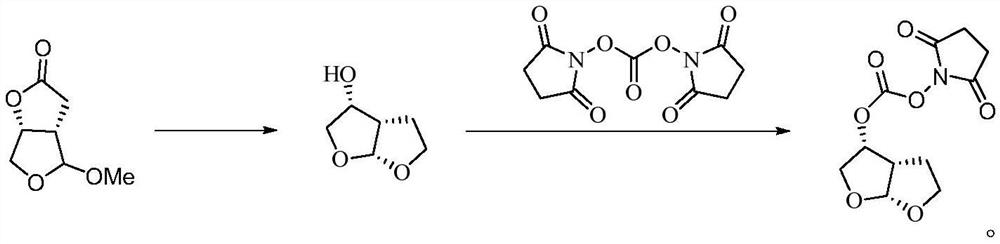
[0027] (1) Under the protection of nitrogen, put 600kg tetrahydrofuran into a clean and dry 2000L reactor, and 100kg racketone (molar weight 158.06, molar number 633mol);
[0028] (2) Control the temperature of the reactor <25°C, add 28.7kg of sodium borohydride (mole weight 37.83, mole number 759mol) in batches, add anhydrous lithium chloride 5.4kg (mole weight 42.39, mole number 127mol) in batches, slowly Warming up to reflux reaction;
[0029] (3) After the reaction of the central control raw material is completed, the temperature of the reactor is lowered to below 10°C, and 175kg of concentrated hydrochloric acid is added dropwise to the reactor, and the pH is adjusted to 1-3. After the addition is completed, the reaction is kept for 2 hours; Neutralize the reaction pH to 6-7 with sodium carbonate;
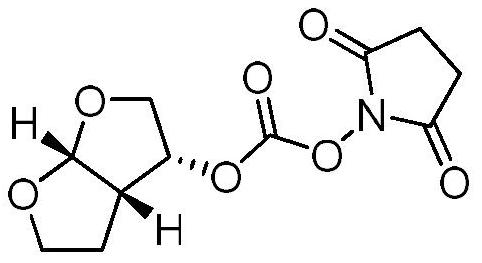
[0030] (4) centrifugation, concentrated to obtain 80kg furan alcohol, GC>95%;
[0031] (5) Under nitrogen protection, drop 800kg dichloromethane into clean, dry 2000L reacto...
Embodiment 2
[0036] (1) Under the protection of nitrogen, put 400kg of acetone and 100kg of racketone into a clean, dry 2000L reactor (molar weight 158.06, molar number 633mol);
[0037] (2) Control the temperature of the reactor <25°C, add 28.8 kg of lithium aluminum hydride (37.95 moles, 759 mol moles) in batches; add 8.4 kg aluminum chloride (133.3 moles, 63.3 moles moles) in batches, and slowly raise the temperature to reflux reaction;
[0038] (3) After the reaction of the central control raw materials is completed, the temperature of the reactor is lowered to below 10°C, and 175kg of concentrated hydrochloric acid is added dropwise to the reactor, and the pH is adjusted to 1-3. After the addition, the temperature is kept for 2 hours. After the incubation, neutralize the reaction pH to 6-7 with sodium carbonate;
[0039] (4) centrifugal, concentration obtains 75.5kg furan alcohol, GC﹥95%;
[0040] (5) Under nitrogen protection, drop 378kg toluene into a clean, dry 2000L reactor, 75....
Embodiment 3
[0045] (1) Under the protection of nitrogen, put 700kg of 2-methyltetrahydrofuran into a clean and dry 2000L reactor, and 100kg of racketone (molar weight 158.06, molar number 633mol);
[0046] (2) Control the temperature of the reactor <25°C, add 26.3kg of sodium borohydride (37.83 moles, 696mol moles) in batches, add 5.4kg anhydrous lithium chloride (42.39 moles, 127mol moles) in batches, and slowly Warming up to reflux reaction;
[0047] (3) After the reaction of the central control raw materials is completed, the temperature of the reactor is lowered to below 10°C, and 175kg of concentrated hydrochloric acid is added dropwise to the reactor, and the pH is adjusted to 1-3. After the addition, the temperature is kept for 2 hours. After the incubation, neutralize the reaction pH to 6-7 with sodium carbonate;
[0048] (4) centrifugation, concentrated to obtain 80kg furan alcohol, GC>95%;
[0049] (5) Under nitrogen protection, drop into 960kg acetonitrile, 80kg furan alcohol (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com